Abstract
In muscle, Ca2+ release from the sarcoplasmic reticulum (SR) into the cytosol is mediated through the ryanodine receptors (RyRs) and sustained by countercurrents that keep the SR membrane potential near 0 mV. Likewise, Ca2+ reuptake by the sarco/endoplasmic reticulum Ca2+ ATPase pump requires countercurrent. Although evidence has suggested that TRIC K+ channels and/or RyR K+ influx provide these countercurrents, the exact sources have not yet been determined. We used an equivalent circuit compartment model of a cardiac SR, the surrounding cytosol, and the dyadic cleft to probe the sources of countercurrent during a complete cardiac cycle. By removing and relocating TRIC K+ channels, as well as limiting when they are active, we explored the various possible sources of SR countercurrent under many conditions. Our simulations indicate that no single channel type is essential for countercurrent. Rather, a cascading network of countercurrents is present with anion fluxes within the SR redistributing charges throughout the full SR volume. This allows ion channels in the entire SR membrane, far from the Ca2+ fluxes through the RyRs in the junctional SR and sarco/endoplasmic reticulum Ca2+ ATPase pump in the nonjunctional SR, to mediate countercurrents that support Ca2+ release and reuptake. This multifactorial network of countercurrents allows Ca2+ release to be remarkably robust.
Introduction
During muscle contraction, Ca2+ is released from the sarcoplasmic reticulum (SR) into the cytosol through the ryanodine receptor (RyR). Countercurrent is necessary to balance the charge moved during RyR-mediated Ca2+ release. Without this countercurrent, the SR membrane potential quickly reaches the Ca2+ equilibrium potential, eliminating the trans-SR Ca2+ driving force and ending Ca2+ release within several milliseconds (1). When the RyRs are closed, countercurrent is also needed to balance the charge moved during SR Ca2+ reuptake by the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pump. The sources of the required SR countercurrents remain a matter of debate.
One important contribution to the nature of countercurrent came when Yazawa et al. (2) identified the K+ selective trimeric intracellular cation (TRIC) channel in the SR membrane, subsequently identified as the SR K+ channel (3) first identified by Miller (4). There are two TRIC channel isoforms, TRIC-A and TRIC-B. Double TRIC knockout (DKO) mice die in utero, whereas mice lacking just TRIC-B channels die as neonates. However, mice lacking just TRIC-A are viable and fertile. Yazawa et al. (2) hypothesized that the TRIC SR K+ channels provide essential countercurrent during SR Ca2+ release. Interestingly, however, caffeine treatment evoked long, robust SR Ca2+ release events in their embryonic DKO cardiomyocytes, suggesting that K+ channels may not be essential to support Ca2+ release and that the SR has a substantial non-K+ channel source of countercurrent.
Exploring this issue, Gillespie and Fill (1) demonstrated that the RyR itself, being only weakly Ca2+ selective, is capable of mediating its own countercurrent during SR Ca2+ release. Specifically, they showed that open RyRs conduct K+ and Mg2+ as countercurrent to fluxed Ca2+. Therefore, they posited that K+ channels may have little or a redundant countercurrent role during SR Ca2+ release, and proposed that K+ channels are likely needed to provide the countercurrent that supports diastolic Ca2+ reuptake. This view was bolstered later by experiments by Guo et al. (5) showing that substituting cytosolic K+ for either Na+ or Cs+ resulted in little to no change to Ca2+ sparks or SR Ca2+ load. This was interesting because the Na+ or Cs+ conductance through SR K+ channels is 35 or 88% less (respectively) than its K+ conductance, and therefore intuitively ought to affect Ca2+ release significantly if K+ were the principle counterion.
Further work on TRIC channels has shown their general importance for SR Ca2+ handling (6, 7, 8), but those experiments only assessed their role in countercurrent indirectly. Moreover, other experiments have also supported both sides. Countercurrent has previously been believed to be mediated by the SR K+ channel (4, 9, 10), but Somlyo et al. (11) showed that both Mg2+ and K+ enter the SR during Ca2+ release in frog skeletal fibers during an onset of tetanus. Because RyR is the only Mg2+ conducting channel in the SR membrane, this indicates some countercurrent role for RyR. Currently, despite all these data, the exact nature of the SR countercurrents has not been resolved.
Here, we investigate SR countercurrent and the role of SR TRIC K+ channels by modeling possible countercurrent sources during cardiac SR Ca2+ release and subsequent reuptake. Our simulations indicate that the SR countercurrent during the cardiac cycle is multifactorial and not carried by any one particular ion or channel type; there is no single essential pathway. Instead, the countercurrent is provided by a complex network of ions and fluxes, involving all open ion channels present and substantial intra-SR ion currents. This ensemble of SR countercurrent cascades down the SR (from the junctional SR through the nonjunctional SR) to diffuse charge buildup across the entire SR unit, not only near the RyRs or SERCA pumps. This assures there is always sufficient countercurrent to support robust Ca2+ release and reuptake even if one of the countercurrent entities is compromised.
Methods
Equivalent circuit model
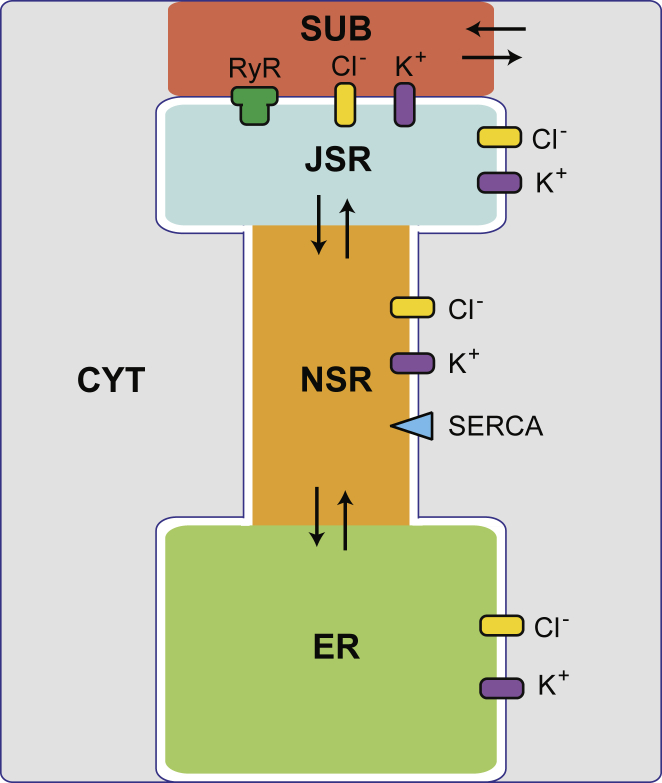
To model ions moving in and out of the SR during one cardiac cycle, we use the equivalent circuit model of a cardiac SR introduced by Berti et al. (12) without changing model parameters unless noted below or in the text. This model contains five compartments: the junctional SR (JSR), nonjunctional SR (NSR), endoplasmic reticulum (ER), junctional cleft subspace (SUB), and cytosol (CYT) (Fig. 1). Each compartment has a homogenous concentration of Ca2+, K+, Mg2+, Cl−, and X−, where the X− represents all the non-Cl− anions (e.g., proteins and phosphates) that neutralize charge in each of the compartments. Ion movements are driven between compartments by concentration and electrical potential gradients through ion channels or between contiguous compartments (e.g., from the JSR to the NSR). The SR membrane is modeled as a capacitor with a uniform capacitance per unit area of 0.01 pF/μm2. The currents through SR K+ and Cl− channels follow Ohm’s Law (both with a conductance of 100 pS) with selectivity for their namesake ion only. Currents through the RyRs follow the Goldman-Hodgkin-Katz formula with permeabilities given by the Gillespie ion permeation model of the RyR (13). The SERCA pump in the NSR membrane is that of Shannon et al. (14). The concentration of each ion species and the voltage in each compartment evolves over time to satisfy Kirchhoff’s voltage and current laws. The resulting algebraic-differential equations were solved numerically using the software Mathematica 10.4 (Wolfram Research, Champaign, IL) using Runge-Kutta methods.
Figure 1.
Schematic of the compartment model. Channels allow the flow of ions through the SR membrane, while the flux of ions between contiguous compartments are indicated with arrows. The largest compartment, labeled as the “ER” here, could also be thought of as the bulk SR and serves as a large reservoir of ions. Not drawn to scale. To see this figure in color, go online.
This model simulates the ion movements into and out of the SR during a cardiac cycle where RyRs open during systole and the SERCA pump restores the released Ca2+ during diastole. Berti et al. (12) showed that the system quickly reached a new homeostatic state whenever the pacing rate (i.e., frequency of the RyR openings) was changed; new, periodic time courses of ion concentrations and voltages were established at each pacing rate so that there was no net loss or gain of ions from any compartment over the course of one cycle. All the results shown in this article are from this steady state with a pacing rate of 60 beats per min (i.e., RyRs open once per s). As described by Berti et al. (12), the opening of the RyRs lasts ∼26 ms with the RyRs opening and closing gradually to produce a Gaussian current versus time profile matched to experimental RyR Ca2+ release currents. Here, we define systole as the window when the RyRs gating function is open >0.001% of its maximum value and diastole as the rest of the cycle.
To understand the dependence of countercurrent during systole on the availability and location of K+ channels, we first consider a baseline case against which we later compare changes resulting from differences in the number or placement of K+ channels in the SR membrane. In the baseline case, 320 K+ channels were distributed in equal numbers across the four SR compartment membranes (i.e., 80 on each): the JSR-subspace, JSR-cytosol, NSR-cytosol, and ER-cytosol interfaces. In other cases, 160, 32, or 0 K+ channels were distributed in equal numbers on these membranes, or else were removed from one or more particular membranes individually, leaving the remaining membranes unchanged. Note that removing K+ channels from the SR membranes is equivalent to reducing the conductance per K+ channel because only the total conductance of all K+ channels in the membrane is used in calculating currents. We chose 320 as the total number of SR K+ channels in the baseline case based on the stoichiometric ratio of K+ channels to RyRs assuming ∼50 RyRs in a SR Ca2+ release unit (2, 6). In the configuration we chose, half the K+ channels are in the JSR, but shifting more channels to the NSR and ER did not qualitatively affect our conclusions.
Caveats and uncertainties
In our model we only consider the total conductance of a membrane (i.e., the product of the number of open K+ channels and the unitary conductance), which is usually 8 nS per membrane. There is substantial ambiguity, however, regarding the properties of the K+ channels that make up this number.
First, a 5:1 TRIC-A to TRIC-B ratio (15) and a 5:1 TRIC-A to RyR ratio (6) were measured only in skeletal muscle. We are unaware of such a measurement in cardiac muscle. This is important because there the amount of Ca2+ released during each contraction is substantially higher than in skeletal muscle, potentially requiring more countercurrent. Therefore, the K+ channel to RyR stoichiometry may be different in cardiac myocytes. Second, we assume that the number of K+ channels that is open is relatively constant. Although a voltage dependence of TRIC-A and B has been described (3), this was measured at ±30 mV and not with physiological voltages close to 0 mV or with physiological ion concentrations (e.g., in the presence of Mg2+ and ATP). Therefore, the ∼10% open probability of TRIC-A channels in light-SR membrane incorporations (and the ∼70% of purified) at +30 mV is difficult to interpret, as is the ∼10-fold reduction in open probability at −30 mV. Third, both TRIC-A and TRIC-B have conductance substates, so it is unclear what their average conductance is. (TRIC-A has a reported full conductance of 192 pS and 129 pS substate and TRIC-B 138 pS full conductance with two substates of 59 and 35 pS (3).) We used 100 pS. To overcome these uncertainties, we will vary the number of K+ channels on each membrane (which is equivalent to varying the channel conductance).
Lastly, we are unaware of any measurement of the number of Cl− channels in the SR. We chose their number to be equal to the number of K+ channels. The Cl− channels, however, do not contribute substantially to the countercurrent. We are also unaware of published Cl− channel conductance measurements. The conductance of 100 pS we used in the model is based on our bilayer measurements (data not shown).
Results
Systole
We first consider systole. Therefore, this section is focused on the JSR and the counterion fluxes that enter or exit the JSR from adjacent compartments during Ca2+ release.
The baseline case
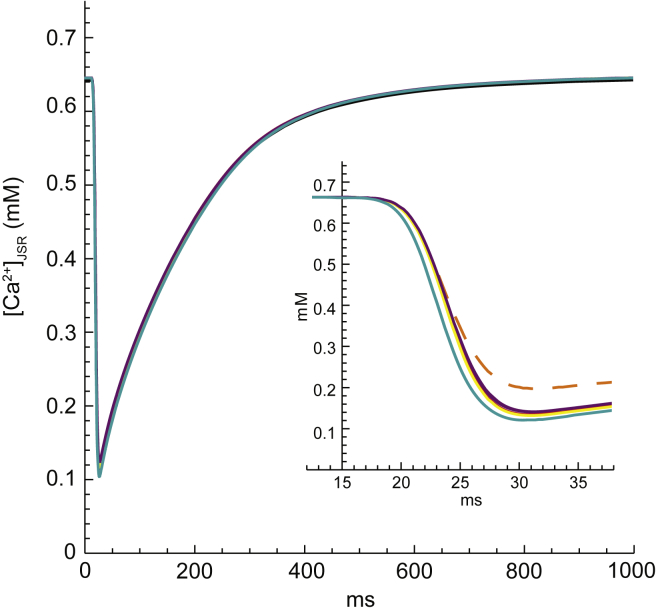
At the start of each cycle, there is a large Ca2+ concentration in the JSR (∼650 μM) and a low concentration in the cytosolic subspace (∼0.1 μM). This represents a large Ca2+ driving force of ∼118 mV across the JSR membranes. When the RyRs open, Ca2+ is released from the JSR into the subspace, reducing the Ca2+ concentration gradient. As shown previously with this model (12), because the Ca2+ concentration is so much larger than any other driving force acting on the Ca2+, Ca2+ release is resilient to most changes of model parameter values. Specifically, unless we directly affect the diastolic Ca2+ gradient (e.g., by suppressing the rate of the SERCA pump), the release of Ca2+ remains unaffected, although other variables in the system will vary. Fig. 2 demonstrates the robustness of Ca2+ release, as every case explored in the Results (the solid lines) is shown to exhibit the same [Ca2+]JSR time course.
Figure 2.
SR Ca2+ load ([Ca2+]JSR) as a function of time for all cases examined in the Results. Specifically, Ca2+ release is unchanged by variations in the number and placement of K+ channels or by the elimination of subspace-cytosol resistance. (Inset) Zoom-in on the systolic timeframe to resolve the small differences between cases. The case with K+ channels turned off during systole (orange, dashed line), which is explored in the Discussion, is included for comparison in the inset only. To see this figure in color, go online.
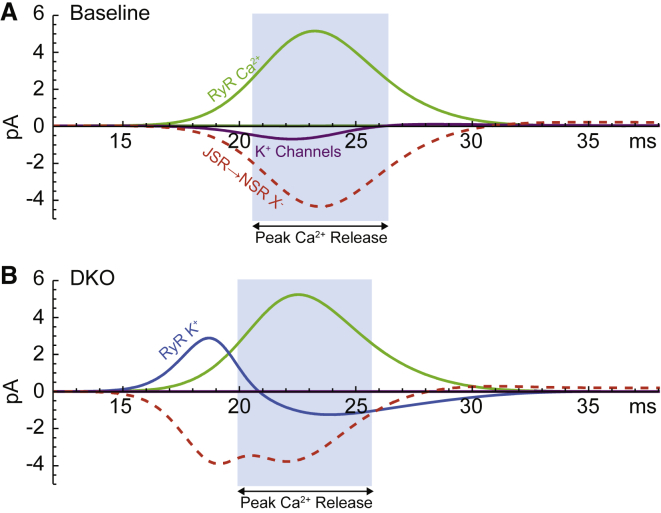
Our interest is to understand the countercurrents that allow for this robust SR Ca2+ release. Therefore, we analyzed countercurrents occurring during peak Ca2+ release, defined as the full width at half-maximum of the Ca2+ current through the RyRs (Fig. 3 A, blue region). In the baseline case, ∼74,000 Ca2+ ions (∼148,000 charges) move through the RyRs during the peak of Ca2+ release (Fig. 3 A). Approximately 88% of all the countercurrent is provided by ions moving between the JSR and NSR compartments, and only 12% from ions that cross the JSR membrane through ion channels. Intuitively, the intra-SR countercurrent contributes much more to total countercurrent than channel-mediated sources because the cross-sectional area between SR compartments is much larger than that of the ion channels. In short, ions move more quickly between SR compartments in reaction to accumulating charge than through channels because ion channels are a higher resistance pathway. Although these intra-SR currents do not lessen the charge buildup across the SR membrane, they quickly redistribute the accumulated charges throughout the entire SR, which limits the growth of the voltage on the JSR-subspace membrane. Approximately 98% of the intra-SR countercurrent comes from X− movement from the JSR into the NSR. The remaining ∼2% comes from Ca2+ moving from the NSR into the JSR because the release of Ca2+ through the RyRs generates a Ca2+ gradient between SR compartments.
Figure 3.
Key JSR currents as a function of time throughout systole for (A) the baseline case and (B) the DKO case. The period of peak Ca2+ release for each case is highlighted in light blue. Anion flow from the JSR to the NSR (red, dashed) provides the majority of countercurrent in the JSR for both cases. In the baseline case, JSR-cytosol K+ channels (purple) move the majority of channel-mediated countercurrent, whereas the complete lack of K+ channels in the DKO case forces the RyRs to mediate this countercurrent (blue). The magnitude of the Ca2+ release current (green) is approximately the same in both cases. To see this figure in color, go online.
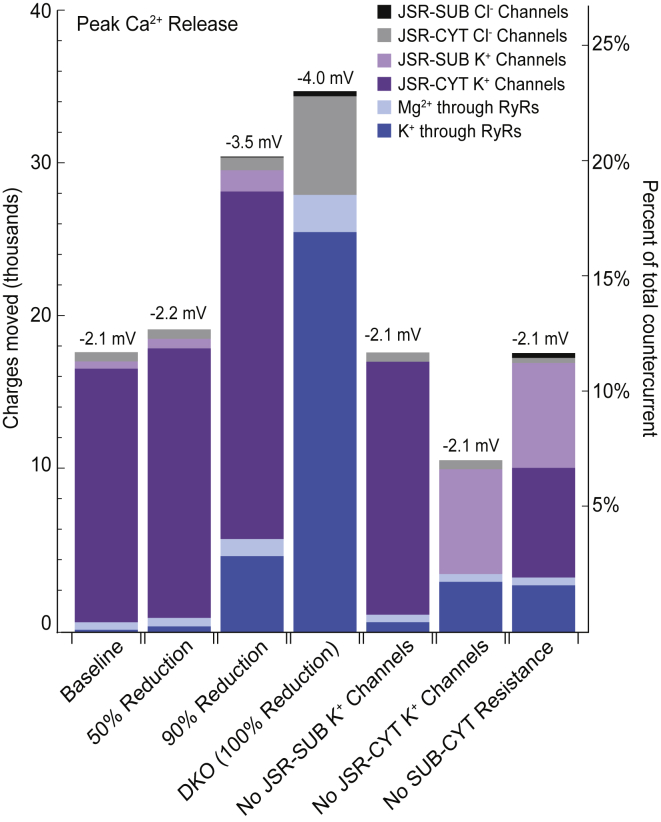
As for channel-mediated countercurrents in the baseline case, each channel type’s contribution to the ∼18,400 charges that moved through ion channels during peak Ca2+ release is very different (Fig. 4, first bar). Countercurrent through K+ channels in the JSR-cytosol membrane accounts for ∼90% of the channel-mediated countercurrent. The rest of the channel-mediated charge movement is mediated by Cl− channels on the JSR-cytosol membrane (∼3.3%), Mg2+ through open RyRs (∼2.7%), K+ channels located on the JSR-subspace interface (∼2.6%), K+ through the RyRs (∼1%), and Cl− channels on the JSR-subspace membrane (∼0.2%).
Figure 4.
Number of charges moved through JSR channels for different numbers of K+ channels and their placement. The peak voltage on the JSR-subspace membrane is listed at the top of each bar. Because Ca2+ release is approximately the same in all cases, the total countercurrent is also approximately the same, and the percent of total countercurrent mediated by JSR channels can be compared across cases (right axis). The first four cases depict the effect of reductions in the number of K+ channels in the SR. For these four cases, the membrane potential increases as the availability of K+ channels is reduced, and the amount of countercurrent mediated by all JSR ion channels increases in step (the total height of the bars). For all seven cases, the K+ channels in the JSR-cytosol membrane (dark purple) mediate the majority of channel-mediated countercurrent when they are available, but cation fluxes through the RyRs (blue and light blue) take over this role in the DKO case. JSR-subspace K+ channels (light purple) can also contribute more significantly in the absence of JSR-cytosol K+ channels. The time course of the currents in each column are shown in Fig. S1. To see this figure in color, go online.
Interestingly, the K+ channels that share the JSR-subspace membrane face with the RyRs conduct significantly less countercurrent than the farther-away K+ channels on the JSR-cytosol membrane. This asymmetry results from a difference in the driving forces on K+ across each of these interfaces, which is caused by the resistance to ion movement between the subspace and cytosol. Because the value of this resistance is unknown, we explored the limiting case of zero resistance. In this case, K+ channels on each JSR interface mediate the same amount of countercurrent, as would be expected, and the RyRs take on a more significant role by mediating ∼20% of the channel-mediated countercurrent with K+ (∼17%) and Mg2+ (∼3%) (Fig. 4, last bar).
The precise location of the K+ channels in the SR membranes is also unknown. We therefore explored cases in which K+ channels are removed from one of the two JSR membranes (JSR-subspace or JSR-cytosol), with the number on the remaining membrane left unchanged. When K+ channels are removed from the subspace-facing membrane, the small amount of K+ carried by these channels in the baseline case moves through open RyRs instead. All other countercurrents were unchanged (Fig. 4, bar 5). The removal of the K+ channels in the JSR-cytosol membrane caused more dramatic changes because ∼90% of JSR channel-mediated countercurrent is carried by these channels in the baseline case. The channel-mediated contribution to total countercurrent was significantly reduced to ∼7% of the total (Fig. 4, bar 6), down from ∼12% in the baseline case. Of this smaller contribution, subspace-facing K+ channels mediated ∼61% of the charge, whereas the RyRs mediated ∼34% (∼29% from K+ and ∼5% from Mg2+). Cl− channels on the JSR-cytosol face mediated the remaining ∼5%. For completeness, we also considered the case where K+ channels were completely removed from the JSR. Channel-mediated countercurrent was again reduced to ∼7% of the total countercurrent, but ∼95% of this went through the RyRs as K+ and Mg2+ currents (data not shown). However, based on experimental observations, it is unlikely that K+ channels are completely absent from both JSR membranes. Interestingly, we found that the K+ channels in the NSR made up the difference when the JSR-cytosol membrane did not have K+ channels, which is why Ca2+ release was unaffected.
These permutations demonstrate that the location of the K+ channels greatly affect which channels provide significant countercurrent, and in some cases, how much countercurrent is mediated by JSR channels in total. Particularly variable is the contribution of the RyR K+ currents, which varies from providing ∼1% of channel-mediated countercurrent to ∼29%, depending on K+ channel locations. However, we find that K+ channels consistently provide the majority of channel-mediated countercurrent when present in the JSR membranes.
Partial reductions of K+ channels
To understand the role of the K+ channels, we modeled two cases of SR K+ channel reductions that have been reported in the experimental literature. A 50% reduction scenario is roughly analogous to the single-knockout cases of Yazawa et al. (2), although our model does not distinguish between the two types of K+ channels, TRIC-A and TRIC-B, that were knocked out independently in their study and were not present in equal proportion. The 50% reduction scenario is also roughly analogous to the Na+ ion substitution performed in Guo et al. (5). Our calculations showed no significant difference between the baseline case and the 50%-reduced-K+-channel case (Fig. 4, bar 2). Deviations for all variables were within ∼10% of the baseline case, with the vast majority within ∼1% (data not shown). Every current, concentration, and driving force for each ion, as well as the voltages, in each compartment exhibited very similar systolic behavior as the baseline case. Moreover, Ca2+ release was unaffected, consistent with experimental findings.
A 90% K+ channel reduction scenario is equivalent to the substitution of Cs+ for K+ in Guo et al. (5). We found that a 90% reduction in the number of K+ channels also did not affect Ca2+ release, which is in agreement with the experiments of Guo et al. (5). However, although adequate countercurrent was provided, the sources of countercurrent in the 90% reduction case were different from the baseline and 50% cases. K+ channels in the JSR-cytosol membrane still conducted the majority of countercurrent during peak Ca2+ release, but their contribution to JSR channel-mediated countercurrent was reduced from ∼90 to ∼73%. K+ current through the RyRs took on a more significant role, contributing ∼16% to channel-mediated countercurrent during peak Ca2+ release, up from ∼1% (Fig. 4, bar 3). Another notable divergence from the baseline case is that the peak systolic JSR membrane potential reaches ∼−3.5 mV, instead of ∼−2.1 mV as in the baseline case (Fig. 5 B). This greater voltage intensifies the driving forces across the JSR membranes, which increases the proportion of countercurrent mediated through JSR ion channels from ∼12 to ∼21% (Fig. 4, bar 3). Interestingly, more systolic JSR channel-mediated countercurrent occurred when 90% of the K+ channels were removed because the electrical driving force across the JSR membrane increased.
Figure 5.
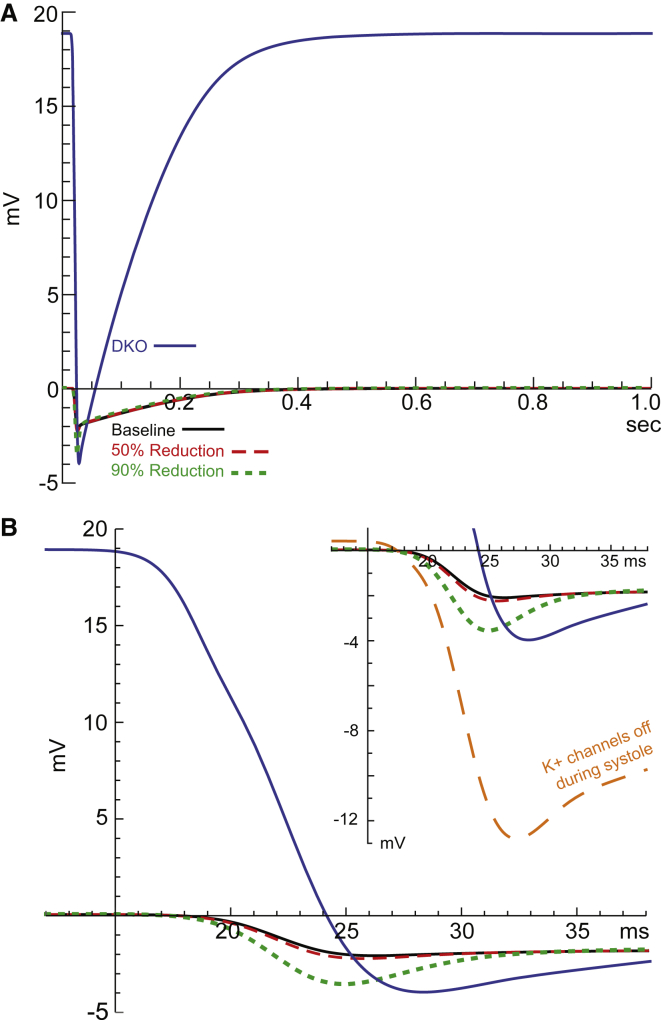
(A) JSR-subspace membrane potential as a function of time through a complete cardiac cycle. The subspace side is grounded. The DKO case (blue line) requires very large diastolic membrane potential (∼19 mV), whereas the baseline case (black line), 50% (red, dashed line), and 90% (green, dotted line) reductions of K+ channels cases are near 0 mV during diastole. (B) Zoom-in on the systolic timeframe. The JSR membrane potentials for these cases seem less affected by the reduction of K+ channels during systole (nadirs are within 2 mV of each other). Note that the membrane potential of the DKO case remains positive for much of systole, which leads to very different behavior compared to the other cases. (Inset) Focus on the nadirs. The case with K+ channels turned off during systole (orange, dashed line), which is explored in the Discussion, is included for comparison in the inset only. To see this figure in color, go online.
The DKO case
Removal of all K+ channels in all membranes of the modeled SR is analogous to the DKO cardiomyocytes presented in Yazawa et al. (2). The ion substitutions of Guo et al. (5) included an ion with zero conductance through K+ channels, Tris+, but because Tris+ also noticeably reduces the conductance of Ca2+ through the RyRs, the resulting conditions are not a good analogy to our DKO case.
As Figs. 2 and 3 demonstrate, Ca2+ release is not substantially affected by the absence of K+ channels. Consequently, the demand for countercurrent during peak Ca2+ release is roughly equivalent to the baseline and K+ channel reduction cases (∼146,000 charges). The primary countercharge movement during peak Ca2+ release is again provided by intra-SR ion movement, but the contribution has decreased from ∼88% of total countercharge in the baseline case to ∼76% in the DKO case. X− ions moving from the JSR into the NSR are again the major contributor to JSR intra-SR countercurrents, accounting for ∼95%. Ca2+, K+, and Mg2+ respond to concentration gradients between SR compartments and move from the NSR into the JSR, together generating the other ∼5% of intra-SR charge movement.
Channel-mediated countercurrents account for ∼24% of the countercurrent during peak Ca2+ release in the DKO case. Because there are no K+ channels, they do not contribute to the channel-mediated countercurrent. Instead, ∼74% of the channel-mediated countercurrent is mediated by the movement of K+ through the RyRs (Fig. 4, bar 4). The Cl− channels in the JSR-cytosol membrane mediate ∼18% of channel-mediated charge movement, whereas Mg2+ current through the RyRs mediates ∼7%.
The contribution of K+ through the RyRs is biphasic over systole; first K+ moves out of the JSR preceding the peak release of Ca2+, then K+ moves back through the RyRs as Ca2+ flux increases to a peak (Fig. 3 B). This surprising biphasic behavior is the result of the system’s need to maintain homeostasis, that is, the need to have zero net flux of each ion species over the course of one cycle. The only channels that conduct K+ in the DKO case are the RyRs, and thus any movement of K+ into or out of the SR must occur during systole. Therefore, to be utilized as a source of channel-mediated countercurrent during Ca2+ release, while also producing a steady state, any K+ that enters the SR must exit the SR within the systolic timeframe (or vice versa) when the RyRs are open. Although the net movement of K+ through the RyRs during systole is zero, the early release of K+ permits K+ to contribute substantially to countercurrent during peak Ca2+ release later on (∼17% of total countercurrent).
The primary driver of this biphasic flux is a very large diastolic JSR-subspace membrane potential of ∼19 mV (compared to ∼0.05 mV in the baseline case) (Fig. 5). The large membrane potential forces positively charged ions (including K+) out of the JSR. Because the membrane potential is held very high, it takes an appreciable amount of time to depolarize after the RyRs open (Fig. 5 B). Consequently, K+ and Mg2+ rush out of the JSR as soon as the RyRs open, but then return to the JSR when the potential depolarizes past their reversal potentials. This is also why the peak Ca2+ release current through the RyRs is shifted slightly earlier in the DKO case compared to release in the baseline case (Fig. 3); Ca2+ is forced out more quickly by the large potential gradient.
Diastole
In the previous section we focused on systole and the countercurrents into and out of the JSR because that is where RyRs release Ca2+. In this section on diastole, we focus on Ca2+ reuptake mediated by the SERCA pump located in the NSR-cytosol membrane. Thus, the focus is on the countercurrents into and out of the NSR.
The baseline case
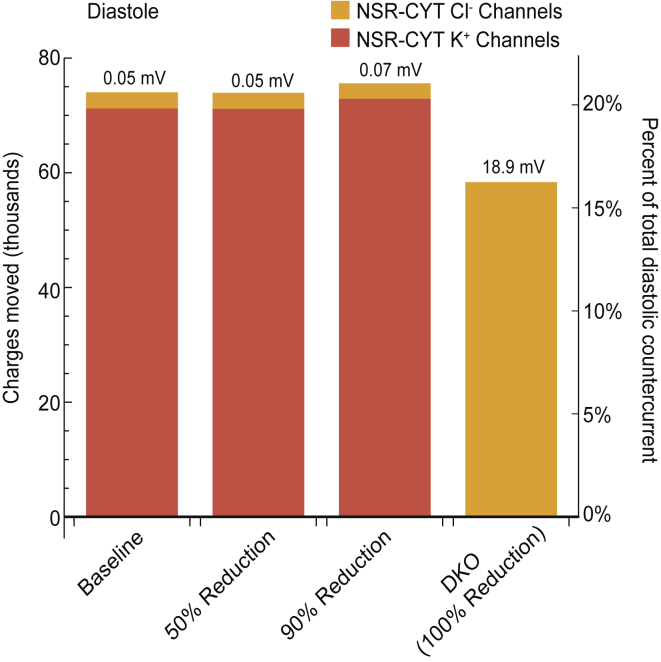
Once the RyRs close, Ca2+ is pumped back into the SR by the SERCA pump. This one-way flux of Ca2+ rebuilds the initial concentration gradient that drove robust release during systole. Countercurrent is needed during diastole to counteract the positive charge accumulation in the NSR resulting from the SERCA-mediated Ca2+ influx (∼185,000 charges over all of diastole). In addition, X− is drawn from the NSR back into the JSR due to the large X− concentration gradient that was generated during systole. This flux of X− out of the NSR also contributes to a net positive charge in the NSR (∼161,000 charges), bringing the total required countercurrent in the NSR during diastole to ∼346,000 charges.
In the baseline case, ∼79% of this needed diastolic countercurrent is provided by intra-SR sources (∼272,000 charges). Specifically, Ca2+ movement from the NSR into the JSR accounts for ∼67% of intra-SR countercurrent (nearly all Ca2+ from the SERCA pump), whereas X− movement from the ER to the NSR accounts for the remaining ∼33%. The other ∼21% of total countercurrent needed during diastole is provided by channel-mediated sources (∼74,000 charges) carried by K+ and Cl− channels in the NSR-cytosol membrane (Fig. 6, first bar). Approximately 96% of channel-mediated countercurrent moves through the NSR K+ channels, and ∼4% is mediated by NSR Cl− channels. The K+ channels are the primary avenue for charges to cross the SR membrane because of the high concentration of K+ (∼120 mM) compared to Cl− (∼5 mM).
Figure 6.
Number of charges moved through NSR ion channels during diastole. When K+ channels are present (first three cases), they mediate roughly the same amount of charge across the NSR membrane (red) with very similar steady-state NSR-cytosol membrane potentials (values shown above the bars). The DKO case varies significantly because it has no K+ channels and therefore must counter the influx of charge by driving the low concentration of Cl− through NSR Cl− channels (orange), which requires a much larger polarization across the NSR-cytosol membrane (Fig. 5, blue line). Because the need for countercurrent is roughly the same for each case, the percent of total diastolic countercurrent mediated by NSR channels can be compared across cases (right axis). To see this figure in color, go online.
To understand how the proximity of K+ channels to the SERCA pump affects the amount and sourcing of diastolic countercurrent, we explored a case where there are no K+ channels alongside the SERCA pump in the NSR-cytosol membrane, but with K+ channels in every other compartment membrane. In this case, almost all of the countercurrent in the NSR is provided by intra-SR countercurrents (∼98.5%). These intra-SR countercurrents move the accumulating charge into adjacent compartments that do have K+ channels, and these ultimately mediate the countercurrent across the SR membrane (data not shown), similar to what we found during systole in the absence of JSR K+ channels.
Partial reductions of K+ channels
As can be expected because there is no substantial difference between the baseline and 50% reduction cases during systole, the diastolic behavior of these two cases are also not substantially different (Figs. 5 and 6). Although the 90% reduction case had noticeably different sources of countercurrent to support normal systolic Ca2+ release (Fig. 4, bar 3), the sources of countercurrent during diastolic Ca2+ reuptake are roughly the same as in the baseline case (Fig. 6, bar 3). The diastolic SR membrane resting potential is not meaningfully different in this case either (Figs. 5 and 6). That is, the severely limited number of K+ channels in this case can still provide the countercurrent needed to facilitate normal Ca2+ reuptake, consistent with the Cs+ substitution experiments from Guo et al. (5).
The DKO case
The DKO case has no K+ channels and the RyRs are closed during diastole. This means that the only sources of channel-mediated countercurrent are the Cl− channels. Yet, the need for countercurrent is roughly the same in this case as in the baseline case: ∼360,000 charges, ∼191,000 of which is needed to counteract Ca2+ reuptake by the SERCA pump, and ∼169,000 is needed to counteract X− movement from the NSR to the JSR. In the absence of K+ channels, intra-SR countercurrents account for ∼84% of overall countercurrent, of which Ca2+ movement from the NSR to the JSR accounts for ∼61% of the intra-SR countercurrents, and X− movement from the ER to the NSR account for ∼38% (∼115,500 charges). The remaining ∼16% of overall countercurrent is mediated by the Cl− channels, which necessarily provide 100% of channel-mediated countercurrent (Fig. 6, last bar).
In this case, Cl− channels provide roughly an equivalent contribution to countercurrent as K+ channels provided in the baseline case (∼16% vs. ∼20%). However, Cl− concentration is much lower than K+, meaning larger driving forces are needed to produce equivalent currents. At the start of diastole, the DKO SR membrane potential is not significantly different from that in the baseline case (Fig. 5 B). However, the NSR-cytosol membrane potential grows rapidly until the driving force on Cl− is high enough to mediate adequate countercurrent and stop the building charge, which occurs at ∼19 mV (Fig. 5 A).
Discussion
We have shown that SR K+ channels are generally the preferred channel-type for mediating countercurrents across the SR membrane. During Ca2+ release and reuptake, the K+ channels moved the majority of the channel-mediated countercurrent, whereas the Cl− channels and RyRs mediated considerably smaller portions. This result held true in every case that we modeled with active K+ channels. During diastole, the absence of all SR K+ channels (the DKO case) generated a membrane potential of ∼19 mV, much larger than the ∼0.05 mV of the baseline or the cases with partial reduction of K+ channels. These changes indicate that SR K+ channels are essential for providing adequate and effective diastolic countercurrent.
During systole, however, countercurrent mediated by the K+ channels under baseline conditions shifts to the RyRs in the DKO case. Even though the peak systolic JSR-subspace membrane polarization increased in the DKO case, it only increased from ∼−2 to ∼−4 mV (Fig. 4), suggesting that the RyRs and K+ channels are redundant pathways for systolic countercurrent, even if K+ channels predominate in this role when present.
These findings support the hypothesis of Gillespie and Fill (1) that K+ channels are essential during Ca2+ reuptake, but are not essential during Ca2+ release because the RyRs can take over with only a small increase to the JSR-subspace membrane potential. To test this idea directly, we modified the baseline case so that all SR K+ channels were closed during systole but open during diastole. One would expect this case to exhibit the diastolic behavior of the baseline case and, with a slight increase of the systolic JSR-subspace membrane polarization (as in the DKO case), the RyRs would take over countercurrent during systole. This is not, however, what happened. Although a significant proportion of the total countercurrent was mediated by the RyRs in this case (∼48%) and the diastolic membrane potentials did not reach extreme values (∼0.4 mV), the systolic JSR-subspace membrane polarization reached ∼−13 mV, much larger than the ∼−4 mV one would anticipate based on the DKO case (Fig. 5 B, inset). Additionally, Ca2+ release was reduced by ∼10% to ∼130,000 charges, which is particularly striking because all other cases we studied resulted in approximately the same Ca2+ release of ∼145,000 charges (Fig. 2, inset).
This suggests that the K+ channels’ contribution during systole has subtler aspects than our initial analysis suggests. Therefore, we reexamine the baseline case in more detail. During Ca2+ release in the baseline case, the largest source of countercurrent is not JSR ion channels, but rather the X− anion intra-SR current that flows from the JSR into the NSR (∼86%). As described in Systole, this serves as an effective source of systolic countercurrent because the anions move more quickly between SR compartments than through a limited number of high resistance ion channels. However, the introduction of ∼126,000 X− charges into the NSR necessitates a countercurrent of its own in the NSR. In the baseline case, roughly half of this countercurrent is mediated by NSR K+ channels, and the other half is offset again by X− flow into the ER (which in turn is countered by K+ channels in the ER). Importantly, these NSR and ER K+ channel currents occur during peak Ca2+ release. By utilizing the high conductance pathways of X− currents to redistribute charge throughout the SR, not only is the charge diffused into a much larger volume, but it can then be shunted across the SR membrane by a larger number of ion channels. The net effect is that ion channels outside the JSR in effect provide countercurrent to support Ca2+ release in the JSR, even while Ca2+ release is occurring.
Therefore, with all K+ channels inactive during systole, anion currents still redistribute the building charge into the NSR and ER, but the only channels that are able to mediate the incoming charges downstream are Cl− channels. To produce the needed channel-mediated countercurrents with the low concentration of Cl−, Cl− must be driven by a large driving force, resulting in large peak systolic potentials across the SR membranes (∼−13 mV). In the DKO case, when K+ channels are inactive during both systole and diastole, the anion currents diffuse the building charge to the NSR and ER during Ca2+ release as well, but the Cl− channels in the downstream compartments are primarily driven by a large Cl− concentration gradient established during diastole under the large diastolic membrane potential (∼19 mV), and the systolic polarization on the membrane does not have to increase as much to produce equivalent countercurrents (∼−4 mV).
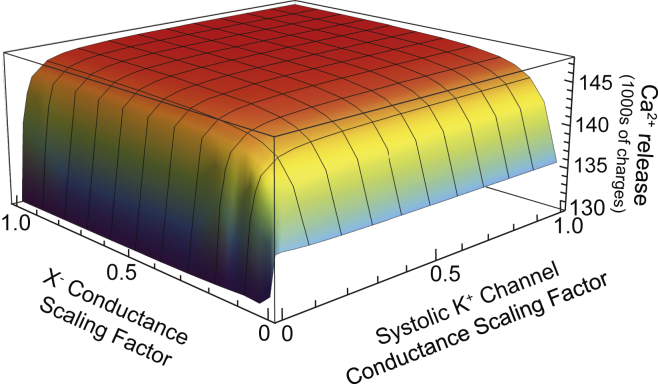
This broader view depicts intra-SR anion currents and ion channels linked together to form a network that produces efficient countercurrent by cascading downstream into non-JSR compartments. Consequently, no single channel type mediates essential countercurrent. To further examine this idea, we looked at a case in which the X− currents were made ineffectual (by setting their conductance to zero everywhere), and found that this also produced large polarizations of all JSR membranes during systole (∼−12 mV) and reduced Ca2+ release (to ∼135,000 charges) (Fig. 7). That is to say, the removal of intra-SR anion currents led to a similar disruption to the SR as removing K+ channels during systole.
Figure 7.
Dependence of SR Ca2+ release on key parameter values. On one axis, the conductance of X− movement within the SR is scaled between zero and its baseline case value. On the other axis, the conductance of K+ channels during systole is scaled between zero and its baseline case value. Note that the scaling factor applies only to K+ channels during systole and the scaling factor of the K+ channel conductance during diastole is 1 in all cases. Ca2+ release is used to illustrate the robustness of the countercurrent network throughout the parameter space because we find that Ca2+ release is largely unchanged in cases where the countercurrent network is active. The stability of Ca2+ release for cases when both conductances are >∼10% of their baseline values (red part) indicates that the countercurrent network is active under these parameters. The key point is that our results are largely independent of the value of these conductances; we could have overestimated the value of both parameters by a factor of >5 and our conclusions would have been qualitatively identical. It is only when one of the key parameters approaches a completely turned-off state that the countercurrent is not effectively provided by the countercurrent network, and Ca2+ release is reduced. To see this figure in color, go online.
Together with the findings described in Systole, our results indicate that: 1) there is not a single essential pathway of systolic countercurrent; 2) intra-SR currents are an integral part of a cooperative countercurrent network, and each of these intra-SR currents requires its own countercurrent downstream; and 3) K+ channels are the most effective pathway to ultimately mediate these downstream countercurrents across the SR membrane because the concentration of K+ is high and K+ channels are abundant.
These three ideas are manifest in all cases that we have modeled and, importantly, are not sensitive to model parameters. As Fig. 7 illustrates, Ca2+ release is minimally impacted by reductions in systolic K+ channel conductance and/or intra-SR X− current conductance; noticeable changes only occur once one of these parameters has been reduced to ∼5–10% of the values that we used. Peak systolic JSR-subspace membrane potentials are also stable with respect to this parameter space (data not shown). These results also help us to deal with having ambiguous characterizations of the K+ channels (e.g., their number and their gating and average conductance of full openings and substates). Figs. 4 and 7 show that just 800 pS of K+ conductance (10% of the baseline 8 nS) through each membrane provides sufficient countercurrent for 5 pA of Ca2+ current (Fig. 3). Put another way, having just four 200 pS TRIC-A channels (3) open (on average) on each membrane can offset the voltage fluctuations produced by ∼50 open RyRs. This suggests that the ambiguity in specific K+ channel properties is unlikely to alter our results.
More generally, the idea of a countercurrent network is independent of the parameters we choose (see also the Supporting Material). It is only when one of the pathways approaches a completely turned-off state (i.e., zero conductance) that other countercurrent options are utilized (e.g., countercurrent mediated by the RyRs or Cl− channels only), which results in much larger SR membrane potentials and/or reduced Ca2+ release. Therefore, the idea of having intra-SR ions carrying large countercurrents that are then dissipated by membrane K+ and Cl− channels is robust. The exact contribution of each channel type cannot be confidently resolved at this stage, however. For example, if we have substantially overestimated the number of open K+ channels, then the role of RyR self-countercurrent would increase (Fig. 4). The same is true for other unknown parameters such as the subspace-to-cytosol conductance; a small conductance will accumulate K+ in the subspace and change the amount of countercurrent provided through both the K+ and RyR channels on the top of the JSR. Even in those rather extreme cases, however, our calculations show that not only will K+ channels likely play a dominant role, but the idea that countercurrent involves substantial intra-SR currents is unchanged. This fundamental mechanism is affected only when the balance between the low-resistance SR interior and high-resistance SR membrane channels is shifted dramatically toward the channels (Fig. 7).
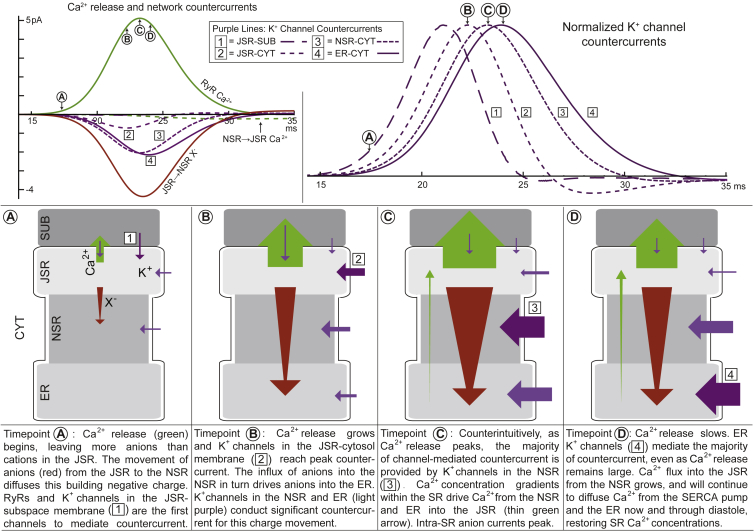
Fig. 8 depicts our concept of a cascading network of countercurrent. As Ca2+ release begins, JSR-subspace K+ channels and the RyRs are the first to start mediating countercurrent (Fig. 8, timepoint A). The high-conductance intra-SR anion flux moves a large portion of the building negative charge out of the JSR to the NSR and later, as Ca2+ release continues to grow, farther down the SR into the ER. Meanwhile, the current through K+ channels on the JSR-cytosol membrane, peaks (Fig. 8, timepoint B). Negative charges quickly entering the NSR and ER are electrically balanced across the SR membrane by large K+ channel currents in these compartments. Of these, the K+ channel currents in the NSR begin and peak first (Fig. 8, timepoint C) and those in the ER peak shortly thereafter (Fig. 8, timepoint D). The cascade of the K+ channel currents through the SR follows the order of the channels’ proximity to the RyRs, which is clearly visible when currents from all membranes are seen on the same scale (Fig. 8, top right).
Figure 8.
The cascading SR countercurrent network during Ca2+ release. (Top row, left) RyR Ca2+ release current (green, solid line) and the countercurrents plotted as a function of time. The countercurrent network is primarily made up of K+ channel countercurrents (purple lines), JSR to NSR anion flux (red line), and NSR to JSR Ca2+ flux (green, dashed line). Four timepoints (A–D) are marked during Ca2+ release, and K+ channel currents through the four SR membranes are numbered (defined in figure); the current through K+ channels on the JSR-subspace face [1], as well as the RyR K+ current (which roughly follows the time course of the K+ channels because they have the same K+ driving force), cannot be resolved on this scale. (Top row, right) The four K+ channel currents normalized by their peak value. Seen on the same scale, the cascade of countercurrent through the SR becomes clear; each set of K+ channels mediates countercurrent in the order of their proximity to the RyRs, with JSR-subspace K+ channels peaking first and ER K+ channels peaking last. (Bottom row) Schematic representation of the countercurrent network. The flow of Ca2+ (green), K+ (purple), and anions (red) are shown for each timepoint identified in the top row, accompanied by a description of the developments between timepoints. The size of the arrows is representative of the amount of charge moved by each current pathway, but should not be taken literally, especially when small amounts of charge are moved (refer to top row, left for exact contributions). The darker purple arrow in each panel is meant to highlight specific K+ channel currents in the cascade of countercurrents from the JSR to the ER throughout time. To see this figure in color, go online.
Very similar networks were at work when the number of K+ channels was reduced by 50 and 90%. Also, in cases where the K+ channels of one SR compartment membrane were removed, the K+ channels of the other SR membranes offset the missing countercurrent, and Ca2+ release remained unaffected. In the extreme case when K+ channels were removed from the SR entirely, Cl− channels took the place of K+ channels in the countercurrent network. In all these cases, the SR anion currents quickly distributed the building JSR charge to whichever channels were available downstream. Therefore, having multiple countercurrent pathways allows movement of building charge out of the JSR to be remarkably robust, even in the face of unusual conditions. Consequently, Ca2+ release is minimally affected across a wide range of conditions in the SR.
Our new description of a cascading network of countercurrent is consistent with and explains the findings of many experiments. For instance, the experiments of Gillespie and Fill (1), which measured currents through the RyR in a planar lipid bilayer, identified the RyR as capable of conducting its own countercurrent while Ca2+ is released. However, the large reservoirs of permeant ions on either side of the membrane did not model interactions between the RyR currents and other SR currents that are ever-present in vivo, which we now propose serve a large role in distributing charge throughout the SR. Moreover, their experiments did not include other channels and therefore all countercurrent was mediated by the RyRs. Our results indicate that this is indeed possible, but not likely to occur. In fact, our simulations suggest that countercurrent may not even be predominantly mediated at the JSR-subspace interface (as assumed by Gillespie and Fill (1)), but rather by the downstream network of countercurrent. From our simulations, however, it is difficult to assess the exact role of this membrane and the exact role of RyR-mediated countercurrent because several parameters that are not precisely known (e.g., the exact locations of the K+ channels, the SUB-CYT resistance) have a large effect on the JSR-subspace channels’ contribution to JSR channel-mediated countercurrent (Fig. 4).
Additionally, our results are consistent with other experiments. Yazawa et al. (2) showed large caffeine-induced Ca2+ transients in DKO cardiomyocytes, which suggests that adequate countercurrent was present despite the absence of K+ channels. In our DKO simulation, we found that countercurrents were able to support normal Ca2+ release, but the driving forces needed to produce adequate countercurrent during Ca2+ reuptake generated very large diastolic membrane potentials (∼19 mV). It is unclear what the precise consequences of these conditions on the SR would be, but it is likely that such large SR voltages are not sustainable in vivo. Additional factors not present in our model, such as osmotic effects and swelling, may become relevant at high and sustained membrane potentials. However, our findings are consistent with large RyR Ca2+ transients existing in a nonviable SR like the enlarged ones found in the DKO mice (2).
Lastly, our findings are also consistent with the experiments of Guo et al. (5). Their substitutions of Na+ and Cs+ for K+ in the SR and cytosol demonstrated that large reductions in K+ channel conductance (by 35 and 88%, respectively) caused no significant change in Ca2+ release. Our simulations are consistent with that outcome. However, contrary to their conclusion, our calculations show that the majority of countercurrent is in fact still mediated by K+ channels, even when K+ channel conductance is low. Guo et al. (5) proposed the possibility that the reduced conductance was perhaps adequate as an alternative explanation, and our simulations support that.
The result that an order-of-magnitude reduction in the number (or conductance) of K+ channels causes no change to Ca2+ release leads to the question as to why there is an apparent overabundance of K+ channels in the SR. One explanation is that we have overestimated their abundance by a factor of >10. However, the cellular spark measurements of Guo et al. (5) strongly suggest that there are naturally more SR K+ channels than needed. Another possibility is that large numbers of K+ channels assure the SR membrane potentials remain low. Although Ca2+ release is unaffected in our 90% K+ channel reduction case, the systolic voltage reaches ∼−3.5 mV, as opposed to ∼−2 mV in the baseline case (Fig. 5 B). On the other hand, if we increase the number of K+ channels from our baseline case, there is no noticeable reduction in membrane polarization (data not shown). This indicates that our estimate for the number of SR K+ channels minimizes SR membrane potentials. Perhaps this is the role of an overabundance of K+ channels.
Conclusion
We used an equivalent circuit model of a cardiac SR, the surrounding cytosol, and the dyadic cleft to explore the sources of countercurrent during a complete cardiac cycle, with particular interest in the role of TRIC K+ channels. We were motivated to probe these relationships because of conflicting conclusions from previous experimental work; some studies indicated that the K+ channels’ contribution to countercurrent is essential, whereas other studies pointed to a nonessential contribution and a primary role for RyR-mediated countercurrent.
Our simulations have led to a more nuanced view of the mechanisms that provide SR countercurrent, where no single channel type is essential for countercurrent. Specifically, we showed that a cascading network of countercurrents throughout the entire SR assures there is efficient countercurrent to support Ca2+ release under a wide variety of conditions. When Ca2+ leaves the SR through the RyRs, all possible sources of channel-mediated countercurrent are engaged. However, because there is always a large concentration of anions (and to a lesser extent, other cations) that move within the SR with much higher conductance than ions through ion channels, the intra-SR flow of these ions quickly diffuses much of the negative charge building in the JSR from the Ca2+ efflux by moving anions from the JSR into the NSR; the rest is countered by JSR channels. The corresponding influx of anions into (and smaller efflux of cations out of) the NSR builds a negative charge there, which in turn demands its own countercurrents. This negative charge is countered by the influx of K+ through the K+ channels in the NSR membrane (as well as some efflux of Cl− through Cl− channels), while other ions continue to diffuse the negative charge downstream into the ER.
These cascading counterion interactions are based on the principle that every movement of charge locally drives its own countercurrent. Thus, it is not only the charges that cross the SR membrane that drive countercurrent, but any charge that moves from one location to another within the SR. As one current counters Ca2+ release, another current is driven to counter the movement of those charges. More concretely, if a current moves charge (ions) from the JSR to the NSR, a countercurrent is needed in the NSR to offset the incoming charge. On the whole-SR scale, these successive local interactions diffuse charge buildup throughout the entire SR volume, and dissipate charge through ion channels everywhere. Ultimately, this mechanism moves charges across every SR membrane, not only the JSR membrane, primarily through K+ channels due to their abundance and high K+ concentration. It is this cascade of currents that supports the remarkably robust Ca2+ release.
Author Contributions
V.Z. performed the simulations, analyzed the data, and wrote the manuscript. M.F. contributed SR ion channel data and discussions. D.G. designed the research.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award No. R01AR054098.
Editor: Eric Sobie.
Footnotes
Supporting Materials and Methods, one figure, and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)35037-3.
Supporting Citations
References (16, 17, 18, 19, 20, 21, 22) appear in the Supporting Material.
Supporting Material
References
- 1.Gillespie D., Fill M. Intracellular calcium release channels mediate their own countercurrent: the ryanodine receptor case study. Biophys. J. 2008;95:3706–3714. doi: 10.1529/biophysj.108.131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yazawa M., Ferrante C., Takeshima H. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature. 2007;448:78–82. doi: 10.1038/nature05928. [DOI] [PubMed] [Google Scholar]
- 3.Pitt S.J., Park K.-H., Sitsapesan R. Charade of the SR K+-channel: two ion-channels, TRIC-A and TRIC-B, masquerade as a single K+-channel. Biophys. J. 2010;99:417–426. doi: 10.1016/j.bpj.2010.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller C. Voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum: steady-state electrical properties. J. Membr. Biol. 1978;40:1–23. doi: 10.1007/BF01909736. [DOI] [PubMed] [Google Scholar]
- 5.Guo T., Nani A., Fill M. Sarcoplasmic reticulum K+ (TRIC) channel does not carry essential countercurrent during Ca2+ release. Biophys. J. 2013;105:1151–1160. doi: 10.1016/j.bpj.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X., Yamazaki D., Ma J. Ca2+ overload and sarcoplasmic reticulum instability in TRIC—a null skeletal muscle. J. Biol. Chem. 2010;285:37370–37376. doi: 10.1074/jbc.M110.170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki D., Tabara Y., Takeshima H. TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab. 2011;14:231–241. doi: 10.1016/j.cmet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Venturi E., Sitsapesan R., Takeshima H. TRIC channels supporting efficient Ca2+ release from intracellular stores. Pflugers Arch. 2013;465:187–195. doi: 10.1007/s00424-012-1197-5. [DOI] [PubMed] [Google Scholar]
- 9.Coronado R., Rosenberg R.L., Miller C. Ionic selectivity, saturation, and block in a K+-selective channel from sarcoplasmic reticulum. J. Gen. Physiol. 1980;76:425–446. doi: 10.1085/jgp.76.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picard L., Côté K., Rousseau E. Sarcoplasmic reticulum K+ channels from human and sheep atrial cells display a specific electro-pharmacological profile. J. Mol. Cell. Cardiol. 2002;34:1163–1172. doi: 10.1006/jmcc.2002.2041. [DOI] [PubMed] [Google Scholar]
- 11.Somlyo A.V., McClellan G., Somlyo A.P. Electron probe x-ray microanalysis of post-tetanic Ca2+ and Mg2+ movements across the sarcoplasmic reticulum in situ. J. Biol. Chem. 1985;260:6801–6807. [PubMed] [Google Scholar]
- 12.Berti C., Zsolnay V., Gillespie D. Sarcoplasmic reticulum Ca2+, Mg2+, K+, and Cl- concentrations adjust quickly as heart rate changes. J. Mol. Cell. Cardiol. 2017;103:31–39. doi: 10.1016/j.yjmcc.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie D. Energetics of divalent selectivity in a calcium channel: the ryanodine receptor case study. Biophys. J. 2008;94:1169–1184. doi: 10.1529/biophysj.107.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shannon T.R., Wang F., Bers D.M. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys. J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Ajouz S., Venturi E., Sitsapesan R. Dampened activity of ryanodine receptor channels in mutant skeletal muscle lacking TRIC-A. J. Physiol. 2017;595:4769–4784. doi: 10.1113/JP273550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soeller C., Crossman D., Cannell M.B. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc. Natl. Acad. Sci. USA. 2007;104:14958–14963. doi: 10.1073/pnas.0703016104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baylor S.M., Chandler W.K., Marshall M.W. Calcium release and sarcoplasmic reticulum membrane potential in frog skeletal muscle fibres. J. Physiol. 1984;348:209–238. doi: 10.1113/jphysiol.1984.sp015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hille B. Sinauer Associates; Sunderland, MA: 2001. Ion Channels of Excitable Membranes; p. 814. [Google Scholar]
- 19.Williams G.S., Chikando A.C., Jafri M.S. Dynamics of calcium sparks and calcium leak in the heart. Biophys. J. 2011;101:1287–1296. doi: 10.1016/j.bpj.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wescott A.P., Jafri M.S., Williams G.S.B. Ryanodine receptor sensitivity governs the stability and synchrony of local calcium release during cardiac excitation-contraction coupling. J. Mol. Cell. Cardiol. 2016;92:82–92. doi: 10.1016/j.yjmcc.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picht E., Zima A.V., Bers D.M. Dynamic calcium movement inside cardiac sarcoplasmic reticulum during release. Circ. Res. 2011;108:847–856. doi: 10.1161/CIRCRESAHA.111.240234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bers D.M. Kluwer Academic; Dordrecht, The Netherlands: 2001. Excitation-Contraction Coupling and Cardiac Contractile Force; p. 427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.