Abstract
Purpose
In human oocytes, sERCs are one of the dysmorphic phenotypes that have been reported. Significantly reduced pregnancy rates and a comparatively higher number of abnormities in live births appear to be associated with the presence of sERCs in oocytes. However, some reports have shown that healthy babies can be born, without any reduced pregnancy rates, from oocytes observed to contain sERCs. Thus, the clinical and scientific significance of oocytes that harbor sERCs remains controversial.
Methods
The presence of sERCs was evaluated using a time-lapse system while studying the dynamic changes within oocytes and embryos. Logistic regression analysis was carried out to explore the independent variables for meiotic and mitotic cleavage failure..
Results
The incidence of mitotic cleavage failure and the incidence of meiotic cleavage failure during the second polar body extrusion in oocytes with sERCs were found to be significantly higher than that in oocytes without sERCs. Furthermore, ICSI was found to have a greater frequency of meiotic failure than IVF.
Conclusions
In cases of cleavage failure, an embryonic cell could become tetraploid and may induce abnormal chromosomal configurations. Some cells exposed to cleavage failure may become trophectoderm cells and form placental abnormalities. Even if they develop into trophectoderm cells, the ICM can be susceptible to further cleavage failure and may in turn cause further aneuploidy. For these reasons, it is important to monitor pregnancies and births derived from oocytes that contained sERCs.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1119-3) contains supplementary material, which is available to authorized users.
Keywords: Smooth endoplasmic reticulum cluster, Time lapse observation, Cleavage failure, Meiosis, Mitosis
Introduction
A smooth endoplasmic reticulum cluster (sERC) is one of the dysmorphic phenotypes which occasionally appears in human oocytes. In our previous report, the first in this specific field of research, we found that pregnancy rates remained low in sERC negative cohort oocytes in sERC positive cycles [1]. Since our first report regarding the poor outcome in sERC positive cycles, many studies have been conducted on the outcome of embryos derived from oocytes with sERCs. Significantly reduced pregnancy rates and a comparatively high number of abnormalities in live birth babies derived from sERC positive oocytes and/or sERC positive cycles have been reported [2–5]. Following these reports, in 2011, the Alpha Scientists in Reproductive Medicine and the European Society of Human Reproduction and Embryology (ESHRE) Special Interest Group on Embryology recommended refraining from inseminating oocytes that contain sERC (2011).
However, following this recommendation to avoid the transfer of affected embryos, the opposite results have also been reported: showing that healthy babies, derived from sERC positive oocytes, can be born, without reduced pregnancy rates [6–8]. Thus, the clinical and scientific impact of sERC positive oocytes remains controversial. This discrepancy may be due to the different techniques employed in each laboratory, such as whether the data comes from fresh embryo transfers or frozen-thawed embryo transfers, different stimulation protocols and the timings of embryo transfers, all of which may influence the implantation rates.
Moreover, using different types of microscopes may have an impact on the ability to detect sERCs. sERC occurrence rates reported in papers are different, ranging from 5.4% [6] to 23.1% (this study). sERC detection rates among embryologists might also have been different depending on their level of experience, training, knowledge, and efforts to record the presence of sERCs. These factors could negatively influence the accuracy of data. Therefore, research on the biological, structural, and physiological features of sERC formation is further required.
We previously reported that sERC formation was related to higher serum estradiol levels detected in sERC positive cycles and this is in agreement with other studies [2, 9]. Very recently, Canto et al. reported that sERC positive oocytes had greater spindle lengths and widths compared with control oocytes. Furthermore, a greater number of sERC positive oocytes had intermittently weak actin staining sections in their sub-oolemmal regions [10].
In human embryos, several abnormal cleavage patterns, such as direct cleavage and reverse cleavage, can be observed during embryo development by the use of time-lapse systems. These abnormalities are reported to be associated with lowered pregnancy rates [11–14]. In the present study, our aim was to explore reproductive outcomes in relation to the presence of sERCs in the cytoplasm of oocytes.
Materials and methods
The presence of sERCs in oocytes was retrospectively reviewed using existing data that was captured by a time-lapse system (EmbryoScope®) between January 2011 and December 2015. Informed consent was obtained from all patients to enable us to use their data for publication and this study received the approval of the institutional review board. During the course of this study, the recordings were restricted to patients aged between 30 and 40 years of age. Moreover, observations were limited only to those who yielded four or more oocytes, to enable the evaluation of the possible effects of the time-lapse system.
IVF and ICSI procedures
During the oocyte retrieval cycles, patients were treated using standard GnRH agonist/FSH protocols or with the antagonist/FSH protocol. Ovulation induction was triggered when the second leading follicle was more than 18 mm in diameter. Ultrasound-guided transvaginal oocyte retrieval was performed 35–36 h later.
The IVF laboratory procedures were as follows. Immediately upon retrieval, oocytes were placed in Universal IVF Medium (Origio a/s, Jyllinge, Denmark) and overlaid with mineral oil (Irvine Scientific, USA). Oocytes were inseminated 3–5 h later using conventional insemination procedures or ICSI, depending on the semen parameters. After a fertilization check, performed approximately 4 h after insemination, the resultant zygotes were placed in Global medium (LifeGlobal, Canada), containing 10% HSA (LifeGlobal, Canada) where they remained until day 5. As sERC disappear in about 5 h after fertilization, it was possible to check the presence of sERC at this point.
Time lapse recordings and embryo evaluations
Time lapse images were captured automatically every 15 min, at seven focal planes using an EmbryoScope®Time-lapse system (Vitrolife, Tokyo, Japan). Any changes in the configuration of the embryos observed by time lapse system were retrospectively analyzed. Unfertilized oocytes and embryos which were cryopreserved on day 2 were excluded from the analyses. In all cases, the second and fourth best quality embryos were chosen for cryopreservation based on Gardner’s embryo morphology criteria. The transfer of fresh best quality embryos, followed by transfer of cryopreserved embryos, in the absence of pregnancy with the fresh embryos, is our standard two-step procedure for improving pregnancy outcomes [15]. Detection of sERCs, using the time-lapse system, was performed by a single embryologist, who has more than 20 years experience in this field. In all cases, the presence of sERC was evaluated by careful observations of seven focal planes, while viewing the dynamic changes within oocytes from the start of time-lapse observations to pronuclear formation.
The definition of reverse cleavage and cleavage/cytokinetic failure
Reverse cleavage
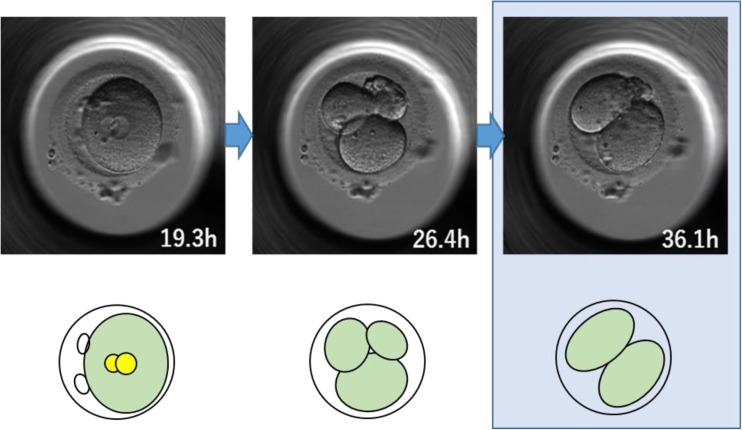
A zygote cleaves into more than three cells. Then the cleaved cells reverse to two cells or to a reduced number of cells from the initial number of cleaved cells (Fig. 1). This pattern is considered to be reverse cleavage with completion of mitosis.
Fig. 1.
An example of reverse cleavage. This 2PN zygote cleaved into an abnormal number of cells (three cells) at 26.4 h, but subsequently the cells reverted to a normal number of two-cell stage at 36.1 h. The time signature in the bottom right-hand corner of each image indicates the time in hours from fertilization
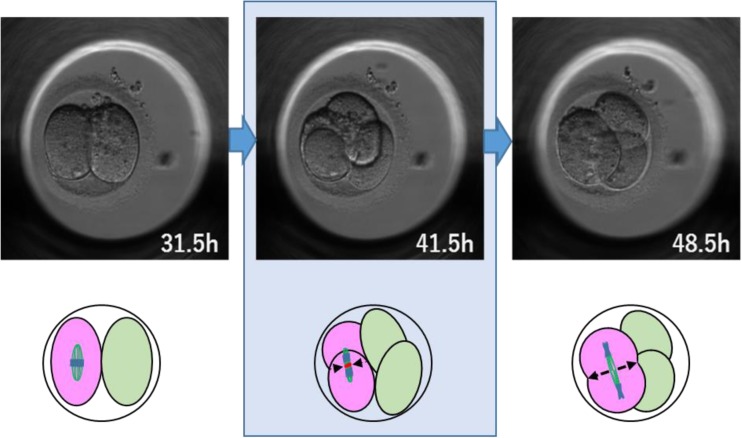
Cleavage/cytokinetic failure
A cell cleaves into two cells or more, but subsequently, the cleaved cells revert to a single cell (Fig. 2). This pattern includes cells in which cleavage has been initiated, but the cells fail to cleave completely. A schematic diagram of cleavage failure is shown in Fig. 3.
Fig. 2.
An example of cleavage failure. This two-cell stage embryo divided into a morphologically normal four cells, but 7 h later, two of the cells (displayed in pink) failed to complete cleavage and reverted to a single cell, which resulted in a three-cell embryo
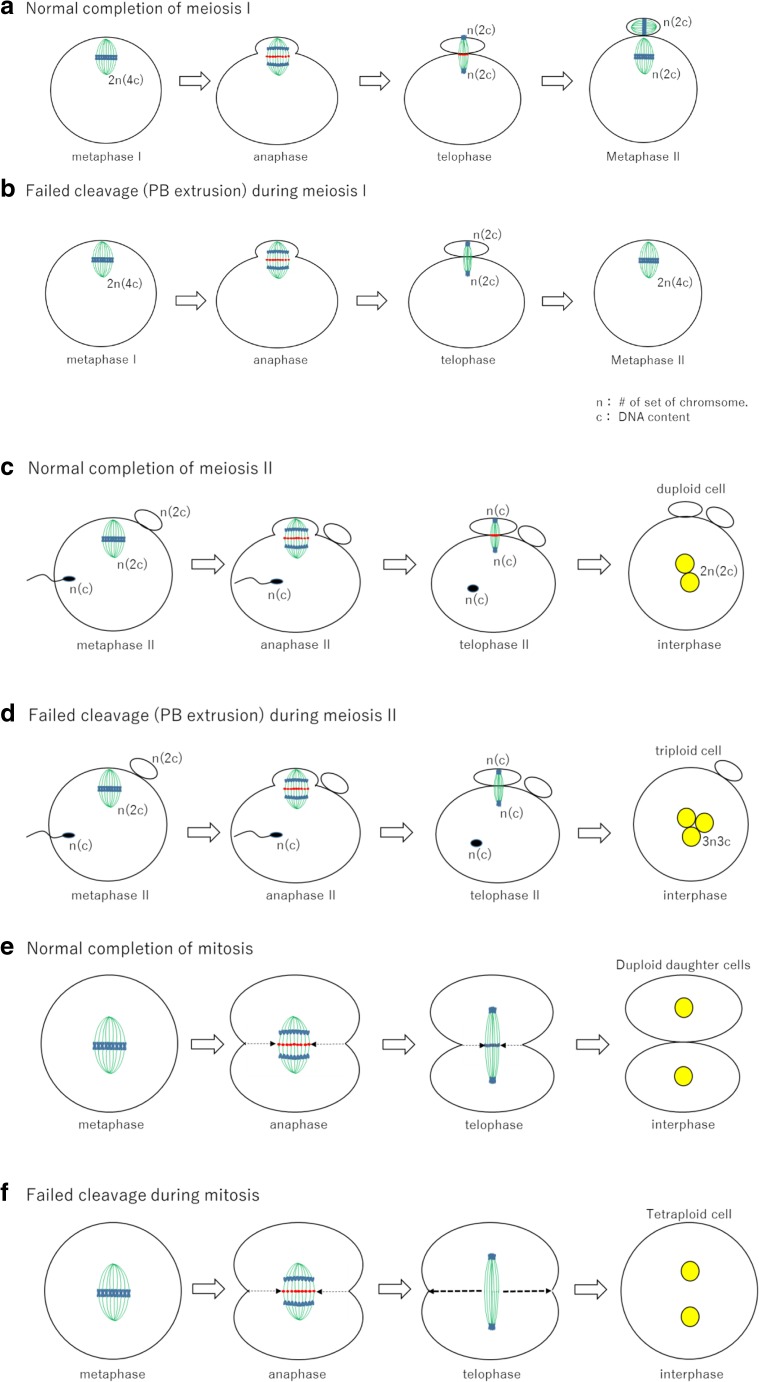
Fig. 3.
A schematic diagram of cleavage failure. a Normal completion of meiosis I. The cleavage furrow corresponds to the plane of the midzone (red) at telophase and polar body extrusion is completed in the plane of the midzone [16]. b Failed cleavage (PB extrusion) during meiosis I. Due to the gradual degradation of midzone particles (red dots), the first polar body extrusion fails. c Normal completion of meiosis II. d Failed cleavage (PB extrusion) during meiosis II. e Normal completion of mitosis. f Failed cleavage during mitosis. When cleavage furrow does not reach the level of the midzone following the disappearance of the midzone particles, the cleavage furrow moves back to the original position (unpublished data)
Statistical analysis
Statistical analysis was carried out to explore the nature of the abnormalities that were found in embryos derived from sERC positive oocytes. Logistic regression analysis was carried out to explore the independent variables for meiotic and mitotic cleavage failures. Independent variables included fertilization procedure (ICSI or IVF), cycle occurrence of sERC, and oocyte-based occurrence of sERC. Odds ratios with 95% confidence intervals were calculated based on the logistic regression analysis. A P < 0.05 was considered statistically significant. EZR software, an open-source statistical software, which is based on “R” was used for statistical analysis.
Results
Time-lapse images were captured automatically and retrospectively analyzed from a total of 1415 MII oocytes, obtained from 43 sERC positive cycles, which had at least one oocyte with sERCs among the retrieved oocytes, and 143 cycles in which no sERCs were detected in the oocytes. During the study period, the occurrence of sERC at seven focal planes, using an EmbryoScope®Time-lapse system, was 23.1% (43/186).
Descriptive analysis of these cycles is shown in Table 1. Among 1415 oocytes, sERCs were found in 5.7% of the oocytes (81/1415) using a time-lapse observation system. The incidence of mitotic cleavage failure in oocytes with sERCs was significantly higher than that in oocytes without sERCs (OR = 2.560, 95% CI 1.210–5.410, P = 0.014) (Table 2). Furthermore, the incidence of meiotic cleavage failure during the second polar body extrusion in oocytes with sERCs was significantly higher than that in oocytes without sERCs (OR = 5.140, 95% CI 1.190–22.200, P = 0.028) (Table 3). ICSI was found to have a greater frequency of meiotic failure than IVF (OR = 0.413, 95% CI 0.174–0.981, P = 0.045) (Table 3). The occurrence of five meiotic failures in sERC positive oocytes was observed in five different patients and was not individual specific.
Table 1.
Descriptive analysis of the cycles
| Outcomes | Fertilization procedure | sERC(+) oocytes in sERC(+) cycles | sERC(−) oocytes in sERC(+) cycles | sERC(−) oocytes in sERC(−) cycles |
|---|---|---|---|---|
| N. of cycles | IVF | 29 | 29 | 80 |
| ICSI | 14 | 14 | 63 | |
| Total | 43 | 43 | 143 | |
| N. of oocytes | IVF | 50 | 186 | 621 |
| ICSI | 31 | 82 | 445 | |
| Total | 81 | 268 | 1066 | |
| N. of MII | IVF | 50 | 165 | 547 |
| ICSI | 31 | 81 | 444 | |
| Total | 81 | 246 | 991 | |
| Fertilization rate/MII | IVF | 90.0% (45/50) | 85.5% (141/165) | 92.5% (506/547) |
| ICSI | 80.6% (25/31) | 82.7% (67/81) | 76.6% (340/444) | |
| Total | 86.4% (70/81) | 84.6% (208/246) | 85.4% (846/991) | |
| 2PN2PB | IVF | 73.3% (33/45) | 85.8% (121/141) | 83.2% (421/506) |
| ICSI | 84.0% (21/25) | 88.1% (59/67) | 84.4% (287/340) | |
| Total | 77.1% (54/70) | 86.5% (180/208) | 83.7% (708/846) | |
| 3PN1PB (meiotic failure) | IVF | 8.9% (4/45) | 0.7% (1/141) | 0.8% (4/506) |
| ICSI | 4.0% (1/25) | 3.0% (2/67) | 2.9% (10/340) | |
| Total | 7.1% (5/70) | 1.4% (3/208) | 1.7% (14/846) | |
| 1PN2PB | IVF | 8.9% (4/45) | 5.7% (8/141) | 6.5% (33/506) |
| ICSI | 8.0% (2/25) | 6.0% (4/67) | 8.8% (30/340) | |
| Total | 8.6% (6/70) | 5.8% (12/208) | 7.4% (63/846) | |
| 3PN2PB | IVF | 6.7% (3/45) | 6.4% (9/141) | 7.9% (40/506) |
| ICSI | 0% (0/25) | 0% (0/67) | 2.4% (8/340) | |
| Total | 4.3% (3/70) | 4.3% (9/208) | 5.7% (48/846) | |
| >4PN2PB | IVF | 2.2% (1/45) | 0.7% (1/141) | 1.4% (7/506) |
| ICSI | 0% (0/25) | 0% (0/67) | 0.6% (2/340) | |
| Total | 1.4% (1/70) | 0.5% (1/208) | 1.1% (9/846) | |
| 1PN1PB | IVF | 0% (0/45) | 0% (0/141) | 0% (0/506) |
| ICSI | 0% (0/25) | 1.5% (1/67) | 0% (0/340) | |
| Total | 0% (0/70) | 0.5% (1/208) | 0% (0/846) | |
| 0PN2PB | IVF | 0% (0/45) | 0% (0/141) | 0.2% (1/506) |
| ICSI | 4.0% (1/25) | 0% (0/67) | 0.3% (1/340) | |
| Total | 1.4% (1/70) | 0% (0/208) | 0.2% (2/846) | |
| 0PN3PB | IVF | 0% (0/45) | 0.7% (1/141) | 0% (0/506) |
| ICSI | 0% (0/25) | 0% (0/67) | 0% (0/340) | |
| Total | 0% (0/70) | 0.5% (1/208) | 0% (0/846) | |
| 2PN1PB | IVF | 0% (0/45) | 0% (0/141) | 0% (0/506) |
| ICSI | 0% (0/25) | 1.5%(1/67) | 0.6% (2/340) | |
| Total | 0% (0/70) | 0.5% (1/208) | 0.2% (2/846) | |
| Cryopreservation on day 2 | IVF | 22.2% (10/45) | 18.4% (26/141) | 20.2%(102/506) |
| ICSI | 16.0% (4/25) | 19.4% (13/67) | 19.4%(66/340) | |
| Total | 20.0% (14/70) | 18.8% (39/208) | 19.9%(168/846) | |
| Mitotic cleavage failure | IVF | 22.9% (8/35) | 8.7% (10/115) | 7.7% (31/404) |
| ICSI | 33.3% (7/21) | 20.4% (11/54) | 5.5% (15/274) | |
| Total | 26.8% (15/56) | 12.4% (21/169) | 6.8% (46/678) |
Table 2.
Multiple logistic regression analysis of the association between the presence of sERCs and mitotic cleavage failure
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| (Intercept) | 0.078 | 0.052–0.117 | < 0.001 |
| Procedures for fertilization (IVF/ICSI) | 0.886 | 0.552–1.420 | 0.614 |
| Presence of sERCs in oocytes sERC(+) oocytes/sERC(−) oocytes |
2.560 | 1.210–5.410 | 0.014 |
| Presence of sERCs in cycles sERC(+) cycles/sERC(−) cycles |
1.970 | 1.140–3.410 | 0.015 |
Table 3.
Multiple logistic regression analysis of the association between the presence of sERC and meiotic failure
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| (Intercept) | 0.026 | 0.014–0.049 | < 0.001 |
| Procedures for fertilization (IVF/ICSI) | 0.413 | 0.174–0.981 | 0.045 |
| Presence of sERCs in oocytes sERC(+) oocytes/sERC(−) oocytes |
5.140 | 1.190–22.200 | 0.028 |
| Presence of sERCs in cycles sERC(+) cycles/sERC(−) cycles |
0.937 | 0.266–3.300 | 0.919 |
During this study period, the formation of sERCs was found only in MII stage oocytes, with the exception of two rare cases. One occurred during mitosis after a prolonged period of metaphase (Supplemental video 1). In the other case, a sERC was detected in an oocyte after a prolonged period of its 2PN stage (Supplemental video 2).
Discussion
We explained in our previous paper that in human oocytes the localization of Ca2+ may change. This phenomenon was detected in the small vesicles beneath the plasma membrane of sERs. In the pronuclear zygotes and blastomeres of cleaving embryos, Ca2+-rich vesicles were no longer present in proximity to the plasma membrane, and the entire cell periphery was poor in Ca2+-containing organelles which, however, were abundant in the perinuclear region [17, 18]. As Ca2+ that can be released from sERs plays a pivotal role in oocyte maturation, fertilization and early embryonic development [19], the unusual distribution pattern of sERC formation may be involved in the abnormal regulation of Ca2+ signaling. Our current data, showing that aberrant cleavage frequently occurs in sERC positive oocytes, suggests that abnormal embryo development could be related to disturbed Ca2+ distribution patterns.
sERCs usually appear in MII stage oocytes rather than in MI and GV stage oocytes, except in a few very rare cases (Supplemental videos 1 and 2), when the metaphase stage is abnormally extended. Supplemental video 1 shows an occurrence of sERC during mitosis after a prolonged period of metaphase. It was also found that sERCs appeared after an extended 2PN stage. Therefore, extended metaphase and/or interphase stages may be predisposed to the occurrence of more easily visible sERCs. In 2004, we reported that during oocyte denuding procedures, in which unfertilized oocytes were cultured for 2–5 days, sERCs frequently appeared in unfertilized oocytes that previously did not contain these structures [1]. In addition to our previous paper, showing that the size of sERCs increased during culture, sERCs move in the cytoplasm, suggesting that connections may exist between all sERs and/or small sERCs and these may combine to form larger clusters in the oocyte (Supplemental video 3). Nuclear maturation is usually suppressed using a GnRH agonist or antagonist. Hence the extended interphase (GV stage) after the completion of follicle maturation and/or prolonged metaphase II, caused by a premature surge of LH, may cause sERC formation in MII oocytes.
The occurrence of sERCs could also be a sign of prolonged cytoplasmic maturation prior to the triggering of LH surges in controlled ovarian stimulation cycles. It is generally considered that the ideal size of pre-ovulatory follicles is around 18–20 mm; however, the negative impact on the cytoplasm in oocytes inside follicles that are larger than 21 mm is still unknown. In fact, we have reported that larger follicles, together with elevated serum estradiol and progesterone concentrations were found in sERC positive cycles [20]. This relates to our previous suggestion that a high concentration of estradiol per oocyte, when ovulation is triggered, may be an indicator of sERC predisposition [1]. It is well known that estradiol levels increase in proportion to the growth of ovarian follicles. From this evidence, the presence of sERCs could be considered to be a sign of excessive cytoplasmic maturation.
The sERC detection rate was higher in this study as compared to previous reports. This may be related to the improved detection of sERCs during their dynamic movement and Z-axis analysis using a time-lapse observation system. These more sensitive techniques were used in this study, whereas they were not in previous studies.
In present study, we found that the incidence of mitotic cleavage failure was higher in embryos derived from sERC positive oocytes than in embryos derived from sERC negative oocytes in both sERC positive and negative cycles. An embryonic cell that experiences cleavage failure during mitosis could become tetraploid and may cause abnormal chromosomal configurations in the embryo. Moreover, it was also found that the occurrence of meiotic errors, during second polar body extrusion, was also higher in sERC positive oocytes than in sERC negative oocytes. The failure of polar body extrusion is also a type of cleavage failure which results in 3PN1PB formation. As these cleavage failures cause chromosomal abnormalities, this may explain the previously reported data showing lower pregnancy rates and higher biochemical pregnancy rates associated with sERCs [1–5]. This may also explain the higher miscarriage rates demonstrated by Akarsu et al. (2009). Some cells exposed to cleavage failure may become trophectoderm cells, and these may be susceptible to forming placental abnormalities. In addition, the cells that arise from sERC oocytes, that form the ICM, may be predisposed to further cleavage failure and may in turn cause further aneuploidy.
More biological, physiological, and structural evidence needs to be accumulated to understand the possible pathological consequences of sERC formation. The mechanism of the frequent failure of cytokinesis in embryos derived from sERC positive oocytes is still unknown. This requires further investigation, as the appearance of sERCs could be a consequence rather than a cause of cleavage failure. Until we achieve a more complete understanding of sERCs origins, composition, and functions, the transfer of embryos derived from sERC positive oocytes should be undertaken with caution. The results of this study do not offer complete conclusions; however, enough data was acquired and analyzed to indicate areas for future research.
Electronic supplementary material
(AVI 5563 kb)
(AVI 6741 kb)
(AVI 14167 kb)
Acknowledgements
We wish to thank Dr. Alex Lopata for editing this paper and rewriting some sections. We would also like to thank Dr. Michio Yamamoto, Associate Professor at Graduate School of Environmental and Life Science, Okayama University for his valuable advice on statistical analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1119-3) contains supplementary material, which is available to authorized users.
References
- 1.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–1597. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 2.Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod BioMed Online. 2008;16:113–118. doi: 10.1016/S1472-6483(10)60563-9. [DOI] [PubMed] [Google Scholar]
- 3.Akarsu C, Caglar G, Vicdan K, Sozen E, Biberoglu K. Smooth endoplasmic reticulum aggregations in all retrieved oocytes causing recurrent multiple anomalies: case report. Fertil Steril. 2009;92:1496. doi: 10.1016/j.fertnstert.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 4.Sá R, Cunha M, Silva J, Luís A, Oliveira C, Teixeira da Silva J, et al. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011;96:143–149. doi: 10.1016/j.fertnstert.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 5.Setti AS, Figueira RC, de Almeida Ferreira Braga DP, Azevedo MC, Iaconelli A, Jr, Borges E., Jr Oocytes with smooth endoplasmic reticulum clusters originate blastocysts with impaired implantation potential. Fertil Steril. 2016;106:1718–1724. doi: 10.1016/j.fertnstert.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Mateizel I, Van Landuyt L, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28:2111–2117. doi: 10.1093/humrep/det241. [DOI] [PubMed] [Google Scholar]
- 7.Hattori H, Nakamura Y, Nakajo Y, Araki Y, Kyono K. Deliveries of babies with normal health derived from oocytes with smooth endoplasmic reticulum clusters. J Assist Reprod Genet. 2014;31:1461–1467. doi: 10.1007/s10815-014-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoi F, Asano Y, Shimizu M, Honnma H, Murata Y. Embryological outcomes in cycles with human oocytes containing large tubular smooth endoplasmic reticulum clusters after conventional in vitro fertilization. Gynecol Endocrinol. 2016;32:315–318. doi: 10.3109/09513590.2015.1115831. [DOI] [PubMed] [Google Scholar]
- 9.Itoi F, Asano Y, Shimizu M, Nagai R, Saitou K, Honnma H, et al. Clinical outcomes after IVF or ICSI using human blastocysts derived from oocytes containing aggregates of smooth endoplasmic reticulum. Reprod BioMed Online. 2017;34:337–344. doi: 10.1016/j.rbmo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Dal Canto M, Guglielmo MC, Mignini Renzini M, Fadini R, Moutier C, Merola M, et al. Dysmorphic patterns are associated with cytoskeletal alterations in human oocytes. Hum Reprod. 2017;32:750–757. doi: 10.1093/humrep/dex041. [DOI] [PubMed] [Google Scholar]
- 11.Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escribá MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–1463. doi: 10.1016/j.fertnstert.2012.07.1135. [DOI] [PubMed] [Google Scholar]
- 12.Basile N, Vime P, Florensa M, Aparicio Ruiz B, García Velasco JA, Remohí J, et al. The use of morphokinetics as a predictor of implantation: a multicentric study to define and validate an algorithm for embryo selection. Hum Reprod. 2015;30:276–283. doi: 10.1093/humrep/deu331. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Chapple V, Feenan K, Roberts P, Matson P. Clinical significance of intercellular contact at the four-cell stage of human embryos, and the use of abnormal cleavage patterns to identify embryos with low implantation potential: a time-lapse study. Fertil Steril. 2015;103:1485–1491. doi: 10.1016/j.fertnstert.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Alpha Scientists in Reproductive Medicine and European Society of Human Reproduction and Embryology (ESHRE) Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 15.Goto S, Shiotani M, Kitagawa M, Kadowaki T, Noda Y. Effectiveness of two-step (consecutive) embryo transfer in patients who have two embryos on day 2: comparison with cleavage-stage embryo transfer. Fertil Steril. 2005;83:721–723. doi: 10.1016/j.fertnstert.2004.07.974. [DOI] [PubMed] [Google Scholar]
- 16.Otsuki J, Nagai Y, Chiba K. Association of spindle midzone particles with polo-like kinase 1 during meiosis in mouse and human oocytes. Reprod BioMed Online. 2009;18:522–528. doi: 10.1016/S1472-6483(10)60129-0. [DOI] [PubMed] [Google Scholar]
- 17.Sousa M, Barros A, Tesarik J. Developmental changes in calcium dynamics, protein kinase C distribution and endoplasmic reticulum organization in human preimplantation embryos. Mol Hum Reprod. 1996;2:967–977. doi: 10.1093/molehr/2.12.967. [DOI] [PubMed] [Google Scholar]
- 18.Sousa M, Barros A, Silva J, Tesarik J. Developmental changes in calcium content of ultrastructurally distinct subcellular compartments of preimplantation human embryos. Mol Hum Reprod. 1997;3:83–90. doi: 10.1093/molehr/3.2.83. [DOI] [PubMed] [Google Scholar]
- 19.Homa ST, Carroll J, Swann K. The role of calcium in mammalian oocyte maturation and egg activation. Hum Reprod. 1993;8:1274–1281. doi: 10.1093/oxfordjournals.humrep.a138240. [DOI] [PubMed] [Google Scholar]
- 20.Otsuki J, Okada A, Momma Y, Takahashi K, Nagai Y, Kubo H. The smooth endoplasmic reticulum clusters in oocytes associated with higher estradiol and progesterone and larger follicles. Hum Reprod. 2003;18(suppl):xviii147. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(AVI 5563 kb)
(AVI 6741 kb)
(AVI 14167 kb)