Abstract
Objective
While prostate cancer does not occur more often in men with diabetes, survival is markedly reduced in this patient group. Androgen signaling is a known and major driver for prostate cancer progression. Therefore, we analyzed major components of the androgen signaling chain and cell proliferation in relation to type 2 diabetes.
Methods
Tumor content of 70 prostate tissue samples of men with type 2 diabetes and 59 samples of patients without diabetes was quantified by an experienced pathologist, and a subset of 51 samples was immunohistochemically stained for androgen receptor (AR). mRNA expression of AR, insulin receptor isoform A (IR-A) and B (IR-B), IGF-1 receptor (IGF1R), Cyp27A1 and Cyp7B1, PSA gene KLK3, PSMA gene FOLH1, Ki-67 gene MKI67, and estrogen receptor beta (ESR2) were analyzed by RT-qPCR.
Results
AR mRNA and protein expression were associated with the tumor content only in men with diabetes. AR expression also correlated with downstream targets PSA (KLK3) and PSMA (FOLH1) and increased cell proliferation. Only in diabetes, AR expression was correlated to higher IR-A/IR-B ratio and lower IR-B/IGF1R ratio, thus, in favor of the mitogenic isoforms. Reduced Cyp27A1 and increased Cyp7B1 expressions in tumor suggest lower levels of protective estrogen receptor ligands in diabetes.
Conclusions
We report elevated androgen receptor signaling and activity presumably due to altered insulin/IGF-1 receptors and decreased levels of protective estrogen receptor ligands in prostate cancer in men with diabetes. Our results reveal new insights why these patients have a worse prognosis. These findings provide the basis for future clinical trials to investigate treatment response in patients with prostate cancer and diabetes.
Keywords: Prostate cancer, Androgen receptor, Insulin receptor, IGF-1 receptor, Cyp27A1, Cyp7B1
Abbreviations: 27HC, 27-hydroxycholesterol; ADT, androgen-deprivation therapy; AR, androgen receptor; Cyp27A1, sterol 27-hydroxylase; Cyp7B1, 25-hydroxycholesterol 7α-hydroxylase; DHT, dihydrotestosterone; ER, estrogen receptor; IGF1R, insulin like growth factor-1 receptor; IR, insulin receptor; IR-A, insulin receptor isoform A; IR-B, insulin receptor isoform B; OGTT, oral glucose tolerance test; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; SERM, selective estrogen receptor modulator; SREBP2, sterol regulatory element-binding protein 2
Highlights
-
•
Androgen receptor expression is elevated in prostate cancer in men with diabetes.
-
•
This correlates with altered IR and IGF-1R and protective estrogen receptor ligands.
-
•
Our results reveal new insights why these patients have worse prognosis.
1. Introduction
In contrast to numerous other malignancies, the incidence of prostate cancer, which is the most common cancer in men, is not increased in case of concurrent type 2 diabetes mellitus; several studies even reported a decreased risk [1]. One of the crucial drivers for prostate cell growth is androgen signaling, paving the way for the androgen-deprivation therapy (ADT) as one standard treatment for prostate cancer [2]. Recently, it was shown that increasing glucose concentrations are able to downregulate androgen receptor (AR) mRNA and protein levels through NF-kB activation in vitro and in an animal model of prostate cancer [3]. Given that men with type 2 diabetes have lower testosterone levels per se, the mentioned changes could be one possible explanation for the lower prostate cancer incidence in this patient group [4]. Nevertheless, according to numerous previous studies, prostate cancer survival is clearly reduced when type 2 diabetes is present [5], [6], [7]. Although strong epidemiological evidence links prostate cancer and type 2 diabetes, the underlying molecular mechanisms are still not understood in detail.
Prostate cell growth and prostate carcinogenesis are not only mediated by androgens, they are also dependent on functional insulin receptor (IR) and insulin-like growth factor-1 (IGF-1) receptor (IGF1R) signaling. Previous studies addressed this issue and reported a correlation between high insulin and IGF-1 levels and prostate cancer cell progression [8], [9], [10]. In addition to the indicated IR overexpression in prostate cancer [11], we demonstrated an isoform configuration showing elevated IR isoform A to B ratio in prostate cancer [12]. In this context, the mitogenic isoform A is differently expressed in various cancer cells, has a high affinity for IGF-2 and can contribute to cell proliferation, whereas the isoform B mainly transmits the regular metabolic effects of insulin [13]. A crosslink between insulin and androgen signaling has been already proposed by several groups, demonstrating increased de novo steroidogenesis in prostate cancer cells by insulin, and vice versa, an increased IR expression, insulin binding, and insulin responsiveness by androgens in Hep-2 larynx carcinoma cells [14], [15]. Moreover, Fan et al. showed an activation of androgen signaling by insulin and IGF-1 through direct interactions of Foxo1 with AR [16].
Of interest, activity of AR in prostate cancer is not only modulated by androgens but also by cholesterol derivates, e.g. oxysterols. These steroids appear to antagonize androgen signaling via estrogen receptor and other pathways [17], [18]. Important estrogen receptor ligands in this context are 27-hydroxycholesterol (27HC), the most abundant oxysterol, and 3β-Adiol, a degradation product of dihydrotestosterone. However, concentrations cannot easily be measured and circulating levels must not necessarily reflect concentrations at the tumor cell. Though, they can be estimated by analyzing the synthesizing and degrading enzymes. 27HC is the most abundant oxidized derivative of cholesterol (oxysterol) in plasma. Cholesterol is converted into 27HC by the enzyme Cyp27A1, a cytochrome P450 oxidase, which is shown to be downregulated in prostate cancer [19], [20]. The rate limiting enzyme in the catabolism of 27HC is Cyp7B1, which is reported to be overexpressed during progression of prostate cancer [21]. Recently, 27HC was shown to inhibit growth of prostate cancer cells by depletion of intracellular cholesterol, representing a negative feedback loop for regulating cholesterol biosynthesis, possibly via inhibition of sterol regulatory element-binding protein 2 (SREBP2) activity [20].
To better understand why prostate cancer survival is reduced in type 2 diabetes, we performed gene expression analysis of key proteins involved in androgen signaling and steroid modulators thereof using prostate tissue samples of men with and without diabetes.
2. Methods
2.1. Study design
70 prostate tissue samples of men with type 2 diabetes and 59 samples of patients without diabetes, all of whom were diagnosed with prostate cancer and underwent a radical prostatectomy at the University of Tübingen between June 2004 and September 2015, were included in the study. All were Caucasians. None of the patients was pre-treated with hormone-altering therapy. Since age (yr) and BMI (kg/m2) were non-normally distributed, they are given as medians [interquartile range]. Age, no diabetes group: 63 [51–83]; diabetes group: 74 [53–87], p < 0.0001; BMI, no diabetes group: 26.5 [20.2–33.7], diabetes group: 28.1 [22.2–41.1], p = 0.0003. Clinical chemistry and hormone measurements for all but 2 of the patients without diabetes and a subgroup of 11 patients with diabetes are reported in Table 1. All patients without diabetes underwent a 75 g oral glucose tolerance test to rule out undiagnosed diabetes (ADA criteria). The group of patients with diabetes consisted of patients with known diabetes prior to operation and patients with newly diagnosed diabetes in our oral glucose tolerance test. Forty-four patients with impaired glucose regulation who did not fulfill the diagnostic criteria for diabetes were included in the “no diabetes” group. Tumor staging was comparable between patients with and without diabetes (Supplementary Table 1). For analyses involving tumor stage, participants were grouped by T-stage into T2 versus T > 2.
Table 1.
Clinical chemistry and hormone measurements from 58 subjects without diabetes and the subgroup of 11 with type 2 diabetes. Insulin sensitivity was estimated according to Matsuda et al., Diabetes Care, 1999. Abbreviations: DHEA-sulfate, dehydroepiandrosterone-sulfate; OGTT, oral glucose tolerance test.
| No diabetes |
Diabetes |
|||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Insulin sensitivityOGTT (1019 l2 mol−2) | 2.33 | 1.29 | 1.35 | 0.68 |
| Fasting insulin (pmol/l) | 82.84 | 36.80 | 109.30 | 33.49 |
| Fasting C-peptide (pmol/l) | 538.43 | 231.37 | 717.50 | 219.56 |
| Fasting glucose (mmol/l) | 103.33 | 9.72 | 131.90 | 33.99 |
| HbA1c (%) | 5.61 | 0.28 | 6.30 | 0.52 |
| Total cholesterol (mg/dl) | 196.69 | 39.23 | 186.18 | 40.62 |
| HDL-cholesterol (mg/dl) | 53.98 | 11.94 | 49.55 | 15.56 |
| LDL-cholesterol (mg/dl) | 111.29 | 27.99 | 102.09 | 32.53 |
| AST (U/l) | 27.17 | 10.13 | 26.82 | 7.11 |
| ALT (U/l) | 29.59 | 12.60 | 28.27 | 9.17 |
| Creatinine (mg/dl) | 0.87 | 0.13 | 0.84 | 0.17 |
| Glomerular filtration rate (ml/min/1.73 m2) | 91.04 | 17.72 | 95.73 | 21.68 |
| Cortisol (nmol/l) | 463.29 | 126.78 | 544.10 | 115.10 |
| DHEA-sulfate (μmol/l) | 4.65 | 2.82 | 4.27 | 1.73 |
| Testosterone (nmol/l) | 13.15 | 5.32 | 10.97 | 4.01 |
| Androstendione (nmol/l) | 14.46 | 55.82 | 6.59 | 2.87 |
| Estradiol (pmol/l) | 125.37 | 31.65 | 134.12 | 37.34 |
| Progesterone (nmol/l) | 1.58 | 1.73 | 1.09 | 0.41 |
| Sex hormone binding globuline (nmol/l) | 41.64 | 16.67 | 36.93 | 9.51 |
Informed written consent was obtained from all participants, and the Ethics Committee of the University of Tübingen approved the protocol.
2.2. Tissue sampling
To ensure optimal quality prostate tissue from patients, we performed a procedure to avoid delayed freezing. Immediately after removing the prostate, the organ was carefully digitally palpated and both an area of peripheral hardness with supposed tumor region and also an area of soft tissue were cut out. Each excised sample comprised an approximately 5 × 5 × 3 mm piece of tissue. It was cut longitudinally into 3 lamellas, from which the two outer lamellas were immediately snap frozen in liquid nitrogen, preserving an as optimal as possible sample quality for the mRNA measurements. Tissues remained frozen at −80 °C prior to analysis.
From every sample, the respective middle slice was formalin fixed and paraffin embedded. On a representative hematoxylin-eosin stained slide along this lamella, an experienced pathologist assessed the slide for malignancy and for tumor content. First, the total area of all glandular structures was defined as 100%, thereby excluding all stromal areas in the slide. Second, all areas of prostate cancer were calculated as total malignant area of the slide. Third, the resulting total area of malignant histology was calculated as percentage share of the whole glandular area. This two-dimensional tumor extent ranged from 0% (nonmalignant samples) to 100%. It was considered as an equivalent for the three-dimensional extent of the two adjacent frozen slices of the sample by adding the third dimension perpendicular to the slide plain and thereby expanding tumor as well as nonmalignant areas to the same scale. For further calculations, this individual value was indexed as ‘tumor content’ of the sample.
2.3. Gene expression analyses
For quantification of mRNA expression in human prostate, tissues were frozen in liquid nitrogen. Total RNA was extracted with AllPrep Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. After treatment with RNase-free DNase I, total RNA was transcribed into cDNA using the first strand cDNA kit from Roche Diagnostics (Mannheim, Germany). RT-qPCR was performed on a LightCycler 480 (Roche Diagnostics) using Probes Master and fluorescent probes from the Universal Probe Library (Roche Diagnostics). Primers were obtained from TIB MOLBIOL (Berlin, Germany). The following primer sequences were used: androgen receptor (AR): forward 5′-GCCTTGCTCTCTAGCCTCAA-3′, reverse 5′-GGTCGTCCACGTGTAAGTTG-3′; insulin receptor isoform A (IR-A): forward 5′-TTTTCGTCCCCAGGCCAT-3′, reverse 5′-CCACCGTCACATTCCCAAC-3′; insulin receptor isoform B (IR-B): forward 5′-TTTCGTCCCCAGAAAAACCTCT-3′, reverse 5′-CCACCGTCACATTCCCAAC-3′; IGF-1 receptor (IGF1R): forward 5′-TCAGCGCTGCTGATGTGT-3′, reverse 5′-GGCTCATGGTGATCTTCTCC-3′; KLK3: forward 5′-CCTGTCCGTGACGTGGAT-3′, reverse 5′-CAGGGTTGGGAATGCTTCT-3′; FOLH1: forward 5′-GATGCACAGAAGCTCCTAGAAAA-3′, reverse 5′-CCAACATTGTAGGGCACTTTG-3′; MKI67: forward 5′-CCAAAAGAAAGTCTCTGGTAATGC3′-, reverse 5′-CCTGATGGTTGAGGCTGTTC-3′; Cyp27A1: forward 5′-CAGTACGGAACGACATGGAG-3′, reverse 5′-GGTACCAGTGGTGTCCTTCC-3′; Cyp7B1: forward 5′-CCTCCAGTCCTACATGGTGAC-3′, reverse 5′-GGTGGTTTTCTTCTTACCATCTTC-3′, ESR2: forward 5′-CATGATCCTGCTCAATTCCA-3′, reverse 5′-ACCAAAGCATCGGTCACG-3′.
Prostate-specific antigen (PSA) is encoded by KLK3, prostate-specific membrane antigen (PSMA) is encoded by FOLH1, Ki-67 is encoded by MKI67, and ER beta is encoded by ESR2. Measurements were performed in duplicates. RNA content was normalized for the housekeeping gene Ubiquitin C (UBC) using the ΔΔCt method, as UBC was neither different between patients with or without diabetes (p = 0.464) nor between cancer and benign samples (p = 0.315) while other commonly applied housekeeping genes showed such differences (e.g. HPRT1 p < 0.0001 or SDHA p = 0.0093).
Data on mRNA and protein expression for prostate cancer from the Cancer Genome Atlas Research Consortium (TCGA) [22] was downloaded via the cBioPortal for Cancer Genomics (http://www.cbioportal.org, accessed 10.11.2017). In this dataset, protein data was available only for AR.
2.4. Immunohistochemistry
Immunohistochemical staining was performed by an automated slide staining instrument BenchMark ULTRA (Ventana Medical System/Roche, Tucson, Arizona, United States). For immunohistochemistry, slides were deparaffinized and rehydrated. AR antibody clone M AR 441 (Dako, Glostrup, Denmark) was used as primary antibody. For detection of the AR, tissues were pretreated by heat antigen retrieval with a Cell Conditioner 1 solution (Roche, Basel, Switzerland) for 64 min with protease 1 (Roche). AR antibodies were diluted 1:200 in an antibody-diluent and incubated for 32 min at 37 °C in the platform. For visualization, the indirect biotin-free OptiView DAB Detection Kit (Roche) was used. The slides were counterstained and mounted. Internal controls served as positive controls for AR.
In microscopic assessment, AR staining was distributed homogeneously. Expression was quantified according to a modified scoring system, which has already been used for assessment of AR immunoreaction. Diversity of positive cells was classified to a score 0–5 [23] by a researcher blinded for diabetes status.
2.5. Statistical analyses
For non-normally distributed parameters, log-transformation was used. For patients with two samples available, one sample was randomly omitted from the analyses. For statistical analysis, we performed multivariate linear regression models adjusted for age and BMI to test differences in the gene expression patterns. Interactions were tested by ANCOVA. Associations with a p-value ≤ 0.05 were considered significant. The statistical software package JMP 11.0 (SAS Institute Inc., Cary, NC) was used.
3. Results
3.1. Elevated androgen receptor expression and activated androgen signaling in diabetes
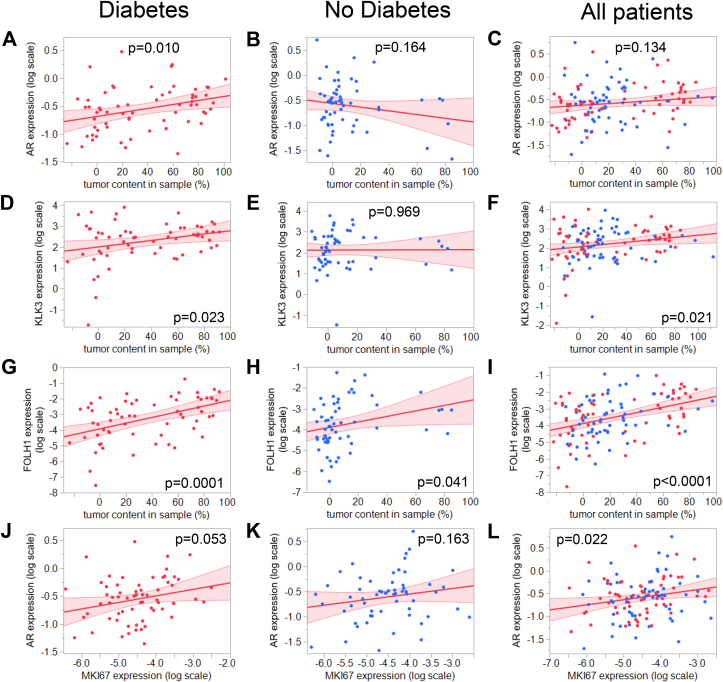
First, we assessed whether androgen receptor (AR) mRNA expression was differentially expressed in tumor depending on diabetes status. There was a significant interaction between diabetes status and tumor status on AR expression (pANCOVA = 0.0151). AR mRNA levels were significantly different between tumor-adjacent benign tissue and prostate cancer only in patients with diabetes (Supplementary Figure 1). In line with this finding, stratification for diabetes status revealed that AR mRNA expression was associated with the tumor content only in the biopsies of men with diabetes (Figure 1A–C). In parallel to AR mRNA, the expression of the AR downstream target gene KLK3, which encodes PSA, was selectively elevated with increasing tumor content only when diabetes was present (Figure 1D–F). Another AR downstream target gene FOLH1, encoding PSMA, was positively correlated with tumor content in both conditions, however, with a larger effect size in diabetes (diabetes: β = 1.81 ± 0.43; no diabetes: β = 1.31 ± 0.63; Figure 1G–I). Further, AR expression was correlated with the expression of MKI67, a gene coding for the proliferation marker Ki-67 (Figure 1J–L). Furthermore, AR mRNA expression in tumor was positively related to high T-score (T > 2) in patients with diabetes (p = 0.027) but not in patients without diabetes (p = 0.441).
Figure 1.
Correlation between the AR mRNA expression and A–C: tumor content in sample; D–F: Correlation between KLK3 mRNA expression (encoding PSA) and tumor content in sample; G–I: Correlation between FOLH1 mRNA expression (encoding PSMA) and tumor content in sample; J–L: Correlation between AR and MKI67 mRNA expression (encoding AR and Ki-67, respectively) in diabetes (left panels, red dots), no diabetes (middle panels, blue dots) and all patients combined (right panels). Samples of men with and without type 2 diabetes who underwent a radical prostatectomy were included in the study. Tumor content was quantified by an experienced pathologist. mRNA expression of target genes was analyzed by RT-qPCR and normalized to UBC mRNA in duplicate. Red line represents fit line ±95% CI. Data were log-transformed where indicated, and associations were tested by multiple linear regression analyses with adjustment for age and BMI. Abbreviations: AR, androgen receptor; Ki-67, cell proliferation marker; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; UBC, ubiquitin C.
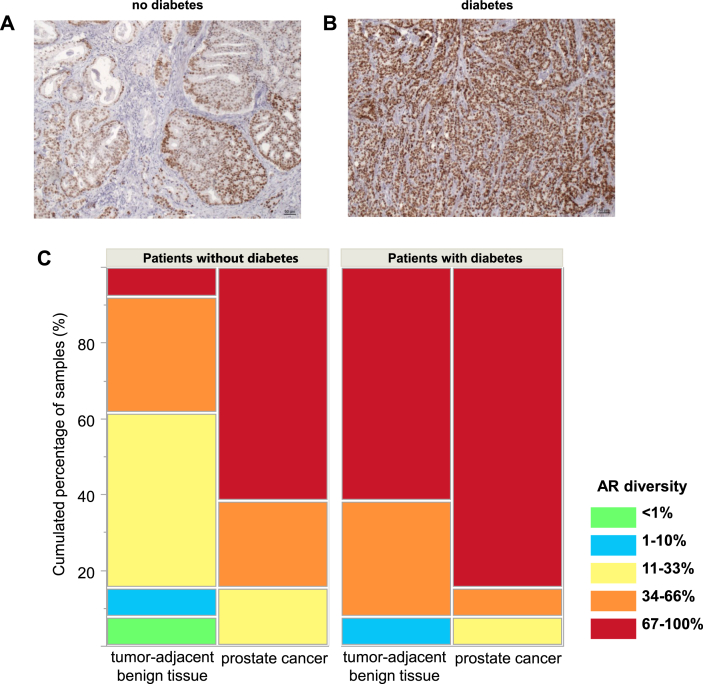
We then quantified immunohistochemical staining for AR in tissue specimens of 51 patients. Staining of the AR was exclusively located in the nucleus to a different extent (Figure 2A,B). Just as with mRNA expression, AR protein expression was significantly positively associated with tumor content in patients with diabetes (p = 0.020) while this did not reach statistical significance in patients without diabetes (p = 0.095). Accordingly, patients with diabetes had more AR protein expression compared to patients without diabetes, both in tumor-adjacent tissue and in prostate cancer (Figure 2C).
Figure 2.
Representative immunohistochemical stainings for AR of prostate carcinoma samples with Gleason scores = 7b in A: no diabetes; B: diabetes. C: AR diversity in immunohistochemical stainings, given in cumulated percentage of samples (%) in patients with and without diabetes, as well as in tumor-adjacent benign tissue and prostate cancer samples. The proportion of cells was scored 0–5 (green: <1%, blue: 1–10%, yellow: 11–33%, orange: 34–66%, red: 67–100%), no sample was scored 0.
Data from the Cancer Genome Atlas Research Consortium (TCGA) also indicated that AR mRNA expression can serve as an estimate of AR protein in prostate cancer (Supplementary Figure 2).
3.2. Association of androgen receptor expression with receptors involved in insulin signaling
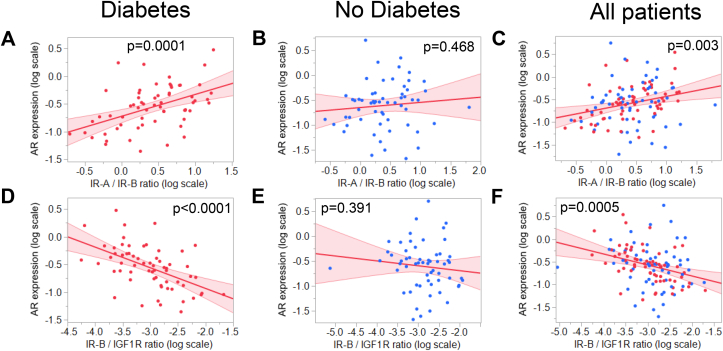
We next asked whether the elevated AR mRNA expression was interrelated to the expression of other receptors involved in insulin signaling. As reported previously [12], we calculated ratios between the two IR isoforms IR-A and IR-B and the IR-B and IGF1R ratio. IR-A/IR-B ratio was correlated to tumor content independent of diabetes status (diabetes: p < 0.0001, no diabetes: p = 0.0003), while IR-B/IGF1R ratio was inversely correlated with the tumor content in the samples (diabetes: p = 0.001, no diabetes: p = 0.025).
AR expression was correlated to higher IR-A/IR-B ratio and lower IR-B/IGF1R ratio when diabetes was present (Figure 3A,D, respectively) but not in patients without diabetes (Figure 3B,E, respectively).
Figure 3.
Correlation between AR mRNA expression and A–C: IR-A/IR-B ratio; D–F: IR-B/IGF1R ratio in diabetes (left panels, red dots), no diabetes (middle panels, blue dots), and all patients combined (right panels). mRNA expression of target genes was analyzed by RT-qPCR and normalized to UBC mRNA in duplicate. Red line represents fit line ±95% CI. Data were log-transformed where indicated, and associations were tested by multiple linear regression analyses with adjustment for age and BMI. Abbreviations: AR, androgen receptor; IGF1R, IGF-1 receptor; IR-A, insulin receptor isoform A; IR-B, insulin receptor isoform B; UBC, ubiquitin C.
3.3. Involvement of Cyp27A1 and Cyp7B1 in androgen signaling, the rate limiting enzymes for 27-hydroxycholesterol synthesis and degradation, depending on diabetes status
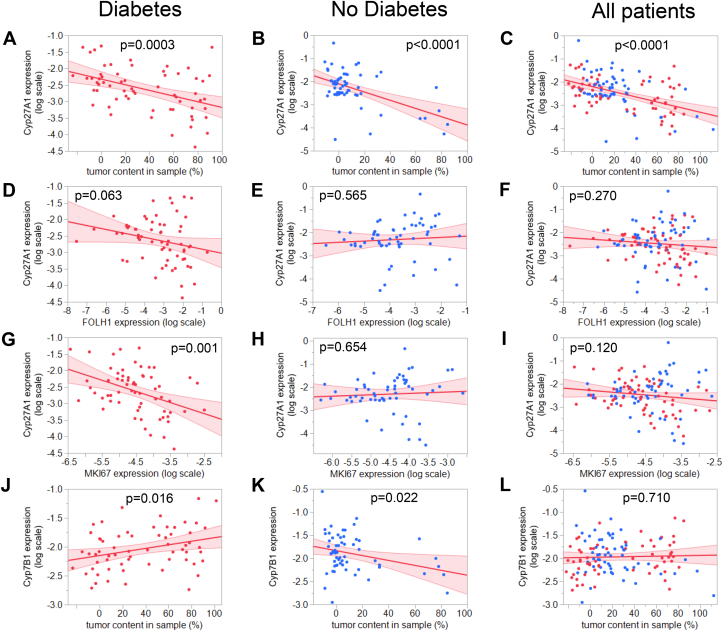
We next assessed Cyp27A1 expression and Cyp7B1 for potential interrelations with the androgen signaling cascade. When correlated to the tumor content in the biopsies, in diabetes Cyp27A1 mRNA levels were significantly reduced with increasing tumor content (Figure 4A). Although Cyp27A1 mRNA correlated inversely to the tumor content in patients without diabetes as well (Figure 4B), the reduction of Cyp27A1 mRNA expression tended to associate with enhanced activation of androgen signaling, as its relation with the AR downstream gene FOLH1 mRNA was only present in diabetes (Figure 4D–F). Moreover, reduced levels of Cyp27A1 expression were associated with elevated cell proliferation solely in men with diabetes, as measured by the expression of MKI67, a gene coding for the proliferation marker Ki-67 (Figure 4G–I).
Figure 4.
Correlation between the Cyp27A1 mRNA expression and A–C: tumor content in sample; D–F: FOLH1 mRNA expression (encoding PSMA); G–I: MKI67 mRNA expression (encoding Ki-67) in diabetes (left panels, red dots), no diabetes (middle panels, blue dots), and all patients combined (right panels). J–L: Correlation between the Cyp7B1 mRNA expression and tumor content in sample in diabetes (left panels, red dots), no diabetes (middle panels, blue dots), and all patients combined (right panels). mRNA expression of target genes was analyzed by RT-qPCR and normalized to UBC mRNA in duplicate. Red line represents fit line ±95% CI. Data were log-transformed where indicated and associations were tested by multiple linear regression analyses with adjustment for age and BMI. Abbreviations: Cyp27A1, sterol 27-hydroxylase; Cyp7B1, 25-hydroxycholesterol 7α-hydroxylase; PSMA, prostate-specific membrane antigen; Ki-67, cell proliferation marker; UBC, ubiquitin C.
Further, stratification for diabetes status revealed a positive correlation of Cyp7B1 expression with tumor content only in the samples in men with diabetes, while men without diabetes showed the opposite direction (Figure 4J–L). Cyp7B1 expression positively correlated with activity of androgen signaling, assessed by KLK3, which encodes PSA (p = 0.003). Furthermore, Cyp7B1 expression was positively associated with cell proliferation, assessed by Ki-67 gene expression (p = 0.0005).
ESR2 encoding ER beta tended to be lower with increasing tumor content (Supplementary Figure 3).
4. Discussion
In this study, we investigated crucial signaling pathways for the progression of prostate cancer on the gene expression level in relation to the patient's diabetes status. Here we report for the first time selectively elevated androgen receptor (AR) and enhanced androgen signaling in tumor tissue of men with diabetes. An augmented gene expression machinery of the AR and downstream target genes underscore enhanced activity in patients with diabetes. As androgen signaling displays one of the most important drivers for prostate cell growth, and since in our study AR expression was strongly correlated with the cell proliferation marker Ki-67 and was associated with higher T-stage, our finding adds a pathomechanism that contributes to the worse cancer-related outcome of prostate cancer patients with type 2 diabetes.
Previous findings reported on the one hand reduced testosterone levels in men with diabetes, and, on the other hand, downregulation of AR mRNA and protein levels through NF-kB activation in vitro and in an animal model of prostate cancer [3], [4]. These mechanisms, which may result in a reduced AR activation, were discussed as one possible explanation for the lower prostate cancer incidence in men with diabetes. However, here we clearly demonstrate an activated AR gene expression machinery selectively under diabetic conditions in prostate cancer patients, paralleled by strengthened cell proliferation and higher tumor stage. Thus, our results argue against AR downregulation in diabetes after occurrence of prostate cancer in vivo.
Possible underlying mechanisms for this AR overexpression could include insulin or IGF-1 signaling as these signaling cascades are known to activate AR [16]. We first confirmed our previous findings [12], as we again detected differential expression patterns of the IR/IGF1R receptors in prostate cancer in the current study. We now addressed this in regard to AR expression. Of note, we detected that higher insulin receptor IR-A/IR-B ratio and lower IR-B/IGF1R ratio, thus, a shift toward the mitogenic isoforms, were correlated with elevated AR expression levels in patients with diabetes. Despite lower testosterone levels in diabetes [4], this shift in receptor composition together with elevated insulin levels could promote upregulation of the AR. In concert with reduction in protective estrogen receptor modulators, this might enhance activity of the androgen signaling machinery. The simultaneously elevated expression of the AR downstream target PSA in tumors of patients with diabetes underline a strictly diabetes-dependent interrelation between androgen and insulin signaling, promoting mitogenic pathways in the cancer cell.
As patients with type 2 diabetes are known to be hyperinsulinemic per se, this relationship between insulin/IGF1 receptor and AR may point towards a causal role of insulin in AR upregulation. Indeed, this is supported by several previous observations. Beyond the already mentioned AR activation by insulin or IGF-1, liganded AR itself may up-regulate IGF1R expression in prostate cancer cells, possibly involving the Src-ERK1/2 pathway, pointing to a vicious cycle once it is activated [16], [24], [25]. In this regard, insulin and IGF-1 may not only activate AR through Foxo1 inactivation, they might also elevate androgen levels (Figure 5). As it was previously shown, insulin is capable of upregulating expression of enzymes necessary for steroidogenesis both at the mRNA and protein levels and, moreover, to directly increase intracellular steroids in prostate cancer cells, which are well-known ligands for the AR, e.g. testosterone [14] (Figure 5). In line with this, in the same work, insulin treatment led to elevated PSA expression and secretion, finally demonstrating a sufficient activation of the AR by insulin. In accordance with these observations, our results point towards a causal role for insulin in AR upregulation especially when type 2 diabetes is present. Of notice, a number of therapeutic strategies in the treatment of diabetes further elevate circulating insulin levels, including all insulin-based therapies and sulfonylureas. Our data might prompt speculation that insulin-independent glucose lowering treatments might be a better option for patients with prostate cancer. One important drug is metformin, which is not only reported to enhance insulin sensitivity and lower circulating insulin levels but also may act on insulin-independent pathways improving cancer-related outcome in prostate cancer [26], [27].
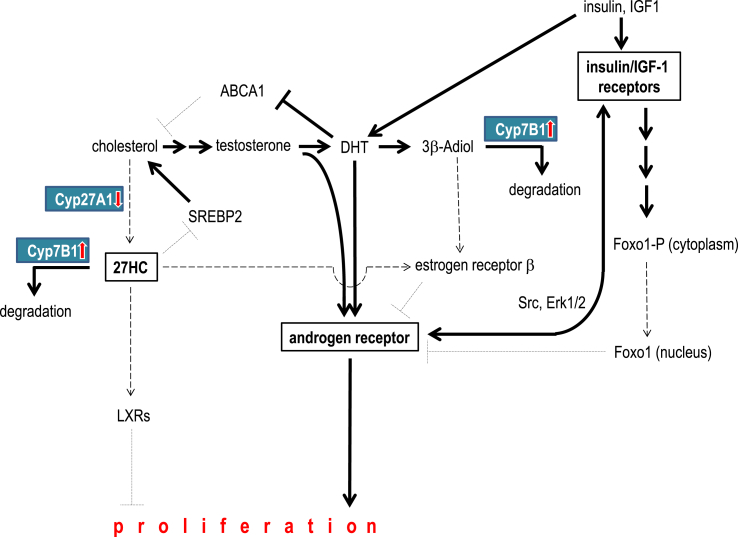
Figure 5.
Cholesterol is the precursor for the steroid hormones testosterone and dihydrotestosterone (DHT). Both activate the androgen receptor, thereby promoting proliferation of prostate tumor cells. DHT also elevates the intracellular cholesterol availability by inhibiting the cholesterol efflux transporter ABCA1 [30]. Insulin and/or IGF-1 induce androgen synthesis in prostate cancer cells. Furthermore, activation of insulin/IGF-1 receptor signaling cascade induces expression of the androgen receptor, presumably via Foxo1 transcription factor [16]. DHT can be further metabolized into 3β-Adiol. This estrogen receptor ligand inhibits androgen signaling via estrogen receptor β. Overexpression of Cyp7B1, the major degrading enzyme of 3β-Adiol was detected in tumors of men with diabetes. This enzyme also degrades 27-hydroxycholesterol (27HC), another cholesterol derivate, that inhibits tumor growth via estrogen receptors as well as Liver X Receptors (LXR). Moreover, 27HC inhibits cholesterol synthesis via SREBP2. Besides enhanced degradation of 27HC, we also detected reduced expression of the synthesizing enzyme Cyp27A1 in prostate tumor tissue in diabetes. Abbreviations: 3β-Adiol, 5α-androstane-3β,17β-diol; 27HC, 27-hydroxycholesterol; ABCA1, cholesterol ATP-binding cassette (ABC) transporter, sub-family A, member 1; DHT, dihydrotestosterone; LXR, Liver X Receptor.
Major activators of the AR are testosterone and dihydrotestosterone (DHT). An important degradation product of DHT is 3β-Adiol. Of notice, 3β-Adiol antagonizes androgen signaling by activating estrogen receptors [18]. In the current work, we investigated the major degrading enzyme of the protective 3β-Adiol, i.e. Cyp7B1. Interestingly, this enzyme also degrades another important selective estrogen receptor modulator (SERM) 27HC [28]. This SERM is synthesized from cholesterol by Cyp27A1. Thus, downregulation of the synthesizing enzyme or overexpression of the degrading enzyme can lead to a decrease in these protective steroids and to a shift from estrogen towards androgen signaling [29]. Besides activation of the estrogen receptor, 27HC is known to contribute to other protective pathways [17], [20]. Interestingly, there are differences in patients with or without diabetes in these metabolic pathways. While the synthesizing enzyme is downregulated in tumor in all patients, the degrading enzyme is upregulated in tumor in patients with diabetes only, while downregulated in patients without diabetes. Even though, the downregulation of the synthesizing enzyme is associated with enhanced tumor cell proliferation only in patients with diabetes. Altogether, these data indicate decreased levels of these important protective estrogen receptor ligands and thus enhanced androgen signaling in tumors of patients with diabetes.
Our results indicate that at least two distinct mechanisms may contribute to the poor prognosis of prostate cancer in men with diabetes: i) upregulation of the androgen receptor, presumably via alteration in the insulin/IGF-1 signaling cascade and ii) disinhibition of androgen signaling due to decreased levels of protective estrogen receptor ligands. Further studies are needed to verify our results on the protein level as most of the proteins addressed were only analyzed on the mRNA level and protein data from TCGA [22] was available only for AR. Further studies are also needed to extend our findings to patients with end-stage disease.
To summarize, we report for the first time enhanced expression of androgen receptor in prostate cancer and stronger activation of androgen signaling in men with type 2 diabetes. Enhanced insulin signaling via either the mitogenic IR-A isoform or IGF-1 receptor might be involved in this upregulation of androgen signaling in tumors of patients with diabetes. Decreased levels of protective estrogen receptor ligands can also contribute to enhanced androgen signaling. Our work provides new insights why men with prostate cancer have worse prognosis in case of coincident diabetes. As the analyzed molecular mechanisms are targets for either antidiabetic or anti-tumor therapy, our results provide the basis for future clinical trials to investigate treatment response to such therapies in patients with prostate cancer and diabetes.
Funding
The study was supported in part by a grant from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
The study was designed by SZL, AF, MH, HUH. Data acquisition was performed by JH, CS, LF, VS, AP, MOS, FF. Data analysis and interpretation was done by SZL, TT, AS, RW, HUH, MH. SZL drafted the manuscript. All authors contributed to the discussion. All authors revised the manuscript and approved the final version to be published.
Acknowledgments
The authors thank all study participants for their cooperation. We gratefully acknowledge the excellent assistance of Alke Guirguis, Dorothee Neuscheler, Anja Dessecker, Ursula Kühs, Tim Neumann, Carmen Salzer, and Dr. Hans Bösmüller.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.11.013.
Conflicts of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Kasper J.S., Liu Y., Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. International Journal of Cancer. 2009;124:1398–1403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todenhofer T., Azad A., Stewart C. AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. Journal of Urology. 2017;197:135–142. doi: 10.1016/j.juro.2016.06.094. [DOI] [PubMed] [Google Scholar]
- 3.Barbosa-Desongles A., Hernandez C., De Torres I. Diabetes protects from prostate cancer by downregulating androgen receptor: new insights from LNCaP cells and PAC120 mouse model. PLoS One. 2013;8:e74179. doi: 10.1371/journal.pone.0074179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. The Journal of Clinical Endocrinology and Metabolism. 2011;96:2341–2353. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Wu F., Saito E. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. 2017;60:1022–1032. doi: 10.1007/s00125-017-4229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currie C.J., Poole C.D., Jenkins-Jones S., Gale E.A., Johnson J.A., Morgan C.L. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh H.C., Platz E.A., Wang N.Y., Visvanathan K., Helzlsouer K.J., Brancati F.L. A prospective study of the associations between treated diabetes and cancer outcomes. Diabetes Care. 2012;35:113–118. doi: 10.2337/dc11-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkateswaran V., Haddad A.Q., Fleshner N.E. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. Journal of the National Cancer Institute. 2007;99:1793–1800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 9.Polychronakos C., Janthly U., Lehoux J.G., Koutsilieris M. Mitogenic effects of insulin and insulin-like growth factors on PA-III rat prostate adenocarcinoma cells: characterization of the receptors involved. Prostate. 1991;19:313–321. doi: 10.1002/pros.2990190405. [DOI] [PubMed] [Google Scholar]
- 10.LeRoith D., Roberts C.T., Jr. The insulin-like growth factor system and cancer. Cancer Letters. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 11.Cox M.E., Gleave M.E., Zakikhani M. Insulin receptor expression by human prostate cancers. Prostate. 2009;69:33–40. doi: 10.1002/pros.20852. [DOI] [PubMed] [Google Scholar]
- 12.Heni M., Hennenlotter J., Scharpf M. Insulin receptor isoforms A and B as well as insulin receptor substrates-1 and -2 are differentially expressed in prostate cancer. PLoS One. 2012;7:e50953. doi: 10.1371/journal.pone.0050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belfiore A., Frasca F., Pandini G., Sciacca L., Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocrine Reviews. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 14.Lubik A.A., Gunter J.H., Hendy S.C. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Research. 2011;71:5754–5764. doi: 10.1158/0008-5472.CAN-10-2470. [DOI] [PubMed] [Google Scholar]
- 15.Sesti G., Marini M.A., Briata P. Androgens increase insulin receptor mRNA levels, insulin binding, and insulin responsiveness in HEp-2 larynx carcinoma cells. Molecular and Cellular Endocrinology. 1992;86:111–118. doi: 10.1016/0303-7207(92)90181-5. [DOI] [PubMed] [Google Scholar]
- 16.Fan W., Yanase T., Morinaga H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. Journal of Biological Chemistry. 2007;282:7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 17.Krycer J.R., Brown A.J. Cross-talk between the androgen receptor and the liver X receptor: implications for cholesterol homeostasis. Journal of Biological Chemistry. 2011;286:20637–20647. doi: 10.1074/jbc.M111.227082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weihua Z., Lathe R., Warner M., Gustafsson J.A. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cali J.J., Russell D.W. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. Journal of Biological Chemistry. 1991;266:7774–7778. [PubMed] [Google Scholar]
- 20.Alfaqih M.A., Nelson E.R., Liu W. CYP27A1 loss dysregulates cholesterol homeostasis in prostate cancer. Cancer Research. 2017;77:1662–1673. doi: 10.1158/0008-5472.CAN-16-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsson M., Gustafsson O., Skogastierna C. Regulation and expression of human CYP7B1 in prostate: overexpression of CYP7B1 during progression of prostatic adenocarcinoma. Prostate. 2007;67:1439–1446. doi: 10.1002/pros.20630. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research Network The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poelaert F., Kumps C., Lumen N. Androgen receptor gene copy number and protein expression in treatment-naive prostate cancer. Urologia Internationalis. 2017;99:222–228. doi: 10.1159/000455158. [DOI] [PubMed] [Google Scholar]
- 24.Pandini G., Mineo R., Frasca F. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Research. 2005;65:1849–1857. doi: 10.1158/0008-5472.CAN-04-1837. [DOI] [PubMed] [Google Scholar]
- 25.Pandini G., Genua M., Frasca F., Vigneri R., Belfiore A. Sex steroids upregulate the IGF-1R in prostate cancer cells through a nongenotropic pathway. Annals of the New York Academy of Sciences. 2009;1155:263–267. doi: 10.1111/j.1749-6632.2009.04361.x. [DOI] [PubMed] [Google Scholar]
- 26.Pollak M.N. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discovery. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 27.Algire C., Moiseeva O., Deschenes-Simard X. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prevention Research (Philadelphia) 2012;5:536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 28.Umetani M., Domoto H., Gormley A.K. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nature Medicine. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 29.Wu W.F., Maneix L., Insunza J. Estrogen receptor beta, a regulator of androgen receptor signaling in the mouse ventral prostate. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E3816–E3822. doi: 10.1073/pnas.1702211114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuchi J., Hiipakka R.A., Kokontis J.M. Androgenic suppression of ATP-binding cassette transporter A1 expression in LNCaP human prostate cancer cells. Cancer Research. 2004;64:7682–7685. doi: 10.1158/0008-5472.CAN-04-2647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.