Abstract
Effect of microparticles and silver nanoparticles was studied on the production of hydrolytic enzymes by a potent phytase-producing mould, Aspergillus oryzae SBS50. Addition of microparticles, viz. talc powder and aluminum oxide enhanced phytase production from 2894 to 3903 and 2847 to 4204 U/L, cellulase from 2529 to 4931 and 2455 to 3444 U/L, xylanase from 9067 to 9642 and 9994 to 14,783 U/L, amylase from 5880 to 11,000 and 6130 to 13,145 U/L, respectively. Fungal morphology was also engineered by the use of microparticles. Fungal pellet size was significantly reduced (~ 90%) by the addition of microparticles. Fermentation time was reduced from 4 to 3 days after the addition of microparticles, thus increasing the productivity of the enzymes significantly. These results confirmed the importance of microparticles in engineering fungal morphology for enhanced production of hydrolytic enzymes.
Keyword: Aspergillus oryzae SBS50, Hydrolytic enzymes, Microparticles, Fungal morphology, Engineering
Introduction
Filamentous fungi have been used for the production of hydrolytic enzymes in submerged (Singh and Satyanarayana 2006; Sapna and Singh 2013) and solid-state fermentations (Singh and Satyanarayana 2008; Sapna and Singh 2014; Kumari et al. 2016). Filamentous fungi are known to secrete a large number of hydrolytic enzymes beside phytases, resulting in decomposition of organic matter (Sapna and Singh 2014). This in turn will help in the management of environmental pollution and recycling of nutrients in the ecosystem. Optimization of fermentation conditions have resulted in improved production of hydrolytic enzymes (Singh and Satyanarayana 2006, 2008; Sapna and Singh 2013, 2014; Kumari et al. 2016). Cultivation of filamentous fungi is commonly accompanied by several problems, like clumpy growth and insufficient mass transfer, which are responsible for lower enzyme yields. Microparticle addition is a novel approach for enhanced production of enzymes by filamentous fungi in submerged culture (Coban et al. 2015a, b; Driouch et al. 2012). Microparticles also control morphology of filamentous fungi during fermentation by preventing bulk fungal growth (Coban et al. 2015a, b; Driouch et al. 2012). Microparticles such as talc powder (magnesium silicate), aluminum oxide, and titanium oxide have been used for enhancing the production of enzymes by filamentous fungi. Driouch et al. (2012) reported enhanced production of fructofuranosidase and glucoamylase by A. niger AB1.13 and A. niger SKAn1015, respectively, in the presence TiSiO4 with concomitant reduction in mycelial pellet diameter. Driouch et al. (2011) also reported that fructofuranosidase production by A. niger was enhanced by 3.5-fold in the presence of microparticles of either 10 g/L of talcum or 20 g/L aluminum oxide in the fermentation medium compared to the control. Coban et al. (2015a, b) reported improved production of phytase by A. niger after addition of microparticles in batch and continuous fermentations. Antecka et al. (2016) reported the enhanced production of laccases by two basidiomycetous fungi, viz. Cerrena unicolor Murr. strain 137 and Pleurotus sapidus DSM 8266 by the addition of microparticles in the medium. β-Mannanase production by A. sojae AsT1 was increased in submerged cultures after the addition of microparticles (Yatmaz et al. 2016). This is the first report studying the effect of microparticles (talc powder, aluminum oxide and Sephadex G-50) and silver nanoparticles on production of hydrolytic enzymes by Aspergillus oryzae SBS50. In this present investigation, effect of microparticles on production of different hydrolytic enzymes (phytase, cellulase, xylanase and amylase) by a phytase-producing mould, A. oryzae SBS50, was studied.
Materials and methods
Microorganism, chemicals and culture conditions
Aspergillus oryzae SBS50 was selected as a potent phytase-producing fungus that secreted various hydrolytic enzymes beside phytase (Sapna and Singh 2014, 2017). The fungus was grown on potato dextrose agar (PDA) slants at 30 °C for 72 h and stored at 4 °C on slants. The culture was routinely cultured on fresh agar slants. Sephadex G-50 (Product #G5080) and silver nanoparticles (Product #730807) were purchased from M/s. Sigma-Aldrich, USA.
Inoculum preparation
Spore suspension of the fungus was prepared from 72-h-old PDA slants by adding 25 mL of normal saline containing Tween 80 (0.1% v/v) followed by shaking and filtration. Spore suspension showing viable spore count of 4 × 107 (CFU/mL) was used for the inoculation of 50 mL medium during submerged fermentation (Sapna and Singh 2013, 2015).
Submerged fermentation
Erlenmeyer flasks (250 mL) containing 50 mL of optimized production medium (Sapna and Singh 2013) were supplemented with 0, 10, 15, 20, and 25 g/L of aluminum oxide (Al2O3) or talc powder. The optimized medium contained (g/L) starch 15, beef extract 8, FeSO4 0.1 MgSO4·7H2O 0.5, KCl 0.1. The pH of the medium was adjusted to 5.0 and flasks were autoclaved at 121 °C, 15 psi for 15 min. Thereafter, flasks were inoculated with spore suspension and incubated at 30 °C and 200 rpm for 96 h. Aliquot samples were collected from each flask and analyzed for phytase activity. Effect of incubation period on the production of hydrolytic enzymes was studied using aluminum oxide (1%) and talc powder (1.5%). A set of flasks was harvested after 24 h and analyzed for the determination of enzyme activities.
Effect of different concentrations of Sephadex G-50 (0.75–3.0%) and silver nanoparticles (0.01–0.1%) was also evaluated on the fungal morphology and the yields of hydrolytic enzymes.
Microscopy of fungal pellets
Fungal pellets collected from the shake flasks with various microparticle concentrations were rinsed with normal saline to remove excessive microparticles and medium components. Thereafter, selected average sizes of pellets from each sample were visually analyzed under inverted microscope (Nikon-TS100) with attached image analysis software. Three samples were analyzed for each treatment and their average values have been used.
Analytical procedure and enzyme assays
Fermented medium was filtered using Whatmann filter paper discs followed by centrifugation at 10,000 rpm for 10 min, and the cell-free supernatant was used as the source of enzymes.
Phytase was assayed by determining the amount of inorganic phosphorus released using calcium phytate (Sapna and Singh 2013, 2014, 2015, 2017). The amount of free phosphate released was determined according to Fiske and Subbarow (1925). One unit of phytase is the amount of enzyme required to liberate one µmole of inorganic phosphate per min under the assay conditions using KH2PO4 as the standard.
Amylase, cellulase (CMCase) and xylanase present in the enzyme extracts were assayed at pH 5.0 and 50 °C using their respective substrates (0.5%) as described earlier (Sapna and Singh 2014). The liberated reducing sugars were determined using dinitrosalicylic acid method (Miller 1959). One unit of each enzyme is defined as the amount of enzyme that liberates 1 µmol of product per ml per min under the assay conditions.
All experiments were carried out in triplicates and their mean values are presented.
Results
Effect of different microparticles on production of hydrolytic enzymes by A. oryzae SBS50 in submerged cultivation
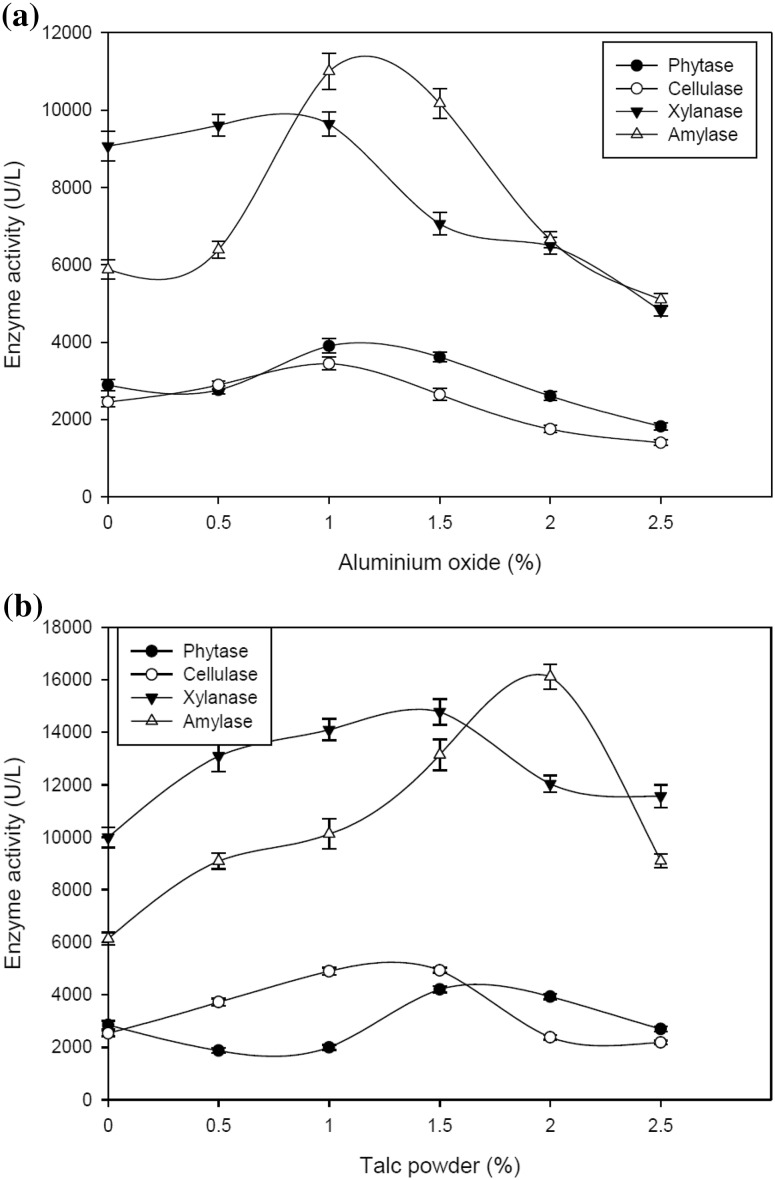
Addition of aluminum oxide increased the production of all the hydrolytic enzymes up to a level of 1.0% followed by decline afterwards (Fig. 1a). An overall 35, 40, 6 and 87% increase in, phytase, cellulase, xylanase and amylase production was attained after the addition of aluminum oxide in the medium, respectively. Addition of talc powder also increased the production of all the hydrolytic enzymes up to a level of 1.5% followed reduction at higher concentrations (Fig. 1b). An overall 48, 95, 48 and 163% increase in phytase, cellulase, xylanase and amylase production was attained after the addition of talc powder in the medium, respectively. These results suggested that talc powder supported higher production of all the hydrolases as compared to aluminum oxide.
Fig. 1.
a Effect of different concentrations of aluminum oxide on the production of hydrolytic enzymes by A. oryzae SBS50 in submerged culture after 4 days. b Effect of different concentrations of talc powder on the production of hydrolytic enzymes by A. oryzae SBS50 in submerged culture after 4 days
Effect of incubation time on the production of hydrolytic enzymes
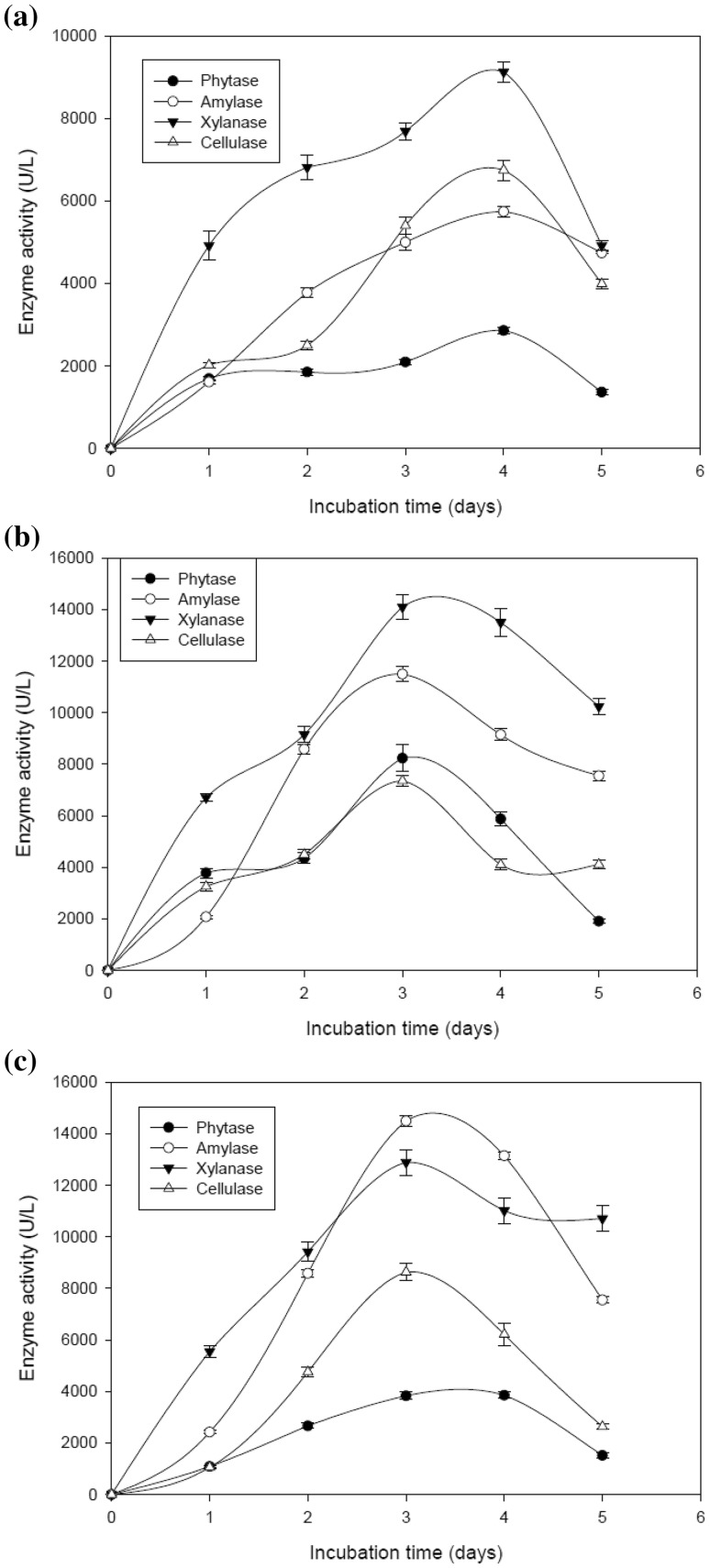
Addition of microparticles (talc powder and aluminum oxide) supported higher production of hydrolytic enzymes as compared to control. Therefore, the effect of incubation time was assessed on the production of hydrolytic enzymes in control and microparticle-supplemented medium. Maximum production of all the enzymes was recorded after 4 days of fermentation followed by decline afterwards in the control medium (Fig. 2a). But addition of microparticle in the medium resulted in the production of all the hydrolytic enzymes after 3 days of incubation (Fig. 2b, c). This in turn increased the productivity of all enzymes as compared to control (Table 1).
Fig. 2.
a Effect of incubation time on the production of hydrolytic enzymes by A. oryzae SBS50 in submerged culture without the addition of microparticles. b Effect of incubation time on the production of hydrolytic enzymes by A. oryzae SBS50 in submerged cultures supplemented with aluminum oxide (1.0%). c Effect of incubation time on the production of hydrolytic enzymes by A. oryzae SBS50 in submerged cultures supplemented with talc powder (1.5%)
Table 1.
Productivity of different hydrolytic enzymes after the addition of microparticles
| Enzyme | Enzyme productivity (U/L/h) | Fold improvement | |||
|---|---|---|---|---|---|
| Control | Aluminum oxide | Talc powder | Aluminum oxide | Talc powder | |
| Phytase | 29.74 | 114.29 | 53.18 | 3.84 | 1.79 |
| Xylanase | 95.07 | 195.83 | 179.01 | 2.06 | 1.88 |
| Cellulase | 70.27 | 102.11 | 119.79 | 1.45 | 1.70 |
| Amylase | 59.78 | 159.63 | 201.29 | 2.67 | 3.37 |
Effect of microparticles on fungal morphology in submerged fermentation
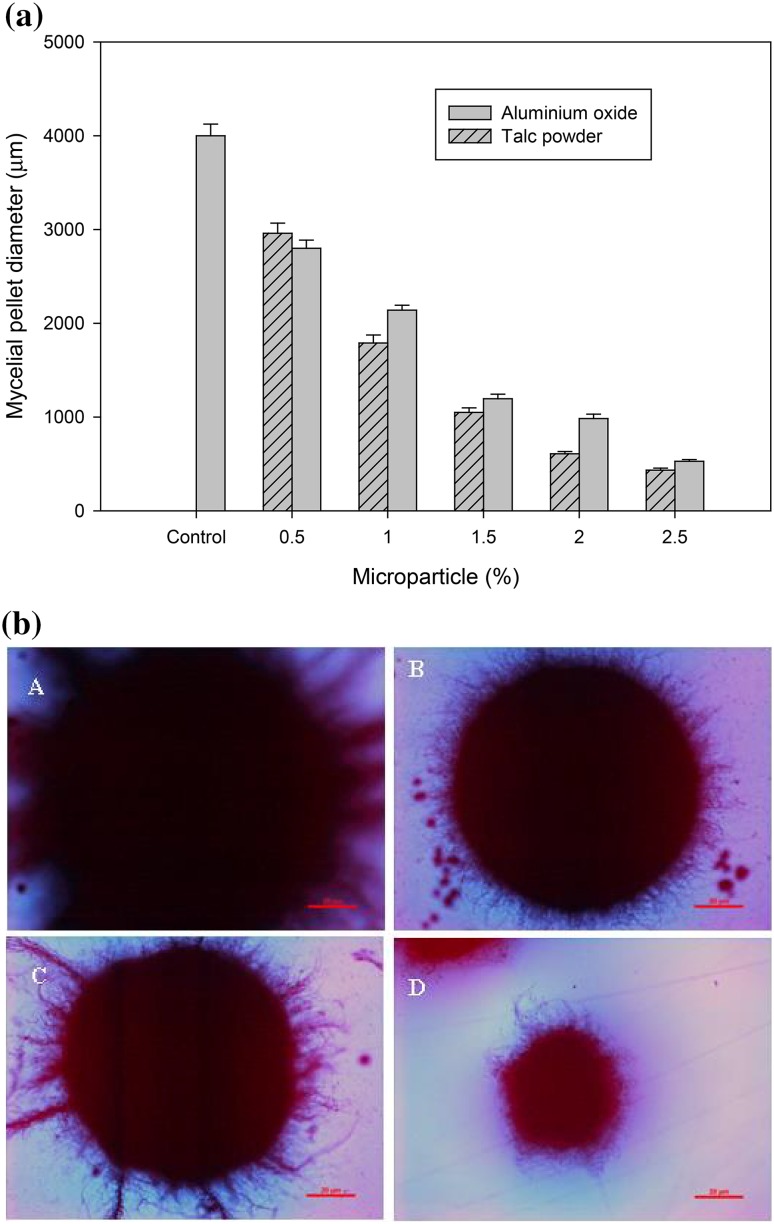
Fungal pellet size was reduced significantly after the addition of microparticles in the production medium (Fig. 3a, b). The average fungal pellet diameter was measured as 4000 ± 124 µm in control fermentations. Size of the fungal pellet was significantly reduced after the addition of 1.5% of microparticles; however, the effect was higher in talc powder as compared to aluminum oxide. Addition of 1.5% of aluminum oxide and talcum powder resulted in reduction of the average fungal pellet size to 1195 ± 49 and 1050 ± 48 µm, respectively (Fig. 3a). About 87–89% reduction in fungal pellet size was observed after addition of both microparticles at a level of 2.5% in the medium. It was observed that fungal growth was homogenously dispersed in the fermentation medium in talcum-added media, whereas clumpy fungal growth was observed in the control medium. Micrographs showed significant decrease in pellet size with increasing concentrations of microparticles (Fig. 3b). Better effect of talc powder than aluminum oxide could be due to differences in mean particle sizes of these two microparticles as the talc powder has a smaller diameter in comparison to aluminum oxide particles that resulted in better mass transfer in the production medium. These observations suggested that microparticles have resulted in smaller pellet size and higher yields of all the hydrolytic enzymes but their higher concentrations negatively affected the production of hydrolases by A. oryzae.
Fig. 3.
a Effect of addition of microparticles on A. oryzae SBS50 fungal pellet size. b Photographs of the fungal pellets under control (A) and different concentrations 1.5% (B), 2.0% (C) and 2.5% (D) of talcum powder
Effect of Sephadex G-50 and silver nanoparticles on production of hydrolytic enzymes by A. oryzae SBS50 in submerged cultures
Among all the concentrations tested, Sephadex G-50 at 1.5% level supported improved production of all the hydrolytic enzymes as compared to control. However, the effect was lower than microparticles, i.e., talc powder and aluminum oxide. Interestingly, xylanase production was higher at 1.0% level followed by decline afterwards. Addition of gel beads also reduced the size of fungal pellets as compared to control. Addition of silver nanoparticles showed differential effects on the production of hydrolytic enzymes. Only phytase production was significantly increased after the addition of nanoparticles in the medium while the production of other hydrolases decreased significantly.
Discussion
Cultivation of filamentous fungi in submerged culture is more complicated as compared to bacteria as moulds show different morphologies dependent on the age of mycelium, cultivation media composition, pH shifting and mechanical stress (Coban et al. 2015a; Antecka et al. 2016). Complex morphology of filamentous fungi is a sophisticated problem during submerged culture as it affects the synthesis of microbial enzymes (Coban et al. 2015a, b; Antecka et al. 2016; Yatmaz et al. 2016). Microparticle addition has increased the production of all the hydrolytic enzymes up to a level of 1.0% followed by decline afterwards. Reduction in the production of hydrolytic enzymes at higher concentrations of microparticles could be due to increased viscosity of the medium as a result of very small fungal structural growth (Coban et al. 2015a, b; Antecka et al. 2016; Yatmaz et al. 2016). Higher concentrations of the particles are also responsible for drastic shift in pH and interact with fungal pellets causing shear stress (Coban et al. 2015a). This in turn negatively affected the mass transfer and consequently fungal growth resulting in lower enzyme yields. Similarly, addition of microparticles (talc powder and aluminum oxide) resulted in higher phytase production by A. ficuum at 1.5% levels as compared to control (Coban et al. 2015a). Talcum powder enhanced chloroperoxidase production by Caldariomyces fumago (Kaup et al. 2008). Addition of aluminum oxide resulted in enhanced production of xylanolytic and cellulolytic enzymes by a thermophilic mould Sporotrichum thermophile BJAMDU5 as compared to talc powder and control (Bala and Singh 2016). Driouch et al. (2011) observed enhanced production of fructofuranosidase and glucoamylase by A. niger after the addition of talc powder in the medium. Antecka et al. (2016) observed the enhanced production of laccases by C. unicolor and P. sapidus by the addition of talcum and aluminum oxide in the medium. Yatmaz et al. (2016) reported the enhanced production of β-mannanase by A. sojae AsT1 in submerged cultures supplemented with talcum and aluminum oxide. Addition of microparticles has resulted in differential effect on the production of various hydrolytic enzymes by different microorganisms (Table 2). Production of microbial enzymes was enhanced in the range from 1.1- to 10.0-fold after the addition of microparticles (Table 2).
Table 2.
Comparison of different studies showing the effect of microparticles on fungal morphology and enzyme production
| Microorganism | Enzyme | Microparticle | Fungal pellet size reduction | Fold improvement in enzyme yield | References |
|---|---|---|---|---|---|
| Aspergillus ficuum | Phytase | Aluminum oxide | 800–500 µm | 2.87 | Coban et al. (2015a) |
| A. ficuum | Phytase | Talc powder | 800–200 µm | 1.97 | Coban et al. (2015b) |
| Caldariomyces fumago | Chloroperoxidase | Aluminum oxide | 4000–500 µm | 6.0 | Kaup et al. (2008) |
| C. fumago | Chloroperoxidase | Talc powder | 4000–500 µm | 10.0 | Kaup et al. (2008) |
| A. niger | Glucoamylase | Aluminum oxide | 1700–100 µm | 4.0 | Driouch et al. (2011) |
| A. niger | Fructofuranosidase | Talc powder | 1700–100 µm | 4.0 | Driouch et al. (2011) |
| Cerrena unicolor Murr. strain 137 | Laccase | Aluminum oxide | 4000–2500 µm | 2.5 | Antecka et al. (2016) |
| Pleurotus sapidus DSM 8266 | Laccase | Aluminum oxide | No effect | 10.0 | Antecka et al. (2016) |
| A. sojae AsT1 | β-Mannanase | Aluminum oxide | 1747.8–774.1 µm | 1.10 | Yatmaz et al. (2016) |
| A. sojae AsT1 | β-Mannanase | Talc powder | 1747.8–520.9 µm | 1.83 | Yatmaz et al. (2016) |
| A. oryzae SBS50 | Phytase, cellulase, xylanase and amylase | Talc powder and aluminum oxide | 4000–434 µm | 1.4–2.88 | Present study |
Addition of microparticles (talc powder and aluminum oxide) resulted in the production of all enzymes after 3 days instead of 4 days thereby, increasing the productivity of all enzymes as compared to control. Similarly, addition of microparticles reduced the fermentation time from 5 to 4 days for phytase production by A. ficuum with concomitant increase in productivity (Coban et al. 2015a). Antecka et al. (2016) also observed reduction in fermentation time for laccase production by C. unicolor and P. sapidus by the addition of talcum and aluminum oxide in the medium.
Fungal pellet size was reduced significantly after addition of microparticles in the production medium resulting into homogenous and dispersed fungal growth. Therefore, microparticle especially talc powder resulted in smaller fungal pellets and more homogenous broth responsible for increasing the nutrient consumption and enzyme production as compared to control (Coban et al. 2015a, b). Microparticles prevent the initial aggregation of conidiospores resulting in the formation of dispersed mycelium instead of large mycelial pellets (Nielsen 1996). Conidiospore aggregation is disrupted by microparticles as a result of collision during shake flask cultivation and thus, minimizing spore–spore interactions (Kaup et al. 2008). Coban et al. (2015a, b) also reported decrease in size of fungal pellets supplemented with microparticles which in turn enhanced phytase production. Kaup et al. (2008) also observed dispersed mycelia of C. fumago due to reduction in the size of fungal pellets after the addition of aluminum oxide and talc powder. Driouch et al. (2011) reported that the physical properties of the microparticles play a significant role in engineering fungal morphology and metabolite production. Kaup et al. (2008) also observed dispersed mycelia of C. fumago when medium was supplemented with microparticles with a diameter of ≤ 42 µm. Antecka et al. (2016) observed reduction in pellets of C. unicolor and P. sapidus for laccase production by the addition of microparticles in the medium. Yatmaz et al. (2016) also reported the enhanced production of β-mannanase by A. sojae AsT1 in submerged cultures supplemented with microparticles with concomitant reduction in size of fungal pellets. These observations suggested that microparticles have resulted in smaller pellet size and higher yields of all the hydrolytic enzymes but their higher concentrations negatively affected the production of hydrolases by A. oryzae.
Sephadex G-50 beads improved production of all the hydrolytic enzymes as compared to control. There is no report in the literature so far on the role of Sephadex G-50 in the production of microbial enzymes. However, Sephadex beads may have shown effect similar to microparticles but the exact mechanism and reason for their action is still unknown. Addition of silver nanoparticles showed differential effects on the production of hydrolytic enzymes. Similarly, addition of nickel–cobaltite nanoparticles enhanced the production of cellulases by Aspergillus fumigatus NS (Srivastava et al. 2014). Mukhopadhyay et al. (2012) reported improved production of pectate lyase by Bacillus megaterium using hydroxyapatite nanoparticles. In contrast, production of β-glucosidase, β-xylosidase and cellobiohydrolase was decreased in the presence of iron and copper nanoparticles while the production of Mn peroxidase remains unaffected (Shah et al. 2010). Production of β-glucosidase by Saccharomyces cerevisiae was enhanced after the addition of zinc oxide nanoparticles in the medium (Ban and Paul 2014).
Conclusions
Addition of microparticles (aluminum oxide and talc powder) enhanced the production of phytase and other hydrolytic enzymes such as phytase, xylanase, cellulase and amylase by A. oryzae SBS50. Microparticle also engineered the fungal morphology resulting in the significant reduction in fungal pellet size with concomitant reduction in fermentation time. Production of all enzymes was also increased by the addition of Sephadex G-50 and silver nanoparticles. Addition of microparticles and nanoparticles revealed their importance in engineering fungal morphology in submerged fermentation.
Acknowledgements
The author acknowledges the financial assistance from the Department of Biotechnology (DBT-IPLS Grant no. BT/PR13563/MED/12/425/2010), New Delhi, during the course of this investigation. Technical support provided by Ms. Divya Choudhary during this work is highly acknowledged.
Compliance with ethical standards
Conflict of interest
The author declares no conflict of interest.
References
- Antecka A, Blatkiewicz M, Bizukojć M, Ledakowicz S. Morphology engineering of basidiomycetes for improved laccase biosynthesis. Biotechnol Lett. 2016;38(4):667–72. doi: 10.1007/s10529-015-2019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala A, Singh B. Cost-effective production of biotechnologically important hydrolytic enzymes by Sporotrichum thermophile. Bioprocess Biosyst Eng. 2016;39(1):181–191. doi: 10.1007/s00449-015-1502-8. [DOI] [PubMed] [Google Scholar]
- Ban DK, Paul S. Zinc oxide nanoparticles modulates the production of β-glucosidase and protects its functional state under alcoholic condition in Saccharomyces cerevisiae. Appl Biochem Biotechnol. 2014;173(1):155–166. doi: 10.1007/s12010-014-0825-2. [DOI] [PubMed] [Google Scholar]
- Coban HB, Demirci A, Turhan I. Enhanced Aspergillus ficuum phytase production in fed-batch and continuous fermentations in the presence of talcum microparticles. Bioprocess Biosyst Eng. 2015;38(8):1431–1436. doi: 10.1007/s00449-015-1384-9. [DOI] [PubMed] [Google Scholar]
- Coban HB, Demirci A, Turhan I. Microparticle enhanced Aspergillus ficuum phytase production in submerged fermentations. Bioprocess Biosyst Eng. 2015;38(6):1075–1080. doi: 10.1007/s00449-014-1349-4. [DOI] [PubMed] [Google Scholar]
- Driouch H, Roth A, Dersch P, Wittmann C. Filamentous fungi in good shape microparticles for tailor-made fungal morphology and enhanced enzyme production. Bioeng Bugs. 2011;2:100–104. doi: 10.4161/bbug.2.2.13757. [DOI] [PubMed] [Google Scholar]
- Driouch H, Wittmann C, Hansch R, Wucherpfennig T, Krull R. Improved enzyme production by bio-pellets Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng. 2012;109:462–471. doi: 10.1002/bit.23313. [DOI] [PubMed] [Google Scholar]
- Fiske CH, Subbarow YP. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–410. [Google Scholar]
- Kaup BA, Ehrich K, Pescheck M, Schrader J. Microparticle-enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng. 2008;99:491–498. doi: 10.1002/bit.21713. [DOI] [PubMed] [Google Scholar]
- Kumari A, Singh B, Satyanarayana T. Mixed substrate fermentation for enhanced phytase production by thermophilic mould Sporotrichum thermophile and its application in beneficiation of poultry feed. Appl Biochem Biotechnol. 2016;178(1):197–210. doi: 10.1007/s12010-015-1868-8. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mukhopadhyay A, Dasgupta AK, Chattopadhyay D, Chakrabarti K. Improvement of thermostability and activity of pectate lyase in the presence of hydroxyapatite nanoparticles. Bioresour Technol. 2012;116:348–354. doi: 10.1016/j.biortech.2012.03.094. [DOI] [PubMed] [Google Scholar]
- Nielsen J. Modelling the morphology of filamentous microorganisms. Trends Biotechnol. 1996;14:438–443. doi: 10.1016/0167-7799(96)10055-X. [DOI] [Google Scholar]
- Sapna, Singh B. Improved production of protease-resistant phytase by Aspergillus oryzae and its applicability in the hydrolysis of insoluble phytates. J Ind Microbiol Biotechnol. 2013;40(8):891–899. doi: 10.1007/s10295-013-1277-3. [DOI] [PubMed] [Google Scholar]
- Sapna, Singh B. Phytase production by Aspergillus oryzae in solid state fermentation and its applicability in dephytinization of wheat bran. Appl Biochem Biotechnol. 2014;173(7):1885–1895. doi: 10.1007/s12010-014-0974-3. [DOI] [PubMed] [Google Scholar]
- Sapna, Singh B. Biocatalytic potential of protease-resistant phytase of Aspergillus oryzae SBS50 in ameliorating food nutrition. Biocatal Biotransform. 2015;33(3):167–174. doi: 10.3109/10242422.2015.1076215. [DOI] [Google Scholar]
- Sapna, Singh B. Purification and characterization of a protease-resistant phytase of Aspergillus oryzae SBS50 whose properties make it exceptionally useful as a feed supplement. Int J Biol Macromol. 2017;103:458–466. doi: 10.1016/j.ijbiomac.2017.05.077. [DOI] [PubMed] [Google Scholar]
- Shah V, Dobiásová P, Baldrian P, Nerud F, Kumar A, Seal S. Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus Trametes versicolor. J Hazard Mater. 2010;178(1–3):1141–1145. doi: 10.1016/j.jhazmat.2010.01.141. [DOI] [PubMed] [Google Scholar]
- Singh B, Satyanarayana T. A marked enhancement in phytase production by a thermophilic mould Sporotrichum thermophile using statistical designs in a cost-effective cane molasses medium. J Appl Microbiol. 2006;101:344–352. doi: 10.1111/j.1365-2672.2006.02921.x. [DOI] [PubMed] [Google Scholar]
- Singh B, Satyanarayana T. Phytase production by a thermophilic mould Sporotrichum thermophile in solid state fermentation and its potential applications. Bioresour Technol. 2008;99:2824–2830. doi: 10.1016/j.biortech.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Rawat R, Sharma R, Oberoi HS, Srivastava M, Singh J. Effect of nickel-cobaltite nanoparticles on production and thermostability of cellulases from newly isolated thermotolerant Aspergillus fumigatus NS (class: Eurotiomycetes) Appl Biochem Biotechnol. 2014;174(3):1092–1103. doi: 10.1007/s12010-014-0940-0. [DOI] [PubMed] [Google Scholar]
- Yatmaz E, Karahalil E, Germec M, Ilgin M, Turhan İ. Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst Eng. 2016;39(9):1391–1399. doi: 10.1007/s00449-016-1615-8. [DOI] [PubMed] [Google Scholar]