Abstract
Purpose
To study the role of individual semen parameters on the offspring birth weight and body mass index (BMI) from a population of men evaluated in an assisted reproduction technology (ART) clinic compared to fertile controls.
Methods
We performed a retrospective study using a cohort with fertile, age-matched controls of men evaluated with semen analysis at the University of Utah Andrology Clinic from 1996 to 2011 and Intermountain Healthcare from 2002 to 2011. We use the offspring from both our sub-fertile cohort and controls using the Utah Population Database. The two main outcomes of interest were offspring birth weight and adolescent BMI.
Results
The offspring of men with impaired sperm parameters had significantly lower birth weight compared to fertile control offspring. Low-concentration offspring weighed 158 g less (95% CI − 278~− 38; p = 0.01), low total count weighed 172 g less (95% CI − 294~− 51; p = 0.005), and low total motility weighed 155 g less (95% CI − 241~− 69; p < 0.001) compared to those of the controls. When we controlled for the use of ART within the sub-fertile group, we found that there was a significant trend of increasing birth weight across levels of total motile count and total sperm count compared to the azoospermic group. We did not find any consistent significant differences between the subject and control adolescence BMI based on semen parameters.
Conclusions
Despite limitations within our population-based dataset, we found that poor quality semen analysis parameters pointed towards an association with low birth weight in the offspring of sub-fertile men compared to the offspring of normal fertile controls. However, in contrast to studies of ART effects on offspring, we did not find evidence of long-term associations between semen quality and offspring BMI.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1154-0) contains supplementary material, which is available to authorized users.
Keywords: Male infertility, Epidemiology, Epigenetics, Semen parameters
Introduction
The prevalence of adult obesity in 2014 internationally was an estimated 13%, which has doubled since 1980 [1]. Multiple studies have noted a parallel decline in semen quality and increased use of assisted reproductive technology (ART) during this same period [2–6]. An estimated 3% of European and 1% of US babies born are conceived with ART [7]. Evidence for the association between obesity and male factor infertility is mounting, but little is known about the impact of semen quality on the birth weight and adolescent body mass index (BMI) (kg/m2) of their offspring. Therefore, we sought to investigate the role of individual semen parameters on the offspring birth weight and BMI from a population of men evaluated in an ART clinic compared to fertile controls.
Offspring health of children born with the use of ART is well studied and important for counseling couples considering ART. Previous studies have demonstrated that singletons conceived with ART have a lower birth weight compared to naturally conceived singletons, and then subsequently these children have a catch up period of growth into adolescence that is associated with increased cardiovascular risk factors [8, 9]. The most common proposed explanations for this are confounding parental characteristics, ART-related epigenetic changes, other technical aspects of ART, early childhood environment, or a combination of all of these factors. Sperm quality and germline inheritance may also play a role.
Our semen analysis data compiled by the Subfertility Health and Assisted Reproduction (SHARE) study at the University of Utah is linked to the Utah Population Database (UPDB), which has the ability to investigate long-term and multigenerational health outcomes. Our primary aim was to determine if there is an association between paternal semen parameters and the birth weight and adolescent BMI of their offspring.
Methods
Data
We used the data compiled by the SHARE study, which combines a unique longitudinal, population-wide data source, the UPDB, with biospecimen data to create a unique resource for the evaluation of transgenerational effects of infertility. The UPDB is a health data repository that collects and integrates data about residents in Utah, a state in the intermountain west with a population of 2.8 million people. It includes biodemographic, health, economic, cancer, and genetic data, by linking sources including Medicare databases, medical records from the two largest healthcare systems in the state, state driver licenses, birth, marriage, and death certificate data. In addition, the UPDB houses extensive pedigree data from the mid-nineteenth century. These linked data allow researchers to study health outcomes across multiple generations. Multiple epidemiologic studies have utilized the comprehensive pedigrees of the UPDB to identify and understand familial diseases [10–12]. This study was approved by the Institutional Review Boards of the University of Utah and Intermountain Healthcare by the Utah Resource for Genetic and Epidemiologic Research (www.research.utah.edu/rge/) #IRB_00069711.
Study design and population
This was a retrospective cohort analysis of the birth weight and adolescence BMI from offspring of men who underwent semen analysis and their matched controls from the UPDB. Semen analysis was performed at the University of Utah Andrology Clinic from 1996 to 2011 and Intermountain Healthcare from 2002 to 2011 as part of an infertility work-up. Together, these two tertiary medical centers’ andrology labs have captured approximately 90% of all semen analyses performed in Utah since 2004.
We started with 491 men with complete semen analysis and complete UPDB follow-up data for both parents and for their offspring. We excluded 11 men due to incomplete data and triplets. Our final sub-fertile cohort consisted of 480 men who underwent semen analysis and ultimately fathered 570 children that had complete UPDB and BMI data available for the men, their partners, and their children. We use the term “sub-fertile group” to denote the men who underwent evaluation and semen analysis as part of their infertility work-up. All of these men ultimately fathered a child and may have had semen parameters within the normal reference range.
We used UPDB birth certificate data to define fertile as having at least one naturally conceived child. Men seen at the IHC or UU clinics were excluded from the pool of potential controls. Controls were required to be residents of the state of Utah and have the same follow-up time as the study subjects. They were matched to men with SA by age and birth year with a matching ratio of 1:1.
Measures
The two main outcomes of interest were birth weight and adolescent BMI. Birth weight of the offspring was collected from UPDB-linked Utah birth certificate records and measured continuously in grams. Utah Driver’s License Division (DLD) records from 1989 to the present have been linked to the UPDB. Adolescent BMI was calculated using the self-reported height and weight from the offspring’s first DLD record between the ages of 15 and 19. Adolescent BMI was dichotomized as overweight/obese (≥ 85th percentile) vs. not overweight/obese for all analyses. This is based on CDC designated gender-age-specific percentile.
Several important confounders were also accounted for in these analyses. Child demographics included were sex, twin status, birth year of the child, and gestational age. Parental demographics included the highest educational level among the parents, maternal smoking status during pregnancy, maternal alcohol use during pregnancy, mother’s age at birth, father’s age at birth, and mother’s pre-pregnancy BMI. Birth year of the child, sex, gestational age, maternal smoking, and maternal alcohol consumption during pregnancy were recorded on the birth certificate records. Maternal pre-pregnancy BMI was calculated using the self-reported DLD height and weight from the DLD record nearest to the time of the child’s birth. BMI was classified as normal (< 25), overweight (25–29), or obese (≥ 30).
Semen analysis was performed according to WHO standards for both centers, and the parameters we included were as follows: sperm count (million), sperm concentration (millions per milliliter), total motile count (TMC), and vitality [13]. TMC and vitality data were only available from the University of Utah database. Semen analyses were performed and processed according to current WHO guidelines based on when analyses were performed for a given patient [14]. If a man had more than one semen analysis on record, we used the mean value for each semen parameter. Sperm concentration (M/ml) was categorized as azoospermia (0), oligozoospermia (< 15), normozoospermia (15–177), and hyperzoospermia that was based on the 90th percentile (≥ 178). Total sperm count was categorized as follows: azoospermia (0), oligozoospermia (< 39), normozoospermia (39–579), and hyperzoospermia that was based on the 90th percentile (> 579). Total motile count and vitality were all categorized based on empirically derived quartiles (azoospermia, Q1–Q4). When applicable, we combined azoospermia and oligozoospermia and referred to as low count or low concentration.
Statistical methods
Categorical variables were summarized as count (%) and continuous variables as mean and standard deviation (SD). Linear generalized estimating equation (GEE) models were used to relate offspring birth weight to each semen quality measure (analyzed individually) adjusting for correlation between children born of the same mother using an unstructured covariance matrix. Models were constructed in two steps: (1) using child demographics only and (2) using child and parent demographics. Coefficients, 95% confidence intervals (CIs), and p values were reported from these models. GEE logistic regression models predicting the odds of being overweight/obese were similarly constructed to analyze offspring adolescent BMI. Odds ratios (ORs), 95% CIs, and p values were reported from these models. Within the sub-fertile group, we performed linear trend analyses across ordered levels of the semen quality measures controlling for use of ART vs. natural birth. Statistical significance was assessed at the 0.05 level and all tests were two-tailed.
Results
Our final sub-fertile cohort consisted of 480 men who underwent semen analysis and ultimately fathered 570 children that had complete UPDB and BMI data available. A total of 119 (21%) of these children were conceived using ART. The 480 control men fathered 1170 naturally conceived children. Our cohort study had 15% multiple gestations compared to only 2% for children of controls. See Table 1 for further details regarding study cohort demographics.
Table 1.
General offspring and cohort demographics
| Subjects | Controls | |
|---|---|---|
| Fathers (N) | 480 | 480 |
| Children (N) | 570 | 1170 |
| Mode of conception | ||
| Control | – | 1170 (100%) |
| IUI | 66 (12%) | – |
| IVF | 53 (9%) | – |
| Natural | 451 (79%) | – |
| Sex (M) | ||
| 296 (52%) | 618 (53%) | |
| Twin | ||
| 84 (15%) | 23 (2%) | |
| Birth weight (GM) | ||
| < 1000 | 3 (1%) | 2 (0%) |
| 1000–1499 | 2 (0%) | 5 (0%) |
| 1500–2499 | 74 (13%) | 51 (4%) |
| ≥ 2500 | 491 (86%) | 1112 (95%) |
| Offspring adolescence BMI (age/gender specific) | ||
| Underweight | 27 (5%) | 47 (4%) |
| Normal | 456 (80%) | 891 (76%) |
| Overweight | 87 (15%) | 232 (20%) |
| Gestational age (weeks) | ||
| < 27 | 1 (0%) | 2 (0%) |
| 28–33 | 12 (2%) | 20 (2%) |
| 34–36 | 75 (13%) | 51 (4%) |
| ≥ 37 | 482 (85%) | 1078 (94%) |
The matching process was based on age and birth year at the time of semen analysis, but we found that the parents of our subjects were on average 5 years older than the controls at time of the child’s birth. There was little difference between subjects and controls in BMI of the parents at either time of birth or at time of offspring adolescence. See Table 2 for paternal and maternal demographics.
Table 2.
Paternal and maternal demographics
| Paternal factors | Subjects | Controls | Maternal factors | Subjects | Controls |
|---|---|---|---|---|---|
| Age at birth, mean (SD) | 32.8 (5.3) | 26.6 (5) | Age at birth, mean (SD) | 30.7 (4.8) | 25.3 (5.1) |
| BMI at time of offspring birth | |||||
| Normal | 191 (34%) | 359 (31%) | Normal | 442 (78%) | 807 (70%) |
| Overweight | 299 (53%) | 637 (55%) | Overweight | 97 (17%) | 258 (22%) |
| Obese | 79 (14%) | 163 (14%) | Obese | 29 (5%) | 89 (8%) |
| BMI at time of offspring adolescence | |||||
| Normal | 125 (22%) | 245 (21%) | Normal | 367 (65%) | 610 (53%) |
| Overweight | 324 (57%) | 638 (55%) | Overweight | 137 (24%) | 388 (34%) |
| Obese | 120 (21%) | 276 (24%) | Obese | 64 (11%) | 156 (14%) |
The four semen parameters we analyzed in our final models were concentration, total count, vitality, and total motile count. See supplemental Table 1 for semen parameter group definitions, categories, and the respective number of children born to sub-fertile men by each semen parameter.
Birth weight
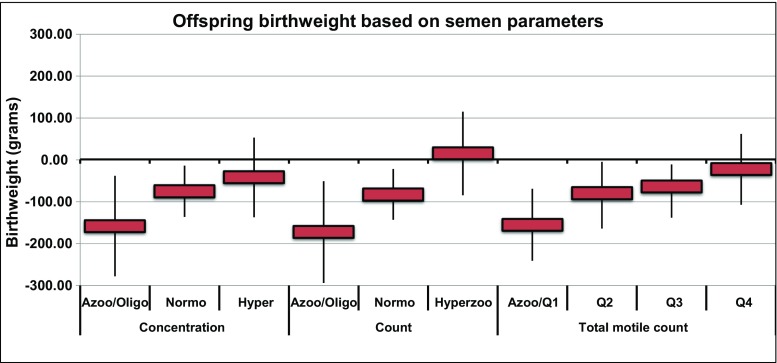
Overall there was a higher proportion of subjects’ offspring that were underweight (< 2500g) compared to controls, 14% vs 5%, respectively. Multivariable models controlling for all of the parental and offspring variables showed that the offspring of men with low sperm concentration had significantly lower birth weight at birth compared to fertile control offspring (− 158 g, 95% CI − 278~− 38; p = 0.01). The offspring of subjects with normal sperm concentration were also significantly underweight compared to fertile control offspring (− 75 g, 95% CI − 136~− 14; p = 0.016). The birth weight of the offspring of men with hyperzoospermic concentration was not significantly different than that of the controls (p = 0.38) (see Fig. 1).
Fig. 1.
Effect of sperm concentration, total count, and total motile count on offspring birth weight (multivariable model)
We observed a similar negative association for total sperm count, where the offspring of men with low and normal sperm counts were underweight compared to control offspring at − 172 g (95% CI − 294~− 51; p = 0.005) and − 83 g (95% CI − 143~− 22; p = 0.008), respectively. The birth weight of the offspring of men with hyperzoospermic count was not significantly different than that of the controls (p = 0.76) (see Fig. 1).
Again, for total motile count, we found that the offspring birth weight was negatively associated with the father’s total motile count. The offspring of men with low count were significantly underweight compared to control offspring (− 155, 95% CI − 241~− 69; p < 0.001). The second and third quartiles of total motile count followed a similar trend towards underweight offspring, but were not significant at − 80 g (95% CI − 164~5; p = 0.06) and − 64 g (95% CI − 138~11; p = 0.09), respectively (see Fig. 1).
We did not observe an association between the offspring’s birth weight and the father’s sperm vitality measures. For all vitality groups, the offspring were underweight compared to fertile control offspring, but only the third quartile reached statistical significance (− 94 g, 95% CI − 165~− 24; p = 0.009).
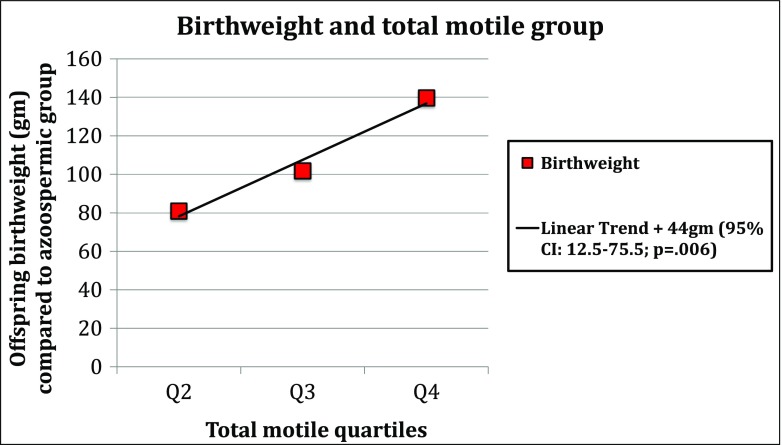
When we controlled for use of ART in the same models as above, within our sub-fertile group, we found that there was a significant trend of increasing birth weight across levels of total motile count and total sperm count compared to the azoospermic group. With each improved total motile count category, we saw a 44-g increased birth weight (95% CI 12.5–75.5; p = 0.006) (see Fig. 2). For each improved total sperm count category, we found a 103.7-g increased birth weight (95% CI 34.4–173; p = 0.003).
Fig. 2.
Linear trend analysis within sub-fertile population only. Azoospermic group was used as a reference (Q1). For each subsequent increase in total motile quartile, the birth weight of the offspring increases by 44 (12.5~75.5) g
Adolescence BMI
We performed the same multivariate generalized estimating equations, controlling for all of the parental and offspring variables, and we did not find any consistent trends of significant difference between the subject and control children adolescence BMI based on the semen parameters we investigated. The highest quartile of total sperm count and the highest quartile of vitality were associated with decreased risk of obesity (OR 0.37, 95% CI 0.15~0.92; p = 0.032; OR 0.5, 95% CI 0.27~0.96; p = 0.038), respectively. Otherwise, we did not find any consistent trends of significant difference between the subject and control children adolescence BMI based on the four semen parameters we investigated.
When we controlled for use of ART in the same models as above, within our sub-fertile group, we found no significant trend across levels of semen quality measures.
Discussion
To our knowledge, this is the first study to examine the relationship between semen parameters and offspring birth weight and BMI. Poor sperm concentration, total count, and total motile count were associated with a significantly lower birth weight compared to fertile control offspring. When controlling for ART in our subgroup analysis, we showed that higher birth weight had a linear association with both total motile count and total sperm count. This suggests that semen quality should be a consideration when studying the birth outcomes of children conceived with ART. These effects may be short-term, as we do not find evidence of a long-term association between semen quality and BMI.
Past studies revealed evidence of singletons and twins conceived through ART, namely in vitro fertilization (IVF), had a greater risk for adverse perinatal outcomes: preterm birth, low birth weight, and perinatal mortality compared to their controls [15–18]. Our study suggests that there may be other factors, such as semen quality, contributing to the short-term health of babies conceived with ART. We have identified two potential mechanisms that may be responsible for such an association and warrant investigation in future studies: epigenetic changes to the male spermatozoa and lack of seminal plasma associated with ART.
Emerging evidence supports the role of epigenetic remodeling of sperm in the infertile male. For instance, Jenkins et al. identified hypermethylated regions of human spermatozoa genes associated with poor motility, viability, and count [19]. These regions correlated with previously identified mammalian genes associated with infertility. In another methylation study, two groups of men with similar semen parameters, but that ultimately displayed differences in fecundity, showed methylation differences in coding regions of genes known to be expressed in sperm [20]. This same author has shown that when sperm is processed by a separation gradient, which results in two fractions of sperm based on quality, and found that the lowest sperm quality demonstrates increased methylation (compared to the higher quality) within the same individual as well as across 20 different men [21]. Klaver et al. performed a meta-analysis of two loci known for their association between infertility and abnormal DNA methylation, H19 and MEST, and found a 14.6- and 3.4-fold increased risk of aberrant DNA methylation in infertile men, respectively [22, 23].
A clear example of a paternal factor influencing offspring development is from a mouse model. Bromfield et al. surgically ligated mouse seminal vesicles and found that the lack of seminal plasma fluid impaired conception, caused placental hypertrophy, and significantly impacted the growth of the male offspring [24]. The authors hypothesize that the seminal fluid may impact the epigenome, the uterine environment, or potentially alter the sperm. While our work examined bulk semen parameters, as opposed to the impact of seminal fluid, it furthers the hypothesis that paternal factors can impact early and late embryogenesis, altering initial offspring phenotype, possibly through epigenetics.
We found that semen quality has an independent effect on offspring birth weight, but not on adolescent BMI. European studies have explored health outcomes beyond the prenatal period of IVF and intra-cytoplasmic sperm injection (ICSI) children. Results from one such study had shown higher peripheral skinfolds, body mass, and total body fat in IVF singletons compared to controls in 8- to 18-year-old singletons born from sub-fertile parents [25]. A more recent study showed pubertal ICSI-conceived girls also had increase peripheral and total adiposity assessed by BMI, as well as central adiposity [26]. Repeated studies have shown a high association of central body fat mass to increased risk for cardiovascular and metabolic health [27–29]. These studies are helpful in understanding how ART affects offspring BMI during pubertal stages, which may negatively impact health outcomes well into adulthood.
The impact of histone modification and RNA packaging on the paternal contribution to early development is another area of active research. The histone bound chromatin and DNA are transferred to the paternal pronucleus following fertilization where they then impact the developing embryo [30]. Furthermore, Hammoud et al. showed that retained histones in sperm often localized to regions involved in embryonic development [31]. When the embryonic development is affected, this can have lasting growth and health consequences for not only the first, but potentially for multiple future generations [32, 33].
In our study, we have identified an association with poor semen quality and offspring birth weight. There are known deleterious effects of obesity on semen quality and fertility, and obesity may also lead to changes in methylation profiles [34, 35]. For instance, Soubry et al. reported that pre-conception maternal and paternal obesity lead to statistically significant differential methylation profiles for multiple growth-related imprinted genes [36]. Donkin et al. showed that sperm DNA methylation and mRNA expression of non-coding regions were different between normal weight and obese males, and also demonstrated that obese males that underwent bariatric surgery significantly changed the sperm DNA methylation levels at loci responsible for appetite [37]. Thus, a clear strength of this study was our ability to control for both maternal and paternal BMI.
In our multivariate analysis, we controlled for maternal and paternal health and socioeconomic factors known to contribute to birth weight of offspring. However, there are several limitations to this study. We attempted to minimize the potential bias of unmeasured variables and the inherent problems of retrospective analysis by utilizing the UPDB’s ability to provide age-matched fertile controls. A limitation of this study is that we do not have SA data on our fertile controls. We have attempted to control this by only choosing controls that have fathered children naturally and no history of an infertility evaluation. Second, we do not have medical comorbidity indexes or smoking status for the men who underwent SA. Third, we were unable to control for subject location within the state, and environmental exposure is a potential confounder for this study or any population-based infertility study. Fourth, this study only involved Utah residents, and the state has less ethnic and racial diversity compared to other regions. Fifth, we do not know the proportion of female partners with diagnosed infertility. Unfortunately, our database does not allow us to control for specific offspring health, disease, or diagnoses. We matched the men with semen analysis to known fertile controls based on age at time of semen analysis. This was due to our original database construction, and both maternal and paternal age was controlled for on multivariable analysis. The use of ART and subtype of ART has been shown to impact birth weight, and we attempted to control for this in our sub-analysis [38]. Due to the constraints of our database only reporting either IUI vs. IVF vs. natural conception, we were unable to control for the different subtypes of ART (IUI, ICSI, IVF) during our analysis. We chose to dichotomize these variables as ART vs. natural conception. Lastly, our final cohort consisted of a small portion of the original group of men with semen analysis due to our rigorous inclusion criteria; this could introduce a potential selection bias.
We did not find an association between adolescent BMI and semen parameters, but this may have been limited by the relative young age of our cohort of offspring. In 15 years, 3500 more children in our cohort will be old enough to be included in a similar study examining BMI at the time of adolescence. This study has identified several potentially interesting areas of future research. We plan to use SHARE and the UPDB to identify unique cohorts of men with their corresponding biospecimens to further investigate the epigenetic changes in sperm and health outcomes of their offspring. Future studies will also investigate the association between hormone profiles of the men and their offspring’s growth.
Conclusion
Despite limitations within our population-based dataset, we found that poor quality semen analysis parameters pointed towards an association with low birth weight in the offspring of sub-fertile men compared to the offspring of normal fertile controls. However, in contrast to studies of ART effects on offspring, we did not find evidence of long-term associations between semen quality and BMI.
Electronic supplementary material
(DOCX 63.6 kb)
Funding information
This investigation was supported in part by from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-02.
Compliance with ethical standards
This study was approved by the Institutional Review Boards of the University of Utah and Intermountain Health Care by the Utah Resource for Genetic and Epidemiologic Research (www.research.utah.edu/rge/) #IRB_00069711.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1154-0) contains supplementary material, which is available to authorized users.
References
- 1.Organization WH. Global status report on noncommunicable diseases 2014. 2014.
- 2.Temporal and geospatial trends in male factor infertility with assisted reproductive technology in the United States from 1999–2010., (2014). [DOI] [PubMed]
- 3.Assisted reproductive technology surveillance—United States, 2011., (2014). [PubMed]
- 4.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jørgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2(4):e000990. doi: 10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect. 2000;108(10):961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ESHRE. ESHRE ART fact sheet. 2016. https://www.eshre.eu/Home/ESHRE-Annual-Report-2016.aspx. Accessed 10/31 2016.
- 8.Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, Henriet S, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413–419. doi: 10.1093/humrep/deh592. [DOI] [PubMed] [Google Scholar]
- 9.Ceelen M, van Weissenbruch MM, Vermeiden JPW, van Leeuwen FE, Delemarre-van de Waal HA. Growth and development of children born after in vitro fertilization. Fertil Steril 2008;90(5):1662–1673. [DOI] [PubMed]
- 10.Gibson SB, Figueroa KP, Bromberg MB, Pulst S-M, Cannon-Albright L. Familial clustering of ALS in a population-based resource. Neurology. 2014;82(1):17–22. doi: 10.1212/01.wnl.0000438219.39061.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuohy TMF, Rowe KG, Mineau GP, Pimentel R, Burt RW, Samadder NJ. Risk of colorectal cancer and adenomas in the families of patients with adenomas: a population-based study in Utah. Cancer. 2014;120(1):35–42. doi: 10.1002/cncr.28227. [DOI] [PubMed] [Google Scholar]
- 12.Hanson HA, Anderson RE, Aston KI, Carrell DT, Smith KR, Hotaling JM. Subfertility increases risk of testicular cancer: evidence from population-based semen samples. Fertil Steril. 2016;105(2):322–328. doi: 10.1016/j.fertnstert.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 2010. http://whqlibdoc.who.int/publications/2010/9789241547789_eng.pdf.
- 14.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 15.Hansen M, Colvin L, Petterson B, Kurinczuk JJ, de Klerk N, Bower C. Twins born following assisted reproductive technology: perinatal outcome and admission to hospital. Hum Reprod. 2009;24(9):2321–2331. doi: 10.1093/humrep/dep173. [DOI] [PubMed] [Google Scholar]
- 16.Ceelen M, van Weissenbruch MM, Roos JC, Vermeiden JPW, van Leeuwen FE, Delemarre-van de Waal HA. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab 2007;92(9):3417–3423. [DOI] [PubMed]
- 17.Halliday J, Wilson C, Hammarberg K, Doyle LW, Bruinsma F, McLachlan R, et al. Comparing indicators of health and development of singleton young adults conceived with and without assisted reproductive technology. Fertil Steril. 2014;101(4):1055–1063. doi: 10.1016/j.fertnstert.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig AK, Sutcliffe AG, Diedrich K, Ludwig M. Post-neonatal health and development of children born after assisted reproduction: a systematic review of controlled studies. Eur J Obstet Gynecol Reprod Biol. 2006;127(1):3–25. doi: 10.1016/j.ejogrb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins TG, Aston KI, Hotaling JM, Shamsi MB, Simon L, Carrell DT. Teratozoospermia and asthenozoospermia are associated with specific epigenetic signatures. Andrology. 2016;4(5):843–849. doi: 10.1111/andr.12231. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins TG, Aston KI, Meyer TD, Hotaling JM, Shamsi MB, Johnstone EB, et al. Decreased fecundity and sperm DNA methylation patterns. Fertil Steril. 2016;105(1):51–57. doi: 10.1016/j.fertnstert.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins TG, Aston KI, Trost C, Farley J, Hotaling JM, Carrell DT. Intra-sample heterogeneity of sperm DNA methylation. Mol Hum Reprod. 2015;21(4):313–319. doi: 10.1093/molehr/gau115. [DOI] [PubMed] [Google Scholar]
- 22.Klaver R, Gromoll J. Bringing epigenetics into the diagnostics of the andrology laboratory: challenges and perspectives. Asian Journal of Andrology. 2014;16(5):669–674. doi: 10.4103/1008-682X.125412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krausz C, Sandoval J, Sayols S, Chianese C, Giachini C, Heyn H, et al. Novel insights into DNA methylation features in spermatozoa: stability and peculiarities. PLoS One. 2012;7(10):e44479. doi: 10.1371/journal.pone.0044479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci U S A. 2014;111(6):2200–2205. doi: 10.1073/pnas.1305609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab 2008;93(5):1682–1688. doi:10.1210/jc.2007-2432. [DOI] [PubMed]
- 26.Belva F, Painter R, Bonduelle M, Roelants M, Devroey P, De Schepper J. Are ICSI adolescents at risk for increased adiposity? 2011;27(1):257–64. doi: 10.1093/humrep/der375. [DOI] [PubMed]
- 27.Hart R, Norman RJ. The longer-term health outcomes for children born as a result of IVF treatment: part I—general health outcomes. Hum Reprod Update. 2013;19(3):232–243. doi: 10.1093/humupd/dms062. [DOI] [PubMed] [Google Scholar]
- 28.Sakka SD, Loutradis D, Kanaka-Gantenbein C, Margeli A, Papastamataki M, Papassotiriou I, et al. Absence of insulin resistance and low-grade inflammation despite early metabolic syndrome manifestations in children born after in vitro fertilization. Fertil Steril. 2010;94(5):1693–1699. doi: 10.1016/j.fertnstert.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 29.Ceelen M, van Weissenbruch MM, Prein J, Smit JJ, Vermeiden JPW, Spreeuwenberg M, et al. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8-18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod. 2009;24(11):2788–2795. doi: 10.1093/humrep/dep273. [DOI] [PubMed] [Google Scholar]
- 30.Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. 2010;16(1):30–36. doi: 10.1093/molehr/gap080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460(7254):473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker DJP. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108(Suppl 3):545–553. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29(2):193–200. doi: 10.1093/humrep/det428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammiche F, Laven JS, Twigt JM, Boellaard WP, Steegers EA, Steegers-Theunissen RP. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum Reprod. 2012;27(8):2365–2372. doi: 10.1093/humrep/des177. [DOI] [PubMed] [Google Scholar]
- 36.Soubry A, Murphy SK, Wang F, Huang Z, Vidal AC, Fuemmeler BF, et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int J Obes. 2015;39(4):650–657. doi: 10.1038/ijo.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donkin I, Versteyhe S, Ingerslev Lars R, Qian K, Mechta M, Nordkap L, et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23(2):369–378. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod. 2014;29(12):2794–2801. doi: 10.1093/humrep/deu246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 63.6 kb)