Abstract
Social anxiety disorder (SAD) is characterized by exaggerated reactivity to social threat, often documented by biased attention to threatening information, and increased activation in brain regions involved in salience/threat processing. Attention training has been developed to ameliorate the attention bias documented in individuals with SAD, with mixed results. We investigated patterns of brain activation underlying acute attention modulation in 41 participants (29 with SAD and 12 health controls). We then investigated how brain activation changed over time in both groups in response to a 4-session attention training protocol (toward threat, away from threat, no-training control). Results revealed diminished pre-training deactivation in the insula in SAD participants during attention modulation. SAD participants also demonstrated an increase in insula deactivation over time, suggestive of an improvement in attention modulation of emotion, and this was associated with a decrease in symptom severity. Attention training did not, itself, lead to clinical improvement, though there was a trend level effect of training toward threat on increased insula deactivation over time. While deficits in attentional control and emotion modulation are documented in individuals with SAD, current attention training protocols are not robustly effective in ameliorating aberrant functioning. Pursuit of training protocols that have more robust impacts on the relevant neural circuitry may have some value.
Keywords: social anxiety, cognition, computer/internet technology, functional MRI, treatment
1. Introduction
Social Anxiety Disorder (SAD) is associated with enhanced threat processing, reflected in enhanced brain activation in regions associated with threat processing/salience (e.g. insula, amygdala, ACC) in response to social information (e.g. facial expressions), compared to controls (Klumpp, Angstadt, & Phan, 2012; Klumpp, Post, Angstadt, Fitzgerald, & Phan, 2013; Phan, Fitzgerald, Nathan, & Tancer, 2006; Stein, Simmons, Feinstein, & Paulus, 2007; Straube, Mentzel, & Miltner, 2005). People with more severe SAD symptoms show greater activation in salience processing regions (Carré et al., 2014; Phan et al., 2006). This exaggerated threat processing could be due to deficits in attention modulation (Bogels & Mansell, 2004). Relative to healthy control participants, some SAD patients and people with subclinical social anxiety attend more to socially negative than neutral information (Amir, Elias, Klumpp, & Przeworski, 2003; Amir, Freshman, & Foa, 2002; Andersson, Westöö, Johansson, & Carlbring, 2006; Becker, Rinck, Margraf, & Roth, 2001; Lazarov, Abend, & Bar-Haim, 2016; Mogg & Bradley, 2002; Mogg, Philippot, & Bradley, 2004; Mueller et al., 2009; Musa, Lepine, Clark, Mansell, & Ehlers, 2003). People with more severe SAD symptoms have a stronger bias toward threat (Bantin, Stevens, Gerlach, & Hermann, 2016) and interpret ambiguous information as negative (Jusyte & Schönenberg, 2014; Peschard & Philippot, 2017).
Attention training was developed on the basis of the well-documented social threat processing bias in individuals with SAD, to train attention away from social threat in an effort to reduce SAD symptoms (Amir, Beard, Taylor, Klumpp, & Jason, 2009; Amir, Weber, Beard, Bomyea, & Taylor, 2008; Heeren, Lievens, & Philippot, 2011; Schmidt, Richey, Buckner, & Timpano, 2009). Attention training has produced reductions in self-reported anxiety symptoms (Amir et al., 2009; Lazarov, Marom, Yahalom, Pine, & Hermesh, 2017; Naim, Kivity, Bar-Haim, & Huppert, 2018; Schmidt et al., 2009), anxious behaviors (Amir et al., 2008; Heeren et al., 2011), and physiological reactivity (Heeren, Reese, McNally, & Philippot, 2012) in response to social threat. Attention training is also associated with reduced activation in brain regions involved in salience processing (e.g. amygdala, insula) and increased activation in emotion modulatory regions (e.g. dorsolateral prefrontal cortex (dlPFC), ventromedial prefrontal cortex (vmPFC), and ventrolateral prefrontal cortex (vlPFC; Taylor et al., 2013)). While most studies train attention away from threat, some have trained in both directions. Findings are mixed - one study reported improvements associated only with training away from threat (Heeren et al., 2012) but others reported improvement regardless of training direction (Heeren, Mogoaşe, McNally, Schmitz, & Philippot, 2015; Klumpp & Amir, 2010).
Despite considerable early promise, there have now been a number of studies showing no specific benefits from attention training, relative to control conditions (Carleton et al., 2015; Carleton, Teale Sapach, Oriet, & LeBouthillier, 2017; Clerkin, Magee, Wells, Beard, & Barnett, 2016; Fitzgerald, Rawdon, & Dooley, 2016; Heeren, Coussement, & McNally, 2016; Heeren, Mogoaşe, et al., 2015; Pergamin-Hight, Pine, Fox, & Bar-Haim, 2016; Yao, Yu, Qian, & Li, 2015). Some of these studies do report improvements in general cognitive processes, like attention and working memory (Heeren et al., 2016; Heeren, Mogoaşe, et al., 2015), and most reported reductions in observed and/or self-reported anxiety symptoms over time (Carleton et al., 2015, 2017; Clerkin et al., 2016; Heeren et al., 2016; Heeren, Mogoaşe, et al., 2015; Pergamin-Hight et al., 2016; Yao et al., 2015). However, when reported, symptom improvements were not significantly different between active training and control paradigms, suggesting that practice effects may have been driving these changes. Recent meta-analyses suggest that attention training benefits are not robust and may be mediated by many variables (Heeren, Mogoașe, Philippot, & McNally, 2015), further suggesting that improvements may be due to non-training factors, like exposure or practice effects (e.g. viewing affective and threatening faces; Carleton et al., 2017).
In light of mixed reports, we sought to identify neural mechanisms underlying attention modulation and identify training-related alterations in relevant neural circuitry that should facilitate development of more targeted and effective training protocols. We sought to (1) replicate evidence that SAD patients have hyperactivation in salience processing regions and/or deficits in emotion modulation compared to HC participants, using an acute attention manipulation task during MRI scanning; (2) confirm that greater salience-related activation and/or diminished attention modulation-related activation is associated with more severe symptoms; and (3) determine whether a computerized attention training protocol can alter either symptoms or aberrant brain activity. To avoid a theory-based bias, we tested both training towards and away from threat against a no-training control condition.
2. Materials and Methods
2.1 Participants
We enrolled forty-two adults (30 with Social Anxiety Disorder (SAD) and 12 Healthy Controls (HC)), ranging in age from 18 to 55 years (MSAD= 25.6, SDSAD = 8.38; MHC= 27.2, SDHC = 6.62). Participants were recruited via internet and paper flyer advertisements throughout the community. We specifically targeted outpatient clinics in the surrounding area, as well as clinicians who provide treatment for SAD. Flyers were hung in clinic waiting rooms, area businesses, and in campus buildings to solicit both SAD and HC participants. We also advertised our study on an online recruitment system, which connects volunteers from the surrounding community to research projects. The SAD group consisted of 25 females and 5 males (67% Caucasian, 3% Black/African American, 6% Asian, 10% Hispanic, 6% other, and 6% not specified). The HC group consisted of 6 females and 6 males (58% Caucasian and 42% not specified). Following the initial MRI scan, one SAD participant was excluded due to a brain abnormality, yielding a final sample of 41 (29 SAD, 12 HC).
Exclusion criteria included significant medical or neurological conditions, left handedness, contraindications to MRI, lifetime history of bipolar I or a psychotic disorder, substance use disorder (past 12 months), non-English speaking, or inability to provide informed consent. SAD participants met criteria for a current primary diagnosis of SAD (currently most severe/causing most distress) based on the Mini International Neuropsychiatric Interview (MINI). The MINI was developed as a brief, but accurate screen for mental health disorders and has been shown to have good reliability and validity in relation to the Structured Clinical Interview for DSM (SCID; Sheehan et al., 1998). The MINI was administered by a clinical psychologist, making diagnoses based on DSM-IV diagnostic criteria (American Psychiatric Association, 2000). Because we did not have separate reliability data verifying our MINI diagnoses against the full SCID, we also utilized the self-report version of the Liebowitz Social Anxiety Scale (LSAS (Liebowitz, 1987)), and required that SAD participants score above an established diagnostic cut-off score of 30 on this measure (Rytwinski et al., 2009). Six participants in the SAD group also screened positive for comorbid diagnoses (2 GAD, 2 OCD, 2 MDD) based on MINI assessment. Fourteen additional participants screened positive for a past psychiatric condition (11 MDD, 2 panic, 1 anorexia). HC participants did not meet current or past criteria for any DSM-IV mental health disorder on the MINI. SAD participants could be on a stable dose (at least 3 months) of an SSRI, but could not be taking any other medications (for psychiatric or medical conditions) or be in active psychotherapy. All inclusion and exclusion decisions were made by a licensed psychologist actively practicing in the area of anxiety disorder assessment and treatment. Participants signed written informed consent and those eligible after evaluation were scheduled for 6 additional visits - a pre-training fMRI scan, four 40-minute attention training sessions, and a post-training fMRI scan.
2.2 Procedures
All procedures were approved by Institutional Review Boards at the University of Michigan and the VA Ann Arbor Healthcare System. The pre-training MRI scan took place one to four weeks following the diagnostic evaluation using the Shifted Attention Emotion Appraisal Task (SEAT (Anderson, Christoff, Panitz, De Rosa, & Gabrieli, 2003; Klumpp et al., 2011)). The SEAT (well established in our laboratory) probes implicit emotional processing, emotion modulation by attention shifting, and emotion modulation by appraisal (Liberzon et al., 2015; Sripada et al., 2013; Wang et al., 2016). The SEAT was introduced to participants outside the scanner prior to the experimental run. The task presents compound images of neutral, threat, and happy faces superimposed on indoor and outdoor scenes (Figure 1). Prior to each image, a cue instructs participants how to respond to the image. Cues include “Male/Female” (identify the gender of the face), to probe implicit emotional processing; “Indoor/Outdoor” (identify whether the scene is indoor or outdoor), to probe attention modulation of emotion; and “Like/Dislike” (tell us whether you like or dislike the face), to probe emotional appraisal. Neutral faces alone and places alone trials are also presented throughout the task. The cue “Face/Place” (identify whether the image is a face or a place) controls for brain activation associated with simply viewing faces and scenes. Trials are randomly presented in an event-related design, with each trial type (response instructions, face expression, type of scene, gender) occurring an equal number of times across three 6-minute runs, totaling 18 mins. Additional attention tasks and a resting state scan were also completed (to be reported elsewhere).
Figure 1.
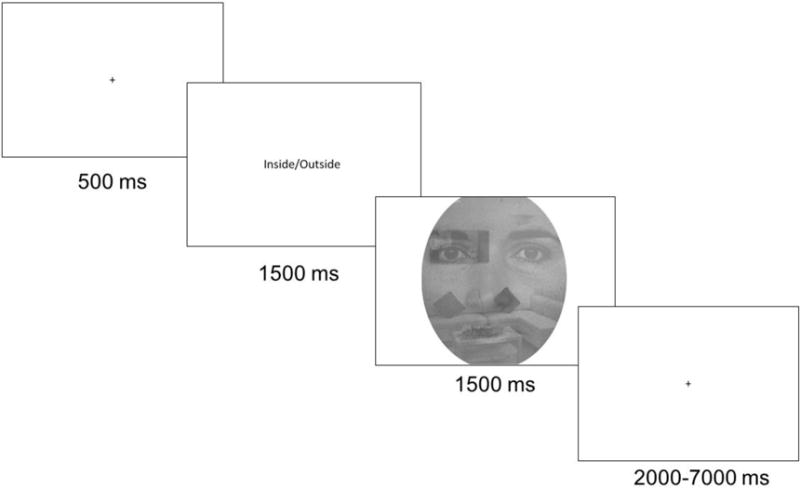
Example trial on the Shifted Attention Emotion Appraisal Task (SEAT).
Scanning was conducted in a Philips 3-T Achieva X-series MRI (Philips Medical Systems) with a SENSE 8ch head coil. T1-weighted anatomic images were acquired with a 3D FFE-TFE sequence (field of view (FOV) = 256 × 256 mm, slice thickness = 1 mm, 0 mm gap). Axial slices aligned with the AC-PC plane were used for slice localization, transformation, and co-registration. Functional scans consisted of gradient echo blood oxygen level dependent (BOLD) scans of contiguous axial slices (repetition time/echo time (TR/TE) = 2000/25ms, flip angle = 90°, FOV = 220×220mm, slice thickness = 2.8mm, 42 data points).
Following initial scans, participants completed four 40-minute computerized attention training tasks in the laboratory, outside the scanner (over 1-6 weeks). Training utilized a modified dot-probe task (MacLeod et al., 1986) similar to protocols used in prior studies (Heeren et al., 2012). Our stimuli consisted of happy and threat faces from the Pictures of Facial Affect (Ekman & Friesen, 1976), an extensively validated set of emotional expressions. We chose happy rather than neutral faces, in contrast to some attention training studies (Amir et al., 2009, 2008; Heeren et al., 2011; Schmidt et al., 2009), because we wanted to replicate Heeren et al. (2012) and to enhance the perceived contrast between face types for SAD participants. Because people with SAD tend to perceive ambiguous or neutral information as negative (Peschard & Philippot, 2017), a neutral face may not be a good non-threat target when trying to train them to attend away from threat cues.
Our attention training protocol began with a centered fixation cross for 500 ms, followed by two affective faces (one positive, one threatening), presented on the left and right side of the screen (random orientation on each trial) for 500 ms. Immediately following disappearance of the faces, a probe (arrow) randomly replaced one of the faces for 1100 ms. Participant were instructed to indicate the direction of the arrow (up or down) with a button press. Each training session had 744 trials with an intertrial interval (ITI) of 1500 ms. SAD participants were assigned to one of three training conditions (away from threat, toward threat, and no-training control) based on a predetermined alternating assignment order, while HC participants were all trained away from threat. SAD participants did not differ in age (F(2,26) < 1, p = .39), pre-training LSAS score (F(2,26) < 1, p = .45), gender distribution (X2 = 2.02, p = .36), or race/ethnicity distribution (X2 = 9.91, p = .27) across the three training groups. The arrow replaced the type of face to be trained towards (threat or positive) on 80% of trials and the other face on the other 20%. In the no-training control condition, the arrow appeared in the center of the screen, and the face pairs were of the same emotion on each trial (two threat or two positive faces) in an attempt to prevent general training of attentional control/shifting. This was a single blind design. Experimenters were aware of training group assignments. Participants were not aware that there were multiple versions of training and were not told that their attention was being directed in any particular way. After attention training, participants repeated the LSAS and a second MRI scan with the SEAT.
2.3 Data Scoring and Analysis
MRI data were analyzed using Statistical Parametric Mapping (SPM8) for MATLAB. Functional images were slice-time corrected with sinc interpolation, realigned and co-registered to the structural images, normalized to the Montreal Neurological Institute (MNI) standard brain, and smoothed with a 6 mm kernel. Runs with more than 3mm of motion in any of the 6 planes (x, y, z, pitch, roll, yaw) were excluded from further analysis. Excessive motion resulted in the exclusion of seven runs of the SEAT across five participants: two from the SAD group lost one run each from pre-training scan, and three (one SAD, two HC) lost four total runs from the post-training scan. All motion parameters and their derivatives were entered into the subject-level analysis as nuisance regressors.
We utilized the SEAT to assess neural activation underlying implicit emotional processing (Male/Female > Face/Place contrast), attention modulation of emotion (Indoor/Outdoor > Male/Female contrast), and emotion modulation by appraisal (Like/Dislike > Male/Female contrast). For all participants, we identified activation in hypothesized regions of interest involved in emotion processing and modulation (insula, amygdala, ACC, dlPFC) for each of the task contrasts. Regions of interest were defined as 3mm-radii spheres centered at the activation peaks that resulted from the three task contrasts across all participants at p < .05 FWE corrected at the whole brain cluster level (after thresholding at p < 0.001 uncorrected). We then extracted average beta weights from each region and submitted them to a series of analyses to examine differences between SAD and HC participants at pre-training (independent sample t-tests), time by group interactions (mixed RM-ANOVA, time within, and group between participants), and time by training interactions in the SAD group (mixed RM-ANOVA, time within, and training between participants). Brain activation and symptom severity were our outcome variables. Correlation analyses examined relationships between neural activation and symptoms.
3. Results
3.1 Psychiatric Symptoms
As expected, participants in the SAD group scored higher on the LSAS (M= 70.66) than HC participants (M= 6.5; t(38.68) = 18.08, p < .001). Age, gender, and race/ethnic distribution did not differ between groups (ps > .28). Participants in the SAD and HC groups were first compared on changes in social anxiety symptoms (LSAS scores) from pre- to post-training (2 × 2 Time by Group ANOVA). We then examined impact of training condition (toward, away, control) over time, within the SAD group (2 × 3 Time by Training ANOVA). Participants in the SAD and HC groups differed in symptom change over time (Time by Group interaction; F(1, 39) = 5.46, p = .025), due to a slight rise in LSAS scores in HC participants (Mtime1= 6.50, Mtime2= 13.00, p= .002); and a small but non-significant decrease in symptoms over time in SAD participants (Mtime1= 70.66, Mtime2= 66.38, p= .148). There was no main effect of time, and no impact of training on symptoms in SAD participants (ps > .16).
3.2 fMRI Findings
3.2.1 Implicit Emotional Processing
Activation patterns on the SEAT replicate previous findings (male/female > face/place contrast (Liberzon et al., 2015; Sripada et al., 2013; Wang et al., 2016)), with activation in regions associated with emotion processing (amygdala, ACC, and insula; Figure 2A). There were no differences in activation between SAD and HC groups (p’s > .10). There was a main effect of time (pre- to post-training) in the ACC, with activation increasing over time in all participants (F(1, 39)= 4.16, p = .048). There were no differences in activation over time between SAD and HC groups (Time by Group interaction p = .31), and no impact of training on brain function in SAD participants (main effect of Training and Time by Training interaction ps > .25).
Figure 2.
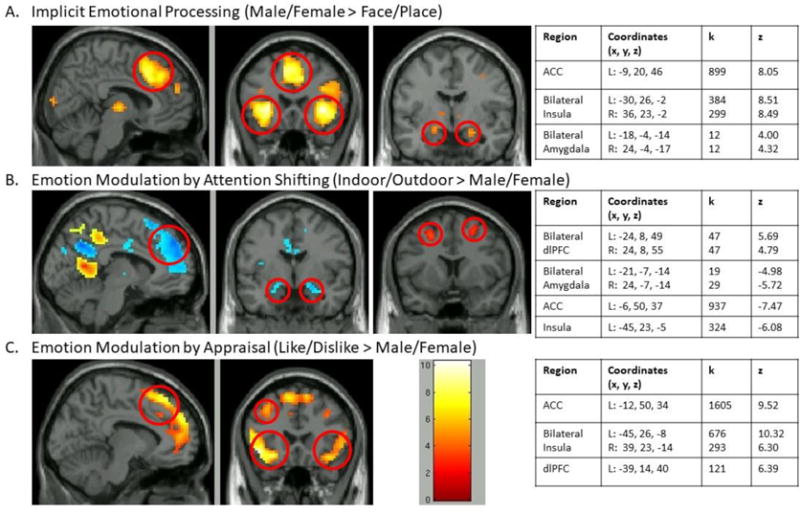
Activation on SEAT across all participants for each of the contrasts of interest, including implicit emotional processing (Panel A), emotion modulation by attention shifting (Panel B), and emotion modulation by appraisal (Panel C). Peak voxels of activation were identified in hypothesized regions of interest. ACC = Anterior Cingulate Cortex, dlPFC = Dorsolateral Prefrontal Cortex. p < .001 uncorrected).
3.2.2 Emotion Modulation by Attention Shifting
SEAT activation patterns replicated previous findings (in/out > male/female contrast (Liberzon et al., 2015; Sripada et al., 2013; Wang et al., 2016)), showing activation in regions associated with attention modulation (dlPFC), and expected deactivation in regions associated with emotional reactivity (amygdala, ACC, and insula; Figure 2B). Prior to training, activation in emotional processing and regulation regions during attention shifting revealed a difference between SAD and HC groups only in the insula. SAD participants showed less deactivation of insula (M = −.35) compared to HC participants (M= −.79; t(39) = 2.78, p = .008; Figure 3A).
Figure 3.
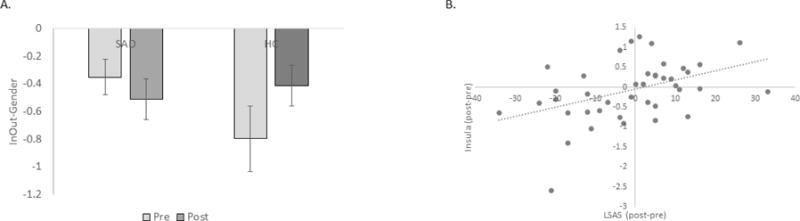
Panel A shows insula activation during emotion modulation by attention shifting (Indoor/Outdoor > Male/Female contrast) in SAD participants and HC participants at pre- and post-training time points. Extracted beta weights are plotted on the y-axis. Panel B shows the relationship between change in insula activation during emotion modulation by attention shifting and change in LSAS scores, across all participants. Error bars indicate standard error of the mean.
Examining changes over time (pre- to post-training) during emotion modulation by attention shifting (2 × 2 Time by Group ANOVA), revealed no main effects of Time or Group (ps > .20), but there was a time by group interaction in the left insula, F(1, 39)= 4.72, p = .036. Relative to HC participants, SAD participants had more deactivation in insula over time (Figure 3B). Participants with greater deactivation in the insula from pre- to post-training also showed greater decreases in symptoms over time. This relationship was present across all participants (r = .43, p = .005) and in the SAD group only (r =.45, p = .021; Figure 3B) and was paralleled by a Time by Group interaction on reaction time (RT), F(1, 38) = 5.27, p = .027. While RT remained stable in the HC group (t < 1, p = .70), SAD participants got faster on attention shifting trials over time (t(27) = 3.84, p = .001).
Finally, we examined the impact of training (toward, away, control) on brain function during attention modulation within SAD participants (2 × 3 Time by Training ANOVA). Training toward threat appeared to deactivate insula more over time (changetoward = −.21) compared to the other two groups (changeaway −.10, changecontrol = −.18; Figure 4), with marginal significance, F(2, 26)= 3.2, p = .057, but pairwise comparisons were not significant (ps > .12).
Figure 4.
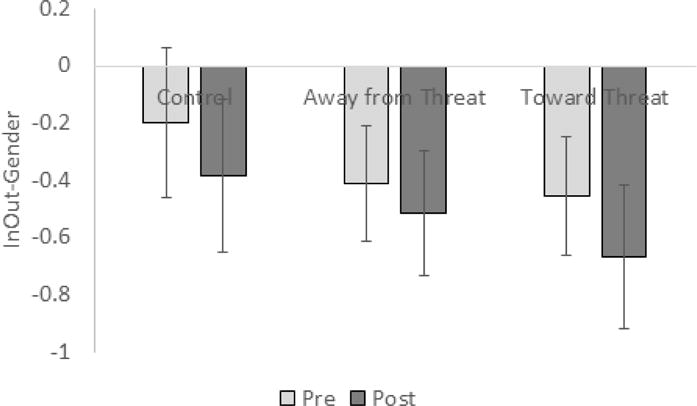
Insula activation during emotion modulation by attention shifting trials (Indoor/Outdoor > Male/Female contrast) in SAD participants pre- and post-training for each training condition (control, away from threat, toward threat). Extracted beta weights are plotted on the y-axis. Error bars indicate standard error of the mean.
3.2.3 Emotion Modulation by Appraisal
Results replicated findings from other samples (like/dislike > male/female (Liberzon et al., 2015; Sripada et al., 2013; Wang et al., 2016)), showing activation in emotion processing and regulatory regions (ACC, bilateral insula/vlPFC, and left dlPFC; Figure 2C).
There were no differences in brain function between SAD and HC participants during appraisal (ps > .09). However, there was a main effect of time in the right insula, with activation decreasing over time in all participants (F(1, 39)= 4.78, p = .035), and a Time by Group interaction in the left insula/vlPFC (F(1, 39)= 463, p = .038). Relative to HC participants, the insula became more deactivated during appraisal over time in SAD participants. This change in activation was significantly correlated with symptom change over time, but only in the HC group (r= .66, p= .02). HC participants with greater deactivation in this region over time reported greater decreases in symptoms over time. In these appraisal analyses, there was no impact of training on brain function in SAD participants (main effect of Training and Time × Training interaction; ps > .23).
4. Discussion
This study examined differences in brain function between SAD and HC participants, identified relationships between brain function and SAD symptoms, and examined whether computerized attention training can alter either symptoms or brain activity. We assessed brain function using an emotion modulation task (SEAT) that uniquely and simultaneously probes neural correlates of emotion processing and emotion modulation (Liberzon et al., 2015; Sripada et al., 2013; Wang et al., 2016). Our data confirmed that this task was an effective tool for probing both emotion processing and modulation.
Our primary finding suggests that insula activation during instructed attention shifting, from an emotional to non-emotional stimulus, has relevance to symptoms and behavior. Specifically, when instructed to shift attention away from threat, SAD participants showed less deactivation in the insula than HC participants, suggesting abnormal “intrusion” of salience processing, consistent with previous literature documenting hyperactivation in the insula in people with SAD (Klumpp et al., 2012, 2013). Additionally, SAD participants showed greater deactivation in insula, relative to the HC group, from pre- to post-training, and the amount of deactivation was linked to improvement in SAD symptoms and enhanced capacity to efficiently shift attention (suggested by reduced reaction time). Our data suggest that difficulty down regulating salience processing regions during tasks requiring attention shifting may mechanistically contribute to SAD symptoms. These findings extend prior literature documenting aberrant attentional processes in response to social threat in people with SAD (Bogels & Mansell, 2004) by showing that individuals with SAD have reduced capacity for emotion modulation in the context of an instructed attention shifting task.
We did not observe expected group differences in activation within attention modulation regions (e.g. dlPFC). This could suggest that activation deficits in modulatory regions is less relevant to symptom expression. SAD participants can perhaps activate control regions normally, but not sufficiently to overcome intensified salience detection. In addition, no group differences were observed during implicit emotional processing. This result contradicts previous findings that salience processing regions are hyperactive in SAD compared to HC participants during affective face processing (Duval, Javanbakht, & Liberzon, 2015; Freitas-Ferrari et al., 2010). Ongoing investigations are needed to further explore these mechanisms.
We were not able to detect any impact of attention training on SAD symptoms, relative to a control condition, though there was a trend that training toward threat resulted in insula deactivation during attention modulation. The lack of impact on symptoms (related to control) adds to a growing body of work reporting no differences between active attention training and control conditions (Carleton et al., 2015, 2017; Clerkin et al., 2016; Heeren et al., 2016; Heeren, Mogoaşe, et al., 2015; Pergamin-Hight et al., 2016; Yao et al., 2015). While our findings, along with prior studies, find reductions in symptoms following attention training, the lack of training-specific effects questions the utility of attention training in its current form. It is possible that directionally manipulating attention modulation with dot-probe paradigms to correct a presumed attentional bias, is not salient to symptom outcomes. It is also possible that the control conditions used in attention training paradigms are producing therapeutic effects through exposure to affective stimuli, or training general attention or cognitive flexibility (Carleton et al., 2017; Clerkin et al., 2016; Pergamin-Hight et al., 2016). Notably, even when studies implemented rigorous control conditions (e.g. no contingency dot probe training, non-emotional probes, lack of probes; Heeren et al., 2016; Yao et al., 2015), symptom reductions did not differ as a function of training. Lack of difference between active and control conditions may be due to the fact that an element of attentional control is still required to effectively complete the task, further suggesting that the use of any task involving attention processing may effectively reduce symptoms. Additional control conditions should be developed and implemented to more effectively examine mechanisms underlying symptom change. In addition, individual differences in the utility of attention training, or the directionality of training needed, may make overall group effects difficult to detect (Pergamin-Hight et al., 2016). Recent preliminary evidence suggests that more dynamic training protocols can reduce SAD symptoms (Amir, Kuckertz, & Strege, 2016). Clearly, more work is needed to determine whether attention training is effective and how to best optimize it. The potential value of ongoing work in this area is highlighted by our findings that attention shifting capacity “improved” over time in patients (reduced insula activation during attention shifting) and that this shift was linked to symptom reductions. These findings raise the possibility that we could develop training protocols that better target attentional control success – marked by reduced insula activation during attention shifting–to develop more clinically effective training.
There are a number of limitations to consider when interpreting our findings. Our sample size was small, which may have led to low power to detect significant group differences. However, post-hoc power analyses (conducted in G*Power v. 3.1.9.2), based on measures of observed effect size and an alpha level of .05, suggested that we had 88.6% power to detect significant group by time interactions, and 88.3% power to detect training by time interactions. The single-blind design of our study does raise the concern that experimenter awareness of the training conditions could have produced measurement bias. Double blind paradigms should be used in the future to rule out this possibility. Our training protocol may not have been fully optimized based on frequency, duration, environment, and match with participant characteristics (Heeren, Mogoașe, et al., 2015; Price et al., 2017). While our study included a control training condition, we did not have a group of participants randomized to receive no training. A no training condition would have allowed us to determine whether changes over time are associated with training (including what we used as a control condition), versus practice effects on the SEAT. Future studies will benefit from inclusion of various controls to identify mechanisms underlying change.
While our findings demonstrate deficits in attention modulation in SAD participants that appear to improve over time, current attention training protocols are not consistently or robustly impacting brain function or symptom severity in individuals with SAD. Additional studies are needed to determine whether attention training can alter neural processes relevant to SAD symptoms, and whether identification of relevant mechanisms will allow us to develop protocols with more consistent clinical impact.
Highlights.
Social anxiety was associated with diminished insula deactivation during attention modulation
Insula deactivation over time was associated with a decrease in social anxiety symptoms
While symptoms decreased over time, attention training did not lead to clinical improvement
Acknowledgments
This project was funded by a Michigan Institute for Clinical Health Research (MICHR) Pilot Grant (UL1TR000433) through an NIH Clinical and Translational Science Award (CTSA). The authors would like to thank Mike Angstadt and the University of Michigan Neuroimaging Methods Core for assistance with data analysis. Cherise White, M.A., Mackenna Hill, B.S., Karlin Stern, Anna Haynes, Annie Rashes, Alyssa Paskow, and Troy Swodzinski assisted with data collection and processing.
Disclosures: This project was funded by a Michigan Institute for Clinical Health Research (MICHR) Grant via an NIH Clinical and Translational Science Award (CTSA) to the University of Michigan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: Author; 2000. [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Jason E. Attention training in individuals with generalized social phobia: A ramdomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77(5):961–973. doi: 10.1037/a0016685. http://doi.org/10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy. 2003;41:1325–1335. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Amir N, Freshman M, Foa E. Enhanced Stroop interference for threat in social phobia. Journal of Anxiety Disorders. 2002;16(1):1–9. doi: 10.1016/s0887-6185(01)00084-6. [DOI] [PubMed] [Google Scholar]
- Amir N, Kuckertz JM, Strege MV. A Pilot Study of an Adaptive, Idiographic, and Multi-Component Attention Bias Modification Program for Social Anxiety Disorder. Cognitive Therapy and Research. 2016;40(5):661–671. doi: 10.1007/s10608-016-9781-1. http://doi.org/10.1007/s10608-016-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. Journal of Abnormal Psychology. 2008;117(4):860–868. doi: 10.1037/a0013445. http://doi.org/10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JDE. Neural correlates of the automatic processing of threat facial signals. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2003;23(13):5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G, Westöö J, Johansson L, Carlbring P. Cognitive bias via the Internet: a comparison of Web-based and standard emotional Stroop tasks in social phobia. Cognitive Behaviour Therapy. 2006;35(1):55–62. doi: 10.1080/16506070500372469. http://doi.org/10.1080/16506070500372469. [DOI] [PubMed] [Google Scholar]
- Bantin T, Stevens S, Gerlach AL, Hermann C. What does the facial dot-probe task tell us about attentional processes in social anxiety? A systematic review. Journal of Behavior Therapy and Experimental Psychiatry. 2016;50:40–51. doi: 10.1016/j.jbtep.2015.04.009. http://doi.org/10.1016/j.jbtep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Becker ES, Rinck M, Margraf J, Roth WT. The emotional Stroop effect in anxiety disorders: General emotionality or disorder specificity? Journal of Anxiety Disorders. 2001;15(3):147–159. doi: 10.1016/s0887-6185(01)00055-x. [DOI] [PubMed] [Google Scholar]
- Bogels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24(7):827–857. doi: 10.1016/j.cpr.2004.06.005. http://doi.org/10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Teale Sapach MJN, Oriet C, Duranceau S, Lix LM, Thibodeau MA, Asmundson GJG. A randomized controlled trial of attention modification for social anxiety disorder. Journal of Anxiety Disorders. 2015;33:35–44. doi: 10.1016/j.janxdis.2015.03.011. http://doi.org/10.1016/j.janxdis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Teale Sapach MJN, Oriet C, LeBouthillier DM. Online attention modification for social anxiety disorder: replication of a randomized controlled trial. Cognitive Behaviour Therapy. 2017;46(1):44–59. doi: 10.1080/16506073.2016.1214173. http://doi.org/10.1080/16506073.2016.1214173. [DOI] [PubMed] [Google Scholar]
- Carré A, Gierski F, Lemogne C, Tran E, Raucher-Chéné D, Béra-Potelle C, Limosin F. Linear association between social anxiety symptoms and neural activations to angry faces: from subclinical to clinical levels. Social Cognitive and Affective Neuroscience. 2014;9(6):880–886. doi: 10.1093/scan/nst061. http://doi.org/10.1093/scan/nst061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin EM, Magee JC, Wells TT, Beard C, Barnett NP. Randomized controlled trial of attention bias modification in a racially diverse, socially anxious, alcohol dependent sample. Behaviour Research and Therapy. 2016;87:58–69. doi: 10.1016/j.brat.2016.08.010. http://doi.org/10.1016/j.brat.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval E, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and Clinical Risk Management. 2015;11:115–126. doi: 10.2147/TCRM.S48528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect (POFA) Human Interaction Laboratory: University of California Medical Center; San Francisco: 1976. [Google Scholar]
- Fitzgerald A, Rawdon C, Dooley B. A randomized controlled trial of attention bias modification training for socially anxious adolescents. Behaviour Research and Therapy. 2016;84:1–8. doi: 10.1016/j.brat.2016.06.003. http://doi.org/10.1016/j.brat.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MHN, Crippa JAS. Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(4):565–80. doi: 10.1016/j.pnpbp.2010.02.028. http://doi.org/10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Heeren A, Coussement C, McNally RJ. Untangling attention bias modification from emotion: A double-blind randomized experiment with individuals with social anxiety disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2016;50:61–67. doi: 10.1016/j.jbtep.2015.05.005. http://doi.org/10.1016/j.jbtep.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Heeren A, Lievens L, Philippot P. How does attention training work in social phobia: Disengagement from threat or re-engagement to non-threat? Journal of Anxiety Disorders. 2011;25(8):1108–1115. doi: 10.1016/j.janxdis.2011.08.001. http://doi.org/10.1016/j.janxdis.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Heeren A, Mogoaşe C, McNally RJ, Schmitz A, Philippot P. Does attention bias modification improve attentional control? A double-blind randomized experiment with individuals with social anxiety disorder. Journal of Anxiety Disorders. 2015;29:35–42. doi: 10.1016/j.janxdis.2014.10.007. http://doi.org/10.1016/j.janxdis.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Heeren A, Mogoașe C, Philippot P, McNally RJ. Attention bias modification for social anxiety: A systematic review and meta-analysis. Clinical Psychology Review. 2015 Jun;40:76–90. doi: 10.1016/j.cpr.2015.06.001. http://doi.org/10.1016/j.cpr.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Heeren A, Reese HE, McNally RJ, Philippot P. Attention training toward and away from threat in social phobia: Effects on subjective, behavioral, and physiological measures of anxiety. Behaviour Research and Therapy. 2012;50(1):30–39. doi: 10.1016/j.brat.2011.10.005. http://doi.org/10.1016/j.brat.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Jusyte A, Schönenberg M. Threat processing in generalized social phobia: AN investigation of interpretation biases in ambiguous facial affect. Psychiatry Research. 2014;217(1–2):100–106. doi: 10.1016/j.psychres.2013.12.031. http://doi.org/10.1016/j.psychres.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Amir N. Preliminary study of attention training to threat and neutral faces on anxious reactivity to a social stressor in social anxiety. Cognitive Therapy and Research. 2010;34(3):263–271. http://doi.org/10.1007/s10608-009-9251-0. [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological Psychology. 2012;89(1):273–276. doi: 10.1016/j.biopsycho.2011.10.010. http://doi.org/10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberzon I. Trait anxiety modulates anterior cingulate activation to threat interference. Depression and Anxiety. 2011;28(3):194–201. doi: 10.1002/da.20802. http://doi.org/10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biology of Mood & Anxiety Disorders. 2013;3(1):7. doi: 10.1186/2045-5380-3-7. http://doi.org/10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov A, Abend R, Bar-Haim Y. Social anxiety is related to increased dwell time on socially threatening faces. Journal of Affective Disorders. 2016;193:282–288. doi: 10.1016/j.jad.2016.01.007. http://doi.org/10.1016/j.jad.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Lazarov A, Marom S, Yahalom N, Pine DS, Hermesh H. Attention bias modification augments cognitive – behavioral group therapy for social anxiety disorder : a randomized controlled trial. 2017 doi: 10.1017/S003329171700366X. http://doi.org/10.1017/S003329171700366X. [DOI] [PMC free article] [PubMed]
- Liberzon I, Ma ST, Okada G, Shaun Ho S, Swain JE, Evans GW. Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Social Cognitive and Affective Neuroscience. 2015 Apr;:1–11. doi: 10.1093/scan/nsv045. http://doi.org/10.1093/scan/nsv045. [DOI] [PMC free article] [PubMed]
- Liebowitz MR. Social Phobia. Modern Problems in Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research and Therapy. 2002;40(12):1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective Attention to Angry Faces in Clinical Social Phobia. Journal of Abnormal Psychology. 2004;113(1):160–165. doi: 10.1037/0021-843X.113.1.160. http://doi.org/10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Mueller EM, Hofmann SG, Santesso DL, Meuret AE, Bitran S, Pizzagalli DA. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine. 2009;39(7):1141. doi: 10.1017/S0033291708004820. http://doi.org/10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa C, Lepine JP, Clark DM, Mansell W, Ehlers A. Selective attention in social phobia and the moderating effect of a concurrent depressive disorder. Behaviour Research and Therapy. 2003;41(9):1043–1054. doi: 10.1016/s0005-7967(02)00212-7. http://doi.org/10.1016/S0005-7967(02)00212-7. [DOI] [PubMed] [Google Scholar]
- Naim R, Kivity Y, Bar-Haim Y, Huppert JD. Attention and interpretation bias modification treatment for social anxiety disorder: A randomized clinical trial of efficacy and synergy. Journal of Behavior Therapy and Experimental Psychiatry. 2018;59:19–30. doi: 10.1016/j.jbtep.2017.10.006. http://doi.org/10.1016/j.jbtep.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Pine DS, Fox NA, Bar-Haim Y. Attention bias modification for youth with social anxiety disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2016;57(11):1317–1325. doi: 10.1111/jcpp.12599. http://doi.org/10.1111/jcpp.12599. [DOI] [PubMed] [Google Scholar]
- Peschard V, Philippot P. Overestimation of threat from neutral faces and voices in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry. 2017;57:206–211. doi: 10.1016/j.jbtep.2017.06.003. http://doi.org/10.1016/j.jbtep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. http://doi.org/10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Amir N, Bar-Haim Y, Carlbring P, Wallace ML. Less is more: Patient-level meta-analysis reveals paradoxical dose-response effects of a computer-based social anxiety intervention targeting attentional bias. Depression and Anxiety. 2017 Apr;:1–10. doi: 10.1002/da.22634. http://doi.org/10.1002/da.22634. [DOI] [PMC free article] [PubMed]
- Rytwinski NK, Fresco DM, Heimberg RG, Coles ME, Liebowitz MR, Cissell S, Hofmann SG. Screening for social anxiety disorder with the self-report version of the liebowitz social anxiety scale. Depression and Anxiety. 2009;26(1):34–38. doi: 10.1002/da.20503. http://doi.org/10.1002/da.20503. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Richey JA, Buckner JD, Timpano KR. Attention training for generalized social anxiety disorder. Journal of Abnormal Psychology. 2009;118(1):5–14. doi: 10.1037/a0013643. http://doi.org/10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl 2):22–57. [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I. Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biological Psychiatry. 2013;73(11):1045–1053. doi: 10.1016/j.biopsych.2012.12.008. http://doi.org/10.1016/j.biopsych.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American Journal of Psychiatry. 2007;164(2):318–27. doi: 10.1176/ajp.2007.164.2.318. http://doi.org/10.1176/appi.ajp.164.2.318. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52(3):163–168. doi: 10.1159/000087987. http://doi.org/10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Aupperle RL, Flagan T, Simmons AN, Amir N, Stein MB, Paulus MP. Neural correlates of a computerized attention modification program in anxious subjects. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst128. http://doi.org/10.1093/scan/nst128. [DOI] [PMC free article] [PubMed]
- Wang X, Xie H, Cotton AS, Duval ER, Tamburrino MB, Brickman KR, Liberzon I. Preliminary study of acute changes in emotion processing in trauma survivors with PTSD symptoms. PLoS ONE. 2016;11(7):1–15. doi: 10.1371/journal.pone.0159065. http://doi.org/10.1371/journal.pone.0159065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Yu H, Qian M, Li S. Does attention redirection contribute to the effectiveness of attention bias modification on social anxiety? Journal of Anxiety Disorders. 2015;36:52–62. doi: 10.1016/j.janxdis.2015.09.006. http://doi.org/10.1016/j.janxdis.2015.09.0066. [DOI] [PubMed] [Google Scholar]


