Abstract
Objective
This study aimed to evaluate the efficacy and safety of acupuncture therapy (AT) for improving functional effects and quality of life in COPD patients.
Methods
PubMed, Embase, Cochrane Library, Web of Science, Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Chongqing VIP (CQVIP), and Wanfang Data were searched. The randomized controlled trials (RCTs) evaluating the effect of AT on COPD patients were included. Primary outcome measures included six-minute walk distance (6MWD) and St. George's Respiratory Questionnaire (SGRQ). Study selection, data extraction, and risk of bias assessment were independently conducted, respectively. Statistical analysis was conducted by RevMan software (version 5.3) and Stata software (version 12.0).
Results
Nineteen studies (1298 participants) were included. 6MWD improved more (MD: 47.84; 95% CI: 23.33 to 72.35; Z = 3.83, P = 0.0001) and effective rate was higher (OR: 2.26; 95% CI: 1.43 to 3.58; Z = 3.48, P = 0.0005) in the experimental group compared to the control group. Symptom domain scores (MD: −24.86; 95% CI: −32.17 to −17.55; Z = 6.66, P < 0.00001), activity domain scores (MD: −16.52; 95% CI: −22.57 to −10.47; Z = 5.36, P < 0.00001) and impact domain scores (MD: −13.07; 95% CI: −17.23 to −8.92; Z = 6.16, P < 0.00001) of SGRQ in the experimental group improved more compared to the control group. There was no significant improvement in SGRQ total scores between two groups. The improvement of FEV1 was not significant between two groups, yet subgroup analysis showed that patients treated with AT adjunctive to other treatments improved more in FEV1 (MD: 0.41; 95% CI: 0.28 to 0.54; Z = 6.01, P < 0.00001) compared to those treated with other treatments alone.
Conclusion
AT may be effective in improving functional effects and quality of life in COPD patients. Besides, AT may also improve pulmonary function of patients with COPD. However, further high-quality RCTs are needed to confirm the efficacy and safety of AT for COPD patients.
1. Introduction
Chronic obstructive pulmonary disease (COPD), a leading cause of morbidity and mortality, is characterized by progressive airflow obstruction, airway inflammation, and systemic effects or comorbidities and is projected to be the third leading cause of death worldwide by 2030 [1, 2]. Since breathlessness, exercise limitation, and health status impairment broadly exist in patients with COPD, effective measures should be taken to improve symptoms, exercise tolerance, and health status based on an individualized assessment of disease [1]. Although appropriate pharmacologic therapy has effect in reducing COPD symptoms and the frequency and severity of exacerbations and improving health status and exercise tolerance [1], its cost and adverse effects can never be ignored.
Acupuncture therapy (AT), one of the most popular treatments in alternative medicine, has been proven to be cost-effective and safe in many conditions [3–5]. However, there is limited evidence concerning its efficacy and safety. One previous review showed that AT might result in clinically important improvements in quality of life and dyspnea of COPD patients, but it is outdated [6]. Moreover, the interventions of included studies involved point application therapy, acupressure, and transcutaneous electrical stimulation over acupuncture points (Acu-TENS), and these techniques may not genuinely reflect the efficacy of AT based on theories of traditional Chinese medicine. Therefore, the current review aims to evaluate the efficacy and safety of AT for improving functional effects and quality of life in COPD patients.
2. Methods
2.1. Inclusion and Exclusion Criteria
We included randomized controlled trials (RCTs) in which the effects of AT on COPD patients were evaluated.
Participants had COPD defined as a clinical diagnosis of COPD, with a postbronchodilator fixed ratio of forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.70 measured by spirometry, and those who had an acute exacerbation within four weeks before the study were excluded.
The intervention included AT, such as manual acupuncture, electroacupuncture, auricular acupuncture, and warm acupuncture, yet noninvasive techniques, such as single moxibustion, acupressure, point application, laser acupuncture, or Acu-TENS, were excluded.
Primary outcome measures included any of the following: (i) six-minute walk test/distance (6MWT/6MWD) [26] and (ii) St. George's Respiratory Questionnaire (SGRQ) [27]. Secondary outcome measures included any of the following: (i) FEV1, (ii) modified Medical Research Council dyspnea scale (mMRC) [28], (iii) effective rate, and (iv) adverse effects.
2.2. Literature Search
PubMed, Embase, Cochrane Library, Web of Science, Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Chongqing VIP (CQVIP), and Wanfang Data were searched from their inception to 31 December 2017. We developed detailed search strategies for each electronic database without language restrictions. Reference lists of eligible studies and previous systematic reviews were also reviewed to identify further eligible studies.
2.3. Study Selection
Two review authors (Yang Xie and Xueqing Yu) independently examined titles and abstracts retrieved from the search and selected all potentially eligible studies. Then these full-text articles were obtained and the same review authors reviewed them independently against the inclusion and exclusion criteria. A third review author (Jiansheng Li) acted as an arbiter when consensus could not be reached.
2.4. Data Extraction
Data extraction was independently conducted by two review authors (Yang Xie and Xueqing Yu) using a standardized data extraction sheet, involving information of authors, year of publication, study design, participants, intervention, comparator, and outcomes, with a third review author (Jiansheng Li) acting as an arbiter when disagreements existed between Yang Xie and Xueqing Yu.
2.5. Assessment of Risk of Bias
Methodological quality was evaluated using the Cochrane tool for assessing risk of bias in RCTs [29]. Two review authors (Yang Xie and Xueqing Yu) independently assessed and scored each study with a third review author (Jiansheng Li) acting as an arbiter when disagreements existed.
2.6. Statistical Analysis
Statistical analysis was conducted by RevMan software (version 5.3) [30] and Stata software (version 12.0; StataCorp LP, USA). We summarized data using odds ratio (OR) with 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) with 95% CI for continuous outcomes. If the data could not be combined into a meta-analysis, we summarized them in the text. We used a χ 2 test to estimate heterogeneity of both the MD and OR. Further analysis was performed using the I 2 test. A random-effect model was used to interpret the results if heterogeneity was statistically significant, whereas a fixed-effect model was used if heterogeneity was not statistically significant. We regarded heterogeneity as substantial when I 2 was greater than 50% or a low P value (P < 0.10) was reported for the χ 2 test [31]. When more than 10 studies were included in the meta-analysis, we would investigate publication bias by funnel plots. In addition, a metaregression analysis was performed to explore potential associations between effect size and covariates of interest (publication year, region, intervention forms, sample size, and treatment period). If necessary, we conducted subgroup analysis to assess whether the treatment effects were different in different subgroups.
3. Results
3.1. Literature Search and Study Selection
We retrieved 600 records using the search strategy specified in our protocol. 223 records were discarded after reviewing the titles and/or abstracts. Thirty-five articles that initially appeared to meet the inclusion criteria were excluded with reasons: (i) not stable COPD (n = 24), (ii) not targeted comparators (n = 9), (iii) not targeted outcomes (n = 1), and (iv) full-text articles unavailable (n = 1). Thus, nineteen studies (1298 participants) finally met our criteria and were included in this review [7–25]. The study selection process was outlined in Figure 1.
Figure 1.
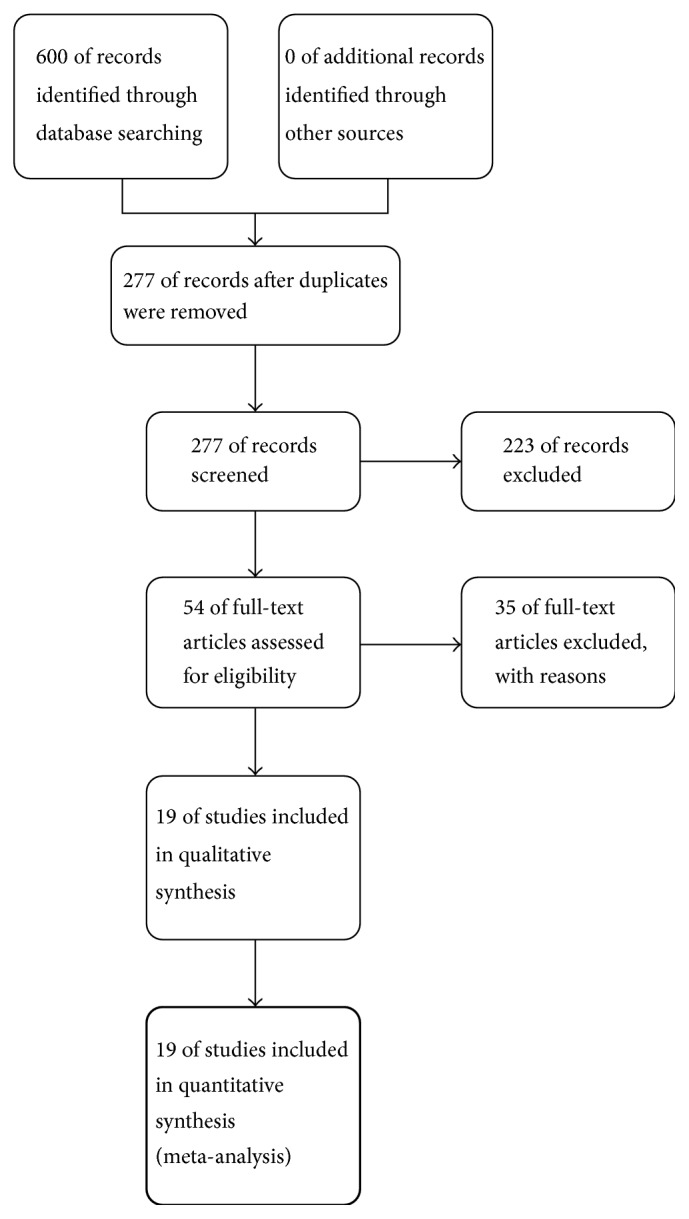
Study flow diagram.
3.2. Data Extraction and Assessment of Risk of Bias
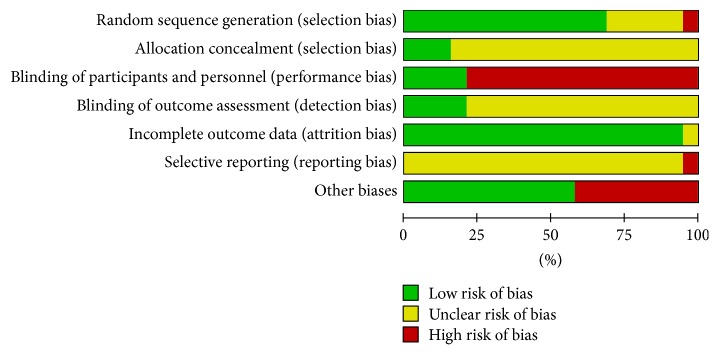
A detailed description of the characteristics of included studies was outlined in Table 1. We determined the Cochrane “risk of bias” score for each study and this information was summarized in Table 2 and Figures 2 and 3.
Table 1.
Characteristics of included studies.
| Study | Country | Study design | Participants | Interventions | Outcomes | Notes |
|---|---|---|---|---|---|---|
| Chu and Cai [7] 2015 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 15/17, CG: 14/18 Age (range, years): from 46 to 80 FEV1% predicted: EG: 56.83 ± 8.96; CG: 52.53 ± 13.96 Course of disease (range): from 5 months to 20 years Participants randomly assigned: 64 participants were randomly assigned Analyzed: EG: 32 CG: 32 |
EG: AT + Chinese medicine + western medicine CG: Chinese medicine + western medicine Duration: treatment for 90 days; follow-up for 6 months |
Spirometry, acute exacerbation frequency, effective rate, adverse effects | |
|
| ||||||
| Deering et al. [8] 2011 | Ireland | RCT, 3 arms |
Participant status: Gender (M/F): PR group: 11/14; AT + PR group: 8/8; CG: 12/7 Age (years): PR group: 67.7 ± 5.3; AT + PR group: 65.1 ± 9.7; CG: 68.6 ± 5.5 FEV1% predicted: PR group: 48.5 ± 16.1; AT + PR group: 48.8 ± 22.7; CG: 45.8 ± 18.3 Participants randomly assigned: 60 participants were randomly assigned Analyzed: AT + PR group: 16 PR group: 25 |
AT + PR group: AT + PR PR group: PR alone CG: no intervention Duration: treatment for 7 weeks; follow-up for 3 months |
Body mass index, mMRC, the modified Borg dyspnea score, systemic inflammation, spirometry, total energy expenditure, physical activity duration, metabolic equivalents, steps per day, sleep time/efficiency, incremental shuttle walk test, SGRQ, EQ-5D | Control group was not used in the analysis |
|
| ||||||
| Deng et al. [9] 2016 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 13/9, CG: 15/7 Age (years): EG: 57.1 ± 5.9; CG: 58.5 ± 6.5 FEV1% predicted: EG: 52.4 ± 2.9; CG: 51.5 ± 2.7 Course of disease (years): EG: 6.4 ± 2.5; CG: 6.3 ± 2.1 Participants randomly assigned: 44 participants were randomly assigned Analyzed: EG: 22 CG: 22 |
EG: abdominal AT + conventional therapy CG: conventional therapy alone Duration: treatment for 24 weeks |
Annual hospital stay, annual acute exacerbation frequency, oxygen saturation level, spirometry, 6MWD | |
|
| ||||||
| Feng et al. [10] 2016 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 33/3, CG: 31/5 Age (years): EG: 67.8 ± 5.4; CG: 67.1 ± 6.1 FEV1% predicted: EG: 47.3 ± 17.1; CG: 43.9 ± 16.8 Participants randomly assigned: 72 participants were randomly assigned Analyzed: EG: 33 CG: 32 |
EG: AT + daily medication CG: Sham acupuncture + daily medication Duration: treatment 3 times weekly for 8 weeks |
6MWD, modified Borg scale score before and after 6MWT, oxygen saturation during the 6MWT, spirometry, SGRQ | |
|
| ||||||
| Gao et al. [11] 2011 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 13/17, CG: 12/18 Age (years): EG: 64.87 ± 8.73; CG: 65.25 ± 10.66 FEV1% predicted: EG: 45.88 ± 5.05; CG: 43.54 ± 6.29 Course of disease (years): EG: 10.31 ± 5.82; CG: 10.78 ± 5.53 Participants randomly assigned: 61 participants were randomly assigned Analyzed: EG: 30 CG: 30 |
EG: warm AT CG: drug therapy Duration: treatment for 8 weeks |
Spirometry, clinical symptoms, SGRQ | |
|
| ||||||
| Hu [12] 2016 | China | RCT, 2 arms |
Participant status: Gender (M/F): not described Age (years): not described FEV1% predicted: not described Course of disease (years): not described Participants randomly assigned: 89 participants were randomly assigned Analyzed: EG: 45 CG: 44 |
EG: warm AT + conventional therapy CG: spreading moxibustion + conventional therapy Duration: treatment for 2 months |
Effective rate, spirometry | |
|
| ||||||
| Jia [13] 2004 | China | RCT, 3 arms |
Participant status: Gender (M/F): AT group: 9/13; PR group: 8/14; AT + PR group: 10/12 Age (years): AT group: 61.0 ± 32.6; PR group: 60.0 ± 34.8; AT + PR group: 61.0 ± 33.2 FEV1% predicted: AT group: 70.9 ± 0.2; PR group: 71.1 ± 0.1; AT + PR group: 70.6 ± 0.7 Course of disease (years): AT group: 11.7 ± 8.9; PR group: 12.1 ± 7.2; AT + PR group: 11.6 ± 9.0 Participants randomly assigned: 66 participants were randomly assigned Analyzed: AT group: 22 PR group: 22 AT + PR group: 22 |
AT group: AT + conventional drugs PR group: PR + conventional drugs AT + PR group: AT + PR + conventional drugs Duration: treatment for 100 days |
Effective rate, spirometry | Data from AT group and AT + PR group were combined in the analysis |
|
| ||||||
| Li [14] 2015 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 19/10, CG: 17/13 Age (years): EG: 57.21 ± 6.68; CG: 55.80 ± 7.23 FEV1% predicted: EG: 66.28 ± 6.86; CG: 65.16 ± 6.16 Course of disease (years): EG: 10.38 ± 4.90; CG: 10.70 ± 4.88 Participants randomly assigned: 60 participants were randomly assigned Analyzed: EG: 29 CG: 30 |
EG: warm AT + drug therapy CG: drug therapy alone Duration: treatment for 1 month |
Safety indicators, CAT, spirometry, clinical symptoms, effective rate, adverse effects | |
|
| ||||||
| Li et al. [15] 2016 | China | RCT, 2 arms |
Participant status: Gender (M/F): two groups were reported to be comparable in gender. Age (years): two groups were reported to be comparable in age. FEV1% predicted: EG: 53.1 ± 10.9; CG: 51.9 ± 11.4 Participants randomly assigned: 60 participants were randomly assigned Analyzed: EG: 30 CG: 30 |
EG: AT + Chinese medicine + western medicine CG: Chinese medicine + western medicine Duration: treatment for 3 weeks |
Clinical symptoms, CAT, spirometry, effective rate, adverse effects | |
|
| ||||||
| Liu et al. [16] 2015 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 24/16, CG: 26/14 Age (years): EG: 58.3 ± 12.4; CG: 63.2 ± 10.7 FEV1% predicted: EG: 35.71 ± 7.28; CG: 36.42 ± 6.42 Participants randomly assigned: 80 participants were randomly assigned Analyzed: EG: 40 CG: 40 |
EG: AT + drug therapy CG: drug therapy alone Duration: treatment for 3 months |
Clinical signs and symptoms, 6MWD, spirometry, effective rate | |
|
| ||||||
| Suzuki et al. [17] 2012 | Japan | RCT, 2 arms |
Participant status: Gender (M/F): EG: 31/3, CG: 32/2 Age (years): EG: 72.7 ± 6.8; CG: 72.5 ± 7.4 FEV1% predicted: EG: 44.5 ± 16.3; CG: 48.0 ± 16.5 Participants randomly assigned: 68 participants were randomly assigned Analyzed: EG: 30 CG: 32 |
EG: AT + daily medication CG: placebo acupuncture + daily medication Duration: treatment once a week for 12 weeks |
6MWD, modified Borg scale score before and after 6MWT, oxygen saturation during the 6MWT, spirometry, SGRQ, arterial blood gas, maximum inspiratory mouth pressure, maximum expiratory mouth pressure, range of motion in the rib cage, body mass index, serum prealbumin levels, MRC score, adverse reactions | |
|
| ||||||
| Tong et al. [18] 2014 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 15/1, CG: 12/2 Age (years): EG: 64 ± 6; CG: 67 ± 6 FEV1% predicted: EG: 41.72 ± 17.95; CG: 36.16 ± 16.29 Participants randomly assigned: 30 participants were randomly assigned Analyzed: EG: 16 CG: 14 |
EG: AT + aerobic exercise CG: placebo acupuncture + aerobic exercise Duration: treatment for 5 weeks |
6MWD, spirometry, maximum oxygen uptake, exercise time, SGRQ | |
|
| ||||||
| Wan et al. [19] 2009 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 47/28, CG: 45/30 Age (years): EG: 62.40 ± 8.56; CG: 61.80 ± 10.10 FEV1% predicted: EG: 48.07 ± 12.77; CG: 47.27 ± 14.02 Course of disease (years): EG: 7.73 ± 3.80; CG: 7.70 ± 2.92 Participants randomly assigned: 150 participants were randomly assigned Analyzed: EG: 75 CG: 75 |
EG: AT + Chinese medicine CG: Chinese medicine alone Duration: treatment for 36 days |
Effective rate, spirometry | |
|
| ||||||
| Xie and Yu [20] 2014 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 22/18, CG: 18/22 Age (years): EG: 68.9 ± 8.7; CG: 68.5 ± 9.6 FEV1% predicted: EG: 45.89 ± 5.06; CG: 43.55 ± 6.30 Course of disease (years): EG: 11.8 ± 6.5; CG: 12.3 ± 5.5 Participants randomly assigned: 80 participants were randomly assigned Analyzed: EG: 40 CG: 40 |
EG: warm AT CG: drug therapy Duration: treatment for 8 weeks |
Spirometry, symptom scores | |
|
| ||||||
| Yang et al. [21] 2009 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 26/14, CG: 28/12 Age (range, years): EG: from 49 to 78; CG: from 45 to 77 FEV1% predicted: EG: 53.5 ± 19.2; CG: 54.3 ± 22.6 Course of disease (mean, years): EG: 6.9; CG: 7.1 Participants randomly assigned: 80 participants were randomly assigned Analyzed: EG: 40 CG: 40 |
EG: AT + PR CG: PR alone Duration: treatment for 40 days |
COPD quality of life questionnaire, 6MWD, spirometry | |
|
| ||||||
| Yu [22] 2014 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 18/12, CG: 17/13 Age (years): EG: 63.0 ± 8.5; CG: 62.0 ± 7.6 FEV1% predicted: EG: 50.23 ± 2.56; CG: 51.33 ± 2.43 Course of disease (years): EG: 8.9 ± 3.7; CG: 8.4 ± 3.5 Participants randomly assigned: 60 participants were randomly assigned Analyzed: EG: 30 CG: 30 |
EG: warm AT + function training CG: conventional drug therapy + function training Duration: treatment for 3 months |
Arterial blood gas, spirometry, SGRQ, effective rate | |
|
| ||||||
| Ge et al. [23] 2017 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 23/1, CG: 15/5 Age (years): EG: 65 ± 6; CG: 65 ± 7 FEV1% predicted: EG: 40.76 ± 16.36; CG: 40.53 ± 17.40 Course of disease (years): EG: 9.1 ± 5.5; CG: 8.6 ± 6.8 Participants randomly assigned: 44 participants were randomly assigned Analyzed: EG: 22 CG: 19 |
EG: AT + conventional drugs + aerobic exercise CG: placebo acupuncture + conventional drugs + aerobic exercise Duration: treatment for 14 times |
Body mass index, average distance and average maximum heart rate during bicycle exercise, 6MWD, maximum power and maximum heart rate during exercise cardiopulmonary function test, spirometry | |
|
| ||||||
| Shi [24] 2017 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 18/12, CG: 17/14 Age (years): EG: 57.77 ± 6.54; CG: 55.90 ± 6.86 FEV1% predicted: EG: 64.11 ± 5.79; CG: 65.85 ± 6.86 Course of disease (years): EG: 11.43 ± 4.37; CG: 11.68 ± 3.64 Participants randomly assigned: 66 participants were randomly assigned Analyzed: EG: 30 CG: 31 |
EG: AT CG: western medicine Duration: treatment for 2 months |
CAT, spirometry, clinical symptoms, effective rate, safety indicators | |
|
| ||||||
| Tang [25] 2017 | China | RCT, 2 arms |
Participant status: Gender (M/F): EG: 19/11, CG: 18/13 Age (years): EG: 57.47 ± 5.33; CG: 56.59 ± 6.34 Course of disease (years): EG: 10.07 ± 2.20; CG: 10.55 ± 2.10 Participants randomly assigned: 64 participants were randomly assigned Analyzed: EG: 30 CG: 29 |
EG: AT + western medicine CG: western medicine alone Duration: treatment for 2 months |
CAT, clinical symptoms, 6MWT, effective rate, safety indicators | |
RCT: randomized controlled trial, EG: experimental group, CG: control group, AT: acupuncture therapy, PR: pulmonary rehabilitation, FEV1: forced expiratory volume in 1 second, 6MWT/MWD: six-minute walk test/distance, SGRQ: St. George's Respiratory Questionnaire, mMRC: modified Medical Research Council dyspnea scale, MRC: Medical Research Council dyspnea scale, CAT: COPD assessment test.
Table 2.
Risks of bias of included studies.
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other biases |
|---|---|---|---|---|---|---|---|
| Chu and Cai [7] 2015 | U | U | H | U | L | U | H |
| Deering et al. [8] 2011 | L | U | H | L | U | U | H |
| Deng et al. [9] 2016 | U | U | H | U | L | U | H |
| Feng et al. [10] 2016 | L | L | L | L | L | U | L |
| Gao et al. [11] 2011 | L | U | H | U | L | U | L |
| Hu [12] 2016 | U | U | H | U | L | U | L |
| Jia [13] 2004 | L | U | H | U | L | U | L |
| Li [14] 2015 | L | U | H | U | L | U | H |
| Li et al. [15] 2016 | L | L | H | U | L | U | L |
| Liu et al. [16] 2015 | U | U | H | U | L | U | H |
| Suzuki et al. [17] 2012 | L | U | L | L | L | U | L |
| Tong et al. [18] 2014 | L | L | L | L | L | U | L |
| Wan et al. [19] 2009 | U | U | H | U | L | U | L |
| Xie and Yu [20] 2014 | L | U | H | U | L | U | L |
| Yang et al. [21] 2009 | H | U | H | U | L | U | H |
| Yu [22] 2014 | L | U | H | U | L | H | L |
| Ge et al. [23] 2017 | L | U | L | U | L | U | L |
| Shi [24] 2017 | L | U | H | U | L | U | H |
| Tang [25] 2017 | L | U | H | U | L | U | H |
Notes. Quality assessment based on the Cochrane tools for assessing risk of bias. L: low (low risk of bias), H: high (high risk of bias), U: unclear (uncertain risk of bias).
Figure 2.
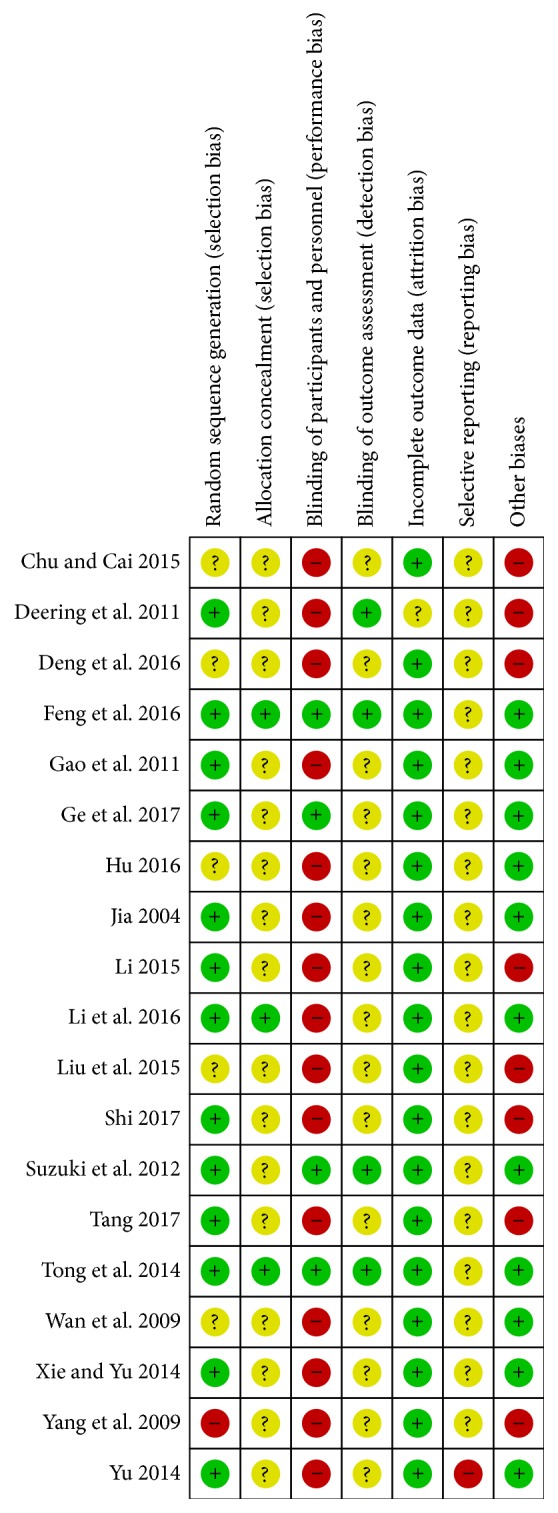
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.3. Effects of Interventions
3.3.1. 6MWD
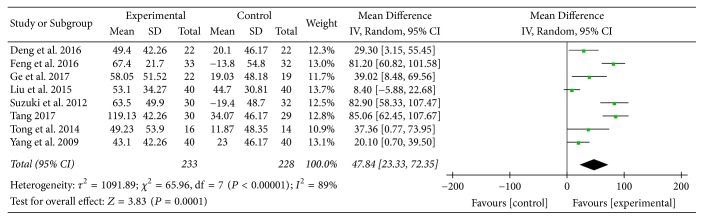
Eight studies [9, 10, 16–18, 21, 23, 25] provided numerical data for 6MWD and were included in the meta-analysis. Analysis of the data indicated that there was heterogeneity (χ 2 = 65.96, P < 0.00001; I 2 = 89%); hence, a random-effect model was used. The pooled results showed that 6MWD in the experimental group improved more compared to the control group (MD: 47.84; 95% CI: 23.33 to 72.35; Z = 3.83, P = 0.0001) (Figure 4).
Figure 4.
Experimental group versus control group, 6MWD.
3.3.2. SGRQ
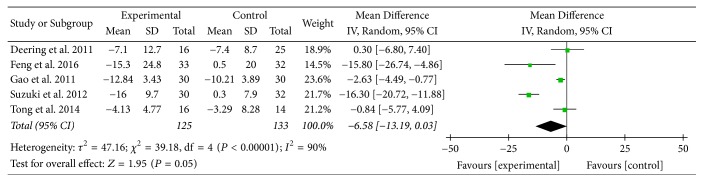
Five studies [8, 10, 11, 17, 18] provided numerical data for SGRQ total scores and were included in the meta-analysis. Analysis of the data indicated that there was heterogeneity (χ 2 = 39.18, P < 0.00001; I 2 = 90%); hence, a random-effect model was used. The pooled results showed that there was no significant improvement in SGRQ total scores between two groups (MD: −6.58; 95% CI: −13.19 to 0.03; Z = 1.95, P = 0.05) (Figure 5).
Figure 5.
Experimental group versus control group, SGRQ total scores.
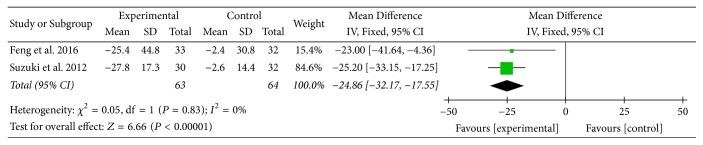
Two studies [10, 17] provided numerical data for symptom domain scores of SGRQ and were included in the meta-analysis. Analysis of the data indicated that heterogeneity was not statistically significant (χ 2 = 0.05, P = 0.83; I 2 = 0%); hence, a fixed-effect model was used. The pooled results showed that symptom domain scores of SGRQ in the experimental group improved more compared to the control group (MD: −24.86; 95% CI: −32.17 to −17.55; Z = 6.66, P < 0.00001) (Figure 6).
Figure 6.
Experimental group versus control group, symptom domain scores of SGRQ.
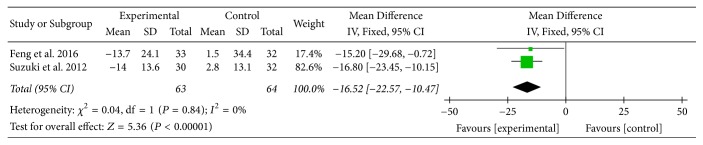
Two studies [10, 17] provided numerical data for activity domain scores of SGRQ and were included in the meta-analysis. Analysis of the data indicated that heterogeneity was not statistically significant (χ 2 = 0.04, P = 0.84; I 2 = 0%); hence, a fixed-effect model was used. The pooled results showed that activity domain scores of SGRQ in the experimental group improved more compared to the control group (MD: −16.52; 95% CI: −22.57 to −10.47; Z = 5.36, P < 0.00001) (Figure 7).
Figure 7.
Experimental group versus control group, activity domain scores of SGRQ.
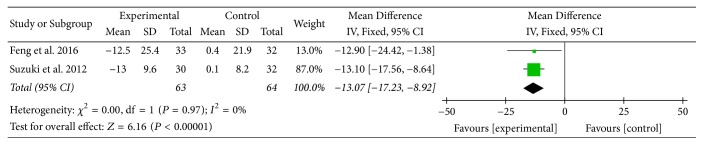
Two studies [10, 17] provided numerical data for impact domain scores of SGRQ and were included in the meta-analysis. Analysis of the data indicated that heterogeneity was not statistically significant (χ 2 = 0.00, P = 0.97; I 2 = 0%); hence, a fixed-effect model was used. The pooled results showed that impact domain scores of SGRQ in the experimental group improved more compared to the control group (MD: −13.07; 95% CI: −17.23 to −8.92; Z = 6.16, P < 0.00001) (Figure 8).
Figure 8.
Experimental group versus control group, impact domain scores of SGRQ.
3.3.3. FEV1
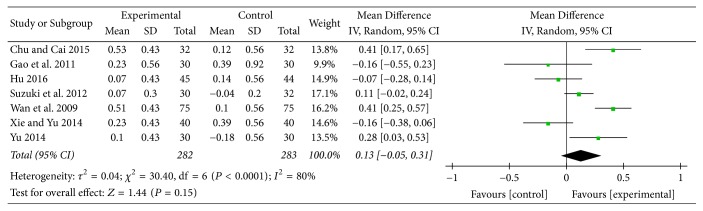
Seven studies [7, 11, 12, 17, 19, 20, 22] provided numerical data for FEV1 and were included in the meta-analysis. Analysis of the data indicated that there was heterogeneity (χ 2 = 30.40, P < 0.0001; I 2 = 80%); hence, a random-effect model was used. The pooled results showed that there was no significant improvement in FEV1 between two groups (MD: 0.13; 95% CI: −0.05 to 0.31; Z = 1.44, P = 0.15) (Figure 9).
Figure 9.
Experimental group versus control group, FEV1.
3.3.4. Effective Rate
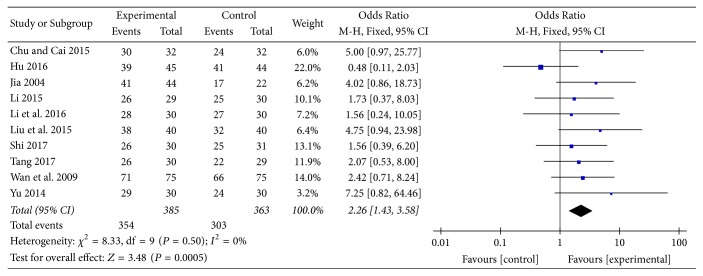
Ten studies [7, 12–16, 19, 22, 24, 25] provided categorical data for effective rate and were included in the meta-analysis. Analysis of the data indicated that heterogeneity was not statistically significant (χ 2 = 8.33, P = 0.50; I 2 = 0%); hence, a fixed-effect model was used. The pooled results showed that effective rate in the experimental group was higher compared to the control group (OR: 2.26; 95% CI: 1.43 to 3.58; Z = 3.48, P = 0.0005) (Figure 10).
Figure 10.
Experimental group versus control group, effective rate.
3.3.5. mMRC
Only one study [8] provided numerical data for mMRC scores; thus, the meta-analysis was not performed. Changes from baseline in mMRC scores in AT plus pulmonary rehabilitation (PR) group and PR group were −0.3 ± 0.5 and −0.3 ± 0.9, respectively. There was significant difference reported within AT plus PR group (P = 0.04).
3.4. Adverse Effects
Six studies [7, 14, 15, 17, 24, 25] provided information about adverse effects. Only one study [17] reported some minor adverse reactions during the trial including fatigue, subcutaneous hemorrhage, dizziness, and needle site pain, and the remaining 5 studies [7, 14, 15, 24, 25] reported no adverse effects.
3.5. Metaregression Analysis
We tried to perform a univariate metaregression analysis to explore potential associations between effect size and covariates of interest (publication year, region, intervention forms, sample size, and treatment period) (see Table 3). However, the results showed that there were no statistically significant associations among them, and this might be due to the insufficient number of studies included [32].
Table 3.
Univariate metaregression analysis of covariates of interest.
| Covariates of interest | Coefficient | Standard error (SE) | t | P | 95% confidence intervals (CI) | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Six-minute walk distance | ||||||
| Publication year | 2.812 | 4.479 | 0.63 | 0.553 | −8.148 | 13.773 |
| Region | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| Intervention forms | 26.947 | 21.520 | 1.25 | 0.257 | −25.710 | 79.603 |
| Sample size | −0.236 | 0.716 | −0.33 | 0.753 | −1.988 | 1.516 |
| Treatment period | −0.115 | 0.292 | −0.39 | 0.707 | −0.829 | 0.599 |
| SGRQ | ||||||
| Publication year | −1.634 | 2.107 | −0.78 | 0.495 | −8.340 | 5.072 |
| Region | 8.595 | 9.520 | 0.90 | 0.433 | −21.702 | 38.891 |
| Intervention forms | 4.605 | 4.389 | 1.05 | 0.371 | −9.363 | 18.573 |
| Sample size | −0.382 | 0.225 | −1.70 | 0.188 | −1.097 | 0.334 |
| Treatment period | −0.342 | 0.139 | −2.47 | 0.090 | −0.783 | 0.099 |
| FEV 1 | ||||||
| Publication year | −0.028 | 0.043 | −0.65 | 0.544 | −0.139 | 0.083 |
| Region | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| Intervention forms | −0.086 | 0.088 | −0.98 | 0.371 | −0.313 | 0.140 |
| Sample size | 0.003 | 0.003 | 0.83 | 0.447 | −0.005 | 0.011 |
| Treatment period | 0.002 | 0.005 | 0.48 | 0.654 | −0.011 | 0.016 |
| Effective rate | ||||||
| Publication year | −0.064 | 0.060 | −1.07 | 0.315 | −0.203 | 0.074 |
| Region | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| Intervention forms | −0.117 | 0.301 | −0.39 | 0.707 | −0.812 | 0.577 |
| Sample size | −0.002 | 0.008 | −0.22 | 0.830 | −0.021 | 0.017 |
| Treatment period | 0.015 | 0.010 | 1.54 | 0.163 | −0.008 | 0.037 |
Note. 6MWD: six-minute walk test/distance, SGRQ: St. George's Respiratory Questionnaire. ∗The region was the same.
3.6. Subgroup Analysis (AT Adjunctive to Other Treatments versus Placebo or Sham Acupuncture Adjunctive to Other Treatments)
3.6.1. 6MWD
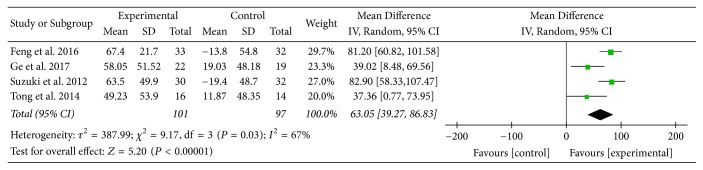
Four studies [10, 17, 18, 23] provided numerical data for 6MWD and were included in the meta-analysis. Analysis of the data indicated that there was heterogeneity (χ 2 = 9.17, P = 0.03; I 2 = 67%); hence, a random-effect model was used. The pooled results showed that 6MWD in the experimental group improved more compared to the control group (MD: 63.05; 95% CI: 39.27 to 86.83; Z = 5.20, P < 0.00001) (Figure 11).
Figure 11.
AT adjunctive to other treatments versus placebo or sham acupuncture adjunctive to other treatments, 6MWD.
3.6.2. SGRQ
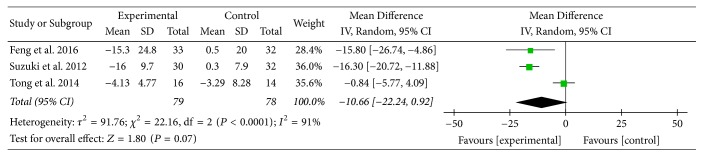
Three studies [10, 17, 18] provided numerical data for SGRQ total scores and were included in the meta-analysis. Analysis of the data indicated that there was heterogeneity (χ 2 = 22.16, P < 0.0001; I 2 = 91%); hence, a random-effect model was used. The pooled results showed that SGRQ total scores in the experimental group improved more compared to the control group (MD: −10.66; 95% CI: −22.24 to 0.92; Z = 1.80, P = 0.07) (Figure 12).
Figure 12.
AT adjunctive to other treatments versus placebo or sham acupuncture adjunctive to other treatments, SGRQ total scores.
3.6.3. FEV1
Only one study [17] provided numerical data for FEV1; thus, the meta-analysis was not performed. Changes from baseline in FEV1 in experimental group and control group were 0.07 ± 0.3 and −0.04 ± 0.2, respectively. However, the P values were not available.
3.7. Subgroup Analysis (AT Adjunctive to Other Treatments versus Other Treatments Alone)
3.7.1. 6MWD
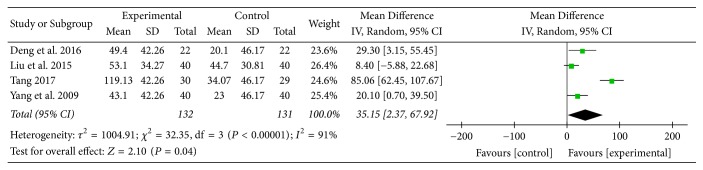
Four studies [9, 16, 21, 25] provided numerical data for 6MWD and were included in the meta-analysis. Analysis of the data indicated that there was heterogeneity (χ 2 = 32.35, P < 0.00001; I 2 = 91%); hence, a random-effect model was used. The pooled results showed that 6MWD in the experimental group improved more compared to the control group (MD: 35.15; 95% CI: 2.37 to 67.92; Z = 2.10, P = 0.04) (Figure 13).
Figure 13.
AT adjunctive to other treatments versus other treatments alone, 6MWD.
3.7.2. SGRQ
Only one study [8] provided numerical data for SGRQ total scores; thus, the meta-analysis was not performed. Compared to the control group (7.0 ± 14.9), both AT plus PR group and PR group demonstrated a significant change for SGRQ total scores (−7.1 ± 12.7, P = 0.01; −7.4 ± 8.7, P = 0.0006). However, there were no data available for symptom domain scores, activity domain scores, and impact domain scores of SGRQ.
3.7.3. FEV1
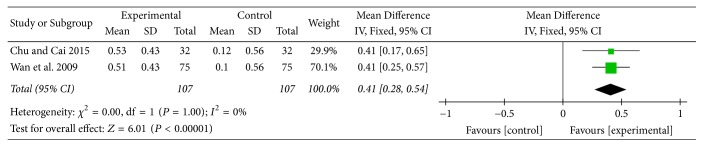
Two studies [7, 19] provided numerical data for FEV1 and were included in the meta-analysis. Analysis of the data indicated that heterogeneity was not statistically significant (χ 2 = 0.00, P = 1.00; I 2 = 0%); hence, a fixed-effect model was used. The pooled results showed that FEV1 in the experimental group improved more compared to the control group (MD: 0.41; 95% CI: 0.28 to 0.54; Z = 6.01, P < 0.00001) (Figure 14).
Figure 14.
AT adjunctive to other treatments versus other treatments alone, FEV1.
3.7.4. Effective Rate
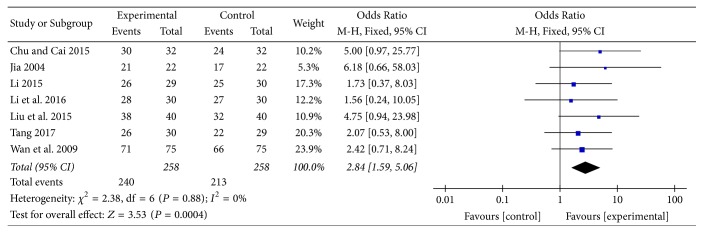
Seven studies [7, 13–16, 19, 25] provided categorical data for effective rate and were included in the meta-analysis. Analysis of the data indicated that heterogeneity was not statistically significant (χ 2 = 2.38, P = 0.88; I 2 = 0%); hence, a fixed-effect model was used. The pooled results showed that effective rate in the experimental group was higher compared to the control group (OR: 2.84; 95% CI: 1.59 to 5.06; Z = 3.53, P = 0.0004) (Figure 15).
Figure 15.
AT adjunctive to other treatments versus other treatments alone, effective rate.
3.8. Reporting Biases
We did not investigate publication biases by funnel plot because each comparison included not more than 10 studies.
4. Discussion
This systematic review provided a detailed summary of the current evidences related to the efficacy and safety of AT for functional effects and quality of life in COPD patients.
6MWD is an important measure of functional exercise capacity of patients with COPD. The distance walked is associated with clinical outcomes such as hospitalization and mortality, and its changes are used to evaluate the efficacy of therapeutic interventions such as pulmonary rehabilitation, surgery, and pharmaceutical management [33, 34]. In this review, 6MWD in the experimental group improved more compared to the control group, and the MD was 47.84 meters, which was greater than 25 meters, the minimal clinically important difference (MCID) of 6MWD for COPD patients [34]. This result might indicate the potential of AT in improving exercise capacity of COPD patients. Two subgroup analyses supported this result as well, and the MD of 6MWD change was 63.05 meters and 35.15 meters, respectively.
SGRQ, another primary outcome measure in this review, is a well-established disease-specific instrument to measure quality of life for asthma and COPD. In this review, there was no statistically significant improvement in SGRQ total scores between two groups. However, MD of symptom domain scores, activity domain scores, and impact domain scores of SGRQ was 24.86 units, 16.52 units, and 13.07 units, respectively. Although there was no MCID available for each domain, each MD was at least three times greater than 4 units, the MCID for SGRQ total scores in COPD patients [35], and this might suggest the effect of AT on different aspects of health status in COPD patients. Subgroup analysis (AT adjunctive to other treatments versus placebo or sham acupuncture adjunctive to other treatments) supported these above results as well.
FEV1 is widely used by physicians in the diagnosis, classification, treatment, monitoring, and establishing prognosis for COPD patients. In this review, there was no statistically significant improvement in FEV1 between two groups. However, subgroup analysis (AT adjunctive to other treatments versus other treatments alone) showed MD of FEV1 change was 410 mL, which was four times greater than 100 mL, the MCID of FEV1 for COPD patients [36]. And this result might suggest the potential of AT in improving pulmonary function in COPD patients.
mMRC is a major instrument to measure breathlessness. In this review, since mMRC scores were only available in one study, the meta-analysis was not performed. According to this study, change from baseline in mMRC scores in AT plus PR group and PR group was 0.3 units in both, and it was reported that there was significant difference within AT plus PR group. However, it was limited to support the effect of AT in improving breathlessness in COPD patients.
Effective rate, an important outcome measure in clinical studies of Chinese medicine, was also evaluated. In this review, effective rate in the experimental group was higher compared to the control group; to some extent, this might suggest that AT was a more effective treatment compared to other treatments. Importantly, subgroup analysis (AT adjunctive to other treatments versus other treatments alone) also supported this result with OR of 2.84.
Adverse effects were poorly reported in included studies. One study reported some minor adverse effects, and 5 studies reported no adverse effects. This might indicate the safety of AT for COPD patients.
There were some limitations in this study. Firstly, methodological quality of the included studies was generally low. For example, most of the included studies had high risk of performance bias. Secondly, most analysis of the data in the meta-analysis indicated that there was heterogeneity. Thirdly, there were various intervention forms of AT, which might make it difficult to evaluate the efficacy of AT alone. Finally, some resources with language other than English and Chinese might not be included in this review.
5. Conclusions
AT may be effective and safe in improving functional effects and quality of life in COPD patients. Besides, AT may also improve pulmonary function of COPD patients. Evidences are inadequate to support the potential of AT in improving breathlessness of COPD patients. These evidences may be useful to clinicians, patients, and health policy-makers with regard to application of AT in COPD. However, further high-quality RCTs are needed to confirm the efficacy and safety of AT for COPD patients.
Acknowledgments
This study was supported by Henan Province Priority and Advantage Discipline Construction Engineering Projects-Traditional Chinese Medicine (no. STS-ZYX-2017003).
Additional Points
Registration Number. This article is registered with PROSPERO 2016 CRD42016054335 (available from http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016054335).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Jiajia Wang searched the literature, conducted the statistical analysis, and drafted the manuscript. Yang Xie and Xueqing Yu screened the studies, extracted the data, and evaluated the risk of bias. Jiansheng Li revised the manuscript. Yang Xie conceived this study.
Supplementary Materials
The methods for this study had been developed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
References
- 1. Global Strategy for the Diagnosis, Management and Prevention of COPD: Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017, http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. [DOI]
- 2.World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/respiratory/copd/burden/en/ [Google Scholar]
- 3.Kaptchuk T. J. Acupuncture: theory, efficacy, and practice. Annals of Internal Medicine. 2002;136(5):374–383. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- 4.Ernst E., White A. R. Prospective studies of the safety of acupuncture: a systematic review. American Journal of Medicine. 2001;110(6):481–485. doi: 10.1016/s0002-9343(01)00651-9. [DOI] [PubMed] [Google Scholar]
- 5.Kim S.-Y., Lee H., Chae Y., Park H.-J. A systematic review of cost-effectiveness analyses alongside randomised controlled trials of acupuncture. Acupuncture in Medicine. 2012;30(4):273–285. doi: 10.1136/acupmed-2012-010178. [DOI] [PubMed] [Google Scholar]
- 6.Coyle M., Shergis J., Huang E. T.-Y., et al. Acupuncture therapies for chronic obstructive pulmonary disease: A systematic review of randomized, controlled trials. Alternative Therapies in Health and Medicine. 2014;20(6):10–23. [PubMed] [Google Scholar]
- 7.Chu E. X., Cai S. C. Observation the efficacy of acupuncture combined with western medicine for the treatment of stable phase of chronic obstructive lung disease. Clinical Journal of Traditional Chinese Medicine. 2015;27(1):82–84. [Google Scholar]
- 8.Deering B. M., Fullen B., Egan C., et al. Acupuncture as an adjunct to pulmonary rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention. 2011;31(6):392–399. doi: 10.1097/HCR.0b013e31822f0f61. [DOI] [PubMed] [Google Scholar]
- 9.Deng C. H., Zhang D. T., Wang N. W. Application of abdominal acupuncture in treating 22 patients with stable chronic obstructive pulmonary disease. Yunnan Journal of Traditional Chinese Medicine and Materia Medica. 2016;37(8):73–74. [Google Scholar]
- 10.Feng J., Wang X., Li X., Zhao D., Xu J. Acupuncture for chronic obstructive pulmonary disease (COPD): A multicenter, randomized, sham-controlled trial. Medicine (United States) 2016;95(40) doi: 10.1097/MD.0000000000004879.e4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J., Ouyang B. S., Sun G., Fan C., Wu Y. J., Ji L. L. Comparative research on effect of warm needling therapy on pulmonary function and life quality of patients with COPD in the stable phase. Chinese Acupuncture and Moxibustion. 2011;31(10):893–893. [PubMed] [Google Scholar]
- 12.Hu N. X. Effect of spreading moxibustion on elderly patients with chronic obstructive pulmonary disease. Chinese Journal of Modern Drug Application. 2016;10(8):254–256. [Google Scholar]
- 13.Jia J. Clinical study on acupuncture combined with rehabilitation training for improvement of pulmonary function in the patient of chronic obstructive pulmonary disease. Chinese Acupuncture and Moxibustion. 2004;24(10):681–683. [Google Scholar]
- 14.Li X. L. Effect of warm acupuncture on stable chronic obstructive pulmonary disease in dog days. Fujian University of Traditional Chinese Medicine; 2015. [Google Scholar]
- 15.Li X. Y., Peng L., Cao J. Z., Yang A. H., Huang C., Liu X. J. Effect of acupuncture combined with medicine on patients with moderate/severe chronic obstructive pulmonary disease. Continuing Medical Education. 2016;30(10):158–160. [Google Scholar]
- 16.Liu L.-J., Shi M.-Y., Song X.-M., Zhang W., Jiang C.-J. Clinical effect observation on acupuncture for chronic obstructive pulmonary disease. Journal of Acupuncture and Tuina Science. 2015;13(5):306–311. doi: 10.1007/s11726-015-0872-4. [DOI] [Google Scholar]
- 17.Suzuki M., Muro S., Ando Y., et al. A randomized, placebo-controlled trial of acupuncture in patients with Chronic Obstructive Pulmonary Disease (COPD) the COPD-acupuncture trial (CAT) JAMA Internal Medicine. 2012;172(11):878–886. doi: 10.1001/archinternmed.2012.1233. [DOI] [PubMed] [Google Scholar]
- 18.Tong J., Guo Y.-M., He Y., Li G.-Y., Chen F., Yao H. Regulatory effects of acupuncture on exercise tolerance in patients with chronic obstructive pulmonary disease at stable phase: a randomized controlled trial. Chinese Acupuncture & Moxibustion. 2014;34(9):846–850. [PubMed] [Google Scholar]
- 19.Wan W. R., Cheng S. L., Zhang W., et al. Clinincal Study on Effect of Lung-Fuction of Patiants with COPD in Remission Stage Treated by Acupuncture and Moxibustion Combining with Chinese Herbs for Warming Kidney-yang and Regulating Qi. Chinese Archives of Traditional Chinese Medicine. 2009;27(1):163–165. [Google Scholar]
- 20.Xie J., Yu J. Effect of warming needle moxibustion on pulmonary function of elderly patients with stable chronic obstructive pulmonary disease. World Journal of Acupuncture - Moxibustion. 2014;24(3):21–24. doi: 10.1016/S1003-5257(15)60006-X. [DOI] [Google Scholar]
- 21.Yang P., Zhang Y. L., Peng M. Effect of Pei Tu Sheng Jin acupuncture method on quality of life in patients with COPD. Journal of Liaoning University of Chinese Medicine. 2009;11(3):149–150. [Google Scholar]
- 22.Yu Z. Influence of Warming Needle Moxibustion on Lung Function of Senile Chronic Obstructive Pulmonary Disease in the Stable Period. China Journal of Chinese Medicine. 2014;29(4):485–486. [Google Scholar]
- 23.Ge Y., Yao H., Tong J., He Y., Li G., Kong X. Effects of acupuncture on peripheral skeletal muscle exercise ability in patients with chronic obstructive pulmonary disease at stable phase. Chinese Acupuncture and Moxibustion. 2017;37(4):366–371. doi: 10.13703/j.0255-2930.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y. Effects of Zong Qi Tonifying Acupuncture on Pulmonary Function of Patients with Chronic Obstructive Pulmonary Disease in Stable Phase. Fujian University of Traditional Chinese Medicine; 2017. [Google Scholar]
- 25.Tang G. D. The effect on the COPD stable-phase patients by the method of Shengsan Acupuncture. Fujian University of Traditional Chinese Medicine; 2017. [Google Scholar]
- 26.Crapo R. O., Casaburi R., Coates A. L., et al. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 27.Jones P. W., Quirk F. H., Baveystock C. M., Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. American Journal of Respiratory and Critical Care Medicine (Salma) 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 28.Perez T., Burgel P. R., Paillasseur J.-L., et al. Modified medical research council scale vs baseline dyspnea index to evaluate dyspnea in chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2015;10(1):1663–1672. doi: 10.2147/COPD.S82408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins J. P. T., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://www.handbook.cochrane.org/
- 30. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 31.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid C. H., Stark P. C., Berlin J. A., Landais P., Lau J. Meta-regression detected associations between heterogeneous treatment effects and study-level, but not patient-level, factors. Journal of Clinical Epidemiology. 2004;57(7):683–697. doi: 10.1016/j.jclinepi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Pinto-Plata V. M., Cote C., Cabral H., Taylor J., Celli B. R. The 6-min walk distance: Change over time and value as a predictor of survival in severe COPD. European Respiratory Journal. 2004;23(1):28–33. doi: 10.1183/09031936.03.00034603. [DOI] [PubMed] [Google Scholar]
- 34.Holland A. E., Hill C. J., Rasekaba T., Lee A., Naughton M. T., McDonald C. F. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation. 2010;91(2):221–225. doi: 10.1016/j.apmr.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Jones P. W. St. George's respiratory questionnaire: MCID. COPD-Journal of chronic obstructive pulmonary disease. 2005;2(1):75–79. doi: 10.1081/COPD-200050513. [DOI] [PubMed] [Google Scholar]
- 36.Donohue J. F. Minimal Clinically Important Differences in COPD Lung Function. COPD-Journal of Chronic Obstructive Pulmonary Disease. 2009;2(1):111–124. doi: 10.1081/COPD-200053377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The methods for this study had been developed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.