Abstract
The combinatorial polymer library approach has been proven to be effective for the optimization of therapeutic delivery systems. The library of polymers with chemical diversity has been synthesized by (i) polymerization of functionalized monomers or (ii) post-polymerization modification of reactive polymers. Most scientists have followed the first approach so far, and the second method has emerged as a versatile approach for combinatorial biomaterials discovery. This review focuses on the second approach, especially discussing the post-modifications that employ reactive polymers as templates for combinatorial synthesis of a library of functional polymers with distinct structural diversity or a combination of different functionalities. In this way, the functional polymers have a consistent chain length and distribution, which allows for systematic optimization of therapeutic delivery polymers for the efficient delivery of genes, small-molecule drugs, and protein therapeutics. In this review, the modification of representative reactive polymers for the delivery of different therapeutic payloads are summarized. The recent advances in rational design and optimization of therapeutic delivery systems based on reactive polymers are highlighted. This review ends with a summary of the current achievements and the prospect on future directions in applying the approach of post-polymerization modification of polymers to accelerate the development of therapeutic delivery systems.
Keywords: reactive polymers, post-modification, combinatorial biomaterials discovery, therapeutic delivery, high-throughput screening
Graphical Abstract
1. Introduction
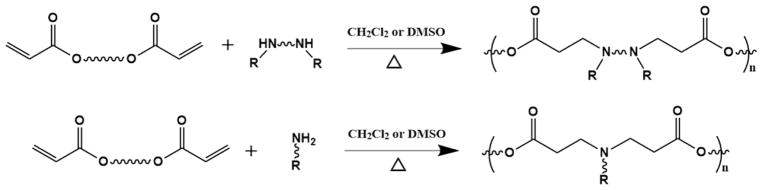
Polymeric delivery systems have been considered to have controllable chemical structures and compositions, relatively low cytotoxicity, and manageable surface chemistry, making them widely useful in the delivery of various kinds of therapeutics, including genes, small-molecule drugs, proteins, and peptides, to treat human diseases.[1–13] The structures of polymeric delivery vehicles, which include micelles, vesicles and dendrimers, have been demonstrated to affect the therapeutic delivery behaviors.[3, 14, 15] For polymeric delivery vehicles having similar structures, the chemical compositions of polymers usually decide the therapeutic binding affinity, release profile, and targeted delivery efficiency.[3, 16, 17] However, a strategy to rationally design and systematically optimize polymers for the efficient delivery of specific therapeutics is lacking. The combinatorial polymer library approach, which was first suggested by Langer and coworkers at the beginning of the 21th century, could be an efficient approach to this end.[18, 19] Based on the Michael addition reaction between monomers containing amines and diacrylates, respectively, an initial library of 3 structurally unique, degradable, cationic poly(β-amino ester)s was synthesized by polymerization.[18] The library was amplified to 140, and then 2350, unique polymers by using a semi-automated synthesis method (the polymer synthesis routes are shown in Fig. 1).[19, 20] Through a high-throughput and cell-based screening, more than 30 polymers were finally identified from the library that transfected the monkey kidney fibroblast COS-7 cells with a higher efficiency than the gold standard poly(ethyleneimine) (PEI) in vitro.[20] This combinatorial approach was further demonstrated to be effective for the optimization of therapeutic delivery polymers in suicide gene therapy for localized cancer treatment in vivo.[21] An account by Green et al. published in 2007 summarized the results of employing structurally diverse, biodegradable poly(β-amino ester)s synthesized by the combinatorial library approach for gene delivery and brought deep insight into the structure-property-function relationships in these gene delivery systems.[22] Combinatorial libraries that consisted of other types of polymers were synthesized by different chemistries, such as epoxide-amine reaction,[23] ring-opening polymerization,[24, 25] and divinylsulfonamide-amine Michael addition reaction[26]. These were also proven to be efficient for polymer optimization for gene delivery, showing improved performance, including higher transfection efficiency and lower cytotoxicity, than PEI and other commercial transfection reagents.[19, 20, 23] It was noticed that the monomer composition often affects the outcome of the polymerization, resulting in a broad range distribution of chain length in the libraries (sometimes more than 20 times in molecular weight difference) (Fig. 2a).[19, 27, 28] The heterogeneous polymeric backbones in molecular weights and polydispersities may underestimate the efficacy of certain functionalities in optimizing the polymers for therapeutic delivery.
Fig. 1.
Synthesis of poly(β-amino ester)s by polymerization of functionalized monomers. The reactions can be done in a combinatorial manner by varying “R” functional groups.
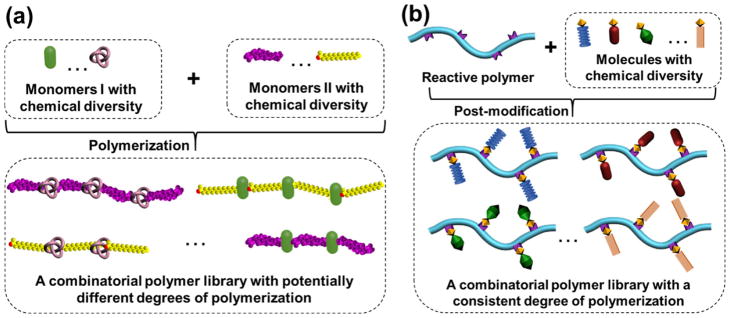
Fig. 2.
Schematic illustration of combinatorial polymer libraries synthesized by two approaches of (a) polymerization of functionalized monomers, and (b) post-modification of reactive polymers for the optimization of therapeutic delivery polymers.
Another approach for combinatorial polymer library synthesis is post-polymerization modification of reactive polymers with diverse functionalities (noted as post-modification).[17, 27–33] The chemistries applied in modifying polymers, including thiol exchange, atom transfer radical addition, and the Huisgen 1,3-dipolar cycloaddition, have been comprehensively reviewed in 2009 by Klok and coworkers.[30] Emerging methodological studies in combinatorial polymer library synthesis via post-modification of reactive polymers for the optimization of therapeutic delivery systems have yet to be systematically summarized and discussed. Reactive polymers are able to be decorated with a variety of molecules in a quantitative manner through post-modification and could be ideal templates for the synthesis of polymers with controlled chemical diversity and molecular weights.[27–29] The key of this approach (of employing reactive polymer precursors for combinatorial polymer library synthesis to optimize therapeutic delivery systems) is to rationally select a series of small molecules with structural comparability and analogous physicochemical properties that can be conjugated to a reactive polymer backbone to yield a combinatorial polymer library for therapeutic encapsulation and delivery. This usually leads to a library of nanocarriers having similar nanostructures/sizes but significantly different therapeutic loading and delivery behaviors.[29, 31] In this way, the relationships between therapeutic-binding moieties and therapeutic loading/delivery behaviors can be simply studied without considering the physical aggregation properties of the self-assembled polymer nanostructures. It is worth noting that this approach is effective for the precise control and systematic optimization of the polymer compositions, however, relatively weak in controlling the self-assembled polymer nanostructures. This approach usually neglects the influence of polymer compositions on the self-assembled nanostructures/sizes. Therefore, it is particularly applicable in the systems having similar self-assembled polymer nanostructures/sizes. The prominent merits of this approach include: (i) Functionalization of reactive polymer precursors by a series of small molecules to produce a library of functional polymers with significant structural diversity and a consistent polymer backbone (Fig. 2b). This allows for parallel comparison of different therapeutic delivery polymers. (ii) The integration of multiple types of functionalities in the therapeutic delivery polymers, which provide opportunities to tailor the encapsulation of therapeutics by multivalent and hybrid interactions. The synergistic combination of hybrid interactions is particularly important for protein encapsulation and delivery,[7, 31] (see Section 3.3). (iii) Screening of small molecules for focused polymer synthesis and evaluation. As the structural diversity of the therapeutic delivery polymers generally originates from the functional small molecules that are used for the post-modification, a simplified screening approach can save time when evaluating and optimizing experimental delivery vehicles, especially when combined with virtual screening.[17, 31] We will further discuss this simplified approach in Section 4.2. These advantages make the post-modification approach greatly useful in combinatorial polymer synthesis for the optimization of therapeutic delivery polymers.
In this review, we discuss the perspectives of the approach of post-modification of reactive polymers in combinatorial polymer synthesis for the optimization of therapeutic delivery systems. The studies on combinatorial polymer synthesis by post-modification approach without therapeutic delivery application have been comprehensively reviewed by Klok and coworkers,[30, 34] and, therefore, are not included or only briefly discussed herein. We summarize representative reactive polymers and their applications in the delivery of different cargoes, e.g., nucleic acids, small-molecule drugs, and protein therapeutics. The principles guiding the selection of functional small molecules for post-modification and the methods to build the combinatorial polymer library are discussed. We also highlight recent advances towards the structure-based approach for polymer design, combinatorial synthesis, and screening using a well-defined reactive polymer template, i.e., telodendrimer. Lastly, we summarize current achievements and give our prospective on the future application of the approach of post-polymerization modification of polymers for the rational development of therapeutic delivery systems.
2. Reactive polymers for combinatorial synthesis of therapeutic delivery polymers
Reactive polymer precursors are the basis for the post-modification-based optimization of therapeutic delivery systems.[27–29] The reactive polymers are isolated from natural sources[35, 36] or synthesized by various methods, including chemical modification,[37] free radical polymerization,[27] atom transfer radical polymerization (ATRP),[38–40] reversible addition-fragmentation chain transfer (RAFT) polymerization,[28, 33, 41, 42] and peptide chemistry[17, 31, 43]. By using controllable synthesis methods, the resulting reactive polymers are able to possess controlled molecular weights and narrow polydispersity.[17, 28] Different kinds of reactive polymers usually have diverse properties, including solubility and reactivity. It is key to rationally utilize the reactivity of these polymers for post-modification. In this section, five categories of typical reactive polymers (Table 1) that have been used as templates for the post-modification-based optimization of therapeutic delivery polymers are detailed. Other potentially useful reactive polymers are also discussed.
Table 1.
Overview of the reactive polymers that have been used as templates for the post-modification-based optimization of therapeutic delivery polymers.
| Category of reactive polymers |
Representative reactive polymers |
Compounds for post- modification |
Conditions and results of post- modification |
Polymer library size |
Properties of functional polymers synthesized by post-modification |
Therapeutic payloads |
Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Need of catalyst or co- reagent |
Reaction byproduct yielded |
Need of additional purificatio n steps |
Well- defined chemical structure |
Cost of polymer and synthesis |
Biocomp atibility/ biodegra dability |
||||||
| N-Hydroxysuccinimide-functionalized polyacrylates | Poly(N-methacryloxysuccinimide) | Primary or secondary amines | No | Yes | Yes | 168 | Fair | Low | Fair | DNA | [29] |
| Azlactone-functionalized polymers | Poly(2-vinyl-4,4-dimethylazlactone) | Primary amines | No | No | Yes† | 12 | Fair | Low | Unknow | DNA | [27] |
|
| |||||||||||
| Hydrazide-functionalized macromolecules | Poly(acryloyl hydrazide) | Aldehydes | No | No | No | 10 | Fair | Low | Unknow | siRNA | [28] |
| Hydrazone-modulated peptide | Aldehydes | No | No | No | 28 | Yes | High | Good | DNA | [43] | |
|
| |||||||||||
| Natural polysaccharides and derivatives | Hyaluronic acid | Amines | Yes | No | Yes | 14 | No | Low | Good | siRNA | [35] |
| O-pentynyl dextran | Azides | Yes | No | Yes | 8 | No | Low | Good | Anticancer drugs | [36] | |
|
| |||||||||||
| Oligo(amino acid)s-based platforms | Hydrazone-modulated peptide | Aldehydes | No | No | No | 28 | Yes | High | Good | DNA | [43] |
| Telodendrimer | Carboxylic acids | Yes | No | Yes | 24, 7 | Yes | High | Good | Doxorubicin, insulin | [17], [31] | |
In ref. [27], the additional purification step was performed to remove the excess, unreacted small-molecule reactants. However, purification steps will become unnecessary if equivalent units of small-molecule reactants are used for post-modification, which will enable high-throughput polymer synthesis for therapeutic delivery.
2.1. N-Hydroxysuccinimide-functionalized polyacrylates
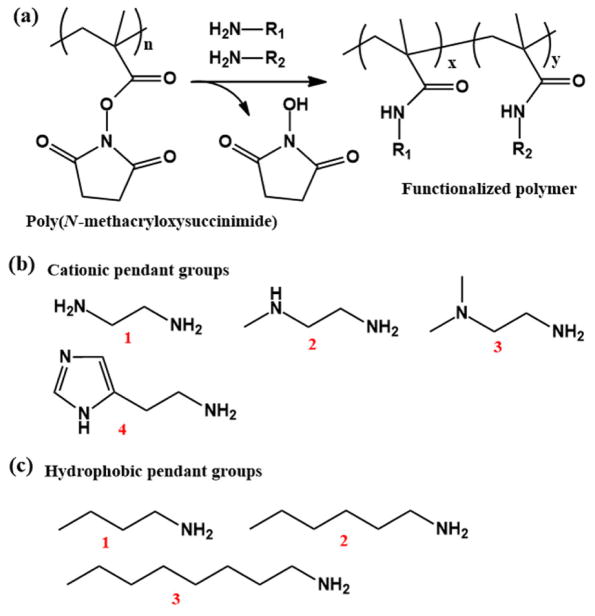
The early application of the approach of post-polymerization modification of polymers for the optimization of therapeutic delivery systems is based on the polymers constructed by polyacrylate backbones and reactive side chains, such as poly(N-methacryloxysuccinimide) (Fig. 3a and Table 1).[29, 44–47] These reactive polymers were first synthesized by Ferruti et al. in the early 1970s, which were proved to be good polymethacrylamide precursors, yielding derivatives by reaction with primary or secondary aliphatic or cycloaliphatic amines.[48] In 2009, Wong et al. reported the screening of combinatorial libraries of functional polymers synthesized by post-modification of poly(N-methacryloxysuccinimide) in order to investigate how the polymer parameters, such as the molecular weights, cationic pendant groups (Fig. 3b), and hydrophobic pendant groups (Fig. 3c) of the functional polymers, affect the gene delivery efficiency.[29] They found the functional polymers with a large molecular weight (30 or 50 kDa) containing primary amino (compound 1 in Fig. 3b) and imidazole (compound 4 in Fig. 3b) groups showed the highest levels of transfection among the combinatorial polymeric delivery vehicle library, comparable to the gold standard PEI. Further, this optimized polymer vector had superb toxicity profiles. The combination of primary amino and imidazole groups was demonstrated to be important for efficient gene delivery: the primary amino groups offered superior charge-neutralizing and size-condensing capacities while the imidazole groups appeared to bind to DNA molecules through non-electrostatically mediated interactions. Thus, stable polyplexes were produced that were resistant to premature dissociation. The hydrophobic pendant groups were found to affect polyplex formation and stability in addition to the interacting with membrane components. This study demonstrated the use of the post-modification method to build combinatorial polymer libraries for the optimization of polymeric delivery vehicles. At the same time, it also provided a versatile and robust platform for the investigation on structure-property-function relationships. However, a limitation of this poly(N-methacryloxysuccinimide) precursor approach is that purification steps are needed to remove reaction byproducts (e.g., 1-hydroxypyrrolidine-2,5-dione, Fig. 3a) and excess reactants, which restricts its application in high-throughput polymer synthesis and screening.
Fig. 3.
(a) Synthesis of mono- and bi-functionalized polymers by the post-modification of poly(N-methacryloxysuccimide). (b) Cationic pendant groups. (c) Hydrophobic pendant groups.
2.2. Azlactone-functionalized polymers
Poly(2-vinyl-4,4-dimethylazlactone) (PVDMA) is the one of the most representative azlactone-functionalized polymer, which is an important reactive polymer and has attracted considerable attention since its chemical modification was pioneered by Heilmann and coworkers in 1984.[49–52] PVDMA can be functionalized by primary amines under facile reaction conditions without added catalysts producing a stable amide linkage without formation of byproducts (Fig. 4a and Table 1). Several reports have detailed the use of PVMDA-containing polymers as reactive matrices. For example, Lynn and coworkers reported the layer-by-layer assembly of PVDMA-based multilayers for DNA delivery.[53] Fontaine and coworkers developed a class of stable azlactone-functionalized thermoresponsive nanoparticles which have potential applications in theranostics.[54] By utilizing the reactivity of PVDMA, Kilbey and coworkers created surface scaffolds for immobilization of biomolecules, manipulated the solution/interface properties, and constructed reactive polymer brushes based on reactive PVDMA homopolymers and dually reactive block copolymers.[55–59] A comprehensive review of azlactone-functionalized polymers as reactive platforms for the design of advanced materials was published in 2012 by Lynn and coworkers.[60]
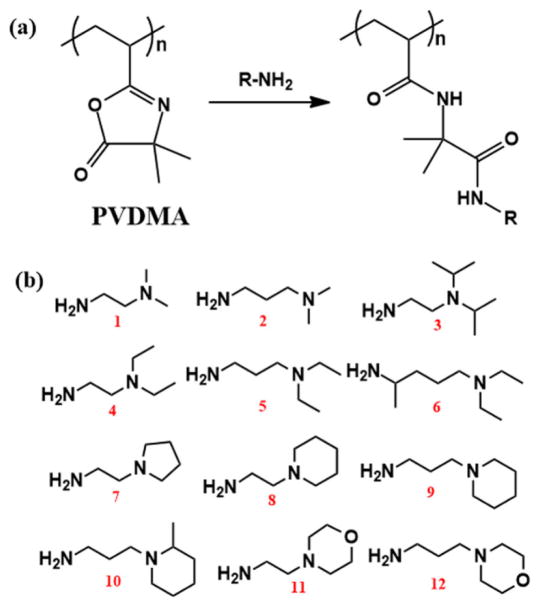
Fig. 4.
(a) Reaction of amines and poly(2-vinyl-4,4-dimethyl azlactone) (PVDMA). (b) Chemical structures of compounds 1–12 possessing both primary and tertiary amine functionalities.
The successful use of PVDMA-based polymers as delivery vectors for efficient gene delivery was presented by Lynn and coworkers in 2010.[27] In their study, PVDMA with a number-average molecular weight of 74000 was used as a reactive polymer precursor to interact with compounds having both primary and tertiary amine functionalities (Fig. 4b) to synthesize a library of 12 cationic polymers. The primary amine groups on each compound could rapidly react with PVDMA while the tertiary amine groups did not react with the azlactone functionality.[27] Exhaustive functionalization of PVDMA in 12 individual reactions resulted in a small library of polymers with a consistent number of repeating units and a consistent polymer chain length. The differences between these 12 polymers are the species of cationic groups and the numbers of positioned carbons from the cationic groups to the polymer backbone. By conducting cell-based screening experiments, two important structural motifs enhancing DNA delivery efficiency were identified: sterically hindered tertiary amine groups and shorter carbon chains leading to the cationic groups (e.g., compounds 3, 7, and 8 in Fig. 4b). In addition, the polymer molecular weights were found to significantly affect the DNA delivery efficiency: A shorter PVDMA with a number-average molecular weight of 5800 was attempted to incorporate compounds 3, 7, and 8, but the resulting polycations showed low levels of transfection. Because the molecular weights of polymers significantly affect the delivery efficiency, the parallel comparison of different polymers with an identical degree of polymerization becomes extremely important, which is, however, challenging to be realized in the polymer systems that synthesized by the approach of polymerization of functionalized monomers (Fig. 2a). In comparison, the reactive polymers are ideal templates for this parallel comparison (Fig. 2b). The study demonstrated the great promise to use azlactone-functionalized polymers as templates in combinatorial polymer synthesis for the optimization of therapeutic delivery systems. Unfortunately, to the best of our knowledge, this is the only example to use azlactone-functionalized polymers in combinatorial polymer synthesis for rational design and optimization of therapeutic delivery vehicles. The use of azlactone-functionalized polymers for combinatorial polymer synthesis and delivery system optimization is neglected probably because the post-modification is often conducted in organic solvents such as tetrahydrofuran (THF) due to poor aqueous solubility of the azlactone-functionalized polymers and additional purification steps are sometimes needed to remove the excess, unreacted small molecules, which may limit the throughput of polymer preparation and screening. However, the post-modification conditions and purification steps can be further optimized to fulfill the requirement of high-throughput polymer screening (e.g., introducing polyethylene glycol (PEG) to prepared PEG-PVDMA block copolymers to improve the aqueous solubility, using equivalent units of small-molecule reactants for post-modification to avoid purification steps). Another concern is the biocompatibility and degradability of PVDMA, which call for further studies on it. Overall, given the easy, controllable, and rapid synthesis procedure, we believe that azlactone-functionalized polymers are promising and convincing candidates for use in the fabrication of novel polymeric biomaterials and as potentially useful templates for the development of screening-based methodologies.
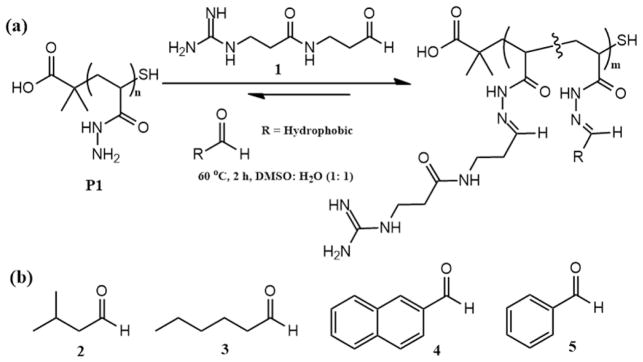
2.3. Hydrazide-functionalized polymers
Hydrazide-functionalized polymers can react readily with various functional aldehydes by post-modification to form acyl hydrazones that are sufficiently stable under physiological conditions.[28, 61–66] Different from PVDMA, the hydrazide-functionalized polymers, e.g., poly(acryloyl hydrazide) (Fig. 5a and Table 1), are usually water soluble, which enables the post-modification reaction(s) to occur in aqueous solutions or dimethyl sulfoxide (DMSO)/water mixtures.[28, 67, 68] In 2016, Montenegro and coworkers reported the in situ functionalization of poly(acryloyl hydrazide) with positively charged and hydrophobic aldehydes (Fig. 5b) and the application for the efficient delivery of small interfering RNA (siRNA) screened in cell culture.[28] This advanced in situ functionalization strategy will be discussed in detail in Section 4.1. It should be mentioned that the small-molecule aldehydes can be consumed completely during the post-modification and no catalyst or co-reagent was used, therefore, no further purification was required for the removal of small molecules. In this work, the cationic aldehyde that contains a guanidinium group was kept constant, and different hydrophobic aldehydes were used for polymer synthesis and screening. It was found in this study that the species and the content of the hydrophobic functionalities significantly affected the membrane transport ability and siRNA transfection efficiency. Similar to PVDMA, the biocompatibility and degradability of poly(acryloyl hydrazide) need to be studied to assess its potential application for therapeutic delivery in vivo.
Fig. 5.
(a) Post-modification of poly(acryloyl hydrazide) with a cationic aldehyde and hydrophobic aldehydes. (b) Representative hydrophobic aldehydes for post-modification of reactive poly(acryloyl hydrazide).
The idea to use hydrazide-functionalized polymers for the optimization of therapeutic delivery polymers could be applied equally to a peptide system (linear oligo(amino acid)s). Arginine and hydrazides were introduced into a peptide system by Montenegro and coworkers, wherein the position and the number of the positively charged arginine still kept constant, and the position of hydrazides also kept constant for the combination of 28 different hydrophobic aldehydes to yield amphiphilic peptides with structural diversity that could form polyplexes for cell-based delivery vehicle screening towards efficient gene delivery.[43] By using this approach, 3 hydrazone-modulated amphiphilic peptides were identified that delivered plasmid DNA into HeLa cells with better efficiency and less cytotoxicity than the standard commercial reagents.
The hydrazide-functionalized macromolecule precursors (polymers and peptides) usually have fine aqueous solubility and the post-modification reactions readily occur in aqueous solution or DMSO/water mixtures. In addition to the aqueous solubility, isolation and purification of the synthesized hydrazone-modulated macromolecules are not required. Given the consistent degree of polymerization, aqueous solubility, and dispensable purification, we believe that hydrazide-functionalized macromolecules are potentially ideal templates for high-throughput screening of polymeric delivery vehicles through an informed choice of aldehydes.
2.4. Natural polysaccharides and derivatives
The natural polysaccharides such as chitosan, dextran, hyaluronic acid, alginic acid have been widely used in the field of biomedical engineering as they are inexpensive, readily available (usually isolated from natural sources), and have excellent biocompatibility and biodegradability.[69–72] In terms of using a combinatorial polymer library approach for delivery vehicle optimization, Amiji and coworkers demonstrated that hyaluronic acid (Table 1) could act as a reactive polymer backbone for post-modification with polyamines and lipids of varying carbon chain lengths and nitrogen content through an amidation reaction for siRNA encapsulation and tumor-targeted delivery.[35] Some natural polysaccharides can be converted into highly reactive derivatives through single-step chemical reactions that would be useful as templates for combinatorial polymer synthesis. For example, Abeylath et al. reported the conjugation of 5-chloro-1-pentyne to a dextran backbone for the synthesis of O-pentynyl dextran that enabled post-modification with varying lengths of lipid chains, thiol groups, and PEG via click chemistry for the construction of a library of functional polymers.[36] This library demonstrated efficacy in self-assembling into nanoparticles for the encapsulation of various small-molecule anticancer drugs with different hydrophobicities. However, the relatively broad molecular weight distribution of the natural polysaccharides and their derivatives, together with the use of coupling reagents or copper in the amidation and click reactions, may limit the application of these materials and chemistries in high-throughput polymer synthesis for the optimization of therapeutic delivery systems. To their benefit, copper-free methods for compound synthesis by click chemistry have been developed,[73] which may circumvent residual copper-associated toxicity that could potentially occur with this click chemistry-based approach.
2.5. Oligo(amino acid)s-based platforms
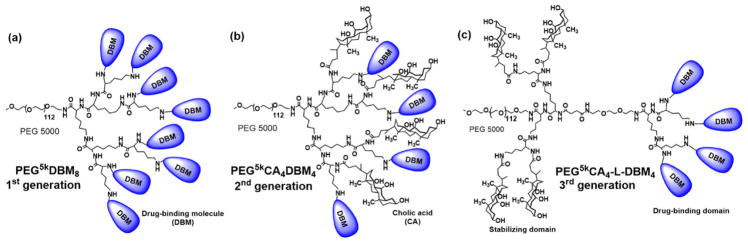
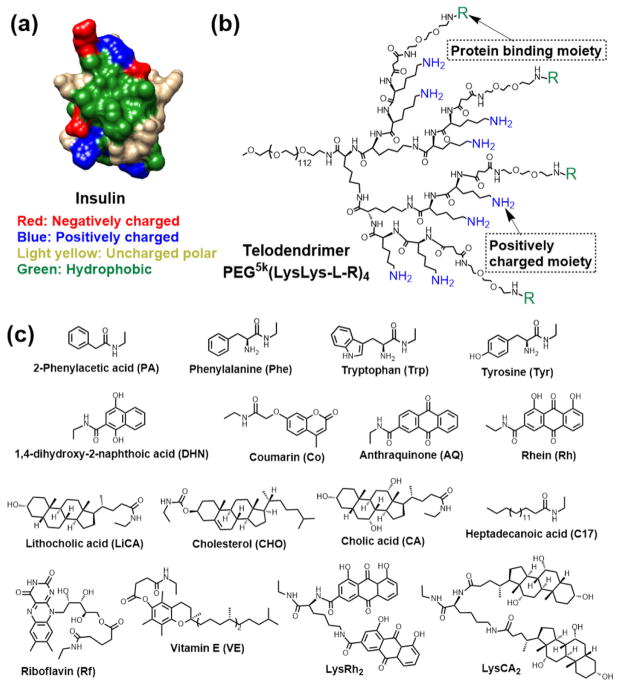
Linear poly(amino acid)s such as polylysine and polyaspartic acid contain reactive primary amine or carboxyl groups, and they have the potential to be useful as templates in combinatorial polymer synthesis. Such poly(amino acid)s have been rarely used in combinatorial polymer synthesis studies, mainly due to their high synthesis costs. However, linear or dendritic oligo(amino acid)s with easy synthesis procedures and low synthesis costs have been well used in combinatorial macromolecule synthesis for the optimization of therapeutic delivery systems.[17, 31, 43] Compared to natural polysaccharides and traditional reactive polymers synthesized by polymerization, the chemical structures of the oligo(amino acid)s-based platforms synthesized via peptide chemistry are well-defined and can be characterized clearly.[7, 17, 31, 43] In addition to the well-defined structures, biocompatible oligo(amino acid)s-based platforms are constructed via peptide bonds, which can be hydrolyzed by peptidases in vivo.[7] The use of linear oligo(amino acid)s with hydrazide-functionalized peptides as reactive templates for combinatorial delivery vehicle synthesis[43] was discussed in Section 2.3. Compared to linear macromolecules that mostly have random coil structures, the more globular shapes in the dendritic segments of dendrimers and linear-dendritic copolymers are generally considered to potentially affect their physiochemical and biological properties. This has led to the discovery of interesting effects related to macromolecular architecture.[74, 75] For instance, Gitsov and coworkers reported that the PEG-b-dendritic poly(benzyl ether) could selectively interact with glycan moieties on a laccase to enhance its enzymatic activity by providing hydrophobic depots for substrates, while the linear PEG-b-polystyrene polymer consistently inhibited the catalytic activity.[76, 77] In terms of dendritic oligo(amino acid)-based systems, we reported the use of a chemically well-defined telodendrimer[78–86] composed of linear PEG-blocking-dendritic oligolysine and capping peripheral building blocks (Fig. 6) for structure-based design of polymers for therapeutic delivery.[17] Efficient peptide chemistry was used in telodendrimer synthesis that allowed for free and precise control over the architecture and the functionality of the telodendrimer. The peripheral groups on the dendritic oligolysine have good flexibility in interacting with drug molecules. Three generations of telodendrimers with distinct architectures were developed: (i) first generation (G1) telodendrimers with 8 drug-binding molecules (DBMs) (Fig. 6a), (ii) second generation (G2) telodendrimers with hybrid peripheral groups of 4 DBMs and 4 amphiphilic cholic acids (CAs) for better stabilization and aqueous solubility of the telodendrimer micelles (Fig. 6b), and (iii) third generation (G3) telodendrimers with functionally segregated peripheral groups of 4 DBMs together for drug binding and 4 CAs together for stabilizing the payload-containing telodendrimer micelles (Fig. 6c). By using these linear-dendritic architectures, various DBMs can be conjugated in a well-defined manner to produce telodendrimers with different species but the same number of DBMs, which is critical for parallel evaluation of different polymeric delivery vehicles. However, compared to the readily available natural polysaccharides and the conventional reactive polymers synthesized by single-step polymerization, the oligo(amino acid)s-based platforms usually have tedious synthetic procedures (Table 1). Employing peptide chemistry for functionalization involves conjugation reactions using coupling reagents, which may necessitate purification steps. This may limit the applications for high-throughput screening of large polymer libraries.
Fig. 6.
Chemical structures of telodendrimers of (a) first generation PEG5k DBM8 (G1), (b) second generation PEG5k CA4 DBM4 (G2), and (c) third generation PEG5k CA4-L-DBM4(G3).
2.6. Other reactive polymers potentially useful in combinatorial polymer synthesis for therapeutic delivery
Along with the five types of classic reactive polymers mentioned above are other reactive polymers that have been used, or have the potential to be used, as templates for combinatorial polymer synthesis. For instance, Bradley and coworkers have demonstrated that poly(allyl glycidyl ethers) can be functionalized through a tetrazine-mediated post-modification, and this modification can be used for triggered release of doxorubicin from the polymer nanoparticles.[87, 88] The inverse electron demand Diels-Alder reaction used in these studies can be conducted in either organic or aqueous solvent, without the need for additives or catalysts,[87] which shows great promise for post-modifications in polymers aimed at biological applications. Poly(pentafluorophenyl methacrylate) (PPFMA) that can react with primary amines has been used for the preparation of a diverse water-soluble polymer library by post-modification without inducing any additional toxicity,[33] indicating the potential to use PPFMA as a reactive polymer precursor in parallel polymer synthesis for the optimization of therapeutic delivery systems. A drawback of using PPFMA as a reactive polymer precursor is that purification steps are needed to remove the functionalization reaction byproducts of 2,3,4,5,6-pentafluorophenol, which may limit the application of PPFMA in high-throughput polymer synthesis. Recently, Li et al. reported the synthesis of reactive polymers with both pentafluorophenyl ester and azlactone functionalities, that can be selectively modified by different amines in a controlled manner to yield polymers with bespoke functionalities.[89] This is realized by fully exploiting the difference in reactivity of these two activated esters toward different amines. Epoxide group-containing polymers, such as poly(glycidyl methacrylate) (PGMA),[58, 59] that can react with functional molecules containing amine, hydroxyl, or carboxyl groups, are promising reactive polymer templates for combinatorial polymer synthesis although the undesired chain-branching reactions may occur when improper nucleophilic reagents (e.g., primary amines) are used. The post-modification via epoxide ring opening can be catalyzed by either tertiary amine small molecules or tertiary amine group-containing polymers, such as poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA).[34, 90] Moreover, the pendant tertiary amine groups in the PGMA-co-PDMAEMA brushes were demonstrated to accelerate the post-modification rate via epoxide ring opening with primary amines in aqueous solution at room temperature.[91] A successful case to use epoxide as a “modification handle” for therapeutic delivery polymer optimization was presented by Anderson and coworkers in 2015.[92] In their study, 1536 structurally distinct nanoparticles with cationic cores and variable shells were synthesized based on the ring-opening reactions between epoxide-functionalized block polymers and amines in a combinatorial fashion for high-throughput screening of polymers toward the efficient delivery of siRNA and DNA.[92] Some other amine- or carboxyl-containing synthetic polymers, such as polyallylamine hydrochloride and poly(acrylic acid), could be useful as templates for combinatorial polymer synthesis. However, similar with the oligo(amino acid)-based platforms, coupling reagents are necessary for the post-modification of these polymers and additional purification is required. Notably, branched PEI with reactive primary amine groups is not a good candidate template for combinatorial polymer synthesis as the chemical structure of PEI is complex and not well-defined. Moreover, the secondary amine groups in PEI may compromise functionalization if small molecules with strong reactivity (e.g., organic acid anhydrides) are used. The design and synthesis of new reactive polymers with high reactivity and good aqueous solubility/stability, in which case the post-modification can be conducted at room temperature in an aqueous solution without catalysts and further purification, will greatly promote the development of reactive polymer-based combinatorial synthesis and screening methods for optimization of therapeutic delivery vehicles.
3. Therapeutic delivery
This section discusses three types of therapeutics used as model payloads in combinatorial polymer synthesis by the post-modification approach for the rational fabrication of therapeutic delivery systems: genes, small-molecule anticancer drugs, and proteins (Table 2). It should be pointed out that the therapeutic cargoes are not limited to these three types. The selection of functional small molecules used for post-modification of reactive polymers relies on the properties of the therapeutic cargoes. Therefore, in this section, we discuss the principles guiding functional small molecule selection along with an introduction on the therapeutic cargoes.
Table 2.
Overview of the model therapeutic payloads used for the optimization of therapeutic delivery polymers synthesized by the post-modification approach.
| Category of therapeutic payloads | Representative therapeutic payloads | Reactive polymers for post-modification | Compounds for post-modification | Delivery efficiency evaluation method | Ref. |
|---|---|---|---|---|---|
| Gene | DNA | Poly(2-vinyl-4,4-dimethylazlactone), poly(N-methacryloxysuccinimide), poly(2-(acryloyloxy)ethyl methacrylate) | Cationic and/or hydrophobic compounds | Cell-based high-throughput screening | [27], [29], [37] |
| Anticancer drug | Doxorubicin | Telodendrimer, O-pentynyl dextran | Hydrophobic compounds | Drug release profile, treatment efficacy | [17], [36] |
| Protein | Insulin | Telodendrimer | Charged and hydrophobic compounds | Binding affinity, treatment efficacy | [31] |
3.1. Genes
Gene therapy is an attractive therapeutic option that holds promise to treat diseases; especially for cancer treatments by targeting vital genes for cancer survival.[21, 44, 93] Nonviral gene delivery systems are under intense investigation due to many advantages over viral systems, such as simple preparation procedures, better safety, higher stability, low immunogenicity, and the possibility for further modification to realize advanced functions (e.g., molecular imaging guided gene delivery).[21, 94, 95] Combinatorial polymer synthesis and screening has been widely used for the optimization of polycation-based gene delivery vehicles.[19–22] In fact, most examples of using a combinatorial polymer synthesis approach to optimize functional polymers synthesized by either polymerization of functionalized monomers or post-modification of reactive polymers focused on the delivery of genes.[19, 20, 23, 27, 44]
While gene therapy is promising to treat diseases, there are other underlying reasons for scientists to focus on the development of efficient gene delivery vectors by combinatorial polymer synthesis and screening. Firstly, unlike small-molecule drugs that can diffuse into diseased cells, the negatively charged, hydrophilic DNA molecules are generally cell-impermeable by themselves, requiring delivery vehicles to condense them and mediate their membrane transport, endosomal escape, and nuclear import. In an account describing the combinatorial polymer library approach for gene delivery, Anderson and coworkers quoted Verma as saying, “…there are only three problems in gene therapy - delivery, delivery and delivery.”, to expound their opinion that the development of efficient delivery vehicles is key for progressing the field of gene therapy.[22, 96] Secondly, compared to small-molecule drugs and proteins with great structural diversity, the structures of genes are relatively simple and fixed. Optimized gene delivery polymers screened using a model gene may have universality for other genes. Electrostatic interactions are the main driving forces for gene loading in the polycationic delivery vehicles, while the hydrophobic functionalities in polymeric delivery vehicles were demonstrated to influence the gene delivery efficiency by affecting membrane transport ability.[43] Lastly, cell level evaluation for the optimization of gene delivery vehicles is reliable, relatively simple, and feasible. Unlike delivery vehicles for small-molecule drug or protein delivery that usually need in vivo treatment efficacy characterizations, high-throughput, cell-based screening has been demonstrated to be reliable and efficient for the identification of optimal polymers for DNA delivery.[20] Incidentally, this cell-based screening method is also suitable for the high-throughput optimization of siRNA delivery vehicles.[24, 28] These three factors make genes the most popular therapeutic cargoes used in combinatorial polymeric delivery vehicle screening studies. We have shown several examples (Tables 1 and 2) of using the reactive polymer-based combinatorial polymer synthesis and screening method to optimize vehicles for efficient gene delivery[27–29, 35, 43] in Sections 2.1–2.4. Here we provide one more example showing how to rationally synthesize and optimize the gene delivery polymers.
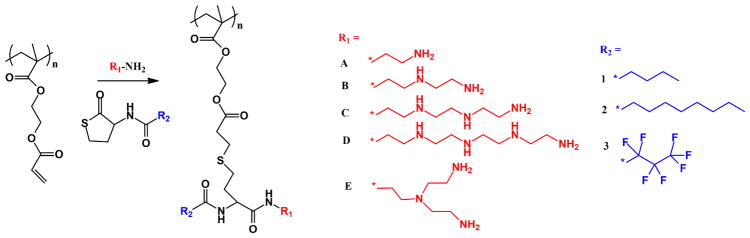
Both amino and hydrophobic functionalities in polymeric nonviral gene vectors seriously affect the gene delivery efficiency.[97–99] On the one hand, the optimization of amino functionalities can efficiently improve the DNA condensation and facilitate the cellular uptake and endosomal escape.[97] On the other hand, the hydrophobic functionalities usually influence the stability of DNA-polymer complexes and the interactions with cell membranes.[98, 99] Ge and coworkers recently presented a one-pot synthetic route for simultaneous optimization of both amino and hydrophobic functionalities in polymeric nonviral gene vectors by employing thiolactone chemistry (Fig. 7).[37] This one-pot combinatorial synthesis of multifunctional polymers was achieved through the aminolysis of the thiolactone unit, followed by the release of a mercapto group for Michael addition reaction with poly(2-(acryloyloxy)-ethyl methacrylate). In this way, both amino and hydrophobic functionalities could be conjugated to the polymer backbones in a facile manner, yielding 15 multifunctional polymers for DNA condensation and cell-based screening. A polymer (R1 = D, R2 = 3, see Fig. 7) based on tetraethylenepentamine and heptafluorobutyric acid-functionalized thiolactone was finally identified to have the highest gene transfection efficiency and low cytotoxicity, which performed better than the gold standard PEI and Lipofectamine 2000 in both HeLa and HepG2 cell lines. In their study, the thiolactone chemistry was used for the first time for combinatorial synthesis of polymers having both amino and hydrophobic functionalities, which presented an effective way for the optimization of gene delivery polymers.
Fig. 7.
One-pot route for thiolactone chemistry-based combinatorial synthesis of multifunctional polymers for efficient gene delivery.
Compared to the more popular research on gene delivery polymers using the combinatorial library approach, the utilization of combinatorial polymer synthesis to optimize polymers for the delivery of small-molecule drugs and protein therapeutics is relatively backward. This is mainly attributed to the largely varied structures and properties of small-molecule drugs and protein therapeutics, which add significant difficulty to the development of universal polymers for the delivery of such cargoes. Structural precision is critical in the combinatorial fabrication of polymers for drug/protein delivery, as slight structural uncertainty may lead to unforeseen difficulties when conducting systematic delivery vehicle evaluation and optimization. Therefore, the development of reactive polymers and functionalized polymers with chemically well-defined structures is essential for combinatorial polymer synthesis and optimization for drug/protein delivery. We will show two examples of employing a well-defined telodendrimer platform (Fig. 6) for polymer optimization by the combinatorial library approach towards the efficient delivery of small-molecule anticancer drugs and protein therapeutics in Sections 3.2 and 3.3, respectively.
3.2. Small-molecule anticancer drugs
As a major public health problem worldwide, many small-molecule drugs have been developed for the treatment of various cancers.[100, 101] A majority of anticancer drugs have undesirable aqueous solubility, poor pharmacokinetics, and may cause severe toxic side effects.[101–107] Polymeric nanoparticle-based drug encapsulation increases drug solubility and stability, minimizes toxic side effects, and, most importantly, delivers drugs specifically to tumor sites by the enhanced permeability and retention (EPR) effects.[104, 108–111] Many factors, including the physicochemical properties of polymer nanoparticles (such as size, shape, charge density, hardness, and surface composition) and the tumor environments, seriously affect (or limit) the extent of the EPR effects.[78, 112] For example, we previously demonstrated that the size of the drug-loaded polymer micelles greatly influenced their in vivo biodistribution, and the smaller ones (hydrodynamic diameter, Dh: 17 and 64 nm) were more readily and efficiently able to carry the loaded anticancer drugs to the tumor sites, when compared to the larger one (Dh: 154 nm).[78] Moreover, the modification of ligands on polymer nanoparticles can further improve the tumor uptake by active targeting effects.[113] Recently, Kobayashi and coworkers showed that the EPR effects could be increased by manipulating either local tumor or systemic conditions, and they suggested three important modifiable parameters that improve the resistance of the tumor capillary wall: (i) modulating tumor blood flow, (ii) modulating tumor vasculature and stroma, and (iii) killing cancer cells to reduce the barrier function.[112]
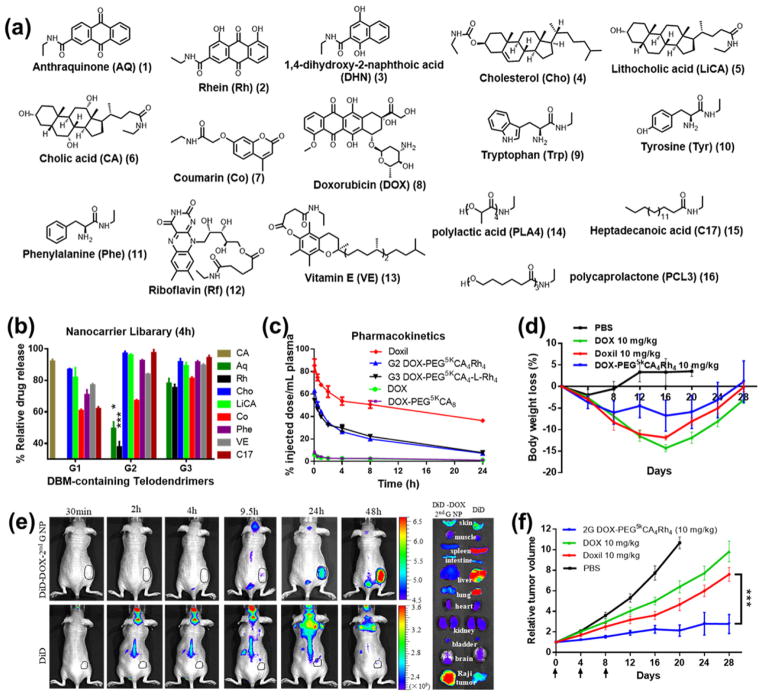
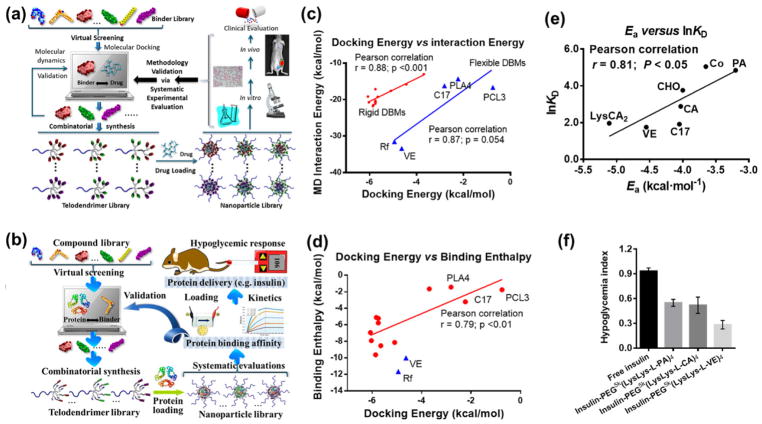
Anticancer drugs have diverse chemical structures and properties, which require rational and customized polymer design for efficient drug encapsulation and delivery. The significance of combinatorial design of polymeric nano-delivery systems and formulation customization for cancer therapy has been reviewed by Amiji and coworkers.[36, 114, 115] Reactive polymer-based combinatorial synthesis and screening can effectively optimize polymer synthesis for targeted drug delivery. By using doxorubicin as a model anticancer drug and telodendrimers (Fig. 6 and Table 2) as a model reactive platform, we presented a study on drug-specific polymer design for efficient anticancer therapy.[17] A number of lipophilic vitamins and natural molecules (Fig. 8a) were collected into a model library for virtual screening, followed by combinatorial polymer synthesis and systematic evaluation/optimization. This computer-aided strategy to facilitate the experimental screening will be discussed in Section 4.2. Rhein (Rh), an aromatic-rich molecule, was identified to be the best DBM for doxorubicin with high binding affinity, as well as to have biocompatibility and bioactivity of a building block. The telodendrimers were synthesized using amphiphilic CAs as co-core forming building blocks with Rh, which efficiently stabilized nanocarriers into small-sized particles and prevented further aggregation. Rh- and CA-containing telodendrimer nanocarriers were demonstrated to have sustained doxorubicin release (Fig. 8b), improved circulation (Fig. 8c), increased maximum tolerated dose, reduced toxic side effects (Fig. 8d), effective tumor targeting (Fig. 8e). The proper stability and drug release profiles of these nanoformulations yielded the significantly improved anticancer effects (Fig. 8f) when compared to both free doxorubicin and Doxil (a stealth liposomal nanoformulation of doxorubicin).
Fig. 8.
(a) Representative drug-binding molecules (DBMs) for doxorubicin (DOX). (b) Percentage of doxorubicin release from telodendrimer micelle library relative to the release rate of free doxorubicin and PEG5k CA8 at 4 h. (c) Pharmacokinetics profiles of doxorubicin concentration in plasma. (d) Body weight change of Raji lymphoma-bearing nude mice treated with different doxorubicin formulations. (e) In vivo and ex vivo near-infrared fluorescence optical images of Raji lymphoma-bearing nude mice injected intravenously with free DiD dye and DiD-doxorubicin-coloaded telodendrimer micelles. (f) In vivo tumor growth inhibition of Raji lymphoma-bearing nude mice treated with different doxorubicin formulations. Data were presented as mean±s.e.m. Student’s t-test: *P<0.05; **P<0.01; ***P<0.001. Reproduced with permission from ref. [17]. Copyright 2015 Nature Publishing Group.
We show this example to illustrate a method for functional small molecule selection when constructing reactive polymer-based systems for the delivery of an anticancer drug. We can study the structure-property relationship of DBMs and drug loading properties in these systems: Firstly, hydrogen bonding and hydrophobic interactions may play important roles for binding the anticancer drugs. For example, aromatic-rich molecules generally had high binding affinity with aromatic-rich doxorubicin. Steric hindrance is another important factor to determine binding affinity, as doxorubicin was not the most favorable binder to itself. Therefore, serious consideration of the drug structure and drug-DBM interactions may help us to narrow the selection of functional small molecules before the screening of polymers. Secondly, the strongest drug binding and encapsulation may not be the best option. For correct function, the payload needs to be released after the nanoparticles reach the tumor site(s). If the nanoformulation (e.g., Doxil) is too stable to release a drug, the anticancer efficiency may be suboptimal (Fig. 8f). Therefore, the goal is to use reactive polymer-based combinatorial polymer synthesis and screening methods to identify the optimal DBM and fabricate drug delivery vehicles to improve the drug release profile, the in vivo pharmacokinetics, and the targeted drug delivery ability. Many amphiphilic polymers have been prepared to optimize the particle size, stability and drug loading capacity via tailoring the balance of hydrophobicity and hydrophilicity of the system.[108, 116–118] We demonstrated an inside-out approach in polymer design through precise control of binding affinity between drugs and polymers, which not only improved drug loading and release profiles, but also optimized the size and stability of nanoformulations, therefore optimizing the in vivo efficacy for disease treatment. Furthermore, we want to emphasize the importance of rational design of polymers based on the structure of a small-molecule anticancer drug. The polymers designed for the delivery of one drug may not be suitable for another drug, therefore an approach for drug-specific polymer design is warranted.
3.3. Proteins
Protein therapy has been considered a safe and direct approach to treat human disease since insulin (Fig. 9a) was used as the first human recombinant protein therapeutic.[119–121] Currently, more than 130 bioactive proteins have been approved to treat human diseases.[122, 123] Delivery systems are able to enhance the therapeutic efficacy of proteins through the modification of their pharmacokinetics. Protein PEGylation has a long-standing history of efficiently prolonging circulation time, increasing stability, and reducing the immunogenicity of protein therapeutics, especially for recombinant proteins.[124, 125] Physical encapsulation of proteins into nano- or micro-particles has been intensively studied for systemic or local administration.[126–128] It is a critical issue to maintain protein structure and activity in such encapsulation/modification processes, especially for processes involving lyophilization or organic solvents. For example, the use of organic solvents in the oil/water emulsion technique[129] for the encapsulation of proteins into biodegradable polymeric microparticles (e.g., polylactic acid and polycaprolactone) usually causes denaturation of proteins and at least partial loss of activity.[130] Encapsulation of proteins in aqueous environments, such as in hydrogels[131, 132] and nanogels[133–135], represent an efficient way to preserve protein structures and activities. However, these processes mostly rely on polymerization or chemical reactions to crosslink bulk or granular hydrogels or within the dispersed nanoaggregates. The chemical process may lead to unexpected complication in control of the physical properties, and the chemicals used in these reactions may remain as toxic impurities that hinder in vivo and clinical applications. Efficient encapsulation of proteins in situ in biologically relevant environments without extra chemicals or procedures required, is ideal for clinical development of protein nanotherapeutics. The interplay of hydrogen bonding, electrostatic interactions, and hydrophobic interactions stabilizes and maintains protein three-dimensional structures. Polar amino acids, including both positively and negatively charged ones, mostly display on the surface of proteins for protein dispersion in aqueous solutions (Fig. 9a). In addition, hydrophobic residues aggregate into hydrophobic grooves to minimize aqueous exposure, as well as for binding of other hydrophobic materials and for the reinforcement of electrostatic interactions by decreasing the static dielectric constants of surroundings.[136–138] The design of a scaffold to target both polar groups and hydrophobic grooves on protein surfaces represents a promising solution for protein coating driven by both enthalpy (electrostatic)- and entropy (hydrophobic)-favored processes.
Fig. 9.
(a) Surface structure of human insulin. (b) Chemical structure of the telodendrimer with both charged and hydrophobic groups. (c) Representative protein binding molecules. Reproduced with permission from ref. [31]. Copyright 2017 American Chemical Society.
Inspired by the cooperative and/or multivalent effects[139–141] in biological systems, we have developed a hybrid telodendrimer composed of a linear PEG and a dendritic polyelectrolyte decorated with different hydrophobic biocompatible protein binding moieties tethered by a flexible linker (Fig. 9b and Table 2), that could encapsulate proteins in aqueous solution without the addition of co-reagents.[7] The decoration of telodendrimers with multiple charged and hydrophobic moieties on the dendritic periphery was demonstrated to be able to match protein surface chemistries by both electrostatic and hydrophobic interactions to facilitate protein binding and stabilize the generated nanoconstructs. The freedom in engineering the telodendrimers allows for generating well-defined structures with modularly tailored functionalities to optimize protein loading efficiency and to fine-tune the physical properties of the nanocomplex. Both charged and hydrophobic groups in the telodendrimers were found to affect the protein encapsulation and delivery. The selection of charged functionalities in telodendrimers should account for the net charges on protein surfaces. However, the species of the charged groups need to be carefully considered based on the protein properties and functions. For example, guanidine or tertiary amine groups may be used to improve the intracellular delivery efficiency of telodendrimers for cell-impermeable proteins, such as cytochrome C and truncated diphtheria toxin, that require delivery into the cytosol to impart their functions.[7, 142] In comparison, primary amino groups in the telodendrimer may be a better choice for the delivery of proteins, such as insulin, that can bind to extracellular receptors.[31] Moreover, the lysine-containing telodendrimer was found to bind to insulin stronger than the equivalent arginine-containing analog in our previous study.[31] This is presumably because the amine groups on lysine have more flexibility in orientation to interact with the carboxylic groups on insulin to form efficient salt bridges, while arginine can form strong bivalent hydrogen bonds only with the planar guanidinium,[143] which may reduce the chance to form stable salt bridges between the telodendrimer and insulin during the assembly process. The hydrophobic moieties in the telodendrimers are largely variable, which have been known to be important for protein encapsulation and delivery.[7] We have presented a computer-aided approach for rational and customized design of telodendrimers for therapeutic protein delivery.[31] Representative hydrophobic protein binding molecules (Fig. 9c) were collected for virtual screening against a model protein of human insulin, followed by precise conjugation on the reactive telodendrimer backbone preinstalled with charged moieties in a combinatorial manner. Protein-loading stability, binding affinity between proteins and telodendrimers, and blood glucose levels controlled by sustained insulin release were studied for systematic evaluation and optimization. D-α-tocopherol (vitamin E)-containing telodendrimers were identified to be optimal by this combinatorial polymer library approach, showing strong binding affinity for insulin loading and leading to improved blood glucose control. This study not only demonstrated the importance of synergetic combinations of rationally selected charged and hydrophobic groups for stable protein loading and efficient protein delivery, but also affirmed the concept of utilizing a combinatorial polymer library for the optimization of therapeutic delivery polymers and validated the approach of structure-based polymer design for protein delivery.
4. Advances to facilitate the reactive polymer-based combinatorial polymer synthesis and screening
The last decade have witnessed the initial development of the post-modification approach in combinatorial polymer synthesis for the optimization of therapeutic delivery systems, and a number of representative therapeutic delivery polymers with desirable properties have been identified by this approach.[17, 27, 28, 31] Despite these achievements, there are still large demands to improve this approach by developing advanced reactive polymer-based systems useful for high-throughput screening of therapeutic delivery polymers and efficient ways to facilitate the rational selection of functional small molecules used for post-modification of reactive polymers. In this section, we introduce recent advances towards the optimization of the post-modification approach for the identification of therapeutic delivery polymers.
4.1. In situ functionalization of reactive polymers towards high-throughput polymer synthesis for therapeutic delivery
Beyond the delivery vehicle evaluation methods, the lack of efficient reactive polymers also limits the use of the post-modification approach for the high-throughput screening of therapeutic delivery polymers. Although the exploration of facile high-throughput evaluation methods is needed for polymer optimization when delivering small-molecule drugs and proteins, we mainly focus on the development of novel strategies to facilitate high-throughput polymer synthesis in this section. Polymer microarray approach is an efficient way to afford a polymer library containing large numbers of biomaterials with diverse structures, which allows for rapid, microscale screening of polymer-cell interactions.[144, 145] Besides, we have showed an example of in situ functionalization of a reactive polymer, poly(acryloyl hydrazide), for combinatorial polymer synthesis and screening[28] in Section 2.3. This in situ functionalization occurred under aqueous conditions and without further purification, which may allow for high-throughput polymer synthesis. Learning from this study, we know that the reaction solvent and further purification steps are the primary barriers that may restrict the high-throughput polymer synthesis by post-modification approach.
4.1.1. Reaction medium
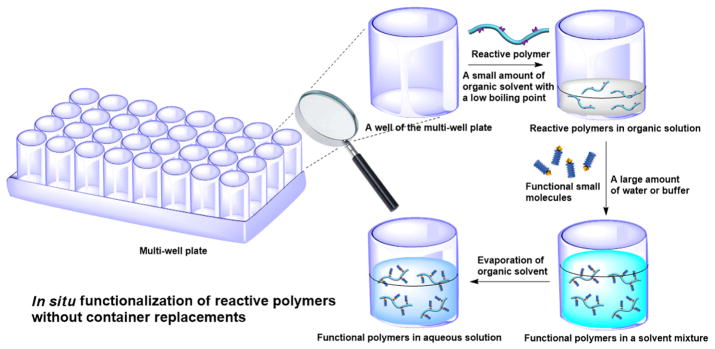
Unlike lipidoids[120, 142, 146, 147] (cationic lipid-like materials frequently used in combinatorial delivery vehicle library studies) that can be synthesized in solvent-free conditions at elevated temperatures, polymers are usually highly viscous and, therefore, require solvents for dissolution and efficient functionalization.[20] Selection of the solvent is critical and directly links to the throughput of functional polymer synthesis.[28] If an unsuitable solvent is used, complicated isolation steps become unavoidable for solvent removal, which significantly limits the functionalization throughput. Indeed, the optimal solvent for post-modification should be water as further delivery efficiency evaluations are usually carried out in aqueous solutions.[28] However, some reactive polymers are not water-soluble or have poor stability in water.[27] A small amount of DMSO or alcohol can be used for functionalization since it has been proved that <5 % (volume percentage) of DMSO or alcohol in aqueous solutions is generally safe in both cell cultures and in vivo studies.[142] Normally, 5% organic solvents are not sufficient for reactive polymer dissolution. We may use a high concentration, but small volume, of organic solvents (e.g., DMSO and alcohol) for the functionalization, followed by dilution with water to reach an organic solvent content of <5%. Alternatively, organic solvents with low boiling points (e.g., methanol and THF) can be used for the functionalization reactions and, after dilution with water, be simply removed by evaporation. These dilution and evaporation steps won’t dramatically limit the throughput of combinatorial polymer functionalization since they can be performed in the same vessel. For example, the functionalization reaction, dilution, and evaporation steps can be completed in sequence in one well of a multi-well plate to yield an aqueous solution of functional polymers ready to be used for further therapeutic loading or delivery efficiency evaluation (Fig. 10).
Fig. 10.
Schematic illustration of in situ functionalization of reactive polymers towards high-throughput polymer synthesis.
4.1.2. Polymer purification
Purification and isolation steps increase the time required and the cost of the discovery process.[28] Therefore, these steps have to be avoided for high-throughput polymer synthesis. Generally, the reactivity of polymers dictates the need to use catalysts or co-reagents and indirectly affects the need for isolation/purification to remove the catalysts or co-reagents. For example, in the reactive polymer systems that employ peptide chemistry for post-modification, the use of coupling reagents usually cannot be avoided.[78] Therefore, isolation/purification steps of the candidate polymers have to be performed to remove the small-molecule coupling reagents. Further, the formation of reaction byproducts also make purification steps necessary, such as in the poly(N-methacryloxysuccinimide) and PPFMA systems.[29, 33] In comparison, polymers with relatively high reactivity, such as PVDMA and poly(acryloyl hydrazide), that are functionalized without added catalysts or co-reagents to yield pure, functional polymers without the formation of byproducts, hold great promise to be used for delivery efficiency evaluation if the functional small molecules used for the post-modification can be exhausted.
Overall, the highly reactive azlactone- and hydrazide-functionalized polymers that can react with functional small molecules without added catalysts or co-reagents in aqueous, DMSO, ethanol, methanol or THF solutions to yield a combinatorial polymer library with a consistent degree of polymerization and significant chemical diversity are the most promising templates for in situ and high-throughput polymer synthesis and screening.
4.2. Computational approach in polymer design for therapeutic delivery
The rational selection of functional small molecules used for post-modification of reactive polymers is essential for combinatorial polymer synthesis and delivery vehicle screening.[17, 31] Although thousands of polymers with structural diversity can be synthesized in parallel via the high-throughput polymer synthesis method in a short time,[20] the cost to purchase so many functional small molecules cannot be neglected. Pre-screening can guide functional small molecule selection and facilitate the efficient fabrication of polymeric delivery vehicles for stable therapeutic loading. Computational chemistries, such as theoretical methods and molecular simulations, have been applied in nanoparticle systems to understand therapeutic-loading properties.[148] The computation of the interactions between therapeutics and large polymers is usually time-consuming. Instead, the binding energy between therapeutics and small molecular building blocks can be rapidly computed by molecular docking, which allows for high-throughput virtual screening of a library of chemically diverse small molecules.[17, 31] In libraries synthesized by post-modification of reactive polymers, the structural diversity of different polymers originates solely from the structural small molecules used for the post-modification. The interactions between therapeutics and structural small molecular building blocks will contribute to the interactions between therapeutics and the resulting polymers, which rationalize the parallel comparison of the polymers conjugated with different structural small molecules. Therefore, the approach of virtual screening of small molecules is particularly suitable for the optimization of polymers synthesized by the post-modification approach. This virtual screening approach could help us to pre-select top-ranking functional small molecules from a large library of thousands of samples for combinatorial polymer synthesis followed by further experimental screening. Such an approach can save time and effort by helping narrow the range of small molecules to be tested at the bench.
The approach of virtual screening of functional small molecules followed by reactive polymer-based combinatorial polymer synthesis and systematic evaluation/optimization was suggested by us in 2015.[17] The initial success of this approach was obtained in a linear-dendritic telodendrimer system for the delivery of an anticancer drug, doxorubicin (Fig. 11a).[17] In a subsequent study, this approach was demonstrated to be effective in structure-based telodendrimer design for the delivery of a therapeutic protein, insulin (Fig. 11b).[31] Reasonable agreement was acquired when employing different computational methods (i.e., molecular docking and molecular dynamic simulation) to evaluate the binding affinity between therapeutics and small-molecule binders (Fig. 11c and d).[17] Moreover, significant correlations between the computational predictions and experimental results were observed (Fig. 11e and f).[17, 31] It should be mentioned that the post-modification-based combinatorial polymer synthesis and characterization are essential for systematic validation of the computational prediction in guiding the design of therapeutic delivery polymers. The optimized formulations identified by this computer-aided approach in these two studies showed affinity-controlled therapeutic release behaviors, leading to superior anticancer effects and improved blood glucose control, respectively.[17, 31] This novel bottom-up approach for the rational design and synthesis of therapeutic delivery polymers may dramatically accelerate the development of polymer-based therapeutics.
Fig. 11.
(a, b) Schematic illustrations of the rational design and combinatorial synthesis of telodendrimers for systematic evaluation and optimization for (a) drug delivery and (b) protein delivery. (c) Unbound interaction energy computed from molecular dynamics simulations plotted against the minimum docking energies. (d) The binding enthalpies analyzed by a solvent-balance method were plotted against the lowest docking energies. (e) Natural logarithm of equilibrium dissociation constant (lnKD) plotted against average docking energy (Ea). Linear regression was fit via Ordinary Least Square to calculate the Pearson correlation coefficient r and the associated P values for c, d and e. (f) Quantification of hypoglycemia index. Reproduced with permissions from ref. [17] and [31]. Copyrights to Nature Publishing Group and the American Chemical Society.
5. Conclusions and future perspectives
Herein, we have summarized the methods and recent advances in combinatorial polymer synthesis by post-modification of reactive polymers for the optimization of therapeutic delivery polymers. By using this post-modification approach, a library of polymers with distinct structural diversity and a consistent degree of polymerization can be synthesized in a combinatorial manner, which is critical for parallel comparison of different polymers for therapeutic delivery. The reactive polymer platforms allow for the integration of multiple kinds of functionalities in a controlled manner for efficient therapeutic encapsulation and delivery. The telodendrimer systems have provided a versatile blueprint for both structure-based polymer design and combinatorial polymer synthesis to synergistically accelerate the development and optimization of therapeutic delivery systems. In comparison to telodendrimers, the polysaccharides isolated from natural sources and conventional reactive polymers synthesized by single-step polymerization are generally more economic in developing vehicles for therapeutic delivery. The use of catalysts or co-reagents, and the formation of reaction byproducts during the post-modification processes in the above mentioned systems make them challenging to be adapted in a high-throughput manner when compared to the development of cationic polymers/lipidoids such as poly(β-amino ester)s for polynucleotide delivery. The in situ formation of functional polymers via traceless post-modification of highly reactive polymers (e.g., the azlactone- and hydrazide-functionalized polymers) provides an opportunity to introduce different building blocks sequentially or simultaneously in a combinatorial manner to generate a library of polymers for the screening and optimization of therapeutic delivery systems. The precise decorations, rational combinations, and tailored chemical components of the polar/nonpolar structural building blocks on these reactive polymers will be the key to the development of optimal delivery vehicles for a therapeutic. More studies on their biocompatibility, chemical and colloidal stability, and in vivo performance are necessary to further evaluate the potential for in vivo therapeutic delivery. Further, an approach of virtual screening of structural molecules followed by combinatorial polymer synthesis and systematic evaluation/optimization of therapeutic delivery polymers shows great promise in accelerating the development of therapeutic delivery systems via the combinatorial post-modification of reactive polymers and high-throughput screening processes.
The development of advanced reactive polymers is promising to increase the throughput of polymer synthesis. The establishment of fast and efficient screening methods for quantitative evaluations of the polymers used for the delivery of small-molecule drugs and protein therapeutics is also essential for high-throughput optimization of drug and protein delivery vehicles. The immobilization of reactive polymers on surfaces by bioprinting to form polymer arrays[149, 150], stepwise reactions to form brushes,[59, 151, 152] or layer-by-layer assembly to form multilayer films[53, 153–155] coupled with fast fluorescence or other optical detection techniques[156] may be another interesting approach toward high-throughput polymer screening of therapeutic loading properties. Although the drug-binding properties on the immobilized surface may be different from the physiochemical properties of drug-loaded nanoparticles in solution, it provides the first criteria to narrow down the drug-binding polymer candidates for focused evaluation. In short, reactive polymers provide an efficient platform greatly applicable for combinatorial polymer synthesis and optimization for therapeutic delivery. It is expected that more useful polymers will be developed and identified for the efficient delivery of nucleic acids, small-molecule drugs, and protein therapeutics in the future by fully exploring the reactive polymer-based combinatorial polymer synthesis methods.
Acknowledgments
This work was supported by the Program of Qilu Young Scholars of Shandong University (XW), National Natural Science Foundation of China (NSFC grant 21704057) (XW), NIH/NIBIB 1R21EB019607 (JL), Napi Family Research Awards (JL), and New York State Department of Health/PETER T. ROWLEY breast cancer research award (JL).
Footnotes
Disclosure
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–1235. doi: 10.1039/c2cs35265f. [DOI] [PubMed] [Google Scholar]
- 2.Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applications. Adv Drug Deliv Rev. 2012;64:866–884. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Roy K. Intracellular delivery of polymeric nanocarriers: a matter of size, shape, charge, elasticity and surface composition. Ther Deliv. 2013;4:705–723. doi: 10.4155/tde.13.37. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther. 2006;112:630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Guan X, Guo Z, Wang T, Lin L, Chen J, Tian H, Chen X. A pH-responsive detachable PEG shielding strategy for gene delivery system in cancer therapy. Biomacromolecules. 2017;18:1342–1349. doi: 10.1021/acs.biomac.7b00080. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Lin T-y, Luo Y, Liu Q, Xiao W, Guo W, Lac D, Zhang H, Feng C, Wachsmann-Hogiu S. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat Commun. 2014;5:4712. doi: 10.1038/ncomms5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Shi C, Zhang L, Bodman A, Guo D, Wang L, Hall WA, Wilkens S, Luo J. Affinity-controlled protein encapsulation into sub-30 nm telodendrimer nanocarriers by multivalent and synergistic interactions. Biomaterials. 2016;101:258–271. doi: 10.1016/j.biomaterials.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X, Zhang Y, Xie Z, Jing X, Bellotti A, Gu Z. Stimuli-responsive polymersomes for biomedical applications. Biomacromolecules. 2017;18:649–673. doi: 10.1021/acs.biomac.6b01704. [DOI] [PubMed] [Google Scholar]
- 9.Cheng R, Meng F, Deng C, Zhong Z. Bioresponsive polymeric nanotherapeutics for targeted cancer chemotherapy. Nano Today. 2015;10:656–670. [Google Scholar]
- 10.He Z, Santos JL, Tian H, Huang H, Hu Y, Liu L, Leong KW, Chen Y, Mao H-Q. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomaterials. 2017;130:28–41. doi: 10.1016/j.biomaterials.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Maheshwari R, Kiick KL. Polymer-based therapeutics. Macromolecules. 2009;42:3–13. doi: 10.1021/ma801782q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen S, Li H-J, Chen K-G, Wang Y-C, Yang X-Z, Lian Z-X, Du J-Z, Wang J. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017;17:3822–3829. doi: 10.1021/acs.nanolett.7b01193. [DOI] [PubMed] [Google Scholar]
- 13.Xiong X-B, Binkhathlan Z, Molavi O, Lavasanifar A. Amphiphilic block co-polymers: preparation and application in nanodrug and gene delivery. Acta Biomater. 2012;8:2017–2033. doi: 10.1016/j.actbio.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Banik BL, Fattahi P, Brown JL. Polymeric nanoparticles: the future of nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:271–299. doi: 10.1002/wnan.1364. [DOI] [PubMed] [Google Scholar]
- 15.Khalid M, El-Sawy HS. Polymeric nanoparticles: promising platform for drug delivery. Int J Pharm. 2017;528:675–691. doi: 10.1016/j.ijpharm.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Fay F, Hak S, Perez-Aguilar JM, Sanchez-Gaytan BL, Goode B, Duivenvoorden R, de Lange Davies C, Bjørkøy A, Weinstein H. Augmenting drug–carrier compatibility improves tumour nanotherapy efficacy. Nat Commun. 2016;7:11221. doi: 10.1038/ncomms11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C, Guo D, Xiao K, Wang X, Wang L, Luo J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat Commun. 2015;6:7449. doi: 10.1038/ncomms8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynn DM, Langer R. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–10768. [Google Scholar]
- 19.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001;123:8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 20.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Ed. 2003;115:3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 21.Anderson DG, Peng W, Akinc A, Hossain N, Kohn A, Padera R, Langer R, Sawicki JA. A polymer library approach to suicide gene therapy for cancer. Proc Natl Acad Sci USA. 2004;101:16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41:749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barua S, Joshi A, Banerjee A, Matthews D, Sharfstein ST, Cramer SM, Kane RS, Rege K. Parallel synthesis and screening of polymers for nonviral gene delivery. Mol Pharm. 2009;6:86–97. doi: 10.1021/mp800151j. [DOI] [PubMed] [Google Scholar]
- 24.Hao J, Kos P, Zhou K, Miller JB, Xue L, Yan Y, Xiong H, Elkassih S, Siegwart DJ. Rapid synthesis of a lipocationic polyester library via ring-opening polymerization of functional valerolactones for efficacious siRNA delivery. J Am Chem Soc. 2015;137:9206–9209. doi: 10.1021/jacs.5b03429. [DOI] [PubMed] [Google Scholar]
- 25.Rinkenauer AC, Tauhardt L, Wendler F, Kempe K, Gottschaldt M, Traeger A, Schubert US. A cationic poly(2-oxazoline) with high in vitro transfection efficiency identified by a library approach. Macromol Biosci. 2015;15:414–425. doi: 10.1002/mabi.201400334. [DOI] [PubMed] [Google Scholar]
- 26.Gan L, Olson JL, Ragsdale CW, Yu L. Poly(β-aminosulfonamides) as gene delivery vectors: synthesis and in vitro screening. Chem Commun. 2008:573–575. doi: 10.1039/b714278a. [DOI] [PubMed] [Google Scholar]
- 27.Sun B, Liu X, Buck ME, Lynn DM. Azlactone-functionalized polymers as reactive templates for parallel polymer synthesis: synthesis and screening of a small library of cationic polymers in the context of DNA delivery. Chem Commun. 2010;46:2016–2018. doi: 10.1039/b921664b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priegue JM, Crisan DN, Martínez-Costas J, Granja JR, Fernandez-Trillo F, Montenegro J. In situ functionalized polymers for siRNA delivery. Angew Chem Int Ed. 2016;128:7618–7621. doi: 10.1002/anie.201601441. [DOI] [PubMed] [Google Scholar]
- 29.Wong SY, Sood N, Putnam D. Combinatorial evaluation of cations, pH-sensitive and hydrophobic moieties for polymeric vector design. Mol Ther. 2009;17:480–490. doi: 10.1038/mt.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gauthier MA, Gibson MI, Klok H-A. Synthesis of functional polymers by post-polymerization modification. Angew Chem Int Ed. 2009;48:48–58. doi: 10.1002/anie.200801951. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Shi C, Zhang L, Lin MY, Guo D, Wang L, Yang Y, Duncan TM, Luo J. Structure-based nanocarrier design for protein delivery. ACS Macro Lett. 2017;6:267–271. doi: 10.1021/acsmacrolett.6b00982. [DOI] [PubMed] [Google Scholar]
- 32.Theato P. Synthesis of well-defined polymeric activated esters. J Polym Sci Pol Chem. 2008;46:6677–6687. [Google Scholar]
- 33.Gibson MI, Fröhlich E, Klok H-A. Postpolymerization modification of poly(pentafluorophenyl methacrylate): Synthesis of a diverse water-soluble polymer library. J Polym Sci Pol Chem. 2009;47:4332–4345. [Google Scholar]
- 34.Günay KA, Theato P, Klok H-A. Standing on the shoulders of Hermann Staudinger: post-polymerization modification from past to present. J Polym Sci Pol Chem. 2013;51:1–28. [Google Scholar]
- 35.Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–3502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abeylath SC, Amiji MM. ‘Click’ synthesis of dextran macrostructures for combinatorial-designed self-assembled nanoparticles encapsulating diverse anticancer therapeutics. Bioorg Med Chem. 2011;19:6167–6173. doi: 10.1016/j.bmc.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zha Z, Hu Y, Mukerabigwi J, Chen W, Wang Y, He C, Ge Z. Thiolactone chemistry-based combinatorial methodology to construct multifunctional polymers for efficacious gene delivery. Bioconjug Chem. 2018;29:23–28. doi: 10.1021/acs.bioconjchem.7b00672. [DOI] [PubMed] [Google Scholar]
- 38.Matyjaszewski K, Xia J. Atom transfer radical polymerization. Chem Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 39.Bontempo D, Heredia KL, Fish BA, Maynard HD. Cysteine-reactive polymers synthesized by atom transfer radical polymerization for conjugation to proteins. J Am Chem Soc. 2004;126:15372–15373. doi: 10.1021/ja045063m. [DOI] [PubMed] [Google Scholar]
- 40.Li A, Ma J, Wooley KL. Two distinct, reactive polymers derived from a single norbornenyl-methacryloyl bifunctional monomer by selective ATRP or ROMP. Macromolecules. 2009;42:5433–5436. [Google Scholar]
- 41.Li H, Bapat AP, Li M, Sumerlin BS. Protein conjugation of thermoresponsive amine-reactive polymers prepared by RAFT. Polym Chem. 2011;2:323–327. [Google Scholar]
- 42.Favier A, D'Agosto F, Charreyre M-T, Pichot C. Synthesis of N-acryloxysuccinimide copolymers by RAFT polymerization, as reactive building blocks with full control of composition and molecular weights. Polymer. 2004;45:7821–7830. [Google Scholar]
- 43.Louzao I, Garcia-Fandino R, Montenegro J. Hydrazone-modulated peptides for efficient gene transfection. J Mater Chem B. 2017;5:4426–4434. doi: 10.1039/c7tb00179g. [DOI] [PubMed] [Google Scholar]
- 44.Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5:439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 45.Mammen M, Dahmann G, Whitesides GM. Effective inhibitors of hemagglutination by influenza virus synthesized from polymers having active ester groups. insight into mechanism of inhibition. J Med Chem. 1995;38:4179–4190. doi: 10.1021/jm00021a007. [DOI] [PubMed] [Google Scholar]
- 46.Godwin A, Hartenstein M, Müller AHE, Brocchini S. Narrow molecular weight distribution precursors for polymer–drug conjugates. Angew Chem Int Ed. 2001;40:594–597. doi: 10.1002/1521-3773(20010202)40:3<594::AID-ANIE594>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 47.Pedone E, Li X, Koseva N, Alpar O, Brocchini S. An information rich biomedical polymer library. J Mater Chem. 2003;13:2825–2837. [Google Scholar]
- 48.Ferruti P, Bettelli A, Feré A. High polymers of acrylic and methacrylic esters of N -hydroxysuccinimide as polyacrylamide and polymethacrylamide precursors. Polymer. 1972;13:462–464. [Google Scholar]
- 49.Heilmann SM, Rasmussen JK, Krepski LR, Smith HK. Chemistry of alkenyl azlactones. IV. Preparation and properties of telechelic acrylamides derived from amine-terminated oligomers. J Polymer Sci Polymer Chem Ed. 1984;22:3149–3160. [Google Scholar]
- 50.Rasmussen JK, Heilmann SM, Krepski LR, Jensen KM, Mickelson J, Johnson K, Coleman PL, Milbrath DS, Walker MM. Crosslinked, hydrophilic, azlactone-functional polymeric beads: A two-step approach. React Polym. 1992;16:199–212. [Google Scholar]
- 51.Drtina GJ, Heilmann SM, Moren DM, Rasmussen JK, Krepski LR, Smith HK, Pranis RA, Turek TC. Highly cross-linked azlactone functional supports of tailorable polarity. Macromolecules. 1996;29:4486–4489. [Google Scholar]
- 52.Heilmann SM, Drtina GJ, Eitzman PD, Haddad LC, Coleman PL, Hyde FW, Johnson TW, Rasmussen JK, Smith HK, Liu JJ, Fitzsimons RT, Williams MG, Moeller SJ, Nakamura MM, Gibbens KJ, Buhl TL. Cartridge filter systems containing immobilized enzymes. J Mol Catal B-Enzym. 2007;45:1–9. [Google Scholar]
- 53.Sun B, Lynn DM. Release of DNA from polyelectrolyte multilayers fabricated using ‘charge-shifting’ cationic polymers: Tunable temporal control and sequential, multi-agent release. J Control Release. 2010;148:91–100. doi: 10.1016/j.jconrel.2010.07.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levere ME, Ho HT, Pascual S, Fontaine L. Stable azlactone-functionalized nanoparticles prepared from thermoresponsive copolymers synthesized by RAFT polymerization. Polym Chem. 2011;2:2878–2887. [Google Scholar]
- 55.Barringer JE, Messman JM, Banaszek AL, Meyer HM, Kilbey SM. Immobilization of biomolecules on poly(vinyldimethylazlactone)-containing surface scaffolds. Langmuir. 2009;25:262–268. doi: 10.1021/la802925g. [DOI] [PubMed] [Google Scholar]
- 56.Lokitz BS, Messman JM, Hinestrosa JP, Alonzo J, Verduzco R, Brown RH, Osa M, Ankner JF, Kilbey SM. Dilute solution properties and surface attachment of RAFT polymerized 2-vinyl-4,4-dimethyl azlactone (VDMA) Macromolecules. 2009;42:9018–9026. [Google Scholar]
- 57.Messman JM, Lokitz BS, Pickel JM, Kilbey SM. Highly tailorable materials based on 2-vinyl-4,4-dimethyl azlactone: (co)polymerization, synthetic manipulation and characterization. Macromolecules. 2009;42:3933–3941. [Google Scholar]
- 58.Lokitz BS, Wei J, Hinestrosa JP, Ivanov I, Browning JF, Ankner JF, Kilbey SM, Messman JM. Manipulating interfaces through surface confinement of poly(glycidyl methacrylate)-block-poly(vinyldimethylazlactone), a dually reactive block copolymer. Macromolecules. 2012;45:6438–6449. [Google Scholar]
- 59.Aden B, Kite CM, Hopkins BW, Zetterberg A, Lokitz BS, Ankner JF, Kilbey SM. Assessing chemical transformation of reactive, interfacial thin films made of end-tethered poly(2-vinyl-4,4-dimethyl azlactone) (PVDMA) chains. Macromolecules. 2017;50:618–630. [Google Scholar]
- 60.Buck ME, Lynn DM. Azlactone-functionalized polymers as reactive platforms for the design of advanced materials: Progress in the last ten years. Polym Chem. 2012;3:66–80. doi: 10.1039/C1PY00314C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godula K, Bertozzi CR. Synthesis of glycopolymers for microarray applications via ligation of reducing sugars to a poly(acryloyl hydrazide) scaffold. J Am Chem Soc. 2010;132:9963–9965. doi: 10.1021/ja103009d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar A, Ujjwal RR, Mittal A, Bansal A, Ojha U. Polyacryloyl hydrazide: an efficient, simple, and cost effective precursor to a range of functional materials through hydrazide based click reactions. ACS Appl Mater Interfaces. 2014;6:1855–1865. doi: 10.1021/am404837f. [DOI] [PubMed] [Google Scholar]
- 63.Mahon CS, Fulton DA. Templation-induced re-equilibration in polymer-scaffolded dynamic combinatorial libraries leads to enhancements in binding affinities. Chem Sci. 2013;4:3661–3666. [Google Scholar]
- 64.Mahon CS, Fascione MA, Sakonsinsiri C, McAllister TE, Bruce Turnbull W, Fulton DA. Templating carbohydrate-functionalised polymer-scaffolded dynamic combinatorial libraries with lectins. Org Biomol Chem. 2015;13:2756–2761. doi: 10.1039/c4ob02587c. [DOI] [PubMed] [Google Scholar]
- 65.Mahon CS, Jackson AW, Murray BS, Fulton DA. Templating a polymer-scaffolded dynamic combinatorial library. Chem Commun. 2011;47:7209–7211. doi: 10.1039/c1cc11998b. [DOI] [PubMed] [Google Scholar]
- 66.Bartolami E, Bessin Y, Gervais V, Dumy P, Ulrich S. Dynamic expression of DNA complexation with self-assembled biomolecular clusters. Angew Chem Int Ed. 2015;54:10183–10187. doi: 10.1002/anie.201504047. [DOI] [PubMed] [Google Scholar]
- 67.Xiong MP, Bae Y, Fukushima S, Forrest ML, Nishiyama N, Kataoka K, Kwon GS. pH-Responsive multi-PEGylated dual cationic nanoparticles enable charge modulations for safe gene delivery. ChemMedChem. 2007;2:1321–1327. doi: 10.1002/cmdc.200700093. [DOI] [PubMed] [Google Scholar]
- 68.Kalia J, Raines RT. Hydrolytic stability of hydrazones and oximes. Angew Chem Int Ed. 2008;47:7523–7526. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliv Rev. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Malafaya PB, Silva GA, Reis RL. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 71.Wang W, Liu X, Xie Y, Zhang Ha, Yu W, Xiong Y, Xie W, Ma X. Microencapsulation using natural polysaccharides for drug delivery and cell implantation. J Mater Chem. 2006;16:3252–3267. [Google Scholar]
- 72.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Jewett JC, Bertozzi CR. Cu-free click cycloaddition reactions in chemical biology. Chem Soc Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gillies ER, Fréchet JM. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 75.Kakkar A, Traverso G, Farokhzad OC, Weissleder R, Langer R. Evolution of macromolecular complexity in drug delivery systems. Nat Rev Chem. 2017;1:0063. doi: 10.1038/s41570-017-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gitsov I, Hamzik J, Ryan J, Simonyan A, Nakas JP, Omori S, Krastanov A, Cohen T, Tanenbaum SW. Enzymatic nanoreactors for environmentally benign biotransformations. 1. Formation and catalytic activity of supramolecular complexes of laccase and linear-dendritic block copolymers. Biomacromolecules. 2008;9:804–811. doi: 10.1021/bm701081m. [DOI] [PubMed] [Google Scholar]
- 77.Gitsov I, Wang L, Vladimirov N, Simonyan A, Kiemle DJ, Schutz A. "Green" synthesis of unnatural poly(amino acid)s with zwitterionic character and pH-responsive solution behavior, mediated by linear-dendritic laccase complexes. Biomacromolecules. 2014;15:4082–4095. doi: 10.1021/bm501126a. [DOI] [PubMed] [Google Scholar]
- 78.Luo J, Xiao K, Li Y, Lee JS, Shi L, Tan Y-H, Xing L, Holland Cheng R, Liu G-Y, Lam KS. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjug Chem. 2010;21:1216–1224. doi: 10.1021/bc1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L, Shi C, Wright FA, Guo D, Wang X, Wang D, Wojcikiewicz RJH, Luo J. Multifunctional telodendrimer nanocarriers restore synergy of bortezomib and doxorubicin in ovarian cancer treatment. Cancer Res. 2017;77:3293–3305. doi: 10.1158/0008-5472.CAN-16-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo D, Shi C, Wang X, Wang L, Zhang S, Luo J. Riboflavin-containing telodendrimer nanocarriers for efficient doxorubicin delivery: High loading capacity, increased stability, and improved anticancer efficacy. Biomaterials. 2017;141:161–175. doi: 10.1016/j.biomaterials.2017.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai L, Xu G, Shi C, Guo D, Wang X, Luo J. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: A synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials. 2015;37:456–468. doi: 10.1016/j.biomaterials.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang W, Wang X, Shi C, Guo D, Xu G, Wang L, Bodman A, Luo J. Fine-tuning vitamin E-containing telodendrimers for efficient delivery of gambogic acid in colon cancer treatment. Mol Pharm. 2015;12:1216–1229. doi: 10.1021/acs.molpharmaceut.5b00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burrer CM, Auburn H, Wang X, Luo J, Abulwerdi FA, Nikolovska-Coleska Z, Chan GC. Mcl-1 small-molecule inhibitors encapsulated into nanoparticles exhibit increased killing efficacy towards HCMV-infected monocytes. Antiviral Res. 2017;138:40–46. doi: 10.1016/j.antiviral.2016.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang W, Wang X, Guo D, Luo J, Nangia S. Drug-specific design of telodendrimer architecture for effective doxorubicin encapsulation. J Phys Chem B. 2016;120:9766–9777. doi: 10.1021/acs.jpcb.6b06070. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Shi C, Wang L, Luo J. Polycation-telodendrimer nanocomplexes for intracellular protein delivery. Colloids Surf B Biointerfaces. 2018;162:405–414. doi: 10.1016/j.colsurfb.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu G, Shi C, Guo D, Wang L, Ling Y, Han X, Luo J. Functional-segregated coumarin-containing telodendrimer nanocarriers for efficient delivery of SN-38 for colon cancer treatment. Acta Biomater. 2015;21:85–98. doi: 10.1016/j.actbio.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jain S, Neumann K, Zhang Y, Geng J, Bradley M. Tetrazine-mediated postpolymerization modification. Macromolecules. 2016;49:5438–5443. [Google Scholar]
- 88.Neumann K, Jain S, Geng J, Bradley M. Nanoparticle “switch-on” by tetrazine triggering. Chem Commun. 2016;52:11223–11226. doi: 10.1039/c6cc05118a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Duong HTT, Jones MW, Basuki JS, Hu J, Boyer C, Davis TP. Selective postmodification of copolymer backbones bearing different activated esters with disparate reactivities. ACS Macro Lett. 2013;2:912–917. doi: 10.1021/mz4004375. [DOI] [PubMed] [Google Scholar]
- 90.Kalal J, Švec F, Maroušek V. J Polym Sci Part C: Polym Symp. Wiley Online Library; 1974. Reactions of epoxide groups of glycidyl methacrylate copolymers; pp. 155–166. [Google Scholar]
- 91.Barbey R, Klok H-A. Room temperature, aqueous post-polymerization modification of glycidyl methacrylate-containing polymer brushes prepared via surface-initiated atom transfer radical polymerization. Langmuir. 2010;26:18219–18230. doi: 10.1021/la102400z. [DOI] [PubMed] [Google Scholar]
- 92.Siegwart DJ, Whitehead KA, Nuhn L, Sahay G, Cheng H, Jiang S, Ma M, Lytton-Jean A, Vegas A, Fenton P. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc Natl Acad Sci USA. 2011;108:12996–13001. doi: 10.1073/pnas.1106379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim J, Kang Y, Tzeng SY, Green JJ. Synthesis and application of poly(ethylene glycol)-co-poly(β-amino ester) copolymers for small cell lung cancer gene therapy. Acta Biomater. 2016;41:293–301. doi: 10.1016/j.actbio.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu G, Swierczewska M, Lee S, Chen X. Functional nanoparticles for molecular imaging guided gene delivery. Nano Today. 2010;5:524–539. doi: 10.1016/j.nantod.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng J, Tang X, Zhao J, Shi T, Zhao P, Lin C. Multifunctional cationic polyurethanes designed for non-viral cancer gene therapy. Acta Biomater. 2016;30:155–167. doi: 10.1016/j.actbio.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 96.Jaroff L. Fixing the genes. Time. 1999;153:68–70. [PubMed] [Google Scholar]
- 97.Uchida H, Miyata K, Oba M, Ishii T, Suma T, Itaka K, Nishiyama N, Kataoka K. Odd–even effect of repeating aminoethylene units in the side chain of N-substituted polyaspartamides on gene transfection profiles. J Am Chem Soc. 2011;133:15524–15532. doi: 10.1021/ja204466y. [DOI] [PubMed] [Google Scholar]
- 98.Zha Z, Li J, Ge Z. Endosomal-escape polymers based on multicomponent reaction-synthesized monomers integrating alkyl and imidazolyl moieties for efficient gene delivery. ACS Macro Lett. 2015;4:1123–1127. doi: 10.1021/acsmacrolett.5b00615. [DOI] [PubMed] [Google Scholar]
- 99.Zhou J, Liu J, Cheng CJ, Patel TR, Weller CE, Piepmeier JM, Jiang Z, Saltzman WM. Biodegradable poly (amine-co-ester) terpolymers for targeted gene delivery. Nat Mater. 2012;11:82–90. doi: 10.1038/nmat3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siegel RL, Miller KD, Jemal A. Cancer statistics 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 101.Le Garrec D, Gori S, Luo L, Lessard D, Smith DC, Yessine MA, Ranger M, Leroux JC. Poly(N-vinylpyrrolidone)-block-poly(d,l-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. J Control Release. 2004;99:83–101. doi: 10.1016/j.jconrel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 102.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small. 2007;3:1341–1346. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 103.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 104.Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991;51:3229–3236. [PubMed] [Google Scholar]
- 105.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 106.Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guan J, Zhou ZQ, Chen MH, Li HY, Tong DN, Yang J, Yao J, Zhang ZY. Folate-conjugated and pH-responsive polymeric micelles for target-cell-specific anticancer drug delivery. Acta Biomater. 2017;60:244–255. doi: 10.1016/j.actbio.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 108.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Nishiyama UMN, Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 109.Zhang X, Huang Y, Zhao W, Chen Y, Zhang P, Li J, Venkataramanan R, Li S. PEG-farnesyl thiosalicylic acid telodendrimer micelles as an improved formulation for targeted delivery of paclitaxel. Mol Pharm. 2014;11:2807–2814. doi: 10.1021/mp500181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu J, Jian Z, Chao D, Meng F, Ru C, Zhong Z. Robust, responsive and targeted PLGA anticancer nanomedicines by combining reductively cleavable surfactant and covalent hyaluronic acid coating. ACS Appl Mater Interfaces. 2017;9:3985–3994. doi: 10.1021/acsami.6b15105. [DOI] [PubMed] [Google Scholar]
- 111.Sun J, Chen Y, Li K, Huang Y, Fu X, Zhang X, Zhao W, Wei Y, Xu L, Zhang P, Venkataramanan R, Li S. A prodrug micellar carrier assembled from polymers with pendant farnesyl thiosalicylic acid moieties for improved delivery of paclitaxel. Acta Biomater. 2016;43:282–291. doi: 10.1016/j.actbio.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakamura Y, Mochida A, Choyke PL, Kobayashi H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem. 2016;27:2225–2238. doi: 10.1021/acs.bioconjchem.6b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao K, Li Y, Lee JS, Gonik AM, Dong T, Fung G, Sanchez E, Xing L, Cheng HR, Luo J. “OA02” peptide facilitates the precise targeting of paclitaxel-loaded micellar nanoparticles to ovarian cancer in vivo. Cancer Res. 2012;72:2100–2110. doi: 10.1158/0008-5472.CAN-11-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh A, Talekar M, Tran T-H, Samanta A, Sundaram R, Amiji M. Combinatorial approach in the design of multifunctional polymeric nano-delivery systems for cancer therapy. J Mater Chem B. 2014;2:8069–8084. doi: 10.1039/c4tb01083c. [DOI] [PubMed] [Google Scholar]
- 115.Abeylath SC, Ganta S, Iyer AK, Amiji M. Combinatorial-designed multifunctional polymeric nanosystems for tumor-targeted therapeutic delivery. Acc Chem Res. 2011;44:1009–1017. doi: 10.1021/ar2000106. [DOI] [PubMed] [Google Scholar]
- 116.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 117.Rösler A, Vandermeulen GWM, Klok H-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev. 2012;64:270–279. doi: 10.1016/s0169-409x(01)00222-8. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, Grayson SM. Approaches for the preparation of non-linear amphiphilic polymers and their applications to drug delivery. Adv Drug Deliv Rev. 2012;64:852–865. doi: 10.1016/j.addr.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 119.Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, Hirose T, Kraszewski A, Itakura K, Riggs AD. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci USA. 1979;76:106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang M, Alberti K, Sun S, Arellano CL, Xu Q. Combinatorially designed lipid-like nanoparticles for intracellular delivery of cytotoxic protein for cancer therapy. Angew Chem Int Ed. 2014;53:2893–2898. doi: 10.1002/anie.201311245. [DOI] [PubMed] [Google Scholar]
- 121.Wu J, Kamaly N, Shi J, Zhao L, Xiao Z, Hollett G, John R, Ray S, Xu X, Zhang X. Development of multinuclear polymeric nanoparticles as robust protein nanocarriers. Angew Chem Int Ed. 2014;53:8975–8979. doi: 10.1002/anie.201404766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 123.Wang C-Y, Mayo MW, Baldwin AS. TNF-and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 124.Chen J, Zhao M, Feng F, Sizovs A, Wang J. Tunable thioesters as “reduction” responsive functionality for traceless reversible protein PEGylation. J Am Chem Soc. 2013;135:10938–10941. doi: 10.1021/ja405261u. [DOI] [PubMed] [Google Scholar]
- 125.Liu M, Johansen P, Zabel F, Leroux J-C, Gauthier MA. Semi-permeable coatings fabricated from comb-polymers efficiently protect proteins in vivo. Nat Commun. 2014;5:5526. doi: 10.1038/ncomms6526. [DOI] [PubMed] [Google Scholar]
- 126.Kobsa S, Saltzman WM. Bioengineering approaches to controlled protein delivery. Pediatr Res. 2008;63:513–519. doi: 10.1203/PDR.0b013e318165f14d. [DOI] [PubMed] [Google Scholar]
- 127.Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43:3595–3629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 128.Nie L, Zhang G, Hou R, Xu H, Li Y, Fu J. Controllable promotion of chondrocyte adhesion and growth on PVA hydrogels by controlled release of TGF-β1 from porous PLGA microspheres. Colloids Surf B Biointerfaces. 2015;125:51–57. doi: 10.1016/j.colsurfb.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 129.Chen J-L, Chiang C-H, Yeh M-K. The mechanism of PLA microparticle formation by waterin-oil-in-water solvent evaporation method. J Microencapsul. 2002;19:333–346. doi: 10.1080/02652040110105373. [DOI] [PubMed] [Google Scholar]
- 130.Lu W, Park TG. Protein release from poly (lactic-co-glycolic acid) microspheres: protein stability problems. PDA J Pharm Sci Tech. 1995;49:13–19. [PubMed] [Google Scholar]
- 131.Peppas NA, Wood KM, Blanchette JO. Hydrogels for oral delivery of therapeutic proteins. Expert Opin Biol Ther. 2004;4:881–887. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 132.Vermonden T, Censi R, Hennink WE. Hydrogels for protein delivery. Chem Rev. 2012;112:2853–2888. doi: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- 133.Ayame H, Morimoto N, Akiyoshi K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjug Chem. 2008;19:882–890. doi: 10.1021/bc700422s. [DOI] [PubMed] [Google Scholar]
- 134.Morimoto N, Hirano S, Takahashi H, Loethen S, Thompson DH, Akiyoshi K. Self-assembled pH-sensitive cholesteryl pullulan nanogel as a protein delivery vehicle. Biomacromolecules. 2012;14:56–63. doi: 10.1021/bm301286h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yan M, Du J, Gu Z, Liang M, Hu Y, Zhang W, Priceman S, Wu L, Zhou ZH, Liu Z. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat Nanotechnol. 2010;5:48–53. doi: 10.1038/nnano.2009.341. [DOI] [PubMed] [Google Scholar]
- 136.Dao-Pin S, Anderson D, Baase W, Dahlquist F, Matthews BW. Structural and thermodynamic consequences of burying a charged residue within the hydrophobic core of T4 lysozyme. Biochemistry. 1991;30:11521–11529. doi: 10.1021/bi00113a006. [DOI] [PubMed] [Google Scholar]
- 137.Robinson AC, Castañeda CA, Schlessman JL. Structural and thermodynamic consequences of burial of an artificial ion pair in the hydrophobic interior of a protein. Proc Natl Acad Sci USA. 2014;111:11685–11690. doi: 10.1073/pnas.1402900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Walther TH, Ulrich AS. Transmembrane helix assembly and the role of salt bridges. Curr Opin Struct Biol. 2014;27:63–68. doi: 10.1016/j.sbi.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 139.Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 140.Caminade A-M, Ouali A, Laurent R, Turrin C-O, Majoral J-P. The dendritic effect illustrated with phosphorus dendrimers. Chem Soc Rev. 2015;44:3890–3899. doi: 10.1039/c4cs00261j. [DOI] [PubMed] [Google Scholar]
- 141.Vonnemann J, Liese S, Kuehne C, Ludwig K, Dernedde J, Böttcher C, Netz RR, Haag R. Size dependence of steric shielding and multivalency effects for globular binding inhibitors. J Am Chem Soc. 2015;137:2572–2579. doi: 10.1021/ja5114084. [DOI] [PubMed] [Google Scholar]
- 142.Wang X, Bodman A, Shi C, Guo D, Wang L, Luo J, Hall WA. Tunable lipidoid-telodendrimer hybrid nanoparticles for intracellular protein delivery in brain tumor treatment. Small. 2016;12:4185–4192. doi: 10.1002/smll.201601234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.White AD, Keefe AJ, Ella-Menye J-R, Nowinski AK, Shao Q, Pfaendtner J, Jiang S. Free energy of solvated salt bridges: a simulation and experimental study. J Phys Chem B. 2013;117:7254–7259. doi: 10.1021/jp4024469. [DOI] [PubMed] [Google Scholar]
- 144.Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. Biomaterial microarrays: rapid, microscale screening of polymer–cell interaction. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 145.Yu H-L, Xu J-H, Wang Y-X, Lu W-Y, Lin G-Q. Assembly of a three-dimensional array of glycoconjugates by combinatorial biocatalysis in nonaqueous media. J Comb Chem. 2007;10:79–87. doi: 10.1021/cc7001606. [DOI] [PubMed] [Google Scholar]
- 146.Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, Jayaprakash KN, Jayaraman M, Rajeev KG, Manoharan M, Koteliansky V, Röhl I, Leshchiner ES, Langer R, Anderson DG. Development of lipidoid–siRNA formulations for systemic delivery to the liver. Mol Ther. 2009;17:872–879. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Whitehead KA, Dorkin JR, Vegas AJ, Chang PH, Veiseh O, Matthews J, Fenton OS, Zhang Y, Olejnik KT, Yesilyurt V, Chen D, Barros S, Klebanov B, Novobrantseva T, Langer R, Anderson DG. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277. doi: 10.1038/ncomms5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huynh L, Neale C, Pomès R, Allen C. Computational approaches to the rational design of nanoemulsions, polymeric micelles, and dendrimers for drug delivery. Nanomed-Nanotechnol. 2012;8:20–36. doi: 10.1016/j.nano.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 149.Zhu XD, Landry JP, Sun YS, Gregg JP, Lam KS, Guo XW. Oblique-incidence reflectivity difference microscope for label-free high-throughput detection of biochemical reactions in microarray format. Appl Opt. 2007;46:1890–1895. doi: 10.1364/ao.46.001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hook AL, Chang CY, Yang J, Scurr DJ, Robert L, Anderson DG, Steve A, Paul W, Davies MC, Alexander MR. Polymer microarrays for high throughput discovery of biomaterials. J Vis Exp. 2012:e3636. doi: 10.3791/3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Deodhar C, Soto-Cantu E, Uhrig D, Bonnesen P, Lokitz BS, Ankner JF, Kilbey SM. Hydration in weak polyelectrolyte brushes. ACS Macro Lett. 2013;2:398–402. doi: 10.1021/mz300615v. [DOI] [PubMed] [Google Scholar]
- 152.Barbey R, Lavanant L, Paripovic D, Schüwer N, Sugnaux C, Tugulu S, Klok H-A. Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications. Chem Rev. 2009;109:5437–5527. doi: 10.1021/cr900045a. [DOI] [PubMed] [Google Scholar]
- 153.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 154.Li Y, Wang X, Sun J. Layer-by-layer assembly for rapid fabrication of thick polymeric films. Chem Soc Rev. 2012;41:5998–6009. doi: 10.1039/c2cs35107b. [DOI] [PubMed] [Google Scholar]
- 155.Wang X, Wang Y, Bi S, Wang Y, Chen X, Qiu L, Sun J. Optically transparent antibacterial films capable of healing multiple scratches. Adv Funct Mater. 2014;24:403–411. [Google Scholar]
- 156.Fei Y, Landry JP, Sun Y, Zhu X, Wang X, Luo J, Wu CY, Lam KS. Screening small-molecule compound microarrays for protein ligands without fluorescence labeling with a high-throughput scanning microscope. J Biomed Opt. 2010;15:016018. doi: 10.1117/1.3309743. [DOI] [PMC free article] [PubMed] [Google Scholar]