Abstract
Mechanical cues from the microenvironments play a regulating role in many physiological and pathological processes, such as stem cell differentiation and cancer cell metastasis. Experiments showed that cells adhered on a compliant substrate may change orientation with an externally applied strain in the substrate. By accounting for actin polymerization, actin retrograde flow, and integrin binding dynamics, here we develop a mechanism-based tensegrity model to study the orientations of polarized cells on a compliant substrate under biaxial stretches. We show that the cell can actively regulate its mechanical state by generating different traction force levels along its polarized direction. Under static or ultralow-frequency cyclic stretches, stretching a softer substrate leads to a higher increase in the traction force and induces a narrower distribution of cell alignment. Compared to static loadings, high-frequency cyclic loadings have a more significant influence on cell reorientation on a stiff substrate. In addition, the width of the cellular angular distribution scales inversely with the stretch amplitude under both static and cyclic stretches. Our results are in agreement with a wide range of experimental observations, and provide fundamental insights into the functioning of cellular mechanosensing systems.
Introduction
The structures, functions, and fates of living cells are subtly regulated by both biochemical compositions and physical properties of the extracellular matrix (1, 2, 3, 4). There is increasing evidence that mechanical microenvironments play a crucial role in the biological processes of cells and their aggregates, e.g., angiogenesis, differentiation, tissue development, and wound healing (5, 6, 7, 8). Many experiments have demonstrated that cells form strong and stable focal adhesions (FAs) on stiff substrates, but weak and dynamic adhesions on soft ones (1), and that cell motion can be guided by the gradient of substrate stiffness (9).
Under physiological conditions (e.g., heart beating, pulsating blood vessels, and breathing), cells are exposed to various substrates under cyclic stretches. It has been shown that cells adhered on a substrate subjected to cyclic stretches can reorient from random orientations to a well-defined angle, depending on the stretch frequency and amplitude (10, 11, 12, 13, 14). Under uniaxial cyclic strains, cells align nearly perpendicular to the loading direction at high frequencies (∼1 Hz) (10, 11). Under biaxial cyclic strains, Livne et al. (14) showed that the most stable cellular orientation can be quantitatively determined by two parameters: the biaxial stretching ratio and the elastic anisotropy index of cells. It was then totally surprising to see that cells exposed to soft collagen gels align themselves parallel to the stretch direction even at high frequencies (15).
These experimental findings pose substantial challenges to theoretical efforts aimed to understand the fundamental mechanisms of cell reorientation under stretching (16, 17, 18, 19, 20, 21). Safran and co-workers (16, 17, 18) developed a force dipole model, in which the cell maintains its minimal stress or strain state under cyclic stretching. Livne et al.'s experimental data has been interpreted by a passively stored elastic energy model (14) or a 2D tensegrity model (21). Chen et al. (19) and Kong et al. (20) examined the binding dynamics of integrins under cyclic loadings and pointed out that the FAs may lose stability under high-frequency loadings. Interestingly, this molecular level model (19) can also reproduce the experimental phenomena of Livne et al.
In contrast to the above studies under cyclic stretches, the cellular responses to static or quasi-static stretches are less understood (22). It remains puzzling why cells exhibit distinct modes by aligning themselves randomly (12) or parallel to the stretching direction (23, 24, 25, 26) under different static strains. Interestingly, the distributions of cellular orientations under static stretches (23, 24, 25, 26) are generally broader than those under cyclic stretches (10, 11, 12, 13, 14). Goli-Malekabadi et al. (27) showed that static stretching is not as influential as dynamic stretching to direct cell reorientation. To date, however, it remains unclear how to integrate the biochemical and mechanical mechanisms that underlie the local dynamics of FAs into a theoretical model at the cellular level. In addition, the previous models for cell reorientation have not accounted for the effect of substrate stiffness on cellular alignment under different loading conditions.
In this article, we develop a mechanism-based tensegrity model, by incorporating the molecular mechanisms of FA dynamics, the actin polymerization and the actin retrograde flow, to investigate the orientations of polarized cells on compliant substrates under different loading conditions. Our analysis shows that stretching the substrate will align cells parallel to the tensile direction but has negligible effects for very stiff substrates under static or ultralow-frequency cyclic stretches. Furthermore, the width of cellular angular distribution scales inversely with the strain amplitude for both static and cyclic strains, whereas cyclic loading is more effective than static loading in directing cell reorientation on a stiff substrate. Compared to other existing models in the literature, our present model is more complete, integrates all known molecular mechanisms, and yields results in good agreement with a broad range of experimental observations.
Materials and Methods
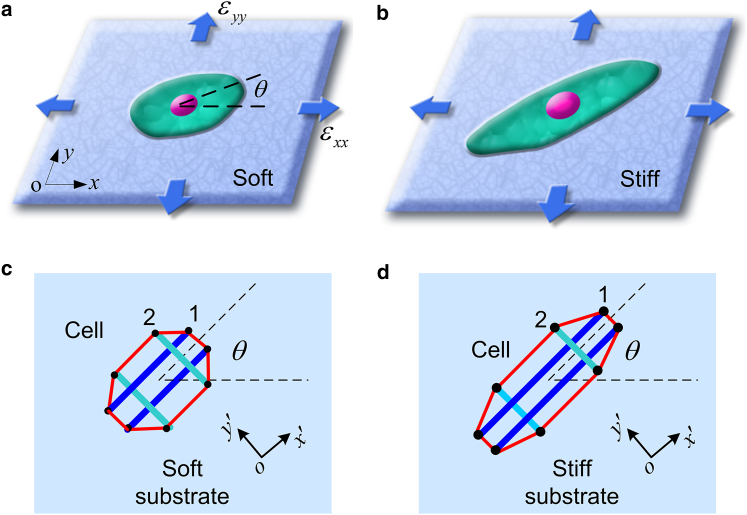
Consider a cell adhering on an elastic substrate subjected to biaxial, either static or cyclic, stretches. Fig. 1, a and b illustrate a polarized cell on a soft and a stiff substrate, respectively, recognizing the fact that substrate elasticity can remarkably affect cellular polarization (28, 29). Chen et al. (19) and Kong et al. (20) have used a simplified model, consisting of only two FAs and a single SF, to explain the experimental results of cell orientations under cyclic stretches. Ingber et al. (30) demonstrated that the cytoskeleton can be well described as a tensegrity. Here, we model the cell as a planar tensegrity structure consisting of four bars and eight strings; see Fig. 1, c and d. The direction of the cell’s long axis corresponds to the orientation of stress fibers (SFs) (13, 14) that are modeled as long bars in the tensegrity. In the lateral direction, the short bars, acting as the lateral support of the cytoskeleton, represent the contributions from microtubules and other biopolymer elements (31) perpendicular to the SF orientation. The strings represent actin microfilaments, which ensure the integrity of the entire cellular structure. At the ends of bars, FAs made of integrins at the cell-substrate interface provide the cellular traction force. As the substrate is stretched, the actins within the cell and the integrins at the cell-substrate interface would reorganize in response to the externally applied mechanical stimuli. In the following, we first investigate the rigidity-dependent dynamics of FAs, then describe the dynamical response of SFs, and finally present the free energy variation of the system under biaxial stretches.
Figure 1.
Schematic of a polarized cell adhered on (a) a soft or (b) a stiff substrate subjected to biaxial strains εxx and εyy. The cell is oriented at angle θ measured from the x direction. The tensegrity model, capturing the essential cell structure on (c) soft or (d) stiff substrates, consists of four bars, corresponding to the longitudinal SFs and lateral supports of the cytoskeleton, and eight strings, representing the microfilaments. To see this figure in color, go online.
Substrate stiffness-dependent traction forces of FAs
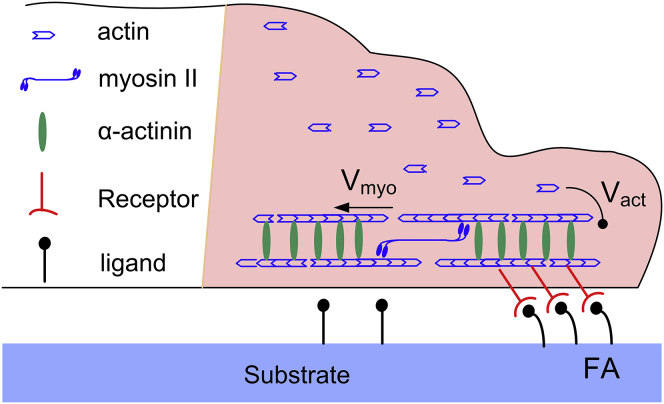
The extension and contraction of the polarized long bars are controlled by the collective dynamics of adhesion bonds (i.e., integrins), nonmuscle myosin II, and actins, as illustrated in Fig. 2. The mobility velocity V = Vact – Vmyo of the cell front is dictated by two simultaneous and oppositely directed mechanisms: actin polymerization with a speed Vact, and actin retrograde flow with a speed Vmyo caused by myosin motors. Experiments showed that at deceleration of cell spreading, the retrograde flow velocity Vmyo increases, whereas the sum Vmyo + V remains constant (32). Similar behaviors have been found in growth cone advancement (33, 34) and filopodia growth (35). Accordingly, Vact remains constant along the polarized direction in this study, as in a previous model for cell spreading (36).
Figure 2.
Schematic of major elements of the mechanosensing device at the front of a polarized cell. Actin polymerization occurs at the cell front. The sarcomeric unit of an SF includes actin filaments, myosin II motors, and cross-linker (α-actinin) proteins. The myosin II motors provide the contractive force with a retrograde flow velocity Vmyo of actins. The relative movement of actin filaments and FAs generates a cell-substrate tangential force that can be determined by the binding dynamics of the receptors (i.e., integrins) and their complementary ligands. To see this figure in color, go online.
The retrograde flow of the actin network can induce local displacements of adhesion bonds in FAs at the cell-substrate interface. Based on the experimental observation of continuous actin retrograde flow (32, 37), the bonds in FAs can collectively give rise to a tangential force, also referred to as the traction force, at the cell-substrate interface. Let s denote the sum of the tangential stretching displacement of the bond and the displacement of the elastic substrate. The probability density function n(s,t) is related to the retrograde speed Vmyo by (36, 38, 39)
| (1) |
where ξb and ξu are the integrin binding and unbind rate functions, respectively. The solution of Eq. 1 at steady state is
| (2) |
For simplicity, we assume the unbinding rate ξu is constant. Let N0 denote the total number of integrins associated within the focal adhesions. The number of bound integrins is . The first and second moments of the tangential deformation s are written as
| (3) |
| (4) |
The tangential force between the FA and substrate is given by , where kfa = Nbkint can be treated as the spring constant of FAs, kint being the elasticity of each bond, and is the viscous coefficient resulting from the dynamics of FAs. The elastic energy of one FA is the sum of contributions from all bonds, that is, .
To account for the effect of the substrate stiffness, we define an effective elastic constant of FAs to describe the elasticity of the FA-substrate system, where ksub ∝ Esub with Esub being the Young’s modulus of the substrate. At the steady state, Vmyo = Vact, and the maximum tangential force, i.e., the traction force of FAs, is
| (5) |
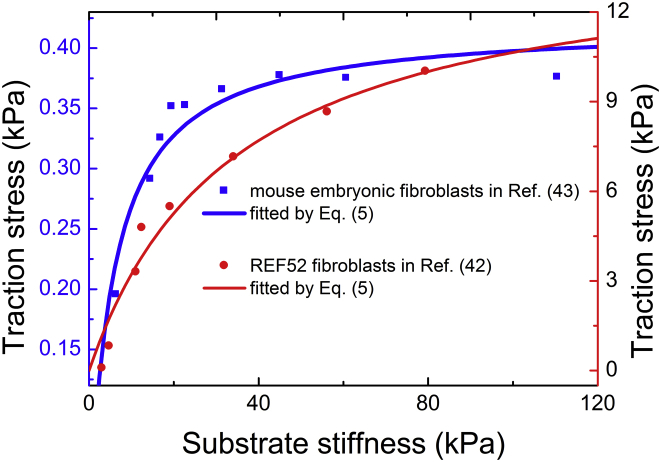
where d0 = Vact/ξu represents an intrinsic length of the cell. Equation 5 shows that cell traction increases monotonously with the substrate stiffness. For softer substrates (kfa ≫ ksub), the traction force is proportional to the stiffness ksub, suggesting that the cell sustains a constant substrate deformation. For stiffer substrates (ksub ≫ kfa), the traction force approaches a constant limited by the intrinsic length d0, indicating that the cell exerts a constant stress on the substrate. This result sheds light on the debate whether the mechanosensing of cells is regulated by deformation or by force (40, 41). Indeed, recent experiments indicate that the cell generates identical deformation on soft substrates, but a constant traction stress on stiff substrates (42, 43). Our results are in perfect agreement with these experimental observations, and can be quantitatively fitted to the data of rigidity-dependent traction forces of FAs, as shown in Fig. 3.
Figure 3.
Traction stress with respect to the substrate stiffness. The theoretical predictions (Eq. 5) fit well with the experimental data reproduced from (43) and (42), respectively. To see this figure in color, go online.
In addition, the integrins within the focal adhesions may form catch bonds (19, 44), whose lifetime increases with the force when it is relatively small. In this case, the unbinding rate could be modeled as , where , , and s0 are constants. Using this unbinding rate function, Nisenholz et al. (36) showed that the number of bound integrins approaches a constant and the mean displacement 〈s〉 increases linearly with Vmyo at relatively high retrograde speeds. This indicates that the tangential force fta = Nbkint〈s〉 also increases linearly with Vmyo, as in the case of a constant unbinding rate. Therefore, our results should hold also for catch bonds.
Dynamics of stress fibers
The dynamics of SFs is regulated by the passive movement of actin filaments and action of myosin II motors. As Kumar et al. (45) reported in their experiments, SFs can be described by a viscoelastic model consisting of a spring of stiffness ksf, a dashpot of passive viscosity ηsf, and an active contractive force fmyo. These forces are balanced by the traction force ffa from FAs. Thus, the force balance of SFs is expressed as
| (6) |
where ΔLsf is the length change of SFs and V is the velocity of SFs. The active contractive force fmyo originates from the action of myosin motors. According to Hill’s law (46),
| (7) |
where f0 is the stall force of the motors, V0 is the zero-load speed of the motors, and ηmyo = f0/V0 represents the active viscosity of motor movements. Using Vmyo = Vact − V, Eq. 6 becomes
| (8) |
Substituting V = dΔLsf/dt into the above equation leads to
| (9) |
where L0 and are the initial and steady lengths of SFs, respectively; and is a characteristic time of SF depending on the dynamics of adhesion bonds, as well as the passive and active viscosities of SFs.
Free energy in the system under biaxial stretches
In the proposed tensegrity model of a cell, the total free energy includes the elastic energies of FAs (Ufa) and tensegrity elements (Uten), as well as the energy of the pulling force (Upull). The total elastic energy of FAs is written as , where ui represents the elastic energy of each FA, subscripts 1 and 2 standing for the x′ and y′ directions, respectively. In the polarized direction, the cell undergoes active actin polymerization and myosin motor contraction. As described above, the elastic energy u1 of one FA is the sum of all bonds: , where f1 is the traction force and Δl1 = 〈s〉 is the mean displacement of the bonds. In the lateral direction, we assume the mechanical response of the adhesion connection is passive, i.e., , where f2 = k2Δl2 denotes the traction force, with k2 and Δl2 being the spring constant and length change of FAs, respectively. The work done by the traction force is , where ΔLx′ and ΔLy′ are the responding length changes of cell structures. This form is also identified as an important constituent of the thermodynamic state of FAs (47). The elastic potential energy Uten includes the contributions of all tensegrity elements. The elastic constant of microfilaments, modeled as strings, is much smaller than that of SFs, because both microfilaments and SFs are composed of actin filaments and the former has a much smaller cross-sectional area. Thus, we will neglect the contribution of strings to the free energy in this study. Noting that and , the free energy variation ΔU in the system induced by the biaxial stretches is
| (10) |
where Ki and ΔLi are the spring constant and length change of bars, respectively; and Δfi is the corresponding change in the traction force. Noticing ΔLi = Δfi/Ki, Eq. 10 can be rewritten as
| (11) |
For convenience, we assume that FAs have the same Young’s modulus E as the long and short bars in the tensegrity (21). Similar analysis can be made when their Young’s moduli are different without changing the main conclusions. Experiments showed that the characteristic size of FAs falls in the range of a few micrometers (14, 41), and the bars are approximately tens of micrometers in length (13, 14). It is known from the relation K = EA/L that k1 ≫ K1 and k2 ≫ K2. Therefore, Eq. 11 can be simplified as or .
Once the change in the traction force (Δfi) or the bar length (ΔLi) has been determined, the most stable cell orientation can be determined from the condition ∂ΔU/∂θ = 0. Here, Δf2 or ΔL2 is easily calculated from the passive mechanical response of the lateral bars; and Δf1 or ΔL1 can be obtained through the active regulation of the cell to its optimal mechanical state in the polarized direction.
Results and Discussion
Orientations of cells under biaxial static stretches
First, consider the case where the substrate is subjected to a biaxial static stretch. As shown in Fig. 1 a, the strains in the x and y directions are denoted as εxx = ε0 and εyy = −νεxx, respectively, where ε0 is the strain amplitude and ν is the biaxial ratio. The strains in the x′ and y′ directions are and , respectively.
The effective substrate stiffness will be increased due to the stretches because of the change of the reference configuration for strain measurement (23). For simplicity, the effective spring constant along the x′ direction is treated as . In this direction, the cell can actively adjust its traction force in response to the increased stiffness. Then, the change in the traction force due to the increased effective stiffness is
| (12) |
and the length change is ΔL1 = Δf1/K1. One can see that the traction force change Δf1 depends on the substrate stiffness, the strain amplitude, and the cellular orientation. For softer substrates (kfa ≫ ksub), significantly increases with the stretch amplitude, whereas for stiffer substrates (ksub ≫ kfa), is very small and could be neglected. In other words, stretching a softer substrate can provide a larger traction force in the tensile direction, whereas stretching a stiffer substrate has no significant effect on the traction force. In the lateral direction, one can calculate and by using the force balance and displacement constraint conditions. Using Eq. 11 and the initial length L1 = f1/K1, the free energy difference ΔU at orientation θ is
| (13) |
From ∂ΔU/∂θ = 0, we obtain three possible steady-state orientations: , π/2, and , where and are defined as the effective stiffnesses of the cell along the two directions under static stretches, respectively. Because the most stable state should have the minimal free energy among the three angles, the steady cellular orientation is determined to be when ξS1 > ξS2, and otherwise. Because the bar in the polarized direction is much longer than that in the lateral direction (14), we have ξS1 > ξS2, and the free energy change in the polarized direction plays a dominant role in the total free energy change. Therefore, the cell prefers aligning along the stretching direction (i.e., ), as observed in experiments (23, 24, 25, 26). However, on very stiff substrates, the variation in ΔU for different orientations may become too small to induce a preferential alignment of cells. This can explain why cells align randomly on stiff substrates under nearly static stretches in experiments (12).
In addition to the stretching strain, the Young’s modulus of some collagen substrate materials can increase with stretching (48, 49), a phenomenon known as strain stiffening. For example, the Young’s modulus of actin gels is nearly a constant under small strains but increases with strain following a power law of exponent ∼3/2 once the strain level is above a threshold value (48). Therefore, for this type of substrate, the effective stiffness may significantly increase along the tensile direction, and drive the cell to align along this direction.
In biological environments, cells do not align perfectly in one direction due to stochastic variations. Based on statistical thermodynamics, we know that the frequency of a given cellular orientation in experiments should depend on its free energy difference via
| (14) |
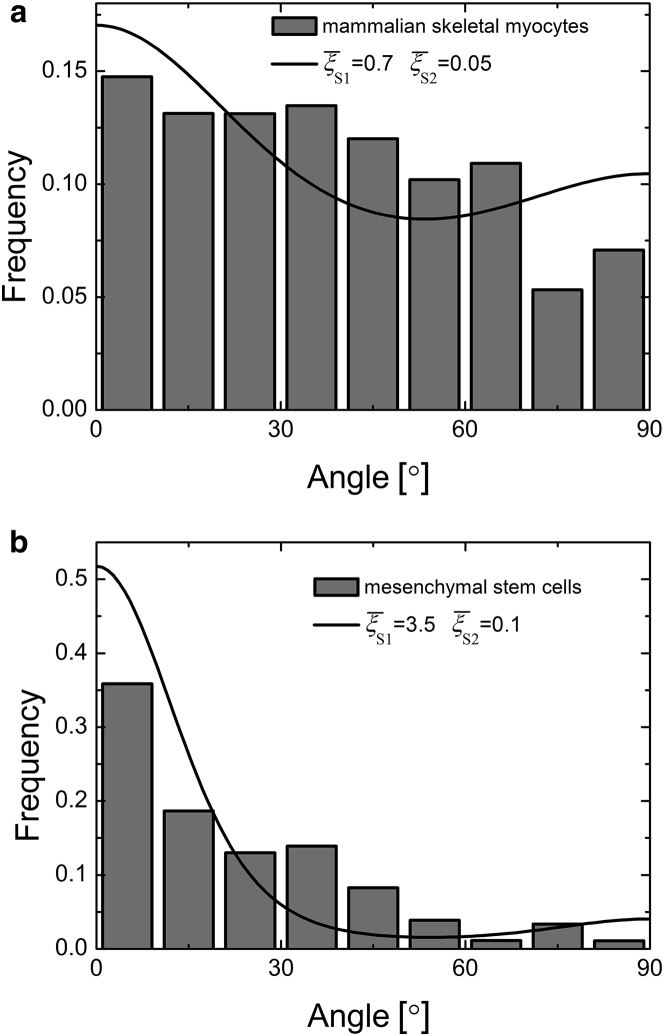
where kB is the Boltzmann constant, T is the effective temperature, and Δθ is the angle interval used in experimental measurements. To verify this model, we compare our predictions with the experimental data of mammalian skeletal myocytes (26) and mesenchymal stem cells (23) under static stretches, and find a good agreement (Fig. 4). Comparing to Fig. 4 a, cells exhibit a narrower orientational distribution and prefer aligning along a well-defined angle (Fig. 4 b), because the scaled effective stiffness is evidently larger than . On the right-hand side of Eq. 13, the two terms represent the energy changes in the polarized and lateral directions, respectively. The contributions from these two directions prefer the orientations θS1 = 0 and θS2 = π/2, respectively. When the scaled effective stiffness is much larger than , the energy contribution in the polarized direction holds a great weight, and thereby cells are prone to aligning along the tensile direction (i.e., θ = 0) and exhibit a narrow orientational distribution.
Figure 4.
Angular distributions of cells under static stretches. The theoretical predictions (red lines) fit well with the experimental data of (a) mammalian skeletal myocytes (26) and (b) mesenchymal stem cells (23). Here, we have taken the strain amplitude ε0 = 0.1 and the angle interval Δθ = 100, as in the experiments (23, 26).
To quantitatively examine the scatter distribution of cell orientations, we use an order parameter S to denote the cell alignment:
| (15) |
where θ is the cellular orientation and 〈···〉 denotes its average. Following this definition, S = 0 implies that the cells are randomly orientated; S = 1 and S = −1 correspond to cells aligning parallel and perpendicular to the stretching direction, respectively. Moreover, we describe the width of the angular distribution by its standard deviation
| (16) |
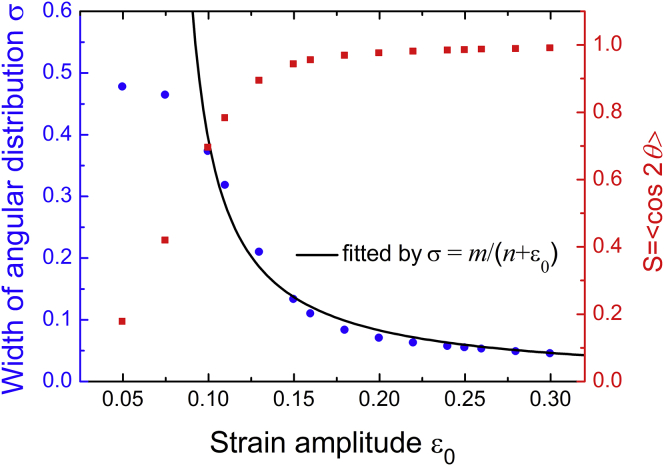
Using the parameters in Fig. 4 b, Fig. 5 plots the order parameter S and the width σ of the distribution as a function of the stretching amplitude ε0. When ε0 is small, the effective stiffness is also small. It then follows from Eq. 14 that the angular distribution will be roughly even, meaning that the cells tend to align randomly. With increasing ε0, the value of S gradually increases and approaches 1, meaning that there are more cells aligning along the stretching direction. This is because the effective stiffness becomes larger and holds more weight in determining the cell alignment. Meanwhile, the width of the angular distribution decreases with ε0, also indicating that the cells should align around a well-defined orientation under large-amplitude stretches. When is large (e.g., under large strains), the cellular orientations will approximately follow a Gaussian distribution, with the standard deviation σ inversely proportional to the strain amplitude. As shown in Fig. 5, the data for relatively large amplitude values can be fitted well by the function σ = m/(ε0 + n). This finding suggests that the angular distribution width scales approximately with under static stretches.
Figure 5.
Order parameter S = 〈cos2θ〉 (red square) and width σ of the cellular angular distribution (blue round) as a function of the strain amplitude ε0 under static stretches. The width calculated by Eq. 16 can be fitted well by the function σ = m/(ε0 + n), indicating that the width scales inversely with the strain amplitude at relatively large strain amplitude. Here, we used the parameters in Fig. 4b. To see this figure in color, go online.
Effects of prestretched substrates on cellular orientations
Most existing experiments focused on the effect of static stretches on cells seeded before stretching. The effect of a prestretch of the substrate has been rarely addressed previously. Recently, Liu et al. (23) observed that prestretched substrates are more effective than poststretched ones to direct cell reorientation. For prestretched substrates, we can calculate the free energy difference as , where L1 and L2 are the longitudinal and lateral lengths, respectively, of cells seeded on the substrate without stretches. Comparing with ΔUpost in Eq. 13 for the poststretched case, ΔUpre is evidently larger. This result can be understood as follows. For the prestretched case, the effective substrate stiffness is largest at the tensile direction. The cells can sense and respond to the largest substrate stiffness from the initial contact to the steady state. This is different from the poststretched case in which the cells respond to stretching from a random orientation to the steady state. In the former case, the cell will lose more energy, as described in the expression of ΔUpre. Therefore, for the prestretched substrate, more cells align at the tensile direction, and the angular distribution is much narrower than those in the poststretched case. These predictions are in agreement with relevant experiments (23).
Orientations of cells under biaxial cyclic stretches
In this subsection, we investigate the orientational response of cells under a biaxial cyclic strain with εxx = 0.5 ε0(1 – cos ωt) and εyy = −νεxx, where ε0 and ω are the strain amplitude and frequency, respectively. If the loading frequency is very low (e.g., ω = 0.0001 Hz), the cell has enough time to actively regulate its deformation and stays in its optimal mechanical state along the polarized direction. In this case, the mechanical response of polarized bars is determined by the traction force varying with the applied strain. Then, the dynamics of SFs can be described by
| (17) |
where η1 = ηfa + ηsf + ηmyo. The solution is
| (18) |
where ω1 = K1/η1 is the characteristic frequency of the SFs. Note that ΔL2 can be easily calculated by using the force balance and displacement constraint conditions in the lateral direction. When t → ∞, the free energy change ΔU per period is
| (19) |
where and denote the effective stiffnesses along the two directions under ultralow-frequency cyclic stretches, respectively, as
| (20) |
At very low frequencies, we have and . Similar to the analysis of static stretches, the effective stiffness is larger than , and the free energy change is dominated by the change in the polarized direction. Thus, the cell will have the minimal energy along the stretching direction (i.e., ).
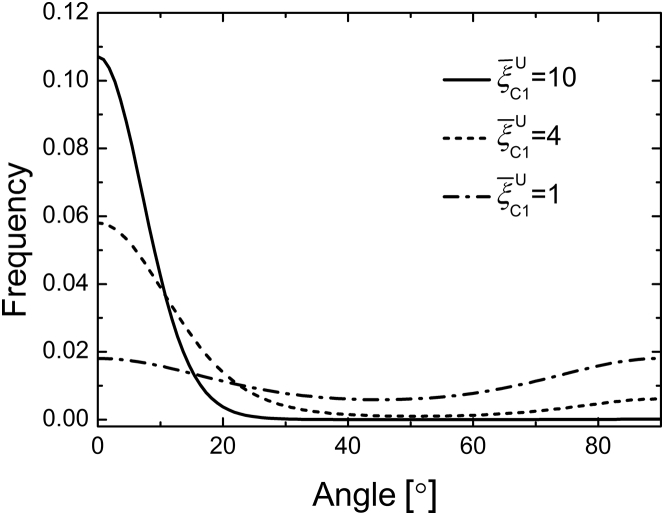
Next, we calculate the angular distributions of cells under different values of scaled effective stiffness by using Eq. 14 (Fig. 6). Here, we fix . It can be seen that as the effective stiffness increases, more cells concentrate along the tensile direction, and meanwhile, the angular distribution becomes narrower. This is because the effective stiffness holds more weight in the orientational distribution and prefers θ = 0. If is small (e.g., on stiff substrates or under small-amplitude stretches), the free energy difference ΔU is small, and thus the angular distribution is quite smooth over all orientations (see Fig. 6), meaning that cells tend to align randomly. This prediction is in agreement with the experiments that cells align randomly on stiff substrates under ultralow-frequency cyclic stretches (12).
Figure 6.
Angular distributions of cells for different scaled effective stiffnesses under ultralow-frequency cyclic stretches. The cells concentrate at the stretching direction for a large value of , whereas they align randomly for a small value of . Here, we have taken .
At high loading frequencies, the cell does not have enough time to attain its optimal state in the polarized direction and, thus, it undergoes the same deformation extent as the applied displacement in the substrate. In this case, as demonstrated in the previous studies (16, 21), the cell only passively responds to the time average of varying stretches. The free energy difference at different orientations is (21)
| (21) |
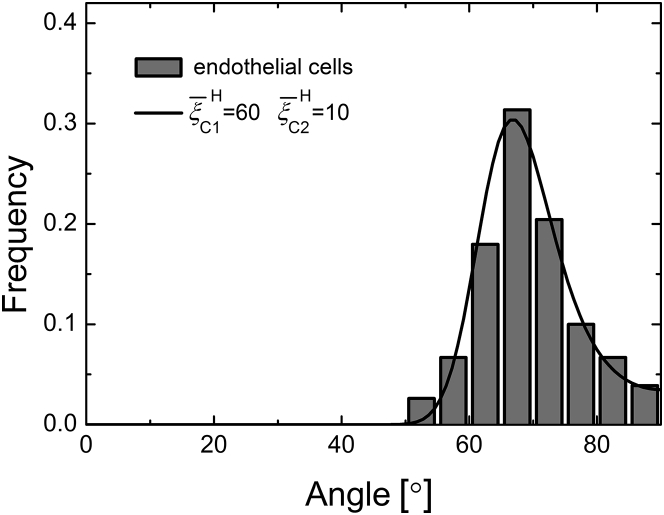
where and . Using Eqs. 14 and 21, our prediction of the cellular angular distribution agrees well with the experimental results of endothelial cells under high-frequency cyclic stretches (11), as plotted in Fig. 7. It should be emphasized that if the FA-substrate system is stiffer than the SF (e.g., ), the first term of ΔU in Eq. 21 is positive, stemming from the oscillating deformations of the polarized bars and the averaging of the external field over a period. The contributions from the longitudinal and lateral directions prefer the orientations and , respectively. At high frequencies (∼1 Hz), the most stable orientation is . When , we can reproduce all experimental data of Livne et al. (14) by this model. Under high-frequency cyclic stretches, the positive energy contribution in the longitudinal direction is different from the negative contributions under static and ultralow-frequency cyclic stretches, which can explain why the most stable orientations are different in these cases.
Figure 7.
Angular distribution of cells under high-frequency cyclic stretches. The theoretical prediction (red lines) fits well with the experimental data of endothelial cells (11). Here, we have taken ε0 = 0.1 and Δθ = 50, as in the experiments.
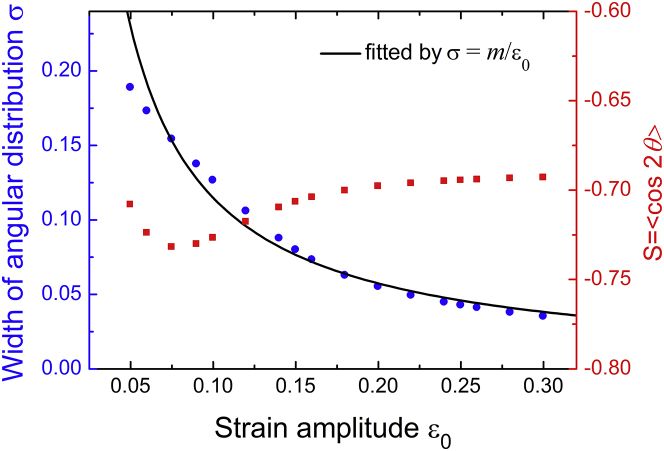
Using the parameters in Fig. 7, Fig. 8 plots the order parameter S and the width σ of the distribution with respect to the stretch amplitude ε0. It can be seen that the order parameter S varies slightly with the amplitude in the range of 0.05–0.3. The steady orientation in this case is independent of the stretch amplitude (see the expression of ), and the orientational distribution is approximately Gaussian, as shown in Fig. 7. Hence, the order parameter S changes slightly with ε0. In addition, Fig. 8 shows that the angular distribution becomes narrower with increasing stretch amplitude, because the free energy difference around the most stable orientation holds more weight in the orientational distribution. The data of the distribution width can be fitted very well by the function σ = m/ε0. This finding suggests that the distribution width scales inversely with the loading amplitude ε0 in the cyclic loading case, as well as in the static loading case.
Figure 8.
Order parameter S = 〈cos2θ 〉 (red square) and width σ of the cell angular distribution (blue round) as a function of the strain amplitude ε0 under high-frequency cyclic stretches. The width obtained by Eq. 16 can be fitted well by the function σ = m/ε0, indicating that the width scales inversely with the strain amplitude. Here, we used the parameters in Fig. 7. To see this figure in color, go online.
Comparison between static and cyclic stretches
We now compare the roles of static and cyclic stretches in cell alignment on compliant substrates. For a very soft substrate, the effective elasticity of FAs is dominated by the substrate elasticity, which may even be smaller than the elasticity K1 of SFs. Under cyclic stretches, the contribution to ΔU in Eq. 21 from the longitudinal direction is negative, similar to the case of static stretches. Then, the effective stiffness in the longitudinal direction plays a dominant role in determining the free energy difference, and the cells prefer aligning along the tensile direction, as in the case of static stretch. This result is consistent with experiments of Tondon and Kaunas (15), who observed that cells on soft gels align along the stretching direction even under high-frequency cyclic stretches.
On stiff substrates (ksub ≫ kfa), is determined by the elasticity of FAs. As discussed in the previous subsection, the energy contributions from the longitudinal direction are positive and negative, respectively, for high-frequency cyclic and static stretches, resulting in different stable orientations in the two cases. Moreover, the longitudinal effective stiffness under high-frequency cyclic stretches is also much larger than the longitudinal effective stiffness under static stretches, although their effective stiffnesses in the lateral direction are comparable. In other words, the free energy change will be significantly larger under high-frequency cyclic stretches than under static stretches. Hence, on a stiff substrate, high-frequency cyclic loadings can more effectively affect cell reorientation than static loadings, and the orientational distribution of cells is much narrower under high-frequency cyclic stretches than under static stretches. These predictions are consistent with the experimental observation that static stretches are not as influential as cyclic stretches at frequency of 1.2 Hz to induce cell reorientation (27). In addition, because the free energy change is smaller under static stretches, our finding also suggests that cells need more time to respond to static loadings.
Conclusions
By incorporating the molecular mechanisms of integrin binding dynamics, actin polymerization, and actin retrograde flow into a cellular tensegrity model, we have investigated the orientational distribution of polarized cells on compliant substrates under biaxial static and cyclic stretches. We show that the cell can actively adjust its mechanical state and generate different traction forces along its polarized direction on substrates of varying rigidities. Under static and ultralow-frequency cyclic stretches, cells prefer aligning along the stretching direction, because the stretched substrates can provide larger traction forces in this direction. The free energy difference becomes smaller on stiffer substrates, whereby the stretching also plays a lesser role. Furthermore, we find that on stiff substrates, the free energy difference under high-frequency cyclic loading is significantly larger than that under static loading, and thus cells exhibit much narrower distributions under high-frequency cyclic stretches. Interestingly, for both static and cyclic loadings, the width of the angular distribution of cells scales inversely with the stretching amplitude. Our present model thus integrates all known molecular mechanisms to explain a wide range of experimental results and to capture the essential features of cellular mechanosensing.
In addition, it should be mentioned that the dynamics (e.g., deadhesion and reformation) of FAs constitutes fundamental molecular mechanisms of cell reorientation. Chen et al. (19, 44) showed that cyclic loadings induce oscillating forces on catch bonds in FAs and can destabilize FAs by reducing the bond lifetime. The resulting sliding and relocation of FAs further cause the SFs to gradually rotate to the most stable orientation with minimal force oscillations. The reorientation process involves complex molecular kinetics of many proteins, which deserves further study.
Author Contributions
G.-K.X., X.-Q.F., and H.G. designed the research. G.-K.X. and X.-Q.F. performed the research. G.-K.X., X.-Q.F., and H.G. analyzed the data and wrote the article.
Acknowledgments
The authors thank Professor S.A. Safran at Weizmann Institute of Science for his valuable suggestions.
Supports from the National Natural Science Foundation of China (grant Nos. 11672227, 11402193, and 11620101001) and the Young Elite Scientist Sponsorship Program by CAST (No. 2015QNRC001) are acknowledged.
Editor: Markus Buehler.
References
- 1.Discher D.E., Janmey P., Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Huebsch N., Arany P.R., Mooney D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher D.T., Alliston T., Weaver V.M. A tense situation: forcing tumour progression. Nat. Rev. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamo L., Naveiras O., Daley G.Q. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huiskes R., Ruimerman R., Janssen J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405:704–706. doi: 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- 7.Behrndt M., Salbreux G., Heisenberg C.P. Forces driving epithelial spreading in zebrafish gastrulation. Science. 2012;338:257–260. doi: 10.1126/science.1224143. [DOI] [PubMed] [Google Scholar]
- 8.Boerckel J.D., Uhrig B.A., Guldberg R.E. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc. Natl. Acad. Sci. USA. 2011;108:E674–E680. doi: 10.1073/pnas.1107019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo C.M., Wang H.B., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa K., Sato N., Obinata T. Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp. Cell Res. 2001;268:104–114. doi: 10.1006/excr.2001.5270. [DOI] [PubMed] [Google Scholar]
- 11.Wang J.H.-C., Goldschmidt-Clermont P., Yin F.C.-P. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 2001;34:1563–1572. doi: 10.1016/s0021-9290(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 12.Jungbauer S., Gao H., Kemkemer R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys. J. 2008;95:3470–3478. doi: 10.1529/biophysj.107.128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faust U., Hampe N., Merkel R. Cyclic stress at mHz frequencies aligns fibroblasts in direction of zero strain. PLoS One. 2011;6:e28963. doi: 10.1371/journal.pone.0028963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livne A., Bouchbinder E., Geiger B. Cell reorientation under cyclic stretching. Nat. Commun. 2014;5:3938. doi: 10.1038/ncomms4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tondon A., Kaunas R. The direction of stretch-induced cell and stress fiber orientation depends on collagen matrix stress. PLoS One. 2014;9:e89592. doi: 10.1371/journal.pone.0089592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De R., Zemel A., Safran S.A. Dynamics of cell orientation. Nat. Phys. 2007;3:655–659. [Google Scholar]
- 17.De R., Safran S.A. Dynamical theory of active cellular response to external stress. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2008;78:031923. doi: 10.1103/PhysRevE.78.031923. [DOI] [PubMed] [Google Scholar]
- 18.Safran S.A., De R. Nonlinear dynamics of cell orientation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2009;80:060901. doi: 10.1103/PhysRevE.80.060901. [DOI] [PubMed] [Google Scholar]
- 19.Chen B., Chen X., Gao H. Dynamics of cellular reorientation on a substrate under biaxial cyclic stretches. Nano Lett. 2015;15:5525–5529. doi: 10.1021/acs.nanolett.5b02095. [DOI] [PubMed] [Google Scholar]
- 20.Kong D., Ji B., Dai L. Stability of adhesion clusters and cell reorientation under lateral cyclic tension. Biophys. J. 2008;95:4034–4044. doi: 10.1529/biophysj.108.131342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu G.K., Li B., Gao H. A tensegrity model of cell reorientation on cyclically stretched substrates. Biophys. J. 2016;111:1478–1486. doi: 10.1016/j.bpj.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz U.S., Safran S.A. Physics of adherent cells. Rev. Mod. Phys. 2013;85:1327–1381. [Google Scholar]
- 23.Liu C., Baek S., Chan C. Effect of static pre-stretch induced surface anisotropy on orientation of mesenchymal stem cells. Cell. Mol. Bioeng. 2014;7:106–121. doi: 10.1007/s12195-013-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steward R.L., Jr., Cheng C.-M., LeDuc P.R. Probing cell structure responses through a shear and stretching mechanical stimulation technique. Cell Biochem. Biophys. 2010;56:115–124. doi: 10.1007/s12013-009-9075-2. [DOI] [PubMed] [Google Scholar]
- 25.Eastwood M., Mudera V.C., Brown R.A. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil. Cytoskeleton. 1998;40:13–21. doi: 10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Collinsworth A.M., Torgan C.E., Truskey G.A. Orientation and length of mammalian skeletal myocytes in response to a unidirectional stretch. Cell Tissue Res. 2000;302:243–251. doi: 10.1007/s004410000224. [DOI] [PubMed] [Google Scholar]
- 27.Goli-Malekabadi Z., Tafazzoli-Shadpour M., Janmaleki M. Effect of uniaxial stretch on morphology and cytoskeleton of human mesenchymal stem cells: static vs. dynamic loading. Biomed. Tech. (Berl.) 2011;56:259–265. doi: 10.1515/BMT.2011.109. [DOI] [PubMed] [Google Scholar]
- 28.Raab M., Discher D.E. Matrix rigidity regulates microtubule network polarization in migration. Cytoskeleton (Hoboken) 2017;74:114–124. doi: 10.1002/cm.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemel A., Rehfeldt F., Safran S.A. Optimal matrix rigidity for stress fiber polarization in stem cells. Nat. Phys. 2010;6:468–473. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingber D.E., Wang N., Stamenović D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014;77:046603. doi: 10.1088/0034-4885/77/4/046603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Civelekoglu-Scholey G., Orr A.W., Mogilner A. Model of coupled transient changes of Rac, Rho, adhesions and stress fibers alignment in endothelial cells responding to shear stress. J. Theor. Biol. 2005;232:569–585. doi: 10.1016/j.jtbi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Giannone G., Dubin-Thaler B.J., Sheetz M.P. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 33.Jurado C., Haserick J.R., Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol. Biol. Cell. 2005;16:507–518. doi: 10.1091/mbc.E04-10-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C.H., Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14:763–771. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 35.Szewczyk D., Yamamoto T., Riveline D. Non-monotonic relationships between cell adhesion and protrusions. New J. Phys. 2013;15:035031. [Google Scholar]
- 36.Nisenholz N., Rajendran K., Zemel A. Active mechanics and dynamics of cell spreading on elastic substrates. Soft Matter. 2014;10:7234–7246. doi: 10.1039/c4sm00780h. [DOI] [PubMed] [Google Scholar]
- 37.Bangasser B.L., Shamsan G.A., Odde D.J. Shifting the optimal stiffness for cell migration. Nat. Commun. 2017;8:15313. doi: 10.1038/ncomms15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walcott S., Sun S.X. A mechanical model of actin stress fiber formation and substrate elasticity sensing in adherent cells. Proc. Natl. Acad. Sci. USA. 2010;107:7757–7762. doi: 10.1073/pnas.0912739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srinivasan M., Walcott S. Binding site models of friction due to the formation and rupture of bonds: state-function formalism, force-velocity relations, response to slip velocity transients, and slip stability. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2009;80:046124. doi: 10.1103/PhysRevE.80.046124. [DOI] [PubMed] [Google Scholar]
- 40.Saez A., Buguin A., Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys. J. 2005;89:L52–L54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balaban N.Q., Schwarz U.S., Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 42.Yip A.K., Iwasaki K., Sawada Y. Cellular response to substrate rigidity is governed by either stress or strain. Biophys. J. 2013;104:19–29. doi: 10.1016/j.bpj.2012.11.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trichet L., Le Digabel J., Ladoux B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc. Natl. Acad. Sci. USA. 2012;109:6933–6938. doi: 10.1073/pnas.1117810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen B., Kemkemer R., Gao H. Cyclic stretch induces cell reorientation on substrates by destabilizing catch bonds in focal adhesions. PLoS One. 2012;7:e48346. doi: 10.1371/journal.pone.0048346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S., Maxwell I.Z., Ingber D.E. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Ser. B. 1938;126:136–195. [Google Scholar]
- 47.Shemesh T., Geiger B., Kozlov M.M. Focal adhesions as mechanosensors: a physical mechanism. Proc. Natl. Acad. Sci. USA. 2005;102:12383–12388. doi: 10.1073/pnas.0500254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardel M.L., Shin J.H., Weitz D.A. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304:1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 49.Vader D., Kabla A., Mahadevan L. Strain-induced alignment in collagen gels. PLoS One. 2009;4:e5902. doi: 10.1371/journal.pone.0005902. [DOI] [PMC free article] [PubMed] [Google Scholar]