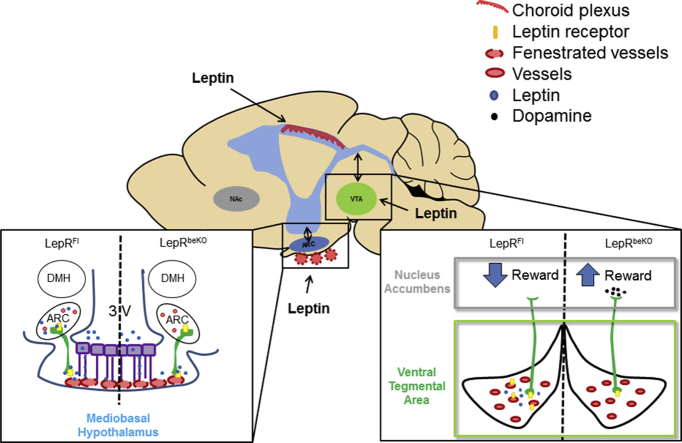
Abstract
Objective
Leptin is a key hormone in the control of appetite and body weight. Predominantly produced by white adipose tissue, it acts on the brain to inhibit homeostatic feeding and food reward. Leptin has free access to circumventricular organs, such as the median eminence, but entry into other brain centers is restricted by the blood–brain and blood–CSF barriers. So far, it is unknown for which of its central effects leptin has to penetrate brain barriers. In addition, the mechanisms mediating the transport across barriers are unclear although high expression in brain barriers suggests an important role of the leptin receptor (LepR).
Methods
We selectively deleted LepR in brain endothelial and epithelial cells of mice (LepRbeKO). The expression of LepR in fenestrated vessels of the periphery and the median eminence as well as in tanycytes was not affected.
Results
Perfusion studies showed that leptin uptake by the brain depended on LepR in brain barriers. When being fed with a rewarding high-fat diet LepRbeKO mice gained more body weight than controls. The aggravated obesity of LepRbeKO mice was due to hyperphagia and a higher sensitivity to food reward.
Conclusions
The LepR-mediated transport of leptin across brain barriers in endothelial cells lining microvessels and in epithelial cells of the choroid plexus controls food reward but is apparently not involved in homeostatic control of feeding.
Keywords: Leptin, Reward, Blood–brain barrier, LepR, Obesity, Endothelial cells
Abbreviations: ARC, arcuate nucleus; BBB, blood–brain barrier; BC, bottle choice test; BSA, bovine serum albumin; CPP, conditioned place preference; CSF, cerebrospinal fluid; DAPI, 4′,6-diamidino-2-phenylindole; HFD, high-fat diet; i.p., intraperitoneal; LepR, leptin receptor; NCD, normal chow diet; PBS, phosphate buffered saline; PFA, paraformaldehyde; qPCR, quantitative polymerase chain reaction; VTA, ventral tegmental area
Graphical abstract
Highlights
-
•
LepR mediates the transport of leptin across brain barriers.
-
•
The LepR-mediated transport of leptin across brain barriers modulates food reward.
-
•
The homeostatic feeding control does not depend on LepR-mediated leptin transport.
1. Introduction
Leptin is a key hormone controlling body weight [1]. With repleted and filled fat depots, adipocytes release leptin into the blood stream. Subsequently, leptin inhibits food intake and stimulates energy expenditure. The important functions of leptin and the leptin receptor (LepR) in body weight regulation are illustrated by the excessive obesity that occurs in mice and humans with inactivating pathogenic variants in the respective genes. For the main metabolic effects of leptin, LepR in neurons is both required and sufficient [2], [3]. LepR in the arcuate nucleus (ARC) and in other nuclei of the mediobasal hypothalamus strongly modulates the homeostatic control of food intake and energy expenditure [4], [5]. In addition, LepR regulates food reward in other brain areas, such as the lateral hypothalamus and the ventral tegmental area (VTA) [6], [7].
Overall, brain access is essential for leptin to exert its physiological actions. This is supported by data showing that brain permeability of leptin is impaired in obesity. Although leptin levels are elevated in plasma of obese subjects, the ratio of CSF to plasma concentrations is decreased in obesity [8], [9]. In preclinical obesity models, leptin only reduces food intake when administered intrathecally but not peripherally [10], [11], [12]. These data suggest that reduced brain permeability may contribute to leptin resistance in obesity and may further elevate body weight.
Due to its size (16-kDa), leptin is not expected to passively diffuse through tight brain barriers. However, it has direct access to circumventricular organs including LepR-expressing neurons in the mediobasal hypothalamus that are not shielded by the blood–brain barrier (BBB) [13]. In addition, there is uptake into the choroid plexus and brain parenchyma [14], [15]. Influx of leptin into the brain is partially saturable, indicating that membrane proteins facilitate the uptake of leptin [14]. The example of other peptides similar to leptin in structure and size that cross the BBB via receptor-mediated transcytosis [16] points to a role of LepR in leptin uptake by the brain. Indeed, LepR is required for the transport of leptin by tanycytes in the mediobasal hypothalamus [17]. Moreover, short isoforms of LepR that lack intracellular signaling domains, mainly LepRa and LepRc, are highly expressed in brain barriers, such as the choroid plexus and brain endothelial cells [18], [19], [20], [21], [22]. In mice lacking all LepR isoforms, leptin uptake by the brain has been reported to be diminished supporting a role of LepR in leptin transport across the BBB [22], although another study using a rat strain deficient of all LepR isoforms found no significant effect of LepR loss on leptin uptake by the brain [23].
Until now, it is unclear whether leptin has to penetrate brain barriers to exert its central effects. Furthermore, current understanding of leptin access to the brain and its role in physiology is potentially confounded by leptin effects on other functional systems. Leptin modulates immunity, the autonomic nervous system, metabolism, and several endocrine axes [24], [25], all of which have major impact on BBB function. To directly assess the role of LepR in leptin uptake by the brain and to elucidate LepR function in CNS barriers for body weight regulation without interfering factors from the periphery, we aimed to delete all LepR isoforms selectively in brain endothelial cells and the choroid plexus. This goal could be achieved with a mouse line, in which exon 1 of LepR is flanked by loxP sites affecting all isoforms [3] and an inducible Cre driver line that mediates recombination in brain endothelial cells and epithelial cells of the plexus [26], [27]. When deleting LepR in brain endothelial and epithelial cells, the uptake of leptin by the brain was reduced and body weight was increased on a high-fat diet (HFD) but not on normal chow (NCD). Further experiments revealed that LepR in brain barriers inhibits food reward but seems to be dispensable for the homeostatic function of leptin mediated by the mediobasal hypothalamus.
2. Material and methods
2.1. Mice
All mice were housed in individually ventilated cages under a 12-h light/12-h dark cycle at 23 °C, with free access to water and food. Mice with an inducible deletion of LepR in brain endothelial and epithelial cells (LepRbeKO) were generated by crossing LepRFl (JAX stock #008327) [3] and Slco1c1-CreERT2 [26] animals. The offspring generated by the first breeding (Slco1c1-CreERT2; LepRFl/+) were crossed back with homozygous LepRFl to obtain Slco1c1-CreERT2; LepRFl and LepRFl animals. To induce recombination, tamoxifen was administered to mice (i.p., 1 mg, Sigma–Aldrich, T5648, dissolved in 90% miglyol 812, 10% ethanol) every 12 h for 5 consecutive days at an age of 5–6 weeks. Cre-negative controls were also treated with tamoxifen. Slco1c1-CreERT2; LepRFl mice that received tamoxifen are referred to as LepRbeKO. Male littermate mice were used for all experiments. Mice with the inducible expression of HA-UPRT in brain endothelial cells were generated by crossing HA-UPRTFl (JAX stock #021469) [28] with Slco1c1-CreERT2 animals. Mice with the expression of Zs-Green were generated by crossing LepR-Cre (JAX stock #008320) [29] with Zs-Green animals (JAX stock #007906) [30].
2.2. Immunohistochemistry
The mice were anesthetized and transcardially perfused with ice-cold Ringer's solution followed by ice-cold paraformaldehyde (PFA, 4% in phosphate-buffered saline, PBS). Brains were post-fixed for 24 h in PFA (4%) and then cryoprotected by overnight immersion in a 30% sucrose solution. Brains were frozen in 2-methylbutane on dry ice. Coronal cryosections (20 μm-thick) were incubated in PBS containing 0.3% triton and 3% bovine serum albumin (BSA) for 1 h to block unspecific binding. Then, sections were incubated with the following primary antibodies diluted in blocking solution overnight at 4 °C: rat anti-CD31 (BD-Pharmingen, 553370, 1:400), mouse anti-leptin receptor (Abcam, ab43406, 1:200), chicken anti-vimentin (Thermo Fischer, PA1-16759, 1:400), rabbit anti-HA (Santa Cruz, sc-805, 1:300), goat anti-collagen IV (MERCK Millipore, AB769, 1:200), and rat anti-PV1 (BD-Pharmingen, 550563, 1:400). On the next day, sections were washed with PBS and subsequently incubated for 3 h at room temperature with the following fluorescently labeled secondary antibodies: donkey anti-rabbit Alexa Fluor 555 (Invitrogen, A-21429, 1:200), donkey anti-rat Alexa Fluor 488 (Invitrogen, A-21208, 1:600), donkey anti-mouse Cy3 (Jackson/Dianova, 715-165-151, 1:600), goat anti chicken cy5 (Abcam, ab97147, 1:500). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, 0.2 μg/ml) in PBS for 5 min at room temperature. Sections were mounted with aqueous mounting medium (Mowiol 4-88, Carl Roth, 0713.2).
For pSTAT3 staining the sections were first incubated in methanol for 20 min at room temperature and then in PBS containing glycerol (0.3%) for 10 min. After permeabilization with sodium dodecyl sulfate (0.03% in PBS) for 30 min, sections were incubated with rabbit anti-pSTAT3 (Y705) (Cell Signaling, 9145S, 1:200 diluted in 3% BSA) for 48 h at 4 °C. Goat anti-rabbit Alexa Fluor 555 (Invitrogen, A-21429, 1:200) secondary antibody was then added for 3 h at room temperature. Cell nuclei were stained with DAPI and the sections were mounted with aqueous mounting medium (Mowiol 4-88, Carl Roth).
2.3. Primary brain endothelial cells and qPCR
For preparation of primary mouse brain endothelial cells from LepRFl and LepRbeKO mice, we used a protocol that has been reported previously [31]. Freshly isolated vessel fragments containing pericytes and endothelial cells were either directly lysed for RNA purification, reverse transcription, and qPCR or they were plated for further purification of brain endothelial cells. After 2 weeks in culture, more than 95% of cells were endothelial cells and less than 5% were pericytes. The culture did not contain astrocytes, microglia, or neurons. RNA was isolated from primary brain endothelial cells using the NucleoSpin kit (Macherey–Nagel), according to the manufacturer's protocol, and transcribed with avian myeloblastosis virus reverse transcription and random hexamer primers (Cloned AMV, First-stand synthesis kit, Invitrogen).
The following primers were used for qPCR: leptin receptor (exon 9) forward 5′-GAC TTG CAG ATG GTC ACC CA-3′, leptin receptor (exon 10) reverse 5′-TGG GCT CAG ACG TAG GAT GA-3′, PCR product 122 bp; beta actin forward (exon 5) 5′-ATG GAA TCC TGT GGC ATC CAT-3′, reverse (exon 5) 5′-TTC TGC ATC CTG TCA GCA ATG-3′, PCR product 140 bp; GADPH forward 5′-ATG TGT CCG TCG TGG ATC TGA-3′, reverse 5′-TGA AGT CGC AGG AGA CAA CCT-3′, PCR product 145 bp. qPCR was performed with the Platinum SYBR Green qPCR superMix (Invitrogen) according to the following protocol: 2 min at 50 °C, 2 min at 95 °C, 15 s at 95 °C, and 1 min at a 60 °C (40 cycles). The results were normalized for Gapdh and β-actin using the ΔΔCt method.
2.4. 125I labeling of leptin
A modified version of the established chloramine-T method [32] employing 125I-NaI was used to bind the radioactive iodine at random tyrosine side-chains of the protein. In brief, 20 μl of the leptin (6.17 μM) was mixed with 50 μl of 0.25 M phosphate buffer pH 7.5. A solution containing 1–5 MBq 125I-NaI in 10 μM NaOH was added and the labeling reaction was started by addition of an aqueous chloramine-T solution (10 mM, 5 μl). After 30 s, the labeling reaction was quenched by adding a saturated aqueous solution of methionine (20 μl). The labeling reaction mixture was passed over a PBS equilibrated buffer exchange column (NAP-5, GE Healthcare) and 200 ml fractions were collected and analyzed for γ-radiation using a γ-counter (LB 2111, Berthold Technologies, Bad Wildbad, Germany). Fraction 2 &3 contained the radioactively labeled protein as determined by thin layer chromatography using ITLC-SG and 0.9% NaCl as the eluent. For in vivo experiments, the solution was used as was.
2.5. 125I-leptin uptake
Mice were anesthetized with urethane (4.0 g/kg i.p., Sigma–Aldrich, U2857). The heart was exposed and the descending part of the aorta was clamped, leaving open only the branches to the upper part of the body. A cannula was inserted in the left ventricle and the brain was transcardially perfused with artificial plasma solution (123 mM NaCl, 4 mM KCl, 2.5 mM CaCl2, 1.8 mM MgCl2, 25 mM NaHCO3, 1.2 mM KH2PO4, 5.5 mM d-glucose, 6% dextran (MW 70,000), 20% washed sheep red blood cells) [33] containing 0.55 nM 125I-leptin (21.6 μCi/μg) for 30 min at a perfusion rate of 1 ml/min, as reported previously [34]. The vascular space was washed out with the same solution without the radiolabeled peptide for 10 min. CSF was sampled by puncturing the cisterna magna as reported previously [35]. Mice were decapitated, and the mediobasal hypothalamus, VTA, and cortex were dissected. After weighing samples 125I activity was measured with a γ-counter (Wallac Wizard2 2470 automatic γ-counter, Perkin Elmer). 125I-leptin uptake was expressed as (radioactivity in brain samples/g sample weight)/(radioactivity in artificial plasma solution/μl) as reported previously [36].
2.6. Feeding studies
Mice were fed with either NCD (Ssniff EF, D12450B) or HFD (Ssniff EF, D12492 (I)) ad libitum. Energy intake was assessed by indirect calorimetry (see below) or by weighing food pellets in the cage. The metabolizeable energy of the HFD (21.6 × 106 J/kg) consisted of fat (60%), proteins (19%), and carbohydrates (21%), while the NCD (15.1 × 106 J/kg) contained fat (13%), proteins (20%), and carbohydrates (67%). Both diets were supplied as 10-mm pellets, which were half discarded and half refilled every 2 days.
2.7. Body composition
The body composition of LepRFl and LepRbeKO mice was measured by magnetic resonance spectroscopy (Bruker Minispec LF110) after 40 and 82 days on HFD or NCD.
2.8. Leptin treatment
Food intake and body weight were measured twice a day (17.30 pm, 30 min before “lights-off”; 8.00 am, 2 h after “lights-on”). After assessing baseline food intake and body weight for 3 days, recombinant mouse leptin (2 mg/kg, PeproTech 450-31) or vehicle were intraperitoneally injected twice a day at the same time points for 5 days. On the last day, mice were fasted overnight and sacrificed 45 min after the injection of leptin (3 mg/kg) or vehicle.
2.9. Indirect calorimetry
Indirect calorimetry was performed with a ventilated, open-circuit system (PhenoMaster System, TSE). The mice were kept in ventilated chambers for 1 week under a 12-h light–dark cycle and at a constant temperature of 23 °C. After allowing animals to adapt to the setup for 4 days, the following parameters were analyzed for 3 consecutive days. Locomotion was measured by a heat sensor. Food and water intake was measured every 10 s. The respiratory exchange ratio was estimated every 20 min as ratio of CO2 produced (ml/h) to O2 consumed (ml/h). Energy expenditure (MJ) was calculated as following: (0.0165 + 0.00463 × RER) × O2 consumed. The average daily energy expenditure over three days was plotted against the lean body mass of the same mouse.
2.10. Conditioned place preference test
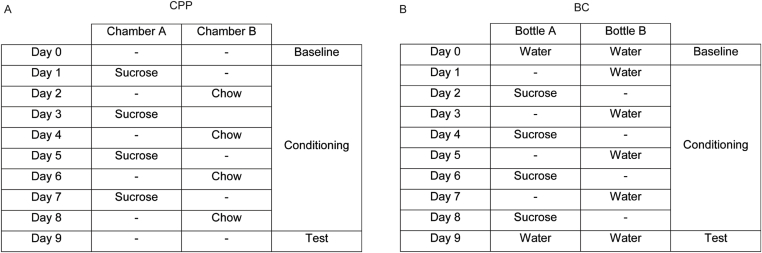
Conditioned place preference (CPP) was tested in a three-chamber apparatus (TSE) consisting of a small middle chamber connected to two larger side chambers that differed in floor and wall texture (Figure 5A). The procedure took 14 days (overview in Supplementary Figure 1A). On day 1 and 2, mice received a free supply of sucrose pellets (65%, Ssniff) in their home cages. Subsequently, mice were food restricted to 60% of individual food intake at baseline from day 3 until the end of the experiment. On day 3, mice were placed in the middle chamber for 10 min to get familiar with the apparatus. The baseline preference for the two side chambers was assessed on day 4, wherein mice were allowed to freely move in the three chambers for 18 min. During the conditioning phase (day 5–13) mice were exposed to either sucrose pellets in the chamber that they preferred less during baseline testing or to normal chow in the preferred chamber. Sucrose pellets were provided on odd days and normal chow on even days for 18 min each. The preference value was evaluated on day 14, while mice had free access to all three chambers. Preference was calculated as: .
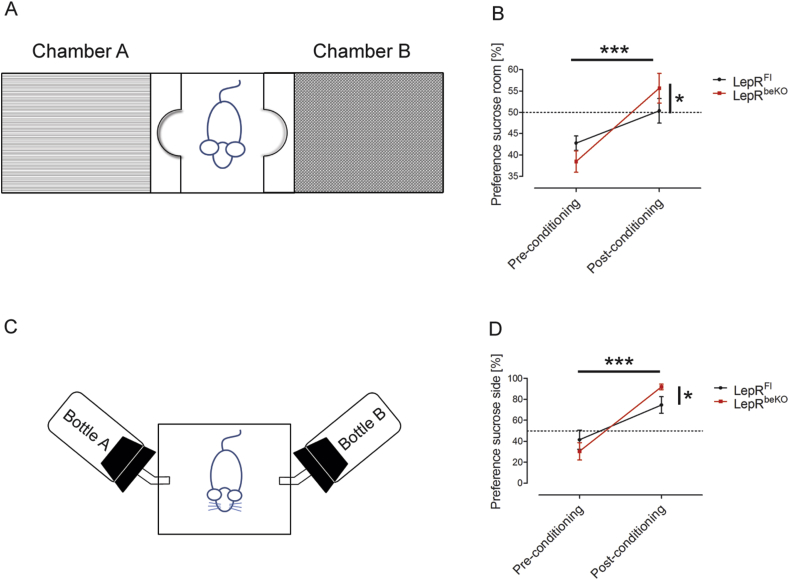
Figure 5.
Mice deficient of LepR in brain barriers (LepRbeKO) are more sensitive to food reward. (A) Schematic illustration of the conditioned place preference setup. The chamber in which mice spent less time at baseline was paired with sucrose pellets. (B) After a conditioning phase of 8 days, mice spent more time in the chamber conditioned to sucrose. Conditioning was more effective in LepRbeKO mice than in LepRFl controls. Values represent means ± SEM. Repeated two-way ANOVA, for interaction F(1, 15) = 6.182, p = 0.0252; for conditioning F(1, 15) = 41.05. *p < 0.05, ***p < 0.0001 (Bonferroni posttest n = 7–10 mice/group). (C) Schematic illustration of the conditioned bottle choice test, wherein sucrose solution was offered in the bottle less preferred by each mouse at baseline. On alternating days, water was provided in the other bottle. (D) After 8 days of conditioning, LepRbeKO mice showed a higher preference for the bottle containing sucrose during the conditioning than LepRFl control animals. Repeated two-way ANOVA, for interaction F(1, 11) = 6.263, p = 0.0294, for conditioning F(1, 11) = 53.78, p < 0.0001. *p < 0.05, ***p < 0.0001 (Bonferroni posttest n = 6–7 mice/group).
2.11. Conditioned two-bottle choice (BC)
During the BC test, the animals received a fixed amount of food and water every day. The test was performed in an operant chamber (Med Associates Inc.) which had two slots for water bottles on opposing sides of the cage (Figure 5C). Each bottle was equipped with a lickometer to detect the number of licks. The baseline preferred bottle position was tested for each mouse by providing a choice of two bottles, both containing water, for 10 min. During the following 8 days (overview in Supplementary Figure 1B), mice were conditioned by providing free access to a bottle containing either water on the baseline preferred side or 0.8 M sucrose on the opposing side for 30 min per day. Sides and content were alternated each day. Mice were tested for 10 min by providing two bottles both containing water [37]. The preference value was calculated as:
3. Results
3.1. LepR is involved in leptin transport into the brain
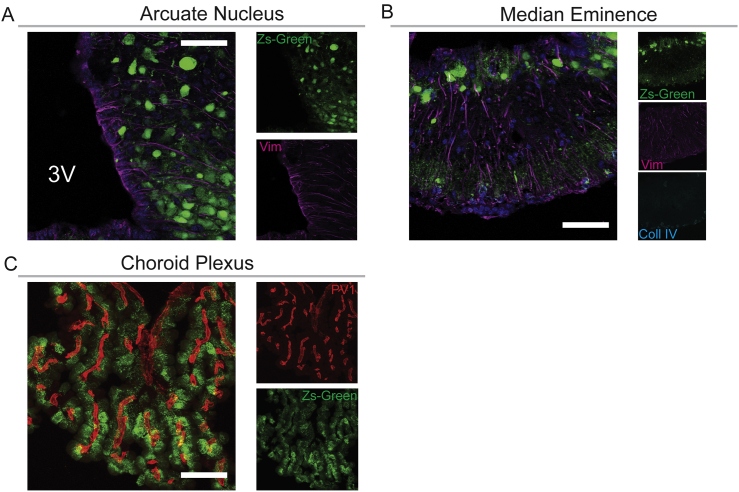
Using LepR-cre;Zs-Green mice in which Zs-Green reflects expression of LepR, we confirmed previous reports that LepR is expressed in crucial access points to the brain [38], including brain capillaries (Figure 1A), cells in the mediobasal hypothalamus (Supplementary Figure 2A and B), and epithelial cells of the choroid plexus (Supplementary Figure 2C).
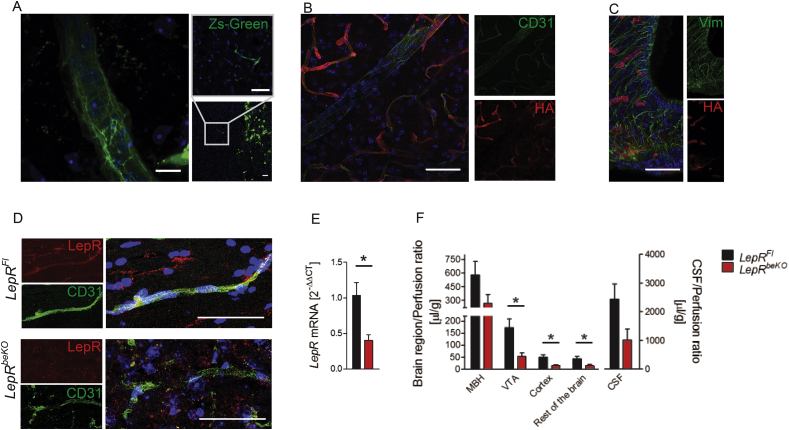
Figure 1.
Deletion of the leptin receptor (LepR) in brain endothelial and epithelial cells reduces leptin uptake by the brain. (A) Zs-Green reflecting expression of LepR was found in brain vessels (left and right panel) and in neurons of the VTA (right lower panel) of LepR-cre;Zs-Green mice. Nuclei are stained by DAPI (blue). Representative images are shown. Scale bar, 10 μm (left panel), 50 μm (right panels). (B and C) We used the reporter line HA-UPRTFl to investigate in which cell types of the median eminence the Slco1c1-CreERT2 allele drives recombination. Immunofluorescent staining showed that HA-UPRT (red) was expressed in CD31-positive endothelial cells (green, B) of the cortex and mediobasal hypothalamus (C). There were no signs of recombination in vimentin-positive tanycytes (green, C). Scale bar, 50 μm. (D) Co-stainings of LepR (red) and CD31 (green) indicated that LepR was reduced in brain capillaries of LeprbeKO mice in comparison to LepRFl controls. Capillaries in the cortex are depicted. (E) Relative LepR mRNA expression in primary brain endothelial cells. Values represent means ± SEM. Unpaired t test, *p < 0.05 (n = 3 mice/group). (F) Leptin uptake in the mediobasal hypothalamus (MBH), ventral tegmental area (VTA), cortex, cerebrospinal fluid (CSF), and the rest of the brain was reduced in LepRbeKO mice compared to LepRFl animals. Mice were perfused with 125I-leptin for 30 min followed by a washout phase of 10 min. Values represent means ± SEM. Two-way ANOVA, for genotype F(41, 95) = 6.122, p < 0.05. *p < 0.05; CSF, p = 0.07 (unpaired t-test, n = 8–13 mice/group).
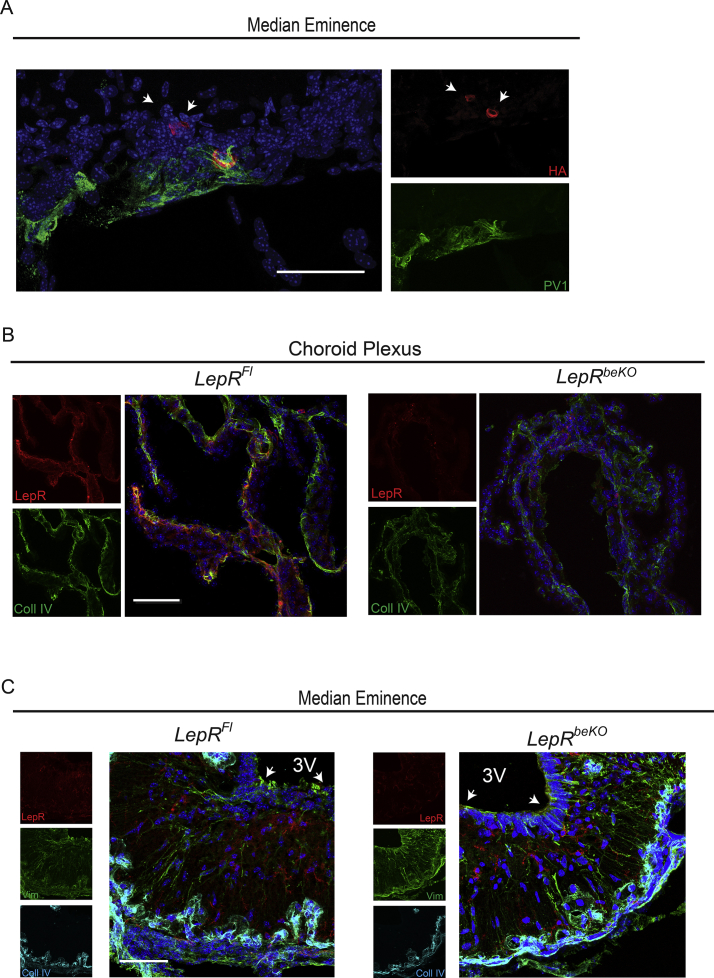
To test the function of LepR at these sites, we aimed to delete LepR in a cell type-specific manner. We used the Slco1c1-CreERT2 Cre driver line, which allows for cell type-specific recombination in brain endothelial cells and in epithelial cells of the choroid plexus but does not cause recombination in endothelial cells of other tissues [26]. Because recombination in the mediobasal hypothalamus of Slco1c1-CreERT2 mice has not been studied so far, we crossed Slco1c1-CreERT2 with HA-UPRTFl mice as a reporter line. Staining of HA-UPRT confirmed recombination in brain endothelial cells (Figure 1B). As reported previously recombination seemed to affect mainly capillaries [39]. There was no co-staining with PV1, a marker of fenestrated vessels in the median eminence (Supplementary Figure 3A) demonstrating that recombination is specific for non-fenestrated vessels of the BBB. Although expression of Slco1c1 has been reported in tanycytes [40], we found no HA-UPRT in these cells (Figure 1C). In accordance with the recombination in the choroid plexus reported previously [26], HA-UPRT was detected in epithelial cells of the choroid plexus. In summary, the Slco1c1-CreERT2 line targets the choroid plexus and endothelial cells of the BBB, two sites where leptin enters the brain, but it spares the fenestrated capillaries and tanycytes of the mediobasal hypothalamus.
We crossed Slco1c1-CreERT2 mice with a LepRFl line, in which exon 1 is flanked by loxP sites [3]. All LepR isoforms include exon 1 and are targeted by this strategy. We assessed LepR expression three weeks after starting the tamoxifen treatment. Immunostainings showed clear reduction in brain endothelial cells (Figure 1D) and epithelial cells of the choroid plexus (Supplementary Figure 3B). In accordance with results obtained in the HA-UPRTFl reporter mouse line, there was no change in the LepR staining in tanycytes (Supplementary Figure 3C). To confirm the deletion of LepR, we cultured primary brain endothelial cells of LepRFl and LepRbeKO mice and measured LepR mRNA expression. For quantification, we used qPCR with primers that detect all splice variants. LepRbeKO endothelial cells expressed significantly less LepR mRNA than LepRFl control cells (Figure 1E).
To investigate whether LepR is involved in leptin transport, we performed in situ brain perfusion with a physiological concentration of 125I-labeled leptin (0.55 nM). This technique allows for the assessment of leptin uptake in vivo without interference of confounding factors present in blood [14]. The results showed a significant reduction in several brain areas of LepRbeKO mice (Figure 1F). In the VTA and cortex, 125I-leptin uptake was more severely impaired (about 60%) than in the mediobasal hypothalamus (about 40%). Thus, LepR in brain endothelial and epithelial cells contributes to leptin transport into the CNS.
3.2. LepR in brain barriers is involved in regulation of body weight and energy intake
After having demonstrated that LepR is involved in leptin transport into the brain, we aimed at characterizing the physiological function of the LepR-mediated leptin transport. For this purpose, we monitored body weight of LepRFl and LepRbeKO mice for 91 days. Body weight gain and energy intake did not significantly differ between the two genotypes (Figure 2A and B). However, elevating leptin levels by administering exogenous leptin (2 mg/kg, i.p., 2 times per day for 5 days) only reduced energy intake in LepRFl controls but not in LepRbeKO mice (Figure 2C). Despite this difference in leptin sensitivity, the number of pSTAT3-positive cells in the ARC was not altered between the genotypes 45 min after the last leptin injection (Figure 2D), suggesting that the difference in energy intake was not driven by leptin-sensitive cells in the ARC.
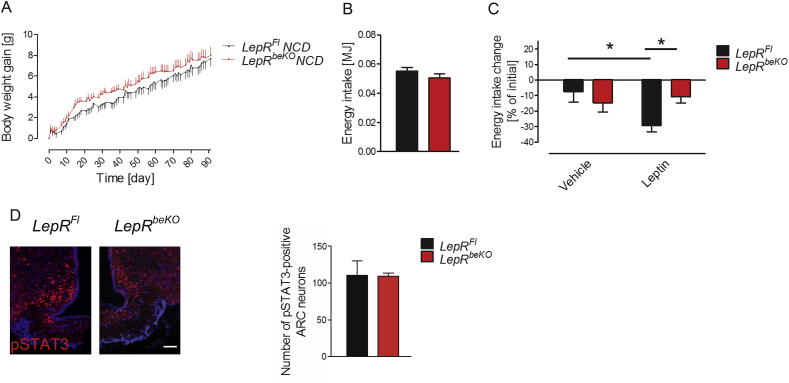
Figure 2.
Mice deficient of LepR in brain barriers (LepRbeKO) do not differ in body weight gain or energy intake but show a diminished response to exogenous leptin. (A) Body weight gain did not differ between LepRFl and LepRbeKO mice on NCD. Values represent means ± SEM (n = 14–15 mice/group). (B) There was also no difference in energy intake of LepRFl mice and LepRbeKO controls after 92 days on NCD (n = 14–15 mice/group). (C) Leptin administration (2 mg/kg, i.p, for 5 days) reduced energy intake in LepRFl animals but not in LepRbeKO mice fed with NCD. Two-way ANOVA, for interaction F(1, 24) = 6.364, p = 0.0187. *p < 0.05 (Bonferroni posttest, n = 6–9 mice/group). (D) Representative image of pSTAT3-positive neurons in the ARC of LepRFl and LepRbeKO mice 45 min after administration of leptin (3 mg/kg, i.p.) Scale bar, 50 μm. The graph depicts mean cell numbers per section ± SEM (n = 3–5).
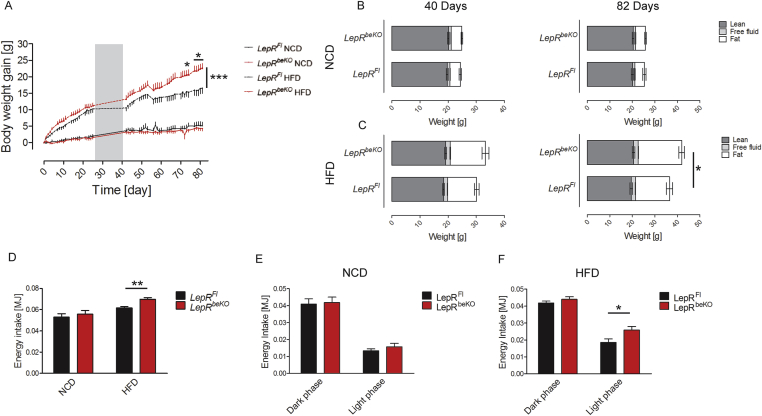
To elevate endogenous leptin levels, we fed mice with HFD or with NCD for comparison. While the two genotypes again did not differ in body weight on NCD, on HFD LepRbeKO animals gained more body weight than LepRFl mice (Figure 3A). Magnetic resonance spectroscopy revealed no difference in body composition of mice fed with NCD (Figure 3B), whereas on HFD LepRbeKO mice showed significantly larger fat depots than LepRFl controls at 82 days (Figure 3C). The energy intake was higher in LepRbeKO mice than in LepRFl controls at 35 days on HFD (Figure 3D), when body weight was still not markedly increased, suggesting altered food ingestion as a cause for the successive increase in body weight. Notably, the diurnal pattern of energy intake was similar between LepRFl controls and LepRbeKO mice on NCD (Figure 3E), but on HFD LepRbeKO mice exhibited a disturbed rhythm with an increased energy intake mainly during the light (resting) phase (Figure 3F).
Figure 3.
Mice deficient of LepR in brain barriers (LepRbeKO) gained more body weight and exhibited an increased energy intake on high-fat diet (HFD) but not on normal control diet (NCD). (A) Body weight gain of LepRbeKO mice and LepRFl controls on HFD and NCD. The gray area represents the time of indirect calorimetry (data in Figure 4A–D). Values are means ± SEM. Two-way ANOVA for interaction of time and genotype, F(49, 686) = 2.986, p < 0.0001. ***p < 0.001, *p < 0.05 (Bonferroni posttest, n = 7–9 mice/group). (B, C) Body composition of LepRFl and LepRbeKO animals after 40 and 82 days on NCD or HFD (n = 7–9 mice/group). *p < 0.05 (unpaired t-test, n = 7–9 mice/group). (D) Energy intake of LepRFl and LepRbeKO mice after 40 days on NCD or HFD. Two-way ANOVA for diet, F(1, 28) = 18.17, p = 0.0002; for genotype, F(1, 28) = 4.205, p = 0.0498. **p < 0.01 (unpaired t-test, n = 7–9 mice/group). (E, F) Diurnal variation of energy intake of LepRFl and LepRbeKO mice on NCD or HFD after 40 days on NCD (E) or HFD (F). Two-way ANOVA for genotype: NCD, not significant; HFD, F(1, 13) = 13.90, p = 0.0025.*p < 0.05. (Bonferroni posttest, n = 7–9 mice/group).
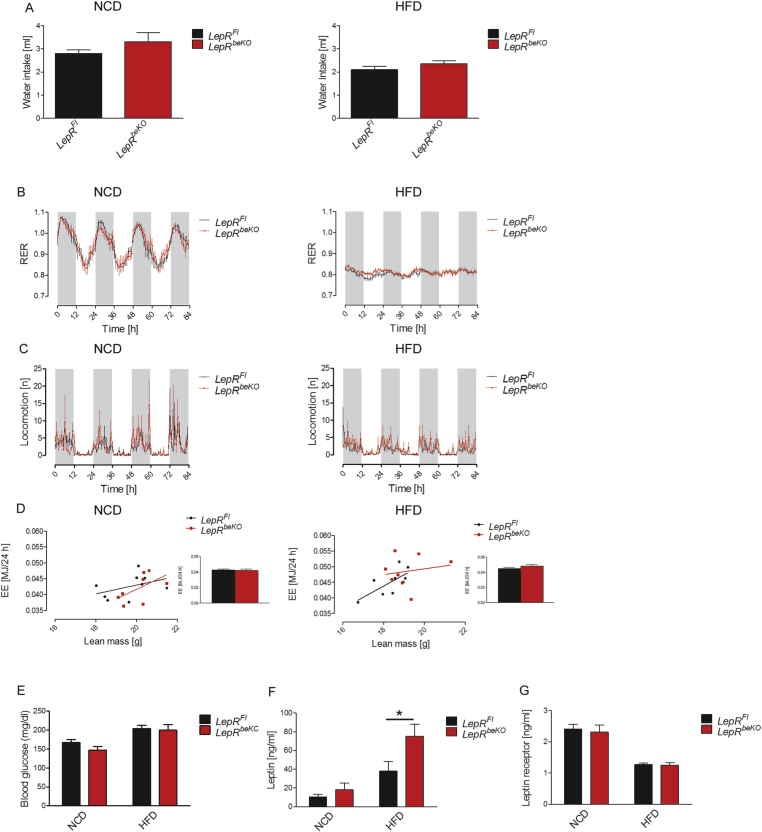
For further characterization of LepR functions in brain barriers, we assessed additional metabolic parameters using indirect calorimetry. As expected from the data on energy intake and body weight, LepRFl and LepRbeKO mice did not differ in water intake, respiratory exchange ratio, locomotion, and energy expenditure when fed NCD (Figure 4A–D). Also on HFD, the two genotypes were similar in these parameters (Figure 4A–D). In addition, blood glucose levels were similar between the genotypes on both diets (Figure 4E). After 82 days of HFD, leptin plasma levels were increased in LepRbeKO mice compared to LepRFL controls reflecting the larger fat depots in mice deficient of LepR in brain barriers (Figure 3, Figure 4C). Elevated plasma leptin levels are associated with reduced release of soluble LepR from tissues [41]. In line with this concept, we found that plasma concentrations of soluble LepR were decreased in HFD-induced obese mice (Figure 4G), although LepR mRNA expression in cerebral vessel fragments was not altered by the diet (NCD, 1.0 ± 0.2 2−ΔΔCT, n = 6; HFD, 0.9 ± 0.4 2−ΔΔCT, n = 5) as reported previously [22]. Interestingly, plasma concentrations of soluble LepR did not differ between genotypes (Figure 3G) in contrast to the elevated soluble LepR levels in a previously reported mouse model of pan-endothelial LepRb deficiency [42]. In summary, LepR in brain endothelial and epithelial cells was required to control food intake under HFD conditions.
Figure 4.
The increased body weight of LepRbeKOmice on HFD is not driven by changes in energy expenditure or locomotion. (A) Water intake did not differ between LepRFl and LepRbeKO mice on NCD or HFD. (B) The respiratory exchange ratio (RER) followed a similar diurnal rhythm in LepRFl and LepRbeKO mice that were fed with NCD (left panel) or HFD (right panel). The gray areas represent the dark phases and the white the light phases. (C) Locomotor activity did not differ between LepRFl and LepRbeKO mice on NCD (left panel) or HFD (right panel). (D) A similar relationship was observed between daily energy expenditure (EE) and lean body mass in LepRFl and LepRbeKO mice fed with NCD (left panel) or HFD (right panel). The bar graphs depict the average daily energy expenditure (n = 7–8). (E) No difference in the fasting plasma glucose concentration of LepRFl and LepRbeKO mice on NCD or HFD (n = 7–9). (F, G) Plasma concentrations of leptin were elevated in LepRbeKO on HFD while levels of the soluble LepR did not significantly differ between genotypes. Leptin plasma concentrations, two-way ANOVA for diet, F(1, 24) = 6.593, p = 0.0169, for genotype F(1, 24) = 24.04, P < 0.0001. *p value < 0.05 (Bonferroni posttest, n = 6–8 mice/group). Leptin receptor concentrations, two-way ANOVA for diet, F(1, 28) = 55.48, p < 0.0001.
3.3. Leptin receptor on brain endothelial cells modulates the response to reward
HFD is associated with food reward [43]. Therefore, we directly investigated whether LepR in brain barriers modulates food reward. In the conditioned place preference (CPP) paradigm (Figure 5A), mice of both genotypes developed a preference for the chamber that was paired with sucrose pellets. However, conditioning was more pronounced in LepRbeKO than in LepRFl mice (Figure 5B), suggesting that LepRbeKO mice were more susceptible to the rewarding effects of palatable food. This conclusion was supported by the conditioned two-bottle choice (BC) test in a separate cohort of animals (Figure 5C). After 8 days of conditioning, mice of both genotypes drank more from the bottle that used to contain sucrose solution, although it was only filled with water during testing. Similar to the observation in the CPP, LepRbeKO mice showed a higher preference for the bottle conditioned to sucrose than LepRFl animals (Figure 5D) confirming the notion that LepR-mediated leptin transport inhibits food reward.
4. Discussion
4.1. Targeting LepR in brain barriers
The routes by which leptin reaches the brain have been debated almost since its discovery more than two decades ago [1]. While leptin may freely access brain regions adjacent to circumventricular organs, the long isoform of LepR that is involved in signaling is also expressed outside of circumventricular organs [44]. This suggests that leptin is transported through barrier forming cells to act on these regions. Three cell types provide potential gates for leptin entry into the brain: (1) endothelial cells forming the BBB [14]; (2) epithelial cells forming the blood-CSF barrier in the choroid plexus [15]; and (3) tanycytes in the mediobasal hypothalamus that form a barrier between median eminence and the CSF by expressing tight junctions [17], [45], [46]. So far, the functional significance of these three access points for body weight regulation has remained largely unclear. Ambiguity also exists with regard to the molecular identity of the leptin transporter. The prime candidate is LepR, as it is expressed in brain endothelial and epithelial cells as well as in tanycytes [17], [18], [19], [20], [21]. However, conflicting data about its role in leptin transport have been obtained from LepR deficient animals [22], [23], probably because these reports solely relied on leptin uptake, a parameter that may be influenced by the increased permeability of the BBB in obesity [47]. Besides LepR, LRP1 and LRP2 qualify as alternative transporters, at least in vitro [48], [49]. Deletion of LRP2 in central and peripheral endothelial cells and in hematopoietic cells triggers obesity, but the phenotype is more complex and includes neurodegenerative and inflammatory changes in the brain making conclusions for body weight regulation difficult [50], [51].
To investigate the role of LepR for leptin transport to the brain without interfering signals from the periphery, we deleted LepR in brain endothelial and epithelial cells (LepRbeKO mice). Two features distinguish the new LepRbeKO mouse model from a similar mouse line that has been reported before [42]. First, all isoforms of LepR are deleted in LepRbeKO mice, whereas previous studies only targeted the long LepR isoform resulting in the compensatory up-regulation of soluble isoforms. Second, by limiting LepR deletion to brain barriers, we aimed to avoid peripheral effects. Fenestrated capillaries in the median eminence as well as tanycytes do not show recombination in LepRbeKO mice. Thus, the approach targets two of the access points of leptin, the BBB and the blood-CSF barrier.
In LepRbeKO mice, LepR mRNA expression in primary brain endothelial cells was reduced by about 60%, in contrast to higher recombination rates that we had observed in previous studies with the Slco1c1-CreERT2 driver line [26], [52]. As noted previously, the floxed locus in LepRFl mice does not seem to be as easily accessible for the Cre recombinase as in other mouse lines [3]. Remaining LepR in the BBB or the blood-CSF barrier may explain why leptin uptake in the cortex or VTA was reduced by about 60%. The reduction of leptin uptake in the mediobasal hypothalamus and CSF of LepRbeKO mice was less (40%) and not statistically significant, probably due to tanycytes and fenestrated capillaries that were not targeted by our approach. In contrast, LepR is efficiently deleted in epithelial cells of the choroid plexus of LepRbeKO mice implying that tanycytes and not epithelial cells of the choroid plexus control leptin uptake into the CSF unless there are other leptin transporters in the choroid plexus. Overall, our study clearly demonstrates that LepR contributes to leptin transport into the brain.
4.2. Functional consequences of reduced leptin transport
The impaired leptin transport in LepRbeKO mice did not affect food intake or body weight on NCD (Figure 2A and B). Only when high doses of leptin were peripherally administered, LepRbeKO mice were less anorexic than controls (Figure 2C). We predict that the sensitivity to intracerebrally administered leptin would be preserved in LepRbeKO mice on NCD. Interestingly, the blunted effect of peripheral leptin on food intake was not associated with a lower number of pSTAT3-positive cells in the ARC, the nucleus that is of primary importance for homeostatic feeding, suggesting that leptin transport across brain barriers modulates feeding behavior through other neuronal circuits. On a rewarding HFD LepRbeKO mice were more susceptible to obesity. Their energy intake was increased, especially during the resting (light) phase (Figure 3F). Notably, a disturbed circadian rhythm of food intake behavior in obese mice may further aggravate obesity [53], [54], [55]. Water intake, locomotion, and energy expenditure clearly did not differ between the genotypes (Figure 4A–D) suggesting that obesity was likely caused by hyperphagia of the rewarding HFD, when leptin uptake in the brain was impaired. Glucose tolerance and insulin plasma concentrations in LepRbeKO mice will have to be investigated by future studies.
More direct evidence for the role of LepR-mediated leptin transport in the regulation of food reward comes from independent experiments studying food preference in lean mice fed with NCD. In both CPP and BC paradigms, mice deficient of LepR in brain barriers developed a stronger preference for the reward stimulus. The enhanced sensitivity to food reward leads to a higher energy intake when mice were fed with the rewarding HFD. Our data indicate that LepR in brain endothelial and epithelial cell is required for leptin to reach its target cells in the reward system of the brain. It is well known that leptin is able to regulate the mesoaccumbens dopamine pathway [56] and that in leptin deficient animals the reward value of palatable food is higher than in wild-type mice [57]. In the absence of LepR in the VTA [6] or in the lateral hypothalamic area [7] dopaminergic neurons in the mesolimbic system show an increased sensitivity to high caloric molecules present in plasma. The present study demonstrates that leptin transport by LepR is required for leptin to exert its inhibitory effects on reward circuits in the brain.
The transport of leptin across the BBB has a Km of about 1 nM [36], a value that is in good accordance with the affinity of both short and long isoforms of LepR [58]. In contrast, leptin binds to LPR2 with a much lower affinity and with a dissociation constant of 200 nM [59] suggesting LepR as the primary binding site. In accordance with this, endothelial and epithelial cells in the brain highly express short isoforms of LepR, including LepRa [18], [19], [20], [21]. Interestingly, deleting LepRa leads to a very similar phenotype to that which we have observed in LepRbeKO mice [60]. Mice deficient of LepRa [60] have a normal body weight on NCD but aggravated obesity on HFD. The latter study and our data converge on the concept that leptin transport across brain barriers that is mediated by LepR modulates food reward but seems to be dispensable for the homeostatic control of feeding.
5. Conclusions
We found that LepR in the BBB and the blood-CSF barrier is at least partially required for leptin uptake by the brain and for the regulation of food intake, particularly of food reward. Previous work demonstrated that LepR is required for leptin transport by tanycytes in the mediobasal hypothalamus [17]. Overall, LepR is essential both for leptin transport into the brain and for its multiple effects in the CNS and the periphery. This dual role of LepR in signaling and transport is attributed to distinct long and short isoforms, respectively, but they share the extracellular leptin-binding domains, a fact that may pose an obstacle for the development of peripheral leptin inhibitors. Such inhibitors have been suggested as a therapeutic principle for some forms of cancer and other diseases [61]. In view of our data, it may be difficult to develop competitive leptin antagonists that do not reach the brain and are free of central metabolic side effects.
In obesity, leptin transport into the brain is impaired [47]. Our data demonstrate that a deficit in leptin transport to the brain is able to enhance food reward, providing a potential explanation for the increased sensitivity to food reward in overweight subjects [62]. In obesity, pathological leptin transport is not due to a simple down-regulation of LepR transcription in brain vessels [22]. However, maintenance of LepR levels at the cell surface, binding of leptin, and the subsequent receptor-mediated transcytosis involve multiple cellular processes, including trafficking, shedding, and glycosylation [63], [64], that may provide targets to optimize leptin transport and to treat obesity.
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft (DFG, GRK1957, T-CRC 134 and PI 379/8-1) and the Deutsches Zentrum für Diabetesforschung (DZD, 82DZD00016G).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.12.001.
Conflicts of interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1.
Protocol of the conditioned place preference (CPP, A) and the bottle choice (BC, B) test. The animals were tested for the most preferred chamber or cage side at day 0. During the conditioning the less preferred chamber/cage side was associated with rewarding stimuli (sucrose pellets or 0.8 M sucrose solution), alternating with non-rewarding stimuli in the preferred chamber/cage side. On the test day mice had to choose between empty chambers or 2 bottles filled with water.
Supplementary Figure 2.
Expression of LepR in crucial access points of leptin to the brain. (A) Zs-Green-positive cells (green) were found in close proximity to the 3rd ventricle (3V) in the arcuate nucleus and in the median eminence (B). Zs-Green reflects the expression of LepR in LepR-cre;Zs-Green mice. Vimentin (Vim) is a marker of tanycytes and collagen IV (Coll IV) of the vascular basement membrane. Scale bar, 50 μm. (C) in the choroid plexus epithelial cells forming the blood-CSF barrier were positive for the Zs-Green; however, the fenestrated PV1-positive capillaries in the plexus (red) were negative for Zs-Green. Scale bar, 50 μm.
Supplementary Figure 3.
Slco1c1-CreERT2 drives recombination in epithelial cells of the choroid plexus but not in fenestrated vessels of the median eminence and in tanycytes. (A) in Slco1c1-CreERT2;HA-UPRTFl mice there was no expression of HA-UPRT (HA, red) in fenestrated PV1-positive vessels (green) in the median eminence suggesting no recombination, in contrast to recombination that occurred in endothelial cells of the blood–brain barrier (arrows). Scale bar, 50 μm. (B) LepRbeKO mice showed a clear reduction of LepR immunoreactivity (red) in the choroid plexus compared to LepRFl controls. Coll IV, collagen IV. (C) in contrast, there was not reduction of LepR (arrows) in vimentin-positive tanycytes (green) in the mediobasal hypothalamus. Vim, vimentin; 3V, 3rd ventricle. Scale bar, 50 μm.
References
- 1.Friedman J.M. A tale of two hormones. Nature Medicine. 2010;16:1100–1106. doi: 10.1038/nm1010-1100. [DOI] [PubMed] [Google Scholar]
- 2.de Luca C., Kowalski T.J., Zhang Y., Elmquist J.K., Lee C., Kilimann M.W. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. Journal of Clinical Investigation. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen P., Zhao C., Cai X., Montez J.M., Rohani S.C., Feinstein P. Selective deletion of leptin receptor in neurons leads to obesity. Journal of Clinical Investigation. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balthasar N., Coppari R., McMinn J., Liu S.M., Lee C.E., Tang V. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon H., Zigman J.M., Ye C., Lee C.E., McGovern R.A., Tang V. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Hommel J.D., Trinko R., Sears R.M., Georgescu D., Liu Z.W., Gao X.B. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Leinninger G.M., Jo Y.H., Leshan R.L., Louis G.W., Yang H., Barrera J.G. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metabolism. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz M.W., Peskind E., Raskind M., Boyko E.J., Porte D., Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nature Medicine. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 9.Caro J.F., Kolaczynski J.W., Nyce M.R., Ohannesian J.P., Opentanova I., Goldman W.H. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 10.Halaas J.L., Boozer C., Blair-West J., Fidahusein N., Denton D.A., Friedman J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Heek M., Compton D.S., France C.F., Tedesco R.P., Fawzi A.B., Graziano M.P. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. Journal of Clinical Investigation. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Haschimi K., Pierroz D.D., Hileman S.M., Bjorbaek C., Flier J.S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. Journal of Clinical Investigation. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faouzi M., Leshan R., Bjornholm M., Hennessey T., Jones J., Munzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- 14.Banks W.A., Kastin A.J., Huang W., Jaspan J.B., Maness L.M. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 15.Zlokovic B.V., Jovanovic S., Miao W., Samara S., Verma S., Farrell C.L. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141:1434–1441. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]
- 16.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiology of Disease. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Balland E., Dam J., Langlet F., Caron E., Steculorum S., Messina A. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metabolism. 2014;19:293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan W., Hsuchou H., He Y., Sakharkar A., Cain C., Yu C. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hileman S.M., Tornoe J., Flier J.S., Bjorbaek C. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby Canine Kidney cells. Endocrinology. 2000;141:1955–1961. doi: 10.1210/endo.141.6.7450. [DOI] [PubMed] [Google Scholar]
- 20.Boado R.J., Golden P.L., Levin N., Pardridge W.M. Up-regulation of blood-brain barrier short-form leptin receptor gene products in rats fed a high fat diet. Journal of Neurochemistry. 1998;71:1761–1764. doi: 10.1046/j.1471-4159.1998.71041761.x. [DOI] [PubMed] [Google Scholar]
- 21.Bjorbaek C., Elmquist J.K., Michl P., Ahima R.S., van Bueren A., McCall A.L. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–3491. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 22.Hileman S.M., Pierroz D.D., Masuzaki H., Bjorbaek C., El-Haschimi K., Banks W.A. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–783. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- 23.Banks W.A., Niehoff M.L., Martin D., Farrell C.L. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Research. 2002;950:130–136. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- 24.Varela L., Horvath T.L. A sympathetic view on fat by leptin. Cell. 2015;163:26–27. doi: 10.1016/j.cell.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Abella V., Scotece M., Conde J., Pino J., Gonzalez-Gay M.A., Gomez-Reino J.J. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nature Reviews Rheumatology. 2017;13:100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 26.Ridder D.A., Lang M.F., Salinin S., Roderer J.P., Struss M., Maser-Gluth C. TAK1 in brain endothelial cells mediates fever and lethargy. Journal of Experimental Medicine. 2011;208:2615–2623. doi: 10.1084/jem.20110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assmann J.C., Korbelin J., Schwaninger M. Genetic manipulation of brain endothelial cells in vivo. Biochimica et Biophysica Acta. 2016;1862:381–394. doi: 10.1016/j.bbadis.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Gay L., Miller M.R., Ventura P.B., Devasthali V., Vue Z., Thompson H.L. Mouse TU tagging: a chemical/genetic intersectional method for purifying cell type-specific nascent RNA. Genes & Development. 2013;27:98–115. doi: 10.1101/gad.205278.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFalco J., Tomishima M., Liu H., Zhao C., Cai X., Marth J.D. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 30.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assmann J.C., Muller K., Wenzel J., Walther T., Brands J., Thornton P. Isolation and cultivation of primary brain endothelial cells from adult mice. Bio-protocol. 2017;7 doi: 10.21769/BioProtoc.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter W.M., Greenwood F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 33.LaRue B., Hogg E., Sagare A., Jovanovic S., Maness L., Maurer C. Method for measurement of the blood-brain barrier permeability in the perfused mouse brain: application to amyloid-beta peptide in wild type and Alzheimer's Tg2576 mice. Journal of Neuroscience Methods. 2004;138:233–242. doi: 10.1016/j.jneumeth.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Banks W.A., Coon A.B., Robinson S.M., Moinuddin A., Shultz J.M., Nakaoke R. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 35.Storck S.E., Meister S., Nahrath J., Meissner J.N., Schubert N., Di Spiezio A. Endothelial LRP1 transports amyloid-beta(1-42) across the blood-brain barrier. Journal of Clinical Investigation. 2016;126:123–136. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banks W.A., Clever C.M., Farrell C.L. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. American Journal of Physiology-Endocrinology and Metabolism. 2000;278:E1158–E1165. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 37.de Araujo I.E., Oliveira-Maia A.J., Sotnikova T.D., Gainetdinov R.R., Caron M.G., Nicolelis M.A. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 38.Leshan R.L., Bjornholm M., Munzberg H., Myers M.G., Jr. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(Suppl. 5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- 39.Eskilsson A., Matsuwaki T., Shionoya K., Mirrasekhian E., Zajdel J., Schwaninger M. Immune-induced fever is dependent on local but not generalized prostaglandin E2 synthesis in the brain. Journal of Neuroscience. 2017;37:5035–5044. doi: 10.1523/JNEUROSCI.3846-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts L.M., Woodford K., Zhou M., Black D.S., Haggerty J.E., Tate E.H. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149:6251–6261. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- 41.Schaab M., Kratzsch J. The soluble leptin receptor. Best Practice & Research Clinical Endocrinology & Metabolism. 2015;29:661–670. doi: 10.1016/j.beem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Hsuchou H., Kastin A.J., Tu H., Markadakis E.N., Stone K.P., Wang Y. Effects of cell-type specific leptin receptor mutation on leptin transport across the BBB. Peptides. 2011;32:1392–1399. doi: 10.1016/j.peptides.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figlewicz D.P., Bennett J., Evans S.B., Kaiyala K., Sipols A.J., Benoit S.C. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behavioral Neuroscience. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- 44.Scott M.M., Lachey J.L., Sternson S.M., Lee C.E., Elias C.F., Friedman J.M. Leptin targets in the mouse brain. Journal of Comparative Neurology. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullier A., Bouret S.G., Prevot V., Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. Journal of Comparative Neurology. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langlet F., Mullier A., Bouret S.G., Prevot V., Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. Journal of Comparative Neurology. 2013;521:3389–3405. doi: 10.1002/cne.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhea E.M., Salameh T.S., Logsdon A.F., Hanson A.J., Erickson M.A., Banks W.A. Blood-brain barriers in obesity. AAPS Journal. 2017;19:921–930. doi: 10.1208/s12248-017-0079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich M.O., Spuch C., Antequera D., Rodal I., de Yebenes J.G., Molina J.A. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiology of Aging. 2008;29:902–912. doi: 10.1016/j.neurobiolaging.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Liu Q., Zhang J., Zerbinatti C., Zhan Y., Kolber B.J., Herz J. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biology. 2011;9:e1000575. doi: 10.1371/journal.pbio.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietrich M., Antequera D., Pascual C., Castro N., Bolos M., Carro E. Alzheimer's disease-like impaired cognition in endothelial-specific megalin-null mice. Journal of Alzheimer's Disease. 2014;39:711–717. doi: 10.3233/JAD-131604. [DOI] [PubMed] [Google Scholar]
- 51.Bartolome F., Antequera D., Tavares E., Pascual C., Maldonado R., Camins A. Obesity and neuroinflammatory phenotype in mice lacking endothelial megalin. Journal of Neuroinflammation. 2017;14:26. doi: 10.1186/s12974-017-0800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ridder D.A., Wenzel J., Muller K., Tollner K., Tong X.K., Assmann J.C. Brain endothelial TAK1 and NEMO safeguard the neurovascular unit. Journal of Experimental Medicine. 2015;212:1529–1549. doi: 10.1084/jem.20150165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barclay J.L., Husse J., Bode B., Naujokat N., Meyer-Kovac J., Schmid S.M. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS One. 2012;7:e37150. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arble D.M., Bass J., Laposky A.D., Vitaterna M.H., Turek F.W. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E.A., Gill S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fulton S., Pissios P., Manchon R.P., Stiles L., Frank L., Pothos E.N. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Domingos A.I., Vaynshteyn J., Sordillo A., Friedman J.M. The reward value of sucrose in leptin-deficient obese mice. Molecular Metabolism. 2014;3:73–80. doi: 10.1016/j.molmet.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fong T.M., Huang R.R., Tota M.R., Mao C., Smith T., Varnerin J. Localization of leptin binding domain in the leptin receptor. Molecular Pharmacology. 1998;53:234–240. doi: 10.1124/mol.53.2.234. [DOI] [PubMed] [Google Scholar]
- 59.Hama H., Saito A., Takeda T., Tanuma A., Xie Y., Sato K. Evidence indicating that renal tubular metabolism of leptin is mediated by megalin but not by the leptin receptors. Endocrinology. 2004;145:3935–3940. doi: 10.1210/en.2004-0074. [DOI] [PubMed] [Google Scholar]
- 60.Li Z., Ceccarini G., Eisenstein M., Tan K., Friedman J.M. Phenotypic effects of an induced mutation of the ObRa isoform of the leptin receptor. Molecular Metabolism. 2013;2:364–375. doi: 10.1016/j.molmet.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leggio A., Catalano S., De Marco R., Barone I., Ando S., Liguori A. Therapeutic potential of leptin receptor modulators. European Journal of Medicinal Chemistry. 2014;78:97–105. doi: 10.1016/j.ejmech.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 62.Davis C., Fox J. Sensitivity to reward and body mass index (BMI): evidence for a non-linear relationship. Appetite. 2008;50:43–49. doi: 10.1016/j.appet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 63.De Bock M., Van Haver V., Vandenbroucke R.E., Decrock E., Wang N., Leybaert L. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia. 2016;64:1097–1123. doi: 10.1002/glia.22960. [DOI] [PubMed] [Google Scholar]
- 64.Wauman J., Zabeau L., Tavernier J. The leptin receptor complex: heavier than expected? Frontiers in Endocrinology (Lausanne) 2017;8:30. doi: 10.3389/fendo.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]