Abstract
Certain cellular components of the eye, such as neural retina, are unable to regenerate and replicate after destructive inflammation. Ocular immune privilege provides the eye with immune protection against intraocular inflammation in order to minimize the risk to vision integrity. The eye and immune system use strategies to maintain the ocular immune privilege by regulating the innate and adaptive immune response, which includes immunological ignorance, peripheral tolerance to eye-derived antigens, and intraocular immunosuppressive microenvironment. In this review, we summarize current knowledge regarding the molecular mechanism responsible for the development and maintenance of ocular immune privilege via regulatory T cells (Tregs), which are generated by the anterior chamber-associated immune deviation (ACAID), and ocular resident cells including corneal endothelial (CE) cells, ocular pigment epithelial (PE) cells, and aqueous humor. Furthermore, we examined the therapeutic potential of Tregs generated by RPE cells that express transforming growth factor beta (TGF-β), cytotoxic T lymphocyte-associated antigen-2 alpha (CTLA-2α), and retinoic acid for autoimmune uveoretinitis and evaluated a new strategy using human RPE-induced Tregs for clinical application in inflammatory ocular disease. We believe that a better understanding of the ocular immune privilege associated with Tregs might offer a new approach with regard to therapeutic interventions for ocular autoimmunity.
1. Introduction
The microenvironment in the eye is both immunosuppressive and anti-inflammatory in nature. This immunosuppressive property by ocular resident cells/tissues is referred to as immune privilege. This phenomenon helps prevent extensive damage caused by infiltrating inflammatory cells that would otherwise lead to blindness. The eye expresses an extensive array of mechanisms through which innate and adaptive immune cells can be regulated, thereby avoiding blindness as a consequence of intraocular inflammation [1–3]. The immunosuppressive mechanisms that have been revealed to date include a microenvironment in the eye, for example, ocular fluids, blood-retina barriers, and ocular resident parenchymal cells. Ocular fluids, which include aqueous humor and vitreous fluids, have anti-inflammatory properties [4–6]. Some ocular resident cells create a blood-retina barrier to limit the ingress of blood cells, while ocular parenchymal cells express the CD95 ligand (CD95L/Fas ligand) that triggers apoptosis of inflammatory cells [7]. In these ocular immune privilege cells, retinal pigment epithelial (RPE) cells contribute to the immune privilege property of the eye. RPE cells form tight junctions and create the blood-retina barriers. Moreover, RPE cells constitutively express immunosuppressive molecules and secrete soluble immunomodulatory factors that are capable of mediating immunogenic inflammation [8, 9]. These mechanisms make it possible for the eye to regulate the intraocular innate and adaptive inflammatory response and accept transplanted tissue grafts for extended periods. In contrast, conventional body sites summarily reject such grafts. This review focuses on the development and maintenance of the immunosuppressive intraocular microenvironment formed via the generation of regulatory T cells (Tregs) by anterior chamber-associated immune deviation (ACAID), and ocular resident cells, which include corneal endothelial (CE) cells, ocular pigment epithelial (PE) cells, and aqueous humor. This review also evaluated the therapeutic potential of Tregs as powerful immunosuppressive cells that can be used for active noninfectious uveitis and corneal allograft transplantation.
2. Generation of Tregs in Eye-Derived Tolerance
To achieve immune privilege, the eye uses several different strategies to prevent and regulate sight-destroying inflammation in the eye [1, 10]. One of the strategies is the induction of the peripheral tolerance of eye-derived antigens referred to as ACAID [1, 11]. Antigenic materials in the anterior chamber generate a systemic immune response that retains primed, clonally expanded cytotoxic T-cell precursors and B cells secreting large concentrations of IgG1, which is a non-complement-fixing antibody. On the other hand, ACAID inhibits CD4+ Th1 and Th2 cells and B cells secreting complement-fixing antibodies [1, 2, 12–16]. The spleens of mice that receive antigen in the anterior chamber acquire three types of antigen-specific Tregs that mediate ACAID [17–19]. One of these populations consists of CD4+ T cells, which are known as the “afferent regulators,” as these CD4+ T cells are able to suppress the initial activation and differentiation of naïve T cells into Th1 effector cells. The second population consists of CD8+ T cells, which are known as “efferent regulators,” as these CD8+ T cells inhibit the expression of Th1 immune responses such as delayed hypersensitivity. The third population consists of CD8+ T cells that inhibit B cells from switching to the IgG isotype that fixes the complement. Efferent CD8+ Tregs in ACAID act in the periphery, including in the eye, whereas afferent CD4+ Tregs act in the secondary lymphoid organs [11, 20]. In ACAID, eye-derived antigen presenting cells (APCs) induce the expansion of tolerogenic B cells in order to induce antigen-specific Tregs [21] and invariant natural killer T cells, which are additionally required for the generation of ACAID [22]. Furthermore, Hare et al. have also demonstrated that the anterior chamber injection of bovine interphotoreceptor retinoid-binding protein (IRBP) impaired the development of IRBP-specific delayed hypersensitivity and prevented the expression of experimental autoimmune uveoretinitis (EAU). This model of human uveitis can be induced by immunization of susceptible animals with a retinal antigen such as IRBP [23–25]. Moreover, the adoptive transfer of spleen cells obtained from mice that received IRBP to the anterior chamber suppressed and eliminated already established intraocular inflammation, which suggests that IRBP-specific, ACAID-inducing Tregs act on the efferent limb of the immune response [23]. A recent study has also shown that retinal antigen-pulsed tolerogenic APCs (ACAID-genic APCs) suppressed ongoing EAU by inducing CD8+ Tregs that, in turn, suppressed the effector activity of IRBP-specific T cells [26]. Thus, ACAID via antigen-specific Tregs suppresses IRBP-induced autoimmune uveoretinitis.
3. Generation of Tregs by an Immunosuppressive Intraocular Microenvironment That Includes Corneal Endothelium, Aqueous Humor, and Pigment Epithelial Cells
There is growing evidence that ocular resident cells, which include CE cells and PE cells, can contribute to the development and maintenance of the immunosuppressive intraocular microenvironment via the generation of Tregs [8]. In addition to the ocular PE cells, the eye also contains resident myeloid cell populations such as macrophages and microglial cells. However, most of the macrophages are restricted to the cornea and uveal tract, where they are responsible for maintaining homeostasis by removing debris and dead cells. Microglial cells also play important roles in retinal development/homeostasis and can mediate local neuroinflammatory reactions [27, 28].
Tregs induced by ocular PE cells, which constitutively express the transcription factor Foxp3, are indispensable for immune tolerance and homeostasis, as they suppress excessive immune responses that are harmful to the host [29]. Since Tregs have been involved in a series of pathologic processes associated with autoimmune disease and cancer [30, 31], Foxp3+ Tregs as well as Tregs in ACAID have been considered to be the key regulators in ocular immune privilege. In the following section, we describe the molecular mechanisms that underlie the Treg induction by ocular resident cells, in addition to evaluating the therapeutic potential of CE and PE-induced Tregs in helping to maintain the ocular immune privilege.
3.1. Strategy for Generation of Tregs by Ocular Resident Cells
We performed in vitro experiments to investigate whether cultured ocular resident cells, including CE, iris PE, ciliary body PE, and retinal PE (RPE) cells, would have the capacity to convert activated T cells into Tregs [8]. To generate Tregs in vitro, naïve CD4+ or CD8+ T cells obtained from C57BL/6 mice were cocultured with ocular PE cells in the presence of anti-CD3 antibody. T cells exposed to CE or PE cells were harvested, x-irradiated, and used as regulators (PE-induced Tregs). CD4+ T cells obtained from C57BL/6 mice were used as responder T cells. The responder T cells and PE-induced Tregs were then cocultured in the presence of anti-CD3 antibody in order to evaluate whether PE-induced Tregs suppressed the proliferation and cytokine production of the responder T cells. If there was suppression of the responder T cell activation, this would confirm that there was induction of Tregs by the ocular resident cells. The molecular mechanism underlying the generation of Tregs differs in accordance with the microenvironment of the ocular resident cells.
3.2. CE Cell-Induced Tregs
CE cells are part of the inner surface of the anterior chamber of the eye and come in contact with the aqueous humor. Human CE cells contribute to local immune tolerance in the human eye, as activated T cells exposed to CE cells fail to acquire effector T-cell function [32–34]. In addition, it has been reported that murine CE cells constitutively express various immunomodulatory molecules such as the Fas ligand, programmed death-ligand 1 (PD-L1/CD274), and glucocorticoid-induced tumor necrosis factor receptor family-related protein ligand, which leads to apoptosis of the effector T cells [35–37]. We have previously demonstrated that cultured human CE cells suppressed the activation of CD4+ Th1 cells in a cell contact-dependent manner via an interaction between the PD-1 and PD-L1 costimulatory molecules in vitro [34]. Subsequently, we then investigated whether human CE cells were capable of inhibiting T cells and generating Tregs in vitro. Cultured human CE cells produced enhanced membrane-bound active transforming growth factor beta 2 (TGF-β2) and suppressed activation of CD8+ T cells via a membrane-bound form of TGF-β [38]. Furthermore, cultured CE cells converted CD8+ T cells into Tregs via their membrane-bound active TGF-β. In addition, CE cell-induced CD8+ Tregs expressed both CD25high and Foxp3 and suppressed activation of bystander effector T cells [38].
In a further experiment, we also examined whether murine CE cells have the capacity to generate Tregs. CD4+ T cells exposed to cultured murine CE cells expressed both CD25high and Foxp3, with these T cells suppressing the activation of the bystander target T cells, which indicates that cultured murine CE cells have the capacity to generate Tregs [39]. Moreover, cytotoxic T lymphocyte-associated antigen-2 alpha (CTLA-2α: cathepsin L inhibitor), which is expressed on murine CE cells, promoted Tregs through TGF-β signaling [39]. Taken together, these findings suggest that cultured CE cells expressing TGF-β and CTLA-2α promote the generation of CD4/CD8+ Tregs that are able to suppress bystander effector T cells, thereby helping to maintain the immunosuppressive intraocular microenvironment.
3.3. Aqueous Humor-Induced Tregs
The aqueous humor participates in the local defense system of the eye and protects the intraocular tissue from immunogenic inflammation [6]. The aqueous humor contains immunosuppressive factors such as α-melanocyte-stimulating hormone (α-MSH), vasoactive intestinal peptide, and TGF-β2 [6]. It has been reported that the aqueous humor is capable of inducing Tregs via α-MSH and TGF-β2 [40, 41]. Furthermore, it has been reported that the aqueous humor obtained from rats recovering from monophasic EAU was able to enhance the regulatory function of ocular Tregs in recurrent EAU [42]. A recent study additionally showed that the aqueous humor promoted the conversion of naïve T cells into Foxp3+ Tregs, while TGF-β and retinoic acid had a synergistic effect on the Treg conversion mediated by the aqueous humor [43].
3.4. Ocular PE Cell-Induced Tregs
Ocular PE cells of the iris, ciliary body, and retina have been identified as important participants in creating and maintaining ocular immune privilege [8, 10, 44]. Iris PE cells have the capacity to suppress anti-CD3-driven activation of primed or naïve T cells [44]. We have previously shown that cultured iris PE cells suppressed TCR-driven T-cell activation in vitro through direct cell contact in which the B7-2 (CD86) expressed by the iris PE cells interacted with CTLA-4 on the responding T cells [45]. B7-2+ iris PE cells in the presence of anti-CD3 agonistic antibody supported selective activation of CTLA-4+CD8+ T cells that express their own B7-2 and secreted enhanced amounts of active TGF-β, leading to the global suppression of entire T-cell populations, including CD4+ T cells [46].
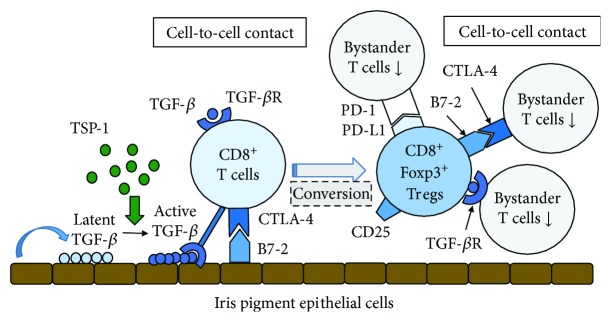
Subsequently, we then examined whether TGF-β was necessary for this process. Our study showed that both the iris PE and T cells exposed to iris PE cells were able to: (1) upregulate their TGF-β and TGF-β receptor genes, (2) convert the latent TGF-β they produced into the active form, and (3) use membrane-bound or soluble TGF-β to suppress bystander T cells. This demonstrated that both iris PE cells and B7-2+CTLA-4+CD8+ iris PE-induced Tregs produce enhanced amounts of active TGF-β, with the membrane-bound form of TGF-β used to suppress T-cell activation [47]. Furthermore, iris PE cells promoted the generation of Foxp3+CD8+CD25+ Tregs with cell contact via the B7-2/CTLA-4 interactions [48, 49]. In addition, iris PE-induced CD8+ Tregs greatly expressed PD-L1 costimulatory molecules and suppressed the activation of bystander Th1 cells that express PD-1 costimulatory receptor via a contact-dependent mechanism [50]. A previous study clearly demonstrated that thrombospondin-1 (TSP-1) binds and activates TGF-β [51]. Furthermore, iris PE cells generated CD8+ Tregs via TSP-1 and iris PE-induced CD8+ Tregs suppressed activation of bystander T cells via TSP-1 [52]. Taken together, these results strongly suggest that iris PE cell-induced CD8+ Tregs play a role in maintaining immune privilege in the anterior segment of the eye (Figure 1).
Figure 1.
Molecular mechanism underlying the generation of regulatory T cells (Tregs) by murine iris pigment epithelial (PE) cells. Cultured iris PE cells suppress anti-CD3-driven T cell activation in vitro by direct cell contact in which B7-2 (CD86) expressed by iris PE cells interacts with cytotoxic T-lymphocyte antigen-4 (CTLA-4) on responding T cells. Furthermore, cultured iris PE cells expressing B7-2 induce the activation of CTLA-4+CD8+ T cells that express their own B7-2 and secrete enhanced amounts of active transforming growth factor beta (TGF-β), leading to the global suppression of entire T-cell populations including CD4+ T cells. Both iris PE cells and T cells exposed to iris PE cells upregulate their TGF-β and TGF-β receptor (TGF-βR) genes and suppress bystander T cells using membrane-bound or soluble TGF-β. In addition, iris PE cell-induced Foxp3+CD8+CD25+ Tregs suppress bystander T cells through cell contact via B7-2/CTLA-4 and/or programmed cell death- (PD-) 1/PD-L1 interactions. Thrombospondin-1 (TSP-1) produced from iris PE cells greatly contributes to the conversion of TGF-β from latent form to active form.
Previous studies have shown that the subretinal space is also an immune privileged site and that RPE cells act as immune privilege tissue [53, 54]. Moreover, RPE cells play pivotal roles in helping to maintain immune privilege in the subretinal space [3]. RPE cells have been shown to secrete soluble factors including TGF-β, TSP-1, and PGE2, which are mediators that alter the innate and adaptive immune responses [55–57]. Depending upon the inflammatory conditions, RPE cells are able to inhibit activated T cells that are regulated by the levels of the MHC class II expression [58]. Moreover, under the presence of inflammatory cytokines such as IL-17 and IFN-γ, RPE cells also highly express PD-L1, which can lead to suppression of the pathogenic activity of IRBP-specific T cells that induce EAU [59].
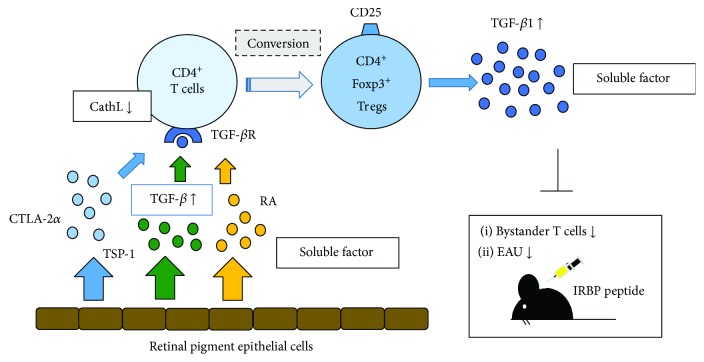
We have also reported that unlike for the iris PE cells, the RPE and ciliary body PE cells can suppress bystander T cells through inhibitory soluble factors and that the soluble form of the active TGF-β1/2 produced by the RPE and ciliary body PE cells demonstrated an immunosuppressive effect on the bystander T cells [56]. Subsequently, we then investigated whether RPE cell-exposed T cells could become Tregs in vitro and if the soluble form of TGF-β produced by the cultured RPE cells could convert T cells into Tregs. Our results showed that cultured RPE cells converted CD4+ T cells into Tregs in the presence of CTLA-2α [60]. RPE cells constitutively expressed CTLA-2α (cathepsin L inhibitor), which promoted the induction of Tregs, and CD4+ T cells exposed to RPE cells predominantly expressed CD25+ and Foxp3 [60]. Furthermore, recombinant CTLA-2α promoted the development of CD4+, CD25+Foxp3+ Tregs through TGF-β signaling in vitro, with these Tregs producing high levels of TGF-β [60]. These findings demonstrated that RPE cell-induced Tregs participated in the establishment of immune tolerance in the posterior segment of the eye (Figure 2). Our recent study also showed that RPE cells that produced retinoic acid and cultured RPE cells from vitamin A-deficient mice were unable to induce Foxp3+ Tregs [61]. These data are compatible with previous studies that have shown that the conversion of naïve T cells into Foxp3+ Tregs in the eye required TGF-β and retinoic acid [43, 61]. Thus, overall, these findings indicate that TGF-β and retinoic acid interact to induce Tregs for immunological regulation in the eye (Figure 2).
Figure 2.
Molecular mechanism underlying the generation of regulatory T cells (Tregs) by murine retinal pigment epithelial (RPE) cells. RPE cells constitutively express cytotoxic T lymphocyte-associated antigen 2 alpha (CTLA-2α), a cathepsin L (CathL) inhibitor, which promotes the induction of Tregs. In addition, CD4+ T cells exposed to RPE cells predominantly express CD25 and Foxp3. CTLA-2α, thrombospondin-1 (TSP-1), and retinoic acid promote the development of CD4+CD25+ Foxp3+ Tregs by transforming growth factor beta (TGF-β) signaling in vitro. These Tregs produce high levels of TGF-β and suppress bystander T cells and experimental autoimmune uveoretinitis (EAU) induced by retinal antigen interphotoreceptor retinoid-binding protein (IRBP).
4. Immunomodulation of Uveitis by Tregs
Thymus-derived naturally occurring Tregs play an essential role in preventing autoimmune disease, with depletion of the naturally occurring Tregs leading to multiorgan autoimmune disease [29, 30]. Indeed, depletion of CD4+CD25+ T cells before immunization has been shown to exacerbate the murine EAU model of human uveitis [62]. A recent study reported that retinal antigen-specific Foxp3+ Tregs play a role in the natural resolution of EAU and the maintenance of remission [63]. Conversely, there is growing evidence that administration of Tregs can effectively suppress uveitis in mice. Antigen-specific Tregs generated by α-MSH and TGF-β2 have also been shown to suppress EAU [64]. In addition, lipopolysaccharide-activated dendritic cell-induced CD4+CD25+Foxp3+ Tregs inhibit CD4+CD25− effector T cells, and when adoptively transferred, these Tregs suppress EAU [65]. Moreover, intravenous administration of antigen-specific Tregs has the capacity to control uveitis in mice [66]. In addition, an intravitreous injection of preactivated polyclonal Tregs was also shown to suppress uveitis in mice [67]. In our own study, we also demonstrated that the adoptive transfer of CD4+CD25+ natural Tregs ameliorated the development of EAU [68]. However, the ability to prepare large numbers of Tregs for adoptive transfer and stable expression of Foxp3 in vivo remains problematic.
Since retinoic acid has been reported to contribute to high and stable Foxp3 expression via the retinoic acid receptor in the presence of TGF-β [69], we investigated whether retinoic acid has the capacity to expand Tregs and ameliorate the development of EAU. The results of our study demonstrated that retinoic acid promoted the generation of CD4+Foxp3+ Tregs in the presence of TGF-β, with systemic administration of retinoic acid during the induction phase reducing the clinical score of EAU [70, 71]. Furthermore, oral administration of a novel synthetic retinoic acid, Am80, not only increased the frequency of Tregs in draining lymph nodes in mice with EAU but also suppressed the Th1/Th17 response [71]. Am80 is more stable to light, heat, and oxidation than retinoic acid, and Am80 is clinically available in Japan for the treatment of relapsed acute promyelocytic leukemia. Thus, systemic administration of retinoid may not only have the potential to promote the expansion of Tregs in vivo, but it appears that it may also have therapeutic possibilities. In addition, since a previous report demonstrated that TGF-β levels were significantly elevated in the aqueous humor from EAU eyes [72], it is conceivable that the expression of Foxp3 on intraocular T cells in Am80-treated mice may be increased, with expansion of Foxp3+ Tregs possibly contributing to the amelioration of murine EAU.
Stabilization of Foxp3 expression is necessary for the generation and maintenance of highly suppressive Tregs in vivo for clinical use. Presently, various reagents and drugs, such as rapamycin, IL-2, and retinoic acid, have been reported to stabilize Foxp3 expression [73]. Furthermore, epigenetic modification of Foxp3 expression may be required in order to generate stable Tregs for clinical application [74].
As described above, we demonstrated that recombinant CTLA-2α (rCTLA-2α) derived from RPE cells has the capacity to generate Tregs through the promotion of TGF-β production [60]. Indeed, rCTLA-2α-treated mice had a high population of Foxp3+ Tregs compared with CD4+ T cells from control EAU mice [75]. Furthermore, the severity of EAU was significantly reduced in rCTLA-2α-treated mice and cathepsin L-deficient mice as compared with wild type mice. Thus, these findings suggest that CTLA-2α secreted from RPE cells converts intraocular effector T cells into Foxp3+ T cells that then acquire regulatory functions and lead to the amelioration of ocular inflammation [75].
We next assessed the ability of murine RPE cell-induced Tregs to suppress EAU in mice through the use of adoptive transfer. Our data revealed that the administration of RPE cell-induced Tregs that greatly expressed Foxp3 were able to suppress ocular inflammation in mice with EAU [76]. Moreover, the retinal antigen-specific cytokine response (IFN-γ and IL-17) was reduced when intraocular T cells were cocultured with RPE cell-induced Tregs in vitro [76]. These findings suggest that RPE cell-induced Tregs might possibly have a therapeutic potential for the treatment of autoimmune uveoretinitis.
Another recent challenge encountered with Treg therapy was reported while using a murine ocular inflammatory model, which included both antigen-specific and nonantigen-specific murine disease models [67]. In the antigen-specific model, TCR-hemagglutinin (HA) transgenic mice and HA-specific effector T cells were used to induce uveitis in mice in which HA is constitutively expressed in the retina. The authors found that Treg transplantation in the systemic circulation significantly suppressed local ocular inflammation. Moreover, polyclonal Tregs that expanded ex vivo also significantly improved ocular inflammation when these Tregs were injected locally, that is, intravitreally. Other recent investigations have additionally shown that several regulatory molecules including IL-22, aryl hydrocarbon receptor, and CD73/adenosine contribute to the generation of Tregs/regulatory mesenchymal stem cells to control EAU in mice [77–79]. These murine study findings support the concept of Treg therapy for ocular inflammation and are the foundation for further human clinical trials.
5. Ocular Surface Disease and Tregs
Dry eye disease (DED) is one of the major ocular surface inflammatory disorders [80, 81]. It is well known that activation and infiltration of pathogenic immune cells, primarily CD4+ T cells, contribute to the development of ocular surface inflammation in DED [82–84]. Increased IL-17 and IFN-γ have been observed in both clinical and experimental DED [85–89]. Recent studies have demonstrated that Th17 cells are the principal effectors actively mediating DED [90, 91]. In fact, Chauhan et al. reported that while Treg frequencies remained unchanged, there was a marked decrease in their potential to suppress the effector Th17 cells in a mouse model of DED. This suggests that dysfunction of Tregs can be presumed to be one of the major causes in ocular anterior segment inflammation such as DED [90, 92]. It has also been reported that in vitro-expanded Foxp3+ Tregs maintain a normal phenotype and are capable of suppressing immune-mediated ocular surface inflammation in animal models, with in vitro-expanded Tregs able to more efficiently reduce tear cytokine levels and conjunctival cellular infiltration compared to freshly isolated Tregs [93]. Antigen specificity is one of important factors required for Tregs in order to more effectively regulate the pathogenic inflammatory cells. Presently, while the specific autoantigen responsible for the induction of dry eye disease has yet to be identified, it has been suggested that α-fodrin might be a candidate autoantigen in primary Sjögren's syndrome [94]. Identification of a specific autoantigen in DED could potentially lead to the generation of antigen-specific Tregs that ultimately could become a promising therapy for immune-mediated ocular surface inflammation.
6. Corneal Transplantation and Tregs
The three fundamental factors that contribute to corneal allograft survival are (1) blocking the induction of the immune response against allograft antigens, (2) generation of Tregs that can suppress the destructive alloimmune reaction, and (3) induction of apoptosis of inflammatory cells at the graft/host interface [95]. Long-term corneal allograft survival leads to an antigen-specific suppression of the delayed type hypersensitivity immune response and resembles the suppression of the delayed type hypersensitivity that is observed in ACAID [96]. Cunnusamy et al. have reported that there are two different Tregs that can promote corneal allograft survival. These include (1) CD4+CD25+ Tregs induced by the corneal allograft act at the efferent arm of the immune response in order to suppress the delayed type hypersensitivity and (2) CD8+ Tregs induced by anterior chamber injection of alloantigens to suppress the efferent phase of the immune response [95]. Furthermore, it has also been demonstrated that the levels of Foxp3 expression in Tregs from corneal allograft acceptors were significantly higher compared to that seen in Tregs from the corneal allograft rejectors, which suggests that dysfunction of Tregs can be presumed to be one of the major causes of corneal allograft rejection [97]. Moreover, Tregs of allograft acceptors during adoptive transfers were reported to significantly increase the allograft survival rate [97]. In addition, it was also shown that the presence of allospecific Tregs in graft recipients primarily suppressed the induction of alloimmunity in the regional draining lymph nodes rather than suppressing the effector phase of the immune response in the periphery [97]. Hori et al. have shown that the expression of the glucocorticoid-induced tumor necrosis factor receptor family-related protein ligand (GITRL) in the cornea led to the local expansion of Foxp3+CD4+CD25+ Tregs, thereby contributing to the immune privilege status for the corneal allografts [98]. As previously described, we have demonstrated that cultured CE cells expressing TGF-β and CTLA-2α promote the generation of CD4/CD8+ Tregs that are able to suppress the bystander effector T cells [39]. Taken together, these findings suggest that cell therapy performed when using Tregs may potentially be able to promote corneal allograft survival during transplantation. However, other recent evidence has shown that there is an increased risk of corneal allograft rejection in mice with allergic conjunctivitis and impaired function of the peripherally induced regulatory T cells in hosts who were at a high risk of graft rejection [99–101]. In fact, increases in corneal graft rejection were found in hosts reported to have previous ocular allergies during routine clinical practice examinations due to allergic inflammatory responses [102, 103]. A recent study demonstrated that systemic treatment of high-risk recipient mice with low-dose IL-2 led to an expansion and improved suppressive function of Tregs, reduced leukocyte infiltration of the graft, and promotion of corneal allograft survival [104]. Further studies that help to better clarify the mechanism of the generation and function of Tregs in corneal allograft transplantation will hopefully lead to the promotion of ocular immune privilege and survival of the corneal allograft in hosts with inflamed or vascularized recipient beds after Treg-based therapy.
7. Current Concept and Strategy of Treg Therapy in Humans
Adoptive transfer of Tregs in humans has been examined and tested in order to treat systemic autoimmune diseases or posttransplant-related complications [105, 106]. These pathologic states are partly caused by the dysfunction of Tregs or due to the relative inferior activity of Tregs to effector T cells. Restoration or reinforcing immune regulation by Tregs is the primary aim of the treatment. Furthermore, there is clear evidence for a relationship between the dysfunction of Tregs and autoimmune disease onset. The Foxp3 gene is mutated in immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome. Thus, Foxp3+ Tregs are thoroughly absent throughout the whole body, which can cause fatal autoimmunity leading to death during the early stages of life if hematopoietic stem cell transplantation is not performed [107, 108]. These examples demonstrate that Treg deficiency or relative dominant proinflammatory cytokines are in fact related to the autoimmune disease onset. Consequently, we believe that adoptive transfer of Tregs into affected patients will be a promising strategy against this pathological inflammation.
In order to achieve new therapeutic strategies for clinical application, the critical issues that need to be addressed include the following: (1) human Treg phenotypes need to be characterized in detail in order for clinical application, (2) techniques need to be standardized for isolating and expanding Tregs in order to avoid contamination, and (3) a method for delivering Tregs into patients will need to be established. With regard to the first issue, appropriate characteristics of Tregs will not be identical for each disease. Presently, the use of antigen-specific Tregs is an ideal choice for cases in which the target antigen is already known. However, the causative self-antigen remains unknown in most autoimmune diseases. Furthermore, it is practically impossible to cover all antigen repertoires in autoimmune diseases. Therefore, a more realistic idea for addressing this issue would be to utilize polyclonal non-antigen-specific Tregs, which may suppress inflammation in a bystander manner. Although polyclonal Tregs may have a relatively broad suppressive function, the effectiveness of polyclonal Tregs is still unclear and could potentially differ for individual organs and disorders.
For the second issue, there are several potential sources of Tregs. Autologous peripheral blood is a straightforward choice, as it is easy to collect. In addition, allogeneic umbilical cord blood, preferably HLA matched, is a favorable alternative choice [109]. Moreover, in the case of graft-versus-host disease (GVHD), allogeneic donor-derived Tregs are also usable material. Regardless of the Treg source, the next critical technical step is the sorting of the polyclonal Tregs according to the characteristic surface markers. CD4+CD25+ selection is the most common method. Although Foxp3 is the most Treg-specific marker, it can only be detected by permeabilization, which, unfortunately, causes cell death. Therefore, this procedure cannot be used for the purpose of selection. In addition to CD4+CD25+ selection, cells are commonly sorted according to CD127low expression for further purification [110].
Human Foxp3+ Tregs have recently been categorized based on CD25 and CD45RA expression, with CD25lowCD45RA+ expression indicating resting Tregs, CD25lowCD45RA− expression indicating nonsuppressive Tregs, and CD25highCD45RA− expression indicating active Tregs. However, for these treatments, it has been suggested that CD25highCD45RA− Tregs might be the best population to use [111, 112]. After sorting the specific populations, cell expansion is essential because the numbers of circulatory Tregs are relatively small (up to 5–7% of CD4+ T cells) [110].
Costimulation with anti-CD3/CD28 and IL-2 stimulation is a popular technique among the general expansion protocols [106]. This protocol enables expansion by a few hundredfold at most. However, contamination with T cells other than Tregs is unpreventable to some extent following this massive expansion. While the acceptable amount of contamination for clinical use remains uncertain, it may be dependent on the target disease. From this point of view, the use of umbilical cord blood-derived T cells, which constitute naïve cells, may be advantageous since natural Tregs are used [113], thereby avoiding contamination of the memory effector T cells in the injected cells.
For the third issue, there are many options for delivering expanded cells. Systemic injection via peripheral circulation is common, while local administration is also possible in some organs. However, careful attention should be paid to potential infusion reactions that could occur following administration via blood circulation. Even so, the eye is one of the best target organs for local administration. Inflammatory disease in the eye, such as uveitis, is the next challenge for targeted Treg therapy [76].
7.1. Application of Tregs in Treatment of Ocular Inflammation
Since the cause of noninfectious uveitis is diverse, most disorders can be treated or well controlled with immunosuppression. As a result, noninfectious uveitis can be viewed as an autoimmune disease of the eye. Systemic or topical administration of steroids has long been used as major immunosuppressive therapies for ocular inflammation. In addition to steroids, immunosuppressive agents or recently introduced monoclonal antibodies against inflammatory cytokines are also frequently administered in these patients [114]. In uveitis, proinflammatory cytokines from pathological T cells play central roles in the inflammation [115, 116]. We previously reported the decreased frequency of peripheral Tregs in patients with active uveitis such as Behçet's disease [117]. In healthy individuals, organ homeostasis is maintained by central and local tolerance [118]. As previously mentioned, the eye is one of the major immune privileged sites [10], where ocular PE cells play a central role in developing local tolerance [9]. The breakdown of immune tolerance leads to unfavorable autologous antigen-specific attacks against organs by the effector T cells. Failure in immune tolerance is partly due to Treg dysfunction and/or dominant effector T cell activity. Since noninfectious uveitis is considered an autoimmune disease, it is logical to assume that adoptive transplantation of Tregs should inhibit ocular inflammation. Thus, restoration of Treg function or artificial transfer of Tregs into noninfectious uveitis patients is likely to be a promising therapeutic choice for treating this disease.
Based on the therapeutic effect of Tregs in animal models of autoimmune uveitis [67], a phase I/II clinical trial has been started in Europe in patients with severe bilateral uveitis who are refractory to standard treatments and presented with a low visual acuity [119, 120]. The objective of this trial is to evaluate the safety of an intravitreal injection of ex vivo-activated polyclonal Tregs in patients with refractory and end-stage noninfectious uveitis. The result of this ongoing clinical trial and further studies on the safety and efficacy of Tregs should provide valuable information for the application of Tregs in patients with refractory uveitis.
7.2. Establishment of Tregs by Ocular Microenvironment
Similar to our previous murine studies, human ocular PE cells have been shown to have immunosuppressive functions, which form the immune privilege in the eye [121]. Primary cultured human iris PE cells are able to suppress the activation of bystander responder T cells in vitro [122]. Human iris PE cells suppress cell proliferation and cytokine production by responder T cells via direct cell-to-cell contact in a TGF-β-dependent manner. Furthermore, responder T cells are not only conventional autogenic activated T cells but also allogeneic activated T cells or T cell clones that have been established from uveitis patients [122]. In addition, human CE cells have an immunoregulatory function equivalent to that of human iris PE cells [38]. Interestingly, human CE cells can inhibit activated PD-1+ helper T cells via the PD-L1–PD-1 interaction [34], while activated T cells are suppressed via membrane-bound TGF-β [38]. Thus, human iris PE cells and CE cells cooperatively create immune privilege in the anterior chamber.
Focusing on the posterior ocular segment, human RPE cells show potent regulatory function in ocular inflammation as well. Inflammatory cells in the retina, where cells cannot freely move, do not always come in direct contact with RPE cells. However, RPE cells can regulate inflammation by secreting soluble inhibitory molecules or generating Tregs [9]. Similar to the results of previous murine studies, human RPE cells have shown a great ability to generate Tregs in vitro [121]. RPE-induced Tregs strongly suppress cytokine production and proliferation of intraocular T-cell clones derived from active uveitis patients. CD4+ T cells express CD25 and Foxp3 after culture with RPE supernatants, especially TGF-β2-pretreated RPE cells. The suppressive mechanism of human RPE-induced Tregs is mediated in a TGF-β-dependent manner, similar to that observed for murine RPE-induced Tregs. Based on these results, practical application of RPE-induced Tregs for treating uveitis and transplantation of a retina/RPE graft in patients with retinal degeneration appears to be a logical approach. However, for future clinical applications of Tregs in inflammatory ocular diseases and retina/RPE transplantation, there needs to be further optimization of the establishing and expanding of Tregs.
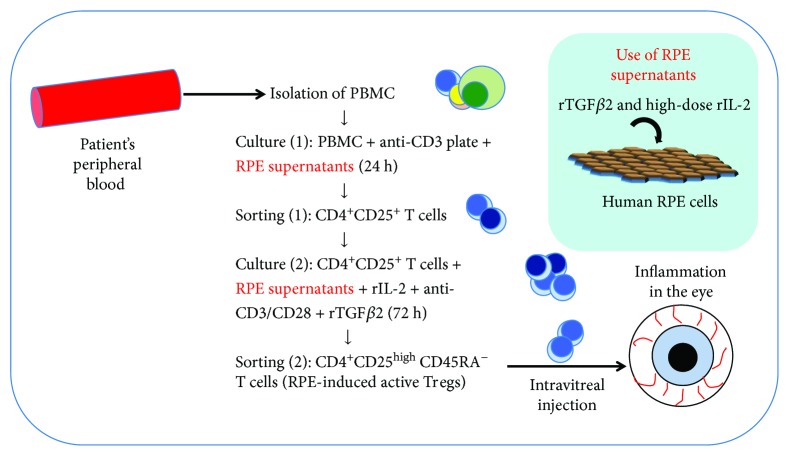
Based on these previous studies, we subsequently developed a method that could be used to more selectively and efficiently obtain RPE-induced Tregs (Figure 3). With this method, PBMCs are first cultured with recombinant TGF-β2-pretreated RPE supernatant on an anti-CD3-coated plate. CD4+CD25+ T cells are then sorted and recultured together with high-dose recombinant IL-2, antihuman CD3/CD28 antibodies, and TGF-β2 for 3 days. Using this method, it is possible to produce a large amount of CD25highCD45RA− active Tregs that highly express Foxp3, CTLA-4 (CD152), and tumor necrosis factor receptor superfamily 18 (TNFRSF18). Furthermore, these RPE-induced Tregs secrete large amounts of suppressive cytokines TGF-β1 and IL-10 and suppress bystander target Th1 cells or Th17 cells [76].
Figure 3.
Regulatory T-cell (Treg) therapy in ocular disease: the original source of Tregs is the patient's peripheral blood. Following isolation of peripheral blood mononuclear cells (PBMCs) from the blood, PBMCs are cultured on anti-CD3-coated plates with RPE supernatants for 24 h. RPE supernatants are collected from culture media of human RPE cell lines with transforming growth factor beta 2 (TGF-β2) and high-dose interleukin 2 (IL-2). CD4+CD25+ T cells are first selected from cultured PBMCs. Sorted CD4+CD25+ T cells are then recultured with RPE supernatants together with recombinant IL-2 (rIL-2) and anti-CD3/CD28 antibodies for 72 h. In the final sort, CD4+CD25highCD45RA− T cells are collected, which are best suited for intravitreal injection into uveitis patients.
8. Conclusions and Future Directions
Although CE and RPE cells are responsible for maintaining the homeostasis of the microenvironment of the eye, they also have unique anti-inflammatory and immunogenic roles in inflammation. Both ocular resident mesenchymal cells and peripheral tolerance of ACAID actively contribute to the regulation of immune responses via the generation of Tregs. These eye-specific Tregs have the therapeutic potential for not only autoimmune uveoretinitis but also promoting allograft survival after transplantation. At present, the therapeutic potential of Tregs in humans has been both examined and tested in order to treat systemic autoimmune diseases or posttransplant-related complications. However, further studies will be required in order to establish Treg therapy for active noninfectious uveitis. In addition, a better understanding of the molecular mechanism that regulates ocular immune privilege may lead to an effective therapeutic strategy that can be used to target individual patients with refractory uveitis.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Streilein J. W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nature Reviews Immunology. 2003;3(11):879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 2.Niederkorn J. Y. Immune privilege in the anterior chamber of the eye. Critical Reviews™ in Immunology. 2002;22(1):13–46. doi: 10.1615/CritRevImmunol.v22.i1.20. [DOI] [PubMed] [Google Scholar]
- 3.Streilein J. W., Ma N., Wenkel H., Fong Ng T., Zamiri P. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Research. 2002;42(4):487–495. doi: 10.1016/S0042-6989(01)00185-7. [DOI] [PubMed] [Google Scholar]
- 4.Streilein J. W., Ksander B. R., Taylor A. W. Immune deviation in relation to ocular immune privilege. The Journal of Immunology. 1997;158(8):3557–3560. [PubMed] [Google Scholar]
- 5.Taylor A. W. Ocular immunosuppressive microenvironment. Chemical Immunology and Allergy. 1999;73:72–89. doi: 10.1159/000058738. [DOI] [PubMed] [Google Scholar]
- 6.Taylor A. W. Ocular immunosuppressive microenvironment. Chemical Immunology and Allergy. 2007;92:71–85. doi: 10.1159/000099255. [DOI] [PubMed] [Google Scholar]
- 7.Griffith T. S., Brunner T., Fletcher S. M., Green D. R., Ferguson T. A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 8.Sugita S. Role of ocular pigment epithelial cells in immune privilege. Archivum Immunologiae et Therapiae Experimentalis. 2009;57(4):263–268. doi: 10.1007/s00005-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 9.Mochizuki M., Sugita S., Kamoi K. Immunological homeostasis of the eye. Progress in Retinal and Eye Research. 2013;33:10–27. doi: 10.1016/j.preteyeres.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Streilein J. W. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Current Opinion in Immunology. 1993;5(3):428–432. doi: 10.1016/0952-7915(93)90064-Y. [DOI] [PubMed] [Google Scholar]
- 11.Stein-Streilein J., Streilein J. W. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. International Reviews of Immunology. 2002;21(2-3):123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan H. J., Streilein J. W. Immune response to immunization via the anterior chamber of the eye: I. F1 Lymphocyte-Induced Immune Deviation. The Journal of Immunology. 1977;118(3):809–814. [PubMed] [Google Scholar]
- 13.Kaplan H. J., Streilein J. W. Immune response to immunization via the anterior chamber of the eye: II. An analysis of F1 lymphocyte-induced immune deviation. The Journal of Immunology. 1978;120(3):689–693. [PubMed] [Google Scholar]
- 14.Streilein J. W., Niederkorn J. Y., Shadduck J. A. Systemic immune unresponsiveness induced in adult mice by anterior chamber presentation of minor histocompatibility antigens. Journal of Experimental Medicine. 1980;152(4):1121–1125. doi: 10.1084/jem.152.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ksander B. R., Streilein J. W. Analysis of cytotoxic T cell responses to intracameral allogeneic tumors. Investigative Ophthalmology & Visual Science. 1989;30(2):323–329. [PubMed] [Google Scholar]
- 16.Wilbanks G. A., Streilein J. W. Distinctive humoral immune responses following anterior chamber and intravenous administration of soluble antigen. Evidence for active suppression of IgG2-secreting B lymphocytes. Immunology. 1990;71(4):566–572. [PMC free article] [PubMed] [Google Scholar]
- 17.Niederkorn J. Y., Streilein J. W. Alloantigens placed into the anterior chamber of the eye induce specific suppression of delayed-type hypersensitivity but normal cytotoxic T lymphocyte and helper T lymphocyte responses. The Journal of Immunology. 1983;131(6):2670–2674. [PubMed] [Google Scholar]
- 18.Streilein J. W., Niederkorn J. Y. Characterization of the suppressor cell(s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. The Journal of Immunology. 1985;134(3):1381–1387. [PubMed] [Google Scholar]
- 19.Wilbanks G. A., Streilein J. W. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen: evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990;71(3):383–389. [PMC free article] [PubMed] [Google Scholar]
- 20.Streilein J. W., Masli S., Takeuchi M., Kezuka T. The eye’s view of antigen presentation. Human Immunology. 2002;63(6):435–443. doi: 10.1016/S0198-8859(02)00393-2. [DOI] [PubMed] [Google Scholar]
- 21.D'Orazio T. J., Mayhew E., Niederkorn J. Y. Ocular immune privilege promoted by the presentation of peptide on tolerogenic B cells in the spleen: II. Evidence for presentation by Qa-1. The Journal of Immunology. 2001;166(1):26–32. doi: 10.4049/jimmunol.166.1.26. [DOI] [PubMed] [Google Scholar]
- 22.Nowak M., Stein-Streilein J. Invariant NKT cells and tolerance. International Reviews of Immunology. 2007;26(1-2):95–119. doi: 10.1080/08830180601070195. [DOI] [PubMed] [Google Scholar]
- 23.Hara Y., Caspi R. R., Wiggert B., Chan C. C., Streilein J. W. Use of ACAID to suppress interphotoreceptor retinoid binding protein-induced experimental autoimmune uveitis. Current Eye Research. 1992;11(Supplement 1):97–100. doi: 10.3109/02713689208999517. [DOI] [PubMed] [Google Scholar]
- 24.Caspi R. R., Roberge F. G., Chan C. C., et al. A new model of autoimmune disease: experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. The Journal of Immunology. 1988;140(5):1490–1495. [PubMed] [Google Scholar]
- 25.Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunologic Research. 2008;42(1–3):41–50. doi: 10.1007/s12026-008-8031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu S. M., Mathew R., Taylor A. W., Stein-Streilein J. Ex-vivo tolerogenic F4/80+ antigen-presenting cells (APC) induce efferent CD8+ regulatory T cell-dependent suppression of experimental autoimmune uveitis. Clinical & Experimental Immunology. 2014;176(1):37–48. doi: 10.1111/cei.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma W., Wong W. T. Aging changes in retinal microglia and their relevance to age-related retinal disease. Advances in Experimental Medicine and Biology. 2016;854:73–78. doi: 10.1007/978-3-319-17121-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinnery H. R., McMenamin P. G., Dando S. J. Macrophage physiology in the eye. Pflügers Archiv - European Journal of Physiology. 2017;469(3-4):501–515. doi: 10.1007/s00424-017-1947-5. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annual Review of Immunology. 2004;22(1):531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka A., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Research. 2017;27(1):109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawashima H., Prasad S. A., Gregerson D. S. Corneal endothelial cells inhibit T cell proliferation by blocking IL-2 production. The Journal of Immunology. 1994;153(5):1982–1989. [PubMed] [Google Scholar]
- 33.Obritsch W. F., Kawashima H., Evangelista A., Ketcham J. M., Holland E. J., Gregerson D. S. Inhibition of in vitro T cell activation by corneal endothelial cells. Cellular Immunology. 1992;144(1):80–94. doi: 10.1016/0008-8749(92)90227-G. [DOI] [PubMed] [Google Scholar]
- 34.Sugita S., Usui Y., Horie S., et al. Human corneal endothelial cells expressing programmed death-ligand 1 (PD-L1) suppress PD-1+ T helper 1 cells by a contact-dependent mechanism. Investigative Opthalmology & Visual Science. 2009;50(1):263–272. doi: 10.1167/iovs.08-2536. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson T. A., Griffith T. S. A vision of cell death: insights into immune privilege. Immunological Reviews. 1997;156(1):167–184. doi: 10.1111/j.1600-065X.1997.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 36.Hori J., Vega J. L., Masli S. Review of ocular immune privilege in the year 2010: modifying the immune privilege of the eye. Ocular Immunology and Inflammation. 2010;18(5):325–333. doi: 10.3109/09273948.2010.512696. [DOI] [PubMed] [Google Scholar]
- 37.Hori J., Wang M., Miyashita M., et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. The Journal of Immunology. 2006;177(9):5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 38.Yamada Y., Sugita S., Horie S., Yamagami S., Mochizuki M. Mechanisms of immune suppression for CD8+ T cells by human corneal endothelial cells via membrane-bound TGFβ. Investigative Ophthalmology & Visual Science. 2010;51(5):2548–2557. doi: 10.1167/iovs.09-4233. [DOI] [PubMed] [Google Scholar]
- 39.Sugita S., Yamada Y., Horie S., et al. Induction of T regulatory cells by cytotoxic T-lymphocyte antigen-2α on corneal endothelial cells. Investigative Opthalmology & Visual Science. 2011;52(5):2598–2605. doi: 10.1167/iovs.10-6322. [DOI] [PubMed] [Google Scholar]
- 40.Taylor A. W., Alard P., Yee D. G., Streilein J. W. Aqueous humor induces transforming growth factor-ß (TGF-ß)-producing regulatory T-cells. Current Eye Research. 1997;16(9):900–908. doi: 10.1076/ceyr.16.9.900.5043. [DOI] [PubMed] [Google Scholar]
- 41.Nishida T., Taylor A. W. Specific aqueous humor factors induce activation of regulatory T cells. Investigative Ophthalmology & Visual Science. 1999;40(10):2268–2274. [PubMed] [Google Scholar]
- 42.Ke Y., Jiang G., Sun D., Kaplan H. J., Shao H. Ocular regulatory T cells distinguish monophasic from recurrent autoimmune uveitis. Investigative Ophthalmology & Visual Science. 2008;49(9):3999–4007. doi: 10.1167/iovs.07-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou R., Horai R., Silver P. B., et al. The living eye “disarms” uncommitted autoreactive T cells by converting them to Foxp3+ regulatory cells following local antigen recognition. The Journal of Immunology. 2012;188(4):1742–1750. doi: 10.4049/jimmunol.1102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida M., Takeuchi M., Streilein J. W. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege: 1. Inhibition of T-cell activation in vitro by direct cell-to-cell contact. Investigative Ophthalmology & Visual Science. 2000;41(3):811–821. [PubMed] [Google Scholar]
- 45.Sugita S., Streilein J. W. Iris pigment epithelium expressing CD86 (B7-2) directly suppresses T cell activation in vitro via binding to cytotoxic T lymphocyte-associated antigen 4. Journal of Experimental Medicine. 2003;198(1):161–171. doi: 10.1084/jem.20030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugita S., Ng T. F., Schwartzkopff J., Streilein J. W. CTLA-4+CD8+ T cells that encounter B7-2+ iris pigment epithelial cells express their own B7-2 to achieve global suppression of T cell activation. The Journal of Immunology. 2004;172(7):4184–4194. doi: 10.4049/jimmunol.172.7.4184. [DOI] [PubMed] [Google Scholar]
- 47.Sugita S., Ng T. F., Lucas P. J., Gress R. E., Streilein J. W. B7+ iris pigment epithelium induce CD8+ T regulatory cells; both suppress CTLA-4+ T cells. The Journal of Immunology. 2006;176(1):118–127. doi: 10.4049/jimmunol.176.1.118. [DOI] [PubMed] [Google Scholar]
- 48.Sugita S., Keino H., Futagami Y., et al. B7+ iris pigment epithelial cells convert T cells into CTLA-4+, B7-expressing CD8+ regulatory T cells. Investigative Opthalmology & Visual Science. 2006;47(12):5376–5384. doi: 10.1167/iovs.05-1354. [DOI] [PubMed] [Google Scholar]
- 49.Sugita S., Futagami Y., Horie S., Mochizuki M. Transforming growth factor β-producing Foxp3+CD8+CD25+ T cells induced by iris pigment epithelial cells display regulatory phenotype and acquire regulatory functions. Experimental Eye Research. 2007;85(5):626–636. doi: 10.1016/j.exer.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 50.Sugita S., Horie S., Yamada Y., et al. Suppression of bystander T helper 1 cells by iris pigment epithelium-inducing regulatory T cells via negative costimulatory signals. Investigative Opthalmology & Visual Science. 2010;51(5):2529–2536. doi: 10.1167/iovs.09-4460. [DOI] [PubMed] [Google Scholar]
- 51.Crawford S. E., Stellmach V., Murphy-Ullrich J. E., et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93(7):1159–1170. doi: 10.1016/S0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 52.Futagami Y., Sugita S., Vega J., et al. Role of thrombospondin-1 in T cell response to ocular pigment epithelial cells. The Journal of Immunology. 2007;178(11):6994–7005. doi: 10.4049/jimmunol.178.11.6994. [DOI] [PubMed] [Google Scholar]
- 53.Wenkel H., Streilein J. W. Analysis of immune deviation elicited by antigens injected into the subretinal space. Investigative Ophthalmology & Visual Science. 1998;39(10):1823–1834. [PubMed] [Google Scholar]
- 54.Wenkel H., Streilein J. W. Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Investigative Ophthalmology & Visual Science. 2000;41(11):3467–3473. [PubMed] [Google Scholar]
- 55.Zamiri P., Masli S., Kitaichi N., Taylor A. W., Streilein J. W. Thrombospondin plays a vital role in the immune privilege of the eye. Investigative Ophthalmology & Visual Science. 2005;46(3):908–919. doi: 10.1167/iovs.04-0362. [DOI] [PubMed] [Google Scholar]
- 56.Sugita S., Futagami Y., Smith S. B., Naggar H., Mochizuki M. Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor beta. Experimental Eye Research. 2006;83(6):1459–1471. doi: 10.1016/j.exer.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Liversidge J., McKay D., Mullen G., Forrester J. V. Retinal pigment epithelial cells modulate lymphocyte function at the blood-retina barrier by autocrine PGE2 and membrane-bound mechanisms. Cellular Immunology. 1993;149(2):315–330. doi: 10.1006/cimm.1993.1158. [DOI] [PubMed] [Google Scholar]
- 58.Sun D., Enzmann V., Lei S., Sun S. L., Kaplan H. J., Shao H. Retinal pigment epithelial cells activate uveitogenic T cells when they express high levels of MHC class II molecules, but inhibit T cell activation when they express restricted levels. Journal of Neuroimmunology. 2003;144(1-2):1–8. doi: 10.1016/S0165-5728(03)00248-0. [DOI] [PubMed] [Google Scholar]
- 59.Ke Y., Sun D., Jiang G., Kaplan H. J., Shao H. PD-L1hi retinal pigment epithelium (RPE) cells elicited by inflammatory cytokines induce regulatory activity in uveitogenic T cells. Journal of Leukocyte Biology. 2010;88(6):1241–1249. doi: 10.1189/jlb.0610332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugita S., Horie S., Nakamura O., et al. Retinal pigment epithelium-derived CTLA-2α induces TGFβ-producing T regulatory cells. The Journal of Immunology. 2008;181(11):7525–7536. doi: 10.4049/jimmunol.181.11.7525. [DOI] [PubMed] [Google Scholar]
- 61.Kawazoe Y., Sugita S., Keino H., et al. Retinoic acid from retinal pigment epithelium induces T regulatory cells. Experimental Eye Research. 2012;94(1):32–40. doi: 10.1016/j.exer.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Grajewski R. S., Silver P. B., Agarwal R. K., et al. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. Journal of Experimental Medicine. 2006;203(4):851–856. doi: 10.1084/jem.20050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silver P., Horai R., Chen J., et al. Retina-specific T regulatory cells bring about resolution and maintain remission of autoimmune uveitis. The Journal of Immunology. 2015;194(7):3011–3019. doi: 10.4049/jimmunol.1402650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Namba K., Kitaichi N., Nishida T., Taylor A. W. Induction of regulatory T cells by the immunomodulating cytokines α-melanocyte-stimulating hormone and transforming growth factor-β2. Journal of Leukocyte Biology. 2002;72(5):946–952. [PubMed] [Google Scholar]
- 65.Siepmann K., Biester S., Plskova J., Muckersie E., Duncan L., Forrester J. V. CD4+CD25+ T regulatory cells induced by LPS-activated bone marrow dendritic cells suppress experimental autoimmune uveoretinitis in vivo. Graefe's Archive for Clinical and Experimental Ophthalmology. 2007;245(2):221–229. doi: 10.1007/s00417-006-0356-9. [DOI] [PubMed] [Google Scholar]
- 66.Terrada C., Fisson S., De Kozak Y., et al. Regulatory T cells control uveoretinitis induced by pathogenic Th1 cells reacting to a specific retinal neoantigen. The Journal of Immunology. 2006;176(12):7171–7179. doi: 10.4049/jimmunol.176.12.7171. [DOI] [PubMed] [Google Scholar]
- 67.Grégoire S., Terrada C., Martin G. H., et al. Treatment of uveitis by in situ administration of ex vivo-activated polyclonal regulatory T cells. The Journal of Immunology. 2016;196(5):2109–2118. doi: 10.4049/jimmunol.1501723. [DOI] [PubMed] [Google Scholar]
- 68.Keino H., Takeuchi M., Usui Y., et al. Supplementation of CD4+ CD25+ regulatory T cells suppresses experimental autoimmune uveoretinitis. British Journal of Ophthalmology. 2006;91(1):105–110. doi: 10.1136/bjo.2006.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mucida D., Park Y., Kim G., et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 70.Keino H., Watanabe T., Sato Y., Okada A. A. Anti-inflammatory effect of retinoic acid on experimental autoimmune uveoretinitis. British Journal of Ophthalmology. 2010;94(6):802–807. doi: 10.1136/bjo.2009.171314. Epub 2009/12/08. [DOI] [PubMed] [Google Scholar]
- 71.Keino H., Watanabe T., Sato Y., Okada A. A. Oral administration of retinoic acid receptor-α/β-specific ligand Am80 suppresses experimental autoimmune uveoretinitis. Investigative Ophthalmology & Visual Science. 2011;52(3):1548–1556. doi: 10.1167/iovs.10-5963. [DOI] [PubMed] [Google Scholar]
- 72.Ohta K., Wiggert B., Yamagami S., Taylor A. W., Streilein J. W. Analysis of immunomodulatory activities of aqueous humor from eyes of mice with experimental autoimmune uveitis. The Journal of Immunology. 2000;164(3):1185–1192. doi: 10.4049/jimmunol.164.3.1185. [DOI] [PubMed] [Google Scholar]
- 73.Manirarora J. N., Wei C. H. Combination therapy using IL-2/IL-2 monoclonal antibody complexes, rapamycin, and islet autoantigen peptides increases regulatory T cell frequency and protects against spontaneous and induced type 1 diabetes in nonobese diabetic mice. The Journal of Immunology. 2015;195(11):5203–5214. doi: 10.4049/jimmunol.1402540. [DOI] [PubMed] [Google Scholar]
- 74.Iizuka-Koga M., Nakatsukasa H., Ito M., Akanuma T., Lu Q., Yoshimura A. Induction and maintenance of regulatory T cells by transcription factors and epigenetic modifications. Journal of Autoimmunity. 2017;83:113–121. doi: 10.1016/j.jaut.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Sugita S., Horie S., Nakamura O., et al. Acquisition of T regulatory function in cathepsin L-inhibited T cells by eye-derived CTLA-2α during inflammatory conditions. The Journal of Immunology. 2009;183(8):5013–5022. doi: 10.4049/jimmunol.0901623. [DOI] [PubMed] [Google Scholar]
- 76.Imai A., Sugita S., Kawazoe Y., et al. Immunosuppressive properties of regulatory T cells generated by incubation of peripheral blood mononuclear cells with supernatants of human RPE cells. Investigative Opthalmology & Visual Science. 2012;53(11):7299–7309. doi: 10.1167/iovs.12-10182. [DOI] [PubMed] [Google Scholar]
- 77.Ke Y., Sun D., Jiang G., Kaplan H. J., Shao H. IL-22-induced regulatory CD11b+ APCs suppress experimental autoimmune uveitis. The Journal of Immunology. 2011;187(5):2130–2139. doi: 10.4049/jimmunol.1100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L., Ma J., Takeuchi M., et al. Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of aryl hydrocarbon receptor. Investigative Ophthalmology & Visual Science. 2010;51(4):2109–2117. doi: 10.1167/iovs.09-3993. [DOI] [PubMed] [Google Scholar]
- 79.Chen X., Shao H., Zhi Y., et al. CD73 pathway contributes to the immunosuppressive ability of mesenchymal stem cells in intraocular autoimmune responses. Stem Cells and Development. 2016;25(4):337–346. doi: 10.1089/scd.2015.0227. [DOI] [PubMed] [Google Scholar]
- 80.Hemady R., Chu W., Foster C. S. Keratoconjunctivitis sicca and corneal ulcers. Cornea. 1990;9(2):170–173. doi: 10.1097/00003226-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 81.Barabino S., Dana M. R. Dry eye syndromes. Chemical Immunology and Allergy. 2007;92:176–184. doi: 10.1159/000099268. [DOI] [PubMed] [Google Scholar]
- 82.Stern M. E., Gao J., Schwalb T. A., et al. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Investigative Ophthalmology & Visual Science. 2002;43(8):2609–2614. [PubMed] [Google Scholar]
- 83.Niederkorn J. Y., Stern M. E., Pflugfelder S. C., et al. Desiccating stress induces T cell-mediated Sjögren’s syndrome-like lacrimal keratoconjunctivitis. The Journal of Immunology. 2006;176(7):3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 84.de Paiva C. S., Villarreal A. L., Corrales R. M., et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Investigative Ophthalmology & Visual Science. 2007;48(6):2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 85.Massingale M. L., Li X., Vallabhajosyula M., Chen D., Wei Y., Asbell P. A. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 86.Kang M. H., Kim M. K., Lee H. J., Lee H. I., Wee W. R., Lee J. H. Interleukin-17 in various ocular surface inflammatory diseases. Journal of Korean Medical Science. 2011;26(7):938–944. doi: 10.3346/jkms.2011.26.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meadows J. F., Dionne K., Nichols K. K. Differential profiling of T-cell cytokines as measured by protein microarray across dry eye subgroups. Cornea. 2016;35(3):329–335. doi: 10.1097/ICO.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 88.de Paiva C. S., Chotikavanich S., Pangelinan S. B., et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunology. 2009;2(3):243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y., Chauhan S. K., Lee H. S., et al. Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Investigative Ophthalmology & Visual Science. 2013;54(4):2457–2464. doi: 10.1167/iovs.12-11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chauhan S. K., el Annan J., Ecoiffier T., et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. The Journal of Immunology. 2009;182(3):1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y., Chauhan S. K., Soo Lee H., Saban D. R., Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunology. 2014;7(1):38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Foulsham W., Marmalidou A., Amouzegar A., Coco G., Chen Y., Dana R. Review: the function of regulatory T cells at the ocular surface. The Ocular Surface. 2017;15(4):652–659. doi: 10.1016/j.jtos.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siemasko K. F., Gao J., Calder V. L., et al. In vitro expanded CD4+ CD25+ Foxp3+ regulatory T cells maintain a normal phenotype and suppress immune-mediated ocular surface inflammation. Investigative Ophthalmology & Visual Science. 2008;49(12):5434–5440. doi: 10.1167/iovs.08-2075. [DOI] [PubMed] [Google Scholar]
- 94.Haneji N., Nakamura T., Takio K., et al. Identification of α-fodrin as a candidate autoantigen in primary Sjogren’s syndrome. Science. 1997;276(5312):604–607. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 95.Cunnusamy K., Paunicka K., Reyes N., Yang W., Chen P. W., Niederkorn J. Y. Two different regulatory T cell populations that promote corneal allograft survival. Investigative Ophthalmology & Visual Science. 2010;51(12):6566–6574. doi: 10.1167/iovs.10-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sonoda Y., Streilein J. W. Orthotopic corneal transplantation in mice—evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54(4):694–703. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 97.Chauhan S. K., Saban D. R., Lee H. K., Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. The Journal of Immunology. 2009;182(1):148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hori J., Taniguchi H., Wang M., Oshima M., Azuma M. GITR ligand-mediated local expansion of regulatory T cells and immune privilege of corneal allografts. Investigative Ophthalmology & Visual Science. 2010;51(12):6556–6565. doi: 10.1167/iovs.09-4959. [DOI] [PubMed] [Google Scholar]
- 99.Niederkorn J. Y., Chen P. W., Mellon J., Stevens C., Mayhew E. Allergic conjunctivitis exacerbates corneal allograft rejection by activating Th1 and th2 alloimmune responses. The Journal of Immunology. 2010;184(11):6076–6083. doi: 10.4049/jimmunol.0902300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niederkorn J. Y. High-risk corneal allografts and why they lose their immune privilege. Current Opinion in Allergy and Clinical Immunology. 2010;10(5):493–497. doi: 10.1097/ACI.0b013e32833dfa11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inomata T., Hua J., Di Zazzo A., Dana R. Impaired function of peripherally induced regulatory T cells in hosts at high risk of graft rejection. Scientific Reports. 2016;6(1, article 39924) doi: 10.1038/srep39924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghoraishi M., Akova Y. A., Tugal-Tutkun I., Foster C. S. Penetrating keratoplasty in atopic keratoconjunctivitis. Cornea. 1995;14(6):610–613. doi: 10.1097/00003226-199511000-00015. [DOI] [PubMed] [Google Scholar]
- 103.Hargrave S., Chu Y., Mendelblatt D., Mayhew E., Niederkorn J. Preliminary findings in corneal allograft rejection in patients with keratoconus. American Journal of Ophthalmology. 2003;135(4):452–460. doi: 10.1016/S0002-9394(02)02055-X. [DOI] [PubMed] [Google Scholar]
- 104.Tahvildari M., Omoto M., Chen Y., et al. In vivo expansion of regulatory T cells by low-dose interleukin-2 treatment increases allograft survival in corneal transplantation. Transplantation. 2016;100(3):525–532. doi: 10.1097/TP.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.June C. H., Blazar B. R. Clinical application of expanded CD4+25+ cells. Seminars in Immunology. 2006;18(2):78–88. doi: 10.1016/j.smim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 106.Riley J. L., June C. H., Blazar B. R. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30(5):656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baud O., Goulet O., Canioni D., et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) by allogeneic bone marrow transplantation. The New England Journal of Medicine. 2001;344(23):1758–1762. doi: 10.1056/NEJM200106073442304. [DOI] [PubMed] [Google Scholar]
- 108.Bennett C. L., Christie J., Ramsdell F., et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nature Genetics. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 109.Seay H. R., Putnam A. L., Cserny J., et al. Expansion of human Tregs from cryopreserved umbilical cord blood for GMP-compliant autologous adoptive cell transfer therapy. Molecular Therapy - Methods & Clinical Development. 2017;4:178–191. doi: 10.1016/j.omtm.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gitelman S. E., Bluestone J. A. Regulatory T cell therapy for type 1 diabetes: may the force be with you. Journal of Autoimmunity. 2016;71:78–87. doi: 10.1016/j.jaut.2016.03.011. Epub 2016/05/03. [DOI] [PubMed] [Google Scholar]
- 111.Miyara M., Yoshioka Y., Kitoh A., et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 112.Sakaguchi S., Miyara M., Costantino C. M., Hafler D. A. FOXP3+ regulatory T cells in the human immune system. Nature Reviews Immunology. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 113.Sawitzki B., Brunstein C., Meisel C., et al. Prevention of graft-versus-host disease by adoptive T regulatory therapy is associated with active repression of peripheral blood toll-like receptor 5 mRNA expression. Biology of Blood and Marrow Transplantation. 2014;20(2):173–182. doi: 10.1016/j.bbmt.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee R. W. J., Dick A. D. Current concepts and future directions in the pathogenesis and treatment of non-infectious intraocular inflammation. Eye. 2012;26(1):17–28. doi: 10.1038/eye.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sugita S., Kawazoe Y., Imai A., et al. Role of IL-22- and TNF-α-producing Th22 cells in uveitis patients with Behçet’s disease. The Journal of Immunology. 2013;190(11):5799–5808. doi: 10.4049/jimmunol.1202677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sugita S., Kawazoe Y., Imai A., Yamada Y., Horie S., Mochizuki M. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behçet’s disease. Arthritis Research & Therapy. 2012;14(3, article R99) doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sugita S., Yamada Y., Kaneko S., Horie S., Mochizuki M. Induction of regulatory T cells by infliximab in Behçet’s disease. Investigative Ophthalmology & Visual Science. 2011;52(1):476–484. doi: 10.1167/iovs.10-5916. [DOI] [PubMed] [Google Scholar]
- 118.Theofilopoulos A. N., Kono D. H., Baccala R. The multiple pathways to autoimmunity. Nature Immunology. 2017;18(7):716–724. doi: 10.1038/ni.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Treatment of the bilateral severe uveitis by IVT of regulator T-cells: study of tolerance of dose (UVEREG) 2018, ClinicalTrials.gov identifier: NCT02494492.
- 120.Foussat A., Gregoire S., Clerget-Chossat N., et al. Regulatory T cell therapy for uveitis: a new promising challenge. Journal of Ocular Pharmacology and Therapeutics. 2017;33(4):278–284. doi: 10.1089/jop.2016.0165. [DOI] [PubMed] [Google Scholar]
- 121.Horie S., Sugita S., Futagami Y., Yamada Y., Mochizuki M. Human retinal pigment epithelium-induced CD4+CD25+ regulatory T cells suppress activation of intraocular effector T cells. Clinical Immunology. 2010;136(1):83–95. doi: 10.1016/j.clim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 122.Horie S., Sugita S., Futagami Y., et al. Human iris pigment epithelium suppresses activation of bystander T cells via TGFβ–TGFβ receptor interaction. Experimental Eye Research. 2009;88(6):1033–1042. doi: 10.1016/j.exer.2009.01.011. [DOI] [PubMed] [Google Scholar]