Abstract
Introduction
Treatment guidelines recommend a stepwise approach to glycemia management in patients with type 2 diabetes (T2D), but this may result in uncontrolled glycated hemoglobin A1c (HbA1c) between steps. This retrospective analysis compared clinical and economic outcomes among patients with uncontrolled T2D initiating two oral antidiabetes drugs (OADs), glucagon-like peptide-1 receptor agonists (GLP-1 RAs), or basal insulin in a real-world setting.
Methods
Adults with T2D on OAD monotherapy were identified in the MarketScan claims database (2007–2014). Those initiating two OADs (simultaneously or sequentially), GLP-1 RAs, or basal insulin were selected (date of initiation was termed the ‘index date’); patients were required to have HbA1c > 7.0% in the 6 months pre-index date. HbA1c was compared from 6 months pre- to 1-year post-index. Annual all-cause healthcare utilization and costs were reported over the 1-year follow-up period.
Results
Data for 6054 patients were analyzed (2-OAD, n = 4442; GLP-1 RA, n = 361; basal insulin, n = 1251). Baseline HbA1c was high in all cohorts, but highest in the basal-insulin cohort. Treatment initiation resulted in reductions in HbA1c in all cohorts, which was generally maintained throughout the follow-up period. Average HbA1c reductions from the 6 months pre- to 1 year post-index date were −1.2% for GLP-1 RA, −1.6% for OADs, and −1.8% for basal insulin. HbA1c < 7.0% at 1 year occurred in 32.6%, 47.5%, and 41.1% of patients, respectively. Annual healthcare costs (mean [SD]) were lowest for OAD (US$10,074 [$22,276]) followed by GLP-1 RA (US$14,052 [$23,829]) and basal insulin (US$18,813 [$37,332]).
Conclusion
Despite robust HbA1c lowering following treatment initiation, many patients did not achieve HbA1c < 7.0%. Basal insulin, generally prescribed for patients with high baseline HbA1c, was associated with a large reduction in HbA1c and with higher costs. Therapy intensification at an appropriate time could lead to clinical and economic benefits and should be investigated further.
Funding
Sanofi U.S., Inc.
Electronic supplementary material
The online version of this article (10.1007/s13300-018-0429-x) contains supplementary material, which is available to authorized users.
Keywords: Basal insulin, Clinical inertia, GLP-1 RA, Oral antidiabetes drugs, Treatment intensification, Type 2 diabetes
Introduction
The American Diabetes Association (ADA) Standards of Medical Care in Diabetes recommend individualized treatment to achieve control of glycated hemoglobin A1c (HbA1c) levels soon after diagnosis [1]. The ADA-recommended HbA1c goal for most people with diabetes is < 7.0%, while that from the American Association of Clinical Endocrinologists and the American College of Endocrinology is ≤ 6.5% [2]. Treatment strategies should be designed to attain and maintain goals to prevent or delay hyperglycemia-related complications [1]. HbA1c targets may be achieved and maintained with a stepwise approach to treatment intensification, based on regular HbA1c testing to identify the need for additional therapy if treatment goals are not met [1]. Often, the recommended first step in pharmaceutical treatment is metformin. Guidelines recommend that if a patient’s HbA1c level is above goal after 3 months, a second agent should be added to metformin. The ADA recommends the addition of one of the following six available treatment options: sulfonylurea, thiazolidinedione, dipeptidyl peptidase-4 (DPP-4) inhibitor, sodium-glucose co-transporter 2 (SGLT-2) inhibitor, glucagon-like peptide-1 receptor agonist (GLP-1 RA), or basal insulin [1]. If the HbA1c goal is not achieved or the patient’s glycemic control is not maintained with dual therapy, triple therapy can be initiated [1]. For patients with marked hyperglycemia (HbA1c ≥ 10.0%) at diagnosis, initial treatment with insulin may be considered [1]. Combination injectable therapy comprising basal insulin plus preferably a GLP-1 RA or rapid-acting insulin (or a change to premixed insulin twice daily) is usually the next recommended step in the treatment course if HbA1c remains above target [1]. The latest algorithm from the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) recommends initiation with combination therapy rather than monotherapy in patients presenting with HbA1c ≥ 7.5% [if the HbA1c goal is ≤ 6.5%] and initiating treatment with insulin, with or without other agents, in symptomatic patients presenting with HbA1c > 9.0% [3]. AACE/ACE recommends antihyperglycemic agents in descending order of preference.
However, the often-used stepwise approach to diabetes treatment may not be ideal, as it can leave patients exposed to periods of uncontrolled HbA1c between treatment steps [4, 5]. In a study by Khunti et al., for example, among type 2 diabetes (T2D) patients with HbA1c ≥ 7.0% who were receiving one oral antidiabetes drug (OAD), patients had 2.9 years between initiation of the first OAD and the second and > 7 years before initiation of basal insulin [5]. Furthermore, a stepwise approach may not be effective for many patients—a previous study observed that only 53% of patients in the USA receiving stepwise therapy achieved and maintained a target HbA1c level of < 7.0% [6].
While having a choice of therapies can be beneficial, the variety of treatment options available to clinicians and patients for initiating intensified regimens can also complicate individualized patient management. Despite elevated HbA1c levels, a number of physician-, patient-, and healthcare delivery system-related resistance factors may contribute to delays in treatment intensification [7–10]. These include senses of loss of control or of personal failure and injection-related anxiety among some patients who have had recommendations for initiating insulin or GLP-1 receptor agonist therapy and concerns about hypoglycemia and/or weight gain and perceived low efficacy of insulin among some patients and providers [7, 10]. This failure of therapy intensification despite an inadequate achievement of target glycemia with the current treatment regimen is known as clinical inertia [11] or clinical myopia [9] and is an established problem contributing to prolonged elevated glycemic levels despite treatment with OADs [4, 5, 12]. Results from published studies continue to demonstrate that clinical inertia, as a delay or a lack of treatment intensification in people with T2D, is common in clinical practice [5, 13, 14].
Alongside the medical consequences for patients with diabetes, clinical inertia also has an impact on healthcare expenditures; overcoming clinical inertia to provide a sustained reduction in HbA1c levels has been associated with significant cost savings and reduced healthcare utilization [15–17]. Thus, overcoming clinical inertia has the potential to improve both clinical and economic outcomes in T2D patients. The Assessment of Clinical Treatment Inertia and Outcomes aNalysis (ACTION) was conducted to compare clinical and economic outcomes in patients with uncontrolled T2D (HbA1c > 7.0%) escalating current OAD therapy to 2-OAD therapy (either simultaneously or sequentially) vs. initiation of GLP-1 RA or basal insulin in a real-world clinical setting.
Methods
Data Source
This was a retrospective analysis of data from the MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits database. This database includes information on > 60 million individuals covered by > 100 large employers and 12 unique health plans as well as retirees with employee-sponsored Medicare supplemental insurance. Information is available on medical and pharmacy claims (including dates and place of service, diagnosis and procedure codes, and costs) as well as patient demographics and periods of continuous health-plan enrollment. Linked laboratory information is also available for approximately 1 million individuals, including laboratory tests (types, results, and dates) ordered by office-based practices. This article does not contain any studies with human participants or animals performed by any of the authors. Research Triangle Institute (RTI) International’s institutional review-board committee determined that this study met all criteria for exemption from full review as the data were retrospective, de-identified, and anonymous [18]. The decision follows Health and Human Services policy detailing which studies can be considered exempt from meeting the requirements of the federal human subjects regulations, including the requirement for initial and annual Institutional Review Board review [19].
Patient Selection Criteria
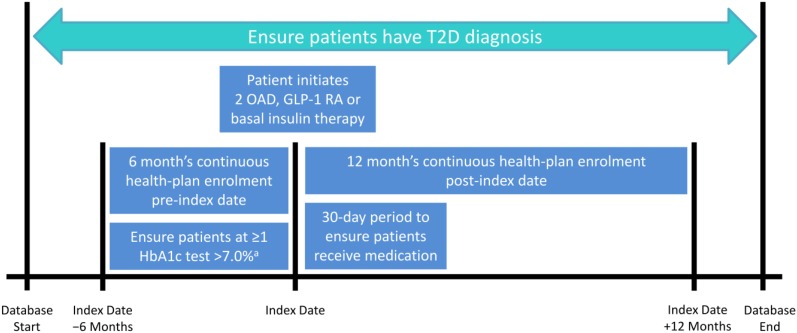
Data were selected from patients aged ≥ 18 years with a diagnosis of T2D (ICD-9-CM codes 250.x0 or 250.x2) between 1 January 2007 and 31 December 2014 who were treated with a single OAD and also had linked laboratory information. The date of initiation of 2-OAD therapy, GLP-1 RA, or basal insulin was designated as the index date. Patients were required to have ≥ 6 months of continuous health-plan enrollment prior to the index date (the baseline period) and ≥ 12 months after the index date (the follow-up period). All patients were required to have ≥ 1 HbA1c test result of > 7.0% in the baseline period (inclusive of the index date).
Patients were selected for inclusion in the 2-OAD cohort if they either simultaneously or sequentially initiated treatment with two OADs; these OADs included the biguanide metformin, sulfonylureas, DPP-4 inhibitors, thiazolidinediones, α-glucosidase inhibitors, amylin analogs, meglitinides, and SGLT-2 inhibitors. Patients could be treatment naive or experienced with prior OAD monotherapy, and were required to remain on both newly initiated OAD medications for a minimum of 30 days. Patients were included in the GLP-1 RA cohort if they received the GLP-1 RA exenatide (short-acting or once-weekly formulation) or liraglutide (other GLP-1 RAs were not available during the study period), regardless of receipt of prior or simultaneous OAD medications. Patients were included in the basal-insulin cohort if they received insulin glargine, insulin detemir, or NPH insulin, regardless of receipt of prior or simultaneous OAD medications; patients receiving premix insulin were excluded. Patients were required to have received their 2-OAD regimen, GLP-1, or basal insulin for a minimum of 30 days.
HbA1c changes were evaluated during each quarter of the follow-up period and for the last available measure of HbA1c among patients with follow-up HbA1c measurements available (note: the last available measure of HbA1c was reported for up to 15 months rather than up to 12 months post-index date). HbA1c was reported among patients with a measurement in the quarter, and it was observed that 38, 38, 34, and 29% of patients had a measurement during quarters 1, 2, 3, and 4, respectively, with 73% of patients having at least one follow-up measurement. The database timeline for patient selection is shown in Fig. 1.
Fig. 1.
Database timeline for patient selection. aMarketScan database not designed to capture clinical outcomes
Study Measures and Data Analyses
Eligible patients were categorized into one of three cohorts (2-OAD, GLP-1 RA, or basal insulin) based on the first observed qualifying treatment or regimen. The following parameters were assessed for each patient cohort and analyzed on an intent-to-treat basis: patient demographics, clinical characteristics, longitudinal HbA1c, HbA1c change from baseline, and total all-cause healthcare resource utilization and costs in the follow-up period. Clinical characteristics included the Charlson Comorbidity Index (CCI) score measured during the baseline period, OADs received during the baseline period, and duration of OAD, GLP-1 RA, or basal-insulin treatment during the follow-up period. Longitudinal HbA1c was recorded at baseline (the closest value to the index date, inclusive of the index date, was reported) for the four quarters of the follow-up period (if more than one measurement was available in each quarter, the measurement closest to the end of the quarter was used) and the last available follow-up (defined as the last available HbA1c test value observed during the 15-month post-index date period, using the last observation carried forward). HbA1c change from baseline included the change from baseline to the last available follow-up. The percentage of patients in the following categories of HbA1c was also reported for the baseline and follow-up measurements: < 7.0%, 7.0 to < 8.0%, 8.0 to < 9.0%, 9.0 to < 10.0%, and ≥ 10.0%. Total all-cause cost data were obtained from medical and pharmacy claims over the 1-year period following the index date, comprising inpatient, emergency-outpatient, physician-office, other ancillary (including laboratory claims and claims in other healthcare settings, such as outpatient hospital), and pharmacy costs. All analyses were descriptive. Cost values represent the total amounts paid to the provider by both health plans and patients (i.e., cost values represent the sum of health plan payments, patient out-of-pocket expenses, and payments by supplemental insurance plans). Cost values were updated to 2014 US dollars using the medical care component of the consumer price index.
Results
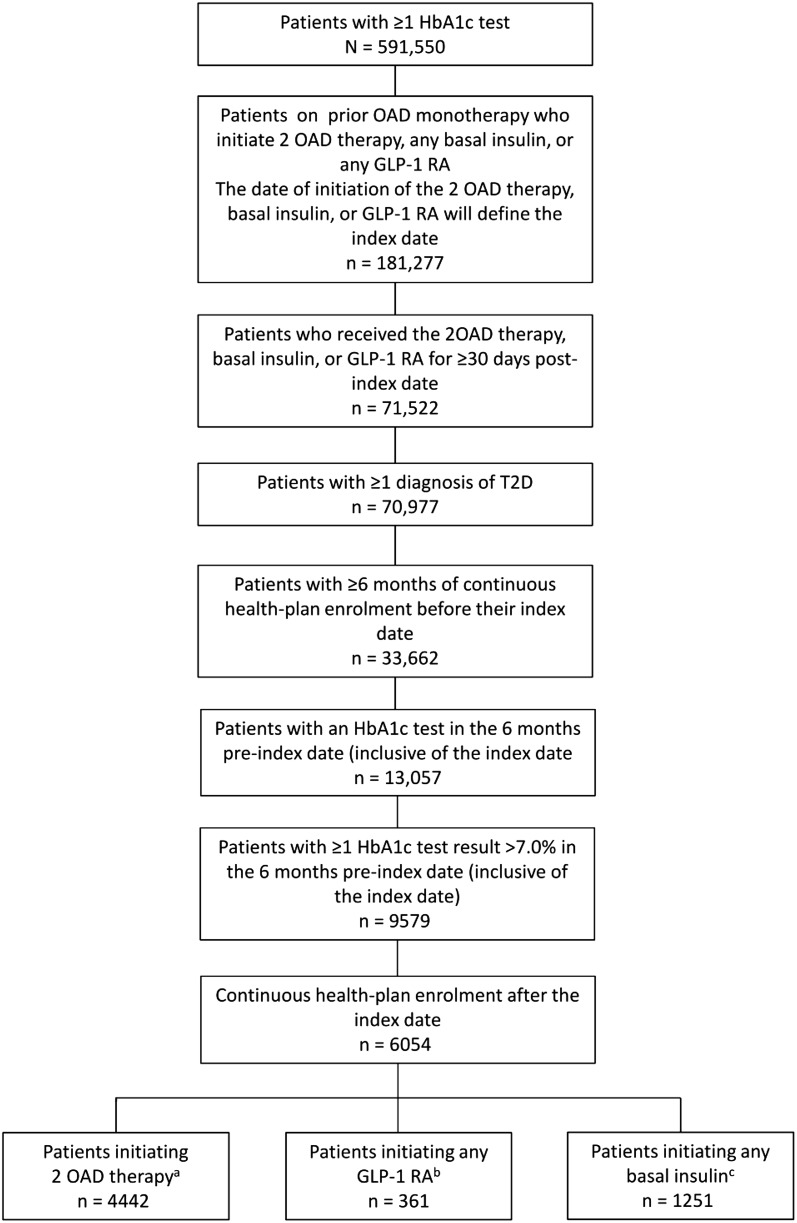
A total of 591,550 T2D patients aged ≥ 18 years were identified as receiving two OADs, GLP-1 RA, or basal insulin in the database. Of these patients, 6054 met all inclusion criteria: 4442 (73.4%) in the 2-OAD cohort, 361 (6.0%) in the GLP-1 RA cohort, and 1251 (20.6%) in the basal-insulin cohort (Fig. 2).
Fig. 2.
Flow chart showing selection of patients for this analysis. Percentages do not add up to 100% because of rounding. aA total of 3897 (74.8%) patients had HbA1c values recorded in the 12-month post-index date period. bA total of 274 (72.9%) patients had HbA1c values recorded in the 12-month post-index date period. cA total of 878 (68.8%) patients had HbA1c values recorded in the 12-month post-index date period
Patients’ demographic and clinical characteristics at baseline are shown in Table 1. Mean HbA1c at baseline was lowest in the GLP-1 RA cohort (8.8%) and highest in the basal-insulin cohort (10.1%). The basal-insulin cohort also had the highest percentage of patients with HbA1c > 10.0% (45.7%). Biguanides were the most common OADs received at baseline; 50.0% of the 2-OAD cohort, 47.4% of the GLP-1 RA cohort, and 25.3% of the basal-insulin cohort were receiving this OAD class. Sulfonylureas were the second most common OAD, with 23.4% of the 2-OAD cohort, 15.8% of the GLP-1 RA cohort, and 15.7% of the basal-insulin cohort receiving sulfonylureas.
Table 1.
Baseline demographic and clinical characteristics of the patients included in this analysis (N = 6054)
| Characteristic | Cohort | ||
|---|---|---|---|
| 2-OAD | GLP-1 RA | Basal insulin | |
| Patients, n (%) | 4442 (73.4) | 361 (6.0) | 1251 (20.6) |
| Demographic characteristics | |||
| Mean (SD) age at index, years | 56.1 (9.8) | 52.5 (10.9) | 53.3 (13.1) |
| Female, n (%) | 1782 (40.1) | 210 (58.3) | 590 (47.2) |
| Region, n (%) | |||
| Northeast | 427 (9.6) | 32 (8.9) | 114(9.1) |
| North central | 591 (13.3) | 59 (16.3) | 202 (16.1) |
| South | 1966 (44.3) | 228 (63.2) | 637 (50.9) |
| West | 1454 (32.7) | 41 (11.4) | 296 (23.7) |
| Missing | 4 (0.1) | 1 (0.3) | 2 (0.2) |
| Commercial payer, n (%) | 3852 (88.1) | 332 (92.7) | 1053 (87.5) |
| Plan type, n (%) | |||
| Preferred provider organization | 2214 (49.8) | 248 (68.7) | 747 (59.7) |
| Health maintenance organization | 1562 (35.2) | 63 (17.5) | 343 (27.4) |
| Other | 636 (14.3) | 50 (13.8) | 151 (12.1) |
| Missing | 30 (0.7) | 0 (0.0) | 10 (0.8) |
| Clinical characteristicsa | |||
| HbA1c, % | |||
| Mean (SD) | 9.1 (1.8) | 8.8 (1.6) | 10.1 (2.38) |
| Median (min, max) | 8.5 (7.1, 17.9) | 8.2 (7.1, 16.1) | 9.7 (7.1, 18.9) |
| HbA1c categories, % patients | |||
| > 7.0–8.0% | 37.1 | 44.3 | 21.7 |
| > 8.0–9.0% | 22.4 | 23.3 | 19.1 |
| > 9.0–10.0% | 14.1 | 12.5 | 13.4 |
| > 10.0% | 26.4 | 19.9 | 45.7 |
| CCI score | |||
| Mean (SD) | 1.04 (1.30) | 1.04 (1.21) | 1.47 (1.91) |
| Median (min, max) | 1.00 (0.00, 14.00) | 1.00 (0.00, 8.00) | 1.00 (0.00, 15.00) |
| OADs received during the baseline period, n (%) | |||
| Biguanides | 2220 (50.0) | 171 (47.4) | 316 (25.3) |
| SUs | 1037 (23.4) | 57 (15.8) | 196 (15.7) |
| DPP-4 inhibitors | 193 (4.3) | 19 (5.3) | 34 (2.7) |
| TZDs | 164 (3.7) | 18 (5.0) | 32 (2.6) |
| Other OAD medication classesb | 8 (0.2) | 3 (0.8) | 14 (1.1) |
| Patients with any OAD treatment during the baseline period, n (%) | 3320 (74.7) | 239 (66.2) | 537 (42.9) |
| Duration of any OAD treatment during the baseline period, daysc | |||
| Mean (SD) | 112.1 (58.4) | 102.8 (58.1) | 87.4 (59.4) |
| Median (min, max) | 127 (1.0, 180.0) | 106 (1.0, 180.0) | 90.0 (1.0, 180.0) |
| OADs received on the index date, n (%) | |||
| α-Glucosidase inhibitor | 9 (0.20) | 0 (0) | 0 (0) |
| Amylin analog | 3 (0.07) | 1 (0.3) | 2 (0.16) |
| Biguanide | 3179 (71.6) | 163 (45.2) | 327 (26.1) |
| DPP-4 inhibitor | 686 (15.4) | 11 (3.0) | 39 (3.1) |
| Meglitinide | 30 (0.68) | 0 (0) | 6 (0.48) |
| SGLT-2 inhibitor | 1 (0.02) | 0 (0) | 0 (0) |
| SU | 2823 (63.6) | 56 (15.5) | 172 (13.7) |
| TZD | 571 (12.9) | 21 (5.8) | 36 (2.9) |
| Biguanide/DPP-4 inhibitor fixed-dose combination | 445 (10.0) | 5 (1.4) | 17 (1.4) |
| Biguanide/meglitinide fixed-dose combination | 1 (0.02) | 0 (0) | 0 (0) |
| Biguanide/SGLT-2 inhibitor fixed-dose combination | 0 (0) | 0 (0) | 0 (0) |
| Biguanide/SU fixed-dose combination | 126 (2.8) | 1 (0.3) | 1 (0.08) |
| Biguanide/TZD fixed-dose combination | 199 (4.5) | 2 (0.55) | 6 (0.48) |
| DPP-4 inhibitor/TZD fixed-dose combination | 0 (0) | 0 (0) | 0 (0) |
| SU/TZD fixed-dose combination | 20 (0.45) | 1 (0.3) | 0 (0) |
SD standard deviation, SU sulfonylurea, TZD thiazolidinedione
aInformation on the duration of diabetes was unavailable because of a lack of information on enrollees’ medical history in the database
bIncludes alphaglucosidase inhibitors, amylin analogs, and meglitinides. No patients received SGLT-2 inhibitors during the baseline period
cDays between index date and last available OAD, basal insulin, and GLP-1 RA
Duration of treatment, measured as the number of days between the index date and last available evidence of a prescription for the index OAD, basal insulin, or GLP-1 RA, was longest for the 2-OAD cohort (338.9 days) followed by 285.0 days for the basal-insulin cohort and 281.1 days for the GLP-1 RA cohort, indicating higher discontinuation rates among patients initiating injectable therapy.
During follow-up, patients may have intensified treatment with additional therapy or switched therapy, as this study used an intent-to-treat approach; patients were not censored at the time of medication discontinuation, switching, or augmentation. Biguanides and sulfonylureas were used more commonly by patients who had initiated injectable therapy—especially those initiating GLP-1 RA therapy—than by those in the 2-OAD cohort. A minority of patients in the basal-insulin cohort and the GLP-1 RA cohort added the other injectable therapy to their index therapy. Rapid-acting insulin use during the follow-up period was more common and used for a longer period of time among patients in the basal-insulin cohort compared with patients in the other two cohorts (Table 2).
Table 2.
Medications used during follow-up among patients initiating 2-OAD therapy, GLP-1 RA, or basal insulin (among ≥ 2.0% of patients)
| Medication class | Cohort | ||
|---|---|---|---|
| 2-OAD (n = 4442) | GLP-1 RA (n = 361) | Basal insulin (n = 1251) | |
| Biguanides | |||
| Patients, n (%) | 175 (3.9) | 69 (19.1) | 208 (16.6) |
| Duration, mean (SD), days | 141.12 (91.24) | 170.35 (99.30) | 163.38 (99.88) |
| Sulfonylureas | |||
| Patients, n (%) | 230 (5.2) | 59 (16.3) | 122 (9.8) |
| Duration, mean (SD), days | 156.83 (99.11) | 170.68 (100.99) | 170.56 (97.64) |
| DPP-4 inhibitors | |||
| Patients, n (%) | 187 (4.2) | 20 (5.5) | 30 (2.4) |
| Duration, mean (SD), days | 131.70 (91.04) | 139.15 (85.34) | 137.20 (105.57) |
| Thiazolidinediones | |||
| Patients, n (%) | 155 (3.5) | 19 (5.3) | 46 (3.7) |
| Duration, mean (SD), days | 133.30 (97.09) | 205.05 (109.80) | 152.30 (99.18) |
| GLP-1 RA | |||
| Patients, n (%) | 133 (3.0) | – | 78 (6.2) |
| Duration, mean (SD), days | 128.89 (90.13) | – | 116.09 (105.74) |
| Basal insulin | |||
| Patients, n (%) | 296 (6.7) | 49 (13.6) | – |
| Duration, mean (SD), days | 120.68 (87.33) | 118.41 (80.43) | – |
| Rapid-acting insulin | |||
| Patients, n (%) | 88 (2.0) | 20 (5.5) | 264 (21.1) |
| Duration, mean (SD), days | 2.13 (18.93) | 8.02 (40.43) | 27.78 (60.50) |
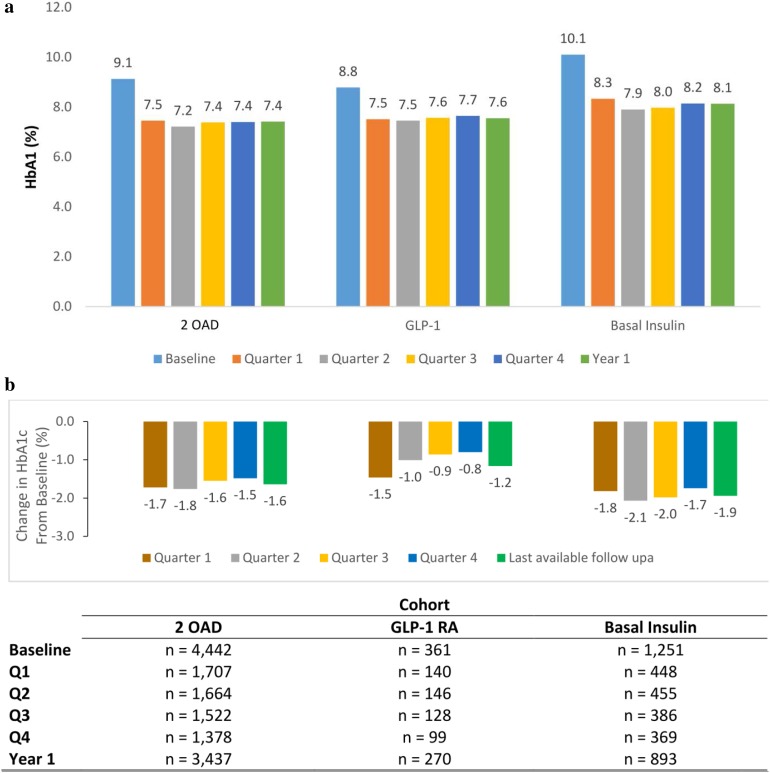
Longitudinal HbA1c data and change in HbA1c from baseline are shown in Fig. 3. HbA1c at baseline was high, indicating the presence of clinical inertia. Following treatment initiation with 2-OAD therapy, GLP-1 RA, or basal insulin, HbA1c levels generally decreased and the decrease was maintained for the duration of the follow-up period (Fig. 3a). The greatest overall HbA1c reductions from baseline were seen in quarter 2 for the basal-insulin (− 2.1%) and 2-OAD (− 1.8%) cohorts (Fig. 3b).
Fig. 3.
Mean HbA1c by cohort and time period among patients with uncontrolled T2D initiating 2-OAD therapy, GLP-1 RA, or basal insulin. a Longitudinal HbA1c, b change from baseline. aLast available follow-up measure defined as the last available HbA1c test value observed during the 15-month post-index period
The percentage of patients achieving HbA1c < 7.0% was lower in the basal-insulin cohort than in the other two cohorts (Table 3). Despite a greater reduction in HbA1c compared with the other two cohorts, patients in the basal-insulin cohort, who had a higher mean HbA1c at baseline, were less likely to reach the target HbA1c.
Table 3.
HbA1c category at last available follow-up measurement (up to 15 months) among patients with uncontrolled T2D initiating 2-OAD therapy, GLP-1 RA, or basal insulin
| HbA1c categories (% patients) | Cohort | ||
|---|---|---|---|
| 2-OAD (n = 4442) | GLP-1 RA (n = 361) | Basal insulin (n = 1251) | |
| < 7.0% | 47.5 | 41.1 | 32.6 |
| > 7.0–8.0% | 27.2 | 29.6 | 21.4 |
| > 8.0–9.0% | 13.1 | 14.1 | 20.5 |
| > 9.0–10.0% | 6.2 | 9.6 | 9.7 |
| > 10.0% | 6.0 | 5.6 | 15.8 |
Patients in the basal-insulin cohort had more inpatient days and emergency-department (ED) visits than patients in the other two cohorts. Patients in the 2-OAD cohort had fewer claims for physician-office visits than patients in the other two cohorts (Supplementary Table 1).
Patients in the 2-OAD cohort had the lowest mean (SD) all-cause total healthcare costs during the 12-month follow-up period, followed by those in the GLP-1 RA cohort, while total healthcare costs were highest in the basal-insulin cohort. Inpatient and ED visit costs were the main drivers of total cost in the basal-insulin cohort, while pharmacy costs (i.e., any costs directly related to drugs, excluding monitoring costs) were the main drivers of total cost for patients in the other two cohorts (Supplementary Table 2).
Discussion
This retrospective analysis of data from a large US claims database was conducted to compare outcomes in patients with uncontrolled T2D intensifying anti-hyperglycemic therapy by initiating treatment with two OADS, GLP-1 RA, or basal insulin. The study results show that many patients are not at optimal glycemic control: HbA1c at baseline was high in all cohorts but highest in patients initiating basal insulin. Average HbA1c levels decreased in all cohorts following treatment intensification; the greatest reduction was seen in the basal-insulin cohort, which also had the highest HbA1c at baseline. Despite robust HbA1c lowering following treatment intensification, many patients still failed to achieve target HbA1c levels. More timely treatment intensification, including earlier use of combination therapy, could help improve the achievement of glycemic control and impact of timely treatment intensification on attaining and maintaining glycemic goals and should be investigated further.
While the recommended stepwise approach for diabetes management [1, 3] is appropriate for many patients, it may not always be effective. For a variety of reasons, the next step in anti-hyperglycemic treatment may often be initiated very late: by the time intensification occurs, HbA1c values may have already reached levels that are so high that it is difficult to effectively achieve the guideline-recommended HbA1c target, despite robust glycemic lowering with a variety of agents—as seen in this study. The greatest overall HbA1c reductions in this study were seen in quarter 2 for both the basal insulin and 2-OAD cohorts. These findings suggest that if patients do not achieve HbA1c < 7% with 2-OAD, basal insulin, or GLP-1 RA therapy within the first 6 months following initiation, they have a low probability of achieving control thereafter. Altering treatment regimens by adding or changing antihyperglycemic agents should be considered when HbA1c goals are not achieved within 3–6 months, as recommended by the ADA [1]. In this analysis, most patients in the basal-insulin cohort did not achieve target HbA1c levels, despite having a greater reduction in HbA1c from baseline compared with the other two cohorts. Potential reasons may be that, on average, they had higher HbA1c at baseline and a greater percentage of patients in the basal-insulin cohort had an HbA1c > 10.0% at follow-up compared with the other cohorts. Patients initiating basal insulin also had a higher CCI score, suggesting a cohort of more difficult-to-treat patients.
In this study we speculate that the high HbA1c levels observed at baseline prior to treatment intensification could be the result of clinical inertia. It is plausible that either clinical inertia or the physician’s perception regarding when treatment goals have been achieved is partly responsible for the observation that a large proportion of patients in the current study had poorly controlled diabetes in the baseline period. This observation is in line with other recently published evidence; based on an analysis of the prevalence of clinical inertia among patients with T2D with personalized HbA1c goals in the US managed-care setting, Lin et al. concluded that clinical inertia—defined as failure to have therapy initiated or intensified despite an inadequate treatment response—is high and has increased in recent years [8]. Results from the current study are in line with those from another real-world retrospective analysis of T2D patients from the MarketScan database, which reported low rates of treatment intensification among patients with elevated HbA1c levels at baseline or during a 4-year follow-up period (8.0–9.2%). The percentage of patients with HbA1c > 7.0% at 4 years after initiation with a new class of anti-hyperglycemic medication ranged from 48% among patients initiating OADs to 74% of patients initiating basal insulin [20]. A retrospective study involving databases from the US and multiple European countries found that after 2 years of basal-insulin therapy, only 27.8% of patients achieved the HbA1c goal of < 7.0%, down from a mean baseline HbA1c level of 9.0% [21]. Fu and Sheehan reported that 52% of adult T2D patients who were uncontrolled on OADs with a baseline HbA1c of ≥ 8.0% had not received treatment intensification after 1 year of follow-up even though these patients were considered eligible for treatment intensification [22]. Likewise, less than half (49%) of older adult T2D patients with a baseline HbA1c of ≥ 8.0% despite treatment with two OADs received intensified treatment during follow-up, with median time to intensification of 18.5 months [14, 23], although the reasons for clinical inertia can be multifactorial [10].
The results from the current study argue for more intensive treatment and the implementation of strategies to overcome clinical inertia. A detailed discussion of the causes of and potential solutions to clinical inertia is outside the scope of this article. Even so, new therapeutic options (with fewer side effects including fewer hypoglycemic events and/or less weight gain), less complex treatment regimens (for example, fewer injections), and earlier treatment regimen intensification in patients with very high HbA1c at diagnosis (e.g., patients who may have been undiagnosed for an extended period of time) may reduce the burden of the treatment and provide ways forward to overcome clinical inertia in T2D.
In our analysis, patients in the basal-insulin cohort had higher total all-cause healthcare costs and more inpatient and ED visits compared with patients in the other two cohorts. These patients had higher HbA1c levels and CCI scores at baseline, and it is possible that they had a longer duration of diabetes and more medical problems overall. Clinicians may have reserved high-efficacy therapy (i.e., basal insulin) for these patients. However, despite basal-insulin therapy, HbA1c remained uncontrolled and patients incurred higher costs. It is possible that overcoming clinical inertia and treating patients earlier would help reduce HbA1c more effectively and could therefore have the potential to contain healthcare costs. The finding of a higher baseline HbA1c and poorer health status of patients initiating basal insulin compared with those initiating a GLP-1 RA was also reported in a real-world clinical and economic outcomes study in T2D patients in the USA [24]. Further studies are needed to better understand the association among earlier treatment intensification, HbA1C goal attainment, and healthcare costs.
A strength of this analysis is that the large administrative claims database included information for US patients across all care settings (e.g., inpatient, ED, physician’s office) and for prescriptions and laboratory tests. Patient baseline characteristics were similar to those found in comparable US studies describing retrospective observational database analyses [16, 25–29].
Several limitations, however, should be taken into account when considering the findings of this study, some of which are due to the limitations and biases inherent to retrospective analyses of claims data [30]. Further, the MarketScan database is not designed to capture clinical information, and this is reflected in the low number of patients with HbA1c values available at all time points in the follow-up period; HbA1c data for quarters 1, 2, 3, and 4 were available for an average of 38%, 38%, 34%, and 29% of patients, respectively, although this could have been due to a failure of clinicians to obtain HbA1C levels at guideline-recommended intervals. Findings of this analysis may also be confounded by the fact that some patients may have received further treatment intensification with additional therapy, may have switched therapy during follow-up, or may have increased medication doses. As it was not possible to randomize patients to treatment groups, the data may be subject to selection bias in terms of representativeness of the study population and their treatment regimens to the overall population of patients with T2D. Patients receiving treatment with GLP-1 RAs, for example, may not be the same as those being treated with basal insulin; those treated with basal insulin will likely have more advanced disease, more comorbidities, and higher HbA1c, while patients treated with a GLP-1 RA may have received these agents rather than basal insulin because of concerns about body weight (exemplified by the higher proportion of female patients in the GLP-1 RA cohort) or a need to minimize weight gain during treatment, or to reduce the risk of hypoglycemia, or as a result of health-plan coverage and co-pay policies. Likewise, the magnitude of the change in HbA1c from baseline is not only influenced by the HbA1c level at baseline, but also by the selection of included patients (e.g., duration of diabetes). Furthermore, the three cohorts compared in this study had different sample sizes: the sample size of the GLP-1 RA cohort was one-third that of the BI cohort and 12 times smaller than the 2-OAD cohort, which hampers between-group comparison and increases the risk of selection bias. An unexpected finding in this study was that the HbA1c reduction from baseline observed in this study was numerically smaller for patients in the GLP-1 RA cohort compared with the 2-OAD cohort, while in clinical trials, GLP-1 RAs have shown an HbA1c-lowering potential comparable to that of basal insulin. The gap between clinical efficacy observed in randomized clinical trials and effectiveness observed in real-world studies in terms of HbA1c reduction with newer antihyperglycemic therapies has been described recently, even when baseline characteristics are comparable. Poor medication adherence in the real world is thought to be a key reason for real-world patients having poorer outcomes than the counterparts participating in randomized clinical trials [31]. In addition to the potential effect of cohort size, this observation could be explained by the relatively better HbA1c control at baseline in the GLP-1 RA cohort. Also, the cohort might have been exposed to slightly less intensive treatment approaches compared with the other cohorts. HbA1c levels at last available follow-up were similar for the 2-OAD and GLP-1 RA cohorts. Further, patients were evaluated based on an intent-to-treat approach and were not censored at the point of medication discontinuation, switching, or augmentation. It was not within the scope of this analysis to measure the effect of a treatment change on HbA1c. Next steps for further research could include an evaluation of HbA1c following treatment changes such as further intensification or treatment switching or an analysis of matched patient cohorts. Additionally, this study did not examine clinical inertia itself or the reasons why glycemic targets were not met. Finally, the current study reported a fairly low use of metformin (25.3%) in combination with basal insulin. Treatment guidelines recommend continuing metformin (unless there are contraindications or intolerance to its use) as background therapy and in combination with insulin and other agents in patients who do not reach their glycemic target on monotherapy [1, 3]. Only 50.1% of patients from the French national health insurance database had co-prescription claims for insulin and metformin (59.6% had a co-prescription of basal insulin only and metformin) [32]. The low use of metformin in the current study may be the result of the fact that baseline treatment utilization for all patients was measured during a 6-month pre-index period (potentially missing earlier prescriptions with long lengths of script). Therefore, the low metformin use could be due to a failure to observe baseline medication history for a longer period or the result of patients not being able to tolerate metformin in the long term.
Conclusions
The high percentage of T2D patients not at optimal glycemic control in this retrospective analysis of data may be the result of clinical inertia in the period prior to treatment intensification, although the study did not look at clinical inertia itself. Despite robust lowering in HbA1c following treatment intensification, many patients still did not achieve guideline-recommended HbA1c levels, suggesting a need for earlier and/or more intensive treatment, including combination pharmacotherapy. Such strategies may be helpful in getting more patients to goal earlier and provide better durability of glycemic control, thus reducing their exposure to hyperglycemia, which may be associated with both clinical and economic benefits.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Sponsorship for this study and article processing charges were funded by Sanofi US, Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Writing and editorial assistance in the preparation of this article was provided by Tessa Hartog, PhD, of Excerpta Medica. Support for this assistance was funded by Sanofi US, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Lawrence Blonde has received grant/research support from AstraZeneca, Janssen Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc., Merck & Co., Novo Nordisk, and Sanofi, speaker honoraria from AstraZeneca, Janssen Pharmaceuticals, Inc., Novo Nordisk, Sanofi, and consultant honoraria from Intarcia Therapeutics, Inc., Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Novo Nordisk, and Sanofi. Denis Raccah has acted as a member of advisory boards and as a speaker during symposia for AstraZeneca, Janssen, Lilly, Novartis, NovoNordisk, and Sanofi. Elisheva Lew is an employee of Sanofi. Juliana Meyers is an employee of RTI Health Solutions, which received funding from Sanofi to conduct this analysis. Elena Nikonova was an employee of Artech Information Systems LLC, under contract with Sanofi, at the time the study was performed. Mayank Ajmera is an employee of RTI Health Solutions, which received funding from Sanofi to conduct this analysis. Keith L. Davis is an employee of RTI Health Solutions, which received funding from Sanofi to conduct this analysis. Monica Bertolini is an employee of Sanofi. Bruno Guerci has acted as an advisory panel/board member for Sanofi, Eli Lilly, NovoNordisk, Novartis, GSK, MSD, Boehringer Ingelheim, AstraZeneca, Abbott, Medtronic, Roche Diagnostics, and as Clinical investigator for Sanofi, Eli Lilly, NovoNordisk, GSK, BMS, AstraZeneca, Medtronic, Abbott, Roche Diagnostics, MSD, Novartis, Janssen, Boehringer Ingelheim; he has received research support from Medtronic, Vitalaire, Sanofi, Eli Lilly, Novo Nordisk; the GetGoal-Duo2 trial (NCT01768559) was sponsored by Sanofi.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors. Research Triangle Institute (RTI) International’s institutional review-board committee determined that this study met all criteria for exemption from full review as the data were retrospective, de-identified, and anonymous [18]. The decision follows Health and Human Services policy detailing which studies can be considered exempt from meeting the requirements of the federal human subjects regulations, including the requirement for initial and annual Institutional Review Board review [19].
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available because of the proprietary nature of the database. Questions regarding the study database should be directed to the corresponding author.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article, go to 10.6084/m9.figshare.6115208.
References
- 1.American Diabetes Association Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl. 1):S1–S135. [Google Scholar]
- 2.Garber AJ, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract. 2018;24(1):91–120. doi: 10.4158/CS-2017-0153. [DOI] [PubMed] [Google Scholar]
- 3.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr Pract. 2017;23:207–238. doi: 10.4158/EP161682.CS. [DOI] [PubMed] [Google Scholar]
- 4.Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care. 2003;9:213–217. [PubMed] [Google Scholar]
- 5.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–3417. doi: 10.2337/dc13-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–2279. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peyrot M, Rubin RR, Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes. 2010;4(Suppl. 1):S11–S18. doi: 10.1016/S1751-9918(10)60004-6. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Zhou S, Wei W, Pan C, Lingohr-Smith M, Levin P. Does clinical inertia vary by personalized A1C goal? A study of predictors and prevalence of clinical inertia in a U.S. managed-care setting. Endocr Pract. 2016;22:151–161. doi: 10.4158/EP15868.OR. [DOI] [PubMed] [Google Scholar]
- 9.Reach G. Patient non-adherence and healthcare-provider inertia are clinical myopia. Diabetes Metab J. 2008;34(4 pt 1):382–385. doi: 10.1016/j.diabet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017 (Epub ahead of print). [DOI] [PubMed]
- 11.Phillips L, Branch W, Cook C, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 12.Grant RW, Cagliero E, Dubey AK, et al. Clinical inertia in the management of type 2 diabetes metabolic risk factors. Diabet Med. 2004;21:150–155. doi: 10.1111/j.1464-5491.2004.01095.x. [DOI] [PubMed] [Google Scholar]
- 13.Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen Intern Med. 2007;22:453–458. doi: 10.1007/s11606-007-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roussel R, Gourdy P, Gautier JF, et al. Low rate of intensification in type 2 diabetic patients inadequately controlled with basal insulin: the INTERDIA study. Diabetes Manag. 2016;6:119–127. [Google Scholar]
- 15.Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285:182–189. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]
- 16.Levin PA, Zhou S, Gill J, Wei W. Health outcomes associated with initiation of basal insulin after 1, 2, or ≥ 3 oral antidiabetes drug(s) among managed care patients with type 2 diabetes. J Manag Care Spec Pharm. 2015;21:1172–1181. doi: 10.18553/jmcp.2015.21.12.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smolen HJ, Murphy DR, Gahn JC, Yu X, Curtis BH. The evaluation of clinical and cost outcomes associated with earlier initiation of insulin in patients with type 2 diabetes mellitus. J Manag Care Spec Pharm. 2014;20:968–984. doi: 10.18553/jmcp.2014.20.9.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Research Triangle Institute. Ethics and Human Research Protection Web site. www.rti.org/ethics-and-human-research-protection. Accessed 19 Oct 2017.
- 19.Health and Human Services. Code of Federal Regulations. Title 45—Public Welfare Department of Health and Human Services. Part 46—Protection of Human Subjects. www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html. Accessed 19 Oct 2017.
- 20.Bonafede M, Chandran A, DiMario S, Saltiel-Berzin R, Saliu D. Medication usage, treatment intensification, and medical cost in patients with type 2 diabetes: a retrospective database study. BMJ Open Diabetes Res Care. 2016;4:e000189. doi: 10.1136/bmjdrc-2015-000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19:1155–1164. doi: 10.1111/dom.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu AZ, Sheehan JJ. Treatment intensification for patients with type 2 diabetes and poor glycaemic control. Diabetes Obes Metab. 2016;18:892–898. doi: 10.1111/dom.12683. [DOI] [PubMed] [Google Scholar]
- 23.Ajmera M, Raval A, Zhou S, et al. A real-world observational study of time to treatment intensification among elderly patients with inadequately controlled Type 2 Diabetes Mellitus. J Manag Care Spec Pharm. 2015;21:1184–1193. doi: 10.18553/jmcp.2015.21.12.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thayer S, Wei W, Buysman E, et al. The INITIATOR study: pilot data on real-world clinical and economic outcomes in US patients with type 2 diabetes initiating injectable therapy. Adv Ther. 2013;30:1128–1140. doi: 10.1007/s12325-013-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin P, Lin J, Wei W, et al. Initiating injectable therapy in US managed care patients with diabetes. Am J Pharm Benefits. 2014;6:e93–e102. [Google Scholar]
- 26.Davis SN, Wei W, Garg S. Clinical impact of initiating insulin glargine therapy with disposable pen versus vial in patients with type 2 diabetes mellitus in a managed care setting. Endocr Pract. 2011;17:845–852. doi: 10.4158/EP10401.OR. [DOI] [PubMed] [Google Scholar]
- 27.Dalal MR, Xie L, Baser O, DiGenio A. Adding rapid-acting insulin or GLP-1 receptor agonist to basal insulin: outcomes in a community setting. Endocr Pract. 2015;21:68–76. doi: 10.4158/EP14290.OR. [DOI] [PubMed] [Google Scholar]
- 28.Bieszk N, Reynolds SL, Wei W, Davis C, Kamble P, Uribe C. “Act on threes” paradigm for treatment intensification of type 2 diabetes in managed care: results of a randomized controlled study with an educational intervention targeting improved glycemic control. J Manag Care Spec Pharm. 2016;22:1028–1038. doi: 10.18553/jmcp.2016.22.9.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayyagari R, Wei W, Cheng D, Pan C, Signorovitch J, Wu EQ. Effect of adherence and insulin delivery system on clinical and economic outcomes among patients with type 2 diabetes initiating insulin treatment. Value Health. 2015;18:198–205. doi: 10.1016/j.jval.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 Suppl. 1):S51–S55. doi: 10.1097/MLR.0b013e31819c95aa. [DOI] [PubMed] [Google Scholar]
- 31.Carls GS, Tuttle E, Tan RD, Huynh J, Yee J, Edelman SV, Polonsky WH. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40(11):1469–1478. doi: 10.2337/dc16-2725. [DOI] [PubMed] [Google Scholar]
- 32.Roussel R, Charbonnel B, Behar M, Gourmelen J, Emery C, Detournay B. Persistence with Insulin therapy in patients with type 2 diabetes in France: an insurance claims study. Diabetes Ther. 2016;7:537–549. doi: 10.1007/s13300-016-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available because of the proprietary nature of the database. Questions regarding the study database should be directed to the corresponding author.