Abstract
The overall survivorship in patients with appendicular osteosarcoma has increased in the past few decades. However, controversies and questions about performing an amputation or a limb salvage procedure still remain. Using three peer-reviewed library databases, a systematic review of the literature was performed to evaluate all studies that have evaluated the outcomes of appendicular osteosarcoma, either with limb salvage or amputation. The mean 5-year overall survivorship was 62% for salvage and 58% for amputation (p > 0.05). At mean 6-year follow-up, the local recurrence rates were 8.2% for salvage and 3.0% for amputation (p > 0.05). Additionally, at mean 6-year follow-up, the rate for metastasis was 33% for salvage and 38% for amputation (p > 0.05). The revision rates were higher with salvage (31 vs. 28%), and there were more complications in the salvage groups (52 vs. 34%; p > 0.05). Despite the heterogeneity of studies available for review, we observed similar survival rates between the two procedures. Although there was no significant statistical difference between rates of recurrence and metastasis, the local recurrence rate and risk of complications were higher for limb salvage as compared to amputation. Cosmetic satisfaction is often higher with limb salvage, whereas long-term expense is higher with amputation. Overall, current literature supports limb salvage procedures when wide surgical margins can be achieved while still retaining a functional limb.
Keywords: Osteosarcoma, Limb salvage, Amputation, Survivorship, Complications, Survival rate
Introduction
Osteosarcoma is the most common primary malignant bone tumor affecting 2–9 persons per 1,000,000 patients [1–5]. Its incidence is highest in the second decade of life during times of accelerated growth in areas with rapid bone turnover. It commonly involves the distal femur (42.8%), proximal tibia (23.2%), and proximal humerus (10.1%) [6]. Although conventional osteosarcoma is the most common one, subtypes have been described based on histological subtype and grade, anatomical location, and the presence of predisposing factors [5, 7, 8]. Most cases present with chromosomal abnormalities upon cytologic analysis [9–17].
The standard of care for the treatment of osteosarcoma has included neoadjuvant chemotherapy followed by wide surgical resection and adjuvant chemotherapy [9, 18]. Recent advancements in neoadjuvant and adjuvant chemotherapy, enhanced diagnostic imaging, improved surgical techniques, and the development of newer tumor prostheses have aided in this change [9, 19–21]. Recently, the standard of surgical care has also changed from limb amputation or resection-arthrodesis to limb salvage [22].
Traditionally, amputations were performed in the context of absolute and relative contraindications to limb salvage surgery. Such conditions included neurovascular compromise, pathologic fractures with an associated hematoma extending beyond compartment boundaries, biopsy site complications, soft tissue coverage difficulties, or severe infection within the surgical field. Other conditions included immature skeletal age with a limb–length discrepancy of > 8 cm or a poor response to preoperative chemotherapy [23–25].
Some studies have examined survivorship rates between patients undergoing limb salvage and those undergoing amputation [24, 26, 27]. However, there is paucity of literature examining other factors associated with these two treatment modalities. The purpose of this study was to systematically evaluate all studies that assessed the outcomes of limb salvage compared to amputation in patients with appendicular conventional osteosarcoma, in order to determine if there are differences in (1) overall and disease-free survival rates, (2) recurrence rates, (3) metastasis, (4) surgical complications, (5) prognostic factors, (6) functional and psychosocial outcomes, and (7) the cost of treatment.
Methods
We evaluated the peer-reviewed databases, PubMed, Medline, Embase, and Scopus, from January 1980 to December 2014 to determine studies that report the results of limb salvage or amputation for patients who had conventional appendicular osteosarcoma. This was performed following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [28]. Our search criteria included the terms osteosarcoma, limb salvage, amputation, cost, pathologic fracture, recurrence, metastasis, and epidemiology. This study focused on appendicular osteosarcoma, in general, and considered rotationplasty, turnplasty, or similar procedures as part of the amputation group.
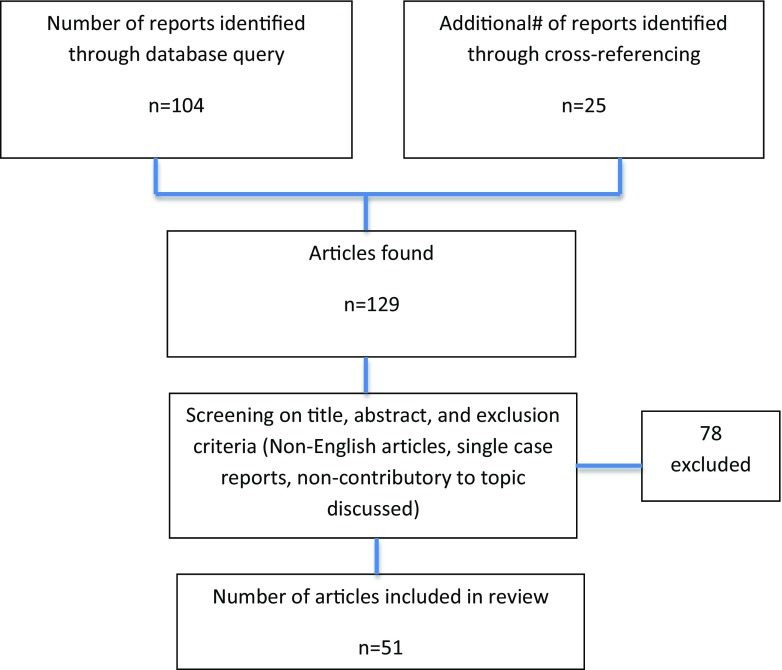
The initial search resulted in 104 articles, with another 25 found from cross-referencing, for a total of 129 articles. We excluded articles that were irrelevant to the study topic based on screening of the title and abstract, that had incomplete or < 12-month follow-up, which did not report on specific outcomes, that were non-English, and those that were single case reports. Fifty-one articles remained for review following application of these exclusion criteria. There were no level I studies included wherein most were lower level studies, including level II (1), level III (27), level IV (19), and level V (4) (Fig. 1).
Fig. 1.
Inclusion criteria flowchart
Clinical outcomes were obtained using surgeon-based objective measures at the latest follow-up. Most studies did not include a functional scoring system but two used the Toronto Extremity Scoring Scale (TESS) and one used the Musculoskeletal Tumor Society scale (MSTS) [27, 29–31]. We evaluated patient survivorship by measuring the 5-year overall (OS) and 5-year disease-free survival (DFS) rates from all studies that provided specific outcome measures. We calculated mean and standard deviation of local recurrence and metastatic rates, complication rates, and the associated costs. Assessment of cosmetic perception and psychological factors could not be directly compared by taking an arithmetic average since each article reported different psychosocial-function measures. We summarized these outcomes.
Cost was analyzed by a formula derived from Grimer et al. [32] which used costs of limb salvage and amputation with historical data to predict the likelihood of further surgery and anticipated related expenses over the patient’s life. Analyses were performed with the use of statistical software (MedCalc, bvba, Ostend, Belgium), wherein descriptive statistics were performed for most outcome measures.
Disease-Free and Overall Survival
Advances in disease-free survival (DFS) and overall survival (OS) for patients with osteosarcoma have been attributed to new developments in both diagnostic and therapeutic measures [3, 33]. Bacci et al. [26] evaluated patient survival for limb salvage and amputation for tumors of the femur, tibia, and other nonspecified sites. They demonstrated a mean 5-year DFS that was significantly higher for patients undergoing limb salvage when compared to those who receive an amputation (63 vs. 49%, p = 0.01). Previous reports in the 1980s have demonstrated contradictory survival rates of 41% and 54% (p = 0.8) [24]. Simon et al. conducted an international multi-institutional, retrospective study of 227 distal femoral osteosarcomas treated with limb salvage, above-knee amputation, or hip disarticulation. The OS at a median of 5.5-years was 41%, 59%, and 46% (p = 0.8), with local recurrences rates of 10.9%, 7.8%, and 0% (p = 0.8), respectively. Although the study was underpowered, the authors noted that the procedure type did not affect the local recurrence rates [24, 34].
Mavrogenis et al. evaluated 42 distal tibia osteosarcomas (23 limb salvage operations and 19 amputations) at a median follow-up of 60 months (8 to 288 months) and noted no significant differences in 10-year survival (84 vs. 74%; p = 0.599) [27]. Furthermore, a significantly higher Musculoskeletal Tumor Society (MSTS) functional score was found in limb salvage patients when compared to those patients who received an amputation (76 vs. 71%, p = 0.044).
Bacci et al. performed an analysis of 560 patients (mean follow-up 10.5 years; range 5 to 17 years) comparing limb salvage (n = 465, 83%) to amputation or rotationplasty (n = 95, 17%) [26]. Surgery type was based on tumor invasion, tumor location, chemotherapy response, age, and desired lifestyle. The mean combined 5-year DFS was 60.3%, with higher survival for patients following limb salvage (63.3%) when compared to amputation or rotationplasty (49.4%). Multivariate analysis demonstrated that a good histological response to chemotherapy was a significant predictor of survivorship seen in 68% of limb salvage and 48% of amputation patients. While the procedure alone was not a statistically significant variable [26], the adequacy of surgical margins was identified to be a significant prognostic variable.
In the series presented by Bacci et al. [26], 17% of limb salvage surgeries had contaminated surgical margins versus only 3.3% in amputations. These discrepancies should be treated with caution as it may represent potential confounders, such as the method of neoadjuvant chemotherapy, which changed during the evaluated period. At a mean follow-up of 10.5 years, 60% of patients remained disease free. The authors found a local recurrence rate of 6.4% and 4.2%, for limb salvage and amputation, respectively. In 2003, they also reported similar results in a group of 46 patients (34 salvage, 11 amputation, 1 rotationplasty). Five-year event-free survival and OS were 59% and 65% [35], respectively, with no significant difference between the groups.
In the present systematic review, we observed a mean OS of 62% for limb salvage (41–84% ± 21.5) and 58% for amputation (49–74% ± 11.8; p > 0.05; Table 1). The literature suggests that the surgery itself is inconsequential regarding the survivorship between these two procedures and that the aforementioned variables (i.e., response to chemo and margins) are what matter. For most surgeons, salvage procedures are viable options when wide surgical margins can be achieved while retaining a functional limb [2].
Table 1.
Comparative survival rates based on the treatment type
| Study name | Number of patients | 5-year disease-free survival | 5-year overall survival | 10-year overall survival | 10-year disease-free survival |
|---|---|---|---|---|---|
| Simon et al. [24] | |||||
| Limb salvage | 227 | N/A | 41% | N/A | N/A |
| Above knee amputation | N/A | 59% | N/A | N/A | |
| Hip disarticulation | N/A | 49% | N/A | N/A | |
| Total | 40% | 55% | |||
| Mavrogenis et al. [27] | |||||
| Limb salvage | 42 | N/A | 84% | 84% | N/A |
| Amputation | N/A | 74% | 74% | N/A | |
| Bacci et al. [26] | |||||
| Limb salvage | 560 | 63% | N/A | N/A | N/A |
| Amputation | 49% | N/A | N/A | N/A | |
| Total | 60.7% | 59.7% | |||
| Bacci et al. [35] | |||||
| Total | 46 | 59% | 65% | N/A | N/A |
| Bielack et al. [6] | |||||
| Total | 1701 | 53.6% | 65.3% | 59.8% | 48.9% |
Recurrence and Metastasis
Studies have demonstrated that there is no significant difference in local recurrence (4.1%) or metastasis (6.2%) between patients undergoing limb salvage versus amputation [24, 26, 27, 34, 35]. When metastases do occur, it is often irrespective of the procedure (amputation 89.5% vs. salvage 85.1%, of all metastases) [26].
In the study by Bacci et al. [26] at 10-year follow-up, a total of 225 of the 560 patients (40%) had recurrent disease, 2 (0.3%) had isolated local recurrences, 191 (34%) had distant metastasis, and 32 (5.7%) were found to have both. Most local recurrences occurred within 2 years. Tumor site, histological response, and margins were predictors for recurrence, wherein osteosarcomas originating from the distal femur had a greater rate of local recurrence than those originating from the tibia, humerus, or other unspecified sites. In contrast, age, gender, tumor volume, surgery type, or presence of pathologic fracture, was not found to be predictors for recurrence.
Mavrogenis et al. showed a higher local recurrence rate for limb salvage compared to previously data [27]. Three of 23 patients (13%) who underwent limb salvage procedures experienced a local recurrence, while none were observed in patients following an amputation. Here, the overall rate of metastasis was 33%.
At a mean follow-up of 6 years (3.8–10.5 years), we observed a local recurrence rate of 8.2% (5.4–13%) for limb salvage and 3.0% (0–7.8%) for amputation, as well as a metastatic rate of 33% and 38%, respectively (Table 2). Current literature shows that there is no significant difference between these procedures when considering metastasis (p > 0.05).
Table 2.
Recurrence and metastasis rates based on the treatment type
| Study name | Number of patients | Local recurrence rate | Metastasis rate | Follow-up mean (range, years) |
|---|---|---|---|---|
| Simon et al. [24] | ||||
| Limb salvage | 227 | 10.9% | 58.9% | 5.54 (not reported) |
| Above knee amputation | 7.8% | 56.5% | ||
| Hip disarticulation | 0% | 53.8% | ||
| Mavrogenis et al. [27] | ||||
| Limb salvage | 46 | 13% | N/A | 5 (0.75–24) |
| Amputation | 0% | N/A | ||
| Total | 6.5% | 9.5% | ||
| Bacci et al. [26] | ||||
| Limb salvage | 560 | 6.4% | N/A | 10.5 (5–17) |
| Amputation | 4.2% | N/A | ||
| Total | 5.3% | 33.5% | ||
| Jeys et al. [36] | ||||
| Limb salvage | 776 | 5.4% | N/A | 9 (10–35) |
| Unwin et al. [41] | ||||
| Limb salvage | 1001 | 5.4% | N/A | 3.8 (not reported) |
| Mean | ||||
| Limb salvage | 2610 | 8.2% (±3.5) | 33% (±24.7) | |
| Amputation | 3% (±3.7) | 38% (±21.7) | ||
Complications of Surgery
The complication rates vary across the literature ranging from 0 to 70% [27, ], wherein no significant difference in complications has been reported comparing limb salvage to amputation [26, 27] (Tables 3 and 4). Unwin et al. evaluated 1001 prostheses with a mean follow-up of 3.8 years. In their series, amputation was performed for patients whose tumors were deemed unresectable [41]. Complications were observed in 616/1001 patients; 7.7% (n = 77) had prosthetic-related complications, 12.4% (n = 124) underwent a revision, and 8.6% (n = 86) ultimately required amputation of the lower limb [41]. The main cause of revision was aseptic loosening (7.1% of the patients), the majority of which required implant removal and implantation of a new prosthesis (n = 65). Infections occurred in 4.6% (n = 46), with 1.7% (n = 17) having a deep infection that required two-staged revision. A total of 28% of patients with infections underwent amputation [41]. Local recurrence occurred in 5.4% of the patients who required revisions (n = 54), with most (52/54, 93%) undergoing amputation while the other two patients underwent rotationplasty. Prosthetic-related complications, such as periprosthetic or implant fracture, were relatively rare and occurred in 2.6% of patients (n = 26), with the majority of patients suffering from implant fractures (17 patients). Jeys et al. [36] studied 776 patients who underwent tumor resection and endoprosthetic placement of which 283 had primary osteosarcoma. The major indication for limb reconstruction was a diagnosis of osteosarcoma, wherein all 283 patients underwent limb salvage [36]. The authors excluded 109 pediatric patients who would require revision for a growing endoprothesis. Although the authors did not stratify the complications according to the tumor type, they found that 227 of 776 patients (34%) underwent revision. Of the 227 revisions, 117 cases were due to mechanical failure (defined as aseptic loosening, implant fracture, rotational loosening), 75 cases due to infection for infection, and 36 cases due to locally recurrent disease [36]. The mean time to revision for any cause was 2.2 years (0 to 34 years), but it increased to a mean of 8.6 years (0 to 34 years) when mechanical failure was defined as the endpoint. Implant survival at 10 years was 75% when only considering mechanical failure and 58% when considering all causes. More specifically, the 10-year implant survival in patients who had osteosarcoma was 50% for all causes [36]. Upper extremity prosthetics had significantly higher 10-year survivorship (85%) than lower extremity or pelvic prosthetics, which had a 53% and 60% survival rate.
Table 3.
Revision rates by study and procedure type
| Study name | Number of patients | Follow-up years (mean, range) | Procedure | Revision rates %, (# of pts)a |
|---|---|---|---|---|
| Jeys et al. [36] | 776 | 15.9 (9, 10–35) | Salvage | 34 (227) |
| Simon et al. [24] | 73 | 5.5 (5.54, N/A) | Salvage | 70 (51) |
| 115 | 5.5 | Above knee amputation | 64 (74) | |
| 39 | 5.5 | Hip disarticulation | 54 (21) | |
| Ghirlizoni et al. [37] | 239 | 4.3 (4.3, 1.6–7.3) | Limb salvage | 7 (17) |
| 116 | Amputation | 7 (8) | ||
| Bacci et al. [26] | 465 | 10.5 (10.5, 5–17) | Salvage | 40 (186) |
| 96 | 10.5 | Amputation or rotationplasty | 40 (38) | |
| Tsuchiya et al. [38] | 107 | 5 (5, 3–8) | Limb salvage | 14 (15) |
| Bacci et al. [35] | 34 | 11 (11, 3–20) | Salvage | 37 (13) |
| 12 | Amputation | 37 (5) | ||
| Abudu et al. [39] | 27 | 4.6 (4.6, 0.8–14.6) | Salvage | 19 (5) |
| 13 | Amputation | 0 (0) | ||
| Marvogenis et al. [27] | 23 | 10 (5, 0.8–24) | Salvage | 13 (3) |
| 18 | Amputation | 0 (0) | ||
| Hegyi et al. [40] | 82 | 5 (N/A, N/A) | Salvage | 38 (31) |
| 30 | Amputation | 38 (11) | ||
| Unwin et al. [41] | 1001 | 3.8 (3.8, N/A) | Salvage | 62 (621) |
| Total | Limb salvage Amputation |
31 (20.3) 28 (24.0) |
||
aAll revision rates are reported as percentage, and standard deviation for the mean revision rate is reported in parenthesis
Table 4.
Complications based on the treatment type
| Study | Unwin et al. [41] | Jeys et al. [36] |
|---|---|---|
| Reason for revision/revision type | Number of patients (%) | Number of patients (%) |
| Aseptic loosening | ||
| Revision implant | 65 (31) | 75 (28.6) |
| Re-cement | 6 (2.9) | |
| Acetabular cup | 3 (1.4) | |
| Total | 74 (35.2) | |
| Infection | ||
| Amputation | 28 (13.3) | 75 (28.6) |
| Revision | 17 (8.1) | |
| Removed implant | 1 (0.5) | |
| Total | 46 (21.9) | |
| Instability | ||
| Total | N/A | 54 (20.6) |
| Recurrence | ||
| Amputation | 52 (24.8) | 36 (13.7) |
| Revision | 1 (0.5) | |
| Rotationplasty | 1 (0.5) | |
| Total | 54 (25.7) | |
| Implant complications | ||
| Fracture | 17 (8.1) | 7 (2.6) |
| Extension complications | 8 (3.8) | |
| Implant fracture | 1 (0.5) | 16 (6.1) |
| Rotational loosening | N/A | 5 (1.9) |
| Total | 26 (12.4) | 28 (10.6) |
| Biological complications | ||
| Bone fracture, revision | 3 (1.4) | N/A |
| Sterile abscess/Ti cyst, | 3 (1.4) | N/A |
| Amputated | ||
| DVT/PE | N/A | 9 (3.4) |
| Circulatory problems | 2 (1.0) | N/A |
| Pain | 2 (1.0) | 4 (1.5) |
| Excessive stiffness | N/A | 5 (1.9) |
| Common peroneal n. palsy | N/A | 7 (2.6) |
| Wrist drop | N/A | 2 (0.7) |
| Hematoma | N/A | 3 (1.1) |
| Total | 10 (4.8) | 30 (11.4) |
| Total | 210 (100) | 262 (100) |
| Total patients per study | 1001 | 776 |
Other studies were not included due to incomplete data
Previous studies demonstrated that the presence of pathologic fractures were negative prognostic factors for patient survival, however, these were most likely related to confounders such as tumor size and location [42]. Due to the conflicting evidence regarding complications following amputation or limb salvage for osteosarcoma, studies evaluating the outcomes following amputation versus salvage are needed.
Patient Function and Satisfaction
Without clear differences between DFS and clinical outcomes between salvage and amputation, some studies focused on assessing functional and psychological outcomes between these groups. Studies using the MSTS system reported better lower extremity function with limb salvage compared to amputation [29, 43, 44]. The MSTS scoring system uses subjective physician ratings, which may therefore be subject to marked bias of these scores.
Robert et al. evaluated 57 patients (33 salvages, 24 amputations) with a mean age of 33.8 years (16–52 years) at a mean follow-up from diagnosis of 18.6 years (3.8–35.6 years) [30]. They assessed function (TESS [31]), quality of life (Quality of Life Cancer-specific scaler [45]), self-esteem, and social support. Multiple linear regression analyses demonstrated no significant differences when evaluating quality of life, self-esteem, or social support between both cohorts. They also reported no difference in the quality of romantic relationships between both treatment groups [30]. The only significant difference between the groups was cosmesis, where limb salvage patients scored higher than those who underwent an amputation. This analysis lost its significance after excluding those who underwent late amputations for local recurrence (n = 7, p = 0.194). The difference in cosmesis remained significant independent of involvement of the pelvis (hemipelvectomy, internal hemipelvectomy, and hip disarticulation; p = 0.046), rotationplasty (p = 0.023), age at diagnosis (p = 0.011), duration from diagnosis to study participation (p = 0.012), or gender (p = 0.011). Positive correlations were found between cosmesis and self-rated physical function, as well as psychological, social, total quality-of-life scores, and self-esteem. This is also correlated to patient satisfaction in multiple areas was the amount of lower limb function post-surgery. Those with higher function reported higher quality of life (p < 0.0001), better cosmesis (p < 0.0001), and greater confidence in romantic relationships (p < 0.0001). Lower limb function did not correlate to social support or self-esteem ratings [4].
Postma et al. evaluated 33 patients (14 salvages, 19 amputations) and assessed psychoneurotic and somatic distress, self-esteem, adjustment to illness, and activities of daily living; they found no significant differences. A trend was found where patients with limb salvage reported more physical complaints compared to those who received an amputation. Amputee’s responses had less physical complaints but focused on lower self-esteem and social isolation [46]. Both groups reported a diminished quality of life and were disabled when compared to their baseline pre-osteosarcoma. Contrary to other series, there was no cosmetic benefit derived from limb salvage when compared to amputation.
Weddington et al. evaluated 35 patients (15 salvages, 20 amputations) for cognitive functioning, affect, mood, cosmetic perception, physical functioning, global psychological adjustment to illness and surgery, and lifetime prevalence of psychiatric disorders before/after surgery. They found no significant differences between the salvage or amputation groups, nor differences when considering age, gender, marital status, surgically involved extremity, chemotherapy status, or social class at the time of surgery or interview [47]. A total of 55% of the patients who underwent either procedure showed good or excellent adjustment to the outcomes of surgery and their disease at a follow-up of 5 years. The results did not demonstrate a psychological advantage for either procedure.
These studies showed that there is no clear difference in the psychosocial and occupational outcomes between the groups [30]. The amount of limb function retained was a positive predictor of cosmetic perception, self-esteem, and romantic confidence regardless of the chosen surgery. The cosmetic advantage associated with limb salvage procedures has not been consistently demonstrated in the current literature [46, 47].
Cost
Compared to amputation, limb salvage procedures were more expensive in the short-term due to implant costs with greater potential for complications. However amputations were associated with higher long-term costs, as these patients often require longer rehabilitation, a lifetime of prosthetic maintenance. Grimer et al. evaluated the length of hospital stay, procedure-related costs, rehabilitation costs, prosthesis charges, as well as additional surgeries [35]. They found that overall the cost of amputation was slightly higher than that of limb salvage, but there was an age distribution affect. Specifically, younger patients who undergo amputations required more expensive, high-performance prosthetics, compared to low-demand elderly patients. Additionally, younger patients will likely require more prosthetic maintenance and may also be at a higher risk for prosthetic-stump interface injuries, which may contribute to increased costs. In 1997, this difference was estimated to be about 70,000 UK pounds (~$166,000 inflation-adjusted 2014 US dollars) more for amputation [32].
Prognostic Factors
While surgical type has not been shown to be a significant prognostic factor for survival or recurrence, many authors reported on other variables that may be implicated in the prognosis of osteosarcoma [24, 26, 27]. These include histological response to chemotherapy (> 90% necrosis), surgical margins, tumor size/location, extent of surgery, and gender [6, 48]. Bielack et al. conducted a retrospective analysis of 1701 patients who had trunk or limb osteosarcoma [6]. They reported a 10-year overall survival and event-free survival of 59.8% and 48.9%. Factors associated with poor prognosis were axial tumor location, male gender, and a history of symptoms longer than 2 months. These factors were also associated with poor response to chemotherapy (defined as < 90% necrosis) [6], in univariate and multivariate analyses. Another variable was age at diagnosis: patients > 40 years of age had lower 10-year survivorship (41.6%) when compared to patients < 40 years of age (60.2%). This was not significant on multivariate analysis.
Multiple studies have demonstrated that response to neoadjuvant chemotherapy was an important prognostic indicator for overall survival [6, 9, 15, 49]. Those with a poor response to chemotherapy had a 5-year survival of 47.2%, while a good response had 73.4%. The extent of surgery was also a predictor of success; procedures that had macroscopically complete tumor resection had a 10-year survival that was higher than patients who did not (64.8 vs. 14.6%, p < 0.0001). Larger tumor size (volume > 100 mL) also had increasingly adverse outcomes [17, 48]. Osteoblastic or chondroblastic histological subtype was associated with an 8-year survival of 54 and 57%, which when compared to patients who had teleangiectatic or fibroblastic subtypes had worse 8-year survivorship (76 and 82%, p = 0.023) [48].
Limitations
This study presents with inherent limitations in study quality, especially with regard to (1) representativeness, (2) reliability and validity of data collection methods, (3) potential for bias, and (4) data analysis. As stated earlier, we used a standardized approach to generate the body of evidence that is presented in this review [28]. However, due to the heterogeneity of studies included in this review, we did not use formal meta-analytic techniques. Furthermore, this review was limited by the quality of included studies, wherein there were no level I studies and only one level II study.
A specific limitation is the underrepresentation of certain populations in the literature regarding limb salvage versus amputation. Numerous factors affect the participation and analysis of underrepresented populations in clinical science research. The most commonly reported barrier factors involve the opportunity for healthcare. Such opportunity factors include individual factors, such as age, socioeconomic status, racial/ethnic minority status, and comorbid conditions. In relation to accounting for almost 17% of the world population, there is a striking lack of Indian studies regarding this subject in health literature. However, the mechanisms that limit opportunities for the study of Indian or other minority populations are not immediately evident. Until the effects of such barriers to healthcare are assessed quantitatively, uncertainty will exist persistently about the bias in study selection.
Conclusion
Limb salvage and amputation are both viable treatment options for patients who have conventional appendicular osteosarcoma. When neoadjuvant chemotherapy is used and adequate surgical margins are achieved, patient outcomes did not differ significantly when comparing limb salvage to amputation in osteosarcoma patients. There was no significant difference between rates of overall survival, recurrence, and metastasis [26, 32, 35–41]. Although the general perception is that patients who have had limb salvage have better cosmetic perception than those who had an amputation, there is discordance in the current literature [30, 46, 47]. The complication and revision rates may be lower with amputation, but the overall cost may be higher in the long-term.
Given the parity between limb salvage and amputation demonstrated in the literature, the type of surgical intervention should be determined based upon the procedure’s ability to fulfill prognostically significant variables, negative margins, and optimized function. Whichever approach maximizes these potential outcomes is likely to result in improved function and patient satisfaction. Advancements in orthotics, prosthetics, and improvement in morbidity from several major surgeries may continue to shift the surgical decision.
Funding Information
This study was performed without external funding.
Compliance with Ethical Standards
Conflicts of Interest
Aditya V. Maheshwari is on the editorial and/or governing board of the World Journal of Orthopedics.
Bhaveen H. Kapadia is a paid consultant and paid presenter and speaker of Sage Products, LLC.
For the remaining authors, they declare that they have no conflicts of interest to disclose.
Contributor Information
Julio J. Jauregui, Phone: (410) 448-6400, Email: juljau@gmail.com
Aditya V. Maheshwari, Phone: (718) 383-8995, Email: aditya.maheshwari@downstate.edu
References
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Grimer RJ. Surgical options for children with osteosarcoma. Lancet Oncol. 2005;6(2):85–92. doi: 10.1016/S1470-2045(05)01734-1. [DOI] [PubMed] [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagarajan R, Neglia JP, Clohisy DR, Robison LL. Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: what are the long-term implications? J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(22):4493–4501. doi: 10.1200/JCO.2002.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006;125(4):555–581. doi: 10.1309/UC6KQHLD9LV2KENN. [DOI] [PubMed] [Google Scholar]
- 6.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 7.Dray MS, Miller MV. Paget’s osteosarcoma and post-radiation osteosarcoma: secondary osteosarcoma at Middlemore Hospital, New Zealand. Pathology. 2008;40(6):604–610. doi: 10.1080/00313020802320663. [DOI] [PubMed] [Google Scholar]
- 8.Ueki H, Maeda N, Sekimizu M, et al. Osteosarcoma after bone marrow transplantation. J Pediatr Hematol Oncol. 2013;35:134–138. doi: 10.1097/MPH.0b013e3182677f19. [DOI] [PubMed] [Google Scholar]
- 9.Dai X, Ma W, He X, Jha RK. Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing’s sarcoma. Med Sci Monit Int Med J Exp Clin Res. 2011;17(8):RA177–RA190. doi: 10.12659/MSM.881893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422–441. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 11.Lindor NM, Furuichi Y, Kitao S, et al. Rothmund-Thomson syndrome due to RECQ4 helicase mutations: report and clinical and molecular comparisons with Bloom syndrome and Werner syndrome. Am J Med Genet. 2000;90:223–228. doi: 10.1002/(SICI)1096-8628(20000131)90:3<223::AID-AJMG7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Hansen MF. Genetic and molecular aspects of osteosarcoma. J Musculoskelet Neuronal Interact. 2002;2(6):554–560. [PubMed] [Google Scholar]
- 13.O’Day K, Gorlick R. Novel therapeutic agents for osteosarcoma. Expert Rev Anticancer Ther. 2009;9(4):511–523. doi: 10.1586/era.09.7. [DOI] [PubMed] [Google Scholar]
- 14.Hughes DP, Thomas DG, Giordano TJ, et al. Cell surface expression of epidermal growth factor receptor and Her-2 with nuclear expression of Her-4 in primary osteosarcoma. Cancer Res. 2004;64(6):2047–2053. doi: 10.1158/0008-5472.CAN-03-3096. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007;19(4):341–346. doi: 10.1097/CCO.0b013e328122d73f. [DOI] [PubMed] [Google Scholar]
- 16.Maris JM, Courtright J, Houghton PJ, Morton CL, Gorlick R, Kolb EA, Lock R, Tajbakhsh M, Reynolds CP, Keir ST, Wu J, Smith MA. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(3):581–587. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 17.Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134(3):281–297. doi: 10.1007/s00432-007-0330-x. [DOI] [PubMed] [Google Scholar]
- 18.Yin K, Liao Q, Zhong D, et al. Meta-analysis of limb salvage versus amputation for treating high-grade and localized osteosarcoma in patients with pathological fracture. Exp Ther Med. 2012;4:889–894. doi: 10.3892/etm.2012.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neel MD, Letson GD. Modular endoprostheses for children with malignant bone tumors. Cancer Control J Moffitt Cancer Center. 2001;8(4):344–348. doi: 10.1177/107327480100800406. [DOI] [PubMed] [Google Scholar]
- 20.Orlic D, Smerdelj M, Kolundzic R, Bergovec M. Lower limb salvage surgery: modular endoprosthesis in bone tumour treatment. Int Orthop. 2006;30(6):458–464. doi: 10.1007/s00264-006-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgart R, Lenze U. Expandable endoprostheses in malignant bone tumors in children: indications and limitations. Recent results in cancer research. Fortschritte der Krebsforschung Progres dans les recherches sur le Cancer. 2009;179:59–73. doi: 10.1007/978-3-540-77960-5_6. [DOI] [PubMed] [Google Scholar]
- 22.Mavrogenis AF, Lenze U, Rechl H, et al. Recent developments in the surgical treatment of bone tumors and their impact on quality of life. Sarcoma. 2013;2013:826432. doi: 10.1155/2013/826432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MR DC, Friedlaender GE. Malignant bone tumors: limb sparing versus amputation. J Am Acad Orthop Surg. 2003;11(1):25–37. doi: 10.5435/00124635-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am Vol. 1986;68(9):1331–1337. doi: 10.2106/00004623-198668090-00005. [DOI] [PubMed] [Google Scholar]
- 25.Malawer MH, Lee JH, O'Sullivan B. Sarcomas of bone. In "DeVita, Hellman, and Rosenberg’s cancer: principles and practice of oncology". Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 1–32. [Google Scholar]
- 26.Bacci G, Ferrari S, Lari S, Mercuri M, Donati D, Longhi A, Forni C, Bertoni F, Versari M, Pignotti E. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br Vol. 2002;84(1):88–92. doi: 10.1302/0301-620X.84B1.12211. [DOI] [PubMed] [Google Scholar]
- 27.Mavrogenis AF, Abati CN, Romagnoli C, Ruggieri P. Similar survival but better function for patients after limb salvage versus amputation for distal tibia osteosarcoma. Clin Orthop Relat Res. 2012;470(6):1735–1748. doi: 10.1007/s11999-011-2238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8:e83138. doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renard AJ, Veth RP, Schreuder HW, et al. Function and complications after ablative and limb-salvage therapy in lower extremity sarcoma of bone. J Surg Oncol. 2000;73(4):198–205. doi: 10.1002/(SICI)1096-9098(200004)73:4<198::AID-JSO3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Robert RS, Ottaviani G, Huh WW, Palla S, Jaffe N. Psychosocial and functional outcomes in long-term survivors of osteosarcoma: a comparison of limb-salvage surgery and amputation. Pediatr Blood Cancer. 2010;54(7):990–999. doi: 10.1002/pbc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5(5):508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 32.Grimer RJ, Carter SR, Pynsent PB. The cost-effectiveness of limb salvage for bone tumours. J Bone Joint Surg Br Vol. 1997;79(4):558–561. doi: 10.1302/0301-620X.79B4.7687. [DOI] [PubMed] [Google Scholar]
- 33.Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17(8):515–527. doi: 10.5435/00124635-200908000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. 1986. J Bone Joint Surg Am Vol. 2005;87:2822. doi: 10.2106/JBJS.8712.cl. [DOI] [PubMed] [Google Scholar]
- 35.Bacci G, Ferrari S, Longhi A, et al. Nonmetastatic osteosarcoma of the extremity with pathologic fracture at presentation: local and systemic control by amputation or limb salvage after preoperative chemotherapy. Acta Orthop Scand. 2003;74:449–454. doi: 10.1080/00016470310017776. [DOI] [PubMed] [Google Scholar]
- 36.Jeys LM, Kulkarni A, Grimer RJ, et al. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am Vol. 2008;90:1265–1271. doi: 10.2106/JBJS.F.01324. [DOI] [PubMed] [Google Scholar]
- 37.Gherlinzoni F, Picci P, Bacci G, Campanacci D (1992) Limb sparing versus amputation in osteosarcoma: Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. Ann Oncol 3(suppl 2):S23–S27 [DOI] [PubMed]
- 38.Tsuchiya H, Tomita K (1992) Prognosis of osteosarcoma treated by limb-salvage surgery: the ten-year intergroup study in Japan. Jpn J Clin Oncol 22:347–353 [PubMed]
- 39.Abudu A, Sferopoulos NK, Tillman RM et al (1996) The surgical treatment and outcome of pathological fractures in localised osteosarcoma. J Bone Joint Surg Br Vol 78:694–698 [PubMed]
- 40.Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss J, Szendroi M, Csoka M, Kovacs G (2011) Good prognosis of localized osteosarcoma in young patients treated with limb-salvage surgery and chemotherapy. Pediatr Blood Cancer 57(3):415–422 [DOI] [PubMed]
- 41.Unwin PS, Cannon SR, Grimer RJ, et al. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br Vol. 1996;78:5–13. doi: 10.1302/0301-620X.78B1.0780005. [DOI] [PubMed] [Google Scholar]
- 42.Kim MS, Lee SY, Lee TR, Cho WH, Song WS, Cho SH, Lee JA, Yoo JY, Jung ST, Jeon DG. Prognostic effect of pathologic fracture in localized osteosarcoma: a cohort/case controlled study at a single institute. J Surg Oncol. 2009;100(3):233–239. doi: 10.1002/jso.21265. [DOI] [PubMed] [Google Scholar]
- 43.Ruggieri P, De Cristofaro R, Picci P et al (1993) Complications and surgical indications in 144 cases of nonmetastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Clin Orthop Relat Res:226–238 [PubMed]
- 44.Capanna R, Morris HG, Campanacci D, et al. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J Bone Joint Surg Br Vol. 1994;76:178–186. doi: 10.1302/0301-620X.76B2.8113272. [DOI] [PubMed] [Google Scholar]
- 45.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4(6):523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 46.Postma A, Kingma A, De Ruiter JH, et al. Quality of life in bone tumor patients comparing limb salvage and amputation of the lower extremity. J Surg Oncol. 1992;51:47–51. doi: 10.1002/jso.2930510113. [DOI] [PubMed] [Google Scholar]
- 47.Weddington WW, Jr, Segraves KB, Simon MA. Psychological outcome of extremity sarcoma survivors undergoing amputation or limb salvage. J Clin Oncol Off J Am Soc Clin Oncol. 1985;3(10):1393–1399. doi: 10.1200/JCO.1985.3.10.1393. [DOI] [PubMed] [Google Scholar]
- 48.Ferrari S, Bertoni F, Mercuri M, et al. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity: an analysis of 300 patients treated at the Rizzoli Institute. Ann Oncol Off J Eur Soc Med Oncol/ESMO. 2001;12:1145–1150. doi: 10.1023/A:1011636912674. [DOI] [PubMed] [Google Scholar]
- 49.Bacci G, Ferrari S, Mercuri M, et al. Neoadjuvant chemotherapy for extremity osteosarcoma—preliminary results of the Rizzoli’s 4th study. Acta Oncol. 1998;37:41–48. doi: 10.1080/028418698423168. [DOI] [PubMed] [Google Scholar]