Abstract
Objective
The very low-density lipoprotein receptor (VLDLR) plays an important role in the development of hepatic steatosis. In this study, we investigated the role of Peroxisome Proliferator-Activated Receptor (PPAR)β/δ and fibroblast growth factor 21 (FGF21) in hepatic VLDLR regulation.
Methods
Studies were conducted in wild-type and Pparβ/δ-null mice, primary mouse hepatocytes, human Huh-7 hepatocytes, and liver biopsies from control subjects and patients with moderate and severe hepatic steatosis.
Results
Increased VLDLR levels were observed in liver of Pparβ/δ-null mice and in Pparβ/δ-knocked down mouse primary hepatocytes through mechanisms involving the heme-regulated eukaryotic translation initiation factor 2α (eIF2α) kinase (HRI), activating transcription factor (ATF) 4 and the oxidative stress-induced nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathways. Moreover, by using a neutralizing antibody against FGF21, Fgf21-null mice and by treating mice with recombinant FGF21, we show that FGF21 may protect against hepatic steatosis by attenuating endoplasmic reticulum (ER) stress-induced VLDLR upregulation. Finally, in liver biopsies from patients with moderate and severe hepatic steatosis, we observed an increase in VLDLR levels that was accompanied by a reduction in PPARβ/δ mRNA abundance and DNA-binding activity compared with control subjects.
Conclusions
Overall, these findings provide new mechanisms by which PPARβ/δ and FGF21 regulate VLDLR levels and influence hepatic steatosis development.
Keywords: VLDLR, PPAR, FGF21, ATF4, ER stress
Abbreviations: ATF4, activating transcription factor 4; Chop, C/EBP homologous protein; eIF2α, eukaryotic translation initiation factor 2α; FGF21, fibroblast growth factor 21; HFD, high-fat diet; HRI, heme-regulated eIF2α kinase; NAFLD, non-alcoholic fatty liver disease; PPAR, peroxisome proliferator-activated receptor
Highlights
-
•
PPARβ/δ deficiency leads to increased levels of hepatic VLDLR levels.
-
•
FGF21 protects against hepatic steatosis by attenuating ER stress-induced VLDLR upregulation.
-
•
Human hepatic steatosis is accompanied by increased levels of VLDLR and reduced expression of PPARβ/δ.
-
•
PPARβ/δ and FGF21 may influence NAFLD development by regulating VLDLR levels.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is currently the most common liver disorder, and its growing prevalence has increased to reach worldwide epidemic proportions [1]. NAFLD encompasses a spectrum of liver injuries ranging from hepatic steatosis, defined by the excessive accumulation of triglycerides in the liver, to the most severe condition of non-alcoholic steatohepatitis (NASH). In addition, NAFLD is an important risk factor for the development of obesity-related pathologies including insulin resistance, type 2 diabetes mellitus (T2DM), and cardiovascular diseases [2].
Hepatic triglyceride levels are regulated by multiple mechanisms such as de novo synthesis, fatty acid oxidation, lipolysis, dietary fat consumption, and the secretion and hepatic delivery of lipoprotein particles [3], [4], [5]. Recently, it has been reported that very low-density lipoprotein receptor (VLDLR) plays an important role in the development of hepatic steatosis [6]. VLDLR belongs to the low-density lipoprotein (LDL) receptor family and is widely expressed in the brain, heart, skeletal muscle, and adipose tissue, whereas its expression is very low in the liver under normal conditions [7], [8]. This receptor binds apolipoprotein E (apoE) triglyceride-rich lipoproteins such as chylomicrons, VLDL, and intermediate density lipoproteins, leading to lipid entry into the cell through lipoprotein lipase (LPL)-dependent lipolysis or receptor-mediated endocytosis [9], [10], [11], [12]. As a result, a link has been established between VLDLR levels and plasma triglyceride levels [13]. VLDLR-null mice are leaner, display normal blood lipids [14] and are protected from obesity induced by HFD feeding or leptin deficiency [15]. However, following fasting or exposure to a HFD, these animals show increased plasma triglyceride levels [15], [16]. In recent years, it has been reported that VLDLR is regulated by several transcription factors, including Peroxisome Proliferator-Activated Receptor (PPAR) γ in adipose tissue [12] and hypoxia-inducible factor 1α (HIF-1α) in the heart [17], contributing to lipid deposition in both tissues. In liver, the upregulation of VLDLR levels has been reported to be dependent on the activation of oxidative stress-induced nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in alcoholic liver disease [18], whereas stimulation of activating transcription factor 4 (ATF4) signaling during endoplasmic reticulum (ER) stress induces hepatic steatosis via increase VLDLR by enhancing lipoprotein delivery to the liver [6]. In addition, hepatic VLDLR upregulation plays an essential role in the triglyceride-lowering effect of fenofibrate through PPARα activation [19]. However, little is known about the effects of PPARβ/δ on VLDLR regulation in the liver. PPARβ/δ is a ligand-activated transcription factor involved in the regulation of glucose and lipid homeostasis [20], and it has been proposed as a therapeutic target for the treatment of metabolic syndrome [21]. Thus, genetic manipulation of PPARβ/δ as well as its activation by agonists attenuate dyslipidemia and hyperglycemia, improve whole-body insulin sensitivity, and prevent diet-induced obesity [22]. In this study, we show that Pparβ/δ deficiency regulates VLDLR levels through Nrf2 and ATF4-dependent mechanisms, whereas fibroblast growth factor 21 (FGF21) deficiency exacerbates ER stress-induced VLDLR levels, contributing to the progression of hepatic steatosis. Finally, our findings show that in humans with severe hepatic steatosis, increased levels of VLDLR accompany a reduction in PPARβ/δ activity.
2. Research design and methods
2.1. Reagents
Control, VLDLR, HRI, ATF4, and Nrf2 siRNA were purchased from Santa Cruz (Dallas, TX) and PPARβ/δ siRNA from GE Dharmacon (Lafayette, CO). Mouse FGF21 neutralizing antibody was purchased from Antibody and Immunoassays Services (Hong Kong, China) and human recombinant FGF21 from R&D Systems (Minneapolis, MN). Triglyceride levels were measured using a commercial kit (Sigma, St. Louis, MO).
2.2. Mice
Male (8–9-wk old) Pparβ/δ knockout (Pparβ/δ−/−) mice and their wild-type littermates (Pparβ/δ+/+) with the same genetic background (C57BL/6X129/SV) [23] and an initial weight of 20–25 g were fed a standard diet. Genotyping was performed as previously described [23]. Wild-type and Pparβ/δ-null mice were treated for 24 h through i.p. injection with DMSO (vehicle) or tunicamycin (3 mg kg−1 body weight). Likewise, male wild-type and Pparβ/δ-null mice at 12 wk. of age were injected intraperitoneally with IgG (9 μg/mouse) or a neutralizing antibody (9 μg/mouse) against FGF21 [24] together with DMSO or tunicamycin (3 mg kg−1 body weight) and were sacrificed at 14 h after treatment. In addition, male wild-type and Pparβ/δ-null mice were either fed a 30% fructose solution or plain tap water for 12 wk, as previously described [25]. The hepatic content of malondialdehyde (MDA) and hydrogen peroxide (H2O2) were determined using the lipid peroxidation (MDA) and Peroxidetect assay kits (Sigma), respectively.
Male (10-wk old) knockout (Fgf21−/−) mice [B6N; 129S5-Fgf21tm1Lex/Mmcd] and their wild-type littermates (Fgf21+/+) were obtained from the Mutant Mouse Regional Resource Center (MMRRC). For examination of the effect of FGF21 on VLDLR levels, male C57BL/6 mice at 12 wk of age were treated with DMSO or tunicamycin (1 mg kg−1 body weight), and starting at 6 h before tunicamycin injection, recombinant mouse FGF21 (PeproTech, London, UK) was administered intraperitoneally at 1 mg kg−1 body weight for a total of five times every 6 h. Mice were sacrificed at 24 h after tunicamycin treatment. All animals were killed under anesthetic conditions, and livers were snap-frozen in liquid nitrogen immediately after resection and stored at −80 °C. The research complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All procedures were approved by the University of Barcelona Bioethics Committee, as stated in Law 5/21 July 1995 passed by the Generalitat de Catalunya.
2.3. Cell culture
Human Huh-7 cells (a kindly gift from Dr. Mayka Sanchez from Josep Carreras Leukaemia Research Institute) were cultured in DMEM supplemented with 10% serum, at 37 °C/5% CO2. Primary mouse hepatocytes were isolated from non-fasting male C57BL/6 mice (10–12 weeks old) by perfusion with collagenase as described elsewhere [26]. siRNA transfections were performed with Lipofectamine 2000 (Life Technologies).
2.4. Human samples
Subjects were recruited by the Gastroenterology Department at the Shahid Beheshti University of Medical Sciences (Tehran, Iran) with the approval of the Ethical Committee of the University Review Board of the participating Taleghani Hospital. NAFLD subjects (Table S1) were diagnosed according to WHO criteria. Patients were considered as having potential liver steatosis if they had abnormal liver blood tests. Alcohol consumption had to be less than 20 g/day for the past 5 years, as assessed using a standard questionnaire. In addition, liver specimen had to be compatible with NAFLD [27], without any pattern suggestive of other cause. Patients were not included if they had another cause of chronic liver disease, complicated cirrhosis, or received putative antifibrotic treatment in the past 6 months. Liver biopsies were performed at Taleghani Hospital in 2012–2013 using a Meghini 16 swg (1.6 mm) × 70 mm syringe. The material consisted of 15 needle biopsies from adult patients. Specimens were immediately sent for pathology assessment by routine procedures; two biopsies were required to obtain an acceptable specimen. All the biopsies were reviewed by the pathologist and the presence of steatosis was graded in grade 0 with <5% of hepatocytes presenting steatosis (control group) (n = 5), grade 2 with 33–66% of hepatocytes presenting steatosis (n = 4), and grade 3 with more than 66% steatotic hepatocytes (n = 6). Written informed consent was obtained from each patient included in the study and the study protocol conformed to the ethical guidelines of the 2013 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
2.5. RNA preparation and quantitative RT-PCR
The relative levels of specific mRNAs were assessed by Real-Time RT-PCR, as previously described [28]. Primer sequences used for Real-Time RT-PCR are displayed in Table S2.
2.6. Immunoblotting
Isolation of total and nuclear extracts was performed as described elsewhere [28]. Proteins (30 μg) were separated by SDS-PAGE on 10% acrylamide separation gels and transferred to Immobilon polyvinylidene difluoride membranes (Millipore). Western blot analysis was performed using antibodies against VLDLR (sc-18824), Nrf2 (sc-722), Nqo1 (sc-393736), ATF4 (sc-200) (Santa Cruz), VLDLR (AF2258) (R&D system), eIF2α (9722), phospho-eIF2α (Ser51) (9721), IgG control (2729S) (Cell Signaling Technology Inc., Danvers, MA), actin (A5441) (Sigma–Aldrich, Madrid, Spain). Detection was achieved using the Western Lightning® Plus-ECL chemiluminescence kit (PerkinElmer, Waltham, MA, USA). The equal loading of proteins was assessed by Ponceau S staining. The size of detected proteins was estimated using protein molecular-mass standards (Bio-Rad).
2.7. Electrophoretic mobility shift assay
The electrophoretic mobility shift assay (EMSA) was performed using double-stranded oligonucleotide for the consensus binding site of PPRE (Santa Cruz Biotechnology). Nuclear extracts (NE) were isolated as previously reported [28]. Oligonucleotides were labeled by incubating the following reaction at 37 °C for 2 h: 2 μL oligonucleotide (1.75 pmol/μL), 2 μL of 5X kinase buffer, 1 μL of T4 polynucleotide kinase (10 U/μL), and 2.5 μL [γ-32P] ATP (3,000 Ci/mmol at 10 mCi/mL). The reaction was stopped by adding 90 μL of TE buffer (10 mmol/L Tris–HCl, pH 7.4, and 1 mmol/L EDTA). To separate the labeled probe from the unbound ATP, the reaction mixture was eluted in a Nick column (GE Healthcare, Barcelona, Spain) according to the manufacturer's instructions. Five micrograms of crude nuclear protein was incubated for 10 min on ice in binding buffer (10 mmol/L Tris–HCl, pH 8.0, 25 mmol/L KCl, 0.5 mmol/L dithiothreitol, 0.1 mmol/L EDTA, pH 8.0, 5% (v:v) glycerol, 5 mg/mL BSA, and 50 μg/ml poly[dI-dC]) in a final volume of 15 μL. Then, specific competitor oligonucleotide or antibody for supershift assays were added and incubated for 15 min on ice. Subsequently, the labeled probe (100,000 cpm) was added and the reaction was incubated for an additional 15 min on ice. Finally, protein-DNA complexes were resolved by electrophoresis at 4 °C on 5% (w:v) polyacrylamide gels in 0.5X Tris-borate-EDTA buffer and subjected to autoradiography.
2.8. Hematoxylin-eosin and Oil Red and staining
We performed hematoxylin-eosin and Oil Red O staining as previously reported [28]. ORO staining was quantified using Image J software.
2.9. Statistical analyses
Results are expressed as means ± S.D. Significant differences were established by two-way ANOVA using the GraphPad Instat program (GraphPad Software V5.01) (GraphPad Software Inc., San Diego, CA). When significant variations were found by two-way ANOVA, the Tukey–Kramer multiple comparison post-test was performed. Differences were considered significant at p < 0.05.
3. Results
3.1. Pparβ/δ−/– mice show increased hepatic VLDLR levels
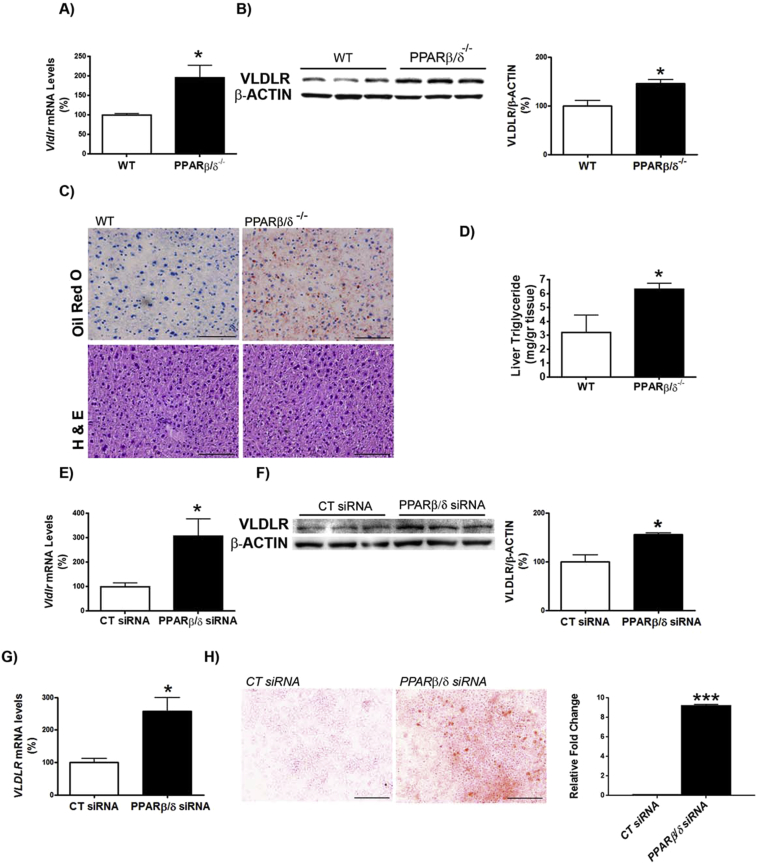
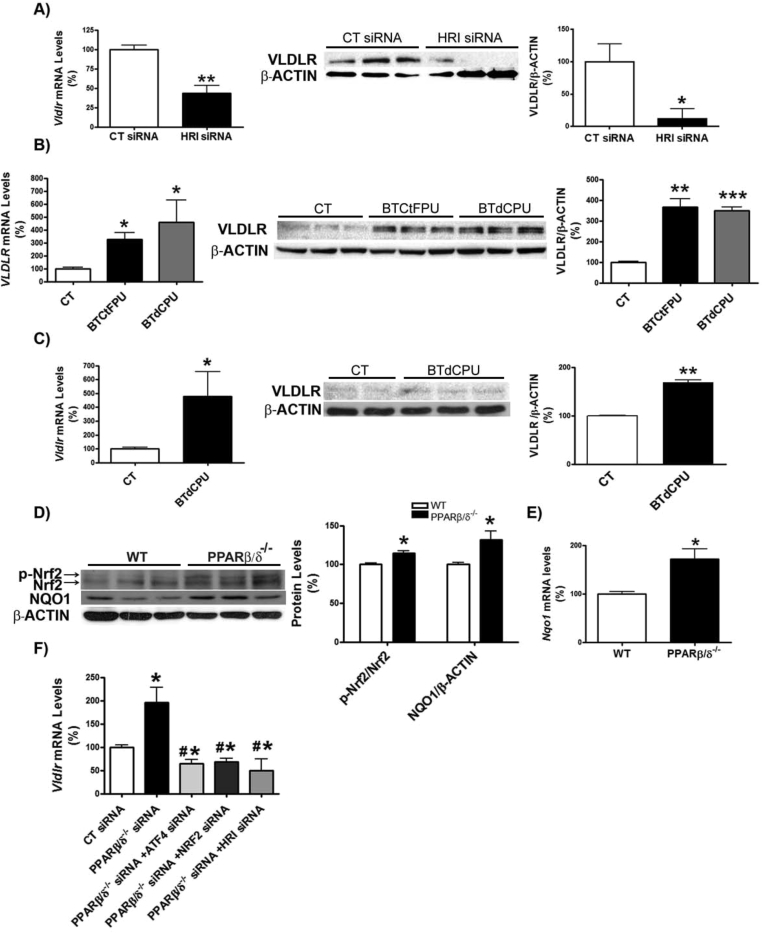
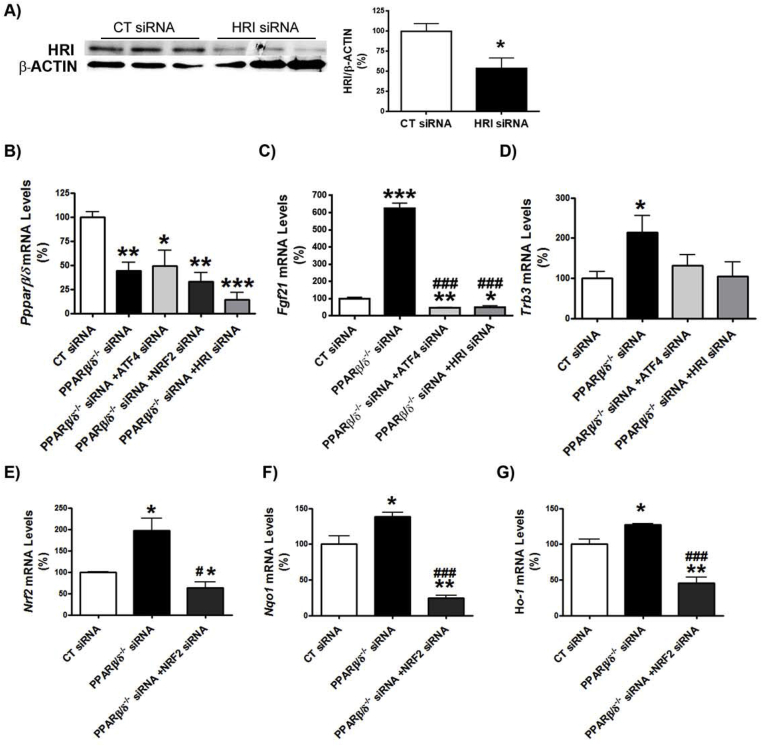
First, we examined whether Pparβ/δ-deficiency affected VLDLR levels. VLDLR mRNA and protein levels were increased in the livers of Pparβ/δ−/− mice compared with wild-type littermates (Figure 1A and B). This increase in VLDLR was accompanied by the presence of hepatic steatosis in Pparβ/δ−/− mice compared to wild-type littermates, as demonstrated by ORO and hematoxylin-eosin staining and hepatic triglyceride quantification (Figure 1C and D), whereas differences in plasma triglyceride levels did not reach statistical significance (data not shown). In accordance with the observations in the liver of Pparβ/δ-deficient mice, siRNA knockdown of Pparβ/δ in primary hepatocytes (Figure S1B) led to enhanced Vldlr mRNA and protein levels (Figure 1E and F). Similarly, transfection of human Huh-7 hepatocytes with siRNA against PPARβ/δ caused a significant increase in VLDLR mRNA levels (Figure 1G) and in cellular lipid accumulation (Figure 1H). Next, we focused on ATF4 as the potential transcription factor responsible for the increase in VLDLR in Pparβ/δ-deficient cells. Although ATF4 is activated by ER stress through eukaryotic translation initiation factor 2α (eIF2α), the increase in phosphorylated eIF2α and activation of its downstream ATF4 signaling pathway can occur independently of ER stress, since eIF2α can also be phosphorylated by other kinases, including the heme-regulated eIF2α kinase (HRI) [29]. Interestingly, Pparβ/δ−/− mice showed increased levels of HRI, which in turn activates the eIF2α-ATF4 pathway [28], suggesting that HRI might also regulate VLDLR levels. In agreement with this, siRNA knockdown of Hri (Figure S1A) in primary hepatocytes caused a reduction in VLDLR mRNA and protein levels (Figure 2A). Moreover, two well-known HRI activators [30], BTCtFPU and BTdCPU, upregulated VLDLR mRNA and protein levels in human Huh-7 hepatocytes (Figure 2B), and BTdCPU also upregulated VLDLR levels in the liver of mice treated with this compound (Figure 2C).
Figure 1.
VLDLR abundance is increased in liver of Pparβ/δ-null mice and in primary hepatocytes following knockdown of Pparβ/δ. Livers from male wild-type (WT) and Pparβ/δ-null mice were used (n = 6 per group). A, Assessment by quantitative real-time RT-PCR of hepatic Vldlr. B, Immunoblot analysis of liver VLDLR. C, Oil Red O and hematoxylin-eosin staining of livers. Scale bar: 100 μm. D, Liver triglyceride levels. Data are presented as the mean ± S.D. (n = 6 per group) relative to the wild-type mice. Vldlr mRNA abundance (E) and protein levels (F) in primary hepatocytes transfected with control siRNA or Pparβ/δ siRNA for 24 h. VLDLR mRNA levels (G) and Oil Red O staining (H) in Huh-7 hepatocytes transfected with control siRNA or Pparβ/δ siRNA for 24 h. Levels are presented as the mean ± S.D. (n = 3–5 per group). *p < 0.05 vs. wild-type mice or control siRNA. Scale bar: 100 μm.
Figure 2.
HRI regulates VLDLR abundance in hepatocytes. A, primary hepatocytes were transfected with control or Hri siRNA for 24 h, and the mRNA abundance and protein levels of VLDLR were assessed. *p < 0.05 and **p < 0.01 vs. control siRNA. B, Huh-7 hepatocytes were incubated for 16 h in the absence (Control, CT) or presence of 10 μmol/L of either BTdCPU or BTCtFPU and the mRNA abundance and protein levels of VLDLR were analyzed. ***p < 0.001, **p < 0.01 and *p < 0.05 vs. control. C, mRNA abundance and protein levels of VLDLR in liver of mice treated with DMSO (vehicle) or BTdCPU (70 mg kg−1 day−1) for 7 days (n = 6 per group). ***p < 0.001, **p < 0.01 and *p < 0.05 vs. control cells or control mice. Immunoblot analyses of total and phospho-Nrf2 and NQO1 (D) and mRNA abundance of Nqo1 (E) in liver from male wild-type (WT) and Pparβ/δ-null mice (n = 6 per group). *p < 0.05 vs. control. F, VLDLR mRNA abundance in primary hepatocytes transfected with control, Pparδ, Atf4, Nrf2 and Hri siRNA for 24 h *p < 0.05 vs. control siRNA. #p < 0.05 vs. Pparδ siRNA.
Next, we checked whether other transcription factors such as Nrf2, known to be involved in the upregulation of hepatic VLDLR [18], might be responsible for the increase in the expression of this gene in the livers of Pparβ/δ-null mice. Interestingly, livers of Pparβ/δ−/− mice showed increased levels of phosphorylated Nrf2, an indicator of the activity of this transcription factor [31], compared with wild-type littermates (Figure 2D). In agreement with this, the mRNA and protein levels of the Nrf2-target gene NAD(P)H quinone dehydrogenase 1 (Nqo1) were also upregulated (Figure 2D and E).
To confirm the involvement of HRI, ATF4, and Nrf2 in the upregulation of Vldlr in the context of Pparβ/δ deficiency, we performed siRNA studies in primary hepatocytes. Knockdown of Pparβ/δ increased the expression of Vldlr, but this increase was prevented by siRNA transfection against Atf4, Nrf2, or Hri (Figure 2F). Two ATF4-target genes, Trb3 [28] and Fgf21 [32], were upregulated by Pparβ/δ knockdown, but this was prevented by transfecting siRNA against either Atf4 or Hri (Figure S1C and D). Similarly, expression of Nrf2 and two of its target genes, Nqo1 and Ho-1, increased following Pparβ/δ knockdown and this was prevented by siRNA transfection against Nrf2 (Figure S1E–G). These findings indicate that Pparβ/δ deficiency increases Vldlr expression through the HRI-eIF2α-ATF4 and Nrf2 pathways.
3.2. Pparβ/δ deficiency exacerbates hepatic steatosis and VLDLR upregulation caused by ER stress
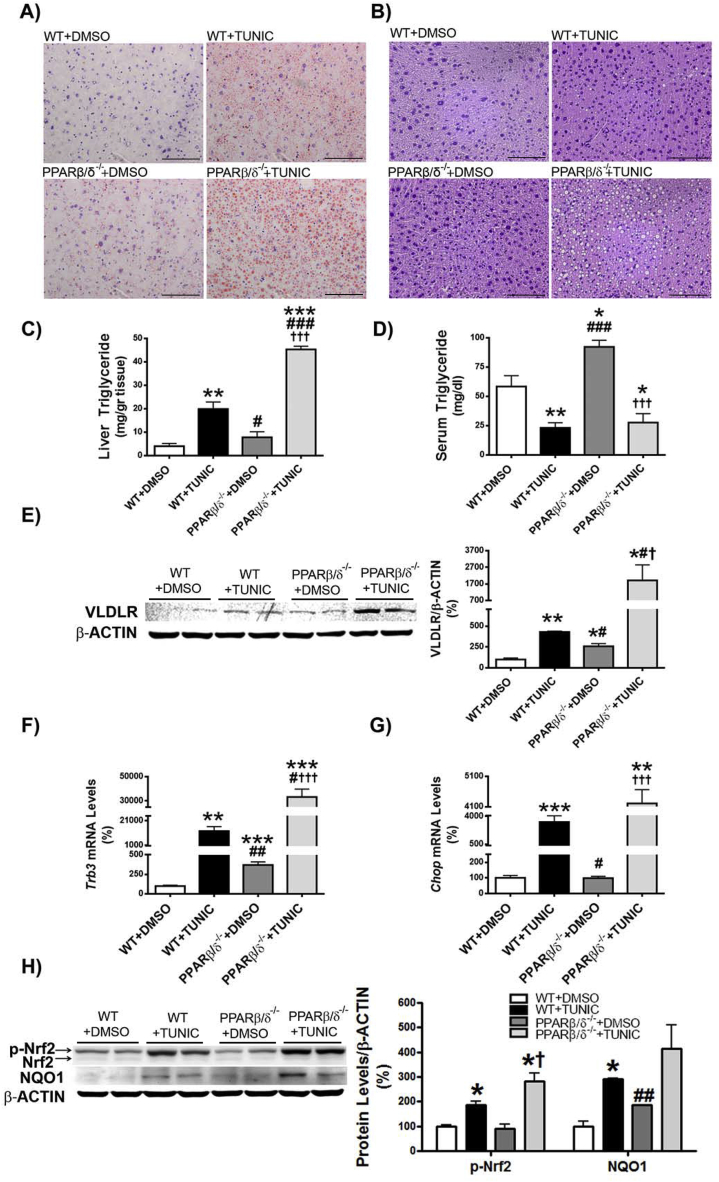
Given that ER stress induces hepatic steatosis via increased expression of VLDLR through eIF2α-ATF4 [6], we hypothesized that Pparβ/δ deficiency may exacerbate liver steatosis in the context of ER stress by potentiating the increase in VLDLR levels. To test this, wild-type and Pparβ/δ−/− mice were treated with the ER stressor tunicamycin for 24 h. Tunicamycin increased hepatic triglyceride accumulation in wild-type mice (approximately 5-fold increase, p < 0.01) compared with vehicle-treated animals, but this accumulation was exacerbated in tunicamycin-treated Pparβ/δ−/− mice (approximately 8-fold increase, p < 0.001 compared with tunicamycin-treated wild-type mice) (Figure 3A–C). In agreement with an increased lipoprotein delivery to the liver through VLDLR as the mechanism responsible for hepatic steatosis, plasma triglyceride levels were reduced in tunicamycin-treated wild-type mice (60% reduction, p < 0.01) compared with vehicle-treated wild-type mice. This reduction was exacerbated in tunicamycin-treated Pparβ/δ−/− mice (70% reduction, p < 0.001) compared with vehicle-treated Pparβ/δ−/− mice (Figure 3D), which is consistent with the higher increase in VLDLR protein levels in the livers of tunicamycin-treated Pparβ/δ−/− mice (Figure 3E). Likewise, the expression of two additional ATF4-target genes, Trb3 and Chop, was significantly higher in tunicamycin-treated Pparβ/δ−/− mice than in tunicamycin-treated wild-type mice, indicating higher ATF4 activity in the former group (Figure 3F and G). Of note, in contrast to Trb3 expression that was increased in PPARβ/δ-null mice compared to wild-type mice, Chop expression was independent of the genotype and it was only increased following tunicamycin treatment.
Figure 3.
Pparβ/δ deficiency exacerbates hepatic steatosis and VLDLR upregulation caused by ER stress. Oil Red O (A) and hematoxylin-eosin (B) staining of livers from male wild-type (WT) and Pparβ/δ-null mice treated for 24 h through i.p. injection with DMSO (vehicle) or tunicamycin (Tunic) (3 mg kg−1 body weight). Scale bar: 100 μm. C, Liver triglyceride levels. D, Serum triglyceride levels. E, Immunoblot analyses of VLDLR. Trb3 (F) and Chop (G) mRNA abundance. H, immunoblot analyses of total and phospho-Nrf2 and NQO1. Data are presented as the mean ± S.D. (n = 6 per group). ***p < 0.001, **p < 0.01 and *p < 0.05 vs. wild-type animals treated with DMSO (vehicle). ###p < 0.001, ##p < 0.01 and #p < 0.05 vs. wild-type animals treated with tunicamycin. †††p < 0.001 and †p < 0.05 vs. Pparβ/δ-null mice treated with DMSO (vehicle).
Since ER stress can also activate Nrf2 [33], we next evaluated whether this antioxidant transcription factor can contribute to the increase in VLDLR in tunicamycin-treated Pparβ/δ−/− mice. Tunicamycin administration led to significant changes in hepatic phospho-Nrf2 and NQO1 protein levels in wild-type mice, and the increase in phospho-Nrf2 was exacerbated in Pparβ/δ-null mice (Figure 3H). Overall, these findings suggest that ATF4 and Nrf2 activation by tunicamycin in the presence of Pparβ/δ deficiency intensifies the increase in hepatic VLDLR.
3.3. FGF21 protects against hepatic steatosis and the upregulation of VLDLR levels caused by ER stress
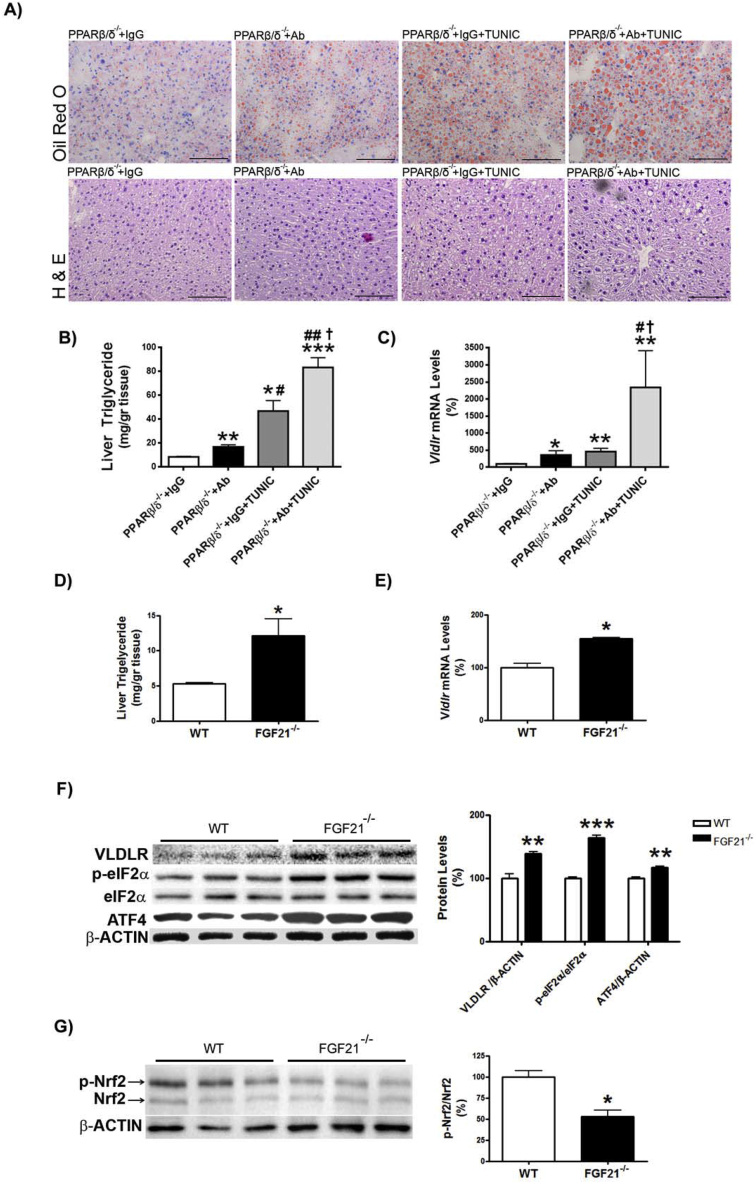
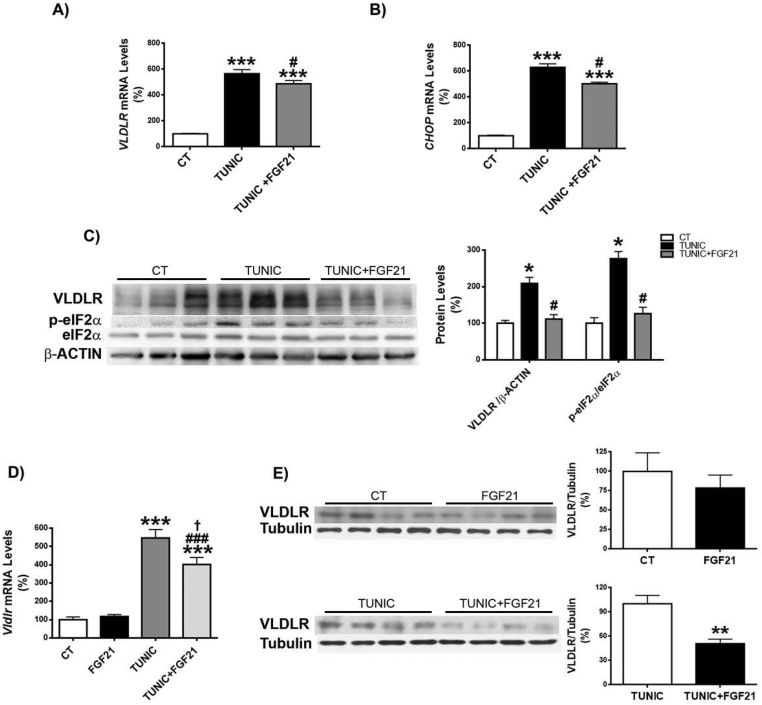
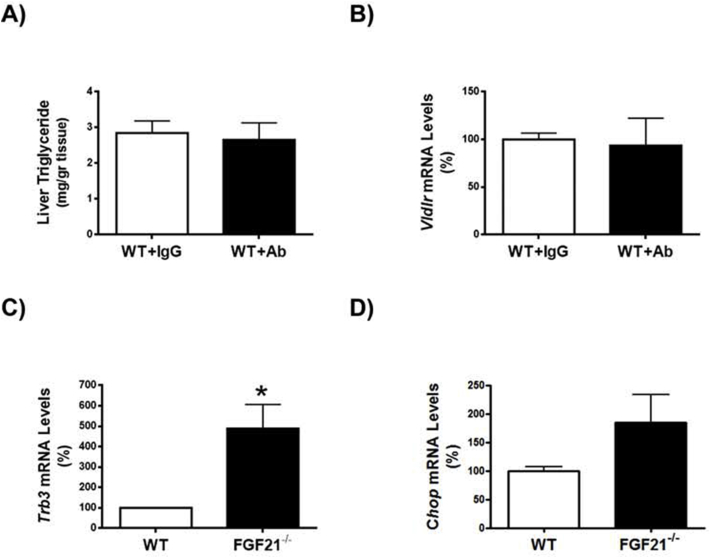
Since FGF21 suppresses the eIF2α-ATF4 pathway through a negative feedback [34], we hypothesized that the reported increase in FGF21 observed in Pparβ/δ-deficient mice [28] might attenuate the increase in the ATF4-target gene Vldlr. To check this, we treated Pparβ/δ-null mice with an FGF21 neutralizing antibody and tunicamycin. Administration of the FGF21 neutralizing antibody to Pparβ/δ−/− mice increased hepatic triglyceride accumulation in both vehicle and tunicamycin-treated mice compared with mice receiving IgG (Figure 4A and B). Consistent with this, Vldlr mRNA levels increased in Pparβ/δ−/− mice injected FGF21 neutralizing antibody compared with mice treated with IgG, especially in tunicamycin-treated mice (Figure 4C). No changes in these parameters were observed in wild-type mice injected with the neutralizing antibody (Figure S2A and B). VLDLR regulation by FGF21 was confirmed in the livers of Fgf21-deficient mice. These mice showed increased hepatic triglyceride accumulation (Figure 4D), a process which has been reported to be age-dependent [35], and Vldlr mRNA (Figure 4E) and protein abundance accompanied by enhanced eIF2α phosphorylation and ATF4 protein levels (Figure 4F). Similarly, the expression levels of two Atf4-target genes, Trb3 and Chop, were also increased in the livers of Fgf21−/− mice compared with wild-type mice (Figure S2C and D), although Chop did not reach statistical significance. In contrast, phosphorylated levels of Nrf2 were reduced in Fgf21−/− mice (Figure 4G), rendering its involvement in VLDLR upregulation unlikely. Finally, we examined the effects of recombinant FGF21 treatment on ER-stress-induced VLDLR levels. In Huh-7 hepatocytes FGF21 significantly reduced the increase in VLDLR and CHOP expression (Figure 5A and B) and in VLDLR protein levels caused by tunicamycin, and these reductions were accompanied by a decrease in the levels of phospho-eIF2α (Figure 5C). Similarly, administration of FGF21 reduced the increase in both the expression and the protein levels of VLDR caused by tunicamycin in liver (Figure 5D and E). These findings suggest that FGF21 may protect against hepatic steatosis by limiting the increase in VLDLR levels via attenuation of the eIF2α-ATF4 pathway.
Figure 4.
Increased Fgf21 expression in liver of Pparβ/δ-null mice attenuates VLDLR abundance. A, Oil Red O and hematoxylin-eosin staining of livers from male wild-type (WT) and Pparβ/δ-null mice injected intraperitoneally with IgG (9 μg/mouse) or a neutralizing antibody (Ab) (9 μg/mouse) against FGF21 together with DMSO or tunicamycin (Tunic) (3 mg kg−1 body weight). Scale bar: 100 μm. Mice were sacrificed at 14 h after treatment. B, Liver triglyceride levels. C, Vldlr mRNA abundance. ***p < 0.001, **p < 0.01 and *p < 0.05 vs. Pparβ/δ-null mice treated with IgG and DMSO. ##p < 0.01 and #p < 0.05 vs. Pparβ/δ-null mice treated with neutralizing antibody against FGF21 and DMSO. †p < 0.05 vs. Pparβ/δ-null mice treated with IgG and tunicamycin. Liver triglyceride levels (D) and Vldlr mRNA abundance (E) in the liver from WT and Fgf21−/− mice. Data are presented as the mean ± S.D. (n = 5 per group). ***p < 0.001, **p < 0.01 and *p < 0.05 vs. wild-type mice. Immunoblot analyses of VLDLR, total and phospho-eIF2α and ATF4 (F) and total and phospho-Nrf2 (G) were performed in liver lysates. Data are presented as the mean ± S.D. (n = 5 per group). ***p < 0.001, **p < 0.01 and *p < 0.05 vs. wild-type mice.
Figure 5.
Recombinant FGF21 protein attenuates the increase in VLDLR levels caused by ER stress. Human Huh-7 hepatocytes were incubated with DMSO (vehicle, control, CT), tunicamycin (TUNIC) (1 μg/ml) or tunicamycin plus recombinant human FGF21 (1 μg/ml) and the mRNA abundance of VLDLR (A) and CHOP (B) and the protein levels of VLDLR and total and phospho-eIF2α (C) were assessed (n = 3 independent experiments). ***p < 0.001 vs. CT cells. #p < 0.05 vs. tunicamycin-treated cells. Analysis of the hepatic levels of VLDLR in mice injected intraperitoneally with vehicle or recombinant mouse FGF21 together with DMSO or tunicamycin. Data are presented as the mean ± S.D. (n = 5 per group). C, Vldlr and Chop mRNA abundance. D, Immunoblot analyses of hepatic VLDLR. ***p < 0.001, **p < 0.01 and *p < 0.05.
3.4. VLDLR upregulation is intensified by fructose feeding in the liver of Pparβ/δ-null mice
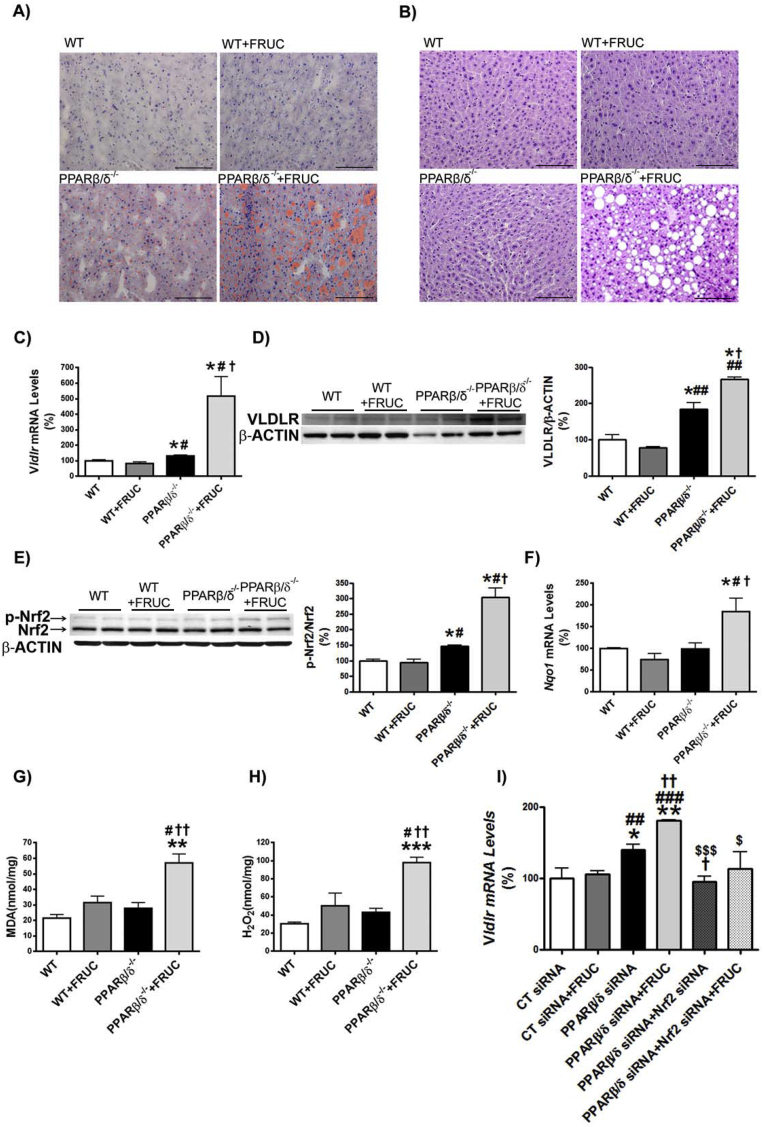
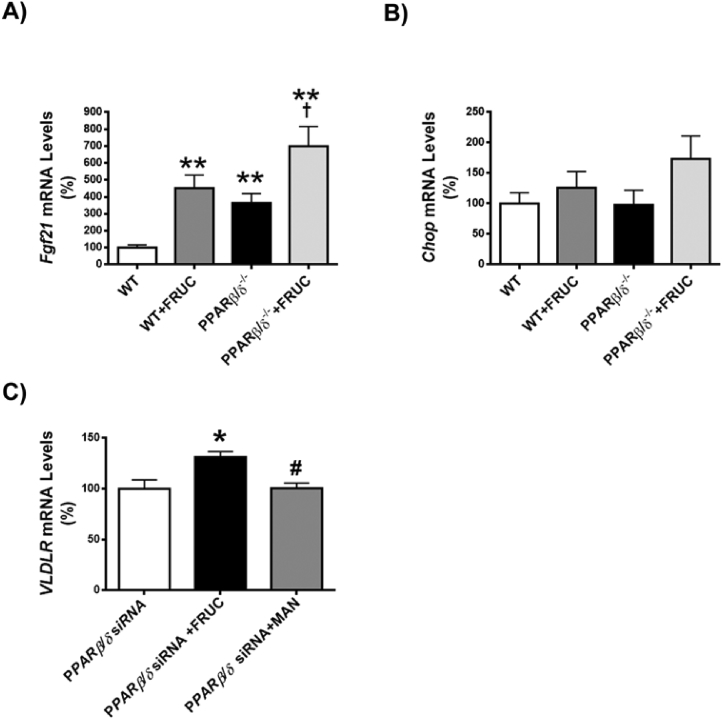
Fructose feeding leads to hepatic steatosis [36]. Consequently, we explored whether VLDLR was involved in the effects of fructose on liver in the context of Pparβ/δ deficiency. As previously shown [25], feeding wild-type mice (C57BL/6X129/SV genetic background) with fructose did not result in hepatic steatosis (Figure 6A and B). However, Pparβ/δ−/− mice exposed to fructose exhibited a clear and intense steatosis. When hepatic VLDLR mRNA and protein levels were assessed, water-fed Pparβ/δ−/− mice showed a significant increase that was intensified by fructose feeding (Figure 6C and D). As previously reported [37], fructose feeding increased Fgf21 expression in liver of wild-type mice (Figure S3A), indicating that despite the lack of induction of triglyceride accumulation in the liver of wild-type mice, fructose feeding was efficacious. This increase was exacerbated in Pparβ/δ-deficient mice. In contrast, expression of the ATF4-target gene Chop was unaffected in fructose-fed Pparβ/δ−/− mice (Figure S3B), rendering it unlikely that this pathway was involved in the VLDLR upregulation observed in the liver of these mice. Consistent with the trend observed in VLDLR, phospho-Nrf2 levels and the expression of its target gene Nqo1 were elevated in livers of fructose-fed Pparβ/δ−/− mice (Figure 6E and F). The oxidative stress status measured by the lipid peroxidized product MDA and H2O2 was significantly increased only in fructose-fed Pparβ/δ−/− mice (Figure 6G and H), suggesting that enhanced ROS levels was the stimulus responsible for the increase in the activity of this redox transcription factor. To clearly demonstrate the involvement of Nrf2, the expression of this gene and that of Pparβ/δ was knocked down by siRNA transfection in mouse primary hepatocytes. As previously shown, Pparβ/δ knockdown increased the expression of Vldlr and this increase was intensified by incubation with fructose (Figure 6I). However, Nrf2 knockdown abrogated the increase caused by the reduction in Pparβ/δ expression and incubation with fructose (Figure 6I). The increase in VLDLR expression was also observed in human hepatocytes transfected with siRNA against PPARβ/δ and this effect was specific for fructose, since it was not observed in hepatocytes exposed to a related carbohydrate such as mannitol (Figure S3C). Overall, these data suggest that in the context of Pparβ/δ deficiency, fructose induces ROS production, a well-known activator of Nrf2, which in turn increases VLDLR levels and ultimately produces hepatic steatosis by increasing lipoprotein delivery to the liver.
Figure 6.
VLDLR upregulation is intensified by fructose feeding in the liver of Pparβ/δ-null mice. Oil Red O (A) and hematoxylin-eosin (B) staining of livers from male wild-type (WT) and Pparβ/δ-deficient mice (PPARβ/δ−/−) fed with either water or water containing 30% fructose for eight weeks. Scale bar: 100 μm. C, Hepatic Vldlr mRNA abundance. Immunoblot analyses of hepatic VLDLR (D) and total and phospho-Nrf2 (E). F, Nqo1 mRNA abundance. MDA (G) and H2O2 (H) levels from liver of wild-type (WT) and Pparβ/δ-deficient mice fed with either water or water containing 30% fructose. Data are presented as the mean ± S.D. (n = 6 per group). **p < 0.01 and *p < 0.05 vs. water-fed WT mice. ##p < 0.01 and #p < 0.05 vs. fructose-fed WT mice. ††p < 0.01 and †p < 0.05 vs. water-fed Pparβ/δ−/− mice. I, Vldlr mRNA abundance in primary hepatocytes transfected with control, Pparδ and Nrf2 siRNA for 24 h in the presence or absence of 25 mM fructose. *p < 0.05 vs. control siRNA. #p < 0.05 vs. Pparδ siRNA.
3.5. VLDLR content is increased in the liver of patients with steatosis
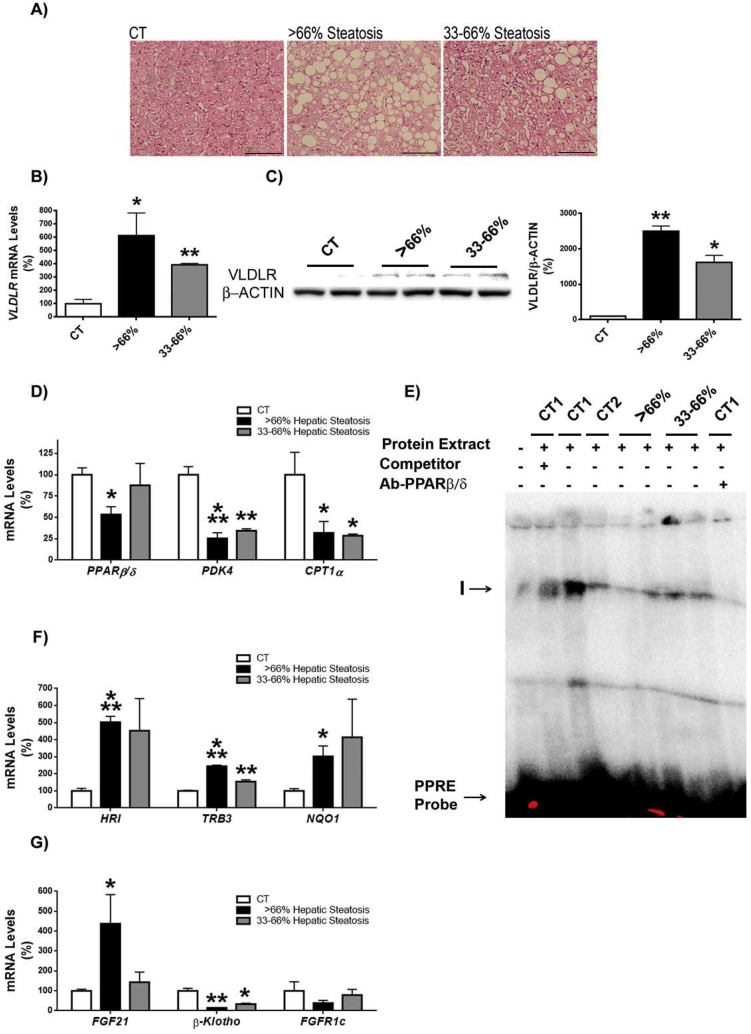
Our findings in in vitro and animal models suggest a new potential pathway that might contribute to NAFLD. In this pathway, a reduction in PPARβ/δ levels might result in activation of both ATF4 and Nrf2, which in turn would enhance VLDLR levels, leading to increased VLDL (triglyceride) delivery to the liver. The contribution of this potential pathway was explored in liver biopsies of patients classified into three grades based on the percentage of hepatocytes presenting steatosis: grade 0 (<5%), grade 2 or moderate steatosis (33–66%) and grade 3 or severe steatosis (>66%) (Figure 7A). VLDLR mRNA expression (Figure 7B) and protein levels (Figure 7C) were increased in patients suffering hepatic steatosis. The increase in VLDLR levels was accompanied by a reduction in PPARβ/δ mRNA abundance that only reached statistical significance in livers of patients with severe hepatic steatosis (Figure 7D). The expression of the PPARβ/δ-target genes involved in fatty acid oxidation, PDK4 and CPT-1α, was reduced in the livers of patients with steatosis (Figure 7D). Consistent with the reduced levels of PPARβ/δ, the DNA-binding activity of this transcription factor assessed by EMSA showed a reduction in patients with hepatic steatosis, especially in those with severe hepatic steatosis (Figure 7E). The reduction in PPARβ/δ expression and activity in patients with severe hepatic steatosis was accompanied by an increase in HRI, TRB3, and NQO1 expression (Figure 7F), suggesting that the increase in VLDLR levels in humans with severe hepatic steatosis in the context of reduced PPARβ/δ levels might be the result of activation of the HRI-eIF2α-ATF4 and Nrf2 pathways. Expression of ATF4-target gene FGF21 was significantly increased only in the patients with severe steatosis (Figure 7G), whereas expression of its receptors, β-klotho and FGFR1c, was reduced, although the reduction of the receptor did not reach significance, suggesting that the effect of this hormone might be attenuated (Figure 7G).
Figure 7.
Liver of patients with steatosis show increased VLDLR content. A, Hematoxylin-eosin staining of liver biopsies from control subjects (Control, CT) and patients with moderate (30–66% of hepatocytes presenting steatosis) and severe (>66%) hepatic steatosis. Scale bar: 100 μm. Hepatic VLDLR mRNA (B) and protein (C) abundance. D, mRNA abundance of PPARβ/δ, PDK4 and CPT1α. E, autoradiograph of EMSA performed with a 32P-labeled PPRE and crude nuclear protein extract (NE) from liver biopsies. One main specific complex (I) based on competition with a molar excess of unlabeled probe is shown. The supershift assay performed by incubating NE with an antibody (Ab) directed against PPARβ/δ shows a reduction in the band. F, mRNA abundance of HRI, TRB3 and NQO1. G, mRNA abundance of FGF21, β-KLOTHO and FGFR1c. Data are presented as the mean ± S.D. (n = 4 per group) relative to the control (CT) group. ***p < 0.001, **p < 0.01 and *p < 0.05 vs. CT group.
4. Discussion
Here we present evidence that VLDLR is regulated by PPARβ/δ and FGF21. The reported increase in VLDLR in Pparβ/δ null macrophages [38], prompted us to examine whether Pparβ/δ-null mice showed increased VLDLR levels and determine its contribution to NAFLD and the mechanisms involved. Our findings indicate that in Pparβ/δ-null mice, HRI activation in liver increases VLDLR levels through the eIF2α-ATF4 pathway (Figure 8). Despite this, HRI activators do not increase hepatic steatosis; on the contrary, these compounds also increased FGF21 levels and this hormonal factor improved glucose intolerance and hepatic triglyceride accumulation induced by feeding a HFD [28]. In addition, Pparβ/δ-deficiency also results in the activation of Nrf2, another transcription factor that contributes to increase VLDLR levels [18]. Under conditions of Pparβ/δ-deficiency, the increase in VLDLR levels would contribute to hepatic steatosis. In addition, stimulation of ER stress induces hepatic steatosis through VLDLR [6], and this process might be exacerbated in Pparβ/δ-null mice through a higher activation of both ATF4 and Nrf2, suggesting that the absence of PPARβ/δ contributes to intensify ER stress-induced fatty liver.
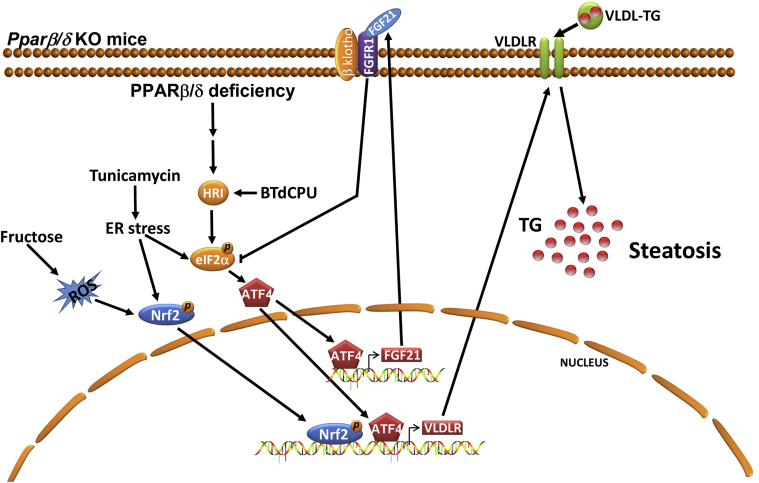
Figure 8.
Proposed mechanisms by which PPARβ/δ regulates VLDLR levels and hepatic steatosis.Pparβ/δ deficiency may result in an increase in VLDLR levels and hepatic steatosis through several mechanisms. The activation of HRI caused by Pparβ/δ deficiency (reference [28]) and by activators of this kinase (BTdCPU) may increase the levels of VLDLR through the eIF2α-ATF4 pathway. ER stress can also activate the eIF2α-ATF4 pathway leading to an increase in the expression of VLDLR and FGF21. This hormone suppresses the eIF2α-ATF4 pathway through a negative feedback mechanism and thereby it also regulates the levels of VLDLR. ER stress also enhances the activity of Nrf2, a transcription factor reported to upregulate the expression of Fgf21 (reference [19]). Fructose feeding increases the levels of ROS and the activity of Nrf2 providing a mechanism for the increase of the levels of VLDLR. All these mechanisms may result in an increase in the levels of VLDLR causing hepatic steatosis. TG: triglyceride.
It has previously been reported that the PPARβ/δ agonist GW501516 promotes glucose flux to the pentose phosphate pathway and fatty acid synthesis in liver [39], establishing a discrepancy with the findings of this manuscript. However, a time-course study demonstrated that GW501516 induces accumulation of liver lipids following 4 wk of treatment, but this turned into a reduction in the content of hepatic lipids after 8 wk. of GW501516 treatment [40], probably as the result of an increase in fatty acid oxidation. Therefore, long treatments with GW501516 reduce hepatic lipids, which is consistent with the increase in liver lipids observed in Pparβ/δ-null mice.
FGF21 has emerged as an important regulator of glucose and lipid metabolism and hence is a promising agent for the treatment of obesity, NAFLD, insulin resistance, and type 2 diabetes mellitus [41]. This study also suggests that the reported enhanced FGF21 levels in Pparβ/δ-deficient mice [28] prevent a higher increase in VLDLR levels and hepatic triglyceride accumulation. This effect of FGF21 is the result of a negative feedback loop by which this hormone suppresses the eIF2α-ATF4 pathway [28], [34]. In fact, we show here that blocking FGF21 increases VLDLR levels and hepatic steatosis. In agreement with this, Fgf21−/− mice showed increased hepatic activation of the eIF2α-ATF4 pathway and VLDLR levels, suggesting that this receptor may account for part of the hepatic steatosis observed in these mice. Thus, FGF21 can prevent NAFLD by increasing hepatic fatty acid oxidation and reducing lipid synthesis [41], and our findings indicate that this hormone may also prevent NAFLD by downregulating VLDLR levels.
Fructose feeding increases hepatic steatosis, but this process is influenced by the genetic background of the mice. In our study, feeding wild-type mice (C57BL/6X129/SV genetic background) with fructose did not result in hepatic steatosis, although fructose feeding was successful, as demonstrated by the increase in Fgf21 expression, probably through carbohydrate response element binding protein (ChREBP)-mediated mechanisms, as previously reported [37]. However, Pparβ/δ-null mice of the same genetic background fed with fructose showed hepatic steatosis, and this was accompanied by higher hepatic VLDLR levels. Our findings demonstrate that in the context of Pparβ/δ deficiency, fructose induces ROS production, leading to an increase in phospho-Nrf2 levels and therefore in its activity. A step further, our siRNA studies demonstrated that this transcription factor was responsible for fructose-induced Vldlr expression in primary hepatocytes.
An analysis of human samples from patients with hepatic steatosis showed an increase in the liver protein content of VLDLR compared with control subjects. Interestingly, a reduction in PPARβ/δ levels and/or activity, as demonstrated by EMSA and the expression of its target genes, was also observed in the liver of these patients, suggesting that the relationship between PPARβ/δ and VLDLR observed in mice might also operate in humans with hepatic steatosis.
Targeting PPAR isotypes for the treatment of fatty liver disease has been extensively studied, mainly in the case of PPARα [42], [43]. However, although the beneficial effect of activating PPARα for NAFLD has been proven in several mouse models [42], [43], fibrates, which are PPARα agonists, do not correct NAFLD in humans [44]. In contrast, long-term activation of PPARβ/δ can improve hepatic steatosis by activating fatty acid oxidation in different mouse models [45], [46], whereas clinical studies have also demonstrated a reduction of hepatic fat content in humans upon treatment with PPARβ/δ agonists [47], [48]. Our findings show that in patients with hepatic steatosis the reduction in PPARβ/δ activity is accompanied by an increase in VLDLR levels. It remains to be studied whether in the clinical setting PPARβ/δ agonists improve hepatic steatosis by regulating VLDLR abundance. Moreover, since we have reported that PPARβ/δ activation in mice increases PPARα expression and activity and enhances the hepatic levels of the endogenous PPARα ligand 16:0/18:1-phosphatidylcholine [49], we cannot discard that the reduction of PPARβ/δ activity may also affect the activity of PPARα. Consistent with this, drugs combining PPARα and PPARβ/δ isotype agonism are a promising treatment for NAFLD. In fact, the dual PPARα/β(δ) agonist elafibranor (GFT505) shows antisteaotic effects in rodent models [50], and, in abdominally obese subjects, it improves hepatic and peripheral insulin sensitivity and probably NAFLD, although liver fat content was not measured [51].
On the other hand, the increase in hepatic FGF21 expression in patients suffering hepatic steatosis is in agreement with previous studies [52], and it has been considered to be the result of a resistance to this hormone [53], implying that the lack of FGF21 activity may also contribute to increase VLDLR levels in hepatic steatosis. It is important to point out that the low number of patients analyzed is a limitation of this part of the study and precludes that VLDLR plays a role in NAFLD although it might correlate with liver fat accumulation.
5. Conclusions
The findings of this study suggest that Pparβ/δ-deficiency results in an increase in VLDLR levels, whereas FGF21 prevents the increase in hepatic VLDLR levels. In patients with hepatic steatosis, a reduction in the levels and/or activity of PPARβ/δ was accompanied by an increase in VLDLR abundance, suggesting that this crosstalk might be involved in fatty liver development. Overall, these data suggest that modulation of PPARβ/δ and/or FGF21 activity might be a key therapeutic target for the treatment of hepatic steatosis by regulating VLDLR abundance.
Author's contribution
MZ, EB, XP, JD, TQL, JCEG, LC, PR, AMV, and MVC performed the experiments; EP and SV synthesized the HRI activators; MR, MM, and RD recruited and obtained human samples; WW, YL, and FV analyzed the data and revised the results; MZ, YL, and MVC designed the experiments and revised the results; MVC was primarily responsible for writing the manuscript. All authors contributed to manuscript editing and approval.
Financial support
This study was partly supported by funds from the Spanish Ministry of the Economy and Competitiveness (SAF2015-64146-R to MVC, SAF2014-55725 to FV, and SAF2015-65267-R to AMV) and European Union ERDF funds. CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) and CIBER Fisiopatologia de la Obesidad y Nutrición (CIBERobn) are Carlos III Health Institute projects. WW is supported by Start-Up Grants from the Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, and by the Région Midi-Pyrénées, France, and TQL is supported by a CONACyT (National Council for Science and Technology in Mexico) Ph.D. scholarship.
Conflict of interest
The authors declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.12.008.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
figs1.
figs2.
figs3.
References
- 1.Satapathy S.K., Sanyal A.J. Epidemiology and natural history of nonalcoholic fatty liver disease. Seminars in Liver Disease. 2015;35:221–235. doi: 10.1055/s-0035-1562943. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musso G., Gambino R., Cassader Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Progress in Lipid Research. 2009;48:1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. Journal of Clinical Investigation. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan R.E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H.S. Regulation of lipolysis in adipocytes. Annual Review of Nutrition. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo H., Choe S.S., Shin K.C., Jang H., Lee J.H., Seong J.K. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57:1366–1377. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 7.Webb J.C., Patel D.D., Jones M.D., Knight B.L., Soutar A.K. Characterization and tissue-specific expression of the human ‘very low density lipoprotein (VLDL) receptor’ mRNA. Human Molecular Genetics. 1994;3:531–537. doi: 10.1093/hmg/3.4.531. [DOI] [PubMed] [Google Scholar]
- 8.Oka K., Ishimura-Oka K., Chu M.J., Sullivan M., Krushkal J., Li W.H. Mouse very-low-density-lipoprotein receptor (VLDLR) cDNA cloning, tissue-specific expression and evolutionary relationship with the low-density-lipoprotein receptor. European Journal of Biochemistry. 1994;224:975–982. doi: 10.1111/j.1432-1033.1994.00975.x. [DOI] [PubMed] [Google Scholar]
- 9.Tacken P.J., Beer F.D., Vark L.C., Havekes L.M., Hofker M.H., Willems Van Dijk K. Very-low-density lipoprotein binding to the apolipoprotein E receptor 2 is enhanced by lipoprotein lipase, and does not require apolipoprotein E. Biochemical Journal. 2000;347:357–361. doi: 10.1042/0264-6021:3470357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi S., Suzuki J., Kohno M., Oida K., Tamai T., Miyabo S. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. Journal of Biological Chemistry. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi S., Sakai J., Fujino T., Hattori H., Zenimaru Y., Suzuki J. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. Journal of Atherosclerosis and Thrombosis. 2004;11:200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- 12.Tao H., Aakula S., Abumrad N.N., Hajri T. Peroxisome proliferator-activated receptor-gamma regulates the expression and function of very-low-density lipoprotein receptor. American Journal of Physiology – Endocrinology and Metabolism. 2010;298:E68–E79. doi: 10.1152/ajpendo.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yagyu H., Lutz E.P., Kako Y., Marks S., Hu Y., Choi S.Y. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. Journal of Biological Chemistry. 2002;277:10037–10043. doi: 10.1074/jbc.M109966200. [DOI] [PubMed] [Google Scholar]
- 14.Frykman P.K., Brown M.S., Yamamoto T., Goldstein J.L., Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8453–8457. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goudriaan J.R., Tacken P.J., Dahlmans V.E., Gijbels M.J., van Dijk K.W., Havekes L.M. Protection from obesity in mice lacking the VLDL receptor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:1488–1493. doi: 10.1161/hq0901.095147. [DOI] [PubMed] [Google Scholar]
- 16.Goudriaan J.R., Espirito Santo S.M., Voshol P.J., Teusink B., van Dijk K.W., van Vlijmen B.J. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. The Journal of Lipid Research. 2004;45:1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Perman J.C., Boström P., Lindbom M., Lidberg U., StÅhlman M., Hägg D. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. Journal of Clinical Investigation. 2011;121:2625–2640. doi: 10.1172/JCI43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z., Dou X., Li S., Zhang X., Sun X., Zhou Z. Nuclear factor (erythroid-derived 2)-like 2 activation-induced hepatic very-low-density lipoprotein receptor overexpression in response to oxidative stress contributes to alcoholic liver disease in mice. Hepatology. 2014;59:1381–1392. doi: 10.1002/hep.26912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Y., Shen W., Lu B., Zhang Q., Hu Y., Chen Y. Upregulation of hepatic VLDLR via PPARα is required for the triglyceride-lowering effect of fenofibrate. The Journal of Lipid Research. 2014;55:1622–1633. doi: 10.1194/jlr.M041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vázquez-Carrera M. Unraveling the effects of PPARβ/δ on insulin resistance and cardiovascular disease. Trends in Endocrinology and Metabolism. 2016;27:319–334. doi: 10.1016/j.tem.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Salvadó L., Serrano-Marco L., Barroso E., Palomer X., Vázquez-Carrera M. Targeting PPARβ/δ for the treatment of type 2 diabetes mellitus. Expert Opinion on Therapeutic Targets. 2012;16:209–223. doi: 10.1517/14728222.2012.658370. [DOI] [PubMed] [Google Scholar]
- 22.Tan N.S., Vázquez-Carrera M., Montagner A., Sng M.K., Guillou H., Wahli W. Transcriptional control of physiological and pathological processes by the nuclear receptor PPARβ/δ. Progress in Lipid Research. 2016;64:98–122. doi: 10.1016/j.plipres.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Nadra K., Anghel S.I., Joye E., Tan N.S., Basu-Modak S., Trono D. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Molecular and Cellular Biology. 2006;26:3266–3281. doi: 10.1128/MCB.26.8.3266-3281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omar B.A., Andersen B., Hald J., Raun K., Nishimura E., Ahrén B. Fibroblast growth factor 21 (FGF21) and glucagon-like peptide 1 contribute to diabetes resistance in glucagon receptor-deficient mice. Diabetes. 2014;63:101–110. doi: 10.2337/db13-0710. [DOI] [PubMed] [Google Scholar]
- 25.Barroso E., Rodríguez-Rodríguez R., Chacón M.R., Maymó-Masip E., Ferrer L., Salvadó L. PPARβ/δ ameliorates fructose-induced insulin resistance in adipocytes by preventing Nrf2 activation. Biochimica et Biophysica Acta. 2015;1852:1049–1058. doi: 10.1016/j.bbadis.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Benveniste R., Danoff T.M., Ilekis J., Craig H.R. Epidermal growth factor receptor numbers in male and female mouse primary hepatocyte cultures. Cell Biochemistry and Function. 1988;6:231–235. doi: 10.1002/cbf.290060403. [DOI] [PubMed] [Google Scholar]
- 27.Brunt E.M. Alcoholic and nonalcoholic steatohepatitis. Clinics in Liver Disease. 2002;6:399–420. doi: 10.1016/s1089-3261(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 28.Zarei M., Barroso E., Leiva R., Barniol-Xicota M., Pujol E., Escolano C. Heme-regulated eIF2α kinase modulates hepatic FGF21 and is activated by PPARβ/δ deficiency. Diabetes. 2016;65:3185–3199. doi: 10.2337/db16-0155. [DOI] [PubMed] [Google Scholar]
- 29.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 30.Chen T., Ozel D., Qiao Y., Harbinski F., Chen L., Denoyelle S. Chemical genetics identify eIF2α kinase heme-regulated inhibitor as an anticancer target. Nature Chemical Biology. 2011;7:610–616. doi: 10.1038/nchembio.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lázaro I., Ferré R., Masana L., Cabré A. Akt and ERK/Nrf2 activation by PUFA oxidation-derived aldehydes upregulates FABP4 expression in human macrophages. Atherosclerosis. 2013;230:216–222. doi: 10.1016/j.atherosclerosis.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 32.De Sousa-Coelho A.L., Marrero P.F., Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochemical Journal. 2012;443:165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- 33.Salvadó L., Palomer X., Barroso E., Vázquez-Carrera M. Targeting endoplasmic reticulum stress in insulin resistance. Trends in Endocrinology and Metabolism. 2015;26:438–448. doi: 10.1016/j.tem.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Jiang S., Yan C., Fang Q.C., Shao M.L., Zhang Y.L., Liu Y. Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. Journal of Biological Chemistry. 2014;289:29751–29765. doi: 10.1074/jbc.M114.565960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X., Zhang P., Martin R.C., Cui G., Wang G., Tan Y. Lack of fibroblast growth factor 21 accelerates metabolic liver injury characterized by steatohepatities in mice. American Journal of Cancer Research. 2016;6:1011–1025. [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez-Calvo R., Barroso E., Serrano L., Coll T., Sánchez R.M., Merlos M. Atorvastatin prevents carbohydrate response element binding protein activation in the fructose-fed rat by activating protein kinase A. Hepatology. 2009;49:106–115. doi: 10.1002/hep.22570. [DOI] [PubMed] [Google Scholar]
- 37.Fisher F.M., Kim M., Doridot L., Cunniff J.C., Parker T.S., Levine D.M. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Molecular Genetics and Metabolism. 2016;6:14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chawla A., Lee C.H., Barak Y., He W., Rosenfeld J., Liao D. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee C.H., Olson P., Hevener A., Mehl I., Chong L.W., Olefsky J.M. PPARdelta regulates glucose metabolism and insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garbacz W.G., Huang J.T., Higgins L.G., Wahli W., Palmer C.N. PPARα is required for PPARδ action in regulation of body weight and hepatic steatosis in mice. PPAR Research. 2015;2015:927057. doi: 10.1155/2015/927057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domouzoglou E.M., Naka K.K., Vlahos A.P., Papafaklis M.I., Michalis L.K., Tsatsoulis A. Fibroblast growth factors in cardiovascular disease: the emerging role of FGF21. American Journal of Physiology – Heart and Circulatory Physiology. 2015;309:H1029–H1038. doi: 10.1152/ajpheart.00527.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka N., Aoyama T., Kimura S., Gonzalez F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacology & Therapeutics. 2017;179:142–157. doi: 10.1016/j.pharmthera.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross B., Pawlak M., Lefebvre P., Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nature Reviews Endocrinology. 2017;13:36–49. doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- 44.Fernández-Miranda C., Pérez-Carreras M., Colina F., López-Alonso G., Vargas C., Solís-Herruzo J.A. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Digestive and Liver Disease. 2008;40:200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Bojic L.A., Telford D.E., Fullerton M.D., Ford R.J., Sutherland B.G., Edwards J.Y. PPARδ activation attenuates hepatic steatosis in Ldlr-/- mice by enhanced fat oxidation, reduced lipogenesis, and improved insulin sensitivity. The Journal of Lipid Research. 2014;55:1254–1266. doi: 10.1194/jlr.M046037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H.T., Chen C.T., Cheng K.C., Li Y.X., Yeh C.H., Cheng J.T. Pharmacological activation of peroxisome proliferator-activated receptor δ improves insulin resistance and hepatic steatosis in high fat diet-induced diabetic mice. Hormone and Metabolic Research. 2011;43:631–635. doi: 10.1055/s-0031-1280781. [DOI] [PubMed] [Google Scholar]
- 47.Bays H.E., Schwartz S., Littlejohn T., 3rd, Kerzner B., Krauss R.M., Karpf D.B. MBX-8025, a novel peroxisome proliferator receptor-delta agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. Journal of Clinical Endocrinology & Metabolism. 2011;96:2889–2897. doi: 10.1210/jc.2011-1061. [DOI] [PubMed] [Google Scholar]
- 48.Risérus U., Sprecher D., Johnson T., Olson E., Hirschberg S., Liu A. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 49.Barroso E., Rodríguez-Calvo R., Serrano-Marco L., Astudillo A.M., Balsinde J., Palomer X. The PPARβ/δ activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1α-Lipin 1-PPARα pathway leading to increased fatty acid oxidation. Endocrinology. 2011;152:1848–1859. doi: 10.1210/en.2010-1468. [DOI] [PubMed] [Google Scholar]
- 50.Staels B., Rubenstrunk A., Noel B., Rigou G., Delataille P., Millatt L.J. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 51.Cariou B., Hanf R., Lambert-Porcheron S., Zaïr Y., Sauvinet V., Noël B. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–2930. doi: 10.2337/dc12-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela-Rey M., Crawley M., Fisher F.M. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher F.M., Chui P.C., Antonellis P.J., Bina H.A., Kharitonenkov A., Flier J.S. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.