Abstract
BACKGROUND
Photodynamic therapy (PDT) is a non-scarring alternative for treating basal cell carcinoma (BCC) in patients with Basal Cell Nevus Syndrome (BCNS), also known as Gorlin syndrome. In Europe, red light (635 nm) is the predominant source for PDT, whereas in the United States blue light (400 nm) is more widely available. The objective of this study was to conduct a head-to-head comparison of blue light and red light PDT in the same BCNS patients.
METHODS
In a pilot study of three patients with 141 BCC lesions, 5-aminolevulinate (20% solution) was applied to all tumors. After 4 hours, half of the tumors were illuminated with blue light and the remainder with red light. To ensure safety while treating this many tumors simultaneously, light doses were escalated gradually. Six treatments were administered in three biweekly sessions over 4 months, with a final evaluation at 6 months. Tumor status was documented with high-resolution photographs. Persistent lesions were biopsied at 6 months.
RESULTS
Clearance rates after blue light (98%) were slightly better than after red light (93%), with blue light shown to be statistically non-inferior to red light. Eight suspicious lesions were biopsied, 5 after red light (5/5 were BCC) and 3 after blue light (1 was BCC). Blue light PDT was reportedly less painful.
CONCLUSION
Blue light and red light PDT appear to be equally safe and perhaps equally effective for treating BCC tumors in BCNS patients. Further studies to evaluate long-term clearance after blue light PDT are needed.
Keywords: Basal cell nevus syndrome, photodynamic therapy, blue light, aminolevulinic acid, basal cell carcinoma
INTRODUCTION
Basal cell nevus syndrome (BCNS; Gorlin; Gorlin-Goltz) is a chronic genetic condition characterized by basal cell carcinoma (BCC) skin tumors and odontogenic jaw cysts before the age of 20 years, palmar/plantar pitting, brain calcifications, various skeletal and soft tissue abnormalities, and a high risk for developing medulloblastoma 1-4. Surgical treatment of multiple BCC tumors, starting in childhood and often numbering into the hundreds over a lifetime, causes extensive scarring that can be highly debilitating both functionally and psychologically 5. New hedgehog pathway inhibitory drugs (e.g. vismodegib) can offer temporary relief, but are not curative because side effects typically limit the time that patients can remain on these medications 6. Therefore, a non-scarring therapeutic alternative for BCC in BCNS patients is greatly needed.
Photodynamic therapy (PDT) is a tumor-selective treatment for cancer 7, 8 that can shrink tumors without leaving a scar 9, 10. A pro-drug, either 5-aminolevulinic acid (ALA) or its methyl ester (MAL) is topically applied, and then protoporphyrin IX that is synthesized from ALA within tumor cell mitochondria is photoactivated by exposure to either blue (400 nm) or red (635 nm) wavelengths of light, thereby killing the cells. Many studies from Europe and Canada have shown that PDT using red light in combination with ALA or MAL can be very effective for superficial BCC or thin nodular BCC that arise either sporadically 11-15 or in BCNS patients 16, 17. Other supportive data were provided by a study from Oseroff’s group in Buffalo, NY, further documenting the utility of red light PDT in BCNS patients 18. Unfortunately for American patients, this evidence has had relatively little impact upon clinical treatment of BCC in the U.S., where PDT is currently only approved for actinic keratoses. The principal reason that PDT is not approved in the U.S. for BCC is because clinical studies with sufficient rigor to convince the U.S. Food and Drug Administration that PDT is safe and efficacious, have not yet been conducted.
Another impediment to the development of PDT as a BCC treatment in America is the fact that blue light sources are much more commonly used than red light in the U.S. at the current time. Only a few studies have examined blue light PDT for the treatment for BCC, most notably a case series by Gilchrest et al. in 2004 which demonstrated that topical 5-ALA and blue light can be effective for treating BCC 19, 20. The goal of our pilot trial was to expand upon those early data of Gilchrest et al. by performing a head-to-head comparison of blue light PDT versus red light PDT. In our comparative design, a red light regimen used in many published studies was employed as a control as we explored less well-characterized responses after blue light PDT. To facilitate the blue/red comparison, our study also incorporated cyclic (biweekly) treatment sessions because these were used in the Gilchrest study and are also routine in European protocols 19, 21. Due to the very large number of tumors to be treated simultaneously at each visit, the initial PDT session in our study was delivered at suboptimal (low) light doses, and then gradually escalated at subsequent visits. This was necessary to avoid potential serious inflammatory side effects and to ensure the safety of our patients.
METHODS
Clinical trial design
The trial was conducted in the outpatient dermatology clinics of the Cleveland Clinic between January and September, 2016. The study scheme is shown in Figure 1. A total of 6 PDT treatments were administered in three biweekly sessions (cycles), spaced one week apart and repeated at two-month intervals. We used the cyclic design as a safety feature, allowing us to observe the inflammatory response after PDT at each light dose before deciding to escalate to a higher dose. For measuring treatment efficacy, it was not practical to confirm tumor clearances histologically by biopsy of every lesion, due to the dozens of BCC tumors per patient. In support of this approach, the vast majority of the BCC encountered in our 3 patients were likely superficial BCC or thin nodular BCC and non-aggressive. Therefore, only tumors that were still clinically detectable at the final study visit were biopsied. Tumor clearance was assessed from high-resolution photographs using a scoring system based upon lesion diameter, surface changes, and erythema (see below).
Fig 1.
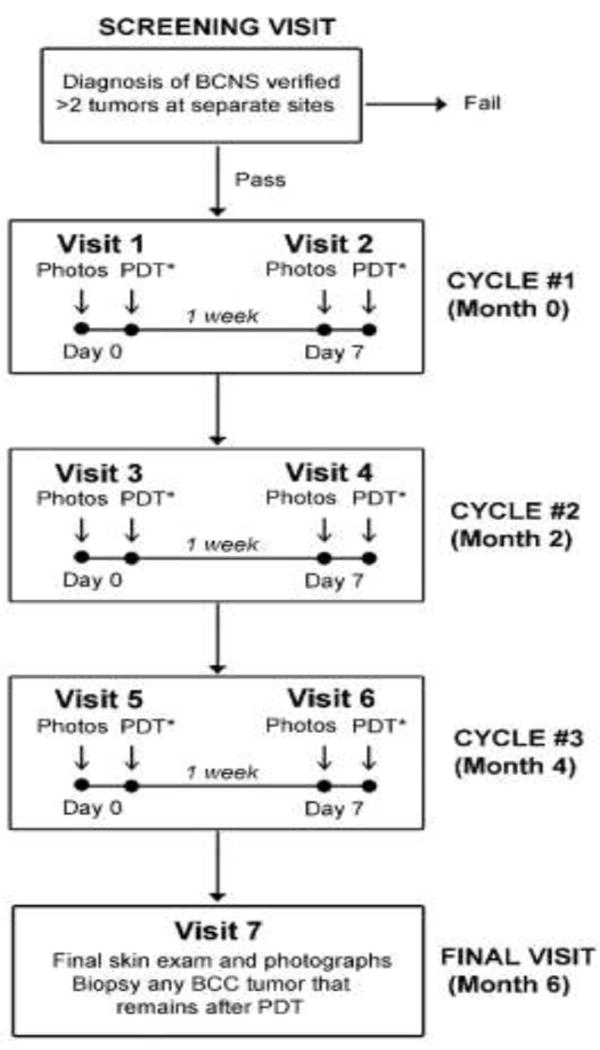
Clinical trial design.
Light sources and light dose escalation
The blue light source used was the Blu-U (fluorescent lamps, 417 nm, DUSA/Sun Pharmaceuticals, power density 10 mW/cm2; 1000 sec to deliver a fluence of 10 J/cm2). The red light source was Aktilite CL128 (LED diode source, Photocure Ltd., 630 nm, power density ~75 mW/cm2, ~8 min to deliver a fluence of 37 J/cm2). Due to safety concerns, i.e. the possibility of an adverse inflammatory reaction when treating >15 tumors per patient, we chose to start with fluences standardly used to treat actinic keratoses (10 J/cm2 for blue light, 37 J/cm2 for red light). This was then escalated by increasing the illumination time by 50% in the second and third cycles. The maximum fluences thus achieved at Visits 5 and 6 were 20 J/cm2 and 75 J/cm2 for blue and red light, respectively.
Study endpoints
The primary endpoint was treatment efficacy, defined as complete tumor clearance (CR). Tolerability of treatments, and overall patient satisfaction, were secondary endpoints.
Patient enrollment
Patients were recruited with assistance from the BCCNS Life Support Network in Burton, Ohio (http://www.gorlinsyndrome.org). Inclusion Criteria: To be eligible, patients had to meet the diagnostic criteria for BCNS; see reference 1 and Table I. Exclusion Criteria: Patients were required to stop BCNS-related medications (e.g., vismodegib) for at least 1 month before the trial.
Table I.
Patient Demographics
| Patient A | Patient B | Patient C | |
|---|---|---|---|
| Age (yr) / Sex | 33 / M | 39 / M | 54 / F |
| BCC tumors, age of onset (yr) | 14 | 15 | 21 |
| Jaw cysts, age of onset (yr) | 12 | >30 | 6 |
| Palmar cysts | Yes | Yes | Yes |
| First degree relative with BCNS | No | Two sons | Father, son |
| Locations of BCC tumors at time of enrollment: | Scalp, face | Scalp, face, neck | Arms, legs, back, face |
| Total BCC tumors at time of enrollment: | 15 | 42 | 86 |
Randomization
All tumors observed at Visit 1 were treated with PDT. These tumors were separated into contralaterally-matched blue or red treatment zones, assigning ~50% of lesions to blue light, ~50% to red light. Sides were chosen by a coin toss, and initial light source assignments were maintained throughout the study.
PDT treatment visits
At each visit, BCC tumors were identified by clinical exam, numbered, and photographed by our professional photographer using studio lighting and a high-resolution digital camera. A millimeter ruler was placed alongside the lesions in every photograph. Each tumor was painted with 20% ALA (Levulan Kerastick™, DUSA/Sun Pharmaceuticals), spotwise, with a margin of 1 cm around the tumor. After 4 h, illumination was administered first to those tumors assigned to receive red light, and then to those assigned to get blue light, using the settings described under “Light sources”. Note that it is not possible to administer two different wavelengths simultaneously because the protective eyewear required for each wavelength are not cross-compatible. During any given illumination session, up to three Blu-U units or three Aktilite units were used simultaneously to cover different body parts (e.g., scalp/face, one arm, and one leg). Pain was controlled with a fan, application of ice-cold wet cloths, and ice-cold ultrasound gel. Standard aftercare included a soothing ointment (equal parts of lidocaine 4%, triamcinolone 0.1%, and aquaphor), sunscreen, and sun avoidance for 48 h 22. Use of antihistamines for itching was allowed. During the week after each PDT treatment, patients recorded their symptoms in a daily questionnaire (log sheet).
Efficacy Assessment
Digital photographic images were assembled in Adobe Photoshop by a medical student (M.I.) and evaluated by an experienced dermatologist (U.K.) on a high-resolution digital monitor (Apple i-Mac with retina display). Each tumor’s location was confirmed by comparison to nearby landmarks such as pigmented nevi or scars. At every visit, tumors were evaluated by measurements of lesion diameter, surface changes, and erythema in the photographs. The three parameters were then multiplied together to create a semiquantitative metric (DSE Composite Score) that was more useful than any individual parameter for describing lesional changes post-PDT. Fig 2 illustrates how the DSE composite score was calculated. More details are provided in Supplementary Methods online.
Fig 2. Determination of DSE composite scores for one illustrative BCC tumor.
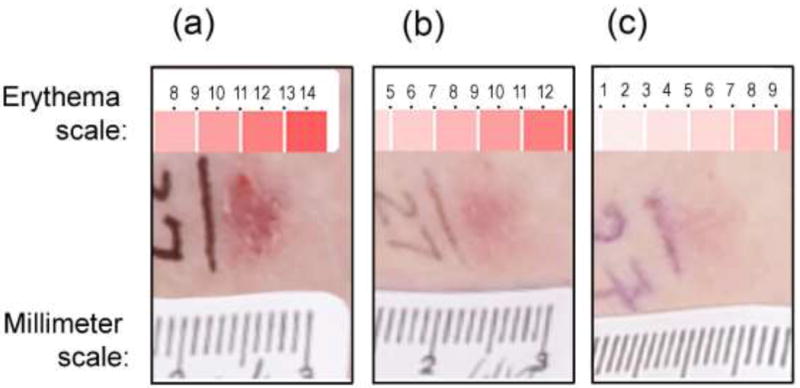
Three parameters were measured from photographs at every visit: Diameter (D), from a millimeter ruler placed on the skin during photography; Erythema (E), matched to a standard color scale; and Surface change (S), set at either 1 (flat) or 2 (scaly/raised). The three parameters were multiplied together to generate the DSE composite score. In this example: (a), At visit 1 the composite score was 121. (b), At visit 4 the composite score was 27. (c), At visit 7 the composite score was 5. For this patient, the DSE threshold for complete clearance was 6.1 (see Supplem Table C), so this particular tumor was rated as completely cleared at visit 7.
In the second step of analysis, two dermatologists (U.K. and E.M.) re-examined every photograph and reached a consensus about each lesion, including whether the BCC was present or absent. DSE scores from all lesions judged to be clinically absent were averaged, and this value (meanca) was used to determine a DSE detection threshold for each patient. This threshold was defined as meanca + 2(SD), a value that appeared to agree very well with lesion clearance as determined by overall clinical impression. For a BCC tumor to be scored as a Complete Response (CR), its DSE score had to fall below the detection threshold, and also had to remain below that threshold at all subsequent study visits. At the last visit, all treated BCC lesions that were still clinically visible were biopsied.
Tolerability Assessment
During PDT treatment, pain was recorded on a 0-to-10 visual-analog scale. In the week following each PDT treatment, patients filled out a score sheet that captured symptoms and signs of inflammation and the healing response.
Patient satisfaction
At the conclusion of the study, patients completed a short questionnaire to rate their opinion of the treatment.
Statistical Analyses
Descriptive statistics were used to summarize the data. A generalized linear mixed-effects model was used to compare the tumor clearance rate between blue and red light PDT (non-inferiority test with a 5% non-inferiority margin).
RESULTS
Demographic and diagnostic features of the subjects are given in Table 1. At Visit 1, a total of 141 BCC tumors were identified. The initial size and DSE composite score for each of these tumors are recorded for each of the patients in Supplementary Tables A-C.
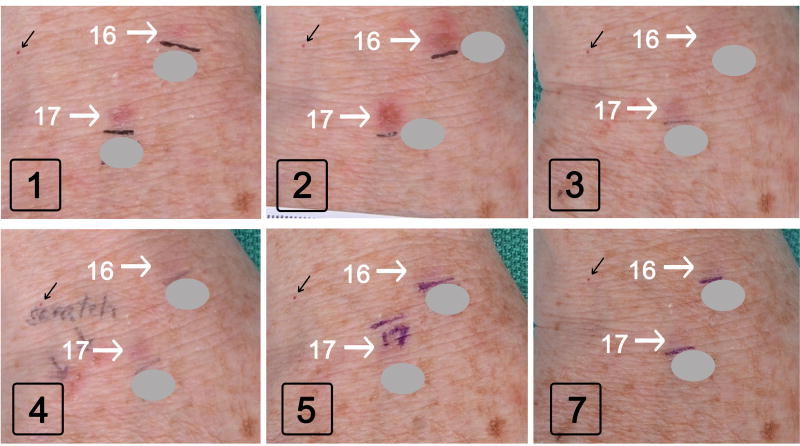
The overall response pattern for BCC tumors after photodynamic treatment was similar following blue or red light PDT. Some examples before and after red light is illustrated in Fig. 3. Compared to baseline (Visit 1), a large increase in erythema and swelling of lesions 14 and 15 was noted at Visit 2, due to inflammation from the PDT treatment given one week prior. At subsequent visits, these tumors shrank and disappeared, whereas lesion 16 persisted and continued to grow (circles in Fig. 3). Biopsy of lesion 16 revealed a nodular BCC.
Fig 3. BCC tumors (on scalp of patient A) after red light PDT sessions.
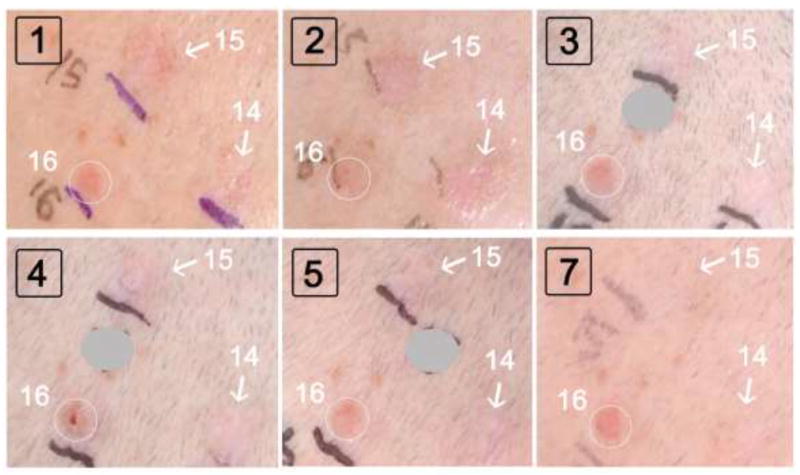
Boxed black numbers indicate the Study Visit. White arrows, lesions that ultimately resolved. White circle, a lesion that did not respond to PDT and was biopsied at visit 7. Gray circles: Numbers drawn on the patient’s skin with a pen, to mark each lesion prior to photography, are masked to reduce clutter and enhance clarity.
The response of two BCC lesions on the dorsal hand during treatment with blue light is illustrated in Fig. 4. Again, a marked inflammatory response was seen at Visit 2. By Visit 4 the lesions were very faint, and by Visit 5 they were completely gone (Visit 7). By contrast, several pigmented moles, lentigines, and a hemangioma in the vicinity did not change after PDT (Fig. 4).
Fig 4. BCC tumors (on dorsal hand of patient C) after blue light PDT sessions.
Black boxed numbers indicate the Study Visit. White arrows, BCC lesions that ultimately resolved. For reference, note the hemangioma (black arrow) and pigmented nevus (lower right corner) that did not respond to PDT.
Using our semiquantitative method of DSE scoring (see Methods), the number of treatments needed to clear each BCC tumor was determined (see Supplementary Tables A-C). These clearance data are summarized in Table 2. Very few tumors cleared after the first treatment cycle (lowest light fluence). After the second cycle (intermediate fluence), between one-third to one-half of the tumors cleared However, nearly all of the tumors cleared after the third cycle at the highest fluence. Interestingly, as seen in Table 2, tumor clearances were very similar for both the blue and red wavelengths of light. A CR rate of >95% was obtained in all patients except one (Patient A after red light, CR = 71%, due to two persistent BCC on the scalp and forehead). Statistical analysis of the data in Table 2 showed that the overall CR for blue light (98%) was non-inferior to red light (93%) at a confidence level of p<0.001, with a 5% non-inferiority margin.
Table 2.
Tumor responses to blue light PDT or red light PDT
| Patient A | Patient B | Patient C | |
|---|---|---|---|
| BLUE | |||
| LIGHT RESPONSES | |||
| Number of tumors before start of treatment: | 8 | 22 | 35 |
| Number (percentage) of original tumors still visible after receiving: | |||
| 2 treatments @ 10 J/cm2 (16 min 30 s) * | 7 (88%) | 22 (100%) | 34 |
| 4 treatments @ 15 J/cm2 (22 min) * | 6 (75%) | 13 (59%) | 24 |
| 6 treatments @ 20 J/cm2 (33 min) * | 0 (0%) | 0 ( 0%) | 1 (3%) |
| Clearance rate after blue light: | 100% | 100% | 97% |
|
| |||
| RED | |||
| LIGHT RESPONSES | |||
| Number of tumors before start of treatment: | 7 | 20 | 49 |
| Number (percentage) of original tumors still visible after receiving: | |||
| 2 treatments @ 37 J/cm2 (~8 min) * | 7 (100%) | 17 (85%) | 44 (90%) |
| 4 treatments @ 50 J/cm2 (~11 min) * | 6 (86%) | 8 (40%) | 39 (80%) |
| 6 treatments @ 75 J/cm2 (~16 min)* | 2 (29%) | 1 ( 5%) | 2 (4%) |
| Clearance rate after red light: | 71% | 95% | 96% |
Comments:
PDT therapy was administered in 3 biweekly treatment cycles, for a total of 6 treatments. Each cycle consisted of two treatments spaced one week apart. Each cycle was spaced 2 months apart. After the first cycle, the light dose for the second cycle was increased by 50%; it was increased another 50% for the third cycle. At each treatment visit, half of the tumors were illuminated at 4 hr after ALA application with blue light; the other half received red light. A final observation was made 2 months after the last PDT treatment. Tumor status was evaluated by photography at every visit (using the DSE scoring method). Because photographic estimation of tumor clearance is most reliable when post-treatment inflammation has subsided, the clearance status of tumors observed at Visit 3, Visit 5, and Visit 7 is recorded here.
Table 3 lists the histological results of skin biopsies performed on all treated lesions still visible at Visit 7. Amongst eight lesions biopsied at the final visit, six were positive for BCC. Interestingly, five of these occurred after red light and only one after blue light.
Table 3.
Histologic diagnoses of PDT-resistant lesions
| Patient-Tumor ID | Location | Histological diagnosis | Comment |
|---|---|---|---|
| BLUE LIGHT TREATED LESIONS: | |||
| C-46 | Leg (posterior calf) | BCC, superficial and nodular | (1) |
| C-11 | Arm | Telangiectasia and hyperkeratosis | (2) |
| C-13 | Forearm | Capillary hemangioma | (2) |
| RED LIGHT TREATED LESIONS: | |||
| A-11 | Scalp | BCC, nodular and micronodular | (1) |
| A-14 | Forehead | BCC, nodular and micronodular | (1) |
| B-26 | Scalp | BCC, nodular | (1) |
| C-25 | Arm (axillary fold) | BCC, micronodular | (1) |
| C-75 | Back | BCC, nodular | (1) |
Comments:
These lesions were raised and clinically suspicious for BCC
These lesions were flat macules with pink erythema
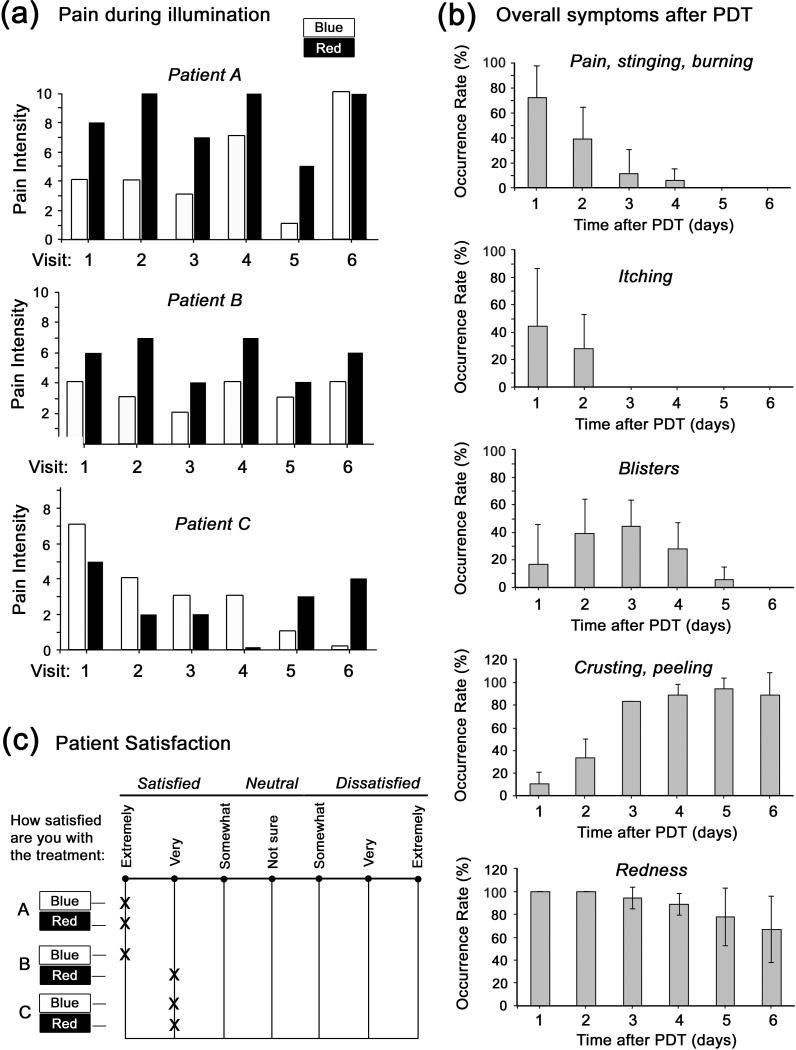
Side effects of the treatments are summarized in Fig. 5. During illumination, patients experienced greater pain with red light than with blue light. In the week following PDT, symptoms due to local skin inflammation (modest pain and itching during the first 2-3 days, occasional blistering at days 1-4, and crusting/peeling at days 3-6) were quite reproducible, and did not differ between blue or red light. At the conclusion of the study, all patients rated both types of treatment (blue and red) very favorably.
Fig 5. Side effects of PDT treatments as reported by the patients.
(a), Pain experienced during illumination, on a 0-10 scale. (b), Symptoms recorded daily during the six days following each PDT treatment. The graphs show the relative frequency of occurrence of each symptom, obtained by recording the simple binary incidence (yes/no) of a given symptom on any given day, averaged over all patients and all treatment visits. (c), Overall satisfaction with the treatment, as reported by each patient (A-C) on a questionnaire depicted in the figure.
DISCUSSION
In this pilot study have shown that PDT with 5-aminolevulinate can be equally effective when blue light (400 nm) or red light (635 nm) are used to treat multiple BCC tumors in BCNS patients. The large number of tumors (141) evaluated, and the bilateral intra-patient study design, suggest some important conclusions. First, PDT with either blue or red light achieved very similar tumor clearance rates (98% CR for blue, 93% CR for red) that were statistically indistinguishable by a test of non-inferiority. Second, the frequency of PDT-resistant tumors was higher in the red light group (5 BCC for red light versus 1 BCC for blue light), supporting the notion that blue light is no less effective than red light. Third, side effect profiles were nearly the same in terms of inflammatory symptoms in the week after treatment. However, patients in the study reported less pain during exposure to blue light as compared to red light (Fig. 5A). One possible reason for this may be that blue light, with a relatively lower fluence rate (energy delivery over a longer duration), may cause less stimulation of PpIX-loaded nerve fibers as compared to red light 23. Results of a recent explorative study showed that daylight PDT (i.e., application of topical MAL followed by 2.5 h of sunlight with an effective mean light dose of 10.8 J/cm2) was completely pain-free during illumination, and >90% of 30 BCC tumors were cleared at 3 months, supporting the notion that low-intensity PDT is less painful while still very effective 24.
During light dose escalation in our study, a blue light dose of 20 J/cm2 appeared to be the most effective since relatively fewer CRs were seen at 10 or 15 J/cm2. Similarly with red light, the majority of CRs occurred after exposure to 75 J/cm2, corresponding to the fluence typically used for non-fractionated illumination in previous studies 15, 25, 26. Fractionated red light delivery has been reported to sometimes provide BCC outcomes exceeding 90% CR after a single PDT session 15, 26, but it was not possible here to add fractionated light as another variable in our study. Instead, blue light and red light were each delivered in a single dose, after the same 4 hr ALA incubation time, as a way to compare the relative efficacy of the two wavelengths.
Relatively few PDT studies have been performed in BCNS patients up to now. In two of those, MAL and 37 J/cm2 of red light were used to treat 12 adults and children with BCNS (103 tumors total) in 3 to 4 treatment sessions 16, 17. Response rates of 79% CR at 1 year 16 and 78% CR at 3 months 17 were reported. Oseroff et al. employed red light and a 20% ALA cream formulation to treat 3 children with BCNS, and achieved 85% to 98% overall clearance after 4 to 7 sessions 18. For those patients, high fluences (150 J/cm2 ) were delivered under general anesthesia 18. In one of the few studies to date using blue light, Gilchrest et al. treated 2 BCNS patients (52 BCC total) with 4 PDT sessions (two biweekly cycles, spaced 2 months apart; 20% ALA Levulan™, 10 J/cm2) to achieve clearance rates of 89% of superficial BCC (sBCC) on the face, 69% of sBCC on the lower extremities, and 31% of nodular BCC (nBCC) on the face at 8 months 19, 20. The 98% CR reported in our study with blue light PDT is almost surely due to our shorter follow-up time (2 months), and would be expected to decline at 8 months. For red light, our results of 93% CR are in general agreement with other published studies in which BCC was treated using 20% ALA and red light, and CRs reported at 2-3 months of follow-up. Examples of such CRs are: (i) 100% (sBCC) and 70% (nBCC) 27; (ii) 92% (nBCC) 28; (iii) 92% (sBCC and nBCC) 29; (iv) 94% (nBCC) 30.
Another important conclusion from our study is that treating large numbers of BCC with PDT is safe. The number of BCC tumors we treated simultaneously per session (e.g., 86 lesions in Patient C) is the highest reported in the literature. When PDT was performed as described here, side effects were well tolerated and patients expressed high satisfaction with the outcome.
Our study has several limitations. First, the final lesion clearance observation was performed 2 months after the last PDT treatment. While many other studies have reported clearance in a 2-3 month time frame (see above), much longer follow-up will be needed for a definitive assessment of treatment durability. Second, our DSE scoring system was based upon clinical and photographic parameters, so that histological proof of the absence of residual tumor nests at each site was lacking. A future study will be needed, requiring much greater time and resources, to establish long-term tumor clearance. Third, light fluences at the beginning of the study were suboptimal (for safety reasons, as described earlier), which meant that many treatments were needed before high clearance rates were observed. Future studies to optimize blue light PDT for BCNS should start with a fluence of 20 J/cm2, shown here to be safe. Using that higher light dose, and considering the results from the earlier Gilchrest study, it seems entirely possible that BCC tumors might clear after only one or two PDT cycles.
Finally, it seems likely that tumor clearance after PDT may involve activation of local and systemic immunological mechanisms 31. If true, then immune activation after PDT with one wavelength may influence tumor responses to the other wavelength, complicating the interpretation of results in our bilateral study. However, despite these caveats, we believe that our data establish the fact that PDT with either blue light or red light is highly effective for treating multiple tumors in BCNS patients. Because it is largely unexplored, blue light PDT should be studied and optimized further to evaluate its potential as an effective nonscarring treatment option for patients with multiple BCC.
Supplementary Material
Supplemental Methods. Analysis of Photographs to Obtain a DSE Composite Tumor Score
Supplemental Fig 1. Lesion Diameters and Composite Scores for Tumors of Patient A.
Supplemental Fig 2. Lesion Diameters and Composite Scores for Tumors of Patient B.
Supplemental Fig 3. Lesion Diameters and Composite Scores for Tumors of Patient C.
HIGHLIGHTS.
Photodynamic therapy (PDT) is a nonscarring approach for basal cell carcinoma (BCC).
Basal cell nevus syndrome (BCNS) patients need a nonscarring alternative to surgery.
In 3 BCNS patients with 141 BCC tumors, blue light PDT was compared to red light PDT.
Tumor clearance rates after blue or red light were >90%, and statistically similar.
Aminolevulinate-PDT with blue or red light represents a promising option for BCNS.
Acknowledgments
We thank Lisa Rittwage, R.N. for study coordination, Janine Sot and Susan Lopez for excellent photography, Christine Warren, M.D. for review of the manuscript, and Prof. Tayyaba Hasan (Wellman Center for Photomedicine, Mass. General Hospital, Boston) for her unflagging support and encouragement. We also thank Stuart Marcus, M.D. Ph.D. a pioneer of dermatological PDT and a great supporter of this project.
FUNDING:
This work was supported by DUSA/Sun Pharmaceuticals [Investigator-Initiated Study, E. Maytin] and by the National Institutes of Health [grant number P01-CA84203, T. Hasan and E. Maytin].
Footnotes
Conflict of interest:The authors declare no conflict of interest.
Ethical Safeguards:
This study was approved by the Protocol Review & Monitoring Committee of the CASE Comprehensive Cancer Center, and the Institutional Review Board of the Cleveland Clinic, and was registered on the National Institutes of Health clinical trial World Wide Web site, www.clinicaltrials.gov (NCT02157623)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bree AF, Shah MR. Consensus statement from the first international colloquium on basal cell nevus syndrome (BCNS) Am J Med Genet A. 2011;155A:2091–7. doi: 10.1002/ajmg.a.34128. [DOI] [PubMed] [Google Scholar]
- 2.Tom WL, Hurley MY, Oliver DS, Shah MR, Bree AF. Features of basal cell carcinomas in basal cell nevus syndrome. Am J Med Genet A. 2011;155A:2098–104. doi: 10.1002/ajmg.a.34127. [DOI] [PubMed] [Google Scholar]
- 3.Kimonis VE, Singh KE, Zhong R, Pastakia B, Digiovanna JJ, Bale SJ. Clinical and radiological features in young individuals with nevoid basal cell carcinoma syndrome. Genet Med. 2013;15:79–83. doi: 10.1038/gim.2012.96. [DOI] [PubMed] [Google Scholar]
- 4.Solis DC, Kwon GP, Ransohoff KJ, Li S, Chahal HS, Ally MS, et al. Risk Factors for Basal Cell Carcinoma Among Patients With Basal Cell Nevus Syndrome: Development of a Basal Cell Nevus Syndrome Patient Registry. JAMA Dermatol. 2017;153:189–92. doi: 10.1001/jamadermatol.2016.4347. [DOI] [PubMed] [Google Scholar]
- 5.Shah M, Mavers M, Bree A, Fosko S, Lents NH. Quality of life and depression assessment in nevoid basal cell carcinoma syndrome. Int J Dermatol. 2011;50:268–76. doi: 10.1111/j.1365-4632.2010.04658.x. [DOI] [PubMed] [Google Scholar]
- 6.Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–8. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacCormack MA. Photodynamic therapy. Adv Dermatol. 2006;22:219–58. doi: 10.1016/j.yadr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 2012;326:8–16. doi: 10.1016/j.canlet.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM. Influence of 5-aminolevulinic acid and red light on collagen metabolism of human dermal fibroblasts. J Invest Dermatol. 2003;120:325–31. doi: 10.1046/j.1523-1747.2003.12037.x. [DOI] [PubMed] [Google Scholar]
- 10.Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM. Keratinocyte-derived cytokines after photodynamic therapy and their paracrine induction of matrix metalloproteinases in fibroblasts. Br J Dermatol. 2004;151:776–83. doi: 10.1111/j.1365-2133.2004.06209.x. [DOI] [PubMed] [Google Scholar]
- 11.Dijkstra AT, Majoie IM, van Dongen JW, van Weelden H, van Vloten WA. Photodynamic therapy with violet light and topical 6-aminolaevulinic acid in the treatment of actinic keratosis, Bowen’s disease and basal cell carcinoma. J Eur Acad Dermatol Venereol. 2001;15:550–4. doi: 10.1046/j.1468-3083.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 12.Karrer S, Szeimies RM, Hohenleutner U, Heine A, Landthaler M. Unilateral localized basaliomatosis: treatment with topical photodynamic therapy after application of 5-aminolevulinic acid. Dermatology. 1995;190:218–22. doi: 10.1159/000246689. [DOI] [PubMed] [Google Scholar]
- 13.Kopera D, Cerroni L, Fink-Puches R, Kerl H. Different treatment modalities for the management of a patient with the nevoid basal cell carcinoma syndrome. J Am Acad Dermatol. 1996;34:937–9. doi: 10.1016/s0190-9622(96)90085-7. [DOI] [PubMed] [Google Scholar]
- 14.Lane JE, Allen JH, Lane TN, Lesher JL., Jr Unilateral Basal cell carcinomas: an unusual entity treated with photodynamic therapy. J Cutan Med Surg. 2005;9:336–40. doi: 10.1007/s10227-005-0118-z. [DOI] [PubMed] [Google Scholar]
- 15.de Vijlder HC, Sterenborg HJ, Neumann HA, Robinson DJ, de Haas ER. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: five-year follow-up of a randomized, prospective trial. Acta Derm Venereol. 2012;92:641–7. doi: 10.2340/00015555-1448. [DOI] [PubMed] [Google Scholar]
- 16.Mougel F, Debarbieux S, Ronger-Savle S, Dalle S, Thomas L. Methylaminolaevulinate photodynamic therapy in patients with multiple basal cell carcinomas in the setting of Gorlin-Goltz syndrome or after radiotherapy. Dermatology. 2009;219:138–42. doi: 10.1159/000228316. [DOI] [PubMed] [Google Scholar]
- 17.Girard C, Debu A, Bessis D, Blatiere V, Dereure O, Guillot B. Treatment of Gorlin syndrome (nevoid basal cell carcinoma syndrome) with methylaminolevulinate photodynamic therapy in seven patients, including two children: interest of tumescent anesthesia for pain control in children. J Eur Acad Dermatol Venereol. 2013;27:e171–5. doi: 10.1111/j.1468-3083.2012.04538.x. [DOI] [PubMed] [Google Scholar]
- 18.Oseroff AR, Shieh S, Frawley NP, Cheney R, Blumenson LE, Pivnick EK, et al. Treatment of diffuse basal cell carcinomas and basaloid follicular hamartomas in nevoid basal cell carcinoma syndrome by wide-area 5-aminolevulinic acid photodynamic therapy. Arch Dermatol. 2005;141:60–7. doi: 10.1001/archderm.141.1.60. [DOI] [PubMed] [Google Scholar]
- 19.Itkin A, Gilchrest BA. delta-Aminolevulinic acid and blue light photodynamic therapy for treatment of multiple basal cell carcinomas in two patients with nevoid basal cell carcinoma syndrome. Dermatol Surg. 2004;30:1054–61. doi: 10.1111/j.1524-4725.2004.30317.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilchrest BA, Brightman LA, Thiele JJ, Wasserman DI. Photodynamic therapy for patients with Basal cell nevus syndrome. Dermatol Surg. 2009;35:1576–81. doi: 10.1111/j.1524-4725.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 21.Haller JC, Cairnduff F, Slack G, Schofield J, Whitehurst C, Tunstall R, et al. Routine double treatments of superficial basal cell carcinomas using aminolaevulinic acid-based photodynamic therapy. Br J Dermatol. 2000;143:1270–5. doi: 10.1046/j.1365-2133.2000.04000.x. [DOI] [PubMed] [Google Scholar]
- 22.Warren CB, Karai LJ, Vidimos A, Maytin EV. Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J Am Acad Dermatol. 2009;61:1033–43. doi: 10.1016/j.jaad.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babes A, Sauer SK, Moparthi L, Kichko TI, Neacsu C, Namer B, et al. Photosensitization in Porphyrias and Photodynamic Therapy Involves TRPA1 and TRPV1. J Neurosci. 2016;36:5264–78. doi: 10.1523/JNEUROSCI.4268-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiegell SR, Skodt V, Wulf HC. Daylight-mediated photodynamic therapy of basal cell carcinomas - an explorative study. J Eur Acad Dermatol Venereol. 2014;28:169–75. doi: 10.1111/jdv.12076. [DOI] [PubMed] [Google Scholar]
- 25.Pariser DM, Lowe NJ, Stewart DM, Jarratt MT, Lucky AW, Pariser RJ, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227–32. doi: 10.1067/mjd.2003.49. [DOI] [PubMed] [Google Scholar]
- 26.de Haas ER, Kruijt B, Sterenborg HJ, Martino Neumann HA, Robinson DJ. Fractionated illumination significantly improves the response of superficial basal cell carcinoma to aminolevulinic acid photodynamic therapy. J Invest Dermatol. 2006;126:2679–86. doi: 10.1038/sj.jid.5700460. [DOI] [PubMed] [Google Scholar]
- 27.Morton CA, MacKie RM, Whitehurst C, Moore JV, McColl JH. Photodynamic therapy for basal cell carcinoma: effect of tumor thickness and duration of photosensitizer application on response. Arch Dermatol. 1998;134:248–9. doi: 10.1001/archderm.134.2.248. [DOI] [PubMed] [Google Scholar]
- 28.Thissen MR, Schroeter CA, Neumann HA. Photodynamic therapy with delta-aminolaevulinic acid for nodular basal cell carcinomas using a prior debulking technique. Br J Dermatol. 2000;142:338–9. doi: 10.1046/j.1365-2133.2000.03404.x. [DOI] [PubMed] [Google Scholar]
- 29.Christensen E, Skogvoll E, Viset T, Warloe T, Sundstrom S. Photodynamic therapy with 5-aminolaevulinic acid, dimethylsulfoxide and curettage in basal cell carcinoma: a 6-year clinical and histological follow-up. J Eur Acad Dermatol Venereol. 2009;23:58–66. doi: 10.1111/j.1468-3083.2008.02946.x. [DOI] [PubMed] [Google Scholar]
- 30.Mosterd K, Thissen MR, Nelemans P, Kelleners-Smeets NW, Janssen RL, Broekhof KG, et al. Fractionated 5-aminolaevulinic acid-photodynamic therapy vs. surgical excision in the treatment of nodular basal cell carcinoma: results of a randomized controlled trial. Br J Dermatol. 2008;159:864–70. doi: 10.1111/j.1365-2133.2008.08787.x. [DOI] [PubMed] [Google Scholar]
- 31.Kabingu E, Oseroff AR, Wilding GE, Gollnick SO. Enhanced systemic immune reactivity to a Basal cell carcinoma associated antigen following photodynamic therapy. Clin Cancer Res. 2009;15:4460–6. doi: 10.1158/1078-0432.CCR-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods. Analysis of Photographs to Obtain a DSE Composite Tumor Score
Supplemental Fig 1. Lesion Diameters and Composite Scores for Tumors of Patient A.
Supplemental Fig 2. Lesion Diameters and Composite Scores for Tumors of Patient B.
Supplemental Fig 3. Lesion Diameters and Composite Scores for Tumors of Patient C.