Abstract
Nearly half of newly diagnosed cases of bladder cancer are low grade, noninvasive, and papillary tumors. The standard treatment for non-muscle-invasive bladder cancer (NMIBC) has been transurethral resection of the bladder tumor (TUR-BT) with or without adjuvant intravesical instillation (IVI) of chemotherapy or Bacillus Calmette-Guerin (BCG) therapy. NMIBC is known to be associated with high rates of recurrence and risk of progression. In this study, we have retrospectively analyzed the clinical outcome of initially diagnosed multiple low-grade Ta tumors, with a special focus on tumor recurrence and worsening progression (WP) pattern. We retrospectively reviewed 42 patients with primary, multiple, low-grade Ta bladder cancer. We defined WP as confirmed high-grade Ta, all T1 or Tis/concomitant CIS of bladder recurrence, upper urinary tract recurrence (UTR), or progression to equal to or more than T2. The associations between clinico-pathological factors and tumor recurrence as well as WP pattern were analyzed. Tumor recurrence and WP occurred in 23 (54.76%) and 8 (19.04%) patients during follow-up (median follow-up: 57.38 months), respectively. WP to high grade/stage was seen in 8 patients. Multivariate analysis demonstrated that use of tobacco (p < 0.0001) and absence of IVI (p < 0.0001) were significant risk factors for tumor recurrence. The 5-year recurrence-free survival rate for non-tobacco users (74.0%) was significantly higher than that for tobacco users (42.5%, p = 0.0001), and also higher for patients receiving intravesical instillation (84.2 vs. 30.0% without IVI, p = 0.0001). Recurrence is common in patients with low-grade, Ta bladder cancer, especially in the setting of multiplicity. Recurrences occurred in 54.76% of patients and WP occurred in 19.04% of patients. Use of tobacco and non-use of IVI were strongly associated with high recurrence rate.
Keywords: Bladder cancer, Low grade, Multiple, Intravesical instillation, Recurrence, Progression
Introduction
Bladder cancer is a common malignancy affecting the urinary tract and is the 7th most common cancer in men and 17th in women [1]. The non-muscle-invasive bladder cancers (NMIBC) which were traditionally known as superficial bladder cancer are malignant urothelial tumors that have not invaded the detrusor (muscle) [2, 3]. Approximately 70% of bladder cancers are non-muscle invasive at presentation. Of these, 70% present as stage Ta, 20% as T1, and 10% as CIS (carcinoma in situ) [4]. The standard treatment for non-muscle-invasive bladder cancer has been transurethral resection of the bladder tumor (TUR-BT) with or without adjuvant intravesical instillation (IVI) of chemotherapy or Bacillus Calmette-Guerin (BCG) therapy [5].
It is estimated that 40 to 80% of non-muscle-invasive bladder cancers recur within 6 to 12 months if managed with a TUR-BT without additional therapy, and 10 to 25% will develop muscle-invasive, regional, or metastatic disease [5]. A more aggressive therapy is often required even though an initial complete transurethral resection is possible in these group of patients. The most important prognostic factors are histologic stage and grade. Other factors that have been assessed include the presence of multicentric disease, the frequency of recurrence, the tumor size, and the presence or absence of concomitant CIS.
Non-muscle-invasive bladder cancers are classified into three groups, Ta, Tis (also referred CIS), and T1 lesions, based upon their growth pattern and depth of invasion. Stage Ta tumors are usually low grade. Although recurrence is common, especially in the setting of multiplicity, progression is rare. However, 2.9 to 18% of Ta tumors are high grade, with an average of 6.9% [6]. CIS is actually a flat, noninvasive urothelial carcinoma (UC) that is high grade by definition and is regarded as a precursor to the development of invasive high-grade cancer [6]. From 40 to 83% of patients with CIS will develop muscle invasion if untreated, especially if associated with papillary tumors [7]. Multicentricity presents another ominous characteristic of CIS [8]. T1 tumors are usually papillary and often have a narrow stalk; a nodular or sessile appearance suggests deeper invasion. Deep penetration into the lamina propria, especially if involving muscularis mucosae, increases the risk of recurrence and progression in some reports. Lymphovascular invasion, [9] pyuria, [10] and bladder neck involvement [11] also increase this risk.
Several large retrospective series have been analyzed, in an attempt to classify patients according to their risk of recurrence and progression to invasive disease [12, 13]. Patients with a Ta lesion at initial presentation, greater than 1 year between tumor recurrences, three or fewer lesions, small (≤3 cm), papillary appearing with a fine stalk, with no invasion of lamina propria and no associated CIS were considered as low risk. However, presence of multiple papillary recurrences within a short period of time, lesions more than three or any lesion >3 cm, sessile with a thick stalk, invasion of lamina propria, high-grade histology, and presence of CIS were considered as poor risk [14].
There is very little information regarding long-term follow-ups in patients with low-grade Ta tumors based on the 2004 WHO classification [15]. Most Ta tumors are known to recur and progress within 5 years of initial treatment; however, recent data support the need for long-term follow-up for more than 10–15 years in such patients even after an initial response to BCG therapy and a recurrence-free period for more than 5 years [16]. In this study, we have analyzed the clinical outcome of initially diagnosed multiple low-grade Ta tumors, with a special focus on tumor recurrence and worsening progression (WP) pattern.
Materials and Methods
We retrospectively reviewed the medical records and imaging (Figs. 1 and 2) of all patients who underwent TUR-BT with complete tumor resection during the period Jan 2007 to Dec 2016 at our hospital for an initially diagnosed multiple low-grade Ta tumors. This study was undertaken with permission obtained from the university/institutional ethical committee. We excluded all patients who had already undergone TUR- BT at another hospital or who had a history of upper tract urothelial cancer. All the pathological specimens were reassessed and reported by a dedicated uro-pathologist, with a special focus on the 2004 WHO grading system. All of the tumors were histologically confirmed as urothelial carcinoma. All patients received adjuvant post-operative (< 6 h) intravesical instillation of Mitomycin C. The use of adjuvant IVI of BCG depended primarily on patient’s consent.
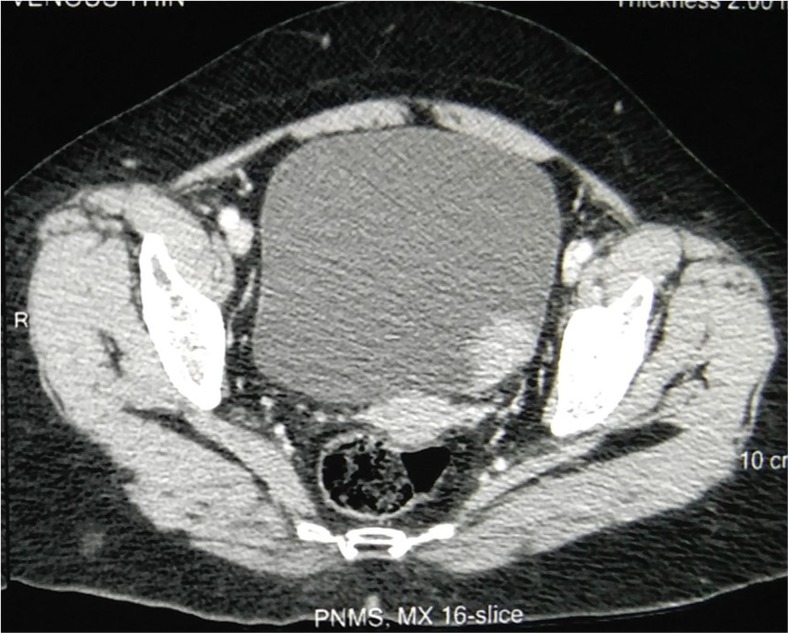
Fig. 1.
Non-contrast CT pelvis showing a space occupying lesion on the left lateral wall of the bladder
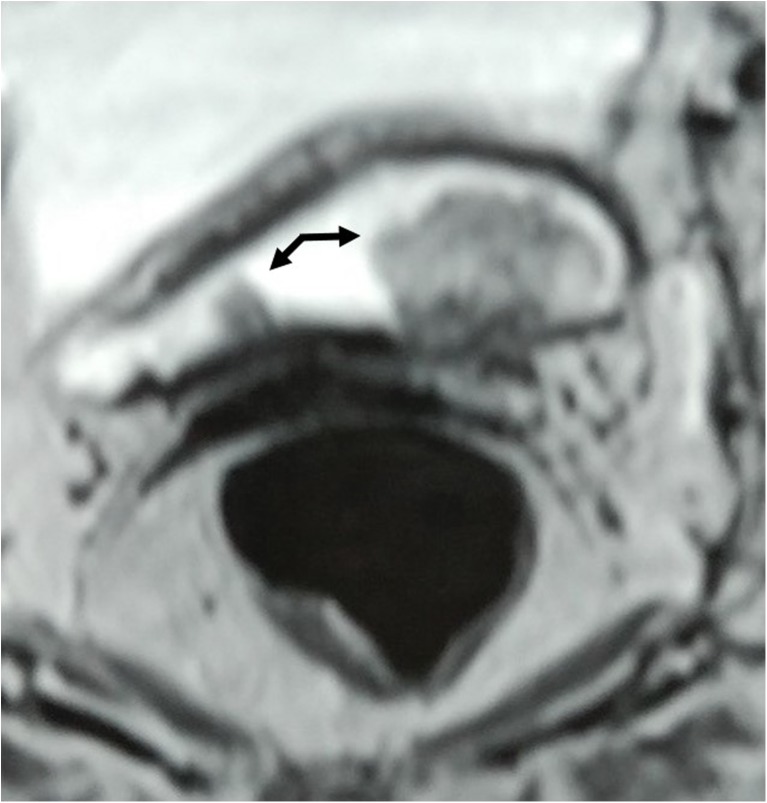
Fig. 2.
MR Urogram showing multiple bladder tumors
All these patients were followed up with urine cytology and cystoscopy at 3-monthly intervals during the initial year, every 6 months for the next 2 years, and then yearly thereafter. Intravenous urography, ultrasonography, and/or CT scanning were used to evaluate distant metastasis and upper urinary tract recurrence (UTR) every year for at least 5 years. Recurrence was defined as the occurrence of a new tumor in the bladder. Worsening progression (WP) was defined as confirmed (1) high-grade Ta, all T1, or Tis/concomitant CIS of bladder recurrence, (2) upper tract recurrence, or (3) progression to ≥ T2.
The following factors were analyzed for each individual patient: age, gender, multiplicity, and smoking status. Smoking status was classified as either nonsmokers, those who had never smoked during their lifetime; or current smokers, those who still smoked regularly at the time of initial TUR-BT. Recurrence-free survival rate curves were constructed using the Kaplan- Meier method and were compared using the log-rank test. Differences among groups were regarded as significant when p < 0.05. Univariate and multivariate analyses of data were performed using the Cox proportional hazards model with stepwise forward selection. These analyses were performed with a statistical software package (IBM SPSS Version 20.0. Armonk, NY: IBM Corp.).
Results
A total of 42 (male 35, female 7) patients with a mean age of 57.3±7.99 years (range, 41–72) underwent TUR-BT for multiple low-grade Ta tumors during the study period and the median follow-up interval was 57.38 ±28.4 months (range, 12 to 118.). Twenty two patients including the 7 female patients gave no history of use of tobacco, whereas the remaining 20 patients used tobacco in the form of either smoking or chewing. The mean number of lesions identified and treated at the initial presentation were 4.73±1.17 (range, 3–7). Histopathological examination confirmed the lesions to be low-grade urothelial carcinoma (2004 WHO grading system) (Fig. 4). All patients received intravesical instillation of mitomycin within 6 h of surgery. As per the departmental policy, all patients were advised intravesical instillation of BCG, only 25 patients had the six, weekly instillations. The remaining 17 either refused or did not turn up for the treatment. Of the 42 patients, 20 patients gave history of using tobacco (chewing or smoking) (Table 1).
Fig. 4.
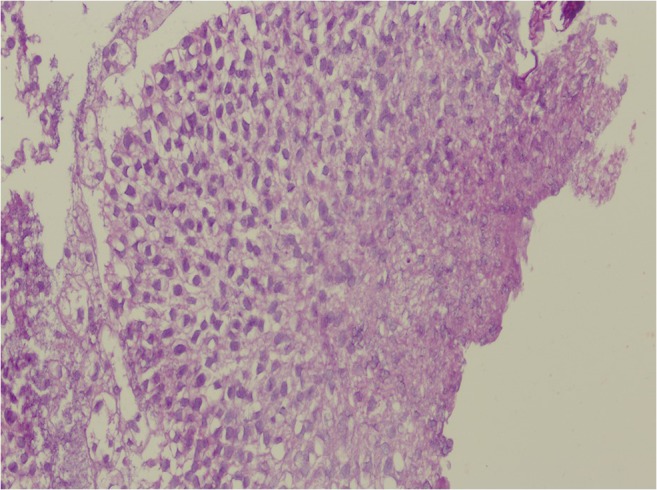
Photomicrograph of low-grade papillary urothelial tumor displaying minimal cytologic and architectural atypia
Table 1.
Recurrences in patients
| Sl. no. | Parameters | Recurrences seen (23) | p value |
|---|---|---|---|
| 1 | Non-tobacco users (20) | 10 | 0.0001 |
| 2 | Tobacco users (22) | 13 | |
| 3 | Intravesical instillation of BCG (25) | 8 | 0.0001 |
| 4 | No instillation of BCG (17) | 12 |
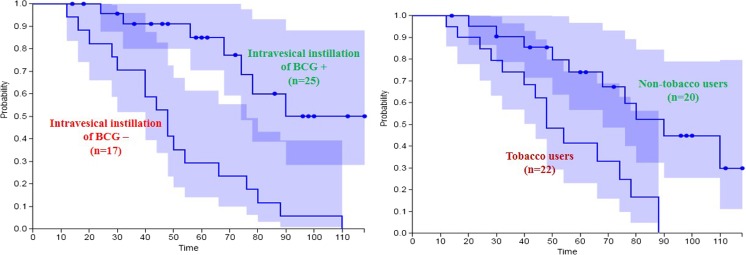
Tumor recurrences occurred in 23 patients (54.76%). Most patients who had tumor recurrence could be diagnosed by the routine follow-up cystoscopic examination. When divided, the patients into two groups, those with or without tumor recurrence, there were no significant differences in age and gender between the two groups. Univariate and multivariate analyses demonstrated that use of tobacco and absence of IVI of BCG were significant risk factors for tumor recurrence. Recurrences developed in 10 and 13 patients belonging to the non-tobacco users and tobacco users group respectively. Similarly, recurrences developed in 8 and 12 patients belonging to IVI and non-IVI group, respectively. The Kaplan-Meier curves demonstrated that the 5-year recurrence-free survival rate for non-tobacco users (74.0%) was significantly higher than that for tobacco users (42.5%, p = 0.0001), and also higher for patients receiving intravesical instillation (84.2 vs. 30.0% without IVI, p = 0.0001) (Fig. 3a, b).
Fig. 3.
Recurrence-free survival rates (a) by use of Tobacco and (b) by presence of adjuvant intravesical instillation of BCG
A total of 8 (19.04%) patients developed worsening progression (WP). Three of the patients developed T1, one developed a high-grade Ta lesion, and two had T2 lesions. The T1 lesions were treated appropriately with TUR-BT and IVI of BCG (induction and maintenance), whereas the two patients with T2 disease and the one with high-grade multiple Ta lesions underwent radical cystectomy. None of the patients developed upper tract disease. Four patients died during the study period of which three died due to causes other than bladder cancer (Fig. 4).
Discussion
Low-grade tumors rarely invade the lamina propria or detrusor. Low-grade Ta lesions recur at a rate of 50 to 70% and progress in approximately 5% of cases, whereas the high-grade T1 lesions recur in more than 80% of cases and progress in 50% of patients within 3 years. This behavior is primarily because of the grade, because it is well known that high-grade tumors progress with similar frequency regardless of whether they are invasive (T1) or noninvasive (Ta) [17]. This biologic behavior of low-grade versus high-grade lesions correlates with the known dual molecular lines of genetic development for these two pathways and supports the concept that high-grade and low-grade cancers may be considered as essentially different diseases [18, 19]. Chromosomal alterations caused by oxidative DNA damage create two separate genetic pathways to the development of urothelial carcinoma [20]. The first and more common (low grade) leads to noninvasive, papillary tumors. These usually follow an indolent course unless they convert to or are associated with a tumor of the second pathway [21]. UC is traditionally considered a field change disease, with tumors arising at different times and sites, rarely low-grade tumors subsequently develop high-grade tumors and nevertheless high-grade and low-grade lesions are known to coexist [16].
Kobayashi et al. evaluated the clinical outcome of 190 patients with low-grade Ta bladder cancer followed up for a long period [15]. Tumor recurrence and worsening progression (WP) occurred in 82 (43.2%) and 21 (11.1%) patients during follow-up (median follow-up 101.5 months), respectively. WP to high-grade Ta, all T1 or Tis/concomitant CIS was seen in 17 patients, and UTR and progression to equal to or more than T2 were seen in 2 and 2 patients, respectively. Multivariate analyses demonstrated that multiple tumor (p < 0.001, HR 2.97) and absence of intravesical instillation (IVI) (p < 0.001, HR 2.88) were significant risk factors for tumor recurrences while multiple tumor was the only risk factor for WP (p = 0.001, HR 5.26). After a 5-year tumor-free period, 9 patients experienced late recurrence in years 5 and 10 and were diagnosed at a follow-up cystoscopy; however, only 2 patients recurred beyond 10 years and were found by gross hematuria. There were no significant risk factors of late recurrence. Multiple tumors are a known risk factor for both tumor recurrence and WP. The authors suggested that routine follow-up of patients with low-grade Ta bladder cancer was needed up to 10 years from the initial diagnosis.
Zieger et al. reported on the natural history of 212 patients initially diagnosed with TaG1-2 tumors for up to 20 years [22]. Only 14 patients received intravesical instillation in their study. Ten of the 212 (4.7%) developed into TaG3 or CIS, 18 (8.5%) developed into T1, and 23 (10.8%) showed muscle invasion or distant metastases. Similarly, Prout et al. followed 178 patients with TaG1 bladder tumors for up to 10 years [23]. They reported that a change in grade or stage progression occurred in 13 (7.3%) patients, while only 14 patients (7.9%) received intravesical chemotherapy. Holmang et al. reported the outcomes in patients treated with BCG intravesical therapy who were tumor-free for more than 5 years (N = 204) [16]. Of the 204 patients, 110 (53.9%) had a G1 or G2 tumor. They stated that patients with TaG1-2 tumors treated with BCG have a very good long-term prognosis, but late recurrences were observed.
Our study clearly shows that patients with multiple low-grade Ta lesions are at high risk to develop recurrences as well as progress. History of use of tobacco carried a high risk of recurrence in these patients and so also non-usage of IVI of BCG carried a high risk. In India, people are known to use tobacco both for smoking as well as for chewing. As of now, we do not know which among these two forms is associated with higher risk of recurrence. Our study clearly shows the beneficial effect of IVI of BCG in these patients. Though IVI of BCG is not indicated in low-grade, solitary Ta lesion, we believe that in cases of multiple tumors at presentation, BCG is useful to reduce recurrences as well as to prevent WP.
This present study of ours has several limitations. This study being a retrospective one with the number of patients being small, it is possible that unknown sources of bias may exist in the findings. Though all patients were given a single immediate post-operative instillation of chemotherapy within 24 h, further IVI of maintenance intravesical therapies with BCG was not carried out in all the patients, as it may have improved the results. Most of the patients in India are not covered by health insurance benefits and it is possible that patients may refuse the advised therapy, investigations, and/or follow-up for financial reasons.
Conclusion
In our study, the tumor recurrence rate and WP rate in patients with primary, multiple, low-grade Ta bladder cancer were 54.76 and 19.04%, respectively. Multiple tumors was a risk factor for both tumor recurrence and WP. Our results suggest that routine follow-up of patients with low-grade multiple Ta bladder cancer is definitely needed for long period after the initial diagnosis.
References
- 1.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Böhle A, Redorta JP, Roupreˆt M. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22(12):1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Smith JA, Jr, Labasky RF, Cockett AT, et al. Bladder cancer clinical guidelines panel summary report on the management of non–muscle-invasive bladder cancer (stages Ta, T1 and TIS). The American Urological Association. J Urol. 1999;162:1697–1701. doi: 10.1016/S0022-5347(05)68208-0. [DOI] [PubMed] [Google Scholar]
- 4.Ro JY, Staerkel GA, Ayala AG. Cytologic and histologic features of superficial bladder cancer. Urol Clin North Am. 1992;19(3):435–453. [PubMed] [Google Scholar]
- 5.Hendricksen K, Witjes JA. Current strategies for first and second line intravesical therapy for nonmuscle invasive bladder cancer. Curr Opin Urol. 2007;17(5):352–357. doi: 10.1097/MOU.0b013e3281c55f2b. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester RJ, van der Meijden A, Witjes JA, et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66(6 Suppl.1):90–107. doi: 10.1016/j.urology.2005.06.135. [DOI] [PubMed] [Google Scholar]
- 7.Althausen AF, Prout GR, Jr, Daly JJ. Non-invasive papillary carcinoma of the bladder associated with carcinoma in situ. J Urol. 1976;116:575–580. doi: 10.1016/S0022-5347(17)58916-8. [DOI] [PubMed] [Google Scholar]
- 8.Koch MO, Smith JA., Jr . Natural history and surgical management of superficial bladder cancer (stages Ta/T1/Tis) In: Vogelzang N, Miles BJ, editors. Comprehensive textbook of genitourinary oncology. Baltimore: Lippincott Williams & Wilkins; 1996. pp. 405–415. [Google Scholar]
- 9.Lotan Y, Goodman PJ, Youssef RF, Svatek RS, Shariat SF, Tangen CM, Thompson IM, Jr, Klein EA. Evaluation of vitamin E and selenium supplementation for the prevention of bladder cancer in SWOG coordinated SELECT. J Urol. 2012;187(6):2005–2010. doi: 10.1016/j.juro.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azuma T, Nagase Y, Oshi M. Pyuria predicts poor prognosis in patients with non–muscle-invasive bladder cancer. Clin Genitourin Cancer. 2013;11(3):331–336. doi: 10.1016/j.clgc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Fujii Y, Koga F, et al. Impact of bladder neck involvement on progression in patients with primary non–muscle invasive bladder cancer: a prospective validation study. Urol Oncol. 2014;32(1):38.e29–38.e36. doi: 10.1016/j.urolonc.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680. doi: 10.1016/S0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 13.Kiemeney LA, Witjes JA, Heijbroek RP, et al. Predictability of recurrent and progressive disease in individual patients with primary superficial bladder cancer. J Urol. 1993;150(1):60–64. doi: 10.1016/S0022-5347(17)35397-1. [DOI] [PubMed] [Google Scholar]
- 14.Pow-Sang JM, Seigne JD. Contemporary management of superficial bladder cancer. Cancer Control. 2000;7(4):335–339. doi: 10.1177/107327480000700402. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Kikuchi E, Mikami S, Maeda T, Tanaka N, Miyajima A, Nakagawa K, Oya M. Long term follow-up in patients with initially diagnosed low grade Ta non-muscle invasive bladder tumors: tumor recurrence and worsening progression. BMC Urol. 2014;14(1):5–12. doi: 10.1186/1471-2490-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmang S, Strock V. Should follow-up cystoscopy in Bacillus Calmette-Guerin-treated patients continue after five tumour-free years? Eur Urol. 2012;61(3):503–507. doi: 10.1016/j.eururo.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol. 2000;163(1):60–61. doi: 10.1016/S0022-5347(05)67972-4. [DOI] [PubMed] [Google Scholar]
- 18.Hasui Y, Osada Y, Kitada S. Significance of invasion to the muscularis mucosae on the progression of superficial bladder cancer. Urology. 1994;43(6):782–786. doi: 10.1016/0090-4295(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 19.Droller MJ. Biological considerations in the assessment of urothelial cancer: a retrospective. Urology. 2005;66(5 Suppl):66–75. doi: 10.1016/j.urology.2005.06.128. [DOI] [PubMed] [Google Scholar]
- 20.Cote R, Chatterjee SJ. Molecular determinants of outcome in bladder cancer. Cancer J Sci Am. 1999;5:1–15. [PubMed] [Google Scholar]
- 21.Kiemeney LA, Witjes JA, Verbeek AL, et al. The clinical epidemiology of superficial bladder cancer. Dutch South-East Cooperative Urological Group. Br J Cancer. 1993;67(4):806–812. doi: 10.1038/bjc.1993.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zieger K, Wolf H, Olsen PR, Hojgaard K. Long-term follow-up of noninvasive bladder tumours (stage Ta): recurrence and progression. BJU Int. 2000;85(7):824–828. doi: 10.1046/j.1464-410x.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- 23.Prout GR, Jr, Barton BA, Griffin PP, Friedell GH. Treated history of noninvasive grade 1 transitional cell carcinoma. The National Bladder Cancer Group. J Urol. 1992;148(5):1413–1419. doi: 10.1016/S0022-5347(17)36924-0. [DOI] [PubMed] [Google Scholar]