Abstract
Phlpp protein phosphatases are abnormally abundant within human osteoarthritic articular chondrocytes and may contribute to the development of osteoarthritis. Mice lacking Phlpp1 were previously shown to be resistant to post-traumatic osteoarthritis. Here a small molecule with therapeutic properties that inhibits Phlpp1 and Phlpp2 was tested for its ability to slow post-traumatic OA in mice and to stimulate anabolic pathways in human articular cartilage from OA joints. PTOA was induced in male C57Bl/6 mice by surgically destabilizing the meniscus. Seven weeks after surgery, mice received a single intra-articular injection of the Phlpp inhibitor NSC117079 or saline. Mechanical allodynia was measured with von Frey assays, mobility was tracked in an open field system, and cartilage damage was assessed histologically. A single intra-articular injection of the Phlpp inhibitor NSC117079 attenuated mechanical allodynia and slowed articular cartilage degradation in joints with a destabilized meniscus. Animals treated with the Phlpp inhibitor seven weeks after injury maintained normal activity levels, while those in the control group traveled shorter distances and were less active three months after the joint injury. NSC117079 also increased production of cartilage extracellular matrix components (glycosaminoglycans and aggrecan) in over 90% of human articular cartilage explants from OA patients and increased phosphorylation of Phlpp1 substrates (AKT2, ERK1/2 and PKC) in human articular chondrocytes. Our results indicate that Phlpp inhibitor NSC117079 is a novel osteoarthritis disease modifying drug candidate that may have palliative affects.
Keywords: Phlpp1, DMM surgery, allodynia, post-traumatic osteoarthritis
Introduction
Osteoarthritis (OA) is a progressive and painful condition characterized by the degradation of articular cartilage within joints. It is a leading cause of disability and hospital visits (1–6). The majority of OA patients are managed conservatively through weight loss, exercise or use of mobility aids. Severe pain is addressed with steroidal and non-steroidal anti-inflammatory drugs or with opioids (7). Although these drugs can temporally provide relief, they are only administered for a limited period of time because these pleiotropic drugs increase the risk of adverse events, such as bone loss, liver toxicity, cardiovascular events and addiction (8). Other compounds, such as injectable hyaluronans and oral glycosaminoglycans (e.g., glucosamine or chondroitin) aim to increase joint lubrication, but their effectiveness is unproven (9). None of the currently available treatment options effectively alter disease progression by slowing articular cartilage degradation (catabolism) and/or promoting its regeneration (anabolism) prior to the onset of arthritis. Alternative therapeutic options are needed to relieve pain and prolong articular cartilage viability.
Post-traumatic OA (PTOA) accounts for 12% of all OA cases and is an ideal condition to test new therapies because the disease initiation and development can be tracked and the effectiveness of therapies that might slow cartilage deterioration can be more accurately evaluated (10). The murine DMM (destabilization of the medial meniscal tibial ligament) model replicates the instability of knee joints caused by some injuries and allows for the testing of potential therapies that address the cellular basis for disease progression (11).
Phlpp1/2 (pleckstrin homology domain leucine-rich protein phosphatases 1/2) are intracellular enzymes that dephosphorylate a number of substrates that promote cell survival and/or protein synthesis, including protein kinase B (PKB/AKT), PKC, and p70 S6K (12–14). We previously showed that Phlpp1-deficient mice are resistant to cartilage loss and mechanical allodynia in the DMM model of PTOA model (15). Phlpp1 KO mice also displayed thicker articular cartilage and more articular chondrocytes. Phlpp staining is aberrantly high in articular cartilage from human OA joints and increases in Phlpp1 transcripts are associated with altered DNA methylation and inflammatory stimulation (15). Phlpp inhibitors stabilize the phosphorylation of AKT2 and PKC on crucial serines and threonines, promoting their activity or stability in chondrocytes, and stimulate matrix production by chondrocytes in culture (15, 16). In the present study, we tested the ability of a small molecule inhibitor of Phlpp1/2 (NSC117079) with therapeutic properties (17) to slow OA progression in mice and promote matrix production in human articular cartilage explants.
Methods
DMM surgeries and intra-articular injections
Twenty-four male mice were purchased from Jackson Labs at 6 to 8 weeks of age. They were acclimated to the new environment for 2 to 3 weeks before beginning the experiment. Fourteen 12-week-old male C57Bl/6 mice underwent a surgery that destabilized the medial meniscus (DMM) by transecting the medial meniscotibial ligament (MMTL) in the right hind limb knee joint as previously described (11, 15). Another 10 mice underwent sham surgeries that were performed by opening the joint space to visualize the MMTL without transection. Mice in the sham group received a single 3 μl intra-articular injection of saline (vehicle) or of 8 μM Phlpp inhibitor (NCS 117079, obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis of the National Cancer Institute) into the right knee joint 8 weeks after surgery (Fig 1A). After hypersensitivity and functional assays revealed no adverse events related to the inhibitor treatment (Fig 2 and 3), we injected inhibitors or saline in the joints of mice that received DMM surgery at 7 weeks post-surgery so the drug effects could be measured for a slightly longer period of time (5 weeks versus 4 weeks). The estimated concentration of the inhibitor in the joint space immediately after injection was approximately 4 μM, which is comparable to the IC50 (17). Intra-articular injections were performed using 50 μl syringes (Hamilton 7637-01) and 30-gauge small hub removable needles (Hamilton 7803-07). Left hind limb knee joints did not receive any surgery or injection and were used as the internal control for each mouse. All animal research was conducted according to National Institute of Health and the Institute of Laboratory Animal Resources, National Research Council guidelines. The Mayo Clinic Institutional Animal Care and Use Committee approved all these rodent studies. Mice were observed daily for adverse reactions in the injected leg (e.g., rash, infection, limping, dragging paws). No adverse events were observed.
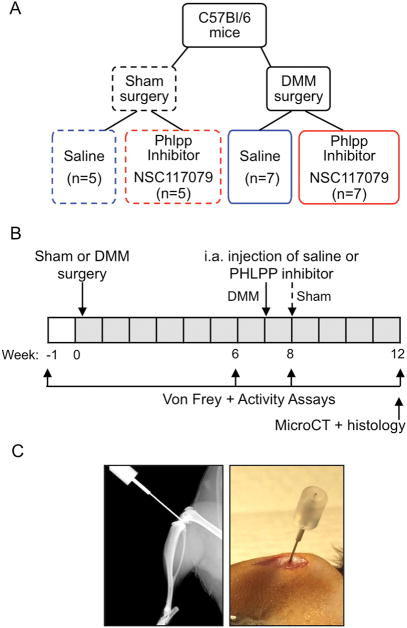
Figure 1. Timeline for surgical, injection and assessment procedures.
A. Four groups of adult male C57Bl/6 mice were studied. Mice underwent either sham or DMM surgery on their right hind knees at 12 weeks of age. These animals were then randomly assigned to one of two treatment groups that received a single intra-articular injection of either saline or 8μM Phlpp inhibitor. B. The timeline of surgeries, intra-articular injections, von Frey and activity assays, and histology and microCT assays is shown. C. An X-ray and photograph showing positioning of needle in mouse knee joint.
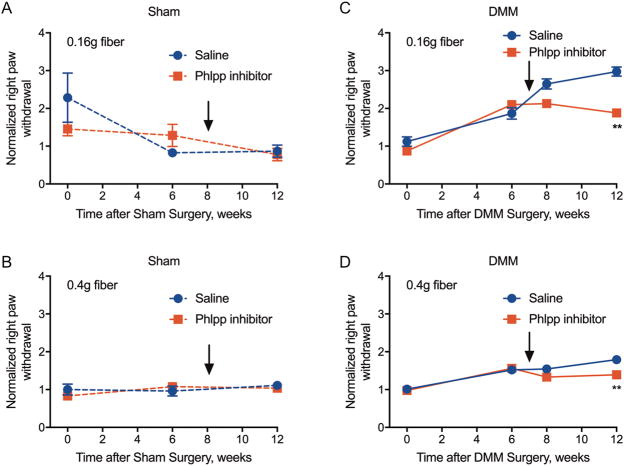
Figure 2. Phlpp inhibitors prevent mechanical allodynia in mice subjected to DMM surgery.
Von Frey assays were performed with flexible monofilaments of 0.16 gram (A,C) or 0.4 gram (B,D). Sham groups had six animals per treatment (A, B). DMM groups had seven animals per treatment (C, D). **p<0.01.
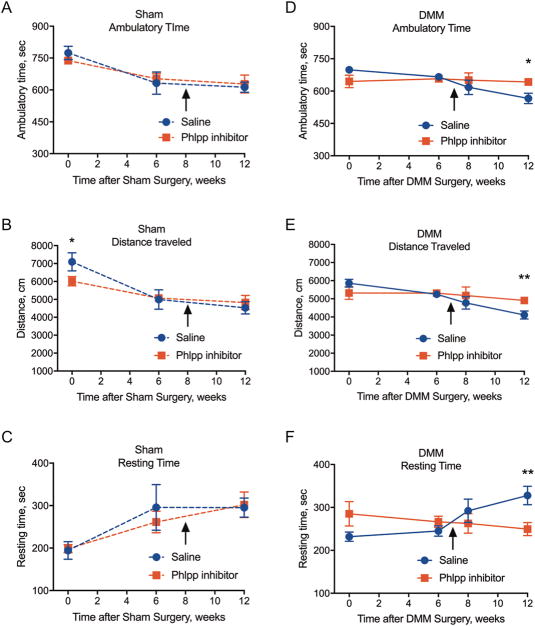
Figure 3. Phlpp inhibitors improve activity in mice subjected to DMM surgery.
Mice were placed into an activity chamber for 20 minutes (1,200 sec). Measurements taken were time with large ambulatory movements (A, D), distance traveled (B, E), and resting time when no movement was detected (C, D). Results represent the mean ± SD from six animals in the sham groups (A–C) and seven animals in the DMM groups (E–H). *p<0.05, **p<0.01.
Behavioral studies
Von Frey assays were performed as previously described (15) using flexible monofilaments (0.16g and 0.4g) (Touch Test Sensory Evaluator Kit, Stoelting, IL) to quantify mechanical allodynia. Mice were habituated to the von Frey chambers for 30 minutes every other day for two weeks preceding the experiment. The number of times each hind paw was withdrawn in response to five applications of von Frey fiber to plantar surface of mouse hind paw was recorded. The assay was repeated six times on two different days. Withdrawal responses in the right paw were divided by the withdrawal responses of left leg based on a total of 30 measurements to achieve normalized values per mouse. Baseline measurements were taken on both hind paws prior to surgery and several times after surgery, before and following intra-articular injection, as indicated in Fig 1B.
Activity assays were performed as previously described (15, 18) using VersaMax Animal Activity Monitors (AccuScan Model RXYZCM-16, Columbus, OH). Mice were habituated to the activity chamber by allowing them to run freely in the unit for 20 minutes four times during the two weeks prior to starting the experiment. Mice were also habituated to the testing room at least two hours before testing and allowed to run freely for 5 minutes in open chambers prior to the 20-minute recording time period. Data were analyzed on a VersaMax Analyzer (AccuScan Model CDA-8, Columbus, OH). Distance traveled (DT), time performing large ambulatory movements (AT), and resting time when no movement was detected (RT) were recorded in one-minute intervals, summed and presented as group means ± SD. All behavioral analyses were performed in the morning (8 am–12 pm local time).
Micro-computed tomography
Mice were sacrificed 12 weeks after surgery for microCT imaging and subsequent histological evaluation. Limbs were fixed in 10% non-buffered formalin for 48 hours, stored in 70% ethanol and analyzed by micro-computed tomography (μCT) (μCT40 scanner; Scanco Medical AG, Basserdorf, Switzerland) to measure bone structural properties in regions of interest (ROI). The distal femur, joint space, and proximal tibia were scanned with 10.5 μm voxel size using an energy setting of 70 kVp and an integration time of 300 msec. The femoral trabecular ROI was initiated just distal to the medial growth plate, whereas the tibial trabecular ROI included only bone proximal to the medial growth plate. The tibial subchondral bone plate was defined as the medial cortical-like bone that overlays the subchondral region. Images were processed using a custom Image Processing Language (IPL) script. Binary images were produced and analyzed using a commercially available 3D Individual Trabecula Segmentation (ITS) Morphological Analysis and Modeling software (Columbia University, NY, USA) (19). Trabeculae were skeletonized, decomposed into individual trabecular units, and categorized as plates and rods based on geometric properties. The software output included standard morphological measures, which were, however, separated into plate and rod groupings. The femoral subchondral bone plate density and thickness could not be determined with these methods because its thickness varies and an accurate ROI could not be drawn.
Histology
Following μCT imaging, the right and left limbs were decalcified for 14 days in 15% EDTA. The joints were then dehydrated and embedded in paraffin for sectioning. Sections (5 micron thick) were cut through the entire right and left knee joints from posterior to anterior aspects. Approximately every 10th slide (every 50 microns) was selected for analysis (8–10 slides per knee per mouse), and stained with Fast Green (Sigma #L5382-10g) and Safranin O (Sigma #S2255-25g) as described (15). Three independent and blinded scorers graded each stained slide following OARSI guidelines (15, 20).
Human articular cartilage explant cultures
Cartilage explants from surgically discarded, de-identified distal femurs were collected from total joint arthroplasties performed at Mayo Clinic as part of the patients’ routine care. The Mayo Clinic Institutional Review Board approved all studies. Articular cartilage cylinders were made from damaged tissue in each specimen using a 4-mm biopsy punch at the site of wear along the articular surface. Excised 4-mm pieces of articular cartilage were placed within wells of a 24-well plate and were cultured in DMEM supplemented with 10% FBS, 1% antibiotic-antimycotic and 1X ITS (Insulin-Transferrin-Selenium) in the presence of 5 μM NSC117079 or vehicle (control) for 14 days. Medium changes were performed every 3 to 4 days with a final medium change on day 13.
Glycosaminoglycan (GAG) assays
The wet weight of each articular cartilage explant was measured after 14 days in culture. Tissue explants were digested in 2 mg/ml pronase in PBS for 1 hour followed by an overnight digestion in 3 mg/ml collagenase. Digested cartilage was diluted 1:100 in PBS and GAG / mg wet tissue weight was assessed using the Glycosaminoglycan Assay Kit (Chondrex #6022).
Immunohistochemistry
Cartilage explant specimens were fixed in formalin for 48 hours, decalcified in 15% EDTA for seven days, embedded in paraffin, and sectioned coronally at a thickness of 5 microns. Immunohistochemistry was performed with antibodies (diluted in 1% bovine serum albumin in Tris-buffered saline) directed to aggrecan (Abcam #ab36861), pPKC (Cell Signaling Technology #9371), or pAKT2 (Cell Signaling Technology #8599), or with a non-specific IgG (control). Chromogens were detected with a polyvalent secondary HRP kit (Abcam ab93697) and 3,3-diaminobenzidine (DAB) (Sigma Aldrich D3939). Sections were counterstained with 0.5% Alcian blue.
Western blots
After explant culture, the tissues were crushed in a dry ice/liquid nitrogen bath using a mortar and pestle. Cell lysates were collected from crushed tissue in a buffered SDS solution (0.1% glycerol, 0.01% SDS, 0.1 M Tris, pH 6.8) on ice. Total protein concentrations were obtained using the Bio-Rad DC assay (Bio-Rad). Proteins (20 μg) were then resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Western blotting was performed with antibodies (1:2000 dilution) for pThr202/Tyr204-ERK1/2 (Cell Signaling Technologies #4370), pSer660-PKC (Cell Signaling Technologies #9371), pSer474-AKT2 (Cell Signaling Technologies #8599), and AKT, (Cell Signaling Technologies #9272) and corresponding secondary antibodies conjugated to horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA). Antibody binding was detected with the Supersignal West Femto Chemiluminescent Substrate (Pierce). Blots were stripped using Restore Western blot Stripping Buffer (Pierce) and reprobed as needed. Each experiment was repeated at least three times, and data from one representative experiment are shown.
RNA isolation and qPCR
Total RNA was isolated from human articular cartilage explants with TRIzol reagent (Invitrogen) and phenol/chloroform. RNA (2 μg) was reverse transcribed to cDNA with the SuperScript III First-Strand Synthesis cDNA kit (Invitrogen) for real-time semi-quantitative PCR (qPCR) with gene-specific primers for ACAN (5′-GTGCCTATCAGGACAAGCTCT-3′, 5′-GATGCCTTTCACCACGACTTC-3′) and HAS2 (5′-GGCCGGTCGTCTCAAATTCA-3′, 5′-TCACAATGCATCTTGTTCAGCTC-3′). Transcript levels were normalized to the reference gene, GAPDH (5′-ATGTTCGTCATGGGTGTGAA-3′, 5′-TGTGGTCATGAGTCCTCGA-3′). Transcript abundance and relative fold changes in gene expression were quantified using the 2−ΔΔCt method relative to control.
Statistical analysis
One-way ANOVA was performed to evaluate the statistical significance of behavioral responses of mice to different drug treatments (saline versus Phlpp inhibitor). OARSI scores were analyzed using the MAX method, which selects the maximum score out of ten slides for each knee per mouse (21), and two-way ANOVA using Tukey’s multiple comparison’s test.
Results
A single intra-articular injection of the Phlpp inhibitor NSC117079 prevents mechanical allodynia and mobility loss in mice with PTOA
Male C57Bl/6 mice were subjected to DMM or sham surgery on the right hind limb. Mice in sham and DMM surgery groups were randomized into subgroups that received an intra-articular injection of either saline or the Phlpp inhibitor (Fig 1). Mechanical allodynia was longitudinally monitored by Von Frey assays using 0.16g and 0.4 g fibers. Right hind limbs in both the sham/saline and sham/Phlpp inhibitor groups maintained baseline levels of paw withdrawal with both fibers, indicating that neither the sham surgery nor the Phlpp inhibitor injection caused long-term mechanical hypersensitivity in a structurally intact joint (Fig 2AB). In contrast, all mice with joints destabilized by DMM surgery exhibited the expected increase in withdrawal response six weeks after surgery (Fig 2CD). Mechanical allodynia continued to increase until the end of the experiment at 12 weeks post-surgery in the saline-treated mice. However, a single intra-articular injection of the Phlpp inhibitor at seven weeks after surgery prevented further increases in mechanical hypersensitivity in the DMM group.
To further test the effects of the Phlpp inhibitor on joint function, the mobility and exploratory activities of mice in both the sham and surgical groups were assessed during 20-minute time periods in an open-field assay. In the animals that underwent sham surgery, no difference was observed in ambulation time, distance traveled, or resting time after saline or Phlpp inhibitor injection (Fig 3A–C). In the mice that received DMM surgery and a saline injection, ambulatory time and distance traveled declined with increasing post-surgery time (Fig 3D–E). In contrast, the mice that underwent DMM surgery and were treated with the Phlpp inhibitor showed greater mobility and traveled greater distances than mice that received saline (Fig 3D–E). Accordingly, resting time was less in the Phlpp inhibitor-treated group (Fig 3F). These data demonstrate that a single intra-articular injection of this Phlpp inhibitor does not cause pain or hamper activity in normal joints but prevents disability and mechanical allodynia in joints with a destabilized meniscus.
The Phlpp inhibitor NSC117079 slows articular cartilage loss in mice with PTOA
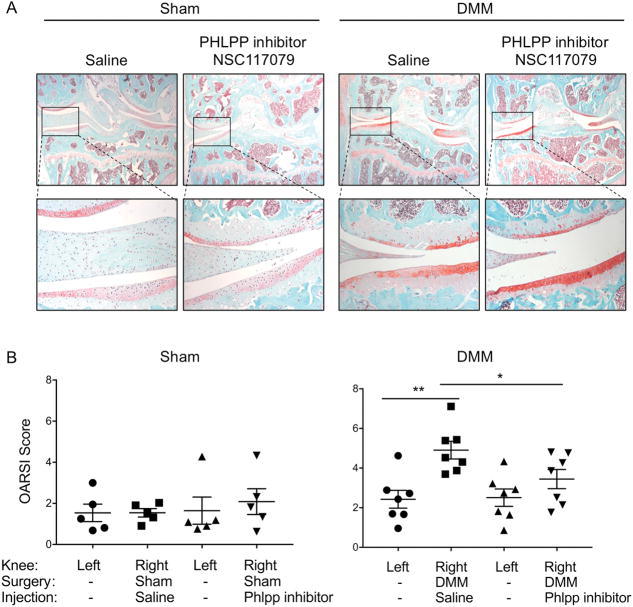
To determine if the Phlpp inhibitor protected articular cartilage from structural damage, de-identified histological sections from the DMM study were evaluated. No structural damage was observed in right hind limbs subjected to sham surgery or in left hind limbs that did not undergo surgery (Fig 4). Importantly, the Phlpp inhibitor did not cause structural damage when injected into a stable knee joint subjected to sham surgery. The DMM surgery caused the expected changes in the articular cartilage, including delamination, clefting, and thinning; however, no difference in subchondral bone structure was detected (Fig 5, Table 1). A single injection of the Phlpp inhibitor significantly reduced the frequency and severity of these structural changes following DMM surgery (Fig 4). These data indicate that the Phlpp inhibitor protects articular cartilage from damage that occurs following knee destabilization.
Figure 4. A Phlpp inhibitor preserves articular cartilage structure and integrity in a destabilized knee.
A. Representative Safranin O/Fast green-stained histological sections from mice in sham or DMM surgical groups that were injected intra-articularly with saline or the Phlpp inhibitor NSC117079. The medial side of the joint is on the left side of the image. Images in the top row are at 2.5X magnification; images in the bottom row are 20X. B. OARSI scores from knees subjected to sham surgery (n= 5 per group) or DMM surgery (n= 7 per group) and injected with saline or the Phlpp inhibitor NSC117079. *p<0.05, **p<0.01.
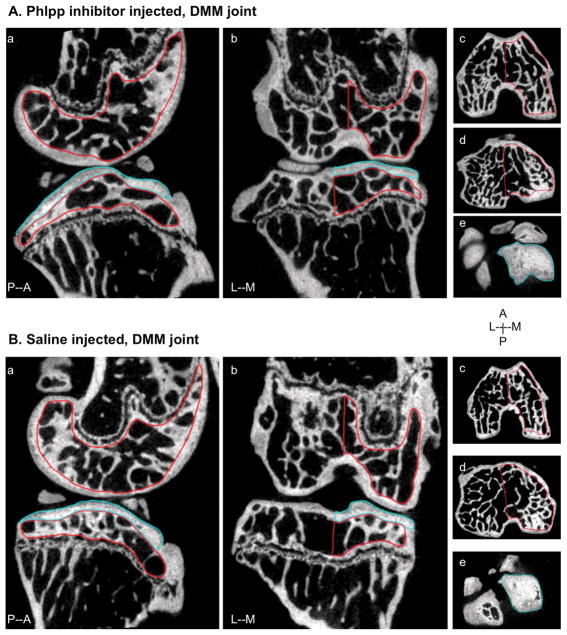
Figure 5. MicroCT images of subchondral bone in mice subjected to DMM.
2D images of DMM knee joints injected with NSC117079 (A) or saline (B) from lateral (a) and frontal (b) views. Regions of subchondral trabecular bone were analyzed from the medial (M) side of the distal femur (c) and proximal tibia (d) (outlined in red). Medial subchondral plate of the tibia was analyzed for mineralization and thickness and outlined in blue (e). Posterior (P), Anterior (A), Medial (M) and Lateral (L) directions are indicated.
Table 1.
Phlpp inhibitors do not affect structural properties of subchondral bone in joints subjected to DMM surgery*
| Bone property | Distal Femur | Proximal Tibia | ||
|---|---|---|---|---|
| Saline | Phlpp inhibitor | Saline | Phlpp inhibitor | |
| Bone mineral density (BMD), mg/mm3 | 342 ± 39.8 | 326 ± 36.4 | 423 ± 49.1 | 379 ± 42.0 |
| Tissue mineral density (TMD), mg/mm3 | 797 ± 16.1 | 785 ± 25.0 | 835 ± 22.1 | 833 ± 32.6 |
| Bone volume fraction (BVF) | 0.38 ± 0.068 | 0.33 ± 0.048 | 0.34 ± 0.074 | 0.32 ± 0.055 |
| Plate bone volume/total volume (pBV/TV) | 0.29 ± 0.070 | 0.24 ± 0.048 | 0.26 ± 0.085 | 0.24 ± 0.069 |
| Rod BV/TV (rBV/TV) | 0.095 ± 0.017 | 0.096 ± 0.031 | 0.082 ± 0.019 | 0.084 ± 0.027 |
| Axially oriented BV/TV (aBV/TV) | 0.010 ± 0.033 | 0.099 ± 0.037 | 0.13 ± 0.044 | 0.13 ± 0.042 |
| Plate volume to bone volume (pBV/BV) | 0.75 ± 0.069 | 0.71 ± 0.083 | 0.74 ± 0.10 | 0.73 ± 0.11 |
| Rod BV/BV (rBV/BV) | 0.26 ± 0.069 | 0.29 ± 0.083 | 0.26 ± 0.10 | 0.27 ± 0.11 |
| Number of trabecular plates (pTb.N), 1/mm | 5.5 ± 0.12 | 5.3 ± 0.25 | 5.5 ± 0.39 | 5.4 ± 0.20 |
| Number of rods (rTb.N), 1/mm | 4.7 ± 0.25 | 4.7 ± 0.47 | 4.6 ± 0.45 | 4.6 ± 0.52 |
| Plate thickness (pTb.Th), mm | 0.067 ± 0.0084 | 0.063 ± 0.0041 | 0.062 ± 0.0097 | 0.0612 ± 0.0075 |
| Rod diameter (rTb.Th), mm | 0.060 ± 0.0025 | 0.060 ± 0.0030 | 0.058 ± 0.0050 | 0.059 ± 0.0030 |
| Spacing between plates (pTb.S), mm2 | 0.026 ± 0.0026 | 0.026 ± 0.0029 | 0.025 ± 0.0026 | 0.025 ± 0.0027 |
| Rod length, rTb.ℓ, mm | 0.29 ± 0.014 | 0.29 ± 0.015 | 0.30 ± 0.021 | 0.28 ± 0.016 |
| Subchondral bone plate BMD, mg/mm3 | ND | ND | 780 ± 27.9 | 767 ± 42.7 |
| Subchondral bone plate thickness (mm) | ND | ND | 0.36 ± 0.062 | 0.35 ± 0.055 |
Mean ± SD, n=7 per group;
ND=not determined
The Phlpp inhibitor NSC117079 increases glycosaminoglycan production in human articular explants
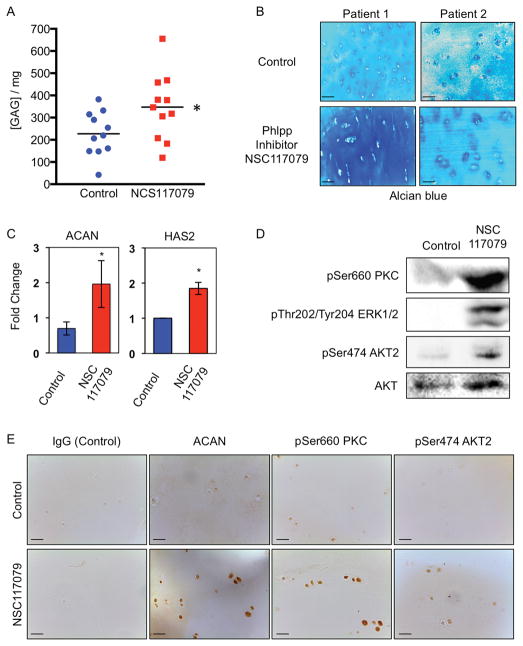
To determine if the Phlpp inhibitor would also affect cellular activities in human chondrocytes, articular cartilage explants from OA patients undergoing joint replacement were incubated with NSC117079 for 14 days. The Phlpp inhibitor increased the production of glycosaminoglycans (GAG) in 10 of 11 specimens (91%) (Fig 6A, 6B, Table 2). The Phlpp inhibitor also increased ACAN and HAS2 transcripts (Fig 6C) and immunostaining for aggrecan was higher in tissue sections of articular cartilage (Fig 6E). No changes in gene expression of Mmp3/9/13 or Adamts4/5 were detected (data not shown). Finally, phosphorylated AKT2, PKC and ERK1/2 were stabilized in human articular chondrocytes treated with the Phlpp inhibitor (Fig 6D, 6E), indicating that the molecule can diffuse through the cartilage matrix to chondrocytes.
Figure 6. The Phlpp inhibitor, NSC117079, induces glycosaminoglycan synthesis, ACAN and HAS2 gene expression and kinase phosphorylation in human articular cartilage explants.
A. Articular cartilage explants from human patients undergoing knee replacement (n=11) were placed in culture with 5μM NSC117079 for 14 days. Medium was changed every 3 to 4 days. GAG content in the medium was measured with the Chondrex Glycoaminoglycans Assay. *p<0.05. B. Human articular cartilage explants treated with the Phlpp inhibitor were embedded in paraffin, sectioned and stained with Alcian blue. Scale bars equal 100 microns. C. RT-qPCR for ACAN and HAS2 mRNA from human articular cartilage chondrocytes that were incubated in chondrogenic medium and the Phlpp inhibitor for 14 days. n=5, *p < 0.05. D. Western blots of proteins extracted from human articular chondrocytes that were incubated in chondrogenic medium and the Phlpp inhibitor NSC117079 for 14 days. E. Human articular cartilage explants treated with the Phlpp inhibitor were embedded in paraffin, sectioned, and subjected to immunohistochemistry with antibodies for the indicated proteins. Scale bars equal 100 microns.
Table 2.
Clinical features of OA patients who donated surgically removed tissue to this study and effects of the Phlpp inhibitor on GAG secretion by articular cartilage explants
| Patient | Age | Gender | Vehicle [GAG]/mg* | NSC117970 [GAG]/mg* |
|---|---|---|---|---|
| OA55 | N/A | N/A | 206.3 | 317.7 |
| OA56 | 59 | Male | 254.4 | 346.2 |
| OA57 | 82 | Female | 382.2 | 467.9 |
| OA58 | 60 | Female | 290.3 | 207.6 |
| OA59 | 80 | Female | 332.5 | 380.7 |
| OA60 | 66 | Female | 314.8 | 458.3 |
| OA61 | 78 | Male | 149.3 | 378.7 |
| OA62 | N/A | Female | 42.2 | 119.3 |
| OA63 | 68 | Female | 219.6 | 655.2 |
| OA64 | 54 | Female | 147.6 | 182.9 |
| OA65 | 72 | Female | 161.3 | 306.2 |
Average of triplicate readings.
N/A= not available
Discussion
After a destabilizing joint injury, articular chondrocytes are exposed to many abnormal biochemical and mechanical signals that alter cellular signaling pathways and transcriptomes, which over time limits their survival and alters the structure and composition of cartilage (22). Surgical repairs can realign joint structures and bones, but complete regeneration of damaged articular cartilage is difficult because it is avascular and chondrocytes have a low turnover rate. Long-held beliefs that articular cartilage cannot be repaired were recently challenged by reports showing that articular cartilage in some human joints, particularly distal joints including knees, are capable of anabolic activity and regeneration (23, 24). Progenitors cells in the superficial layer may be key to this natural repair (25). Thus, an attractive therapeutic strategy is to slow disease progression and facilitate regeneration by intervening as soon as possible after injury to allow cells within the joint to heal damaged tissues through reactivation of developmental programs. DMOADs would ideally affect one or more anabolic and/or catabolic cellular process (i.e., proliferation, matrix synthesis and degradation, senescence, and/or apoptosis) if they were to be effective OA therapies and reduce pain for longer than a few days.
The protein phosphatases, Phlpp1/2, are emerging as facilitators of degenerative diseases such as OA and cardiac ischemia because they repress repair and pro-survival pathways, particularly those regulated by receptor tyrosine kinases (15, 26–28). Consequently, Phlpp inhibitors are candidate therapeutics for tissue regeneration. In this study we demonstrated that a single injection of the Phlpp inhibitor, NSC117079, preserves mobility and prevents pain and cartilage degradation in a model of post-traumatic OA in mice. Moreover, human articular chondrocytes incubated with the Phlpp inhibitor had higher GAG and aggrecan levels, which are important extracellular matrix proteins supporting tissue lubrication, hydration, and shock-absorption by the joint. Human chondrocytes responded to the Phlpp inhibitor by increasing phosphorylation of AKT2, PKC, and ERK1/2. These molecules control proliferative, metabolic and survival pathways that promote regeneration and/or slow tissue degradation, although the experiments used in this study were not able to discriminate between these two processes. Phosphorylated forms of these molecules were not detected in joint sections from the mouse study by immunohistochemistry, most likely because of the five-week interval between drug administration and tissue harvest.
Phlpp inhibitors may directly influence these anabolic pathways, but they may also sensitize cells to Igf1 and other ligands that stimulate signaling pathways that are reduced in chondrocytes (29). We previously showed that Phlpp1 inhibition increases Fgf18 expression (16), which has anabolic effects on articular cartilage (30, 31) and is an advanced DMOAD candidate (32). Future studies are needed to probe these anabolic pathways more deeply and to test the effects of Phlpp inhibitors on catabolic events like senescence and inflammation. The effectiveness of Phlpp inhibitors on other forms of OA and other degenerative joint diseases is also of interest.
The mechanism(s) by which a single local injection of a Phlpp inhibitor can attenuate mechanical allodynia for up to five weeks requires further study of cellular, inflammatory and neurological pathways active in the injured joint. The dogma is that articular cartilage is aneural and pain is transmitted via other soft tissues in the joint or by bone. We did not observe any structural changes in subchondral bone density that may affect nerve function. The pharmacokinetics and pharmacodynamics of NSC117079 in osteoarthritic synovial fluid also need to be determined and are under investigation.
A perplexing question is why mice with a destabilized meniscus and greater mobility do not develop OA faster than mice that move less. We hypothesize that several factors may be contributing to these results. One is that the Phlpp inhibitor is enhancing cartilage matrix production and thus cartilage that would normally undergo degradation is actually be replenished in real time. Thus, less cartilage destruction is observed. A limitation of this study is that cartilage integrity was not measured at the time of injection (7 weeks), so we do not know if any damage occurred after the injection. Another reason may be that the relative short period of time between injury and assessment of cartilage destruction in this model precludes the development of loading induced cartilage damage, at least during the 12-week monitoring period post-injury. Recent mechanical loading studies revealed few kinematic changes in DMM joints (33). Future studies will determine how long Phlpp inhibitor treatment is effective and if inhibitors block the initiation of cartilage damage and/or promote regeneration after the onset of cartilage degeneration.
Overall, the data presented indicate for the first time that Phlpp inhibitors may alleviate mechanical pain and slow cartilage degradation in osteoarthritic joints. The mechanisms for these phenotypes remain to be elucidated and may be mutually exclusive. The results reported within are consistent with data generated in Phlpp1 deficient mice (15). Since PHLPP1 levels are aberrantly high in osteoarthritic cartilage, NSC117079 is a DMOAD candidate. For any DMOAD to be successful, we believe that a comprehensive treatment strategy that includes early surgical intervention and rehabilitation will be needed to stabilize joints and prevent long-term wear-and-tear that may work against the cellular activities of DMOADs.
Acknowledgments
The authors would like to thank Dr. David Razidlo and Mr. Xiaodong Li for technical assistance. We are grateful to Mr. Dirk Larson for statistical support, Dr. Alexandra C. Newton for helpful discussions, and Drs. Daniel Berry and Michael Stuart for providing surgical specimens. This work was supported by research and training grants from the National Institutes of Health (R01 AR68103, T32 AR56950, K01 AR65397), Regenerative Medicine Minnesota 2015 6272, and the Orthopedic Research and Education Foundation in collaboration with the Howard Hughes Medical Institute.
Footnotes
Author Contributions Statement: SMH, MF, DLB, LCS, SK, and EWB performed experiments and acquired and analyzed data. SMH, KLC, MO, MBG, LET, EWB and JJW designed the experiments and provided essential technical expertise. SMH, MBG, LET, EWB, and JJW wrote and/or critically revised the manuscript. All authors have read and approved the final submitted manuscript.
Competing interests: The authors declare that they have no competing interests.
References
- 1.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(8 Suppl):S230–5. [PubMed] [Google Scholar]
- 2.Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis and rheumatism. 2011;63(10):2974–82. doi: 10.1002/art.30498. [DOI] [PubMed] [Google Scholar]
- 3.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis and rheumatism. 2009;60(12):3546–53. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 4.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. British medical bulletin. 2013;105:185–99. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 6.St Sauver JL, Warner DO, Yawn BP, Jacobson DJ, McGree ME, Pankratz JJ, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56–67. doi: 10.1016/j.mayocp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. doi: 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahn S, Seror J, Klein J. Lubrication of Articular Cartilage. Annu Rev Biomed Eng. 2016;18:235–58. doi: 10.1146/annurev-bioeng-081514-123305. [DOI] [PubMed] [Google Scholar]
- 10.Carbone A, Rodeo S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res. 2017;35(3):397–405. doi: 10.1002/jor.23341. [DOI] [PubMed] [Google Scholar]
- 11.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill AK, Niederst MJ, Newton AC. Suppression of survival signalling pathways by the phosphatase PHLPP. Febs J. 2013;280(2):572–83. doi: 10.1111/j.1742-4658.2012.08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grzechnik AT, Newton AC. PHLPPing through history: a decade in the life of PHLPP phosphatases. Biochem Soc Trans. 2016;44(6):1675–82. doi: 10.1042/BST20160170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley EW, Carpio LR, McGee-Lawrence ME, Castillejo Becerra C, Amanatullah DF, Ta LE, et al. Phlpp1 facilitates post-traumatic osteoarthritis and is induced by inflammation and promoter demethylation in human osteoarthritis. Osteoarthritis Cartilage. 2016;24(6):1021–8. doi: 10.1016/j.joca.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley EW, Carpio LR, Newton AC, Westendorf JJ. Deletion of the PH-domain and Leucine-rich Repeat Protein Phosphatase 1 (Phlpp1) Increases Fibroblast Growth Factor (Fgf) 18 Expression and Promotes Chondrocyte Proliferation. J Biol Chem. 2015;290(26):16272–80. doi: 10.1074/jbc.M114.612937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sierecki E, Sinko W, McCammon JA, Newton AC. Discovery of small molecule inhibitors of the PH domain leucine-rich repeat protein phosphatase (PHLPP) by chemical and virtual screening. Journal of Medicinal Chemistry. 2010;53(19):6899–911. doi: 10.1021/jm100331d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Molecular pain. 2009;5:9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XS, Sajda P, Saha PK, Wehrli FW, Bevill G, Keaveny TM, et al. Complete volumetric decomposition of individual trabecular plates and rods and its morphological correlations with anisotropic elastic moduli in human trabecular bone. J Bone Miner Res. 2008;23(2):223–35. doi: 10.1359/JBMR.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Del Carlo M, Jr, Loeser RF. Cell death in osteoarthritis. Current rheumatology reports. 2008;10(1):37–42. doi: 10.1007/s11926-008-0007-8. [DOI] [PubMed] [Google Scholar]
- 23.Catterall JB, Hsueh MF, Stabler TV, McCudden CR, Bolognesi M, Zura R, et al. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J Biol Chem. 2012;287(7):4640–51. doi: 10.1074/jbc.M111.249649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catterall JB, Zura RD, Bolognesi MP, Kraus VB. Aspartic acid racemization reveals a high turnover state in knee compared with hip osteoarthritic cartilage. Osteoarthritis Cartilage. 2016;24(2):374–81. doi: 10.1016/j.joca.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Newton PT, Bouderlique T, Sejnohova M, Zikmund T, Kozhemyakina E, et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017;31(3):1067–84. doi: 10.1096/fj.201600918R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto S, Purcell NH, Smith JM, Gao T, Whittaker R, Huang K, et al. PHLPP-1 negatively regulates Akt activity and survival in the heart. Circ Res. 2010;107(4):476–84. doi: 10.1161/CIRCRESAHA.109.215020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Y, Sun W, Wang Y, Gao F, Ma H. Mutual inhibition of insulin signaling and PHLPP-1 determines cardioprotective efficiency of Akt in aged heart. Aging. 2016;8(5):873–88. doi: 10.18632/aging.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes G, Niederst M, Cohen-Katsenelson K, Stender JD, Kunkel MT, Chen M, et al. Pleckstrin homology domain leucine-rich repeat protein phosphatases set the amplitude of receptor tyrosine kinase output. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(38):E3957–65. doi: 10.1073/pnas.1404221111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis and rheumatism. 2000;43(9):2110–20. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.Davidson D, Blanc A, Filion D, Wang H, Plut P, Pfeffer G, et al. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280(21):20509–15. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- 31.Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S, et al. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage. 2002;10(4):308–20. doi: 10.1053/joca.2002.0514. [DOI] [PubMed] [Google Scholar]
- 32.Lohmander LS, Hellot S, Dreher D, Krantz EF, Kruger DS, Guermazi A, et al. Intra-articular Sprifermin (Recombinant Human Fibroblast Growth Factor 18) in Knee Osteoarthritis: Randomized, Double-blind, Placebo-controlled Trial. Arthritis Rheumatol. 2014;66(7):1820–31. doi: 10.1002/art.38614. [DOI] [PubMed] [Google Scholar]
- 33.Adebayo OO, Ko FC, Goldring SR, Goldring MB, Wright TM, van der Meulen MC. Kinematics of meniscal- and ACL-transected mouse knees during controlled tibial compressive loading captured using roentgen stereophotogrammetry. J Orthop Res. 2017;35(2):353–60. doi: 10.1002/jor.23285. [DOI] [PMC free article] [PubMed] [Google Scholar]