Abstract
Objective
Adult human kidneys produce erythropoietin (EPO), which regulates red blood cell formation; however, whether EPO also functions directly on kidney development and controls diabetic kidney disease remains unknown. Here we analyzed the role of EPO in kidney development and under hyperglycemic conditions in zebrafish and in humans.
Methods
Diabetic patients and respective controls were enrolled in two cohorts. Serum EPO level and urine protein change upon human EPO administration were then analyzed. Transient knockdown and permanent knockout of EPO and EPOR in renal TG(WT1B:EGFP) zebrafish were established using the morpholino technology and CRISPR/Cas9 technology. Zebrafish embryos were phenotypically analyzed using fluorescence microscopy, and functional assays were carried out with the help of TexasRed labeled 70 kDa Dextran. Apoptosis was determined using the TUNEL assay and Annexin V staining, and caspase inhibitor zVADfmk was used for rescue experiments.
Results
In type 2 diabetic patients, serum EPO level decreased with the duration of diabetes, which was linked to reduced kidney function. Human recombinant EPO supplementation ameliorated proteinuria in diabetic nephropathy patients. In zebrafish, loss-of-function studies for EPO and EPOR, showed morphological and functional alterations within the pronephros, adversely affecting pronephric structure, leading to slit diaphragm dysfunction by increasing apoptosis within the pronephros. Induction of hyperglycemia in zebrafish embryos induced pronephros alterations which were further worsened upon silencing of EPO expression.
Conclusions
EPO was identified as a direct renal protective factor, promoting renal embryonic development and protecting kidneys from hyperglycemia induced nephropathy.
Keywords: Erythropoietin, Type 2 diabetes, Diabetic nephropathy, Zebrafish
Graphical abstract
Highlights
-
•
EPO exhibited renal protective and proteinuria ameliorating function in type 2 DM patients and in hyperglycemic zebrafish embryos.
-
•
Enhanced co-expression of EPO and EPOR was identified in both glomeruli and tubuli of DN patients.
-
•
EPO and its receptor directly regulate physiological kidney development via repressing apoptosis.
Abbreviations and Acronyms
- DM
diabetes mellitus
- DN
diabetic nephropathy
- EPO
erythropoietin
- EPOR
erythropoietin receptor
- hEPO
human recombinant erythropoietin
- hpf
hour post fertilization
- dpf
day post fertilization
- hpi
hour post injection
1. Introduction
Diabetic nephropathy (DN) [1], a common diabetes microvascular complication, ranks first as the cause of end-stage renal disease (ESRD) globally [2]. Accumulating evidence suggests that renal hyperfiltration and renal injury, oxidative stress, and apoptotic signals are all involved in the pathogenesis of DN [3], [4], [5], [6]. It was also shown that polymorphisms in some genes were associated with a heightened incidence of diabetic nephropathy [7], [8]. However, genetic mechanisms for the onset of diabetic nephropathy are still poorly understood and thus limit the therapeutic options for patients.
Erythropoietin (EPO) is a glycoprotein hormone traditionally considered essential for erythropoiesis [9], [10]. Recent studies revealed a relationship between EPO and the progression of diabetic complications [11], [12], [13]. In addition, single nucleotide polymorphisms (SNPs) in the human EPO gene were suggested to be a potential contributing factor for the development of DN in various global populations [14]. Functional EPO and EPOR expression have been demonstrated in both human and murine kidney tubular epithelial, glomerular endothelial, and mesangial cells, suggesting that EPO may play a physiological role in both renal development and renal disease states through interactions with EPOR [15]. In adult human, EPO is mainly produced in kidney interstitial fibroblast and then released into circulation to exert its subsequent effects [16], [17]. As a result, global EPO silencing animal models are required to explore its underlying functions. However, studies using EPO knockout or knockdown animal models beyond the embryonic stage have rarely been reported, likely due to embryonic lethality [18], [19], [20], [21]. In addition, the mechanisms by which EPO functions in renal system in vivo remains largely unknown.
With the development of novel genome-editing techniques and renal reporter lines and due to its translucency [22], the zebrafish is an attractive model for carrying out research in various biomedical fields, including renal and diabetes studies [23]. The two to three day-old zebrafish pronephros consists simply of two glomeruli, which fuse at the embryonic midline and are connected by pronephric tubules to the bilateral pronephric ducts [24]. The simplicity and rapid development of pronephros in zebrafish make it an useful model for investigating the morphology and function of kidney in the context of a developing organism [25]. In addition, it was reported that pronephros cell types are also highly conserved between zebrafish and mammals [24]. Thus, the present studies took advantage of the zebrafish model to dissect the developmental pathways and regulations in renal development and pathogenesis under hyperglycemic conditions.
In this study, we provide evidence that EPO is a clinically-protective factor in the progression of diabetic complications. In addition, by analyzing transient and permanent loss-of-function models for EPO and EPOR in zebrafish, we have identified EPO as an essential regulator of pronephros development and function by interacting with its receptor EPOR and thereby repressing apoptosis. In summary, the present study identifies EPO as an active renal antiapoptotic factor, protecting kidney from hyperglycemia-induced damage and proteinuria in an EPOR-dependent manner both in zebrafish and in type 2 diabetic patients.
2. Material and methods
2.1. Serum EPO concentrations from DM and non-DM patients
Blood samples from DM and non-DM patients were collected on admission after overnight fasting and stored at −70 °C prior to analysis. Type 2 diabetes was diagnosed on initial admission, and the diabetes duration was recorded accordingly. The diagnosis of Type 2 diabetes was made according to AACE Diagnostic Criteria for Glucose Abnormalities [26]. Exclusion criteria included patients with uncorrected anemia, active EPO supplementary treatment, dialysis, cardiovascular disease, cancer, and GFR <30 ml/min. The demographic data are listed in Supplementary Table 1. Erythropoietin was measured using a sandwich enzyme-linked immunoassay (Quantikine® IVD EPO ELISA and Quantikine® Immunoassay R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions. The study protocol was approved beforehand by the Medical Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University, and the procedures followed were in accordance with the institutional guidelines. This study complied with the Declaration of Helsinki, and formal consent was obtained from all patients.
2.2. Study design and participants of hEPO injection in end-stage diabetic nephropathy patients
Consecutive patients (Supplementary Table 2) admitted to the nephrology department of the First Affiliated Hospital of Xi'an Jiaotong University for diabetic nephropathy between May 2013 and March 2017 were selected. The inclusion criteria were: 1) confirmed admission diagnosis of Type 2 diabetic nephropathy, and 2) continuous follow-up measurements of urine protein concentration and 24 h urine protein collection. The exclusion criteria were: 1) diabetic ketosis or nonketotic hyperosmolar coma, and 2) severe nondiabetic disease with expected survival of less than 1 year and unwillingness to participate. A patient was included only once. Information about present medication and a detailed medical history were obtained via hospital medical records. Diabetic nephropathy and proteinuria were defined according to the universal definition criteria by the American Diabetes Association criteria [26]. Twenty-four h urine protein was collected from the first morning urine and urine protein concentration were measured using biuret techniques [27]. Urine protein concentration change and 24 h urine protein change was defined as the subtracted level of urine protein or 24 h urine protein by 2 follow-ups. Written informed consent was obtained from all study participants, with ethnic committee approval at the First Affiliated Hospital of Xi'an Jiaotong University.
2.3. Gene profile analysis and co-expression analysis
The gene expression data GSE30528 [28], including 9 diabetic kidney disease and 13 control glomeruli samples from micro dissected human kidney, and the microarray gene expression data GSE30529 [28], including 10 diabetic kidney disease and 12 control tubule samples from micro dissected human kidney, were obtained from the Gene Expression Omnibus [29] database. Pearson's correlation coefficient method [30] was utilized to identify the co-expression as well as differential co-expression of EPO and EPOR along with kidney pathogenesis related genes, including Notch1, WT1, Pax1, NPHS1, and NPHS2 in the kidney section from DN patients and control. For co-expression analysis of EPO and proteinuria related genes, GeneMANIA (http://genemania.org/; accessed April, 2017), a real-time multiple association network integration algorithm for predicting gene function, was used for analyzing gene–gene interactions in the study [31].
2.4. Zebrafish lines
Embryos of the TG(WT1B:EGFP) line were raised and staged as described. Embryos were kept in E3 medium (5 mM NaCl, 0.17 mM KCL, 0.33 mM CaCl2, 5–10% methylene blue) at 28.5 °C with 0.003% 1-phenyl-2-thiourea (PTU) (Sigma) to suppress pigmentation and staged according to somite number or hours post-fertilization (hpf).
2.5. Inhibitors and reagents
Texas-Red® tagged 70 kDa dextran (Molecular Probes) was used for renal functional assays. Proteinase K (10 mg/ml stock) was utilized for embryo digestion and linearized plasmid treatment (Roche Recombinant PCR grade). Zebrafish embryos were subjected to 300 μM of the pan caspase inhibitor, zVAD-fmk (Sigma–Aldrich) incubation at 24 hpf for 24 h.
2.6. Injections of morpholinos and intracardiac injection of human EPO in zebrafish
EPO (ENSDARG00000055163.7, HYPERLINK: http://www.ensembl.org/Danio_rerio/Gene/Summary?db=core;g=ENSDARG00000055163;r=7:21624135-21652094) and EPOR (ENSDARG00000090834.4, HYPERLINK: http://www.ensembl.org/Danio_rerio/Gene/Summary?db=core;g=ENSDARG00000090834;r=3:14394007-14414315) morpholinos were selected as previously described [32], [33] and produced by Gene Tools™ (Philomath, OR, USA). Morpholino sequences are listed in Supplementary Table 3. Dose escalation studies were performed to determine submaximal morpholino concentrations (Supplementary Figure 2C, D). EPO, EPOR and Pdx1 morpholino were diluted to 4 or 6 μg/μl in 0.1 M KCL respectively. One nanoliter of morpholino was injected into the embryos through the chorion of one-cell or two-cell stage embryos. Intracardiac injections at 24 hpf were performed as previously described [34]. One nanoliter of human recombinant EPO (4 IU/μl, Sanshengzhiyao™, S19980073) was injected into the heart of the anesthetized embryo to enter the blood circulation at the 24 hpf stage.
2.7. CRISPR/Cas9 zebrafish mosaic mutant generation
CRISPR target sites were identified and selected using ZiFiT Targeter. CRISPR target sites are denoted in Figure 4. Oligonucleotide sequences for cloning of CRISPR gRNAs are listed in Supplementary Table 4. Equal amounts of control CRISPR gRNA (derived from original pT7-gRNA) were used as the negative control. EPO gRNA, EPOR gRNA, control, and Cas9 mRNA were diluted to 200 pg/nl respectively, in 0.1 M KCL. One nanoliter of a mixture of CRISPR gRNAs and Cas9 was injected into the embryos at the 1-cell stage. Sanger sequence was used to detect mutations at CRISPR-target loci, and qPCR was used to check the relative expression levels as previously described [35], [36]. Oligonucleotide sequences for sequencing of CRISPRants are listed in Supplementary Table 5.
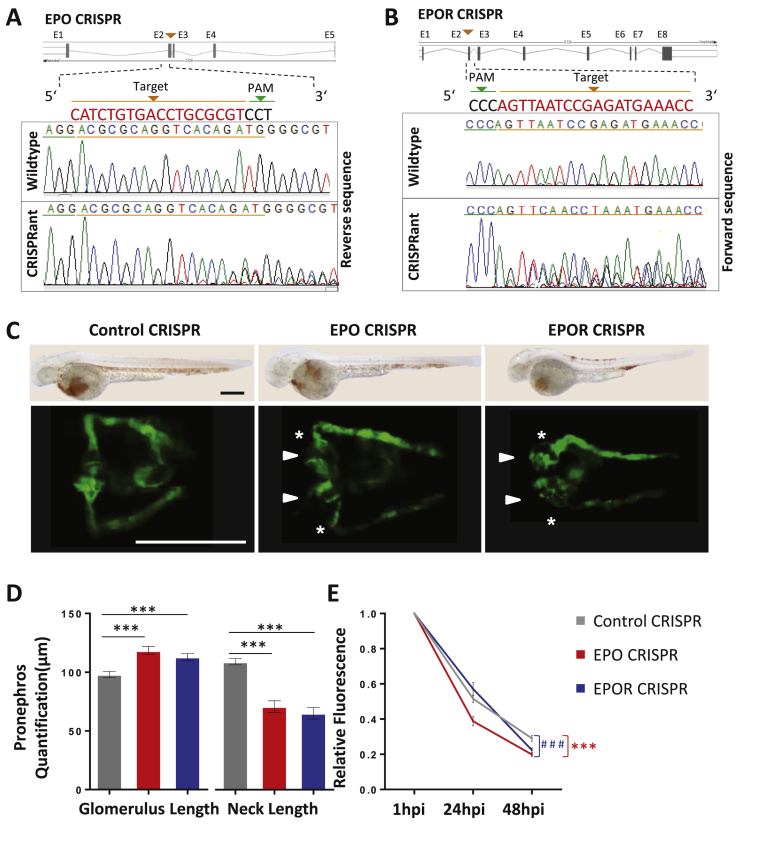
Figure 4.
EPO and EPOR CRIPSRants phenocopies EPO and EPOR morphants in zebrafish. A, B. Schematic illustration of EPO and EPOR CRISPR target regions and sequence results of wildtype, EPO CRISPRant, and EPOR CRISPRants. C. Similar to morphants, EPO CRISPR, and EPOR CRISPR injected zebrafish embryos (EPO CRISPR and EPOR CRISPR) also showed an enlarged glomerulus (white arrow head) and a highly shortened pronephric neck (white asterixis) at 48 hpf in TG(WT1B:EGFP) zebrafish embryos. Light microscopy images above show strongly reduced hemoglobin concentrations using O-dianiside stain in EPO CRISPR and EPOR CRISPR injected zebrafish embryos as compared to Control CRISPR injected embryos at 48 hpf indicating efficient knockout of both genes. D. Quantification of data shown in C in three independent experiments for each group. (n = 43–47 embryos per group). E. Increased loss of intracardiac injected 70 kDa dextran–FITC at 24 hpi and 48 hpi in EPO CRISPR and EPOR CRISPR injected zebrafish embryos as compared to its respective control performed in three independent experiments. (n = 40–48 embryos per group). All data were analyzed using the Student‘s t-test. Mean ± s.e.m. *** or ###p < 0.001.
2.8. O-dianiside stain
O-Dianiside staining was performed on 48 hpf TG(WT1B:EGFP) embryos as previously described [32], [33].
2.9. TUNEL assay
Forty-eight hpf TG(WT1B:EGFP) embryos were stained by using the ApopTag® Red InSitu Apoptosis Detection Kit (Millipore™). The embryos were deyolked and fixed over-night at 4 °C in freshly prepared 4% PFA/PBS. After washing in PBST (1× PBS/0.1% Tween-20), sequential dehydration with increasing concentrations of methanol was done to store embryos in 100% methanol overnight at −20 °C. Embryos were rehydrated and treated with Proteinase K (10 μg/ml, Roche) for 21 min. The TUNEL protocol was followed as previously described [35], [37].
2.10. Flow cytometry
To analyze the expression of EPO and EPOR in EGFP positive cells, 48 hpf anesthetized TG(WT1B:EGFP) embryos were dechorionated, incubated 15 min in modified Ringer solution (116 mM NaCl, 2.9 mM KCL, 5 mM HEPES, pH 7.2), deyolked, washed in PBS, and lysed in 0.25% trypsin for 15–20 min. The reaction was stopped with PBS containing 10% FCS, and 2 mM CaCl2, cells were centrifuged and resuspended in buffer (0.5% FCS, 1 mM EDTA in PBS). EGFP positive cells were sorted using a FACScan flow cytometer running CellQuest software (Becton Dickinson). EGFP positive and negative cells were then used for RT-PCR [35].
2.11. FACS analysis of Annexin V-positive cell fractions
To analyze zebrafish morphants undergoing apoptosis by FACS, cells from 48 hpf zebrafish were stained with APC-conjugated Annexin V (BD Pharmingen). To this end, cells were harvested, washed twice with PBS, resuspended in 1× Binding Buffer (Bender MedSystems), and incubated with Annexin V-APC and 7-AAD (Beckman–Coulter). FACS analysis was performed by the Mannheim Cell Sorting Core Facility of the Medical Faculty Mannheim using a BD FACS Canto II. For quantification, Annexin V-positive and 7-AAD-negative cell fraction was considered as the early apoptotic cell fraction, and Annexin V-positive and 7-AAD-positive cell fraction was considered as the late apoptotic cell fraction. The apoptotic cell fraction was set in relation to control and was displayed as relative fraction [37].
2.12. RNA isolation and real-time quantitative PCR analysis of zebrafish
Total RNA was isolated from TG(WT1B:EGFP) zebrafish embryos, EGFP positive and negative zebrafish cells using the RNeasy Mini-Kit (Qiagen) following the manufacturer's protocol. First-strand cDNA was generated from normalized RNA amounts using random hexamer primers and the Superscript II kit (Invitrogen). RT-PCR was performed with specific primer pairs as listed in Supplementary Table 6. Primer design for zebrafish was done by Roche Universal Probe Library Assay Design Center. 2× sentiFAST probe No-ROX mix (Bioline) was used in each 96-well reaction plate (Axon). A Roche light cycler® 480 was used for the qPCR. The real-time quantitative PCR was repeated three times for each condition [35].
2.13. Ultrafiltration assay
At 72 h post-fertilization, 5 nl of Texas-Red® tagged 70 kDa dextran (2 ng/ml in PBS) was injected into the heart of zebrafish embryos. Sequential images of the living fish were taken 1, 24, and 48 h post injection (hpi) using an inverted microscope (Leica DMI 6000 B) with a camera (Leica DFC420 C) and the Leica LAS application suite 3.8 software [35].
2.14. Microscopy and analysis
TG(WT1B:EGFP) embryos were manually dechorionated and anesthetized with 0.05% tricaine (Sigma™). For in vivo imaging of pronephros structure, TG(WT1B:EGFP) embryos were embedded in 1% low melting point agarose (Promega™) dissolved in E3 medium. Images were taken with a Leica DFC420 C camera, attached to a Leica MZ10 F modular stereo microscope. Quantification of the pronephros was done using the Leica LAS V4.8 software. Confocal imaging was performed using a TCS SP5 system (Leica) for TUNEL stain.
2.15. Statistics
The regression analysis of clinical data reported in Figure 1 was performed using the simple linear regression (SPSS 17.0). Because serum EPO values were not normally distributed, EPO was log-transformed to the base 2, and analyses were reported per doubling of serum EPO. The TUNEL assay confocal images were processed using NIH's ImageJ application. Apoptotic cell number/area was determined by the Analyze Particles tool of ImageJ, within the glomerulus area as well as the entire pronephric structure. Alterations in the pronephric structure were measured and quantified by measuring size in μm of glomerular width and neck. Results are expressed as mean ± s.e.m. Statistical significance between different groups was analyzed using Student's t-test or Mann–Whitney-U-test as indicated (SPSS 17.0). P-values <0.05 were considered as significant * <0.05, ** <0.01, and *** <0.001.
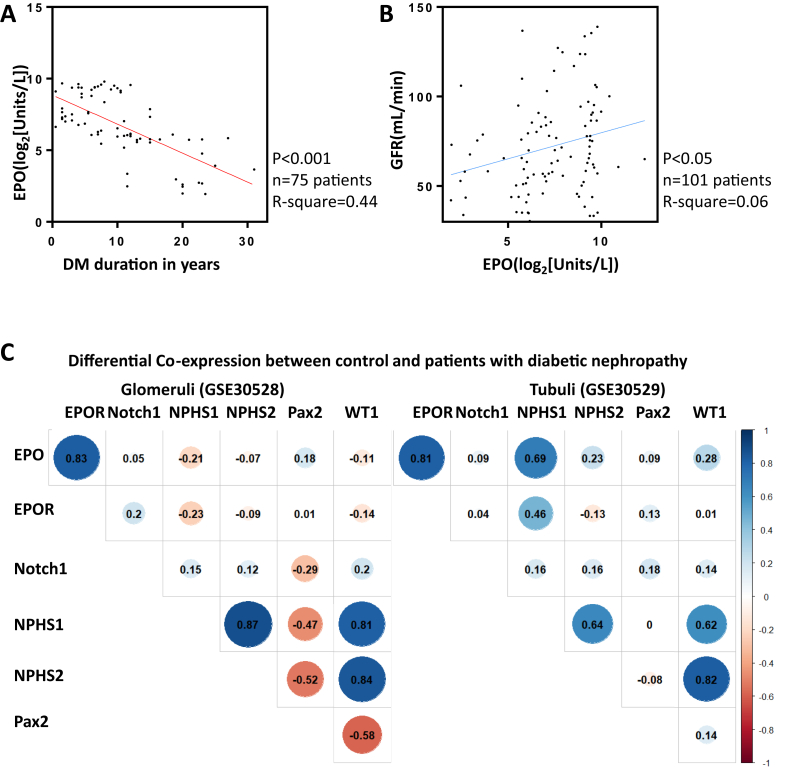
Figure 1.
EPO loss induced kidney function deterioration in Type 2 diabetic patients. A. Simple linear regression model with DM duration in years in relation to log 2 transformed serum EPO level. The y-axis represented serum EPO level (Log2(Unit/L)), and the x-axis represented DM duration in years. Black dots stood for log 2 transformed serum EPO level in each patient. 75 diabetic patients were enrolled in the analysis. R square is 0.44, and 95% CI is −0.25 to −0.14. B. Simple linear regression model with log 2 transformed serum EPO level in relation to GFR (n = 101). The y-axis represented GFR (ml/min), and the x-axis represented serum EPO level (Log2(Unit/L)). Black dots represent GFR level in each patient. 101 diabetic and non-diabetic patients were enrolled in the analysis. R square is 0.06, and 95% CI is 0.59–5.24. C. Differential co-expression of EPO and EPOR along with kidney pathogenesis related genes, including Notch1, WT1, Pax1, NPHS1, and NPHS2 in the kidney glomeruli and tubuli section from DN patients and control. The figures within each crossover represented the co-expression value between the respective genes. Blue color indicates enhanced co-expression and red color indicates decreased co-expression. Differential threshold was set as 0.8.
3. Results
3.1. EPO protected type 2 diabetic patients from kidney function alteration and proteinuria
Previous studies revealed an association between a functional EPO promoter polymorphism and diabetic microvascular complications [14]. Observational studies also noted that DM patients frequently manifest anemia, which might be associated with decreased endogenous EPO [38]. Although there is emerging evidence about the protective functions of EPO in diabetic microvascular complications, few clinical studies have addressed serum EPO levels and renal function in DM patients.
To address this question, we evaluated serum EPO concentrations from plasma samples collected from 26 healthy and 75 diabetic patients who underwent routine health examination. Baseline factors were collected and listed in Supplementary Table 1. Patients suffering from anemia (defined as hemoglobin below 12 g/dL for women and 14 g/dL for men) and end stage kidney failure (defined as Glomerulus Filtration Rate (GFR) below 30 ml/min) were not selected in order to exclude possible misleading factors. Notably, serum EPO concentrations were significantly negatively correlated with DM duration in years (Figure 1A), suggesting that EPO was a potential modulator for the progression of diabetes. Likewise, GFR positively correlated with serum EPO concentrations in a significant manner, although diabetes by itself had a much stronger influence on GFR (Figure 1B). This suggests that loss of EPO expression might contribute to the deteriorating kidney function under diabetic conditions.
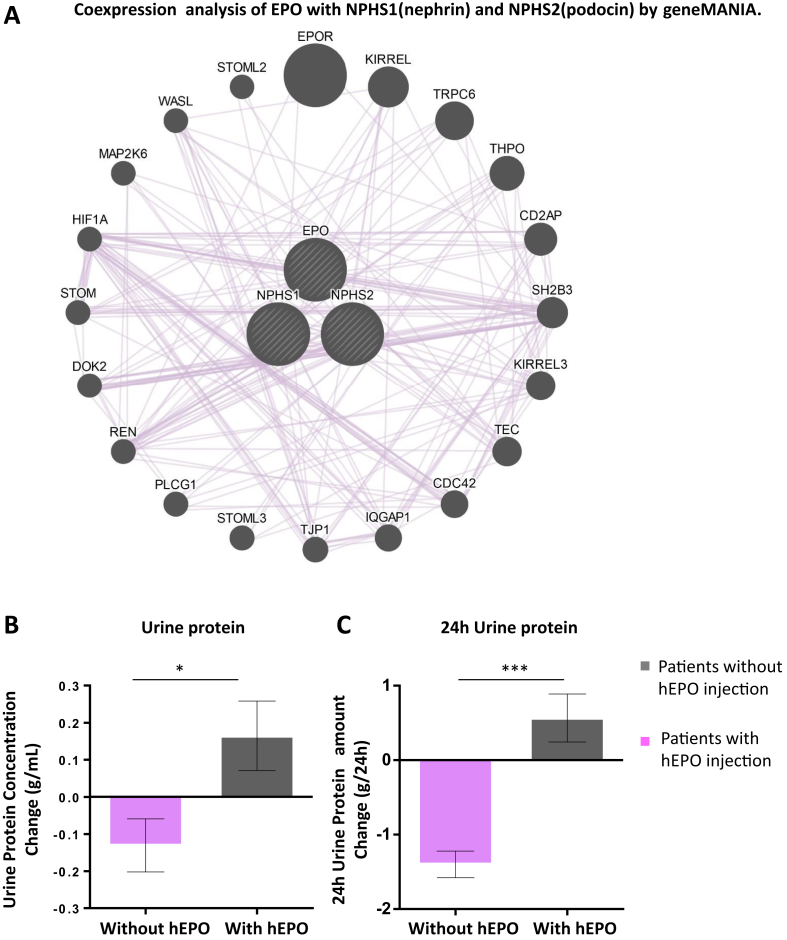
To further investigate EPO function in diabetic nephropathy, microarray data GSE30528 [28] from micro dissected human kidney glomeruli, and GSE30529 [28] from tubule, were obtained from the GEO [29] database. Pearson's correlation [30] was used to measure the co-expression of EPO and EPOR along with kidney pathogenesis related genes, including Notch1, WT1, Pax1, NPHS1, and NPHS2, and differential co-expression between control and diabetic patients was analyzed. Enhanced co-expression of EPO and EPOR was identified in the glomeruli and tubule of DN patients as compared to controls (Figure 1C and Supplementary Figure 1). Next, we utilized GeneMANIA [31], to predict the possible underlying pathological alterations with the loss of EPO in DN. Integration of EPO and NPHS1 and NPHS2, two genes important for glomerulus filtration barrier formation in humans [39], [40], into a single unified network enabled functional prediction of previously uncharacterized co-expressions, indicating possible function of EPO in filtration barrier damage and proteinuria (Figure 2A).
Figure 2.
hEPO supplement ameliorates proteinuria in Type 2 diabetic nephropathy patients and co-expression analysis of EPO and proteinuria related genes by Genemania. A. Co-expression of EPO and proteinuria related genes (NPHS1, also nephrin in zebrafish; and NPHS2, also podocin in zebrafish) by GeneMANIA. B. Urine protein concentration change in Type 2 diabetic nephropathy patients with or without hEPO supplement. The urine protein concentration change was defined as urine protein concentration at follow-up minus urine protein in initial evaluation. C. 24 h urine protein amount change in Type 2 diabetic nephropathy patients with or without hEPO supplement. The 24 h urine protein amount change was defined as 24 h urine protein amount at follow-up minus 24 h urine protein amount in initial evaluation. Data were analyzed using the Student‘s t-test. Mean ± s.e.m. *p < 0.05, ***p < 0.001.
To directly investigate EPO function in proteinuria in DN patients, we collected urine samples from DN patients, 51 with hEPO injection to treat renal anemia and 41 without as control. Baseline factors were collected and are listed in Supplementary Table 2. Both urine protein concentration and 24 h urine protein amount were measured two times during enrollment and follow-up. Urine protein concentration change and 24 h urine protein amount change were then compared between patients with or without hEPO injection (Figure 2B, C). Patients with hEPO supplementation exhibited a significant reduction of urine protein, suggesting a proteinuria ameliorating function of EPO in DN patients. In summary, EPO was lowered with DM progression and was positively correlated with GFR in DM patients. In addition, enhanced EPO and EPOR co-expression was identified in DN glomeruli and tubule samples, and hEPO supplement ameliorated proteinuria in DN patients.
3.2. EPO and EPOR regulate pronephros morphogenesis and pronephros filtration barrier function in zebrafish embryos
From the clinical investigation, a potential protective function for EPO in DM progression and proteinuria was identified. We therefore asked the question whether and how EPO was related to kidney function in experimental animal models, which could explain the observations in diabetic patients. Due to embryonic lethality of EPO knockout mice [18], [19], [20], zebrafish was employed as an experimental model system because zebrafish could survive several days of embryonic development without having a functional vascular and erythroid system by simply by taking up oxygen from the environment by diffusion. Thus, we then addressed the question whether loss of EPO and EPOR had a physiological role in renal development and function.
EPO and EPOR expression were identified in embryonic and adult zebrafish [32], [33]. In order to further analyze expression of EPO and EPOR in zebrafish pronephros, we took advantage of a transgenic TG(WT1B:EGFP) zebrafish line where the WT1B promotor drives expression of EGFP in pronephros (Figure 3A) [41]. Quantitative expression of EPO and EPOR mRNA at 48 hpf was evaluated by real time PCR using RNA isolated by FACS sorting from EGFP pronephric cells of 48 hpf TG(WT1B:EGFP) zebrafish embryos. While EPO was weakly expressed in EGFP positive pronephric cells, most abundant expression of EPO mRNA was identified in EGFP negative cells (Figure 3B) indicating different sources from the pronephros of EPO expression in zebrafish embryos at 48 hpf, which also confirmed previously published data [32], [33]. In contrast, EPOR mRNA expression was mostly identified in EGFP positive pronephric cells, but non-pronephric were almost negative for EPOR mRNA expression (Figure 3C). Thus, expression of EPOR at 48 hpf in pronephric cells suggested a function for EPOR in pronephros development and function in zebrafish embryos. In order to prove this hypothesis, we have subsequently performed several EPO and EPOR loss-of-function experiments.
Figure 3.
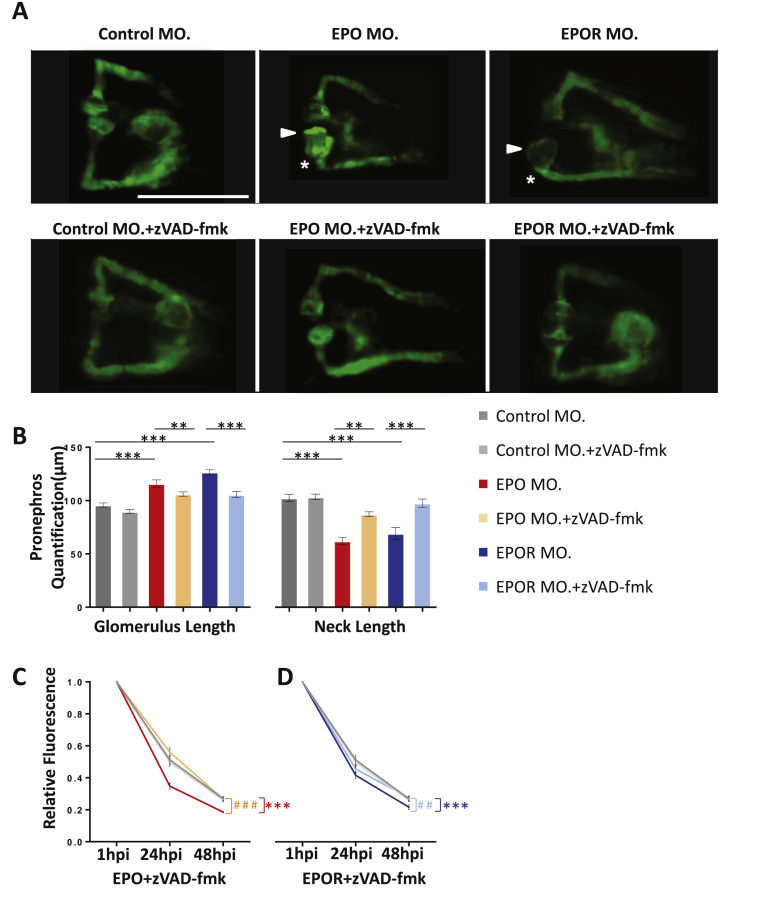
Genetic inactivation of EPO signaling induced pronephros structure alteration and proteinuria in zebrafish. A. Left: zebrafish embryo, shown in a dorsal view, with pronephros depicted in green fluorescence. Right: pronephros drawing showed segmental organization (P = podoycytes/glomerulus; N = neck; PCT = Proximal convoluted tubule; PST = Proximal straight tubule). Below: expression of indicated genes, podocin and nephrin, related to split diagram formation in zebrafish. B, C. Relative expression of EPO and EPOR at 48 hpf analyzed by RT-PCR from RNA isolated from whole zebrafish embryos, EGFP negative (EGFP−) cell fraction (entire embryo excluding pronephros), and pronephros (EGFP+) cell fraction. While EPO was mostly expressed in EGFP− cell traction, EPOR was preferentially expressed in pronephros (EGFP+). D. As compared to control morphants (Control MO.), EPO morphants (EPO MO.) and EPOR morphants (EPOR MO.) showed an enlarged glomerulus (white arrow head) and a highly shortened pronephric neck (white asterisks). The fluorescence microscopy images were taken at 48 hpf of TG(WT1B:EGFP) zebrafish embryos. White scale bar: 200 μm. The light microscopy images above showed a large percentage or nearly completely loss of hemoglobin, i.e. almost absence of red blood cells in EPO MO. and EPOR MO. at 48 hpf, indicating functionality of EPO and EPOR mopholinos. In contrast, Control MO. injected embryos had a high abundance of red blood cells. Black scale bar in O-dianiside stain: 500 μm. E. Altered pronephros structure in EPO morphants and EPOR morphants as indicated by length of the neck and length of glomerulus was quantified in three independent experiments. (n = 56–78 embryos per group). F. Increased loss of intracardiac injected 70 kDa dextran–FITC at 24 hpi and 48 hpi in EPO morphants and EPOR morphants as compared to control morphants showing three independent experiments. (n = 41–50 embryos per group). G. Relative expression of Podocin at 48 hpf analyzed by RT-PCR from RNA isolated from EPO MO., EPOR MO., and Control MO. H. Relative expression of Nephrin at 48 hpf analyzed by RT-PCR from RNA isolated from EPO MO., EPOR MO., and Control MO. Data were analyzed using the Student‘s t-test (E and F) or Mann–Whitney-U-test (G and H). Mean ± s.e.m. ns. Not significant. **p < 0.01, *** or ###p < 0.001.
First, we used an antisense approach with a splice-blocking morpholino targeting zebrafish EPO (Supplementary Figure 2A, B), which had already been previously validated [33]. To confirm functionality of the published EPO morpholino, we injected 4 ng of EPO morpholino into the one cell stage embryo, and we performed at 48 hpf an O-dianiside stain, which showed loss of red blood cell formation as indicated by strong reduction of hemoglobin in EPO-injected zebrafish embryos as compared to its controls (Figure 3D). Thus, this experiment additionally confirmed functionality of used EPO morpholino. Next, we analyzed formation of pronephric structures in TG(WT1B:EGFP) at 48 hpf (Figure 3A), which, at this stage, consist of a fused glomerulus connected on both sides by the neck which later forms tubular structures [24]. Injection of the EPO morpholino resulted at 48 hpf in dose dependent alterations with a significant increase of glomerulus length and a decrease of neck length (Figure 3D, E and Supplementary Figure 2C). In order to address the question whether these structural alterations also resulted in kidney function changes, an established assay for evaluating slit diaphragm function in zebrafish embryos was applied [35]. Upon intracardiac injection of 70 kDa Texas-Red® dextran, a significantly increased loss of heart fluorescence over time was observed in EPO morphants as compared to its controls, which suggested proteinuria resulting from disrupted slit diaphragm in EPO morphants (Figure 3F). Since co-expression network analysis revealed a relationship between EPO, NPHS1 and NPHS2 in humans (Figure 2A), we then checked their expression of their homologues in zebrafish. Relative podocin expression was found to be decreased in EPO morphants (Figure 3G)
To further verify whether loss of EPO indeed caused a disruption in pronephros development in zebrafish, we used a reverse genetics approach applying the CRISPR/Cas9 technology [36]. CRISPR guided RNA individually targeting zebrafish EPO exon 2 was generated and efficiency was validated using qPCR and sequencing (Figure 4A, B and Supplementary Figure 3). Injecting EPO CRISPR gRNA together with Cas9 mRNA also led to reduced hemoglobin formation and therefore reduced red blood cell formation in zebrafish embryos at 48 hpf (Figure 4C). Likewise, in EPO CRISPRant zebrafish embryos similar pronephric structural alterations as seen in EPO morphants were observed (Figure 4C, D) and slit diaphragm alterations, as again indicated by 70 kDa Texas-Red® dextran clearance, were similar to EPO morphants (Figure 4E).
Next we asked the question, whether the receptor for EPO, EPOR, which regulates survival of precursor of red blood cells and thereby exerts its hematopoietic functions [9], also regulates pronephric formation and function in zebrafish embryos. A previously reported splice-blocking morpholino targeting zebrafish EPOR was used (Supplementary Figure 2A, B), and zebrafish embryos were analyzed at 48 hpf [32]. Similar to EPO morphants and EPO CRISPRants, EPOR knockdown in zebrafish embryos caused a nearly complete hemoglobin loss at 48 hpf (Figure 3D), once again indicating the functionality of the tool used. Moreover, injections of different concentrations of the EPOR morpholino dose dependently induced pronephric alterations showing increased glomerulus length and decrease of neck length at 48 hpf (Figure 3D, E and Supplementary Figure 2D). Likewise, upon intracardiac injection of 70 kDa Texas-Red® dextran, an increased loss of heart fluorescence over time was also observed in EPOR morphants as compared to the control (Figure 3F), and glomerular podocin expression was also decreased in EPOR morphants (Figure 3G). Lastly, a gRNA targeting zebrafish EPOR exon 2 was created to further verify pronephric phenotype caused by EPOR silencing (Figure 4B and Supplementary Figure 3). Similar to EPOR morphants, although less pronounced, EPOR CRISPRant showed reduced hemoglobin staining, pronephric structural, and functional alteration (Figure 4C–E). Electron microscopy further identified that podocyte foot processes were less developed and Bowman space was almost absent upon EPOR knockdown (Supplementary Figure 4). Together, the data indicated that EPOR knockdown and knockout resulted in similar pronephric structural and functional changes in 48 h zebrafish embryos as seen for EPO morphants and CRISPRants.
In order to establish the specificity that EPO exerted its pronephric function in an EPOR dependent manner, we performed rescue experiments in EPO and EPOR morphants by using human recombinant EPO protein (hEPO). Intracardiac injections of hEPO at 24 hpf increased red blood cells number and thus hemoglobin concentration in EPO morphants, but not in EPOR morphants, at 48 hpf (Supplementary Figure 5A), which again proved that hEPO exerts its hematopoietic function via EPOR in zebrafish embryos. Importantly, hEPO also rescued pronephric alterations and functions in EPO morphants (Supplementary Figure 5A–C), while no rescue of pronephric structural and function changes was observed in EPOR morphants upon hEPO injection (Supplementary Figure 5A, B, D). Taken together, experiments performed in zebrafish identified EPO and EPOR as novel regulators of zebrafish nephrogenesis and function.
3.3. EPO and EPOR support pronephros development and function in zebrafish embryos via anti-apoptotic activities
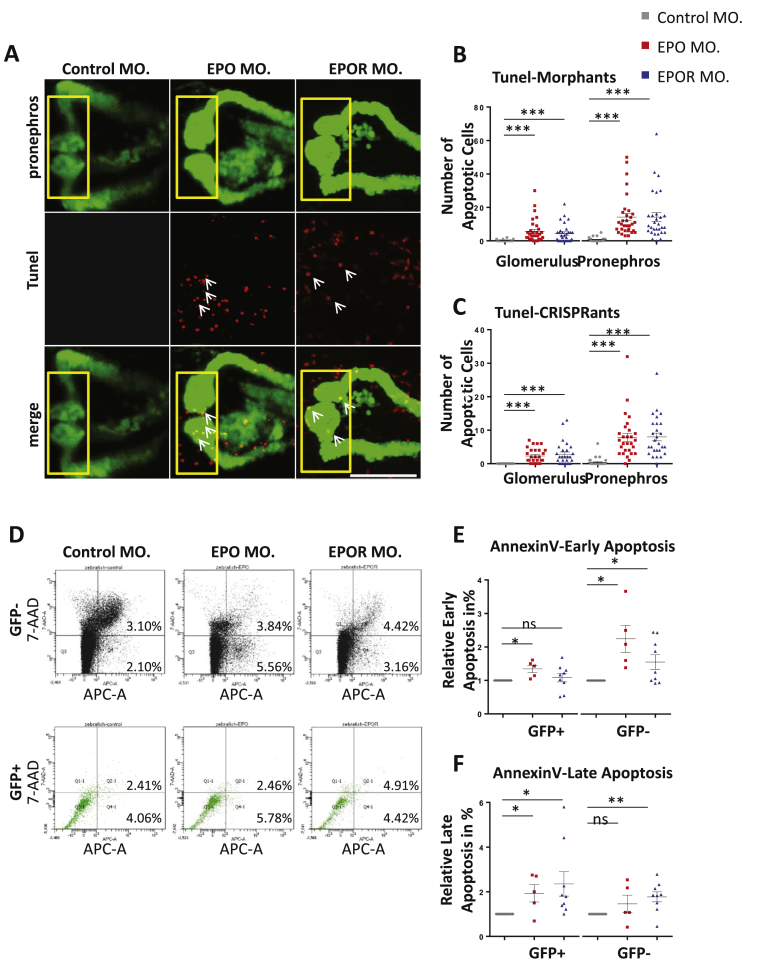
Since EPO is well known for binding EPOR and preventing apoptosis of precursor red blood cells [9], we next addressed the question of whether EPO maintains cell survival in pronephros development in zebrafish. To this end, we performed experiments in zebrafish embryos at 48 hpf which aimed to detect apoptotic cells within EPO and EPOR morphants and CRISPRants using TUNEL and Annexin V assays [37]. Injections of EPO morpholino and EPO gRNA + CAS led to a significantly higher incidence of apoptotic cells as compared to their controls at 48 hpf (Figure 5A–C, Supplementary Figures 6 and 7). As already shown in Figure 3, Figure 4C, altered pronephros structure was observed in EPO morphants and EPO CRISPRants. Importantly, apoptotic cell number was increased in the entire zebrafish embryo as well as in the pronephros. A similar pattern was observed in EPOR morphants and EPOR CRISPRants (Figure 5A–C, Supplementary Figures 6 and 7), indicating that EPO and EPOR protect pronephros during embryonic development from cell death. In order to validate the TUNEL data, apoptotic assay Annexin V for FACS analysis [37] was used which detects both early and late stages within the apoptotic cascade. We also identified within pronephros of EPO morphants an increased cell fraction of early and late apoptotic cells, while only the late apoptotic cell fraction was significant increased within EPOR morphants (Figure 5D–F). These results further supported the idea that prevention of apoptosis driven by EPO and EPOR was also essential for development of the pronephric system in zebrafish embryos. Yet, these experiments did not conclusively show that increased apoptotic cell number within pronephros of EPO and EPOR morphants and CRISPRants was the cause for observed pronephric structural and functional alterations. In order to address this question, we performed anti-apoptosis rescue experiments using zVAD-fmk, which previously has been reported as an apoptotic inhibitory compound on zebrafish embryos [35]. EPO and EPOR morpholinos were injected into zebrafish embryos, which were then incubated in 20 μM zVAD-fmk as compared to egg water as control, starting from 24 hpf. Embryos were incubated for additional 24 h and pronephros structure and function were analyzed at 48 hpf and 72 hpf accordingly. Upon treatment with zVAD-fmk, EPO and EPOR morphants showed a significant rescue of pronephric structures as compared to those embryos without zVAD-fmk treatment (Figure 6A, B). Likewise, a pronephric functional assay was also analyzed in EPO and EPOR morphants upon zVAD-fmk treatment and a significant reduction of loss of fluorescence was found in zVAD-fmk treated EPO and EPOR morphants (Figure 6C, D). To finally prove that EPO driven pronephros development and protection of apoptosis was indeed EPOR dependent, hEPO was intracardially injected at 24 hpf in EPO and EPOR morphants, and TUNEL assay to detect apoptotic cells assay was performed at 48 hpf. As shown earlier in EPO and EPOR morphants, an increased number of apoptotic cells were observed, which could be reduced upon hEPO injection in EPO morphants only (Supplementary Figure 6). Taken together, these data identified EPO as an important anti-apoptosis regulator within the zebrafish pronephros via binding of EPOR and exerting its downstream function.
Figure 5.
Knockdown of EPO and EPOR increased apoptosis within zebrafish pronephros. A. Increased apoptotic cell number (white arrows) and altered pronephros structure in EPO MO. and EPOR MO. as compared to Control MO. Confocal images of TUNEL-stained TG(WT1B:EGFP) zebrafish embryos at 48 hpf are shown. The yellow square highlighted glomerulus of zebrafish pronephros. White arrows labeled apoptotic cells shown in red color; pronephros was labeled in green. White scale bar: 100 μm. B. Quantification of glomerulus and total pronephros apoptotic cells (TUNEL) in EPO MO., EPOR MO. and Control MO. performed in three independent experiments as shown in Figure A (n = 28–32 embryos per group). C. Quantification of glomerulus and total pronephros apoptotic cells (TUNEL) in EPO CRISPRants, EPOR CRISPRants, and Control CRISPRants shown in Supplementary Figures performed in three independent experiments. (n = 28–30 embryos per group). D. FACS scan results for Annexin V stain in EPO MO. and EPOR MO. as compared to Control MO. E. Increased relative Annexin V-positive/7-AAD-negative cell fraction as an indicator of early apoptotic cells in EPO MO. and EPOR MO. as compared to Control MO. performed in at least three different experiments. Cells from 48 hpf zebrafish were harvested, stained with Annexin V-APC and 7-AAD and analyzed by FACS. F. Increased relative Annexin V-positive/7-AAD-positive cell fraction indicating late apoptotic cells in EPO MO. and EPOR MO. as compared to Control MO. performed in at least three different experiments. Data were analyzed using the Student‘s t-test (C and D). Mean ± s.e.m. ns not significant. ***p < 0.001.
Figure 6.
Apoptosis inhibition treatment partially rescued pronephros phenotype induced by EPO inactivation. A. Normalization of enlarged glomerulus (white arrow head) and shortened pronephric neck (white asterisks) in EPO MO. and EPOR MO. zebrafish embryos treated at 24 hpf with zVAD-fmk (300 μM) for 24 h. White scale bar: 200 μm. B. Quantification of data shown in Figure A performed in three independent experiments. (n = 40–52 embryos per group). **p ≤ 0.01 or ***p < 0.001 as indicated. C. Elevated loss of 70 kDa dextran–FITC fluorescence at 24 hpi and 48 hpi in EPO MO. zebrafish embryos was rescued by zVAD-fmk treatment (n = 41–43 embryos per group). Significance was given for EPO MO. against Control MO. as ***p < 0.001, and EPO MO. + zVAD-fmk against EPO MO. as ###p < 0.001. D. Elevated loss of 70 kDa dextran–FITC fluorescence at 24 hpi and 48 hpi in EPOR MO. zebrafish embryos was rescued by zVAD-fmk incubation (n = 41–47 embryos per group). Same Control MO. and Control MO. + zVAD-fmk were applied. Significance was given for EPOR MO. against Control MO. as ***p < 0.001, and EPOR MO. + zVAD-fmk against EPOR MO. as ##p < 0.01 as indicated. All data were analyzed using the Student‘s t-test. Mean ± s.e.m.
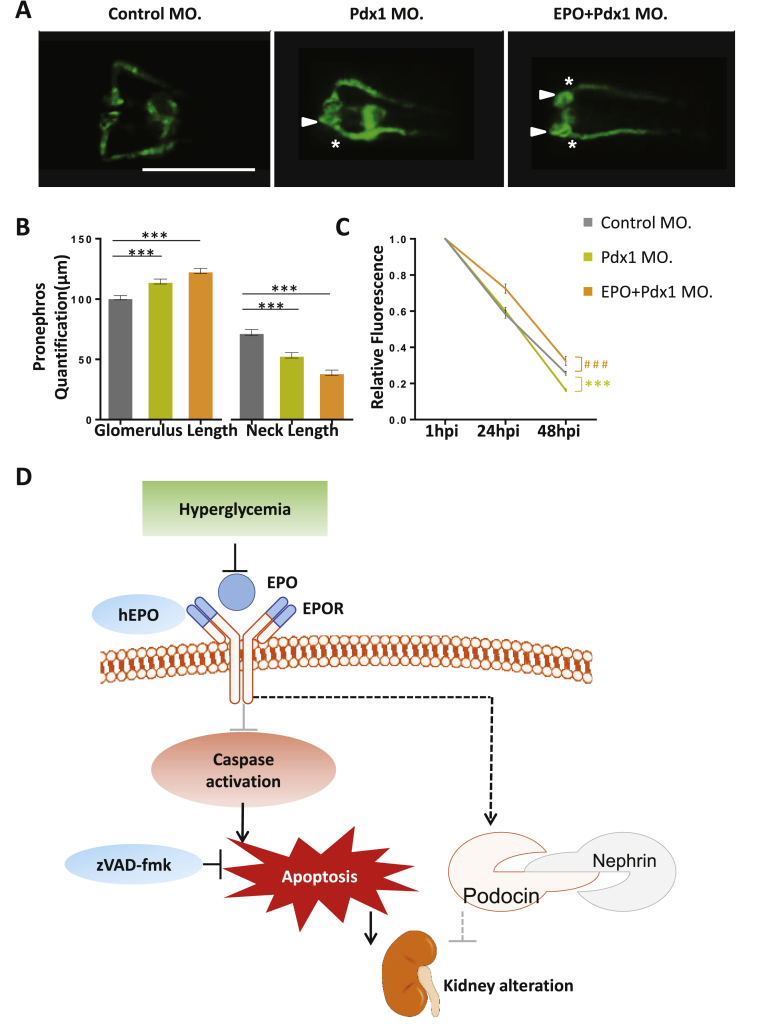
3.4. Loss of EPO in zebrafish aggravated hyperglycaemia induced renal damage
To finally test our hypothesis that EPO protects kidneys from hyperglycemia mediated renal deterioration, we took advantage of a Pdx1 knockdown strategy as a hyperglycemic zebrafish model, which has previously been described [35]. As compared to controls, Pdx1 morphants at 48 hpf exhibited an enlarged glomerulus and shortened neck (Figure 7A, B). Notably, pronephros structural alterations were further worsened in hyperglycemic zebrafish upon EPO knockdown, as shown by further increased glomerulus length and decreased neck length (Figure 7A, B). Increased loss of fluorescence was observed in Pdx1 morphants as compared to control, which correlated with previously published results [35] (Figure 7C). However, at 48 hpi, loss of fluorescence in EPO/Pdx1 double morphants was less increased as compared to Pdx1 morphants and even to control morphants, which was likely explained by further severed glomerulus filtration barrier formation in PDX1/EPO double morphants (Figure 7B, C). Taken together, these data suggested that EPO cooperated with EPOR to protect renal function by repressing apoptosis under hyperglycemia condition (Figure 7D).
Figure 7.
EPO silencing aggravated pronephros structure and function in hyperglycemic zebrafish. A. Further enlarged glomerulus (white arrow head) and shortened pronephric neck (white asterixis) in EPO + Pdx1 morphants (EPO + Pdx1 MO.) as compared to Pdx1 morphants (Pdx1 MO.) alone. White scale bar: 200 μm. B. Quantification of data in Figure A performed in three independent experiments. (n = 55–69 embryos per group). ***p < 0.001 as indicated. C. Elevated loss of 70 kDa dextran–FITC fluorescence at 24 hpi and 48 hpi in Pdx1 MO. as compared to Control MO. but decreased loss of 70 kDa dextran–FITC fluorescence at 24 hpi and 48 hpi in EPO + Pdx1 MO. as compared to Control MO. (n = 39–44 embryos per group). Significance was given for Pdx1 MO. against Control MO. as ***p < 0.001, and for EPO + Pdx1 MO. against Control MO. as ###p < 0.001. All data were analyzed using the Student‘s t-test. Mean ± s.e.m. D. Schematic illustration that EPO and EPOR maintain kidney structure and function through repressing apoptosis and protect kidney pathogenesis under hyperglycemia condition.
4. Discussion
In this study, EPO was identified as a protective factor both in early kidney development and hyperglycemia-induced kidney alterations in zebrafish embryos and in human diabetics setting based on the following observations: 1) EPO exhibited renal protective and proteinuria ameliorating function in type 2 DM patients and in hyperglycemic zebrafish embryos. 2) Bioinformatic analysis revealed enhanced co-expression of EPO and EPOR in the glomeruli and tubule of DN patients. 3) Loss of function studies for EPO and EPOR showed renal structure and functional alteration in zebrafish embryos. 4) EPO and EPOR knockdown or knockout resulted in increased apoptosis within zebrafish pronephros, which could be rescued by zVAD-fmk anti-apoptosis treatment.
The important implication of the present study is that EPO is a renal protective modulator among diabetic patients. GWAS suggested that EPO plays an important role in diabetes [14]. To further investigate the function of EPO in diabetes, serum samples from DM and non-DM patients were collected and analyzed for EPO levels and a negative correlation between serum EPO concentrations and DM duration was uncovered, indicating that EPO levels gradually decrease with the progression and duration of diabetes. Furthermore, GFR is also lower when EPO decreases. The above results indicate that EPO loss is an activator for function deterioration of the kidney in diabetes, unveiling a renal protection mechanism for EPO in diabetes. This conclusion is further proven by the fact that EPO knockdown further aggravated kidney alterations in hyperglycemia zebrafish, indicating an important protective function for EPO in diabetes and diabetic nephropathy.
Bioinformatic analysis of data from clinical DN patients receiving hEPO treatment further identified a role for EPO in protecting against proteinuria in DN patients. Enhanced co-expression of EPO and EPOR was identified in both glomeruli and tubule of DN patients, suggesting that the protective function of EPO is further enhanced by binding to EPOR in diabetic nephropathy, although the overall serum EPO level is decreased with DM progression. Further co-expression prediction analysis by geneMANIA indicates that EPO could co-express with NPHS1 and NPHS2, which are two genes crucially important for glomerulus filtration barrier formation in humans [39], [40]. As podocyte loss and thus glomerulus filtration barrier damage proceeds pathological alterations in diabetic nephropathy, proteinuria develops with the progression of diabetic nephropathy [42], [43]. The potential link between EPO and proteinuria was further suggested by the fact that end stage DN patients receiving hEPO supplementation show decreased proteinuria as compared to patients without EPO supplement. One thing worth noting is that patients with or without hEPO injection exhibit no difference in angiotensin blocking medication and hemoglobin level, excluding other potential proteinuria ameliorating factors in those patients. In zebrafish, it is further proven that both EPO and EPOR silencing result in proteinuria-like pronephros function alteration by 70KD dextran functional assay. Lastly, podocin, the zebrafish homologue of NPHS2, is also down regulated in its expression level upon EPO and EPOR knockdown, correlates with the gene co-expression analysis in human.
Although reduced EPO concentrations are traditionally considered the result of kidney failure due to the fact that EPO is mainly produced in human kidney interstitial cells [9], our study indicates for the first time that low EPO concentrations could be responsible for the deterioration of kidney structure and function. This is strongly supported by our observations in zebrafish embryonic development that expression of the EPOR receptor is mostly seen in renal cells which suggests a cell autonomous function for EPOR in renal development. Mechanistically, we demonstrated that increased apoptosis is detected within whole embryo as well as in pronephros, which further explains the alterations in renal structural and functional. This is further proven by the fact that the apoptosis inhibitor zVAD-fmk rescued pronephros structure and function in EPO and EPOR silenced zebrafish embryos. Thus, our study reveals that EPO plays a direct and critical renal protective function during development by binding and activating EPOR and repressing apoptosis.
The current study also highlights zebrafish as a model organism to analyze common and hyperglycemia-induced renal pathogenesis and its mechanism in the embryonic stages. The pronephros alterations in EPO silencing and hyperglycemic zebrafish embryos correlate with the clinical result that EPO is lowered within diabetes duration and is protective for kidney function. This is further strengthened by several advantages of zebrafish as compared to other model organisms in diabetes research, which includes visualization of early pronephros alterations in a translucent, living vertebrate and simple and effective manipulation of gene expression. Thus, zebrafish represent a powerful tool for the fast induction of hyperglycemia or EPO-induced pronephros pathologies, simple and fast analyses of genetic and biochemical mechanisms that are involved in these pathologies, as well as mechanism investigations for visible pathological alterations. This study is, to the best of our knowledge, the first global EPO knockout observation in vivo studying renal development.
The major limitation for the present study is that the evaluation of the urine protein change in patients receiving hEPO supplement is only a retrospective and observational study, in which the selection bias could not be easily excluded. It was shown by our data that decreased EPO during diabetes might contribute to the progression of nephropathy. However, further experiments are required to clarify the mechanism whether EPO is the cause or the result of kidney damage. In addition, there is conflicting evidence regarding clinical mortality and morbidity in diabetic patients with anemia when treated with hEpo [44], [45], [46]. Although our study suggested that EPO exerts reno-protective and proteinuria-ameliorating functions in diabetes, we could not overlook the potential toxicity of human recombinant EPO. As a result, randomized control clinical trials are required over the long run to further elucidate the proteinuria ameliorating function of EPO in clinical practice. Meanwhile, it must be determined when and how much EPO should be administered in order to minimize the potential detrimental side effects. Developing novel and safer forms of recombinant EPO might be also advisable in the future.
In conclusion, the current work establishes a protective function for EPO and EPOR in renal development and diabetic kidney pathogenesis on multiple levels. Data from the model organism zebrafish can be further translated into human type 2 DM patients, indicating a link between EPO loss and kidney function deterioration as well as proteinuria under hyperglycemic condition. As a perspective, our data strongly suggest that therapeutic strategies to block EPO loss or EPO supplementation could be considered as a potential renal protective treatment for type 2 DM patients, even before renal anemia treatment in end stage kidney disease.
Author contributions
JQ.S., ZY.Y., Y.W., and J.C. conducted the experiments, J.K. and JQ.S. designed the experiments and wrote the paper.
Acknowledgments
The authors would like to thank Marlene Hauser, Karen Bieback, Stefanie Uhlig, and Christel Weiss for their contributions and technical assistance in plasmid cloning, qPCR, FACS, and statistical analysis. The authors further acknowledge support of China Scholarship Council (CSC) and Deutscher Akademischer Austauschdienst (DAAD). This study was supported by Deutsche Forschungsgemeinschaft (International Research Training Group 1874/1 “DIAMICOM”, project SP9; Collaborative Research Centre SFB1118, project B1; Collaborative Research Centre SFB/TR23 project Z5); Central University Basic Science Foundation of China (1191329724) and by National Natural Science Foundation of China (81570406). Dr. Jens Kroll is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.11.006.
Conflicts of interest
The authors have no conflicts of interest to report.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Figure 1. A. Coexpression analysis of EPO and EPOR along with kidney pathogenesis related genes, including Notch1, WT1, Pax1, NPHS1, and NPHS2 in the kidney glomeruli section from DN patients and control. B. Coexpression analysis of EPO and EPOR along with kidney pathogenesis related genes, including Notch1, WT1, Pax1, NPHS1, and NPHS2 in the kidney tubuli section from DN patients and control. The figures within each crossover represented the coexpression value between the respective genes. Blue color indicated a positive coexpression and red color indicated a negative coexpression. Differential threshold was set as 0.8.
Supplementary Figure 2. Morpholino efficiencies and target sequences. A. Reduced expression of EPO and EPOR in 48 hpf zebrafish embryos upon indicated morpholino injections as compared to their respective controls in 3 independent experiments (n = 30–50 embryos per group). B. Schematic illustration of EPO and EPOR morpholino sequences. C. Dose dependently increased glomerulus length and reduced neck length upon 2 μg/μl and 4 μg/μl EPO morpholino injections in n = 51–78 embryos. D. Dose dependently increased glomerulus length and reduced neck length upon 4 μg/μl and 6 μg/μl EPOR morpholino injections (n = 28–31 embryos). Data were analyzed using the Mann–Whitney-U-test (A) or the Student's t-test (C and D). Mean ± s.e.m. *p < 0.05. **p < 0.01. ***p < 0.001.
Supplementary Figure 3. CRISPR constructs efficiencies. Reduced expression of EPO, and EPOR in 48 hpf zebrafish embryos upon CRISPR injections as compared to their respect controls in 3 independent experiments (n = 30–50 embryos per group). Data were analyzed using the Mann–Whitney-U-test. Mean ± s.e.m. *p < 0.05.
Supplementary Figure 4. Ultramicroscopic pronephros structure alteration upon genetic inactivation of EPO signaling in zebrafish. A. Podocytes foot processes (PFP) and bowman space (BS) in Control Mo. are well developed. B. Upon EPOR knockdown, foot processes are immature and are unable to interdigitate to form an appropriate filtration barrier. Bowman space (BS) is completely lost. The white line indicates the scale bar corresponding to 1 μm length.
Supplementary Figure 5. EPO supplement attenuate kidney structure and split diagram function alteration via a EPOR-dependent manner in zebrafish. A. Intracardiac injection of hEPO normalized enlarged glomerulus (white arrow head) and shortened pronephric neck (white asterixis) in EPO MO. In contrast, pronephros alterations in EPOR MO. could not be rescued by hEPO injections. Morpholino injected zebrafish embryos were injected at 24 hpf with 1 nl control or 4 IU/mL hEPO solution into the heart. Light microscopy images show increased hemoglobin concentrations in EPO MO. embryos injected with hEPO as compared to EPO MO. embryos injected with control solution only. In EPOR MO. embryos, hEPO could not increase hemoglobin concentrations. White scale bar in pronephros structure: 200 μm. Black scale bar in O-dianiside stain: 500μm. B. Quantification of data shown in A done in three independent experiments for each group. (n = 34–51 embryos per group). ns = not significant, **p ≤ 0.01, ***p < 0.001. C. Elevated loss of 70 kDa dextran–FITC fluorescence at 24 hpi and 48 hpi could be normalized in EPO morphants injected with hEPO (n = 42–50 embryos per group). Mean ± standard error. Significance was given for EPO MO. + KCL against Control MO. + KCL as ***p < 0.001, and EPO MO. + KCL against EPO MO + hEPO as #p < 0.05. D. Elevated loss of 70 kDa dextran–FITC fluorescence at 24 hpi and 48 hpi could not be rescued in EPOR MO zebrafish embryos injected with hEPO (n = 40–44 embryos per group). The same Control MO. + KCL and Control MO. + hEPO were applied. Significance was given for EPOR MO. + KCL against Control MO. + KCL as ***p < 0.001, and EPOR MO. + KCL against EPOR MO + hEPO as not significant (ns). All data were analyzed using the Student's t-test. Mean ± s.e.m.
Supplementary Figure 6. hEPO partially reduced apoptotic cell number in EPO morphants but not in EPOR morphants. A. Normalization of apoptotic cell number (white arrows) and pronephros structure in EPO morphants, but not in EPOR morphants injected with hEPO at 24 hpf. Confocal images of TUNEL-stained TG(WT1B:EGFP) zebrafish embryos at 48 hpf were shown. Arrows labelled apoptotic cells in red; pronephros was labelled in green. White scale bar: 100 μm. B. Quantification of data for glomerulus (top) and pronephros (below) performed in three independent experiments. (n = 26–31 embryos per group). All data were analyzed using the Student's t-test. Mean ± s.e.m. ns not significant. *p < 0.05. **p < 0.01. ***p < 0.001.
Supplementary Figure 7. Increased apoptotic cell number (white arrows) and altered pronephros structure in EPO CRISPRants and EPOR CRISPRants as compared to Control CRISPRants. Confocal images of TUNEL-stained TG(WT1B:EGFP) zebrafish embryos at 48 hpf are shown. The yellow square highlighted glomerulus of zebrafish pronephros. White arrows labelled apoptotic cells shown in red color; pronephros was labelled in green. White scale bar: 100 μm.
References
- 1.Xie C., Kim H.J., Haw J.G., Kalbasi A., Gardner B.K., Li G. A novel multiplex assay combining autoantibodies plus PSA has potential implications for classification of prostate cancer from non-malignant cases. Journal of Translational Medicine. 2011;9:43. doi: 10.1186/1479-5876-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badal S.S., Danesh F.R. Diabetic nephropathy: emerging biomarkers for risk assessment. Diabetes. 2015;64:3063–3065. doi: 10.2337/db15-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. Preventing kidney cell suicide. Nature Medicine. 2007;13:1284–1285. doi: 10.1038/nm1107-1284. [DOI] [PubMed] [Google Scholar]
- 4.Isermann B., Vinnikov I.A., Madhusudhan T., Herzog S. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nature Medicine. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 5.Long J., Badal S.S., Ye Z., Wang Y. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. The Journal of Clinical Investigation. 2016;126:4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Cohrs C.M., Stertmann J., Bozsak R., Speier S. Human beta cell mass and function in diabetes: recent advances in knowledge and technologies to understand disease pathogenesis. Molecular Metabolism. 2017;6:943–957. doi: 10.1016/j.molmet.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlqvist E., van Zuydam N.R., Groop L.C., McCarthy M.I. The genetics of diabetic complications. Nature Reviews. Nephrology. 2015;11:277–287. doi: 10.1038/nrneph.2015.37. [DOI] [PubMed] [Google Scholar]
- 8.Ndiaye F.K., Ortalli A., Canouil M., Huyvaert M. Expression and functional assessment of candidate type 2 diabetes susceptibility genes identify four new genes contributing to human insulin secretion. Molecular Metabolism. 2017;6:459–470. doi: 10.1016/j.molmet.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jelkmann W. Erythropoietin: back to basics. Blood. 2010;115:4151–4152. doi: 10.1182/blood-2010-03-271395. [DOI] [PubMed] [Google Scholar]
- 10.Farsijani N.M., Liu Q., Kobayashi H., Davidoff O. Renal epithelium regulates erythropoiesis via HIF-dependent suppression of erythropoietin. The Journal of Clinical Investigation. 2016;126:1425–1437. doi: 10.1172/JCI74997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo M., Hawkins M. Beyond erythropoiesis: emerging metabolic roles of erythropoietin. Diabetes. 2014;63:2229–2231. doi: 10.2337/db14-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradinaru D., Margina D., Ilie M., Borsa C. Correlation between erythropoietin serum levels and erythrocyte susceptibility to lipid peroxidation in elderly with type 2 diabetes. Acta Physiologica Hungarica. 2015;102:400–408. doi: 10.1556/036.102.2015.4.7. [DOI] [PubMed] [Google Scholar]
- 13.Loeffler I., Ruster C., Franke S., Liebisch M., Wolf G. Erythropoietin ameliorates podocyte injury in advanced diabetic nephropathy in the db/db mouse. American Journal of Physiology. Renal Physiology. 2013;305:F911–F918. doi: 10.1152/ajprenal.00643.2012. [DOI] [PubMed] [Google Scholar]
- 14.Tong Z., Yang Z., Patel S., Chen H. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6998–7003. doi: 10.1073/pnas.0800454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Echigoya M.H., Obikane K., Nakashima T., Sasaki S. Glomerular localization of erythropoietin receptor mRNA and protein in neonatal and mature mouse kidney. Nephron. Experimental Nephrology. 2005;100:e21–29. doi: 10.1159/000084109. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson L.O., Goldwasser E., Fried W., Plzak L. Role of the kidney in erythropoiesis. Nature. 1957;179:633–634. doi: 10.1038/179633a0. [DOI] [PubMed] [Google Scholar]
- 17.Fisher J.W., Koury S., Ducey T., Mendel S. Erythropoietin production by interstitial cells of hypoxic monkey kidneys. British Journal of Haematology. 1996;95:27–32. doi: 10.1046/j.1365-2141.1996.d01-1864.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu H., Liu X., Jaenisch R., Lodish H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 19.Wu H., Lee S.H., Gao J., Liu X., Iruela-Arispe M.L. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–3605. doi: 10.1242/dev.126.16.3597. [DOI] [PubMed] [Google Scholar]
- 20.Yu X., Lin C.S., Costantini F., Noguchi C.T. The human erythropoietin receptor gene rescues erythropoiesis and developmental defects in the erythropoietin receptor null mouse. Blood. 2001;98:475–477. doi: 10.1182/blood.v98.2.475. [DOI] [PubMed] [Google Scholar]
- 21.Zeigler B.M., Vajdos J., Qin W., Loverro L., Niss K. A mouse model for an erythropoietin-deficiency anemia. Disease Models & Mechanisms. 2010;3:763–772. doi: 10.1242/dmm.004788. [DOI] [PubMed] [Google Scholar]
- 22.Howe K., Clark M.D., Torroja C.F., Torrance J. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heckler K., Kroll J. Zebrafish as a model for the study of microvascular complications of diabetes and their mechanisms. International Journal of Molecular Sciences. 2017;18 doi: 10.3390/ijms18092002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond I. Making a zebrafish kidney: a tale of two tubes. Trends in Cell Biology. 2003;13:357–365. doi: 10.1016/s0962-8924(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 25.Wingert R.A., Davidson A.J. The zebrafish pronephros: a model to study nephron segmentation. Kidney International. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- 26.Handelsman Y., Mechanick J.I., Blonde L., Grunberger G. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocrine practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17(Suppl. 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 27.Tarantino M., Marchi S. [Determination of urinary total proteins and some protein fractions] Quaderni Sclavo di diagnostica clinica e di laboratorio. 1975;11:82–102. [PubMed] [Google Scholar]
- 28.Woroniecka K.I., Park A.S., Mohtat D., Thomas D.B. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354–2369. doi: 10.2337/db10-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez P.E., Fares R.P., Risso J.J., Bonnet C. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9848–9853. doi: 10.1073/pnas.0901840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., Zheng N. A novel fractal image compression scheme with block classification and sorting based on Pearson's correlation coefficient. IEEE Transactions on Image Processing: A Publication of the IEEE Signal Processing Society. 2013;22:3690–3702. doi: 10.1109/TIP.2013.2268977. [DOI] [PubMed] [Google Scholar]
- 31.Warde-Farley D., Donaldson S.L., Comes O., Zuberi K. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Research. 2010;38:W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paffett-Lugassy N., Hsia N., Fraenkel P.G., Paw B. Functional conservation of erythropoietin signaling in zebrafish. Blood. 2007;110:2718–2726. doi: 10.1182/blood-2006-04-016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu C.Y., Cheng C.H., Chen G.D., Chen Y.C. The zebrafish erythropoietin: functional identification and biochemical characterization. FEBS Letters. 2007;581:4265–4271. doi: 10.1016/j.febslet.2007.07.073. [DOI] [PubMed] [Google Scholar]
- 34.Stoll S.J., Bartsch S., Augustin H.G., Kroll J. The transcription factor HOXC9 regulates endothelial cell quiescence and vascular morphogenesis in zebrafish via inhibition of interleukin 8. Circulation Research. 2011;108:1367–1377. doi: 10.1161/CIRCRESAHA.111.244095. [DOI] [PubMed] [Google Scholar]
- 35.Sharma K.R., Heckler K., Stoll S.J., Hillebrands J.L. ELMO1 protects renal structure and ultrafiltration in kidney development and under diabetic conditions. Scientific Reports. 2016;6:37172. doi: 10.1038/srep37172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiesow K., Bennewitz K., Miranda L.G., Stoll S.J. Junb controls lymphatic vascular development in zebrafish via miR-182. Scientific Reports. 2015;5:15007. doi: 10.1038/srep15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaker K., Bartsch S., Patry C., Stoll S.J. The bipartite rac1 Guanine nucleotide exchange factor engulfment and cell motility 1/dedicator of cytokinesis 180 (elmo1/dock180) protects endothelial cells from apoptosis in blood vessel development. Journal of Biological Chemistry. 2015;290:6408–6418. doi: 10.1074/jbc.M114.633701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbieri J., Fontela P.C., Winkelmann E.R., Zimmermann C.E. Anemia in patients with type 2 diabetes mellitus. Anemia. 2015;2015:354737. doi: 10.1155/2015/354737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kestila M., Lenkkeri U., Mannikko M., Lamerdin J. Positionally cloned gene for a novel glomerular protein–nephrin – is mutated in congenital nephrotic syndrome. Molecular Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 40.Hinkes B., Wiggins R.C., Gbadegesin R., Vlangos C.N. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nature Genetics. 2006;38:1397–1405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 41.Zhou W., Boucher R.C., Bollig F., Englert C., Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. American Journal of Physiology-Renal Physiology. 2010;299:F1040–F1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiological Reviews. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 43.Mogensen C.E., Christensen C.K., Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl. 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 44.Garimella P.S., Katz R., Patel K.V., Kritchevsky S.B. Association of serum erythropoietin with cardiovascular events, kidney function decline, and mortality: the Health Aging and Body Composition Study. Circulation. Heart Failure. 2016;9:e002124. doi: 10.1161/CIRCHEARTFAILURE.115.002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Briet M., Barhoumi T., Mian M.O., Sierra C. Effects of recombinant human erythropoietin on resistance artery endothelial function in stage 4 chronic kidney disease. Journal of the American Heart Association. 2013;2:e000128. doi: 10.1161/JAHA.113.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos P.R., Melo A.D., Lima M.M., Negreiros I.M. Mortality risk in hemodialysis patients according to anemia control and erythropoietin dosing. Hemodialysis International. International Symposium on Home Hemodialysis. 2011;15:493–500. doi: 10.1111/j.1542-4758.2011.00607.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.