Abstract
With the rapidly expanding role of immune checkpoint inhibitor therapy in advanced cancer treatment, an increasing number of new immune-related adverse events (irAEs) are being reported. The present report describes sarcoid-like granulomatosis of the lung as a distinct type of irAE with characteristic clinical, imaging, and histologic features. In patients treated with immune checkpoint inhibitors, sarcoid-like granulomatosis of the lung presented with a focal area of consolidation in the lung, which was often nodular or round, in the absence of new or enlarging lymphadenopathy on imaging. Histologic examination demonstrated non-necrotizing granulomas and an absence of malignant cells. The patients were free of new or worsening respiratory symptoms, despite the development of lung parenchymal consolidations. Holding the immune checkpoint inhibitors led to the spontaneous resolution of the findings, without any specific treatment for the abnormality. Awareness of the manifestations of sarcoid-like granulomatosis of the lung as a distinct type of irAE will improve management of patients treated with immune checkpoint inhibitors.
Keywords: immune-checkpoint inhibitors, immune-related adverse event, sarcoid-like granulomatosis, cancer, drug toxicity
INTRODUCTION
Immune-checkpoint blockade using PD-1/PD-L1 inhibitors and CTLA-4 inhibitors has brought a paradigm shift for the treatment of a number of advanced cancers. Several agents have been approved for the treatment of a variety of solid and hematologic malignancies, and indications of these agents continue to rapidly expand.
The unique immunologic toxicities noted in cancer patients treated with immune checkpoint blockade are often referred to as immune-related adverse events (irAEs)(1–3). Among various irAEs, sarcoid-like granulomatosis and lymphadenopathy has been noted in up to 5-7% of patients. The underlying pathophysiology of the entity is not fully understood. However, as in the cases of idiopathic sarcoidosis, it is speculated to be due to uncontrolled T-helper 1 mediated immune responses, which may result from enhanced immune responses by immune checkpoint blockade in the setting of cancer immunotherapy (4). Sarcoid-like granulomatosis and lymphadenopathy present mostly in the form of mediastinal and hilar lymphadenopathy, sometimes with accompanying multifocal pulmonary nodular abnormalities resembling parenchymal sarcoidosis with a propensity to involve lymphatic systems (2, 4, 5). However, the development of a focal consolidative opacity in the lung in the absence of new or enlarging lymphadenopathy has not been previously defined as a form of irAE. We report a series of four cases of sarcoid-like granulomatosis of the lung with distinct clinical and imaging features, which will contribute to the increased awareness and appropriate management of this under-recognized entity.
MATERIALS AND METHODS
The study included four patients with advanced cancers treated with immune-checkpoint inhibitor therapy who developed sarcoid-like granulomatosis of the lung as an irAE. The medical records and imaging studies of the patients were retrospectively reviewed under the approval of the institutional review board with the waiver for the informed consent. The study was in compliance with Health Insurance Portability and Accountability Act.
Review of the imaging studies was performed using clinical chest CT scans obtained as a part of a standard care for oncologic follow-up, by a board-certified chest radiologist (M.N.) with 10 years of experience in thoracic and oncologic imaging. The lung findings of sarcoid-like granulomatosis are assessed for distributions, morphological patterns of abnormalities, and bi-dimensional diameters measured for a dominant lesion. On follow-up imaging scans, the changes of the findings in comparison with the prior scans were qualitatively evaluated.
Review of the histologic findings was performed by a board-certified pathologist (L.S.) with 10 years of experience in thoracic pathology. The specimens were obtained as a part of standard care and were stained with hematoxylin and eosin (H&E), and were evaluated to exclude the presence of malignancy and to document patterns of lung parenchymal changes, dominant cell populations, and the presence of non-necrotizing granulomas.
CASE SERIES
The clinical characteristics of the patients and the imaging and histologic findings of sarcoid-like granulomatosis of the lung are summarized in Table 1. One patient with stage IV lung adenocarcinoma and two patients with advanced melanoma were treated with pembrolizumab monotherapy. One patient had stage IV classical Hodgkin lymphoma and was treated with nivolumab and lirilumab combination therapy on a phase I trial. The remaining two patients with advanced melanoma were treated with pembrolizumab monotherapy. The time from the initiation of immune checkpoint inhibitor therapy to the onset of sarcoid-like granulomatosis of the lung ranged from 8.0 to 20.7 months, with a median of 12.8 months. None of the patients had new or worsening respiratory symptoms. All patients initially responded to their immune-checkpoint inhibitor therapy. Two patients (Patient 1 and 4) had continued response, whereas the remaining two patients have had tumor recurrence (in lymph nodes for Patient 2 and in brain for Patient 4) at the time of development of sarcoid-like granulomatosis.
Table 1.
Clinical characteristics of the patients and the imaging and histologic findings of sarcoid-like granulomatosis of the lung
| Baseline Patient Characteristics | ||||
|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Age/Sex | 61/Female | 29/Male | 65/Female | 74/Male |
| Tumor type | Lung adenocarcinoma | Hodgkin lymphoma | Melanoma | Melanoma |
| Prior therapy | Carboplatin, pemetrexed, plus bevacizumab | ABVD; ICE; GND; autologous stem cell transplant with BEAM; brentuximab | Ipilimumab plus nivolumab | Carboplatin plus etoposide; ipilimumab; temozolomide; |
| Immunotherapy agent | Pembrolizumab | Nivolumab and Lirilumab | Pembrolizumab | Pembrolizumab |
| Treatment regimen | 200 mg every 3 weeks | Nivolumab: 3mg/kg every 2 weeks; Lirilumab: 3mg/kg, every 4 weeks | 2mg/kg every 3 weeks | 2mg/kg every 3 weeks |
| Clinical Characteristics of Sarcoid-like Granulomatosis of the Lung | ||||
| Onset since therapy initiation | 8.0 months | 20.7 months | 11.3 months | 14.3 months |
| New or worsening respiratory symptom | None | None | None | None |
| Treatment for sarcoid-like granulomatosis | Hold pembrolizumab | Hold nivolumab and lirilumab | Hold pembrolizumab | Hold pembrolizumab |
| Imaging Features of Sarcoid-like Granulomatosis of the Lung (Fig. 1) | ||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Lung |
|
|
|
|
| New or enlarging lymph nodes | None | None | None | None |
| Follow-up imaging |
|
|
|
|
| Histologic Findings of Sarcoid-like Granulomatosis of the Lung (Fig. 2) | ||||
| Patient 1 | Patient 2* | Patient 3 | Patient 4 | |
|
|
|
|
|
The patient also underwent biopsy of supraclavicular node that has been involved by lymphoma, which showed distorted architecture by nodules containing loose aggregates of histiocytes and giant cells with admixed lymphocytes rimmed by circumferential fibrosis, representing a pattern reminiscent of sarcoidosis.
ABVD: Doxorubicin Hydrochloride, Bleomycin, Vincristine Sulfate, Dacarbazine
ICE: Ifosfamide, Carboplatin, and Etoposide
GND: Gemcitabine, Navelbine, and Doxorubicin
BEAM: Carmustine, Etoposide, Cytarabine, and Melphalan
GGO: ground glass opacity
FDG: fluorodeoxyglucose
PET: positron emission tomography
The imaging features of sarcoid-like granulomatosis of the lung were characteristic in that it presented with a focal area of consolidation in the lung parenchyma, often round or nodular with a surrounding halo of ground glass opacities (GGO) (Fig. 1). In all patients, the lung findings appeared in the lung regions that were not previously involved by their primary tumor. In Patient 1, a development of focal nodular consolidation with surrounding halo of GGO in the superior segment of the right lower lobe was noted at eight months of therapy. A near complete resolution of the finding was noted on follow-up CT scans performed two and four months after holding pembrolizumab (Fig. 1). In Patient 2, a small nodular opacity with a GGO halo was initially noted, which increased to a focal consolidation with a GGO halo in one month. The lesion regressed two months after holding nivolumab and lirilumab therapy, while the patient started another regimen of immune checkpoint inhibitors (Fig. 1). Patient 3 presented with a focal round consolidation with a GGO halo in the left upper lobe that developed at 11.3 months of therapy. The finding spontaneously resolved one month after holding pembrolizumab (Fig. 1). Patient 4 had a different course and originally developed a focal area of subcentimeter centrilobular nodular opacities, which gradually developed into a larger focal consolidation over the course of 16 months (Fig. 1). Follow-up CT scan performed ten months after holding pembrolizumab demonstrated decrease of the lesion. None of the patients demonstrated new or enlarging mediastinal, hilar, or other lymphadenopathy, which are often considered to be the hallmarks of sarcoid-like granulomatosis and lymphadenopathy in the setting of irAE (1, 2, 4, 5).
Figure 1. Imaging characteristics of sarcoid-like granulomatosis of the lung on chest CT.
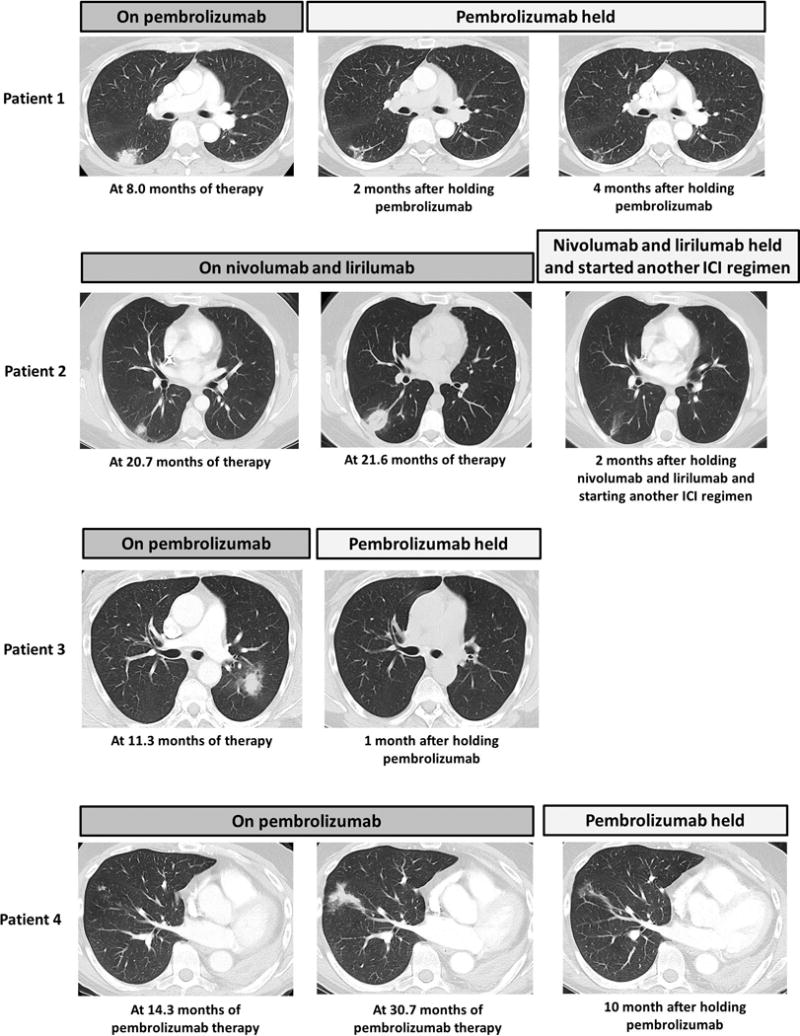
Patient 1: CT scan of the lung at 8 months of therapy and follow-up CT scans 2 and 4 months after holding pembrolizumab. Patient 2: CT scans of the lung at 20.7 and 21.6 months of therapy and a follow-up CT scan 2 months after holding nivolumab and lirilumab, while starting another immune checkpoint inhibitor (ICI) treatment. Patient 3: CT scan of the lung at 11.3 months of therapy and a follow-up CT scan 1 month after holding pembrolizumab. Patient 4: CT scans of the lung at 14.3 and 30.7 months of therapy and follow-up CT scan 10 months after holding pembrolizumab.
Histologic findings were available in three of the four patients who underwent biopsy of the lung lesions (Fig. 2). Non-necrotizing granulomas were present in two of the cases (Patient 1 and Patient 4), which were histologically consistent with sarcoid-like granulomatosis of the lung. In Patient 1, the alveolated parenchyma was diffusely replaced by large aggregates of epithelioid histiocytes, forming non-necrotizing granulomas. Scattered acute inflammatory cells, predominantly polymorphonuclear cells are admixed with the granulomas (Fig. 2). In Patient 2, the lung specimen showed alveolar spaces were filled by organizing fibroblasts, alveolar macrophages, numerous lymphocytes focally forming aggregates, and scattered eosinophils. Rare epithelioid giant cells were identified (Fig. 2), without definitive granulomas. However, a biopsy of a supraclavicular lymph node, known to be involved in lymphoma, showed a distorted architecture representing a pattern reminiscent of sarcoidosis. Although histologic sampling was not performed in Patient 3, the clinical and imaging characteristics and the follow-up course were similar with other cases with histologic confirmation. In Patient 4, core biopsy specimen of the lung lesion demonstrated interstitial non-necrotizing granulomas with lymphocytic infiltrates, as well as peri-airway granulomas with prominent giant cells (Fig. 2).
Figure 2. Histologic characteristics of sarcoid-like granulomatosis of the lung.
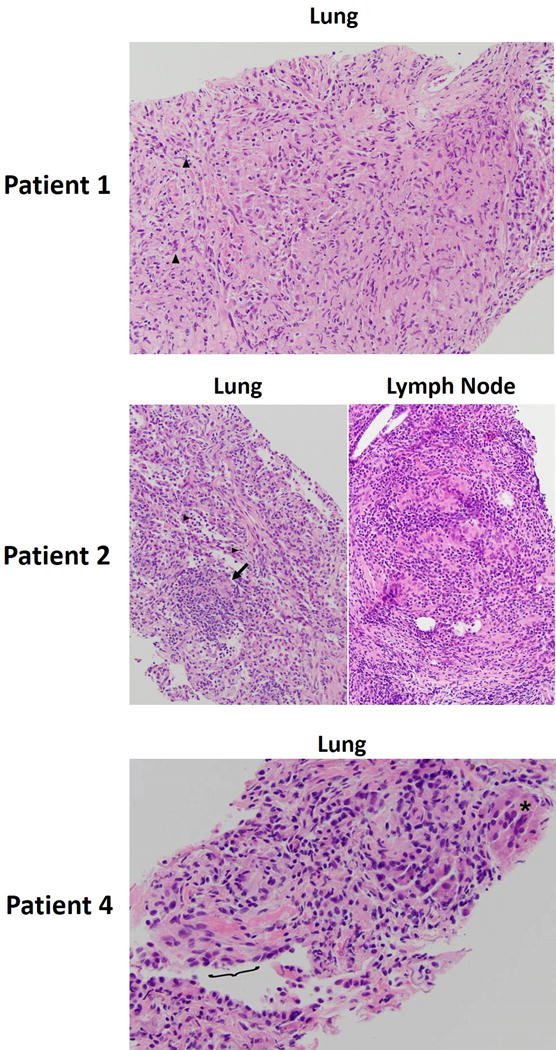
Patient 1: Hematoxylin and eosin (H&E) stain of the alveolated parenchyma of the lung. Inflammatory cells (predominantly polymorphonuclear cells) indicated by arrowheads. Patient 2: Left - H&E stain of the lung alveolar spaces. Eosinophils are indicated by arrowheads, and epithelioid giant cells indicated by the arrow. Right – H&E stain of the supraclavicular lymph node biopsy. Patient 4: core biopsy of the lung lesion. Peri-airway granulomas with prominent giant cells are indicated (asterisk). Terminal respiratory epithelium is denoted by the bracket.
Immune checkpoint inhibitor therapy was held in all patients, and no other specific treatment was given for sarcoid-like granulomatosis of the lung. In all patients, the lung findings have resolved or decreased on follow-up CT scans performed at 1-4 months after holding the original therapy (Table 1 and Fig. 1). At the time of review, two patients (Patient 1 and 4) were observed without restarting pembrolizumab or other therapy and remained progression-free from their tumor. Patient 2 started another regimen of immune checkpoint inhibitors immediately after the development of sarcoid-like granulomatosis. Patient 3 restarted pembrolizumab, given a spontaneous resolution of the lung findings on CT performed one month after holding pembrolizumab, and remains on therapy without recurrent sarcoid-like granulomatosis for three months.
DISCUSSION
The present report describes the clinical, radiologic, and histologic characteristics of sarcoid-like granulomatosis of the lung, which can occur in the absence of new or enlarging lymphadenopathy, as a distinct irAE in a variety of tumor types in patients treated with immune checkpoint inhibitors. Recognition of this entity as an immunologic reaction, and not a sign of disease progression, will improved appropriate management of patients treated with immunotherapy.
Radiographically, sarcoid-like granulomatosis of the lung presented with focal areas of consolidation in the lung, often nodular or round, in the absence of new or enlarging lymphadenopathy. In all cases presented here, the parenchymal lesions were noted as a new abnormality on chest CT scans performed for oncology follow-up and response assessments. Appearance of new lung abnormalities in patients treated with immune checkpoint inhibitors often raises the suspicion of drug-related pneumonitis, which is rare but clinically significant and potentially life-threatening (6–8). Whereas pneumonitis most commonly presents with diffuse or multifocal lung disease with new or worsening respiratory symptoms (6–8), sarcoid-like granulomatosis of the lung, by contrast, most commonly appears as a focal consolidation with a lack of clinical symptoms. Recognition of a focal consolidation with surrounding GGO as a characteristic feature of sarcoid-like granulomatosis of the lung is important because it is distinct from the imaging features of pneumonitis, which most commonly presents bilateral multifocal areas of consolidations (cryptogenic organizing pneumonia (COP) pattern) or interstitial lung disease with interlobular septal thickening and GGO in a subpleural and basilar distribution (non-specific interstitial pneumonia (NSIP) pattern) (6–8).
Additionally, the appearance of a “new lesion” on imaging often indicates disease progression in the setting of tumor response evaluations.(9) Therefore, familiarity with the characteristic appearance of the lung abnormalities seen in sarcoid-like granulomatosis of the lung, including a focal round consolidation with surrounding GGO, is important to accurately interpret the imaging studies and adequately direct patient management. Two cases were evaluated with FDG-PET and demonstrated FDG avidity of the lesions, which may also be misleading. It is important to be reminded that FDG avidity is non-specific and can be noted in infectious and inflammatory lesions. Awareness of sarcoid-like granulomatosis as a differential diagnosis of a new lung lesion during immune checkpoint inhibitor therapy and interpretation of imaging findings referring to the characteristic features are critical to avoid misdiagnosis of the entity as tumor progression, which may mislead patient management and subsequent treatment. When differentiating with tumor progression, the assessment of systemic tumor burden and evaluation of tumor progression in other sites beyond lungs can be helpful, along with correlation with overall clinical performance status.
Clinically, all cases of sarcoid-like granulomatosis of the lung were free of new or worsening respiratory symptoms, which is similar to the cases with sarcoid-like lymphadenopathy (2, 4, 5). However, in sarcoid-like granulomatosis of the lung, the clinically silent nature of the entity, in spite of the recognizable new lung abnormality on imaging, provides an important clue to accurately diagnose this particular entity because the feature makes other differential diagnoses, including pneumonitis and infectious pneumonia, much less likely. Excluding infectious pneumonia can be challenging based on imaging alone, and clinical correlation with infectious symptoms is crucial. It should also be emphasized that a dialogue between clinical providers and radiologists is essential to reach the accurate diagnosis of the entity, while evaluating the possibilities of major differential diagnoses, including pneumonitis, tumor progression, and pneumonia.
In terms of the treatment, in our series, holding the immune checkpoint inhibitors led to the resolution of the findings, without any specific treatment such as corticosteroids, which is also important when considering an adequate patient management plan for this specific type of irAE. The entity is also distinct in this regard from pneumonitis, which often requires corticosteroids therapy or sometimes even more intensive therapy, indicating the importance of accurate distinction of the two phenomena.
Histologically, non-necrotizing granulomas were present in the lung specimen in 2 of the 3 cases that underwent biopsy, confirming the granulomatous nature of the phenomenon. In the remaining case, the lung specimen showed organizing fibroblasts with inflammatory changes, although a sarcoid-like reaction was present in the specimen from the lymph node with ongoing tumoral involvement. The lymph nodes of the patient did not show new or further size increase in images at the time of the development of lung abnormalities. Although the histologic sampling helped to understand the biologic nature of the under-recognized entity in these initial cases, increased awareness of the distinct features may limit the need for invasive procedures for this clinically silent and self-limited entity upon holding of immune checkpoint inhibitors.
In conclusion, sarcoid-like granulomatosis of the lung is a distinct form of irAE, characterized by development of a focal consolidation in the lung in the absence of new or enlarging lymphadenopathy on imaging in patients treated with immune checkpoint inhibitors. The entity was clinically silent, and often resolved after holding immune checkpoint inhibitors, without a need for specific treatment. Increased recognition of the distinct features of sarcoid-like granulomatosis of the lung allows for accurate diagnosis and appropriate patient management and helps to maximize the benefit of immune checkpoint inhibitor therapy.
Acknowledgments
The investigator, M.N., was supported by 1R01CA203636 (NCI).
References
- 1.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment. European journal of radiology. 2015;84(7):1259–68. doi: 10.1016/j.ejrad.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tirumani SH, Ramaiya NH, Keraliya A, et al. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer immunology research. 2015;3(10):1185–92. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. European journal of cancer. 2016;54:139–48. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Berthod G, Lazor R, Letovanec I, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(17):e156–9. doi: 10.1200/jco.2011.39.3298. [DOI] [PubMed] [Google Scholar]
- 5.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR. American journal of roentgenology. 2011;197(6):W992–W1000. doi: 10.2214/ajr.10.6198. [DOI] [PubMed] [Google Scholar]
- 6.Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer immunology research. 2016;4(4):289–93. doi: 10.1158/2326-6066.CIR-15-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. The New England journal of medicine. 2015;373(3):288–90. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishino M, Ramaiya NH, Awad MM, et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(24):6051–60. doi: 10.1158/1078-0432.CCR-16-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR. American journal of roentgenology. 2010;195(2):281–9. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]


