Abstract
An equilibrium needs to be established by the cellular and acellular components of the ovarian follicle if developmental competence is to be acquired by the oocyte. Both cumulus cells (CCs) and follicular fluid (FF) are critical determinants for oocyte quality. Understanding how CCs and FF influence oocyte quality in the presence of deleterious systemic or pelvic conditions may impact clinical decisions in the course of managing infertility. Given that the functional integrities of FF and CCs are susceptible to concurrent pathological conditions, it is important to understand how pathophysiological factors influence natural fertility and the outcomes of pregnancy arising from the use of assisted reproduction technologies (ARTs). Accordingly, this review discusses the roles of CCs and FF in ensuring oocyte competence and present new insights on pathological conditions that may interfere with oocyte quality by altering the intrafollicular environment.
Keywords: Oocyte, Follicular fluid, Cumulus cells, Developmental competence
Introduction
A healthy intrafollicular environment supports the acquisition of developmental competence in oocytes [1], largely via the coordinated contributions of cumulus cells (CCs) and follicular fluid (FF) [2]. An oocyte is considered competent when it is able to complete meiosis and undergo fertilization, embryogenesis, and term development [1]. An understanding of how CCs and FF influence the acquisition of oocyte competence and protect the oocyte from deleterious systemic and pelvic conditions is important for clinical decision-making in the management of infertility. Disruption of the intrafollicular environment under different pathological conditions [3–12] has the potential to impact the chance of pregnancy if not treated and/or adjusted in a timely manner. This paper discusses how CCs and FF (and their functional integrity) affect oocyte quality in the context of commonly encountered pathological conditions that are likely to compromise the sustainable environment that is needed to obtain pregnancy. Understanding how these pathological conditions may interfere with natural fertility and pregnancy/live birth rates in assisted reproduction technologies (ARTs) may inform future clinical practice.
Cumulus cells-oocyte interactions and their role in the acquisition of oocyte competence
During folliculogenesis, granulosa cells are in constant communication with the oocyte, and the two cell types undergo bi-directional nutrient transfer and paracrine signaling [13–15]. This intercellular communication is essential for follicular compartment development, oocyte maturation, and competence acquisition [2, 16, 17]. The oocyte becomes fully competent when it completes meiosis (from prophase I to metaphase II) and is able to be fertilized to generate a viable embryo [1]. In antral follicles, CCs contribute to metabolic support and maintaining meiotic arrest in the growing oocyte [13, 15, 18]. In vitro culture of denuded oocytes causes abnormalities in nuclear and cytoplasmic maturation, but these changes are prevented when denuded oocytes are co-cultured with CCs [19]. At the same time, some oocyte-secreted factors mediate the metabolism, maturation, and survival of CCs [17]. The intercellular dialog occurs through two major mechanisms: gap junctions and paracrine signals [18] (summarized in Table 1 and Fig. 1).
Table 1.
Cumulus cells-oocyte interactions and their role in the acquisition of oocyte quality
| Factor | Signal source | Affected cell type | Function |
|---|---|---|---|
| Gap junctions | Cumulus cells (CCs) | Oocyte | Intercellular channels responsible for small molecules (ions, pyruvate, cyclic nucleotides, metabolites, amino acids, and RNA transcripts) transfer from CCs to the oocyte, which contribute to meiosis, ATP production, and pH balance into the oocyte |
| KIT pathway | Oocyte | CCs | This pathway induces molecular events that dictate oocyte growth and induces the production of oocyte factors which stimulate granulosa cell proliferation |
| Growth Differentiation Factor 9 (GDF-9) Bone Morphogenetic Protein 15 (BMP-15) |
Oocyte | CCs | Induce expansion, metabolism, differentiation, proliferation, apoptosis, and luteinization of CCs |
| Enzymatic (e.g., SOD, CAT, GPx) and non-enzymatic (e.g., GSH, VitE, VitC) antioxidant defense | CCs | Oocyte | Metabolize and/or neutralize ROS and protect the oocyte from oxidative stress-induced apoptosis |
SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase, GSH reduced gluthatione, VitE vitamin E, VitC vitamin C
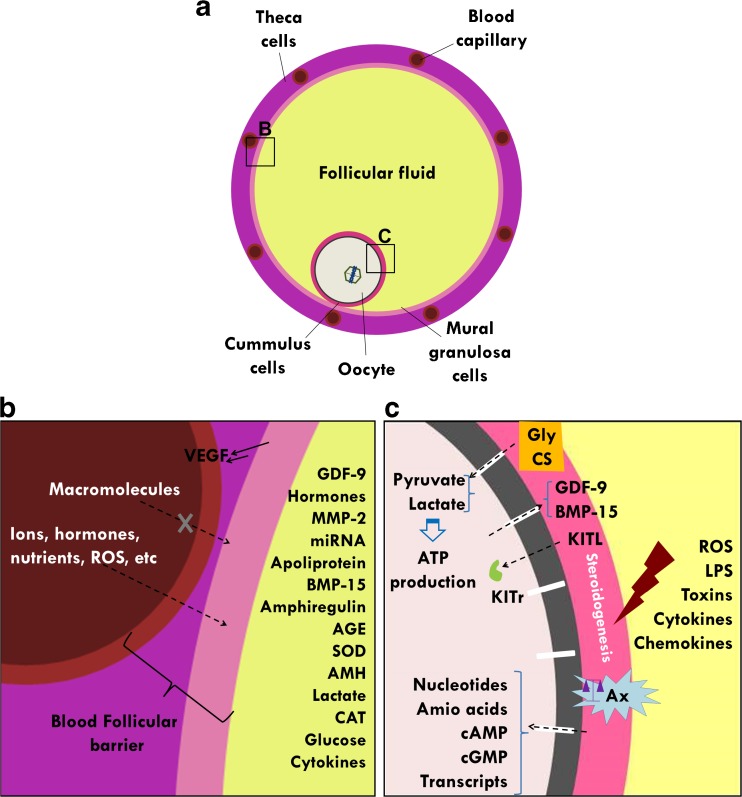
Fig. 1.
Schematic diagram showing the role of the blood-follicle barrier and cumulus cells in follicular physiology. a General view of the antral follicle. b During follicular development, granulosa and thecal cells secrete VEGF, which stimulates follicular angiogenesis to form a complex vascular network. The endothelium, sub-endothelial basement membrane, thecal interstitium, follicular basement membrane, and membrane granulosa constitute the blood-follicle barrier, which restricts the transcellular transport of molecules between the blood and follicular fluid based on molecular size. The constitution of follicular fluid is altered under many conditions (e.g., age, oocyte maturation stage, and blood supply/flow), and this can negatively impact oocyte maturation and oocyte quality. c Cumulus cells (CCs) express the soluble and membrane-bound isoforms of KIT ligand (KITL), which acts on oocyte KIT tyrosine kinase receptors (KITr) to enhance oocyte growth. The oocyte secretes growth differentiation factor 9 (GDF-9) and bone morphogenic protein 15 (BMP-15), which regulate CC proliferation, differentiation, glycolysis (Gly), and cholesterol synthesis (CS). Meanwhile, CCs provide pyruvate, lactate, and products of the cholesterol biosynthetic pathway to the oocyte via gap junctions to support the production of ATP in the oocyte. CCs also transfer ions, cyclic nucleotides (cAMP and cGMP), metabolites, amino acids, and RNA transcripts via gap junctions to the oocyte to support meiosis and help maintain the pH balance of the gamete. As the metabolism of the oocyte and CCs come to resemble one another, any alteration in the somatic cells may affect the competence of the gamete. CCs also defend the oocyte against metabolites, ROS, toxins, and inflammatory markers present in the follicular fluid. Under adverse conditions (e.g., pathologies, infections, or inflammatory processes), the protective effects of CCs may be affected, leading to downstream compromises in steroidogenesis and oocyte development. Maternal aging may also impair oocyte competence, such by perturbing the antioxidant defenses and energy metabolism of CCs and inducing apoptosis in these cells, thereby compromising the oocyte meiotic spindle. Abbreviations: AGE (advanced glycation end-products), AMH (anti-Müllerian hormone), ATP (adenosine triphosphate), Ax (antioxidants), BMP-15 (bone morphogenetic protein-15), cAMP (cyclic adenosine monophosphate), cGMP (cyclic guanosine monophosphate), CAT (catalase), CS (cholesterol synthesis), GDF-9 (growth differentiation factor-9), Gly (glycolysis), KITL (KIT ligand), KITr (KIT receptors), LPS (lipopolysaccharide), miRNA (micro RNA), MMP-2 (metalloproteinase-2), ROS (reactive oxygen species), SOD (superoxide dismutase)
Gap junctions are intercellular membrane channels that are formed by connexins; when connected to connexins of an adjacent cell, they form solute-permeable channels [20]. In the follicle, CCs are connected to the oocyte by connexin 37 (Cx37)-formed gap junctions that reach through the zona pellucida and to each other by connexin 43 (Cx43)-formed gap junctions [20, 21]. These gap junctions enable the transfer of small molecules from CCs to the oocyte; the relevant small molecules include ions, cyclic nucleotides (cAMP, cGMP), metabolites, amino acids, and RNA transcripts, all of which contribute to meiosis, ATP production, and the pH balance in the oocyte [18, 21, 22]. This communication is also important for the differentiation of CCs [23].
Intrafollicular paracrine signaling is active throughout oogenesis and plays a critical role during follicular growth and maturation [13]. It occurs through specific receptors and signaling pathways [24–27] and is modulated by circulating endocrine factors, such as follicle stimulating hormone (FSH) [28], and by growth factors, including members of the transforming growth factor beta (TGF-β) superfamily [29]. The paracrine signaling that moves from CCs to the oocyte supports meiotic resumption, enables nuclear and cytoplasmic maturation, and controls transcriptional activity [18, 30]. In this sense, the growth of the follicle and oocyte is enhanced by the KIT pathway, which consists of a soluble KIT ligand isoform (KITLG1) and the CC-expressed membrane-bound isoform (KITLG2), which acts on oocyte KIT tyrosine kinase receptors in the plasma membrane [18, 24, 25, 31]. The activation of these receptors induces molecular events that dictate oocyte growth and granulosa cell proliferation [23]. The paracrine signaling that moves from the oocyte to the CCs was first demonstrated in vitro by experiments in which oocytes were removed from cumulus-oocyte complexes (COCs) and the somatic cells were cultured alone. This resulted in granulosa cell luteinization, altered steroid production [32], reduced hyaluronic acid synthesis [33–35], reduced CC mucification [34], and reduced cumulus expansion [33] compared to intact COCs subjected to in vitro culture. Moreover, the evidence suggests that the female gamete induces CC gene expression, regulates follicle growth and atresia, and modulates the metabolism, differentiation, proliferation, apoptosis, and luteinization of CCs. This occurs through secreted factors that act on CCs. The two best-studied oocyte-secreted factors are growth differentiation factor 9 (GDF-9) and bone morphogenetic protein 15 (BMP-15) [24, 27, 36, 37]. GDF-9 is present in mouse follicles from the primary to ovulatory stages [16] and affects CC expansion by inducing the expression of genes such as Has2, Tnfip6, Ptx3, and Ptgs2 [38, 39] in the adjacent somatic cells. The involvement of BMP-15 in CC proliferation and metabolism is generally accepted; given that BMP-15 plays a role in ovulation, however, there is some question as to whether its main action occurs before [26, 40] or after [41, 42] the LH surge. Studies have shown that, in rodents, BMP-15 acts together with GDF-9 and is involved in cell proliferation [43, 44], glycolysis, cholesterol biosynthesis [26, 45], and the regulation of cGMP levels [15]. Interestingly, the paracrine interaction between oocytes and CCs appears to represent a negative feedback system between the oocyte and the granulosa cells: oocyte-derived BMP-15 induces KITL expression in rat granulosa cells, while granulosa cell-derived KITL inhibits the secretion of BMP-15 by the oocyte [46]. GDF-9 mutant ovaries were found to exhibit increased mRNA levels of Kitl, suggesting that GDF-9 downregulates Kitl [39]. Another interesting observation is that there is an interaction between gap junctions and paracrine signaling in the ovaries. It seems that the arrangement of connexins may alter the response of CCs to paracrine signals, and that paracrine factors may influence the organization of connexins between CCs [47, 48], thereby potentially impacting folliculogenesis and oocyte competency. Gittens et al. (2005) [48] found that mice with Cx43-null mutant ovaries and consequent loss of gap junctional coupling among granulosa cells (GCs) exhibited an alteration in their in vitro response to exogenous GDF-9 and a subsequent reduction in GC proliferation. Chang et al. (2014) [47] evaluated the effects of GDF-9 and BMP-15 on Cx43 expression. The authors found that BMP-15 decreased Cx43 at the mRNA and protein levels in a human granulosa cell line and further confirmed this finding in primary human granulosa-lutein cells from infertile patients undergoing in vitro fertilization (IVF). These data suggest that oocyte-derived BMP-15 may promote the downregulation of Cx43 expression, consequently decreasing gap junctions between GCs. Given some authors have reported that BMP-15 seems to have greater effects after the LH peak [41, 42], the relationship between BMP-15 and Cx43 downregulation, perhaps, could explain how the gap junctions between CCs decrease at that moment.
Importantly, CCs support energy production in the COC [49–51]. Oocytes have a poor capacity for glycolysis and cholesterol synthesis [52], but work in a mouse model showed that they secrete GDF-9 and BMP-15 to regulate these events in CCs [26, 27]. CCs carry pyruvate, lactate, and products of the cholesterol biosynthetic pathway to the oocyte via gap junctions [18, 51]. These molecules are metabolized to produce adenosine triphosphate (ATP) via the tricarboxylic acid cycle (TCA) and oxidative phosphorylation, which is the predominant pathway for ATP generation [51, 53]. Within the COC, other pathways are also responsible for a small proportion of glucose metabolism, including the pentose phosphate pathway (PPP) and the hexosamine biosynthesis pathway (HBP). The PPP importantly contributes to maturation by generating substrates for purine synthesis and maintaining the redox state. The HBP is responsible for generating substrates that are important for matrix production and cumulus expansion [53, 54]. Thus, once the oocyte and CCs resemble one another in terms of metabolism, any metabolic alteration in the somatic follicular cells may also affect the oocyte’s development [53]. For example, glucose concentration-dependent effects may perturb nuclear and cytoplasmic maturation [53]. A low-glucose environment seems to affect nucleic acid synthesis and energy production by decreasing the PPP, the HBP, and glycolysis, as observed in mouse oocytes [54], reducing cytoplasmic maturation, the resumption/completion of nuclear maturation, and granulosa cell mucification [53]. In contrast, a high-glucose environment has been associated with increased production of reactive oxygen species (ROS), increased activity of the HBP, and decreased concentrations of reduced glutathione (GSH, an antioxidant) [55]. Thus, pathologic conditions that alter the metabolic balance, such as obesity and diabetes mellitus, may have negative repercussions on the follicular environment [53].
Influence of follicular fluid on oocyte quality
FF is produced by granulosa cells during the later phases of secondary follicle development [56]; it is in intimate contact with the COC and is comprised of a plasma exudate and secretory products from granulosa cells (mural and CCs) and thecal cells [56]. The main components of FF are steroid hormones, metabolites, polysaccharides, proteins, ROS, and antioxidants [57]. The components of FF may be directly altered by hormonal, paracrine, and autocrine signaling pathways, and they may be indirectly altered by systemic conditions [58–62]. Such changes in the components of FF have been suggested to influence oocyte quality (from maturation to fertilization), early embryo development, and subsequent pregnancy [60, 63, 64].
The composition of FF differs from that of serum and undergoes physiological alterations during follicular development [56], suggesting that it may be adequate to supply the necessities of developing oocytes [65]. Variations in the follicular levels of ROS/antioxidants, hormones, and metabolites have been reported in distinct stages of follicular development [65]. Although ROS are essential for ovulation in mice [66], excessive ROS levels may cause aberrations in microtubule organization and the chromosomal alignment of metaphase II (MII) meiotic spindles in mouse oocytes [67–70].
Hormone concentrations in FF can both directly (via genomic and non-genomic actions) and indirectly (via somatic cells within the follicle) influence oocyte differentiation [65]. Lower concentrations of progesterone and higher concentrations of testosterone were observed in follicles containing germinal vesicle (GV) oocytes compared to MII oocytes [71]. Women undergoing controlled ovarian stimulation (COS) for IVF had lower follicular levels of AMH, testosterone, androstenedione, estradiol, and LH but a higher level of FSH, compared to women undergoing natural cycle IVF, suggesting that COS significantly alters the hormone milieu of FF [72].
FF also contains metabolites that accumulate within the oocyte and provide the necessary intracellular materials for oocyte differentiation [65]. These metabolites include amino acids, lipids, nucleotides, and other small molecules and are derived from serum and the metabolic activity of follicular cells [56].
Other molecules fluctuate during follicular development, as summarized in Table 2. These include GDF-9, BMP-15, amphiregulin, metalloproteinase-2 (MMP-2), and various microRNAs (miRNAs; e.g., hsa-miR-27b, hsa-miR-29b, hsa-miR-139-3p, hsa-miR-339-3p, hsa-miR-451, hsa-miR-483-3p, hsa-miR-520d-3p, hsa-miR-563, hsa-miR-572, hsa-miR-630, hsa-miR-663, hsa-miR-720, and hsa-miR-940) [59] (Fig. 1). During a stimulated ovarian cycle, higher concentrations of GDF-9 in FF were correlated with oocyte nuclear maturation and embryo quality [63], while higher BMP-15 levels were reported in FF whose oocytes underwent fertilization/cleavage and later exhibited the best (grade I) embryonic morphology [78]. Epidermal growth factor (EGF)-like growth factors, such as amphiregulin, are mediators that propagate the LH stimulus for oocyte maturation. The level of amphiregulin in FF was correlated with increased number of retrieved oocytes, number of formed embryo, and pregnancy rate in women undergoing COS for ART [60]. In addition, a higher concentration of MMP-2 [64], a zinc endopeptidase family member that can degrade all components of the extracellular matrix [79], was reported in human FF from groups that had 100% MII oocytes compared to a group with less than 100% MII oocytes [64]. We suggest that MMP-2 contributes to the progression of meiosis, possibly by signaling the oocyte regarding the imminence of ovulation. Consistent with this, upregulation of MMP-2 in FF is required for ovulation in Drosophila [80]. As little is known about this connection in mammals, MMP-2 knockout studies are needed to help us understand the roles of MMP-2 in oocyte maturation and fertility.
Table 2.
Influence of follicular fluid (FF) constituents on oocyte quality
| Follicular fluid constituents | Impact | Reference |
|---|---|---|
| ROS and oxidative stress markers | ROS and oxidative stress affect microtubule organization and chromosomal alignment of metaphase II (MII) meiotic spindles in mouse oocytes | [67–70] |
| Progesterone (P4) | Lower P4 levels in follicular fluid are associated with more germinal vesicle and less MI and MII oocytes than higher P4 levels | [71] |
| Growth differentiation factor 9 (GDF-9) | Higher GDF-9 levels in the FF are correlated with oocyte nuclear maturation and high-embryo-quality | [63] |
| Bone morphogenetic protein 15 (BMP-15) | Higher BMP-15 levels in FF are associated with oocyte fertilization and cleavage and with the best embryo morphology | [78] |
| Amphiregulin | Amphiregulin levels in FF are positively correlated with the number of available embryos | [60] |
| Matrix metalloproteinase 2 (MMP-2) | Increased activity of MMP-2 in FF is related to 100% of matured oocytes | [63] |
| Tumor necrosis factor α (TNF-α) | FFs with higher TNF-α concentration originate poor quality oocytes | [74, 75] |
| Interleukins (IL-6, IL-1, IL-15) | IL-6 level in FF is associated with decreased chance of clinical pregnancy; higher follicular levels of IL-1 are related to higher chance of embryo implantation after IVF; lower IL-15 levels are related to clinical pregnancy after IVF | [76, 91, 93] |
MiRNAs are small single-stranded noncoding RNA molecules that act as important regulators of diverse biological processes, including proliferation, differentiation, migration, and apoptosis [81]. Comparison of human FF from MII versus GV oocytes enabled researchers to identify 13 differentially expressed miRNAs, while comparison of FF from MII versus MI oocytes identified seven differentially expressed miRNAs [59]. In silico analysis showed that these miRNAs appeared to target in the GnRH signaling pathway (their potential gene targets included kinases and members of the calcium and MAPK signaling pathways) and thus might modulate the timing of oocyte meiosis and maturation [59]. In the future, studies utilizing animal models should be performed to examine the in vivo effect of those deregulated miRNAs on oocyte maturation. If the alteration of such miRNAs is found to impair oocyte development, researchers could seek to modulate the deregulated pathways in the hopes of developing a therapeutic strategy to address some cases of infertility.
The composition of FF may also be influenced by age. In experimental studies, increased advanced glycation end-products (AGEs) were observed in FF from older cows compared to younger cows [82]. Moreover, the addition of FF from aged cows to the in vitro maturation (IVM) medium accelerated nuclear maturation, increased the ROS content, promoted abnormal oocyte fertilization, and inhibited blastulation compared to the addition of FF from younger cows [82]. Alterations in follicular miRNA levels have been observed in aged mares (by exosome miRNA profiling) [83] and aged women [84]. Increased concentrations of superoxide dismutase (SOD) [85], AMH [86], lactate, and progesterone [87], and decreased concentrations of catalase (CAT) [85] and glucose [87] were observed in older FF compared to younger FF. The aging process is related to oxidative stress, which can be divided into three distinct stages: increased production of reactive species, the mobilization of antioxidants, and oxidative damage to major targets (lipids, proteins, and nucleic acids) [88, 89]. Decreased CAT activity may indicate exacerbated ROS production; this can promote damage to DNA, proteins, and lipids, thereby compromising ovarian follicle development. On the other hand, increased SOD may be understood as a mobilization of antioxidative forces intended to prevent the damage that may be caused by excessive ROS (second stage). Mitochondria are the main generator of ROS, and errors in these organelles can lead to apoptosis, which can compromise follicle development. In older women, decreased follicular glucose levels and increased lactate levels may indicate that follicular glycolysis is upregulated; moreover, excessive levels of lactate can decrease the pH of FF and thus reduce oocyte fertilization [87].
Progesterone levels were found higher in the FF of older women, suggesting that there may be disruptions in their steroidogenesis and CC differentiation. Interestingly, more progesterone is produced by granulosa cells than by CCs in young women, but this pattern reverses with age until CCs produce more progesterone than mural granulosa cells [87]. The mechanisms underlying this alteration are not yet known, but it may indicate that older women experience an incomplete differentiation of CCs that could interfere with overall steroidogenesis. The follicular levels of different kinds of apolipoproteins also vary with age, and this correlates with a lower number of retrieved mature oocytes in intracytoplasmic sperm injection (ICSI) cycles [58].
Increased inflammatory factors in FF may also disrupt oocyte quality [73, 90]. Higher follicular TNF-α levels are related to poor oocyte quality [74] and fewer fertilized oocytes [75]. Higher follicular interleukin (IL)-6 levels are observed in older IVF patients compared to younger patients, and the IL-6 level has been associated with a decreased chance of clinical pregnancy [76]. The same study also found a linear correlation between FF IL-6 levels and estradiol on the day of hCG administration, but IL-6 did not appear to have any negative effect on parameters of folliculogenesis (e.g., ovarian response) [76]. Thus, we do not yet clearly understand the relationship between IL-6 levels and clinical pregnancy in older women. Higher follicular levels of another interleukin, IL-1, are related to a higher chance of embryo implantation following IVF [91]. IL-1 seems to regulate the expression of the FSH receptor [92] and to be regulated by FSH [77], but we currently lack a well-designed study assessing the impact of follicular IL-1 on pregnancy success. The levels of the pleiotropic cytokine, IL-15, are decreased in patients who achieve clinical pregnancy compared with patients who do not have a successful IVF-embryo transfer outcome [93]. However, no published report has assessed the role of IL-15 in oocyte development. Further studies are needed to investigate the role of follicular cytokines levels in predicting pregnancy after ART.
Oxidative stress in FF can also be related to compromised oocyte quality [3, 61, 82]. For example, high ROS levels and low antioxidant capacity in FF can predict a reduced pregnancy rate in IVF cycles [94, 95]. Our group recently demonstrated that the level of 8-hydroxy-2′-deoxyguanosine (8OHdG) is higher in FF of infertile women with endometriosis compared to other infertile controls, suggesting oxidative DNA damage in this reproductive microenvironment. Such damage may compromise oocyte quality [3]. In the same study, we demonstrated that 8OHdG concentrations higher than 28.02 ng/ml in FF showed a high accuracy to predict clinical pregnancy following COS for ICSI in infertile women with endometriosis [3].
Role of the vasculature in determining FF constituents and oocyte competence acquisition
A growing follicle (comprising somatic follicular cells and the oocyte) is surrounded by a capillary network that is responsible for follicular vascularization. Along with follicular cells, this vasculature constitutes the blood-follicle barrier, which is important for oocyte protection and FF formation [96].
In the ovarian cortex, angiogenesis is related to follicular development and is independently regulated in each follicle [97]. Primordial and primary follicles receive nutrients and oxygen by passive diffusion from stromal blood vessels; during follicular development, however, the synthesis of vascular endothelial growth factor (VEGF) by granulosa and thecal cells induces the formation of a vascular network between the layers of thecal cells [98]. This focuses the nutrient resources toward the follicle.
Researchers assessed the dynamics of the ovarian blood supply to preovulatory follicles by injecting radioactive microspheres into the ovarian artery of sheep and found that the follicular blood supply was higher at the preovulatory LH surge and fell off near the time of follicular rupture [99]. A study assessing the effect of a progesterone receptor antagonist (RU486) on VEGF expression in follicular cells showed that the progesterone receptor affects VEGF expression through thecal cells, vascularization, endothelial cell proliferation, and the recruitment of perivascular mural cells [100]. VEGF is also essentially regulated by BMP-7; this cytokine, which is mainly expressed in thecal cells [101], stimulates endothelial cells to form vasculature in the follicle by inducing VEGF expression in granulosa cells and increasing the sensitivity of endothelial cells to VEGF [102].
In mice, hCG administration causes a 15% enlargement in the pore radius of ovarian follicle capillaries [103]. In equines, nitric oxide (NO) is detectable in preovulatory FFs and its concentration increases after administration of hCG [104]. Thus, we suggest that hCG stimulates NO production in the ovarian follicle and is likely to act on the blood-follicle barrier by increasing capillary permeability. Mice subjected to knockout of the endothelial NO synthase gene (eNOS, which produces NO in the endothelium) showed fewer ovulated oocytes, a lower MII oocytes rate, fewer pups born per litter, and a higher pup mortality rate compared to wild-type mice [105]. Thus, we hypothesize that alterations in NO production and eNOS can contribute to infertility by altering FF composition via errors in capillary permeability, thereby impairing oocyte maturation and ovulation.
The formations of the antrum and FF depend on the presence of an osmotic gradient and the degree of vascular permeability. It is believed that the granulosa cell-derived productions of hyaluronan and versican (a chondroitin sulfate proteoglycan) generate an osmotic gradient, leading to cavity formation [106]. Aquaporins, which are found on the basal and apical surfaces of granulosa cells, allow water to move across cells to form a plasma transudate in follicles [107, 108].
The vascular endothelium, sub-endothelial basement membrane, thecal interstitium, follicular basement membrane, and granulosa membrane together constitute the blood-follicle barrier [96] (Fig. 1). Similar to other blood-tissue barriers, the blood-follicle barrier restricts the transcellular transport of solutes and macromolecules based on their molecular sizes; it acts as an important ultrastructure in the ovary by limiting the access of foreign compounds and/or harmful substances to developing follicles [96] and allowing the formation of two distinct compartments that allow the FF and serum to differ in their compositions [3, 58, 62].
Some studies have assessed the relationship between vascularity and follicular development or oocyte quality. Experiments using Doppler ultrasonography in cows showed that follicles with perifollicular blood flow had a higher chance of producing top-quality COCs compared to follicles without perifollicular blood flow [109]. In women, a positive correlation was observed between the Doppler index of ovarian follicles and oocyte quality and better reproductive outcomes [110–113]. Furthermore, VEGF levels in FF have been correlated with perifollicular blood flow in patients undergoing COS for IVF [114, 115].
The protective role of cumulus cells against harmful conditions that affect the follicular environment
CCs play a protective role that critically ensures oocyte competency and thus may be considered to act as both a bridge and a barrier between the oocyte and the extra follicular microenvironment [116, 117]. CCs support the oocyte’s needs, but they are also responsible for isolating the gamete from harmful conditions [30, 116–118]. It has been well established that the proper regulation of lipid content and fatty acids is important for oocyte competency and early embryonic development, as excessive lipid accumulation inside the oocyte can exert toxicity on cell functions [11]. Free fatty acids (FFAs) induce ROS accumulation, which promotes endoplasmic reticulum stress and cell death [119]. In the follicular microenvironment, increased FFAs are associated with poor COC morphology due to lipotoxicity [120]. CCs protect the oocyte against toxic metabolites and thus play an important metabolic function in protecting the developmental competence of the oocyte [118].
FF may reflect the composition of the plasma, and harmful compounds can accumulate in the FF to influence oocyte maturation. Some reports have described the presence of unhealthy compounds in FF (inflammatory mediators, oxidative stress metabolites, reactive oxygen species, toxins, and others) under pathological conditions [3, 12, 61, 121–127]. Such compounds may affect the resumption and/or appropriate progression of meiosis, thereby hindering oocyte development [117]. Beyond metabolic protection, CCs also contribute to the enzymatic and nonenzymatic antioxidant defenses that protect the oocyte from ROS [128] and oxidative stress-induced apoptosis [129]. Our group recently demonstrated that infertile patients with stage III/IV endometriosis who achieved clinical pregnancy after COS for ICSI showed higher SOD1 expression in CCs, suggesting that SOD1 may protect the oocyte from oxidative damage [5].
Under adverse situations, CCs may be unable to protect the oocyte against FF alterations, which can disrupt CC functions and decrease oocyte quality. As an example of this, a study evaluating the ability of ROS (e.g., H2O2, •OH and HOCl) to overcome the protective functions of CCs and affect oocyte quality found that CCs could neutralize lower concentrations of H2O2 and •OH, but that higher concentrations decreased the number and vitality of CCs, reducing the antioxidant response and rendering oocytes more susceptible to damage [117]. The same study found that any concentration of HOCl negatively affected both oocytes and CCs [117]. These data suggest that when harmful factors exceed the ability of CCs to metabolize and/or neutralize them, such factors may negatively affect the oocyte.
Even pathologic conditions in sites far removed from the ovaries (e.g., infectious and inflammatory processes) may affect the follicle, damage oocyte quality, and decrease fertility [12]. Studies have suggested that toxins, inflammatory molecules, and ROS may all reach the circulation and either enter the oocyte or be metabolized (and thus defended against) by CCs [12, 121, 124]. During the advanced antral phase, when the follicle becomes highly vascularized and the FF mirrors the systemic environment, these molecules may have several particularly important repercussions in the follicle, such as by interfering with the pituitary axis, affecting CCs functions, and dysregulating steroidogenesis [12, 121, 124].
The effects of age on follicular cells may also contribute to the failure of CCs to protect oocytes against harmful factors. The effects of senescence have been widely studied in female gametes. It is well established that human oocyte quality decreases with age, and changes in functions of somatic follicular cells have been associated with this fertility impairment [130–132]. Alterations in granulosa cells, such as double-strand breaks and a reduced ability to repair DNA errors, may be involved in ovarian aging [132]. It is believed that, with aging, increased reactive species production and inappropriate antioxidant defenses may compromise the functions of CCs and oocytes by inducing mitochondrial alterations, reducing ATP production, affecting the meiotic spindle, and promoting genomic instability, leading to oocyte incompetence [2, 133, 134].
Telomere attrition has also been related to oocyte senescence, and the telomere length of CCs has been proposed as a marker of embryo quality (good quality: grade 1 and 2 embryos, with shaped blastomeres, uniform cytoplasm, and up to 20% fragmentation) [135]. Oocytes may also be affected by the shortening of CC telomeres, which is related to senescence and apoptosis. CCs from older women undergoing IVF seem to exhibit increased apoptosis, and this is related to lower fertilization and pregnancy rates [136]. Moreover, CCs from older women exhibited altered oxidative phosphorylation and increases in the enzymes responsible for the metabolism of amino acids, carbohydrates, and fatty acids [137]. These findings suggest that metabolic alterations in aging CCs may be a compensatory mechanism that counteracts age-related ATP deficiencies, such as those caused by compromised mitochondrial activity and increased oxidative phosphorylation.
Aged oocytes and their surrounding CCs arising from older female animals or women reportedly exhibit alterations in gene expression [137–139]. In the CCs of older goats, researchers found altered expression of genes related to mitochondrial function, metabolism, apoptosis, and antioxidant defense [138]. In cows, aged FF seems to accelerate nuclear maturation, accelerate the closure of gap junctions between oocytes and CCs, increase ROS, and increase the abnormal fertilization rate of oocytes, as compared with young FF [82]. In human CCs, those of older women showed upregulation of genes involved in cellular energy maintenance and antioxidant defense, and these increases were associated with decreased embryo quality [139]. A recent RNA sequencing study of human CCs showed that senescent cells exhibit activation of gene pathways associated with oxidative stress and hypoxia; this may disrupt intracellular pH and reduce oocyte metabolism [140]. Together, the data suggest that maternal aging may affect crucial functions in surrounding somatic follicular cells, such as by altering antioxidant defenses and perturbing energy metabolism, thereby affecting the meiotic spindle and oocyte competence.
Systemic and pelvic conditions may compromise oocyte quality by interfering with the oocyte environment
Endometriosis
Endometriosis is associated with a higher risk of infertility [141, 142], but the mechanisms involved in the etiology of endometriosis-related infertility are poorly understood. Some authors have suggested that infertility in women with this disease might reflect decreases in oocyte quality [3, 5, 126, 143–145].
Endometriosis is associated with chronic inflammation, and ROS are inflammatory mediators known to modulate cell proliferation [122]. Therefore, some authors have suggested that endometriosis is associated with oxidative stress [122, 146–148] via a pathophysiology that may be related to an inflammatory response to ectopic endometrial implants [149]. Thus, endometriotic cells might promote oxidative stress by increasing ROS production and/or altering detoxification pathways in the peritoneal cavity [150]. Mansour et al. (2009) demonstrated that peritoneal fluid (PF) from women with endometriosis promotes microtubule and chromosome abnormalities in mature mouse oocytes, and that these effects were reduced when the medium was supplemented with the antioxidant l-carnitine. These findings suggest that substances present in the PF of women with this disease may compromise oocyte and embryo quality, and that oxidative stress is likely to mediate this process [151]. Moreover, the chronic inflammation and oxidative stress in the peritoneal environment of women with endometriosis may be reflected in their systemic circulation [3, 152]. The increased degree of ovarian vascularization at the late stages of folliculogenesis can allow ROS from blood plasma to reach the FF and affect the follicular microenvironment. For example, Singh et al. (2016) found that the serum and FF concentrations of some cytokines and angiogenic factors were correlated in women with endometriosis, and that the levels of IL-8, IL-12, and adrenomedullin (ADM) were negatively correlated with oocyte maturity and embryo quality in these women [153]. Other studies have found evidence of oxidative stress in FF from infertile women with endometriosis [3, 62, 154]. Our group recently demonstrated that FF from infertile women with mild endometriosis has a deleterious effect on the spindle morphology and chromosome distribution of IVM bovine oocytes [144], and that this effect could be diminished or completely prevented by the addition of antioxidants, especially l-carnitine [145]. This suggests that the oxidative stress in the FF of these women may contribute to compromising their oocyte quality. As mentioned above, our group recently demonstrated that SOD1 expression is significantly higher in CCs from infertile women with moderate and advanced pelvic endometriosis compared to CCs from infertile women with early-stage endometriosis or without the disease [5]. The highest level of SOD1 gene expression was observed in patients with stage III/IV endometriosis who achieved clinical pregnancy after COS for ICSI, suggesting when the expression of the SOD1 gene in CCs is sufficiently high to prevent oxidative damage to the oocyte, oocyte quality is not compromised and the occurrence of clinical pregnancy is favored [5]. Although these data are preliminary and studies with larger sample sizes are needed to better evaluate the external validity of our results, this work offers a good example of a pelvic disease that may impact FF and deleteriously affect oocyte quality when CCs are not capable of protecting the oocyte against oxidative damage.
Pelvic infection
Pelvic infection, as demonstrated by positivity to Chlamydia antibody testing and/or tubal damage, is associated with poor ovarian response (POR) [155, 156]. It is also a risk factor for POR according to the Bologna’s Consensus [157]. POR may be an early sign of ovarian aging (i.e., altered oocyte quality) or a reduced ovarian reserve, which is associated with decreased live birth rates following IVF [158–161] and an increased risk of premature menopause [162]. Hence, ovarian stimulation can be viewed as a dynamic test for the resting ovarian follicular pool [158], and the size of the cohort of recruitable follicles may reflect the actual resting follicle pool [163]. It is intriguing to question why pelvic infection is a risk factor for POR and whether compromised oocyte quality may contribute to the infertility diagnosed in these women.
No published study has specifically evaluated these questions in women, and the mechanisms underlying the continued infertility that may be seen following resolution of uterine infection and the resultant inflammation remain to be elucidated. A recent review by Bromfield et al. (2015) focused on work in cows and offers some insights [12]. The authors hypothesize that three factors link postpartum uterine infection of dairy cows with infertility, even following clearance of infectious agents: (1) disruption of endocrine signaling and the hypothalamic-pituitary-gonadal axis, (2) negative effects on the ability of the endometrium to support embryonic development and implantation, and (3) ovarian dysregulation resulting in reduced oocyte quality (i.e., reduced abilities to complete meiosis, undergo fertilization, and develop into a viable embryo) [12]. A key relevant change in the ovary is the presence of lipopolysaccharides (LPS) within the FF of diseased cows [164]. Ovarian granulosa cells respond to bacterial LPS in a Toll-like receptor 4 (TLR4)-dependent manner by increasing inflammatory mediators and reducing aromatase and estradiol, leading to impaired oocyte competence [12, 164, 165]. LPS negatively affects the oocytes developing in the dominant follicle. This may explain the infertility seen shortly after infection, but not the long-term infertility seen in animals following uterine infection. Bromfield et al. (2013) proposed that infection also perturbs smaller developing follicles, including primordial-stage follicles, which could affect long-term fertility by compromising the ovarian reserve [166]. In the presence of LPS, primordial follicle activation is increased, enlarging the pool of primary follicles and depleting the primordial follicle reserve [166]. However, the precise mechanisms by which LPS exposure reduces the primordial follicle pool and inhibits the ability of the oocyte to acquire developmental competence remain to be elucidated, and studies evaluating animal models are needed.
Obesity and diabetes
Obesity is associated with infertility and poor ART outcomes [167]. Although the pathophysiology behind these effects is not fully known, some evidence suggests that obesity impairs oocyte quality [168]. For example, analysis of a large dataset from the Society of Assisted Reproductive Technology Clinic Online Reporting System revealed that obese women using autologous oocytes were less likely to achieve a clinical pregnancy than their normal-weight peers, whereas obese women who used donor oocytes had pregnancy rates similar to their normal-weight peers [168]. Research investigating the effects of obesity on human oocytes has shown that mature oocytes from obese women are smaller and have more abnormal spindles and chromosome misalignment than oocytes from women with a normal body mass index (BMI) [169, 170].
Since adipose tissue is deeply involved in steroid hormone production, obesity may affect oocyte competence and maturation by disturbing the hormones involved in the maturation process [171]. Moreover, the characteristic increased serum insulin concentration and cell insulin resistance in obese women was associated with decreased levels of steroid hormone-binding globulin (SHBG); this increase is associated with upregulations of testosterone, dihydrotestosterone, and androstenediol, which may affect follicular steroidogenesis [172]. Hyperinsulinemia upregulates thecal LH receptors [173] and, together with LH hypersecretion, perturbs ovulation and the resumption of oocyte maturation, compromising oocyte competence in these women [171, 174].
Within FF, glucose and insulin levels are positively correlated with BMI, offering a link between disordered insulin-glucose homeostasis and impaired oocyte quality [175, 176]. Increased glucose levels enhance ROS production, alter the glucose metabolism pathway, and lead to antioxidant deficiency in the follicular environment [55]. Obesity and insulin resistance are frequently related to type II diabetes, which is an endocrine disorder that is characterized by hyperglycemia and hyperinsulinemia [171]. In diabetic obese mice, hyperglycemia is known to affect oocyte development (growth and maturation), promote mitochondrial dysfunction among CCs and oocytes, induce granulosa cell apoptosis, compromise gap junction communication within the COC [10, 177, 178], and induce metabolic alterations, such as by decreasing the glucose flux in the PPP, affecting purine synthesis and cAMP production [179].
Elevated levels of FFA in the FF are associated with abnormal COC morphology [120]. The high lipid content in COCs induces lipotoxicity, which affects oocyte mitochondrial membrane potential and promotes CC apoptosis in obese mice, consequently impairing fertilization and natural conception rates [11]. Thus, obesity-related hyperinsulinemia, hyperglycemia, and/or increased FFAs seem to affect COC steroidogenesis and oocyte metabolism and promote meiotic defects, impairing oocyte maturation and competence acquisition.
A recent study in an obese mouse model demonstrated that co-enzyme Q10 (CoQ10), an antioxidant component of the electron transport chain, improved the mitochondrial function of oocytes [180]. CoQ10 acts in mitochondria by regulating beta oxidation, which uses cytosolic FFA to produce acetyl-CoA, that is a substrate for citric acid cycle, generating NADH.FADH2 to respiratory chain, which produce ATP and generate free radicals. Thus, mitochondrial errors could explain the alterations in the follicular levels of FFA and ROS among obese women. Clinical studies are needed to evaluate the therapeutic potential of CoQ10 in obese women and women with diabetes who present infertility.
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is another metabolic disorder that is related to infertility and may negatively impact the follicular environment to affect oocyte competence. PCOS is frequently associated with obesity, hyperandrogenism, insulin resistance, luteinizing hormone (LH) hypersecretion, and anovulation [181]. These associated conditions may contribute to impairing the follicular balance that is crucial to oocyte development.
Women with PCOS seem to undergo altered folliculogenesis with arrested small follicles; they can also exhibit an altered follicular hormonal environment [181–183], which may interfere with oocyte maturation, metabolism, and competence acquisition. Altered expressions of various transcripts, such as those encoding insulin receptors [175], IGF-binding proteins and receptors [184], and genes related to meiotic cell cycle regulation [185] have been observed in CCs from women with PCOS, suggesting that these women experience disturbances or delays in CC maturation and differentiation [185] and/or compromises in CC function [175]. Other studies focusing on CCs from these women found changes in long noncoding RNAs (lncRNAs; e.g., XLOC_011402, ENST00000454271, ENST00000433673, ENST00000450294, and ENST00000432431) [7], miRNAs (17 were differentially expressed; hsa-miRNA-135b-5p was the most highly upregulated and hsa-miRNA-3940-5p was the only example of downregulation) [186, 187], and the methylation patterns of specific gene [188] when compared to controls, indicating that these cells undergo epigenetic deregulation. Together, the data indicate that the CCs from women with PCOS exhibit various alterations that are likely to negatively affect oocyte development.
PCOS is a complex condition, with patients showing different degrees of obesity, dyslipidemia, insulin resistance, abnormal glucose metabolism, metabolic syndrome, and other metabolic abnormalities [189] that may interact to interfere with the follicular environment. In this sense, obese PCOS patients with hyperinsulinemia and impaired glucose tolerance have decreased fertilization and implantation rates compared to non-obese women with PCOS [190]. Moreover, hyperandrogenism is intimately related to the metabolic disorders in PCOS patients: androgen may directly or indirectly alter glucose metabolism, increase FFA formation, and inhibit intrahepatic insulin clearance, leading to insulin resistance [191]. When researchers evaluated the intermediate metabolites of FF from classic PCOS patients, they found that these molecules were altered by hyperandrogenism but not obesity [8]. Evaluation of CCs from these patients revealed the presence of mitochondrial dysfunction, imbalanced redox potential, and increased oxidative stress [8], suggesting that their defenses against ROS were compromised.
Finally, CCs from PCOS patients were found to exhibit increased levels of ribosomal RNA compared to CCs from healthy women; this suggests that CCs from PCOS patients undergo activation of ribosomal gene expression, which may disturb oocyte-CCs communication [192].
Conclusions
The healthy Graafian follicle is the result of a protracted developmental process designed to achieve two functions: (1) support the acquisition and maintenance of a developmentally competent oocyte and (2) fulfill the steroidogenic demands of the follicular and luteal phases of the menstrual cycle. CCs and FF directly contribute to oocyte quality, together forming a niche that protects the gamete against local or systemic pathologic conditions capable of compromising its developmental potential. Here, we reviewed how CCs and FF may help maintain follicular health and how oocyte quality may be impacted by systemic conditions, including obesity, diabetes, and PCOS, or those of pelvic origin, such as endometriosis and pelvic/STD infections. We addressed a set of intrafollicular properties that may be negatively impacted during folliculogenesis and predicted their consequences with respect to the acquisition and maintenance of oocyte developmental competencies. For example, alterations in the physico-chemical and cellular status of FF are suggested to trigger a series of downstream events that may contribute to imbalanced steroidogenesis, inhibited luteinization, and disruption of the symbiotic oocyte-CCs relationship that determines oocyte quality. Despite the extensive literature that we reviewed herein, however, some of the available knowledge is incomplete and we must be careful in interpreting the data. Our current challenge is to connect the crucial findings obtained from experimental, animal, and in vitro studies and to reflect on how clinical conditions may impact follicular health. Understanding the complex interplay between homeostatic and pathologic processes should provide a practical framework for designing clinical interventions that are well suited for the patient-specific management of infertility. Further studies investigating new therapies that can modulate the mechanisms (e.g., oxidative stress, altered gene expression, epimutation, pathway deregulation, metabolic imbalance, and disrupted follicular homeostasis) that appear to decrease oocyte quality in the discussed clinical conditions will help us determine better and individualized approaches for improving the natural fertility of such patients.
Funding
This work was supported in part by a scholarship the Foundation for Research Support of the State of São Paulo (FAPESP, grant number 2015/21907-0, Brazil) to PAN.
Contributor Information
D. L. Keefe, Email: david.keefe@nyumc.org
D. Albertini, Email: dalbertini@thechr.com
P. A. Navarro, Phone: +55-16-3602-2821, Email: pnavarro@fmrp.usp.br
References
- 1.Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164(1):1–9. doi: 10.1006/dbio.1994.1175. [DOI] [PubMed] [Google Scholar]
- 2.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103(2):303–316. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Da Broi MG, de Albuquerque FO, de Andrade AZ, Cardoso RL, Jordão Junior AA, Navarro PA. Increased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016; 10.1007/s00441-016-2428-4. [DOI] [PubMed]
- 4.Barcelos ID, Donabella FC, Ribas CP, Meola J, Ferriani RA, de Paz CC, et al. Down-regulation of the CYP19A1 gene in cumulus cells of infertile women with endometriosis. Reprod BioMed Online. 2015;30(5):532–541. doi: 10.1016/j.rbmo.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Donabela FC, Meola J, Padovan CC, de Paz CC, Navarro PA. Higher SOD1 gene expression in cumulus cells from infertile women with moderate and severe endometriosis. Reprod Sci. 2015;22(11):1452–1460. doi: 10.1177/1933719115585146. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Liu C, Hao C, Xue Q, Huang X, Zhang N, et al. Aberrant expression of angiopoietin-like proteins 1 and 2 in cumulus cells is potentially associated with impaired oocyte developmental competence in polycystic ovary syndrome. Gynecol Endocrinol. 2016;32(7):557–561. doi: 10.3109/09513590.2016.1138463. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Hao C, Bao H, Wang M, Dai H. Aberrant expression of long noncoding RNAs in cumulus cells isolated from PCOS patients. J Assist Reprod Genet. 2016;33(1):111–121. doi: 10.1007/s10815-015-0630-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Zhao Y, Li T, Li M, Li J, Li R, et al. Metabolism alteration in follicular niche: the nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic Biol Med. 2015;86:295–307. doi: 10.1016/j.freeradbiomed.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Hao C, Shen X, Liu X, Shan Y, Zhang Y, et al. Differences in the transcriptional profiles of human cumulus cells isolated from MI and MII oocytes of patients with polycystic ovary syndrome. Reproduction. 2013;145(6):597–608. doi: 10.1530/REP-13-0005. [DOI] [PubMed] [Google Scholar]
- 10.Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, et al. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142(4):681–691. doi: 10.1242/dev.114850. [DOI] [PubMed] [Google Scholar]
- 11.Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151(11):5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- 12.Bromfield JJ, Santos JE, Block J, Williams RS, Sheldon IM. Physiology and endocrinology symposium: uterine infection: linking infection and innate immunity with infertility in the high-producing dairy cow. J Anim Sci. 2015;93(5):2021–2033. doi: 10.2527/jas.2014-8496. [DOI] [PubMed] [Google Scholar]
- 13.Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21(4):427–454. doi: 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- 14.Russell DL, Gilchrist RB, Brown HM, Thompson JG. Bidirectional communication between cumulus cells and the oocyte: old hands and new players? Theriogenology. 2016;86(1):62–68. doi: 10.1016/j.theriogenology.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Wigglesworth K, Lee KB, O'Brien MJ, Peng J, Matzuk MM, Eppig JJ. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc Natl Acad Sci U S A. 2013;110(39):E3723–E3729. doi: 10.1073/pnas.1314829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol. 2010;88(4):399–413. doi: 10.1139/y10-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 18.Monniaux D. Driving folliculogenesis by the oocyte-somatic cell dialog: lessons from genetic models. Theriogenology. 2016;86(1):41–53. doi: 10.1016/j.theriogenology.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Ge L, Sui HS, Lan GC, Liu N, Wang JZ, Tan JH. Coculture with cumulus cells improves maturation of mouse oocytes denuded of the cumulus oophorus: observations of nuclear and cytoplasmic events. Fertil Steril. 2008;90(6):2376–2388. doi: 10.1016/j.fertnstert.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Winterhager E, Kidder GM. Gap junction connexins in female reproductive organs: implications for women's reproductive health. Hum Reprod Update. 2015;21(3):340–352. doi: 10.1093/humupd/dmv007. [DOI] [PubMed] [Google Scholar]
- 21.Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123(5):613–620. doi: 10.1530/rep.0.1230613. [DOI] [PubMed] [Google Scholar]
- 22.FitzHarris G, Siyanov V, Baltz JM. Granulosa cells regulate oocyte intracellular pH against acidosis in preantral follicles by multiple mechanisms. Development. 2007;134(23):4283–4295. doi: 10.1242/dev.005272. [DOI] [PubMed] [Google Scholar]
- 23.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385(6616):525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- 24.Joyce IM, Pendola FL, Wigglesworth K, Eppig JJ. Oocyte regulation of kit ligand expression in mouse ovarian follicles. Dev Biol. 1999;214(2):342–353. doi: 10.1006/dbio.1999.9437. [DOI] [PubMed] [Google Scholar]
- 25.Thomas FH, Vanderhyden BC. Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol. 2006;4:19. doi: 10.1186/1477-7827-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135(1):111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 27.Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134(14):2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 28.Thomas FH, Ethier JF, Shimasaki S, Vanderhyden BC. Follicle-stimulating hormone regulates oocyte growth by modulation of expression of oocyte and granulosa cell factors. Endocrinology. 2005;146(2):941–949. doi: 10.1210/en.2004-0826. [DOI] [PubMed] [Google Scholar]
- 29.Williams CJ, Erickson GF. Morphology and physiology of the ovary. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM et al., editors. Endotext. South Dartmouth (MA); 2000.
- 30.Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 31.Buratini J, Price CA. Follicular somatic cell factors and follicle development. Reprod Fertil Dev. 2011;23(1):32–39. doi: 10.1071/RD10224. [DOI] [PubMed] [Google Scholar]
- 32.Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro : I. The activation of ovarian eggs. J Exp Med. 1935;62(5):665–675. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol. 1990;138(1):16–25. doi: 10.1016/0012-1606(90)90172-f. [DOI] [PubMed] [Google Scholar]
- 34.Salustri A, Yanagishita M, Hascall VC. Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH-stimulated cumulus cells. Dev Biol. 1990;138(1):26–32. doi: 10.1016/0012-1606(90)90173-g. [DOI] [PubMed] [Google Scholar]
- 35.Salustri A, Ulisse S, Yanagishita M, Hascall VC. Hyaluronic acid synthesis by mural granulosa cells and cumulus cells in vitro is selectively stimulated by a factor produced by oocytes and by transforming growth factor-beta. J Biol Chem. 1990;265(32):19517–19523. [PubMed] [Google Scholar]
- 36.Miyoshi T, Otsuka F, Nakamura E, Inagaki K, Ogura-Ochi K, Tsukamoto N, et al. Regulatory role of kit ligand-c-kit interaction and oocyte factors in steroidogenesis by rat granulosa cells. Mol Cell Endocrinol. 2012;358(1):18–26. doi: 10.1016/j.mce.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122(6):829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 38.Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16(6):1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- 39.Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999;13(6):1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 40.Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, et al. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol. 2004;276(1):64–73. doi: 10.1016/j.ydbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Gueripel X, Brun V, Gougeon A. Oocyte bone morphogenetic protein 15, but not growth differentiation factor 9, is increased during gonadotropin-induced follicular development in the immature mouse and is associated with cumulus oophorus expansion. Biol Reprod. 2006;75(6):836–843. doi: 10.1095/biolreprod.106.055574. [DOI] [PubMed] [Google Scholar]
- 42.Yoshino O, McMahon HE, Sharma S, Shimasaki S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci U S A. 2006;103(28):10678–10683. doi: 10.1073/pnas.0600507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore RK, Otsuka F, Shimasaki S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem. 2003;278(1):304–310. doi: 10.1074/jbc.M207362200. [DOI] [PubMed] [Google Scholar]
- 44.McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, et al. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction. 2005;129(4):481–487. doi: 10.1530/rep.1.00517. [DOI] [PubMed] [Google Scholar]
- 45.Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci U S A. 2002;99(12):8060–8065. doi: 10.1073/pnas.122066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang HM, Cheng JC, Taylor E, Leung PC. Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells. Mol Hum Reprod. 2014;20(5):373–383. doi: 10.1093/molehr/gau001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gittens JE, Barr KJ, Vanderhyden BC, Kidder GM. Interplay between paracrine signaling and gap junctional communication in ovarian follicles. J Cell Sci. 2005;118(Pt 1):113–122. doi: 10.1242/jcs.01587. [DOI] [PubMed] [Google Scholar]
- 49.Saito T, Hiroi M, Kato T. Development of glucose utilization studied in single oocytes and preimplantation embryos from mice. Biol Reprod. 1994;50(2):266–270. doi: 10.1095/biolreprod50.2.266. [DOI] [PubMed] [Google Scholar]
- 50.Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod. 1999;60(6):1446–1452. doi: 10.1095/biolreprod60.6.1446. [DOI] [PubMed] [Google Scholar]
- 51.Paczkowski M, Silva E, Schoolcraft WB, Krisher RL. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol Reprod. 2013;88(5):111. doi: 10.1095/biolreprod.113.108548. [DOI] [PubMed] [Google Scholar]
- 52.Cetica P, Pintos L, Dalvit G, Beconi M. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction. 2002;124(5):675–681. [PubMed] [Google Scholar]
- 53.Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139(4):685–695. doi: 10.1530/REP-09-0345. [DOI] [PubMed] [Google Scholar]
- 54.Downs SM, Humpherson PG, Leese HJ. Meiotic induction in cumulus cell-enclosed mouse oocytes: involvement of the pentose phosphate pathway. Biol Reprod. 1998;58(4):1084–1094. doi: 10.1095/biolreprod58.4.1084. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto S, Minami N, Yamada M, Imai H. Excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: relevance to intracellular reactive oxygen species and glutathione contents. Mol Reprod Dev. 2000;56(4):520–526. doi: 10.1002/1098-2795(200008)56:4<520::AID-MRD10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 56.Edwards RG. Follicular fluid. J Reprod Fertil. 1974;37(1):189–219. doi: 10.1530/jrf.0.0370189. [DOI] [PubMed] [Google Scholar]
- 57.Ambekar AS, Nirujogi RS, Srikanth SM, Chavan S, Kelkar DS, Hinduja I, et al. Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. J Proteome. 2013;87:68–77. doi: 10.1016/j.jprot.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 58.Von Wald T, Monisova Y, Hacker MR, Yoo SW, Penzias AS, Reindollar RR, et al. Age-related variations in follicular apolipoproteins may influence human oocyte maturation and fertility potential. Fertil Steril. 2010;93(7):2354–2361. doi: 10.1016/j.fertnstert.2008.12.129. [DOI] [PubMed] [Google Scholar]
- 59.Moreno JM, Núñez MJ, Quiñonero A, Martínez S, de la Orden M, Simón C, et al. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil Steril. 2015;104(4):1037–46.e1. doi: 10.1016/j.fertnstert.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Liu N, Ma Y, Li R, Jin H, Li M, Huang X, et al. Comparison of follicular fluid amphiregulin and EGF concentrations in patients undergoing IVF with different stimulation protocols. Endocrine. 2012;42(3):708–716. doi: 10.1007/s12020-012-9706-z. [DOI] [PubMed] [Google Scholar]
- 61.Chattopadhayay R, Ganesh A, Samanta J, Jana SK, Chakravarty BN, Chaudhury K. Effect of follicular fluid oxidative stress on meiotic spindle formation in infertile women with polycystic ovarian syndrome. Gynecol Obstet Investig. 2010;69(3):197–202. doi: 10.1159/000270900. [DOI] [PubMed] [Google Scholar]
- 62.Prieto L, Quesada JF, Cambero O, Pacheco A, Pellicer A, Codoceo R, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil Steril. 2012;98(1):126–130. doi: 10.1016/j.fertnstert.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 63.Gode F, Gulekli B, Dogan E, Korhan P, Dogan S, Bige O, et al. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil Steril. 2011;95(7):2274–2278. doi: 10.1016/j.fertnstert.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 64.Yang WJ, Liu FC, Hsieh JS, Chen CH, Hsiao SY, Lin CS. Matrix metalloproteinase 2 level in human follicular fluid is a reliable marker of human oocyte maturation in in vitro fertilization and intracytoplasmic sperm injection cycles. Reprod Biol Endocrinol. 2015;13:102. doi: 10.1186/s12958-015-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hennet ML, Combelles CM. The antral follicle: a microenvironment for oocyte differentiation. Int J Dev Biol. 2012;56(10–12):819–831. doi: 10.1387/ijdb.120133cc. [DOI] [PubMed] [Google Scholar]
- 66.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011;108(4):1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi WJ, Banerjee J, Falcone T, Bena J, Agarwal A, Sharma RK. Oxidative stress and tumor necrosis factor-alpha-induced alterations in metaphase II mouse oocyte spindle structure. Fertil Steril. 2007;88(4 Suppl):1220–1231. doi: 10.1016/j.fertnstert.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 68.Tarín JJ, Vendrell FJ, Ten J, Blanes R, van Blerkom J, Cano A. The oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytes. Mol Hum Reprod. 1996;2(12):895–901. doi: 10.1093/molehr/2.12.895. [DOI] [PubMed] [Google Scholar]
- 69.Hu Y, Betzendahl I, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Effects of low O2 and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Hum Reprod. 2001;16(4):737–748. doi: 10.1093/humrep/16.4.737. [DOI] [PubMed] [Google Scholar]
- 70.Navarro PA, Liu L, Keefe DL. In vivo effects of arsenite on meiosis, preimplantation development, and apoptosis in the mouse. Biol Reprod. 2004;70(4):980–985. doi: 10.1095/biolreprod.103.020586. [DOI] [PubMed] [Google Scholar]
- 71.Seibel MM, Smith D, Dlugi AM, Levesque L. Periovulatory follicular fluid hormone levels in spontaneous human cycles. J Clin Endocrinol Metab. 1989;68(6):1073–1077. doi: 10.1210/jcem-68-6-1073. [DOI] [PubMed] [Google Scholar]
- 72.von Wolff M, Kollmann Z, Flück CE, Stute P, Marti U, Weiss B, et al. Gonadotrophin stimulation for in vitro fertilization significantly alters the hormone milieu in follicular fluid: a comparative study between natural cycle IVF and conventional IVF. Hum Reprod. 2014;29(5):1049–1057. doi: 10.1093/humrep/deu044. [DOI] [PubMed] [Google Scholar]
- 73.Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88(2):142–148. doi: 10.1016/j.jri.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Relationships between concentrations of tumor necrosis factor-alpha and nitric oxide in follicular fluid and oocyte quality. J Assist Reprod Genet. 2000;17(4):222–228. doi: 10.1023/A:1009495913119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaafar TM, Hanna MO, Hammady MR, Amr HM, Osman OM, Nasef A, et al. Evaluation of cytokines in follicular fluid and their effect on fertilization and pregnancy outcome. Immunol Investig. 2014;43(6):572–584. doi: 10.3109/08820139.2014.901974. [DOI] [PubMed] [Google Scholar]
- 76.Altun T, Jindal S, Greenseid K, Shu J, Pal L. Low follicular fluid IL-6 levels in IVF patients are associated with increased likelihood of clinical pregnancy. J Assist Reprod Genet. 2011;28(3):245–251. doi: 10.1007/s10815-010-9502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hurwitz A, Ricciarelli E, Botero L, Rohan RM, Hernandez ER, Adashi EY. Endocrine- and autocrine-mediated regulation of rat ovarian (theca-interstitial) interleukin-1 beta gene expression: gonadotropin-dependent preovulatory acquisition. Endocrinology. 1991;129(6):3427–3429. doi: 10.1210/endo-129-6-3427. [DOI] [PubMed] [Google Scholar]
- 78.Wu YT, Tang L, Cai J, Lu XE, Xu J, Zhu XM, et al. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum Reprod. 2007;22(6):1526–1531. doi: 10.1093/humrep/dem029. [DOI] [PubMed] [Google Scholar]
- 79.Emonard H, Grimaud JA. Matrix metalloproteinases. A review. Cell Mol Biol. 1990;36(2):131–153. [PubMed] [Google Scholar]
- 80.Deady LD, Shen W, Mosure SA, Spradling AC, Sun J. Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila. PLoS Genet. 2015;11(2):e1004989. doi: 10.1371/journal.pgen.1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moreno-Moya JM, Vilella F, Simón C. MicroRNA: key gene expression regulators. Fertil Steril. 2014;101(6):1516–1523. doi: 10.1016/j.fertnstert.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 82.Takeo S, Kimura K, Shirasuna K, Kuwayama T, Iwata H. Age-associated deterioration in follicular fluid induces a decline in bovine oocyte quality. Reprod Fertil Dev. 2016; 10.1071/RD15228. [DOI] [PubMed]
- 83.da Silveira JC, de Andrade GM, Nogueira MF, Meirelles FV, Perecin F. Involvement of miRNAs and cell-secreted vesicles in mammalian ovarian antral follicle development. Reprod Sci. 2015;22(12):1474–1483. doi: 10.1177/1933719115574344. [DOI] [PubMed] [Google Scholar]
- 84.Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, et al. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil (Camb) 2014;17(2):90–98. doi: 10.3109/14647273.2014.897006. [DOI] [PubMed] [Google Scholar]
- 85.Wdowiak A. Comparing antioxidant enzyme levels in follicular fluid in ICSI-treated patients. Gynecol Obstet Fertil. 2015;43(7–8):515–521. doi: 10.1016/j.gyobfe.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Kedem A, Yung Y, Yerushalmi GM, Haas J, Maman E, Hanochi M, et al. Anti Müllerian hormone (AMH) level and expression in mural and cumulus cells in relation to age. J Ovarian Res. 2014;7:113. doi: 10.1186/s13048-014-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pacella L, Zander-Fox DL, Armstrong DT, Lane M. Women with reduced ovarian reserve or advanced maternal age have an altered follicular environment. Fertil Steril. 2012;98(4):986–94.e1-2. doi: 10.1016/j.fertnstert.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 88.Fujii J, Iuchi Y, Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol. 2005;3:43. doi: 10.1186/1477-7827-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006;86(3):503–512. doi: 10.1016/j.fertnstert.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 90.Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004;1034:300–315. doi: 10.1196/annals.1335.032. [DOI] [PubMed] [Google Scholar]
- 91.Karagouni EE, Chryssikopoulos A, Mantzavinos T, Kanakas N, Dotsika EN. Interleukin-1beta and interleukin-1alpha may affect the implantation rate of patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 1998;70(3):553–559. doi: 10.1016/s0015-0282(98)00243-x. [DOI] [PubMed] [Google Scholar]
- 92.Uri-Belapolsky S, Miller I, Shaish A, Levi M, Harats D, Ninio-Many L, et al. Interleukin 1-alpha deficiency increases the expression of follicle-stimulating hormone receptors in granulosa cells. Mol Reprod Dev. 2017;84(6):460–467. doi: 10.1002/mrd.22799. [DOI] [PubMed] [Google Scholar]
- 93.Vujisic S, Lepej SZ, Emedi I, Bauman R, Remenar A, Tiljak MK. Ovarian follicular concentration of IL-12, IL-15, IL-18 and p40 subunit of IL-12 and IL-23. Hum Reprod. 2006;21(10):2650–2655. doi: 10.1093/humrep/del217. [DOI] [PubMed] [Google Scholar]
- 94.Palini S, Benedetti S, Tagliamonte MC, De Stefani S, Primiterra M, Polli V, et al. Influence of ovarian stimulation for IVF/ICSI on the antioxidant defence system and relationship to outcome. Reprod BioMed Online. 2014;29(1):65–71. doi: 10.1016/j.rbmo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Bedaiwy MA, Elnashar SA, Goldberg JM, Sharma R, Mascha EJ, Arrigain S, et al. Effect of follicular fluid oxidative stress parameters on intracytoplasmic sperm injection outcome. Gynecol Endocrinol. 2012;28(1):51–55. doi: 10.3109/09513590.2011.579652. [DOI] [PubMed] [Google Scholar]
- 96.Siu MK, Cheng CY. The blood-follicle barrier (BFB) in disease and in ovarian function. Adv Exp Med Biol. 2012;763:186–192. doi: 10.1007/978-1-4614-4711-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tamanini C, De Ambrogi M. Angiogenesis in developing follicle and corpus luteum. Reprod Domest Anim. 2004;39(4):206–216. doi: 10.1111/j.1439-0531.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 98.Berisha B, Schams D, Kosmann M, Amselgruber W, Einspanier R. Expression and localisation of vascular endothelial growth factor and basic fibroblast growth factor during the final growth of bovine ovarian follicles. J Endocrinol. 2000;167(3):371–382. doi: 10.1677/joe.0.1670371. [DOI] [PubMed] [Google Scholar]
- 99.Murdoch WJ, Nix KJ, Dunn TG. Dynamics of ovarian blood supply to periovulatory follicles of the ewe. Biol Reprod. 1983;28(4):1001–1006. doi: 10.1095/biolreprod28.4.1001. [DOI] [PubMed] [Google Scholar]
- 100.Mauro A, Martelli A, Berardinelli P, Russo V, Bernabò N, Di Giacinto O, et al. Effect of antiprogesterone RU486 on VEGF expression and blood vessel remodeling on ovarian follicles before ovulation. PLoS One. 2014;9(4):e95910. doi: 10.1371/journal.pone.0095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25(1):72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 102.Akiyama I, Yoshino O, Osuga Y, Shi J, Harada M, Koga K, et al. Bone morphogenetic protein 7 increased vascular endothelial growth factor (VEGF)-a expression in human granulosa cells and VEGF receptor expression in endothelial cells. Reprod Sci. 2014;21(4):477–482. doi: 10.1177/1933719113503411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitsube K, Brannstrom M, Haraldsson B. Modulation of microvascular permeability in the preovulatory rat ovary by an ovulatory gonadotropin stimulus. Fertil Steril. 2013;99(3):903–909. doi: 10.1016/j.fertnstert.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 104.Pinto CR, Paccamonti DL, Eilts BE, Venugopal CS, Short CR, Gentry LR, et al. Concentrations of nitric oxide in equine preovulatory follicles before and after administration of human chorionic gonadotropin. Theriogenology. 2003;60(5):819–827. doi: 10.1016/s0093-691x(03)00096-7. [DOI] [PubMed] [Google Scholar]
- 105.Jablonka-Shariff A, Olson LM. The role of nitric oxide in oocyte meiotic maturation and ovulation: meiotic abnormalities of endothelial nitric oxide synthase knock-out mouse oocytes. Endocrinology. 1998;139(6):2944–2954. doi: 10.1210/endo.139.6.6054. [DOI] [PubMed] [Google Scholar]
- 106.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82(6):1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 107.McConnell NA, Yunus RS, Gross SA, Bost KL, Clemens MG, Hughes FM., Jr Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology. 2002;143(8):2905–2912. doi: 10.1210/endo.143.8.8953. [DOI] [PubMed] [Google Scholar]
- 108.Skowronski MT, Kwon TH, Nielsen S. Immunolocalization of aquaporin 1, 5, and 9 in the female pig reproductive system. J Histochem Cytochem. 2009;57(1):61–67. doi: 10.1369/jhc.2008.952499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pancarci SM, Ari U, Atakisi O, Güngör O, Ciğremiş Y, Bollwein H. Nitric oxide concentrations, estradiol-17β progesterone ratio in follicular fluid, and COC quality with respect to perifollicular blood flow in cows. Anim Reprod Sci. 2012;130(1–2):9–15. doi: 10.1016/j.anireprosci.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 110.Nargund G, Bourne T, Doyle P, Parsons J, Cheng W, Campbell S, et al. Associations between ultrasound indices of follicular blood flow, oocyte recovery and preimplantation embryo quality. Hum Reprod. 1996;11(1):109–113. doi: 10.1093/oxfordjournals.humrep.a019000. [DOI] [PubMed] [Google Scholar]
- 111.Nargund G, Doyle PE, Bourne TH, Parsons JH, Cheng WC, Campbell S, et al. Ultrasound derived indices of follicular blood flow before HCG administration and the prediction of oocyte recovery and preimplantation embryo quality. Hum Reprod. 1996;11(11):2512–2517. doi: 10.1093/oxfordjournals.humrep.a019150. [DOI] [PubMed] [Google Scholar]
- 112.Huey S, Abuhamad A, Barroso G, Hsu MI, Kolm P, Mayer J, et al. Perifollicular blood flow Doppler indices, but not follicular pO2, pCO2, or pH, predict oocyte developmental competence in in vitro fertilization. Fertil Steril. 1999;72(4):707–712. doi: 10.1016/s0015-0282(99)00327-1. [DOI] [PubMed] [Google Scholar]
- 113.Coulam CB, Goodman C, Rinehart JS. Colour Doppler indices of follicular blood flow as predictors of pregnancy after in-vitro fertilization and embryo transfer. Hum Reprod. 1999;14(8):1979–1982. doi: 10.1093/humrep/14.8.1979. [DOI] [PubMed] [Google Scholar]
- 114.Monteleone P, Giovanni Artini P, Simi G, Casarosa E, Cela V, Genazzani AR. Follicular fluid VEGF levels directly correlate with perifollicular blood flow in normoresponder patients undergoing IVF. J Assist Reprod Genet. 2008;25(5):183–186. doi: 10.1007/s10815-008-9218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vural F, Vural B, Doğer E, Çakıroğlu Y, Çekmen M. Perifollicular blood flow and its relationship with endometrial vascularity, follicular fluid EG-VEGF, IGF-1, and inhibin-a levels and IVF outcomes. J Assist Reprod Genet. 2016; 10.1007/s10815-016-0780-7. [DOI] [PMC free article] [PubMed]
- 116.Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev. 2002;61(3):414–424. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- 117.Shaeib F, Khan SN, Ali I, Thakur M, Saed MG, Dai J, et al. The defensive role of cumulus cells against reactive oxygen species insult in metaphase II mouse oocytes. Reprod Sci. 2016;23(4):498–507. doi: 10.1177/1933719115607993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lolicato F, Brouwers JF, de Lest CH, Wubbolts R, Aardema H, Priore P, et al. The cumulus cell layer protects the bovine maturing oocyte against fatty acid-induced lipotoxicity. Biol Reprod. 2015;92(1):16. doi: 10.1095/biolreprod.114.120634. [DOI] [PubMed] [Google Scholar]
- 119.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47(12):2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 120.Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS, et al. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril. 2011;95(6):1970–1974. doi: 10.1016/j.fertnstert.2011.01.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Price JC, Bromfield JJ, Sheldon IM. Pathogen-associated molecular patterns initiate inflammation and perturb the endocrine function of bovine granulosa cells from ovarian dominant follicles via TLR2 and TLR4 pathways. Endocrinology. 2013;154(9):3377–3386. doi: 10.1210/en.2013-1102. [DOI] [PubMed] [Google Scholar]
- 122.Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90(2):247–257. doi: 10.1016/j.fertnstert.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 123.Ibrahim LA, Kramer JM, Williams RS, Bromfield JJ. Human granulosa-luteal cells initiate an innate immune response to pathogen-associated molecules. Reproduction. 2016;152(4):261–270. doi: 10.1530/REP-15-0573. [DOI] [PubMed] [Google Scholar]
- 124.Carneiro LC, Cronin JG, Sheldon IM. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod Biol. 2016;16(1):1–7. doi: 10.1016/j.repbio.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 125.Neuer A, Lam KN, Tiller FW, Kiesel L, Witkin SS. Humoral immune response to membrane components of Chlamydia trachomatis and expression of human 60 kDa heat shock protein in follicular fluid of in-vitro fertilization patients. Hum Reprod. 1997;12(5):925–929. doi: 10.1093/humrep/12.5.925. [DOI] [PubMed] [Google Scholar]
- 126.Garrido N, Navarro J, Remohí J, Simón C, Pellicer A. Follicular hormonal environment and embryo quality in women with endometriosis. Hum Reprod Update. 2000;6(1):67–74. doi: 10.1093/humupd/6.1.67. [DOI] [PubMed] [Google Scholar]
- 127.Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohí J, Simón C. The follicular and endocrine environment in women with endometriosis: local and systemic cytokine production. Fertil Steril. 1998;70(3):425–431. doi: 10.1016/s0015-0282(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 128.Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86(2):27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod. 2000;63(3):805–810. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- 130.Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil Steril. 2016;105(3):548–559. doi: 10.1016/j.fertnstert.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 131.Broekmans FJ, Knauff EA, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18(2):58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 132.Zhang D, Zhang X, Zeng M, Yuan J, Liu M, Yin Y, et al. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J Assist Reprod Genet. 2015;32(7):1069–1078. doi: 10.1007/s10815-015-0483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril. 2015;103(2):317–322. doi: 10.1016/j.fertnstert.2014.12.115. [DOI] [PubMed] [Google Scholar]
- 134.Eichenlaub-Ritter U, Wieczorek M, Luke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11(5):783–796. doi: 10.1016/j.mito.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 135.Cheng EH, Chen SU, Lee TH, Pai YP, Huang LS, Huang CC, et al. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum Reprod. 2013;28(4):929–936. doi: 10.1093/humrep/det004. [DOI] [PubMed] [Google Scholar]
- 136.Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J Assist Reprod Genet. 2001;18(9):490–498. doi: 10.1023/A:1016649026353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McReynolds S, Dzieciatkowska M, McCallie BR, Mitchell SD, Stevens J, Hansen K, et al. Impact of maternal aging on the molecular signature of human cumulus cells. Fertil Steril. 2012;98(6):1574–1580. doi: 10.1016/j.fertnstert.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 138.Zhang GM, Gu CH, Zhang YL, Sun HY, Qian WP, Zhou ZR, et al. Age-associated changes in gene expression of goat oocytes. Theriogenology. 2013;80(4):328–336. doi: 10.1016/j.theriogenology.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 139.Lee MS, Liu CH, Lee TH, Wu HM, Huang CC, Huang LS, et al. Association of creatin kinase B and peroxiredoxin 2 expression with age and embryo quality in cumulus cells. J Assist Reprod Genet. 2010;27(11):629–639. doi: 10.1007/s10815-010-9459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Molinari E, Bar H, Pyle AM, Patrizio P. Transcriptome analysis of human cumulus cells reveals hypoxia as the main determinant of follicular senescence. Mol Hum Reprod. 2016;22(8):866–876. doi: 10.1093/molehr/gaw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod. 2016;31(7):1475–1482. doi: 10.1093/humrep/dew085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Medicine PCotASfR Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598. doi: 10.1016/j.fertnstert.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 143.Barcelos ID, Vieira RC, Ferreira EM, Martins WP, Ferriani RA, Navarro PA. Comparative analysis of the spindle and chromosome configurations of in vitro-matured oocytes from patients with endometriosis and from control subjects: a pilot study. Fertil Steril. 2009;92(5):1749–1752. doi: 10.1016/j.fertnstert.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 144.Da Broi MG, Malvezzi H, Paz CC, Ferriani RA, Navarro PA. Follicular fluid from infertile women with mild endometriosis may compromise the meiotic spindles of bovine metaphase II oocytes. Hum Reprod. 2014;29(2):315–323. doi: 10.1093/humrep/det378. [DOI] [PubMed] [Google Scholar]
- 145.Giorgi VS, Da Broi MG, Paz CC, Ferriani RA, Navarro PA. N-acetyl-cysteine and L-carnitine prevent meiotic oocyte damage induced by follicular fluid from infertile women with mild endometriosis. Reprod Sci. 2016;23(3):342–351. doi: 10.1177/1933719115602772. [DOI] [PubMed] [Google Scholar]
- 146.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 147.Carvalho LF, Samadder AN, Agarwal A, Fernandes LF, Abrão MS. Oxidative stress biomarkers in patients with endometriosis: systematic review. Arch Gynecol Obstet. 2012;286(4):1033–1040. doi: 10.1007/s00404-012-2439-7. [DOI] [PubMed] [Google Scholar]
- 148.Szczepańska M, Koźlik J, Skrzypczak J, Mikołajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril. 2003;79(6):1288–1293. doi: 10.1016/s0015-0282(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 149.Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab. 1998;83(6):2110–2113. [Google Scholar]
- 150.Donnez J, Binda MM, Donnez O, Dolmans MM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. 2016; 10.1016/j.fertnstert.2016.07.1075. [DOI] [PubMed]
- 151.Mansour G, Abdelrazik H, Sharma RK, Radwan E, Falcone T, Agarwal A. L-carnitine supplementation reduces oocyte cytoskeleton damage and embryo apoptosis induced by incubation in peritoneal fluid from patients with endometriosis. Fertil Steril. 2009;91(5 Suppl):2079–2086. doi: 10.1016/j.fertnstert.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 152.Andrade AZ, Rodrigues JK, Dib LA, Romão GS, Ferriani RA, Jordão Junior AA, et al. Serum markers of oxidative stress in infertile women with endometriosis. Rev Bras Ginecol Obstet. 2010;32(6):279–285. doi: 10.1590/s0100-72032010000600005. [DOI] [PubMed] [Google Scholar]
- 153.Singh AK, Dutta M, Chattopadhyay R, Chakravarty B, Chaudhury K. Intrafollicular interleukin-8, interleukin-12, and adrenomedullin are the promising prognostic markers of oocyte and embryo quality in women with endometriosis. J Assist Reprod Genet. 2016; 10.1007/s10815-016-0782-5. [DOI] [PMC free article] [PubMed]
- 154.Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod Toxicol. 2013;42:116–124. doi: 10.1016/j.reprotox.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 155.Molloy D, Martin M, Speirs A, Lopata A, Clarke G, McBain J, et al. Performance of patients with a “frozen pelvis” in an in vitro fertilization program. Fertil Steril. 1987;47(3):450–455. doi: 10.1016/s0015-0282(16)59054-2. [DOI] [PubMed] [Google Scholar]
- 156.Keay SD, Barlow R, Eley A, Masson GM, Anthony FW, Jenkins JM. The relation between immunoglobulin G antibodies to Chlamydia trachomatis and poor ovarian response to gonadotropin stimulation before in vitro fertilization. Fertil Steril. 1998;70(2):214–218. doi: 10.1016/s0015-0282(98)00145-9. [DOI] [PubMed] [Google Scholar]
- 157.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 158.Beckers NG, Macklon NS, Eijkemans MJ, Fauser BC. Women with regular menstrual cycles and a poor response to ovarian hyperstimulation for in vitro fertilization exhibit follicular phase characteristics suggestive of ovarian aging. Fertil Steril. 2002;78(2):291–297. doi: 10.1016/s0015-0282(02)03227-2. [DOI] [PubMed] [Google Scholar]
- 159.de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, Klip H, van Leeuwen FE, et al. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77(5):978–985. doi: 10.1016/s0015-0282(02)02972-2. [DOI] [PubMed] [Google Scholar]
- 160.Lawson R, El-Toukhy T, Kassab A, Taylor A, Braude P, Parsons J, et al. Poor response to ovulation induction is a stronger predictor of early menopause than elevated basal FSH: a life table analysis. Hum Reprod. 2003;18(3):527–533. doi: 10.1093/humrep/deg101. [DOI] [PubMed] [Google Scholar]
- 161.Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod. 2015;30(2):315–322. doi: 10.1093/humrep/deu319. [DOI] [PubMed] [Google Scholar]
- 162.Szmidt NA, Bhattacharya S, Maheshwari A. Does poor ovarian response to gonadotrophins predict early menopause? A retrospective cohort study with minimum of 10-year follow-up. Hum Fertil (Camb) 2016;19(3):212–219. doi: 10.1080/14647273.2016.1221149. [DOI] [PubMed] [Google Scholar]
- 163.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 164.Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, et al. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007;134(5):683–693. doi: 10.1530/REP-07-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bromfield JJ, Sheldon IM. Lipopolysaccharide initiates inflammation in bovine granulosa cells via the TLR4 pathway and perturbs oocyte meiotic progression in vitro. Endocrinology. 2011;152(12):5029–5040. doi: 10.1210/en.2011-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bromfield JJ, Sheldon IM. Lipopolysaccharide reduces the primordial follicle pool in the bovine ovarian cortex ex vivo and in the murine ovary in vivo. Biol Reprod. 2013;88(4):98. doi: 10.1095/biolreprod.112.106914. [DOI] [PubMed] [Google Scholar]
- 167.Bellver J, Busso C, Pellicer A, Remohí J, Simón C. Obesity and assisted reproductive technology outcomes. Reprod BioMed Online. 2006;12(5):562–568. doi: 10.1016/s1472-6483(10)61181-9. [DOI] [PubMed] [Google Scholar]
- 168.Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R, et al. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011;26(1):245–252. doi: 10.1093/humrep/deq306. [DOI] [PubMed] [Google Scholar]
- 169.Marquard KL, Stephens SM, Jungheim ES, Ratts VS, Odem RR, Lanzendorf S, et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2011;95(6):2146–9, 9.e1. doi: 10.1016/j.fertnstert.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Machtinger R, Combelles CM, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod. 2012;27(11):3198–3207. doi: 10.1093/humrep/des308. [DOI] [PubMed] [Google Scholar]
- 171.Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28(6):517–524. doi: 10.1007/s10815-011-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140(3):347–364. doi: 10.1530/REP-09-0568. [DOI] [PubMed] [Google Scholar]
- 173.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 174.Bützow TL, Lehtovirta M, Siegberg R, Hovatta O, Koistinen R, Seppäla M, et al. The decrease in luteinizing hormone secretion in response to weight reduction is inversely related to the severity of insulin resistance in overweight women. J Clin Endocrinol Metab. 2000;85(9):3271–3275. doi: 10.1210/jcem.85.9.6821. [DOI] [PubMed] [Google Scholar]
- 175.Phy JL, Conover CA, Abbott DH, Zschunke MA, Walker DL, Session DR, et al. Insulin and messenger ribonucleic acid expression of insulin receptor isoforms in ovarian follicles from nonhirsute ovulatory women and polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89(7):3561–3566. doi: 10.1210/jc.2003-031888. [DOI] [PubMed] [Google Scholar]
- 176.Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94(5):1533–1540. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- 177.Wang Q, Frolova AI, Purcell S, Adastra K, Schoeller E, Chi MM, et al. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One. 2010;5(12):e15901. doi: 10.1371/journal.pone.0015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ratchford AM, Esguerra CR, Moley KH. Decreased oocyte-granulosa cell gap junction communication and connexin expression in a type 1 diabetic mouse model. Mol Endocrinol. 2008;22(12):2643–2654. doi: 10.1210/me.2007-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Colton SA, Humpherson PG, Leese HJ, Downs SM. Physiological changes in oocyte-cumulus cell complexes from diabetic mice that potentially influence meiotic regulation. Biol Reprod. 2003;69(3):761–770. doi: 10.1095/biolreprod.102.013649. [DOI] [PubMed] [Google Scholar]
- 180.Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod. 2016;31(9):2090–2097. doi: 10.1093/humrep/dew181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dumesic DA, Abbott DH. Implications of polycystic ovary syndrome on oocyte development. Semin Reprod Med. 2008;26(1):53–61. doi: 10.1055/s-2007-992925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Franks S, Mason H, Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163(1–2):49–52. doi: 10.1016/s0303-7207(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 183.Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91(6):2327–2333. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- 184.Kwon H, Choi DH, Bae JH, Kim JH, Kim YS. mRNA expression pattern of insulin-like growth factor components of granulosa cells and cumulus cells in women with and without polycystic ovary syndrome according to oocyte maturity. Fertil Steril. 2010;94(6):2417–2420. doi: 10.1016/j.fertnstert.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 185.Wissing ML, Sonne SB, Westergaard D, Nguyen K, Belling K, Høst T, et al. The transcriptome of corona radiata cells from individual MІІ oocytes that after ICSI developed to embryos selected for transfer: PCOS women compared to healthy women. J Ovarian Res. 2014;7:110. doi: 10.1186/s13048-014-0110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang X, Liu C, Hao C, Tang Q, Liu R, Lin S, et al. Identification of altered microRNAs and mRNAs in the cumulus cells of PCOS patients: miRNA-509-3p promotes oestradiol secretion by targeting MAP3K8. Reproduction. 2016;151(6):643–655. doi: 10.1530/REP-16-0071. [DOI] [PubMed] [Google Scholar]
- 187.Liu S, Zhang X, Shi C, Lin J, Chen G, Wu B, et al. Altered microRNAs expression profiling in cumulus cells from patients with polycystic ovary syndrome. J Transl Med. 2015;13:238. doi: 10.1186/s12967-015-0605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pruksananonda K, Wasinarom A, Sereepapong W, Sirayapiwat P, Rattanatanyong P, Mutirangura A. Epigenetic modification of long interspersed elements-1 in cumulus cells of mature and immature oocytes from patients with polycystic ovary syndrome. Clin Exp Reprod Med. 2016;43(2):82–89. doi: 10.5653/cerm.2016.43.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cano F, García-Velasco JA, Millet A, Remohí J, Simón C, Pellicer A. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet. 1997;14(5):254–261. doi: 10.1007/BF02765826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang R, Yang S, Li R, Liu P, Qiao J, Zhang Y. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: a meta-analysis. Reprod Biol Endocrinol. 2016;14(1):67. doi: 10.1186/s12958-016-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Polzikov M, Yakovenko S, Voznesenskaya J, Troshina M, Zatsepina O. Overexpression of ribosomal RNA in cumulus cells of patients with polycystic ovary syndrome. J Assist Reprod Genet. 2012;29(10):1141–1145. doi: 10.1007/s10815-012-9827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]