Abstract
Background
Amylin is a pancreatic β-cell hormone that produces effects in several different organ systems. One of its best-characterized effects is the reduction in eating and body weight seen in preclinical and clinical studies. Amylin activates specific receptors, a portion of which it shares with calcitonin gene-related peptide (CGRP). Amylin's role in the control of energy metabolism relates to its satiating effect, but recent data indicate that amylin may also affect hedonic aspects in the control of eating, including a reduction of the rewarding value of food. Recently, several amylin-based peptides have been characterized. Pramlintide (Symlin®) is currently the only one being used clinically to treat type 1 and type 2 diabetes. However other amylin analogs with improved pharmacokinetic properties are being considered as anti-obesity treatment strategies. Several other studies in obesity have shown that amylin agonists could also be useful for weight loss, especially in combination with other agents.
Scope of review
This review will briefly summarize amylin physiology and pharmacology and then focus on amylin's role in food reward and the effects of amylin analogs in pre-clinical testing for anti-obesity drugs.
Conclusion
We propose here that the effects of amylin may be homeostatic and hedonic in nature.
Keywords: Amylin, CGRP, Homeostatic, Hedonic, Reward, Analog
1. Amylin in the control of energy metabolism
The pancreatic hormone amylin is co-synthesized and co-released with insulin from pancreatic beta-cells [1], [2], [3]. It has long been thought that pancreas-derived amylin is the only relevant source of amylin to control metabolism. However, recent evidence has shown that amylin is also expressed in the central nervous system, in particular in parts involved in metabolic control, such as the lateral hypothalamus (LH) [4]. Globally, amylin controls nutrient fluxes by reducing energy intake, modulating nutrient utilization and increasing energy expenditure. The best-investigated function of amylin is its role as satiation signal. Indeed, chronic administration of amylin reduces total energy intake, which eventually results in the reduction of body weight [5], [6]. These findings were the basis for the development of amylin analogs that may represent a new approach for the treatment of overweight in obese individuals [7], [8].
The caudal hindbrain area postrema (AP) and nucleus of the solitary tract (NTS) are critically involved in mediating the effects of amylin on eating [9]. However, recent data indicate that other areas of the brain, including the hypothalamic arcuate (ARC) or ventromedial (VMN) nucleus [10], [11], ventral tegmental area (VTA) [12], [13], and lateral dorsal tegmental nucleus (LDTg) [14], may be directly or indirectly targeted by amylin to influence hedonic aspects of eating such as reward-guided behaviors that may contribute to the food selection [15], [16]. This review will briefly summarize amylin physiology and pharmacology and then focus on the amylin's role in food reward and the effects of amylin analogs in pre-clinical testing for anti-obesity drugs. We have also included some previously unpublished, original data, because we believe that these data are important to introduce and emphasize certain aspects covered in this review article. These points are important because they have not been covered in other, recently published review articles on amylin. Experimental details in respect to these unpublished data are provided in the Supplementary part of this review.
2. Amylin receptor structure and function
The amylin receptor consists of a heterodimer of the calcitonin receptor (CTR) core protein combined with one or several receptor activity modifying proteins (RAMPs) to yield specific amylin receptors [8], [17], [18], [19]. Two splice variants of the CTR and three RAMPs are known, resulting in at least 6 different subtypes of amylin receptors. Recent data from the caudal hindbrain indicate that individual neurons may in fact express more than a single RAMP, theoretically increasing the number of possible amylin receptor subtypes per cell [20]. Amylin receptor components are widely distributed throughout the central nervous system, and a high density of both the CTR and RAMPs is found in the AP of the caudal hindbrain, other circumventricular organs (e.g., the subfornical organ), the hypothalamus (ARC, VMN) and other brain areas (VTA, LDTg, nucleus accumbens [NAc]) [21], [22], [23], [24]. So far, the co-expression of the CTR and RAMPs in single neurons of native tissue has only been shown in the AP of the caudal hindbrain [20], but our work has also shown that non-neuronal cells, in particular microglia, also seem to mediate amylin's effects [10], [25], [26].
Study of amylin receptor function is complicated by the fact that antagonists that specifically block certain subtypes of amylin receptors are not available [8], [27], and prototypical amylin receptors (in particular the amylin-1 receptor resulting from the combination of CTR and RAMP1) also seem to mediate the effects of the related peptide calcitonin gene-related peptide (CGRP) [27], [28]. Hence, we currently have no clear picture of the role of specific amylin receptor subtypes for certain amylin functions, or of the importance of the expression of more than one RAMP in single cells.
Upon amylin receptor activation, various intracellular signaling systems are activated. Specifically, amylin increases the expression of cyclic GMP (cGMP) in activated AP neurons [29] and leads to a phosphorylation of ERK [30]. In both cases, there is evidence for a functional relevance of these systems in amylin's effect to reduce eating. On the other hand, transfected cell system studies show that amylin signaling also involves cAMP, intracellular Ca2+, and beta-arrestin [17], [22]. But none of these has been linked to specific amylin actions as yet.
3. Sites of amylin action
3.1. Amylin activation of the brainstem and neuroaxis
The AP is critically implicated in mediating amylin's satiating effect. Local AP administration of amylin decreases eating, while local AP amylin antagonist injection increases it, and blocks the eating inhibitory effect of peripheral amylin [31]. Further, surgical lesion [32] or a specific deletion of noradrenergic AP neurons (see also below; [33]) block the effect of peripheral amylin on eating. In addition, a large array of electrophysiological and imaging experiments provide confirmatory evidence for an important and most likely direct effect of circulating amylin on AP neurons (reviewed in [9], [34]).
The amylin-induced activation of AP neurons occurred to a large extent in neurons expressing the noradrenalin synthetizing enzyme, dopamine-beta-hydroxylase (DBH), and presumably the subsequent enhanced release of noradrenaline, possibly in the NTS or the lateral parabrachial nucleus (LPB) [33]. These AP neurons are necessary for peripheral amylin to reduce eating, because even a partial chemical lesion of these neurons is sufficient to abolish the eating inhibitory effect of peripheral amylin; this type of lesion had no effect on baseline food intake [33] and therefore circumvents the problem of a surgical destruction of the entire AP, which itself has a negative influence on eating and body weight gain [16], [32].
The activation of AP neurons is the first step in the subsequent activation of a neural pathway that projects rostrally to the forebrain and includes the NTS, LPB, and possibly the central amygdala (CeA). Lesions of the respective brain areas (AP, NTS, LPB) abolish amylin's effect on eating and the expression of the neuronal activation marker c-Fos, indicating that activation was absent in brain areas rostral to the lesion, e.g. in the NTS, LPB, and CeA in AP-lesioned rats, or in the CeA in LPB-lesioned rats [9], [35], [36]. Indeed using anterograde and retrograde tracing, the AP was shown to project to the NTS, LPB, CeA, and the bed nucleus of the stria terminalis [37].
Furthermore, a recent study suggested that glutamatergic neurotransmission in the AP seems to play a role in mediating amylin effects on eating, and that the amylin receptors appear to be located mainly on presynaptic glutamatergic terminals synapsing with AP neurons [38]; interestingly, our own studies also showed a close apposition of amylin-activated neurons that expressed DBH with VGLUT2-positive boutons [39]. How these effects may be linked mechanistically, and whether this mechanism is physiologically relevant, is currently unknown.
3.2. Amylin action in other brain areas
As mentioned, amylin binding sites have widespread distribution throughout the brain [21]. Similarly, the expression of all critical amylin receptor components has also been described in many brain areas [23], [24], and amylin itself may also be expressed selectively in the lateral hypothalamic area [4], although the contribution of the latter to the physiological control of eating remains largely unknown. Recent experiments focused on how amylin receptors in other brain areas outside the AP mediate the physiological actions of peripheral amylin.
Among the most prominent of these is the VTA, where components forming the active amylin receptor complex are expressed and where the peripheral administration of the amylin receptor agonist salmon calcitonin (sCT) reduces eating by activating amylin receptors. Importantly, this effect was blocked by the VTA administration of the amylin antagonist AC187 [12], [13].
The LDTg has been implicated in processing signals related to the homeostatic but also the hedonic control of eating. It expresses all components of the amylin receptor, and administration of amylin or sCT into the LDTg reduces eating primarily by reducing meal size, similar to amylin's satiating action in the AP [14], [40]. Furthermore, administration of the amylin receptor antagonist AC187 into the LDTg reduces the inhibitory effect of peripheral sCT on eating, and depletion of the CTR component leads to increased body weight gain suggesting a physiological relevance of these findings [14].
The relationship between the effects mediated by intra-VTA or intra-LDTg administration of sCT and the previously described AP-mediated effects of amylin and its receptor agonists is unclear at present. Indeed, it is not clear if amylin and its agonists activate several brain areas in parallel. With the exception of the circumventricular organs, including the AP, it is also unclear how much peripheral amylin actually reaches these respective receptor populations as a function of transport across the blood brain barrier [41], [42], [43]. It needs to be tested whether similarly to leptin [44], amylin crosses the tanycyte layer around the 3rd ventricle in particular to reach hypothalamic areas. Finally, available data do not distinguish between a direct activation of the pertinent receptors by peripheral amylin or sCT or activation elsewhere (possibly in the AP) and mediation of this effect via projection to the VTA or LDTg, both of which may use CGRP as neurotransmitter interacting with the amylin-1 receptor [27].
4. Amylin action on food reward
Conceptually, controls of eating have often been classified as homeostatic, acting via the caudal hindbrain, including the AP, NTS and LPB, and the hypothalamus, and hedonic, acting via reward pathways in the brain (e.g. [45], [46], [47], [48]). However, this distinction is probably over-simplistic since hedonic controls of eating through reward pathways interact with homeostatic control pathways and often can rely on the same signaling molecules. For example, gastrointestinal hormones such as glucagon-like peptide-1 (GLP-1) not only act as homeostatic signals that are involved in the control of meal size or body weight but also influence reward–based aspects of ingestive behavior [49], [50]. Food reward is often divided into two components: “liking” and “wanting.” In particular, wanting seems to be modulated by the mesolimbic dopamine system that includes neurons in the VTA, which release dopamine from axon terminals in the NAc of the ventral striatum [46], [51]. Recent data indicate that amylin may be one of the signals influencing the rewarding properties of food, and that the mesolimbic reward pathway may be involved in these effects.
4.1. Amylin's effect on food choices
Direct activation of amylin receptors using sCT in the VTA has been shown to reduce not only the intake of chow, which has low palatability, but also the intake of a palatable sucrose solution in rats [13]. Similar to the effect of peripheral amylin, these effects appear to be mainly due to a reduction in meal size. Further, administration of amylin agonists into the VTA also reduces sucrose self-administration on a progressive ratio schedule [52]. These studies were recently extended by showing that sCT administered into the VTA greatly reduced the intake of fat in fat-preferring rats [13], [52]. Sucrose-preferring rats did reduce sucrose intake after sCT, but the selectivity was more variable than in fat-preferring rats. Interestingly, administration of amylin or sCT into the LDTg also reduced motivated behaviors such as lever pressing for sucrose solution in rats, suggesting that not only the consummatory phase, but also the appetitive phase of eating is affected [14].
4.2. Amylin's effect on highly palatable diet intake
Our own studies indicated that the effectiveness of amylin to reduce energy intake may actually depend on the palatability of the diet offered to animals, but there is also an important influence of the diet that is context specific [53], [54], [55]. In recent unpublished experiments (see Figure 1), we used rats that were exposed for different periods of time to Ensure®, a highly palatable, liquid high-fat, and high-carbohydrate diet. Please see Supplementary part of this review for experimental details.
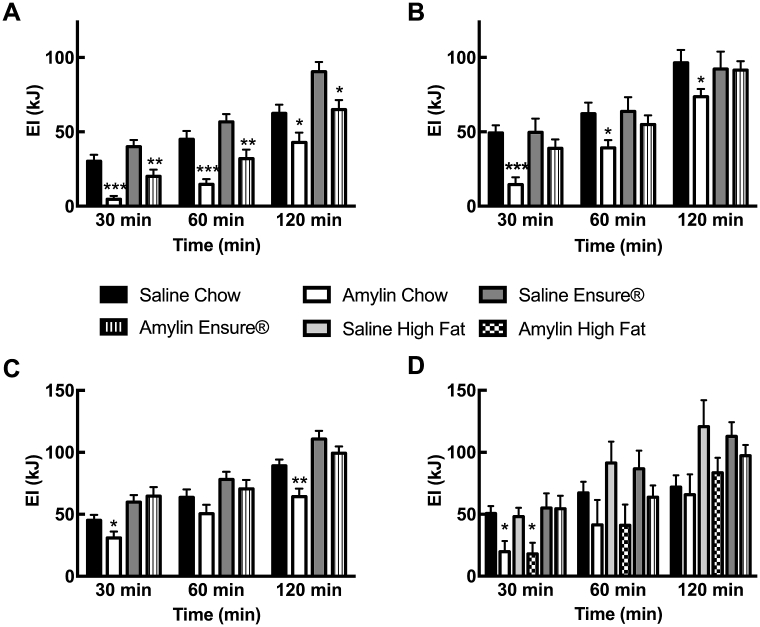
Figure 1.
(A) Cumulative energy intake after saline or amylin (5 pmol; i3vt injection at dark onset in undeprived rats) in rats maintained for three weeks on Ensure® cacao plus chow or on chow only; n = 18–19/group. Cumulative energy intake after saline or amylin (5 pmol; i3vt injection at dark onset in undeprived rats (B, n = 10/group) or 5 μg/kg s.c. injection at dark onset after 3 h-food deprivation (C, n = 32/group), respectively) in rats maintained for three days on Ensure® cacao only or on chow only; (D) Cumulative energy intake after saline or amylin (5 pmol; i3vt injection at dark onset in undeprived rats) in rats maintained for three days on Ensure® cacao, a pelleted high-fat diet, or chow only; n = 6–10/group. Values are mean ± SEM, symbols denote significant differences between saline- and amylin-treated groups within the respective diet groups; *p < 0.05, **p < 0.01, ***p < 0.001.
Rats that were adapted to chocolate-flavored Ensure® in addition to standard rat chow ingested approximately 90% of energy as Ensure® and only 10% in the form of chow. Animals that were exposed to either chocolate Ensure® or chow for 3 weeks both decreased eating in an acute feeding test when amylin was injected into the 3rd cerebral ventricle (i3vt; Figure 1A). Hence, long-term access to Ensure® did not reduce the sensitivity of obese rats to central amylin. Interestingly, the outcome was very different when rats were tested after only 3 days of exposure to chocolate Ensure®. Presumably due to very strong drive to eat early after exposure to a novel, highly palatable diet, rats appeared to be insensitive to centrally or peripherally injected amylin (Figure 1B, C). In contrast, amylin reduced acute intake of a high fat diet, which was provided for 3 days and had a similar nutrient composition to Ensure® (Figure 1D). The “insensitivity” to amylin after Ensure® appeared to be linked to the presence of the chocolate flavor in the diet, which produced a very strong drive to eat, because rats with brief exposure to unflavored Ensure® reduced eating after amylin, similar to amylin's effect on intake of high fat diet-exposed rats (data not shown).
Thus, amylin insensitivity early after exposure to the highly palatable chocolate Ensure® seemed to be more dependent on the palatable flavor and duration of exposure rather than diet composition. These data also suggest that specific taste preference, rather than diet composition, appeared to play the major role, indicating that homeostatic signals may fail to influence eating when the rewarding effect is too strong. Finally, the data showed that the “insensitivity” towards amylin waned once the animals had had been exposed to the highly palatable diet for an extended period.
4.3. Mechanism of amylin's action on food reward
A possible direct implication of the mesolimbic reward system in amylin action, and in particular of the VTA and the NAc, was indicated by studies showing that infusion of sCT into the VTA reduced the release of dopamine in the NAc and that dopamine receptor activation in the NAc overcame the inhibitory effect of sCT on eating [15]. Along these same lines, we recently reported that peripheral sCT was also able to reduce the VTA-stimulated release of dopamine in the NAc of rats. Interestingly, amylin itself had no such effect, suggesting that this difference might relate more to the duration of amylin receptor activation by amylin, which is short lasting, versus sCT, which is long lasting, than a true difference in pharmacodynamics [16].
Most importantly, like the meal-terminating properties of amylin, the effect of peripheral sCT to reduce evoked dopamine release was absent in rats with AP or LPB lesions [16]. This suggests that, as opposed to what had been suggested before [13], [15], the effect of sCT on reducing dopamine release in the NAc may be mediated in the reward system through the AP and LPB, rather than by direct action on VTA amylin receptors. Our data can be reconciled with the previously published work by assuming that peripheral sCT (and presumably also amylin, at least when given chronically) activates a caudal hindbrain network including CGRP-positive projections to the VTA, where CGRP acting on the amylin-1 receptor may reduce the activity of dopaminergic neurons projecting to the NAc. This model opens up the possibility that the AP and LPB are also important in mediating hedonic and motivational processes and that homeostatic signals also interact directly with the hedonic system and the perception of food reward. Hence, similar to GLP-1, amylin would be another example of a negative feedback signal acting in the hindbrain that also plays a role in the mediation of food reward [49], [50], [56], [57].
These studies raise a number of questions. For example, is the sensitivity of this system and its responsiveness to amylin due to differences in the circumstances of varying feeding situations? Does the effect of chronic exposure to highly palatable diets differ from the effect of short-term exposure, and if so, which is the critical component? Is amylin “insensitivity” linked to changes in the dopaminergic reward pathway between the VTA and NAc?
5. Clinical use of amylin and amylin analogs
Experience with pramlintide (Symlin®) and other clinical studies in obesity have shown that amylin agonists could also be useful for weight loss, especially in combination with other agents [58], [59], [60], [61], [62]. One of the most promising and clinically relevant combinations seems to be amylin plus leptin [63], [64], [65]. Amylin can improve leptin sensitivity and overcome leptin resistance in obese individuals [62]. In the following paragraphs, the development and effects of new agonists will be summarized, because, even though pramlintide is a useful amylin mimetic, other amylin agonists with improved potency and pharmacokinetics may provide optimized therapeutics for the treatment of obesity. Based on the findings discussed above that amylin seems to affect homeostatic and hedonic controls of eating and that a disturbance of both aspects of eating is responsible for the increase in obesity rates, amylin-based pharmacotherapy may be a particularly promising approach for future treatment options.
Since next to its satiating action, amylin has an effect on the reward system [15], [16] and since alterations in the function of the reward processing brain areas have been linked to mood disorders [66], it is important to examine if amylin analogs do not induce anxiety- and depression-like behaviors. Currently, there is no indication that this might be the case. For example, it has been shown that rats treated with sCT did not display an increase in anxiety type behaviors when tested during an open field and social interaction test [52]. Further, chronic amylin improved the recovery from social stress in a visible burrow system in rats [67], and long term administration of pramlintide did not induce signs of anxiety or depression in people [62]. Some studies indicate that amylin has anxiolytic and anti-depression like properties (see Ref. [68] for review).
5.1. Next generation drugs for the amylin system
5.1.1. Davalintide
Davalintide is an amylinomimetic with increased potency, efficacy, and duration of action. It binds similarly to rat amylin to amylin receptors but has greater affinity than amylin at the human CGRP receptor or the rat calcitonin receptor, at least under in vitro conditions [69]. In general, davalintide exhibits a greatly enhanced duration of action and is more potent than amylin in reducing food intake and body weight in rats. Thus, davalintide shares similarities with sCT. Apart from differences in pharmacokinetics, davalintide's mode of action seems to be more similar to that of amylin, because both preferentially reduced intake of a palatable high fat diet, and the effect of both peptides on eating appears to depend on an intact AP [69].
5.1.2. PEGylated or glycosylated amylin
Given the short half-life of the native amylin peptide (13 min), one strategy to improve the effectiveness of amylin mimetics is to modify the peptide chain by coupling it to molecular scaffolds such as polyethylene glycol (PEG) or by glycosylation. Compared to native amylin, PEGylated amylin has a prolonged glucose-lowering effect, but no in-depth data are available for the effect of these compounds on eating and body weight regulation. Further, glycosylation of the amylin analog pramlintide also provides increased half-life compared to the native peptide (10–15 vs. 45 min) [70], [71], [72], [73].
5.1.3. Dual amylin and calcitonin receptor agonist (DACRA)
As discussed before, sCT is an amylin mimetic that differs from native amylin by a prolonged activation of the amylin receptor, which leads to enhanced and prolonged in vivo effects. Further, sCT activates not only amylin receptors but also the classical calcitonin receptor. Based on these findings, several dual amylin- and calcitonin receptor agonists (DACRA) have been designed and tested for their anti-obesity and anti-diabetes effects.
The best investigated compounds with published structures are KBP-042, KBP-088, and KBP-089 [74], [75], [76], [77], [78], [79]. KBP-042 exhibited stronger binding affinity and receptor activation at amylin and calcitonin receptors and also produced a stronger eating-inhibitory effect than sCT. Further, KBP-042 reduced body weight more than in pair-fed controls, indicating a potential effect on energy expenditure, effects which had also been reported for amylin and sCT [6]. Interestingly, despite its peptide structure, KBP-042 was also active when given orally, albeit at much higher doses [74], [77], [78], [79].
In a direct comparison between KBP-088 and davalintide, KBP-088 produced stronger effects on eating and body weight than davalintide, which may be associated with the extended receptor activation under in vitro conditions [75]. Finally, KBP-089 was shown to reduce eating and body weight and significantly reduced total energy intake by reducing the intake of energy rich diets and a relative shift towards the intake of low energy diets in chronically treated rats [76], [80].
5.1.4. Oral amylin agonists
Several studies have tested the usefulness of the oral administration of amylin agonists. Some studies used the carrier Intravail® which is a transmucosal absorption enhancement agent. Pramlintide has been formulated in Intravail, and the effects on energy balance and glycemic control corresponded to the known effects of injected pramlintide [81]. Further studies showed that orally administered sCT which was formulated in N-(5-chlorosalicyloyl)-8-aminocaprylicacid (5CNAC-sCT) also had reduced weight and improved glucose tolerance [82], [83], [84].
5.2. Integrated circuits for the release of amylin agonists
In recent years, various approaches led to the development of engineered mammalian cell systems that allow an “automated” release of bioactive peptides to treat metabolic diseases. The underlying idea of such systems is that synthetic gene networks are designed in a way that allow the systems to respond to certain physiological stimuli in a well-balanced manner to produce an appropriate amount of bioactive molecules in a specific metabolic situation [85], [86], [87], [88]. Next to glucose-sensing cell systems that release insulin and that may be used for the treatment of diabetes mellitus, a novel method has been described recently to deliver the amylin analog pramlintide in a diet-specific manner in mice. The engineered closed-loop synthetic gene circuit is able to monitor blood free fatty acid levels (FFA) and to produce pramlintide in response to elevated fatty acids. Interestingly, intraperitoneal implantation of the engineered cell circuits in mice led to a reduced food intake and body weight, and it also decreased blood FFA levels compared to respective controls [89].
Please see Supplementary part of this review for experimental details of these unpublished data. Plasmids for lipid sensing and peptide synthesis were co-transfected into human fibrosarcoma cells, microencapsulated in inert porous alginate-poly-(l-lysine)-alginate beads, and then used in vivo studies in rats. The genetically engineered lipid sensor is based on the nuclear lipid receptor peroxisome proliferator-activated receptor-α (PPARα) which causes the fibrosarcoma cells to release pramlintide in a dose-dependent manner in presence of FFA [89].
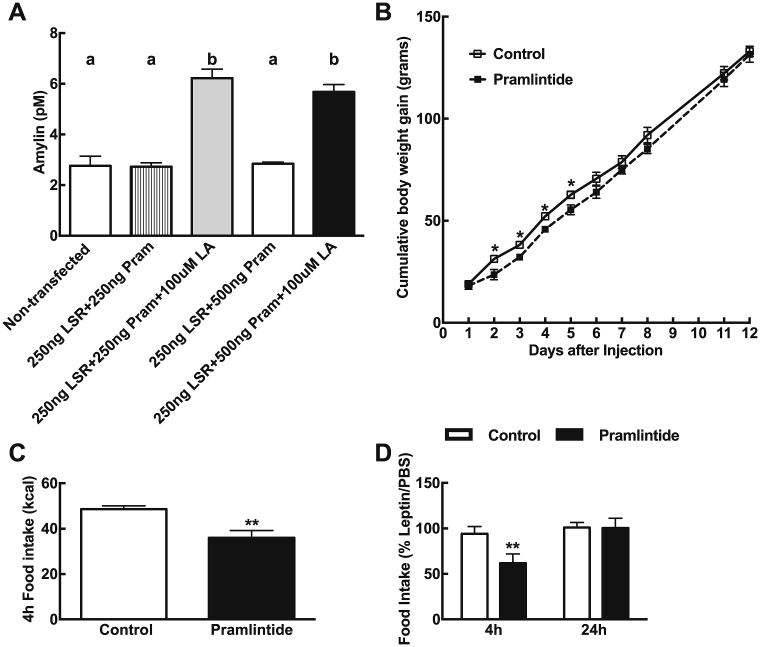
First, we confirmed that pramlintide was released from co-transfected cells in vitro and them showed that they released pramlintide into the incubation medium upon exposure to linoleic acid (Figure 2A). We then tested the efficacy of the pramlintide-releasing microcapsules under in vivo conditions in rats. These microencapsulated cells were injected intraperitoneally in male Sprague–Dawley rats maintained on a 60% high fat diet (D12492, Research Diet); 20 × 106 cells were injected per rats, which is 10-times more than what has been previously used in mice [89]. High-fat diet fed rats displayed a temporary effect of fatty acid-induced pramlintide production as compared to control animals. Indeed, high-fat fed rats receiving pramlintide exhibited a reduction in body weight gain although the effect waned approximately 5 days after microcapsule implantation (Figure 2B). In a fasting–refeeding test, rats injected i.p. with the pramlintide secreting cells ate about 26% less than control animals (Figure 2C). Further, when tested 5 and 7 days after injection, the leptin induced anorexia was amplified at 4 h by 34% in rats injected with pramlintide secreting cells. The effect of leptin on food intake at 24 h was no longer different between groups (Figure 2D).
Figure 2.
(A) Linoleic acid (LA)-induced pramlintide production in HT1080 cells at 48 h. Non-transfected cells were incubated with transfection solution without plasmids, non-treated cells were incubated with transfection solution and 250 ng pKR135 (LSR construct) + 250 ng pKR146 (pramlintide construct) or 250 ng pKR135 + 500 ng pKR146, and treated cells were transfected with 250 ng pKR135 + 250 ng pKR146 or 250 ng pKR135 + 500 ng pKR146 and treated daily with 100 μM linoleic acid. Values are mean ± SEM, n = 4/group. Data with differing superscripts differ from each other by P = 0.05 or less when assessed by two way ANOVA followed by post-hoc Bonferroni t-test. LSR-construct alginate beads or LSR-pramlintide alginate beads were then injected i.p in male Sprague–Dawley rats fed HF 60% (B) Cumulative body weight gain over the 12-day period. *P < 0.05 after a two-way repeated measures ANOVA and post-hoc Bonferroni test. (C) Food intake in kcal after a 4 h meal test on day 3 and (D) leptin-induced anorexia at 4 h and 24 h tested in a cross-over design on day 5 and 7 post-injection. Values are mean ± SEM, n = 6/group, **P < 0.01 or less, using unpaired Student's t-test.
When tested about 2 weeks after the implantation of the pramlintide secreting cells, there was no enhancement of leptin signaling (leptin-induced pSTAT3) in the hypothalamus of rats receiving pramlintide versus control (data not shown). Possibly, this lack of effect was due to an insufficient number of cells that were still functional at this time point. Hence, our data showed that when genetically-engineered pramlintide-releasing cells were stimulated via the LSR plasmid reacting to an increase in lipids in plasma, we saw effects on body weight and eating that mimicked those observed after repeated peripheral injection of pramlintide itself. Our study extended previous findings and demonstrated the successful but transient effect of an autonomous genetic system providing monitoring, production and secretion of pramlintide in rats put on a high fat diet.
6. Summary
In this review, we summarized recent findings on the physiological effects of amylin on the control of eating. We briefly discussed the potential relevance of different amylin receptor subtypes, but a better understanding of the role of specific amylin receptor subtypes is needed. We also summarized recent evidence that amylin, similar to other gastrointestinal hormones, modulates both homeostatic and hedonic feedback systems regulating ingestive behavior. Finally, we summarized recent reports on the development of amylin analogs that may have the potential for the development of anti-obesity drugs.
Acknowledgment
The continued financial support by the Swiss National Science Foundation (several grants) is highly appreciated. Further substantial funding was obtained from the Novartis Foundation, the Olga Mayenfisch Foundation, the Vontobel Foundation and the University of Zurich (Forschungskredit and Zurich Center for Integrative Human Physiology). We also thank M. Fussenegger (ETHZ, Basel) for the generous supply of plasmids and the introduction to the microencapsulation technique.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.11.009.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Butler P.C., Chou J., Carter W.B., Wang Y.N., Bu B.H., Chang D. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- 2.Cooper G.J. Amylin compared with calcitonin gene-related peptide: structure, biology, and relevance to metabolic disease. Endocrine Reviews. 1994;15:163–201. doi: 10.1210/edrv-15-2-163. [DOI] [PubMed] [Google Scholar]
- 3.Hartter E., Svoboda T., Ludvik B., Schuller M., Lell B., Kuenburg E. Basal and stimulated plasma levels of pancreatic amylin indicate its co-secretion with insulin in humans. Diabetologia. 1991;34:52–54. doi: 10.1007/BF00404025. [DOI] [PubMed] [Google Scholar]
- 4.Li Z., Kelly L., Heiman M., Greengard P., Friedman J.M. Hypothalamic amylin acts in concert with leptin to regulate food intake. Cell Metabolism. 2015;22:1059–1067. doi: 10.1016/j.cmet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Young A.A. Brainstem sensing of meal-related signals in energy homeostasis. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Lutz T.A. Control of energy homeostasis by amylin. Cellular and Molecular Life Sciences. 2012;69:1947–1965. doi: 10.1007/s00018-011-0905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorsal T., Rungby J., Knop F.K., Vilsboll T. GLP-1 and amylin in the treatment of obesity. Current Diabetes Reports. 2016;16:1. doi: 10.1007/s11892-015-0693-3. [DOI] [PubMed] [Google Scholar]
- 8.Hay D.L., Chen S., Lutz T.A., Parkes D.G., Roth J.D. Amylin: pharmacology, physiology, and clinical potential. Pharmacological Reviews. 2015;67:564–600. doi: 10.1124/pr.115.010629. [DOI] [PubMed] [Google Scholar]
- 9.Potes C.S., Lutz T.A. Brainstem mechanisms of amylin-induced anorexia. Physiology & Behavior. 2010;100:511–518. doi: 10.1016/j.physbeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Le Foll C., Johnson M.D., Dunn-Meynell A., Boyle C.N., Lutz T.A., Levin B.E. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes. 2015;64:1621–1631. doi: 10.2337/db14-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson M.D., Bouret S.G., Dunn-Meynell A.A., Boyle C.N., Lutz T.A., Levin B.E. Early postnatal amylin treatment enhances hypothalamic leptin signaling and neural development in the selectively bred diet-induced obese rat. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2016 doi: 10.1152/ajpregu.00326.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mietlicki-Baase E.G., Olivos D.R., Jeffrey B.A., Hayes M.R. Cooperative interaction between leptin and amylin signaling in the ventral tegmental area for the control of food intake. American Journal of Physiology: Endocrinology and Metabolism. 2015;308:E1116–E1122. doi: 10.1152/ajpendo.00087.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mietlicki-Baase E.G., Rupprecht L.E., Olivos D.R., Zimmer D.J., Alter M.D., Pierce R.C. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology. 2013;38:1685–1697. doi: 10.1038/npp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiner D.J., Mietlicki-Baase E.G., Olivos D.R., McGrath L.E., Zimmer D.J., Koch-Laskowski K. Amylin acts in the lateral dorsal tegmental nucleus to regulate energy balance through gamma-aminobutyric acid signaling. Biological Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mietlicki-Baase E.G., Reiner D.J., Cone J.J., Olivos D.R., McGrath L.E., Zimmer D.J. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology. 2015;40:372–385. doi: 10.1038/npp.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiting L., McCutcheon J.E., Boyle C.N., Roitman M.F., Lutz T.A. The area postrema (AP) and the parabrachial nucleus (PBN) are important sites for salmon calcitonin (sCT) to decrease evoked phasic dopamine release in the nucleus accumbens (NAc) Physiology & Behavior. 2017;176:9–16. doi: 10.1016/j.physbeh.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower R.L., Hay D.L. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. British Journal of Pharmacology. 2016;173:1883–1898. doi: 10.1111/bph.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopoulos G., Perry K.J., Morfis M., Tilakaratne N., Gao Y., Fraser N.J. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Molecular Pharmacology. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 19.McLatchie L.M., Fraser N.J., Main M.J., Wise A., Brown J., Thompson N. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 20.Liberini C.G., Boyle C.N., Cifani C., Venniro M., Hope B.T., Lutz T.A. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. The European Journal of Neuroscience. 2016 doi: 10.1111/ejn.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sexton P.M., Paxinos G., Kenney M.A., Wookey P.J., Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience. 1994;62:553–567. doi: 10.1016/0306-4522(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 22.Hay D.L., Christopoulos G., Christopoulos A., Poyner D.R., Sexton P.M. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Molecular Pharmacology. 2005;67:1655–1665. doi: 10.1124/mol.104.008615. [DOI] [PubMed] [Google Scholar]
- 23.Ueda T., Ugawa S., Saishin Y., Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Research. Molecular Brain Research. 2001;93:36–45. doi: 10.1016/s0169-328x(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 24.Becskei C., Riediger T., Zund D., Wookey P., Lutz T.A. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Research. 2004;1030:221–233. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Dunn-Meynell A.A., Le Foll C., Johnson M.D., Lutz T.A., Hayes M.R., Levin B.E. Endogenous VMH amylin signaling is required for full leptin signaling and protection from diet-induced obesity. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2015 doi: 10.1152/ajpregu.00462.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin B.E., Lutz T.A. Amylin and leptin: Co-Regulators of energy homeostasis and neuronal development. Trends in Endocrinology and Metabolism. 2017;28:153–164. doi: 10.1016/j.tem.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Bailey R., Walker C., Ferner A., Loomes K., Prijic G., Halim A. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. British Journal of Pharmacology. 2012;166:151–167. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hay D.L., Walker C.S., Gingell J.J., Ladds G., Reynolds C.A., Poyner D.R. Receptor activity-modifying proteins; multifunctional G protein-coupled receptor accessory proteins. Biochemical Society Transactions. 2016;44:568–573. doi: 10.1042/BST20150237. [DOI] [PubMed] [Google Scholar]
- 29.Riediger T., Schmid H.A., Lutz T., Simon E. Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2001;281:R1833–R1843. doi: 10.1152/ajpregu.2001.281.6.R1833. [DOI] [PubMed] [Google Scholar]
- 30.Potes C.S., Boyle C.N., Wookey P.J., Riediger T., Lutz T.A. Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin's eating inhibitory effect. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2012;302:R340–R351. doi: 10.1152/ajpregu.00380.2011. [DOI] [PubMed] [Google Scholar]
- 31.Mollet A., Gilg S., Riediger T., Lutz T.A. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiology & Behavior. 2004;81:149–155. doi: 10.1016/j.physbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Lutz T.A., Senn M., Althaus J., Del Prete E., Ehrensperger F., Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides. 1998;19:309–317. doi: 10.1016/s0196-9781(97)00292-1. [DOI] [PubMed] [Google Scholar]
- 33.Potes C.S., Turek V.F., Cole R.L., Vu C., Roland B.L., Roth J.D. Noradrenergic neurons of the area postrema mediate amylin's hypophagic action. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2010;299:R623–R631. doi: 10.1152/ajpregu.00791.2009. [DOI] [PubMed] [Google Scholar]
- 34.Lutz T.A. Control of energy homeostasis by amylin. Cellular and Molecular Life Sciences. 2011 doi: 10.1007/s00018-011-0905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becskei C., Grabler V., Edwards G.L., Riediger T., Lutz T.A. Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Research. 2007;1162:76–84. doi: 10.1016/j.brainres.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Riediger T., Zuend D., Becskei C., Lutz T.A. The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2004;286:R114–R122. doi: 10.1152/ajpregu.00333.2003. [DOI] [PubMed] [Google Scholar]
- 37.Potes C.S., Lutz T.A., Riediger T. Identification of central projections from amylin-activated neurons to the lateral hypothalamus. Brain Research. 2010;1334:31–44. doi: 10.1016/j.brainres.2010.03.114. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda T., Hirai Y., Maezawa H., Kitagawa Y., Funahashi M. Electrophysiologically identified presynaptic mechanisms underlying amylinergic modulation of area postrema neuronal excitability in rat brain slices. Brain Research. 2013;1494:9–16. doi: 10.1016/j.brainres.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 39.Braegger F.E., Asarian L., Dahl K., Lutz T.A., Boyle C.N. The role of the area postrema in the anorectic effects of amylin and salmon calcitonin: behavioral and neuronal phenotyping. The European Journal of Neuroscience. 2014;40:3055–3066. doi: 10.1111/ejn.12672. [DOI] [PubMed] [Google Scholar]
- 40.Lutz T.A., Geary N., Szabady M.M., Del P.E., Scharrer E. Amylin decreases meal size in rats. Physiology & Behavior. 1995;58:1197–1202. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- 41.Banks W.A., Kastin A.J. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 42.Banks W.A., Kastin A.J., Maness L.M., Huang W., Jaspan J.B. Permeability of the blood-brain barrier to amylin. Life Sciences. 1995;57:1993–2001. doi: 10.1016/0024-3205(95)02197-q. [DOI] [PubMed] [Google Scholar]
- 43.Kastin A.J., Pan W. Involvement of the blood-brain barrier in metabolic regulation. CNS Neurol Disord Drug Targets. 2016;15:1118–1128. doi: 10.2174/1871527315666160920124928. [DOI] [PubMed] [Google Scholar]
- 44.Balland E., Dam J., Langlet F., Caron E., Steculorum S., Messina A. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metabolism. 2014;19:293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berthoud H.R. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity (Silver Spring) 2006;14(Suppl 5):197s–200s. doi: 10.1038/oby.2006.308. [DOI] [PubMed] [Google Scholar]
- 46.Berridge K.C., Kringelbach M.L. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finlayson G., King N., Blundell J.E. Liking vs. wanting food: importance for human appetite control and weight regulation. Neuroscience & Biobehavioral Reviews. 2007;31:987–1002. doi: 10.1016/j.neubiorev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Munzberg H., Qualls-Creekmore E., Yu S., Morrison C.D., Berthoud H.R. Hedonics act in unison with the homeostatic system to unconsciously control body weight. Frontiers in Nutrition. 2016;3:6. doi: 10.3389/fnut.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickson S.L., Shirazi R.H., Hansson C., Bergquist F., Nissbrandt H., Skibicka K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. Journal of Neuroscience. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skibicka K.P. The central GLP-1: implications for food and drug reward. Frontiers in Neuroscience. 2013;7:181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berridge K.C. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiology & Behavior. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mietlicki-Baase E.G., McGrath L.E., Koch-Laskowski K., Krawczyk J., Reiner D.J., Pham T. Amylin receptor activation in the ventral tegmental area reduces motivated ingestive behavior. Neuropharmacology. 2017;123:67–79. doi: 10.1016/j.neuropharm.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyle C.N., Lutz T.A. Amylinergic control of food intake in lean and obese rodents. Physiology & Behavior. 2011;105:129–137. doi: 10.1016/j.physbeh.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Boyle C.N., Munz M., Wielinga P.Y., Stöcker D., Lutz T.A. Short-term, but not extended, access to palatable diet diminishes amylin responsiveness in rat. Appetite. 2010;54:636. [Google Scholar]
- 55.Boyle C.N., Rossier M.M., Lutz T.A. Influence of high-fat feeding, diet-induced obesity, and hyperamylinemia on the sensitivity to acute amylin. Physiology & Behavior. 2011;104:20–28. doi: 10.1016/j.physbeh.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 56.Anderberg R.H., Anefors C., Bergquist F., Nissbrandt H., Skibicka K.P. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiology & Behavior. 2014 doi: 10.1016/j.physbeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 57.Mietlicki-Baase E.G., Ortinski P.I., Rupprecht L.E., Olivos D.R., Alhadeff A.L., Pierce R.C. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. American Journal of Physiology: Endocrinology and Metabolism. 2013;305:E1367–E1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aronne L., Fujioka K., Aroda V., Chen K., Halseth A., Kesty N.C. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. The Journal of Clinical Endocrinology & Metabolism. 2007;92:2977–2983. doi: 10.1210/jc.2006-2003. [DOI] [PubMed] [Google Scholar]
- 59.Hollander P., Maggs D.G., Ruggles J.A., Fineman M., Shen L., Kolterman O.G. Effect of pramlintide on weight in overweight and obese insulin-treated type 2 diabetes patients. Obesity Research. 2004;12:661–668. doi: 10.1038/oby.2004.76. [DOI] [PubMed] [Google Scholar]
- 60.Smith S.R., Aronne L.J., Burns C.M., Kesty N.C., Halseth A.E., Weyer C. Sustained weight loss following 12-month pramlintide treatment as an adjunct to lifestyle intervention in obesity. Diabetes Care. 2008;31:1816–1823. doi: 10.2337/dc08-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weyer C., Maggs D.G., Young A.A., Kolterman O.G. Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Current Pharmaceutical Design. 2001;7:1353–1373. doi: 10.2174/1381612013397357. [DOI] [PubMed] [Google Scholar]
- 62.Ravussin E., Smith S.R., Mitchell J.A., Shringarpure R., Shan K., Maier H. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roth J.D., Roland B.L., Cole R.L., Trevaskis J.L., Weyer C., Koda J.E. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trevaskis J.L., Lei C., Koda J.E., Weyer C., Parkes D.G., Roth J.D. Interaction of leptin and amylin in the long-term maintenance of weight loss in diet-induced obese rats. Obesity (Silver Spring) 2010;18:21–26. doi: 10.1038/oby.2009.187. [DOI] [PubMed] [Google Scholar]
- 65.Turek V.F., Trevaskis J.L., Levin B.E., Dunn-Meynell A.A., Irani B., Gu G. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology. 2010;151:143–152. doi: 10.1210/en.2009-0546. [DOI] [PubMed] [Google Scholar]
- 66.Manning C.E., Williams E.S., Robison A.J. Reward network immediate early gene expression in mood disorders. Frontiers in Behavioral Neuroscience. 2017;11:77. doi: 10.3389/fnbeh.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smeltzer M., Scott K., Melhorn S., Krause E., Sakai R. Amylin blunts hyperphagia and reduces weight and fat gain during recovery in socially stressed rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2012;303:R676–R682. doi: 10.1152/ajpregu.00090.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roth J.D., Maier H., Chen S., Roland B.L. Implications of amylin receptor agonism: integrated neurohormonal mechanisms and therapeutic applications. Archives of Neurology. 2009;66:306–310. doi: 10.1001/archneurol.2008.581. [DOI] [PubMed] [Google Scholar]
- 69.Mack C.M., Soares C.J., Wilson J.K., Athanacio J.R., Turek V.F., Trevaskis J.L. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. International Journal of Obesity (London) 2010;34:385–395. doi: 10.1038/ijo.2009.238. [DOI] [PubMed] [Google Scholar]
- 70.Guerreiro L.H., Guterres M.F., Melo-Ferreira B., Erthal L.C., Rosa Mda S., Lourenco D. Preparation and characterization of PEGylated amylin. AAPS PharmSciTech. 2013;14:1083–1097. doi: 10.1208/s12249-013-9987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun C., Trevaskis J.L., Jodka C.M., Neravetla S., Griffin P., Xu K. Bifunctional PEGylated exenatide-amylinomimetic hybrids to treat metabolic disorders: an example of long-acting dual hormonal therapeutics. Journal of Medicinal Chemistry. 2013;56:9328–9341. doi: 10.1021/jm401418s. [DOI] [PubMed] [Google Scholar]
- 72.Kowalczyk R., Brimble M.A., Tomabechi Y., Fairbanks A.J., Fletcher M., Hay D.L. Convergent chemoenzymatic synthesis of a library of glycosylated analogues of pramlintide: structure-activity relationships for amylin receptor agonism. Organic & Biomolecular Chemistry. 2014;12:8142–8151. doi: 10.1039/c4ob01208a. [DOI] [PubMed] [Google Scholar]
- 73.Tomabechi Y., Krippner G., Rendle P.M., Squire M.A., Fairbanks A.J. Glycosylation of pramlintide: synthetic glycopeptides that display in vitro and in vivo activities as amylin receptor agonists. Chemistry (Weinheim an der Bergstrasse, Germany) 2013;19:15084–15088. doi: 10.1002/chem.201303303. [DOI] [PubMed] [Google Scholar]
- 74.Andreassen K.V., Feigh M., Hjuler S.T., Gydesen S., Henriksen J.E., Beck-Nielsen H. A novel oral dual amylin and calcitonin receptor agonist (KBP-042) exerts antiobesity and antidiabetic effects in rats. American Journal of Physiology: Endocrinology and Metabolism. 2014;307:E24–E33. doi: 10.1152/ajpendo.00121.2014. [DOI] [PubMed] [Google Scholar]
- 75.Gydesen S., Andreassen K.V., Hjuler S.T., Christensen J.M., Karsdal M.A., Henriksen K. KBP-088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. American Journal of Physiology: Endocrinology and Metabolism. 2016;310:E821–E827. doi: 10.1152/ajpendo.00514.2015. [DOI] [PubMed] [Google Scholar]
- 76.Gydesen S., Andreassen K.V., Hjuler S.T., Hellgren L.I., Karsdal M.A., Henriksen K. Optimization of tolerability and efficacy of dual amylin and calcitonin receptor agonist, KBP-089, through dose escalation and combination with a GLP-1 analogue. American Journal of Physiology: Endocrinology and Metabolism. 2017 doi: 10.1152/ajpendo.00419.2016. [DOI] [PubMed] [Google Scholar]
- 77.Hjuler S.T., Andreassen K.V., Gydesen S., Karsdal M.A., Henriksen K. KBP-042 improves bodyweight and glucose homeostasis with indices of increased insulin sensitivity irrespective of route of administration. European Journal of Pharmacology. 2015;762:229–238. doi: 10.1016/j.ejphar.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 78.Hjuler S.T., Gydesen S., Andreassen K.V., Karsdal M.A., Henriksen K. The dual amylin- and calcitonin-receptor agonist KBP-042 works as adjunct to Metformin on fasting hyperglycemia and HbA1c in a rat model of type 2 diabetes. Journal of Pharmacology and Experimental Therapeutics. 2017;362:24–30. doi: 10.1124/jpet.117.241281. [DOI] [PubMed] [Google Scholar]
- 79.Hjuler S.T., Gydesen S., Andreassen K.V., Pedersen S.L., Hellgren L.I., Karsdal M.A. The dual amylin- and calcitonin-receptor agonist KBP-042 increases insulin sensitivity and induces weight loss in rats with obesity. Obesity (Silver Spring) 2016;24:1712–1722. doi: 10.1002/oby.21563. [DOI] [PubMed] [Google Scholar]
- 80.Gydesen S., Hjuler S.T., Freving Z., Andreassen K.V., Sonne N., Hellgren L.I. A novel dual amylin and calcitonin receptor agonist, KBP-089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. British Journal of Pharmacology. 2017 doi: 10.1111/bph.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leinung M.C., Grasso P. [D-Leu-4]-OB3, a synthetic peptide amide with leptin-like activity, augments the effects of orally delivered exenatide and pramlintide acetate on energy balance and glycemic control in insulin-resistant male C57BLK/6-m db/db mice. Regulatory Peptides. 2012;179:33–38. doi: 10.1016/j.regpep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 82.Karsdal M.A., Henriksen K., Bay-Jensen A.C., Molloy B., Arnold M., John M.R. Lessons learned from the development of oral calcitonin: the first tablet formulation of a protein in phase III clinical trials. Journal of Clinical Pharmacology. 2011;51:460–471. doi: 10.1177/0091270010372625. [DOI] [PubMed] [Google Scholar]
- 83.Feigh M., Andreassen K.V., Hjuler S.T., Nielsen R.H., Christiansen C., Henriksen K. Oral salmon calcitonin protects against impaired fasting glycemia, glucose intolerance, and obesity induced by high-fat diet and ovariectomy in rats. Menopause (New York, N.Y.) 2013;20:785–794. doi: 10.1097/GME.0b013e31827c58ab. [DOI] [PubMed] [Google Scholar]
- 84.Feigh M., Hjuler S.T., Andreassen K.V., Gydesen S., Ottosen I., Henriksen J.E. Oral salmon calcitonin enhances insulin action and glucose metabolism in diet-induced obese streptozotocin-diabetic rats. European Journal of Pharmacology. 2014;737:91–96. doi: 10.1016/j.ejphar.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 85.Auslander S., Fussenegger M. Synthetic RNA-based switches for mammalian gene expression control. Current Opinion in Biotechnology. 2017;48:54–60. doi: 10.1016/j.copbio.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 86.Saxena P., Bojar D., Fussenegger M. Design of synthetic promoters for gene circuits in mammalian cells. Methods in Molecular Biology (Clifton, N.J.) 2017;1651:263–273. doi: 10.1007/978-1-4939-7223-4_19. [DOI] [PubMed] [Google Scholar]
- 87.Teixeira A.P., Fussenegger M. Synthetic biology-inspired therapies for metabolic diseases. Current Opinion in Biotechnology. 2017;47:59–66. doi: 10.1016/j.copbio.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Xie M., Ye H., Wang H., Charpin-El Hamri G., Lormeau C., Saxena P. beta-cell-mimetic designer cells provide closed-loop glycemic control. Science. 2016;354:1296–1301. doi: 10.1126/science.aaf4006. [DOI] [PubMed] [Google Scholar]
- 89.Rossger K., Charpin-El-Hamri G., Fussenegger M. A closed-loop synthetic gene circuit for the treatment of diet-induced obesity in mice. Nature Communications. 2013;4:2825. doi: 10.1038/ncomms3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.