Abstract
Introduction
Basal insulin (BI) plays an important role in treating type 2 diabetes (T2D), especially when oral antidiabetic (OAD) medications are insufficient for glycemic control. We conducted a retrospective, observational study using electronic medical records (EMR) data from the IBM® Explorys database to evaluate the probability of achieving glycemic control over 24 months after BI initiation in patients with T2D in the USA.
Methods
A cohort of 6597 patients with T2D who started BI following OAD(s) and had at least one valid glycated hemoglobin (HbA1c) result recorded both within 90 days before and 720 days after BI initiation were selected. We estimated the changes from baseline in HbA1c every 6 months, the quarterly conditional probabilities of reaching HbA1c < 7% if a patient had not achieved glycemic control prior to each quarter (Q), and the cumulative probability of reaching glycemic control over 24 months.
Results
Our cohort was representative of patients with T2D who initiated BI from OADs in the USA. The average HbA1c was 9.1% at BI initiation, and decreased robustly (1.5%) in the first 6 months after initiation with no further reductions thereafter. The conditional probability of reaching glycemic control decreased rapidly in the first year (26.6% in Q2; 17.6% in Q3; 8.6% in Q4), and then remained low (≤ 6.1%) for each quarter in the second year. Cumulatively, about 38% of patients reached HbA1c < 7% in the first year; only approximately 8% more did so in the second year.
Conclusion
Our study of real-world data from a large US EMR database suggested that among patients with T2D who initiated BI after OADs, the likelihood of reaching glycemic control diminished over time, and remained low from 12 months onwards. Additional treatment options should be considered if patients do not reach glycemic control within 12 months of BI initiation.
Funding
Sanofi Corporation.
Keywords: Basal insulin, IBM Explorys database, Real-world evidence, Type 2 diabetes
Introduction
Type 2 diabetes (T2D) affects 30.3 million people (~ 9.4% of the population) in the USA as of 2017 [1]. The economic and social costs of T2D are considerable because of its associated serious short- and long-term complications, particularly in patients who do not achieve and/or maintain glycemic control [2, 3], as well as its significant contribution to overall mortality (approximately 12% of all deaths) [4]. Hyperglycemia, which may result from insufficient treatment intensification, significantly increases the risks of microvascular complications, such as retinopathy and nephropathy, as well as macrovascular complications including myocardial infarction, heart failure, stroke, and their composite [3, 5]. The American Diabetes Association (ADA) Standards of Medical Care in Diabetes recommend that most adult patients with T2D maintain a glycated hemoglobin (HbA1c) level at < 7% [6].
Over the past three decades, there has been considerable progress in the development of new antihyperglycemic medications for patients with T2D. Therapeutic options now include novel agents such as dipeptidyl peptidase 4 (DPP4) inhibitors, sodium–glucose cotransporter 2 (SGLT-2) inhibitors, as well as injectable glucagon-like peptide 1 receptor agonists (GLP-1 RAs). While oral antidiabetics (OADs) are usually the first- or second-line options, many patients with T2D will eventually require therapy with injectables including insulin therapy. Basal insulin (BI) has been shown to be effective in reducing HbA1c levels and attaining glycemic control, with between 40% and 70% of patients reaching a target of < 7% in randomized controlled trials (RCTs) [7, 8]. Initiating insulin replacement with BI has been endorsed in professional guidelines for patients who have not attained target HbA1c levels with the use of non-insulin therapies [9, 10].
Studies of the effectiveness of BI in real-world practice have shown mixed results. The proportions of patients achieving target HbA1c levels (HbA1c < 7%) in these studies have ranged from as low as 11% after 1 year to as high as 58% over 2.5 years [11–19]. The likelihood of reaching glycemic control with BI treatment in the real world remains a question of interest to patients, clinicians, healthcare systems, and payers.
The IBM® Watson Health™ Explorys database (later referred to as Explorys) is a large, US population-based, commercial database that contains an aggregate of electronic medical record (EMR) data from over 54 million unique patients from 39 major integrated healthcare systems covering over 344,000 clinicians across all 50 US states. It contains de-identified, structured, longitudinal patient data (including diagnoses, laboratory results, biometric measures, and procedures) from clinical encounters at participating institutions. Collected data are standardized and normalized by IBM Watson Health. The data are automatically updated at least once every 24 h. This database offers a good means of studying the real-world evidence for the effectiveness of different treatment modalities (including BI) in the USA.
We conducted a retrospective, observational study using the Explorys database to evaluate the probability of achieving glycemic control over 24 months after BI initiation in patients with T2D.
Methods
Study Cohort Construction
Within the Explorys dataset (from 2000 to November 3, 2017), we created a diabetes cohort by selecting all patients with at least one encounter with a diagnostic code (primary or secondary) for T2D (ICD9 codes 250.x0, 250.x2, and ICD10 code E11.*), an HbA1c measure ≥ 6.5%, or a prescription for an antidiabetic medication. We excluded those who had a diagnosis of type 1 diabetes, gestational diabetes, or polycystic ovarian syndrome. This resulted in a set of ~ 4.27 million patients, among whom ~ 2.3 million had at least one valid prescription for antidiabetic medication. Among the ~ 2.3 million patients, 69% had at least one OAD prescription and 24% had at least one prescription of BI [neutral protamine Hagedorn (NPH), glargine, detemir, or degludec]. Approximately 85% of BI prescriptions identified for this study were filled after 2011.
For this analysis, we were interested in patients who progressed from any OAD regimen to BI. To assess treatment regimens we calculated prescription length as the time between the prescription start date and prescription end date. Where a valid prescription end date was given, that date was used as provided. An end date was considered valid if it occurred:
Between 2000 and the analysis date and
After the prescription start date and
A maximum of 12 months after the prescription date.
For oral prescriptions without end dates, we used a proxy of twice as many days as the median population valid prescription duration past the start date. A valid prescription is a prescription with a start date which is not the same as the end date (if it exists), and where the prescription is not flagged as erroneous or cancelled. For insulin prescriptions without valid end dates, we inferred the end date to be 1 year after the prescription start date.
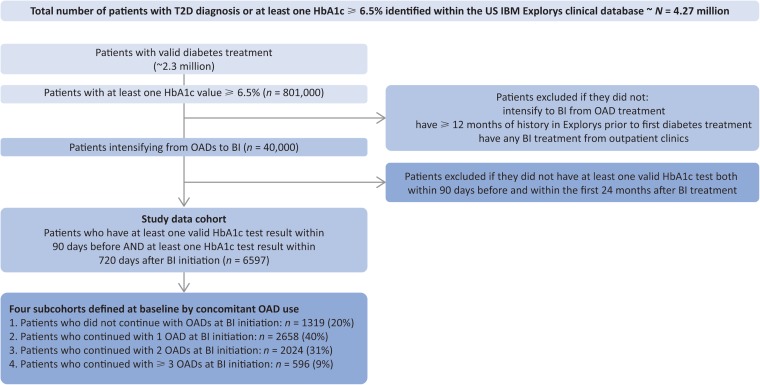
Our analysis cohort included patients who had progressed to BI from one or more OAD(s). Patients who did not have any encounters recorded within Explorys for the year prior to the first diabetes prescription were excluded. The date of first prescription of BI was defined as the index date. Patients were included if they had at least one valid HbA1c test recorded (i.e., positive HbA1c value) within 90 days prior to and including the index date AND at least one valid HbA1c test recorded within 24 months (720 days) after the index date. If a patient had multiple valid HbA1c records prior to (and including) the index date, the last value was used as the baseline. Patient follow-up continued until the first BI regimen ended or was changed, until they reached 2 years after BI initiation, or until the database cutoff date for this analysis was reached. Overlapping BI prescriptions or BI prescriptions with < 90 days of gap between the end of one prescription and the start of another were collapsed into the same regimen. Only outpatient BI prescriptions were included in the analysis. Data were stratified by the number of concomitant OADs at the time of BI initiation (i.e., BI only, BI + 1 OAD, BI + 2 OADs, and BI + ≥ 3 OADs), creating four subcohorts. A flow diagram showing inclusion and exclusion of the patients in the study cohort is shown in Fig. 1.
Fig. 1.
Patient selection from the database. BI basal insulin, HbA1c glycated hemoglobin, OAD oral antidiabetic, T2D type 2 diabetes
This article is based on the existing EMR database and does not contain any studies with human participants or animals performed by any of the authors.
Statistical Approach
Descriptive statistics were calculated to describe the baseline demographics and clinical characteristics of the study cohort and subcohorts. Means and standard deviations (SD) are reported for continuous variables and percentages are provided for categorical variables.
Each quarter post-index date was defined to be 90 days, i.e., 0–90 days as the first quarter (0–3 months); 91–180 days as the second quarter (3–6 months), etc. The descriptive statistics of HbA1c change from baseline were calculated semi-annually (i.e., every 180 days) following BI initiation.
To assess the patients’ response to BI treatment within 24 months after the index date, we defined the target goal of glycemic control as achieving an HbA1c < 7%. We calculated the percentage of patients who reached glycemic control for the first time among those who had not reached glycemic control and were still on BI and had any EMR record in the corresponding periods.
We estimated the probability of achieving glycemic control after BI initiation in two ways:
The conditional probability was estimated as the proportion of patients who reached their first glycemic control within a specific quarter among those patients who had not previously achieved glycemic control, who were still taking their BI regimen, and who had a valid HbA1c test recorded in that quarter. As this denominator changed at each quarter, conditional probability was calculated quarterly, not cumulatively over time.
The cumulative probability of patients reaching first glycemic control over time was estimated via Kaplan–Meier curves for the whole study cohort as well as the four subcohorts. Log-rank tests were done to compare the subcohorts. Censoring occurred at the end of the BI regimen (including switching to a new non-BI regimen), loss of record in the database, or the cutoff date for the analysis.
Results
Baseline Demographics and Clinical Characteristics
Our study cohort included a total of 6597 patients selected from the clinical EMR database (Fig. 1). Patient demographic and clinical characteristics data at baseline (i.e., last value within 90 days prior to and including the index date) are shown in Table 1. The study cohort was representative of the US population with T2D in terms of age (62 ± 12.7 years), race (75% white, 13% African-American), insurance coverage (47% private, 36% Medicare, 7% Medicaid) and most common comorbidities (81% hypertension, 70% obesity, 50% dyslipidemia, 24% heart disease, and 20% anemia, which may be associated with diabetic chronic kidney disease). The most common OADs taken were metformin (79%), sulfonylureas (63%), DPP4 inhibitors (30%), and thiazolidinediones (24%). At BI initiation, the mean (SD) of HbA1c was 9.1% (2.1%), with 3219 (48.8%) of the 6597 patients having an HbA1c > 9%. Before BI initiation, 3856 (58.5%) of the 6597 patients were on one OAD only, 2032 (30.8%) were on two OADs, and 709 (10.7%) were on at least three OADs. At BI initiation, about 20% of patients were prescribed BI alone, while 40%, 31%, and 9% were prescribed BI together with one OAD, two OADs, and at least three OADs, respectively (Table 2). The four subcohorts appeared to be similar in terms of age, sex, HbA1c, and body mass index at baseline. Diabetes duration could not be provided, as the diagnosis of T2D for some patients predated their entry into the Explorys database.
Table 1.
Baseline demographics and characteristics of the study cohort and overall T2D cohorts in the Explorys database
| T2D patients in the US IBM Explorys database as of November 3, 2017 (N = 4.27 M) | Study cohort at time of BI initiation (n = 6597) | |
|---|---|---|
| Female, n (%) | 2.27 M (53) | 3042 (46) |
| Mean age ± SD, years | 58 ± 15.7 | 62 ± 12.7 |
| Median age, years | 60 | 62 |
| ≥65 years, n (%) | 1.65 M (38.7) | 3042 (43) |
| Race, n (%) | ||
| White | 2.6 M (61) | 4979 (75) |
| African-American | 574 K (14) | 882 (13) |
| Asian | 85 K (2) | 67 (1) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 243 K (6) | 466 (7) |
| Unknown | 1.1 M (27) | 318 (5) |
| Insurance, n (%) | ||
| Private | 1.6 M (38) | 3099 (47) |
| Medicare | 1.1 M (27) | 2377 (36) |
| Self-pay | 185 K (5) | 192 (3) |
| Medicaid | 241 K (6) | 425 (7) |
| Unknown | 1 M (23) | 205 (3) |
| Comorbidity, n (%) | ||
| Hypertension | 2.6 M (62) | 5336 (81) |
| Dyslipidemia | 1.3 M (30) | 3308 (50) |
| Obesity | 2.1 M (50) | 4671 (70) |
| Anemia | 1 M (24) | 1352 (20) |
| Heart disease | 1 M (23) | 1584 (24) |
| Prescription medication, n (%) | ||
| OADs | N/A | 6597 (100) |
| Metformin | N/A | 5206 (79) |
| Sulfonylureas | N/A | 4135 (63) |
| DPP4 inhibitors | N/A | 2006 (30) |
| TZDs | N/A | 1561 (24) |
| SGLT-2 inhibitors | N/A | 206 (3) |
| BI | N/A | 6597 (100) |
BI basal insulin, DPP4 dipeptidyl peptidase 4, K thousand, M million, OAD oral antidiabetic, SD standard deviation, SGLT-2 sodium–glucose cotransporter 2, T2D type 2 diabetes, TZD thiazolidinedione
Table 2.
Baseline characteristics of subcohorts at time of BI initiation
| BI only | BI + 1 OAD | BI + 2 OADs | BI + ≥3 OADs | Total | |
|---|---|---|---|---|---|
| Patients, n (%) | 1319 (20) | 2658 (40) | 2024 (31) | 596 (9) | 6597 (100) |
| Female, % | 50 | 48 | 44 | 38 | 46 |
| Mean ± SD age, years | 64.5 ± 12.5 | 61.6 ± 13.1 | 60.8 ± 12.4 | 61.3 ± 11.5 | 61.9 ± 12.7 |
| Mean ± SD HbA1c at BI initiation, % | 8.7 ± 2.0 | 9.3 ± 2.2 | 9.3 ± 2.0 | 9.1 ± 2.0 | 9.1a ± 2.1 |
| Mean ± SD BMI at BI initiation, kg/m2 | 33.2 ± 7.8 | 33.5 ± 7.6 | 33.8 ± 7.6 | 33.8 ± 7.3 | 33.6 ± 7.6 |
BI basal insulin, BMI body mass index, HbA1c glycated hemoglobin, OAD oral antidiabetic, SD standard deviation
aAt baseline, 3219 (48.8%) of the 6597 patients had an HbA1c > 9.0%
HbA1c Change Over Time
Based on the available HbA1c data in the cohort in each 6-month interval, the change from baseline in HbA1c was estimated. Mean (SD) HbA1c decreased 1.49 (2.63) percentage points from baseline to 6 months, with no further reductions thereafter (Table 3). As not all patients had an HbA1c value in each period, the members of the cohort differed in each period.
Table 3.
HbA1c change over time in the first 2 years post-BI initiation
| Duration post-BI initiation | Number of patients with HbA1c records in this period | HbA1c change from baseline, mean (SD) |
|---|---|---|
| 0–6 months | 5679 | − 1.49 (2.63) |
| 6–12 months | 3600 | − 1.43 (2.69) |
| 12–18 months | 861 | − 1.44 (2.69) |
| 18–24 months | 331 | − 1.49 (2.88) |
Only those with a valid HbA1c record at baseline and the corresponding time period were included for the calculations. Few patients had multiple HbA1c records across two or more periods post-BI initiation
BI basal insulin, HbA1c glycated hemoglobin, SD standard deviation
Patients Achieving Glycemic Control (HbA1c < 7%)
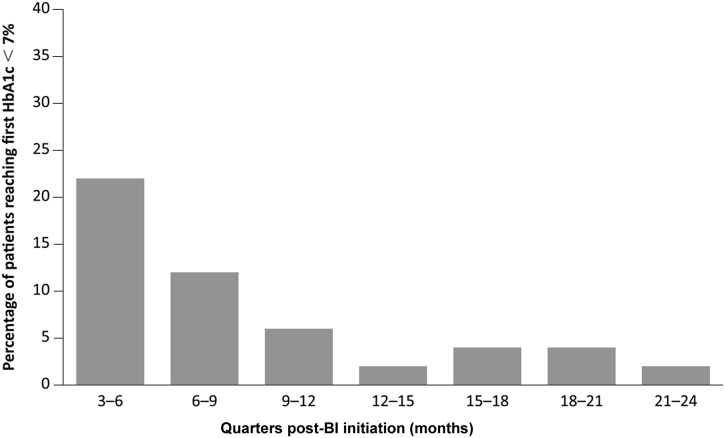
The percentage of patients who registered an HbA1c < 7% for the first time, out of the number of those patients who were still in the cohort, was calculated for each quarter after the index date (Fig. 2, Table 4). During the second quarter after the index date, there were 6086 patients who had HbA1c ≥ 7% prior to the beginning of the period and were continuing on BI treatment, among whom a total of 1311 (21.5%) patients had reached HbA1c < 7% for the first time in that quarter. Similar calculations were done for every subsequent quarter until 24 months. The percentages of patients reaching HbA1c < 7% for the first time in that quarter are shown in Fig. 2 and Table 4, column E.
Fig. 2.
Percentage of patients reaching their first HbA1c < 7% post-BI initiation. The numerator is defined as the number of patients who reached glycemic control (HbA1c < 7%) for the first time during each corresponding quarter; the denominator is defined as the number of patients who did not reach glycemic control prior to that quarter AND were still on BI treatment AND had at least one valid electronic medical records entry in that quarter. BI basal insulin, HbA1c glycated hemoglobin
Table 4.
Conditional probability of reaching first glycemic control (HbA1c < 7%)
| A: time after BI initiation | B: number of patients who had not reached glycemic control previously AND were still on BI treatment within this quarter | C: number of patients who had not reached glycemic control previously AND were still on BI treatment AND had at least one valid HbA1c record within this quarter | D: number of patients who reached their first glycemic control within this quarter | E: percentage (%) of patients in column D among the patients in column B | F: estimated conditional probability (% of patients in column D among patients in column C), % (95% CI) |
|---|---|---|---|---|---|
| 3–6 months | 6086 | 4933 | 1311 | 21.5 | 26.6 (25.4, 27.8) |
| 6–9 months | 4102 | 2767 | 487 | 11.9 | 17.6 (16.2, 19.0) |
| 9–12 months | 2423 | 1668 | 143 | 5.9 | 8.6 (7.3, 9.9) |
| 12–15 months | 1679 | 684 | 37 | 2.2 | 5.4 (3.7, 7.1) |
| 15–18 months | 597 | 361 | 22 | 3.7 | 6.1 (3.6, 8.6) |
| 18–21 months | 365 | 260 | 14 | 3.8 | 5.4 (2.7, 8.1) |
| 21–24 months | 216 | 147 | 5 | 2.3 | 3.4 (0.5, 6.3) |
BI basal insulin, CI confidence interval, HbA1c glycated hemoglobin
Estimation of Conditional Probabilities
In each quarter post-index date, approximately 2/3 of the patients had a valid HbA1c measurement (Table 4, columns B and C). From patients who had a valid HbA1c measurement, we estimated the conditional probabilities of reaching the glycemic target of HbA1c < 7% for the first time beginning at the second quarter after BI initiation (Table 4, column F). For those patients who had not reached HbA1c < 7% in the first quarter (90 days) following BI initiation, the probability of reaching first glycemic control in the second quarter was 26.6%. This conditional probability decreased to 17.6% during the third quarter and to 8.6% in the fourth quarter after BI initiation. After 12 months post-BI initiation, it diminished further to ≤ 6.1% during any quarter in the second year.
Time to Reach First Glycemic Control Analyses
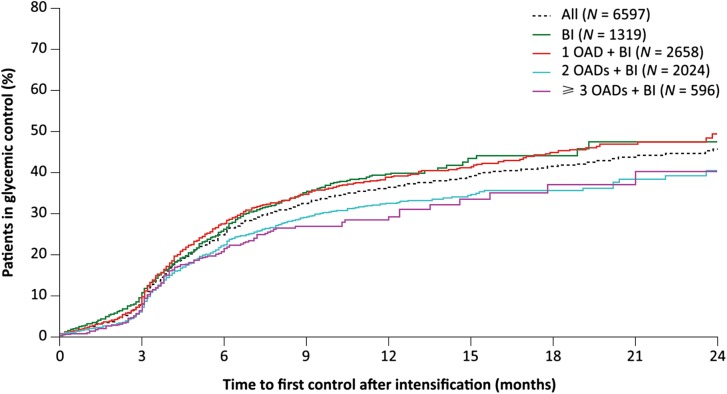
The Kaplan–Meier curves of the time to reach HbA1c < 7% showed similar trends for the overall cohort (Fig. 3, dotted line) as well as across all four subcohorts of concomitant OAD usage (Fig. 3, solid lines). All curves rose gradually over the first 3 months, with a sharper increase over the remainder of the first year, followed by a much more gradual increase over the second year. Overall, approximately 25%, 38%, 42%, and 46% of eligible patients achieved their first HbA1c < 7% by 6, 12, 18, and 24 months after BI initiation, respectively (estimated from the overall cohort Kaplan–Meier curve). Patients in the two subcohorts treated with BI only or BI + 1 OAD appeared to have a slightly higher rate of achieving HbA1c < 7% than those treated with BI + 2 or at least 3 OADs, but the differences did not reach statistical significance (p = 0.27).
Fig. 3.
Kaplan–Meier curves for time to reach glycemic control (HbA1c < 7%) for the overall study cohort and the four subcohorts. BI basal insulin, HbA1c glycated hemoglobin, OAD oral antidiabetic
Discussion
In this real-world study based on a large US EMR database, our study cohort of patients initiating BI from OAD(s) had a mean HbA1c of 9.1%, and approximately 49% had an HbA1c > 9.0% at baseline. This underscores that patients may remain on OAD(s) for extended periods, despite being significantly above recommended glycemic control targets. Further, it indicates that the patients selected for RCTs of BI are generally in better glycemic control than patients who initiate BI in real life; in fact, the average baseline HbA1c was higher in our study than in 36 of 44 RCTs of BI included in a systematic review [7]. However, the results presented here are in line with previous real-world studies in which HbA1c at initiation of BI was well above the recommended target, showing that early intensification to the recommended BI treatment is uncommon in routine clinical care [11, 17, 18]. Factors underlying such delays are complex [20–22] and may reflect physicians’ perceptions of patients’ lifestyles and non-adherence to existing medications; and patients’ concerns about out-of-pocket expenses, and reluctance to consider BI, as well as fear of hypoglycemia and concern about weight gain [11, 23].
After initiation of BI (with or without concomitant OADs), there was a robust drop in mean HbA1c of about 1.5% over the first 6 months, but with no additional change from baseline over subsequent quarters. Few real-world studies have examined both HbA1c change after 3–6 months of BI treatment and after long-term follow-up [11, 12, 17]. A US retrospective analysis of patients with T2D found that in 4387 new initiators of BI with an average HbA1c of 9.5% at baseline, the HbA1c decrease of 1.3% at 3 months was the same as the decrease from baseline at 12 months [11]. Similarly, a retrospective longitudinal analysis of EMR from five European countries and the USA showed a similar trend, with the majority of the mean HbA1c decline within the initial 6 months, and no further improvement after 12 months [17]. As these data represent a cross-sectional analysis of a cohort, no consideration is given to the impact of patients who may achieve control initially, and subsequently return to an HbA1c ≥ 7% again.
To better mimic the situation of the healthcare practitioner (HCP), we estimated the conditional probability of reaching glycemic control if continuing BI treatment, beginning in the second quarter post-BI initiation until 2 years, given the condition that glycemic control has not been achieved up to the beginning of the specific quarter. This provides a potentially useful perspective for prescribers who initiate their patients on BI and need to estimate the likelihood over time that a patient will achieve glycemic goals. Our study results suggest that if a patient has been using the same BI regimen for 6 or 12 months and has not yet reached their glycemic target, the likelihood of achieving success on the same regimen is low, which should prompt consideration of treatment modification or intensification. While the estimated probabilities can be affected by the relative robustness of the EMR database and need further confirmation, the overall decreasing trend and the very low probabilities of reaching HbA1c targets after the first year highlight the need for paying attention to patients who have failed to achieve glycemic targets in the first year following BI initiation. There is often a delay in treatment intensification despite persistently elevated glucose levels [13, 14, 24]. In a real-world observational study published in 2016, the median time to treatment intensification in patients with elevated HbA1c following BI initiation was 4.3 years [24]. Multiple factors may contribute to such delays [25] including concerns related to some treatment options beyond BI. Basal-bolus and premix insulin regimens are potential options, but hypoglycemia, weight gain, and the need to take multiple daily injections can be major concerns [26–28]. With the recent development of medications such as GLP-1 RAs, fixed-ratio combinations of BIs and GLP-1 RAs, and SGLT2 inhibitors, which do not increase the risk of hypoglycemia or induce weight gain [29–31], prescribers now have more options to consider.
The cumulative probability of reaching glycemic control over time (Fig. 3) revealed that about 38% of patients reached glycemic control in the first 12 months but only about 8% more did so in the second year. These results are also in line with other real-world research [11, 17, 18] which has generally found little further increase in rates of patients with HbA1c below the glycemic target with extended treatment. The previously mentioned US-based retrospective analysis [11] found an increase in patients below target for new initiators of BI, from 11% at baseline to 27% at 3 months after BI initiation; however, this fell to 25% after 12 months. The aforementioned retrospective longitudinal EMR analysis from five European countries and the USA [17] found that 20.9% had an HbA1c ≤ 7% at 3 months after BI initiation; by 24 months post-initiation this had only increased to 27.8%. An additional US EMR analysis found that 44% of its cohort achieved HbA1c ≤ 7% within 1 year after BI initiation, with 58% reaching this goal over the entire 2.5-year follow-up [18]. Differences in the patient group (39.2% of this cohort had HbA1c > 9% at baseline, compared with 48.8% in our study) and the specific glycemic goal may play a role in the results.
In the current study, the subcohorts of patients taking no or one concomitant OAD at BI initiation had a slightly higher rate of achieving HbA1c < 7% than those taking two or more OADs, which might reflect differences in stage of disease progression. While the Explorys database cannot definitively provide disease duration for the majority of patients, it is possible that those patients who continued treatment with two or more OADs while initiating BI were considered more progressed and therefore had lower residual beta cell function, which could impact glycemic response to BI treatment. Corresponding broadly to results seen here, a retrospective database analysis of 1830 patients found greater achievement of glycemic targets among those patients taking fewer OADs at baseline (38.2%, 26.7%, and 19.6% for patients taking one, two, and at least three OADs, respectively; p < 0.0001) [15]. There could have been other contributing factors; for example, patients on more medications may have more challenges to be compliant with the prescriptions.
Our study has some important limitations. In the USA, patients change insurance coverage and/or HCPs from time to time because of employer decisions, job changes, relocations, personal choices, etc.; the Health Insurance Portability and Accountability Act rules typically preclude the database from linking multiple records from different sources using personal information. The EMR data collected in the Explorys database provides only a snapshot of patients’ medical histories, and cannot provide the rigor and completeness of data that is typically expected from a prospective longitudinal clinical trial. Laboratory variables are assessed for clinical practice instead of research purposes, and are from multiple sources, obtained as per local clinical practices. HbA1c measurements were not consistently performed every 3 months in many patients, reducing the assessable number of patients included in the analysis. In addition, it is possible that HbA1c measurements may have occurred outside of the data capture infrastructure, and it cannot be guaranteed that those patients with missing HbA1c records behave the same as those with available HbA1c results. The paucity of fasting plasma glucose data and of dosing information in the database are other limitations which make it difficult to evaluate titration practice after BI initiation, or any possible relationship between insulin dose and glycemic response. Given the difficulty of achieving glycemic goals reported in real-world literature, clinicians may find value in understanding which factors predict achievement of an HbA1c target. For example, assessing durability of glycemic control in those treated with BI, along with treatment patterns after BI initiation, can add important further information on factors associated with significantly higher rates of reaching the goal of HbA1c ≤ 7% [18]. We did not do this analysis in our current study because of limitations of the data, but it is our plan to do so in ongoing studies.
Conclusions
Our study of real-world data from a large US database suggests that, among patients with T2D who initiated BI after the use of OADs, the likelihood of newly reaching glycemic control diminished over time, regardless of whether BI was taken alone or together with OADs. The ADA or American Association of Clinical Endocrinologists guidelines recommend some change to treatment if patients do not achieve control within 3 months. Our data suggest that maintaining a BI regimen over 12 or 24 months in a patient who has not yet reached the HbA1c < 7% target provides little additional benefit towards reaching this goal, and that prescribers should consider additional medication options if a patient does not reach glycemic control within 12 months of BI initiation. Information generated from a large real-world EMR database offers complementary information to that provided by RCTs, with the potential for additional insights of use for HCPs, healthcare systems, payers, and industry.
Acknowledgements
The authors would like to acknowledge Sharon Hensley Alford for her contributions to the manuscript.
Funding
This study and article processing charges for this journal article were sponsored by Sanofi.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Rob Coover of Caudex (New York, NY, USA). Support for this assistance was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Lawrence Blonde has received grant/research support from AstraZeneca, Janssen, Lexicon, Merck, Novo Nordisk, and Sanofi; speaker honoraria from AstraZeneca, Janssen, and Merck; and consultancy fees from AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Janssen, Merck, and Novo Nordisk. Luigi Meneghini has received advisory board/consultancy fees from Novo Nordisk, Sanofi, and Intarcia. Anders Boss is an employee of Sanofi. Alka Shaunik is an employee of Sanofi. Claire Brulle-Wohlhueter is an employee of Sanofi. Xuejun Victor Peng is an employee of Sanofi. Kyu Rhee is an employee of Watson Health, IBM. Supriya Kumar is an employee of Watson Health, IBM. Sidhartha Balodi is an employee of Watson Health, IBM. Rory J McCrimmon has received consultancy fees from Lilly, Novo Nordisk, and Sanofi; and speaker bureau fees from Novo Nordisk and Sanofi.
Compliance with Ethics Guidelines
This article is based on an existing EMR database and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
These analyses were conducted on medical records data provided under a commercial licence, which the authors are unable to share.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to 10.6084/m9.figshare.5986948.
References
- 1.Centers for Disease Control. National diabetes statistics report, 2017. [article online], 2018. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 22 Jan 2018.
- 2.Dandona P. Minimizing glycemic fluctuations in patients with type 2 diabetes: approaches and importance. Diabetes Technol Ther. 2017;19(9):498–506. doi: 10.1089/dia.2016.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed]
- 4.Stokes A, Preston SH. Deaths attributable to diabetes in the United States: comparison of data sources and estimation approaches. PLoS One. 2017;12(1):e0170219. doi: 10.1371/journal.pone.0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S55–S64. doi: 10.2337/dc18-S006. [DOI] [PubMed] [Google Scholar]
- 7.Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target < 7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab. 2012;14(3):228–233. doi: 10.1111/j.1463-1326.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 8.Hermansen K, Davies M, Derezinski T, Martinez RG, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29(6):1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 9.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract. 2016;22(1):84–113. doi: 10.4158/EP151126.CS. [DOI] [PubMed] [Google Scholar]
- 10.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalal MR, Grabner M, Bonine N, Stephenson JJ, DiGenio A, Bieszk N. Are patients on basal insulin attaining glycemic targets? Characteristics and goal achievement of patients with type 2 diabetes mellitus treated with basal insulin and physician-perceived barriers to achieving glycemic targets. Diabetes Res Clin Pract. 2016;121:17–26. [DOI] [PubMed]
- 12.Gordon J, Pockett RD, Tetlow AP, McEwan P, Home PD. A comparison of intermediate and long-acting insulins in people with type 2 diabetes starting insulin: an observational database study. Int J Clin Pract. 2010;64(12):1609–1618. doi: 10.1111/j.1742-1241.2010.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418–e424. [PMC free article] [PubMed] [Google Scholar]
- 14.Heintjes EM, Thomsen TL, Penning-van Beest FJ, Christensen TE, Herings RM. Glycemic control and long-acting insulin analog utilization in patients with type 2 diabetes. Adv Ther. 2010;27(4):211–222. doi: 10.1007/s12325-010-0020-y. [DOI] [PubMed] [Google Scholar]
- 15.Levin PA, Zhou S, Durden E, Farr AM, Gill J, Wei W. Clinical and economic outcomes associated with the timing of initiation of basal insulin in patients with type 2 diabetes mellitus previously treated with oral antidiabetes drugs. Clin Ther. 2016;38(1):110–121. doi: 10.1016/j.clinthera.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Lin SD, Tsai ST, Tu ST, et al. Glycosylated hemoglobin level and number of oral antidiabetic drugs predict whether or not glycemic target is achieved in insulin-requiring type 2 diabetes. Prim Care Diabetes. 2015;9(2):135–141. doi: 10.1016/j.pcd.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19(19):1155–1164. doi: 10.1111/dom.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu N, Aagren M, Boulanger L, Friedman M, Wilkey K. Assessing achievement and maintenance of glycemic control by patients initiating basal insulin. Curr Med Res Opin. 2012;28(10):1647–1656. doi: 10.1185/03007995.2012.722989. [DOI] [PubMed] [Google Scholar]
- 19.Kostev K, Dippel FW, Rathmann W. Glycemic control after initiating basal insulin therapy in patients with type 2 diabetes: a primary care database analysis. Diabetes Metab Syndr Obes. 2015;8:45–8. [DOI] [PMC free article] [PubMed]
- 20.Kim SG, Kim NH, Ku BJ, et al. Delay of insulin initiation in patients with type 2 diabetes mellitus inadequately controlled with oral hypoglycemic agents (analysis of patient- and physician-related factors): a prospective observational DIPP-FACTOR study in Korea. J Diabetes Investig. 2017;8(3):346–353. doi: 10.1111/jdi.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28(11):2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 22.Strain WD, Cos X, Hirst M, et al. Time to do more: addressing clinical inertia in the management of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2014;105(3):302–312. doi: 10.1016/j.diabres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12. doi: 10.1016/j.pcd.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401–409. doi: 10.1111/dom.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berard L, Bonnemaire M, Mical M, Edelman S. Insights into optimal basal insulin titration in type 2 diabetes: results of a quantitative survey. Diabetes Obes Metab. 2018;20(2):301–308. doi: 10.1111/dom.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFarlane SI. Insulin therapy and type 2 diabetes: management of weight gain. J Clin Hypertens (Greenwich). 2009;11(10):601–607. doi: 10.1111/j.1751-7176.2009.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polonsky WH, Thompson S, Wei W, et al. Greater fear of hypoglycaemia with premixed insulin than with basal-bolus insulin glargine and glulisine: patient-reported outcomes from a 60-week randomised study. Diabetes Obes Metab. 2014;16(11):1121–1127. doi: 10.1111/dom.12328. [DOI] [PubMed] [Google Scholar]
- 28.Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med. 2005;20(5):479–482. doi: 10.1111/j.1525-1497.2005.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai X, Gao X, Yang W, Ji L. Comparison between insulin degludec/liraglutide treatment and insulin glargine/lixisenatide treatment in type 2 diabetes: a systematic review and meta-analysis. Expert Opin Pharmacother. 2017;18(17):1789–1798. doi: 10.1080/14656566.2017.1400011. [DOI] [PubMed] [Google Scholar]
- 30.Steen O, Goldenberg RM. The role of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Can J Diabetes. 2017;41(5):517–523. doi: 10.1016/j.jcjd.2017.08.241. [DOI] [PubMed] [Google Scholar]
- 31.Valentine V, Goldman J, Shubrook JH. Rationale for, initiation and titration of the basal insulin/GLP-1RA fixed-ratio combination products, IDegLira and iGlarLixi, for the management of type 2 diabetes. Diabetes Ther. 2017;8(4):739–752. doi: 10.1007/s13300-017-0287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These analyses were conducted on medical records data provided under a commercial licence, which the authors are unable to share.