Abstract
This Viewpoint Article describes major advances pertaining to perfunctionalized boron clusters in synthesis and their respective applications. The first portion of this work highlights key synthetic methods allowing one to access a wide range of polyhedral boranes (B4 and B6 – B12 cluster cores) that contain exhaustively functionalized vertices. The second portion of this Viewpoint showcases the historical developments in using these molecules for applications ranging from materials science to medicine. Lastly, we suggest potential new directions for these clusters as they apply to both synthetic methods and applications.
Graphical abstract
The present Viewpoint Article summarizes the syntheses and applications of perfunctionalized boron clusters with particular highlights on foundational works as well as the most up-to-date examples. We also provide a brief outlook on the field as it stands in the context of potential further development and application of boron cluster chemistry to solve pressing problems in chemistry, materials science and medicine.
Introduction
Carbon-based chemistry has been at the forefront of chemical research and has occupied the thoughts and efforts of its leading exponents for over two centuries. Despite its proximity to carbon in the periodic table, boron-based chemistry (though rich) has lagged far behind its first-row neighbor and in most cases has been used in service of carbon-based chemistry,1 rather than as a building block in its own right.
For boron chemistry, the history of borane (or boron hydride) chemistry began with the pioneering work of Alfred Stock, who with his co-workers isolated a series of simple boranes, including the classic diborane(6) B2H6.2 However, it was not until the theoretical prediction of the icosahedral borane [B12H12] by Lipscomb and co-workers in 1954 that the unusual nature of the bonding in boranes become apparent. Here, the concept of boron clusters was introduced,3 with subsequent studies by Longuet-Higgins and Roberts suggesting that the stable form of this icosahedron would have an overall 2- charge.4 These theoretical studies were then validated by Hawthorne and Pitochelli in a series of reports highlighting the first synthesis of dodecaborate [B12H12]2− as well as decaborate [B10H10]2− species.5,6
Contemporary books and reviews have broadly showcased recent advances in boron cluster chemistry and their applications,7–19 but in this Viewpoint we hope to reveal to the wider inorganic chemistry community the immense potential of boron clusters by focusing on a particular class of molecules, namely perfunctionalized clusters whose cores are composed entirely of boron atoms. We will discuss their synthesis and unique attributes and then present examples of their use in some unusual and unexpected arenas, ranging from photo-activation of olefins to hybrid nanomolecules. Finally, we will discuss potential future directions for these unique molecules and hope to provide inspiration for the wider community to engage in boron cluster chemistry.
Definition of a Cluster in this Viewpoint
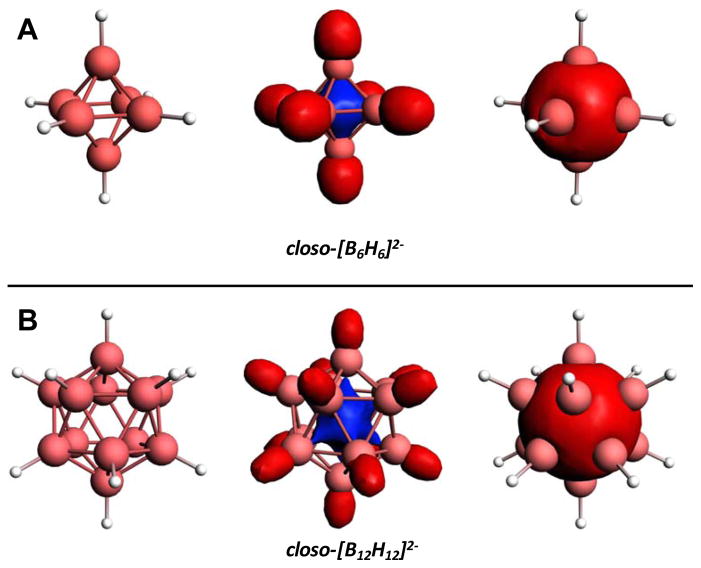
In this Viewpoint, we will be discussing the synthesis and applications of perfunctionalized all-boron clusters. Here the clusters are in their closo polyhedral forms as defined by Wade,20 where n number of boron atoms contain (n+1) skeletal bonding electron pairs, typically resulting in anions of the type closo-[BxHx]2− (x = 6 – 12 and above). These molecules exhibit unique bonding situations characterized by multicenter, two electron bonding and three-dimensional aromaticity and electron delocalization. These characteristics are believed to contribute to the high stability of these species and have been reviewed in the literature.21 This bonding situation is qualitatively visualized in 1) one set of atomic orbitals whose radial extension toward the center of the cluster results in the in-phase overlap of n boron-based orbitals of a cluster of the general formula BxHxy−, forming a molecular orbital in the center of the cluster shared by all boron atoms and 2) one set of atomic orbitals whose in-phase contributions result in a molecular orbital delocalized over the surface of the pseudo-spherical cage (Fig. 1A–B). For thoroughness, some perfunctionalized boron cluster classes will be addressed that deviate from Wade’s rules, such as the 4-membered polyhedral boron clusters and charge-neutral boron subhalides that have been reported. Furthermore, in addition to the expected closo-[BxHx]2− boron cluster forms (x = 6 – 12), we will show that perfunctionalization of such molecules can also lead to hypocloso-[BxHx]1− and hypercloso-[BxHx]0 derivatives. Insofar as the parentage of these monoanionic and neutral species lie in boron clusters that abide by Wade’s rules, they will be included in this Viewpoint.
Figure 1.
Calculated structures (based on X-ray crystal structures) and representative Kohn-Sham molecular orbitals of A1g symmetry demonstrating the delocalization of electron density in A. closo-[B6H6]2− and B. closo-[B12H12]2− as described by King.21 This bonding arrangement gives rise to high kinetic stability relative to tri-coordinate and non-polyhedral boranes.
Synthesis of Perfunctionalized Boron Clusters
B4 and B6 Clusters
The smallest boron-based clusters known are the deltahedral tetraboranes.22,23 In contrast to higher-membered congeners (e.g., B6 or B12), these clusters do not display the expected 2n+2 bonding electron count that is commonly encountered in 3D-aromatic boron clusters.21,24 The first known boron tetrahedrane, tetraboron tetrachloride,25 was reported in 1952 as a thermal decomposition product of B2Cl4 (Fig. 2), obtained only in small yields; its solid-state X-ray structure was subsequently solved in 1953.26 The early procedures for synthesizing this molecule involved either electrical or microwave discharges in the presence of BCl3 and mercury,23 with more recent procedures that produce workable yields involving the reduction of R–BF2 fragments by Na/K alloy in alkane solvents.27,28 Alternatively, B4RnCl4-n (n = 1, 2, 4) analogues have been synthesized through treatment of the halogenated B4Cl4 cluster with alkyllithium reagents.29,30 The reactivity of B4Cl4 has been described by Morrison and is summarized in Fig. 2.30
Figure 2.
Summary of reactivity of tetrachloro-closo-tetraborate.
From haloaminoborane precursors, Siebert and co-workers synthesized tetraborane compounds of the type B4(NR2)4 (R = Me, Et, iPr, or NR2 = 2,2,6,6-tetramethylpiperidinyl (TMP)).31 However only in the cases of R = Et or TMP were closo-tetraborane products obtained; the other derivatives were isolated as cage-opened species. Mention has also been made of a tetramesityltetraborane, though to our knowledge this molecule has not been published.32 Finally, Paetzold and co-workers have reported the synthesis of neopentyl and mixed alkyl tetraborate derivatives, however these neutral compounds were obtained in yields of 5% or less.33
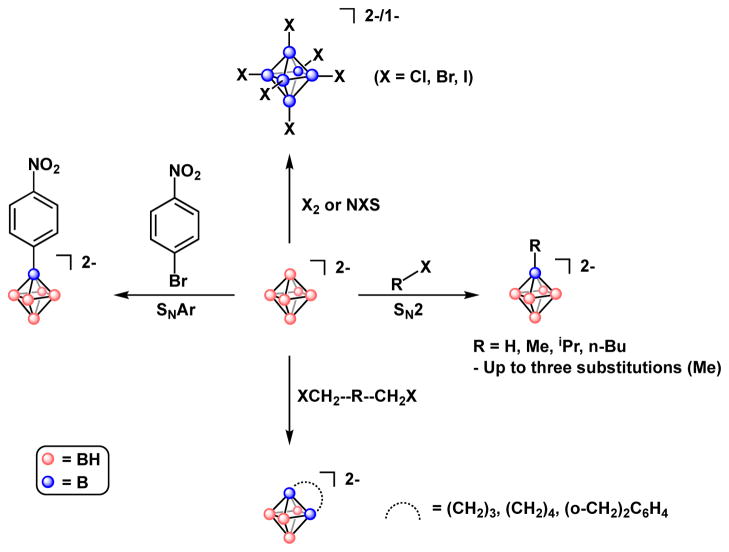
In comparison to larger cluster congeners, the functionalization and applications of 6-membered, closo-[B6H6]2− clusters is largely underdeveloped. Most of chemistry conducted with these species was performed by Preetz and co-workers, who showed that in contrast to the typical Lewis acidity and high electrophilicity of tricoordinate boron, the hexaborate dianion is in fact nucleophilic at boron. It is generally accepted that this nucleophilicity is a result of the maxima of electron-density residing at each face of the hexaborate octahedron. A Microreview by Preetz and Peters describes the majority of the synthesis and substitution chemistry of closo-[B6H6]2− clusters.34 It is noteworthy, however, that the issue of hexaborate peralkylation was addressed briefly in this account, in which it was suggested that only up to three vertex substitutions by alkyl substituents were possible as a consequence of the steric crowding of alkyl substituents and difficulty in facial proton removal prior to further substitution (vide supra). We have recently discovered, however, that under appropriate conditions, perfunctionalization is in fact possible (vide infra).
The first mention of a perfunctionalized closo-[B6H6]2− species was made by Klandberg and Muetterties in 1966.35 However, not until almost 20 years later was a directed preparation of perhalogenated hexaborate dianions disclosed by Preetz and Fritze via treatment of Na2[B6H6] with halogen in basic, aqueous media to produce closo-[B6X6]2− (X = Cl, Br or I).36 The resulting compounds were thoroughly studied by IR and Raman spectroscopy,37 as well as 11B NMR spectroscopy, which revealed an upfield shift of boron resonances from closo-[B6Cl6]2− to closo-[B6Br6]2− to closo-[B6I6]2−.38 Subsequently, it was discovered that halogenation could be performed using succinimide-based reagents, however these conditions were employed largely for synthesizing only partially halogenated derivatives (Fig. 3).39
Figure 3.
Reactivity of closo-[B6H6]2−, as described in the literature.
In cases of either diatomic halogen or NXS-based halogenation, species of the type closo-[B6XnH6-n]2− (n = 1 – 5) were generally observed, opening the possibility for heteroleptic perhalogenated closo-hexaborates. Interestingly, the synthesis of the disclosed bis-heteroleptic perhalogenated hexaborates, which form under analogous conditions to homoleptic perhalogenated derivatives, require a specific, sequential treatment with halogen, in which the more electronegative halogen must first be charged to the hexaborate cluster, followed by the less electronegative halogen.39,40 In this way, halogenated hexaborate clusters of the types closo-[B6ClnBr6-n]2−, closo-[B6ClnI6-n]2−, and closo-[B6BrnI6-n]2− may be synthesized. To the best of our knowledge, the chemistry of these bis-heteroleptic clusters has not been further explored.
Prior to our involvement, the only non-halogen containing perfunctionalized six-membered closo-boron clusters were the neutral hypercloso-B6(NEt2)6,41,42 with Berndt and co-workers also disclosing the synthesis and structure of the dimethyl analogue, hypercloso-B6(NMe2)6.43 Given our interest in the development of chemistry and applications of perfunctionalized boron clusters,44–46 we reexamined the reactivity of the closo-[B6H7]1− anion. Under microwave heating conditions, the hexaborate anion can be substituted with benzyl bromide electrophiles (Fig. 4A), affording a perfunctionalized cluster of the type closo-[B6(CH2Ar)6Hfac]1− (Ar = C6H5, 4-Br-C6H4), containing six newly formed B–C bonds. Importantly, these cluster molecules can be isolated as moisture and air-stable solids. Crystallographic characterization of one derivative confirmed the formation of six B–C linkages. Interestingly, in contrast to the perhalogenated hexaborates reported by Preetz (vide supra), these alkylated clusters are marked by their relative oxidative instability, distinguishing them from the known redox-reversible substituted dodecaborate clusters (vide infra). Overall, it is clear that the original observations by Preetz suggesting that closo-[B6H6]2− cluster features nucleophilic reactivity at boron sites can be extended towards building complex perfunctionalized cluster scaffolds.
Figure 4.
A. Synthesis of perfunctionalized B6 clusters featuring direct B–C bonds. B. X-ray crystal structure of closo-[B6(CH2-4-Br-C6H4)6Hfac]1−.
B7–B11 Clusters
Moving beyond the small octahedral B6 cluster, several additional three-dimensional BnHn2− clusters (n = 7 – 11) have been studied,6,47,48 though with the exception of B10 decaborate clusters, there has been relatively little exploration of their functionalization. The perfunctionalization chemistry of closo-[B10H10]2− was developed somewhat in parallel to that of the closo-[B12H12]2− dodecaborate cluster in the early 1960s,49–53 with several routes to diverse functionalized derivatives resulting in partial substitutions of the cage-bound hydrogens.54 Despite this early progress, in the subsequent decades only halogenation resulted in successful substitution of all B–H vertices to achieve perfunctionalization of the BnHn2− (n = 7 – 11) clusters to form species of the type BnXn2− (X = Cl, Br and I).30,53,55–59
In 1964, the first fully halogenated cluster in this series was the decaborate dianion, with [B10Cl10]2−, [B10Br10]2−, and [B10I10]2− all synthesized by treating [B10H10]2− with chlorine, bromine, and iodine, respectively.53 Several other perhalogenated clusters were synthesized via the thermal decomposition of diboron tetrahalide B2X4 or boron trihalide BX3, thus forming the perhalogenated clusters directly from these non-polyhedral precursors55 rather than subjecting the boron hydride BnHn clusters to halogens directly. There is some mass spectral evidence suggesting that B9(t-Bu)9 was formed from the reaction of [B8Cl8]2− with tert-butyl lithium,60 but the supposed product was neither isolated nor fully characterized.
A more exhaustive history of the functionalization chemistry possible with these clusters that fall short of full persubstitution can be found in excellent reviews by Morrison30 (for B7–9,11) and Sivaev54 (for B10). The limited perfunctionalization observed over the past few decades can be largely attributed to lower stability of these clusters compared to closo-[B12H12]2−, which precludes harsher functionalization strategies. The stability of the boron subhalide clusters was predicted from their valence state index to be 9>11>8>10≈7,56 though experimental thermal stability of the neutral clusters BnXn was found to be 9>10>8≈7,55 showing poor agreement between the two methods but confirming the significantly lower stability of these clusters compared to [B12H12]2−. More recent investigations of these clusters have resulted in the X-ray crystallographic analysis of the structures as well as further functionalization, however the halogenated derivatives remain the only perfunctionalized clusters in the BnHn (n = 7–11) series to date.57,61–64
B12 Clusters
Due to its stability and commercial availability, the vast majority of reports on perfunctionalized boron clusters concern the deltahedral closo-dodecaborate dianion, closo-[B12H12]2−. For clarity, the following sections will be partitioned into those concerning homoperfunctionalized (Fig. 5) and heteroperfunctionalized (Fig. 6) B12-based clusters.
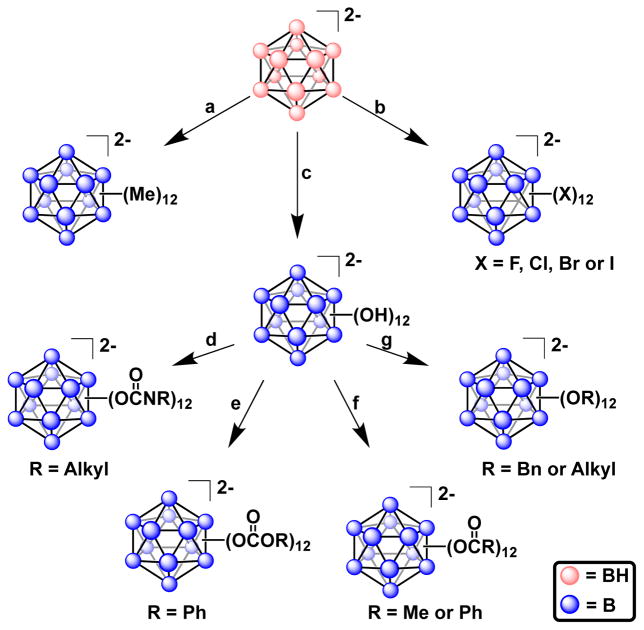
Figure 5.
Homoperfunctionalization of closo-[B12H12]2−. Cations omitted for clarity. a. Alkylation. Peralkylation of dodecaborate can be achieved by refluxing [NnBu4]2[B12H12] with neat AlMe3 and iodomethane over eleven days to form [NnBu4]2[B12Me12].80 This remains the only reported peralkylation of dodecaborate, and the only homopersubstituted dodecaborate that contains B–C linkages. b) Halogenation52,65–68 c) Hydroxylation69–71,81 d) Carbamation82 e) Carbonation82 f) Esterification72 g) Etherification71.
Figure 6.
Established routes for the heteroperfunctionalization of closo-[B12H12]2−. Cations have been omitted for clarity.
Homoperfunctionalized B12 Clusters
Halogenation
The first reported perfunctionalization of closo-[B12H12]2− involved direct perhalogenation with elemental chlorine, bromine and iodine, resulting in molecules of the type closo-[B12X12]2− (X = Cl, Br or I) that could be handled without decomposition under ambient conditions.53 To avoid the use of chlorine gas, Ozerov and co-workers recently showed that perchlorination of closo-[B12H12]2− may be achieved using SO2Cl2 as a chlorinating agent, leading to a more operationally simple synthesis.65 Perfluorination of closo-[B12H12]2− proved more difficult and was first achieved by Solntsev and co-workers in the early 1990’s through the action of supercritical HF at 550°C, producing Cs2[B12F12] in 38 % yield.66 Later, work by Strauss and co-workers described improved yields of closo-[B12F12]2− salts up to 74 % and could be carried out on multigram scale using non-specialized glassware.67,68
Hydroxylation
Hawthorne and co-workers reported the first successful perhydroxylation of dodecaborate by refluxing Cs2[B12H12] in 30 % H2O2 for several days to form Cs2[B12(OH)12].69 Through cation exchange protocols, the B12(OH)122− dianion can be isolated as a variety of metal, acid, or organic salts M2[B12(OH)12] (where M = Li+, Na+, K+, Rb+, Cs+, [H3O]+, [NH4]+, [NnBu4]+, [(PPh3)2N]+).70,71 Unlike their organic analogues, most perhydroxylated dodecaborate salts show low solubility in water, with only Cs2[B12(OH)12] showing appreciable solubility and [NnBu4]2[B12(OH)12] showing excellent solubility at slightly elevated temperatures. In addition, the tetra-n-butyl ammonium salt is soluble in organic solvents, making it a valuable precursor for further derivatization.
Esterification
[NnBu4]2[B12(OH)12] can be transformed into esters of the type [NnBu4]2[B12(OC(O)R)12] (R = Me or Ph) using acetic anhydride or benzoyl chloride, as reported by Hawthorne and co-workers. Later reports from the same laboratory further expanded the scope of substituents that could be installed on the B12(OH)122− core through ester linkages.72–74
Etherification
closo-[B12(OH)12]2− reacts with a variety of alkyl or benzyl halides in the presence of base to form per-etherated clusters. The first such compound reported involved the reaction of [(PPh3)2N]2[B12(OH)12] with benzyl chloride and iPr2NEt in refluxing acetonitrile for several days to form (after workup) Na[(PPh3)2N][B12(OCH2Ph)12] in 48 % yield.75 A variety of other peretherated clusters were also reported using similar conditions.71 Although these methods were effective, the length of time required for the reactions reduced their utility. To address this issue, our laboratory has reported a rapid microwave-assisted method to prepare peretherated clusters.44 This method drastically reduces the reaction time from an average of multiple days to minutes to hours. Etherification of the perhydroxylated clusters has a profound effect on the redox properties of B12-clusters and is discussed separately in the text (vide infra).
Heteroperfunctionalized B12 clusters
B–X/B–N functionalization
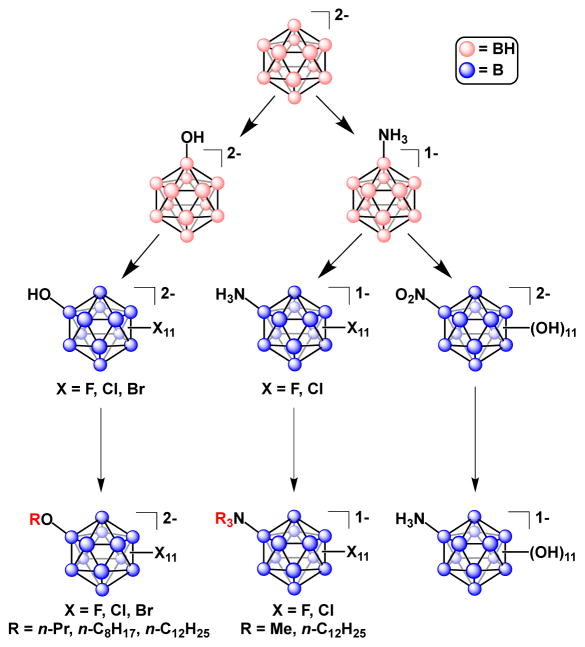
While Muetterties and co-workers reported a variety of B12 clusters containing B–X and B–N bonds as early as 1965, none of the molecules described were perfunctionalized.76 Strauss and co-workers were successful in perfluorinating closo-[H3NB12H11]1− to form closo-[H3NB12F11]1− in situ, which could then be isolated via precipitation as the ammonium salt [NnBu4]2[H2NB12F11].77 An important feature of closo-[H3NB12F11]1− is the reduction of the overall charge on the cluster from 2- to 1- as a result of charge compensation by the ammonium fragment. In principle, this allows the cluster to be treated as an analogue of the significantly more expensive polyhedral carba-closo-dodecaborate closo-[CB11F11]1−, an exceptional weakly coordinating anion. Further functionalization of closo-[H3NB12F11]1− at nitrogen affords dodecaborate anions of the type closo-[R3NB12F11]1− (R = Me and n-C12H25). Jenne and co-workers were successful in the synthesis of the perchlorinated derivative closo-[H3NB12Cl11]1− using SbCl5 as a chlorinating reagent,78 with further substitution then carried out at nitrogen to produce closo-[Me3NB12Cl11]1−. Duttwyler and co-workers later reported a simplified synthesis whereby SbCl5 was replaced with SO2Cl2.79
B–X/B–O functionalization
Muetterties and co-workers reported the first example of a mixed halogenated/hydroxylated B12 cluster, closo-[B12F11OH]2−, as a byproduct of the attempted perfluorination of closo-[B12H12]2− with elemental fluorine in water.53 Jenne and Kirsch reported the synthesis of the heteroperfunctionalized cluster closo-[B12X11OH]2− and subsequent alkoxylation to form a variety of compounds of the type closo-[B12X11OR]2− (where X = Cl or Br; R = n-C3H7, n-C8H17 or n-C12H25).83 As with the formation of closo-[H3NB12Cl11]1−, Duttwyler and co-workers described a simplified synthesis of closo-[B12Cl11OH]2− using SO2Cl2 instead of chlorine gas and showed additional reactivity at the hydroxyl group to produce closo-[B12Cl11O(SO2CF3)]2− and closo-[B12Cl11OTs]2− respectively.82 A summary of these functionalization methods is shown in Figure 5.
B–N/B–O functionalization
Hawthorne and co-workers synthesized the mixed B–N/B–O functionalized cluster closo-[B12(OH)11(NH3)]1− via amination of closo-[B12H12]2− to form closo-[B12H11(NH3)]1− followed by hydroxylation to form closo-[B12(OH)11(NO2)]2− and a final reduction step with Raney nickel to afford the desired closo-[B12(OH)11(NH3)]1− species.84 The authors report that nitro derivative closo-[B12(OH)11(NO2)]2− may be used directly for further functionalization of the hydroxyl group, with subsequent reduction to yield products of the type closo-[B12(OR)11(NH3)]1− (see Figure 6).
Redox Chemistry of Perfunctionalized Clusters
Upon perfunctionalization, boron clusters can access electronic states that do not conform to Wade’s rules. This gives rise to molecules that potentially have access to three distinct oxidation states: closo-[BxRx]2−, hypocloso-[BxRx]1− and hypercloso-[BxRx]0. For hexaborate clusters, this redox chemistry is readily accessible for the homoleptic perhalogenated cluster derivatives closo-[B6X6]2− (X = Cl, Br or I). Use of choice oxidants sees their transformation to their highly colored open shell radical monoanions, hypocloso-[B6X6]1− (X = Cl, Br or I).85,86 In comparison to the oxidation potential of the parent closo-[B6H6]2− (0.86V vs. Ag/AgCl/LiCl), closo-[B6Cl6]2− and closo-[B6Br6]2− are more easily oxidized, with oxidation potentials lying at 0.58V and 0.77V, respectively; in contrast, closo-[B6I6]2− demonstrates approximately the same oxidation potential as closo-[B6H6]2− at 0.88V. The perhalogenated derivatives B9X9 (X = Cl, Br, I) were found to have two highly reversible, well-separated one-electron steps between the dianionic and neutral states, with significantly lower oxidation potentials compared to the B12X12 species (−0.63 V to −0.74 V vs Fc/Fc+ for the 2- to 1- oxidation step for B9X9 clusters, compared to 1.68 V to 2.27 V vs Fc/Fc+ for the same redox event with B12X12).87,88
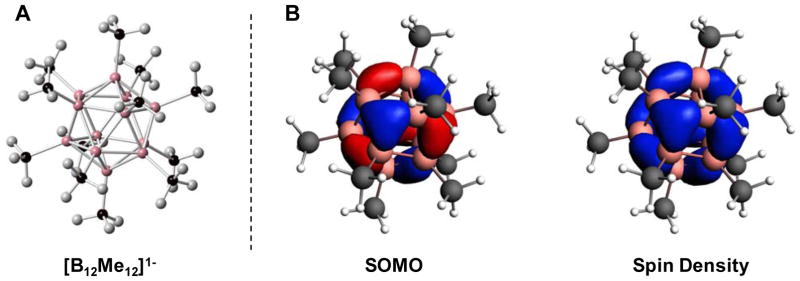
As mentioned, the vast majority of reports on perfunctionalized boron clusters concern the 12-vertex polyhedral closo-[B12H12]2−. This particular class of molecule has a rich redox chemistry that has only recently been exploited. Without modification, boron hydride clusters such as closo-[B12H12]2− have very high redox potentials, which can result in irreversible cluster degradation or the formation of B–B linked dimers when subjected to controlled oxidiation.89 However, perfunctionalization of the closo-[B12H12]2− scaffold to form halogenated or other derivatives engenders reversible redox behavior.11 Early examples of this phenomenon include reports that the perhalogenated dodecaborates could undergo single electron oxidation, forming a stable hypocloso-[B12X12]1− radical.90 Attempted isolation of one such species, [B12Cl12]1−, instead resulted in the isolation of neutral B12Cl12, evidencing the structural stability but potentially high reactivity of these radicals.91 However, other dodecaborate radicals, such as salts of perhydroxylated closo-[B12(OH)12]1− 92 as well as permethylated [B12Me12]1− 93 have been isolated as stable anions. Their stability is largely due to the 3-dimensional delocalization of electron density throughout the cluster core, which mitigates the potent reactivity of a localized radical (Fig. 7). Furthermore, steric protection of the cluster core through B-bound substituents also kinetically stabilizes the hypocloso species.
Figure 7.
A. X-ray crystal structure of [(PPh3)2N][B12Me12] ([(PPh3)2N]+ counterion omitted for clarity). B. The singly occupied molecular orbital (SOMO) and the spin density localization (PBE/D3BJ:TZP).
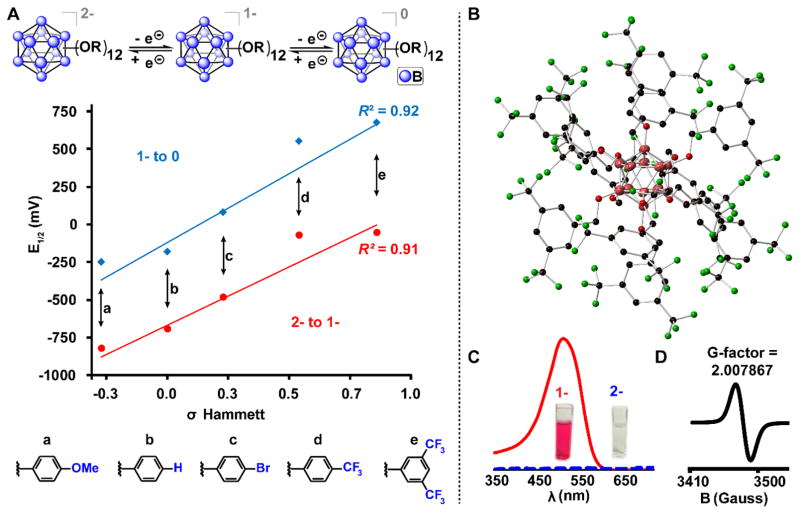
More recent explorations of this reversible redox behavior have been conducted with perfunctionalized derivatives of closo-[B12(OH)12]2−. It was demonstrated that the ether-linked benzyl closo-[B12(OCH2Ph)12]2− cluster can undergo two sequential, quasi-reversible one-electron oxidation reactions, enabling access to three distinct redox states: closo-[B12(OCH2Ph)12]2−, the stable radical hypocloso-[B12(OCH2Ph)12]1−, and the neutral hypercloso-B12(OCH2Ph)12. In all three oxidation states, the dodecaborates were stable, isolable products that were fully structurally characterized.75 The most useful feature of this redox behavior was the discovery that the individual redox potentials of the closo-[B12(OR)12]2− clusters could be rationally tuned — in a manner reminiscent of metal-based inorganic complexes — as a function of the O-bound alkyl or benzyl substituent.11,71,94 Recent work has demonstrated even higher oxidation potentials for some newer closo-[B12(OR)12]2− derivatives, continuing the correlation between the predicted effects of the substituents (Fig. 8) while expanding the tunable window for the same redox event to a full 1 V (−0.61 V vs Fc/Fc+ for the 1-/0 redox couple oxidation when R = n-hexyl, rising to +0.68 V vs Fc/Fc+ for the same redox event when R = (3,5-bis)trifluoromethylbenzyl).44,94
Figure 8.
A. Redox potentials of [B12(OR)12] clusters plotted vs Hammett constants (top/blue = 1- to 0, bottom/red = 2- to 1-). B. Single crystal X-ray structure of cluster [e]1−. C. UV-vis spectra and photos of the air-stable radical cluster [e]1− (red line) and the dianionic cluster [e]2− (blue dashed line). D. EPR spectrum of the radical cluster [e]1−. These highlight the tunable nature of the perfunctionalized clusters.10,44,95
Applications in Catalysis and Materials Chemistry
While boron clusters may have existed as chemical curiosities over the last century, they became a topic of intense interest for scientists during the 1950s, who, at the height of the space race, were keen to apply them towards the development of ever more powerful energetic materials for rocket fuels. Since interest quelled in the use of boron clusters in that arena, applications for boron clusters have been few and far between. Having outlined the state-of-the-art with regards to the synthesis of perfunctionalized boron clusters and extolled the virtues of their signature features, in this section of the Viewpoint we will highlight select examples of areas in which perfunctionalized clusters may be exploited.
Photoredox Behavior
Perfunctionalized carba-closo-dodecaborates of the type closo-M[CB11X11] have found exceptional utility in homogeneous catalysis due to their ability to act as inert, extremely weakly coordinating anions.96,97 While dodecaborates composed entirely of boron, which bear a charge of 2-, may be reminiscent of these weakly coordinating carboranes, the ability to functionalize these molecules and the properties that manifest as a result present a powerful opportunity to move perfunctionalized boron clusters away from a spectator role and towards being an active participant in reaction chemistry. In particular, the unique ability to tune the redox window of dodecaborates through perfunctionalization via judicious choice of functional group (vide supra) provides straightforward access to air- and moisture-stable molecules that have well-defined one-electron redox chemistry across a range of potentials. Such redox chemistry could then be exploited to mediate a plethora of transformations across a range of substrates, irrespective of substrate reactivity.
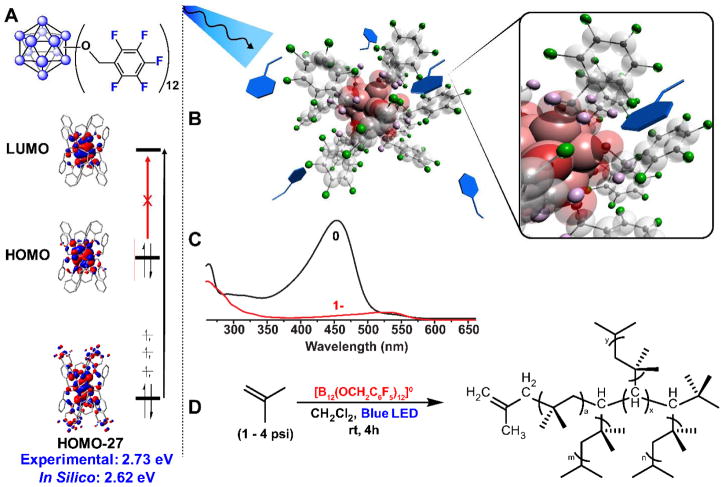
During the course of our studies on improving the synthesis of ether linked perfunctionalized clusters,44 we observed that solutions of hypercloso-B12(OCH2Ph)12 left to stand in ambient light in the presence of with 4-methoxystyrene led to the formation of viscous mixtures; in the dark, no such mixture was obtained. By utilizing blue LEDs as a light source under controlled conditions, we demonstrated that the perfunctionalized cluster hypercloso-B12(OCH2Ph)12 can behave as a powerful photooxidant in the visible light-assisted polymerization of olefins.46 The proposed mechanism involves the generation of a potent photooxidant by visible light promotion of an electron from a low lying occupied orbital localized largely on the aryl rings and oxygens to the cluster-based LUMO (Fig. 9A). Unlike metal-initiated polymerizations of olefins which generally require an open coordination site for monomer approach, these clusters are devoid of well-defined coordination sites. It was therefore hypothesized that favorable, non-covalent interactions of the monomer and the aryl ring(s) of the benzyl substituents on the cluster periphery facilitate electron transfer from monomer to the dodecaborate core (Fig. 9B). Time-dependent Density Functional Theory (TD-DFT) studies carried out on both hypercloso-B12(OCH2Ph)12 and hypercloso-B12(OCH2C6F5)12 suggest that this charge-transfer excitation pathway is favorable and leads to an excited state species with a redox potential sufficiently anodic for the one-electron oxidation of styrene. Importantly, the energy of this excitation calculated in silico corresponds to the energy provided by a photon of blue (~450nm) light, which is consistent with the ~450nm absorption band observed for the benzyloxy-substituted hypercloso-B12-based species (Fig. 9C). In order to render more electronically deactivated olefins reactive toward the B12-based photoinitiators, enhancing the photooxidizing power of the hypercloso-cluster was required. Since the redox potential of perfunctionalized clusters can be tuned by varying the substituents bound to the cluster, more electron-withdrawing C6F5 groups were installed to form hypercloso-B12(OCH2C6F5)12. The photooxidizing power was sufficiently increased to enable the activation of a large variety of olefins, including isobutylene, a notoriously difficult monomer that typically requires metal-based activators or harsh conditions (Fig. 9D).98 Most impressively, these reactions were observed to proceed with as little as 0.005 mol % initiator loadings.
Figure 9.
A. TD-DFT calculation describing the charge-transfer excitation pathway in hypercloso-B12(OCH2C6F5)12. B. Schematic representation of hypothesized docking of styrene monomers among the pentafluoroaryl groups of hypercloso-B12(OCH2C6F5)12 which helps facilitate electron transfer. C. UV-vis absorption spectra for neutral hypercloso-B12(OCH2C6F5)12 (black) and radical hypocloso-[B12(OCH2C6F5)12]1− (red). D. Production of branched poly(isobutylene) initiated by B12(OCH2C6F5)12 in the presence of blue LED light.
Applications as Weakly Coordinating Ions
Catalysis
Ozerov and co-workers have leveraged the weakly coordinating nature of closo-[B12X12]2− dianions in the catalytic hydrodefluorination (HDF) of sp3 C–F bonds in the presence of silylium cations generated in-situ from H–SiEt3.65 In dichlorobenzene solvent, >97% HDF of 4-fluorobenzotrifluoride occurred at room temperature in 1h; the more challenging substrate, perfluorotoluene, likewise underwent HDF in 1h at 80°C. In both cases, perfect selectivity was observed for sp3 C–F bonds and arene C–F bonds remained intact. This method is a clear proof-of-concept that perfunctionalized dodecaborates can be successfully employed as weakly coordinating anions under catalytic conditions. Furthermore, this example shows that these dianions can behave as competent substitutes for carba-closo-dodecaborate analogues (CB11H111− and functionalized derivatives) which, despite their well-established utility, are more difficult to synthesize, more expensive to purchase, and generally more difficult to perfunctionalize.
More recently, Kirsch and co-workers successfully employed a perfunctionalized boron cluster under gold catalysis conditions to effect a variety of ene-yne cyclization reactions. Single crystal X-ray structural data was also obtained which revealed a dimeric active catalyst and the weakly coordinating nature of the dodecaborate counterion.99 The kinetic stability of these perfuctionalized boron clusters is particularly relevant to (cationic) transition metal catalysis, since even the widely used BArF anion ([B(C6F5)4]−) has been shown to engage in aryl ring transfer in the presence of transition metal cations.100,101,102
Reagents
The weakly coordinating nature of these dianions prompted Knapp and co-workers to employ [CH3]2[B12Cl12] as a methylating agent. Me+–Cl interactions stabilize the methyl cation however the dodecaborate framework remains intact. This compound is capable of methylating SO2 as well as benzene to form [CH3OSO]2[B12Cl12] and [CH3C6H6]2[B12Cl12], respectively.103 The same group later disclosed the stabilization of [Al(CH3)2]+ using this dodecaborate anion.104 It should be noted that Wehmschulte and co-workers reported a similar complexes employing a perfunctionalized dodecaborate as a counterion, including [Ag(CH3CN)][B12Cl11(NMe3)], [CPh3][B12Cl11(NMe3)], and [Et2Al][B12Cl11(NMe3)].105
Superacids
Superacids have been used for many years to probe extremes of chemical behavior and engage interesting reactivity. Among the strongest are those based on carba-closo-dodecaborates, which like their neutral carborane and dianionic dodecaborate analogues exhibit chemical stability toward acidic solutions as well as charge delocalization which is thought to enhance acidic properties. And while most attention has been given to CB11-based superacids, comparatively fewer studies have examined the acidity of dianionic dodecaborate derivatives. Muetterties and co-workers noted the extreme acidity of B12Cl12 and B12Br12 in the mid-1960s in which the authors disclosed their superior acidity to sulfuric acid.53 More recently, experimental106,107 and theoretical107,108 efforts have been made to further quantify the (potential) acidity of such derivatives. In particular, Reed and co-workers showed106 that B12Cl12 and B12Br12 exhibit comparable acidity to carborane acids and are sufficiently acidic to protonate benzene, which even trifluoromethanesulfonic acid and fluorosulfonic acid cannot do. Furthermore, both protons from H2B12X12 are capable of arene protonation, not simply the first. This is a fundamental limitation of carborane acids and suggests potential for applications where superacidic conditions are necessary.
Ionic Liquids
Ionic liquids are gaining increased attention due to their unique chemical properties and potential applications.109 Polyhedral boron clusters stand as viable candidates for such an application due to their known chemical and electrochemical stability as well as their tunability (vide supra). While usually encountered as solids, recent work has shown that these species may be appropriately functionalized to give low-melting (< 100°C) ionic liquids. Jenne and Kirsch reported that perfunctionalized clusters of the type [C6mim]2[B12X11OR] ([C6mim]+ = 1-hexyl-3-imidazolium) displayed decomposition temperatures well beyond 300°C for five of six derivatives. One of these, [C6mim]2[B12Cl11(O-nPr)] gave a melting point under 100°C.83 More recently, the same group showed improved melting properties of dodecaborate-based ionic liquids using [B12X6H5NR2]2− and [B12X6H5NR3]− anions.110 These compounds show low (as low as 57°C) melting points and high (> 2V) electrochemical stability, showing promise not only as ionic liquids but also as electrolytes. Notably, [C6mim][B12Cl6H5N(nPr)3] shows a liquid range of over 300°C between melting (65°C) and decomposition (371°C).
Materials with cationic conductivity
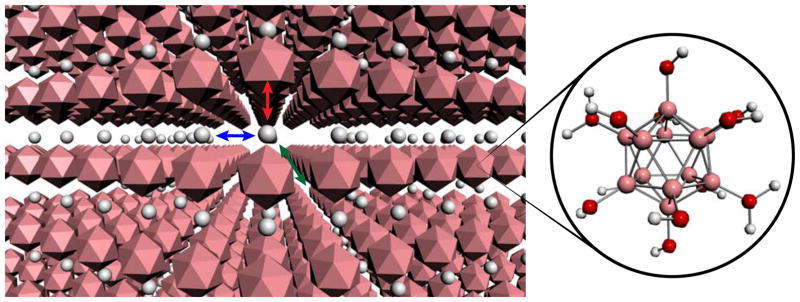
In early works on Li-ion batteries, several research groups evaluated anionic boron clusters including closo-[B12Cl12]2− and closo-[B10Cl10]2− as electrolyte components.111 For example, primary liquid cathode Li/SOCl2 cells, which utilized Li2[B10Cl10] and Li2[B12Cl12] salts were reported in 1979.112 Later, these electrolytes were tested in Li/TiS2 cells by Exxon.113,114 More recently, fluorinated all boron nanoclusters Li2[B10F10] and Li2[B12F12] salts were developed at Air Products (commercial name – “Stabilife fluorinated electrolyte salts”). The electrolyte solvent/salt formulation can be tuned to obtain promising cell cycling performance, especially at elevated temperature (60°C).115,116 The high electrochemical stability of these anions has been shown to provide overcharge protection for 4V Li-ion batteries, shown with MCMB/spinel cells. Perfunctionalized cluster anions containing protons as counterions have been investigated for their potential use in proton conductor membranes. Early efforts testing H2[B12Cl12] however proved unsatisfactory for this application given significant decomposition of this cluster observed at elevated temperatures in aqueous media resulted from B-Cl bond hydrolysis.90 In 2004, Stasko and co-workers reported the solid-state proton conductivity study of closo-H2[B12(OH)12], the conjugate acid of the B12(OH)12 dianion (Fig. 10).117 The title compound was generated by treating closo-Cs2[B12(OH)12] with concentrated HCl at 150°C for 3d at ~8.5 atm. As with other dodecaborate based anions, closo-H2[B12(OH)12] displayed excellent thermal stability (>400°C in air or N2 atmosphere). A proton conductivity of 1.5 × 10−5 Ω−1 cm−1 was measured for H2[B12(OH)12], which is an order of magnitude higher than that of known solid-state proton conductor CsHSO4. This conductivity was shown to increase with increasing temperature. Distinct changes in proton dynamics at varying temperatures were also observed by solid-state 1H NMR, corroborating the proton mobility hypothesis.
Figure 10.
Schematic representation of the crystal lattice of closo-H2[B12(OH)12] synthesized by Stasko and co-workers which was shown to conduct protons in the solid state.
Very recently, Strauss and co-workers disclosed detailed studies of the coordination of Na2[B12X12] (X = F, Cl) and their hydrated forms to Na+ ions in the solid state.118 Interestingly, they concluded that B12Cl122− coordinates Na+ less strongly than B12F122− and ultimately suggest that both perfunctionalized dodecaborate derivatives, in their dehydrated forms, may be viable candidates for ion conductivity in energy storage applications.
Biological Applications
A number of boron cluster derivatives are attractive candidates for a broad range of biomedical applications due to their exceptional stability and biocompatibility, availability of 10B nuclei (20% natural abundance), and tunability.52,119–121 Over the past few decades, many biological applications of boron clusters have been explored, most notably in the areas of drug delivery and imaging for the diagnosis and treatment of cancer,7,119 and only most recently in the area of probing protein-biomolecule interactions.45
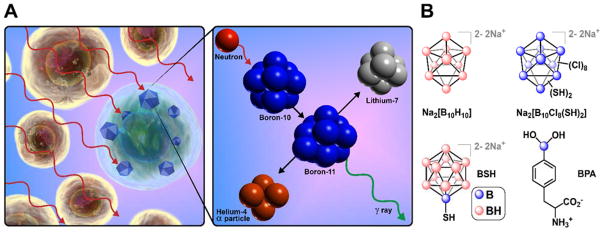
Delivery of 10B for BNCT
The most widely investigated research area within boron cluster-based drug delivery is undoubtedly boron neutron capture therapy (BNCT), a binary therapeutic strategy for the treatment of cancer (Fig. 11A). The concept of BNCT was first proposed in 1936 by Locher,122 and has since captured the attention of many researchers due to its elegant design. As described by Hawthorne and others, the beauty of BNCT lies in the fact that both of its essential components, nonradioactive 10B nuclei and low-energy thermal neutrons, are nontoxic by themselves, however together they initiate an energetic nuclear decomposition reaction that typically cannot extend past the diameter of a cell.10,119,123 Based on this principle, BNCT was proposed as a safer alternative to many of the single-component radiation and chemotherapy approaches for the treatment of cancer. Many boron-containing compounds have been investigated for BNCT with generally unsuccessful results, with the exception of L-p-dihydroxyborylphenylalanine (BPA) (Fig. 11B), which is still being used in the clinical settings today.119,124,125 Between 1959 and 1960, the discovery of polyhedral borane anions closo-[B10H10]2− and closo-[B12H12]2− 5,6,126 sparked renewed interest in BNCT given the exceptional stability of these species and their high boron content. The sodium salt of [B10H10]2− (Fig. 11B) was tested in animals, and while it showed promising results, it later failed in a clinical trial.127,128 Two thiol-containing derivatives, Na2[B10Cl8(SH)2] and Na2[B12H11SH] (BSH) (Fig. 11B), were identified as potential drug candidates from studies using mouse tumor models.128,129 Although Na2[B10Cl8(SH)2] proved too toxic in mice, BSH exhibited more favorable properties and has since been extensively used in the clinical trials.96,121,130
Figure 11.
A. An illustration of BNCT, a binary strategy for the treatment of cancer. B. Some BNCT candidates used in the preclinical and clinical settings.
However, despite the relative success of BPA and BSH in the clinical trials, an important challenge remains to selectively deliver sufficiently large amounts of 10B nuclei to the tumor tissue (approximately 20–35 μg/g tumor or 109 atoms/cell), which is critical to the success of BNCT.10,131 Therefore, two general approaches have been taken to tackle this issue. The first strategy involves the incorporation of boron into biomolecules with tumor-targeting abilities such as peptides and proteins, nucleic acids, carbohydrates, and porphyrins,132,133 while the second approach uses liposomes and nanoparticles to deliver 10B atoms to tumor tissues.133–137 In particular, many boron-encapsulated and boron-lipid liposomes have shown promising results in their ability to selectively deliver therapeutic quantities of 10B atoms into tumor cells for effective BNCT.138–146
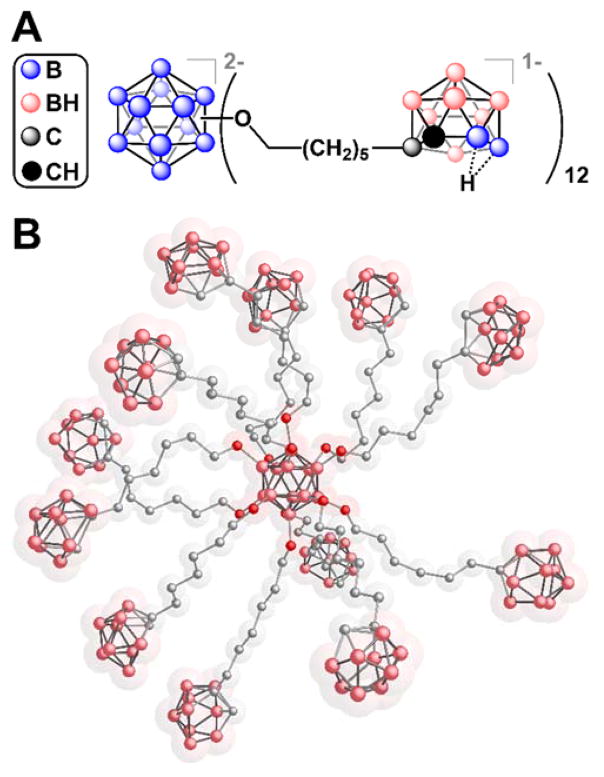
However, while the liposome-based approach has been successful, this system is inherently inefficient, as only around 5–10 % of the total mass arises from boron.73,147 In an attempt to improve the efficiency, boron cluster-based nanoparticles have been proposed as alternative BNCT agents. Toward this end, Hawthorne and colleagues synthesized ester-linked73 and ether-linked147 derivatives of the dodecaborate core with pendant nido-o-carboranyl substituents, which in total contain roughly 40% boron content by mass. The sodium salt of the ether-linked cluster (Fig. 12) was found to be water-soluble and was subsequently evaluated for its suitability as a BNCT agent in a series of toxicity and biodistribution studies in animals.147 The results from these studies indicate that the compound is non-toxic to mice at a therapeutic dose and tumor-selective (the tumor-to-blood ratio is 9.4), however in this particular study its accumulation in tumor tissues (10.5 μg/g tumor) was half of the therapeutic amount necessary for BNCT. Nevertheless, this strategy of using cluster-based nanoparticles as BNCT agents is appealing due to its high boron content, modularity, and low toxicity.
Figure 12.
A. The chemical structure and B. A 3D representation of the dodeca(nido-o-carboranyl)-substituted dodecaborate cluster.
Very recently, Kovalska, Gumienna-Kontecka, and co-workers reported a spectroscopic and calorimetric binding study of perhalogenated decaborate ([B10X10]2−, X = Cl, Br, I) and dodecaborate ([B12X12]2−, X = Cl, I) dianions to bovine and human serum albumins (BSA and HSA, respectively) toward potential use in BNCT.148 It was shown through isothermal titration calorimetry (ITC) and fluorescence quenching experiments that the halogenated boron clusters displayed a greater affinity toward albumin binding (~ 4–5 clusters per protein, K ~ 104 – 106 M−1) than the parent, [BnHn]2− (n = 10 or 12) cluster (2 clusters per protein, K ~ 103 M−1). Circular dichroism studies suggested that no significant change in the secondary protein structure had occurred even at the highest concentrations of cluster species tested, which is consistent with the inefficient fluorescence quenching of the HSA, which contains a Tyr residue buried within the protein manifold, relative to BSA, which contains both buried and surface Tyr residues, by all clusters examined. In a similar study, Losytskyy et al. studied the binding of oligoether- and ammonium-substituted decaborate anions to BSA, HSA, immunoglobin IgG, b-lactoglobulin, and lysozyme. It was found that the functionalized, charge-compensated B10-based monoanions formed stronger interactions with these protein substrates than with unfunctionalized decaborate and dodecaborate dianions.149 These studies present an additional delivery system for high boron content molecules in vitro and suggest that perhalogenated boron clusters may be potentially useful in BNCT applications.
Delivery of Chemotherapy Drugs
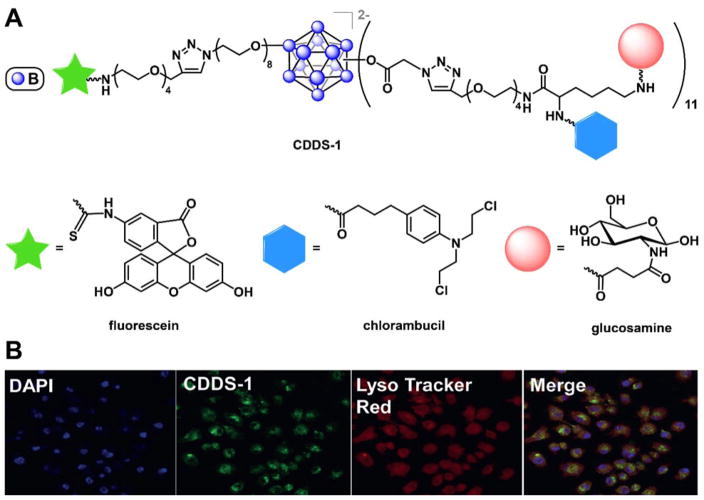
In addition to their function as BNCT agents, boron clusters have recently been developed as nanocarriers for the delivery of various chemotherapy drugs. Within the past few years, Hawthorne and colleagues have constructed several heterofunctional cluster-based drug delivery systems. For example, Bondarev et al. demonstrated the synthesis of a vertex-differentiated dodecaborate cluster scaffold, closo-[B12(OH)11NH3]1–, that is further functionalized with an amide-bound fluorescein and eleven carboplatin analogues bound through carbamate linkages.84 This species is found to localize in the nuclei of A549 cancer cells and shows potent cytotoxicity against both platinum-sensitive and platinum-resistant cell lines.84 In another example by Sarma et al., a trimodal targeted drug delivery system was assembled from a hetero-perfunctionalized dodecaborate dianion, closo-[B12(OH)11)(OR)]2–, by conjugating one vertex to a fluorescein molecule via click chemistry and the eleven other vertices to a branched linker featuring both the targeting molecule glucosamine and the anticancer drug chlorambucil (Fig. 13A).150 Interestingly, the targeted cluster co-localized with the lysosomes (Fig. 13B) and mitochondria within Jurkat cancer cells and exhibited 15-fold enhanced cytotoxicity compared to the non-targeted cluster against cells overexpressing the receptor for glucosamine.150 In a different approach to assembling the cluster-based nanocarriers, encapsulation of hydrophobic chemotherapy agent doxorubicin by the dendritic dodecaborate clusters in a manner reminiscent to its encapsulation by the poly(amidoamine) (PAMAM) dendrimers was disclosed,151,152 thus laying the groundwork for potential delivery into cancer cells using funtionalized dodecaborates.
Figure 13.
A. A trimodal closomer drug delivery system, CDDS-1, features targeting, imaging, and cell-killing abilities. B. Confocal microscopy images of CDDS-1 show its co-localization with lysosomes. Reprinted (adapted) with permission from Sarma, S. J.; Khan, A. A.; Goswami, L. N.; Jalisatgi, S. S.; Hawthorne, M. F. Chem. Eur. J. 2016, 22, 12715. Copyright 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Diagnostic Imaging
In addition to their applications in drug delivery for various types of cancer treatments, many derivatives of boron clusters have also been investigated for potential applications in diagnostic imaging. In fact, for decades, radiolabeled boron clusters have been studied as X-ray contrast agents because the incorporation of radiolabels on boron clusters can lead to higher chemical stability of the radiolabels.7 However, while there have been numerous reports on the radiolabeling of boron clusters,7,13,153 the vast majority have focused on the radiolabeled carborane derivatives and therefore will not be discussed here.
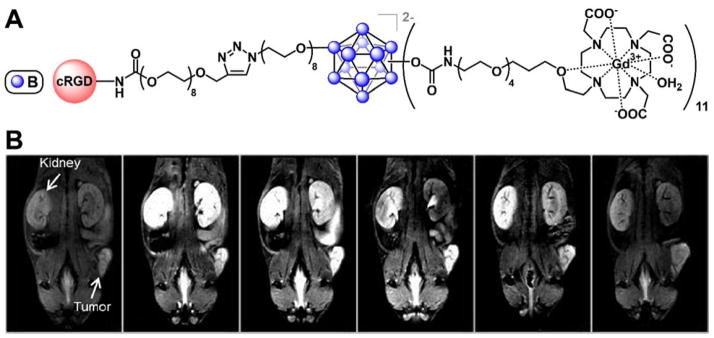
We will instead examine the development of high-performance all-boron cluster-based contrast agents for a more modern imaging technique, magnetic resonance imaging (MRI). MRI has emerged as one of the most important tools for the diagnosis of diseases, however there is a need to develop larger MRI contrast agents that can overcome the issues of low relaxivity values, short plasma half-lives, and low contrast typically associated with low-molecular weight contrast agents, while remaining well-defined.154–157 To tackle this challenge, Hawthorne and colleagues reported the synthesis of both the non-targeted154 and targeted157 (Fig. 14A) dodecaborate cluster-based MRI contrast agents that feature multiple gadolinium(III) complexes, and further demonstrated that these compounds provided significantly enhanced image contrast in addition to tumor-targeting ability provided by the cyclic RGD (cRGD) peptide (Fig. 14B).
Figure 14.
A. The cRGD peptide-labeled, Gd3+-DOTA chelates-carrying dodecaborate cluster MRI contrast agent and B. the resulting strong contrast enhancement in T1-weighted MRI scans of mice for up to 4 hours after injection. Reprinted (adapted) with permission from Goswami, L. N.; Ma, L.; Cai, Q.; Sarma, S. J.; Jalisatgi, S. S.; Hawthorne, M. F. Inorg. Chem. 2013, 52, 1701. Copyright 2013 American Chemical Society.
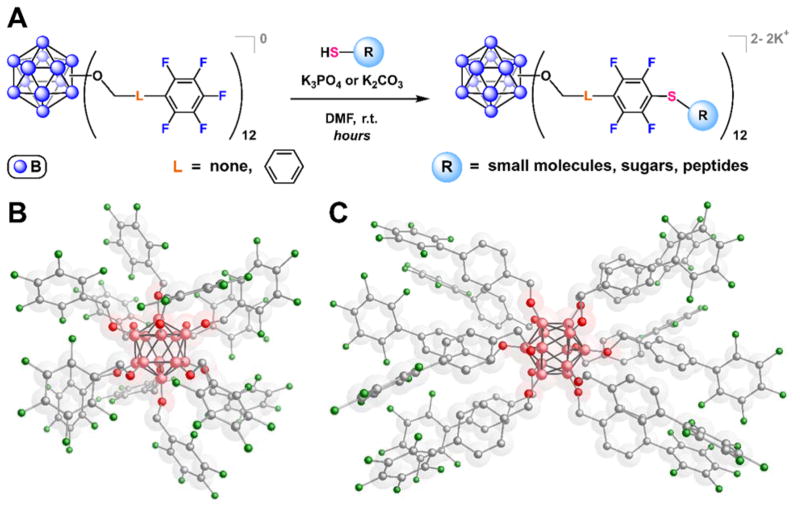
Multivalent Protein Binders
We recently reported a strategy to rapidly assemble atomically precise, highly tunable organomimetic cluster nanomolecules (OCNs) via perfluoroaryl-thiol SNAr chemistry (Fig. 15).45 This approach is reminiscent of the facile assembly method of the thiol-capped gold nanoparticles (AuNPs), however the resulting OCNs are monodisperse and structurally robust under a range of biologically relevant conditions due to covalently-linked substituents on the cluster core.
Figure 15.
A. The pentafluoroaryl-based dodecaborate clusters can undergo efficient SNAr reactions with a variety of thiolated molecules. B–C. Single X-ray crystal structures of hypercloso-B12(OCH2C6F5)12 (B) and hypercloso-B12(OCH2-4-(C6F5)-C6H4)12 (C).
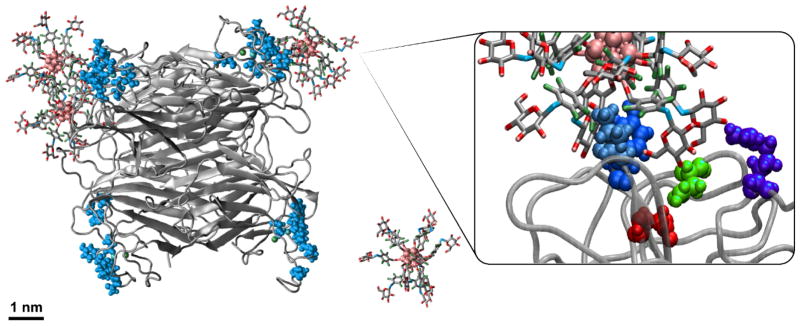
Employing this strategy, the pentafluoroarylated dodecaborate clusters were perfunctionalized with a broad scope of thiolated (macro)molecules, including unprotected peptides, saccharides, and polymers. Furthermore, a glycosylated OCN was shown to be capable of multivalent interactions with Concanavalin A (Con A) (Fig. 16), resulting in binding affinities that are orders of magnitude higher than those observed for a single carbohydrate-lectin interaction. Taken together, this work showcases how one might rationally design and synthesize well-defined nanomolecules that can be useful in 1) elucidating multivalent binding mechanisms and 2) potentially probing or inhibiting protein-biomolecule interactions.
Figure 16.
A snapshot after 20 ns of a molecular dynamics simulation between a glycosylated OCN and the tetrameric lectin ConA. The carbohydrate-binding sites of ConA are highlighted.
Outlook and Conclusions
Beyond the applications mentioned above, perfunctionalized boron clusters remain relatively unexplored entities. Yet, these molecules represent a very unique class of building blocks for potential applications in materials science. For example, polymeric materials produced from boron-rich clusters have been disclosed as early as 1968,158 but surprisingly, studies focused on producing functional, polymer-based materials featuring perfunctionalized boron cluster species have been extremely scarce. This is in contrast to a number of studies addressing the use of various carborane-based scaffolds for applications ranging from coatings to porous materials.159,160 Thus, polymeric materials and surfaces featuring some of the halogenated clusters can expand the scope of existing materials and provide resins and supports featuring bulk “weakly coordinating” properties reminiscent of zeolites and other “weakly-coordinating” supports.161 Similarly, polymers featuring anionic boron clusters as pendant functional groups should produce a unique class of polyelectrolytes. These materials can potentially show enhanced stabilities and tunable affinities toward cations, which in turn should allow one to tune ion mobility in the solid state providing access to materials with rationally tuned compositions and morphologies.162 Furthermore, given the inherent three-dimensionality of substituted clusters, these should possess attractive properties as cross-linkers for existing classes of polymeric materials.163
Clusters exhibiting pseudo-metallic properties manifested in their photoredox properties also have many unexplored avenues. First, understanding how functionalization affects the redox chemistry and the radical states of these highly delocalized systems is fundamentally important. The physical properties associated with unpaired electrons of functionalized species need to be studied further, both in the solid and solution states. These species can provide an entry to a unique chemistry of stable radicals, which can be used as building blocks with strong magnetic coupling properties to design molecular magnets.164 Furthermore, these molecules can potentially exhibit useful properties for spin labeling and nuclear polarization transfer.165,166 Given the potential materials-favorable properties of many perfunctionalized redox-active boron clusters, we expect that these species can be used as charge carrier scaffolds with good stability, cyclability, and potentially enhanced voltage characteristics in devices including polymer-based and redox-flow batteries.167,168 Likewise, the ability to control geometry and electronic delocalization between the cluster and tethered substituents should allow one to tune charge-transfer properties of the resulting systems and ultimately design metal-free chromophores with tunable absorption/emission colors and excited state lifetimes.
Perfunctionalized boron clusters can also be envisioned as building blocks for hierarchical hybrid materials. Recent developments in the field of hybrid porous solids169 clearly showcase the need for rigid, tunable, and robust three-dimensional building blocks. Furthermore, atomically precise 3D hierarchical materials containing nano-sized building blocks can be also used for assembly of nanoparticles into hierarchical extended structures. For example, cluster-based molecules can be synthesized with a densely packed corona of functional groups that will bind to the surface of nanoparticles. Tuning the aspect ratios of these clusters and nanoparticles may provide a method for creating assemblies with tunable plasmonic170 and catalytic properties.171
Finally, in contrast to carbon-based aromatic building blocks where there exist numerous chemical transformations enabling the functionalization of C–H bonds, methods targeting heterofunctionalized boron clusters are still currently very limited. Metal-catalyzed transformations will likely emerge in the future targeting synthesis of these molecules.172 Significantly, many opportunities can be envisioned that stem directly from the ability to generate complex structures that contain multiple functional groups appended to its vertices (vide supra). Beyond metal-based catalysis, several studies indicate that perhalogenated carboranes and boron clusters can undergo substitution at B–Cl and B–F vertices,173,174 suggesting it should be possible to design more well-behaved reaction protocols for creating vertex-differentiated systems via this relatively unexplored pathway. We are strongly convinced that expanding a molecular toolbox of existing organic and hybrid monomers for polymer synthesis through introduction of new perfunctionalized boron-rich cluster architectures will create a fundamentally new class molecular materials.
Acknowledgments
Funding Sources
A.M.S. acknowledges the University of California, Los Angeles (UCLA) Department of Chemistry and Biochemistry for start-up funds, 3M for a Non-Tenured Faculty Award, the American Chemical Society Petroleum Research Fund (56562-DNI3) for the Doctoral New Investigator Grant, Alfred P. Sloan Foundation for Research Fellowship in Chemistry and NIGMS for the Maximizing Investigator’s Research Award (R35GM124746). E.A.Q. is a recipient of a NIH Predoctoral Training Fellowship through the UCLA Chemistry-Biology Interface Training Program under the National Research Service Award (T32GM008496).
Authors thank Rafal M. Dziedzic for assistance with computational work.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Miyaura N, Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem Rev. 1995;95:2457–2483. [Google Scholar]
- 2.For details see: Spokoyny AM. New Ligand Platforms Featuring Boron-Rich Clusters as Organomimetic Substituents. Pure & Appl Chem. 2013;85:903–919. doi: 10.1351/PAC-CON-13-01-13.
- 3.Crawford B, Jr, Eberhardt WH, Lipscomb WN. The Valence Structure of the Boron Hydrides. J Chem Phys. 1954;22:989–1001. [Google Scholar]
- 4.Longuet-Higgins HC, Roberts M, de V. The Electronic Structure of an Icosahedron of Boron Atoms. Proc R Soc London, Ser A. 1955;230:110–119. [Google Scholar]
- 5.Pitochelli AR, Hawthorne MF. The Isolation of the Icosahedral B12H122− Ion. J Am Chem Soc. 1960;82:3228–3229. [Google Scholar]
- 6.Hawthorne MF, Pitochelli AR. The Reactions of Bis-Acetonitrile Decaborane with Amines. J Am Chem Soc. 1959;81:5519–5519. [Google Scholar]
- 7.Hawthorne MF, Maderna A. Applications of Radiolabeled Boron Clusters to the Diagnosis and Treatment of Cancer. Chem Rev. 1999;99:3421–3434. doi: 10.1021/cr980442h. [DOI] [PubMed] [Google Scholar]
- 8.Valiant JF, Guenther KJ, King AS, Morel P, Schaffer P, Sogbein OO, Stephenson KA. The Medicinal Chemistry of Carboranes. Coord Chem Rev. 2002;232:173–230. [Google Scholar]
- 9.Sivaev IB, Bregadze VI, Sjöberg S. Chemistry of closo-Dodecaborate Anion B12H122−: A Review. Collect Czech Chem Commun. 2002;67:679–727. [Google Scholar]
- 10.Sivaev IB, Bregadze VI. Polyhedral Boranes for Medical Applications: Current Status and Perspectives. Eur J Inorg Chem. 2009:1433–1450. [Google Scholar]
- 11.Kaim W, Hosmane NS, Záliš S, Maguire JA, Lipscomb WN. Boron Atoms as Spin Carriers in Two- and Three-Dimensional Systems. Angew Chem, Int Ed. 2009;48:5082–5091. doi: 10.1002/anie.200803493. [DOI] [PubMed] [Google Scholar]
- 12.Issa F, Kassiou M, Rendina LM. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem Rev. 2011;111:5701–5722. doi: 10.1021/cr2000866. [DOI] [PubMed] [Google Scholar]
- 13.Hosmane NS. Boron Science: New Technologies and Applications. CRC Press; Boca Raton, FL: 2011. [Google Scholar]
- 14.Scholz M, Hey-Hawkins E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem Rev. 2011;111:7035–7062. doi: 10.1021/cr200038x. [DOI] [PubMed] [Google Scholar]
- 15.Grimes RN. Carboranes. 3. Academic Press; 2016. [Google Scholar]
- 16.Olid D, Núñez R, Viñas C, Teixidor F. Methods to Produce B–C, B–P, B–N and B–S Bonds in Boron Clusters. Chem Soc Rev. 2013;42:3318–3336. doi: 10.1039/c2cs35441a. [DOI] [PubMed] [Google Scholar]
- 17.Douvris C, Michl J. Chemistry of the Carba-closo-Dodecaborate(-) Anion, CB11H12−. Chem Rev. 2013;113:PR179–PR233. doi: 10.1021/cr400059k. [DOI] [PubMed] [Google Scholar]
- 18.Núñez R, Romero I, Teixidor F, Viñas C. Icosahedral Boron Clusters: A Perfect Tool for the Enhancement of Polymer Features. Chem Soc Rev. 2016;45:5147–5173. doi: 10.1039/c6cs00159a. [DOI] [PubMed] [Google Scholar]
- 19.Núñez R, Tarrés M, Ferrer-Ugalde A, Fabrizi de Biani F, Teixidor F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem Rev. 2016;116:14307–14378. doi: 10.1021/acs.chemrev.6b00198. [DOI] [PubMed] [Google Scholar]
- 20.Welch AJ. The Significance and Impact of Wade’s Rules. Chem Commun. 2013;49:3615–3616. doi: 10.1039/c3cc00069a. (and references therein) [DOI] [PubMed] [Google Scholar]
- 21.King RB. Three-Dimensional Aromaticity in Polyhedral Boranes and Related Molecules. Chem Rev. 2001;101:1119–1152. doi: 10.1021/cr000442t. [DOI] [PubMed] [Google Scholar]
- 22.Braunschweig H, Dewhurst RD. Single, Double, Triple Bonds and Chains: The Formation of Electron-Precise B–B Bonds. Angew Chem, Int Ed. 2013;52:3574–3583. doi: 10.1002/anie.201208189. [DOI] [PubMed] [Google Scholar]
- 23.Braunschweig H, Dewhurst RD, Mozo S. Bulding Electron-Precise Boron–Boron Single Bonds: Imposing Monogamy on a Promiscuous Element. ChemCatChem. 2015;7:1630–1638. [Google Scholar]
- 24.For references, see: Davan T, Morrison JA. Chemistry of the Boron Subhalides: Preparation, Boron Nuclear Magnetic Resonance, and Thermal Stability of Tetraboron Tetrachloride. Inorg Chem. 1979;18:3194–3197.
- 25.Urry G, Wartik T, Schlesinger HI. A New Sub-Chloride of Boron, B4Cl4. J Am Chem Soc. 1952;74:5809–5809. [Google Scholar]
- 26.Ajoti A, Lipscomb WN. The Crystal and Molecular Structure of B4Cl4. Acta Crystallogr. 1953;6:547–550. [Google Scholar]
- 27.Mennekes T, Paetzold P, Boese R, Bläser D. Tetra-tert-butyltetraboratetrahedrane. Angew Chem, Int Ed Engl. 1991;30:173–175. [Google Scholar]
- 28.Hnyk D. T Symmetrical Gaseous Tetra-tert-butyltetrahoratetrahedrane: An Electron Diffraction Study. Polyhedron. 1997;16:603–606. [Google Scholar]
- 29.Davan T, Morrison JA. Tetrakis(t-butyl)tetraborane(4), ; Synthesis of the First Peralkyl Derivative of a 2N Framework Electron Count Deltahedral Borane. J Chem Soc, Chem Comm. 1981:250–251. [Google Scholar]
- 30.B4Cl4 reactivity: Morrison JA. Chemistry of the Polyhedral Boron Halides and the Diboron Tetrahalides. Chem Rev. 1991;91:35–48.
- 31.Maier CJ, Pritzkow H, Siebert W. Blue Tetrakis(diisopropylamino)-cyclo-tetraborane and Yellow Tetrakis(tetramethylpiperidino)tetraboratetrahedrane. Angew Chem, Int Ed. 1999;38:1666–1668. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1666::AID-ANIE1666>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 32.See ref. 2 in: Präsang C, Hofmann M, Geiseler G, Massa W, Berndt A. Topomerization of a Distorted Diamond-Shaped Tetraborane(4) and Its Hydroboration to a closo-Pentaborane(7) with a nido Structure. Angew Chem, Int Ed. 2003;42:1049–1052. doi: 10.1002/anie.200390271.
- 33.Neu A, Mennekes T, Paetzold P, Englert U, Hofmann M, von Ragué Schleyer P. Novel Tetralkyltetraboranes of the Type B4R4, B4H2R4 and B4H4R4. Inorg Chim Acta. 1999;289:58–69. [Google Scholar]
- 34.Preetz W, Peters G. The Hexahydro-closo-hexaborate Dianion [B6H6]2− and Its Derivatives. Eur J Inorg Chem. 1999:1831–1846. [Google Scholar]
- 35.Klandberg F, Muetterties EL. Chemistry of Boranes. XXVII. New Polyhedral Borane Anions, B9H92− and B11H112−. Inorg Chem. 1966;5:1955–1960. [Google Scholar]
- 36.Preetz W, Fritze J. Preparation, 11B NMR and Vibrational Spectra of the Octahedral closo-Borate Anions B6X62−; X = H, Cl, Br, I. Z Naturforsch. 1984;39b:1472–1477. [Google Scholar]
- 37.Fritze J, Preetz W, Marsmann HC. closo-Halogenohydrohexaborate, II 11B NMR Spectra of the closo-Halogenohydrohexaborates XnB6B6-n2−, n = 0–6; X = Cl, Br, I. Z Naturforsch. 1987;42b:287–292. [Google Scholar]
- 38.Fritze J, Preetz W. closo-Halogenohydrohexaborate, III Vibrational Spectra of the closo-Halogenohydrohexaborates XnB6B6-n2−, n = 1–5; X = Cl, Br, I. Z Naturforsch. 1987;42b:293–300. [Google Scholar]
- 39.Preetz W, Stallbaum M. Preparation and 11B NMR Spectra of the Perhalogenated closo-Hexaborates B6XnY6-n2−, n = 0–6; X ≠ Y = Cl, Br, I. Z Naturforsch. 1990;45b:1113–1117. [Google Scholar]
- 40.Thesing J, Stallbaum M, Preetz W. Vibrational Spectra and Noraml Coordinate Analysis of the Heteroleptic Halogenohexaborates B6XnY6-n2−, n = 1–5; X ≠ Y = Cl, Br, I. Z Naturforsch. 1991;46b:602–608. [Google Scholar]
- 41.Baudler M, Rockstein K, Oehlert W. Tris(diethylamino)cyclotriborane and Constitutional Isomerism Between cyclo- and closo-Hexakis(diethylamino)hexaborane(6) Chem Ber. 1991;124:1149–1152. [Google Scholar]
- 42.Siebert W, Maier CJ, Greiwe P, Bayer MJ, Jofmann M, Pritzkow H. Small Boranes, Carboranes, and Heterocarboranes. Pure Appl Chem. 2003;75:1277–1286. [Google Scholar]
- 43.Mesbah W, Soleimani M, Kianfar E, Geiseler G, Massa W, Hofmann M, Berndt A. hypercloso-Hexa(amino)hexaboranes: Structurally Related to Known hypercloso-Dodecaboranes, Metastable with Regard to Their Classical Cycloisomers. Eur J Inorg Chem. 2009:5577–5582. [Google Scholar]
- 44.Wixtrom AI, Shao Y, Jung D, Machan CW, Kevork SN, Qian EA, Axtell JC, Khan SI, Kubiak CP, Spokoyny AM. Rapid Synthesis of Redox-Active Dodecaborane B12(OR)12 Clusters under Ambient Conditions. Inorg Chem Front. 2016;3:711–717. doi: 10.1039/c5qi00263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian EA, Wixtrom AI, Axtell JC, Saebi A, Jung D, Rehak P, Han Y, Moully EH, Mosallaei D, Chow S, Messina MS, Wang JY, Royappa AT, Rheingold AL, Maynard HD, Král P, Spokoyny AM. Atomically Precise Organomimetic Cluster Nanomolecules Assembled via Perfluoroaryl-Thiol SNAr Chemistry. Nature Chem. 2017;9:333–340. doi: 10.1038/nchem.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Messina MS, Axtell JC, Wang Y, Chong P, Wixtrom AI, Kirlikovali KO, Upton BM, Hunter BM, Shafaat OS, Khan SI, Winkler JR, Gray HB, Alexandrova AN, Maynard HD, Spokoyny AM. Visible-Light-Induced Olefin Activation Using 3D Aromatic Boron-Rich Cluster Photooxidants. J Am Chem Soc. 2016;138:6952–6955. doi: 10.1021/jacs.6b03568. [DOI] [PubMed] [Google Scholar]
- 47.Bykov AY, Zhdanov AP, Zhizhin KY, Kuznetsov NT. Structure, Physicochemical Properties, and Reactivity of the [B9H9]2− Anion. Russ J Inorg Chem. 2016;61:1629–1648. [Google Scholar]
- 48.Klanberg F, Eaton DR, Guggenberger LJ, Muetterties EL. Chemistry of Boranes. XXVIII. New Polyhedral Borane Anions, B8H82−, B8H8.−, and B7H72−. Inorg Chem. 1967;6:1271–1281. [Google Scholar]
- 49.Knoth WH, Miller HC, England DC, Parshall GW, Muetterties EL. Derivative Chemistry of B10H10− and B12H12−. J Am Chem Soc. 1962;84:1056–1057. [Google Scholar]
- 50.Hoffmann R, Lipscomb WN. Sequential Substitution Reactions on B10H10−2 and B12H12−2. J Chem Phys. 1962;37:520–523. [Google Scholar]
- 51.Hertler WR, Raasch MS. Chemistry of Boranes. XIV. Amination of B10H10−2 and B12H12−2 with Hydroxalamine-O-Sulfonic Acid. J Am Chem Soc. 1964;86:3661–3668. [Google Scholar]
- 52.Muetterties EL, Balthis JH, Chia YT, Knoth WH, Miller HC. Chemistry of Boranes. VIII. Salts and Acids of B10H10−2 and B12H12−2. Inorg Chem. 1964;3:444–451. [Google Scholar]
- 53.Knoth WH, Miller HC, Sauer JC, Balthis JH, Chia YT, Muetterties EL. Chemistry of Boranes. IX. Halogenation of B10H10−2 and B12H12−2. Inorg Chem. 1964;3:159–167. [Google Scholar]
- 54.Sivaev IB, Prikaznov AV, Naoufal D. Fifty Years of the closo-Decaborate Anion Chemistry. Collect Czechoslov Chem Commun. 2010;75:1149–1199. [Google Scholar]
- 55.Kutz NA, Morrison JA. 2N Framework Electron Clusters: Proparation and Relative Thermal Stabilitites of the Polyhedran Boron Subbromides. Inorg Chem. 1980;19:3295–3299. [Google Scholar]
- 56.Gielen M. Does the Valence State Index Reflect the Stabililty of Neutral Boron Subhalide Clusters? Theor Chim Acta. 1982;61:21–28. [Google Scholar]
- 57.Hönle W, Grin Y, Burkhardt A, Wedig U, Schultheiss M, von Schnering HG, Kellner R, Binder H. Syntheses, Crystal Structures, and Electronic Structure of the Boron Halides B9X9 (X = Cl, Br, I) J Solid State Chem. 1997;133:59–67. [Google Scholar]
- 58.Speiser B, Wizemann T, Würde M. Two-Electron-Transfer Redox Systems Part 7: Two-Step Electrochemical Oxidation of the Boron Subhalide Cluster Dianions B6X62− (X = Cl, Br, I) Inorg Chem. 2003;42:4018–4028. doi: 10.1021/ic034101k. [DOI] [PubMed] [Google Scholar]
- 59.Kabbani RM, Wong EH. Selective Synthesis of the Nonachlorononaborate Clusters B9Cl9 and B9Cl92−. J Chem Soc Chem Commun. 1978:462–463. [Google Scholar]
- 60.Emery SL, Morrison JA. 2n Framework Electron Clusters: Directed Synthesis of Octachlorooctaborane, B8Cl8. The Activation of Carbon-Hydrogen Bonds. J Am Chem Soc. 1982;104:6790–6791. [Google Scholar]
- 61.Volkov O, Dirk W, Englert U, Paetzold P. Undecaborates M2[B11H11]: Facile Synthesis, Crystal Structure, and Reactions. Z Anorg Allg Chem. 1999;625:1193–1200. [Google Scholar]
- 62.Schlüter F, Bernhardt E. Syntheses and Crystal Structures of the closo-Borates M2[B7H7] and M[B7H8] (M = PPh4, PNP, and N(n-Bu4)): the Missing Crystal Structure in the Series [BnHn]2− (n = 6 – 12) Inorg Chem. 2011;50:2580–2589. doi: 10.1021/ic102434t. [DOI] [PubMed] [Google Scholar]
- 63.Schlüter F, Bernhardt E. Syntheses and Crystal Structures of the closo-Borate M[B8H9] (M = [PPh4]+ and [N(n-Bu4)]+) Inorg Chem. 2012;51:511–517. doi: 10.1021/ic201973b. [DOI] [PubMed] [Google Scholar]
- 64.Zhizhin KY, Zhdanov AP, Kuznetsov NT. Derivatives of closo-Decaborate Anion [B10H10]2− with exo-Polyhedral Substituents. Russ J Inorg Chem. 2010;55:2089–2127. [Google Scholar]
- 65.Gu X, Ozerov OV. Exhaustive Chlorination of [B12H12]2− without Chlorine Gas and the Use of [B12Cl12]2− as a Supporting Anion in Catalytic Hydrodefluorination of Aliphatic C–F Bonds. Inorg Chem. 2011;50:2726–2728. doi: 10.1021/ic200024u. [DOI] [PubMed] [Google Scholar]
- 66.Solntsev KA, Mebel AM, Votinova NA, Kuznetsov NT, Charkin OP. Dodecahydrododecaborate(2-) Polyhedral Anion as a Spacial Aromatic System. Koord Khim. 1992;18:340–364. [Google Scholar]
- 67.Ivanov SV, Miller SM, Anderson OP, Solntsev KA, Strauss SH. Synthesis and Stability of Reactive Salts of Dodecafluoro-closo-dodecaborate(2-) J Am Chem Soc. 2003;125:4694–4695. doi: 10.1021/ja0296374. [DOI] [PubMed] [Google Scholar]
- 68.Peryshkov DV, Popov AA, Strauss SH. Direct Perfluorination of K2B12H12 in acetonitrile Occurs at the Gas Bubble-Solution Interface and Is Inhibited by HF. Experimental and DFT Study of Inhibition by Protic Acids and Soft, Polarizable Anions. J Am Chem Soc. 2009;131:18393–18403. doi: 10.1021/ja9069437. [DOI] [PubMed] [Google Scholar]
- 69.Peymann T, Herzog A, Knobler CB, Hawthorne MF. Aromatic Polyhedral Hydroxyborates: Briding Boron Oxides and Boron Hydrides. Angew Chem, Int Ed. 1999;38:1061–1064. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1061::AID-ANIE1061>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 70.Peymann T, Knobler CB, Khan SI, Hawthorne MF. Dodecahydroxy-closo-dodecaborate(2-) J Am Chem Soc. 2001;123:2182–2185. doi: 10.1021/ja0014887. [DOI] [PubMed] [Google Scholar]
- 71.Farha OK, Julius RL, Lee MW, Huertas RE, Knobler CB, Hawthorne MF. Synthesis of Stable Dodecaalkoxy Derivatives of hypercloso-B12H12. J Am Chem Soc. 2005;127:18243–18251. doi: 10.1021/ja0556373. [DOI] [PubMed] [Google Scholar]
- 72.Maderna A, Knobler CB, Hawthorne MF. Twelvefold Functionalization of an Icosahedral Surface by Total Esterification of [B12(OH)12]2−: 12(12)-Closomers. Angew Chem, Int Ed. 2001;40:1661–1664. [PubMed] [Google Scholar]
- 73.Thomas J, Hawthorne MF. Dodeca(carboranyl)-Substituted Closomers: Toward Unimolecular Nanoparticles as Delivery Vehicles for BNCT. Chem Commun. 2001:1884–1885. doi: 10.1039/b105716m. [DOI] [PubMed] [Google Scholar]
- 74.Li T, Jalisatgi SS, Bayer MJ, Maderna A, Khan SI, Hawthorne MF. Organic Syntheses on an Icosahedral Borane Surface: Closomer Structures with Twelvefold Functionality. J Am Chem Soc. 2005;127:17832–17841. doi: 10.1021/ja055226m. [DOI] [PubMed] [Google Scholar]
- 75.Peymann T, Knobler CB, Khan SI, Hawthorne MF. Dodeca(benzyloxy)dodecaborane, B12(OCH2Ph)12: A Stable Derivative of hypercloso-B12H12. Angew Chem, Int Ed. 2001;40:1664–1667. [PubMed] [Google Scholar]
- 76.Miller EC, Hertler WR, Muetterties EL, Knoth WH, Miller NE. Chemistry of Boranes. XXV. Synthesis and Chemistry of Base Derivatives of B10H102− and B12H122−. Inorg Chem. 1965;4:1216–1221. [Google Scholar]
- 77.Ivanov SV, Davis JA, Miller SM, Anderson OP, Strauss SH. Synthesis and Characterization of Ammonioundecafluoro-closo-dodecaborates(1-). New Superweak Anions. Inorg Chem. 2003;42:4489–4491. doi: 10.1021/ic0344160. [DOI] [PubMed] [Google Scholar]
- 78.Bolli C, Derendorf J, Jenne C, Scherer H, Sindlinger CP, Wegener B. Synthesis and Properties of the Weakly Coordinating Anion [Me3NB12Cl11] Chem Eur J. 2014;20:13783–13792. doi: 10.1002/chem.201403625. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Liu J, Duttwyler S. Synthesis and Structural Characterization of Ammonio/Hydroxo Undecachloro-closo-Dodecaborates [B12Cl11NH3]−/[B12Cl11OH]2− and Their Derivatives. Eur J Inorg Chem. 2015:5158–5162. [Google Scholar]
- 80.Peymann T, Knobler CB, Hawthorne MF. An Icosahedral Array of Methyl Groups Supported by an Aromatic Borane Scaffold: The [closo-B12(CH3)12]2− Ion. J Am Chem Soc. 1999;121:5601–5602. [Google Scholar]
- 81.Bondarev O, Hawthorne MF. Catalytic Hydroxylation of [Closo-B12H12]2− – Adaptation of the Periana Reaction to a Polyhedral Borane. Chem Commun. 2011;47:6978–6980. doi: 10.1039/c1cc11722j. [DOI] [PubMed] [Google Scholar]
- 82.Jalisatgi SS, Kulkarni VS, Tang B, Houston ZH, Lee MW, Hawthorne MF. A Convenient Route to Diversely Substituted Icosahedral Closomer Nanoscaffolds. J Am Chem Soc. 2011;133:12382–12385. doi: 10.1021/ja204488p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jenne C, Kirsch C. Alkoxy Substituted Halogenated closo-Dodecaborates as Anions for Ionic Liquids. Dalton Trans. 2015;44:13119–13124. doi: 10.1039/c5dt01633a. [DOI] [PubMed] [Google Scholar]
- 84.Bondarev O, Khan AA, Tu X, Sevryugina YV, Jalisatgi SS, Hawthorne MF. Synthesis of [closo-B12(OH)11NH3]−: A New Heterobifunctional Dodecaborane Scaffold for Drug Delivery Applications. J Am Chem Soc. 2013;135:13204–13211. doi: 10.1021/ja4069613. [DOI] [PubMed] [Google Scholar]
- 85.Heinrich A, Keller HL, Preetz W. Oxidation Reactions on XnB6H6-n2−, X = Cl, Br, I; n = 1 – 6: and Crystal Structures of [(n-C4H9)4N]B6I6 and [(n-C4H9)4N]2B6I6. Z Naturforsch. 1990;45b:184–190. [Google Scholar]
- 86.Lorenzen V, Preetz W. Crystal Structures of (n-Bu4N)[B6Cl6], (n-Bu4N)[B6Br6], and (Ph3P=N=PPh3)[B6I6], and Vibrational Spectra and Normal Coordinate Analysis of the Hexahalogeno-closo-hexaborate Radical Anions [B6X6]−, X = Cl, Br. I. Z Naturforsch. 1997;52b:565–572. [Google Scholar]
- 87.Binder H, Kellner R, Vaas K, Hein M, Baumann F, Wanner M, Winter R, Kaim W, Hönle W, Grin Y, Wedig U, Schultheiss M, Kremer RK, von Schnering HG, Groeger O, Engelhardt G. The closo-Cluster Triad: B9X9, [B9X9].−, and [B9X9]2− with Tricapped Trigonal Prisms (X = Cl, Br, I). Syntheses, Crystal and Electronic Structures. Z Anorg Allg Chem. 1999;625:1059–1072. [Google Scholar]
- 88.Boeré RT, Derendorf J, Jenne C, Kacprzak S, Keßler M, Riebau R, Riedel S, Roemmele TL, Rühle M, Scherer H, Vent-Schmidt T, Warneke J, Weber S. On the Oxidation of the Three-Dimensional Aromatics [B12X12]2− (X = F, Cl, Br, I) Chem Eur J. 2014;20:4447–4459. doi: 10.1002/chem.201304405. [DOI] [PubMed] [Google Scholar]
- 89.Wiersema RJ, Middaugh RL. Electrochemical Preparation and halogenation of 1,1′-μ-Hydro-bis(undecahydro-closo-dodecaborate)(3-), B24H233−. Inorg Chem. 1969;8:2074–2079. [Google Scholar]
- 90.Rupich MW, Foos JS, Brummer SB. Characterization of Chloroclosoborane Acids as Electrolytes for Acid Fuel Cells. J Electrochem Soc. 1985;132:119–122. [Google Scholar]
- 91.Boeré RT, Kacprzak S, Keßler M, Knapp C, Riebau R, Riedel S, Roemmele TL, Rühle M, Scherer H, Weber S. Oxidation of closo-[B12Cl12]2− to the Radical Anion [B12Cl12].− and to Neutral B12Cl12. Angew Chem, Int Ed. 2011;50:549–552. doi: 10.1002/anie.201004755. [DOI] [PubMed] [Google Scholar]
- 92.Van N, Tiritiris I, Winter RF, Sarkar B, Singh P, Duboc C, Muñoz-Castro A, Arratia-Pérez R, Kaim W, Schleid T. Oxidative Perhydroxylation of [closo-B12H12]2− to the Stable Inorganic Cluster Redox System [B12(OH)12]2−/.−: Experiment and Theory. Chem Eur J. 2010;16:11242–11245. doi: 10.1002/chem.201001374. [DOI] [PubMed] [Google Scholar]
- 93.Peymann T, Knobler CB, Hawthorne MF. An Unpaired Electron Incarcerated with an Icosahedral Borane Cage: Syntehsis and Crystal Structure of the Blue, Air-Stable {[closo-B12(CH3)12].}− Radical. Chem Commun. 1999:2039–2040. [Google Scholar]
- 94.Lee MW, Farha OK, Hawthorne MF, Hansch CH. Alkoxy Derivatives of Dodecaborate: Discrete Nanomolecular Ions with Tunable Pseudometallic Properties. Angew Chem, Int Ed. 2007;46:3018–3022. doi: 10.1002/anie.200605126. [DOI] [PubMed] [Google Scholar]
- 95.Hansch C, Leo A, Taft RW. A Survey of Hamett Substituent Constances and Resonance and Field Parameters. Chem Rev. 1991;91:165–195. [Google Scholar]
- 96.Douvris C, Ozerov OV. Hydrodefluorination of Perfluoroalkyl Groups Using Silylium-Carborane Catalysts. Science. 2008;321:1188–1190. doi: 10.1126/science.1159979. [DOI] [PubMed] [Google Scholar]
- 97.Shao B, Bagdasarian AL, Popov S, Nelson HM. Arylation of Hydrocarbons Enabled by Organosilicon Reagents and Weakly Coordinating Anions. Science. 2017;355:1403–1407. doi: 10.1126/science.aam7975. [DOI] [PubMed] [Google Scholar]
- 98.Kostjuk SV. Recent Progress in the Lewis Acid Co-Initiated Cationic Polymerization of Isobutylene and 1,3-Dienes. RSC Adv. 2015;5:13125–13144. [Google Scholar]
- 99.Wegener M, Huber F, Bolli C, Jenne C, Kirsch SF. Silver-Free Activation of Ligated Gold(I) Chlorides: The Use of [Me3NB12Cl11] as a Weakly Coordinating Anion in Homogeneous Gold Catalysis. Chem Eur J. 2015;21:1328–1336. doi: 10.1002/chem.201404487. [DOI] [PubMed] [Google Scholar]
- 100.Konze WV, Scott BL, Kubas GJ. First Example of B–C Bond Cleavage in the BArF (B[C6H3(CF3)2-3,5]4) Anion Mediated by a Transition Metal Species, trans-[(Ph3P)2Pt(Me)(OEt2)]+ Chem Commun. 1999:1807–1808. [Google Scholar]
- 101.Salem H, Shimon LJW, Leitus G, Weiner L, Milstein D. B–C Bond Cleavage of BArF Anion Upon Oxidation of Rhodium(I) with AgBArF. Phosphinite Rhodium(I), Rhodium(II), and Rhodium(III) Pincer Complexes. Organometallics. 2008;27:2293–2299. [Google Scholar]
- 102.Weber SG, Zahner D, Rominger F, Straub BF. A Cationic Gold Complex Cleaves BArF24. Chem Commun. 2012;48:11325–11327. doi: 10.1039/c2cc36171j. [DOI] [PubMed] [Google Scholar]
- 103.Bolli C, Derendorf J, Keßler M, Knapp C, Scherer H, Schulz C, Warneke J. Synthesis, Crystal Structure, and Reactivity of the Strongly Methylating Agent Me2B12Cl12. Angew Chem Int Ed. 2010;49:3536–2528. doi: 10.1002/anie.200906627. [DOI] [PubMed] [Google Scholar]
- 104.Kessler M, Knapp C, Zogaj A. Cationic Dialkyl Metal Compounds of Group 13 Elements (E = Al, Ga, In) Stabilized by the Weakly Coordinating Dianion [B12Cl12]2−. Organometallics. 2011;30:3786–3792. [Google Scholar]
- 105.Saleh M, Powell DR, Wehmschulte RJ. Chlorination of 1-Carba-closo-dodecaborate and 1-Ammonio-closo-dodecaborate Anions. Inorg Chem. 2016;55:10617–10627. doi: 10.1021/acs.inorgchem.6b01867. [DOI] [PubMed] [Google Scholar]
- 106.Avelar A, Tham FS, Reed CA. Superacidity of Boron Acids H 2(B12X12) (X = Cl, Br) Angew Chem Int Ed. 2009;48:3491–3493. doi: 10.1002/anie.200900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jenne C, Keßler M, Warneke J. Protic Anions [H(B12X12)]− That Act as Brønsted Acids in the Gas Phase. Chem Eur J. 2015;21:5887–5891. doi: 10.1002/chem.201500034. [DOI] [PubMed] [Google Scholar]
- 108.Lipping L, Leito I, Koppel I, Krossing I, Himmel D, Koppel IA. Superacidity of closo-Dodecaborate-Based Brønsted Acids: a DFT Study. J Phys Chem A. 2015;119:735–743. doi: 10.1021/jp506485x. [DOI] [PubMed] [Google Scholar]
- 109.Rogers RD, Sneddon KR. Ionic Liquids – Solvents of the Future? Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- 110.Bertocco P, Derendorf J, Jenne C, Kirsch C. Improving the Solubility of 1-Ammonio-closo-dodecaborate Anions. Inorg Chem. 2017;56:3459–3466. doi: 10.1021/acs.inorgchem.6b03006. [DOI] [PubMed] [Google Scholar]
- 111.Johnson JW, Brody JF. Lithium Closoborane Electrolytes: III. Preparation and Characterization. J Electrochem Soc. 1982;129:2213–2219. [Google Scholar]
- 112.Dey AN, Miller J. Primary Li/SOCl2 Cells: VII. Effect of Li2B10Cl10 and Li2B12Cl12 Electrolyte Salts on the Performance. J Electrochem Soc. 1979;126:1445–1451. [Google Scholar]
- 113.Johnson JW, Whittingham MS. Lithium Closoboranes as Electrolytes in Solid Cathode Lithium Cells. J Electrochem Soc. 1980;127:1653–1654. [Google Scholar]
- 114.Johnson JW, Thompson AH. Lithium Closoboranes II. Stable Nonaqueous Electrolytes for Elevated Temperature Lithium Cells. J Electrochem Soc. 1981;128:932–933. [Google Scholar]
- 115.GirishKumar G, Bailey WH, III, Peterson BK, Casteel WJ., Jr Electrochemical and Spectroscopic Investigations of the Overcharge Behavior of StabiLife Electrolyte Salts in Lithium-Ion Batteries. J Electrochem Soc. 2011;158:A146–A153. [Google Scholar]
- 116.Ionica-Bousquet CM, Muñoz-Rojas D, Casteel WJ, Pearlstein RM, GirishKumar G, Pez GP, Palacín MR. Polyfluorinated Boron Cluster-Based Salts: A New Electrolyte for Application in Li4Ti5O12/LiMn2O4 Rechargeable Lithium-Ion Batteries. J Power Sources. 2010;195:1479–1485. [Google Scholar]
- 117.Stasko DJ, Perzynski KJ, Wasil MA, Brodbeck JK, Kirschbaum K, Kim YW, Lind C. An Addition to the Oxoacid Family: H2B12(OH)12. Inorg Chem. 2004;43:3786–3788. doi: 10.1021/ic049564k. [DOI] [PubMed] [Google Scholar]
- 118.Bukovsky EV, Peryshkov DV, Wu H, Zhou W, Tang WS, Jones WM, Stavila V, Udovic TJ, Strauss SH. Comparison of the Coordination of B12F122−, B12Cl122−, and B12H122− to Na+ in the Solid State: Crystal Structures and Thermal Behavior of Na2(B12F12), Na2(H2O)4(B12F12), Na2(B12Cl12), and Na2(H2O)6(B12Cl12) Inorg Chem. 2017;56:4369–4379. doi: 10.1021/acs.inorgchem.6b02920. [DOI] [PubMed] [Google Scholar]
- 119.Hawthorne MF. The Role of Chemistry in the Development of Boron Neutron Capture Therapy of Cancer. Angew Chem, Int Ed Engl. 1993;32:950–984. [Google Scholar]
- 120.Wsek J. Potential Applications of the Boron Cluster Compounds. Chem Rev. 1992;92:269–278. [Google Scholar]
- 121.Grimes RN. Boron Clusters Come of Age. J Chem Educ. 2004;81:657–672. [Google Scholar]
- 122.Locher GL. Bioloical Effects and Therapeutic Possibilties of Netrons. Am J Roentgenol Radium Therapy. 1936;36:1–13. [Google Scholar]
- 123.Hawthorne MF. New Horizons for Therapy Based on the Boron Neutraon Capture Reaction. Mol Med Today. 1998;4:174–181. doi: 10.1016/s1357-4310(98)01226-x. [DOI] [PubMed] [Google Scholar]
- 124.Moss RL. Critical Review, with an Optimistic Outlook, on Boron Neutron Capture Therapy (BNCT) Appl Radiat Isotopes. 2014;88:2–11. doi: 10.1016/j.apradiso.2013.11.109. [DOI] [PubMed] [Google Scholar]
- 125.Slatkin DN. A History of Boron Netron Capture Therapy of Brain Tumours: Postulation of a Brain radiation Dose Tolerance Limit. Brain. 1991;114:1609–1629. doi: 10.1093/brain/114.4.1609. [DOI] [PubMed] [Google Scholar]
- 126.Lipscomb WN, Pitochelli AR, Hawthorne MF. Probable Structure of the B10H102− Ion. J Am Chem Soc. 1959;81:5833–5834. [Google Scholar]
- 127.Asbury AK, Ojemann RG, Nielsen SL. Neuropathologic Study of Fourteen Cases of Malignant Brain Tumor Treated by Boron-10 Slow Neutron Capture Radiation. J Neuropathol Exp Neurol. 1972;31:278–303. doi: 10.1097/00005072-197204000-00005. [DOI] [PubMed] [Google Scholar]
- 128.Barth RF, Soloway AH, Fairchild RG, Brugger RM. Boron Neutron Capture Therapy for Cancer. Realities and Prospects. Cancer. 1992;70:2995–3007. doi: 10.1002/1097-0142(19921215)70:12<2995::aid-cncr2820701243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 129.Soloway AH, Hatanaka H, Davis MA. Penetration of Brain and Brain Tumor. VII. Tumor-Binding Sulfhydryl Boron Compounds. J Med Chem. 1967;10:714–717. doi: 10.1021/jm00316a042. [DOI] [PubMed] [Google Scholar]
- 130.Ciani L, Ristori S. Boron as a Platform for New Drug Design. Expert Opin Drug Discov. 2012;7:1017–1027. doi: 10.1517/17460441.2012.717530. [DOI] [PubMed] [Google Scholar]
- 131.Barth RF, Coderre JA, Vicente MGH, Blue TE. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 132.Nakamura H, Kirihata M. In: Neutron Capture Therapy. Sauerwein W, Wittig A, Moss R, Nakagawa Y, editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2012. [Google Scholar]
- 133.Soloway AH, Tjarks W, Barnum BA, Rong FG, Barth RF, Codogni IM, Wilson JG. The Chemistry of Neutron Capture Therapy. Chem Rev. 1998;98:1515–1562. doi: 10.1021/cr941195u. [DOI] [PubMed] [Google Scholar]
- 134.Sumitani S, Nagasaki Y. Boron Neutron Capture Therapy Assisted by Boron-Conjugated Nanoparticles. Polym J. 2012;44:522–530. [Google Scholar]
- 135.Zhu YZ, Lin Y, Zhu YZ, Lu J, Maguire JA, Hosmane NS. Boron Drug Delivery via Encapsulated Magnetic Nanocomposites: A New Approach for BNCT in Cancer Treatment. J Nanomater. 2010;2010:1–8. [Google Scholar]
- 136.Mandal S, Bakeine GJ, Krol S, Ferrari C, Clerici AM, Zonta C, Cansolino L, Ballarini F, Bortolussi S, Stella S, Protti N, Bruschi P, Altieri S. Design, Development, and Characterization of Multi-Functional Gold Nanoparticles for Biodetection and Targeted Boron Delivery in BNCT Applications. Appl Radiat Isotopes. 2011;69:1692–1697. doi: 10.1016/j.apradiso.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 137.Umano M, Uechi K, Uriuda T, Murayama S, Azuma H, Shinohara A, Liu Y, Ono K, Kirihata M, Yanagie H, Nagasaki T. Tumor Accumulation of ε-poly-lysines-based Polyamines Conjugated with Boron Clusters. Appl Radiat Isotopes. 2011;69:1765–1767. doi: 10.1016/j.apradiso.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 138.Yanagië H, Tomita T, Kobayashi H, Fujii Y, Takahashi T, Hasumi K, Nariuchi H, Sekiguchi M. Application of Boronated Anti-CEA Immunoliposome to Tumour Cell Growth Inhibition in in vitro Boron Neutron Capture Therapy Model. Br J Cancer. 1991;63:522–526. doi: 10.1038/bjc.1991.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shelly K, Feakes DA, Hawthorne MF, Schmidt PG, Krisch TA, Bauer WF. Model Studies Directed Toward the Boron Neutron-Capture Therapy of Cancer: Boron Delivery to Murine Tumors with Liposomes. Proc Natl Acad Sci US A. 1992;89:9039–9043. doi: 10.1073/pnas.89.19.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pan XQ, Wang H, Shukla S, Sekido M, Adams DM, Tjarks W, Barth RF, Lee RJ. Boron-Containing Folate Receptor-Targeted Liposomes as Potential Delivery Agents for Neutron Capture Therapy. Bioconjug Chem. 2002;13:435–442. doi: 10.1021/bc015557y. [DOI] [PubMed] [Google Scholar]
- 141.Maruyama K, Ishida O, Kasaoka S, Takizawa T, Utoguchi N, Shinohara A, Chiba M, Kobayashi H, Eriguchi M, Yanagie H. Intracellular Targeting of Sodium Mercaptoundecahydrododecaborate (BSH) to Solid Tumors by Transferrin-PEG Liposomes, for Boron Neutron-Capture Therapy (BNCT) J Control Release. 2004;98:195–207. doi: 10.1016/j.jconrel.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 142.Feakes DA, Shelly K, Hawthorne MF. Selective Boron Delivery to Murine Tumors by Lipophilic Species Incorporated in the membranes of Unilamellar Liposomes. Proc Natl Acad Sci US A. 1995;92:1367–1370. doi: 10.1073/pnas.92.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hawthorne MF, Shelly K. Liposomes as Drug Delivery Vehicles for Boron Agents. J Neurooncol. 1997;33:53–58. doi: 10.1023/a:1005713113990. [DOI] [PubMed] [Google Scholar]
- 144.Feakes DA, Spinler JK, Harris FR. Synthesis of Boron-Containing Cholesterol Derivatives for Incorporation into Unilamellar Liposomes and Evaluation as Potential Agents for BNCT. Tetrahedron. 1999;55:11177–11186. [Google Scholar]
- 145.Lee JD, Ueno M, Miyajima Y, Nakamura H. Synthesis of Boron Cluster Lipids: closo-Dodecaborate as an Alternative Hydrophilic Function of Boronated Liposomes for Neutron Capture Therapy. Org Lett. 2007;9:323–326. doi: 10.1021/ol062840+. [DOI] [PubMed] [Google Scholar]
- 146.Heber EM, Hawthorne MF, Kueffer PJ, Garabalino MA, Thorp SI, Pozzi ECC, Hughes AM, Maitz CA, Jalisatgi SS, Nigg DW, Curotto P, Trivillin VA, Schwint AE. Therapeutic Efficacy of Boron Neutron Capture Therapy Mediated by Boron-Rich Liposomes for Oral Cancer in the Hamster Cheek Pouch Model. Proc Natl Acad Sci. 2014;111:16077–16081. doi: 10.1073/pnas.1410865111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ma L, Hamdi J, Wong F, Hawthorne MF. Closomers of High Boron Content: Synthesis, Characterization, and Potential Application as Unimolecular Nanoparticle Delivery Vehicles for Boron Neutron Capture Therapy. Inorg Chem. 2006;45:278–285. doi: 10.1021/ic051214q. [DOI] [PubMed] [Google Scholar]
- 148.Kuperman MV, Losytskyy MY, Bykov AY, Yarmoluk SM, Zhizhin KY, Kuznetsov NT, Varzatskii OA, Gumienna-Kontecka E, Kovalska VB. Effective Binding of Perhalogenated closo-Borates to Serum Albumins Revealed by Spectroscopic and ITC Studies. J Mol Struct. 2017;1141:75–80. [Google Scholar]
- 149.Losytskyy MY, Kovalska VB, Varzatskii OA, Kuperman MV, Potocki S, Gumienna-Kontecka E, Zhdanov AP, Yarmoluk SM, Voloshin YZ, Zhizhin KY, Kuznetsov NT, Elskaya AV. An Interaction of the Functionalized closo-Borates with Albumins: The Protein Fluorescence Quencing and Calorimetry Study. J Luminescence. 2016;169:51–60. [Google Scholar]
- 150.Sarma SJ, Khan AA, Goswami LN, Jalisatgi SS, Hawthorne MF. A Trimodal Closomer Drug-Delivery System Tailored with Tracing and Targeting Capabilities. Chem Eur J. 2016;22:12715–12723. doi: 10.1002/chem.201602413. [DOI] [PubMed] [Google Scholar]
- 151.Pushechnikov A, Jalisatgi SS, Hawthorne MF. Dendritic Closomers: Novel Spherical Hybrid Dendrimers. Chem Commun. 2013;49:3579–3581. doi: 10.1039/c3cc40597d. [DOI] [PubMed] [Google Scholar]
- 152.Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of Polyamidoamine Dendrimers Having Poly(ethylene glycol) Grafts and Their Ability to Encapsulate Anticancer Drugs. Bioconjug Chem. 2000;11:910–917. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]
- 153.Armstrong AF, Valliant JF. The Bioinorganic and Medicinal Chemistry of Carboranes: From New Drug Discovery to Molecular Imaging and Therapy. Dalton Trans. 2007;38:4240–4251. doi: 10.1039/b709843j. [DOI] [PubMed] [Google Scholar]
- 154.Goswami LN, Ma L, Chakravarty S, Cai Q, Jalisatgi SS, Hawthorne MF. Discrete Nanomolecular Polyhedral Borane Scaffold Supporting Multiple Gadolinium(III) Complexes as a High Performance MRI Contrast Agent. Inorg Chem. 2013;52:1694–1700. doi: 10.1021/ic3017613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Caravan P. Protein-Targeted Gadolinium-Based Magnetic Resonance Imaging (MRI) Contrast Agents: Design and Mechanism of Action. Acc Chem Res. 2009;42:851–862. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 156.Langereis S, de Lussanet QG, van Genderen MHP, Backes WH, Meijer EW. Multivalent Contrast Agents Based on Gadolinium-Diethylenetriaminepentaacetic Acid-Terminated Poly(propylene imine) Dendrimers for Magnetic Resonance Imaging. Macromolecules. 2004;37:3084–3091. [Google Scholar]
- 157.Goswami LN, Ma L, Cai Q, Sarma SJ, Jalisatgi SS, Hawthorne MF. cRGD Peptide-Conjugated Icosahedral closo-B122− Core Carrying Multiple Gd3+-DOTA Chelates for αvβ3 Integrin-Targeted Tumor Imaging (MRI) Inorg Chem. 2013;52:1701–1709. doi: 10.1021/ic302340c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Graham B. Polymers Derived from Metal Decaborates and Dodecaborates. 3368878. US Patent. 1968 Feb 13;
- 159.Schaefgen JR, Miller HC, Sauer JC, Sharkey WH. Polymers Containing Polyhedral Borane Rings I. Condensation Polymers. Journal of Polymer Science Part A-1: Polymer Chemistry. 1971;9:51–68. [Google Scholar]
- 160.Spokoyny AM, Farha OK, Mulfort KL, Hupp JT, Mirkin CA. Porosity Tuning of Carborane-Based Metal-Organic Frameworks (MOFs) via Coordination Chemistry and Ligand Design. Inorg Chim Acta. 2011;364:266–271. [Google Scholar]
- 161.Arata K. Preparation of Superacidic Metal Oxides and Their Catalytic Action. In: Jackson SD, Hargreaves JSJ, editors. Metal Oxide Catalysis. Wiley-VCH; Weinheim, Germany: 2009. [Google Scholar]
- 162.Mortimer DA. Synthetic Polyelectrolytes – A Review. Polym Int. 1991;25:29–41. [Google Scholar]
- 163.Zhou N, Cao Z, Xu B. Functional Hyper-Crosslinkers. Chem Eur J. 2017;23:15844–15851. doi: 10.1002/chem.201703671. [DOI] [PubMed] [Google Scholar]
- 164.DeGayner JA, Jeon IR, Sun L, Dinca M, Harris TD. 2D Conductive Iron-Quinoid Magnets Ordering up to Tc = 105K via Heterogenous Redox Chemistry. J Am Chem Soc. 2017;139:4175–4184. doi: 10.1021/jacs.7b00705. [DOI] [PubMed] [Google Scholar]
- 165.Michaelis VK, Smith AA, Corzilius B, Haze O, Swager TM, Griffin RG. High-Field 13C Dynamic Nuclear Polarization with a Radical Mixture. J Am Chem Soc. 2013;135:2935–2938. doi: 10.1021/ja312265x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jagtap AP, Krstic I, Kunjir NC, Hänsel R, Prisner TF, Sigurdsson ST. Sterically Shielded Spin Labels for In-Cell EPR Spectroscopy: Analysis of Stability in Reducing Environment. Free Radic Res. 2015;49:78–85. doi: 10.3109/10715762.2014.979409. [DOI] [PubMed] [Google Scholar]
- 167.Nishide H, Oyaizu K. Toward Flexible Batteries. Science. 2008;319:737–738. doi: 10.1126/science.1151831. [DOI] [PubMed] [Google Scholar]
- 168.Dmello R, Milshtein JD, Brushett FR, Smith KC. Cost-Driven Materials Selection Criteria for Redox Flow Battery Electrolytes. J Power Sources. 2016;330:261–272. [Google Scholar]
- 169.Popp TMO, Yaghi OM. Sequence-Dependent Materials. Acc Chem Res. 2017;50:532–534. doi: 10.1021/acs.accounts.6b00529. [DOI] [PubMed] [Google Scholar]
- 170.Jones MR, Osberg KD, MacFarlane RJ, Langille MR, Mirkin CA. Templated Techniques for the Synthesis and Assembly of Plasmonic Nanostructures. Chem Rev. 2011;111:3736–3827. doi: 10.1021/cr1004452. [DOI] [PubMed] [Google Scholar]
- 171.Auyeung E, Morris W, Mondloch JE, Hupp JT, Farha OK, Mirkin CA. Controlling Structure and Porosity in Catalytic nanoparticle Superlattices with DNA. J Am Chem Soc. 2015;137:1658–1662. doi: 10.1021/ja512116p. [DOI] [PubMed] [Google Scholar]
- 172.Zhang Y, Sun Y, Lin F, Liu J, Duttwyler S. Rhodium(III)-Catalyzed Alkynylation-Annulation of closo-Dodecaborate Anions through Double B–H Activation at Room Temperature. Angew Chem. 2016;128:15838–15843. doi: 10.1002/anie.201607867. [DOI] [PubMed] [Google Scholar]
- 173.Fajardo J, Jr, Chan AL, Tham FS, Lavallo V. Synthesis and Characterization of Anionic Polybrominated Carboranyl Azides. Inorg Chim Acta. 2014;422:206–208. [Google Scholar]
- 174.Trofimenko S. Photochemical Method for the Preparation of Substituted Polyhedral Borane Anions. 3373098. US Patent. 1968 Mar 12;