Abstract
Breast cancers detected after a negative breast screening examination and prior to the next screening are referred to as interval cancers. These cancers generally have poor clinical characteristics compared to screen-detected cancers, but associations between interval cancer and genomic cancer characteristics are not well understood. Mammographically-screened women diagnosed with primary invasive breast cancer from 1993-2013 (n=370) were identified by linking the Carolina Breast Cancer Study and the Carolina Mammography Registry. Among women with a registry-identified screening mammogram 0-24 months before diagnosis, cancers were classified as screen-detected (N=165) or interval-detected (N=205). Using logistic regression, we examined the association of mode of detection with cancer characteristics (clinical, IHC, and genomic), overall, and in analyses stratified on mammographic density and race. Interval cancer was associated with large tumors (> 2 cm) (OR=2.3; 95% C.I.: 1.5, 3.7), positive nodal status (OR=1.8; 95% C.I.: 1.1, 2.8), and triple negative subtype (OR=2.5; 95% C.I.: 1.1, 5.5). Interval cancers were more likely to have non-Luminal A subtype (OR=2.9; 95% C.I.: 1.5, 5.7), while screen-detected cancers tended to be more indolent (96% had low risk of recurrence genomic scores; 71% were PAM50 Luminal A). When stratifying by mammographic density and race, associations between interval detection and poor prognostic features were similar by race and density status. Strong associations between interval cancers and poor-prognosis genomic features (non-Luminal A subtype and high risk of recurrence score) suggest that aggressive tumor biology is an important contributor to interval cancer rates.
Keywords: breast cancer, mammography, interval cancer, PAM50
INTRODUCTION
The purpose of screening is to diagnose cancer at an earlier more treatable stage, thereby reducing mortality(1,2). Mammography, the most widely used breast cancer screening method, has been shown to reduce breast cancer mortality in both randomized control trials(3,4) and population-based screening programs(5,6). Interval cancers, which represent a weakness of mammographic screening, are defined as cancers detected after a negative mammogram in the interval between regular screenings. These cancers tend to be higher stage and grade at the time of diagnosis whereas screen-detected cancers have been reported to have more indolent molecular characteristics(7–10). The proportion of interval cancers in screened populations varies from 14% to 38%(11–14), depending on screening interval and underlying population breast cancer incidence rates(15).
Interval cancers are believed to arise from multiple scenarios. First, interval cancers may be cancers that existed at the time of screening but were not detected (false negatives). Some missed tumors are believed to be caused by masking bias, wherein high mammographic density can conceal a tumor from being detected(16,17); it is also possible that radiographic features of the cancer may influence detection(18). Second, interval cancers may represent cancers that were not present at the time of screening, but possess aggressive tumor characteristics that enable them to grow to a detectable level before the next screening. Understanding how biologic characteristics and masking contribute to the rate of interval cancer could help in the development of new technologies such as 3D-mammography.
In this study, we examined the molecular characteristics (immunohistochemical and RNA-based) of interval cancers. Previous studies have shown that interval cancers have a more aggressive profile with respect to clinical factors such as estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor (HER)2-status(8,13), but only one study has reported associations between interval cancer and RNA-based genomic subtype such as the PAM50 intrinsic subtype(19). No study to our knowledge has reported associations for the genomic risk of recurrence (ROR-PT) score based on PAM50. Given that genomic tests are increasingly utilized in clinical settings, it is important to understand the relationship of interval detection to these genomic characteristics.
METHODS
Data Sources
Carolina Breast Cancer Study
The Carolina Breast Cancer Study (CBCS) is a population-based study designed to identify both genetic and environmental risk factors for breast cancer among North Carolina women(20). The current analysis uses data from all three study phases of CBCS (Phase 1, 1993-1996; Phase 2, 1996-2001; and Phase 3, 2008-2013). Randomized recruitment was used to oversample both African American and younger cases (under age 50)(21,22) in all phases. The first two phases of CBCS recruited both cases and controls from 24 counties of eastern and central NC(20). Cases were women aged 20 to 74 diagnosed with a primary invasive breast cancer between May 1, 1993 and December 31, 2000 and identified through rapid case ascertainment from the North Carolina Central Cancer Registry. Cases of in situ cancer were also enrolled in Phase 2. There was a total of 2311 cases (1803 invasive cases, 508 in situ cases) enrolled in Phases 1&2. Phase 3 recruited invasive cases only (N=3000) from 44 counties in NC(22).
CBCS Variables
Women in CBCS were interviewed at baseline by a nurse, at which point they also provided written consent for medical record requests. All measures were self-reported, except BMI, which was nurse-measured. All demographic/patient (age at diagnosis, race, menopausal status, education, income, first degree family history of breast cancer, marital status, and hormone replacement (HRT) use), clinical (tumor size, nodal status, and stage), and molecular data used in this study came from CBCS.
The following IHC markers were used to distinguish intrinsic subtype: ER, PR HER2, human epidermal growth factor-1 (HER1), and cytokeratin 5/6 (CK5/6), and tumor suppressor p53. For Phases 1&2 of CBCS, previously described assays were used for these IHC markers(23–25). ER and PR status were determined from medical records for the 80% of women who had these data available from medical records(23); for the remaining cases with paraffin-embedded tissue available, IHC analysis was performed at the University of North Carolina Translational Pathology Laboratory (TPL). Positivity for ER and PR status were defined as having more than 5% of cells showing nuclei-specific staining(24). Tumors with HER2 staining in more than 10% of cells were considered HER2 positive(25). Positivity of EGFR was defined as any HER1 staining and positivity for CK 5/6 was defined as any cytoplasmic and/or membranous staining. Methods to distinguish intrinsic subtypes in CBCS Phase 3 are described in detail by Allott et al.(26). Briefly, tissue microarrays (TMAs) were constructed and stained by TPL and were digitally imaged using the Aperio ScanScope XT (Aperio Technologies, Vista CA). Automated digital image analysis was performed to quantify IHC staining using a Genie classifier and the Nuclear V9 algorithm (Aperio Technologies, Vista CA), for ER and PR and a Genie classifier and Membrane V9 algorithm for HER2. Three-marker intrinsic subtypes were defined using ER, PR, and HER2 status as described by Allott et al.(26): Luminal A (ER+ and/or PR+, HER2−), Luminal B (ER+ and/or PR+, HER2+), HER2+ (ER−, PR−, HER2+) and triple negative (ER−, PR−, HER2−). Five-marker intrinsic subtypes were assigned to women who had complete data for ER, PR, HER2, CK5/6 and HER1 as described by Carey et al.(24), where the three markers were used for Luminal and HER2 cancers, but basal-like cancer required positivity for either HER1 or Ck5/6 (ER−, PR−, HER2−, HER1+ or CK5/6+). p53 positivity for IHC was defined as dark nuclear protein staining present in 10% or more of invasive cells, all other cases were considered p53 negative(27).
PAM50 gene expression subtyping was performed on a subset (n=2007) of samples with available formalin-fixed paraffin embedded cores or unstained slides from CBCS Phases 1-3. For samples from CBCS 1&2 (N=188), RNA was extracted from two unstained 10-μm FFPE slides per patient. For women in CBCS Phase 3, RNA was extracted from two 1-mm cores (N=377) or two 10 μm slides (N=79) as described previously(26). RNA was isolated using the RNeasy FFPE Kit (Qiagen) and Nanostring analyses were performed in the Rapid Adoption Molecular laboratory and the Translational Genomics laboratory at UNC. Tumors were classified as Luminal A, Luminal B, HER2-enriched, Basal-like, and normal-like using the PAM50 predictor(28). RNA gene expression for p53 mutation status was determined using a previously published 48-gene p53 signature(29). A subset of the PAM50 genes were also used to construct the risk of recurrence score, taking into account proliferation and tumor size (ROR-PT)(30). The ROR-PT is the research correlate to the clinically used Prosigna assay (NanoString Technologies Inc., Seattle, WA, USA), which has been clinically validated(31). The ROR-PT is a continuous score, but can be categorized (Low/Medium/High) using published protocols(28).
Carolina Mammography Registry
The Carolina Mammography Registry (CMR)(32) is a large community-based mammography registry that has studied the performance and outcomes of mammography in North Carolina since 1994 and participates in the Breast Cancer Surveillance Consortium (BCSC)(33). The CMR collects data from breast imaging facilities across North Carolina. Data from patients and radiologists include patient demographics, prior screening history, breast cancer risk factors including family history of breast cancer, radiologist-reported breast density using Breast Imaging Reporting and Data System (BI-RADS) classifications, reason for the visit, screening and diagnostic procedures performed, and radiologists’ interpretation of the examination using BI-RADS assessment categories and the recommended follow-up. Mammographic modality was available for the majority of patients (74%, N=186), and among these women, 75% had digital mammography and 25% had screen-film mammography.
CMR Variables
All mammography data used in this analysis, including mammographic density, type of examination, screening dates, and screening outcomes came from the CMR. In the CMR, mammographic density is recorded at each mammogram by the interpreting radiologist using BI-RADS. For all analyses, mammographic density was categorized as non-dense (BI-RADS 1 and 2) and dense (BI-RADS 3 and 4)(34).
Mammogram findings were reported by the radiologists in CMR using BI-RADS assessment categories(35). Screening mammograms and results were defined using BCSC definitions(36). A mammogram was considered to be screening if: the woman was 18 or older, had no breast implants or prior mastectomy, no history of breast cancer, the indication for the examination was routine screening, it was the first examination sequence of the day, bilateral screening views were done, there was no imaging in the previous 9 months, and the overall assessment code was not BI-RADS 6. A positive screening mammogram is defined as a screening mammogram with a BI-RADS assessment code of 4 (suspicious abnormality) or 5 (highly suggestive of malignancy). Screening mammograms with a BI-RADS assessment code of 0 (incomplete) or 3 (probably benign finding) with a recommendation for biopsy, fine needle aspiration (FNA), or surgery were also considered positive. A negative screening mammogram was defined as a screening mammogram with a BI-RADS assessment category of 1, 2, or 3 with no recommendation for biopsy, FNA, or surgery.
CBCS-CMR Linkage
Study approval was granted by the University of North Carolina Institutional Review Board. All cases and controls from Phases 1, 2 and 3 of CBCS (N=7331) were matched to women in CMR from 1994-2014 inclusive (N=657,060) using probabilistic linkage. The following identifiers were used to match records: last four digits of social security number (SSN), first name, last name, middle initial, date of birth, and address. Because some women in CBCS Phase 3 did not consent to use of SSNs, Phase 3 of CBCS was linked separately for those with and those without SSN.
Matches (women that were in both CBCS and CMR) were determined using thresholds set on linking probabilities of the identifiers chosen. The final linked dataset included 2,614 women (871 controls and 1,743 cases of DCIS or invasive breast cancer). The sensitivity of linkage (100%) was the same for women linked with SSN information and those linked without, but specificity was higher (97.1% vs. 95.2%) for those with SSN. Linkage was performed by the Cancer Information and Population Health Resource (CIPHR) at the University of North Carolina(37). Consistent with screening patterns in the general population, CBCS women with records in the CMR were more likely to be cancer cases, older, post-menopausal, and have used hormone replacement therapy.
Eligibility Criteria
The eligibility criteria applied in this study are shown in Supplemental Figure 1. As a secondary quality control measure for the linkage, information from one commonly collected variable between the two data sets, date of diagnosis, was compared. Both CBCS and CMR collected data for this variable from the NC Central Cancer Registry; date of diagnosis should therefore be the same if the match from the linkage was correct. There were 15 of 1512 (0.1%) women where dates of diagnosis did not match. After manual review, it was determined that these women represented false matches and these women were excluded from analysis. The linked dataset contained 1497 women. 43% of these women (N=644) had genomic data available.
Defining Interval vs. Screen-detected Cases
Invasive breast cancer cases were classified as interval- or screen-detected based on the date of the most recent pre-diagnostic screening mammogram and the date of breast cancer diagnosis. Screening interval recommendations varied from 1-2 years(38–41) during the study period (1993-2013). Mode of detection was defined using both a 12 and 24 month screening interval (Figure 1). For example, using the 24 month screening interval, if a positive screening mammogram was recorded in the 24 months before the diagnosis date, the cancer was classified as screen-detected. If a negative screening mammogram was recorded in the 24 months before diagnosis, the cancer was classified as interval cancers. The 24 month interval was chosen for the main analysis to reflect current screening recommendations and to enhance comparability with other studies(8,12,16,42,43).
Figure 1.
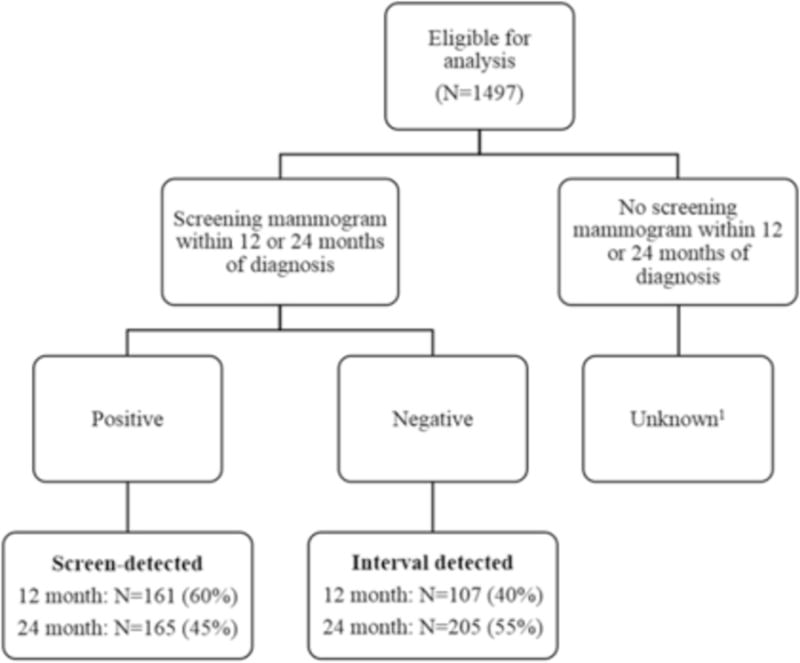
Mode of detection categorization, using 12 or 24 month screening interval.
1Women who had unknown mode of detection were excluded from this study.
Of the 1,497 women with a primary invasive breast cancer in the CMR-CBCS data set, we identified 165 women who were screen-detected and 205 women who were interval-detected within two years of a negative screening mammogram. Sensitivity analyses that decreased the screening interval to 12 months were also performed; using this shorter interval, 161 women were classified as screen-detected and 107 women were classified as interval-detected. Women who met neither screen-detected nor interval-detected definitions were classified as “unknown”. Compared to screen-detected women, women with unknown mode of detection had less screening history in the linked dataset, were more likely to be <50 years old and premenopausal. Women with unknown mode of detection were excluded from all analyses.
Statistical Analysis
Logistic regression was used to calculate univariate odds ratios for associations for each of the demographic/patient variables (age, race, BMI, CBCS Phase, menopausal status, education, marital status, income, family history, hormone replacement therapy use, and mammographic density) with mode of detection, with screen-detected cancers being used as the referent group. Potential confounders were chosen a priori based on a review of the literature. Adjusted odds ratios were calculated for the association between clinical and molecular variables (tumor size, nodal status, cancer stage, ER, PR, and HER2 positivity, 3-marker subtype, 5-marker subtype IHC p53, PAM50 subtype, genomic p53) and mode of detection; odds ratios were adjusted for demographic/patient variables found to be strongly associated with mode of detection. For subtype analyses, Luminal A was used as the referent group. For genomic analyses, an additional analysis was done comparing Luminal A vs. non-Luminal A cancers (Luminal B, HER2, and basal-like).
We considered mammographie density and race as potential effect measure modifiers of the relationship between patient and clinical characteristics and mode of detection; as such, analyses for demographic/personal and clinical characteristics were repeated stratifying for mammographic density and race separately. In this study, we define ‘aggressive’ cancer characteristics according to well-established prognostic associations, such that ‘aggressive’ characteristics include: large tumor size (> 2 cm), high stage (Stage III/IV), positive nodal status, ER−, PR−, triple negative, p53 mutant, basal-like, or high ROR-PT score. All analyses were done in SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
The final analytic population contained 370 women. As described in Table 1, the majority of women were ≥ 50 years of age (60%), White (53%), postmenopausal (64%), had no first degree family history of breast cancer (79%), were never users of hormone replacement therapy (68%), and had non-dense breast tissue (55%). To assess patterns of mammography use, we evaluated mean number of mammography visits, mammographic exams (screening and diagnostic exams), and screening mammograms among all participants with at least one screening mammogram recorded during a prediagnostic screening interval (defined as more than two years before diagnosis, Table 1). Older and postmenopausal women had a greater number of visits, exams, and screening mammograms compared to younger and premenopausal women. There were no differences in mammography use by any of the other demographic/patient factors examined.
Table 1.
Characteristics and pre-diagnosis mammography use of women with mammography recorded in CMR >2 years before diagnosis of invasive breast cancer (N=209).
| Full analysis data set (N=370) |
Women with prediagnosis mammography (N=209) |
Mean number of visits (SD) |
p | Mean mammographic exams (SD) |
p | Mean screening mammograms (SD) |
p | |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| <50 | 148 (40) | 70 (33) | 4.7 (4.0) | 3.6 (3.1) | 2.9 (2.7) | |||
| ≥50 | 222 (60) | 139 (66) | 7.1 (5.2) | 0.02 | 5.8 (4.0) | <0.0001 | 5.3 (3.8) | <0.0001 |
| Race | ||||||||
| White | 197 (53) | 115 (55) | 6.5 (5.3) | 5.2 (4.0) | 4.5 (3.8) | |||
| African American | 173 (47) | 94 (45) | 6.1 (4.6) | 0.5 | 4.8 (3.7) | 0.5 | 4.4 (3.4) | 0.8 |
| Menopausal status | ||||||||
| Pre | 134 (36) | 62 (30) | 4.7 (3.7) | 3.7 (3.0) | 3.1 (2.8) | |||
| Post | 236 (64) | 147 (70) | 7.0 (5.3) | 0.003 | 5.6 (4.1) | 0.001 | 5.1 (3.8) | <0.0001 |
| Education | ||||||||
| ≤ High school | 194 (52) | 112 (54) | 6.7 (5.4) | 5.4 (4.1) | 4.9 (3.9) | |||
| ≥ High school | 176 (48) | 97 (46) | 5.8 (4.5) | 0.2 | 4.6 (3.6) | 0.1 | 4.0 (3.3) | 0.1 |
| Income | ||||||||
| < 30,000 | 137 (40) | 81 (42) | 6.9 (5.4) | 5.6 (4.3) | 5.0 (3.7) | |||
| > 30,000 | 211 (61) | 114 (58) | 5.9 (4.6) | 0.2 | 4.6 (3.6) | 0.1 | 4.1 (3.6) | 0.1 |
| Missing | 22 | 14 | ||||||
| Family historya | ||||||||
| No | 282 (79) | 156 (77) | 6.3 (4.9) | 5.0 (3.8) | 4.3 (3.5) | |||
| Yes | 76 (21) | 47 (23) | 6.6 (5.5) | 0.7 | 5.0 (4.2) | 1.0 | 4.9 (4.0) | 0.4 |
| Missing | 12 | 6 | ||||||
| HRT use | ||||||||
| Never | 244 (68) | 126 (63) | 5.8 (4.4) | 4.7 (3.6) | 3.9 (3.4) | |||
| Current/former | 117 (32) | 75 (37) | 6.6 (5.1) | 0.2 | 5.2 (3.8) | 0.3 | 5.2 (3.9) | 0.03 |
| Missing | 9 | 8 | ||||||
| Breast densityb | ||||||||
| Non-dense | 178 (55) | 109 (64) | 6.2 (5.4) | 5.1 (4.2) | 4.5 (3.9) | |||
| Dense | 145 (45) | 61 (36) | 5.7 (4.4) | 0.5 | 4.5 (3.4) | 0.4 | 3.8 (2.9) | 0.2 |
| Missing | 47 | 39 | ||||||
| Mode of detection | ||||||||
| Screen | 165 (45) | 85 (41) | 6.0 (4.8) | 4.9 (3.7) | 4.0 (3.3) | |||
| Interval | 205 (55) | 124 (59) | 6.5 (5.1) | 0.5 | 5.1 (4.0) | 0.7 | 4.8 (3.9) | 0.1 |
First degree family history of breast cancer.
BI-RADS mammographic breast density: Non-dense= BI-RADS categories 1&2; Dense= BI-RADS categories 3&4.
Table 2 shows associations between interval-detected vs. screen-detected cancers and clinical characteristics. Younger age (<50 years old, OR=1.44; 95% C.I.: 0.95, 2.20), premenopausal status (OR= 1.14; 95% C.I.: 0.94, 1.75), and dense breast tissue (OR=2.02; 95% C.I.: 1.29, 3.16) were associated with interval detection (Supplemental Table 1), and all analyses for clinical characteristics are adjusted for age and menopausal status. Interval cancers were associated with aggressiveness as measured by tumor size, stage, and nodal status. Interval cancers were also more commonly hormone receptor negative, but these results were not significant, nor was an association with p53 status. However, interval cancers were statistically significantly associated with triple negative status (OR= 2.45; 95% C.I: 1.10, 5.47) and with basal-like cancer (OR=2.06; 95% C.I: 1.07, 3.95) (Table 3). Associations between mode of detection and molecular variables (ER, PR, HER2, triple negative, basal-subtype) were unchanged after adjusting for tumor size, stage, and nodal status. Interval cancers were strongly associated with genomic markers (Table 3), including PAM50 non-Luminal A subtype (OR=2.94; 95% C.I.: 1.52, 5.71) and PAM50 basal-like subtype (OR=2.68; 95% C.I.: 1.21, 5.94). Mean ROR-PT score was significantly higher in interval than screen-detected cancers (mean =41.0 vs. 26.0; p <0.001). As shown in Figure 2, the kernel density distribution is shifted toward higher risk tumors among interval cancers and a higher proportion of ROR-PT high risk tumors, (24/105, 23%) were detected among interval-detected cancers (vs. 3/71, 4% among screen-detected). Associations between interval detection and tumor characteristics were not markedly changed when stratified by density (Table 2) or race (Supplemental Table 2), or by screening interval (Supplemental Table 3). Furthermore, to evaluate the impact of length-bias on differences between screen- and interval-detected cancers, we performed sensitivity analyses restricting the screen-detected population to only those women for whom we had multiple mammograms >2 years prior to diagnosis (N=76). The results in this group were not substantially different in magnitude, but had reduced precision.
Table 2.
Interval vs. Screen-detected cancers: Associations with clinical characteristics stratified by mammographie density.
| Overall | Non-dense | Dense | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SDC (N=165) |
Interval (N=205) |
Adjusted OR (95% CI)a |
SDC (N=93) |
Interval (N=85) |
Adjusted OR (95% CI)a |
SDC (N=51) |
Interval (N=94) |
Adjusted OR (95% CI)a |
|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||||
| Age | |||||||||
| ≥50 | 107 (65) | 115 (56) | 1.0 | 65 (70) | 52 (61) | 1.00 | 30 (59) | 46 (49) | 1.00 |
| ≤50 | 58 (35) | 90 (44) | 1.44 (0.95, 2.20) | 28 (30) | 33 (39) | 1.47 (0.79, 2.74) | 21 (41) | 48 (51) | 1.49 (0.75, 2.97) |
| Tumor size | |||||||||
| ≤ 2 cm | 115 (70) | 103 (52) | 1.0 | 69 (78) | 42 (51) | 1.00 | 33 (66) | 44 (49) | 1.00 |
| ≥ 2 cm | 44 (27) | 94 (46) | 2.33 (1.48, 3.65) | 20 (22) | 41 (49) | 3.22 (1.66, 6.26) | 17 (34) | 45 (51) | 2.00 (0.97, 4.12) |
| Missing | 6 | 8 | 4 | 2 | 1 | 5 | |||
| Nodal status | |||||||||
| Negative | 123 (75) | 127 (62) | 68 (74) | 51 (60) | 1.00 | 38 (75) | 61 (66) | 1.00 | |
| Positive | 41 (25) | 77 (38) | 1.78 (1.13, 2.81) | 24 (26) | 34 (40) | 1.78 (0.94, 3.39) | 13 (25) | 32 (34) | 1.58 (0.73, 3.42) |
| Missing | 1 | 1 | 1 | 0 | 0 | 1 | |||
| Stage | |||||||||
| I/II | 151 (94) | 172 (86) | 85 (94) | 69 (83) | 1.00 | 46 (92) | 81 (89) | 1.00 | |
| III/IV | 9 (6) | 28 (14) | 3.22 (1.43, 7.25) | 5 (6) | 14 (17) | 3.21 (1.09, 9.44) | 4 (8) | 10 (11) | 1.38 (0.40, 4.74) |
| Missing | 5 | 5 | 3 | 2 | 1 | 3 | |||
| ER | |||||||||
| Positive | 112 (71) | 124 (65) | 1.0 | 62 (70) | 48 (61) | 1.00 | 35 (70) | 58 (67) | 1.00 |
| Negative | 46 (29) | 66 (35) | 1.25 (0.79, 1.98) | 26 (30) | 31 (39) | 1.44 (0.75, 2.77) | 15 (30) | 29 (33) | 1.15 (0.54, 2.45) |
| Missing | 7 | 17 | 5 | 6 | 1 | 7 | |||
| PR | |||||||||
| Positive | 94 (61) | 96 (50) | 1.0 | 48 (57) | 35 (45) | 1.00 | 33 (66) | 50 (56) | 1.00 |
| Negative | 60 (39) | 96 (50) | 1.53 (0.99, 2.37) | 36 (43) | 43 (55) | 1.57 (0.84, 2.95) | 17 (34) | 39 (44) | 1.58 (0.76, 3.28) |
| Missing | 11 | 13 | 9 | 7 | 1 | 5 | |||
| HER2 | |||||||||
| Positive | 20 (14) | 23 (12) | 1.0 | 11 (13) | 6 (8) | 1.00 | 6 (13) | 11 (13) | 1.00 |
| Negative | 127 (86) | 162 (88) | 1.24 (0.64, 2.38) | 72 (87) | 69 (92) | 1.84 (0.63, 5.33) | 39 (87) | 75 (87) | 1.32 (0.43, 4.00) |
| Missing | 18 | 20 | 10 | 10 | 6 | 8 | |||
| p53 IHC | |||||||||
| Wild-type | 75 (71) | 78 (67) | 1.0 | 43 (72) | 31 (66) | 1.00 | 23 (70) | 38 (68) | 1.00 |
| Mutant | 30 (29) | 39 (33) | 1.23 (0.69, 2.18) | 17 (28) | 16 (34) | 1.28 (0.55, 2.95) | 10 (30) | 18 (32) | 1.08 (0.41, 2.81) |
| Missing | 60 | 88 | 33 | 38 | 18 | 38 | |||
All odds ratios, except those for age, are adjusted for age and menopausal status
Table 3.
Odds ratios for molecular characteristics for linked invasive cases.
| Screen-detected (N=165) |
Interval (N=205) |
Adjusted OR (95% CI)a |
|
|---|---|---|---|
| N (%) | N (%) | ||
| 3-marker subtype | |||
| Luminal A | 99 (68) | 102 (55) | 1.00 |
| Luminal B | 14 (10) | 16 (9) | 0.95 (0.43, 2.08) |
| HER2 | 6 (4) | 7 (4) | 1.12 (0.36, 3.45) |
| Triple negative | 26 (18) | 60 (32) | 2.45 (1.10, 5.47) |
| Missing | 20 | 20 | |
| 5-marker subtype | |||
| Luminal A | 67 (64) | 64 (47) | 1.00 |
| Luminal B | 12 (12) | 30 (22) | 2.45 (1.14, 5.25) |
| HER2 | 6 (6) | 4 (3) | NRb |
| Basal-like | 19 (18) | 38 (28) | 2.06 (1.07, 3.95) |
| Missing | 61 | 69 | |
| PAM50 | |||
| Luminal A | 51 (71) | 46 (46) | 1.00 |
| Luminal B | 4 (6) | 18 (18) | NRb |
| HER2 | 5 (7) | 8 (8) | 1.82 (0.54, 6.15) |
| Basal-like | 12 (17) | 29 (29) | 2.68 (1.21, 5.94) |
| Missing | 93 | 104 | |
| PAM50 | |||
| Luminal A | 51 (71) | 46 (46) | 1.00 |
| Non-Luminal A | 21 (29) | 55 (54) | 2.94 (1.52, 5.71) |
| Missing | 93 | 104 | |
| p53 | |||
| Wild type | 42 (55) | 55 (52) | 1.00 |
| Mutant | 34 (45) | 51 (48) | 1.13 (0.63, 2.05) |
| Missing | 89 | 99 | |
| ROR-PTc | |||
| Low/Medium | 68 (96) | 81 (77) | NRb |
| High | 3 (4) | 24 (23) | NRb |
| Missing | 94 | 100 |
All odds ratios are adjusted for age and menopausal status.
Odd ratios are not reported where cell size < 5 observations.
PAM50 risk of recurrence score.
Figure 2.
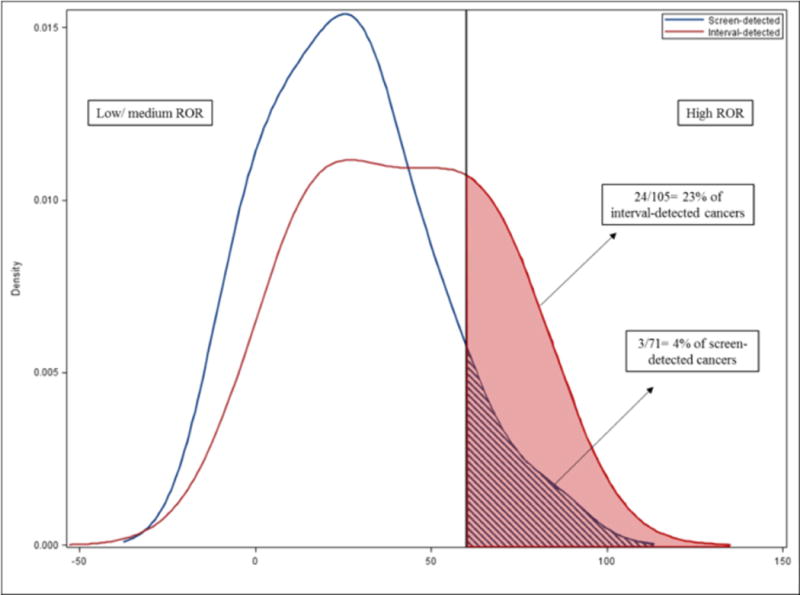
Kernel density distribution of PAM50 risk of recurrence (ROR-PT) score for interval- and screen-detected cancers. ROR distributions of screen-detected and interval-detected cancers are blue and red, respectively. Vertical line marks the threshold for high ROR-PT. The area shaded under the curve represents the proportion of cancers that have high risk of recurrence score. Of 105 interval-detected cancers that had genomic data available, 24 cancers (23%) had high ROR score. Of 71 screen-detected cancers that had genomic data available, 3 cancers (4%) had high ROR score.
DISCUSSION
Identification of the predictors and characteristics of interval cancers contributes to our knowledge of the risks and benefits of mammography. We found that standard clinical prognosis features are associated with interval cancers, and that genomic tests indicative of poor prognosis are more common among interval cancers. Previous literature has shown that interval cancers tend to have negative prognostic characteristics (8,12,13,16,42,43), however we found associations to be weaker than reported previously for ER− or PR−(12,43), triple negative(8,42,43), and p53 mutant(43). With the exception of triple negative subtype, none of these were significantly associated with interval detection.
While multi-gene classification methods have become more prominent clinically, genomic characteristics of interval cancers are not well studied. The only study that has reported associations between PAM50 results and mode of detection was based within a clinical cancer sequencing study in Sweden with 173 patients. That study had similar findings, showing that interval cancer was associated with basal-like subtype(19). Higher ROR-PT among interval cancers has not been assessed previously. It is striking that only 4% of screen-detected cancers had high ROR-PT, in parallel with high frequency of Luminal A subtype (71%).
While our findings strongly support biologic determinants of interval cancers, masking bias may nonetheless contribute to interval cancer rates. Multiple studies have shown high mammographic density to be associated with interval cancers(44–46), including our own findings herein. However, it is difficult to disentangle tumor biology and mammographic density because younger women have both higher density and more aggressive tumor characteristics(47,48). We were unable to consider the independent contributions of age, race, and mammographic density due to sample size.
Some limitations of the study should be noted. CMR does not include all breast imaging facilities in North Carolina, so only ~30% of women enrolled in CBCS were linked to CMR. Furthermore, CBCS oversampled younger and African American women, and therefore the proportion of screen and interval detected cases may vary as a function of the demographic and selection characteristics of CBCS(47). Therefore our study is not designed to estimate the proportion of screen and interval-detected cases in the general population. Notably, among screened women, we classified 45% of invasive cases as screen-detected. Previous studies based on CMR have reported higher proportions of screen detected cases (e.g. Henderson et al. reported 80% of cases were screen detected using a 1 year-interval(14); Hofvind et al. reported 60% of cases were screen detected given the 24-month definitions used herein(11)). We were unable to retrospectively review mammographic images to confirm which interval cases arose from false negatives, but we minimized misclassification within screen and interval-detected groups by classifying women with missing screening data as ‘unknown’. We note that the unknown category likely includes true screen- and interval-detected cases along with true clinically detected cases. We also acknowledge that use of BI-RADS categories to assess masking may be less sensitive than use of quantitative mammographic density, which was unavailable in this analysis. Despite these limitations, this study does provide novel data on genomic characteristics in a racially diverse population.
The goal of mammography is to find aggressive cancers at an earlier stage to increase survivorship and reduce mortality. Our research shows that a high proportion of interval cancers are associated with aggressive biology. Our work also suggests that genomic tests may be useful in distinguishing indolent vs. aggressive screen-detected cancers, given the high prevalence of low-risk tumors among screen-detected cases. If confirmed, these findings indicate that continued evaluation of genomic tools in combination with mammography could help to increase the benefit and reduce negative consequences of screening.
Supplementary Material
Acknowledgments
SP was supported in part by the Komen Graduate Training in Disparities Research Grant (Komen OGUNC1202). SJN was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, (Grant KL2TR001109). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Funding was also provided by the National Institutes of Health, National Cancer Institute P50-CA058223, U54-CA156733, U01-CA179715, U01-CA070040, HHSN261201100031C, and P01-CA154292.
Footnotes
Conflict of Interest Disclosures: Dr Perou reported being an equity stockholder in BioClassifier, LLC, and University Genomics and reported filing a patent on the PAM50 subtyping assay.
References
- 1.Day NE, Williams DR, Khaw KT. Breast cancer screening programmes: the development of a monitoring and evaluation system. British Journal of Cancer. 1989;59(6):954–8. doi: 10.1038/bjc.1989.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vainio H, Bianchini F. IARC Handbook of Cancer Prevention Volume 7. IARC Press; 2002. Apr 17, < http://www.iarc.fr/en/publications/pdfs-online/prev/handbook7/index.php>. Accessed 2016 April 17. [Google Scholar]
- 3.Kerlikowske K. Efficacy of screening mammography among women aged 40 to 49 years and 50 to 69 years: comparison of relative and absolute benefit. Journal of the National Cancer Institute Monographs. 1997;(22):79–86. doi: 10.1093/jncimono/1997.22.79. [DOI] [PubMed] [Google Scholar]
- 4.Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. The Lancet. 2002;359(9310):909–19. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl CK, Kuhn W, Schild H. Management of women at high risk for breast cancer: New imaging beyond mammography. Breast (Edinburgh, Scotland) 2005;14(6):480–6. doi: 10.1016/j.breast.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Andersson I, Aspegren K, Janzon L, Landberg T, Lindholm K, Linell F, et al. Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ (Clinical Research Ed) 1988;297(6654):943–8. doi: 10.1136/bmj.297.6654.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitak B, Olsen KE, Manson JC, Arnesson LG, Stal O. Tumour characteristics and survival in patients with invasive interval breast cancer classified according to mammographic findings at the latest screening: a comparison of true interval and missed interval cancers. Eur Radiol. 1999:460–9. doi: 10.1007/s003300050693. [DOI] [PubMed] [Google Scholar]
- 8.Rayson D, Payne JI, Abdolell M, Barnes PJ, MacIntosh RF, Foley T, et al. Comparison of Clinical-Pathologic Characteristics and Outcomes of True Interval and Screen-Detected Invasive Breast Cancer Among Participants of a Canadian Breast Screening Program: A Nested Case-Control Study. Clinical Breast Cancer. 2011;11(1):27–32. doi: 10.3816/CBC.2011.n.005. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Lee S, Bae S, Choi M-Y, Lee J, Jung S, et al. Comparison between screen-detected and symptomatic breast cancers according to molecular subtypes. Breast Cancer Res Treat. 2012;131(2):527–40. doi: 10.1007/s10549-011-1836-0. [DOI] [PubMed] [Google Scholar]
- 10.Chuang SL, Chen SL, Yu CP, Chang KJ, Yen AM, Chiu SY, et al. Using tumor phenotype, histological tumor distribution, and mammographic appearance to explain the survival differences between screen-detected and clinically detected breast cancers. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2014;122(8):699–707. doi: 10.1111/apm.12294. [DOI] [PubMed] [Google Scholar]
- 11.Hofvind S, Yankaskas BC, Bulliard J-L, Klabunde CN, Fracheboud J. Comparing interval breast cancer rates in Norway and North Carolina: results and challenges. Journal of Medical Screening. 2009;16(3):131–9. doi: 10.1258/jms.2009.009012. [DOI] [PubMed] [Google Scholar]
- 12.Kirsh VA, Chiarelli AM, Edwards SA, O’Malley FP, Shumak RS, Yaffe MJ, et al. Tumor characteristics associated with mammographic detection of breast cancer in the Ontario breast screening program. J Natl Cancer Inst. 2011;103(12):942–50. doi: 10.1093/jnci/djr138. [DOI] [PubMed] [Google Scholar]
- 13.Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, et al. Breast Tumor Characteristics as Predictors of Mammographic Detection: Comparison of Interval- and Screen-Detected Cancers. Journal of the National Cancer Institute. 1999;91(23):2020–8. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- 14.Henderson LM, Benefield T, Nyante SJ, Marsh MW, Greenwood-Hickman MA, Schroeder BF. Performance of digital screening mammography in a population-based cohort of black and white women. Cancer Causes & Control : CCC. 2015;26(10):1495–9. doi: 10.1007/s10552-015-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houssami N, Hunter K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ Breast Cancer. 2017;3(1):12. doi: 10.1038/s41523-017-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domingo L, Salas D, Zubizarreta R, Baré M, Sarriugarte G, Barata T, et al. Tumor phenotype and breast density in distinct categories of interval cancer: results of population-based mammography screening in Spain. Breast Cancer Research. 2014;16(1):1–11. doi: 10.1186/bcr3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanch J, Sala M, Ibáñez J, Domingo L, Fernandez B, Otegi A, et al. Impact of Risk Factors on Different Interval Cancer Subtypes in a Population-Based Breast Cancer Screening Programme. PLoS ONE. 2014;9(10):1–10. doi: 10.1371/journal.pone.0110207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bare M, Tora N, Salas D, Sentis M, Ferrer J, Ibanez J, et al. Mammographic and clinical characteristics of different phenotypes of screen-detected and interval breast cancers in a nationwide screening program. Breast Cancer Res Treat. 2015;154(2):403–15. doi: 10.1007/s10549-015-3623-9. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Ivansson E, Klevebring D, Tobin NP, Lindström LS, Holm J, et al. Molecular Differences between Screen-Detected and Interval Breast Cancers Are Largely Explained by PAM50 Subtypes. Clinical Cancer Research. 2017;23(10):2584–92. doi: 10.1158/1078-0432.CCR-16-0967. [DOI] [PubMed] [Google Scholar]
- 20.Newman B, Moorman P, Millikan R, Qaqish B, Geradts J, Aldrich T, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35(1):51–60. doi: 10.1007/BF00694745. [DOI] [PubMed] [Google Scholar]
- 21.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGee SA, Durham DD, Tse C-K, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiology Biomarkers & Prevention. 2013;22(7):1227–38. doi: 10.1158/1055-9965.EPI-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG. Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. American Journal of Epidemiology. 2000;151(7):703–14. doi: 10.1093/oxfordjournals.aje.a010265. [DOI] [PubMed] [Google Scholar]
- 24.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 25.Livasy CA, Perou CM, Karaca G, Cowan DW, Maia D, Jackson S, et al. Identification of a basal-like subtype of breast ductal carcinoma in situ. Human Pathology. 2007;38(2):197–204. doi: 10.1016/j.humpath.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Allott EH, Cohen SM, Geradts J, Sun X, Khoury T, Bshara W, et al. Performance of Three-Biomarker Immunohistochemistry for Intrinsic Breast Cancer Subtyping in the AMBER Consortium. Cancer Epidemiology Biomarkers & Prevention. 2016;25(3):470–8. doi: 10.1158/1055-9965.EPI-15-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furberg H, Millikan RC, Geradts J, Gammon MD, Dressler LG, Ambrosone CB, et al. Reproductive factors in relation to breast cancer characterized by p53 protein expression (United States) Cancer Causes & Control : CCC. 2003;14(7):609–18. doi: 10.1023/a:1025682410937. [DOI] [PubMed] [Google Scholar]
- 28.Parker JS, Mullins M, Cheang MCU, Leung S, Voduc D, Vickery T, et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. Journal of Clinical Oncology. 2009;27(8):1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troester MA, Herschkowitz JI, Oh DS, He X, Hoadley KA, Barbier CS, et al. Gene expression patterns associated with p53 status in breast cancer. BMC Cancer. 2006;6:276. doi: 10.1186/1471-2407-6-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor positive breast cancer. Clinical Cancer Research. 2010;16(21):5222–32. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnant M, Filipits M, Greil R, Stoeger H, Rudas M, Bago-Horvath Z, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Annals of Oncology. 2014;25(2):339–45. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]
- 32.Carolina Mammography Registry. < http://cmr.unc.edu/>. Accessed 2014 February 25.
- 33.Ballard-Barbash R, Taplin SH, Yankaskas BC, Ernster VL, Rosenberg RD, Carney PA, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR American Journal of Roentgenology. 1997;169(4):1001–8. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 34.American College of Radiology. Breast imaging and reporting data system (BI-RADS) 3rd. Reston, Va: American College of Radiology; 2003. [Google Scholar]
- 35.D’Orsi C, Bassett LW, Berg W. BI-RADS: Mammography, 4th edition in: D’Orsi CJ, Mendelson EB, Ikeda DM, et al: Breast Imaging Reporting and Data System: ACR BI-RADS – Breast Imaging Atlas. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 36.Breast Cancer Surveillance Consortium (BCSC) BCSC Glossary of Terms [Google Scholar]
- 37.Meyer AM, Olshan AF, Green L, Meyer A, Wheeler SB, Basch E, et al. Big data for population-based cancer research: the integrated cancer information and surveillance system. North Carolina Medical Journal. 2014;75(4):265–9. doi: 10.18043/ncm.75.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Cancer Society. History of ACS Recommendations for the Early Detection of Cancer in People without Symptoms. 2015 Apr 1; < http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/chronological-history-of-acs-recommendations>. Accessed 2016 April 1.
- 39.ACOG committee opinion. Routine Cancer Screening. Int J Gynecol Obstet. 1997;59:157–61. [PubMed] [Google Scholar]
- 40.United States Preventive Services Task Force. Breast Cancer: Screening, 2002. 2010 Mar 29; < http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening-2002>. Accessed 2016 March 29.
- 41.United States Preventive Services Task Force. Breast Cancer: Screening, 2009. 2016 Mar 30; < http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/br_east-cancer-screening>. Accessed 2016 March 30.
- 42.Caldarella A, Puliti D, Crocetti E, Bianchi S, Vezzosi V, Apicella P, et al. Biological characteristics of interval cancers: a role for biomarkers in the breast cancer screening. J Cancer Res Clin Oncol. 2013;139(2):181–5. doi: 10.1007/s00432-012-1304-1. [DOI] [PubMed] [Google Scholar]
- 43.Collett K, Stefansson IM, Eide J, Braaten A, Wang H, Eide GE, et al. A Basal Epithelial Phenotype Is More Frequent in Interval Breast Cancers Compared with Screen Detected Tumors. Cancer Epidemiology Biomarkers & Prevention. 2005;14(5):1108–12. doi: 10.1158/1055-9965.EPI-04-0394. [DOI] [PubMed] [Google Scholar]
- 44.Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. Breast Density as a Predictor of Mammographic Detection: Comparison of Interval- and Screen-Detected Cancers. JNCI: Journal of the National Cancer Institute. 2000;92(13):1081–7. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 45.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic Density and the Risk and Detection of Breast Cancer. New England Journal of Medicine. 2007;356(3):227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 46.Henderson LM, Miglioretti DL, Kerlikowske K, Wernli KJ, Sprague BL, Lehman CD. Breast Cancer Characteristics Associated With Digital Versus Film-Screen Mammography for Screen-Detected and Interval Cancers. AJR American Journal of Roentgenology. 2015;205(3):676–84. doi: 10.2214/AJR.14.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chollet-Hinton L, Anders CK, Tse CK, Bell MB, Yang YC, Carey LA, et al. Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: a case-control study. Breast Cancer Research. 2016;18(1):79. doi: 10.1186/s13058-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burton A, Maskarinec G, Perez-Gomez B, Vachon C, Miao H, Lajous M, et al. Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLoS Medicine. 2017;14(6) doi: 10.1371/journal.pmed.1002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


