Abstract
Background
Pulmonary arterial hypertension (PAH) is a potentially serious cause of dyspnea and exercise limitation in patients with HIV infection. In this trial, we propose using exercise MRI in conjunction with cardiopulmonary testing to delineate PAH from other causes of cardiovascular dysfunction, identify individuals with exercise-induced PAH who are at high risk of developing resting PAH, and provide longitudinal estimates of progression of PAH and right ventricular function.
Methods
In this prospective observational study, HIV patients with dyspnea and exercise limitation in the absence of identifiable causes and those who meet the inclusion criteria will be enrolled based on resting pulmonary artery pressure (≤ or >40 mmHg) on a screening echocardiogram and exercise limitation on the Modified Medical Research Council dyspnea scale. Patients without evidence of resting PAH will be enrolled into both rest and exercise MRI and cardiopulmonary testing protocol, whereas patients with evidence of PAH on resting echocardiograms will undergo only resting cardiac MRI studies to evaluate right ventricular function and fibrosis. Both patient subgroups will be followed for 24 months to obtain longitudinal progression of the disease. In a sub-study, we will further analyze inflammatory variables that may predict these changes, thus allowing early identification of these patients.
Implications and conclusions
This trial will be the first study to provide an understanding of the mechanisms underpinning the functional deterioration of the right ventricle in patients with HIV and will impart insight into the immune mediators of PAH progression and right ventricular functional deterioration in patients with HIV-PAH.
Keywords: cardiac MRI, cardiopulmonary testing, exercise limitation, exercise MRI, HIV, pulmonary hypertension
Introduction
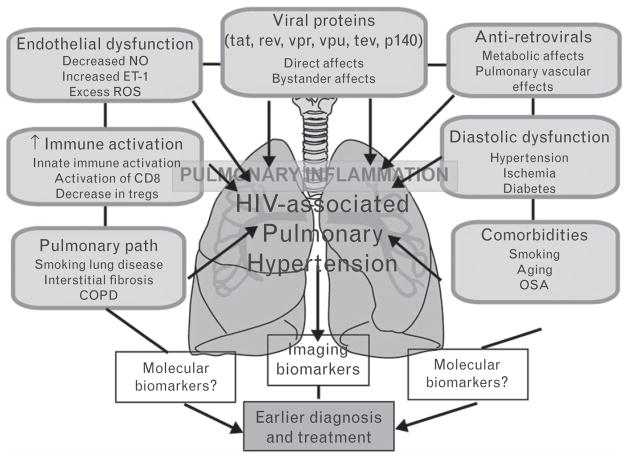
The advent of antiretroviral therapy (ART) has markedly improved survival rates in patients with HIV infection, converting HIV from a fatal disease to a chronic condition1,2; indeed, long-term cardiopulmonary complications have emerged as the primary source of morbidity and mortality.3–5 Pulmonary artery hypertension (PAH) is one such condition and presents with nonspecific symptoms of dyspnea and exercise intolerance.6 The diagnosis of PAH in HIV portends a poor prognosis, with the median survival being only 12 months.7–9 In most cases of HIV-PAH, death is a consequence of PAH rather than complications of HIV itself.10 PAH therapies are most effective in the early stages, before the onset of right heart failure (RHF). Thus, recognition of subclinical right ventricular dysfunction and PAH in HIV patients may have major implications for prognosis, insofar as prompt initiation of PAH-specific therapy could potentially improve outcomes. However, the nonspecificity of symptoms in PAH such as dyspnea makes the early diagnosis of PAH a challenge, as it may mimic multiple disorders, and a variety of mechanisms may contribute to dyspnea and exercise limitation in HIV (Fig. 1).11,12 Distinguishing between these various causes in a patient with HIV is often difficult, time-consuming, and involves complex care coordination across medical specialties.
Fig. 1.
Potential causative mechanisms of HIV-associated pulmonary hypertension: COPD, chronic obstructive pulmonary disease; ET-1, endothelin 1; NO, nitric oxide; OSA, obstructive sleep apnea; ROS, reactive oxygen species.
Similarly to other secondary forms of PAH, it is widely believed that a good proportion of HIV patients may have normal pulmonary artery pressures at rest, but exhibit exercise-induced increases in PAH.13–17 The recent American College of Cardiology/American Heart Association consensus guidelines for PAH acknowledge that the relevance and impact of exercise hemodynamic studies in symptomatic patients with risk factors for PAH and normal resting pulmonary artery pressures warrant further investigation.9 It has been hypothesized that exercise-induced PAH (Ex-PAH) may represent an intermediate phase in the PAH spectrum that may predict future development of resting PAH.13–17
Cardiac MRI (CMR) is currently a preferred imaging modality to assess patients with PAH,18,19 due to its high accuracy, safety, easy reproducibility, low observer variability, and ability to provide structural and functional data.20–22 This single-center prospective study will study patients with both resting PAH, and also those with no evidence of PAH at rest by echocardiogram, but who present with symptoms of dyspnea and exercise limitation, and study longitudinal progression in both groups. As there is also evidence to suggest a role of immune response in vascular disorders,6,23–26 we will also examine the link between PAH progression and markers of immune activation. The overarching hypothesis of this study is that the delineation of exercise PAH in patients with normal resting right ventricular function and pulmonary pressures may identify a patient population at risk for progression, and potentially allow appropriate intervention at an early stage of this disease.
Methods
This is a prospective observational study that will enroll patients with HIV and dyspnea rate 1–3 on the Modified Medical Research Council (MMRC) dyspnea scale having no other identifiable cause.27 The inclusion/exclusion criteria for the study are detailed as follows:
Inclusion criteria:
Adults aged 18–65 years with confirmed HIV-1 who intend to remain in current geographical area of residence during duration of study.
Unexplained persistent or progressively worsened dyspnea for at least 6 weeks or previously documented systolic pulmonary artery pressure (PAP) above 35 mmHg, and/or abnormal right ventricle on echocardiogram or established diagnosis of HIV-PAH.
Karnofsky score above 70 on at least one occasion within past 2 weeks prior to entry.
Exclusion criteria:
Contraindications to MRI (metallic implants, severe claustrophobia).
Acute coronary syndrome, transient ischemic attack, CVA, or critical limb ischemia during the past 6 months, or coronary/peripheral revascularization within the past 3 months.
Concurrent potentially life-threatening arrhythmia or symptomatic arrhythmia.
Documented left ventricular ejection fraction less than 45%.
Evidence of pulmonary infection in the past 4 weeks.
Evidence of another cause of individual’s dyspnea such as reactive airway disease, congestive heart failure, and decompensated cirrhosis.
Glomerular fltration rate (GFR) (modification of diet in renal disease) of less than 40 at visit 1.
hemoglobin <8 g/dl.
Platelet count below 50 000/mm.
Pregnancy or intent of becoming pregnant during duration of study.
Active drug use, either by history or urine toxicology screen.
Any surgical or medical conditions which places the patient at higher risk derived from his/her participation into the study, or likely to prevent patient from complying with requirements.
Eligible participants will undergo initial screening with a complete medical history, physical examination, chest radiograph, 12-lead electrocardiogram (EKG), pulmonary function test (PFT), and various transthoracic echocardiogram end-points of relevance to pulmonary hypertension (PAH) (Appendix, http://links.lww.com/JCM/A113). Two sets of patients will be recruited: those with dyspnea and no evidence of PAH at rest, and patients with previously known PAH attributable to HIV. Patients who do not have resting PAH will be enrolled into the exercise MRI (ExMR) and cardiopulmonary testing (CPT) testing protocols, whereas patients with established or newly diagnosed resting PAH will not undergo exercise testing, but will only undergo baseline and repeat CMR at 12 and 24 months to longitudinally assess serial changes in right ventricular function. Individuals with newly diagnosed PAH will undergo clinically driven right heart catheterization to confirm this diagnosis (study flow outline in Fig. 1). Both groups of patients will also undergo muscle strength testing at baseline, which includes handgrip and knee extensor strength, and muscle girth measurements.
Exercise cardiac MRI protocol with cardiopulmonary testing
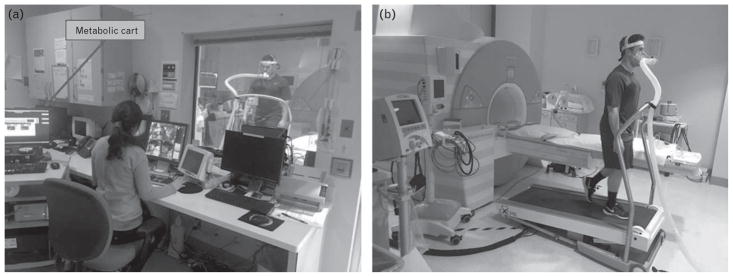
The combination of ExMR and CPT may provide vital cardiopulmonary information, including an accurate assessment of function and exercise capacity. In order to utilize ExMR in combination with CPT, modification of conventional exercise stress testing equipment to ensure magnetic resonance (MR) compatibility is required. The MR-compatible treadmill is placed immediately adjacent to the CMR system and is previously been utilized in single-center studies and multisite investigations (Fig. 2). We will utilize a CMR-compatible gas exchange system to measure ventilator effort and gas exchange during a maximal effort stress test, performed immediately adjacent to the MRI system as described previously by our group.28 Using standard corrugated plastic tubes, the gas analyzer and metabolic cart will be positioned immediately outside the CMR room for safety to avoid any electronic interference that may corrupt the CMR images and to reduce any impact that a strong magnetic field may have on the gas analyzer. Oxygen consumption (VO2), carbon dioxide production (VCO2), minute ventilation, fractional concentrations of oxygen and carbon dioxide, and respiratory rate will be measured during exercise. Ventilatory efficiency slope (VE/VCO2) will be calculated from metabolic data. Breath-by-breath oxygen utilization, carbon dioxide production, and minute ventilation values will be averaged at rolling 10-s intervals. VO2max will be recorded as the average of the last two VO2 values during exercise.
Fig. 2.
(a) The metabolic cart is outside the CMR room. (b) The MR-compatible treadmill sits safely in the MR examination room as part of system required to perform the exercise CMR examination without any significant delay. CMR, cardiac MRI.
A 1.5-T scanner (Siemens, Espree) with a 32-channel phased array coil will be used for CMR. Patients will abstain from ingesting caffeine for 12 h and/or eating within 3 h prior to ExMR. In the MRI room, volunteers will be positioned on the gantry using individualized molds created from deflatable cushions that allow easy return to the same position postexercise. Resting cine images consisting of five short-axis and four long-axis slices will be performed using a nontriggered real-time steady-state free precession (SSFP) acquisition using a radial scheme for k-space acquisition using the following parameters: repetition time TR = 2.74 ms, ms, echo time TE = 1.37 ms, bandwidth =1115 Hz/px, flip angle =70°, field of view (FoV) =300 mm2, temporal resolution =43.8 ms, in-plane resolution =2.3 mm2, acceleration factor of 8; slice thickness =6–8 mm, number of slices =12–16, slice gap =0–20%, cardiac phases =13–33. A total of 60 accelerated short-axis images were acquired for each slice in order to ensure capture of a complete cardiac cycle (approximately 2.5 s of imaging per slice) as described previously.29,30 A real-time, through-plane velocity encoding sequence based on gradient-echo echo-planar imaging (GRE-EPI) with shared velocity encoding (SVE) reconstruction will be implemented.31 An echo train length of 7 and linear k-space acquisition order, aTE of 5 ms and a TR of 10 ms will be used to assess velocities across the main pulmonary artery. Four shots per image were used to collect a total of 28 k-space lines resulting in an acquisition time of 40 ms for each full k-space dataset. Parallel imaging technique TGRAPPA with acceleration rate 2–3 will be used to reconstruct 84 lines per image. Other imaging parameters: 2405 Hz/pixel readout bandwidth, 84 ×128 pixel reconstructed matrix, 10 mm slice, 268 ×350 mm rectangular FoV (3.2 ×2.7 mm pixels). A 25° rapid water-excitation pulse will be used to suppress fat, and Maxwell correction used to account for the effect of concomitant gradients on the phase maps. Three orthogonal slices across the pulmonary artery will be prescribed with the highest velocity vector within these slices and the time velocity integral calculated. The latter will be taken to represent mean pulmonary artery velocity.
After obtaining a resting MR exam, the patient will be placed on the MR-compatible treadmill and connected to MR-compatible EKG leads. Patients will be stressed using an exercise protocol appropriately chosen by the performing exercise physiologist and in accordance with institutional review board protocols. Exercise protocols will be chosen to account for the hydraulic treadmill requiring a starting treadmill angle of at least 2% and starting speed of 1.5 mph to function appropriately.32 Exercise time will be measured from the beginning of the protocol until voluntary exhaustion or safety criteria for termination are reached including: symptoms at maximal exercise including chest pain, dizziness or fainting, a decrease in systolic blood pressure below the resting pressure or severe hypertension, significant oxygen desaturation, ischemic ECG changes, significant tachyarrhythmia’s, second or third degree heart block. Immediately after peak exercise, the patient will be immediately returned to the scanner isocenter based on prestress positioning. Nonbreath-held real-time cine imaging will be performed using a protocol identical to that described at rest and with assessment of pulmonary artery velocities using phase contrast imaging. At the completion of imaging, the patient will be brought out of the scanner and allowed to recover with close monitoring of EKG and blood pressure. Gadobenate Meglumine (Multihance, 0.15 mmol/kg) will be injected, and after a delay of 10 min, placed back in scanner with inversion time estimated followed by a segmented inversion recovery sequence as described for late gadolinium enhancement (LGE). During follow-up visits, echocardiography will be obtained at 12 and 24 months to allow comparison of CMR volumes and velocities (see below) with a historic gold standard.
Analysis of cardiac MRI
Using validated software (Argus, Siemens, Erlangen, Germany) myocardial volumetric measurements at rest and stress will be obtained by manually tracing the end-diastolic and end-systolic myocardial boundary for all short-axis slices. Papillary muscles will be excluded as described by us previously.33 Contours of the main pulmonary artery will be traced in each reconstructed phase-contrast MR image in all three slices, and average pulmonary artery velocity was calculated by integrating over time, the values of pixel velocity within the traced region of interest. For measurement of LGE and fibrosis of the right ventricle, we have made significant improvements in spatial resolution required for imaging LGE in the right ventricle, and will employ a three-dimensional (3D) single breath-hold sequence at high spatial resolution. LGE will be quantified using a signal intensity threshold of more than 2 SD above a remote reference region and also using regions defined as above 50% of maximal signal intensity of the enhanced area [full-width at half maximum (FWHM)] using previously validated methods.34,35 We will also divide the right ventricle into seven segments as described by Babu-Narayan et al.36 on fibrosis for descriptive purposes. The magnitude of right ventricular enhancement will be expressed in mm2.
Differentiation of dyspnea causes and identification of exercise pulmonary arterial hypertension
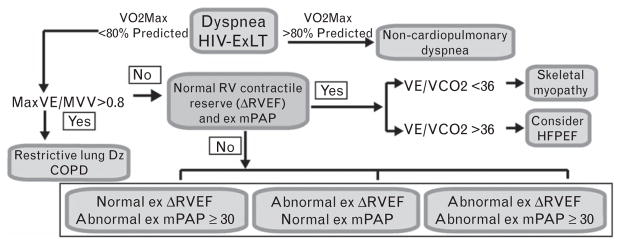
We will employ a previously described and clinically accepted approach, based on previous descriptions using invasive exercise hemodynamics, modified for MR purposes (illustrated in Fig. 3) to assign and differentiate the diagnoses of skeletal myopathy, heart failure with preserved ejection fraction (HFPEF), and HIV Ex-PAH.37 Based on this approach, patients who are found to have abnormal Ex ΔRVEF (Δ=change) (<5%) and/or abnormal ExPAP response [mean pulmonary artery pressure (mPAP) ≥30 mmHg], as measured by ExMR, will receive a diagnosis of HIV Ex-PAH. Those with normal ΔRVEF and Ex mPAP, but with VE/VCO2 below 36, will receive a diagnosis of skeletal myopathy, whereas those with VE/VCO2 above 36 will be labeled HFpEF.28,37,38 Although we acknowledge that there are many limitations with such a simplistic categorization of patients, especially given the overlap of these conditions, such an approach is reasonable from the standpoint of initial categorization of patients.39
Fig. 3.
Approach to distinguishing PAH from other causes of dyspnea that may be highly prevalent in HIV-exercise limitation (HIV-ExLT). Abnormal exercise ΔRVEF will be defined as less than 5% improvement in RVEF with exercise. PAH, pulmonary artery hypertension; RVEF, right ventricular ejection fraction.
Evaluation of progression of right ventricular dysfunction and exercise-induced right ventricular contractile reserve
This will be defined as a decrease of at least 5% in right ventricular ejection fraction (RVEF); this value has been shown to be clinically relevant among a variety of patients.40 Similarly, a change of 10% or more in right ventricular end-diastolic volume (RVEDV) has been shown to clinically correlate with change in functional outcomes, as shown by us previously in a clinical trial in patients with end-stage renal disease at risk for heart failure.33,41 On the basis of our preliminary data and published literature, we will define a normal right ventricular contractile reserve as at least 10%.42 Responses less than 5% will be categorized as abnormal, whereas increase in 5–10% will be categorized as flat. Longitudinal changes (Δ) in the magnitude of these primary endpoints including ΔRVEF (contractile function), and also VO2max will be determined and compared across different time-points within a study participant and between study participants using paired t test.
Overall data analysis
Demographical, clinical, and laboratory characteristics between groups will be compared using t test (for comparing means), Wilcoxon test (for comparing medians), and chi-square test (for comparing binary and ordinal variables). As a primary goal, we will use CMR information in conjunction with CPT study information in HIV patients to assess the prevalence of Ex-PAH and to identify subsets of PAH at risk (e.g. those with alteration in right ventricular function) and finally to differentiate PAH from other prevalent causes of dyspnea in this patient population. Use of right ventricular contractile function, mPAP measurements, and cardiopulmonary indices (VO2max and VE/VCO2) by ExMR will allow separation of patients with exercise limitation and importantly identify and follow a subset of Ex-PAH patients. CD4 and viral load will also be measured to assess viral load suppression and immune restoration. The distribution of HIV plasma viral load and CD4+ lymphocyte count will be examined for normality using Kolmogorov–Smirnov one-sample test with non-normal variables. We will also measure various immune parameters to correlate with CMR parameters to understand the immune dysregulation in PAH (see below).
To investigate the longitudinal change in right ventricular function and volumes, we will study the progression of right ventricular function and volume (as defined above) at the end of 12 and 24 months in patients with Ex-PAH. We will also model these progression outcomes using generalized estimating equation (GEE) approach, which takes into account the correlation between repeated measurements, and will also be used to model changes in our end-points.43,44 To allow for directionality in ΔRVEF between individuals, we will include parameters for random slope and random intercept. We will also determine the association between progression of right ventricular indices and VO2max defined above, and immune activation and immune exhaustion markers as mentioned above. At the bivariate analysis level, association between these markers and changes in RVEF and VO2max at 12 and 24 months from baseline will be tested by chi-square test for categorical variables and by t test or Mann–Whitney U test for continuous variables.
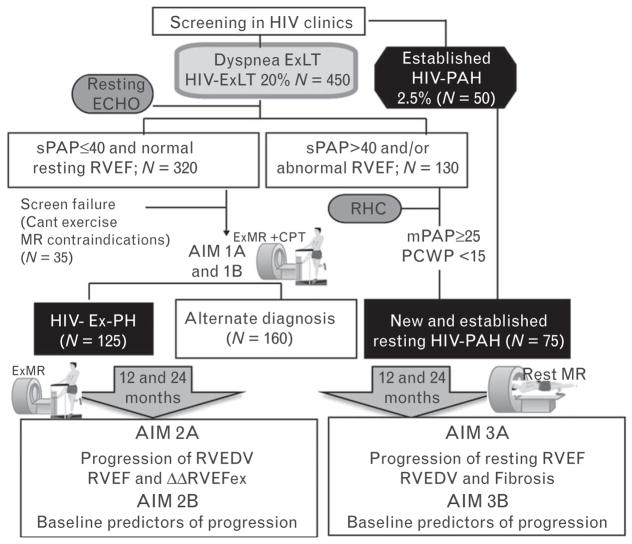
In patients with resting PAH, we will focus on assessing longitudinal changes in ΔRVEF and ΔRVEDV, and also ΔRV-LGE (progression of fibrosis) at baseline, and 12 and 24 months (Fig. 4). Pearson’s correlation method will be used to evaluate these longitudinal changes. Differences and changes in hemodynamic parameters between study participants with prevalent and newly diagnosed PAH will be compared using unpaired and paired t test, respectively. We shall also perform a cross-sectional analysis, to compare baseline immune activation markers and plasma viral antigens (vpr, nef) between patients with HIV Ex-PAH and patients with established PAH.
Fig. 4.
Study flow with sample size. ExLT, exercise limitation; mPAP, mean pulmonary artery pressure; RHC, right heart catheterization; RVEDV, right ventricular end-diastolic volume. mPAP, mean pulmonary artery pressure.
Power and sample size calculation
Based on our experience, we estimate approximately a 10% screen failure due to claustrophobia and stage IV chronic kidney disease [estimated GFR (eGFR) <30]. Based on a true prevalence of abnormal ExMR of 39.0% [95% confidence interval (CI) 34–45%], our sample size of N =320 will have at a maximum of ±8% level of precision, even assuming a prevalence estimate at the lower boundary of the CI. We determined power for 125 study participants to account for 20% attrition rate. For RVEF and RVEDV change, assuming a σ =3–4% and σ =6%, respectively, our study has power of 87 and 100% to detect the hypothesized changes in these end-points, respectively.33
In patients with resting PAH, we anticipate recruiting 75 study participants to account for 20% attrition rate. We estimate a power of 84% to detect ΔRVEF decrease of 5% or more and a power of 93% to detect ΔRVEDV for 10% or more. These changes are relevant and correlate with clinical progression in PAH.40 Our GEE analysis will have above 80% power to detect differences in outcomes by various risk factors ranging from 40% and above intracorrelation coefficient between 0.3 and 0.4.
Evaluating the immune dysregulation in HIV-PAH
There is increasing evidence supporting a role for inflammation in the lung and pathogenesis of PAH.45,46 Whereas innate immune activation in PAH has received the most attention, there are emerging data suggesting that the adaptive immune response may be important in disorders associated with alteration of vascular health.23–26,47,48 Baseline clinical characteristics, including rest MRI measures, CD8 cytotoxic potential (CD3+CD8+NK56+), immune exhaustion (CD3+CD8+Tim3+), and elevated measures of dendritic cell activation (CD14loCD16hiTim3+, including cytokine expression in these cells), will be analyzed and compared among study groups. Flow sorted cells from CD14loCD16hi populations will be analyzed for IFN-gamma/interleukin (IL)-1, the levels of which may also predict progression to resting PAH. We will additionally test the effects of plasma nef/vpr (viral proteins which have also been implicated in the development of PH) on outcomes and relationships between immune measures and worsening right ventricular volume indices and contractile function; this will serve as a foundation to pursue further investigations into the mechanisms underlying progression in HIV-PAH.
All flow cytometry will be done with whole blood. Cells (5 ×105) will be labeled with appropriate antibodies at 4°C for 30 min. The following monoclonal antibodies (from BD Biosciences; San Jose, California, USA) will be used in this procedure: anti-CD8, anti-CD16, anti-CD14, anti-CD3, anti-CD56, anti-Tim-3 (Biolegend, San Diego, California, USA), anti-CD38, anti-CD69 and the appropriate isotype controls. After staining, cells will be washed and fixed with 1% paraformaldehyde. For intra-cellular IFN-gamma and IL-1 staining, cells will be fixed after staining for surface markers, then permeabilized using the BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit and stained with the appropriate antibodies. Data from at least 2 ×104 cells in the lymphocyte gate will be collected on a FACSCalibur flow cytometer (BD Biosciences). Flow cytometry data will be analyzed using FlowJo software (Tree Star, California, USA). Analysis will initially enumerate the quantity of CD3 and CD8 gates. These will then be further analyzed according to CD56 and Tim-3 expression. CD3+CD8+ + cells will also be analyzed using the markers CD38, CD69. To analyze the expression of Tim-3 in monocytes, CD14, CD16 cells will be analyzed according to CD14hiCD16lo, CD14hiCD16hi and CD14loCD16hi populations. These cells will be gated according to Tim-3 positivity. CD14loCD16hiTim3+ cells will be permeabilized and examined for expression of inflammatory cytokines such as IFN-gamma and IL-1.
Discussion
This study will be the first systematic, prospective, and longitudinal evaluation of exercise limitation in HIV patients measuring exercise capacity and comprehensive MR evaluation including right ventricular function, pulmonary artery pressures, and cardiopulmonary variables with longitudinal assessments and measurements of immune factors that predict progression of PAH in the HIV population. It is designed as a ‘proof-of-concept’ study to test the utility of an exercise CMR approach to diagnose exercise-induced alterations in PAP and right ventricular function. A novel aspect of the protocol is the incorporation of exercise CMR with CPT to understand the nature of exercise limitation in HIV (which may be multifactorial) and provide a rational basis for the recognition of unique pathophysiological entities that may contribute to dyspnea and exercise limitation. In this regard, the incorporation of CMR in the diagnosis of HIV-PAH is consistent with its increased utility in the diagnosis and surveillance of PAH, given its ability to reliably and reproducibly quantitate right ventricular function, hypertrophy, and fibrosis. Further evaluation of functional response of the right ventricle to exercise using real-time MR approaches is unique and will allow unprecedented ability to quantify exercise-induced alterations in right ventricular contractile and hemodynamic performance, a hitherto unknown aspect of right ventricular function in PAH in general and specifically for HIV-PAH. CMR is an imaging modality associated with low risks, low user-dependent variability, and high accuracy and reproducibility to assess longitudinal changes in ventricular function and may be valuable in this population. Moreover, combining ExMR with CPT allows the development of novel diagnostic algorithms that could differentiate between various causes of dyspnea in this patient population (Fig. 3).
An important secondary goal of this study is to understand the pathophysiologic significance of markers of immune exhaustion and activation with measures of PAH progression. HIV-specific cytotoxic lymphocytes are present in elevated numbers in the lung with increased levels of CD8 T cells, regardless of coinfection with other respiratory pathogens, in both the peripheral blood and bronchoalveolar lavage samples.49 While it is widely recognized that HIV dysregulates multiple lymphocyte subsets with decreases in antiviral effector immune responses, there is evidence for additional reduction in cytotoxic potential of CD8+ T cells.50–52,53 The loss of CD8+ cytotoxic effector function may explain, in part, why HIV persists for many years, despite suppression of viremia by ART therapy. The diminished cytotoxic function of CD8 T cells, combined with paradoxically increased activation51,54 and increased expression of programmed death-1 (PD-1: molecule that inhibits co-stimulation), results in a state of immune unresponsiveness (‘immune exhaustion’) in chronically infected individuals with viral suppression.55,56 Moreover, HIV infection leads to accumulation of ‘effector T cells’ with an enhanced expression of Tim-3, another marker of immune exhaustion that is suggested to play a dual role in promoting pro-inflammatory responses via Toll-like receptor (TLR) signaling.57 Therefore, a reduced, more exhausted pool of cytotoxic T cells may lead to impaired immune reconstitution, despite plasma virological suppression. Assessing the longitudinal relationship between the immune markers, viral proteins, and PAH progression may provide the foundation for future comprehensive mechanistic approaches in order to understand the complex and heterogeneous relationship between HIV and PAH involving various hemodynamic, immune, and endothelial mechanisms.
In summary, this will be the first single-center systematic prospective observational study to evaluate PAH in HIV patients that may provide new insights in to the mechanisms of dyspnea in PAH, magnitude of exercise limitations, and finally alteration of right ventricular function (including contractile reserve with exercise) and scar in patients with established PAH. By doing so, we hope to better characterize the HIV-PAH patient population and hopefully identify parameters which may predict development of resting PAH in symptomatic adults.
Supplementary Material
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Barbaro G. Heart and HAART: two sides of the coin for HIV-associated cardiology issues. World J Cardiol. 2010;2:53–57. doi: 10.4330/wjc.v2.i3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J. 2013;34:3538–3546. doi: 10.1093/eurheartj/eht388. [DOI] [PubMed] [Google Scholar]
- 3.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boccara F. Cardiovascular complications and atherosclerotic manifestations in the HIV-infected population: type, incidence and associated risk factors. AIDS Lond Engl. 2008;22(Suppl 3):S19–S26. doi: 10.1097/01.aids.0000327512.76126.6e. [DOI] [PubMed] [Google Scholar]
- 5.Cannillo M, D’Ascenzo F, Grosso Marra W, et al. Heart failure in patients with human immunodeficiency virus: a review of the literature. J Cardiovasc Med (Hagerstown) 2015;16:383–389. doi: 10.2459/JCM.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 6.Morris A, Gingo MR, George MP, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS Lond Engl. 2012;26:731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Presberg KW, Doyle RL, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 8.Pellicelli AM, Barbaro G, Palmieri F, et al. Primary pulmonary hypertension in HIV patients: a systematic review. Angiology. 2001;52:31–41. doi: 10.1177/000331970105200105. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 10.Nunes H, Humbert M, Sitbon O, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167:1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- 11.Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006;22:1113–1121. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 12.Scott WB, Oursler KK, Katzel LI, et al. Central activation, muscle performance, and physical function in men infected with human immunodeficiency virus. Muscle Nerve. 2007;36:374–383. doi: 10.1002/mus.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolle JJ, Waxman AB, Van Horn TL, et al. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118:2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doukky R, Lee WY, Ravilla M, et al. A novel expression of exercise induced pulmonary hypertension in human immunodeficiency virus patients: a pilot study. Open Cardiovasc Med J. 2012;6:44–49. doi: 10.2174/1874192401206010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkotob ML, Soltani P, Sheatt MA, et al. Reduced exercise capacity and stress-induced pulmonary hypertension in patients with scleroderma. Chest. 2006;130:176–181. doi: 10.1378/chest.130.1.176. [DOI] [PubMed] [Google Scholar]
- 16.Saggar R, Sitbon O. Hemodynamics in pulmonary arterial hypertension: current and future perspectives. Am J Cardiol. 2012;110(6 Suppl):9S–15S. doi: 10.1016/j.amjcard.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Gomberg-Maitland M. Prognostication in pulmonary arterial hypertension. Heart Fail Clin. 2012;8:373–383. doi: 10.1016/j.hfc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Stevens GR, Fida N, Sanz J. Computed tomography and cardiac magnetic resonance imaging in pulmonary hypertension. Prog Cardiovasc Dis. 2012;55:161–171. doi: 10.1016/j.pcad.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Grothues F, Moon JC, Bellenger NG, et al. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, et al. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. Chest. 2001;119:1761–1765. doi: 10.1378/chest.119.6.1761. [DOI] [PubMed] [Google Scholar]
- 22.Bradlow WM, Assomull R, Kilner PJ, et al. Understanding late gadolinium enhancement in pulmonary hypertension. Circ Cardiovasc Imaging. 2010;3:501–503. doi: 10.1161/CIRCIMAGING.109.919779. [DOI] [PubMed] [Google Scholar]
- 23.Savai R, Pullamsetti SS, Kolbe J, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:897–908. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 24.Huertas A, Tu L, Gambaryan N, et al. Leptin and regulatory T-lymphocytes in idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;40:895–904. doi: 10.1183/09031936.00159911. [DOI] [PubMed] [Google Scholar]
- 25.Tamosiuniene R, Tian W, Dhillon G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res. 2011;109:867–879. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daley E, Emson C, Guignabert C, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez T, Burgel PR, Paillasseur J-L, et al. Modified Medical Research Council scale vs Baseline Dyspnea Index to evaluate dyspnea in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1663–1672. doi: 10.2147/COPD.S82408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lafountain RA, da Silveira JS, Varghese J, et al. Cardiopulmonary exercise testing in the MRI environment. Physiol Meas. 2016;37:N11–N25. doi: 10.1088/0967-3334/37/4/N11. [DOI] [PubMed] [Google Scholar]
- 29.Seiberlich N, Lee G, Ehses P, et al. Improved temporal resolution in cardiac imaging using through-time spiral GRAPPA. Magn Reson Med. 2011;66:1682–1688. doi: 10.1002/mrm.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aandal G, Nadig V, Yeh V, et al. Evaluation of left ventricular ejection fraction using through-time radial GRAPPA. J Cardiovasc Magn Reson. 2014;16:79. doi: 10.1186/s12968-014-0079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H-Y, Bender JA, Ding Y, et al. Shared velocity encoding: a method to improve the temporal resolution of phase-contrast velocity measurements. Magn Reson Med. 2012;68:703–710. doi: 10.1002/mrm.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handler CE, Sowton E. A comparison of the Naughton and modified Bruce treadmill exercise protocols in their ability to detect ischaemic abnormalities six weeks after myocardial infarction. Eur Heart J. 1984;5:752–755. doi: 10.1093/oxfordjournals.eurheartj.a061737. [DOI] [PubMed] [Google Scholar]
- 33.Chan CT, Greene T, Chertow GM, et al. Effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling. Clin J Am Soc Nephrol. 2013;8:2106–2116. doi: 10.2215/CJN.03280313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neilan TG, Coelho-Filho OR, Danik SB, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944–954. doi: 10.1016/j.jcmg.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383–2389. doi: 10.1016/j.jacc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Babu-Narayan SV, Goktekin O, Moon JC, et al. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111:2091–2098. doi: 10.1161/01.CIR.0000162463.61626.3B. [DOI] [PubMed] [Google Scholar]
- 37.Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127:1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 38.Groepenhoff H, Vonk-Noordegraaf A, Boonstra A, et al. Exercise testing to estimate survival in pulmonary hypertension. Med Sci Sports Exerc. 2008;40:1725–1732. doi: 10.1249/MSS.0b013e31817c92c0. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4:607–616. doi: 10.1016/j.jchf.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 40.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 41.Rocco MV, Lockridge RS, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusunose K, Popović ZB, Motoki H, Marwick TH. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ Cardiovasc Imaging. 2013;6:167–176. doi: 10.1161/CIRCIMAGING.112.000162. [DOI] [PubMed] [Google Scholar]
- 43.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 44.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 45.Price LC, Wort SJ, Perros F, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–221. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 46.El Chami H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2012;55:218–228. doi: 10.1016/j.pcad.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki C, Takahashi M, Morimoto H, et al. Mycophenolate mofetil attenuates pulmonary arterial hypertension in rats. Biochem Biophys Res Commun. 2006;349:781–788. doi: 10.1016/j.bbrc.2006.08.109. [DOI] [PubMed] [Google Scholar]
- 48.Cuttica MJ, Langenickel T, Noguchi A, et al. Perivascular T-cell infiltration leads to sustained pulmonary artery remodeling after endothelial cell damage. Am J Respir Cell Mol Biol. 2011;45:62–71. doi: 10.1165/rcmb.2009-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barry SM, Johnson MA, Janossy G. Increased proportions of activated and proliferating memory CD8+ T lymphocytes in both blood and lung are associated with blood HIV viral load. J Acquir Immune Defic Syndr 1999. 2003;34:351–357. doi: 10.1097/00126334-200312010-00001. [DOI] [PubMed] [Google Scholar]
- 50.Trabattoni D, Piconi S, Biasin M, et al. Granule-dependent mechanisms of lysis are defective in CD8 T cells of HIV-infected, antiretroviral therapy-treated individuals. AIDS Lond Engl. 2004;18:859–869. doi: 10.1097/00002030-200404090-00003. [DOI] [PubMed] [Google Scholar]
- 51.Trimble LA, Shankar P, Patterson M, et al. Human immunodeficiency virus-specific circulating CD8 T lymphocytes have down-modulated CD3zeta and CD28, key signaling molecules for T-cell activation. J Virol. 2000;74:7320–7330. doi: 10.1128/jvi.74.16.7320-7330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hay CM, Ruhl DJ, Basgoz NO, et al. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J Virol. 1999;73:5509–5519. doi: 10.1128/jvi.73.7.5509-5519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trabattoni D, Fossati S, Biasin M, et al. Functional analysis of HIV-specific cytotoxic T lymphocytes in antiviral-treated- and-naive patients: a preliminary report. J Biol Regul Homeost Agents. 2002;16:25–29. [PubMed] [Google Scholar]
- 54.López M, Rallón N, Soriano V, et al. HIV rebound after discontinuation of antiretroviral therapy increases and expands HIV-specific CD8+responses but has no impact on its functionality. AIDS Res Hum Retroviruses. 2008;24:1197–1201. doi: 10.1089/aid.2008.0088. [DOI] [PubMed] [Google Scholar]
- 55.Hong JJ, Amancha PK, Rogers K, et al. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PloS One. 2013;8:e60186. doi: 10.1371/journal.pone.0060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sachdeva M, Fischl MA, Pahwa R, et al. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr 1999. 2010;54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol. 2013;4:449. doi: 10.3389/fimmu.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.