Abstract
Background
Regularly transfused people with sickle cell disease (SCD) and people with thalassaemia (who are transfusion‐dependent or non‐transfusion‐dependent) are at risk of iron overload. Iron overload can lead to iron toxicity in vulnerable organs such as the heart, liver and endocrine glands; which can be prevented and treated with iron chelating agents. The intensive demands and uncomfortable side effects of therapy can have a negative impact on daily activities and well‐being, which may affect adherence.
Objectives
To identify and assess the effectiveness of interventions (psychological and psychosocial, educational, medication interventions, or multi‐component interventions) to improve adherence to iron chelation therapy in people with SCD or thalassaemia.
Search methods
We searched CENTRAL (the Cochrane Library), MEDLINE, Embase, CINAHL, PsycINFO, Psychology and Behavioral Sciences Collection, Web of Science Science & Social Sciences Conference Proceedings Indexes and ongoing trial databases (01 February 2017). We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register (12 December 2017).
Selection criteria
For trials comparing medications or medication changes, only randomised controlled trials (RCTs) were eligible for inclusion.
For studies including psychological and psychosocial interventions, educational Interventions, or multi‐component interventions, non‐RCTs, controlled before‐after studies, and interrupted time series studies with adherence as a primary outcome were also eligible for inclusion.
Data collection and analysis
Three authors independently assessed trial eligibility, risk of bias and extracted data. The quality of the evidence was assessed using GRADE.
Main results
We included 16 RCTs (1525 participants) published between 1997 and 2017. Most participants had β‐thalassaemia major; 195 had SCD and 88 had β‐thalassaemia intermedia. Mean age ranged from 11 to 41 years. One trial was of medication management and 15 RCTs were of medication interventions. Medications assessed were subcutaneous deferoxamine, and two oral‐chelating agents, deferiprone and deferasirox.
We rated the quality of evidence as low to very low across all outcomes identified in this review.
Three trials measured quality of life (QoL) with validated instruments, but provided no analysable data and reported no difference in QoL.
Deferiprone versus deferoxamine
We are uncertain whether deferiprone increases adherence to iron chelation therapy (four trials, very low‐quality evidence). Results could not be combined due to considerable heterogeneity (participants' age and different medication regimens). Medication adherence was high (deferiprone (85% to 94.9%); deferoxamine (71.6% to 93%)).
We are uncertain whether deferiprone increases the risk of agranulocytosis, risk ratio (RR) 7.88 (99% confidence interval (CI) 0.18 to 352.39); or has any effect on all‐cause mortality, RR 0.44 (95% CI 0.12 to 1.63) (one trial; 88 participants; very low‐quality evidence).
Deferasirox versus deferoxamine
We are uncertain whether deferasirox increases adherence to iron chelation therapy, mean difference (MD) ‐1.40 (95% CI ‐3.66 to 0.86) (one trial; 197 participants; very‐low quality evidence). Medication adherence was high (deferasirox (99%); deferoxamine (100%)). We are uncertain whether deferasirox decreases the risk of thalassaemia‐related serious adverse events (SAEs), RR 0.95 (95% CI 0.41 to 2.17); or all‐cause mortality, RR 0.96 (95% CI 0.06 to 15.06) (two trials; 240 participants; very low‐quality evidence).
We are uncertain whether deferasirox decreases the risk of SCD‐related pain crises, RR 1.05 (95% CI 0.68 to 1.62); or other SCD‐related SAEs, RR 1.08 (95% CI 0.77 to 1.51) (one trial; 195 participants; very low‐quality evidence).
Deferasirox film‐coated tablet (FCT) versus deferasirox dispersible tablet (DT)
Deferasirox FCT may make little or no difference to adherence, RR 1.10 (95% CI 0.99 to 1.22) (one trial; 173 participants; low‐quality evidence). Medication adherence was high (FCT (92.9%); DT (85.3%)).
We are uncertain if deferasirox FCT increases the incidence of SAEs, RR 1.22 (95% CI 0.62 to 2.37); or all‐cause mortality, RR 2.97 (95% CI 0.12 to 71.81) (one trial; 173 participants; very low‐quality evidence).
Deferiprone and deferoxamine combined versus deferiprone alone
We are uncertain if deferiprone and deferoxamine combined increases adherence to iron chelation therapy (very low‐quality evidence). Medication adherence was high (deferiprone 92.7% (range 37% to 100%) to 93.6% (range 56% to 100%); deferoxamine 70.6% (range 25% to 100%).
Combination therapy may make little or no difference to the risk of SAEs, RR 0.15 (95% CI 0.01 to 2.81) (one trial; 213 participants; low‐quality evidence).
We are uncertain if combination therapy decreases all‐cause mortality, RR 0.77 (95% CI 0.18 to 3.35) (two trials; 237 participants; very low‐quality evidence).
Deferiprone and deferoxamine combined versus deferoxamine alone
Deferiprone and deferoxamine combined may have little or no effect on adherence to iron chelation therapy (four trials; 216 participants; low‐quality evidence). Medication adherence was high (deferoxamine 91.4% to 96.1%; deferiprone: 82.4%)
Deferiprone and deferoxamine combined, may have little or no difference in SAEs or mortality (low‐quality evidence). No SAEs occurred in three trials and were not reported in one trial. No deaths occurred in two trials and were not reported in two trials.
Deferiprone and deferoxamine combined versus deferiprone and deferasirox combined
Deferiprone and deferasirox combined may improve adherence to iron chelation therapy, RR 0.84 (95% CI 0.72 to 0.99) (one trial; 96 participants; low‐quality evidence). Medication adherence was high (deferiprone and deferoxamine: 80%; deferiprone and deferasirox: 95%).
We are uncertain if deferiprone and deferasirox decreases the incidence of SAEs, RR 1.00 (95% CI 0.06 to 15.53) (one trial; 96 participants; very low‐quality evidence).
There were no deaths in the trial (low‐quality evidence).
Medication management versus standard care
We are uncertain if medication management improves health‐related QoL (one trial; 48 participants; very low‐quality evidence). Adherence was only measured in one arm of the trial.
Authors' conclusions
The medication comparisons included in this review had higher than average adherence rates not accounted for by differences in medication administration or side effects.
Participants may have been selected based on higher adherence to trial medications at baseline. Also, within the clinical trial context, there is increased attention and involvement of clinicians, thus high adherence rates may be an artefact of trial participation.
Real‐world, pragmatic trials in community and clinic settings are needed that examine both confirmed or unconfirmed adherence strategies that may increase adherence to iron chelation therapy.
Due to lack of evidence this review cannot comment on intervention strategies for different age groups.
Plain language summary
Strategies to increase adherence to iron chelation therapy in people with sickle cell disease or thalassaemia
Review question
We wanted to determine if there are any interventions (medication, psychological or educational) that would help people adhere to their iron chelation therapy.
Background
People with sickle cell disease or thalassaemia who receive regular transfusions, are exposed to iron overload which can result in toxicity to organs and death. Iron chelation therapy is used to prevent or treat iron overload, but it can be a demanding regimen, and have unwanted side effects. There are three types of iron chelators being used to treat iron overload: deferoxamine given subcutaneously (by injecting a drug into the tissue layer between the skin and the muscle); and two agents that are taken orally, deferiprone and deferasirox.
Search date
The evidence is current to 12 December 2017.
Study characteristics
We searched the literature for both randomised and non‐randomised studies, and found 16 randomised trials with 1525 participants, published between 1997 and 2017. Most people had β‐thalassaemia major; one trial included people with SCD and one included people with a milder form of thalassaemia (thalassaemia intermedia). Mean age ranged from 11 years to 41 years. We included one trial of medication management and 15 trials comparing different drug treatments.
Key results
Trials included comparisons of individual agents to each other or a combination of drugs compared to one drug alone or to other combinations of drugs.
We were uncertain if single agents or combined agents made any difference in adherence rates, serious adverse events or mortality. Quality of life, measured using validated questionnaires, was only reported in two trials, but not enough data were reported to determine any differences between treatments.
There was no evidence on intervention strategies for different age groups.
We found that there was an unusually high adherence rate to all drugs and combinations of drugs in all the trials. This may be because participants may have been selected based on their ability to stick to medication regimens. Also, adherence may increase in trial participants when there is a higher level of clinician involvement in care.
We concluded that real‐world randomised and non‐randomised trials, run in both the community and in clinics, are needed to examine a variety of proven and unproven strategies that may be useful for increasing adherence to iron chelation therapy.
Quality of evidence
We rated the quality of evidence as low to very low across all of the outcomes in this review. This was due to trials being at serious or very serious risk of bias; outcome estimates being imprecise (wide confidence intervals); and not widely applicable (with some trials conducted only in children of a specific age and meeting specific criteria).
Summary of findings
Background
Description of the condition
Haemoglobinopathies are a range of inherited disorders resulting from mutations of the globin genes (the protein component of haemoglobin). Two of the most common of these disorders are sickle cell disease (SCD) and thalassaemia.
Sickle cell disease
SCD is an inheritable blood disorder, which can lead to life‐threatening complications. People with SCD experience episodes of severe pain, and other complications including anaemia, end‐organ damage, pulmonary complications, kidney disease, and increased susceptibility to infections and stroke (Pleasants 2014). It is one of the most common severe monogenic disorders in the world, due to the inheritance of two abnormal haemoglobin (beta globin) genes (Rees 2010). Populations originating from sub‐Saharan Africa, Spanish‐speaking regions in the western hemisphere (South America, the Caribbean, and Central America), the Middle East, India and parts of the Mediterranean are predominantly affected. Reductions in infant and child mortality and increasing migration from highly affected countries have made this a worldwide problem (Piel 2012). Over 12,500 people in the UK and 100,000 in the USA suffer from the disease (NICE 2010; Pleasants 2014).
The term SCD refers to all mutations that cause the disease, of which there are three main types. Sickle cell anaemia is the most common form of the disease (up to 70% of cases of SCD in people of African origin) and is due to the inheritance of two beta globin S (βS) alleles (haemoglobin (Hb)SS). The second most common genotype (up to 30% of cases in people of African origin) is haemoglobin SC disease (HbSC disease) and is due to the co‐inheritance of the βS and βC alleles; this tends to be a more moderate form of the disease. The third major type of SCD occurs when βS is inherited with a β‐thalassaemia allele, causing HbS/β‐thalassaemia (Rees 2010). People who have inherited a thalassaemia null mutation (HbSβº) have a disease that is clinically indistinguishable from sickle cell anaemia, whereas people with HbSβ+ thalassaemia have a milder disorder. In high‐income nations, people with SCD are expected to live into their 40s, 50s and beyond; whereas in low‐income countries, including some African nations, it is estimated that between 50% to 90% of children born with HbSS die before their fifth birthday (Gravitz 2014; Grosse 2011).
Red blood cell transfusions can be given to treat complications of SCD (e.g. acute chest syndrome), this often involves a single transfusion episode, or they can be part of a regular long‐term transfusion programme to prevent complications of SCD such as stroke in children (Yawn 2014).
Thalassaemia
The term thalassaemia describes a group of inheritable disorders caused by the absence or reduction in globin chain production. This results in ineffective red blood cell production, anaemia and poor oxygen delivery. The genetic defect can be in the α or β globin chain (α‐thalassaemia, β‐thalassaemia or H disease). In β‐thalassaemia, reduced or absent β globulin production leads to an excess of free α‐globin chains resulting in severe anaemia and bone marrow hyperplasia (abnormal cell growth) preventing normal development. In H disease and α‐thalassaemia, the α‐globin chains are affected and disease can vary from mild (where reduced, but adequate, amounts of the functional globin chains are produced) to severe (where no effective haemoglobin is produced) (UK Thalassaemia Society 2008). Complications that may occur include infections, bone diseases, enlarged spleen, slowed growth rates, cardiomyopathy, venous thrombosis, pulmonary hypertension, and hypothyroidism (Rund 2005).
Thalassaemia is common in people from the Mediterranean, the Middle East, Southeast Asia, the Indian subcontinent, and Africa (Piel 2014; UK Thalassaemia Society 2008). It is estimated that there are over 1000 people with thalassaemia in the UK (APPG 2009). In high‐income countries most affected children survive with a chronic disorder; however, most children born with thalassaemia are in low‐income countries die before the age of five years (Modell 2008). Nevertheless, the thalassaemias are a global health burden due to population migration and growth and improved survival leading to an increase in the incidence of the disorder (Piel 2014).
Regular red blood cell transfusion is the standard treatment to correct anaemia and to enable growth and development, normal activities and to inhibit bone marrow expansion. People with severe forms, β‐thalassaemia major, require life‐long transfusions from the first year of life.
Iron chelation therapy and adherence
Regularly transfused people with SCD, as well as transfusion‐dependent, and non‐transfusion‐dependent people with thalassaemia, are exposed to transfusion‐related iron overload. Transfusion‐related iron overload can lead to iron toxicity, with organs such as the heart, liver and endocrine glands being particularly vulnerable. Iron overload is the major cause of morbidity and mortality in thalassaemia (Aydinok 2014; Rund 2005; Trachtenberg 2012).
Iron chelating agents are used for preventing and treating iron overload. Deferoxamine (DFO) has been the standard treatment for the last 40 years; it is administered subcutaneously or intravenously usually over eight to 12 hours, up to seven days a week. More recently two oral chelating agents, deferiprone (DFP) and then deferasirox (DFX), have been licensed. These were initially introduced as second‐line agents in children six years and older with β‐thalassaemia major, or in people when DFO is contraindicated or found to be inadequate (Fisher 2013). These oral agents are becoming more commonly used, particularly DFX, because of the ease of administration compared to subcutaneous or intravenous DFO (Aydinok 2014).
Licensed iron chelating agents are effective at iron removal; however, the treatment is not without side effects (Telfer 2006). Side effects with DFO include pain or skin reactions at the injection site, retinal toxicity and hearing loss. Side effects with DFX include skin rashes, gastroenteritis, an increase in liver enzymes, and reduced kidney function. Adverse events reported in people taking DFP include gastrointestinal disturbances, arthropathy (joint disease), raised liver enzymes, neutropenia (a decrease in neutrophils, a type of white blood cell, in the blood stream) and agranulocytosis (lowered white blood cell count). Regular blood sampling is recommended to monitor neutropenia, renal function and liver enzymes in people taking oral chelating agents (Fisher 2013).
Adherence to medications is defined as the extent to which a person's use of the medicine matches the agreed prescription from the healthcare provider (NICE 2009;Walsh 2014). Moderate adherence is defined as taking 60% to 80% of a prescribed dose, while high adherence can include the continued use of the medicine or taking at least 80% of the recommended dose. There are several ways to measure adherence including the self‐reporting of medication use or more objective factors such as pill counts, prescription refills, urinary assays or in the case of iron chelation, signs of iron overload (Ryan 2014;Walsh 2014). Adherence rates can vary widely, a recent review reported that adherence rates to the iron chelator deferasirox ranged between 22% and 89% (Loiselle 2016).
Research suggests that iron chelation therapies impact on a person's quality of life (QoL) and result in low levels of personal satisfaction. The intensive demands and uncomfortable side effects of iron chelation therapy can have a negative impact on daily activities and well‐being, which may affect adherence to therapy (Abetz 2006; Payne 2008; Rofail 2010). Other factors affecting adherence to medications include inappropriate use, the quality of information provided to the individual, complex treatment regimens, as well as intolerance to the harms caused by the medications (Ryan 2014). Non‐adherence can be both intentional and unintentional, with intentional non‐adherence being influenced by such factors as poor communication, adverse effects, personal preferences or beliefs and disagreement with the need for treatment; whereas unintentional non‐adherence is influenced by factors generally beyond the person's control such as forgetfulness or difficulties in understanding instructions (NICE 2009; Ryan 2014; Trachtenberg 2012). Sub‐optimal adherence can increase adverse events associated with iron overload and result in increased cost of care, hospitalisations, and severe morbidity and mortality (Payne 2008; Vekeman 2016; WHO 2003).
Description of the intervention
The research on adherence and appropriate use of medicines is vast and complex and comprises a number of studies targeting people taking the medication, clinicians, indications and specific classes of medications. This research has also been reviewed in many systematic reviews as well as overviews of systematic reviews and in guidelines (Costello 2004; NCCPC 2009; NICE 2009; Ryan 2014; WHO 2003).
For this review we focus on the individual with SCD or thalassaemia, with interventions to increase adherence to iron chelation therapy being divided into three main categories. These are psychological and psychosocial interventions, educational interventions and medication interventions. These interventions may be delivered alone or in combination (as a complex intervention). For instance, combining psychological with psychosocial interventions such as symptom self‐management with peer support; or medication changes implemented with reconciliation strategies or complemented with medication information and education.
Psychological and psychosocial interventions
Psychological and psychosocial therapies that may promote medication adherence include interventions to promote behavioural change such as cognitive behavioural therapy (CBT), as well as peer support, counselling and skills development (communication, social, emotional). In addition there is an increasing emphasis on health‐system interventions that may influence adherence such as patient‐centred care and shared decision‐making (NCCPC 2009; Ryan 2014; WHO 2003).
In an outpatient clinic survey of 328 people with SCD using the Patient Health Questionnaire 9, up to 60% of people with SCD experienced mild to severe depressive symptoms. Interventions to address depression and other co‐morbidities may promote medication adherence, and depending on the degree of depression or other co‐morbidities can include medications, guided self‐help, individual or group CBT or peer support (NCCMH 2010; NICE 2009; Thomas 2013).
Education interventions
Educational interventions may include disease and medication information, and assistance with communication skills to facilitate communication with healthcare providers (Haywood 2009;Ryan 2014). Interventions in the form of personal communication, structured presentations, and formal educational activities delivered by clinicians or non‐medical personnel are included in this category.
Medication interventions
The identification and correction of medication issues such as under‐utilisation, dosing and scheduling, allergies and contraindications, financial issues and inadequate monitoring may impact on adherence and health outcomes. Additional strategies such as positive medication changes to reduce burden or increase effectiveness, route of administration, risk minimisation and medication reconciliation may be used to promote improved medication adherence (NCCPC 2009; Ryan 2014).
How the intervention might work
Psychological and psychosocial interventions
People with chronic illness face a variety of psychological and psychosocial problems including depression, anxiety disorders, disease burden and restrictions on social and occupational functioning. Research suggests that skill development to help people with chronic illnesses cope with adverse effects of medication and any co‐morbidities will decrease disease burden, and improve their health‐related QoL (NCCMH 2010; NCCPC 2009). The use of cognitive aids, clear instructions and realistic expectations can improve adherence (Wertheimer 2003). Person‐centred psychological and psychosocial interventions encourage self‐management skills, shared decision‐making and self‐efficacy (NCCPC 2009; NICE 2009).
Educational interventions
Tailored educational interventions can be delivered to individuals or groups and can be delivered face‐to‐face or remotely. Educational interventions may include both a simple approach, such as evidence‐based plain language information, by written or verbal communication, or a multi‐faceted approach that considers the wider environment, management, decision making, lifestyle and communication roles taken on by the person taking the medication (Ryan 2014). Each approach should be tailored to the individual (NCCPC 2009;WHO 2003).
Medication interventions
Iron levels are monitored in people receiving regular transfusions. An increasing iron burden may necessitate medication changes or more aggressive iron chelation therapy such as increasing doses or combination therapy. People may also change medications multiple times due to worsening iron overload, side effects, or personal preferences (Trachtenberg 2014). Medication changes that reflect personal preferences or minimise harms and improve outcomes, combined with medication reconciliation strategies including audit and feedback, prescription and medication help lines, counselling and age‐appropriate discharge instructions, may help to address and improve adherence (NCCPC 2009; Ryan 2014). Medication interventions also include medication management which is a person‐centred intervention by a clinician (often a pharmacist) to optimise drug therapy in order to improve outcomes for the person (American Pharmacists Association 2008).
Why it is important to do this review
Adherence to iron chelation therapy is necessary to decrease the risk of morbidity and mortality associated with iron overload. Poor adherence can also result in increased healthcare costs. It is therefore important to understand the effectiveness and limitations of interventions which can be used to influence adherence in people receiving iron chelation therapy for SCD or thalassaemia.
Objectives
To identify and assess the effectiveness of interventions to improve adherence to iron chelation therapy compared to standard care in people with SCD or thalassaemia including:
identifying and assessing the effectiveness of different types of interventions (psychological and psychosocial, educational, medication interventions (which include comparisons of adherence between different iron chelators), or multi‐component interventions);
identifying and assessing the effectiveness of interventions specific to different age groups (children, adolescents, adults).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing one or more adherence interventions, to standard care.
For studies comparing medications or medication changes, we only included RCTs.
If no RCTs were available, we planned to include non‐randomised studies of interventions (NRSIs), controlled before‐after (CBA) studies, and interrupted time series (ITS) studies including repeated measures designs for those studies including psychological and psychosocial interventions, educational Interventions, or multi‐component interventions. We used the Cochrane Effective Practice and Organisation of Care (EPOC) Group's definition of study designs to consider studies for inclusion (EPOC 2015).
We planned to include cluster‐randomised trials, non‐randomised cluster trials, and CBA studies if they had at least two intervention sites and two control sites. We excluded cluster‐randomised trials, non‐randomised cluster trials, and CBA studies that had only one intervention or control site because the intervention (or comparison) may be confounded by study site making it difficult to attribute any observed differences to the intervention rather than to other site‐specific variables (EPOC 2015).
We planned to include ITS and repeated measures studies which had a clearly defined point in time when the intervention occurred and at least three data points before and after the intervention. We excluded ITS studies that did not have a clearly defined point in time when the intervention occurred, or fewer than three data points before and after the intervention, or the ITS study ignored secular (trend) changes, performed a simple t‐test of the pre‐ versus post‐intervention periods and re‐analysis of the data was not possible (in accordance with EPOC 2015 recommendations).
Types of participants
Children, adolescents, or their caregivers, and adults with SCD or transfusion‐dependent or non‐transfusion‐dependent thalassaemia.
Types of interventions
Psychological and psychosocial Interventions
Educational interventions
Medication interventions
Multi‐component interventions (combining aspects of the above interventions)
versus
Standard care (as defined in the trial)
Types of outcome measures
Primary outcomes
Adherence to iron chelation therapy rates (defined as per cent of doses administered (number of doses of the iron chelator taken, out of number prescribed), measured for a minimum of three months
Serious adverse events (SAEs) (including complications from the therapy, the disease itself, and non‐adherence to chelation therapy)
All‐cause mortality
We categorised all‐cause mortality and SAEs according to short‐, medium‐, and long‐term outcomes. We reported the exact definition of these time frames over time periods that are common to as many trials as possible (e.g. zero to one year, one to five years, over five years).
Secondary outcomes
Sustained adherence to therapy (measured for a minimum of six months)
Health‐related QoL (as measured by validated instruments)
Iron overload (defined by ferritin over 1000 µg/L, or clinical symptoms, or signs of iron overload, e.g. magnetic resonance imaging (MRI) T2* cardiac iron content, MRI R2* liver iron content, liver biopsy, or the need for medically indicated additional or change in chelation therapy)
Organ damage (including cardiac failure, endocrine disease, surrogate markers of organ damage (creatinine), histologic evidence of hepatic fibrosis)
Other adverse events related to iron chelation
We categorised health‐related QoL, iron overload and organ damage according to short‐, medium‐, and long‐term outcomes. We reported the exact definition of these time frames over time periods that are common to as many studies as possible (e.g. up to six months, six to 12 months, over 12 months).
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
We identified studies from the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR thalassaemia) AND iron chelation.
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register: 12 December 2017.
In addition to the above, we conducted a search of the following databases to include RCTs, NRSIs, CBA and ITS studies:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) and Other Reviews (DARE; 2015, Issue 2) (www.cochranelibrary.com/) searched 01 February 2017;
PubMed (Epub Ahead of Print, In‐Process and Other Non‐Indexed Citations, for recent records not yet added to MEDLINE) (www.ncbi.nlm.nih.gov/sites/entrez) searched 01 February 2017;
MEDLINE (OvidSP, Epub Ahead of Print, In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE, 1946 to 01 February 2017);
Embase (OvidSP, 1974 to 01 February 2017);
CINAHL (EBSCOHost, 1937 to 01 February 2017);
PsycINFO (EBSCOHost, 1900 to 01 February 2017);
ProQuest Dissertations & Theses Global (ProQuest, 1861 to 01 February 2017);
Psychology and Behavioral Sciences Collection (EBSCOHost, 1930 to 01 February 2017);
Web of Science Science & Social Sciences Conference Proceedings Indexes (CPSI‐S & CPSSI, 1990 to 01 February 2017).
We also searched the following trial registries for ongoing trials:
ClinicalTrials.gov (clinicaltrials.gov/) searched on 01 February 2017;
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) searched on 01 February 2017;
ISRCTN registry (www.isrctn.com/) searched on 01 February 2017.
Search strategies can be found in an appendix (Appendix 1).
Searching other resources
We handsearched reference lists of included trials in order to identify further relevant trials.
Data collection and analysis
Selection of studies
We selected trials according to chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Three review authors (PF, KM, LE) independently screened all electronically‐derived citations and abstracts of papers identified by the search strategy for relevance. We excluded studies that were clearly irrelevant at this stage based on the abstract. Three review authors (PF, KM, LE) independently assessed the full texts of all potentially‐relevant studies for eligibility against the criteria outlined above. We resolved disagreements by discussion, if we did not reach a consensus or if we were unsure of trial eligibility, we consulted a third review author (LE or SH). We sought further information from trial investigators if the trial report or abstract contained insufficient data to make a decision about eligibility. We used Covidence software to assess trial eligibility, which included ascertaining whether the participants had SCD or thalassaemia, if the trial addressed interventions to improve adherence to iron chelation therapy, and whether the trial was randomised or a NRSI or a CBA or an ITS study (Covidence). We recorded the reasons why potentially‐relevant studies failed to meet the eligibility criteria.
Data extraction and management
Three review authors (PF, SF, KM) extracted the data according to Cochrane guidelines (Higgins 2011a). We resolved disagreements by consensus or we consulted a fourth review author (LE). We extracted data independently for all of the trials using Covidence modified to reflect the outcomes in this review (Covidence). In addition, we used the available tables in Review Manager 5 to extract data on trial characteristics as below (RevMan 2014).
General information
Review author's name, date of data extraction, study ID, first author of study, author's contact address (if available), citation of paper, objectives of the study.
Study details
Design, location, setting, sample size, power calculation, treatment allocation, inclusion and exclusion criteria, reasons for exclusion, comparability of groups, length of follow‐up, stratification, stopping rules described, statistical analysis, results, conclusion, and funding.
Characteristics of participants
Age, gender, total number recruited, total number randomised, total number analysed, types of underlying disease, loss to follow‐up numbers, dropouts (percentage in each arm) with reasons, protocol violations, iron chelating agent, previous treatments, current treatment, prognostic factors, co‐morbidities, ferritin levels.
Interventions
Details of the interventions including type of intervention whether psychological and psychosocial or educational or medication or multi‐component interventions, how the intervention is being delivered (i.e. group, face‐to‐face, written information, electronically) and by whom (i.e. clinicians, peers) and where the intervention is being delivered (i.e. hospital, clinic, home).
Outcomes measured
Adherence rates, SAEs, all‐cause mortality, sustained adherence to therapy, health‐related QoL, iron overload defined by ferritin over 1000 µg/L or clinical symptoms or signs of iron overload or need for medically indicated additional or change in chelation therapy (or any combination of these), evidence of organ damage, other adverse events.
We used both full‐text versions and abstracts as data sources and used one data extraction form for each unique study. Where sources did not provide sufficient information, we contacted authors for additional details.
Three review authors (PF, SF, KM) entered data and we resolved disagreements by consensus.
If NRSIs had been identified we planned to extract data according to the criteria developed for NRSIs as recommended in Chapter 13 of the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011). In addition to the items above, for NRSIs, CBA and ITS studies we also planned to collect data on: confounding factors; the comparability of groups on confounding factors; methods used to control for confounding and on multiple effect estimates (both unadjusted and adjusted estimates) as recommended in chapter 13 of the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011).
Assessment of risk of bias in included studies
Three review authors (PF, KM, SF) assessed all included trials for possible risks of bias as described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011c).
The assessment included information about the design, the conduct and the analysis of the trial. We assessed each criterion using Cochrane's tool for assessing the risk of bias for RCTs (classed as 'low', 'high' or 'unclear' risk) in the following areas.
Selection bias (random sequence generation and allocation concealment)
Performance bias (blinding of participants and personnel)
Detection bias (blinding of outcome assessment)
Attrition bias (incomplete outcome data)
Reporting bias (selective reporting)
Other bias
We resolved disagreements on the assessment of quality of an included trial by discussion until we reached consensus or failing that by consulting a fourth review author (LE).
The only included trials were RCTs. In future updates of this review, we plan to use the ROBINS‐I tool (Risk Of Bias In Non‐randomized Studies of Interventions) to rate the quality of NRSIs and CBA studies (Sterne 2016). The tool, uses signalling questions and covers seven domains (listed below) where the quality of evidence is rated as 'low', 'moderate', 'serious', 'critical' or 'no information'. Please refer to an appendix for a copy of the tool (Appendix 2).
Bias due to confounding
Bias in the selection of participants
Bias in measurement of interventions
Bias due to departure from intended interventions
Bias due to missing data
Bias in measurement of outcomes
Bias in the selection of the reported result
In future updates of this review, for ITS studies we plan to use the risk of bias criteria below as suggested for EPOC reviews (EPOC 2015).
Was the intervention independent of other changes?
Was the shape of the intervention effect pre‐specified?
Was the intervention unlikely to affect data collection?
Was knowledge of the allocated interventions adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Was the study free from selective outcome reporting?
Was the study free from other risks of bias?
Measures of treatment effect
RCTs
For RCTs of continuous outcomes we recorded the mean, standard deviation (SD) and total number of participants in both the treatment and control groups. For those using the same scale, we performed analyses using the mean difference (MD) with 95% confidence intervals (CIs); for those reported using different scales, we would have used standardised mean difference (SMD).
For RCTs of dichotomous outcomes we recorded the number of events and the total number of participants in both the treatment and control groups and reported the pooled risk ratio (RR) with a 95% CI (Deeks 2011). Where the number of observed events is small (less than 5% of sample per group), and where trials have balanced treatment groups, we would have reported the Peto odds ratio (OR) with 95% CI (Deeks 2011).
There were no eligible cluster randomised trials, if such trials are included in future updates of this review, we plan to extract and report direct estimates of the effect measure (e.g. RR with a 95% CI) from an analysis that accounts for the clustered design. We will obtain statistical advice (MT) to ensure the analysis is appropriate. If appropriate analyses are not available, we will make every effort to approximate the analysis following the recommendations in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d).
Non‐randomised studies
There were no eligible NRSIs, if such studies are included in future updates of this review, we plan to extract and report the RR with a 95% CI for dichotomous outcomes, adjusting for baseline differences (such as Poisson regressions or logistic regressions) or the ratio of RRs (i.e. the RR post intervention / RR pre intervention).
For continuous variables we will extract and report the absolute change from a statistical analysis adjusting for baseline differences (e.g. regression models, mixed models or hierarchical models) or the relative change adjusted for baseline differences in the outcome measures (i.e. the absolute post‐intervention difference between the intervention and control groups, as well as the absolute pre‐intervention difference between the intervention and control groups / the post‐intervention level in the control group) (EPOC 2015).
ITS studies
There were no eligible ITS studies, if such studies are included in future updates, we plan to standardise data by dividing the level (or time slope) and standard error (SE) by the SD of the pre‐intervention slope, in order to obtain the effect sizes.
Where appropriate, we plan to report the number‐needed‐to‐treat‐to‐benefit (NNTB) and the number‐needed‐to‐treat‐to‐harm (NNTH) with CIs.
If we are unable to report the available data in any of the formats described above, we will perform a narrative report, and if appropriate, present the data in tables.
Unit of analysis issues
For trials with multiple treatment groups or interventions, we included subgroups that were considered relevant to the analysis. If appropriate, we combined groups to create a single pair‐wise comparison. If this was not possible, we selected the most appropriate pair of interventions and excluded the others (Higgins 2011d). No trials randomised participants more than once.
There were no included cluster randomised studies or NRSIs. If these are included in future updates of this review, we plan to treat any unit of analysis issues that arise in accordance with the advice given in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d). .
There were no included ITS studies. If these are included in future updates of this review, we plan to deal with any unit of analysis issues arising from their inclusion according to the EPOC recommendations (EPOC 2015).
Dealing with missing data
Where we identified data as being missing or unclear in the published literature, we contacted trial authors directly. We contacted three authors for additional trial information (Antmen 2013; Badawy 2010; Elalfy 2015) and have received one response stating that the trial data were not available at this time (Badawy 2010).
We recorded the number of participants lost to follow‐up for each trial. Where possible, we analysed data on an intention‐to‐treat (ITT) basis, but if insufficient data were available, we also presented a per protocol analyses (Higgins 2011c).
Assessment of heterogeneity
If the clinical and methodological characteristics of individual trials were sufficiently homogeneous, we combined the data to perform a meta‐analysis. We planned to analyse the data from RCTs, NRSIs, CBA and ITS studies separately, but we only included RCTs.
We assessed statistical heterogeneity of treatment effects between trials using a Chi² test with a significance level at P < 0.1. We used the I² statistic to quantify the degree of potential heterogeneity and classified it as moderate if I² is greater than 50%, or considerable if I² is greater than 75%. We used the random‐effects model as we anticipated that we would identify at least moderate clinical and methodological heterogeneity within the trials selected for inclusion. If statistical heterogeneity was considerable, we did not report the overall summary statistic. We assessed potential causes of heterogeneity by sensitivity and subgroup analyses (Deeks 2011).
Assessment of reporting biases
No meta‐analysis in this review included at least 10 trials, we therefore could not perform a formal assessment of publication bias (Sterne 2011).
Data synthesis
If trials were sufficiently homogenous in their design, we conducted a meta‐analysis according to the recommendations of Cochrane (Deeks 2011). We used the random‐effects model for all analyses as we anticipated that true effects would be related but not the same for included trials. If we could not perform a meta‐analysis we commented on the results as a narrative.
For RCTs where meta‐analysis was feasible, we used the Mantel‐Haenszel method for dichotomous outcomes and the inverse variance method for continuous outcomes. We did not have outcomes that included data from cluster‐RCTs. Where heterogeneity was above 75%, and we identified a cause for the heterogeneity, we explored this with subgroup analyses. If we did not find a cause for the heterogeneity then we did not perform a meta‐analysis.
If identified, we planned to analyse NRSIs or CBA studies separately. We planned to analyse outcomes with adjusted effect estimates if these were adjusted for the same factors using the inverse variance method as recommended in chapter 13 of the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011). For ITS studies, we would have used the effect sizes (if reported in the included studies or obtained (as described earlier)) and pooled them using the generic inverse variance method in Review Manager 5 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We reported results for the different types of disease separately (SCD or thalassaemia). Only one trial included participants with SCD (Vichinsky 2007).
There were insufficient data to perform some of the planned subgroup analyses. We planned to perform subgroup analyses according to Cochrane's recommendations (Deeks 2011) for each of the following criteria, and separately for the different study design types included in the review in order to assess the effect on heterogeneity.
Age of participant (child (one to 12 years), adolescent (13 to 17 years) adult (18+ years))
Route of administration of iron chelating agents (oral, intravenous or subcutaneous)
Sensitivity analysis
There were insufficient data to perform the planned sensitivity analyses. If adequate data were available, we planned to assess the robustness of our findings by performing the following sensitivity analyses according to Cochrane recommendations where appropriate (Deeks 2011).
Including only those trials with a 'low' risk of bias (e.g. RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation)
Including only those studies with less than a 20% dropout rate
Duration of follow‐up (up to and including six months compared to over six months)
Summary of findings table
We used the GRADE approach to generate a 'Summary of Findings' table as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a). We used the GRADE approach to rate the quality of the evidence as 'high', 'moderate', 'low', or 'very low' using the five GRADE considerations.
Risk of bias (serious or very serious)
Inconsistency (serious or very serious)
Indirectness (serious or very serious)
Imprecision (serious or very serious)
Publication bias (likely or very likely)
For NRSIs or CBA or ITS studies, we planned to consider the following factors.
Dose response (yes or no)
Size of effect (large or very large)
Confounding either reduces the demonstrated effect or increases the effect if no effect was observed (yes or no)
In GRADE NRSIs or CBA or ITS studies are rated initially as low quality and upgraded according to GRADE guidelines if appropriate. We planned to present outcomes for these studies in separate tables from outcomes for the results of RCTs.
We reported the following outcomes in each 'Summary of findings' table.
Adherence rates (minimum of three months)
Serious adverse events (most common time frame used in most studies)
All‐cause mortality (most common time frame used in most studies)
Sustained adherence (six months or more)
QoL (most common time frame used in most studies)
Results
Description of studies
See also Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
See PRISMA flow diagram (Figure 1).
1.
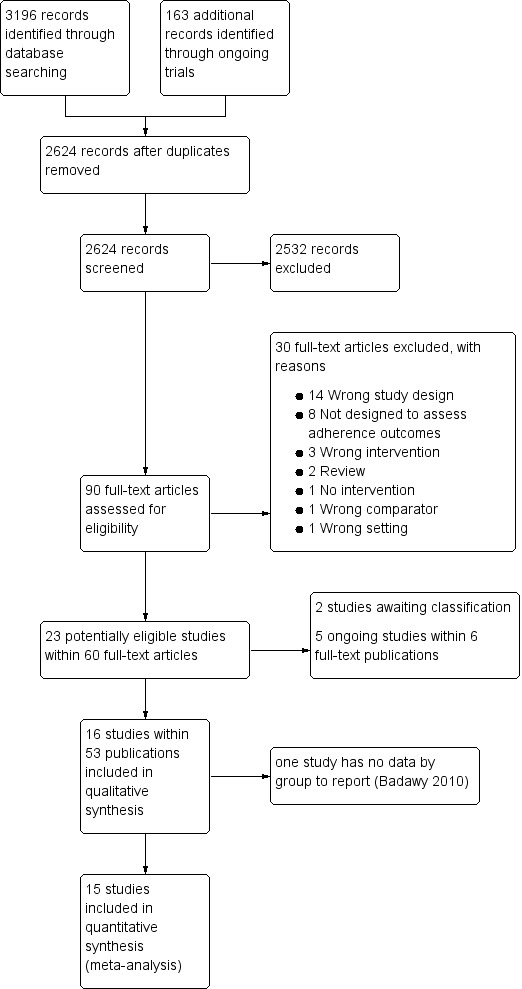
Study flow diagram.
In the searches for this review we identified a total of 3359 potentially relevant references. There were 2624 references after we removed duplicates and three review authors (PF, KM and LE) excluded 2533 references on the basis of abstract and three authors (PF, KM, LE) reviewed 90 full‐text articles for relevance.
We excluded 30 studies that were not relevant and identified 16 studies within 53 publications ‐ all were RCTs (Aydinok 2007; Badawy 2010; Bahnasawy 2017; Calvaruso 2015; El Beshlawy 2008; Galanello 2006; Hassan 2016; Maggio 2009; Mourad 2003; Olivieri 1997; Pennell 2006; Pennell 2014; Taher 2017; Tanner 2007; Vichinsky 2007).
We also identified five ongoing RCTs ( IRCT2015101218603N2; EudraCT 2012‐000353‐31; Madderom 2016; NCT02173951; NCT02435212), and two studies awaiting classification (Antmen 2013; NCT00004982). We did not identify any cluster‐randomised trials, NRSIs, CBA or ITS studies that met the inclusion criteria.
Included studies
Sixteen RCTs including 1525 participants met the pre‐defined inclusion criteria (Aydinok 2007; Badawy 2010; Bahnasawy 2017; Calvaruso 2015; Elalfy 2015; El Beshlawy 2008; Galanello 2006; Hassan 2016; Maggio 2009; Mourad 2003; Olivieri 1997; Pennell 2006; Pennell 2014; Taher 2017; Tanner 2007; Vichinsky 2007).
Two of the included trials were abstract reports only (Badawy 2010; Olivieri 1997). One abstract did not report outcomes by intervention and therefore is not included in the quantitative reporting of the effects of interventions (Badawy 2010).
Trial design
There were 15 RCTs of medication interventions (Aydinok 2007; Badawy 2010; Calvaruso 2015; Elalfy 2015; El Beshlawy 2008; Galanello 2006; Hassan 2016; Maggio 2009; Mourad 2003; Olivieri 1997; Pennell 2006; Pennell 2014; Taher 2017; Tanner 2007; Vichinsky 2007); while one was an RCT on medication management (Bahnasawy 2017).
Ten were multicentre trials (Calvaruso 2015; Elalfy 2015; Galanello 2006; Maggio 2009; Olivieri 1997; Pennell 2006; Pennell 2014; Taher 2017; Tanner 2007; Vichinsky 2007) and ranged from two centres in one country (Calvaruso 2015; Elalfy 2015; Olivieri 1997) to 44 centres in multiple countries (Vichinsky 2007). Six were single‐centre trials (Aydinok 2007; Bahnasawy 2017; Badawy 2010; El Beshlawy 2008; Hassan 2016, Mourad 2003).
Follow‐up ranged from six months in two trials (Bahnasawy 2017; Taher 2017) to five years in two trials (Calvaruso 2015; Maggio 2009). The remainder of the trials were of 12 months duration, except in the Badawy trial, which did not report follow‐up time (Badawy 2010); and the Olivieri trial, which had 24 months follow‐up (Olivieri 1997).
Trial size
The number of participants enrolled in the trials ranged from 24 (Aydinok 2007) to 213 (Maggio 2009). Sample‐size calculations were reported in eight trials (Calvaruso 2015; Elalfy 2015; El Beshlawy 2008; Maggio 2009; Pennell 2006; Pennell 2014; Tanner 2007; Vichinsky 2007).
Setting
Trials were published between 1997 and 2017. Five were conducted in Egypt (Badawy 2010; Bahnasawy 2017; Elalfy 2015; El Beshlawy 2008, Hassan 2016); five in Italy (Calvaruso 2015; Galanello 2006; Maggio 2009; Pennell 2006; Tanner 2007); and three were international multicentre trials conducted in several countries (Pennell 2014; Taher 2017; Vichinsky 2007). One trial was conducted in each of the following countries: Turkey (Aydinok 2007); Lebanon (Mourad 2003); and Canada (Olivieri 1997).
Participants
Fourteen trials included only participants with β‐thalassaemia major (Aydinok 2007; Badawy 2010; Bahnasawy 2017; Elalfy 2015; El Beshlawy 2008; Galanello 2006; Hassan 2016; Maggio 2009; Mourad 2003; Olivieri 1997; Pennell 2006; Pennell 2014; Taher 2017; Tanner 2007). One trial included only participants with SCD (Vichinsky 2007); and one trial included only participants with thalassaemia intermedia (Calvaruso 2015).
The mean age ranged from 11 years (El Beshlawy 2008) to 41 years (Calvaruso 2015). Two trials only provided the minimum age of enrolment into the RCT, at least eight years old in the Badawy trial (Badawy 2010); and at least 10 years old in the Olivieri trial (Olivieri 1997).
Participants tended to be equally divided between males and females with the lowest percentage of males at 38% (Bahnasawy 2017) to a high of 66% (Elalfy 2015).
Intervention
In this review we report the Effects of interventions by the various comparisons in the different trials. All trials included medication interventions except for one, which was a medication management intervention by a clinical pharmacist (Bahnasawy 2017).
The comparisons and studies included:
DFP versus DFO: five trials (Badawy 2010; Calvaruso 2015; El Beshlawy 2008; Olivieri 1997; Pennell 2006);
DFX versus DFO: three trials (Hassan 2016; Pennell 2014; Vichinsky 2007);
DFX (film‐coated tablet (FCT) versus DFX (dispersible tablet (DT)): one trial (Taher 2017);
DFP and DFO combined versus DFP alone: four trials (Aydinok 2007; Badawy 2010; El Beshlawy 2008; Maggio 2009);
DFP and DFO combined versus DFO alone: five trials (Badawy 2010; El Beshlawy 2008; Galanello 2006; Mourad 2003; Tanner 2007);
DFP and DFO combined versus DFP and DFX combined: one trial (Elalfy 2015);
Medication management versus standard care: one trial (Bahnasawy 2017).
Outcomes
Outcomes varied across trials depending on the objectives. All trials measured adherence, although this was usually as a secondary, rather than a primary outcome. Reduction in serum ferritin or LIC were the primary outcomes in most trials; however, in three trials the primary outcome was myocardial T2* MRI results (Pennell 2006; Pennell 2014; Tanner 2007) and in one trial was overall safety (Taher 2017). Safety (including both SAEs and AEs) was included as a secondary outcome in all trials. QoL was reported in three trials (Aydinok 2007; Bahnasawy 2017; Elalfy 2015).
Source
Four trials identified non‐profit organisations as their source of support, including universities, foundations and societies (Badawy 2010; Calvaruso 2015; Elalfy 2015; Maggio 2009).
Five trials identified industry sponsorships (Galanello 2006; Pennell 2006; Pennell 2014; Taher 2017; Vichinsky 2007). Six trials did not state their source of funding (Aydinok 2007; Bahnasawy 2017; El Beshlawy 2008; Hassan 2016; Mourad 2003; Olivieri 1997); but of these, three may have had industry funding. In one trial, drugs were supplied by the manufacturer (Aydinok 2007); one trial was halted by the manufacturer (Olivieri 1997); and one trial included industry employees as authors (El Beshlawy 2008).
One trial had a mix of non‐profit and industry funding (Tanner 2007).
Excluded studies
We excluded 30 trials:
in 14 trials the trial design did not meet the inclusion criteria (Abu 2015; Al Kloub 2014; Al Kloub 2014a; Al Refaie 1995; Alvarez 2009; Kidson Gerber 2008; Kolnagou 2008; Leonard 2014; NCT02133560; NCT02466555; Pakbaz 2004; Pakbaz 2005; Porter 2009; Porter 2012);
eight trials were not designed to assess adherence (Berkovitch 1995; Chakrabarti 2013; NCT01709032; NCT01825512; Vichinsky 2005; Vichinsky 2008; Waheed 2014; Yarali 2006);
three trials assessed the wrong intervention (Armstrong 2011, Belgrave 1989; Gomber 2004);
one trial had no interventions (Bala 2014);
one trial had a wrong comparator (Mazzone 2009);
one trial was in the wrong setting (Daar 2010);
two were reviews (Loiselle 2016; Walsh 2014).
Risk of bias in included studies
Refer to the figures section of the review for visual representations of the assessments of risk of bias across all trials and for each item in the included trials (Figure 2; Figure 3). See the risk of bias section in the Characteristics of included studies section for further information about the bias identified within individual trials.
2.
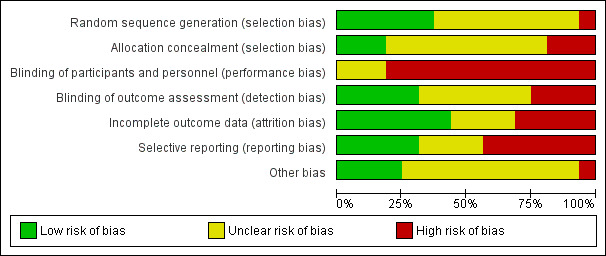
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
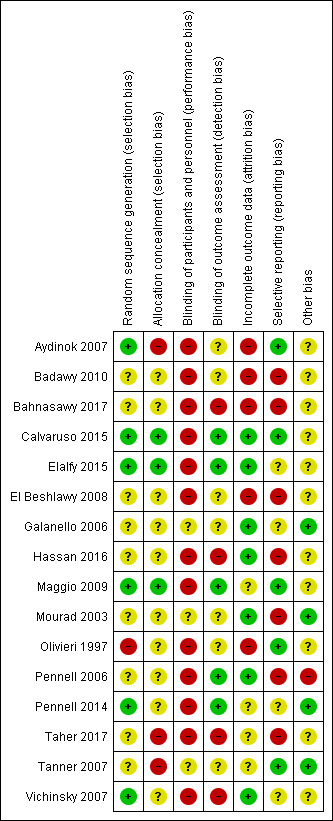
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered six trials to be at a low risk of bias for random sequence generation as randomisation was clearly described and done centrally, in permuted blocks, or computer‐generated (Aydinok 2007; Calvaruso 2015; Elalfy 2015; Maggio 2009; Pennell 2014; Vichinsky 2007).
We considered nine trials to be at an unclear risk of bias. Although one trial used permuted blocks there were several imbalances in baseline characteristics between groups (Hassan 2016). We judged the remaining eight trials to have an unclear risk of bias as there was no description of randomisation and the report only stated that participants were randomised (Badawy 2010; Bahnasawy 2017; El Beshlawy 2008; Galanello 2006; Mourad 2003; Pennell 2006; Taher 2017; Tanner 2007).
We considered one trial to be at a high risk of bias as participants were "assigned" to treatment groups by a research pharmacist and there was no description of how it was done (Olivieri 1997).
Allocation concealment (selection bias)
We considered three trials to be at low risk for selection bias as participants were allocated by telephone contact from a co‐ordinating centre (Calvaruso 2015; Elalfy 2015; Maggio 2009).
We considered 10 trials to be at an unclear risk as there was no description of how allocation was concealed (Badawy 2010; Bahnasawy 2017; El Beshlawy 2008; Galanello 2006; Hassan 2016; Mourad 2003; Olivieri 1997; Pennell 2006: Pennell 2014; Vichinsky 2007).
We considered three trials to be at a high risk for selection bias as there was no allocation concealment (Aydinok 2007; Taher 2017; Tanner 2007).
Blinding
Blinding of participants and personnel (performance bias)
We considered three trials to be at an unclear risk for performance bias as there was no description of blinding (Galanello 2006; Mourad 2003; Tanner 2007).
We considered 13 trials to be at a high risk for performance bias. Trials were either open label, did not mention blinding, or blinding was difficult due to type of treatment: a subcutaneous injection compared to an oral intervention or combination of both (Aydinok 2007; Badawy 2010; Bahnasawy 2017; El Beshlawy 2008; Calvaruso 2015; Elalfy 2015; Hassan 2016; Maggio 2009; Olivieri 1997; Pennell 2006; Pennell 2014; Taher 2017; Vichinsky 2007).
Blinding of outcome assessment (detection bias)
We considered five trials to be at a low risk of detection bias for all outcomes as data management and analysis were carried out by assessors who were blinded to interventions (Calvaruso 2015; Elalfy 2015; Maggio 2009; Pennell 2006; Pennell 2014).
We considered seven trials to be at an unclear risk of detection bias for all outcomes except mortality as there was no mention of blinding (Aydinok 2007; Badawy 2010; El Beshlawy 2008; Galanello 2006; Mourad 2003; Olivieri 1997; Tanner 2007).
We considered four trials to be at a high risk of detection bias as there was no description of blinding of outcome assessment and it appears that investigators who were not blinded were also involved in outcome assessment (Bahnasawy 2017; Hassan 2016; Taher 2017; Vichinsky 2007).
Incomplete outcome data
We considered seven trials to be at a low risk for attrition bias as all outcomes were reported and either no participants or few participants were lost to follow‐up and flow of participants was reported (Calvaruso 2015; Elalfy 2015; Galanello 2006; Hassan 2016; Mourad 2003; Pennell 2006; Vichinsky 2007).
We considered four trials to be at an unclear risk of attrition bias as there was no indication of the number of participants included in the different outcome analyses; there was substantial attrition towards the end of the trial, a per protocol analysis was conducted for some outcomes; or there was high attrition or vague reporting with no specific results (Maggio 2009; Pennell 2014; Taher 2017; Tanner 2007).
We considered the rest of the trials to be at a high risk for attrition bias as there was no data on the flow and number of participants completing the trial; no participant numbers on adverse events or compliance; no comparative data reported; per protocol analysis only; or large attrition bias in outcome analysis (Aydinok 2007; Badawy 2010; Bahnasawy 2017; El Beshlawy 2008; Olivieri 1997).
Selective reporting
We considered five trials to be at a low risk of reporting bias as all identified outcomes were reported (Aydinok 2007; Calvaruso 2015; Maggio 2009; Olivieri 1997; Tanner 2007).
We considered four trials to be at an unclear risk of reporting bias because of either: minimal reporting of participant satisfaction and compliance; or no report of compliance with DFP; or unclear and selective reporting of adverse events (Elalfy 2015; Galanello 2006; Pennell 2014; Vichinsky 2007).
We considered seven trials to be at a high risk of reporting bias due to: the incomplete reporting of adverse events or a lack of reporting of adverse events by treatment groups; or a lack of detailed or incomplete reporting of compliance and serum ferritin and LIC; or non‐reporting of some pre‐specified outcomes (Badawy 2010, Bahnasawy 2017; El Beshlawy 2008; Hassan 2016, Mourad 2003; Pennell 2006; Taher 2017).
Other potential sources of bias
We considered four trials to be at a low risk as no other potential sources of bias were identified (Galanello 2006; Mourad 2003; Pennell 2014; Tanner 2007).
We considered 11 trials to be at an unclear risk of other bias for various reasons including: baseline imbalances; abstract reports with insufficient details; no comparative numbers in control group; incomplete reporting of AEs; dose amendments after the start of the trial (Aydinok 2007; Badawy 2010; Bahnasawy 2017; Calvaruso 2015; Elalfy 2015; El Beshlawy 2008; Hassan 2016; Maggio 2009; Olivieri 1997; Taher 2017; Vichinsky 2007).
We considered one trial to be at a high risk of other sources of bias due to a serious imbalance in baseline characteristics of participants, particularly serum ferritin levels (Pennell 2006).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. DFP compared to DFO for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.
| DFP compared to DFO for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia | ||||||
| Patient or population: improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia Setting: outpatients Intervention: DFP Comparison: DFO | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with DFO | Risk with DFP | |||||
| Adherence to iron chelation therapy (per cent, SD) | ‐ | 242 (4 RCTs) |
⊕⊝⊝⊝ VERY LOW 1 2 | We found considerable heterogeneity and identified age as possible cause: 1 trial in children 10 years or older and 1 conducted in participants 18 or older | ||
| SAEs (from therapy, disease, non‐adherence) Agranulocytosis** | Study population | RR 7.88 (99% CI 0.18 to 352.39) | 88 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 4 | No SAEs were reported in the second trial reporting this outcome | |
| 15 per 1000 | 118 per 1,000 (7 to 1000) |
|||||
| All‐cause mortality | Study population | RR 0.44 (95% CI 0.12 to 1.63) | 88 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 4 | No deaths occurred in the second trial reporting this outcome | |
| 146 per 1000 | 64 per 1000 (18 to 239) | |||||
| Sustained adherence ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Sustained adherence is reported as adherence as all trials were longer than 6 months and only end of trial adherence numbers were provided |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFO: deferoxamine; DFP: deferiprone; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by 1 for risk of bias due to high or uncertain risk of bias due to lack of blinding of participants and personnel in all four RCTs, as well as selection bias (Olivieri 1997), attrition bias (El Beshlawy 2008; Olivieri 1997), reporting bias (El Beshlawy 2008; Pennell 2006), and other bias (Pennell 2006). 2 We downgraded the quality of evidence by 2 for inconsistency due to considerable heterogeneity in comparison. 3 We downgraded the quality of evidence by 2 for imprecision due to very wide CIs that included clinically important benefits and harms. 4 We downgraded the quality of evidence by 1 for indirectness as the trial was conducted in participants with thalassaemia intermedia only; a milder form of thalassaemia
** Risk estimate based on: Tricta F, Uetrecht J, Galanello R, et al. Deferiprone‐induced agranulocytosis: 20 years of clinical observations. American Journal of Hematology. 2016;91(10):1026‐1031. doi:10.1002/ajh.24479.
Summary of findings 2. DFX compared to DFO for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.
| DFX compared to DFO for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia | ||||||
| Patient or population: improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia Setting: outpatients Intervention: DFX Comparison: DFO | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with DFO | Risk with DFX | |||||
| Adherence to iron chelation therapy (per cent, SD) | The mean adherence to iron chelation therapy (per cent, SD) was 0 | MD 1.4 lower (3.66 lower to 0.86 higher) | ‐ | 197 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Narrative report of adherence for 2 trials as either no or incompatible data to enable comparisons |
| SAEs ‐ thalassaemia‐related SAEs | Study population | ‐ | 247 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | There were no SAEs to report in one trial so no estimate of effect | |
| see comment | see comment | |||||
| SAEs ‐ SCD‐related SAEs | Study population | RR 1.08 (95% CI 0.77 to 1.51) | 195 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 429 per 1000 | 463 per 1000 (330 to 647) | |||||
| Incidence of SCD‐related SAEs ‐pain crisis | Study population | RR 1.05 (95% CI 0.68 to 1.62) | 195 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 317 per 1000 | 333 per 1000 (216 to 514) | |||||
| All‐cause mortality (thalassaemia) | Study population | RR 0.96 (95%CI 0.06 to 15.06) | 240 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 8 per 1000 | 8 per 1000 (1 to 128) | |||||
| Sustained adherence ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Sustained adherence is reported as adherence as all trials were longer than 6 months and only end of trial adherence reported |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFO: deferoxamine; DFX: deferasirox; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by 2 due to high or uncertain risk of bias in several domains 2 We downgraded the quality of evidence by 1 due to imprecision as CIs are wide and only 1 trial with data in comparison
Summary of findings 3. DFX film‐coated tablet compared to DFX dispersible tablet for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.
| DFX film‐coated tablet compared to DFX dispersible tablet for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia | ||||||
| Patient or population: improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia Setting: outpatients Intervention: DFX film‐coated tablet Comparison: DFX dispersible tablet | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with DFX dispersible tablet | Risk with DFX film‐coated tablet | |||||
| Adherence to iron chelation therapy (n, N) | Study population | RR 1.10 (95% CI 0.99 to 1.22) | 173 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 849 per 1000 | 934 per 1000 (840 to 1000) | |||||
| Incidence of SAEs | Study population | RR 1.22 (95% CI 0.62 to 2.37) | 173 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 151 per 1,000 | 184 per 1000 (94 to 358) | |||||
| All‐cause mortality | Study population | RR 2.97 (95% CI 0.12 to 71.81) | 173 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Sustained adherence ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Reported as adherence as trial was 6 months in duration and end of trial adherence reported |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFX: deferasirox; RCT: randomised controlled trial; RR: risk ratio; SAEs: serious adverse events | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by 2 for risk of bias due to high or unclear risk of bias in all domains 2 We downgraded the quality of evidence by 1 for imprecision due to wide CIs
Summary of findings 4. DFP and DFO compared to DFP for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.
| DFP and DFO compared to DFP for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia | ||||||
| Patient or population: improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia Setting: outpatients Intervention: DFP and DFO Comparison: DFP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with DFP | Risk with DFP and DFO | |||||
| Adherence to iron chelation therapy (per cent, SD) | see comment | see comment | ‐ | 289 (3 RCTs) |
⊕⊝⊝⊝ VERY LOW 1 2 | Reported as narrative as no comparisons possible |
| Incidence of SAEs | Study population | RR 0.15 (95% CI 0.01 to 2.81) | 213 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 | ||
| 28 per 1,000 | 4 per 1,000 (0 to 78) | |||||
| All‐cause mortality | Study population | RR 0.77 (95% CI 0.18 to 3.35) | 237 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | ||
| 33 per 1,000 | 26 per 1,000 (6 to 112) | |||||
| Sustained adherence ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Sustained adherence is reported as adherence as trial duration longer than 6 months and reports adherence for length of trial |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Quality of life was either not reported or no validated instruments were used |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFO: deferoxamine DFP: deferiprone; RCT: randomised controlled trial; RR: risk ratio; SAEs: serious adverse events; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by 2 for risk of bias as there was high or uncertain risk of bias in most domains in 3 out of 4 trials 2 We downgraded the quality of evidence by 1 due to high or unclear risk of bias in 3 domains 3 We downgraded the quality of evidence by 1 for imprecision due to wide CIs 4 We downgraded the quality of evidence by 2 for risk of bias as there was high or uncertain risk of bias in 1 of the trials in the comparison
Summary of findings 5. DFP and DFO compared to DFO for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.
| DFP and DFO compared to DFO for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia | ||||||
| Patient or population: improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia Setting: outpatients Intervention: DFP and DFO Comparison: DFO | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with DFO | Risk with DFP and DFO | |||||
| Adherence to iron chelation therapy (per cent, SD) | see comment | see comment | ‐ | 205 (4 RCTs) |
⊕⊕⊝⊝ LOW 1 | Reported as narrative only as adherence in combined group not reported for combination therapy |
| Incidence of SAEs | Study population | ‐ | 205 (4 RCTs) |
⊕⊕⊝⊝ LOW 1 | 3 trials report no SAEs; SAES are not reported in one trial | |
| see comment | see comment | |||||
| All‐cause mortality | Study population | ‐ | 205 (4 RCTs) |
⊕⊕⊝⊝ LOW 1 | no deaths reported | |
| see comment | see comment | |||||
| Sustained adherence ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Sustained adherence reported as adherence as trial duration was longer than 6 months and adherence reported at end of trial |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). DFO: deferoxamine; DFP: deferiprone; SAEs: serious adverse events. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by 2 for risk of bias as high or unclear risk of bias in all domains
Summary of findings 6. DFP and DFO compared to DFP and DFX for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.
| DFP/DFO compared to DFP/DFX for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia | ||||||
| Patient or population: improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia Setting: outpatients Intervention: DFP/DFO Comparison: DFP/DFX | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with DFP/DFX | Risk with DFP/DFO | |||||
| Adherence to iron chelation therapy rates (n,N) ‐ 1 year | Study population | RR 0.84 (95% CI 0.72 to 0.99) | 96 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 938 per 1000 | 788 per 1000 (675 to 928) | |||||
| Incidence of SAE | Study population | RR 1.00 (95% CI 0.06 to 15.53) | 96 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 2 3 | ||
| 21 per 1,000 | 21 per 1000 (1 to 324) | |||||
| All‐cause mortality ‐ at 1 year ‐ trial end | Study population | Not estimable | 96 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | No deaths were reported | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Sustained adherence ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Sustained adherence is reported as adherence as trial was 1 year in duration and end of trial adherence reported |
| Quality of life see comment | ‐ | ‐ | ‐ | ‐ | ‐ | The study uses SF36 to measure quality of life, the results are presented as a graph. Quality of life increased in both trial arms with no significant difference between trial arms P = 0.860 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFO: deferoxamine; DFP: deferiprone; DFX: deferasirox; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by 1 for risk of bias as there was high or unclear risk of bias in 3 domains 2 We downgraded the quality of evidence by 1 for indirectness as the trial included children 10 ‐ 18 with severe iron overload 3 We downgraded the quality of evidence by 1 for imprecision as the comparison has wide CIs
Summary of findings 7. Medication management compared to standard care for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.
| Medication management compared to standard care for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia | ||||||
| Patient or population: improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia Setting: outpatient Intervention: medication management Comparison: standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with medication management | |||||
| Adherence to iron chelation therapy ‐ not reported | ‐ | ‐ | ‐ | Adherence was only reported in the intervention group and therefore no comparative data | ||
| SAEs ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Sustained adherence | ‐ | ‐ | ‐ | ‐ | ‐ | Adherence was only reported in the intervention group and therefore no comparative data |
| Quality of life assessed with: PedsQLTM HRQoL total score | ‐ | 48 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Medication management: 63.51 (51.75 – 84.54); standard care: 49.84 (41.9 – 60.81) | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). RCT: randomised controlled trial; SAEs: serious adverse events. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence for indirectness by 2 because most outcomes were only reported in the medication management group 2 We downgraded the quality of evidence by 2 for risk of bias due to high or uncertain risk of bias in all domains
Results are presented for each of the main comparisons.
The main focus of our review is on compliance and effects of compliance (or non‐compliance) on participant outcomes. For more detailed estimates of effectiveness of different iron chelators please refer to another Cochrane Review (Fisher 2013).
One abstract of a trial that included three review comparisons (DFP versus DFO; combination DFP and DFO versus DFP; combination DFP and DFO versus DFO) did not report any outcomes by intervention group and did not include counts of events (i.e. adverse events); therefore we did not include this trial in the quantitative analysis (Badawy 2010). Thus we have included 15 trials within the quantitative analysis.
See Table 8 and also the outcomes section in the Characteristics of included studies section for summary information on results and how adherence was measured in the individual trials. Adherence rates were mostly measured by pill or vial count (either automated or manual).
1. Adherence Measurement and Results Table.
| STUDY | HOW ADHERENCE MEASURED | RESULTS |
| Aydinok 2007 |
|
|
| Badawy 2010 |
|
|
| Bahnasawy 2017 |
|
|
| Calvaruso 2015 |
|
|
| El Beshlawy 2008 |
|
|
| Elalfy 2015 |
|
|
| Galanello 2006 |
|
|
| Hassan 2016 |
|
|
| Maggio 2009 |
|
|
| Mourad 2003 |
|
|
| Olivieri 1997 |
|
|
| Pennell 2006 |
|
|
| Pennell 2014 |
|
|
| Taher 2017 |
|
|
| Tanner 2007 |
|
|
| Vichinsky 2007 |
|
|
DFO: deferoxamine DFP: deferiprone DFX: deferasirox DT: dispersible tablet FCT: film‐coated tablet SD: standard deviation SF: serum ferritin
The quality of the evidence has been graded for those outcomes included in the summary of findings table. For the definitions of these gradings, please refer to the summary of findings tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7).
DFP (deferiprone) alone versus DFO (deferoxamine) alone
Four trials of thalassaemia met the inclusion criteria for this comparison (Calvaruso 2015; El Beshlawy 2008; Olivieri 1997; Pennell 2006). See Table 1.
Primary outcomes
1. Adherence to iron chelation therapy rates
We are uncertain whether oral DFP increases adherence to iron chelation therapy more than subcutaneous DFO (very low‐quality evidence). Results could not be combined due to both a lack of data to report as well as considerable heterogeneity between comparisons (I² = 99%) (Analysis 1.1). We identified the age of participants and differences in the medication regimens as possible explanations for heterogeneity. We provide a narrative review of the data on compliance below.
1.1. Analysis.
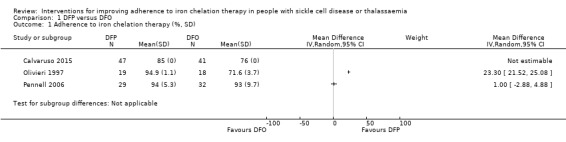
Comparison 1 DFP versus DFO, Outcome 1 Adherence to iron chelation therapy (%, SD).
Calvaruso 2015: compliance with DFP: 85% (47 participants) versus compliance with DFO: 76% (41 participants).
El Beshlawy 2008: "four patients, all treated with DFO‐based regimen, were excluded from the study due to lack of compliance. Compliance was otherwise excellent during the entire study period".
Olivieri 1997: compliance with DFP: 94.9% ± 1.1% (19 participants) versus compliance with DFO: 71.6% ± 3.9% (18 participants).
Pennell 2006: compliance with DFP: 94% ± 5.3% (29 participants) versus compliance with DFO: 93% ± 9.7% (32 participants).
2. Serious adverse events (SAEs)
Two trials reported this outcome (Calvaruso 2015; Pennell 2006). One trial reported on the risk of developing agranulocytosis: we are uncertain if switching to oral DFP increases the risk of agranulocytosis compared to subcutaneous DFO, RR 7.88 (95% CI 0.18 to 352.39) (one trial; 88 participants; very low‐quality evidence) (Calvaruso 2015) (Analysis 1.2). No SAEs occurred in the second trial (Pennell 2006).
1.2. Analysis.
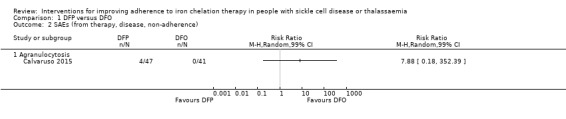
Comparison 1 DFP versus DFO, Outcome 2 SAEs (from therapy, disease, non‐adherence).
3. All‐cause mortality
Two trials reported this outcome (Calvaruso 2015; Pennell 2006). Oral DFP may have little or no difference on mortality compared to subcutaneous DFO, RR 0.44 (95% CI 0.12 to 1.63) (88 participants; one trial; low‐quality evidence) (Calvaruso 2015) (Analysis 1.3). No deaths occurred in the second trial (Pennell 2006).
1.3. Analysis.
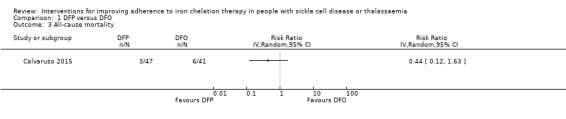
Comparison 1 DFP versus DFO, Outcome 3 All‐cause mortality.
Secondary outcomes
1. Sustained adherence to therapy (measured for a minimum of six months)
All trials reported more than six months follow‐up, sustained adherence is reported in the primary outcome (adherence to iron chelation therapy rates), as only end‐of‐trial adherence numbers were provided.
2. Health‐related quality of life (QoL)
No trials measured QoL.
3. Iron overload
One trial reported the proportion of participants with iron overload (Calvaruso 2015). We are uncertain if DFP reduces iron overload compared to DFO: iron levels greater or equal to 800 (µg/L), RR 1.31 (95% CI 0.49 to 3.48) (one trial; 38 participants; very low quality evidence) (Analysis 1.4).
1.4. Analysis.
Comparison 1 DFP versus DFO, Outcome 4 Iron overload: defined as proportion of participants with serum ferritin ≥ 800 (µg/L).
4. Organ damage
One trial reported the proportion of participants with organ damage (Calvaruso 2015). We are uncertain if DFP increases the risk of liver damage compared to DFO, RR 4.36 (95% CI 0.53 to 35.82) (one trial; 88 participants; very low‐quality evidence) (Analysis 1.5).
1.5. Analysis.
Comparison 1 DFP versus DFO, Outcome 5 Organ damage.
5. Other adverse events (AEs) related to iron chelation
Three trials reported this outcome (Calvaruso 2015; El Beshlawy 2008; Pennell 2006). In people with thalassaemia taking DFP, we are uncertain if there is a difference in the risk of AEs compared to people taking DFO (Analysis 1.6).
1.6. Analysis.
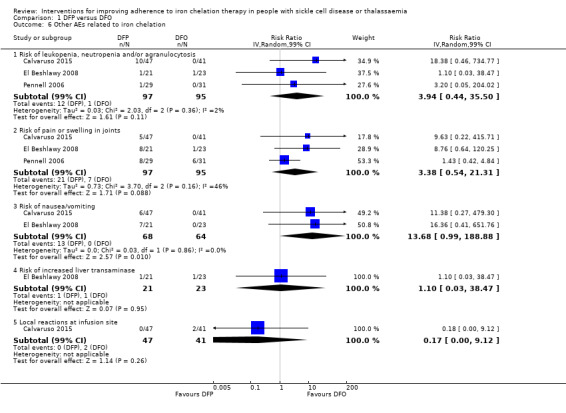
Comparison 1 DFP versus DFO, Outcome 6 Other AEs related to iron chelation.
Risk of leukopenia: RR 3.94 (99% CI 0.44 to 35.50) (three trials; 192 participants; very low‐quality evidence) (Calvaruso 2015; El Beshlawy 2008; Pennell 2006).
Risk of pain or swelling in joints: RR 3.38 (99% CI 0.54 to 21.31) (three trials; 192 participants; very low‐quality evidence) (Calvaruso 2015; El Beshlawy 2008; Pennell 2006).
Risk of nausea or vomiting: RR 13.68 (99% CI 0.99 to 188.88) (two trials; 132 participants; very low‐quality evidence) (Calvaruso 2015; El Beshlawy 2008).
Risk of increased liver transaminase: RR 1.10 (99% CI 0.03 to 38.47) (one trial; 44 participants; very low‐quality evidence) (El Beshlawy 2008).
Local reactions at infusions site: RR 0.17 (99% CI 0.00 to 9.12) (one trial; 88 participants; very low‐quality evidence) (Calvaruso 2015).
In all trials we downgraded the quality of evidence by one for risk of bias (due to high or unclear risk of bias in several domains) and in one trial we downgraded by two due to imprecision, the effect estimates have wide CIs (Calvaruso 2015).
DFX (deferasirox) alone versus DFO (deferoxamine) alone
Three trials met the inclusion criteria for this comparison; two in thalassaemia (Hassan 2016; Pennell 2014); and one in SCD (Vichinsky 2007). See Table 2.
Primary outcomes
1. Adherence to iron chelation therapy rates
All three trials reported on this outcome. Only one trial reported data in a format that could be incorporated into the analysis (Pennell 2014). We are uncertain if DFX increases the rate of adherence compared to people taking DFO, MD ‐1.40 (95% CI ‐3.66 to 0.86) (one trial; 197 participants; very‐low quality evidence) (Analysis 2.1).
2.1. Analysis.
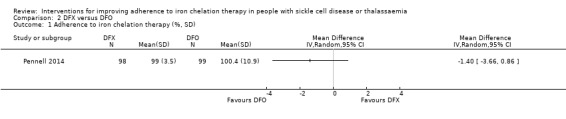
Comparison 2 DFX versus DFO, Outcome 1 Adherence to iron chelation therapy (%, SD).
Regarding the remaining two trials:
Hassan 2016 stated that "throughout the study, all patients were compliant with the prescribed doses, and no discontinuation of drugs or drop‐out of follow‐up occurred."
Vichinsky 2007 reported that "the ratios of the administered to intended doses of therapy were high (1.16 for deferasirox and 0.97 for deferoxamine), indicating high adherence to the prescribed treatment regimens."
2. Serious adverse events (SAEs)
All three trials reported the effect on disease‐related SAEs (Hassan 2016; Pennell 2014; Vichinsky 2007); two in thalassaemia (Hassan 2016; Pennell 2014), and one in SCD (Vichinsky 2007).
We are uncertain whether DFX decreases risk of disease‐related SAEs in thalassaemia compared to DFO, RR 0.95 (95% CI 0.41 to 2.17) (two trials; 247 participants; very low‐quality evidence) (Analysis 2.2).
2.2. Analysis.
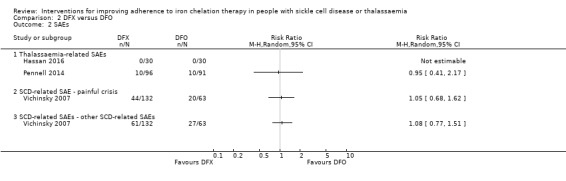
Comparison 2 DFX versus DFO, Outcome 2 SAEs.
We are uncertain whether DFX decreases the risk of SCD‐related pain crisis, RR 1.05 (95% CI 0.68 to 1.62) (one trial; 195 participants; very low‐quality evidence); or other SCD‐related SAEs compared to DFO, RR 1.08 (95% CI 0.77 to 1.51) (one trial; 195 participants; very low‐quality evidence) (Analysis 2.2).
3. All‐cause mortality
Two trials report mortality (Hassan 2016; Pennell 2014). We are uncertain whether DFX decreases the risk of mortality in people with thalassaemia compared to DFO, RR 0.96 (95% CI 0.06 to 15.06) (two trials; 240 participants; very low‐quality evidence) (Analysis 2.3).
2.3. Analysis.
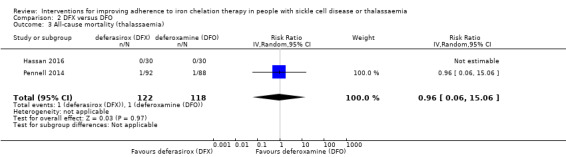
Comparison 2 DFX versus DFO, Outcome 3 All‐cause mortality (thalassaemia).
Secondary outcomes
1. Sustained adherence to therapy (measured for a minimum of six months)
All trials reported more than six months follow‐up, sustained adherence is reported in the primary outcome (adherence to iron chelation therapy rates), as only end‐of‐trial adherence numbers were provided.
2. Health‐related quality of life (QoL)
No trials measured quality of life.
3. Iron overload
In people with thalassaemia, we are uncertain whether DFX reduces the proportion of participants with serum ferritin of 1500 (µg/l) or higher, RR 1.18 (95% CI 0.63 to 2.20) (one trial; 60 participants; very low‐quality evidence) (Hassan 2016) (Analysis 2.4). Furthermore, we are uncertain whether DFX reduces the proportion of participants with severe LIC (15 mg Fe/g dw or higher), RR 1.00 (95% CI 0.83 to 1.20); or myocardial T2* < 10 ms, RR 1.10 (95% CI 0.72 to 1.70) (one trial; 172 participants; very low‐quality evidence)* (Pennell 2014) (Analysis 2.4).
2.4. Analysis.
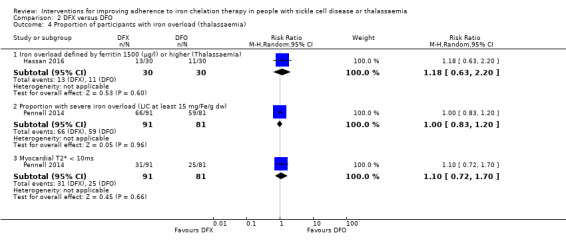
Comparison 2 DFX versus DFO, Outcome 4 Proportion of participants with iron overload (thalassaemia).
*LIC and myocardial T2*analyses from Pennell 2014 were based on the per protocol population.
In people with SCD, Vichinsky reported LIC mean changes from baseline and no data on proportion of participants with end‐of‐trial iron overload (Vichinsky 2007).
4. Organ damage
No trial reported any other organ damage.
5. Other adverse events (AEs) related to iron chelation
In people with thalassaemia taking DFX, we are uncertain if there is a difference in the risk of iron chelation therapy‐related AEs compared to people taking DFO (Analysis 2.5).
2.5. Analysis.
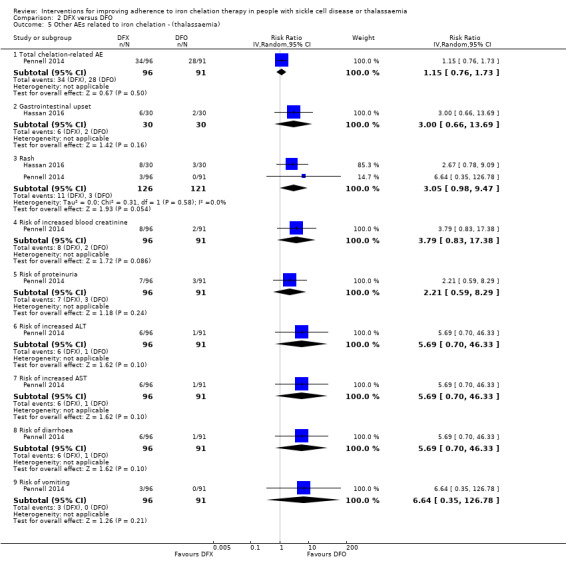
Comparison 2 DFX versus DFO, Outcome 5 Other AEs related to iron chelation ‐ (thalassaemia).
Risk of total iron chelation therapy‐related AE: RR 1.15 (95% CI 0.76 to 1.73); (one trial; 187 participants; very low‐quality evidence) (Pennell 2014).
Risk of gastrointestinal upset: RR 3.00 (95% CI 0.66 to 13.69); (one trial; 60 participants; very low‐quality evidence) (Hassan 2016).
Risk of rash: RR 3.05 (95% CI 0.98 to 9.47); (two trials; 247 participants; very low‐quality evidence) (Hassan 2016; Pennell 2014).
Risk of increased blood creatinine: RR 3.79 (95% CI 0.83 to 17.38); (one trial;187 participants; very low‐quality evidence) (Pennell 2014).
Risk of proteinuria: RR 2.21 (95% CI 0.59 to 8.29); (one trial; 187 participants; very low‐quality evidence) (Pennell 2014).
Risk of increased ALT: RR 5.69 (95% CI 0.70 to 46.33); (one trial; 187 participants; very low‐quality evidence); (Pennell 2014).
Risk of increased AST: RR 5.69 (95% CI 0.70 to 46.33); (one trial; 187 participants; very low‐quality evidence); (Pennell 2014).
Risk of diarrhoea: RR 5.69 (95% CI 0.70 to 46.33); (one trial; 187 participant; very low‐quality evidence); (Pennell 2014).
Risk of vomiting: RR 6.64 (95% CI 0.35 to 126.78); (one trial; 187 participants; very low‐quality evidence); (Pennell 2014).
In people with thalassaemia, we are uncertain whether DFX reduces the incidence of total AEs as compared to DFO, RR 0.89 (95% CI 0.75 to 1.07) (one trial; 187 participants; very low‐quality evidence) (Pennell 2014) (Analysis 2.6). We downgraded the quality of evidence either by two due to high or uncertain risk of bias in several domains, or by one due to imprecision as CIs are wide and only one trial with data in comparison, or both.
2.6. Analysis.
Comparison 2 DFX versus DFO, Outcome 6 Total AEs (thalassaemia).
In people with SCD, DFX compared to DFO, may increase slightly the risk of abdominal pain, RR 1.91(99% CI 0.80 to 4.58); the risk of diarrhoea, RR 4.14 (99% CI 0.90 to 18.92); and the risk of nausea or vomiting, RR 1.63 (99% CI 0.90 to 2.94) (one trial; 195 participants; low‐quality evidence) (Vichinsky 2007) (Analysis 2.7). We are uncertain if DFX compared to DFO increases the risk of an increase in ALT, RR 5.29 (99% CI 0.12 to 232.98) or the risk of pain or swelling in joints, RR 1.06 (99% CI 0.41 to 2.76) (one trial; 195 participants; very low‐quality evidence) (Vichinsky 2007) (Analysis 2.7). We downgraded the quality of evidence either by two due to high or uncertain risk of bias in several domains, or by one due to imprecision as CIs are wide and only one trial with data in comparison, or both.
2.7. Analysis.
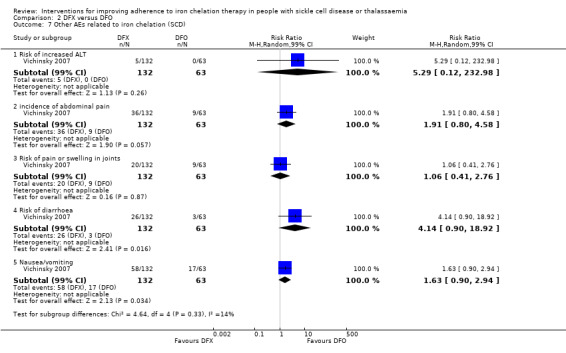
Comparison 2 DFX versus DFO, Outcome 7 Other AEs related to iron chelation (SCD).
DFX film‐coated Tablet (FCT) versus DFX (deferasirox) dispersible tablet (DT)
One trial in thalassaemia met the inclusion criteria for this comparison (Taher 2017). See Table 3.
Primary outcomes
Adherence to iron chelation therapy rates
DFX FCT may have little or no difference on adherence as compared to DFX DT, RR 1.10 (95% CI 0.99 to 1.22) (one trial; 173 participants; low‐quality evidence) (Analysis 3.1).
3.1. Analysis.
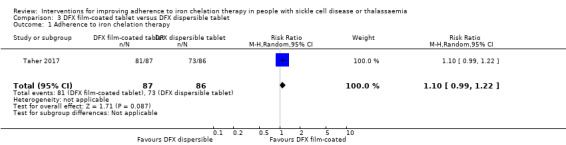
Comparison 3 DFX film‐coated tablet versus DFX dispersible tablet, Outcome 1 Adherence to iron chelation therapy.
Serious adverse events (SAEs)
We are uncertain if DFX FCT increases SAEs as compared to DFX DT, RR 1.22 (95% CI 0.62 to 2.37) (one trial; 173 participants; very low‐quality evidence) (Analysis 3.2).
3.2. Analysis.
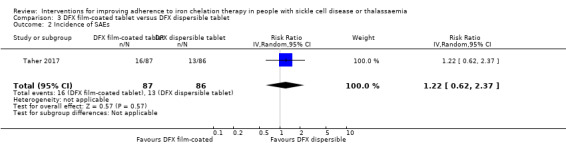
Comparison 3 DFX film‐coated tablet versus DFX dispersible tablet, Outcome 2 Incidence of SAEs.
All‐cause mortality
We are uncertain if DFX FCT increases all‐cause mortality as compared to DFX DT, RR 2.97 (95% CI 0.12 to 71.81) (one trial; 173 participants; very low‐quality evidence) (Analysis 3.3).
3.3. Analysis.
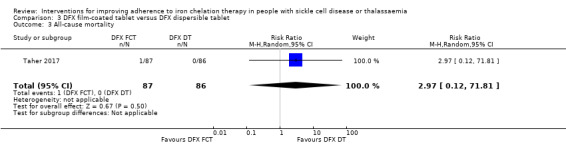
Comparison 3 DFX film‐coated tablet versus DFX dispersible tablet, Outcome 3 All‐cause mortality.
Secondary outcomes
1. Sustained adherence to therapy
This trial reported more than six months follow‐up, sustained adherence is reported in the primary outcome (adherence to iron chelation therapy rates), as only end of trial adherence numbers were provided.
2. Health‐related quality of life (QoL)
This outcome was not measured with a validated instrument.
3. Iron overload
The trial did not report the proportion of participants with iron overload at the end of the trial.
4. Organ damage
We are uncertain if DFX FCT increases the incidence of renal events as compared to DFX DT, RR 1.25 ( 95% CI 0.83 to 1.91) (one trial; 173 participants; very low‐quality evidence) (Analysis 3.4).
3.4. Analysis.
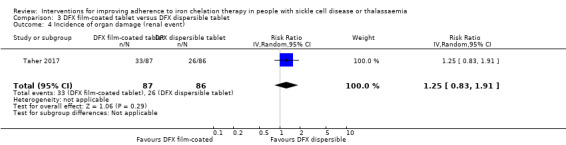
Comparison 3 DFX film‐coated tablet versus DFX dispersible tablet, Outcome 4 Incidence of organ damage (renal event).
5. Other adverse events (AEs) related to iron chelation
DFX FCT, compared to DFX DT, may improve slightly the incidence of all chelation‐related AEs, RR 0.75 (99% CI 0.52 to 1.08); and the incidence of vomiting, RR 0.28 (99% CI 0.07 to 1.15) (one trial; 173 participants; low‐quality evidence) (Analysis 3.5).
3.5. Analysis.
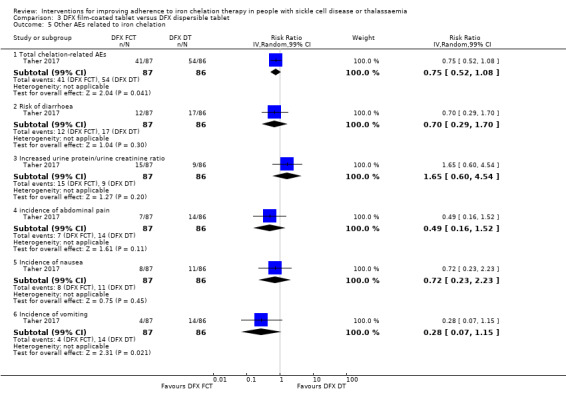
Comparison 3 DFX film‐coated tablet versus DFX dispersible tablet, Outcome 5 Other AEs related to iron chelation.
We are uncertain if DFX FCT, compared to DFX DT, improves: the risk of diarrhoea, RR 0.70 (99% CI 0.29 to 1.70); the incidence of abdominal pain, RR 0.49 (99% CI 0.16 to 1.52); the incidence of nausea, RR 0.72 (99% CI 0.23 to 2.23) or increases urine protein/urine creatinine ratio, RR 1.65 (99% CI 0.60 to 4.54) (one trial; 173 participants; very low‐quality evidence) (Analysis 3.5).
We downgraded the quality of evidence by either two for risk of bias due to high or unclear risk of bias in all domains or by one for imprecision due to wide confidence intervals, or both.
DFP (deferiprone) and DFO (deferoxamine) combination therapy versus DFP (deferiprone) alone
Three trials in thalassaemia met the inclusion criteria for this comparison (Aydinok 2007; El Beshlawy 2008; Maggio 2009). See Table 4.
Primary outcomes
1. Adherence to iron chelation therapy rates
All trials reported on this outcome. We are uncertain if DFP and DFO increases adherence compared to DFP alone (very low‐quality evidence).
Aydinok 2007: "Compliance was generally excellent during the entire study period. There was only one patient in the DFP treatment arm who missed more than one chelation dose per week because of problems with swallowing."
El Beshlawy 2008: "four patients, all treated with DFO‐based regimen, were excluded from the study due to lack of compliance. Compliance was otherwise excellent during the entire study period."
Maggio 2009: "In the sequential DFP–DFO group, compliance was 92.7% (SD ± 15.2%; range 37–100%) with DFP treatment and 70.6% (SD ± 24.1%; range 25–100%) with DFO treatment (105 participants). Compliance with DFP was 93.6% (SD ± 9.7%; range 56–100%) in the DFP‐alone patients (108 participants)."
2. Serious adverse events (SAEs)
Only one trial reported this outcome (Maggio 2009). In people with thalassaemia, combination therapy with DFP and DFO may have little or no difference on the incidence of SAEs as compared to DFP alone, RR 0.15 ( 95% CI 0.01 to 2.81) (one trial; 213 participants; low‐quality evidence) (Maggio 2009) (Analysis 4.1).
4.1. Analysis.
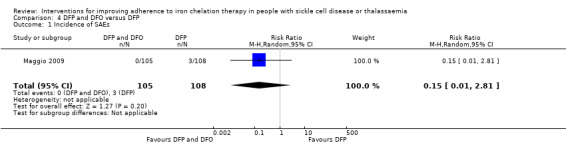
Comparison 4 DFP and DFO versus DFP, Outcome 1 Incidence of SAEs.
3. All‐cause mortality
Two trials reported on this outcome (Aydinok 2007; Maggio 2009). We are uncertain if combination therapy with DFP and DFO decreases mortality as compared to DFP alone, RR 0.77 (95% CI 0.18 to 3.35) (two trials; 237 participants; very low‐quality evidence) (Analysis 4.2).
4.2. Analysis.
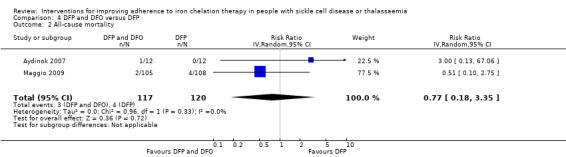
Comparison 4 DFP and DFO versus DFP, Outcome 2 All‐cause mortality.
Secondary outcomes
1. Sustained adherence to therapy
Sustained adherence is reported under the primary outcome (adherence to iron chelation rates), as all trials are longer than six months and end‐of‐trial adherence is reported.
2. Health‐related quality of life (QoL)
One trial assessed QoL, but did not use a validated questionnaire (Aydinok 2007).
3. Iron overload
No trial reported the proportion of participants with iron overload.
4. Organ damage
No trial reported the proportion of participants with organ damage.
5. Other adverse events (AEs) related to iron chelation
All three trials reported AEs. We are uncertain if combination DFP and DFO reduces the incidence of adverse events compared to DFP alone in people with thalassaemia (Analysis 4.3).
4.3. Analysis.
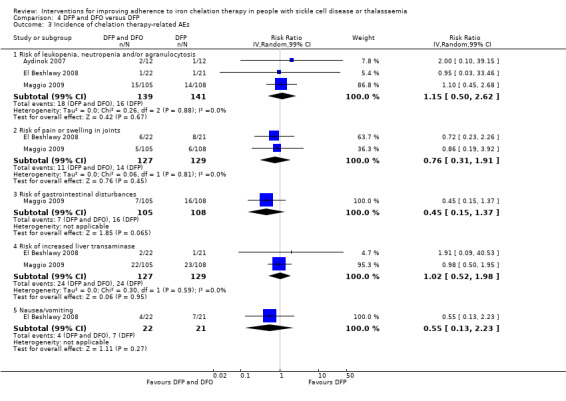
Comparison 4 DFP and DFO versus DFP, Outcome 3 Incidence of chelation therapy‐related AEs.
Risk of leukopenia, neutropenia or agranulocytosis (or a combination of): RR 1.15 (99% CI 0.50 to 2.62) (three trials; 280 participants; very low‐quality evidence) (Aydinok 2007; El Beshlawy 2008; Maggio 2009).
Risk of pain or swelling in joints: RR 0.76 (99% CI 0.31 to 1.91) (two trials; 256 participants; very low‐quality evidence) (El Beshlawy 2008; Maggio 2009).
Risk of increased liver transaminase: RR 1.02 (99% CI 0.52 to 1.98) (two trials; 256 participants; very low‐quality evidence) (El Beshlawy 2008; Maggio 2009).
Risk of nausea or vomiting: RR 0.55 (99% CI 0.13 to 2.23) (one trial; 43 participants; very low‐quality evidence) (El Beshlawy 2008).
One trial reported on this outcome (Maggio 2009). Combination therapy with DFP and DFO may have little or no difference on the risk of gastrointestinal disorders as compare to DFP alone: RR 0.45 (95% CI 0.15 to 1.37) (one trial; 213 participants; low‐quality evidence) (Analysis 4.3).
We downgraded the quality of evidence by either two for risk of bias due to high or unclear risk of bias in several domains in all trials, or by one due to imprecision, the effect estimates have wide confidence intervals, or both.
DFP (deferiprone) and DFO (deferoxamine) combination therapy versus DFO (deferoxamine) alone
Four trials in thalassaemia met the inclusion criteria for this comparison (El Beshlawy 2008; Galanello 2006; Mourad 2003; Tanner 2007). See Table 5.
Primary outcomes
1. Adherence to iron chelation therapy rates
In people with thalassaemia, combined therapy with DFP and DFO versus DFO alone, may have little or no difference in adherence rates (low‐quality evidence). We could not combine any data for an effect estimate.
El Beshlawy 2008: "four patients, all treated with DFO‐based regimen, were excluded from the study due to lack of compliance. Compliance was otherwise excellent during the entire study period".
Galanello 2006: DFP/DFO: DFO: 96.1 ±5.0 (29 participants); DFP compliance was not reported; DFO: 95.7 ± 5.7 (30 participants).
Mourad 2003: "In patients receiving the combined therapy, compliance was excellent (arbitrarily defined as taking > 90% of the recommended doses) in 10 patients and good (75% to 90% of recommended doses) in one patient, as assessed by the patient’s history, parental evidence and usage of tablets provided in just sufficient quantities between check‐up visits. In patients receiving DFX alone, compliance was considered to be excellent in 11 patients and good in three patients, as assessed mainly by counting the vials given to, and returned by, the patients".
Tanner 2007: "Compliance with deferoxamine was similar in both groups (combined 91.4 ± 2.7% versus deferoxamine 92.6 ± 2.7%; P = 0.7). Compliance with deferiprone was less than compliance with placebo (82.4 ± 18.1% versus 89.8 ± 7.2%; P = 0.04)".
2. Serious adverse events (SAEs)
Three trials reported SAEs (Galanello 2006; Mourad 2003; Tanner 2007). In people with thalassaemia, combined therapy with DFP and DFO versus DFO alone, may have little or no difference in SAEs (low‐quality evidence). No SAEs occurred in the three trials.
3. All‐cause mortality
Only one trial reported on this outcome and no deaths occurred (Tanner 2007). Combined therapy with DFP and DFO versus DFO alone, may have little or no difference in morality (one trial; 65 participants; low‐quality evidence).
Secondary outcome
1. Sustained adherence to therapy
All trials reported more than six months follow‐up, sustained adherence is reported in the primary outcome (adherence to iron chelation therapy rates), as only end‐of‐trial adherence numbers were provided.
2. Health‐related quality of life (QoL)
No trials measured QoL.
3. Iron overload
No trials reported the proportion of participants with iron overload.
4. Organ damage
No trials reported the proportion of participants with organ damage.
5. Other adverse events (AEs) related to iron chelation
All four trials reported some AEs. We are uncertain if DFP combined with DFO reduces other chelation‐related AEs compared to DFO alone in people with thalassaemia (Analysis 5.1).
5.1. Analysis.
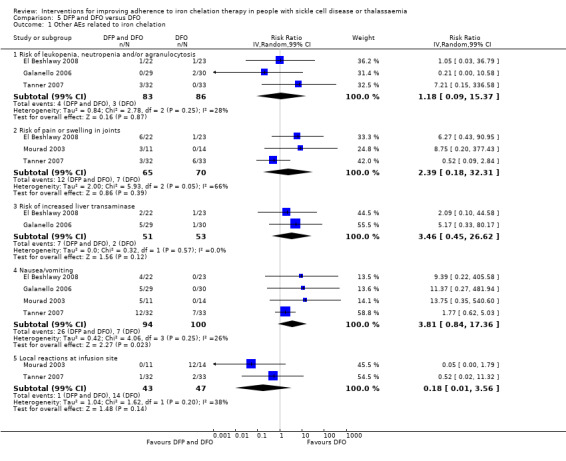
Comparison 5 DFP and DFO versus DFO, Outcome 1 Other AEs related to iron chelation.
Risk of leukopenia, neutropenia or agranulocytosis (or a combination of): RR 1.18 (99% CI 0.09 to 15.37) (three trials; 169 participants; very low‐quality evidence) (El Beshlawy 2008; Galanello 2006; Tanner 2007).
Risk of pain or swelling in joints: RR 2.39 (99% CI 0.18 to 32.31) (three trials; 135 participants; very low‐quality evidence I² = 66%) (El Beshlawy 2008; Mourad 2003; Tanner 2007).
Risk of increased liver transaminase: RR 3.46 (99% CI 0.45 to 26.62) (two trials; 104 participants; very low‐quality evidence) (El Beshlawy 2008; Galanello 2006).
Risk of nausea or vomiting: RR 3.81 (99% CI 0.84 to 17.36) (four trials; 194 participants; very low‐quality evidence) (El Beshlawy 2008; Galanello 2006; Mourad 2003; Tanner 2007).
Risk of local reactions at infusion site: RR 0.18 (99% CI 0.01 to 3.56) (two trials; 90 participants; very low‐quality evidence) (Mourad 2003; Tanner 2007).
We downgraded the quality of evidence by two for risk of bias due to high or unclear risk of bias in several domains in all trials and by one due to imprecision, the effect estimates have wide CIs.
Combination DFP (deferiprone) and DFO (deferoxamine) versus combination DFP (deferiprone) and DFX (deferasirox)
One trial in thalassaemia met the inclusion criteria for this comparison (Elalfy 2015). See Table 6.
Primary outcomes
1. Adherence to iron chelation therapy rates
In children with thalassaemia, combination therapy with DFP and DFX may improve adherence to iron chelation therapy compared to combination therapy with DFP and DFO, RR 0.84 (95% CI 0.72 to 0.99) (one trial; 96 participants; low‐quality evidence) (Analysis 6.1).
6.1. Analysis.
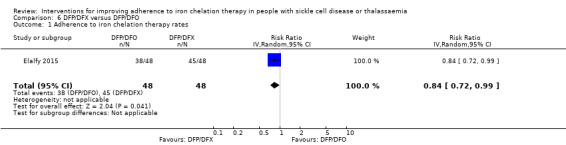
Comparison 6 DFP/DFX versus DFP/DFO, Outcome 1 Adherence to iron chelation therapy rates.
2. Serious adverse events (SAEs)
In children with thalassaemia, we are uncertain if combination therapy with DFP and DFX decreases the incidence of SAEs compared to combination therapy with DFP and DFO, RR 1.00 (95% CI 0.06 to 15.53) (one trial; 96 participants; very low‐quality evidence) (Analysis 6.2).
6.2. Analysis.
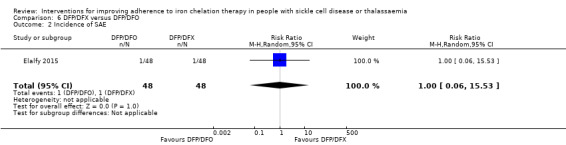
Comparison 6 DFP/DFX versus DFP/DFO, Outcome 2 Incidence of SAE.
3. All‐cause mortality
In children with thalassaemia, combination therapy with DFP and DFX may make little or no difference in mortality compared to combination therapy with DFP and DFO. There were no deaths in the trial (one trial; 96 participants; low‐quality evidence).
Secondary outcomes
1. Sustained adherence to therapy
The trial reported more than six months follow‐up, sustained adherence is reported in the primary outcome (adherence to iron chelation therapy rates), as only end‐of‐trial adherence numbers were provided.
2. Health‐related quality of life (QoL)
In children with thalassaemia we are unclear if combination therapy with DFP and DFX improves QoL compared to combination therapy with DFP and DFO (very low‐quality evidence). Authors state that "significant improvement in quality of life was observed in both groups at study end compared to baseline"; no comparative data were provided.
3. Iron overload
Proportion of participants with iron overload was not reported.
4. Organ damage
In children with thalassaemia, combination therapy with DFP and DFX as compared to DFP and DFO may have little or no difference in the incidence of increased creatinine, RR 3.00 (99% CI 0.16 to 56.04) (one trial; 96 participants; low‐quality evidence) (Analysis 6.4).
6.4. Analysis.
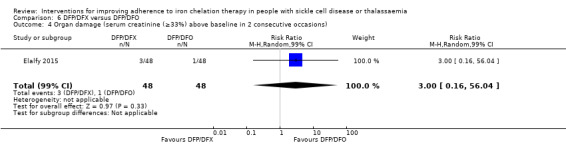
Comparison 6 DFP/DFX versus DFP/DFO, Outcome 4 Organ damage (serum creatinine (≥33%) above baseline in 2 consecutive occasions).
5. Other adverse events (AEs) related to iron chelation
In children with thalassaemia, we are unclear if combination therapy with DFP and DFX as compared to DFP and DFO reduces the incidence of AEs (one trial; 96 participants; very low‐quality evidence) (Analysis 6.5).
6.5. Analysis.
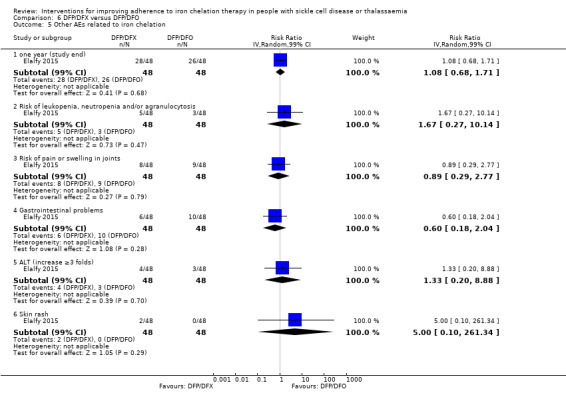
Comparison 6 DFP/DFX versus DFP/DFO, Outcome 5 Other AEs related to iron chelation.
Total drug‐related AEs: RR 1.08 (99% CI 0.68 to 1.71).
Risk of leukopenia, neutropenia, or agranulocytosis: RR 1.67 (99% CI 0.27 to 10.14).
Risk of pain or swelling in joints: RR 0.89 (99% CI 0.29 to 2.77).
Gastrointestinal problems: RR 0.60 (99% CI 0.18 to 2.04).
Liver transaminase increased: RR 1.33 (99% CI 0.20 to 8.88).
Skin rash: RR 5.00 (99% CI 0.10 to 261.34).
We downgraded the quality of evidence by one for risk of bias as there was a high or unclear risk of bias in three domains; by one for indirectness, as the trial was conducted in children aged 10 to 18 with years with severe iron overload; and by one due to imprecision, the effect estimates have wide CIs.
Medication management versus standard care
One trial in thalassaemia met the inclusion criteria for this comparison (Bahnasawy 2017). See Table 7.
Primary outcomes
1. Adherence to iron chelation therapy rates
Adherence was only reported in the intervention group and not in the control group.
2. Serious adverse events (SAEs)
SAEs were not reported.
3. All‐cause mortality
All‐cause mortality was not reported.
Secondary outcomes
1. Sustained adherence to therapy
Adherence was only reported in the intervention group and not in the control group.
2. Health‐related quality of life (QoL)
We are uncertain if medication management improves health‐related QoL: PedsQLTM HRQoL total score median (IQR): test group: 63.51 (51.75 to 84.54); control group: 49.84 (41.9 to 60.81) (one trial; 48 participants; very low‐quality evidence).
3. Iron overload
Proportion of participants with iron overload was not reported.
4. Organ damage
Proportion of participants with organ damage was not reported.
5. Other adverse events (AEs) related to iron chelation
AEs were not reported.
Discussion
Regularly transfused people with SCD, as well as transfusion‐dependent, and non‐transfusion‐dependent people with thalassaemia, are at risk of iron overload. Iron overload can lead to iron toxicity, with organs such as the heart, liver and endocrine glands being particularly vulnerable.
In this review we reviewed the evidence for improving adherence to iron chelation therapy in people with SCD or thalassaemia.
Sixteen RCTs with a total of 1525 participants met our inclusion criteria. Fourteen trials included people with β‐thalassaemia major, one trial was conducted in people with SCD and another in people with β‐thalassaemia intermedia, a milder form of β‐thalassaemia. Trials were conducted between 1997 and 2017 and all included trials were medication interventions, except for one, which was a medication management intervention.
We also identified an additional five ongoing RCTs, and two studies awaiting classification (one RCT and one prospective cohort study).
We did not identify any cluster randomised trials, NRSIs, CBA or ITS studies that met the inclusion criteria.
Summary of main results
The findings of the review led to the following main conclusions regarding medication interventions to improve adherence to iron chelation.
DFP versus DFO
Based on results from four trials in thalassaemia, we are uncertain whether oral DFP increases adherence to iron chelation therapy more than subcutaneous DFO (Calvaruso 2015; El Beshlawy 2008; Olivieri 1997; Pennell 2006). Results could not be combined due to a lack of data to report as well as the considerable heterogeneity between comparisons (I² = 99%). There was high adherence in all trials. We are uncertain if switching to oral DFP increases the risk of agranulocytosis compared to subcutaneous DFO. Oral DFP may have little or no effect on mortality compared to subcutaneous DFO. Quality of life was not measured in any trial in this comparison.
DFX versus DFO
Based on results from three trials, two in thalassaemia (Hassan 2016; Pennell 2014) and one in SCD (Vichinsky 2007), we are uncertain if DFX increases the rate of adherence compared to people taking DFO; participants had high adherence in all trials. We are uncertain whether DFX decreases risk of thalassaemia‐related SAEs or decreases the risk of mortality in people with thalassaemia compared to DFO. We are uncertain whether DFX decreases the risk of SCD‐related pain crisis or other SCD‐related SAEs compared to DFO. QoL was not reported in any trial in this comparison.
DFX (film‐coated tablet (FCT)) versus DFX (dispersible tablet (DT))
Based on results from a single trial in thalassaemia, DFX FCT may make little or no difference to adherence as compared to DFX DT (Taher 2017). There was high adherence in both arms of the trial. We are uncertain if DFX FCT increases SAEs or all‐cause mortality as compared to DFX DT. QoL was not measured using a validated instrument.
DFP and DFO combined versus DFP alone
Based on results from three trials in thalassaemia, we are uncertain if DFP and DFO combined increases adherence compared to DFP alone (Aydinok 2007; El Beshlawy 2008; Maggio 2009). There was high adherence in all trials. Combination therapy with DFP and DFO may make little or no difference to the incidence of SAEs as compared to DFP alone. We are uncertain if combination therapy with DFP and DFO decreases mortality as compared to DFP alone. QoL was not measured using a validated instrument.
DFP and DFO combined versus DFO alone
Based on results from four trials in people with thalassaemia, combined therapy with DFP and DFO versus DFO alone, may make little or no difference to adherence rates, SAEs, or mortality (El Beshlawy 2008; Galanello 2006; Mourad 2003; Tanner 2007). There was high adherence in all trials. QoL was not measured in any trial in this comparison.
DFP and DFO combined versus DFP and DFX combined
Based on the results of a single trial in children with thalassaemia, combination therapy with DFP and DFX may improve adherence to iron chelation therapy compared to combination therapy with DFP and DFO (Elalfy 2015). There was high adherence in both arms. We are uncertain if DFP and DFX reduces the incidence of SAEs, and may make little or no difference in mortality or QoL, compared to combination therapy with DFP and DFO.
Medication management versus standard care
A single trial on thalassaemia reported on this comparison (Bahnasawy 2017). Adherence rates were only reported in the intervention arm and therefore there are no comparative data to report. We are uncertain if medication management improves health‐related QoL.
Overall completeness and applicability of evidence
This review provides the most up‐to‐date assessment of interventions to improve adherence to iron chelation therapy in people with SCD and thalassaemia. We have also identified five ongoing trials and two trials that are awaiting classification.
Of the five ongoing trials, two compare medication interventions in thalassaemia and two in SCD and thalassaemia (EudraCT 2012‐000353‐31; IRCT2015101218603N2; NCT02173951; NCT02435212), and one assesses the effectiveness of group medical appointments on self‐efficacy and adherence in SCD (Madderom 2016). Of the two studies awaiting classification, one is an educational study (Antmen 2013), and one is a medication intervention (NCT00004982).
The results of this review can only be interpreted in consideration of the following factors.
Adherence is not the primary outcome in any of the included trials.
All trials, except for one medication management trial, are medication interventions and participants were often selected based on their anticipated compliance. Lack of adherence was a reason for exclusion from some trials or analyses of results.
Within the context of a clinical trial, there is increased attention by, and involvement of, clinicians and specialist nurses with participants which may impact and increase rates of adherence not seen in a community setting.
Research has shown that up to 50% of people do not take medications as prescribed and over 85% of people are occasionally non‐adherent to prescribed medications (Ryan 2014). The reported adherence rates in the trials included in this review are substantially higher than average, despite the substantial side effects and demanding administration regimen of iron chelators. This may be indicative of high adherence rates being an artefact created by participant involvement in a clinical trial.
We did not identify any cluster randomised trials, NRSIs, CBA or ITS studies with adherence as a primary outcome, that met the inclusion criteria.
Due to a lack of evidence this review cannot comment on intervention strategies for different age groups.
Quality of the evidence
Overall the quality of the evidence according to GRADE methodology across all comparisons for the outcomes of adherence, SAEs, and mortality was rated as low to very low (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7). This was due to trials being at serious or very serious risk of bias; outcome estimates being imprecise (wide CIs); and indirectness with some trials conducted only in children of a specific age and meeting specific criteria. QoL was mostly not reported or reported using non‐validated measurements or sparsely reported with no data.
Potential biases in the review process
To our knowledge, our review process was free from bias. We conducted a comprehensive search: searching data sources (including multiple databases, and clinical trial registries) to ensure that all relevant studies would be captured. There were no restrictions for the language in which the paper was originally published. The relevance of each paper was carefully assessed and all screening and data extractions were performed in duplicate. We pre‐specified all outcomes and subgroups prior to analysis. There were insufficient numbers of included trials within the meta‐analyses for us to use a funnel plot to examine the risk of publication bias.
Agreements and disagreements with other studies or reviews
Adherence rates can vary widely, a recent review reported that adherence rates to the oral iron chelator DFX ranged between 22% and 89% (Loiselle 2016). Another review of medication adherence in SCD reports adherence rates ranging from 16% to 89%; but most included studies reported moderate adherences (Walsh 2014). In this Cochrane Review, we found adherence rates across trials and for all comparisons of different chelators to be quite high in individual trial reports (predominantly at least 80%). Indeed the results of this review are in disagreement with most literature that identifies major issues with compliance across indications, people and setting (NICE 2009; Ryan 2014; WHO 2003). We suggest that selection bias for compliance into the chelation trials as a possible reason for high adherence; as well, the additional time and attention received by participants make high adherence an artefact of trial participation.
Ryan identifies several strategies that may help to promote adherence including self‐management; self‐monitoring; simplified dosing regimens; or interventions involving pharmacists in medication management (Ryan 2014). Other identified interventions that need further research include pragmatic interventions (such as reminders); educational interventions, and financial incentives. One RCT on pharmacist‐led medication management was included in this review, but the trial had few participants, was of short duration and poorly reported (Bahnasawy 2017). The remaining trials in this review measured compliance primarily as a secondary outcome and did not identify any specific strategies that may have led to increased compliance, thus supporting the contention that high compliance is an artefact of participation in these trials and not the result of change or improvement in medication regimens.
Authors' conclusions
Implications for practice.
Adherence to iron chelation regimens can reduce morbidity and mortality in people with transfusion‐ and non‐transfusion‐dependent thalassaemia and sickle cell disease. Iron chelation regimens can be demanding and also have unpleasant side effects that reduce adherence to these medications. In this review we did not identify any specific medication intervention that increased adherence with iron chelators and suggest that adherence was high due to the artefact of participation in these trials. Due to a lack of evidence, this review cannot comment on intervention strategies for different age groups.
Overviews of systematic reviews that identify intervention strategies that have been successful for other indications and medications may be more useful to clinicians who want to improve compliance with iron chelation therapy. However, the successful translation of these interventions to iron chelation regimens would still need to be confirmed in appropriate trials.
Implications for research.
Real‐world, pragmatic trials in community and clinic settings are needed to examine a variety of confirmed or unconfirmed adherence strategies that may be useful to increase adherence to iron chelation therapy. High‐quality, non‐randomised trials that measure compliance over multiple time points, before and after an intervention, as well as non‐randomised studies that test interventions in multiple settings could help to identify evidence‐based strategies that increase compliance with iron chelation therapy. Finally, appropriate measurements of compliance are needed that include both patient‐oriented, such as quality of life measurements, as well as objective measurements that link iron levels and morbidity due to iron overload to levels of adherence. Targeted strategies that increase adherence in different age groups, particularly in adolescents, are also needed.
Acknowledgements
We thank the National Institute for Health Research for supporting this project, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
This review is part of a series of reviews that have been partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Appendices
Appendix 1. Search strategies
The following databases will be searched using the strategies below (without study filters):
CENTRAL & DARE, (The Cochrane Library) #1 MeSH descriptor: [Patient Acceptance of Health Care] explode all trees #2 MeSH descriptor: [Patient Education as Topic] this term only #3 MeSH descriptor: [Data Collection] explode all trees #4 (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*):ti #5 ((adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*) near/6 (patient* or treatment* or therapy or therapies or medication* or drug*)):ab #6 (patient* near/3 (dropout* or drop* out*)) #7 MeSH descriptor: [Treatment Refusal] this term only #8 (treatment* near/3 refus*) #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 MeSH descriptor: [Iron Chelating Agents] explode all trees #11 MeSH descriptor: [Chelation Therapy] this term only #12 (chelat* near/3 (treatment* or therap*)) #13 (deferoxamine* or deferoximine* or deferrioxamine* or desferioximine* or desferrioxamine* or desferroxamine* or desferal* or desferral* or DFO or desferin* or desferol* or dfom) #14 (deferiprone or L1* or kelfer or DMHP or ferriprox or CP20 or dmohpo or hdmpp CPD or hdpp) #15 (exjade* or deferasirox* or ICL 670* or icl670* or "CGP 72670") #16 (iron near/5 (chelat* or reduc*)) #17 #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 MeSH descriptor: [Thalassemia] explode all trees #19 (thalassemi* or thalassaemi* or lepore or hydrops fetalis) #20 ((hemoglobin or haemoglobin) near/3 disease) #21 (hemochromatosis or haemochromatosis or hemosiderosis or haemosiderosis) #22 ((mediterranean or erythroblastic or cooley*) next (anemi* or anaemi*)) #23 MeSH descriptor: [Iron Overload] explode all trees #24 (iron near/3 (overload* or over‐load*)) #25 MeSH descriptor: [Hemoglobinopathies] this term only #26 MeSH descriptor: [Hemoglobin C Disease] this term only #27 (hemoglobinopath* or haemoglobinopath*) #28 MeSH descriptor: [Anemia, Sickle Cell] explode all trees #29 (barts and (blood or plasma)) #30 (sickle cell or sicklemi* or sickled or sickling or meniscocyt* or drepanocyt*) #31 (hemoglobin S or hemoglobin SC or hemoglobin SE or hemoglobin SS or hemoglobin C or hemoglobin D or haemoglobin S or haemoglobin SC or haemoglobin SE or haemoglobin SS or haemoglobin C or haemoglobin D Hb S or Hb SC or Hb SE or Hb SS or Hb C or Hb D or SC disease) #32 #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 #33 #9 and #17 and #32 #34 ((thalassemi* or thalassaemi* or sickle or hemoglobinopath* or haemoglobinopath*) and (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or educat*)):ti #35 #33 or #34
PubMed (for Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations only) #1 ((adher* OR nonadher* OR complian* OR comply* OR noncomplian* OR noncomply* OR complier* OR noncomplier* OR accept* OR nonaccept* OR abandon* OR co‐operat* OR cooperat* OR unco‐operative* OR uncooperative* OR nonco‐operat* OR noncooperat* OR satisfaction OR dissatisfaction OR persist* OR educat* OR questionnaire*) AND (patient OR patients OR treatment* OR therapy OR therapies OR medication* OR drug*)) #2 (patient dropout* OR patient drop* outs OR patients drop* out OR treatment* refus* OR refus* treatment*) #3 #1 OR #2 #4 (deferoxamine* OR deferoximine* OR deferrioxamine* OR desferioximine* OR desferrioxamine* OR desferroxamine* OR desferal* OR desferral* OR DFO OR desferin* OR desferol* OR dfom OR deferiprone OR L1 OR kelfer OR DMHP OR ferriprox OR CP20 OR dmohpo OR hdmpp CPD OR hdpp OR exjade* OR deferasirox* OR ICL 670* OR icl670* OR CGP "72670" OR iron chelat* OR iron reduc* OR chelat* treatment* OR chelat* therapy) #5 (thalassemi* OR thalassaemi* OR lepore OR hydrops fetalis OR cooley* anemi* OR cooley* anaemi*) #6 (hemoglobin disease OR haemoglobin disease OR hemochromatosis OR haemochromatosis OR hemosiderosis OR haemosiderosis) #7 (mediterranean anemi* OR mediterranean anaemi* OR erythroblastic anemi* OR erythroblastic anaemi*) #8 hemoglobinopath* OR haemoglobinopath* OR iron overload* OR iron over‐load* #9 ("sickle cell" OR sicklemi* OR sickled OR sickling OR meniscocyt* OR drepanocyt* OR "hemoglobin S" OR "hemoglobin SC" OR "hemoglobin SE" OR "hemoglobin SS" OR "hemoglobin C" OR "hemoglobin D" OR "haemoglobin S" OR "haemoglobin SC" OR "haemoglobin SE" OR "haemoglobin SS" OR "haemoglobin C" OR "haemoglobin D" OR "Hb S" OR "Hb SC" OR "Hb SE" OR "Hb SS" OR "Hb C" OR "Hb D" OR "SC disease") #10 #5 OR #6 OR #7 OR #8 OR #9 #11 #3 AND 4 AND #10 #12 ((adher*[TI] OR nonadher*[TI] OR complian*[TI] OR comply*[TI] OR noncomplian*[TI] OR noncomply*[TI] OR complier*[TI] OR noncomplier*[TI] OR accept*[TI] OR nonaccept*[TI] OR abandon*[TI] OR co‐operat*[TI] OR cooperat*[TI] OR unco‐operative*[TI] OR uncooperative*[TI] OR nonco‐operat*[TI] OR noncooperat*[TI] OR satisfaction[TI] OR dissatisfaction[TI] OR persist*[TI] OR educat*[TI] OR questionnaire*[TI]) AND (thalassemia*[TI] OR thalassaemia*[TI] OR sickle[TI] OR iron overload*[TI])) #13 #11 OR #12 #14 (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb]) #15 #13 AND #14
MEDLINE (OvidSP) 1. exp "Patient Acceptance of Health Care"/ 2. (px or ed).fs. 3. "Patient Education as Topic"/ 4. exp Data Collection/ 5. (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*).ti. 6. ((adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*) adj6 (patient* or treatment* or therapy or therapies or medication* or drug*)).ab,kf. 7. (patient* adj3 (dropout* or drop* out*)).tw,kf. 8. Treatment Refusal/ 9. (treatment* adj3 refus*).tw,kf. 10. or/1‐9 11. exp IRON CHELATING AGENTS/ 12. CHELATION THERAPY/ 13. (chelation adj3 (treatment* or therap*)).tw,kf. 14. (deferoxamine* or deferoximine* or deferrioxamine* or desferioximine* or desferrioxamine* or desferroxamine* or desferal* or desferral* or DFO or desferin* or desferol* or dfom).mp. 15. (deferiprone or L1* or kelfer or DMHP or ferriprox or CP20 or dmohpo or hdmpp CPD or hdpp).mp. 16. (exjade* or deferasirox* or ICL 670* or icl670* or "CGP 72670").mp. 17. (iron adj5 (chelat* or reduc*)).tw,kf. 18. or/11‐17 19. exp THALASSEMIA/ 20. (thalass?emi* or lepore or hydrops fetalis).tw,kf. 21. ((hemoglobin or haemoglobin) adj3 disease).tw,kf. 22. (hemochromatosis or haemochromatosis or hemosiderosis or haemosiderosis).tw,kf. 23. ((mediterranean or erythroblastic or cooley*) adj (anemi* or anaemi*)).tw,kf. 24. exp IRON OVERLOAD/ 25. (iron adj3 (overload* or over‐load*)).tw,kf. 26. exp HEMOGLOBINOPATHIES/ 27. exp HEMOGLOBIN, SICKLE/ 28. (hemoglobinopath* or haemoglobinopath*).tw,kf. 29. exp ANEMIA, SICKLE CELL/ 30. (barts and (blood or plasma)).tw,kf. 31. (sickle or sicklemi* or sickled or sickling or meniscocyt* or drepanocyt*).tw,kf. 32. (h?emoglobin s or h?emoglobin sc or h?emoglobin se or h?emoglobin ss or h?emoglobin c or h?emoglobin d or Hb s or Hb sc or Hb se or Hb ss or Hb c or Hb d or sc disease*).tw,kf. 33. or/19‐32 34. 10 and 18 and 33 35. exp *Hemoglobinopathies/ or (thalass?emi* or sickle or hemoglobinopath* or haemoglobinopath*).ti. 36. exp *Patient Compliance/ or (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or educat*).ti. 37. 35 and 36 38. 34 or 37
Embase (OvidSP) 1. exp THALASSEMIA/ 2. (thalass?emi* or lepore or hydrops fetalis).tw,kf. 3. ((hemoglobin or haemoglobin) adj3 disease).tw,kf. 4. (hemochromatosis or haemochromatosis or hemosiderosis or haemosiderosis).tw,kf. 5. ((mediterranean or erythroblastic or cooley*) adj (anemi* or anaemi*)).tw,kf. 6. IRON OVERLOAD/ 7. (iron adj3 (overload* or over‐load*)).tw,kf. 8. HEMOGLOBINOPATHY/ 9. HEMOGLOBIN S/ 10. (hemoglobinopath* or haemoglobinopath*).tw,kf. 11. exp SICKLE CELL ANEMIA/ 12. (barts and (blood or plasma)).tw,kf. 13. (sickle or sicklemi* or sickled or sickling or meniscocyt* or drepanocyt*).tw,kf. 14. (h?emoglobin s or h?emoglobin sc or h?emoglobin se or h?emoglobin ss or h?emoglobin c or h?emoglobin d or Hb s or Hb sc or Hb se or Hb ss or Hb c or Hb d or sc disease*).tw,kf. 15. or/1‐14 16. exp PATIENT ATTITUDE/ 17. PATIENT EDUCATION/ 18. "PATIENT EDUCATION AS TOPIC"/ 19. exp DATA COLLECTION METHOD/ 20. (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*).ti. 21. ((adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*) adj6 (patient* or treatment* or therapy or therapies or medication* or drug*)).ab,kf. 22. (patient* adj3 (dropout* or drop* out*)).tw. 23. (treatment* adj3 refus*).tw. 24. or/16‐23 25. IRON CHELATING AGENT/ 26. CHELATION THERAPY/ 27. (chelation adj3 (treatment* or therap*)).tw,kf. 28. (deferoxamine* or deferoximine* or deferrioxamine* or desferioximine* or desferrioxamine* or desferroxamine* or desferal* or desferral* or DFO or desferin* or desferol* or dfom).mp. 29. (deferiprone or L1* or kelfer or DMHP or ferriprox or cp20 or dmohpo or hdmpp CPD or hdpp).mp. 30. (exjade* or deferasirox* or (icl adj 670*) or icl670* or (cgp adj "72670")).mp. 31. (iron adj5 (chelat* or reduc*)).tw. 32. or/25‐31 33. 15 and 24 and 32 34. exp *Hemoglobinopathy/ or (thalass?emi* or sickle or hemoglobinopath* or haemoglobinopath*).ti. 35. exp *Patient Compliance/ or (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or educat*).ti. 36. 34 and 35 37. 33 or 36
CINAHL (EBSCOHost) S1 (MH "Patient Compliance+") S2 (MH "Patient Education") S3 (MH "Instrument by Type+") S4 TI (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*) S5 AB ((adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*) N6 (patient* or treatment* or therapy or therapies or medication* or drug*)) S6 TX (patient* N3 (dropout* or drop* out*)) S7 MH Treatment Refusal S8 TX (treatment* N3 refus*) 9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10 (MH "Chelating Agents+") S11 (MH "Chelation Therapy") S12 TX (deferoxamine* or deferoximine* or deferrioxamine* or desferioximine* or desferrioxamine* or desferroxamine* or desferal* or desferral* or DFO or desferin* or desferol* or dfom) S13 TX (deferiprone or L1* or kelfer or DMHP or ferriprox or CP20 or dmohpo or hdmpp CPD or hdpp) S14 TX (exjade* or deferasirox* or ICL 670* or icl670* or "CGP 72670") S15 TX (iron N5 (chelat* or reduc*)) OR TX (chelat* N3 (treatment* or therap*)) S16 S10 OR S11 OR S12 OR S13 OR S14 OR S15 S17 (MH "Thalassemia+") S18 TX (thalassemi* or thalassaemi* or lepore or hydrops fetalis) S19 TX ((hemoglobin or haemoglobin) N3 disease) S20 TX (hemochromatosis or haemochromatosis or hemosiderosis or haemosiderosis) S21 TX ((mediterranean or erythroblastic or cooley*) N1 (anemi* or anaemi*)) S22 (MH "Iron Overload+") S23 TX (iron N3 (overload* or over‐load*)) S24 (MH "Hemoglobinopathies") S25 TX (hemoglobinopath* or haemoglobinopath*) S26 (MH "Anemia, Sickle Cell+") S27 TX (barts and (blood or plasma)) S28 TX (sickle OR sicklemi* OR sickled OR sickling OR meniscocyt* OR drepanocyt* OR "hemoglobin S" OR "hemoglobin SC" OR "hemoglobin SE" OR "hemoglobin SS" OR "hemoglobin C" OR "hemoglobin D" OR "haemoglobin S" OR "haemoglobin SC" OR "haemoglobin SE" OR "haemoglobin SS" OR "haemoglobin C" OR "haemoglobin D" OR "Hb S" OR "Hb SC" OR "Hb SE" OR "Hb SS" OR "Hb C" OR "Hb D" OR "SC disease") S29 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 S30 S9 AND S16 AND S29 S31 (MM "Patient Compliance+") S32 TI (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or educat*) S33 S31 OR S32 S34 (MM "Hemoglobinopathies+") S35 TI (thalassemi* or thalassaemi* or sickle or hemoglobinopath* or haemoglobinopath*) S36 S34 OR S35 S37 S33 AND S36 S38 S30 OR S37
ProQuest Dissertations & Theses Global ti(adher* OR nonadher* OR complian* OR comply* OR noncomplian* OR noncomply* OR complier* OR noncomplier* OR accept* OR nonaccept* OR abandon* OR co‐operat* OR cooperat* OR unco‐operative* OR uncooperative* OR nonco‐operat* OR noncooperat* OR satisfaction OR dissatisfaction OR refus* OR persist* OR educat* OR questionnaire*) AND ti(thalassemia OR thalassaemia OR sickle OR sickled OR sickling OR iron overload OR hemoglobinopath*) AND (chelation OR chelating OR deferiprone OR deferoxamine OR deferasirox OR DFO OR ferriprox OR exjade OR iron reduction) PsycINFO (EBSCOHost) & Psychology and Behavioral Sciences Collection (EBSCOHost) S1 DE "Treatment Compliance" OR DE "Compliance" OR DE "Treatment Refusal" OR DE "Treatment Dropouts" OR DE "Treatment Termination" S2 DE "Client Education" S3 DE "Questionnaires" OR DE "General Health Questionnaire" S4 TI (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*) S5 AB ((adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or abandon* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or persist* or educat* or questionnaire*) N6 (patient* or treatment* or therapy or therapies or medication* or drug*)) S6 TX (patient* N3 (dropout* or drop* out*)) S7 DE Treatment Refusal S8 TX (treatment* N3 refus*) S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10 TX (deferoxamine* or deferoximine* or deferrioxamine* or desferioximine* or desferrioxamine* or desferroxamine* or desferal* or desferral* or DFO or desferin* or desferol* or dfom) S11 TX (deferiprone or L1* or kelfer or DMHP or ferriprox or CP20 or dmohpo or hdmpp CPD or hdpp) S12 TX (exjade* or deferasirox* or ICL 670* or icl670* or "CGP 72670") S13 TX (iron N5 (chelat* or reduc*)) OR TX (chelat* N3 (treatment* or therap*)) S14 S10 OR S11 OR S12 OR S13 S15 TX (thalassemi* or thalassaemi* or lepore or hydrops fetalis) S16 TX ((hemoglobin or haemoglobin) N3 disease) S17 TX (hemochromatosis or haemochromatosis or hemosiderosis or haemosiderosis) S18 TX ((mediterranean or erythroblastic or cooley*) N1 (anemi* or anaemi*)) S19 TX (iron N3 (overload* or over‐load*)) S20 TX (hemoglobinopath* or haemoglobinopath*) S21 DE "Sickle Cell Disease" S22 TX (barts and (blood or plasma)) S23 TX (sickle OR sicklemi* OR sickled OR sickling OR meniscocyt* OR drepanocyt* OR "hemoglobin S" OR "hemoglobin SC" OR "hemoglobin SE" OR "hemoglobin SS" OR "hemoglobin C" OR "hemoglobin D" OR "haemoglobin S" OR "haemoglobin SC" OR "haemoglobin SE" OR "haemoglobin SS" OR "haemoglobin C" OR "haemoglobin D" OR "Hb S" OR "Hb SC" OR "Hb SE" OR "Hb SS" OR "Hb C" OR "Hb D" OR "SC disease") S24 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 S25 S9 AND S14 AND S24 S26 MM "Treatment Compliance" S27 TI (adher* or nonadher* or complian* or comply* or noncomplian* or noncomply* or complier* or noncomplier* or accept* or nonaccept* or co‐operat* or cooperat* or unco‐operative* or uncooperative* or nonco‐operat* or noncooperat* or satisfaction or dissatisfaction or educat*) S28 S26 OR S27 S29 MM "Sickle Cell Disease" S30 TI (thalassemi* or thalassaemi* or sickle or hemoglobinopath* or haemoglobinopath*) 31 S29 OR S30 S32 S28 AND S31 S33 S25 OR S32
Web of Science CPCI‐S & CPSSI #1 TS=((adher* OR nonadher* OR complian* OR comply* OR noncomplian* OR noncomply* OR complier* OR noncomplier* OR accept* OR nonaccept* OR abandon* OR co‐operat* OR cooperat* OR unco‐operative* OR uncooperative* OR nonco‐operat* OR noncooperat* OR satisfaction OR dissatisfaction OR persist* OR educat* OR questionnaire*) AND (patient* OR treatment* OR therapy OR therapies OR medication* OR drug*)) #2 TS=(patient dropout* OR patient drop* outs OR patients drop* out OR treatment* refus* OR refus* treatment*) #3 #1 OR #2 #4 TS=(deferoxamine* OR deferoximine* OR deferrioxamine* OR desferioximine* OR desferrioxamine* OR desferroxamine* OR desferal* OR desferral* OR DFO OR desferin* OR desferol* OR dfom OR deferiprone OR L1 OR kelfer OR DMHP OR ferriprox OR CP20 OR dmohpo OR hdmpp CPD OR hdpp OR exjade* OR deferasirox* OR ICL 670* OR icl670* OR CGP "72670" OR iron chelat* OR iron reduc* OR chelat* treatment* OR chelat* therap*) #5 TS=(thalassemi* OR thalassaemi* OR lepore OR hydrops fetalis OR cooley* anemi* OR cooley* anaemi* OR hemoglobin disease OR haemoglobin disease OR hemochromatosis OR haemochromatosis OR hemosiderosis OR haemosiderosis OR mediterranean anemi* OR mediterranean anaemi* OR erythroblastic anemi* OR erythroblastic anaemi* OR iron overload* OR iron over‐load* OR hemoglobinopath* OR haemoglobinopath*) #6 TS=(sickle OR sicklemi* OR sickled OR sickling OR meniscocyt* OR drepanocyt* OR "hemoglobin S" OR "hemoglobin SC" OR "hemoglobin SE" OR "hemoglobin SS" OR "hemoglobin C" OR "hemoglobin D" OR "haemoglobin S" OR "haemoglobin SC" OR "haemoglobin SE" OR "haemoglobin SS" OR "haemoglobin C" OR "haemoglobin D" OR "Hb S" OR "Hb SC" OR "Hb SE" OR "Hb SS" OR "Hb C" OR "Hb D" OR "SC disease") #7 #5 OR #6 #8 #3 AND #4 AND #7
ClinicalTrials.gov Other Terms: (thalassemia OR sickle cell anemia OR iron overload OR hemoglobinopathies) AND (iron chelation OR chelation therapy OR deferiprone OR deferoxamine OR deferasirox OR DFO OR iron reduction)
WHO ICTRP Condition: thalassemia OR sickle cell anemia OR iron overload OR hemoglobinopathies Intervention: iron chelation OR chelation therapy OR deferiprone OR deferoxamine OR deferasirox OR DFO OR iron reduction
ISRCTN Condition: thalassemia OR sickle cell anemia OR iron overload OR hemoglobinopathies Interventions: iron chelation OR chelation therapy OR deferiprone OR deferoxamine OR deferasirox OR DFO OR iron reduction
Appendix 2. The Risk Of Bias In Non‐randomised Studies of Interventions (ROBINS‐I) assessment tool
ROBINS‐I tool (Stage I)
Specify the review question
| Participants | |
| Experimental intervention | |
| Control intervention | |
| Outcomes |
List the confounding areas relevant to all or most studies
List the possible co‐interventions that could be different between intervention groups and could have an impact on outcomes
The ROBINS‐I tool (Stage II): For each study
Specify a target trial specific to the study.
| Design | Individually randomised or cluster randomised or matched |
| Participants | |
| Experimental intervention | |
| Control intervention |
Is your aim for this study...?
□ to assess the effect of initiating intervention (as in an intention‐to‐treat analysis)
□ to assess the effect of initiating and adhering to intervention (as in a per protocol analysis)
Specify the outcome
Specify which outcome is being assessed for risk of bias (typically from among those earmarked for the Summary of Findings table). Specify whether this is a proposed benefit or harm of intervention.
Specify the numerical result being assessed
In case of multiple alternative analyses being presented, specify the numeric result (e.g. RR = 1.52 (95% CI 0.83 to 2.77) or a reference (e.g. to a table, figure or paragraph) that uniquely defines the result being assessed (or both).
Preliminary consideration of confounders
Complete a row for each important confounding area (i) listed in the review protocol; and (ii) relevant to the setting of this particular study, or which the study authors identified as potentially important.
'Important' confounding areas are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention. 'Validity' refers to whether the confounding variable or variables fully measure the area, while 'reliability' refers to the precision of the measurement (more measurement error means less reliability).
| (i) Confounding areas listed in the review protocol | ||||
| Confounding area | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?* | Is the confounding area measured validly and reliably by this variable (or these variables)? | OPTIONAL: is adjusting for this variable (alone) expected to favour the experimental or the control group? |
| Yes / No / No information | Favour intervention / Favour control / No information | |||
| (ii) Additional confounding areas relevant to the setting of this particular study, or which the study authors identified as important | ||||
| Confounding area | Measured Variable(s) | Is there evidence that controlling for this variable was unnecessary?* | Is the confounding area measured validly and reliably by this variable (or these variables)? | OPTIONAL: is adjusting for this variable (alone) expected to favour the experimental or the control group? |
| Yes / No / No information | Favour intervention / Favour control / No information | |||
* In the context of a particular study, variables can be demonstrated not to be confounders and so not included in the analysis: (a) if they are not predictive of the outcome; (b) if they are not predictive of intervention; or (c) because adjustment makes no or minimal difference to the estimated effect of the primary parameter. Note that “no statistically significant association” is not the same as “not predictive”.
Preliminary consideration of co‐interventions
Complete a row for each important co‐intervention (i) listed in the review protocol; and (ii) relevant to the setting of this particular study, or which the study authors identified as important.
'Important' co‐interventions are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention.
| (i) Co‐interventions listed in the review protocol | ||
| Co‐intervention | Is there evidence that controlling for this co‐intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co‐intervention likely to favour outcomes in the experimental or the control group |
| Favour experimental / Favour comparator / No information | ||
| Favour experimental / Favour comparator / No information | ||
| Favour experimental / Favour comparator / No information | ||
| (ii) Additional co‐interventions relevant to the setting of this particular study, or which the study authors identified as important | ||
| Co‐intervention | Is there evidence that controlling for this co‐intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co‐intervention likely to favour outcomes in the experimental or the control group |
| Favour experimental / Favour comparator / No information | ||
| Favour experimental / Favour comparator / No information | ||
| Favour experimental / Favour comparator / No information | ||
Risk of bias assessment (cohort‐type studies)
| Bias domain | Signalling questions | Elaboration | Response options |
| Bias due to confounding | 1.1 Is there potential for confounding of the effect of intervention in this study? IfN or PN to1.1: the study can be considered to be at low risk of bias due to confounding and no further signalling questions need be considered |
In rare situations, such as when studying harms that are very unlikely to be related to factors that influence treatment decisions, no confounding is expected and the study can be considered to be at low risk of bias due to confounding, equivalent to a fully randomised trial. There is no NI (No information) option for this signalling question. |
Y / PY / PN / N |
| If Y or PY to 1.1: determine whether there is a need to assess time‐varying confounding: | |||
| 1.2. Was the analysis based on splitting participants’ follow up time according to intervention received? If N orPN, answer questions relating to baseline confounding (1.4 to 1.6) If Y orPY, proceed to question 1.3. |
If participants could switch between intervention groups then associations between intervention and outcome may be biased by time‐varying confounding. This occurs when prognostic factors influence switches between intended interventions. | NA / Y / PY / PN / N / NI | |
| 1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? If N or PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y orPY, answer questions relating to both baseline and time‐varying confounding (1.7 and 1.8) |
If intervention switches are unrelated to the outcome, for example when the outcome is an unexpected harm, then time‐varying confounding will not be present and only control for baseline confounding is required. | NA / Y / PY / PN / N / NI | |
| Questions relating to baseline confounding only | |||
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding areas? | Appropriate methods to control for measured confounders include stratification, regression, matching, standardization, and inverse probability weighting. They may control for individual variables or for the estimated propensity score. Inverse probability weighting is based on a function of the propensity score. Each method depends on the assumption that there is no unmeasured or residual confounding. | NA / Y / PY / PN / N / NI | |
| 1.5.If Y or PY to1.4: were confounding areas that were controlled for measured validly and reliably by the variables available in this study? | Appropriate control of confounding requires that the variables adjusted for are valid and reliable measures of the confounding domains. For some topics, a list of valid and reliable measures of confounding domains will be specified in the review protocol but for others such a list may not be available. Study authors may cite references to support the use of a particular measure. If authors control for confounding variables with no indication of their validity or reliability pay attention to the subjectivity of the measure. Subjective measures (e.g. based on self‐report) may have lower validity and reliability than objective measures such as lab findings. | NA / Y / PY / PN / N / NI | |
| 1.6. Did the authors control for any post‐intervention variables? | Controlling for post‐intervention variables is not appropriate. Controlling for mediating variables estimates the direct effect of intervention and may introduce confounding. Controlling for common effects of intervention and outcome causes bias. | NA / Y / PY / PN / N / NI | |
| Questions relating to baseline and time‐varying confounding | |||
| 1.7. Did the authors use an appropriate analysis method that adjusted for all the important confounding areas and for time‐varying confounding? | Adjustment for time‐varying confounding is necessary to estimate per‐protocol effects in both randomised trials and NRSI. Appropriate methods include those based on inverse‐probability weighting. Standard regression models that include time‐updated confounders may be problematic if time‐varying confounding is present. | NA / Y / PY / PN / N / NI | |
| 1.8. IfY orPY to1.7: Were confounding areas that were adjusted for measured validly and reliably by the variables available in this study? | See 1.5 above. | NA / Y / PY / PN / N / NI | |
| Risk of bias judgement | Low ‐ no confounding expected. | Low / Moderate / Serious / Critical / NI | |
|
Moderate ‐ confounding expected, all known important confounding domains appropriately measured and controlled for; and Reliability and validity of measurement of important domains were sufficient, such that we do not expect serious residual confounding. | |||
|
Serious ‐ at least one known important domain was not appropriately measured, or not controlled for; or Reliability or validity of measurement of a important domain was low enough that we expect serious residual confounding. | |||
| Critical ‐ confounding inherently not controllable, or the use of negative controls strongly suggests unmeasured confounding. | |||
| Optional: what is the predicted direction of bias due to confounding? | Can the true effect estimate be predicted to be greater or less than the estimated effect in the study because one or more of the important confounding domains was not controlled for? Answering this question will be based on expert knowledge and results in other studies and therefore can only be completed after all of the studies in the body of evidence have been reviewed. Consider the potential effect of each of the unmeasured domains and whether all important confounding domains not controlled for in the analysis would be likely to change the estimate in the same direction, or if one important confounding domain that was not controlled for in the analysis is likely to have a dominant impact. | Favours experimental / Favours comparator / Unpredictable | |
| Bias in selection of participants into the study | 2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? | This domain is concerned only with selection into the study based on participant characteristics observed after the start of intervention. Selection based on characteristics observed before the start of intervention can be addressed by controlling for imbalances between intervention and control groups in baseline characteristics that are prognostic for the outcome (baseline confounding). | Y / PY / PN / N / NI |
| IfN orPN to2.1: go to 2.4 | |||
| 2.2. IfY orPY to2.1: were the post‐intervention variables that influenced selection likely to be associated with intervention | Selection bias occurs when selection is related to an effect of either intervention or a cause of intervention and an effect of either the outcome or a cause of the outcome. Therefore, the result is at risk of selection bias if selection into the study is related to both the intervention and the outcome. | NA / Y / PY / PN / N / NI | |
| 2.3 If Y orPY to2.2: were the post‐intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? | NA / Y / PY / PN / N / NI | ||
| 2.4. Do start of follow up and start of intervention coincide for most participants? | If participants are not followed from the start of the intervention then a period of follow up has been excluded, and individuals who experienced the outcome soon after intervention will be missing from analyses. This problem may occur when prevalent, rather than new (incident), users of the intervention are included in analyses. | Y / PY / PN / N / NI | |
| 2.5. IfY orPY to2.2 and2.3, or N orPN to 2.4: were adjustment techniques used that are likely to correct for the presence of selection biases? | It is in principle possible to correct for selection biases, for example by using inverse probability weights to create a pseudo‐population in which the selection bias has been removed, or by modelling the distributions of the missing participants or follow up times and outcome events and including them using missing data methodology. However such methods are rarely used and the answer to this question will usually be “No” | NA / Y / PY / PN / N / NI | |
| Risk of bias judgement | Low ‐ all participants who would have been eligible for the target trial were included in the study and start of follow up and start of intervention coincide for all subjects. | Low / Moderate / Serious / Critical / NI | |
| Moderate ‐ selection into the study may have been related to intervention and outcome, but the authors used appropriate methods to adjust for the selection bias; or Start of follow up and start of intervention do not coincide for all participants, but (a) the proportion of participants for which this was the case was too low to induce important bias; (b) the authors used appropriate methods to adjust for the selection bias; or (c) the review authors are confident that the rate (hazard) ratio for the effect of intervention remains constant over time. | |||
|
Serious ‐ selection into the study was related to intervention and outcome; or Start of follow up and start of intervention do not coincide, and a potentially important amount of follow‐up time is missing from analyses, and the rate ratio is not constant over time. | |||
|
Critical ‐ selection into the study was strongly related to intervention and outcome; or A substantial amount of follow‐up time is likely to be missing from analyses, and the rate ratio is not constant over time. | |||
| Optional: what is the predicted direction of bias due to selection of participants into the study? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias in classification of interventions | 3.1 Were intervention groups clearly defined? | A pre‐requisite for an appropriate comparison of interventions is that the interventions are well defined. Ambiguity in the definition may lead to bias in the classification of participants. For individual‐level interventions, criteria for considering individuals to have received each intervention should be clear and explicit, covering issues such as type, setting, dose, frequency, intensity and/or timing of intervention. For population‐level interventions (e.g. measures to control air pollution), the question relates to whether the population is clearly defined, and the answer is likely to be ‘Yes’. | Y / PY / PN / N / NI |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | In general, if information about interventions received is available from sources that could not have been affected by subsequent outcomes, then differential misclassification of intervention status is unlikely. Collection of the information at the time of the intervention makes it easier to avoid such misclassification. For population‐level interventions (e.g. measures to control air pollution), the answer to this question is likely to be ‘Yes’. | Y / PY / PN / N / NI | |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | Collection of the information at the time of the intervention may not be sufficient to avoid bias. The way in which the data are collected for the purposes of the NRSI should also avoid misclassification. | Y / PY / PN / N / NI | |
| Risk of bias judgement | Low ‐ intervention status is well defined and based solely on information collected at the time of intervention. | Low / Moderate / Serious / Critical / NI | |
| Moderate ‐ intervention status is well defined but some aspects of the assignments of intervention status were determined retrospectively | |||
| Serious ‐ intervention status is not well defined, or major aspects of the assignments of intervention status were determined in a way that could have been affected by knowledge of the outcome. | |||
| Critical ‐ (unusual) An extremely high amount of misclassification of intervention status, e.g. because of unusually strong recall biases. | |||
| Optional: what is the predicted direction of bias due to measurement of outcomes or interventions? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias due to departures from intended interventions | 4.1. Was the intervention implemented successfully for most participants? | Consider the success of implementation of the intervention in the context of its complexity. Was recommended practice followed by those administering the intervention? | Y / PY / PN / N / NI |
| If your aim for this study is to assess the effect of initiating and adhering to intervention (as in a per‐protocol analysis), answer questions 4.2 to 4.4 | |||
| 4.2. Did study participants adhere to the assigned intervention regimen? | Lack of adherence to assigned intervention includes cessation of intervention, crossovers to the comparator intervention and switches to another active intervention. We distinguish between analyses where: (1) intervention switches led to follow up time being assigned to the new intervention; and (2) intervention switches (including cessation of intervention) where follow up time remained allocated to the original intervention; (3) is addressed under time‐varying confounding, and should not be considered further here. Consider available information on the proportion of study participants who continued with their assigned intervention throughout follow up. Was lack of adherence sufficient to impact the intervention effect estimate? |
NA/ Y / PY / PN / N / NI | |
| 4.3. Were important co‐interventions balanced across intervention groups? | Consider the co‐interventions that are likely to affect the outcome and to have been administered in the context of this study, based on the preliminary consideration of co‐interventions and available literature. Consider whether these co‐interventions are balanced between intervention groups. | NA/ Y / PY / PN / N / NI | |
| 4.4. IfN orPN to4.1, 4.2 or4.3: were adjustment techniques used that are likely to correct for these issues? | Such adjustment techniques include inverse‐probability weighting to adjust for censoring at deviation from intended intervention, or inverse probability weighting of marginal structural models to adjust for time‐varying confounding. Specialist advice may be needed to assess studies that used these approaches. | NA / Y / PY / PN / N / NI | |
| Risk of bias judgement | Low ‐ no bias due to deviation from the intended intervention is expected, for example if both the intervention and comparator are implemented over a short time period, and subsequent interventions are part of routine medical care, or if the specified comparison relates to initiation of intervention regardless of whether it is continued. | Low / Moderate / Serious / Critical / NI | |
| Moderate ‐ bias due to deviation from the intended intervention is expected, and switches, co‐interventions, and some problems with intervention fidelity are appropriately measured and adjusted for in the analyses. Alternatively, most (but not all) deviations from intended intervention reflect the natural course of events after initiation of intervention. | |||
| Serious ‐ switches in treatment, co‐interventions, or problems with implementation fidelity are apparent and are not adjusted for in the analyses. | |||
| Critical ‐ substantial deviations from the intended intervention are present and are not adjusted for in the analysis. | |||
| Optional: what is the predicted direction of bias due to departures from the intended interventions? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias due to missing data | 5.1 Were there missing outcome data? | This aims to elicit whether the proportion of missing observations is likely to result in missing information that could substantially impact our ability to answer the question being addressed. Guidance will be needed on what is meant by ‘reasonably complete’. One aspect of this is that review authors would ideally try and locate an analysis plan for the study. | Y / PY / PN / N / NI |
| 5.2 Were participants excluded due to missing data on intervention status? | Missing intervention status may be a problem. This requires that the intended study sample is clear, which it may not be in practice. | Y / PY / PN / N / NI | |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | This question relates particularly to participants excluded from the analysis because of missing information on confounders that were controlled for in the analysis. | Y / PY / PN / N / NI | |
| 5.4 If Y orPY to 5.1, 5.2 or5.3: are the proportion of participants and reasons for missing data similar across interventions? | This aims to elicit whether either (i) differential proportion of missing observations or (ii) differences in reasons for missing observations could substantially impact on our ability to answer the question being addressed. | NA / Y / PY / PN / N / NI | |
| 5.5If Y or PY to5.1, 5.2 or5.3: were appropriate statistical methods used to account for missing data? | It is important to assess whether assumptions employed in analyses are clear and plausible. Both content knowledge and statistical expertise will often be required for this. For instance, use of a statistical method such as multiple imputation does not guarantee an appropriate answer. Review authors should seek naïve (complete‐case) analyses for comparison, and clear differences between complete‐case and multiple imputation‐based findings should lead to careful assessment of the validity of the methods used. | NA / Y / PY / PN / N / NI | |
| Risk of bias judgement | Low ‐ data were reasonably complete; or Proportions of and reasons for missing participants were similar across intervention groups; or Analyses that addressed missing data are likely to have removed any risk of bias. | Low / Moderate / Serious / Critical / NI | |
| Moderate ‐ proportions of missing participants differ across interventions; or Reasons for missingness differ minimally across interventions; and Missing data were not addressed in the analysis. | |||
| Serious ‐ proportions of missing participants differ substantially across interventions; or Reasons for missingness differ substantially across interventions; and Missing data were addressed inappropriately in the analysis; or The nature of the missing data means that the risk of bias cannot be removed through appropriate analysis. | |||
| Critical ‐ (unusual) There were critical differences between interventions in participants with missing data that were not, or could not, be addressed through appropriate analysis. | |||
| Optional: what is the predicted direction of bias due to missing data? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias in measurement of outcomes | 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | Some outcome measures involve negligible assessor judgment, e.g. all‐cause mortality or non‐repeatable automated laboratory assessments. Risk of bias due to measurement of these outcomes would be expected to be low. | Y / PY / PN / N / NI |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | If outcome assessors were blinded to intervention status, the answer to this question would be ‘No’. In other situations, outcome assessors may be unaware of the interventions being received by participants despite there being no active blinding by the study investigators; the answer this question would then also be ‘No’. In studies where participants report their outcomes themselves, for example in a questionnaire, the outcome assessor is the study participant. In an observational study, the answer to this question will usually be ‘Yes’ when the participants report their outcomes themselves. | Y / PY / PN / N / NI | |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | Comparable assessment methods (i.e. data collection) would involve the same outcome detection methods and thresholds, same time point, same definition, and same measurements | Y / PY / PN / N / NI | |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | This question refers to differential misclassification of outcomes. Systematic errors in measuring the outcome, if present, could cause bias if they are related to intervention or to a confounder of the intervention‐outcome relationship. This will usually be due either to outcome assessors being aware of the intervention received or to non‐comparability of outcome assessment methods, but there are examples of differential misclassification arising despite these controls being in place. | Y / PY / PN / N / NI | |
| Risk of bias judgement |
Low ‐ the methods of outcome assessment were comparable across intervention groups; and The outcome measure was unlikely to be influenced by knowledge of the intervention received by study participants (i.e. is objective) or the outcome assessors were unaware of the intervention received by study participants; and Any error in measuring the outcome is unrelated to intervention status. |
Low / Moderate / Serious / Critical / NI | |
|
Moderate ‐ the methods of outcome assessment were comparable across intervention groups; and The outcome measure is only minimally influenced by knowledge of the intervention received by study participants; and Any error in measuring the outcome is only minimally related to intervention status. | |||
|
Serious ‐ the methods of outcome assessment were not comparable across intervention groups; or The outcome measure was subjective (i.e. likely to be influenced by knowledge of the intervention received by study participants) and was assessed by outcome assessors aware of the intervention received by study participants; or Error in measuring the outcome was related to intervention status. | |||
| Critical ‐ the methods of outcome assessment were so different that they cannot reasonably be compared across intervention groups. | |||
| Optional: what is the predicted direction of bias due to measurement of outcomes? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias in selection of the reported result | Is the reported effect estimate unlikely to be selected, on the basis of the results, from... | ||
| 7.1. ... multiple outcome measurements within the outcome domain? | For a specified outcome domain, it is possible to generate multiple effect estimates for different measurements. If multiple measurements were made, but only one or a subset is reported, there is a risk of selective reporting on the basis of results. | Y / PY / PN / N / NI | |
| 7.2 ... multiple analyses of the intervention‐outcome relationship? | Because of the limitations of using data from non‐randomized studies for analyses of effectiveness (need to control confounding, substantial missing data, etc), analysts may implement different analytic methods to address these limitations. Examples include unadjusted and adjusted models; use of final value vs change from baseline vs analysis of covariance; different transformations of variables; a continuously scaled outcome converted to categorical data with different cutpoints; different sets of covariates used for adjustment; and different analytic strategies for dealing with missing data. Application of such methods generates multiple effect estimates for a specific outcome metric. If the analyst does not prespecify the methods to be applied, and multiple estimates are generated but only one or a subset is reported, there is a risk of selective reporting on the basis of results. | Y / PY / PN / N / NI | |
| 7.3 ... different subgroups? | Particularly with large cohorts often available from routine data sources, it is possible to generate multiple effect estimates for different subgroups or simply to omit varying proportions of the original cohort. If multiple estimates are generated but only one or a subset is reported, there is a risk of selective reporting on the basis of results. | Y / PY / PN / N / NI | |
| Risk of bias judgement | Low ‐ there is clear evidence (usually through examination of a pre‐registered protocol or statistical analysis plan) that all reported results correspond to all intended outcomes, analyses and sub‐cohorts. | Low / Moderate / Serious / Critical / NI | |
|
Moderate ‐ the outcome measurements and analyses are consistent with an a priori plan; or are clearly defined and both internally and externally consistent; and there is no indication of selection of the reported analysis from among multiple analyses; and there is no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. | |||
| Serious ‐ outcome measurements or analyses are internally or externally inconsistent; or There is a high risk of selective reporting from among multiple analyses; or The cohort or subgroup is selected from a larger study for analysis and appears to be reported on the basis of the results. | |||
| Critical ‐ there is evidence or strong suspicion of selective reporting of results, and the unreported results are likely to be substantially different from the reported results. | |||
| Optional: What is the predicted direction of bias due to selection of the reported result? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Overall bias | Risk of bias judgement | Low ‐ the study is judged to be at low risk of bias for all domains. | Low / Moderate / Serious / Critical / NI |
| Moderate ‐ the study is judged to be at low or moderate risk of bias for all domains. | |||
| Serious ‐ the study is judged to be at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. | |||
| Critical ‐ the study is judged to be at critical risk of bias in at least one domain. | |||
| No information ‐ there is no clear indication that the study is at serious or critical risk of bias and there is a lack of information in one or more key domains of bias (a judgement is required for this). | |||
| Optional: what is the overall predicted direction of bias for this outcome? |
Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | ||
Data and analyses
Comparison 1. DFP versus DFO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to iron chelation therapy (%, SD) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 SAEs (from therapy, disease, non‐adherence) | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 2.1 Agranulocytosis | 1 | Risk Ratio (M‐H, Random, 99% CI) | 0.0 [0.0, 0.0] | |
| 3 All‐cause mortality | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4 Iron overload: defined as proportion of participants with serum ferritin ≥ 800 (µg/L) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Organ damage | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Liver damage | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Other AEs related to iron chelation | 3 | Risk Ratio (IV, Random, 99% CI) | Subtotals only | |
| 6.1 Risk of leukopenia, neutropenia and/or agranulocytosis | 3 | 192 | Risk Ratio (IV, Random, 99% CI) | 3.94 [0.44, 35.50] |
| 6.2 Risk of pain or swelling in joints | 3 | 192 | Risk Ratio (IV, Random, 99% CI) | 3.38 [0.54, 21.31] |
| 6.3 Risk of nausea/vomiting | 2 | 132 | Risk Ratio (IV, Random, 99% CI) | 13.68 [0.99, 188.88] |
| 6.4 Risk of increased liver transaminase | 1 | 44 | Risk Ratio (IV, Random, 99% CI) | 1.10 [0.03, 38.47] |
| 6.5 Local reactions at infusion site | 1 | 88 | Risk Ratio (IV, Random, 99% CI) | 0.17 [0.00, 9.12] |
Comparison 2. DFX versus DFO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to iron chelation therapy (%, SD) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 SAEs | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Thalassaemia‐related SAEs | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 SCD‐related SAE ‐ painful crisis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 SCD‐related SAEs ‐ other SCD‐related SAEs | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 All‐cause mortality (thalassaemia) | 2 | 240 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.06, 15.06] |
| 4 Proportion of participants with iron overload (thalassaemia) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Iron overload defined by ferritin 1500 (µg/l) or higher (Thalassaemia) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.63, 2.20] |
| 4.2 Proportion with severe iron overload (LIC at least 15 mg/Fe/g dw) | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.20] |
| 4.3 Myocardial T2* < 10ms | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.72, 1.70] |
| 5 Other AEs related to iron chelation ‐ (thalassaemia) | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Total chelation‐related AE | 1 | 187 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.76, 1.73] |
| 5.2 Gastrointestinal upset | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.66, 13.69] |
| 5.3 Rash | 2 | 247 | Risk Ratio (IV, Random, 95% CI) | 3.05 [0.98, 9.47] |
| 5.4 Risk of increased blood creatinine | 1 | 187 | Risk Ratio (IV, Random, 95% CI) | 3.79 [0.83, 17.38] |
| 5.5 Risk of proteinuria | 1 | 187 | Risk Ratio (IV, Random, 95% CI) | 2.21 [0.59, 8.29] |
| 5.6 Risk of increased ALT | 1 | 187 | Risk Ratio (IV, Random, 95% CI) | 5.69 [0.70, 46.33] |
| 5.7 Risk of increased AST | 1 | 187 | Risk Ratio (IV, Random, 95% CI) | 5.69 [0.70, 46.33] |
| 5.8 Risk of diarrhoea | 1 | 187 | Risk Ratio (IV, Random, 95% CI) | 5.69 [0.70, 46.33] |
| 5.9 Risk of vomiting | 1 | 187 | Risk Ratio (IV, Random, 95% CI) | 6.64 [0.35, 126.78] |
| 6 Total AEs (thalassaemia) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7 Other AEs related to iron chelation (SCD) | 1 | Risk Ratio (M‐H, Random, 99% CI) | Subtotals only | |
| 7.1 Risk of increased ALT | 1 | 195 | Risk Ratio (M‐H, Random, 99% CI) | 5.29 [0.12, 232.98] |
| 7.2 incidence of abdominal pain | 1 | 195 | Risk Ratio (M‐H, Random, 99% CI) | 1.91 [0.80, 4.58] |
| 7.3 Risk of pain or swelling in joints | 1 | 195 | Risk Ratio (M‐H, Random, 99% CI) | 1.06 [0.41, 2.76] |
| 7.4 Risk of diarrhoea | 1 | 195 | Risk Ratio (M‐H, Random, 99% CI) | 4.14 [0.90, 18.92] |
| 7.5 Nausea/vomiting | 1 | 195 | Risk Ratio (M‐H, Random, 99% CI) | 1.63 [0.90, 2.94] |
Comparison 3. DFX film‐coated tablet versus DFX dispersible tablet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to iron chelation therapy | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.99, 1.22] |
| 2 Incidence of SAEs | 1 | 173 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.62, 2.37] |
| 3 All‐cause mortality | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.12, 71.81] |
| 4 Incidence of organ damage (renal event) | 1 | 173 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.83, 1.91] |
| 5 Other AEs related to iron chelation | 1 | Risk Ratio (IV, Random, 99% CI) | Subtotals only | |
| 5.1 Total chelation‐related AEs | 1 | 173 | Risk Ratio (IV, Random, 99% CI) | 0.75 [0.52, 1.08] |
| 5.2 Risk of diarrhoea | 1 | 173 | Risk Ratio (IV, Random, 99% CI) | 0.70 [0.29, 1.70] |
| 5.3 Increased urine protein/urine creatinine ratio | 1 | 173 | Risk Ratio (IV, Random, 99% CI) | 1.65 [0.60, 4.54] |
| 5.4 incidence of abdominal pain | 1 | 173 | Risk Ratio (IV, Random, 99% CI) | 0.49 [0.16, 1.52] |
| 5.5 Incidence of nausea | 1 | 173 | Risk Ratio (IV, Random, 99% CI) | 0.72 [0.23, 2.23] |
| 5.6 Incidence of vomiting | 1 | 173 | Risk Ratio (IV, Random, 99% CI) | 0.28 [0.07, 1.15] |
Comparison 4. DFP and DFO versus DFP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of SAEs | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 2.81] |
| 2 All‐cause mortality | 2 | 237 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.18, 3.35] |
| 3 Incidence of chelation therapy‐related AEs | 3 | Risk Ratio (IV, Random, 99% CI) | Subtotals only | |
| 3.1 Risk of leukopenia, neutropenia and/or agranulocytosis | 3 | 280 | Risk Ratio (IV, Random, 99% CI) | 1.15 [0.50, 2.62] |
| 3.2 Risk of pain or swelling in joints | 2 | 256 | Risk Ratio (IV, Random, 99% CI) | 0.76 [0.31, 1.91] |
| 3.3 Risk of gastrointestinal disturbances | 1 | 213 | Risk Ratio (IV, Random, 99% CI) | 0.45 [0.15, 1.37] |
| 3.4 Risk of increased liver transaminase | 2 | 256 | Risk Ratio (IV, Random, 99% CI) | 1.02 [0.52, 1.98] |
| 3.5 Nausea/vomiting | 1 | 43 | Risk Ratio (IV, Random, 99% CI) | 0.55 [0.13, 2.23] |
Comparison 5. DFP and DFO versus DFO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other AEs related to iron chelation | 4 | Risk Ratio (IV, Random, 99% CI) | Subtotals only | |
| 1.1 Risk of leukopenia, neutropenia and/or agranulocytosis | 3 | 169 | Risk Ratio (IV, Random, 99% CI) | 1.18 [0.09, 15.37] |
| 1.2 Risk of pain or swelling in joints | 3 | 135 | Risk Ratio (IV, Random, 99% CI) | 2.39 [0.18, 32.31] |
| 1.3 Risk of increased liver transaminase | 2 | 104 | Risk Ratio (IV, Random, 99% CI) | 3.46 [0.45, 26.62] |
| 1.4 Nausea/vomiting | 4 | 194 | Risk Ratio (IV, Random, 99% CI) | 3.81 [0.84, 17.36] |
| 1.5 Local reactions at infusion site | 2 | 90 | Risk Ratio (IV, Random, 99% CI) | 0.18 [0.01, 3.56] |
Comparison 6. DFP/DFX versus DFP/DFO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to iron chelation therapy rates | 1 | 96 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.72, 0.99] |
| 2 Incidence of SAE | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.53] |
| 3 All‐cause mortality | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4 Organ damage (serum creatinine (≥33%) above baseline in 2 consecutive occasions) | 1 | 96 | Risk Ratio (M‐H, Random, 99% CI) | 3.0 [0.16, 56.04] |
| 5 Other AEs related to iron chelation | 1 | Risk Ratio (IV, Random, 99% CI) | Subtotals only | |
| 5.1 one year (study end) | 1 | 96 | Risk Ratio (IV, Random, 99% CI) | 1.08 [0.68, 1.71] |
| 5.2 Risk of leukopenia, neutropenia and/or agranulocytosis | 1 | 96 | Risk Ratio (IV, Random, 99% CI) | 1.67 [0.27, 10.14] |
| 5.3 Risk of pain or swelling in joints | 1 | 96 | Risk Ratio (IV, Random, 99% CI) | 0.89 [0.29, 2.77] |
| 5.4 Gastrointestinal problems | 1 | 96 | Risk Ratio (IV, Random, 99% CI) | 0.6 [0.18, 2.04] |
| 5.5 ALT (increase ≥3 folds) | 1 | 96 | Risk Ratio (IV, Random, 99% CI) | 1.33 [0.20, 8.88] |
| 5.6 Skin rash | 1 | 96 | Risk Ratio (IV, Random, 99% CI) | 5.0 [0.10, 261.34] |
6.3. Analysis.
Comparison 6 DFP/DFX versus DFP/DFO, Outcome 3 All‐cause mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aydinok 2007.
| Methods |
Study design: single‐centre RCT Study grouping: parallel group Study duration: treatment duration 12 months; follow‐up: not stated |
|
| Participants |
Baseline characteristics DFP, DFO
DFP
Inclusion criteria: iron‐overloaded people with thalassaemia at least 4 years old Exclusion criteria: lack of compliance, known toxicity or intolerance preventing therapy with DFO and DFP, neutropenia (neutrophils < 1.5×109/L), thrombocytopenia (platelets < 100×109/L), renal, hepatic or decompensated heart failure, active viral illness being treated with interferon‐α/ribavirin, repeated Yersinia infections, HIV–positivity, pregnancy or nursing, and patients of reproductive age not taking adequate contraceptive precautions |
|
| Interventions | Treatment arm: DFO (50 mg/kg/day subcutaneously twice weekly (mean (SD) dose: 43.8 (2.8) mg/kg)) combined with DFP (75 mg/kg/day, daily (mean (SD) dose: 78.2 (1.4) mg/kg/day)) Comparator arm: DFP (75 mg/kg/day, daily (mean (SD) dose: 78.2 (2.6) mg/kg/day)) | |
| Outcomes |
Adherence: compliance was assessed by drug accounting at each visit (by counting the returned empty blisters of DFP and used vials of DFO) as well as by a trial‐specific questionnaire completed by the participants and/or their legal representative/guardian at quarterly intervals. The same questionnaire also served for the assessment of tolerance to treatment and QoL Trial‐reported outcomes 1. Changes in LIC and SF (primary outcome) 2. Total iron excretion 3. Urinary iron excretion 4. Iron balance 5. Cardiac function (Echo) 6. Toxicity 7. Assessment of tolerance to treatment and QoL |
|
| Identification | Source of funding: none stated although the drugs were supplied by Lipomed AG, Switzerland | |
| Notes | All participants had prior exposure to DFO (dose, schedule and duration were not reported) and all had a washout period of 2 weeks with no iron chelation before initiating trial treatment
Sample‐size calculation not reported Country: Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was generated by the Department of Mathematical Statistics at the University of Berne, Switzerland according to local policy". Following central registration of a subject by the investigator, the trial co‐ordinator assigned the intervention according to the randomisation sequence |
| Allocation concealment (selection bias) | High risk | The trial report states that the intervention was assigned according to the randomisation sequence “without concealing the sequence prior to allocation” |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | The authors did not report any information as to whether participants, personnel were blinded to treatment allocation but one treatment subcutaneous and other oral so difficult to blind |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Unclear risk | The authors did not report any information as to whether outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was an imbalance in missing data across the treatment arms. 4 participants from the comparator group (DFO) were not included in the outcome analysis: 2 withdrew consent due to refusal to take DFO; 1 died from arrhythmia induced congestive heart failure at start of trial; and 1 developed agranulocytosis at week 14 |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | There is an imbalance in baseline LIC and Ferritin between groups |
Badawy 2010.
| Methods |
Study design: RCT Study grouping: parallel group Length of trial or follow‐up not stated. Not stated if open label; but no mention of blinding and DFO is infusion versus tablet |
|
| Participants |
Baseline characteristics DFP, DFO
DFP
DFO
Inclusion criteria: 8 years, RBC transfusion every 3 to 4 weeks, on DFO prior to study as single therapy. Exclusion criteria: not stated Participants PRBCs /3 – 4 weeks to maintain Hb > 9 g/dL |
|
| Interventions |
DFP, DFO
DFP
DFO
|
|
| Outcomes |
Adherence to iron chelation therapy rates Questionnaire on chelation therapy, reasons for non‐compliance, side effects, life activities, transfusion regimen Trial‐reported outcomes 1. CBC monthly 2. SF levels 3. liver and kidney functions 4. blood glucose level 5. serum calcium and phosphorus/3 months and T3, T4,TSH, LH, FSH 6. echocardiography 7. bone density 8. auditory and visual examination twice |
|
| Identification |
Sponsorship source: Zagazig University Hospital, Zagazig Country: Egypt Setting: University Hospital Comments: Abstract Poster 124 Authors name: Sherif Badawy Institution: Ann Robert H. Lurie Children’s Hospital of Chicago Email: sbadawy@luriechildrens.org Address: Ann Robert H. Lurie Children’s Hospital of Chicago Northwestern University Feinberg School of Medicine225 East Chicago Avenue, Box 30, Chicago, Illinois 60611‐2605 |
|
| Notes | Contacted author and study data not available at this time. Sample‐size calculation not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: no description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Judgement comment no description, but one drug is subcutaneous injection (DFO). Open label |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Unclear risk | Judgement comment: no description of blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: no data on number of participants who completed the study and how many in each group experienced complications. Lack of detail on number of compliant or non‐compliant participants |
| Selective reporting (reporting bias) | High risk | Judgement comment: not clear which groups and how many experienced adverse events. No data reported on SF or other outcomes |
| Other bias | Unclear risk | Judgement comment: results of the trial were not published in detail and no data available when authors were contacted |
Bahnasawy 2017.
| Methods |
Study design: single‐centre RCT Study grouping: parallel group Study duration: 6 months |
|
| Participants |
Baseline characteristics Comprehensive medication management
Standard care (as defined in the trial)
Inclusion criteria: transfusion‐dependent children with β‐thalassaemia major aged 8 to 18 years with SF level of more than 1000 µg/L Exclusion criteria: people with cognitive impairment |
|
| Interventions |
Comprehensive medication management
Standard care (as defined in the trial)
|
|
| Outcomes |
Adherence to iron chelation therapy rates "DRP identification: The clinical pharmacist analysed the collected data to detect whether any DRPs existed and allocated them to one of the seven categories as classified by Cipolle et al. [18]: unnecessary drug therapy, need for additional drug therapy, ineffective drug product, dosage too low, adverse drug reaction, dosage too high, non‐compliance" Trial‐reported outcomes 1. SF levels were measured at baseline, 3 months and after 6 months 2. CBC with WBC differential was assessed at every visit, and SCr and ALT were measured routinely for all the participants every 3 months 3. Health‐related QoL was assessed at baseline and at the end of the trial (after 6 months) using PedsQL™ 4.0 Generic Core Scale questionnaire. PedsQL is a 23‐item multidimensional model with 4 domains for paediatric health‐related QoL measurement: physical functioning (8 items), emotional functioning (5 items), social functioning (5 items) and school functioning (5 items) (19). |
|
| Identification |
Sponsorship source: not stated Country: Egypt Setting: Hematology clinic Authors name: Lamia El Wakeel Institution: Pediatric Hematology Clinic, Children’s Hospital, Ain Shams University, Email: lamywak@yahoo.com Address: Lamia El Wakeel, Pediatric Hematology Clinic, Children’s Hospital, AinShams University, 4, Street 292 New Maadi, Cairo, Egypt |
|
| Notes | Sample‐size calculation not reported. Drug‐related outcomes do not have any comparable data reported. Only outcomes with comparable data reported are SF levels and health‐related QoL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a prospective, randomized, controlled study. It was conducted on pediatric BTM patients admitted to the Pediatric Hematology Clinic," Stratified randomization was used considering the iron chelation therapy as the stratification factor Judgement comment: no description of how randomisation was done or by whom |
| Allocation concealment (selection bias) | Unclear risk | The control group (n = 24) received standard medical care by a physician while the intervention group received standard medical care plus clinical pharmacist‐provided services. Judgement comment: no description of how participants were allocated to the pharmacist intervention or standard care |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Judgement comment: not possible to blind a pharmacist intervention versus no pharmacist intervention |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | High risk | Judgement comment: no indication that outcome assessors where different from pharmacists who implemented the intervention. Also most outcomes were reported only in the intervention group except for ferritin levels and health‐related QoL |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: all drug‐related outcomes were only reported in the intervention group including adherence ‐ no comparative data available. Multiple interventions in small number of participants |
| Selective reporting (reporting bias) | High risk | Judgement comment: drug‐related outcomes reported only in intervention group. No comparative data. The participants within the intervention arm seem to have complex and multiple changes. Difficult to tease out the actual intervention that effected a change |
| Other bias | Unclear risk | Judgement comment: small sample size and only report intervention group |
Calvaruso 2015.
| Methods |
Study design: RCT Study grouping: parallel group This trial was designed as a 5‐year, multicentre, randomised, open‐label trial with blinded data management and data analyses to evaluate whether the DFP treatment is superior to the DFO treatment Follow‐up after trial. An additional 5 years of follow‐up after the end of the trial was planned to collect data on the survival, cause of death and chelation treatment of this cohort of participants. During this period, the participants were allowed to change their chelation treatment |
|
| Participants |
Baseline characteristics DFP
DFO
Inclusion criteria: people with thalassaemia intermedia (based on clinical and molecular criteria), SF between 800 and 3000 µg/L, 13 years of age, consent from patient or parent or guardian (if 13 to 18) Exclusion criteria: known intolerance to treatment, platelet count < 100 ×109/L, white cell count of < 3 ×109/L, severe liver damage, sepsis or heart failure (or both) Pretreatment: none of the participants in the DFP group and 8 in the DFO group withdrew from the trial. 1 participant in the DFP group and 3 in the DFO group changed their chelation therapy (P value = 0.357) If the participants were treated with a subcutaneous administration of DFO (30 ‐ 50 mg/kg per day, 8 – 12 hours for 5 days a week) before inclusion in the trial, a DFO washout was executed for 1 week before randomisation.The minimum number of participants required for each treatment group was calculated, assuming equal allocation under the hypothesis of equality between the 2 treatment groups at each point during the course.The recommended number of participants was 30. One participant in the DFP group and 3 in the DFO group changed their chelation therapy |
|
| Interventions |
DFP
DFO
Treatment failure was defined as an increase in the SF level to greater than 1000 lg/L from baseline, confirmed by at least 2 consecutive determinations. Participants who failed were switched to the alternative treatment and followed until the end of the trial. The criteria for a dosage reduction to 50 mg/kg of DFP per day were arthralgia and nausea, and the criterion for a reduction to 30 mg/kg of DFO per day was a local reaction at the site of infusion. Both treatments were reduced if the ferritin levels for 2 consecutive determinations were less than 400 lg/L. The treatment was resumed when the ferritin levels were greater than 700 lg/L for at least 2 determinations |
|
| Outcomes |
Adherence to iron chelation therapy rates Compliance was assessed by counting the number of DFP pills in each returned bag and by assessing the number of infusions of DFO registered on the electronic pump Trial‐reported outcomes 1. The primary endpoint was treatment effectiveness, evaluated as the mean change in the SF level over the 5‐year period. This type of evaluation strengthened the power of the test for the sample‐size calculation compared with the standard. 2. The secondary endpoints were safety and survival analysis after 5 years |
|
| Identification |
Sponsorship source: contract grant sponsor: Franco and Piera Cutino Foundation Country: Italy (17 centres) Setting: haematology and thalassaemia clinical centres at institutions Recruitment: January 2001 to January 2006 Trial registration: NCT00733811 Authors name: Aurelio Maggio Institution: Unita Operativa Complessa Ematologia II, Email: md.amaggio@gmail.com Address: U.O.C. Ematologia II, A.O.R. “Villa Sofia – V. Cervello”, Palermo, Italy |
|
| Notes | Sample‐size calculation reported for primary outcome Notes: 9 participants changed from DFP therapy 5 to DFO 2 to none 1 to DFX 1 to DFP‐DFO 6 participants changed from DFO therapy 4 to DFP 1 to DFX 1 to DFP‐DFO |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was based on a computer‐ randomized list arranged in permuted blocks of 10 with a 1:1 ratio." |
| Allocation concealment (selection bias) | Low risk | To ensure for allocation concealment, treatments were assigned by telephone contact from the coordinating centre. The sequence was concealed until the interventions were assigned. Randomization was performed for each consecutive patient after verification of the exclusion criteria |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Quote: "open‐label trial" Judgement comment: 1 of 2 arms was desferal pump infusers, participants would know. Participants on DFO attended for weekly blood tests. |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Low risk | Quote: "with blinded data management and data analysis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up for 5‐year trial |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Unclear how participant variation relating to SF levels may have had effect on results. Although all outcomes were reported for the 5 year trial in the 5 years of follow‐up only mortality was reported |
El Beshlawy 2008.
| Methods |
Study design: single‐centre RCT Study grouping: parallel group, follow‐up for 54 weeks |
|
| Participants |
Baseline characteristics DFP/DFO
DFP
DFO
Inclusion criteria: males or females with thalassaemia major attending the Hematology Clinic at Cairo University Children Hospital; participants had to be iron overloaded with transfusion dependency and older than 4 years of age Exclusion criteria: known to have DFP or DFO toxicity; neutrophil count less than 1.5×109/L; platelet count less than 100×109/L; renal or hepatic insufficiency; decompensated heart failure; without contraceptive precaution; pregnant or nursing |
|
| Interventions |
DFP/DFO
DFP
DFO
|
|
| Outcomes |
Adherence to iron chelation therapy rates Compliance was assessed by performing a drug accounting at each patient visit by counting the returned empty blisters of DFP and used vials of DFO Trial‐reported outcomes 1. Incidence of chelation therapy‐related SAEs (reported in AEs) 2. Iron overload defined by ferritin over 1000 µg/L and/or clinical symptoms and/or signs of iron overload and/or need for medically indicated additional or change in chelation therapy (mean ferritin levels extrapolated from graph ‐ no SD provided) 3. Other AEs related to iron chelation (in this trial participants with an event are reported. 1 person could experience more than 1 event) 4. LIC mg/g dry weight (change from baseline (extrapolated from graph Least squares means / lower and upper value)) |
|
| Identification |
Sponsorship source Country: Egypt Setting: Hematology Clinic at Cairo University Children Hospital, Egypt Comments: 2 authors from Lipomed (DFP): C. Manz : C. Tarabishi Clinical Research Development, Lipomed AG, Arlesheim, Switzerland Authors name: A. El‐Beshlawy Institution: Faculty of Medicine, Cairo University, Email: amalelbeshlawy@yahoo.com Address: Faculty of Medicine, Cairo University, 32 Falaky Street, Bab El‐Louk, Cairo, Egypt |
|
| Notes | Sample‐size calculation reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: no description of how randomisation was accomplished: The participants were randomly assigned into 1 of 3 treatment arms |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | No mention of blinding ‐ since DFO is an injection and DFP is oral likely participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Unclear risk | Judgement comment: no blinding mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: a total of 10 participants dropped out of the trial as a result of several complications. Only 56 participants completed 54 weeks of treatment. Evaluation of LIC could not be done in another 8 participants. Reports on per protocol participants |
| Selective reporting (reporting bias) | High risk | Compliance not reported as number or percentage of participants compliant throughout trial: "Four patients, all treated with DFO‐based regimen, were excluded from the study due to lack of compliance. Compliance was otherwise excellent during the entire study. The majority of patients had no problems with the intake and swallowing of the DFP tablets. By contrast, 80% of patients in the combination arm and 76% of patients in the DFO monotherapy arm complained about difficulties in the parenteral use of DFO or problems to insert a needle", SF and LIC are partially reported in charts and no actual numbers are provided in the text. Also the focus on UIE over LIC and SF measures is misleading as DFP is known to have a higher UIE but this can be highly variable over multiple measurements. LIC is the gold standard and there was no difference in this outcome between groups. |
| Other bias | Unclear risk | There was a higher incidence of AEs in the combined group and the DFP group versus the DFO group |
Elalfy 2015.
| Methods |
Study design: RCT in 2 treatment centres Study grouping: parallel group Study duration: 1 year |
|
| Participants |
Baseline characteristics Group A: DFP/DFO
Group B: DFP/DFX
Inclusion criteria: people with β‐thalassaemia major aged 10 – 18 years with severe iron overload defined as: ferritin > 2500 μg/L on maximum tolerated dose of a single iron chelator with up trend of ferritin over the last 12 months prior to the study. People with LIC more than 7 mg/g by MRI R2* and mean cardiac T2* less than 20 and more than 6 ms calculated as geometric mean without clinical symptoms of cardiac dysfunction (shortness of breath at rest or exertion, orthopnoea, exercise intolerance, lower extremity oedema, arrhythmias). Adequacy of prior chelation defined as taking 75% of the calculated dose/month on maximum tolerated dose with upward ferritin trend Exclusion criteria: past history of agranulocytosis, clinically significant GI or renal disease, clinical cardiac disease, or with LVEF < 50% on baseline echocardiography; evidence of active hepatitis or serum transaminases > 3 times above ULN or renal impairment (serum creatinine > ULN) participation in a previous investigational drug study within the 30 days preceding screening, known allergy to DFX, DFP, and DFO. Pre‐treatment: baseline difference in mean Hb (P 0.004) |
|
| Interventions |
DFP/DFO
DFP/DFX
To achieve an acceptable treatment washout, chelation therapy was withdrawn for 2 weeks before randomisation, after verifying inclusion and exclusion criteria. The transfusion regimen aimed to maintain the participants pre‐transfusion Hb ≥80 g/L by receiving approximately 15 mL/kg packed RBCs every 3 – 4 weeks |
|
| Outcomes |
Adherence to iron chelation therapy rates Compliance was evaluated by counting of returned tablets for the oral chelators and of the vials for DFO. The percentage of actual dose that participant had taken in relation to the total prescribed dose was calculated Trial‐reported outcomes 1. % change in SF (from baseline to the end of trial) 2. % change in LIC (from baseline to the end of trial) 3. % change in cardiac MRI (from baseline to the end of trial) 4. SAEs and AEs (safety assessment) 5. Compliance 6. Satisfaction 7. QoL |
|
| Identification |
Sponsorship source: Ain Shams University Country: Egypt and Oman Setting: Thalassemia treatment centres (Ain Shams University, Egypt and Sultan Qaboos University Hospital, Oman) Comments: Government Clinical Trial NCT01511848 Authors name: Amira Abdel Moneam Adly, Institution: Department of Pediatrics, Ain Shams University, Cairo, Egypt Email: amiradiabetes@yahoo.com Address: 6 A ElSheshini street, Shoubra, Soudia buildings, Cairo, Egypt |
|
| Notes | The chelation regimens in the last year prior to the trial were daily DFX (14 participants), daily DFP (29 participants), and DFP 4 days/week alternating with subcutaneous DFO 3 days/week (53 participants) Sample‐size calculation reported Author contacted for additional info on SF 36 mean (SD) 6 months and end of trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was based on a computer randomised list in permuted blocks of 10 with a 1 : 1 ratio, generated at both University of Ain Shams and Sultan Qaboos" |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure no allocation bias, treatment group was assigned by telephone contact from the coordinating center in Ain Shams" |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Oral versus subcutaneous medication therefore participants would be aware which medication arm they had been randomised to |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Low risk | Quote: "open‐label study with blinded data management and data analyses" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: treatment was started within the following 24 hr, and all the included participants continued till the end of study with no participants were lost follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: provide only P values for patient satisfaction, satisfaction with ICT self‐reported satisfaction and all 'significantly' higher in group B; no actual end of trial data provided (mean (SD)). All outcomes are reported |
| Other bias | Unclear risk | Judgement comment: it is not clear how the investigators would have known that infections, GI disorders or skin disorders were not related to the drug therapies |
Galanello 2006.
| Methods | Study Design: 2‐arm parallel RCT conducted in Italy and Greece Number of centres: multicentre (3 centres) Duration of treatment: 12 month Follow‐up: not stated. | |
| Participants |
DFP/DFO
DFP/DFO
Inclusion criteria: participants were 10 years or older with a diagnosis of thalassaemia major undergoing iron chelation therapy with subcutaneous DFO, with a SF value between 1000 ‐ 4000 μg/L over the previous year. Exclusion criteria: not reported |
|
| Interventions | DFO: 20 ‐ 60 mg/kg/day subcutaneously on 5 ‐ 7 days a week (mean (SD) dose at baseline: 34.8 (8.9) mg/kg/day and at end of trial: 37.8 (8.9) mg/kg/day)) DFO/DFP: DFO 20 ‐ 60 mg/kg/day subcutaneously on 2 days a week (mean (SD) dose DFO for the 29 participants who completed the trial at baseline: 36.0 (5.8) mg/kg/day and at end of trial: 33.3 (6.64) mg/kg/day) with DFP 25 mg/kg/ body weight 3 x daily for 5 days a week) | |
| Outcomes |
Adherence see compliance below Trial‐reported outcomes 1. SF change at 1 year 2. LIC (measured by SQUID) change at 1 year 3. ALT 4. FBC 5. Zinc levels 6. AEs 7. Participant compliance: compliance with DFP was assessed by pill counts, diary cards and an electronic cap that recorded the time and date of each opening of the tablet container. Compliance with DFO was assessed by diary cards, weekly physical examination of infusion sites, and by the Crono™ infusion pump that recorded the number of completed infusions Primary outcome: not identified |
|
| Identification | Source of funding: Apotex Research Inc, Toronto, Canada. The last author of the study is an Apotex employee | |
| Notes | The trial inferred that participants had previously received DFO treatment but no details as to dose, schedule or duration were reported Sample‐size calculation not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not report any information about how randomisation was undertaken |
| Allocation concealment (selection bias) | Unclear risk | The authors did not report any information about how treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | Unclear risk | The authors did not report any information as to whether participants, personnel or outcome assessors were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Unclear risk | The authors did not report any information as to whether outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although 1 participant in the treatment group was withdrawn due to intolerance to DFP, this is unlikely to effect the findings of the trial |
| Selective reporting (reporting bias) | Unclear risk | Compliance to DFP was pre‐specified as an outcome but was not measured or reported in the manuscript |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Hassan 2016.
| Methods |
Study design: single‐centre RCT Study grouping: parallel group Trial duration: September 2014 to September 2015 |
|
| Participants |
Baseline characteristics DFX
DFO
Inclusion criteria: transfusion‐dependent β‐thalassaemia major, ages were ≥ 6 years, and they had SF levels greater than 1500 µg/L and were on irregular subcutaneous DFO chelation therapy Exclusion criteria: serum creatinine above the upper age‐related normal range, significant proteinuria (urinary protein/creatinine ratio 1.0 in a non–first‐void urine sample at baseline), elevated ALT more than 3‐fold of the ULN, GI diseases, clinically relevant auditory and/or ocular toxicity related to iron chelation therapy, cardiac disease, and/or SAEs with DFO or DFX, and absolute heutrophilic count 1500/mm3 or platelet count 100,000/mm3 Pre‐treatment: significant difference between the 2 groups with participants having splenectomy 4 in DFX group compared to 17 in DFO group (P = 0.001), hepatitis C status 2 in DFX group compared to 11 in DFO group (P = 0.005) and baseline ALT baseline mean of 28.2 in the DFX group compared to 46.1 in the DFO group (P = 0.001) |
|
| Interventions |
DFX
DFO
7‐day washout phase |
|
| Outcomes |
Adherence to iron chelation therapy rates During the study, we kept records of all dosages administered, all study medications that were dispensed and returned, and intervals between visits to determine compliance with the treatment. The patients’ parents were instructed to contact the investigator if the patients were unable to take the study drug as prescribed Trial‐reported outcomes 1. decrease in the SF level to < 1500 μg/L 2. Safety of the drugs that were used |
|
| Identification |
Sponsorship source: not stated Country: Egypt Setting: out‐patient paediatric hematology clinic Al‐ Hussein University Hospital, Al‐Azhar University, Cairo, Egypt Comments: no conflict of interest. Authors name: Dr Omar Atef Tolba Institution: Cairo University Children's Hospital Email: omartolba80@yahoo.com Address: Dr Omar Atef Tolba, Cairo University Children's Hospital, Department of Pediatrics, Cairo University, Egypt. Tel: +201222101717, +20233025539, Fax: +20233025539 There is no conflict of interest declared |
|
| Notes | Sample‐size calculation not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomized in a 1:1 ratio based on permuted blocks to receive deferasirox (DFX) or deferoxamine (DFO) for one year." Judgement comment: it is unclear risk as there is imbalance in the groups on several variables |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described and imbalance between groups |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Judgement comment: oral tablet versus subcutaneous infusion ‐ unable to blind participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | High risk | Quote: "During the study, we kept records of all dosages administered, all study medications that were dispensed and returned, and intervals between visits to determine compliance with the treatment." Judgement Comment: Does not state if outcome assessors were blinded. Assessors would be aware the treatment participants were on. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "no discontinuation of drugs or drop‐out of follow‐up occurred." |
| Selective reporting (reporting bias) | High risk | Quote: "Post‐treatment levels of ALT and AST were significantly higher in the DFO group (p = 0.022, p = 0.020, respectively), both drugs have comparable safety profiles, as the adverse effects noted did not reach clinical significance or lead to discontinuation of treatment with either agent. In the light of the comparable efficacy and safety of both agents for the reduction of iron overload, as was reported in the monotherapy of patients with transfusion‐dependent thalassaemia (31, 32), the oral preparation merits convenience and therefore patient compliance and adherence to treatment regimen that needs to be taken on a long‐term basis." "The oral DFX is recommended due to more convenience to assure adherence to treatment regimen." Judgement comment: the data within this trial do not provide evidence that DFX assures adherence. Pre‐treatment ALT, AST were also higher in the DFO group ‐ and also reflects imbalance in randomisation. Most outcomes vaguely reported (i.e. compliance ‐ not percentages even though did a count and closely monitored). Also not clear if all drug‐related AEs reported (i.e. agranulocytosis). Further the evidence is uncertain from this trial that both drugs of comparable efficacy and safety |
| Other bias | Unclear risk | Small trial N = 60 and short‐term follow‐up. Sample‐size calculation not reported, and single‐centre trial |
Maggio 2009.
| Methods |
Study design: multicentre RCT Study grouping: parallel group Consecutive thalassaemia major participants (n = 275) were observed at the 25 SoSTE centres from September 30, 2000 to January 31, 2008 9 participants did not meet inclusion criteria and 53 patients declined to participate. The remaining 213 participants were included; 105 and 108 respectively, were randomly allocated to DFP–DFO sequential treatment or DFP alone (Fig 1). None of the participants were lost to follow‐up Study duration: 5 year follow‐up |
|
| Participants |
Baseline characteristics DFP/DFO
DFP
Inclusion criteria: thalassaemia major, SF between 800 and 3000 ug/L over 13 years of age Exclusion criteria: known intolerance treatment, platelet count 100 x 109/l or leucocyte count 3.0 x 109/l, severe liver damage, heart failure |
|
| Interventions |
DFP/DFO
DFP
|
|
| Outcomes |
Adherence Compliance was assessed by counting the pills in each returned bag of DFP and by assessing the number of infusions of DFO registered on the electronic pump Trial‐reported outcomes 1. Difference between multiple observations of SF concentrations during the 5‐year treatment. A correlation between LIC and SF levels has previously been shown in cohort of people with thalassaemia major treated with DFP (Olivieri et al, 1995). 2. Survival analysis 3. AEs 4. Costs 5. Multislice‐multiecho T2* MRI scan, available since June 2004, was used in a subgroup of participants to evaluate variations in the iron content of the heart and liver during the trial |
|
| Identification |
Sponsorship source: Italian Society for the Study of Thalassaemia and Haemoglobinopathies (SoSTE) Country: Italy Setting: 25 SoSTE centres in Italy Comments: NCT 00733811 Authors name: Aurelio Maggio Institution: A.O.V. Cervello, U.O.C. di Ematologia Email: aureliomaggio@virgilio.it Address: A.O.V. Cervello, U.O.C. di Ematologia II,Cervello’’, Palermo, Italy |
|
| Notes | Follow‐up was planned for 5 years; however, because of the beneficial effects, in terms of SF levels reduction in the sequential DFP–DFO group, observed after the interim analysis performed at 31 January 2008 the trial was stopped before the planned 5 years of treatment were completed for all participants years but mean (SD) duration of treatment was 2.5 (2.2) and 2.9 ( 2.1) years for DFP and sequential DFP–DFO groups, respectively Sample‐size calculation reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was based on a computer‐randomized list in permuted blocks of 10 with a 1:1 ratio," Judgement comment: the randomization sequence was based on a computer‐randomized list in permuted blocks of 10 with a 1:1 ratio. The sequence was concealed until interventions were assigned. Randomization was performed per each consecutive participant after verification of the exclusion criteria |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure allocation concealment, treatment was assigned by telephone contact from the coordinating centre" |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Trial was open‐label |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Low risk | Quote: "All outcome assessments were done under code by physicians blinded to the trial treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The statistical analysis was based on the 'intention‐to‐treat' principle. None of the participants were lost to follow‐up. However, SF measurements were only complete for all participants in the first year of the trial and decrease substantially thereafter to n = 32 in the combined group and n = 26 in the DFP group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | "Only 21 (35%) subjects in the DFP‐alone and 12 (24%) in the sequential DFP–DFO group withdrew definitely from the trial (Table V). The mean time for definitive withdrawal was 152 ± 103 (days) in DFP‐alone versus 112 ± 76 (days) in the sequential DFP–DFO group respectively." "The planned duration of treatment was 5 years. However, because of the beneficial effects, in terms of serum ferritin levels reduction in the sequential DFP–DFO group, observed after the interim analysis performed at January 31, 2008 the trial was stopped before the planned 5 years of treatment were completed for all patients. Therefore, the mean duration of treatment was 2.5 ± 2.2 and 2.9 ± 2.1 years for DFP and sequential DFP–DFO group respectively" Judgement comment: withdrawal rate is high and the trial stopped early |
Mourad 2003.
| Methods | 2‐arm parallel RCT.
Number of centres: 1.
Trial dates: not stated.
Duration of treatment: 1 year.
Follow‐up: none. Trial undertaken: Chronic Care Centre, Beirut, Lebanon. |
|
| Participants | Number randomised: 25 (treatment group: 14; comparator group: 11)
Number analysed: 25 (treatment group: 14; comparator group: 11) β‐thalassaemia participants, severely iron overloaded and previously poorly chelated Age range: 12 ‐ 40 years Sex: treatment: 43% male, comparator: 64% male Ethnicity: not stated |
|
| Interventions |
DFO
DFP/DFO
|
|
| Outcomes |
Adherence see compliance below Trial‐reported outcomes 1. Mean serum iron concentration at baseline, 6 & 12 months (primary outcome) 2. Number RBC units during the trial 3. Iron excretion at 1 & 12 months 4. Hb level measured weekly for 3 months then monthly for 9 months 5. Liver function measured weekly for 3 months then monthly for 9 months 6. Renal function measured weekly for 3 months then monthly for 9 months 7. Side effects 8. Participant compliance: compliance was assessed by the number of vials of DFX or tablets of DFP used. Safety was determined by detailed clinical and laboratory examination. Participants were also asked to complete questionnaires about any side‐effects they experienced. |
|
| Identification | Source of funding: not stated. | |
| Notes | Prior exposure to iron chelators: DFO, less than 4 times a week, dose and duration not reported. Sample‐size calculation not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not report any information about how randomisation was undertaken |
| Allocation concealment (selection bias) | Unclear risk | The authors did not report any information about how treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | Unclear risk | The authors did not report any information as to whether participants, personnel were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Unclear risk | The authors did not report any information as to whether outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis for all outcomes: there were no missing outcome data |
| Selective reporting (reporting bias) | High risk | Data for 2 pre‐specified outcomes were not reported in the paper: iron excretion at 1 and 12 months and renal function. Both are important clinical markers of the efficacy of iron chelation therapy |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Olivieri 1997.
| Methods | 2‐arm parallel RCT
Number of centres: 2
Trial dates: November 1993 ‐ September 1995
Duration of treatment: analysis undertaken after 24 months (mean (SD) duration 33 (1.0) months, range 24 ‐ 43 months)
Follow‐up: none Trial undertaken: Hospital Centres in Toronto and Montreal, Canada. These data are from the Toronto participants only |
|
| Participants |
Baseline characteristics Number randomised: 64 (DFO: 32; DFP: 32) Number analysed: 37 (DFO: 18; DFP: 19). The trial reports details for why 6 and 7 participants respectively were not included in the analysis. The remaining participants had not completed 24 months treatment at the time of analysis for this trial report DFP (L1)
DFO
Inclusion criteria: diagnosed with homozygous β‐thalassaemia, 10 years of age or older, willing to participate in the trial Exclusion criteria:
Pre‐treatment:
|
|
| Interventions |
DFP (L1)
DFO
|
|
| Outcomes |
Adherence see adherence below Trial‐reported outcomes 1. Change in LIC (measured by SQUID or biopsy) between 12 months prior to randomisation & 24 months duration on trial treatment 2. Adherence to iron chelation therapy rates defined as per cent of doses administered (number of doses of the iron chelator taken, out of number prescribed), measured for a minimum of 3 months |
|
| Identification |
Sponsorship source: no sponsorship stated Country: Canada Setting: Transfusion Clinic Authors name: Nancy Olivieri Institution: University of Toronto Source of funding: not stated |
|
| Notes | Prior exposure to iron chelators: not reported
Abstract publication. Some data from Pope 1995 thesis included for baseline characteristics Sample‐size calculation not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "After stratification patients by LIC (>7mg Fe/g; < 7mg Fe/g) 'patients were assigned by a research pharmacist who did not know the patients" |
| Allocation concealment (selection bias) | Unclear risk | The authors did not report any information about how treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | 1 treatment a pump and 1 treatment a tablet, participants and researchers would not be blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Unclear risk | The authors did not report any information as to whether outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The trial analysed data from 58% of randomised participants. Of the 42% randomised participants who were not available for outcome analysis:
• 22% randomised participants had not completed the required 24 months treatment at the time of analysis for this trial report;
• 16% DFP‐treated participants and 5% DFO treated participants were withdrawn due to treatment induced side effects This missing data may inappropriately affect the statistical findings of the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified were reported in the manuscript |
| Other bias | Unclear risk | The trial was reported in an abstract, thus there are few data available to make an assessment of whether the trial was free of other bias. Trial stopped early by manufacturer |
Pennell 2006.
| Methods | 2‐arm parallel RCT
Number of centres: 4
Trial dates: December 2002 ‐ March 2005
Duration of treatment: 1 year
Follow‐up: outcome data recorded for duration of treatment Trial undertaken: 4 participating centres in Italy and Greece |
|
| Participants | Number randomised: 61 DFO: 32; DFP: 29
Number analysed: variable across outcomes. Minimum and maximum numbers analysed were: treatment group: 30 ‐ 32; comparator group: 27 ‐ 29. Trial reported details as to why data from 1 participant in the treatment group and 2 in the comparator group were withdrawn from treatment Transfusion‐dependent homozygous participants with β‐thalassaemia major Age: mean (SD) treatment group: 26.2 (4.7) years; mean (SD) comparator group: 25.1 (5.8) years Sex: treatment group: 50% male; comparator group: 52% male Ethnicity: Greek/Italian: treatment group: 18/14; comparator group: 16/13 |
|
| Interventions |
DFO
DFP
|
|
| Outcomes |
Adherence rates: DFP compliance was measured using the Medication Event Monitoring System device (Aardex, Zug, Switzerland) and calculated as the percent of openings with an interval longer than 4 hours recorded, divided by number of doses prescribed. DFO compliance was calculated as the percentage of completed infusions, as determined by the Crono pumps, divided by the number of infusions prescribed. Trial‐reported outcomes 1. Change over 1 year in myocardial T2* (primary outcome) 2. Cardiac volumes and function 3. LIC 4. SF 5. ANC 6. AEs 7. ALT 8. Serum zinc levels 9. Serum creatinine levels |
|
| Identification | Trial sponsor: Apotex (manufacturer of DFP) | |
| Notes | Prior exposure to iron chelators: DFO at a mean (SD) dose of 39 (8) mg/kg/day for 5 ‐ 7 days/week Sample‐size calculation reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not report any information about how randomisation was undertaken |
| Allocation concealment (selection bias) | Unclear risk | The authors did not report any information about whether treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Open label one treatment subcutaneous and the other oral so not possible to mask treatments |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Low risk | The primary outcome was independently measured in a different country (UK) to where the trial took place and the findings were not communicated back to the clinicians during the course of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis of the outcomes SF and AEs Data from 1 participant in the treatment (DFO) group were not included in the analysis of the cardiac outcomes (primary outcome) and last observation carried forward method was used to accommodate the missing data from 3 other participants (1 treatment group and 2 from the comparator group) in the cardiac outcomes (primary outcome) 2 participants in each treatment group did not have a LIC assessment at 12 months and the data from these participants were missing from the analysis |
| Selective reporting (reporting bias) | High risk | The following pre‐specified outcomes were not reported in the manuscript: ANC; ALT; serum zinc levels; and serum creatinine levels |
| Other bias | High risk | There are several imbalances in baseline characteristics between the 2 interventions including a major imbalance in SF measures with the DFO group having much higher levels as well as a greater proportion of participants with severe iron overload (above 2500 µg/L) |
Pennell 2014.
| Methods |
Study design: RCT Study grouping: parallel group CORDELIA was a prospective, multinational, randomised, open‐label, parallel‐group, phase 2 trial. A total of 81.2% of participants (n = 160) completed 1 year of treatment |
|
| Participants | "Overall, 925 patients were screened and 197 randomized. The majority of patients screened were β‐thalassemia major patients (902/925; 99.1%). Other patients who were screened and for whom underlying anaemia was captured had low/intermediate 1 myelodysplastic syndrome (n = 4), Diamond–Blackfan anaemia, β‐thalassemia intermedia, congenital dyserythropoietic anaemia, and paroxysmal nocturnal haemoglobinuria (all n = 1). Only β‐thalassemia major patients fulfilled the inclusion criteria and were enrolled in the study. A total of 81.2% of patients (n = 160) completed 1 year of treatment" Baseline characteristics DFX (Exjade)
DFO (Desferal)
Inclusion criteria: people with β‐thalassemia major, Diamond–Blackfan anaemia, low/intermediate myelodysplastic syndromes, or sideroblastic anaemia, aged ≥ 10 years with myocardial T2* 6 ‐ 20 ms, LVEF ≥ 56%, R2 MRI LIC ≥ 3 mg Fe/g dw, lifetime history of ≥ 50 units RBC transfusions, and receiving ≥10 unit/year of RBC transfusions Exclusion criteria: participants with serum creatinine above the ULN or significant proteinuria (urinary protein/creatinine ratio ≥1.0 mg/mg in a non–first‐void urine sample at baseline; people with ALT 5 x the ULN only if their LIC was 10 mg Fe/g dw; considerable impaired GI function or GI disease; history of clinically relevant ocular and/or auditory toxicity related to iron chelation; therapy, and history of HIV seropositivity or malignancy within the past 5 years; clinical symptoms of cardiac dysfunction (shortness of breath at rest or exertion, orthopnoea, exercise intolerance, lower‐extremity edema, arrhythmias) |
|
| Interventions |
DFX (Exjade)
DFO (Desferal)
Mean actual dose over 1‐year treatment was 36.7 6 4.2 mg/kg per day DFX (range, 19.7‐ 43.3 mg/kg per day). Mean actual dose of DFO was 41.5 6 8.7 (13.2 ‐ 60.2) mg/kg per day, when normalized to a 7‐day regimen |
|
| Outcomes |
Adherence to iron chelation therapy rates: not stated how adherence was measured Trial‐reported outcomes 1. Ratio of Gmean myocardial T2* after 1 year of treatment with DFX divided by the ratio of Gmean for DFO 2. Change in LVEF after 1 year of treatment, assessed by absolute change from baseline CMR 3. Absolute change from baseline in LIC after 1‐year treatment 4. Absolute change from baseline in SF after 1‐year treatment |
|
| Identification |
Sponsorship source: Novartis Pharma AG Country: multinational, 11 countries Setting: 22 centres across 11 countries Comments: the authors thank Debbi Gorman of Mudskipper Business Ltd for medical editorial assistance. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Authors name: Dudley J. Pennell Institution: National Institute for Health, Research Cardiovascular Biomedical Research Unit Email: d.pennell@ic.ac.uk Address: National Institute for Health Research Cardiovascular Biomedical Research Unit, Royal Brompton Hospital, Sydney Street, London, SW3 6NP, UK |
|
| Notes | Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA) co‐ordinated the design and execution of this trial and contributed to the analysis and interpretation of the trial data. Novartis Pharmaceuticals Corporation also collaborated with the external authors to assist in the development and approval of the manuscript for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "22 centers across 11 countries. Following a 35‐day screening phase, patients were randomized in a 1:1 ratio" Randomisation was based on permuted blocks; stratification by centre was not conducted |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no description of allocation concealment except that randomisation was based on permuted blocks |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Judgement comment: open‐label trial ‐ subcutaneous pump versus oral tablet ‐ difficult to blind |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Low risk | Quote: "Core laboratories were blinded to treatment allocation.In order to eliminate potential unrecognized biases, the core clinical trial team was blinded to the treatment assignment prior to the database lock for the primary analysis." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: 21 withdrawn DFO arm 16 in DFX (78 to 82 completed trial) Efficacy outcomes reported in per protocol and safety in the participants who received the trial drug |
| Selective reporting (reporting bias) | Unclear risk | Investigator‐reported AEs, regardless of causality, were reported in 65 (67.7%) DFX participants and 69 (75.8%) DFO participants (supplemental Table 2). AEs suspected to be related to trial drug occurred in 35.4% of DFX participants and 30.8% of DFO participants Judgement comments: It is unclear if investigator‐reported AEs and those suspected to be related to trial drug include the same AEs. Also, they only report the end of trial LIC value for the DFX group |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Taher 2017.
| Methods |
Study design: multicentre RCT conducted in several countries Study grouping: parallel group Study duration: 24 weeks |
|
| Participants |
Baseline characteristics DFX film‐coated tablet
DFX dispersible tablet
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
DFX film‐coated tablets
DFX dispersible tablet
|
|
| Outcomes |
Adherence to iron chelation therapy rates Compliance with medication as assessed by relative consumed tablet count Trial‐reported outcomes 1. Overall safety of both DFX formulations, measured by frequency and severity of AEs and changes in laboratory values from baseline to 24 weeks. 2. Evaluation of both formulations on selected GI AEs (diarrhoea, constipation, nausea, vomiting, and abdominal pain) during treatment 3. Estimation of treatment compliance 4. Evaluation of both formulations on participant satisfaction, palatability, and GI symptoms using PROs 5. Evaluation of the pharmacokinetics of both formulations 6. Reported % compliant with upper and lower percentages |
|
| Identification |
Sponsorship source: Novartis Pharmaceuticals Country: USA Comments: NCT02125877 Authors name: Ali Taher Institution: American University of Beirut Medical Center Email: ataher@aub.edu.lb Address: Haematology and Oncology, Department of Internal Medicine, Faculty of Medicine, American University of Beirut Medical Center, Beirut, Lebanon |
|
| Notes | Sample‐size calculation not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was stratified by underlying disease and previous chelation treatment." No clear description of randomisation or if participants were randomised centrally |
| Allocation concealment (selection bias) | High risk | Quote: "Post‐ hoc analyses identified that 23 patients on FCT (26%) were started on a dose that was higher than recommended in the protocol compared with 8 patients (9.3%) on DT (not recognized or reported by the investigators as dosing error)." Judgement comment: the trial was open label and most participants had been on 1 or the other of the trial drugs prior to the trial ‐ doses may have corresponded to prior dosing since there was no description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Judgement comment: open‐label |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | High risk | No description of how outcome assessment was performed ‐ centrally or blinded open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Overall, all patients were satisfied with their medicine during the study period; satisfaction scores were higher with deferasirox FCT compared with DT at all visits." Judgement comment: no data provided on number of participants or scores just general statements |
| Selective reporting (reporting bias) | High risk | Quote: "patients discontinued treatment because of AEs (n = 10), protocol deviation (n = 5), withdrawal of consent (n = 3), patient guardian decision (n = 2), and other reasons (administrative problems, death, and physician’s decision, n = 1 each)." Judgement comment: investigators do not report all outcomes by treatment assignment, and AEs and SAEs are reported as suspected relationship to trial drug and occurring in > or equal to 10% |
| Other bias | Unclear risk | "The absolute reduction in median serum ferritin (range) in patients receiving FCT was –350 (–4440–3572) ng/mL and in those receiving DT was –85.5 (–2146–8250) ng/mL); these correspond to a relative change of –14.0% with FCT and –4.1% with DT." Judgement comment: some of difference in change could be accounted for more participants starting on a higher dose of film‐coated tablet |
Tanner 2007.
| Methods | 2‐arm parallel RCT
Number of centres: multicentre (12 centres)
Duration of treatment: 12 months
Follow‐up: not stated Trial undertaken: thalassaemia out‐patient clinics in Sardinia |
|
| Participants | Number randomised: 65 (treatment group: 33; comparator group: 32)
Number analysed: not reported Number completing treatment: 60 (treatment group: 32; comparator group: 28). The reason for the withdrawal was not fully reported by the trial authors Participants aged 18 years or older with a diagnosis of β‐thalassaemia, currently maintained on subcutaneous DFO and with a myocardial T2* between 8 ‐ 20 ms Age: treatment group: mean (SD) 28.7 (5.3) years; comparator group: mean (SD) 28.8 (4.2) years Age range for both arms was 18 ‐ 42 years Sex: treatment group: 39% male; comparator group: 44% male Ethnicity: not stated |
|
| Interventions |
DFO
DFO/DFP
|
|
| Outcomes |
Adherence see compliance below Trial‐reported outcomes 1. Change over 1 year in myocardial T2* (primary outcome) 2. Change in liver T2* at 12 months 3. SF 4. Left ventricular volume & function 5. Brachial artery reactivity as a marker of heart failure 6. Participant compliance with chelation treatments: DFO compliance was calculated as the percentage of completed infusions, as determined by the Crono pumps, divided by the number of infusions prescribed. DFP/placebo compliance was measured through pill counting at the bi‐monthly visits 7. AEs 8. BNP test |
|
| Identification | Source of funding: CORDA, Royal Brompton & Harefield Hospitals Charitable funds, Cooley’s Anemia Foundation, Apotex, UK Thalassaemia Society, University College London Special trustees Chairty | |
| Notes | Prior exposure to iron chelation: DFO mean (SD) dose 36.4 (11.1) mg/kg per day for 5.5 day/week (equivalent to 40.5 mg/kg for 5 day/week). Participants were excluded if they had previously received DFP Sample‐size calculation reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not report any information about how randomisation was undertaken |
| Allocation concealment (selection bias) | High risk | Trial reports that the participants and clinicians were aware of how treatment was to be allocated |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | Unclear risk | The authors did not report any information as to whether participants or personnel were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | Unclear risk | The authors did not report any information as to whether outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | As the trial does not report the number of participants included in each outcome assessment. The trial reports the number completing treatment and the reasons why 3 participants in the treatment group (1 adverse event & 2 participant requests) and 4 participants in the comparator group (3 adverse events & 1 participant request) were withdrawn from the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified were reported in the manuscript |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Vichinsky 2007.
| Methods |
Study design: RCT Study grouping: parallel group The study duration was 52 weeks. Participants were recruited by investigators at 44 sites in the USA, France, Italy, UK and Canada |
|
| Participants |
Baseline characteristics DFX
DFO
Age group (% DFX, DFO) < 6 years: 3.0, 4.8 6 to < 12 years: 22.7, 23.8 12 to <16 years: 25.0, 20.6 16 to < 50 years: 47.7, 49.2 50 to < 65 years: 1.5, 1.6 Inclusion criteria:
Exclusion criteria
|
|
| Interventions |
DFX
DFO
|
|
| Outcomes |
Adherence to iron chelation therapy rates Compliance. For DFX, compliance was assessed by counting the number of tablets returned in bottles at each visit. For DFO, the numbers of vials returned at each visit were counted Trial‐reported outcomes 1. Safety assessments 2. Laboratory assessments were performed at least monthly and included complete blood counts with differential counts. Biochemistry testing included electrolytes, glucose, liver function tests, gamma‐glutaryl‐transferase, lactate dehydrogenase, cholesterol, triglycerides, uric acid, total protein, C‐reactive protein, copper and zinc levels. Iron parameters included total iron, transferrin, transferrin saturation and ferritin. Urinary testing performed on random collections included determination of creatinine, total protein and albumin 3. Physical examinations, ECGs, audiometry and ophthalmological tests were performed at baseline, 12, 24, 36 and 52 weeks. In participants less than 16 years of age, additional assessments included growth velocity and pubertal stage 4. Efficacy assessments. LIC was determined by SQUID biospectrometry at baseline, 24 and 52 weeks. The 24‐week assessment was performed primarily for safety purposes, and the change in LIC was calculated between baseline and 52 weeks. SF was assessed monthly during the trial and the change was determined using the baseline and final ferritin level |
|
| Identification |
Sponsorship source: Novartis Pharmaceuticals Country: international (Canada, France, Italy, UK and USA) Setting: medical centre outpatient Authors name: Elliott Vichinsky Institution: Children’s Hospital and Research Center at Oakland, Email: evichinsky@mail.cho.org Address: Children’s Hospital and Research Center at Oakland, 747 52nd Street, Oakland, CA 94609, USA Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA) co‐ordinated the design and execution of this trial and contributed to the analysis and interpretation of the trial data. Novartis Pharmaceuticals Corporation also collaborated with the external authors to assist in the development and approval of the manuscript for publication |
|
| Notes | Sample‐size calculation reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was performed using an interactive voice response system" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "stratified according to the following age groups: 2 to < 6 years, 6 to < 12 years, 12 to < 16 years and 16 years and older. The randomisation sequence included permuted block groups of six patients for each of the three age strata." Judgement comment: some of the age groups had few participants and unclear if allocation would remain concealed with permuted block groups of 6 participants |
| Blinding of participants and personnel (performance bias) All outcomes except mortality or other objective outcomes | High risk | Judgement comment: no mention of blinding, but DFO is delivered by infusion pumps and DFX is a solution in water, so blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes except mortality | High risk | Judgement comment: no description of blinding: Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA) co‐ordinated the design and execution of this trial and contributed to the analysis and interpretation of the trial data. The data were analysed under supervision of the trial statistician and were reviewed by the investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported. 8 participants did not complete and were not included. 6 DFX arm withdraw consent, one in DFO arm. 3 DFO non compliant, 2 DFX and 1 DFO lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Adverse events, irrespective of the relationship to study medication, which occurred in more than 10% of patients receiving either treatment, are shown in Table III. As arbitrarily defined by an increased frequency of at least 5% indicating a potential relationship to drug administration." Judgement comment: do not report the total number of AEs in all participants, as well there was a substantial number of participants experience SAEs and there is no list of the type except for pain crisis: The number of participants receiving DFX and DFO that reported SAEs was similar (46.2% and 42.9% respectively) and the most common SAE in both groups was sickle cell anaemia with crisis (33.3% and 31.7% respectively). Also table of AEs report % and no totals so impossible to determine total number of participants with an AE |
| Other bias | Unclear risk | Quote: "The reasons for withdrawal of consent were not included in the database." Quote: "The initial 24 patients enrolled were randomised to receive deferasirox 10 mg/kg or deferoxamine at recommended doses of 20–60 mg/kg based on initial LIC. Subsequently, additional safety information became available for deferasirox suggesting a need to modify the starting dose (Cappellini et al, 2006). Therefore, following the enrolment of the first 24 patients, the study was amended so that all subsequent patients randomised to deferasirox were dosed at 10–30 mg/kg according to baseline LIC" Judgement comment: it is important to understand reasons for withdrawals and also the nature of the missing safety information which may have implications for dosing and effects of the dosing amendment |
ADRs: adverse drug reactions AEs: adverse events ALT: alanine aminotransferase ANC: absolute neutrophil count BNP: brain natriuretic peptide CBC: complete blood count CMR: cardiovascular magnetic resonance imaging DFO: deferoxamine DFP: deferiprone DFX: deferasirox dw: dry weight ECGs: electrocardiograms FBC: full blood count Hb: haemoglobin HRQoL: health‐related quality of life ICT: iron chelation therapies IQR: interquartile range LVEF: left ventricular ejection fraction LIC: liver iron concentration MRI: magnetic resonance imaging PK: pharmacokinetic PRBC: packed red blood cell QoL: quality of life RBCs: red blood cells RCT: randomised controlled trial SAEs: serious adverse events SCr: sickle cell retinopathy SD: standard deviation SF: serum ferritin SGPT: serum glutamate‐pyruvate transaminase SQUID: Superconducting Quantum Interference Device UIE: urinary iron excretion ULN: upper limit of normal WBC: white blood count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu 2015 | Wrong study design ‐ qualitative interview questionnaire used. |
| Al Kloub 2014 | Wrong study design ‐ qualitative interview questionnaire used. |
| Al Kloub 2014a | Wrong study design ‐ cross‐sectional study. |
| Al Refaie 1995 | Wrong study design ‐ medication study ‐ not an RCT. |
| Alvarez 2009 | Wrong study design ‐ medication study ‐ not an RCT. |
| Armstrong 2011 | No intervention. |
| Bala 2014 | No intervention. |
| Belgrave 1989 | No intervention. |
| Berkovitch 1995 | Not designed to measure adherence to iron chelation therapy. |
| Chakrabarti 2013 | Not designed to measure adherence to iron chelation therapy. |
| Daar 2010 | Wrong setting ‐ single‐centre study. |
| Gomber 2004 | No intervention. |
| Kidson Gerber 2008 | Wrong study design ‐ clinical audit of medication use. |
| Kolnagou 2008 | Wrong study design ‐ medication study not RCT. |
| Leonard 2014 | Wrong study design ‐ single‐treatment study. |
| Loiselle 2016 | Review. |
| Mazzone 2009 | Wrong comparator ‐ healthy children not taking iron chelation therapy. |
| NCT01709032 | Not designed to measure adherence to iron chelation therapy. |
| NCT01825512 | Not designed to measure adherence to iron chelation therapy. |
| NCT02133560 | Wrong study design ‐ single‐centre study with no control. |
| NCT02466555 | Wrong study design ‐ single‐centre study with no control. |
| Pakbaz 2004 | Wrong study design ‐ single‐centre study with no control. |
| Pakbaz 2005 | Wrong study design ‐ single‐centre study with no control. |
| Porter 2009 | Wrong study design ‐ medication intervention not a RCT. |
| Porter 2012 | Wrong study design ‐ medication intervention not a RCT. |
| Vichinsky 2005 | Not designed to measure adherence to iron chelation therapy. |
| Vichinsky 2008 | Not designed to measure adherence to iron chelation therapy. |
| Waheed 2014 | Not designed to measure adherence to iron chelation therapy. |
| Walsh 2014 | Review. |
| Yarali 2006 | Not designed to measure adherence to iron chelation therapy. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Antmen 2013.
| Methods | Prospective cohort study; parallel group |
| Participants | Participants using DFX ‐ we do not know the disease diagnosis and therefore awaiting classification Exclusion criteria: not stated |
| Interventions | Educational intervention, standard care (as defined in the study) |
| Outcomes | Exjade Patient Compliance Program (EX‐PAT) was established to increase patients’ knowledge about DFX usage. This abstract aimed to represent the results of the pilot EX‐PAT program It is highly recommended to educate the patients under iron chelating treatment about possible complication and usage of chelating agent |
| Notes | Email sent to author asking for the following information so we could include the study: a full study report of this abstract? If this is not available would it be possible to have more information on: 1. The disease diagnosis of the participants (were they sickle cell (phenotypes) or thalassaemia (phenotypes) or other); 2. How participants were assigned to intervention or control; 3. Any inclusion/exclusion criteria; 4. Any group differences; 5. Is the age range for the whole group or is it for the intervention group only? If so could we have the age range for the control group; 6. Baseline and end of study ferritin levels; 7. SAEs or any AEs |
NCT00004982.
| Methods | RCT; parallel group |
| Participants | Inclusion criteria: ages eligible for trial: 7 years and older (child, adult, senior); genders eligible for study: both Exclusion criteria: overt cardiac disease |
| Interventions | Combination iron chelation therapy, standard care (as defined in the trial) |
| Outcomes | This small trial is testing the premise that a combination of drugs as a new approach to iron chelation therapy may reduce side effects and increase efficacy. If both drugs can be given orally, there may be a better chance of finding a suitable alternative to Desferal. Several combinations of experimental iron chelating drugs are being used in this trial |
| Notes | This trial has been completed. Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). No study results posted NCT00004982: scant information about the trial was documented on the clinicaltrials.gov web site. We have been unable to identify any publications from this trial and despite repeated emails to the trial co‐ordinator and searching the funders web site, we have been unable to identify any further details about the trial. Start date: December 1998; estimated completion November 2002 |
AEs: adverse events DFX: deferasirox RCT: randomised controlled trial SAEs: serious adverse events
Characteristics of ongoing studies [ordered by study ID]
EudraCT 2012‐000353‐31.
| Trial name or title | Multicentre, randomised, open‐label, non‐inferiority active‐controlled trial to evaluate the efficacy and safety of DFP compared to DFX in paediatric patients aged from 1 month to less than 18 years of age affected by transfusion‐dependent haemoglobinopathies |
| Methods | Randomised trial, parallel group |
| Participants | 1. Children on current treatment with DFO or DFX or DFP in a chronic transfusion program receiving at least 150 mL/kg/year of packed RBCs (corresponding approximately to 12 transfusions); 2. For those naive to chelation treatment: participants that have received at least 150 mL/kg of packed RBCs (corresponding to approximately 12 transfusions) in a chronic‐transfusion program and with SF levels ≥ 800 ng/mL; 3. For children aged from 1 month to less than 6 years: known intolerance or contraindication to DFO; 4. Written informed consent and patient's informed assent to child’s maturity and understanding |
| Interventions | DFP compared to DFX |
| Outcomes | Percentage of successfully chelated children assessed by SF levels (all participants) and cardiac MRI T2* (children above 10 years of age able to have an MRI scan without sedation) 1. LlC as measured by MRI in those able to undergo MRI scan without sedation 2. Safety and tolerability assessments 3. QoL |
| Starting date | Not stated |
| Contact information | Consorzio per le Valutazioni Biologiche e Farmacologiche via Luigi Porta, 14 Pavia 27100 Italy deep.2@deep‐project.net |
| Notes |
IRCT2015101218603N2.
| Trial name or title | To assess compliance, efficacy and satisfaction with two different formulation of deferasirox in people with transfusion‐dependent beta‐thalassaemia |
| Methods | RCT; parallel group |
| Participants | Inclusion criteria: signing informed consent; male or female aged ≥ 2 years at screening; people with transfusion‐dependent thalassaemia major; regular transfusion indicated by a blood requirement ≥ 8 blood transfusions per year at screening. Exclusion criteria: people with mean levels of ALT above 5‐fold the ULN; people with serum creatinine above ULN; significant proteinuria as indicated by a urinary protein/creatinine ratio > 0.6 (mg/mg); creatinine clearance ≤ 60 mL/min; chronic hepatitis B infection; active hepatitis C infection; pregnancy or breastfeeding; non‐transfusion dependent thalassaemia |
| Interventions | DFX (new formulation JadenuTM), DFX (Exjade®) |
| Outcomes | Participants compliance and satisfaction; 3 months after drug consumption; designed questionnaire to assess participant compliance and satisfaction; ferritin serum amount; safety; possible GI side effects, including diarrhoea, and dermatologic symptoms |
| Starting date | 22 December 2015 |
| Contact information | Vice chancellor of research, Shiaz Univeisity of Medical Sciences COUNTRY: Iran SETTING: multicentre (outpatient) Dr. Sezaneh Haghpanah INSTITUTION:Hematology Research Center, Nemazee Hospital, Shiraz, Iran EMAIL: haghpanah@sums.ac.ir ADDRESS: Dr Sezaneh Haghpan Professor of community medicine Hematology Research Center, Nemazee Hospital, Zand Street, Shiraz, Ira |
| Notes |
Madderom 2016.
| Trial name or title | A randomised controlled trial studying the effectiveness of group medical appointments on self‐efficacy and adherence in sickle cell disease (TEAM study): study protocol |
| Methods | RCT; parallel group |
| Participants | Inclusion criteria: individuals with homozygous or compound heterozygous SCD Exclusion criteria: individuals with a first visit to the outpatient clinic, patients who cannot communicate adequately due to language difficulties and/or hearing problems or patients who have behavioral problems which will limit group functioning |
| Interventions | Group Medical Appointment, Individual Medical Appointment (IMA; care‐as‐usual) |
| Outcomes | Primary and secondary endpoints will be measured at baseline (start of the study), after 1.5 years (after two GMA visits) and after 3 years (after four GMA visits), in both groups. Assessments are performed at the hospital, directly before the outpatient visit and in presence of a psychologist. Primary endpoint: 1. Self‐efficacy as measured by the validated Sickle Cell Self‐ Efficacy Scale; Secondary endpoints; 2. Adherence to prescribed treatment by (paediatric) hematologist; 3. QoL as measured with the validated Pediatric Quality of Life Inventory for children and SF‐36 for adults. 4. Emergency visits and hospital admissions for SCD related symptoms and complications. 5. Satisfaction with treating physician and nurse (by visual analogue scale: score 1 – 10); 6. Measurement of costs and effects in the GMA and IMA group by an economic analysis according to Dutch guidelines and with respect to an increase in self‐ efficacy |
| Starting date | The trial opened to recruitment in January 2013 for the children and in September 2015 for the adults and is still ongoing. |
| Contact information | Marjon H. Cnossen INSTITUTION: Department of Pediatric Hematology, Erasmus University Medical Center ‐ Sophia Children’s Hospital EMAIL: m.cnossen@erasmusmc.nl ADDRESS: Department of Pediatric Hematology, Erasmus University Medical Center ‐ Sophia Children’s Hospital, Wytemaweg 80, PO Box 2060, 3000 CB Rotterdam, The NetherlandsAdditional data |
| Notes | Trial registration: NTR4750 (NL42182.000.12) |
NCT02173951.
| Trial name or title | An algorithm to start iron chelation in minimally transfused young beta‐thalassaemia major patients |
| Methods | RCT; parallel group |
| Participants | Inclusion criteria: young individuals with β‐thalassaemia major (diagnosed by HPLC, CBC) who started transfusion therapy who received 5 ‐ 7 transfusions or less, aged more than 6 months. Pre‐transfusional Hb should be >9 g/dL. Serum ferritin should be ≤ 500 ng/mL, transferrin saturation ≤ 50%. Exclusion criteria: 1. individuals with β‐thalassaemia intermedia, those with other transfusion‐dependent anemias (myelodysplasia, other chronic haemolytic anemias, pure red cell aplasia, aplastic anaemia); 2. Individuals with levels of ALT > 5 the ULN, serum creatinine > ULN on 2 measurements; 3. Indiviudals with history of agranulocytosis (ANC < 0.5×109/L). 4. Non‐complaint individuals acknowledged by reviewing the patient's records. |
| Interventions | DFP, placebo |
| Outcomes | Primary outcome measures: determine the time and number of transfusion units as well as amount of infused iron that will lead to appearance of LPI > 0.2 or TSAT > 50 % , serum ferritin ≥ 500 ng/mL in the studied thalassaemic patients which warrant start of iron chelation Time frame: 12 months To determine the time as well as amount of transfused iron (calculated in mg iron/kg) at which there is LPI appearance of > 0.2 as well as TSAT reaching 70 %, a serum ferritin ≥ 500 in order to start iron chelation therapy Secondary outcome measures: Evaluation of safety of early use of iron chelation therapy in terms of drug related AEs or SAEs Time frame: 12 months To determine the tolerability and safety of early low dose DFP 50mg/kg and effectiveness to postpone or prevent SF from reaching 1000 ng/mL or LPI > 0.6 or TSAT > 70% in comparison to participants not starting chelation therapy |
| Starting date | July 2014 |
| Contact information | Amira AM Adly, INSTITUTION: Pediatric Hematology clinic, Ain Shams University Cairo, Egypt EMAIL: amiradiabetes@yahoo.com |
| Notes |
NCT02435212.
| Trial name or title | Study to evaluate treatment compliance, efficacy and safety of an improved deferasirox formulation (granules) in paediatric patients (2 ‐ < 18 years old) with iron overload |
| Methods | RCT; parallel group |
| Participants | Inclusion criteria: written informed consent/assent before any study‐specific procedures. Consent will be obtained from parent(s) or legal guardians. Investigators will also obtain assent of patients according to local guidelines. Male and female children and adolescents aged ≥ 2 and < 18 years. Any transfusion‐dependent anaemia associated with iron overload requiring iron chelation therapy and with a history of transfusion of approximately 20 PRBC units and a treatment goal to reduce iron burden (300 mL PRBC = 1 unit in adults whereas 4 mL/kg PRBC is considered 1 unit for children). Serum ferritin > 1000 ng/mL, measured at screening visit 1 and screening visit 2 (the mean value will be used for eligibility criteria). Exclusion criteria: creatinine clearance below the contraindication limit in the locally approved prescribing information. Creatinine clearance will be estimated from serum creatinine (using the Schwartz formula) at screening visit 1 and screening visit 2 and the mean value will be used for eligibility criteria. Serum creatinine > 1.5 x ULN at screening measured at screening visit 1 and screening visit 2 (the mean value will be used for eligibility criteria). ALT and/or AST > 3.0 x ULN (Criterion no longer applicable, removed as part of amendment 1): prior iron chelation therapy. Liver disease with severity of Child‐Pugh class B or C. Significant proteinuria as indicated by a urinary protein/creatinine ratio > 0.5 mg/mg in a non‐first void urine sample at screening visit 1 or screening visit 2. Those with significant impaired GI function or GI disease that may significantly alter the absorption of oral DFX (e.g. ulcerative diseases, uncontrolled nausea, vomiting, diarrhoea, malabsorption syndrome or small bowel resection |
| Interventions | DFX granule formulation, DFX DT formulation |
| Outcomes | Primary outcome measures: compliance
Change in SF in iron chelation therapy‐naive participants.
Secondary outcome measures: domain scores of treatment satisfaction and palatability over time
Overall safety, as measured by frequency and severity of adverse.This includes active monitoring for renal toxicity; including renal failure, hepatic toxicity; including hepatic failure, and gastrointestinal haemorrhage), and changes in laboratory values from baseline (serum creatinine, creatinine clearance, ALT, AST, RBC and WBC). In addition, vital signs, physical, ophthalmological, audiometric, cardiac, and growth and development evaluations will be assessed.
Rate of dosing instructions deviations ('Compliance', using a questionnaire) .
Pre‐dose DFX concentrations in all patients. Pre‐dose PK data from all patients will be analysed to support the assessment of compliance. Post‐dose DFX concentrations between 2 and 4 hours post‐dose Change in SF in iron chelation therapy naive and pre‐treated participants PK/PD relationship to explore exposure‐response relationships for measures of safety and effectiveness: serum creatinine change from baseline, notable serum creatinine values, serum creatinine clearance change from baseline and notable serum creatinine clearance categories, SF change from baseline, in relationship to derived PK parameters for pre‐ and post‐dose DFX concentrations. Assess additional safety, as measured by frequency and severity of adverse for granules during extension phase includes active monitoring for renal toxicity; including renal failure, hepatic toxicity; including hepatic failure, and gastrointestinal haemorrhage), and changes in laboratory values from baseline (serum creatinine, creatinine clearance, ALT, AST, RBC and WBC). In addition, vital signs, physical, ophthalmological, audiometric, and growth and development evaluations will be assessed |
| Starting date | 21 October 2015 |
| Contact information | Principal Investigator: Janet L. KwiatkowskiI; NSTITUTION: Children's Hospital of Philadelphia Onc. Dept; EMAILContact: John Hammond 267‐426‐5602 hammondjh@email.chop.edu ADDRESS: Children's Hospital of Philadelphia, Oncology Dept, Philadelphia, Pennsylvania, USA, 19104‐4399 |
| Notes | March 30, 2023 (Final data collection date for primary outcome measure) |
AEs: adverse events ALT: alanine transaminase ANC: absolute neutrophil count AST: aspartate transaminase CBC: complete blood count DFO: deferoxamine DFP: deferiprone DFX: deferasirox DT: dispersible tablet GI: gastrointestinal HPLC: high‐performance liquid chromatography LIC: liver iron concentration LPI: labile plasma iron MRI: magnetic resonance imaging PK/PD: pharmacokinetic/pharmacodynamic QoL: quality of life RBCs: red blood cells RCT: randomised controlled trial SAEs: serious adverse events SF: serum ferritin TSAT: transferrin saturation ULN: upper limit of normal WBC: white blood cell
Differences between protocol and review
See Fortin 2016.
Confidence intervals
In most studies we were unable to report total adverse events due to participants having one or more of the listed adverse events. We therefore use the 99% CI to report estimates of effects in subgroups of adverse events.
Assessment of reporting biases
We could not assess reporting bias as there were fewer than 10 trials for each comparison
Subgroup analysis
Due to insufficient data we could not undertake subgroup analyses as planned in the protocol (see below). From the outset, we also reported separately on the SCD trial.
Age of participant (child (one to 12 years), adolescent (13 to 17 years) adult (18+ years))
Type of disease (SCD or thalassaemia)
Route of administration of iron chelating agents (oral, intravenous or subcutaneous)
Sensitivity analysis
We could not undertake sensitivity analyses due to a lack of data.
Contributions of authors
Lise Estcourt: searching; selection of trials; eligibility assessment; content expert, and review content development
Patricia Fortin: searching; selection of trials; eligibility assessment; data extraction, risk of bias assessment, and review content development.
Karen Madgwick: selection of trials; eligibility assessment; data extraction, risk of bias assessment, content expert.
Sally Hopewell: methodological expert and review development.
Marialena Trivella: statistical and methodological expert and review development
Sheila Fisher: data extraction, risk of bias assessment, review content development.
Sources of support
Internal sources
-
NHS Blood and Transplant, Research and Development, UK.
To fund the work of the Systematic Review Initiative (SRI)
External sources
-
National Institute for Health Research (NIHR) Cochrane Programme Grant, UK.
To provide funding for systematic reviewers and methodological support from the Centre for Statistics in Medicine, Oxford
Declarations of interest
Lise Estcourt: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Patricia Fortin: funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Karen Madgwick: none to declare.
Sally Hopewell: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Marialena Trivella: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Sheila Fisher: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
New
References
References to studies included in this review
Aydinok 2007 {published data only}
- Aydinok Y, El‐Beshlawy A, Orelli‐Leber C, Czarnecki‐Tarabishi C, Manz C Y. A randomized controlled trial comparing the combination therapy of deferiprone (DFP) and desferrioxamine (DFO) versus DFP or DFO monotherapy in patients with thalassemia major. Blood 2006;108(11). [Abstract no.: 557] [Google Scholar]
- Aydinok Y, Ulger Z, Nart D, Terzi A, Cetiner N, Ellis G, et al. A randomized controlled 1‐year study of daily deferiprone plus twice weekly desferrioxamine compared with daily deferiprone monotherapy in patients with thalassemia major. Haematologica 2007;92(12):1599‐606. [DOI] [PubMed] [Google Scholar]
- Manz CH, El‐Beshlawy A, Aydinok Y, Leber C, Czarnecki‐Tarabishi C. A randomized controlled prospective clinical study comparing the combination therapy of deferiprone (L1) and desferrioxamine with L1 and DFO monotherapy in patients with thalassemia major. Haematologica 2006;91(S1):190. [Abstract no.: 0515] [Google Scholar]
Badawy 2010 {published data only}
- Badawy S, Hassan TH, Hesham MA, Badr MA. Evaluation of iron chelation therapy in B‐thalassemic patients in Zagazig University Hospital. ASPHO abstracts (The American Society of Pediatric Hematology/Oncology) 2010; Vol. 54, issue 6:799‐800.
Bahnasawy 2017 {published data only}
- Bahnasawy SM, Wakeel LM, Beblawy N, El‐Hamamsy M. Clinical pharmacist‐provided services in iron overloaded Beta‐thalassemia major children; A new insight to patient care. Basic & Clinical Pharmacology & Toxicology 2017;120(4):354‐9. [DOI] [PubMed] [Google Scholar]
Calvaruso 2015 {published data only}
- Calvaruso G, Vitrano A, Maggio R, Ballas S, Steinberg MH, Rigano P, et al. Deferiprone versus deferoxamine in thalassemia intermedia: results from a 5‐year long‐term Italian multicenter randomized clinical trial. American Journal of Hematology 2015;90(7):634‐8. [DOI] [PubMed] [Google Scholar]
- Vitrano A, Calvaruso G, Maggio G, Romeo MA, Cianciulli P, Lai ME, et al. Deferiprone versus deferoxamine in thalassemia intermedia: results from 5‐year long‐term Italian multi‐center randomized clinical trial. 56th ASH Annual Meeting and Exposition; 2014 Dec 6‐9; San Francisco, California 2014.
Elalfy 2015 {published data only}
- Elalfy M, Walli Y, Adly A, Henawy Y. 18 months data of a randomized controlled trial of combined deferiprone (DFP) and deferasirox (DFX) versus combined deferiprone and deferoxamine (DFO), in young B‐thalassemia major. Haematologica 2014;99(S1):443‐4. [Google Scholar]
- Elalfy MS, Adly AM, Wali Y, Tony S, Samir A, Elhenawy YI. Efficacy and safety of a novel combination of two oral chelators deferasirox/deferiprone over deferoxamine/deferiprone in severely iron overloaded young beta thalassemia major patients. European Journal of Haematology 2015;95(5):411‐20. [DOI] [PubMed] [Google Scholar]
- Elalfy MS, Wali Y, Tony S, Samir, Adly A. Comparison of two combination iron chelation regimens, deferiprone and deferasirox versus deferiprone and deferoxamine, in pediatric patients with beta‐thalassemia major. Blood 2013;122(21). [Abstract no.: 559] [Google Scholar]
El Beshlawy 2008 {published data only}
- Study With Deferiprone and/or Desferrioxamine in Iron Overloaded Patients. ClinicalTrials.gov 2006; Vol. NCT00350662.
- El‐Beshlawy A, Manz C, Naja M, Eltagui M, Tarabishi C, Youssry I, et al. Iron chelation in thalassemia: combined or monotherapy? The Egyptian experience. Annals of Hematology 2008;87(7):545‐50. [DOI] [PubMed] [Google Scholar]
Galanello 2006 {published data only}
- Galanello R, Kattamis A, Piga A, Tricta F. Safety and efficacy of alternate desferrioxamine and deferiprone compared to desferrioxamine alone in the treatment of iron overload in transfusion‐dependent thalassemia patients. Blood 2004;104(11 Pt 1). [Abstract no.: 3611] [Google Scholar]
- Galanello R, Piga A, Forni G L, Bertrand Y, Foschini M L, Bordone E, et al. Phase II clinical evaluation of deferasirox, a once‐daily oral chelating agent, in pediatric patients with beta‐thalassemia major. Haematologica 2006;91(10):1343‐51. [PubMed] [Google Scholar]
Hassan 2016 {published data only}
- Hassan MA, Tolba OA. Iron chelation monotherapy in transfusion‐dependent beta‐thalassemia major patients: A comparative study of deferasirox and deferoxamine. Electronic physician 2016;8(5):2425‐2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maggio 2009 {published data only}
- Maggio A, Capra M, Cuccia L, Gagliardotto F, Magnano C, Caruso V, et al. Deferiprone versus sequential deferiprone‐deferoxamine treatment in thalassemia major: a five years multicenter randomized clinical trial under the auspices of the society for the study of thalassemia and hemogobinopathies (SoST). Blood 2007;110(11). [Abstract no.: 575] [Google Scholar]
- Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, Filosa A, et al. Decrease of mortality during deferiprone treatments: results from a large randomised cohort of thalassemia major patients under the auspices of the Italian society for thalassemia and hemogobinopathies. Blood 2008;112 Suppl:Abstract no: 3885. [Google Scholar]
- Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, Filosa A, et al. Long‐term sequential deferiprone‐deferoxamine versus deferiprone alone for thalassaemia major patients: A randomized clinical trial. British Journal of Haematology 2009;145(2):245‐54. [DOI] [PubMed] [Google Scholar]
- NCT00733811. Efficacy Study of the Use of Sequential DFP‐DFO Versus DFP. https://clinicaltrials.gov/ct2/show/NCT00733811 (accessed 11 April 2018).
- Pantalone GR, Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, et al. Sequential alternating deferiprone and deferoxamine treatment compared to deferiprone monotherapy: Main findings and clinical follow‐up of a large multicenter randomized clinical trial in ‐thalassemia major patients. Hemoglobin 2011;35(3):206‐16. [DOI] [PubMed] [Google Scholar]
Mourad 2003 {published data only}
- Mourad FH, Hoffbrand AV, Sheikh‐Taha M, Koussa S, Khoriaty AI, Taher A. Comparison between desferrioxamine and combined therapy with desferrioxamine and deferiprone in iron overloaded thalassaemia patients. British Journal of Haematology 2003;121(1):187‐9. [DOI] [PubMed] [Google Scholar]
Olivieri 1997 {published data only}
- Olivieri N, the Iron Chelation Research Group. Randomized trial of deferiprone (LI) and deferoxamine (DFO) in thalassemia major. Blood 1996; Vol. 88, issue 10 Suppl 1:651a.
- Olivieri NF, Brittenham GM. Evidence of progression of myocardial iron loading as determined by magnetic resonance imaging (MRI) in thalassemia patients during treatment with deferiprone (L1) and deferoxamine (DFO). Blood 1999; Vol. 94, issue 10 Suppl 1:35b.
- Olivieri NF, Brittenham GM. Final results of the randomized trial of deferiprone (L1) and deferoxamine (DFO). Blood 1997; Vol. 90, issue 10 Suppl 1:264a.
- Olivieri NF, Brittenham GM, Armstrong SAM, Basran RK, Daneman R, Daneman N, et al. First prospective randomized trial of the iron chelators deferiprone (L1) and deferoxamine. Blood 1995; Vol. 86, issue 10 Suppl 1:249a.
- Pope, Elena. Critical review of standard and new methods of assessing compliance with chelation therapy in thalassemic patients. Proquest Dissertations Publishing 1995.
Pennell 2006 {published data only}
- Pennell D J, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, et al. Randomized controlled trial of deferiprone or deferoxamine in beta‐thalassemia major patients with asymptomatic myocardial siderosis. Blood 2006;107(9):3738‐44. [DOI] [PubMed] [Google Scholar]
- Smith GC, Alpendurada F, Carpenter JP, Alam MH, Berdoukas V, Kargiorga M, et al. Effect of deferiprone or deferoxamine on right ventricular function in thalassemia major patients with myocardial iron overload. Journal of Cardiovascular Magnetic Resonance 2011; Vol. 13, issue 1:34. [DOI] [PMC free article] [PubMed]
Pennell 2014 {published data only}
- Aydinok Y, Porter JB, Piga A, Elalfy M, El‐Beshlawy A, Kilinc Y, et al. Prevalence and distribution of iron overload in patients with transfusion‐dependent anemias differs across geographic regions: results from the CORDELIA study. European Journal of Haematology 2015;95(3):244‐53. [DOI] [PubMed] [Google Scholar]
- Pennell D, Porter J, Piga A, El‐Alfy M, El‐Beshlawy A, Kilinc Y, et al. Prevalence of cardiac iron overload in patients with transfusion‐dependent anemias: data from the randomized, active‐controlled deferasirox CORDELIA trial. Haematologica 2012;97 Suppl 1:384. [Abstract no.: 0928] [Google Scholar]
- Pennell D, Porter JB, Piga A, Lai Y, El‐Beshlawy A, Beloul K, et al. A multicenter, randomized, open‐label trial evaluating deferasirox compared with deferoxamine for the removal of cardiac iron in patients with beta‐thalassemia major and iron overload (CORDELIA). Blood 2012;120(21). [Abstract no.: 2124] [Google Scholar]
- Pennell DJ, Porter JB, Piga A, Lai Y, El‐Beshlawy A, Belhoul K M, et al. A 1‐year randomized controlled trial of deferasirox vs deferoxamine for myocardial iron removal in beta‐thalassemia major (CORDELIA). Blood 2014;123(10):1447‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taher 2017 {published data only}
- A randomized, open‐label, multicenter, two arm, phase II study to investigate the benefits of an improved deferasirox formulation (Film‐coated Tablet). ClinicalTrials.go 2014; Vol. NCT02125877.
- A randomized, open‐label, multicenter, two arm, phase II study to investigate the benefits of an improved deferasirox formulation (film‐coated tablet). EU Clinical Trials Register issue https://www.clinicaltrialsregister.eu/ctr‐search/trial/2013‐004167‐32/AT.
- Huang VW, Banderas B, Sen R. Psychometric evaluation of clinical outcomes assessments in a phase II trial. Value in Health 2016; Vol. 19, issue 7:A746.
- Taher AT, Origa R, Perrotta S, Kourakli A, Ruffo GB, Kattamis A, et al. New film‐coated tablet formulaion of deferasirox is well tolerated in patients with thalassemia or lower‐risk MDS: Results of the randomized, Phase II ECLIPSE study. American Journal of Hematology 2017;92(5):420‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanner 2007 {published data only}
- Tanner MA, Galanello R, Dessi C, Agus A, Smith GC, Westwood MA, et al. Improved endothelial function combined chelation therapy in thalassaemia major. Blood 2006;108(11). [Abstract no.: 1770] [Google Scholar]
- Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, et al. A randomized, placebo‐controlled, double‐blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 2007;115(14):1876‐84. [DOI] [PubMed] [Google Scholar]
- Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, et al. The effect of combined therapy with deferoxamine and deferiprone on myocardial iron and endothelial function in thalassaemia major: a randomized controlled trials using cardiovascular magnetic resonance. Haematologica 2006; Vol. 91, issue Suppl 1:191.
- Tanner MA, Galanello R, Dessi C, Westwood MA, Smith GC, Khan M, et al. A randomized, placebo controlled, double blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassaemia major using cardiovascular magnetic resonance. Blood 2005;106(11 Pt 1). [Abstract no.: 3655] [DOI] [PubMed] [Google Scholar]
Vichinsky 2007 {published data only}
- Vichinsky E. Patient reported outcomes with chelation therapy in patients with sickle cell disease (SCD) on either deferasirox (Exjade®, ICL670) or deferoxamine (DFO). 29th annual meeting of the National Sickle Cell Disease Program; April 8‐12; Memphis, USA 2006. [Abstract no.: 174]
- Vichinsky E. Results of a randomized, controlled phase two trials of deferasirox (Exjade®, ICL670) in sickle cell disease patients with chronic overload. 29th Annual Meeting of the National Sickle Cell Disease Program; April 8‐12; Memphis, USA 2006. [Abstract no.: 175]
- Vichinsky E, Bernaudin F, Forni GL, Gardner R, Hassell K, Heeney MM, et al. Long‐term safety and efficacy of deferasirox (Exjade) for up to 5 years in transfusional iron‐overloaded patients with sickle cell disease. British Journal of Haematology 2011;154(3):387‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky E, Bernaudin F, Forni GL, Gardner R, Hassell KL, Heeney MM, et al. Long‐term safety and efficacy of deferasirox (Exjade®) in transfused patients with sickle cell disease treated for up to 5 years. Blood 2011;118(21). [Abstract no.: 845] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky E, Coates T, Thompson A, Bernaudin F, Lagrone D, Dong V, et al. Safety and efficacy of iron chelation therapy with deferasirox in patients with sickle cell disease (SCD): 3.5‐year follow‐up. 14th Congress of the European Haematology Association; Jun 4‐7; Berlin, Germany 2009.
- Vichinsky E, Coates T, Thompson AA, Bernaudin F, Rodriguez M, Rojkjaer L, et al. Deferasirox (Exjade®), the once‐daily oral iron chelator, demonstrates safety and efficacy in patients with sickle cell disease (SCD): 3.5‐year follow‐up. Blood 2008;112(11). [Abstract no.: 1420] [Google Scholar]
- Vichinsky E, Coates T, Thompson AA, Bernaudin F, Rodriguez M, Rojkjaer L, et al. Deferasorix (Exjade®), the once‐daily oral iron chelator, demonstrates safety and efficacy in patients with sickle cell disease (SCD): 3.5‐year follow‐up. 3rd Annual Sickle Cell Disease Research and Educational Symposium and Annual Sickle Cell Disease Scientific Meeting; Feb 18‐20; Florida, USA 2009. [Abstract no.: 225]
- Vichinsky E, Coates T, Thompson AA, Mueller BU, Lagrone D, Heeney MM. Long‐term efficacy and safety of deferasirox (Exjade®, ICL670), a once‐daily oral iron chelator, in patients with sickle cell disease (SCD). Blood 2007;110(11 Pt 1):995A. [Abstract no.: 3395] [Google Scholar]
- Vichinsky E, Fischer R, Fung E, Onyekwere O, Porter J, Swerdlow P, et al. A randomized, controlled phase two trial in sickle cell disease patients with chronic iron overload demonstrates that the once‐daily oral iron chelator deferasirox (Exjade®, ICL670) is well tolerated and reduces iron burden. Blood 2005;106. [Abstract no.: 313] [Google Scholar]
- Vichinsky E, Fischer R, Pakbaz Z, Onyekwere O, Porter J, Swerdlow P, et al. Satisfaction and convenience of chelation therapy in patients with sickle cell disease (SCD): Comparison between deferasirox (Exjade®, ICL670) and deferoxamine (DFO). Blood 2005;106(11 Pt 1). [Abstract no.: 2334] [Google Scholar]
- Vichinsky E, Onyekwere O, Porter J, Swerdlow P, Eckman J, Lane P, et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. British Journal of Haematology 2007;136(3):501‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky E, Pakbaz Z, Onyekwere O, Porter J, Swerdlow P, Coates T, et al. Patient‐reported outcomes of deferasirox (Exjade, ICL670) versus deferoxamine in sickle cell disease patients with transfusional hemosiderosis. Substudy of a randomized open‐label phase II trial. Acta Haematologica 2008;119(3):133‐141. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abu 2015 {published data only}
- Abu SO, Auda W, Kamhawy H, Al‐Tonbary Y. Impact of educational programme regarding chelation therapy on the quality of life for B‐thalassemia major children. Hematology 2015;20(5):297‐303. [DOI] [PubMed] [Google Scholar]
Al Kloub 2014 {published data only}
- Al‐Kloub MI, A Bed MA, Al Khawaldeh OA, Al Tawarah YM, Froelicher ES. Predictors of non‐adherence to follow‐up visits and deferasirox chelation therapy among Jordanian adolescents with Thalassemia major. Pediatric Hematology and Oncology 2014;31(7):624‐37. [DOI] [PubMed] [Google Scholar]
Al Kloub 2014a {published data only}
- Al‐Kloub, MI, TN, Salameh, ES, Froelicher. Impact of psychosocial status and disease knowledge on deferoxamine adherence among thalassaemia major adolescents. International Journal of Nursing Practice 2014;20(3):265‐74. [DOI] [PubMed] [Google Scholar]
Al Refaie 1995 {published data only}
- Al‐Refaie FN, Hershko C, Hoffbrand AV, Kosaryan M, Olivier NF, Tondury P, et al. Results of long‐term deferiprone (L1) therapy: a report by the International Study Group on Oral Iron Chelators. British Journal of Haematology 1995;91(1):224‐9. [DOI] [PubMed] [Google Scholar]
Alvarez 2009 {published data only}
- Alvarez O, Rodriguez‐Cortes H, Robinson N, Lewis N, Pow Sang CD, Lopez‐Mitnik G, et al. Adherence to deferasirox in children and adolescents with sickle cell disease during 1‐year of therapy. Journal of Pediatric Hematology/Oncology 2009;31(10):739‐44. [DOI] [PubMed] [Google Scholar]
Armstrong 2011 {published data only}
- Armstrong EP, Skrepnek GH, Ballas SK, Kwok P, Snodgrass S, Sasane M. Costs, persistence, and hospitalizations associated with the use of iron‐chelating therapies in sickle cell disease in medicaid patients. Blood 2011; Vol. 118, issue 21.
Bala 2014 {published data only}
- Bala J, Sarin J. Treatment adherence and quality of life of thalassemic children. International Journal of Nursing Education 2014;6(2):151‐2. [Google Scholar]
Belgrave 1989 {published data only}
- Belgrave FZ, Gilbert SK. Health care adherence of persons with sickle cell disease. The role of social support. Annals of the New York Academy of Sciences 1989;565:369‐70. [Google Scholar]
Berkovitch 1995 {published data only}
- Berkovitch M, Davis S, Matsui D, DonskyJ, Koren G, Olivieri NF. Use of a eutectic mixture of local anesthetics for prolonged subcutaneous drug administration. Journal of Clinical Pharmacology 1995;35(3):295‐7. [DOI] [PubMed] [Google Scholar]
Chakrabarti 2013 {published data only}
- Chakrabarti P, Bohara V, Ray S, Sankar Ray S, Kumar, NU, Chaudhuri U. Can the availability of unrestricted financial support improve the quality of care of thalassemics in a center with limited resources? A single center study from India. Thalassemia Reports 2013;3(1):6‐10. [Google Scholar]
Daar 2010 {published data only}
- Daar S, Al, Salmi F, Ableen V, Jacob W, Jabeen Z, Pathare A. T2*MRI ‐ An effective tool to increase chelation compliance in thalassemia major. Haematologica 2010, issue 95:698.
Gomber 2004 {published data only}
- Gomber S, Saxena R, Madan N. Comparative efficacy of desferrioxamine, deferiprone and in combination on iron chelation in thalassemic children. Indian Pediatrics 2004;41(1):21‐7. [PubMed] [Google Scholar]
Kidson Gerber 2008 {published data only}
- Kidson‐Gerber G, Lindeman R. Adherence to desferrioxamine and deferiprone and the impact of deferiprone co‐prescription in thalassaemia major patients. Does the addition of deferiprone improve adherence?. British Journal of Haematology 2008;142(4):679‐80. [DOI] [PubMed] [Google Scholar]
Kolnagou 2008 {published data only}
- Kolnagou A, Economides C, Eracleous E, Kontoghiorghes GJ. Long term comparative studies in thalassemia patients treated with deferoxamine or a deferoxamine/deferiprone combination. Identification of effective chelation therapy protocols. Hemoglobin 2008;32(1‐2):41‐7. [DOI] [PubMed] [Google Scholar]
Leonard 2014 {published data only}
- Leonard S, Jonassaint J, Anderson L, Shah N. The use of mobile technology for intensive training in medication management in the pediatric population. Blood 2014; Vol. 124, issue 21.
Loiselle 2016 {published data only}
- Loiselle K, Lee JL, Szulczewski L, Drake S, Crosby LE, Pai AL. Systematic and meta‐analytic review: medication adherence among pediatric patients With sickle cell disease. Journal of Pediatric Psychology 2016;41(4):406‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzone 2009 {published data only}
- Mazzone L, Battaglia L, Andreozzi F, Romeo MA, Mazzone D. Emotional impact in beta‐thalassaemia major children following cognitive‐behavioural family therapy and quality of life of caregiving mothers. Clinical Practice and Epidemiology in Mental Health 2009;5(5):Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01709032 {published data only}
- NCT01709032. Combination deferasirox and deferiprone for severe iron overload in thalassemia. https://clinicaltrials.gov/ct2/show/NCT01709032 (accessed 11 April 2018).
NCT01825512 {published data only}
- NCT01825512. Multicentre, randomised, open label, non‐inferiority active‐controlled trial to evaluate the efficacy and safety of deferiprone compared to deferasirox in paediatric patients aged from 1 month to less than 18 years of age affected by transfusion‐dependent haemoglobinopathies. https://clinicaltrials.gov/ct2/show/NCT01825512 (accessed 11 April 2018).
NCT02133560 {published data only}
- NCT02133560. Use of mobile technology for intensive training in medication management. https://clinicaltrials.gov/ct2/show/NCT02133560 (accessed 14 April 2018).
NCT02466555 {published data only}
- NCT02466555. Music Therapy in Sickle Cell Transition Study. https://clinicaltrials.gov/ct2/show/NCT02466555 (accessed 14 April 2018). [Sponsors: University Hospital Case Medical Center|Kulas FoundationOther IDs: 03‐15‐30]
Pakbaz 2004 {published data only}
- Pakbaz Z, Fischer R, Gamino R, Quirolo K, Yamashita R, Treadwell M, et al. Assessing compliance to iron chelation therapy in patients with thalassemia. Blood 2004; Vol. 104, issue 11:33B.
Pakbaz 2005 {published data only}
- Pakbaz ZR, Fischer M, Treadwell R, Yamashita EB, Fung L, Calvell K, et al. A simple model to assess and improve adherence to iron chelation therapy with deferoxamine in patients with thalassemia. Annals of the New York Academy of Sciences 2005;1054:486‐91. [DOI] [PubMed] [Google Scholar]
Porter 2009 {published data only}
- Porter JB, Athanasiou‐Metaxa M, Bowden DK, Troncy, J, Habr D, Domokos G, et al. Improved patient satisfaction, adherence and health‐related quality of life with deferasirox (Exjade) in beta‐thalassemia patients previously receiving other iron chelation therapies. Blood 2009; Vol. 114, issue 22.
Porter 2012 {published data only}
- Porter J, Bowden DK, Economou M, Troncy J, Ganser A, Habr D, et al. Health‐ related quality of life, treatment satisfaction, adherence and persistence in beta‐thalassemia and myelodysplastic syndrome patients with iron overload receiving deferasirox: results from the epic clinical trial. Anemia 2012;297:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vichinsky 2005 {published data only}
- Vichinsky E, Fischer R, Pakbaz Z, Onyekwere O, Porte, J, Swerdlow P, et al. Satisfaction and convenience of chelation therapy in patients with sickle cell disease (SCD): Comparison between deferasirox (Exjade®, ICL670) and deferoxamine (DFO). Blood 2005;106(11 Pt 1). [Abstract no.: 2334] [Google Scholar]
Vichinsky 2008 {published data only}
- Vichinsky E, Pakbaz Z, Onyekwere O, Porter J, Swerdlow P, Coates T, et al. Patient‐reported outcomes of deferasirox (Exjade, ICL670) versus deferoxamine in sickle cell disease patients with transfusional hemosiderosis. Substudy of a randomized open‐label phase II trial. Acta Haematologica 2008;119(3):133‐41. [DOI] [PubMed] [Google Scholar]
Waheed 2014 {published data only}
- Waheed N, Ali S, Butt MA. Comparison of deferiprone and deferrioxamine for the treatment of transfusional iron overload in children with beta thalassemia major. Journal of Ayub Medical College, Abbottabad: JAMC 2014;26(3):297‐300. [PubMed] [Google Scholar]
Walsh 2014 {published data only}
- Walsh KE, Cutrona SL, Kavanagh PL, Crosby LE, Malone C, Lobner K, et al. Medication adherence among pediatric patients with sickle cell disease: a systematic review. Pediatrics 2014;134(6):1175‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yarali 2006 {published data only}
- Yarali N, Fisgin T, Duru F, Kara A, Ecin N, Fitoz S, Erden I. Subcutaneous bolus injection of deferoxamine is an alternative method to subcutaneous continuous infusion. Journal of Pediatric Hematology/Oncology 2006;28(1):11‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Antmen 2013 {published data only}
- Antmen B, Organ K, Sasmaz I, Berktas M, Kilinc Y. A cohort study to assess the contribution of patient compliance program on persistence to deferasirox in patients with chronic iron overload in turkey (ex‐pat program). Haematologica/the Hematology Journal 2013; Vol. 98:713‐4.
NCT00004982 {published data only}
- NCT00004982. Combination iron chelation therapy. https://clinicaltrials.gov/ct2/show/NCT00004982 (accessed 18 April 2018).
References to ongoing studies
EudraCT 2012‐000353‐31 {published data only}
- Consorzio per le Valutazioni Biologiche e Farmacologiche. Multicentre, randomised, open label, non‐inferiority active‐controlled trial to evaluate the efficacy and safety of deferiprone compared to deferasirox in paediatric patients aged from 1 month to less than 18 years of age affected by transfusion‐dependent haemoglobinopathies. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2012‐000353‐31/IT (EU Clinical Trials Register) (accessed 18 April 2018).
IRCT2015101218603N2 {published data only}
- Amirmoezi F. To assess compliance, efficacy and satisfaction with two different formulation of deferasirox in patients with transfusion‐dependent beta‐thalassemia. http://en.irct.ir/trial/16826 (Iranian Registry of Clinical Trails) (accessed 18 April 2018).
Madderom 2016 {published data only}
- Cnossen MH. A randomized trial evaluaTing the Effects of group medical AppointMents on self‐efficacy and adherence in Sickle Cell Disease (TEAM study). ‐ TEAM study [Effecten van GroepsconsulTen op zElfmAnageMent en therapietrouw in sikkelcelziekte (TEAM studie)]. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=4750 (Erasmus Medical Center, Sophia Children's Hospital) (accessed 18 April 2018), issue NTR4750.
- Madderom MJ, Heijdra J, Utens EM, Polinder S, Rijneveld AW, Cnossen MH. A randomized controlled trial studying the effectiveness of group medical appointments on self‐efficacy and adherence in sickle cell disease (TEAM study): study protocol. BMC Hematology 2016;16(21):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02173951 {published data only}
- NCT02173951. An algorithm to start iron chelation in minimally transfused young beta‐thalassemia major patients. https://clinicaltrials.gov/ct2/show/NCT02173951 (accessed 18 April 2018):ClinicalTrials.gov.
NCT02435212 {published data only}
- NCT02435212. Study to evaluate treatment compliance, efficacy and safety of an improved deferasirox formulation (granules) in pediatric patients (2‐<18 years old) with iron overload. https://clinicaltrials.gov/ct2/show/NCT02435212 (accessed 18 April 2018). [Sponsors: Novartis Pharmaceuticals|NovartisOther IDs: CICL670F2202|2013‐004739‐55]
Additional references
Abetz 2006
- Abetz l, Baladi JF, Jones P, Rafail D. The impact of iron overload and its treatment on quality of life: results from a literature review. Health and Quality of Life Outcomes 2006;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
American Pharmacists Association 2008
APPG 2009
- All‐Party Parliamentary Group on Sickle Cell and Thalassaemia (APPG). Sickle cell disease and thalassaemia: A health check. http://ukts.org/pdfs/awareness/appg.pdf (accessed 20 June 2016).
Aydinok 2014
- Aydinok Y, Kattamis A, Viprakasit V. Current approach to iron chelation in children. British Journal of Haematology 2014;165(6):745‐55. [DOI] [PubMed] [Google Scholar]
Costello 2004
- Costello I, Wong ICK, Nunn AJ. A literature review to identify interventions to improve the use of medicines in children. Child: Care, Health and Development 2004;30(6):647‐65. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2017.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group, editor(s). Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). EPOC Resources for review authors. http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 21 September 2017).
Fisher 2013
- Fisher SA, Brunskill SD, Doree C, Gooding S, Chowdbury O, Roberts DJ. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion‐dependent thalassaemia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004450.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gravitz 2014
- Gravitz L, Pincock S. Sickle‐cell disease. Nature 2014;515:S1. [DOI] [PubMed] [Google Scholar]
Grosse 2011
- Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa. A neglected cause of early childhood mortality. American Journal of Preventive Medicine 2011;41(6 S4):S298‐S405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haywood 2009
- Haywood, C, Beach M, Lanzkron S, Strouse, J, Wilson, R, Park, H, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. Journal of the National Medical Association 2009;10(10):1022‐33. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011d
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group, editor (s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Modell 2008
- Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bulletin of the World Health Organization 2008;86(6):480‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCMH 2010
- National Collaborating Centre for Mental Health. Depression in adults with a chronic physical health problem: treatment and management. Clinical guideline [CG91]. www.nice.org.uk/guidance/cg91 (accessed 16 April 2018). [ISBN:: 13: 978‐1‐904671‐86‐2]
NCCPC 2009
- The National Collaborating Centre for Primary Care. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. NICE Guideline CG76. https://www.nice.org.uk/guidance/cg76 (accessed 20 June 2016).
NICE 2009
- National Institute for Health and Care Excellence. Depression in adults with chronic physical health problem: recognition and management. NICE guideline CG91. www.nice.org.uk/guidance/cg91 (accessed 20 June 2016).
NICE 2010
- NICE National Institute for health and Care Excellence. Sickle cell disease. http://cks.nice.org.uk/sickle‐celldisease#!backgroundsub:3 (accessed 20 June 2016).
Payne 2008
- Payne KA, Rofail D, Baladi JF, Viala M, Abetz L, Desrosiers MP, et al. Iron chelation therapy: clinical effectiveness, economic burden and quality of life in patients with iron overload. Advances in Therapy 2008;25(8):725‐42. [DOI] [PubMed] [Google Scholar]
Piel 2012
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model‐based map and population estimates. Lancet 2012;381(9861):142‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piel 2014
- Piel, FB, Weatherall DJ. The α‐thalassemias. New England Journal of Medicine 2014;371(20):1908‐16. [DOI] [PubMed] [Google Scholar]
Pleasants 2014
- Pleasants S. Epidemiology: a moving target. Nature 2014;515:S2‐3. [DOI] [PubMed] [Google Scholar]
Rees 2010
- Rees DC, Williams TN, Gladwin MT. Sickle‐cell disease. Lancet 2010;376(9757):2018‐31. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA on behalf of the Cochrane Non‐Randomised Studies Methods Group. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rofail 2010
- Rofail D, Viala M, Gater A, Abetz‐Webb L, Baladi JF, Cappellini MD. An instrument assessing satisfaction with iron chelation therapy: Psychometric testing from an open‐label clinical trial. Advances in Therapy 2010;27(8):533‐46. [DOI] [PubMed] [Google Scholar]
Rund 2005
- Rund D, Rachmilewitz E. β‐Thalassemia. New England Journal of Medicine 2005;353(11):1135‐46. [DOI] [PubMed] [Google Scholar]
Ryan 2014
- Ryan R, Santesso N, Lowe D, Hill S, Grimshaw J, Prictor M, Kaufman C, Cowie G, Taylor M. Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007768.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2011
- Sterne JAC, Egger M, Moher D on behalf of the Cochrane Bias Methods Group, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2016
- Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ROBINS‐I. ROBINS‐I: a tool for assessing Risk Of Bias In Non‐randomized Studies of Interventions, Version 7 March 2016. www.riskofbias.info (accessed 19 April 2016).
Telfer 2006
- Telfer P, Coen PG, Christou S, Hadjigavriel M, Kolnakou A, Pangalou E, et al. Survival of medically treated thalassemia patients in Cyprus. Trends and risk factors over the period 1980‐2004. Haematologica 2006;91(9):1187‐92. [PubMed] [Google Scholar]
Thomas 2013
- Thomas, V, Rawle, H, Abedian, M, Ferguson, A. Implementing the NICE clinical guideline 91 ('Depression in adults with a chronic physical health problem') in a haematology department. Clinical Psychology Forum 2013;246:41‐45. [Google Scholar]
Trachtenberg 2012
- Trachtenberg FL, Mednick L, Kwiatkowski JL, Neufeld EJ, Haines D, Pakbaz Z, et al. Beliefs about chelation among thalassemia patients. Health and Quality of Life Outcomes 2012;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trachtenberg 2014
- Trachtenberg, F, Gerstenberger, E, Xu, Y Mednick, L, Sobota, A, Ware, H, et al. Relationship among chelator adherence, change in chelators, and quality of life in Thalassemia. Quality of Life Research 2014;23:2277‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK Thalassaemia Society 2008
- United Kingdom Thalassaemia Society. Standards for the clinical care of children and adults with thalassaemia in the UK. UK Thalassaemia Society 2008; Vol. 2nd edition:120.
Vekeman 2016
- Vekeman F, Sasane M, Cheng WY, Ramanakumar AV, Fortier J, Qiu Y, et al. Adherence to iron chelation therapy and associated healthcare resource utilization and costs in Medicaid patients with sickle cell disease and thalassemia. Journal of Medical Economics 2016;19(3):292‐303. [DOI] [PubMed] [Google Scholar]
Wertheimer 2003
- Wertheimer A, Santella T. Medication compliance research. Journal of Applied Research in Clinical and Experimental Therapeutics 2003;3(3):254‐61. [Google Scholar]
WHO 2003
- World Health Organization. Adherence to long‐term therapies: Evidence for action. WHO 2003:194.
Yawn 2014
- Yawn BP, Buchanan GR, Afenyi‐Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease:summary of the 2014 evidence‐based report by expert panel members. JAMA 2014;312(10):1033‐48. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fortin 2016
- Fortin PM, Madgwick KV, Trivella M, Hopewell S, Doree C, Estcourt LJ. Interventions for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012349] [DOI] [PMC free article] [PubMed] [Google Scholar]


