Abstract
Purpose
To evaluate the effect of 12-month DHEA supplementation on menstrual pattern and ovarian reserve markers in women with premature ovarian insufficiency (POI)
Methods
This is a prospective observational study. Women with POI were given DHEA supplements (25 mg three times daily) for 12 months. Sonographic assessment for ovarian volume and antral follicle count (AFC) and serum measurement for anti-Mullerian hormone (AMH), follicle stimulating hormone (FSH), estradiol, testosterone, liver function, and hemoglobin level were performed at baseline and monthly for 13 months after the supplementation. Menstrual pattern, ovarian reserve markers, and side-effects were recorded.
Results
Between August 2011 and July 2014, 38 women with POI were recruited and 31 completed the study. The median age of women was 36 years, and the median baseline FSH and AMH concentrations were 82.2 IU/L and 0.01 ng/ml, respectively. No women had resumption of regular menstruation after DHEA supplementation. AMH, FSH, and AFC did not change significantly. No serious side effects were reported.
Conclusions
Our results do not support any significant improvement in ovarian function by 12-month DHEA supplementation in women with POI.
Keywords: Dehydroepiandrosterone, Premature ovarian insufficiency, Anti-Mullerian hormone, Follicle-stimulating hormone, Antral follicle count
Introduction
Premature ovarian insufficiency (POI) occurs in approximately 1% of the population [1]. Problems faced by women with POI include infertility and long-term risks of estrogen deficiency. Fertility treatment in these women has been one of the biggest challenges in the clinical management of these women. Only about 5–10% of women may become pregnant after being diagnosed with POI, and there is no effective treatment which may effectively enhance pregnancy rate in these women [2].
At birth, there are approximately 700,000 primordial follicles in the ovaries, and the number decreases to 300,000 by the onset of puberty. Most primordial follicles are kept dominant, and only a proportion will be activated and join the growing pool of follicles. It takes approximately 270 days for primordial follicles to reach the pre-antral stage, and this process is gonadotrophin independent [3, 4]. Under the influence of pituitary gonadotrophin, antral follicles will further mature, and one will become a dominant follicle and ovulate.
DHEA has been suggested to reduce follicular atresia [5] and to potentiate the effect of gonadotrophin on folliculogenesis by increasing follicular insulin-like growth factor-1 [6] and upregulating the FSH receptor expression in granulosa cells [7]. It is also an important prohormone for ovarian follicular steroidogenesis as it is an important substrate in the formation of testosterone and estradiol in peripheral tissues.
The beneficial effect of DHEA on ovarian stimulation in women with diminished ovarian reserve was first suggested by Casson et al. [7]. The case series of five patients with history of poor ovarian response showed that after 2 months of DHEA supplementation, there was increased responsiveness to gonadotrophin stimulation and peak estradiol concentration in all five patients and one had a successful twin pregnancy.
In women with POI, Mamas and Mamas [8, 9] showed that after 2 to 6 months of DHEA supplementation, serum follicle-stimulating hormone (FSH) levels dropped, and spontaneous pregnancy had been reported along with successful pregnancy following intrauterine insemination combined with mild ovarian stimulation. Our double-blind randomized trial on the effect of 16 weeks of DHEA supplementation on ovarian response markers in women with POI demonstrated a higher antral follicle count at 12 weeks and ovarian volume at 20 weeks in the DHEA treatment group [8].
At present, the published studies involved women who had taken 2 to 6 months of DHEA. Further trials using a longer duration of DHEA should be considered to evaluate the long-term effect of DHEA in women with POI based on the time needed for the primordial follicles to reach the antral stage. This study aims to evaluate the menstrual pattern and ovarian response markers as well as the side-effects of a 12-month course of DHEA supplementation in women with POI.
Methods
This was a prospective observational study carried out in the Department of Obstetrics and Gynecology, the University of Hong Kong, Queen Mary Hospital, Hong Kong. The study was approved by the Institutional Review Broad of the University of Hong Kong/Hospital Authority Hong Kong West Cluster and was registered under the University of Hong Kong Clinical Trials Registry (reference number: HKUCTR-1411).
Women aged under 40 years, who were diagnosed to have POI as defined by amenorrhoea or oligomenorrhoea together with serum FSH levels greater than 40 IU/L on two separate occasions 6 weeks apart, and who had normal karyotype (46XX) were recruited from August 2011 to July 2014. Some of these women had previously participated in the shorter course study on DHEA supplementation [10]. Women who had abnormal karyotype, screened positive for FMR1 gene mutation and diagnosis of autoimmune condition; screened positive for anti-thyroid antibodies and anti-adrenal antibodies, previous ovarian surgery, history of chemotherapy, or pelvic irradiation; and those who were on current androgen supplementation for other reasons were excluded. Written consent was obtained from all women prior to their participation in the study.
Baseline investigations carried out on the women included transvaginal pelvic ultrasound scan for antral follicle count (AFC) and ovarian volume, as well as blood tests for serum FSH, anti-Mullerian hormone (AMH), estradiol, testosterone, hemoglobin level, and liver function. Hormonal replacement therapy, if being taken by the women, was withheld for at least 2 months before starting DHEA.
Every participating woman received DHEA (GNC LiveWellTM) 25 mg three times a day for 12 months. They were assessed at recruitment, and monthly for 13 months, i.e., up till 1 month after stopping DHEA treatment. At each follow-up, AFC and ovarian volume were measured, and blood was collected for serum AMH, FSH, estradiol, testosterone, hemoglobin level, and liver function. Any return of menstruation and any adverse events after DHEA supplementation were documented.
The transvaginal ultrasound scans were performed using a 7-MHz transvaginal probe on the Voluson 730 system (GE Healthcare, Madison, WI, USA). Serum samples were stored at − 200C for hormonal assays in batches. Serum AMH, estradiol, and testosterone were measured using the Beckman-Coulter Access 2 Immunoassay system (Beckman-Coulter Inc., Fullerton, CA, USA). The AMH assay had stable intra- and inter-assay coefficients of variation 0.7–2.2 and 0.9–2.5%, respectively, and a sensitivity of 0.02 ng/ml.
Statistical analysis
Data analyses were performed using the SPSS Statistics software version 22 (IBM Corporation, New York, USA) and MedCalc Statistical Software version 17.8 (MedCalc Software bvba, Ostend, Belgium). For descriptive analysis, continuous data are presented as median (interquartile range). Non-normally distributed continuous data at different time points were compared using Friedman’s test.
According to data from previous study on women with newly diagnosed POI, the mean serum AMH level was 2.65 ± 1.8 pmol/l, i.e., 0.37 ± 0.25 ng/ml (mean ± SD). Assuming a 50% rise of AMH level in the DHEA group would be a clinically significant change; 22 women would be required to give a test of significance of 0.05 and a power of 0.9. Thirty-eight women were recruited in this study to allow for 40% of possible dropouts.
Results
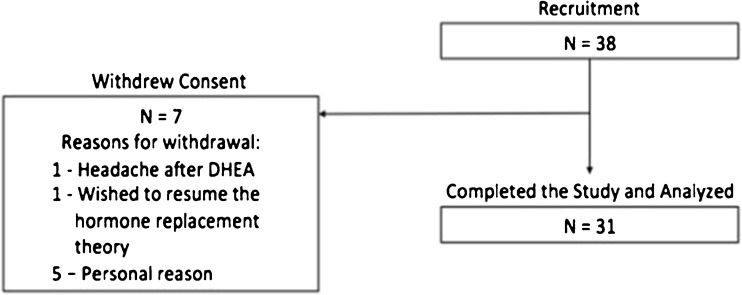
Between August 2011 and July 2014, 38 women were recruited and seven women withdrew from the study (Fig. 1). One woman withdrew after her second month assessment as she would like to resume the hormonal replacement therapy. One woman withdrew due to headache after taking DHEA supplement for 7 months, and the other five women withdrew their consents due to personal reasons. The remaining 31 women completed the 12 months of DHEA supplementation and were finally included for analysis. Five out of the 31 women had history of taking DHEA as they joined the previous DHEA study [10]. They have stopped DHEA for 37 to 280 days (mean, 165.2 days) before entering the present study.
Fig. 1.
Flow chart of the study
Baseline characteristics
Table 1 shows the baseline characteristics, ultrasound findings, and hormonal profile of ovarian response markers. The median age of the women was 36 years and the median duration of POI was 20 months. Out of the 31 women, 18 (58.1%) of them had used hormone replacement therapy (combined estrogen and progestogen) previously which had been stopped for at least 2 months; 24 (77.4%) of them were amenorrhoeic, while seven (22.6%) were oligomenorrhoeic.
Table 1.
Baseline anthropometric, sonographic, and hormonal characteristics of subjects (N = 31)
| Median (25–75 percentile) or N (%) | |
|---|---|
| Age at recruitment (years) | 36 (31–38) |
| BMI (kg/m2) | 22.2 (20.2–24.4) |
| Duration of POI (months) | 20 (2–180) |
| Previous hormone replacement therapy | |
| • Yes | 19 (61.3%) |
| • No | 12 (38.7%) |
| Duration of stopping hormone replacement therapy before the start of the study (days) | 168 (63–320) |
| Menstrual pattern | |
| • Oligomenorrhoea | 7 (22.6%) |
| • Amenorrhoea | 24 (77.4%) |
| FSH level at diagnosis (IU/L) | 82.2 (50.0–5.3) |
| Baseline FSH level before DHEA (IU/L) | 68.9 (57.4–108.9) |
| Total ovarian volume (cm3) | 1.1 (0.7–1.7) |
| Antral follicle count | 0 (0–0.5) |
| AMH (ng/ml) | 0.01 (0–0.01) |
| Serum estradiol (pmol/L) | 73 (42–179) |
The serum testosterone level was significantly higher during the 12 months of DHEA supplementation when compared with baseline testosterone level (p < 0.05), and it returned to baseline level 1 month after stopping the DHEA supplementation (Fig. 2). This is an indirect evidence for compliance of the women in taking the DHEA supplementation.
Fig. 2.
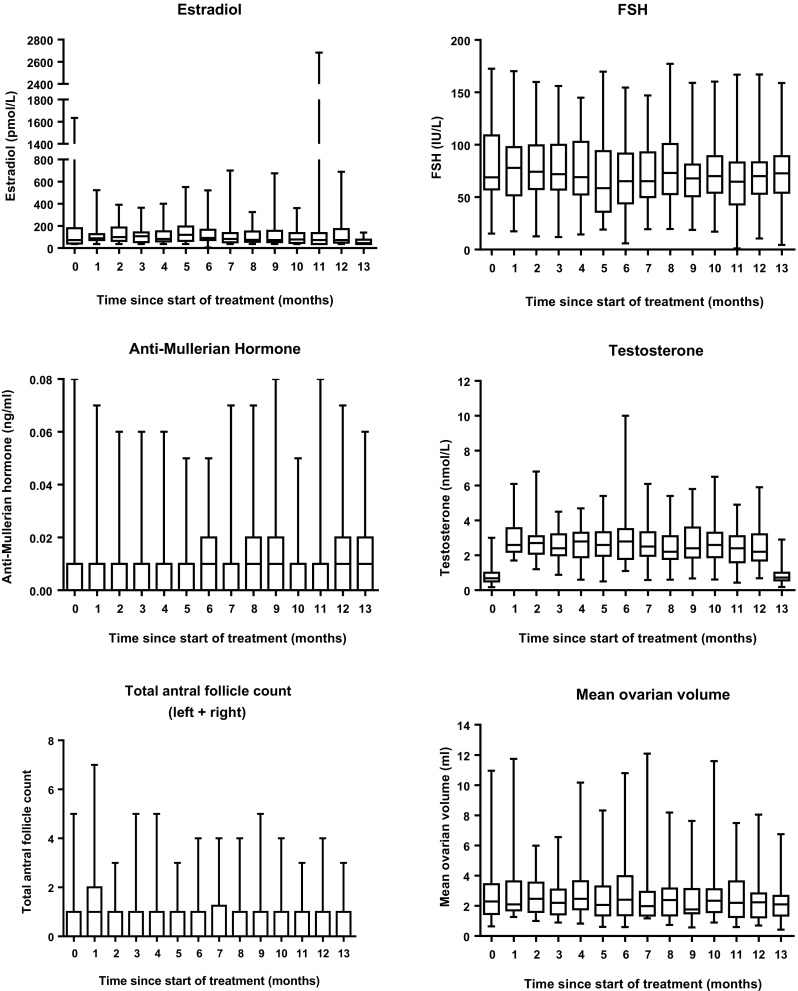
Box-and-whisker plots of serum hormone levels of subjects at monthly intervals up to 13 months of follow-up. The boxes indicate the 25th and 75th percentiles, with the middle lines representing the median. The whiskers span the range. The X-axis indicated the time after commencement of DHEA treatment
Menstrual pattern
Seven women were oligomenorrhoeic at baseline. Five of them continued to have irregular menstruation throughout the study period while the other two had amenorrhoea. Eleven out of the 24 women who were amenorrhoeic at baseline remained so. The other 13 women had irregular menstruation, and the mean number of period in 13-month period was 3.2 (range 1–8).
Ovarian response markers (Fig. 2)
Serum AMH level was undetectable in ten women before the start of DHEA supplementation and was detectable in very low levels in the remaining 20 women (up to 0.08 ng/ml).
AFC, ovarian volume, serum FSH, AMH, and estradiol levels did not change significantly throughout the entire 13-month study period.
Side effects
Five out of the 31 (16.1%) women complaint of acne during the treatment period. There were no significant changes in hemoglobin concentration and liver function of the women throughout the entire study period. There were two women with transient polycythemia (defined as hemoglobin level of more than 16 g/dL). One had hemoglobin concentration of 16.6 g/dL at the 10-month follow-up; one had hemoglobin concentration of 16.0 and 16.4 g/dL at the 11th and 12th month, respectively. Both remained asymptomatic, and the hemoglobin concentration normalized subsequently without any adverse clinical consequence. No subject had deranged liver function throughout the entire study period.
Discussion
There were only a few reports on the use of DHEA in women with POI. To the best of our knowledge, this is the first study reporting menstrual pattern, ovarian response markers, and side effects after 12 months of DHEA in women with POI. In the present study, we did not find any significant changes in AFC, ovarian volume, serum FSH, AMH, and estradiol levels after 12 months of DHEA supplementation in women with POI, nor any persistent change in menstrual pattern. There were no significant side effects, although the sample size is probably too small to detect any occurrence of rare side effects such as seizures associated with the use of DHEA [11].
DHEA is an important precursor of androgen and estrogen and declines with age. The effects of DHEA supplementation on menopausal symptoms, sexual function, bone health, and its effects on lipids and carbohydrates metabolism in post-menopausal women had been studied previously [12, 13]. The use of DHEA 50 mg daily for 52 weeks had not been found to have adverse effects on the endometrium [14].
Narkwichean et al. [15] had suggested that DHEA might influence in vivo ovine ovarian folliculogenesis by increasing the antral follicle population through increasing both the rate of primordial follicle initiation and the rate of preantral follicle development. POI is at the extreme end where the primordial follicular pool is more severely depleted. This is supported by the extremely low or undetectable serum level of AMH, which is a hormone exclusively produced by ovarian granulosa cells, in our subjects. Therefore, pharmacological manipulation like DHEA supplementation (an androgen precursor that might only help to stimulate early folliculogenesis but not to replenish the depleted follicular pool) is unlikely to restore ovarian activity in women with established POI whose ovarian reserve has been largely burnt-out.
Mamas and Mamas [8] reported a patient with POI having her menstruation returned and normalization of FSH and subsequent had a spontaneous conception after DHEA treatment. The same group [9] had further published a case series of five patients with POI who became pregnant after 6 to 27 weeks of DHEA treatment. Isolated sporadic ovulations were also documented in some women in the present study, and yet there was no persistent improvement in ovarian function based on antral follicle count and hormonal profile. Such intermittent spontaneous resumption of ovarian activity is largely due to the presence of residual follicles [16, 17], which occurs in some women with POI, yet this is unlikely to be sustained by DHEA supplementation.
The strength of our study was that the study population was homogenous and only included women with idiopathic POI, hence obviating the confounding effects which may otherwise associate with other iatrogenic causes of POI. The negative findings of DHEA on ovarian reserve markers in the present study, in contrast to the results of our previous study, may be due to the larger sample size. The median duration of POI was 20 months and was shorter than those in our previous trial [10].
One limitation of the present study is that five women were not naïve to DHEA supplementation but had stopped DHEA for a mean of more than 165 days. Given the half-life of DHEA in blood after oral supplementation is 15.1 to 35.2 h [18], the washout period in our studied population should be sufficed to reduce the effect of previous DHEA exposure on results of current study. The results of the present observational study seems to contradict our previous randomized trial with serum AMH level as the primary outcome [10], which found no significant change in serum AMH and FSH levels throughout the study period, although AFC and ovarian volume were significantly higher at weeks 12 and 20, respectively, in the DHEA group. We cautioned the interpretation of the results because AFC or ovarian volume was not the primary outcome and the sample size was very small. The present study serves as a pilot study for the sample size calculation in a subsequent randomized controlled trial if a significant difference in the primary outcome is found. The diagnosis of POI in the present study is different from that suggested by the 2015 European guidelines on management of POI [19], which used FSH higher than 25 IU/L on two occasions at least 4 weeks apart as the cutoff. This difference is unlikely to affect the results of the effects of DHEA on ovarian reserve markers based on studies on women with poor ovarian reserve [20, 21].
In conclusion, our results do not support any significant improvement in ovarian function by 12-month DHEA supplementation in women with POI.
References
- 1.Meskhi A, Seif MW. Premature ovarian failure. Curr Opio Obstet Gynecol. 2006;18:418–426. doi: 10.1097/01.gco.0000233937.36554.d3. [DOI] [PubMed] [Google Scholar]
- 2.van Kasterne YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–492. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 3.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1(2):81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 4.Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian Folliculogenesis. Results Probl Cell Differ. 2016;58:167–190. doi: 10.1007/978-3-319-31973-5_7. [DOI] [PubMed] [Google Scholar]
- 5.Barad D, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil Steril. 2005;84:756. doi: 10.1016/j.fertnstert.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15(10):2129–2132. doi: 10.1093/humrep/15.10.2129. [DOI] [PubMed] [Google Scholar]
- 8.Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertil Steril. 2009;91:644–646. doi: 10.1016/j.fertnstert.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 9.Mamas L, Mamas E. Dehydroepiandrosterone supplementation in assisted reproduction: rationale and results. Curr Opin Obstet Gynecol. 2009;21:306–308. doi: 10.1097/GCO.0b013e32832e0785. [DOI] [PubMed] [Google Scholar]
- 10.Yeung T, Li R, Lee V, Ho P, Ng E. A randomized double-blinded placebo-controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98:380–388. doi: 10.1210/jc.2012-3071. [DOI] [PubMed] [Google Scholar]
- 11.Karp G, Bentov Y, Masalha R, Ifergane G: Onset of posttraumatic seizure after dehydroepiandrosterone treatment. Fertil Steril. 2009, 91: e1–2. 931. [DOI] [PubMed]
- 12.Panjari N, Davis SR. DHEA for postmenopausal women: a review of the evidence. Maturitas. 2010;66:172–179. doi: 10.1016/j.maturitas.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for post-menopausal women. J Clin Endocrinol Metab. 2011;96:1642–1653. doi: 10.1210/jc.2010-2888. [DOI] [PubMed] [Google Scholar]
- 14.Panjari M, Bell RJ, Jane F, Adams J, Morrow C, Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas. 2009;63:240–245. doi: 10.1016/j.maturitas.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Narkwichean A, et al. Effects of dehydroepiandrosterone on in vivo ovine follicular development. Hum Reprod. 2013;29(1):146–154. doi: 10.1093/humrep/det408. [DOI] [PubMed] [Google Scholar]
- 16.Nelson LM, Anasti JN, Kimzey LM, et al. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79:1470–1475. doi: 10.1210/jcem.79.5.7962345. [DOI] [PubMed] [Google Scholar]
- 17.Kalantaridou SN, Nelson LM. Premature ovarian failure is not premature menopause. Ann N Y Acad Sci. 2000;900:393–302. doi: 10.1111/j.1749-6632.2000.tb06251.x. [DOI] [PubMed] [Google Scholar]
- 18.Legrain S, Massien C, Lahlou N, et al. Dehydroepiandrosterone replacement administration: pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab. 2000;85:3208–3217. doi: 10.1210/jcem.85.9.6805. [DOI] [PubMed] [Google Scholar]
- 19.Eshre Guideline Group on Premature ovarian insufficiency. Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 20.Yeung T, Chai J, Li RH, Lee VC, Ho PC, Ng EH. A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertil Steril. 2014;102(1):108–115. doi: 10.1016/j.fertnstert.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Qin JC, Fan L, Qin AP. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: evidence from a meta-analysis. J Gynecol Obstet Hum Reprod. 2017;46:1–7. doi: 10.1016/j.jgyn.2016.01.002. [DOI] [PubMed] [Google Scholar]