Abstract
Purpose
Annexin A5 (ANXA5) is a protein abundantly expressed in normal placenta where it contributes to the healthy outcome of a pregnancy. Lower ANXA5 levels have been observed in M2/ANXA5 haplotype carrying chorion. Consequently, this study aimed to assess the potential association of M2 maternal carrier status with the risk of recurrent pregnancy loss (RPL), the timing of miscarriages, and other obstetric complications, for the first time in a population from Latin America.
Methods
This study was designed as a prospective recruitment of RPL patients with post hoc analysis. The distribution of the M2/ANXA5 haplotype was compared between a group of 229 Argentine women with RPL and 100 parous controls, and was further analyzed in subgroups of patients stratified according to the timing of miscarriages and in relation to other obstetric complications.
Results
No significant differences were found in the distribution of M2 haplotype among either RPL patients or the subgroups with embryonic, early fetal, or late fetal losses compared to parous controls. Notwithstanding, maternal M2/ANXA5 was found to be independently associated with a higher risk of suffering intrauterine growth restriction (IUGR) and/or preeclampsia (PE). Simultaneously, the presence of inherited and/or acquired thrombophilia also proved to be an independent risk factor for these.
Conclusions
The association found between the maternal carriage of the M2/ANXA5 haplotype and an elevated risk of IUGR and/or PE supports the hypothesis that carrier status of this haplotype and the consequently reduced placental ANXA5 expression might be responsible, at least partially, for the onset of these gestational vascular complications.
Keywords: Annexin A5, M2/ANXA5, Recurrent pregnancy loss, IUGR, Pre-eclampsia, Risk factor
Introduction
Among women attempting to conceive, 1–3% of them suffer from recurrent pregnancy loss (RPL). Although there are some well-described risk factors for RPL, such as parental chromosomal anomalies, anatomical uterine abnormalities, endocrine dysfunctions, and high levels of antiphospholipid antibodies, up to 50% of the cases still remain unexplained [1, 2]. Inherited thrombophilia has been also associated with RPL and other obstetric complications, and it has been proposed that the increased obstetric risk might be mediated by an impairment of placental perfusion [3–6].
Annexin A5 (ANXA5) is a protein that is abundantly expressed in normal placenta where it was proposed to exert an anticoagulant function [7]. Upon calcium loading, ANXA5 avidly binds to phosphatidylserine (PS) that is naturally exposed on the apical surface of placental syncytiotrophoblasts, setting up a bidimensional shield that interferes with phospholipid dependent clotting reactions [8–10]. Furthermore, it has been also proposed that ANXA5 displays an essential role promoting membrane repair that might be crucial for the integrity of a healthy placenta and very recent research ascribes facilitating function in the fusion of villous trophoblasts [11]. In pathophysiological scenarios, like the presence of anti-phospholipid antibodies, anti-annexin antibodies, or even when ANXA5 protein levels are relatively decreased, this ANXA5 shield undergoes a disruption that has been suggested to be a considerable risk factor affecting the normal outcome of a pregnancy due to the occurrence of a hypercoagulable state in the intervillous space [12–14].
Almost a decade ago, Bogdanova et al. described the presence of different ANXA5 promoter haplotypes that, in reporter gene assays, showed to accordingly affect the expression levels of this protein. Among them, the carrier status of the M2 haplotype resulted in 60% reduction of ANXA5 promoter activity making it a logical candidate to have an impact on the etiology of RPL and/or other obstetric complications [15]. Further studies demonstrated that the M2 haplotype was responsible for a reduced expression of ANXA5 in chorionic placenta compared to the normal haplotype [16, 17], and that M2 carriers have an increased risk of suffering RPL and placenta-mediated pregnancy complications (PMPCs) in various European, Asian, and an Austronesian populations [15, 18–25].
The aims of this study were to assess the potential association of M2 maternal carrier status with the risk of RPL, to analyze its proposed influence according to the timing of miscarriages and to trace the link with other PMPC, for the first time in a population from Latin America.
Patients and methods
This field study to verify possible association of M2/ANXA5 was designed as a prospective recruitment of RPL patients with post hoc analysis that was approved by the ethical committees of the institutions involved and was performed according to the principles of the Declaration of Helsinki. Informed consent was obtained from all participants.
Initially, the prevalence of the ANXA5 M2 haplotype was estimated in groups of 100 RPL patients and 50 strictly selected controls accordingly. Since the issuing comparison lacked on statistical power, recruitment continued until 329 subjects, including 229 cases and 100 controls, were selected for this study after meeting the corresponding inclusion criteria. Patients were enrolled between June 2010 and March 2016 in the Autoimmune, Thrombophilic Diseases and Pregnancy Section, Acute Care Hospital “Dr. Carlos G. Durand,” Buenos Aires, Argentina. During this time, 229 women who were presented with RPL, fulfilled all inclusion criteria, and agreed to participate in the study were selected from a large cohort of 1185 consecutive miscarriage couples that attended to the medical center. RPL was defined, according to a slight modification of the statement of the Practice Committee of the American Society for Reproductive Medicine, as two or more unexplained failed pregnancies with the same partner [26]. Pregnancies were confirmed by sonography showing vital heartbeat, and pregnancy losses were identified when transvaginal ultrasound showed no heartbeat of an embryo with more than 7-mm crown to rump length, no embryo in a gestational sac having a mean sac diameter of more than 25 mm, or no appearance of an embryo within 7–10 days after the primary examination [27]. Patients were divided into three different groups according to fetal development at the time of pregnancy loss: embryonic (between 5 and 10 weeks of gestation), early fetal (between 10 and 15 weeks of gestation), and late fetal losses (after 15 weeks) [28–30].
Study participants were additionally stratified to the status of placental-mediated pregnancy complications: pre-eclampsia (PE), placental abruption, and intrauterine growth restriction (IUGR). PE was defined as high blood pressure (systolic blood pressure > 140 mmHg or diastolic blood pressure ≥ 90 mmHg) and 24-h proteinuria ≥ 0.3 g [31]. Placental abruption was defined as bleeding associated with partial or total separation of the placenta from its normal insertion site corresponding to the uterine fundus, this occurring from the 20 weeks of gestation until before delivery [32]. Fetuses with an estimated fetal weight < 3rd percentile or fetuses with a combination of estimated fetal weight < 10th percentile and with abnormal umbilical Doppler sonography were classified as IUGR [33].
RPL patients who presented with either endocrine disorders (clinical hypothyroidism [34], diabetes mellitus, polycystic ovary syndrome according to the Rotterdam criteria [35] and hyperprolactinemia), related infections, immunologic alterations (except from obstetric antiphospholipid syndrome (APS)), anatomic abnormalities of the uterus (detected by ultrasonography), premature rupture of membranes, or a history suggestive of cervical incompetency were excluded. Carrier status of fetal and/or parental chromosomal abnormalities, diagnosed through cytogenetic analyses, was also considered as an exclusion criterion.
Inherited thrombophilia, deficiencies in circulation levels of either Antithrombin (AT), Protein C (PC), or free Protein S (PS) and carrier status of Factor V Leiden or Prothrombin G20210A and acquired thrombophilia were evaluated in all patients. Factor V Leiden (FVL) and Prothrombin G20210A (PTm) genotypes were determined by real-time polymerase chain reaction (RT-PCR) using FV Leiden and FII G20210A specific reagents (Roche Diagnostics GmbH). PC, AT, and free PS levels were measured on a Destiny Max Coagulometer (Tcoag, Ireland). PC and AT were determined through a chromogenic method (Stago, France, and Xa-Chromogenix, Mölndal, Sweden, respectively) and free PS was determined through an immunoturbidimetric method (Liatest Stago, France). PC < 70%, AT < 80%, and free PS < 60% were considered as deficiencies in their circulation levels. As free PS levels exhibit variations during pregnancy, the free PS cutoff levels according to week of gestation were considered, if it was necessary, as suggested by Szecsi et al. [36]. PC and AT levels do not show significant changes during pregnancy.
Obstetric APS was diagnosed according to updated international consensus classification criteria [37]. Clinical criteria included only obstetric morbidity (three pregnancy losses before the 10th week, and/or one pregnancy loss at or after the 10th week, and/or premature delivery before the 34th week because of PE or placental insufficiency). Laboratory criteria included positive test results for antiphospholipid antibodies, represented by positive test for lupus anticoagulant, and/or moderated or high titers for IgG and/or IgM anti-β2 glycoprotein I antibodies, and/or moderated or high titers for IgG and/or IgM anticardiolipin antibodies. Laboratory criteria were confirmed positive on two or more occasions at least 12 weeks after first positivity. Clinical characteristics of RPL patients are summarized in Table 1.
Table 1.
Clinical characteristics of patients with recurrent pregnancy loss
| Age | 32 years [26–35]a |
| BMI | 24.2 kg/m2 [21.9–27.1] |
| Number of losses | 3 [2–4] |
| Embryonic losses | 55 (24.0%) |
| Early fetal losses | 63 (27.5%) |
| Late fetal losses | 111 (48.5%) |
| IUGR | 33 (14.4%) |
| Placental abruption | 14 (6.1%) |
| Pre-eclampsia | 16 (7.0%) |
| Obesity | 9 (3.8%) |
| Obstetric APS | 37 (16.1%) |
| FVL carriers | 6 (2.6%) |
| PTm carriers | 12 (5.2%) |
aValues are expressed as median [interquartile range] or number (percentage)
The parous control group consisted of 100 women with a history of normal pregnancies, at menopausal or post menopausal age (in order to be able to obtain complete obstetric history for each one of them), with two or more normal term deliveries of healthy and normal weight singletons and with no gestational pathology in any of their pregnancies.
All of the subjects were of Argentinian descent and shared common ethno-geographic and social origins, thus representative of the urban admixed population of Buenos Aires, Argentina. This population is the result of genetic admixture processes principally involving Europeans (mainly Spaniards and Italians) and Native Americans [38–40].
Genomic DNA was extracted from peripheral leukocytes using the High Pure PCR Template Preparation Kit for genomic DNA (Roche Diagnostics, GmbH, Mannheim, Germany). Genotyping of the proximal core promoter region of the ANXA5 gene was conducted by amplicon sequencing as described by Bogdanova et al. [15].
Statistical analyses were performed using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA). Genotype distributions among patients and control groups were assessed as carrier rates (percentage), according to a dominant model. Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were estimated by binary logistic regression to evaluate the strength of the association between maternal carrier status of M2/ANXA5 and the risk of IUGR and/or PE; p < 0.05 was considered statistically significant.
Results
The proximal core promoter region of the ANXA5 gene was genotyped in 229 Argentine women with RPL and in 100 parous controls. Both patient and control groups fulfilled Hardy-Weinberg equilibrium (HWE), and in fact, the parous women group was in perfect HWE with p = 1, as estimated through complete enumeration of genotypes (Table 2).
Table 2.
Genotype frequencies of ANXA5 promoter haplotypes in control groups and RPL patients
| Genotype | Parous controls, n = 100 | RPL, n = 229 | Embryoniclosses, n = 55 | Early fetal losses, n = 63 | Late fetal losses, n = 111 | IUGR/PE, n = 41 | RPL-healthy delivery, n = 109 | RPL NO IUGR/PE, n = 188 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Expecteda | Observed | Expected | |||||||
| N/N | 71 (71.0) | 72 (72.0) | 159 (69.5) | 161 (70.3) | 46 (83.6) | 41 (65.1) | 74 (66.7) | 22 (53.7) | 74 (67.9) | 137 (72.9) |
| N/M1 | 12 (12.0) | 11 (11.0) | 27 (11.8) | 24 (10.5) | 6 (10.9) | 7 (11.1) | 14 (12.6) | 5 (12.2) | 16 (14.7) | 22 (11.7) |
| M1/M1 | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.4) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | (0.0) | 1 (0.5) |
| N/M2; M1/M2b | 17 (17.0) | 16 (16.0) | 39 (17.0) | 41 (17.9) | 4 (7.3) | 13 (20.6) | 22 (19.8) | 14 (34.1) | 18 (16.5) | 25 (13.3) |
| M2/M2 | 0 (0.0) | 1 (1.0) | 3 (1.3) | 2 (0.8) | 1 (1.8) | 1 (1.6) | 1 (0.9) | 0 (0.0) | 1 (0.9) | 3 (1.6) |
| M2 carriers | 17 (17.0%) | 17 (17.0%) | 42 (18.3%) | 43 (18.3%) | 6 (10.9%) | 14 (22.2%) | 23 (20.7%) | 14 (34.1%) | 19 (17.4%) | 28 (14.9) |
Values are expressed as number (percentage)
RPL recurrent pregnancy loss, IUGR/PE intrauterine growth restriction/pre-eclampsia
aGenotype frequencies expected under Hardy-Weinberg equilibrium
bA genotype M1/M2 was observed only once in the parous control group
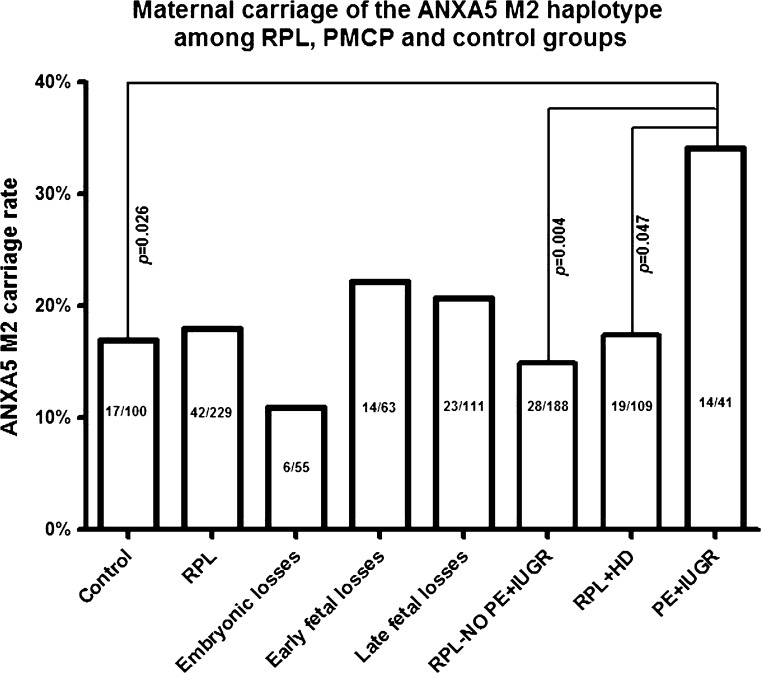
In order to assess the potential RPL association in maternal M2/ANXA5 carriers, its distribution was compared among the RPL and parous control groups and no significant difference was found (p > 0.05) (Fig. 1). Next, aiming to relate a possible influence to the timing of the losses, the M2 carrier rates were individually assessed in RPL subgroups stratified according to fetal development at the time of pregnancy loss (Table 2). There were no significant differences (p > 0.05) in the distribution of the M2 haplotype among RPL subgroups with either embryonic, early fetal or late fetal losses compared to parous controls. A higher M2 carrier rate in the subgroup of patients with early fetal losses was noted, although not of statistical significance (22.2%; 14/63).
Fig. 1.
Distribution of M2/ANXA5 carriers (%) in fertile controls, RPL patients, and additionally in clinical subgroups of patients stratified according to the timing of losses (embryonic, early fetal and late fetal), with or without obstetric complications, pre-eclampsia and/or intrauterine growth restriction (PE+IUGR, RPL-NO PE+IUGR) and a subgroup of RPL patients with healthy delivery (RPL+HD). Patients with PE+IUGR have significantly higher M2/ANXA5 carrier rate compared to fertile controls, to RPL patients without PE+IUGR and to RPL patients with healthy deliveries
Subsequently, a subgroup of patients with PMPC was tested for M2 association. Consequently, the potential impact of M2/ANXA5 was assessed in the subgroups of patients that suffered from either IUGR and/or PE (n = 41). These patients had significantly higher M2 carrier rates as compared to parous controls, who have not experienced any of these gestational vascular complications (34.1%; 14/41 vs. 17.0%; 17/100; p = 0.026) (Fig. 1). Next, in order to exclude the RPL phenotype from the statistical analysis, PMPC patients were compared to the rest of RPL patients without obstetric complications (RPL - NO PE+IUGR). This comparison also yielded a significant difference in the distribution of M2/ANXA5 (34.1%; 14/41 vs. 14.9%; 28/188; p = 0.004) (Fig. 1). Moreover, when evaluating “miscarriages” but not RPL as phenotype category, similar results were obtained when comparing RPL patients with IUGR and/or PE to a subgroup of RPL patients with at least one healthy delivery without experiencing any gestational vascular complications (RPL+HD) (34.1%; 14/41 vs. 17.4%; 19/109; p = 0.047) (Fig. 1).
Hence, in order to assess the strength of the association between maternal M2/ANXA5 with a higher IUGR and/or PE risk in RPL patients with greater confidence, two different logistic binary regression analyses were performed considering the presence of either hereditary and/or acquired thrombophilia as covariates. The analyses were conducted in comparison to the RPL patients without obstetric complications (RPL - NO PE+IUGR) and to the RPL patients with healthy delivery (RPL+HD) subgroups. Among the 41 RPL with IUGR and/or PE patients, 10 presented with obstetric APS, 3 with FVL, and 1 with obstetric APS+FVL. Despite that approximately 35% of these patients already presented with a known risk factor, the logistic analysis indicated maternal M2/ANXA5 is independently associated, OR = 2.84 (1.31 to 6.16, p = 0.008) and OR = 2.38 (1.04 to 5.45, p = 0.040, respectively), with a higher risk of these gestational vascular complications. Parallel to this, the presence of inherited and/or acquired thrombophilia was also an independent risk factor for IUGR and/or PE in RPL patients with OR = 2.51 (1.20 to 5.26, p = 0.015) and OR = 2.58 (1.10 to 6.05; p = 0.03, respectively).
Finally, the association between maternal carriage of the M2/ANXA5 haplotype and the risk of obstetric APS was assessed, and there was no significant difference (p > 0.05) in M2 distribution among RPL patients who presented with obstetric APS (24.3%; 9/37) and those who did not (17.1%; 33/193).
Discussion
In previous studies from various European and Asian, and an Austronesian population, the M2/ANXA5 haplotype was found to be associated with an increased risk of RPL as well as of other PMPCs like PE, IUGR, and small for gestational age newborns [15, 18–24]. Superficially, the results of this study, the first from a Latin-American population so far, would indicate that maternal carriers of M2/ANXA5 would not suffer a greater RPL risk. Nevertheless, the findings indicate that maternal carriage of this haplotype would be associated with a higher risk of IUGR and/or PE, which are gestational vascular complications partly with underlying thrombotic pathology among other etiologies that have been described [41].
As previously stated, RPL is of multifactorial etiology but it has been fairly well documented that certain risk factors are more strongly associated with pregnancy loss at different gestational times [42]. From this point of view, despite having excluded those patients who presented with known risk factors for RPL (except of APS), the resulting sample of RPL women might still represent an etiologically heterogeneous group. This could be one of the main reasons for the discrepancies between results published so far and the results of this study, regarding the role of M2/ANXA5 as RPL predisposition. Furthermore, different studies have suggested that the placental rather than the maternal genotype would be determinant in the ANXA5 placental expression levels [16, 17]. Besides, as it is well known, the genetic footprints can have an impact in the understanding of population-level differences in biomedical traits [43]. Therefore, the lack of data on paternal genotype and the ethnic genetic footprints might also help to explain the discrepancies with regard to the implication of the M2/ANXA5 haplotype as a risk factor responsible, at least in part, for RPL.
As a consequence of the possible etiological heterogeneity in the composition of the RPL group, maternal carriage rates of the M2 haplotype in RPL subgroups stratified according to the timing of losses were compared. Obtained results indicated that the M2 haplotype would not be an independent risk factor associated with a higher predisposition to either embryonic, early fetal or late fetal losses. The highest rate of maternal M2/ANXA5 carriage was detected in patients with early fetal losses though its difference was not significant with regard to parous controls. In conclusion, the solely maternal M2/ANXA5 carrier status did not appear independently associated with the timing of losses.
Among placental-mediated pregnancy complications, IUGR and PE are two separate gestational vascular pathologies that might share similar pathophysiological mechanisms. It is well known that obstetric APS and maternal carrier status of FVL have been associated with a higher risk of PE and, in addition, other inherited thrombophilia has also been associated with the risk of suffering IUGR [44–47]. Based on the literature, carrier status of the M2/ANXA5 haplotype might well be a risk factor for the occurrence of these gestational vascular pathologies. Consequently, three different analyses were performed to address this possibility. The results from the first, where M2 carrier rates of RPL patients with IUGR and/or PE were compared to parous controls superficially, suggested that the M2 haplotype might be a risk factor for IUGR and/or PE, excluding RPL, but not miscarriage, which may be a compound of a contiguous specter of thrombophilia related placental complications including PE, IUGR and, ultimately, miscarriage. As there were no complete data on thrombophilia for the parous control group, two additional analyses were performed. The results obtained from both analyses further confirmed the association between maternal M2/ANXA5 carriage and PMPC after adjusting for the presence of either hereditary and/or acquired thrombophilia.
Consequently, even though approximately 35% of the patients with IUGR and/or PE already presented with a known risk factor, the results of performed comparisons confirmed that maternal carriage of M2/ANXA5 would be independently associated with a more than two times higher risk of suffering from these gestational vascular complications. Notwithstanding, the results also indicated that the maternal presence of inherited and/or acquired thrombophilia would be an independent risk factor for IUGR and/or PE in RPL patients, being in agreement with previously performed studies. It could be argued that in this logistic model the assumption that APS, FVL, or PTm carrier status would have a similar impact on the risk of PMPC was taken. After all, this might not have been the most accurate analysis but based on sample sizes in subgroups, it has been the most informative statistical approach. Therefore, making this assumption and considering the results of the regression analysis, maternal carrier status of either the M2/ANXA5 haplotype or acquired and/or inherited thrombophilia would be independent risk factors for IUGR and/or PE with a similar strength of association. This is in line with the notion about the proposed thrombophilic role of M2/ANXA5.
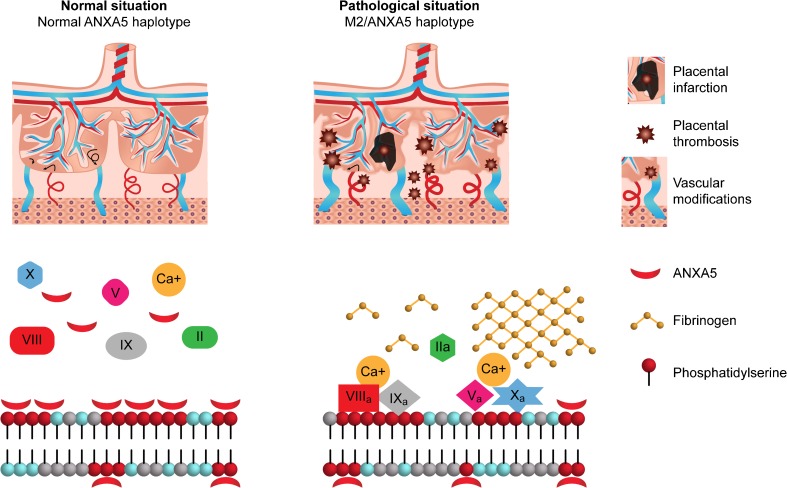
The results of this study are in agreement with previous findings about the role of M2/ANXA5 in gestational vascular complications. Ota et al. found that the carrier rate of this haplotype was significantly higher in placentae from pre-eclamptic patients than in controls, the placental expression of ANXA5 mRNA was lower in M2 carriers, the ANXA5 placental protein levels were also slightly but significantly lower and the placental M2 carrier status correlated with the severity of perivillous fibrin deposition [25]. They also found that these effects were determined by the placental genotype, suggesting that placental carriage of the M2 haplotype, whether transmitted maternally or paternally, might play a key role increasing the risk of PE contributing to localized thrombosis at the feto-maternal interface. In this regard, we acknowledge that it would have been important to further analyze the paternal M2/ANXA5 carrier status to better assess its association with a higher risk of suffering from gestational vascular complications. Moreover, Tiscia et al. had previously documented that maternal M2/ANXA5 carrier status would be associated with a twofold higher risk of pregnancy-related hypertensive disorders [18]. Meanwhile, Sifakis et al. found decreased ANXA5 mRNA expression in placentas from IUGR-affected compared to uncomplicated pregnancies. However, they found similar placental ANXA5 protein levels in both groups [48]. Considering the afore mentioned evidence, results of this study are consistent with a model proposing that the reduction in ANXA5 placental expression due to M2 carrier status negatively affects its anticoagulant capacity promoting fibrin/fibrinoid deposition both in the intervillous and perivillous space that are responsible, at least in part, for the onset of either IUGR and/or PE (Fig. 2). Notwithstanding, there cannot be certainty that the enhancement of local thrombosis at the feto-maternal interface is the only way the reduction of ANXA5 levels exerts its pathophysiological mechanism. Impairment in trophoblastic invasion, a process involved in the etiology of both gestational vascular complications during embryonic implantation, has been suggested as another plausible opportunity [41, 49–51].
Fig. 2.
A model of pathophysiological expression of ANXA5 deficiency in placentae carrying the M2/ANXA5 haplotype
Those infants that suffered from IUGR are at increased risk of fetal/neonatal morbidity and mortality and during child and adulthood they might be still at risk of experiencing neurosensory disability, cognitive impairment, short stature, hypertension, diabetes, and long-term cardiovascular disease [52, 53]. Besides, PE still remains as a major complication in pregnancy that poses a severe risk to both the mother and the fetus and often requires preterm delivery. As previously mentioned, different etiologies and risk factors for both IUGR and PE have been identified but few contribute significantly to risk prediction. In this context, the results of this study might be of practical importance for the management of PMPC with similar impact to the successful IVF outcome management after proper diagnostic workup [54].
In conclusion, this study generated evidence of an association between the maternal carrier status of M2/ANXA5 haplotype and an elevated risk of IUGR and/or PE, supporting the hypothesis that carrier status of this haplotype and the consequently reduced placental ANXA5 expression might be responsible, at least in part, for the onset of these PMPC. If further analyses confirm these initial findings, genotyping for M2/ANXA5 could be considered as a suitable prognostic marker for any one of these gestational vascular complications.
Compliance with ethical standards
This field study to verify possible association of M2/ANXA5 was designed as a prospective recruitment of RPL patients with post hoc analysis that was approved by the ethical committees of the institutions involved and was performed according to the principles of the Declaration of Helsinki. Informed consent was obtained from all participants.
Footnotes
Arseni Markoff and Gabriela de Larrañaga contributed equally to this work.
References
- 1.Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2:76–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Egerup P, Kolte AM, Larsen EC, Krog M, Nielsen HS, Christiansen OB. Recurrent pregnancy loss: what is the impact of consecutive versus non-consecutive losses? Hum Reprod. 2016;31:2428–34. [DOI] [PubMed]
- 3.Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet. 2003;361:901–908. doi: 10.1016/S0140-6736(03)12771-7. [DOI] [PubMed] [Google Scholar]
- 4.Middeldorp S. Pregnancy failure and heritable thrombophilia. Semin Hematol. 2007;44:93–97. doi: 10.1053/j.seminhematol.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Rodger MA, Betancourt MT, Clark P, Lindqvist PG, Dizon-Townson D, Said J, et al. The association of factor V Leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: a systematic review and meta-analysis of prospective cohort studies. PLoS Med. 2010;7:e1000292. doi: 10.1371/journal.pmed.1000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandone E, Margaglione M. Inherited thrombophilia and gestational vascular complications. Best Pract Res Clin Haematol. 2003;16:321–332. doi: 10.1016/S1521-6926(03)00017-3. [DOI] [PubMed] [Google Scholar]
- 7.Krikun G, Lockwood CJ, Wu XX, Zhou XD, Guller S, Calandri C, et al. The expression of the placental anticoagulant protein, annexin V, by villous trophoblasts: immunolocalization and in vitro regulation. Placenta. 1994;15:601–612. doi: 10.1016/S0143-4004(05)80407-2. [DOI] [PubMed] [Google Scholar]
- 8.Andree HA, Stuart MC, Hermens WT, Reutelingsperger CP, Hemker HC, Frederik PM, et al. Clustering of lipid-bound annexin V may explain its anticoagulant effect. J Biol Chem. 1992;267:17907–17912. [PubMed] [Google Scholar]
- 9.Rand JH, Wu XX. Antibody-mediated interference with annexins in the antiphospholipid syndrome. Thromb Res. 2004;114:383–389. doi: 10.1016/j.thromres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Ueki H, Mizushina T, Laoharatchatathanin T, Terashima R, Nishimura Y, Rieanrakwong D, et al. Loss of maternal annexin A5 increases the likelihood of placental platelet thrombosis and foetal loss. Sci Rep. 2012;2:827. doi: 10.1038/srep00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouter A, Carmeille R, Gounou C, Bouvet F, Degrelle SA, Evain-Brion D, et al. Review: Annexin-A5 and cell membrane repair. Placenta. 2015;36(Suppl 1):S43–S49. doi: 10.1016/j.placenta.2015.01.193. [DOI] [PubMed] [Google Scholar]
- 12.Rand JH, Wu XX, Guller S, Gil J, Guha A, Scher J, et al. Reduction of annexin-V (placental anticoagulant protein-I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;171:1566–1572. doi: 10.1016/0002-9378(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 13.Matsubayashi H, Arai T, Izumi S, Sugi T, McIntyre JA, Makino T. Anti-annexin V antibodies in patients with early pregnancy loss or implantation failures. Fertil Steril. 2001;76:694–699. doi: 10.1016/S0015-0282(01)02009-X. [DOI] [PubMed] [Google Scholar]
- 14.Gourvas V, Soulitzis N, Konstantinidou A, Dalpa E, Koukoura O, Koutroulakis D, et al. Reduced ANXA5 mRNA and protein expression in pregnancies complicated by preeclampsia. Thromb Res. 2014;133:495–500. doi: 10.1016/j.thromres.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Bogdanova N, Horst J, Chlystun M, Croucher PJ, Nebel A, Bohring A, et al. A common haplotype of the annexin A5 (ANXA5) gene promoter is associated with recurrent pregnancy loss. Hum Mol Genet. 2007;16:573–578. doi: 10.1093/hmg/ddm017. [DOI] [PubMed] [Google Scholar]
- 16.Chinni E, Tiscia GL, Colaizzo D, Vergura P, Margaglione M, Grandone E. Annexin V expression in human placenta is influenced by the carriership of the common haplotype M2. Fertil Steril. 2009;91:940–942. doi: 10.1016/j.fertnstert.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 17.Markoff A, Gerdes S, Feldner S, Bogdanova N, Gerke V, Grandone E. Reduced allele specific annexin A5 mRNA levels in placentas carrying the M2/ANXA5 allele. Placenta. 2010;31:937–940. doi: 10.1016/j.placenta.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Tiscia G, Colaizzo D, Chinni E, Pisanelli D, Scianname N, Favuzzi G, et al. Haplotype M2 in the annexin A5 (ANXA5) gene and the occurrence of obstetric complications. Thromb Haemost. 2009;102:309–313. doi: 10.1160/TH09-02-0123. [DOI] [PubMed] [Google Scholar]
- 19.Miyamura H, Nishizawa H, Ota S, Suzuki M, Inagaki A, Egusa H, et al. Polymorphisms in the annexin A5 gene promoter in Japanese women with recurrent pregnancy loss. Mol Hum Reprod. 2011;17:447–452. doi: 10.1093/molehr/gar008. [DOI] [PubMed] [Google Scholar]
- 20.Rogenhofer N, Engels L, Bogdanova N, Tuttelmann F, Markoff A, Thaler C. Paternal and maternal carriage of the annexin A5 M2 haplotype are equal risk factors for recurrent pregnancy loss: a pilot study. Fertil Steril. 2012;98:383–388. doi: 10.1016/j.fertnstert.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Tuttelmann F, Ivanov P, Dietzel C, Sofroniou A, Tsvyatkovska TM, Komsa-Penkova RS, et al. Further insights into the role of the annexin A5 M2 haplotype as recurrent pregnancy loss factor, assessing timing of miscarriage and partner risk. Fertil Steril. 2013;100:1321–1325. doi: 10.1016/j.fertnstert.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 22.Demetriou C, Abu-Amero S, White S, Peskett E, Markoff A, Stanier P, et al. Investigation of the Annexin A5 M2 haplotype in 500 white European couples who have experienced recurrent spontaneous abortion. Reprod BioMed Online. 2015;31:681–688. doi: 10.1016/j.rbmo.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Thean Hock T, Bogdanova N, Kai Cheen A, Kathirgamanathan S, Bin Abdullah R, Mohd Yusoff N, et al. M2/ANXA5 haplotype as a predisposition factor in Malay women and couples experiencing recurrent spontaneous abortion: a pilot study. Reprod BioMed Online. 2015;30:434–439. doi: 10.1016/j.rbmo.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Tiscia G, Colaizzo D, Favuzzi G, Vergura P, Martinelli P, Margaglione M, et al. The M2 haplotype in the ANXA5 gene is an independent risk factor for idiopathic small-for-gestational age newborns. Mol Hum Reprod. 2012;18:510–513. doi: 10.1093/molehr/gas023. [DOI] [PubMed] [Google Scholar]
- 25.Ota S, Miyamura H, Nishizawa H, Inagaki H, Inagaki A, Inuzuka H, et al. Contribution of fetal ANXA5 gene promoter polymorphisms to the onset of pre-eclampsia. Placenta. 2013;34:1202–1210. doi: 10.1016/j.placenta.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Practice Committee of American Society for Reproductive M Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99:63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Hamza A, Meyberg-Solomayer G, Juhasz-Boss I, Joukhadar R, Takacs Z, Solomayer EF, et al. Diagnostic methods of ectopic pregnancy and early pregnancy loss: a review of the literature. Geburtshilfe Frauenheilkd. 2016;76:377–382. doi: 10.1055/s-0041-110204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolte AM, Bernardi LA, Christiansen OB, Quenby S, Farquharson RG, Goddijn M, et al. Terminology for pregnancy loss prior to viability: a consensus statement from the ESHRE early pregnancy special interest group. Hum Reprod. 2015;30:495–498. doi: 10.1093/humrep/deu299. [DOI] [PubMed] [Google Scholar]
- 29.ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009;113:748–61. [DOI] [PubMed]
- 30.Heuser C, Dalton J, Macpherson C, Branch DW, Porter TF, Silver RM. Idiopathic recurrent pregnancy loss recurs at similar gestational ages. Am J Obstet Gynecol. 2010;203:343 e1–343 e5. doi: 10.1016/j.ajog.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–474. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand. 2011;90:140–149. doi: 10.1111/j.1600-0412.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 33.Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O'Donoghue K, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol. 2013;208:290 e1–290 e6. doi: 10.1016/j.ajog.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. [DOI] [PubMed]
- 36.Szecsi PB, Jorgensen M, Klajnbard A, Andersen MR, Colov NP, Stender S. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010;103:718–727. doi: 10.1160/TH09-10-0704. [DOI] [PubMed] [Google Scholar]
- 37.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 38.Corach D, Lao O, Bobillo C, van Der Gaag K, Zuniga S, Vermeulen M, et al. Inferring continental ancestry of argentineans from autosomal, Y-chromosomal and mitochondrial DNA. Ann Hum Genet. 2010;74:65–76. doi: 10.1111/j.1469-1809.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 39.Catelli ML, Alvarez-Iglesias V, Gomez-Carballa A, Mosquera-Miguel A, Romanini C, Borosky A, et al. The impact of modern migrations on present-day multi-ethnic Argentina as recorded on the mitochondrial DNA genome. BMC Genet. 2011;12:77. doi: 10.1186/1471-2156-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avena S, Via M, Ziv E, Perez-Stable EJ, Gignoux CR, Dejean C, et al. Heterogeneity in genetic admixture across different regions of Argentina. PLoS One. 2012;7:e34695. doi: 10.1371/journal.pone.0034695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61:1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 42.Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11:154. doi: 10.1186/1741-7015-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Homburger JR, Moreno-Estrada A, Gignoux CR, Nelson D, Sanchez E, Ortiz-Tello P, et al. Genomic insights into the ancestry and demographic history of South America. PLoS Genet. 2015;11:e1005602. doi: 10.1371/journal.pgen.1005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Branch DW, Porter TF, Rittenhouse L, Caritis S, Sibai B, Hogg B, et al. Antiphospholipid antibodies in women at risk for preeclampsia. Am J Obstet Gynecol. 2001;184:825–832. doi: 10.1067/mob.2001.113846. [DOI] [PubMed] [Google Scholar]
- 45.Dizon-Townson DS, Nelson LM, Easton K, Ward K. The factor V Leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol. 1996;175:902–905. doi: 10.1016/S0002-9378(96)80022-6. [DOI] [PubMed] [Google Scholar]
- 46.Kupferminc MJ, Eldor A, Steinman N, Many A, Bar-Am A, Jaffa A, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340:9–13. doi: 10.1056/NEJM199901073400102. [DOI] [PubMed] [Google Scholar]
- 47.Dudding T, Heron J, Thakkinstian A, Nurk E, Golding J, Pembrey M, et al. Factor V Leiden is associated with pre-eclampsia but not with fetal growth restriction: a genetic association study and meta-analysis. J Thromb Haemost. 2008;6:1869–1875. doi: 10.1111/j.1538-7836.2008.03134.x. [DOI] [PubMed] [Google Scholar]
- 48.Sifakis S, Soufla G, Koukoura O, Soulitzis N, Koutroulakis D, Maiz N, et al. Decreased annexin A5 mRNA placental expression in pregnancies complicated by fetal growth restriction. Thromb Res. 2010;125:326–331. doi: 10.1016/j.thromres.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 49.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovo M, Schreiber L, Bar J. Placental vascular pathology as a mechanism of disease in pregnancy complications. Thromb Res. 2013;131(Suppl 1):S18–S21. doi: 10.1016/S0049-3848(13)70013-6. [DOI] [PubMed] [Google Scholar]
- 51.Huppertz B. Placental pathology in pregnancy complications. Thromb Res. 2011;127(Suppl 3):S96–S99. doi: 10.1016/S0049-3848(11)70026-3. [DOI] [PubMed] [Google Scholar]
- 52.Chernausek SD. Update: consequences of abnormal fetal growth. J Clin Endocrinol Metab. 2012;97:689–695. doi: 10.1210/jc.2011-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozanne SE, Fernandez-Twinn D, Hales CN. Fetal growth and adult diseases. Semin Perinatol. 2004;28:81–87. doi: 10.1053/j.semperi.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Fishel S, Baker D, Elson J, Ragunath M, Atkinson G, Shaker A, et al. Precision medicine in assisted conception: a multicenter observational treatment cohort study of the annexin A5 M2 haplotype as a biomarker for antithrombotic treatment to improve pregnancy outcome. EBioMedicine. 2016;10:298–304. [DOI] [PMC free article] [PubMed]