Abstract
Objective
To characterize the EndoC-βH1 cell line as a model for human beta cells and evaluate its beta cell functionality, focusing on insulin secretion, proliferation, apoptosis and ER stress, with the objective to assess its potential as a screening platform for identification of novel anti-diabetic drug candidates.
Methods
EndoC-βH1 was transplanted into mice for validation of in vivo functionality. Insulin secretion was evaluated in cells cultured as monolayer and as pseudoislets, as well as in diabetic mice. Cytokine induced apoptosis, glucolipotoxicity, and ER stress responses were assessed. Beta cell relevant mRNA and protein expression were investigated by qPCR and antibody staining. Hundreds of proteins or peptides were tested for their effect on insulin secretion and proliferation.
Results
Transplantation of EndoC-βH1 cells restored normoglycemia in streptozotocin induced diabetic mice. Both in vitro and in vivo, we observed a clear insulin response to glucose, and, in vitro, we found a significant increase in insulin secretion from EndoC-βH1 pseudoislets compared to monolayer cultures for both glucose and incretins.
Apoptosis and ER stress were inducible in the cells and caspase 3/7 activity was elevated in response to cytokines, but not affected by the saturated fatty acid palmitate.
By screening of various proteins and peptides, we found Bombesin (BB) receptor agonists and Pituitary Adenylate Cyclase-Activating Polypeptides (PACAP) to significantly induce insulin secretion and the proteins SerpinA6, STC1, and APOH to significantly stimulate proliferation.
ER stress was readily induced by Tunicamycin and resulted in a reduction of insulin mRNA. Somatostatin (SST) was found to be expressed by 1% of the cells and manipulation of the SST receptors was found to significantly affect insulin secretion.
Conclusions
Overall, the EndoC-βH1 cells strongly resemble human islet beta cells in terms of glucose and incretin stimulated insulin secretion capabilities. The cell line has an active cytokine induced caspase 3/7 apoptotic pathway and is responsive to ER stress initiation factors. The cells' ability to proliferate can be further increased by already known compounds as well as by novel peptides and proteins. Based on its robust performance during the functionality assessment assays, the EndoC-βH1 cell line was successfully used as a screening platform for identification of novel anti-diabetic drug candidates.
Keywords: EndoC-βH1, Pseudoislets, Glucose stimulated insulin secretion, Somatostatin signaling, Proliferation
Abbreviations: BB, Bombesin; PACAP, Pituitary Adenylate Cyclase-Activating Polypeptides; SST, Somatostatin; Bxv1, B10 xenotropic virus 1; GLP1R, GLP1 receptor; GSIS, Glucose stimulated insulin secretion; STZ, Streptozotocin; IPGTT, Intraperitoneal glucose tolerance test; SI, Stimulation index; EdU, 5-ethynyl-2′-deoxyuridine; CytMix, Cytokine mixture; SEM, Standard error of the mean; Ex4, Exendin-4; SSTR, Somatostatin receptor; sXBP1, spliced XBP1
Highlights
-
•
The EndoC-βH1 cell line responds robustly to glucose and incretins and restores normoglycaemia in diabetic mice.
-
•
EndoC-βH1 cells assembled in pseudoislets show an improved insulin secretion profile compared with monolayer cells.
-
•
Bombesin receptor agonists as well as the neuropeptides PACAP27 and PACAP38 significantly increase insulin secretion.
-
•
The proteins SerpineA6, STC1 and APOH significantly increase the rate of proliferation.
-
•
EndoC-βH1 cells are affected in a biological relevant way by cytokines and are sensitive to Tunicamycin induced ER stress.
1. Introduction
The insulin producing beta cell is central in the etiology of human diabetes as beta cell failure is the major determining factor for progression from impaired glucose tolerance to overt diabetes [1]. The beta cell is by nature a highly metabolic active cell type and thus particularly sensitive to further stress, e.g. dietary stress with excessive glucose and fatty acids. Such high levels of glucose and lipids initially cause stress that may escalate to toxicity, especially if these factors act in combination with the high oxidative environment inside the beta cell. The cellular mechanism for this glucolipotoxicity is initially believed to be stress of the endoplasmic reticulum, but, if sustained, it can also lead to apoptosis mediated beta cell death [2].
Given the current increase in the number of obese people, more type 2 diabetic patients are emerging. To treat this condition, increased knowledge of the cellular and molecular mechanisms causing human beta cell failure is warranted.
To date, the major drugs for diabetes treatment are metformin, sulfonylurea, insulin, insulin analogs, GLP1-analogs, as well as DPP4 and SGLT2 inhibitors. As these drugs are so efficient in blood glucose regulation, the next innovative step could be towards a cure for diabetes. Therefore, new potent antidiabetic drugs are needed, and analyses using human beta cells are a necessity as the majority of in vitro research so far has been done on islets and cell lines from rodents with only sporadic follow-up using human islets, a natural consequence of the scarcity and the variable quality of human islets available for research [3]. Excitingly, with the human beta cell line EndoC-βH1 [4], it is becoming clear that we may have a robust, valid and useful human beta cell line available for studying human beta cell physiology [5], [6], [7], [8]. Accordingly, all in vitro data from the original publication by Philippe Ravassard et al. have now been confirmed by independent laboratories. Thus the identified infection with the B10 xenotropic virus 1 (Bxv1), which is a xenotropic endogenous murine leukemia virus, does not appear to hamper proper functionality of the cell line [9].
However, the studies using EndoC-βH1 have so far been focused on general characterization and comparison to the commonly used beta cell models [10] and much less on the applicability of the cell line for screening purposes.
At Novo Nordisk A/S, we performed a thorough phenotypic validation of the cells including: transplantation to diabetic mice, static and dynamic insulin secretion assays using both standard adherent cultures and pseudoislet aggregates, validation of GLP1 receptor (GLP1R) functionality, mRNA expression of selected beta and non-beta cell genes in single cells and in pools over time, as well as assessing the protein levels of the pancreatic hormones. Subsequently, we used the cell line to establish medium through-put screening assays for the identification of drugs enhancing beta cell functionalities: glucose stimulated insulin secretion (GSIS), proliferation, resistance to cytokine or glucolipotoxicity induced apoptosis and ER stress. We observe that this human background is a major step forward for all assays but especially important for proliferation given the substantial lack of correlation between data obtained in rodent versus human beta cells [11], [12].
To generate a prioritized list of potential novel drug candidates, we developed a bioinformatic pipeline exploiting both public and in-house generated datasets (for details see Suppl. M&M). We then produced or acquired more than 200 proteins and peptides and performed an arrayed screen where each of the drug candidates was tested in at least four independent biological replicates at three different concentrations. Overall, we identified several peptides and proteins that increase insulin secretion and proliferation, and we report that insulin secretion is increased by the PACAP as well as four different BB receptor agonistic peptides. Moreover, that the proteins SerpineA6, STC1, and APOH stimulate proliferation of the EndoC-βH1 cell line.
2. Materials and methods
2.1. In vivo experiments
SCID/beige mice were used for the in vivo experiments and transplantation was performed when the mice were 8–10 weeks of age. The animals were bred by Taconic Biosciences and kept at Novo Nordisk in accordance with our standard animal unit procedures. All experiments were approved by the Danish ethical committee for animal experiments.
EndoC-βH1 cells or human islets were transplanted under the kidney capsule of diabetic and non-diabetic mice. Diabetes was induced by multiple low dose streptozotocin (STZ) injections. Glucose tolerance in non-diabetic animals was tested by intraperitoneal glucose tolerance test (IPGTT) using 3 g/Kg glucose. Blood glucose and human C-peptide were measured in all animals. After the experiments, the animals were euthanized by cervical dislocation; kidneys were isolated, fixed, and analyzed by histology and immunohistochemistry. For detailed information, see Supplementary Materials and Methods.
2.2. Immunohistochemical staining of kidneys grafted with EndoC-cells
The isolated grafted kidneys were fixed in 10% natural buffered formalin for 24 h and processed to paraffin. Graft morphology was visualized with hematoxylin and eosin staining on 3 μm sections. The slides were scanned on a Nanozoomer 2.0-HT (Hamamatsu) at 40× magnification. The sections were stained with primary and secondary antibodies, counterstained with DAPI and scanned. For detailed information, see Supplementary Materials and Methods.
2.3. Human islets
Human islets from cadaver donors were provided by Prodo Laboratories Inc., CA, USA, with informed consent from the relatives of the deceased individuals. All experiments were done in agreement with national legislation and institutional ethical rights. The islets were cultured in CMRL1066 medium (Gibco) supplemented with 2 mM Glutamine, 10% FCS (Gibco) and 1% Penicillin/Streptomycin (Gibco) at 37 °C and 5% CO2.
2.4. EndoC-βH1 monolayer cell culture and pseudoislets
The cell line was cultured as previously described [4]. To generate EndoC-βH1 pseudoislets, 10 μM ROCK inhibitor (Sigma), and 50 μg/ml DNase (Roche) were added to 7.5 × 106 EndoC-βH1 cells in 10 ml culture medium, then placed in 10 cm Corning Ultra Low attachment dishes and aggregated for 4–5 days at 37 °C and 5% CO2 on an orbital shaker running at 50 rpm. The pseudoislets were continuously kept like this and used for experiments until day 7.
2.5. Live-dead assay on EndoC-βH1 pseudoislets
Pseudoislets were stained with calcein for detection of esterase activity and ethidium homodimer-1 to indicate loss of plasma membrane integrity (Thermo Fisher Scientific). Scanning was performed on a fluorescent microscope (Olympus IX71) at 20× magnification.
2.6. Glucose stimulated insulin secretion (GSIS)
EndoC-βH1 cells were seeded at 7 × 104 cells per well in 96-well plate (Thermo Scientific) and cultured for 5 days. EndoC-βH1 pseudoislets were aggregated for 5 days and seeded at 50 islets per well in 96-well plates. Both secreted and intracellular amounts of insulin were measured. For detailed information, see Supplementary Materials and Methods.
2.7. Perifusion
Perifusion was done on EndoC-βH1 pseudoislets using the Biorep Perifusion System, version 4.2 (BIOREP technologies, FL, USA). For detailed information, see Supplementary Materials and Methods.
2.8. Calculations of insulin amounts
To determine the amount of secreted insulin as % of total insulin, the total insulin content was calculated as the sum of the secreted and the intracellular insulin after the respective stimulations; ultimately the value for secreted insulin was divided by the value for total insulin content. The stimulation index (SI) was calculated as the fold change between insulin secretion during treatments and the corresponding insulin secretion at stimulation with low glucose, i.e. 0.5 mM glucose. For calculation of absolute values of secreted insulin and insulin content in EndoC-βH1 and human islets, see Supplementary Materials and Methods.
2.9. Bioinformatics analysis
The bioinformatics analysis performed in this project consisted of many different parts and while the full details of the in-house drug target pipeline are classified, a general overview of the main features is given here. A bioinformatics toolbox was developed (in perl) that could store (Oracle) and process all relevant processed data, including a visual inspection of the most promising candidates (perl cgi). We started with a comprehensive survey of publicly available expression data (microarray data, RNA-seq, proteomics, GWAS) from pancreas, islets, and beta cells as well as tissue profiling experiments of biological relevant conditions, which included samples from humans, animal models, and cell lines. Thereafter, we performed extensive quality control on each experiment; both of a technical nature (e.g. FastQC, arrayQualityMetrics) and a biological nature. Only about 50% of the public experiments were included, and in the majority of the remaining, at least one sample was excluded. This left us with 19 relevant public datasets that were used in the down-stream analysis as a complement to our own in-house generated data sets. The data was then, among others, via textmining sorted by annotations for each particular protein, e.g. was there a known link to Type 2 Diabetes, or diabetes/insulin in general, or any of its co-morbidities (Non-Alcoholic Fatty Liver Disease, cardiovascular disorders, kidney disease, or hyperlipidemia). Candidates and the diseases were then subjected to an in depth manual curation in which abstracts from PubMed was read to determine if the link appeared real. Well over 100.000 abstracts where read in order to ensure the best possible automatic textmining solution. In addition, traditional sources of biological annotations, such as Gene Ontology (GO) terms and UniProt functional descriptions, were also used for confirmation. In all cases, we integrated all the normalized QCed data on a gene level (aggregating the signal across multiple lines of evidence) followed by a Systems Biology/Network Biology interpretation using a high-quality Protein–Protein interaction network (InWeb_InBioMap) [13] as the scaffold for the analysis.
2.10. RNA purification, cDNA reverse transcription and real time PCR
For detailed information, see Supplementary Materials and Methods.
2.11. Single-cell qPCR
EndoC-βH1 (passage number 101) and human islet cells were sorted by FACS and single cell qPCR was performed on microfluidic chips (Biomark, Fluidigm). Data were analyzed in R (version 3.1.1) and the analysis consisted of 44 EndoC cells and 17 human islet beta cells. For detailed information, see Supplementary Materials and Methods.
2.12. Immunocytochemical staining of monolayer cultures and pseudoislets
For detailed information, see Supplementary Materials and Methods.
2.13. SST-ELISA
SST-ELISA was carried out according to the manufacturer's procedure (Antibodies-online GmbH, Cat# E0592h).
2.14. Knock down of GLP1R in EndoC-βH1
To generate a cell line with knock down of GLP1R, five different shRNA sequences from the TRC library (Sigma–Aldrich, SHCLNV-NM_002062) were delivered individually by lentiviral transduction and subsequently grown into stable cell lines using 3 μg/ml Puromycin. The best knockdown was obtained using the TRC clone NM_002062.2-963s1c1, harboring the shRNA sequence CCTCATCTTTGTTCGGGTCAT; this cell line was used for the subsequent analysis. For comparison, we used a stable cell line expressing a negative control shRNA from the same lentiviral vector (pLKO.1) with the shRNA sequence CAACAAGATGAAGAGCACCAA (Sigma, SHC002V) which targets turboGFP and hence not any known human mRNA.
2.15. Proliferation assays
The cells were seeded at a density of 4 × 104 cells per well and allowed to adhere for 24 h prior to addition of the experimental compounds: CHIR99021 (Cayman Chemical) or the in-house produced proteins APOH, SerpineA6 and STC1 (Novo Nordisk). Cells were stimulated for 24–48 h.
For the 14C-Thymidine incorporation assay, the cells were plated in scintillation plates (CytoStar-T 96-well, Perkin Elmer) and following change to experimental media 14C-Thymidine was supplemented to a final concentration 0.5 μCi/ml. The scintillation signal was measured in a TopCounter NXT HTS instrument (Perkin Elmer).
For the 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay the cells were plated in black 96-well culture plates (BD-Falcon) and following change to experimental media the amount of incorporated EdU was analyzed using the Click-iT EdU Alexa Fluor 488 Imaging Kit (Life Technologies) according to the manufacturer's procedure. The cells were incubated with 0.5 μM EdU for 2 h and subsequently fixed and stained with DAPI. The EdU signal was quantified using the InCell Analyzer 2000 Imaging system (GE Healthcare).
2.16. Apoptosis assay
For the cytokine induced apoptosis assay, the cells were seeded at 4 × 104 cells per well in 96-well plates and cultured for 48 h prior to treatment with a cytokine mixture (CytMix) consisting of 1 ng/ml IL-1β, 20 ng/ml IFNγ and 20 ng/ml TNFα for 24 h. Caspase 3/7 activity was measured by use of the ApoTox Triplex or Apo-ONE Caspase 3/7 assay (Promega) according to the manufacturer's instructions. All data were normalized to CytMix treated cells.
For the glucolipotoxicity (palmitate) assay, the cells were seeded at a density of 5.5 × 104 cells per well in 96-well plates and 24 h later treated with increasing amounts of BSA-conjugated palmitate (Echelon Biosciences) in 20 mM glucose. After 72 h caspase 3/7 activity was measured using the Apo-ONE Caspase 3/7 assay (Promega).
2.17. Statistics
All statistical analyses are represented an initial unpaired ANOVA followed by Student's t-test. The analysis was performed in GraphPad Prism version 6.0 and all depicted columns are means and the error bars are standard error of the mean (SEM), +SEM is shown on top of each column.
3. Results
3.1. In vivo validation of the EndoC-βH1 cells
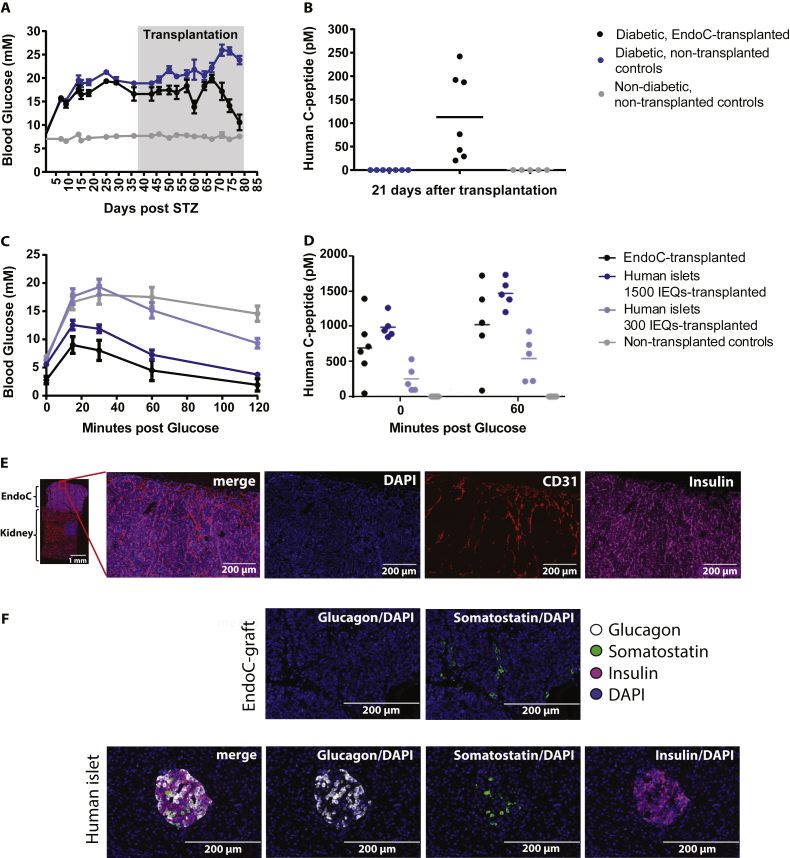
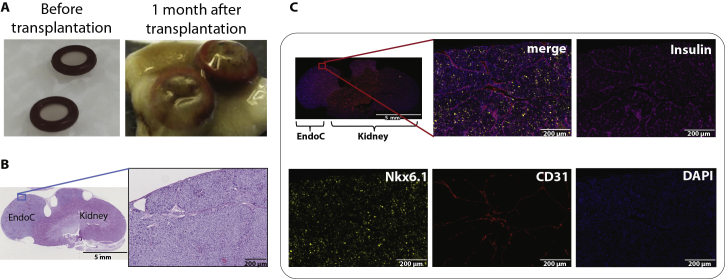
To validate the functional capability of the human beta cell line EndoC-βH1 in vivo, we reproduced the original setup from Ravassard et al. and transplanted two million cells under the kidney capsule of SCID/beige mice made diabetic by STZ mediated beta cell ablation. During the 42 days long experiment, the transplanted cells proliferated substantially and protracted out of the boundaries of the originally transplanted silicone rings (Figure S1A). About six weeks after transplantation, blood glucose levels in the animals grafted with EndoC-βH1 decreased to normoglycemic levels (Figure 1A). Human C-peptide measured three weeks after transplantation was only detected in the animals with EndoC-βH1 cell transplants (Figure 1B).
Figure 1.
Blood glucose and C-peptide in diabetic and non-diabetic SCID beige mice after transplantation of EndoC-βH1 cells or human islets under the kidney capsule. A) Blood glucose measurements in STZ induced diabetic SCID beige mice after transplantation of 2 million EndoC-βH1 cells, n = 7 in each group. B) Circulating human C-peptide measured 3 weeks after transplantation, n = 7 in each group. Data shown as mean + SEM. Measurement of C) blood glucose (at 0, 15, 30, 60, and 120 min) and D) circulating human C-peptide (at 0 and 60 min) in non-diabetic SCID beige mice during IPGTT performed 6 weeks after transplantation of EndoC-βH1 cells or human islets, n = 5–7 in each group. Histological analysis: E) Immunohistochemical staining of EndoC-βH1 kidney grafts isolated from diabetic SCID beige mice: Insulin (magenta), CD31 (red) and DAPI (blue). Scale bar: 1 mm (left), 200 μm (four magnified images on the right). F) Immunohistochemical staining of EndoC-βH1 kidney grafts isolated from diabetic SCID beige mice and human pancreatic islet section: Insulin (magenta), Glucagon (white), Somatostatin (green) and DAPI (blue). Scale bar: 200 μm.
Glucose clearance was studied by IPGTT in non-diabetic mice seven weeks post-transplantation with EndoC-βH1. The cells performed comparable to 1500 human islet IEQs and much better than 300 IEQs (Figure 1C). Blood glucose levels in the groups with EndoC-βH1 and 1500 human islet IEQs increased with 5–7 mM after 15 min and then decreased to 2–4 mM 2 h post glucose administration. In the non-transplanted animals as well as those transplanted with 300 IEQs, the blood glucose levels increased at least 2-fold at 30 min prior to decreasing to a final level of 15 mM and 10 mM, respectively. Likewise, human C-peptide levels in animals with EndoC-βH1 transplants or 1500 human IEQs were elevated before the IPGTT and further increased by the glucose load (Figure 1D).
By histological and immunohistochemical analysis of the EndoC-βH1 grafts from both diabetic (Figure 1E) and non-diabetic (Figure S1B and C) animals, the transplanted cells were clearly present on top of the kidney and stained positive for insulin as well as for the vascular marker CD31 and the beta cell specific transcription factor Nkx6.1 (data shown for non-diabetic animals in Figure S1C). EndoC-grafts from diabetic animals stained negative for glucagon while sporadic SST expression was observed (Figure 1F, top row). The SST positive cells appeared to be clustered together. Sections from human islets were stained in parallel as positive controls (Figure 1F, bottom row).
3.2. Dynamic and static GSIS on EndoC-βH1 monolayers and pseudoislets
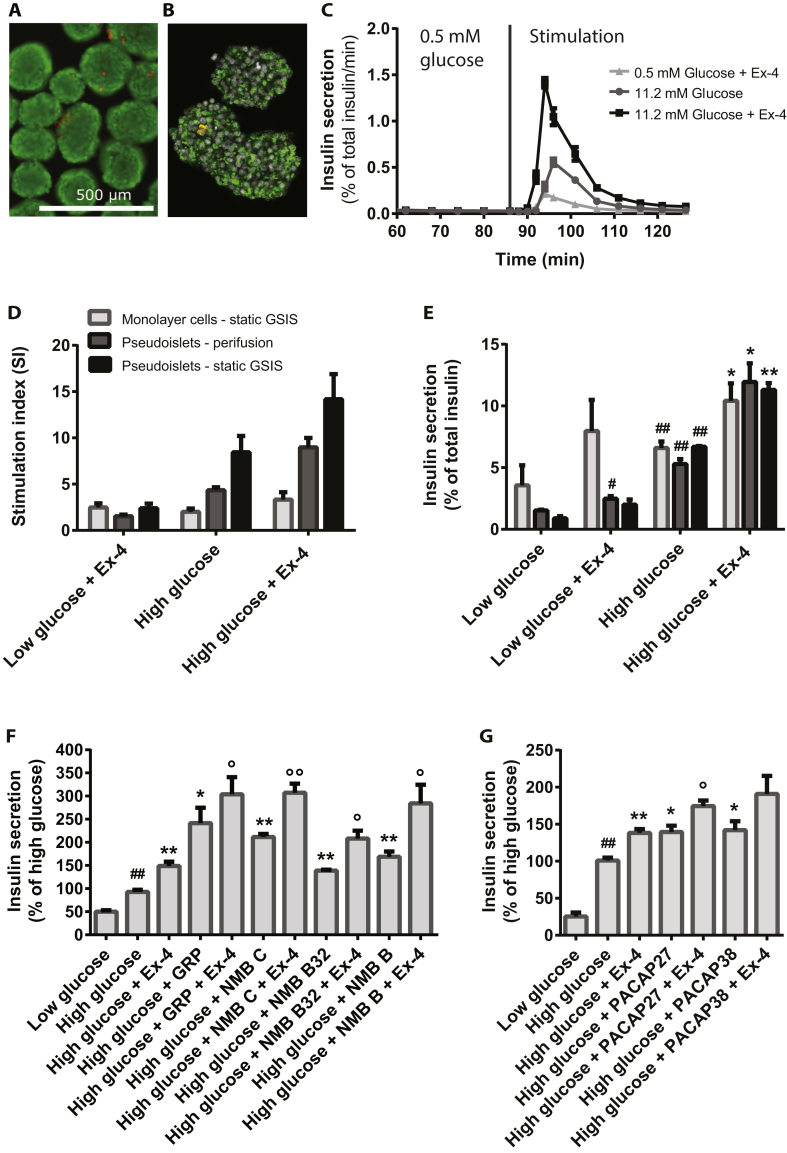
The insulin secretion of EndoC-βH1 monolayer cultures and pseudoislets was assessed by perifusion and static GSIS. The appearance of the generated pseudoislets was uniform, and the cells were highly viable as evaluated by staining for live (green) and dead (red) cells (Figure 2A). In addition, they were stained strongly positive for insulin as demonstrated by immunocytochemistry and subsequent confocal imaging (Figure 2B).
Figure 2.
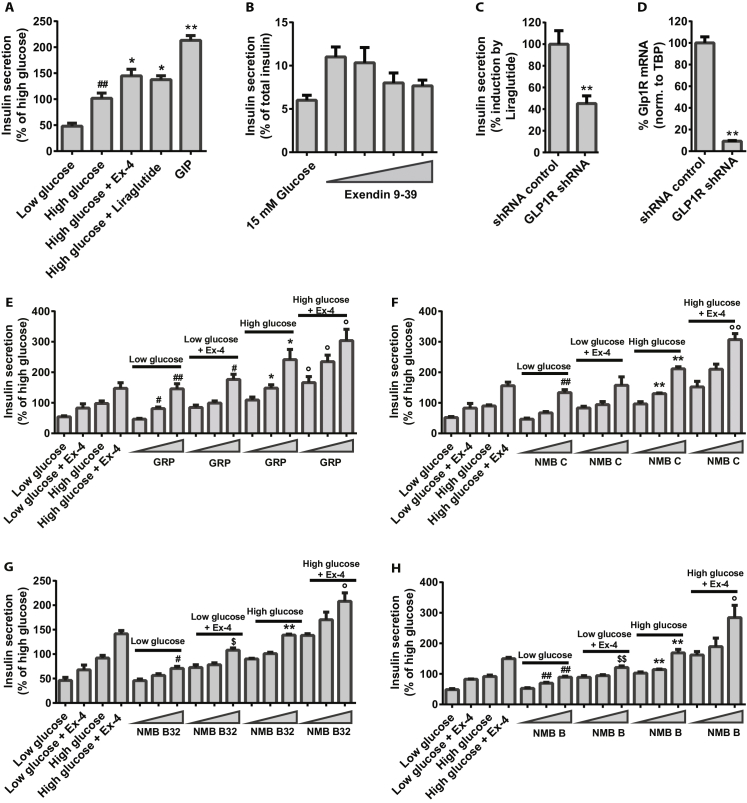
Insulin secretion measured by perifusion and static GSIS on EndoC-βH1 pseudoislets and monolayer cells after stimulation with 0.5 mM glucose (low glucose), 11.2 mM glucose (high glucose), or in combination with the GLP-1R agonist Ex4, used at 100 nM. A) Staining of EndoC-βH1 pseudoislets with calcein (green) and ethidium homodimer-1 (red), scale bar 500 μm. B) Immunocytochemical staining and confocal imaging of insulin (green), SST (red) and DAPI (grey) in EndoC-βH1 pseudoislets. C) Insulin secretion measured by perifusion of EndoC-βH1 pseudoislets, 1000 islets per condition; starvation in low glucose performed for 86 min prior to stimulation for 40 min, n = 3. D) SI for perifusion of EndoC-βH1 pseudoislets (n = 3), for static GSIS in EndoC-βH1 monolayer (n = 9) and pseudoislets (n = 3) based on percent of secreted insulin of total insulin content; for perifusion SI-calculations were based on AUC/min, for the 38 min stimulation period. E) Insulin secretion measured by perifusion on pseudoislets and static GSIS on EndoC-βH1 monolayer cells and pseudoislets, n = 3–9. F) Effect of BBs; 10 nM GRP, 10 nM NMB C, 10 nm NMB B32 and 10 nM NMB B on insulin secretion in EndoC-βH1 monolayer cells measured by GSIS, n = 3. G) Effect of 100 nM PACAP 27 and 10 nM PACAP 38 on insulin secretion in EndoC-βH1 monolayer cells measured by static GSIS, n = 3. Data are shown as mean + SEM. P-values determined by unpaired One Way ANOVA and Student's t-test. The # symbol illustrates significant difference from low glucose; #∼p < 0.05; ##∼p < 0.01. The $ symbol illustrates significant difference from low glucose + Ex-4, $∼p < 0.05; $$∼p < 0.01. The * symbol illustrates significant difference from high glucose, *∼p < 0.05; **∼p < 0.01. The ° symbol illustrates significant difference from high glucose + Ex-4, °∼p < 0.05; °°∼p < 0.01.
Perifusion of EndoC-βH1 pseudoislets with low or high glucose resulted in a single burst of insulin secretion, which declined to baseline after 30 min despite the continued perifusion with high glucose (Figure 2C). Addition of the GLP1R agonist Exendin-4 (Ex4), as expected, led to increased insulin secretion and also led to faster secretory kinetics compared to glucose alone. This phenomenon was observed in all experiments. Re-stimulation of the pseudoislets following a rest period in low glucose resulted in a very modest insulin secretion response (data not shown). The obtained SI during perifusion was 4-fold between low and high glucose and upon stimulation with Ex4 and high glucose it increased to 9-fold (Figure 2D; Table 1).
Table 1.
Summary table on SI and insulin secretion (presented as % of total insulin content) in EndoC-βH1 pseudoislets and monolayer cells during stimulation with low or high glucose with and without addition of Ex4. SI in EndoC-βH1 pseudoislets is calculated as AUC/min for a 38 min stimulation by perifusion. The presented values are averages from 3 to 9 independent experiments with 3–4 biological replicates per experiment. Values for amount of secreted insulin in human islets are obtained during stimulation with low glucose (range 1.8–3.5 mM) and high glucose (range 11.2–27 mM).
| EndoC-βH1/human islets | Low glucose |
Low glucose + Ex-4 |
High glucose |
High glucose + Ex-4 |
||||
|---|---|---|---|---|---|---|---|---|
| SI | Secreted insulin (%) | SI | Secreted insulin (%) | SI | Secreted insulin (%) | SI | Secreted insulin (%) | |
| EndoC-βH1 pseudoislets Perifusion |
– | 1.5 ± 0.1 | 1.5 ± 0.3 | 2.5 ± 0.4 | 4.4 ± 0.5 | 5.3 ± 0.7 | 9.0 ± 1.7 | 11.9 ± 2.6 |
| EndoC-βH1 pseudoislets Static GSIS |
– | 0.9 ± 0.3 | 2.4 ± 0.9 | 2.0 ± 0.7 | 8.5 ± 3.1 | 6.7 ± 0.1 | 14.2 ± 4.7 | 11.3 ± 1.0 |
| EndoC-βH1 monolayer Static GSIS |
– | 4.0 ± 2.0 | 2.5 ± 0.8 | 8.0 ± 4.4 | 2.0 ± 1.1 | 6.6 ± 1.6 | 3.3 ± 2.4 | 10.4 ± 4.2 |
| Human islets (6 studies) | – | 0.9–2.7 | – | – | – | 4.3–8.9 | – | – |
In regular static GSIS using monolayer cultures, the observed SIs were 2 and 3 for high glucose and high glucose + Ex4 (Figure 2D, Table 1), thus the SI using perifusion of pseudoislets was 2–3 folds higher and this increase in SI was only due to lower insulin secretion in low glucose and not to higher insulin secretion in high glucose (Figure 2E, Table 1). To evaluate whether this increased SI obtained during perifusion was due to the perifusion or to the pseudoislet formation, a static GSIS assay was performed on pseudoislets. We observed a further decrease in insulin secretion at the low glucose concentration (Figure 2E) resulting in a further increase of the SI to 2-fold higher than the SI observed during perifusion and 4-fold higher than what was observed in monolayer cultures. The obtained SIs and insulin secretion values are summarized in Table 1.
As reported previously by other groups [4], [5], [6], [7], [8], we also found that the EndoC-βH1 cells secrete insulin in response to glucose and to incretins (Figure S2A), and that this occurs both under low and high glucose conditions. However, we were not able to detect the GLP1R protein despite a substantial effort using several validated antibodies and mass spectrometry. Thus, in order to show that the cell line contains a functional GLP1R signaling system, we used the GLP1R antagonist Exendin9-39 to inhibit the effect of Ex4 (Figure S2B) and by use of shRNA we reduced the GLP1R mRNA level by 90%, which resulted in a significant decrease of the stimulatory effect of Ex4 (Figure S2C and D), providing strong evidence for a functional GLP1R pathway in the EndoC-βH1 cell line.
3.3. Absolute values of secreted insulin and insulin content in EndoC-βH1 and human islets
The GSIS data obtained in this study were compared to all other studies reporting the amount of insulin secretion and intracellular content in EndoC-βH1 monolayer cultures [4], [6], [7] as well as to a range of data from human islets [14], [15], [16], [17], [18], [19] (Table 2). To do this comparison, we did three approximations regarding cell numbers in the various assay setups (for details please refer to Supplementary Materials and Methods). In summary, we find that the EndoC-βH1 cells secrete approximately 5–10% of the insulin amount secreted by human islets and that the cell line contains around 5% of the insulin content present in a human islet beta cell.
Table 2.
Amount of secreted insulin (ng/106 β-cells/stimulation period) and insulin content (μg/106 β-cells/stimulation period) in EndoC-βH1 pseudoislets and monolayer cultures as well as in human islets during static GSIS and/or perifusion. Stimulation with low glucose, high glucose, or high glucose plus 100 nM Ex4 performed for 1 h (static GSIS) or 40 min (perifusion). Each value is based on results from 2 to 3 independent experiments, with 3–4 biological replicates per experiment. The insulin amount in EndoC-βH1 monolayer cells and human islets reported from other research groups is shown for comparison. The calculations regarding human islets are based on the assumption that an islet consists of 1000 cells with 50% of those being beta cells.
| Beta cells | Secreted insulin (ng/106 β-cells/hr) |
Insulin content (μg/106 β-cells) | |
|---|---|---|---|
| Low glucose (0.5–5 mM) | High glucose (11.2–27 mM) | ||
| EndoC-βH1 Monolayer cells (this study) |
33 | 60 | 0.7–1.1 |
| EndoC-βH1 Pseudoislets – perifusion (this study) |
9 | 37 | 0.6 |
| EndoC-βH1 Pseudoislets – static GSIS (this study) |
9 | 84 | 1.2 |
| EndoC-βH1 Monolayer cells (Ravassard et al., 2011) |
6 | 19 | 0.5–0.6 |
| EndoC-βH1 Monolayer cells (Gurgul-Convey et al., 2015) |
0.1 | 1 | 0.045–0.064 |
| EndoC-βH1 Monolayer cells (Krishnan et al., 2015) |
0.1 | 0.4 | 8.8 |
| Human islets (6 studies) | 250–1300 | 950–4000 | 25–95 |
Moreover, we noted that the cell line secretes a substantial amount of pro-insulin especially under low glucose conditions (data not shown).
3.4. Selection of protein and peptide drug candidates for screening
To select for protein and peptides for screening, a comprehensive bioinformatics analysis was performed with the aim of producing a prioritized list of target candidates with the potential of affecting beta cell functionality, with a focus on GSIS and proliferation. Please refer to the materials & methods part for further details. In general, a protein was only considered a target if it was secreted. It was considered relevant for Type 2 Diabetes biology, for example if it: 1) interacted with a receptor expressed in beta cells, 2) interacted with a receptor that is differentially expressed in relevant conditions, 3) was a novel protein expressed in beta cells, 4) was specifically expressed in islets/beta cells. Then, from each category, we ranked the proteins by different criteria, for example by the fold change in beta vs alpha cells. Positive selection of candidates was thus based on known relevance in diabetes for either the target itself, or its interaction partner(s). In addition, we performed negative filtering to remove proteins that were not suitable candidates for protein therapy. These included proteins that in UniProt were annotated as being expressed in the mitochondria, the endoplasmic reticulum or that were enzymes, blood factors, extracellular matrix proteins, major structural proteins, or core immune system proteins (e.g. the HLA family). Lastly, an evaluation for complexity with regards to production of the candidate protein was performed. The final list contained well over 300 proteins, of which 228 was tested in vitro, resulting in identification of 16 proteins or peptides that showed a significant biological effect on beta cells.
3.5. GSIS screening for insulin secretagogues in EndoC-βH1 monolayer cultures
Among the hundreds of proteins and peptides screened in GSIS (data not shown), we identified several compounds with a stimulatory effect. The most potent induction was observed by the BB receptor agonists GRP and NMB B32 as well as their concomitant cleavage products NMB C and NMB B. This induction was evident both as a single treatment and when administered on top of Ex4 (Figure 2F). Also, clear dose dependent insulin responses were observed for each of these four peptides both with and without Ex4 in high glucose but also in low glucose (Figure S2E–H).
The vasoactive intestinal polypeptides PACAP27 and PACAP38 were also found to significantly stimulate insulin secretion in EndoC-βH1 cells (Figure 2G). Both peptides stimulated insulin secretion already at 10 nM when applied in media with high glucose, alone, and, for PACAP27, this secretion was further increased by addition of Ex4 (Figure S2I and J).
3.6. Gene and protein expression analysis in EndoC-βH1 compared to human islets
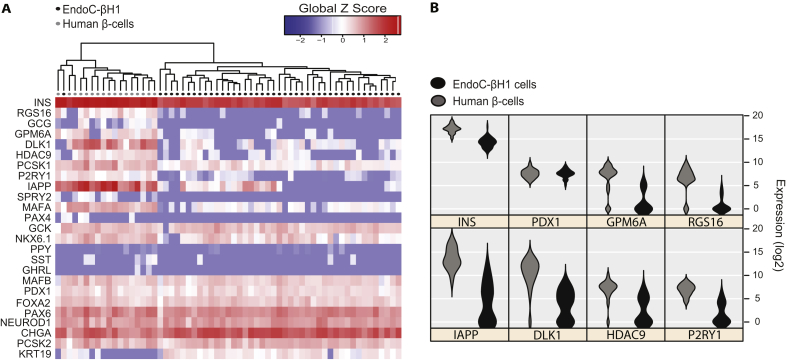
To investigate potential heterogeneity in EndoC-βH1 and to evaluate the similarity to human beta cells, mRNA expression in 44 EndoC-βH1 single cells was compared with 17 previously published [20] human beta cells obtained from the islets of one human donor (Figure 3A). To determine overall gene expression in the EndoC-βH1 population, standard qPCR analyses was performed and mRNA levels quantified in two different passages of monolayer cultures as well as in pseudoislets. These levels were subsequently compared to mRNA levels obtained from four independent preparations of human islets (Figure S3).
Figure 3.
qPCR profiling and protein expression in EndoC-βH1. A) Heat map for the expression of 25 selected genes in individual EndoC-βH1 cells (n = 44) and primary human beta cells (n = 17). Genes are clustered by Pearson Correlation and samples by normalized Euclidian distance. Gene expression values are normalized to the global mean and global SD. B) mRNA expression at single-cell level for 8 selected genes in EndoC-βH1 cells and primary human beta cells are presented by violin plots.
We found that insulin mRNA expression in EndoC-βH1 cells is lower than the level in human islets both on single beta cell and islet level, whereas the important beta cell transcription factors PDX1, PAX6, FOXA2, NEUROD1, MAFB, NKX6.1, and genes involved in the insulin secretory machinery like PCSK2 as well as CHGA were expressed at similar levels or slightly higher compared to human beta cells and islet preparations (Figure 3A, S3). The glucose transporters SLC2A1-4 were expressed at low levels in the low passage number EndoC-βH1 monolayer cultures, but, interestingly, SLC2A1 and SCL2A4 were clearly upregulated in pseudoislets and were found in levels comparable to those in human islets (Figure S3). The expression of GCK in the EndoC-βH1 monolayer cultures and pseudoislets was found to be 6–7 folds higher than the expression in human islets, while at single cell level this difference was not noticeable.
Different from the mature human primary beta cells, the ductal cell marker KRT19 was highly expressed in most of the studied EndoC-βH1 single cells (Figure 3A).
Some of the genes characteristic of adult beta cells were expressed at very low levels or not detected at all in EndoC-βH1 cells, while these were readily observed in the studied human beta cells: P2RY1, HDAC9, GPM6A, RGS16, DLK1, and IAPP (Figure 3B). Overall, the single cell analysis showed that although many markers in the EndoC-βH1 cells do not reveal differences between cells, IAP, DLK1, HDAC9, and P2RY1 reveal that distinct subpopulations do exist (Figure 3B).
The so-called beta cell disallowed genes including PDGFRA, ITIH5, SMAD3, ZYX, IGFBP4, and CAT were expressed at similar or lower levels compared to human islets, while SLC16A1 (MCT1) and LDHA were significantly higher expressed in the EndoC-βH1 cells [21]. LMO4, a disallowed gene associated with increased cell proliferation was also found at high levels compared to human islets. As expected, the proliferation marker Ki67 was highly expressed in EndoC-βH1 monolayer cultures and pseudoislets.
3.7. Expression of non-beta cell markers in EndoC-βH1 cells and significant effect of SST signaling
Transcripts for the non-beta cell markers GCG, PPY, GHRL, AMY2A, and CFTR were not detected in the EndoC-βH1 cells and immunocytochemical staining of monolayer cell cultures for GCG, PPY, and GHRL were negative (data not shown).
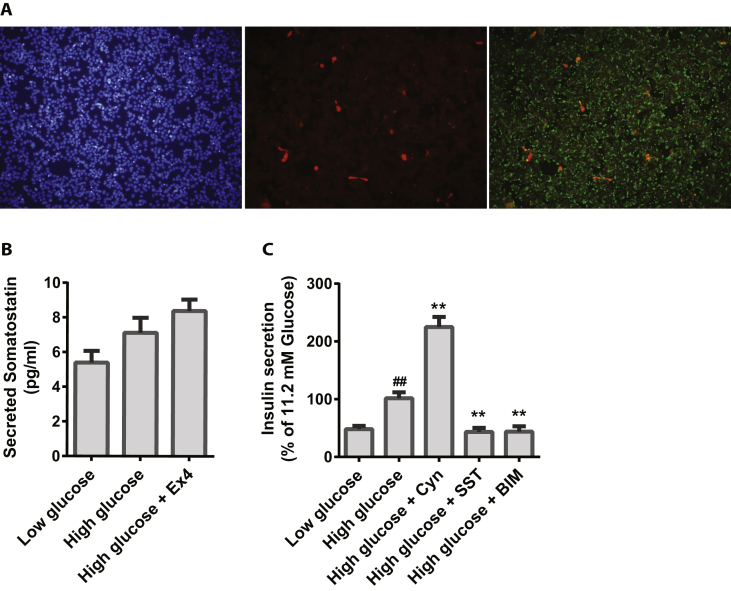
SST mRNA was observed in a few single cells (Figure 3A) and at low level in the pooled cultures compared to human islets (Figure S3). All five SST receptors were detected in the pools, with somatostatin receptor 2 (SSTR2) and SSTR5 being expressed at the highest levels (Figure S3). The SST hormone was clearly detected at the protein level in approximately 1% of both monolayer cultures (Figure 4A), pseudoislets (Figure 2B) and in retrieved grafts (Figure 1F). SST was also found to be secreted into the cell media, and this appeared to be in a glucose and incretin dependent manner (Figure 4C). As expected, exogenous SST inhibited the GSIS response (Figure 4D) but interestingly, inhibition of SSTR2 using the compound Cyn154806 resulted in a significant increase in GSIS, whereas inhibition of SSTR5 by BIM23056 led to a significant decrease in GSIS (Figure 4D).
Figure 4.
SST expression in EndoC-βH1 cells and EndoC-βH1 pseudoislets. A) Immunocytochemical staining for SST (red), insulin (green) and DAPI (blue) in EndoC-βH1 cells, n = 3. B) SST ELISA in EndoC-βH1 cells, n = 3. C) Effect of 100 nM SST, 100 nM SST-receptor 2 (SSTR2) antagonist Cyn154806, and 1 μM SSTR5-antagonist BIM23056 in addition to high glucose on insulin secretion in EndoC-βH1 cells, n = 3–8. Data are shown as mean + SEM, P-values are determined by unpaired One Way ANOVA and Student's t-test. The # symbol illustrates significant difference from low glucose; #∼p < 0.05; ##∼p < 0.01. The * symbol illustrates significant difference from high glucose, *∼p < 0.05; **∼p < 0.01.
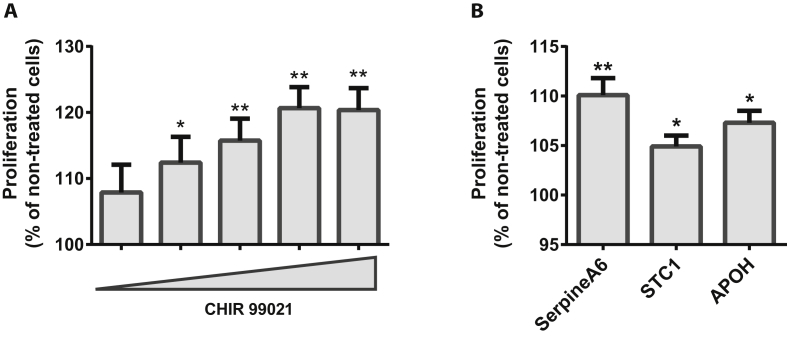
3.8. Proliferation screening to identify mitogenic compounds in the EndoC-βH1 cells
The major difference between EndoC-βH1 cells and mature human beta cells is their ability to proliferate. The cells have a doubling time of approximately 7 days, and, before we screened our library of peptides and proteins, we tested a number of compounds reported to induce proliferation in human and mouse beta cells in order to find a positive control for induction of EndoC-βH1 cell proliferation.
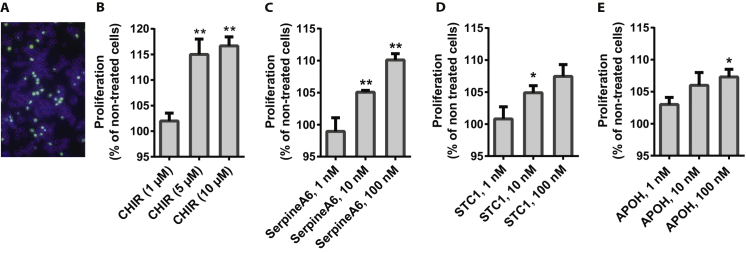
We identified CHIR99021, a GSK3-inhibitor, as the most potent stimulator resulting in a robust, dose-dependent increase in proliferation rate of EndoC-βH1 by 10–20%. This was observed using two different proliferation assay setups, 14C-Thymidine- and EdU-incorporation (Figure 5A, S4A and B).
Figure 5.
Stimulation of proliferation in EndoC-βH1 cells using the small molecule CHIR99021 (A) and the proteins SerpinA6, STC1, and APOH (B). A) Dose response of the GSK3-inhibitor CHIR99021 in concentrations between 0.625 μM and 10 μM with 2-fold increase of dose between each concentration as measured by 14C-Thymidine incorporation after 48 h treatment, n = 3; each experiment is based on 5–10 replicates for each condition. B) Effect of 100 nM SerpinA6, 10 nM STC1 and 100 nM APOH determined by 14C-Thymidine incorporation after 48 h treatment, n = 2–3. Data are shown as mean + SEM. P-values determined by unpaired One Way ANOVA and Student's t-test. The * symbol illustrates significant difference from non-treated control cells *∼p < 0.05; **∼p < 0.01.
Upon applying our peptide and protein library, we found the proteins SerpinA6, STC1, and APOH induced a 10% increase in proliferation rate of the EndoC-βH1 cells (Figure 5B). A dose titration with these proteins clearly showed that the effect of SerpinA6 is dose dependent, and even though the experiment was only performed twice with STC1 and APOH, a similar trend was observed (Figure S4C–E).
3.9. Apoptosis and ER stress assays in EndoC-βH1 cells
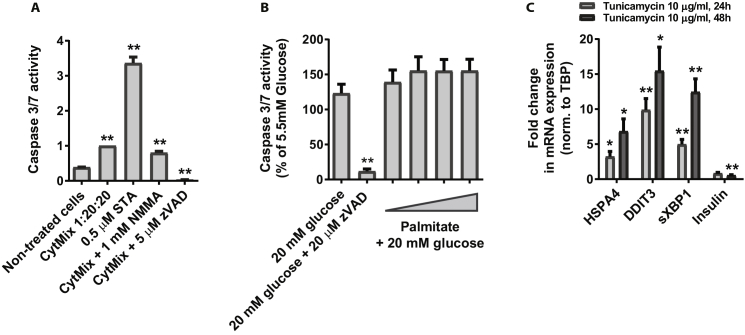
Beta cell apoptosis and ER stress are observed in many cases of type 1 and type 2 diabetes [22], and much attention has been focused on identifying inhibitors of this process for drug development. A classic mix of cytokines consisting of 1 ng/ml IL-1β, 20 ng/ml IFNγ and 20 ng/ml TNFα significantly increased the apoptotic rate in EndoC-βH1 cells by 3-fold (Figure S5A). The apoptosis inducing effect was confirmed by use of NMMA and zVAD as partial and complete inhibitors of apoptosis, respectively. However, upon screening of selected proteins and peptides, we did not observe compounds with protective effect (data not shown).
The apoptotic effect of palmitate on EndoC-βH1 cells was also investigated and showed that 3-days treatment with high glucose (20 mM) and palmitate at concentrations between 0.1 and 0.7 mM do not have an effect on apoptosis in the EndoC-βH1 cells (Figure S5B).
ER stress was induced by 10 μg/ml tunicamycin for 24 or 48 h and this resulted in an ample mRNA induction of the classic ER stress genes HSPA4, DDIT3/CHOP, spliced XBP1 (sXBP1) and also in significant down regulation of insulin mRNA levels (Figure S5C).
4. Discussion
Human beta cells are not recapitulated well by current rodent models, and more knowledge on the human beta cell is needed in order to identify novel drug targets as well as potential drug candidates. To evaluate whether the EndoC-βH1 cells could be useful for identification of proteins and peptides with anti-diabetic properties, we initially verified the functionality of the cells both in vitro and in vivo as well as performed a basic evaluation of mRNA and protein expression of typical beta and non-beta cell genes. Overall, we found that the cell line performed satisfactorily and in accordance with data reported in the original publication [4].
The response of the cell line was tested in four classic assays for beta cell functionality, namely GSIS, proliferation, apoptosis induced by cytokines or glucolipotoxic treatment, and induction of ER stress. To identify positive controls for each assay, we tested the most obvious candidates and found that Ex4 induced GSIS, that CHIR99021 induced proliferation and that apoptosis was induced by a mixture of cytokines (IL-1β, IFNγ, TNFα), but not by incubation with the glucolipotoxic mixture of palmitate and high glucose. For the ER stress assay, the cells were clearly sensitive to the glycosylation inhibitor Tunicamycin, as treatment for 24 h resulted in upregulation of the ER stress genes HSPA4, CHOP and sXBP1, accompanied by a 50% reduction in insulin mRNA.
4.1. Insulin secretion – validation of the cell line and subsequent screening
The key measure of beta cell functionality is the GSIS assay. Therefore, we initially did a thorough examination of the cell line's ability to secrete insulin in response to glucose and incretins with regards to robustness, degree of stimulation (SI) and the stability of this response over time.
The obtained results clearly showed that in our hands the EndoC-βH1 cell line also reacts robustly to both glucose and incretins, and, when grown as regular monolayers, the difference in insulin secretion between low glucose and high glucose plus incretin is on average 3-fold (Figure 2D, E, Table 1, Table 2). Thus, our data are in line with the original data from Ravassard et al., 2011 [4]. The absolute amount of secreted insulin ranges from 10 to 60 ng/106 cells/hr, and the absolute insulin content is around 1000 ng/106 cells. In comparison to beta cells from human islets, this is slightly less as they secrete 250–4000 ng insulin/106 cells/hr and contain around 50,000 ng/106 cells. Thus, in our hands the EndoC-βH1 cells contain and secrete insulin in the range of 1–5% of the levels observed in beta cells from human islets.
To initiate a screening for novel proteins and peptides that could improve beta cell functionality, we performed a bioinformatics analysis identifying more than 300 proteins and peptides of which we produced or procured and then tested 228 (refer to Materials and Methods section for further details). A similar approach based on GWAS data was published recently and resulted in identification of novel roles for several proteins in human beta cell functionality [23].
Using the GSIS assays, we identified agonists of the BB receptors as extremely potent inducers of insulin secretion both under high and low glucose conditions alone as well as in combination with the GLP1R agonist Ex4 (Figure 2G). Also, the PACAP peptides stimulate insulin secretion in high glucose both alone and when co-administered with Ex4 (Figure 2F), although to a lesser degree thereby more resembling Ex4 mono therapy.
BB agonist and PACAP are well known secretagogues, which have been reported to either induce or inhibit insulin as well as glucagon and SST secretion from human, canine and rodent islets [24], [25], [26], [27], [28], [29]. BB agonists are also known to induce gastrin, CCK and amylase secretion from the exocrine pancreas [30], [31] and have also been shown to induce GLP1 expression in gut cells [32]. Thus, we substantiate the already known mode of action of BB agonists and show that the EndoC-βH1 cells harbor a functional BB receptor signaling system. The fact that these agonists also induce insulin secretion under low glucose conditions makes them unattractive as drug candidates. Interestingly, there was no effect of the PACAP peptides in low glucose indicating that these peptides may be true glucose sensitive secretagogues. However, in our hands neither the four BB agonists nor the two PACAP peptides showed an effect on blood glucose levels when administered i.p. to db/db mice (data not shown). It is plausible that this lack of effect could be due to induction of simultaneous secretion of glucagon as well as insulin in the db/db model. Others are currently attempting to develop PACAP analogs without glucagon secreting properties as a potential drug for treatment of diabetes [33]. Otherwise, the lack of effect on blood glucose could be due to a difference in functionality of these particular peptides between human and mouse species. Future studies could investigate the effect of the peptides in transplanted human islets.
Moreover, PACAP has been reported to induce beta cell proliferation and mediate protection against apoptosis [34], but we did not find proliferative or apoptosis protecting effects of the PACAP peptides in the EndoC-βH1 cells. Lastly, PACAP peptides have been reported to have undesirable secretory effects on the exocrine pancreas, which could lead to increased risk of pancreatitis [35].
4.2. Proliferation – search of a positive control and subsequent screening
To induce proliferation in the EndoC-βH1 cells, we initially tested a number of published molecules (CHIR99021, BIO, 1-AKP, Harmine, INDY, WS6 and Denosumab) and found CHIR99021 to exert the most robust effect, leading to a 10–20% induction of growth rate. Whether compounds that induce proliferation in the EndoC-βH1 cells have a similar effect on human islets remains an important question to pursue in the future. Since GSK3 inhibition has been shown to induce proliferation in both rodent [36] and human islet beta cells [16], this may also be the case for other compounds despite inhibition of GSK3 alone may not always lead to increased proliferation in human islet beta cells [37].
In our screening using the thymidine incorporation assay, we found the proteins SerpinA6, STC1 and APOH to marginally but significantly induce proliferation (Figure 5). Serpins are a highly conserved superfamily of serine protease inhibitors consisting of 16 clades of proteins (named from A to P). SerpinB1, a member of the B clade, has been shown to increase beta cell proliferation in human islets [38], thereby suggesting a role for the serine protease inhibitor superfamily in pancreatic beta cell proliferation. Furthermore, SerpinA6 has been associated with resistance to chemotherapy in patients with breast cancer [39] and human hepatocellular carcinoma [40], also indicative of a role in proliferation. Future studies should investigate whether this protein also has a mitogenic effect on beta cells from human islets.
STC1 is a known oncogene [41] and APOH is widely expressed in the body and involved in numerous biological processes that we did not find these proteins relevant for further investigations in the context of drug discovery.
4.3. Apoptosis – investigation of potential assays and subsequent screening
To assess the level of apoptosis in the EndoC-βH1 cells under diabetes-like conditions, we treated the cells with either palmitate and high glucose (glucolipotox) or pro-inflammatory cytokines and then measured the induction of caspase 3/7 activity. For the glucolipotoxic stress, three days of treatment with 0.1–0.7 mM palmitate in the presence of 20 mM glucose did not initiate apoptotic cell death in the EndoC-βH1 cells. Similar results were obtained in a recent study using 1 mM palmitate and 22 mM glucose [42]. Interestingly, in the same study it was found that the use of 1.5–2 mM palmitate resulted in an increase in cell death already at day 1 and that use of the more nutrient rich culture medium DMEM/Ham's F12 significantly increased palmitate induced cell death even at normal glucose level (5.5 mM). Similarly, glucolipotoxicity studies on human islets have shown no effect of 0.5 mM or 1 mM palmitate after 2–3 days of treatment [43], [44], whereas a significant effect was observed after 6–7 days of treatment [43], [45]. This indicates that a longer treatment period is necessary to induce an apoptotic effect in human islets and therefore potentially also in EndoC-βH1 cells. However, from a screening point of view, we conclude that an assay duration of 7–10 days is too long to be useful. Nevertheless, the use of DMEM/F12 and further optimization of the culture conditions seem very promising for the use of EndoC-βH1 cells for lipotoxicity studies.
Interestingly, the cells are clearly responsive to cytokines as 24 h of treatment with a mixture of IL1β, IFNγ, and TNFα resulted in a 3-fold increase in caspase-3/7 activity. We used this assay to screen more than 100 proteins and peptides, but without observing significant protective effects of the compounds tested. Nevertheless, the EndoC-βH1 cell line reacts to cytokines in a manner similar to human beta cells and the assay is valuable for further investigations.
Induction of ER stress with Tunicamycin resulted in significant effects comparable to what is observed in human and mouse islet beta cells [46], [47]. Moreover, the assay is fast and reproducible and therefore highly compatible with high-through put screening, and we are currently testing our compounds in this assay. However, this assay may be less biological relevant due to the harsh effect of Tunicamycin, and it may also represent an earlier stage of diabetes and hence be more relevant for identification of drug candidates to be used for prevention of diabetes rather than for treatment of the disease.
4.4. Perifusion of pseudoislets
Perifusion of pseudoislets resulted in a dramatic increase of the GSIS window, i.e. at the point of maximal secretion; a GSIS window of approximately 20- and 45-fold was observed for high glucose and high glucose + Ex4 compared to low glucose (Figure 2C), respectively. This increase was even more noticeable in static GSIS on the pseudoislets (Figure 2D, Table 1), and, from analysis of the total amount of secreted insulin (Figure 2E), it became apparent that the increased GSIS window is due to decreased insulin secretion at low glucose in the pseudo-islets compared to monolayer cultures and not related to the perifusion method. Thus, in our hands aggregation of EndoC-βH1 cells into pseudoislets results in a highly reproducible drop in insulin secretion under low glucose conditions, which leads to an increase in the static GSIS assay window of approximately 4-fold (Figure 2D, Table 1). Pseudoislets from beta cell lines have been generated previously, and a similar 4-fold increase in response to glucose was observed in the EndoC-βH3 cell line following aggregation into pseudoislets. This occurred despite their method of aggregation was quite different from ours [48]. Also, equivalent observations have been reported using the mouse beta cell line βTC3 [49].
Overall, the possibility of having a large GSIS assay window in a human setting is desirable for identification of potential drug candidates with more modest stimulatory effect on insulin secretion. Handling of the pseudoislets is more challenging compared to regular monolayer cultures, and, hence, we did not find it applicable for the screening assays.
4.5. Gene expression – markers of maturity and expression homogeneity
Single-cell qPCR was used to evaluate the degree of homogeneity of the EndoC-βH1 cell line and to make a direct comparison of mRNA expression levels to beta cells from human islets (Figure 3). To analyze the EndoC-βH1 cells for expression of genes known to be important for beta cell identity, function, and maturity, we performed qPCR analysis of selected mRNAs on regular pools of EndoC-βH1 cells, both as monolayer cultures at early and late passage numbers and as pseudoislets. To compare directly to the mRNA levels found in human islets, we performed parallel qPCR on four different preparations of human islets (Figure S3).
Overall, we found the general expression pattern of the investigated mRNAs to be quite similar between EndoC-βH1 and human islets. However, with expression levels in the EndoC-βH1 cells being a bit lower for some of genes linked to beta cell identity. For example: PDX1, PAX4, PAX6, FOXA2, NeuroD1, MAFB, NKX6.1, KCNJ11, PCSK1/2, and CHGA, an observation that is supported by the original publication [4]. Most mRNAs were expressed at a stable level over time (low vs. high passage number) and when aggregated to pseudoislets. However, there were also some interesting changes, such as the glucose transporters SLC2A1/GLUT1 and SLC2A4/GLUT4, which were expressed at higher levels in pseudoislets, potentially explaining the reduction in insulin secretion under low glucose in the pseudoislets. Also, the MafB mRNA was expressed at higher levels in EndoC-βH1 pseudoislets, which is in accordance with the known presence of MafB in adult human beta cells [50], [51]. In contrast, MafA did not change and remained low at all times. Moreover, we found clear expression of the so-called disallowed genes [21] SLC16A1/MCT1 and LDHA in EndoC-βH1 compared to human islets (Figure S3). SLC16A1 and LDHA are important for nutrient sensing in non-beta cells and hence repressed in adult beta cells to prevent inappropriate insulin secretion [52]. The aberrant expression of these genes may be involved in the observed insulin secretion in low glucose and the relatively small GSIS window of EndoC-βH1 monolayer cultures.
By single cell qPCR, we found EndoC-βH1 cells to be quite homogeneous (Figure 3A), despite some variation in expression between cells was also observed for some genes (Figure 3B). For example the mRNA expression level of the genes IAPP, DLK1, HDAC9, P2RY1, GPM6A, and RGS16 were lower and more heterogeneous in EndoC-βH1 cells than in beta cells from human islets (Figure 3B). These genes are known to be expressed in adult, and thus non-proliferating, beta cells [53] and to be fully absent or expressed at lower levels in young or fetal beta cells [54]. Also, most of the EndoC-βH1 cells express the ductal marker KRT19 at single cell level, which could also be indicative of a beta cell in an earlier stage of development [55]. Interestingly, HDAC9 was also recently been found to be differentially expressed between subpopulations of beta cells within individual human donors [56].
Overall, these findings likely reflect the proliferative status of the EndoC-βH1 cell line and hence the expected fate towards a slightly immature genotype due to this forced proliferation.
4.6. SST – expression and functional consequence
We found the SST protein to be expressed in approximately 1% of the EndoC-βH1 cells as well as secreted in significant amounts. Also, mRNA from all five SST receptors (SSTR1-5) were detected with SSTR2 and SSTR5 being the most abundant. In accordance with SST being an inhibitory hormone, treatment with exogenous SST leads to a clear reduction in GSIS. Interestingly, inhibition of SSTR2 or SSTR5 using the small modified peptides CYN154806 and BIM23056, respectively led to strong but opposite effects on GSIS (Figure 4D). Furthermore, treatment with the pan SSTR1-5 inhibitor peptide cyclo-SST also resulted in inhibition of GSIS (data not shown), i.e. similar to the natural SST agonist. This suggests that a strong differential effect on signaling exists between the five SST receptors in the EndoC-βH1 cells and this phenomenon warrants further exploration.
In an attempt to reduce or remove this aberrant expression, we generated several stable cell lines with knockdown of SST and/or SSTR2. Even with a 70% knockdown of SST or SSTR2 mRNA we did not observe any change in GSIS response (data not shown). This is likely due to the remaining SST being able to sustain adequate signaling and future studies could apply gene editing to completely remove the SST expression. Finally, we did not find SST or CYN154806 to modulate proliferation or apoptosis.
The fact that EndoC-βH1 cells express SST could be mediated by incomplete recruitment of transcription factors during differentiation due to the relatively short rat insulin promotor sequence used to drive proliferation of the fetal pancreatic cell buds used to develop the EndoC-βH1 cell line.
4.7. In vivo functionality
Upon transplantation of two million EndoC-βH1 cells to STZ induced diabetic SCID/Beige mice, the grafted cells clearly produce sufficient insulin to restore normoglycemia, and human C-peptide is evident only in the animals with EndoC-βH1 transplants. This demonstrates both production and function of human insulin in the transplanted mice with an outcome equivalent to 1500 human islets. The normalization of blood glucose occurs around six weeks post-transplantation and, thus, vascularization as well as proliferation of the cells in vivo is likely a prerequisite. In an i.p. glucose challenge performed six weeks after transplantation, the grafted cells cleared the glucose in a manner comparable to 1500 human islet IEQs, which was accompanied by an increase in C-peptide secretion. However, we had to terminate the mice 7–8 weeks after the transplantation due to hypoglycemia and we speculate that the insulin secretion in vivo is ongoing in a constant fashion and that the amount of secreted insulin may be more related to the number of transplanted cells present in the mouse rather than the actual glucose level. Regarding hormone expression, the grafted cells appeared similar post-transplantation as compared to standard in vitro cultured cells.
5. Conclusions
We have investigated the EndoC-βH1 cell line with regard to functionality and general beta and non-beta cell marker expression and conclude that it is a valid cell line model of human beta cells. We have successfully used it as a screening platform for early drug discovery with regards to insulin secretion and beta cell proliferation and identified several interesting peptides and proteins. Furthermore, the cell line is also of interest for screening compounds with beneficial effect on ER stress and to assess more in depth the importance of the five SST receptors and their interplay in the context of insulin secretion. Thus, we conclude that the EndoC-βH1 cell line is a functional and valid human beta cell line that can be efficiently applied as a screening platform for novel anti-diabetic drug candidates with the aim of achieving higher probability of the candidates to show an effect on real human beta cells.
Author contributions
Violeta Georgieva Tsonkova: Main executor of all cell based experiments, biology exploration, co-writer of the manuscript. Fredrik Wolfhagen Sand, Xenia Asbæk Wolf: Transplantation studies. Anna Kirstine Ringgaard, Lars Groth Grunnet: Perifusion and data analysis. Camilla Ingvorsen: Histology on transplants. Louise Winkel: Pseudoislet generation. Mark Kalisz: Cell culture, RNAi knockdown, beta cell biology. Kevin Dolowy Dalgaard: db/db mouse experiments. Christine Bruun: Human islets. Johannes Josef Fels: Development of antibody based HTS assay to detect human insulin and all subsequent insulin measurements. Charlotte Helgstrand, Sven Hastrup, Fredrik Kryh Öberg, Erik Vernet, Michael Paolo Bastner Sandrini, Allan Christian Shaw: Protein production including plasmid design, protein expression, purification and characterization. Carsten Jessen: Peptide production. Mads Grønborg: Proteomics. Jacob Hald, Hanni Willenbrock Thomsen, Dennis Madsen, Rasmus Wernersson, Lena Hansson, Jan Nygaard Jensen: Bioinformatics analysis. Annette Plesner: Cytokine induced apoptosis. Tomas Alanentalo: Confocal imaging. Maja Borup Kjær Petersen, Anne Grapin-Botton, Christian Honoré: Single cell qPCR and data analysis. Jonas Ahnfelt-Rønne: Bioinformatics analysis, beta cell biology and histology. Jacob Hecksher-Sørensen: Histology, beta cell biology and funding of part of the work. Philippe Ravassard: Technical assistance with EndoC-bH1 cell culture, assays and transplantations, scientific review of the manuscript. Ole D. Madsen: Beta cell biology, interpretation of data, scientific review of the manuscript. Claude Rescan: EndoC-bH1 cell culture, assay development, biology exploration, design of experiments, interpretation of data. Thomas Frogne: Main driver of EndoC-bH1 validation and usage at Novo Nordisk (2010–2016), assay development, biology exploration, design of experiments, interpretation of data, main writer of the manuscript, corresponding author.
Acknowledgements
We wish to thank Chloe Munk Cleland Meier Larsen, Henrik Olsen, Rikke Bonne, Lin Chen, Marianne Vollmond, Tove Dannemann, and Jytte Nielsen for excellent technical assistance. This work was supported by grants from Innovation Fund Denmark.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.12.007.
Conflict of interest
All Novo Nordisk employees hold shares in Novo Nordisk A/S, but otherwise all authors declare no conflict of interests.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure S1.
Histological analysis on EndoC-βH1 kidney grafts isolated from non-diabetic SCID beige mice. A) Silicone rings with EndoC-βH1 cells transplanted under the kidney capsule of mice. B) HE staining. Scale bar: 5 mm (left), 200 μm (right). C) Immunohistochemical staining for insulin (purple), Nkx6.1 (yellow) and CD31 (red) and DAPI (blue). Scale bar: 5 mm (upper left image), 200 μm (five magnified images).
Supplementary Figure S2.
Insulin secretion in EndoC-βH1 monolayer cells in response to incretins, incretin inhibitors and neuropeptides. A) Insulin secretion in response to 100 nM Ex4, 100 nM Liraglutide, and 100 nM GIP in addition to 11.2 mM glucose (high glucose), n = 4–8; B) Effect of the GLP1R antagonist Exendin9-39 used in concentrations at 0.1, 1, 10, 100 nM in addition to 15 mM glucose and 10 nM Ex4, n = 3. C) Inhibition of the incretin effect by shRNA mediated knockdown of the GLP1R in EndoC-βH1 cells. Insulin secretion is calculated as percent of total insulin and presented as percent induction by 100 nM Liraglutide, n = 3. D) qPCR measurement of GLP1R mRNA from cells treated with a shRNA control or a shRNA against the GLP1R; data normalized to TBP, n = 3. E) Dose response of GRP in concentrations 1 pM, 100 pM and 10 nM; n = 3. F) Dose response of NMB C in concentrations 1 pM, 100 pM and 10 nM; n = 3. G) Dose response of NMB B32 in concentrations 0.1 nM, 1 nM and 10 nM; n = 3. H) Dose response of NMB B in concentrations 0.1 nM, 1 nM and 10 nM; n = 3. I) Dose response of PACAP 27 in concentrations 1 nM, 10 nM and 100 nM, n = 3. J) Dose response of PACAP 38 in concentrations 1 nM, 10 nM and 100 nM, n = 3. Data are shown as mean + SEM, P-values are determined by unpaired One Way ANOVA and Student's t-test. The # symbol illustrates significant difference from low glucose; #∼p < 0.05; ##∼p < 0.01. The $ symbol illustrates significant difference from low glucose + Ex-4; $∼p < 0.05; $$∼p < 0.01. The * symbol illustrates significant difference from high glucose, *∼p < 0.05; **∼p < 0.01. The ° symbol illustrates significant difference from high glucose + Ex-4, °∼p < 0.05; °°∼p < 0.01.
Supplementary Figure S3.
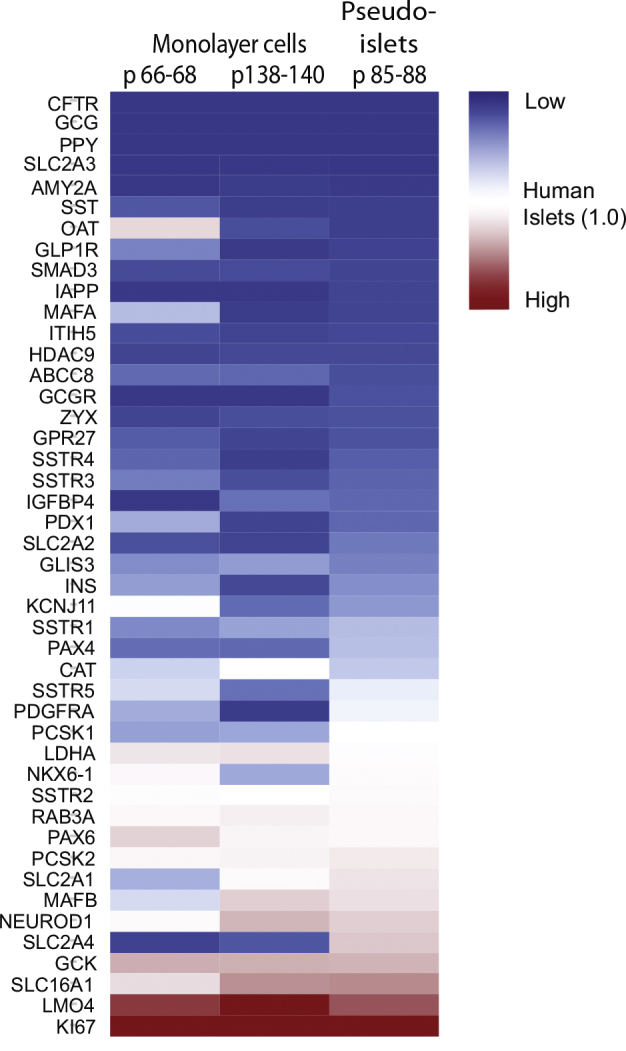
mRNA-expression in EndoC-βH1 monolayer cells (two different passage groups) and pseudoislets. All mRNA levels are shown as fold change relative to the levels obtained from 4 independent preparations of human islets. The bar on the right side of the figure shows the lowest (blue) and the highest (red) expression values compared with the levels in human islets set at one (white). The Ct-values for mRNA expression of the following genes is above 32: AMY2A, CFTR, GCG, GCGR, IGFBP4, ITIH5, MAFA, OAT, PDGFRA, PPY, SLC2A2, SLC2A3, SSTR4, and ZYX.
Supplementary Figure S4.
Stimulation of EndoC-βH1 cell proliferation by the small molecule CHIR99021 and the proteins SerpinA6, STC1 and APOH. A) An image of the cells after EdU incorporation assay, EdU-positive cells (green) and DAPI (blue). B) Stimulatory effect of CHIR99021 after 24h treatment measured by EdU incorporation, n = 2–3; at least 10K cells were analyzed for each treatment. Dose response effect of SerpinA6, n = 3 (C); STC1, n = 2 (D) and APOH, n = 2 (E), measured by 14C-Thymidine incorporation after 48h treatment. Data are shown as mean + SEM. P-values determined by unpaired One Way ANOVA and Student's t-test. The * symbol illustrates significant difference from non-treated control cells, *∼p < 0.05; **∼p < 0.01.
Supplementary Figure S5.
Apoptosis and ER-stress in EndoC-βH1 cells. A) Cytokine induced apoptosis measured by caspase 3/7 activity assay after 24h treatment. The cytokine mixture (CytMix) consists of 1 ng/ml IL-1β, 20 ng/ml IFNγ and 20 ng/ml TNFα and were applied for 24 h 0.5 μM Staurosporine (STA) was used as positive control. 1 mM NMMA and 5 μM zVAD–FMK as partial and complete inhibitors of apoptosis, respectively; n = 12. Data are shown as fold change of CytMix and are means + SEM; *, p < 0.05; **, p < 0.01; B) Caspase 3/7 activity measured in EndoC-βH1 cells treated for 72h with 20 mM glucose and increasing amount of palmitate (0.1, 0.3, 0.5, 0.7 mM); 20 μM zVAD is used as positive control; n = 5. Data are shown as mean + SEM; P-values determined by unpaired One Way ANOVA and Student's t-test. The * symbol illustrates significant difference from non-treated control cells, *∼p < 0.05; **∼p < 0.01; C) Tunicamycin induced ER-stress in EndoC-βH1 cells. Expression (mRNA) of the genes HSPA4, DDIT3 and spliced XBP1 (sXBP1) associated with ER-stress in human beta cells, as well as insulin, are presented as fold change relative to levels in non-treated control cells; n = 3. Data are normalized to TBP and shown as mean + SEM.
References
- 1.Levy J., Atkinson A.B., Bell P.M., Mccance D.R., Hadden D.R. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow- up of the belfast diet study. Diabetes Medicine. 1998;15:290–296. doi: 10.1002/(SICI)1096-9136(199804)15:4<290::AID-DIA570>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 2.Poitout V., Amyot J., Semache M., Zarrouki B., Hagman D., Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids. 2011;1801:289–298. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayton N.S., Poffenberger G., Henske J., Dai C., Thompson C., Aramandla R. Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. American Journal of Physiology –Endocrinology and Metabolism. 2015;308:E592–E602. doi: 10.1152/ajpendo.00437.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravassard P., Hazhouz Y., Pechberty S., Bricout-neveu E., Armanet M., Czernichow P. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. The Journal of Clinical Investigation. 2011;121:3589–3597. doi: 10.1172/JCI58447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson L.E., Valtat B., Bagge A., Sharoyko V.V., Nicholls D.G., Ravassard P. Characterization of stimulus-secretion coupling in the human pancreatic EndoC-βH1 beta cell line. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurgul-Convey E., Kaminski M.T., Lenzen S. Physiological characterization of the human EndoC-bH1 b-cell line. Biochemical and Biophysical Research Communications. 2015;464:13–19. doi: 10.1016/j.bbrc.2015.05.072. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan K., Ma Z., Björklund A., Islam M.S. Calcium signaling in a genetically engineered human pancreatic β -cell line. Pancreas. 2015;44:773–777. doi: 10.1097/MPA.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 8.Grieco F.A., Moore F., Vigneron F., Santin I., Villate O., Marselli L. IL-17A increases the expression of proinflammatory chemokines in human pancreatic islets. Diabetologia. 2014;57:502–511. doi: 10.1007/s00125-013-3135-2. [DOI] [PubMed] [Google Scholar]
- 9.Kirkegaard J.S., Ravassard P., Ingvarsen S., Diedisheim M., Bricout-neveu E., Grønborg M. Xenotropic retrovirus Bxv1 in human pancreatic β cell lines. 2016;126:1109–1113. doi: 10.1172/JCI83573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scharfmann R., Didiesheim M., Richards P., Chandra V., Oshima M., Albagli O. Mass production of functional human pancreatic β-cells: why and how? Diabetes, Obesity and Metabolism. 2016;18:128–136. doi: 10.1111/dom.12728. [DOI] [PubMed] [Google Scholar]
- 11.Wang P., Fiaschi-Taesch N.M., Vasavada R.C., Scott D.K., García-Ocaña A., Stewart A.F. Diabetes mellitus—advances and challenges in human β-cell proliferation. Nature Reviews Endocrinology Nature Publishing Group. 2015;11:201–212. doi: 10.1038/nrendo.2015.9. [DOI] [PubMed] [Google Scholar]
- 12.Chen C., Cohrs C.M., Stertmann J., Bozsak R., Speier S. Human beta cell mass and function in diabetes: recent advances in knowledge and technologies to understand disease pathogenesis. Molecular Metabolism Elsevier GmbH. 2017;6:943–957. doi: 10.1016/j.molmet.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T., Wernersson R., Hansen R.B., Horn H., Mercer J., Slodkowicz G. A scored human protein – protein interaction network to catalyze genomic interpretation. Nature Methods Nature Publishing Group. 2016;14:61–64. doi: 10.1038/nmeth.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Björklund A., Lansner A., Grill V.E. Glucose-induced [Ca2+]i abnormalities in human pancreatic islets. Diabetes. 2000;49:1840–1848. doi: 10.2337/diabetes.49.11.1840. [DOI] [PubMed] [Google Scholar]
- 15.Eizirik D.L., Korbutt G.S., Hellerstrom C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the b-cell function. The Journal of Clinical Investigation. 1992;90:1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H., Remedi M.S., Pappan K.L., Kwon G., Rohatgi N., Marshall C.A. Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes. 2009;58:663–672. doi: 10.2337/db07-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandhorst D., Brandhorst H., Maataoui V., Maataoui A., Johnson P.R.V. Anti-caspase-3 preconditioning increases proinsulin secretion and deteriorates posttransplant function of isolated human islets. Apoptosis. 2013;18:681–688. doi: 10.1007/s10495-013-0834-6. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti P., Lupi R., Federici M., Marselli L., Masini M., Boggi U. Insulin secretory function is impaired in isolated human islets carrying the Gly972 -{>}Arg IRS-1 polymorphism. Diabetes. 2002;51:1419–1424. doi: 10.2337/diabetes.51.5.1419. [DOI] [PubMed] [Google Scholar]
- 19.Hodson D.J., Mitchell R.K., Marselli L., Pullen T.J., Brias S.G., Semplici F. ADCY5 couples glucose to insulin secretion in human islets. Diabetes. 2014;63:3009–3021. doi: 10.2337/db13-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen M.B.K., Azad A., Ingvorsen C., Hess K., Hansson M., Grapin-Botton A. Single-cell gene expression analysis of a human ESC model of pancreatic endocrine development reveals different paths to β-cell differentiation. Stem Cell Reports. 2017;9:1246–1261. doi: 10.1016/j.stemcr.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuit F., Van Lommel L., Granvik M., Goyvaerts L., De Faudeur G., Schraenen A. b-cell – specific gene repression: a mechanism to protect against inappropriate or maladjusted insulin secretion? Diabetes. 2012;61:969–975. doi: 10.2337/db11-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonseca S.G., Gromada J., Urano F. Endoplasmic reticulum stress and pancreatic beta cell death. Trends in Endocrinology and Metabolism. 2011;22:266–274. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndiaye F.K., Ortalli A., Canouil M., Huyvaert M., Salazar-Cardozo C., Lecoeur C. Expression and functional assessment of candidate type 2 diabetes susceptibility genes identify four new genes contributing to human insulin secretion. Molecular Metabolism Elsevier GmbH. 2017;6:459–470. doi: 10.1016/j.molmet.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruzzone R., Tamburrano G., Lala A., Mauceri M., Annibale B., Roma S. Effect of Bombesin on plasma insulin, pancreatic glucagon, and gut glucagon in man. Journal of Clinical Endocrinology and Metabolism. 1983;56:643–647. doi: 10.1210/jcem-56-4-643. [DOI] [PubMed] [Google Scholar]
- 25.Ipp E., Unger R.H. Bombesin stimulates the release of insulin and glucagon, but not pancreatic somatostatin, from the isolated perfused dog pancreas. Endocrine Research Communications. 1979;6:37–42. doi: 10.3109/07435807909070882. [DOI] [PubMed] [Google Scholar]
- 26.Pettersson M., Ahren B. Gastrin releasing peptide (GRP): effects on basal and stimulated insulin and glucagon secretion in the mouse. Peptides. 1987;8:55–60. doi: 10.1016/0196-9781(87)90165-3. [DOI] [PubMed] [Google Scholar]
- 27.Taminato T., Seino Y., Goto Y., Matsukura S., Imura H., Sakura N. Bombesin inhibits insulin release from isolated pancreatic islets of rats in vitro. Endocrinology. 1978;25:305–307. doi: 10.1507/endocrj1954.25.305. [DOI] [PubMed] [Google Scholar]
- 28.Filipsson K., Tornøe K., Holst J., Ahren B. Pituitary adenylate cyclase-activating polypeptide stimulates insulin and glucagon secretion in humans *. Journal of Clinical Endocrinology and Metabolism. 1997;82:3093–3098. doi: 10.1210/jcem.82.9.4230. [DOI] [PubMed] [Google Scholar]
- 29.Yada T., Sakurada M., Filipsson K., Kikuchi M., Ahren B. Intraperitoneal PACAP administration decreases blood glucose in GK rats, and in normal and high fat diet mice. Annals of the New York Academy of Sciences. 2000;921:259–263. doi: 10.1111/j.1749-6632.2000.tb06974.x. [DOI] [PubMed] [Google Scholar]
- 30.Herzig K.H., Louie D.S., Owyang C. In vivo action of Bombesin on exocrine pancreatic secretion in the Rat: independent of cholecystokinin and cholinergic mediation. Pancreas. 1988;3:292–296. doi: 10.1097/00006676-198805000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Howard J.M., Jensen R.T., Gardner J.D. Bombesin-induced residual stimulation release from mouse pancreatic acini of amylase. American Journal of Physiology. 1985;248:196–199. doi: 10.1152/ajpgi.1985.248.2.G196. [DOI] [PubMed] [Google Scholar]
- 32.Hansen L., Deacon C.F., Ørskov C., Holst J.J. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine *. Endocrinology. 1999;140:5356–5363. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y., Fang S., Zhao S., Wang X., Wang D., Ma M. A recombinant slow-release PACAP-derived peptide alleviates diabetes by promoting both insulin secretion and actions. Biomaterials. 2015;51:80–90. doi: 10.1016/j.biomaterials.2015.01.064. [DOI] [PubMed] [Google Scholar]
- 34.Sakurai Y., Shintani N., Hayata A., Hashimoto H., Baba A. Trophic effects of PACAP on pancreatic Islets: a mini-review. Journal of Molecular Neuroscience. 2011;43:3–7. doi: 10.1007/s12031-010-9424-z. [DOI] [PubMed] [Google Scholar]
- 35.Hamagami K. ichi, Sakurai Y., Shintani N., Higuchi N., Ikeda K., Hashimoto H. Over-expression of pancreatic pituitary adenylate cyclase – activating polypeptide (PACAP) aggravates cerulein-induced acute pancreatitis in mice. Journal of Pharmacological Sciences. 2009;110:451–458. doi: 10.1254/jphs.09119fp. [DOI] [PubMed] [Google Scholar]
- 36.Mussmann R., Geese M., Harder F., Kegel S., Andag U., Lomow A. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells *. Journal of Biological Chemistry. 2007;282:12030–12037. doi: 10.1074/jbc.M609637200. [DOI] [PubMed] [Google Scholar]
- 37.Shen W., Taylor B., Jin Q., Nguyen-Tran V., Meeusen S., Zhang Y.Q. Inhibition of DYRK1A and GSK3B induces human b-cell proliferation. Nature Communications. 2015;6 doi: 10.1038/ncomms9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Ouaamari A., Dirice E., Gedeon N., Qian W.J., Remold-O'Donnell E., Kulkarni R.N. SerpinB1 promotes pancreatic b cell proliferation article SerpinB1 promotes pancreatic b cell proliferation. Cell Metabolism. 2016;23:194–205. doi: 10.1016/j.cmet.2015.12.001. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Ronde J.J., Lips E.H., Mulder L., Vincent A.D., Wesseling J., Nieuwland M. SERPINA6, BEX1, AGTR1, SLC26A3, and LAPTM4B are markers of resistance to neoadjuvant chemotherapy in HER2-negative breast cancer. Breast Cancer Research and Treatment. 2013;137:213–223. doi: 10.1007/s10549-012-2340-x. [DOI] [PubMed] [Google Scholar]
- 40.Shen N., Gong J., Wang Y., Tian J., Qian J., Zou L. Integrative genomic analysis identifies that SERPINA6- rs1998056 regulated by FOXA/ER a is associated with female hepatocellular carcinoma. PLoS One. 2014;9:1–7. doi: 10.1371/journal.pone.0107246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang A.C.M., Doherty J., Huschtscha L.I., Redvers R., Restall C., Reddel R.R. STC1 expression is associated with tumor growth and metastasis in breast cancer. Clinical & Experimental Metastasis. 2015;32:15–27. doi: 10.1007/s10585-014-9687-9. [DOI] [PubMed] [Google Scholar]
- 42.Krizhanovskii C., Kristinsson H., Elksnis A., Wang X., Bergsten P., Scharfmann R. EndoC-βH1 cells display increased sensitivity to sodium palmitate when cultured in DMEM/F12 medium. Islets Taylor & Francis. 2017;0:1–6. doi: 10.1080/19382014.2017.1296995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staaf J., Ubhayasekera S.J.K.A., Sargsyan E., Chowdhury A., Kristinsson H., Manell H. Initial hyperinsulinemia and subsequent β-cell dysfunction is associated with elevated palmitate levels. Pediatric Research IOP Publishing. 2016;80:264–274. doi: 10.1038/pr.2016.80. [DOI] [PubMed] [Google Scholar]
- 44.Hall E., Volkov P., Dayeh T., Bacos K., Rönn T., Nitert M.D. Effect of palmitate on genome-wide mRNA expression and DNA methylation patterns in human pancreatic islet Effects of palmitate on genome-wide mRNA expression and DNA methylation patterns in human pancreatic islets. BMC Medicine. 2014;12 doi: 10.1186/1741-7015-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunha D.A., Hekerman P., Ladrière L., Bazarra-castro A., Ortis F., Wakeham M.C. Initiation and execution of lipotoxic ER stress in pancreatic β-cells. Journal of Cell Science. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oslowski C.M., Hara T., O'Sullivan-Murphy B., Lu S., Hara M., Ishigaki S. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metabolism. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasnain S.Z., Borg D.J., Harcourt B.E., Tong H., Sheng Y.H., Ng C.P. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nature Medicine Nature Publishing Group. 2014;20:1417–1428. doi: 10.1038/nm.3705. [DOI] [PubMed] [Google Scholar]
- 48.Lecomte M.J., Pechberty S., Machado C., Da Barroca S., Ravassard P., Scharfmann R. Aggregation of engineered human β -cells into pseudoislets: insulin secretion and gene expression profile in normoxic and hypoxic milieu. Cell Medicine. 2016;8:99–112. doi: 10.3727/215517916X692843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spelios M.G., Kenna L.A., Wall B., Akirav E.M. In vitro formation of b cell pseudoislets using islet- derived endothelial cells. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0072260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai C., Brissova M., Hang Y., Thompson C., Poffenberger G., Shostak A. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55:707–718. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benner C., van der Meulen T., Cacéres E., Tigyi K., Donaldson C.J., Huising M.O. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorrez L., Laudadio I., Van Deun K., Thorrez L., Laudadio I., Van Deun K. Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Research. 2011;21:95–105. doi: 10.1101/gr.109173.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muraro M.J., Dharmadhikari G., Grün D., Groen N., Dielen T., Jansen E. A single-cell transcriptome atlas of the human pancreas. Cell Systems. 2016;3:385–394. doi: 10.1016/j.cels.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y.J., Schug J., Won K.J., Liu C., Naji A., Avrahami D. Single cell transcriptomics of the human endocrine pancreas. Diabetes. 2016:1–49. doi: 10.2337/db16-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao R., Ustinov J., Pulkkinen M.A., Lundin K., Korsgren O., Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- 56.Dorrell C., Schug J., Canaday P.S., Russ H.A., Tarlow B.D., Grompe M.T. Human islets contain four distinct subtypes of β cells. Nature Communications Nature Publishing Group. 2016;7:1–9. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.