Main Text
Cells have three sources of energy: chemical, electrical, and mechanical. We know a fair amount about the first two, and very little about the third. The study of mechanical transduction is difficult because the stimulus at the site of a transducer (in this case an ion channel) is generally unknown (1, 2). The mechanics of cells involves the interaction of many structural components including the cytoskeleton, the lipid bilayer, and the extracellular matrix and intracellular organelles (3). This sharing of stress means that a mechanical stimulus supplied by an experimenter is split between many components, only some of which may affect the channels. The best data to date suggests that mechanosensitive ion channels (MSCs) respond primarily to tension in in the bilayer (4, 5, 6, 7, 8), although channels in specialized sensory organs may utilize a direct linkage to the cytoskeleton (9, 10). Patch experiments have shown that stress in the bilayer is comparable to the stress in the cortical cytoskeleton (11), but both of the components are viscoelastic and often plastic, hence the forces directly acting on MSCs are not accurately defined. Even channels reconstituted in lipid bilayers have protein-bound lipids that effectively alter the line tension of the domain so the magnitude of the local stimulus is different from that predicted from the macroscopic stimulus parameters (12). This review focuses on the factors that affect the stimulus at the channels, but does not deal with the molecular structure or the genetics of MSCs; note that there are many such reviews available (5, 9, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25).
Let us consider different types of mechanical stimulation (2). The most common stimulus is a patch pipette where the applied pressure is varied to change tension in the cortical membrane of the patch. As is well known, tension in the bilayer is shared with the proteins of the cortical cytoskeleton (11). The patch cytoskeleton is different from that of the cell it was taken from (26). The forces involved in patch formation disrupt the normal cytoskeleton, yielding new cortical structures. The adherence of the patch to the walls of the pipette creates the gigaseal but also generates large tensile forces (∼3 mN/m) in the patch so that all published patch recordings are made under conditions of high membrane stress. The stress in resting cells is very small: 0.02–0.04 mN/m (27). The stress in patches is variable in space and time (26) even if the applied pressure is constant, so we should expect nonstationary scatter in patch data. The unknown time dependence of local stimulus complicates kinetic studies of the channels. How much of a channel’s activation or inactivation or adaptation kinetics reflects relaxation of the local stimulus? How much of the kinetics depends on the flow of the lipids (28)? Let us consider a system other than a patch.
Channels in a reconstituted planar membrane are under considerable resting tension due to adhesion of the lipids to the Plateau-Gibbs border where lipids meet the supporting structure (29). It is not possible to observe channel kinetics at low tension in a planar bilayer because the bilayer tension is buffered by the Plateau-Gibbs border, which also means that bilayer bending with hydrostatic pressure does not increase the bilayer tension (30). Reconstitution into lipid vesicles avoids the problems of membrane binding to the glass, but studying patches made from vesicles reintroduces those stresses (31, 32, 33). Whole-vesicle patching removes those problems because most of the membrane that contributes current is not close to the pipette (34).
Another popular mechanical stimulus is osmotic pressure, which assumes that osmotic stress is borne by the cell’s cortex. However, in general, this is not found to be true, because most cells are filled with a cross-linked gel-like cytoskeleton that bears most of the stress, much like a sponge (35, 36, 37). AFM experiments have shown that cells with a 3D cytoskeleton get softer with swelling, which is in contradiction to the cortical stress model (38). Direct measurements of tension in cytoskeletal proteins in hypotonically swollen cells show that the stress is distributed and not concentrated in the cortex (39, 40, 41, 42). If one is working with a cell lacking a full cytoskeleton, such as a red blood cell, then the cortical tension will increase with osmotic pressure. Equivalently, if the bilayer separates from the deeper cytoskeleton as in a bleb (43), then bleb tension will increase with osmotic pressure. The tension in a cell’s bilayer due to osmotic pressure is unknown although it is probably monotonic with cell volume.
The bilayer contains many domains with different physical properties, so where the channels are located will affect their mechanical sensitivity. Electrophysiology data of Piezo1 show the presence of functional domains. We found that we could record currents from many channels in a patch, and with repeated stimulation the channels lost inactivation, not one by one, but all at once. How do many channels communicate simultaneously? One method is if they were driven through a domain boundary that could fracture (44) (Fig. 1). We found that channel activation was unaffected by a putative fracture, but inactivation was affected and seemed to require that the channels be close to each other. When the channels are physically separate, as in reconstitution experiments, they do not inactivate; therefore, inactivation is not an intrinsic state of the protein, but it does seem to require channel/channel interactions. Activation of WT human Piezo1 has no measurable latency to the generation of current, so there are unlikely to be many subreactions in the opening process. However, we have found that mutations that slow inactivation often also produce random latencies to activation (45, 46). What is going on during the latency is unknown, although it may reflect the time required for a domain to fracture.
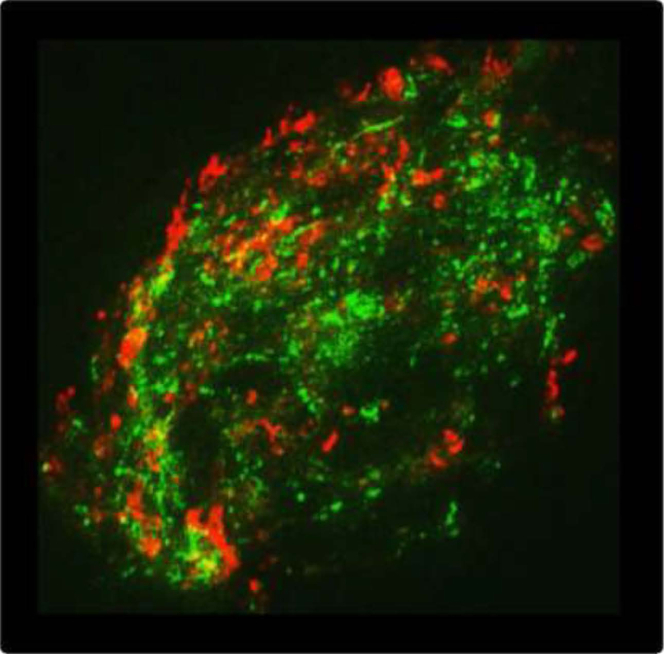
Figure 1.
MSCs form discrete domains. Given here is a single HEK cell cotransfected with Piezo1 in red and TREK-1 in green. Both types of channels form domains that are separate and the tension in each type of domain may be different. To see this figure in color, go online.
How can you measure the bilayer tension (not cortical membrane tension) in a patch? The best solution we have found is to use a cotransfected precalibrated ion channel. We used the eukaryotic-expressing bacterial channel, MscL (47), that has been calibrated in lipid membranes to have a Boltzmann relationship between open probability and tension (4, 48, 49). The midpoint of that curve is ∼11 mN/m (4, 49). We cotransfected HEK cells with a noninactivating mutant of human Piezo1 and MscL and varied the patch suction to vary the tension and traced out a dose-response curve of Popen versus pipette pressure for both sets of channels (44). If we assume that both channels, Piezo1 and MscL, are in similar bilayer domains, we then extract the free energy between closed and open. Approximating the channel as a right circular cylinder embedded in the bilayer, the energy between closed and open would be ΔG = TΔA, where T is tension and ΔA is the change of in-plane area between closed and open. This calculation suggests that MscL has an ΔA value of ∼20 nm2 in agreement with published data (22). Surprisingly, hP1 had a similar ΔA, despite a radically different structure. As a control, we measured ΔA for the K+-selective MSC, TREK-1, and this was only ∼10 nm2 (44).
Piezo is the largest known membrane protein, but a large size is not necessary to make an MSC; e.g., TREK is much smaller than Piezo. So why did nature make Piezo into such a large structure? Possibly it was to intertwine the channels to form domains (Fig. 2). Or, because the channel has such a wide span, ∼100 nm, the channel may become sensitive to local curvature (50, 51, 52). Does curvature matter? In experiments we did with TREK-1, we found that we could create changes in local patch curvature with a sudden change from negative pressure to zero pressure (7). Under suction the patch area is close to a spherical cap with an area of 2Πr2, and when the pressure goes to 0 mmHg, the equilibrium patch geometry is close to a planar disk of area Πr2 (33). This transient excess area of Πr2 caused a wrinkling of the patch that produced no change in TREK-1 current (7). The wrinkles disappeared as the membrane spontaneously reannealed to the pipette in ∼0.5 s. The wrinkles appeared to be folds <0.5 μm wide with a radius of curvature at the vertex that was much smaller than the mean dome curvature (7) (Fig. 3). For curvature to be an effective mechanical stimulus, the radius of curvature should be comparable to the channel dimensions (53, 54). Syeda et al. (55) estimated a gradient of tension across a bent bilayer in their supporting material.
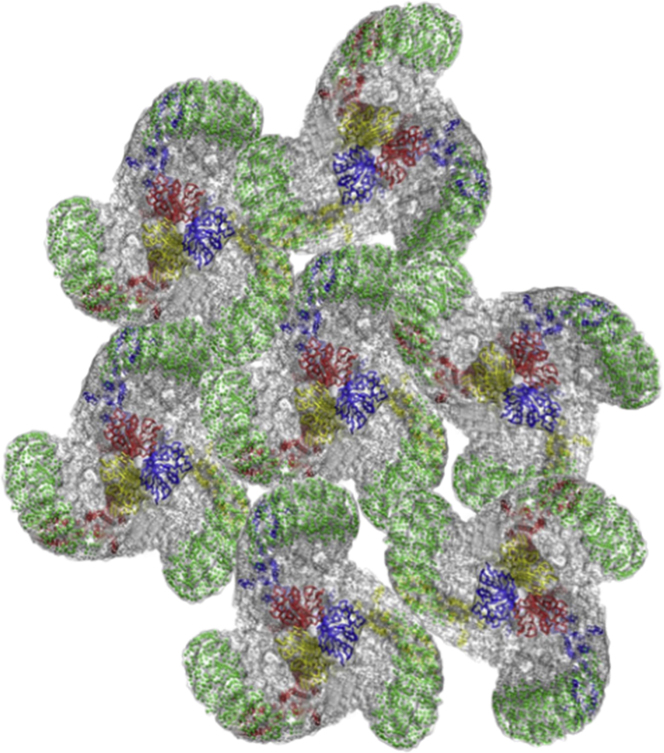
Figure 2.
Putative model of a Piezo domain formed by bonding of channels to each other. Individual channel images are modified from (1). To see this figure in color, go online.
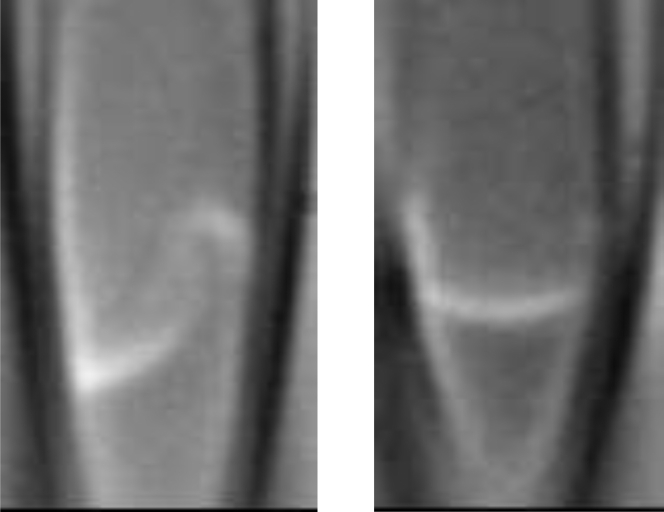
Figure 3.
Two differential interference contrast microscopy images of patches in a pipette. In the left panel, the change in curvature emphasizes the presence of mechanical domains in a patch. In the right panel, an inside-out patch has been pushed toward the tip with positive pressure. Note that on the left side, the membrane has been partially peeled off the glass, and there is a kink in the membrane where the fold occurs. These are images from a video; in steady state, the local curvature tends to disappear (2).
This wrinkling idea makes me think of the effect of positive and negative patch pressure. There are reports of rectifying gating that only works with positive or negative pressure (56, 57). When an excised or cell-attached patch is exposed to positive pressure, the membrane peels off the wall, forming a spherical cap that points toward the pipette tip (Figs. 3 and 4). However, at the junction of the membrane with the glass, there is a very tight radius of curvature. We do not know where the active channels are located in a patch; could they be in the bends where the membrane meets the glass?
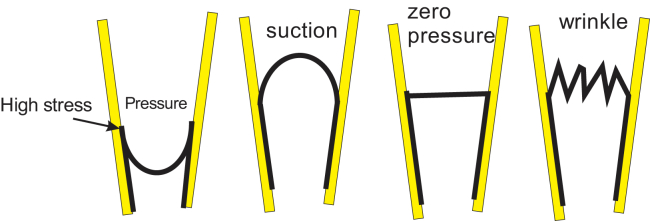
Figure 4.
Cartoon of patch geometry under different conditions. Positive pressure generates high curvature where the membrane leaves the glass and begins pointing downward. Because dome tension is symmetric with respect to pressure, there seems to be no intrinsic way for channels in the dome to sense a particular sign of the pressure. Images of inside-out patches revealed that positive pressure peels the membrane off the wall (4, 26). The channels that are seen with positive pressure may represent channels activated in the region of extreme curvature, such as where the bound membrane is peeled off the glass and pushed toward the tip (33) (Figs. 3 and 4). It is also possible that the enormous stresses caused by positive-pressure extrusion rip loose the cytoskeleton to transfer local mean tension to the bilayer. To see this figure in color, go online.
Physiological roles of MSCs
There are many physiological processes that involve mechanical transduction: blood pressure regulation, filling of other hollow organs (58, 59), proprioception (60), touch (61, 62), and hearing (63, 64). There are undoubtedly many more processes involving MSCs, because any process that involves changes in cell shape involves change in force, notably in development (65). In prokaryotes, MSCs serve to regulate osmotically active solute concentrations, but eukaryotic MSCs are cation-selective (66, 67, 68), so by themselves they cannot regulate volume. In red blood cells, MSCs are sensitive to the specific inhibitor, GsMTx4 (44, 69, 70, 71). In sickled cells, crystals of hemoglobin may expand sufficiently to press against the membrane, thereby activating MSCs and generating GsMTx4 sensitivity. MSCs have a strong influence on blood vessel function (72, 73, 74). Also involving mechanical stress, GsMTx4 is a potent stimulator of neurite growth, probably by inhibiting Ca+2 entry (75, 76).
Biophysics of the channels
For a channel to be mechanosensitive, it has to change dimensions under stress. Any membrane-bound protein that changes shape will be sensitive to changes in membrane tension. The voltage-sensitive channels such as K+, Na+, and Ca+2 are all modulated by membrane tension (77, 78, 79, 80, 81), as is the NMDA channel (82). Using the term “mechanical modulation” suggests that mechanics cannot transmit enough energy to cover the whole dynamic range of a channel but can alter rates (22). To simplify discussion, I will refer to MSCs as those channels that can be driven over their whole dynamic range with mechanical stress alone.
The simplest model for the energy of gating is to treat the channel as a right circular cylinder in the membrane with two states (closed and open) with different in-plane areas (25). The energy difference between closed and open states is then ΔG = TΔA, where T is the tension in the bilayer and ΔA is the difference of in-plane area between closed and open. For a given tension, larger changes in area produce higher sensitivity. However, there are other potential energy terms. For example, if the channel changes thickness upon opening, the bilayer and the channel thickness may deviate, so energy should be added or subtracted from the reaction. This process is called “hydrophobic mismatch” (83, 84, 85). AFM data suggests that if a channel like Shaker opens as a wedge, with the cytoplasmic monolayer expanding more than the extracellular monolayer, it causes buckling of the bilayer—thus contributing mechanical potential energy to the reaction (51, 86). Changes in membrane curvature can drive gating (87), and this is particularly relevant to endo- or exocytosis. Curvatures of 1/40 nm−1 have been shown to add ∼7 kBT to a channel relative to the flat membrane (87). More differentiated MSCs can be linked in series with elements of the cytoskeleton, and this seems to be characteristic of sensory organs (10).
Pharmacology
MSCs can be inhibited by Gd3+ (88, 89, 90, 91), but that is a highly nonspecific inhibitor (91). It may inhibit by condensing the negative lipids (89) and reducing the free volume of the bilayer (92). The only known specific inhibitor of any MSC is GsMTx4 (93), a 34-amino-acid ICK peptide found in tarantula venom (94), which was later synthesized (95). We have shown that the D- and L-forms of GsMTx4 are equally active, suggesting that there are no protein-protein interactions involved (95). The interaction of GsMTx4 with lipid membranes shows an association at the extracellular aqueous boundary (96, 97). Recent unpublished results using an environmentally sensitive, fluorescently labeled GsMTx4, suggest that the peptide does not enter the bilayer at resting tension, but enters with increased tension (T.M. Suchyna, personal communication). The resulting increased free volume with an increase of tension provides space for peptide penetration. The space taken up by the inserted GsMTx4 compresses the surrounding lipids, causing a drop in local tension. At saturating concentrations (∼10 μM), GsMTx4 is a gating inhibitor equivalent to an offset of ∼60 mmHg of patch pressure. Because GsMTx4 does not appear to act on membranes at resting tension, its effect as an inhibitor can only be tested when the membrane is under pathologic stress, i.e., when MSCs are active.
In situ experiments suggest that GsMTx4 has (98) minimal side effects on animals despite its wide efficacy in a variety of in vitro systems (14). This lack of toxicity in situ may represent the inability of GsMTx4 to bind to resting membranes. Its effects are confined to cell membranes under pathologic tension. In dystrophic skeletal muscle cells, GsMTx4 protects against the loss of contractile strength and rundown with repeated stimulation (99). Its use as a drug for dystrophy is further assisted by the fact that the pharmacologic half-life (in mice) is ∼1 week, and it does not penetrate the blood brain barrier. However, like many other drugs, GsMTx4 binds to blood plasma proteins so if intravenous administration is used, blood proteins will bind a good deal of the applied drug (76). That partitioning may explain why recent experiments on the effect of GsMTx4 on cardiac arrhythmias in pigs found little effect (100). In contrast, the cardiac sensitivity to GsMTx4 in saline was demonstrated in Langendorff rabbit hearts, where it reversibly suppressed inflation-stimulated atrial fibrillation at ∼170 nM (101). GsMTx4 has shown no toxic effects on human cardiac muscle (102) or in free roaming ferrets (98). Mild side effects of GsMTx4 at high concentrations have been seen in hair cells (63, 103) and possibly TRPC channels (104).
MSCs are traditionally sensitive to amphipaths (19, 47, 95, 96, 97, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115). The amphipath effects seem to be local to the channel (say within three lipids of the protein) rather than correlated with changes in global bilayer properties. Lots of drugs in clinical use are amphipathic, so some of their side effects could be caused by interactions with MSCs. An interesting aspect of this amphipath sensitivity is that general anesthetics activate the 2P MSCs in the concentration ranges used for clinical dosing (111, 116). We might speculate that these channels are the responsible for people getting knocked out by a blow to the head; the 2P channels are activated by mechanical stress, and that leads to hyperpolarization of neurons with a resulting loss of excitability. As the channels inactivate (7), normal operating voltages return and the client wakes up.
Cautions
Although Patapoutian’s lab originally found Piezo in N2A cells (117), others have found no activity in WT N2A cells (118). Variable expression was also emphasized by the report that HEK cells had little MSC activity (67), despite the fact that we cloned human Piezo1 and 2 from HEK cells (119). The presence of channel RNA clearly is not equivalent to the functional expression of channels. A key surprising observation affecting the interpretation of physiological effects of MSCs stems from the work of Lauritzen et al. (120). They showed that expression of 2P channels had gross effects on structure of the actin cytoskeleton, even if the channels were nonconducting. We have seen similar histological effects. Changes in the cytoskeleton will affect tension in the bilayer, so we expect that any drugs affecting the cytoskeleton will also affect MSC activity (121, 122, 123). We and others (124) have also seen strong correlations between MSC activity and cell motility (125, 126).
Acknowledgments
I thank the reviewers for catching my many typos and other errors.
The work was supported by National Institutes of Health grant (HL054887) to F.S. and National Institute of Health grant (NS085517) and National Science Foundation (CMMI-1537239).
Editor: Brian Salzberg.
References
- 1.Sachs F. Mechanical transduction by ion channels: a cautionary tale. World J. Neurol. 2015;5:74–87. doi: 10.5316/wjn.v5.i3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu A.P. Biophysical tools for cellular and subcellular mechanical actuation of cell signaling. Biophys. J. 2016;111:1112–1118. doi: 10.1016/j.bpj.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedl P., Wolf K., Lammerding J. Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox C.D., Bae C., Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016;7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brohawn S.G., Su Z., MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. USA. 2014;111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrier C., Pozza A., Ghazi A. The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. J. Biol. Chem. 2013;288:27307–27314. doi: 10.1074/jbc.M113.478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honoré E., Patel A.J., Sachs F. Desensitization of mechano-gated K2P channels. Proc. Natl. Acad. Sci. USA. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brohawn S.G., Campbell E.B., MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature. 2014;516:126–130. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinac B. The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. Biochim. Biophys. Acta. 2014;1838:682–691. doi: 10.1016/j.bbamem.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Arnadóttir J., O’Hagan R., Chalfie M. The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 2011;31:12695–12704. doi: 10.1523/JNEUROSCI.4580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinlaja J., Sachs F. The breakdown of cell membranes by electrical and mechanical stress. Biophys. J. 1998;75:247–254. doi: 10.1016/S0006-3495(98)77511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian A., Johnson C., Baumgart T. Line tension at fluid membrane domain boundaries measured by micropipette aspiration. Phys. Rev. Lett. 2007;98:208102. doi: 10.1103/PhysRevLett.98.208102. [DOI] [PubMed] [Google Scholar]
- 13.Ranade S.S., Syeda R., Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb P.A. Elsevier; London, UK: 2017. Piezo Channels. [Google Scholar]
- 15.Martinac B., Nomura T., Landsberg M.J. Bacterial mechanosensitive channels: models for studying mechanosensory transduction. Antioxid. Redox Signal. 2014;20:952–969. doi: 10.1089/ars.2013.5471. [DOI] [PubMed] [Google Scholar]
- 16.Grage S.L., Afonin S., Ulrich A.S. Crowding of membrane proteins and peptides. Eur. Biophys. J. 2011;40:47. [Google Scholar]
- 17.Kung C., Martinac B., Sukharev S. Mechanosensitive channels in microbes. In: Gottesman S., Harwood C.S., editors. Annual Review of Microbiology. Vol 64. Kung; Palo Alto, CA: 2010. pp. 313–329. [DOI] [PubMed] [Google Scholar]
- 18.Teng J., Loukin S., Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch. 2015;467:27–37. doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anishkin A., Loukin S.H., Kung C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl. Acad. Sci. USA. 2014;111:7898–7905. doi: 10.1073/pnas.1313364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heureaux J., Chen D., Liu A.P. Activation of a bacterial mechanosensitive channel in mammalian cells by cytoskeletal stress. Cell. Mol. Bioeng. 2014;7:307–319. doi: 10.1007/s12195-014-0337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Çetiner U., Rowe I., Sukharev S. Tension-activated channels in the mechanism of osmotic fitness in Pseudomonas aeruginosa. J. Gen. Physiol. 2017:201611699. doi: 10.1085/jgp.201611699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sukharev S., Sachs F. Molecular force transduction by ion channels: diversity and unifying principles. J. Cell Sci. 2012;125:3075–3083. doi: 10.1242/jcs.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anishkin A., Yoshimura K., Sukharev S. Membrane tension and cytoplasmic crowding pressure: the multimodal mechanism of the mechanosensitive channel MscS. Biophys. J. 2011;100:279. [Google Scholar]
- 24.Gottlieb P.A. A tour de force: the discovery, properties, and function of piezo channels. Curr. Top. Membr. 2017;79:1–36. doi: 10.1016/bs.ctm.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Soattin L., Fiore M., Vassalli M. The biophysics of piezo1 and piezo2 mechanosensitive channels. Biophys. Chem. 2016;208:26–33. doi: 10.1016/j.bpc.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Suchyna T.M., Markin V.S., Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys. J. 2009;97:738–747. doi: 10.1016/j.bpj.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai J.W., Sheetz M.P., Morris C.E. Membrane tension in swelling and shrinking molluscan neurons. Mol. Biol. Cell. 1996;7:2614. doi: 10.1523/JNEUROSCI.18-17-06681.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans E., Yeung A. Hidden dynamics in rapid changes of bilayer shape. Chem. Phys. Lipids. 1994;73:39–56. [Google Scholar]
- 29.Nikoleli G.-P., Nikolelis D.P., Hianik T. Advances in lipid film based biosensors. Trends Analyt. Chem. 2016;79:210–221. [Google Scholar]
- 30.Awayda M.S., Ismailov I.I., Benos D.J. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am. J. Physiol. 1995;268:C1450–C1459. doi: 10.1152/ajpcell.1995.268.6.C1450. [DOI] [PubMed] [Google Scholar]
- 31.Bae C., Markin V., Sachs F. Modeling ion channels in the gigaseal. Biophys. J. 2011;101:2645–2651. doi: 10.1016/j.bpj.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battle A., Ridone P., Martinac B. Lipid-protein interactions: lessons learned from stress. Biochim. Biophys. Acta. 2015;1848:1744–1756. doi: 10.1016/j.bbamem.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Slavchov R.I., Nomura T., Sachs F. Gigaseal mechanics: creep of the gigaseal under the action of pressure, adhesion, and voltage. J. Phys. Chem. B. 2014;118:12660–12672. doi: 10.1021/jp506965v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garten M., Mosgaard L.D., Toombes G.E. Whole-GUV patch-clamping. Proc. Natl. Acad. Sci. USA. 2016;114:328–333. doi: 10.1073/pnas.1609142114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sachs F., Sivaselvan M.V. Cell volume control in three dimensions: water movement without solute movement. J. Gen. Physiol. 2015;145:373–380. doi: 10.1085/jgp.201411297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charras G.T., Mitchison T.J., Mahadevan L. Animal cell hydraulics. J. Cell Sci. 2009;122:3233–3241. doi: 10.1242/jcs.049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchison T., Charras G., Mahadevan L. Seminars in Cell & Developmental Biology. Elsevier; Amsterdam, the Netherlands: 2008. Implications of a poroelastic cytoplasm for the dynamics of animal cell shape; pp. 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spagnoli C., Beyder A., Sachs F. Atomic force microscopy analysis of cell volume regulation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2008;78:031916. doi: 10.1103/PhysRevE.78.031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang C., Zhang X., Guo J. Mechanical dynamics in live cells and fluorescence-based force/tension sensors. Biochim. Biophys. Acta. 2015;1853:1889–1904. doi: 10.1016/j.bbamcr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J., Wang Y., Meng F. Actin stress in cell reprogramming. Proc. Natl. Acad. Sci. USA. 2014;111:E5252–E5261. doi: 10.1073/pnas.1411683111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng F., Sachs F. Orientation-based FRET sensor for real-time imaging of cellular forces. J. Cell Sci. 2012;125:743–750. doi: 10.1242/jcs.093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng F. State University of New York at Buffalo; Ann Arbor, MI: 2008. stFRET, A Novel Tool to Study Molecular Force in Living Cells and Animals. p. 143. [Google Scholar]
- 43.Charras G.T., Coughlin M., Mahadevan L. Life and times of a cellular bleb. Biophys. J. 2008;94:1836–1853. doi: 10.1529/biophysj.107.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae C., Gnanasambandam R., Gottlieb P.A. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc. Natl. Acad. Sci. USA. 2013;110:E1162–E1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae C., Sachs F., Gottlieb P.A. Protonation of the human PIEZO1 ion channel stabilizes inactivation. J. Biol. Chem. 2015;290:5167–5173. doi: 10.1074/jbc.M114.604033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bae C., Gottlieb P.A., Sachs F. Human PIEZO1: removing inactivation. Biophys. J. 2013;105:880–886. doi: 10.1016/j.bpj.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doerner J.F., Febvay S., Clapham D.E. Controlled delivery of bioactive molecules into live cells using the bacterial mechanosensitive channel MscL. Nat. Commun. 2012;3:990. doi: 10.1038/ncomms1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moe P., Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 49.Sukharev S.I., Sigurdson W.J., Sachs F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 1999;113:525–540. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svetina S. Curvature-dependent protein-lipid bilayer interaction and cell mechanosensitivity. Eur. Biophys. J. 2015;44:513–519. doi: 10.1007/s00249-015-1046-5. [DOI] [PubMed] [Google Scholar]
- 51.Bavi N., Nakayama Y., Martinac B. Biophysical implications of lipid bilayer rheometry for mechanosensitive channels. Proc. Natl. Acad. Sci. USA. 2014;111:13864–13869. doi: 10.1073/pnas.1409011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J., Goyal R., Grandl J. Localized force application reveals mechanically sensitive domains of Piezo1. Nat. Commun. 2016;7:12939. doi: 10.1038/ncomms12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ursell T., Agrawal A., Phillips R. Lipid bilayer mechanics in a pipette with glass-bilayer adhesion. Biophys. J. 2011;101:1913–1920. doi: 10.1016/j.bpj.2011.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips R., Ursell T., Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Syeda R., Florendo M.N., Patapoutian A. Piezo1 channels are inherently mechanosensitive. Cell Reports. 2016;17:1739–1746. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franco-Obregón A., Lansman J.B. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J. Physiol. 2002;481:299–309. doi: 10.1113/jphysiol.1994.sp020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franco-Obregon Jr., A., and J. B. Lansman. 1994. Mechanosensitive gating of ion channels in muscle cells from the mdx mouse: implications for dystrophin function. Abstract:1–27.
- 58.Wang S., Chennupati R., Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Invest. 2016;126:4527–4536. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alcaino C., Knutson K., Beyder A. Mechanosensitive ion channel Piezo2 is inhibited by D-GsMTx4. Channels (Austin) 2017;11:245–253. doi: 10.1080/19336950.2017.1279370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo S.-H., Lukacs V., Patapoutian A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015;18:1756–1762. doi: 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walsh C.M., Bautista D.M., Lumpkin E.A. Mammalian touch catches up. Curr. Opin. Neurobiol. 2015;34:133–139. doi: 10.1016/j.conb.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lumpkin E.A., Marshall K.L., Nelson A.M. The cell biology of touch. J. Cell Biol. 2010;191:237–248. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang J., Iwasa K.H. Effects of tarantula toxin GsMTx4 on the membrane motor of outer hair cells. Neurosci. Lett. 2006;404:213–216. doi: 10.1016/j.neulet.2006.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hudspeth A.J., Choe Y., Martin P. Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc. Natl. Acad. Sci. USA. 2000;97:11765–11772. doi: 10.1073/pnas.97.22.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranade S.S., Qiu Z., Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA. 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coste B., Murthy S.E., Patapoutian A. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat. Commun. 2015;6:7223. doi: 10.1038/ncomms8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coste B., Xiao B., Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gnanasambandam R., Bae C., Sachs F. Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS One. 2015;10:e0125503. doi: 10.1371/journal.pone.0125503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andolfo I., Alper S.L., Iolascon A. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood. 2013;121:3925–3935. doi: 10.1182/blood-2013-02-482489. S3921–3912. [DOI] [PubMed] [Google Scholar]
- 70.Vandorpe D.H., Xu C., Alper S.L. Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLoS One. 2010;5:e8732. doi: 10.1371/journal.pone.0008732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cinar E., Zhou S., Wan J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc. Natl. Acad. Sci. USA. 2015;112:11783–11788. doi: 10.1073/pnas.1507309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Retailleau K., Duprat F., Honoré E. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Reports. 2015;13:1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 73.Honoré E., Martins J.R., Demolombe S. The piezo mechanosensitive ion channels: may the force be with you! Rev. Physiol. Biochem. Pharmacol. 2015;169:25–41. doi: 10.1007/112_2015_26. [DOI] [PubMed] [Google Scholar]
- 74.Sharif-Naeini R., Folgering J.H., Honoré E. Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J. Mol. Cell. Cardiol. 2010;48:83–89. doi: 10.1016/j.yjmcc.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 75.Jacques-Fricke B.T., Seow Y., Gomez T.M. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J. Neurosci. 2006;26:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gottlieb P.A., Barone T., Plunkett R. Neurite outgrowth from PC12 cells is enhanced by an inhibitor of mechanical channels. Neurosci. Lett. 2010;481:115–119. doi: 10.1016/j.neulet.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morris C.E. Why are so many ion channels mechanosensitive? In: Sperelakis N., editor. Cell Physiology Source Book. Elsevier; Amsterdam, the Netherlands: 2012. pp. 493–505. [Google Scholar]
- 78.Morris C.E. Voltage-gated channel mechanosensitivity: fact or friction? Front. Physiol. 2011;2:25. doi: 10.3389/fphys.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banderali U., Clark R.B., Giles W.R. Effects of applied stretch on native and recombinant cardiac Na+ currents. In: Kamkin A., Kiseleva I., editors. Mechanosensitivity of the Heart. Mechanosensitivity in Cells and Tissues. Vol. 3. Springer; Dordrecht, the Netherlands: 2010. [Google Scholar]
- 80.Morris C.E., Juranka P.F. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys. J. 2007;93:822–833. doi: 10.1529/biophysj.106.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beyder A., Strege P.R., Farrugia G. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel Nav1.5: a novel mechanism of drug action. Circulation. 2012;125:2698–2706. doi: 10.1161/CIRCULATIONAHA.112.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maneshi M.M., Maki B., Hua S.Z. Mechanical stress activates NMDA receptors in the absence of agonists. Sci. Rep. 2017;7:39610. doi: 10.1038/srep39610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nomura T., Cranfield C.G., Martinac B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc. Natl. Acad. Sci. USA. 2012;109:8770–8775. doi: 10.1073/pnas.1200051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Markin V.S., Sachs F. Thermodynamics of mechanosensitivity. Mechanosensitive ion channels. Part A. 2007;58:87–119. [Google Scholar]
- 85.Lee K.J. Energetics of rotational gating mechanisms of an ion channel induced by membrane deformation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2006;73:021909. doi: 10.1103/PhysRevE.73.021909. [DOI] [PubMed] [Google Scholar]
- 86.Beyder A., Sachs F. Electromechanical coupling in the membranes of Shaker-transfected HEK cells. Proc. Natl. Acad. Sci. USA. 2009;106:6626–6631. doi: 10.1073/pnas.0808045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tonnesen A., Christensen S.M., Stamou D. Geometrical membrane curvature as an allosteric regulator of membrane protein structure and function. Biophys. J. 2014;106:201–209. doi: 10.1016/j.bpj.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dhein S., Schreiber A., Mohr F.W. Mechanical control of cell biology. Effects of cyclic mechanical stretch on cardiomyocyte cellular organization. Prog. Biophys. Mol. Biol. 2014;115:93–102. doi: 10.1016/j.pbiomolbio.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Ermakov Y.A., Kamaraju K., Sukharev S. Gadolinium ions block mechanosensitive channels by altering the packing and lateral pressure of anionic lipids. Biophys. J. 2010;98:1018–1027. doi: 10.1016/j.bpj.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang X.C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- 91.Caldwell R.A., Clemo H.F., Baumgarten C.M. Using gadolinium to identify stretch-activated channels: technical considerations. Am. J. Physiol. 1998;275:C619–C621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- 92.Markin V.S., Sachs F. Free volume in membranes: viscosity or tension? Open J. Biophys. 2015;5:80–83. doi: 10.4236/ojbiphy.2015.53007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tape S.E., Suchyna T.M., Gottlieb P.A. Nonchiral effects of a peptide inhibitor of mechanosensitive channels: evidence for a bilayer-dependent mechanism. J. Gen. Physiol. 2004;124:11a–12a. [Google Scholar]
- 94.Bowman C.L., Gottlieb P.A., Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon. 2007;49:249–270. doi: 10.1016/j.toxicon.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suchyna T.M., Tape S.E., Gottlieb P.A. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 96.Nishizawa K., Nishizawa M., Suchyna T.M. Effects of Lys to Glu mutations in GsMTx4 on membrane binding, peptide orientation, and self-association propensity, as analyzed by molecular dynamics simulations. Biochim. Biophys. Acta. 2015;1848(11 Pt A):2767–2778. doi: 10.1016/j.bbamem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gnanasambandam R., Nishizawa K., Suchyna T. Positively charged residues on GsMTx4 are crucial for inhibition of the mechanosensitive ion channel piezo1. Biophys. J. 2013;104:467a. [Google Scholar]
- 98.Wang J., Ma Y., Suchyna T.M. GsMTx4-D is a cardioprotectant against myocardial infarction during ischemia and reperfusion. J. Mol. Cell. Cardiol. 2016;98:83–94. doi: 10.1016/j.yjmcc.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yeung E.W., Whitehead N.P., Allen D.G. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J. Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barrabés J.A., Inserte J., Garcia-Dorado D. Effects of the selective stretch-activated channel blocker GsMtx4 on stretch-induced changes in refractoriness in isolated rat hearts and on ventricular premature beats and arrhythmias after coronary occlusion in swine. PLoS One. 2015;10:e0125753. doi: 10.1371/journal.pone.0125753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bode F., Sachs F., Franz M.R. Tarantula peptide inhibits atrial fibrillation. Nature. 2001;409:35–36. doi: 10.1038/35051165. [DOI] [PubMed] [Google Scholar]
- 102.Kockskämper J., von Lewinski D., Pieske B. The slow force response to stretch in atrial and ventricular myocardium from human heart: functional relevance and subcellular mechanisms. Prog. Biophys. Mol. Biol. 2008;97:250–267. doi: 10.1016/j.pbiomolbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peng A.W., Gnanasambandam R., Ricci A.J. Adaptation independent modulation of auditory hair cell mechanotransduction channel open probability implicates a role for the lipid bilayer. J. Neurosci. 2016;36:2945–2956. doi: 10.1523/JNEUROSCI.3011-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gottlieb P., Suchyna T., Sachs F. The mechanosensitive TRPC1 ion channel activity is modulated by cytoskeleton and inhibited by the peptide GsMTx4. Biophys. J. 2006;90:1545. [Google Scholar]
- 105.Kamaraju K., Sukharev S. The membrane lateral pressure-perturbing capacity of parabens and their effects on the mechanosensitive channel directly correlate with hydrophobicity. Biochemistry. 2008;47:10540–10550. doi: 10.1021/bi801092g. [DOI] [PubMed] [Google Scholar]
- 106.Qi Z., Naruse K., Sokabe M. Ionic amphipaths affect the gating of a stretch activated BK channel (SAKCa) cloned from chick heart. Biophys. J. 2003;84:234A. [Google Scholar]
- 107.Hamill O.P., McBride D.W.J., Jr. The pharmacology of mechano-gated membrane ion channels. Pharmacol. Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- 108.Sokabe M., Hasegawa N., Yamanori K. Aminoglycoside blockade and amphipath activation of stretch activated ion channels from chick skeletal muscle. Biophys. J. 1994;66:A167. [Google Scholar]
- 109.Martinac B., Adler J., Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 110.Chemin J., Patel A., Honoré E. Lysophosphatidic acid-operated K+ channels. J. Biol. Chem. 2005;280:4415–4421. doi: 10.1074/jbc.M408246200. [DOI] [PubMed] [Google Scholar]
- 111.Patel A.J., Lazdunski M., Honoré E. Lipid and mechano-gated 2P domain K+ channels. Curr. Opin. Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 112.Maingret F., Patel A.J., Honoré E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J. Biol. Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 113.Patel A.J., Honoré E., Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 114.Lundbaek J.A., Collingwood S.A., Andersen O.S. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J. R. Soc. Interface. 2010;7:373–395. doi: 10.1098/rsif.2009.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hwang T.C., Koeppe R.E., 2nd, Andersen O.S. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- 116.Patel A.J., Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- 117.Coste B., Mathur J., Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee W., Leddy H.A., Liedtke W.B. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA. 2014;111:E5114–E5122. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bae C., Sachs F., Gottlieb P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295–6300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lauritzen I., Chemin J., Jane Patel A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gottlieb P.A., Bae C., Sachs F. Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin) 2012;6:282–289. doi: 10.4161/chan.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sokabe M., Sigurdson W.S., Sachs F. Effect of excision and cytochalasin on the viscoelastic properties of patch clamped membranes in heart and skeletal muscle. Biophys. J. 1993;61:A513. [Google Scholar]
- 123.Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J. Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maroto R., Kurosky A., Hamill O.P. Mechanosensitive Ca2+ permeant cation channels in human prostate tumor cells. Channels (Austin) 2012;6:290–307. doi: 10.4161/chan.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kamkin A., Kiseleva I., Scholz H. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Prog. Biophys. Mol. Biol. 2003;82:111–120. doi: 10.1016/s0079-6107(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 126.Fabian A., Bertrand J., Schwab A. Transient receptor potential canonical channel 1 impacts on mechanosignaling during cell migration. Pflugers Arch. 2012;464:623–630. doi: 10.1007/s00424-012-1169-9. [DOI] [PubMed] [Google Scholar]