Abstract
Objectives
Type 2 diabetes (T2D) is associated with chronic, low grade inflammation. Activation of the NLRP3 inflammasome and secretion of its target interleukin-1β (IL-1β) have been implicated in pancreatic β cell failure in T2D. Specific targeting of the NLRP3 inflammasome to prevent pancreatic β cell death could allow for selective T2D treatment without compromising all IL-1β-associated immune responses. We hypothesized that treating a mouse model of T2D with MCC950, a compound that specifically inhibits NLRP3, would prevent pancreatic β cell death, thereby preventing the onset of T2D.
Methods
Diabetic db/db mice were treated with MCC950 via drinking water for 8 weeks from 6 to 14 weeks of age, a period over which they developed pancreatic β cell failure. We assessed metabolic parameters such as body composition, glucose tolerance, or insulin secretion over the course of the intervention.
Results
MCC950 was a potent inhibitor of NLRP3-induced IL-1β in vitro and was detected at high levels in the plasma of treated db/db mice. Treatment of pre-diabetic db/db mice with MCC950, however, did not prevent pancreatic dysfunction and full onset of the T2D pathology. When examining the NLRP3 pathway in the pancreas of db/db mice, we could not detect an activation of this pathway nor increased levels of its target IL-1β.
Conclusions
NLRP3 driven-pancreatic IL-1β inflammation does not play a key role in the pathogenesis of the db/db murine model of T2D.
Keywords: Type 2 diabetes, Inflammasome, Interleukin-1β, MCC950, db/db mice
Abbreviations: T2D, Type 2 Diabetes; IL-1β, interleukin-1β; NLRP3, Nod-like receptor family pyrin domain-containing protein 3; DAMPs, danger-associated molecular patterns
Highlights
-
•
Inhibition of NLRP3 via MCC950 in db/db mice did not improve glucose tolerance.
-
•
MCC950 treatment did not prevent beta cell loss of function.
-
•
Expression of IL1beta and NLRP3 does not appear increased in db/db islets.
-
•
We conclude against a role for NLRP3 in db/db pancreatic dysfunction.
1. Introduction
Diabetes mellitus is growing world-wide and is expected to affect almost 600 million people by 2035 [1]. Type 2 Diabetes (T2D) is recognized as a chronic inflammatory disease, and it is now established that inflammation and immune cell dysfunction are strongly associated with both insulin resistance and insulin secretion dysfunction [2]. The ability of immune cells to affect pancreatic function and, more specifically, β cell insulin-secretory activity in islets, is well established in the context of type 1 diabetes [3], [4]. In contrast, the role of inflammation and immune cells in the pathology of T2D is a relatively recent phenomenon (for review see [5]). Specifically, macrophages have been shown to infiltrate pancreatic islets both in T2D patients as well as in rodent models of insulin resistance and diabetes [6], [7]. This infiltration, in addition to the nutrient-stressed β cells, contributes to elevated cytokine levels in the diabetic islet [8], [9]. In particular, the pro-inflammatory cytokine interleukin-1β (IL-1β) has been highlighted as a potent driver of beta cell dysfunction [9], [10]. IL-1β secretion is triggered in the islet in response to high level of glucose, initiating a pro-inflammatory milieu that contributes to the recruitment and activation of macrophages, which, in turn, sustain islet inflammation [9]. Indeed, suppressing macrophage recruitment in db/db mice (that harbor a mutation of the leptin receptor leading to hyperphagia, obesity and eventually β cell failure) using clodronate liposomes improved insulin secretion [11]. Mature IL-1β is mainly produced through the multi-protein inflammasome complexes such as NOD-like receptor pyrin domain containing protein 3 (NLRP3) inflammasome. Pro-inflammatory danger-associated molecular patterns (DAMPs), endogenous molecules such as extracellular ATP, or uric acid crystals are detected by the NLR scaffolding protein leading to the recruitment of the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase-1. The assembly of the NLRP3 inflammasome results in the cleavage and activation of caspase-1 which in turn activates the precursor form of IL-1β through proteolytic cleavage [9]. In the context of T2D, several different stimuli have been proposed to trigger pancreatic NLRP3 activation and increased IL-1β. We have shown that accumulation of islet amyloid polypeptide (IAPP), a protein known to accumulate into amyloid deposits in the pancreas of T2D patients, could activate the NLRP3 inflammasome and promote IL-1β production [12]. In addition to IAPP, saturated fatty acids, ATP from apoptotic cells, endocannabinoids, ER stress, and oxidative stress have also been reported to potentiate NLRP3-induced IL-1β production and contribute to islet inflammation [9]. Targeting IL-1β signaling using Anakinra (IL-1 receptor antagonist) and Canakinumab (anti-IL-1β antibody) to treat T2D patients yielded modest but promising results [13], [14], [15]. Specific targeting of the NLRP3 inflammasome to prevent pancreatic cell death, however, could be a viable treatment strategy for T2D, because it would not compromise IL-1β-associated immune function, initiated by pathways other than NLRP3. We have shown that the orally available small molecule MCC950 potently and specifically inhibits NLRP3 activation and that it is efficacious in several pre-clinical models of inflammatory diseases including NLRP3-driven auto-inflammatory conditions [16], [17], [18], [19], [20], [21]. We hypothesized that treating a diabetic mouse with MCC950 would prevent the pancreatic islets death and delay, if not completely block, the onset of T2D.
2. Material and methods
2.1. Mouse models and drug treatment
db/db and db/+ littermate control mice (BKS.Cg-Dock7<m> +/+ Lepr<db>/J) were purchased from Jackson laboratories (USA) at 4 weeks of age and left to acclimatize for 2 weeks. All mice were housed at the Alfred Medical Research and Education Precinct Animal Centre in a pathogen free facility under controlled environmental conditions and exposed to a 12:12 h light:dark cycle. Mice were fed a normal chow diet (Specialty Feeds, Australia) and provided with food and water ad libitum. From 6 weeks of age, mice received MCC950 (40 mg/kg) via drinking water for a period of 8 weeks. Water intake was recorded daily for each cage. The dose of MCC950 was adjusted every 3 days according to mice weight gain/loss and averaged water intake per cage. Animal experiments were approved by the Alfred Medical Research and Education Precinct Animal Ethics Committee and conducted in accordance with the National Health and Medical Research Council of Australia Guidelines for Animal Experimentation.
2.2. Metabolic measurements
2.2.1. Plasma insulin measurements
Insulin concentrations were measured using a Mouse Ultrasensitive Insulin ELISA kit (ALPCO, Salem, NH, USA) according to manufacturer's instructions.
2.2.2. Body composition
Mouse body composition (fat mass (FM) and lean body mass (LBM)) were measured weekly with a 4-in-1 EchoMRI body composition analyzer (Columbus Instruments, USA) and standard laboratory scales.
2.2.3. oral Glucose Tolerance Test (oGTT)
OGTT (2 g/kg LBM) were performed in 5 h (for week 2 oGTT) or 12 h (for week 7 oGTT) fasted mice as previously described [22].
2.3. RNA extraction and real time quantitative PCR
Pancreatic islets from 16 weeks old db/+ and db/db mice were isolated as previously described [23]. Total RNA was isolated from tissues with Tri Reagent® (Sigma Aldrich) and reverse transcribed to cDNA with the use of random hexamers. Gene expression analysis was performed by Real-time PCR on a 7500 fast sequence detector (Applied Biosystems) using TaqMan gene expression assays (Applied Biosystems), including an 18S probe and primers for housekeeping measurements (Taqman references are included in Supplementary Fig. 3).
2.4. Blood parameters
For hematological assessment, a small volume (20 μL) of whole blood was diluted 1:7 in Sysmex CELLPACK™ (Sysmex, Japan) diluent and assessed using an automated hematology analyzer (Sysmex XS-1000i, Japan).
2.5. MCC950 quantification
MCC950 was measured in the plasma by Liquid chromatography-tandem mass spectrometry as previously described [16] for plasma. For tissue analysis, fat, liver and kidney were weighed and homogenised in saline using a Qiagen tissuelyser. The quantification was performed as described in the Supplementary Material.
2.6. Western blotting
Renal cortex was isolated from frozen kidney samples and western blotting was performed on tissue homogenates as previously described [24] and quantified relative to relevant loading controls.
2.7. In vitro experiment
Bone marrow derived macrophages were obtained and cultured as previously described [25]. Cells were treated with LPS, ATP and MCC950 as described in the figure legend before the supernatant was harvested and used to measure IL-1β using the R&D Duoset Mouse ELISA.
2.8. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Two-way analysis of variance (ANOVA) was used to detect main effects for genotype (db/+ vs. db/db) and treatment (vehicle vs. MCC950), Post-hoc analyses (Holm-Sidak) were performed when a significant interaction was detected. Analyses were performed using a statistical computer program (Sigma Stat Version 3.5). p < 0.05 was considered statistically significant.
3. Results
3.1. MCC950 inhibits IL-1β secretion in BMDM stimulated with NLRP3 inflammasome activators
We first aimed to verify the potency of MCC950 to inhibit the NLRP3 inflammasome in our experimental conditions. We pre-treated bone marrow derived macrophages with LPS in order to induce Il1b gene expression and pro-IL-1β production. We next treated these cells with MCC950 before inducing the NLRP3 inflammasome activation with ATP. We observed a near complete prevention of ATP-induced NLRP3-mediated IL-1β release in the cells treated with the two doses of MCC950 confirming its strong inhibitory potency (Supplementary Fig. 1)
3.2. MCC950 does not impact body weight or fat and lean mass in db/db mice
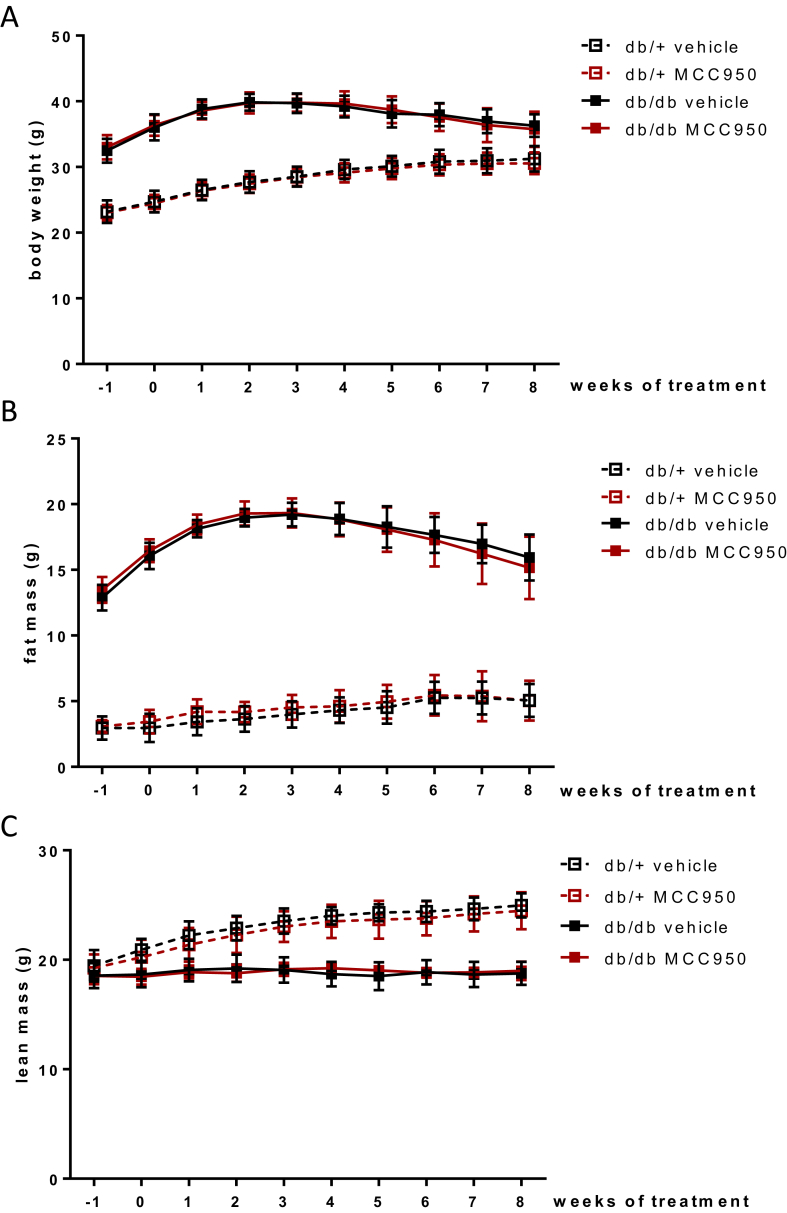
We next proceeded to test the in vivo effect of MCC950 in a mouse model of T2D. db/db and db/+ (lean littermate) mice were fed a chow diet for 8 weeks while receiving MCC950 (40 mg/kg) or plain water through their drinking water. As expected, the db/db mice became severely obese with a markedly increased fat mass, and decreased lean mass, compared with db/+ mice (Figure 1). Treatment of both groups of mice with MCC950 did not, however, affect these parameters (Figure 1).
Figure 1.
MCC950 does not impact body weight or fat and lean mass in db/db mice. 6 weeks old db/db mice were given 40 mg/kg MCC950 through their drinking water for an 8 weeks period. (A) Body weight (B) fat mass and (C) lean mass were assessed by EchoMRI a week before and weekly throughout the treatment period. Data presented as Mean ± SEM, n = 10.
3.3. MCC950 is bioavailable in plasma and tissues but does not affect immune cell profile in db/db mice
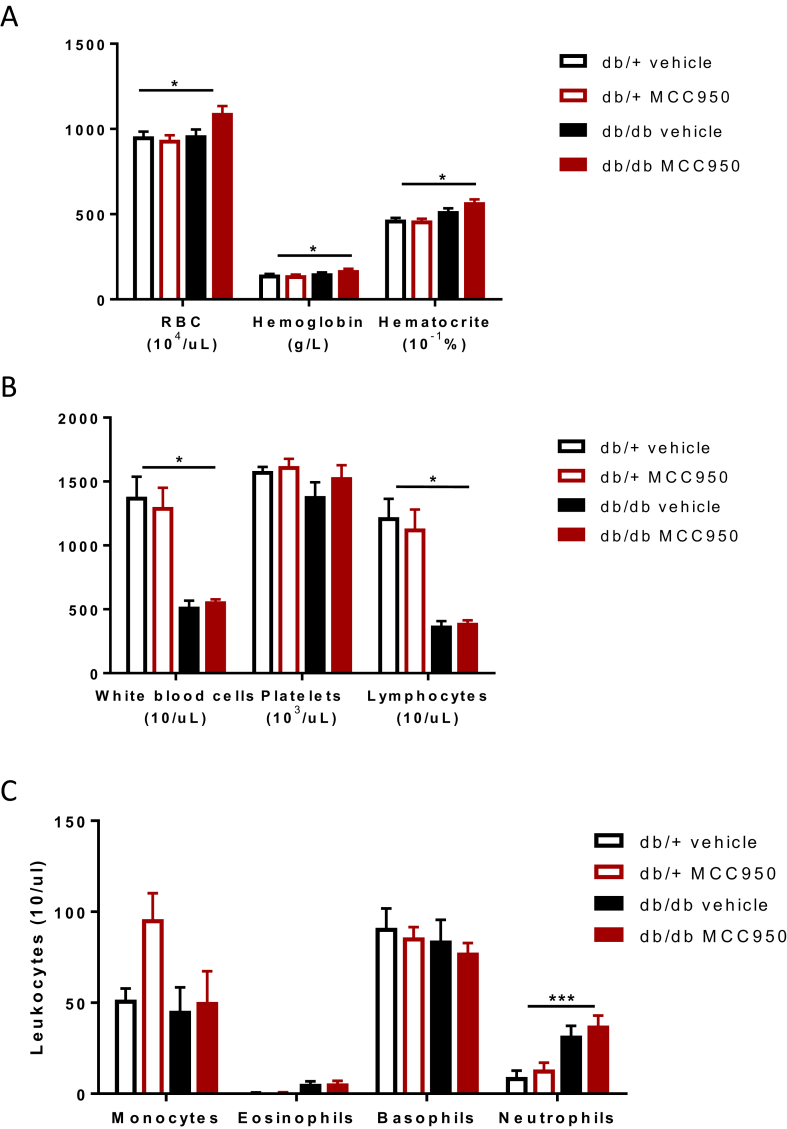
After observing no impact on the animals' body composition in response to MCC950 treatment, we aimed to verify the bioavailability of MCC950. We analyzed plasma from all mice at termination of the intervention and assessed circulating levels of MCC950 using liquid chromatography-tandem mass spectrometry. As expected, we detected high circulating levels of our compound in both db/+ and db/db treated groups as expected (Supplementary Fig. 2) consistent with previous pharmacokinetic studies that have shown efficacy of the compound at this dose [16]. Interestingly, we observed a ∼5 fold higher (P < 0.05) plasma MCC950 concentration in db/+ compared with db/db mice (Supplementary Fig. 2), suggesting that some of the compound might get trapped in the large fat stores in the obese db/db mice. Hence, we set to investigate the amount of compound in several tissues as well as in urine (Supplementary Fig. 2). Surprisingly, the compound was found in negligible quantities in the visceral fat depot of both db/+ and db/db and the liver and kidney presented with the same pattern as the plasma with up to 10 times more compound being located in the liver of the db/+ compared to the db/db mice. However, a major result arise from the urine analysis. db/db mice excreted MCC950 metabolite at a higher rate (1.5 fold versus db/+) and given that their urine output was almost 10 times higher (data not shown), it reveals that the discrepancy between the groups in relation to circulating levels was due to the compound being excreted in db/db mice at a higher rate. Importantly, the circulating concentration in the db/db mice was nonetheless >1000 ng/mL, which was proven sufficient to inhibit NLRP3 in vivo [16]. We next determined whether MCC950 administration could result in any side effects by measuring several blood markers. MCC950 did not affect either hemoglobin, or hematocrit concentrations, nor did it affect red blood cells number (Figure 2A). White blood cells, including platelets and lymphocytes (Figure 2B) or circulating monocytes, neutrophils, eosinophils, and basophils (Figure 2C) were also unaffected by the treatment. Of note, the db/db mice displayed lymphocytopenia (Figure 2B) and mild neutrophilia, consistent with previous reports [26], [27].
Figure 2.
MCC950 is bioavailable in plasma but does not affect immune cell profile indb/dbmice. 6 weeks old db/db mice were given 40 mg/kg MCC950 through their drinking water for an 8 weeks period. (A) Red blood cell parameters (B) White blood cell, platelets, lymphocyte counts, and (C) leukocyte counts were measured in the blood at the 8 weeks endpoint. Data presented as Mean ± SEM, n = 10. Statistical analysis by 2 way ANOVA, *p < 0.05; ***p < 0.001 Main effect for db/db vs db/+.
3.4. MCC950 does not improve glucose metabolism nor prevent pancreatic islet failure in db/db mice
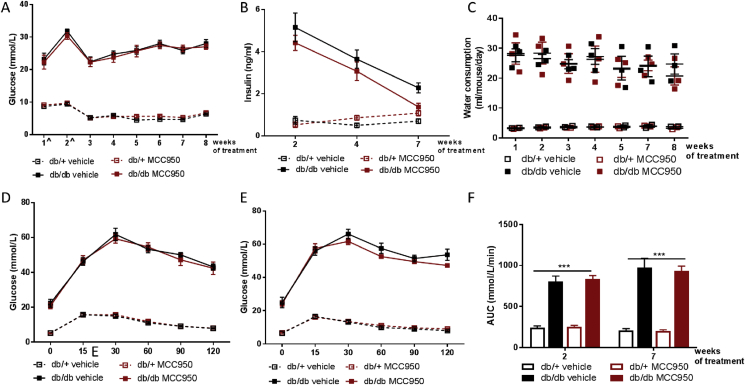
We next determined whether MCC950 could better maintain glucose homeostasis in db/db mice. Six week old db/db mice, displayed both frank hyperglycemia (Figure 3A) and markedly increased water consumption (Figure 3C) throughout the intervention period compared with db/+ mice. Importantly, MCC950 treatment did not affect these parameters (Figure 3A,C). The failure of MCC950 to affect hyperglycemia was associated with a lack of effect on insulin secretion (Figure 3B). Indeed, we measured fasting insulin levels along the course of the intervention and MCC950 treatment was unable to prevent the progressive decrease of insulin secretion observed in the db/db mice, an indication of the transition from β cell compensation to β cell failure (Figure 3B).
Figure 3.
MCC950 does not improve glucose metabolism nor prevent pancreatic islet failure in db/db mice. 6 weeks old db/db mice were given 40 mg/kg MCC950 through their drinking water for an 8 week period. Glycemia (A) and circulating insulin levels (B) were measured after a 5 h (time points marked ˆ) or 12 h (all other time points) fasting period. (C) Water consumption per mouse per day was measured in the 3 different cages of each experimental group. (D–F) Glucose tolerance was assessed through an oGTT following a 5 h fast at 2 weeks of treatment (D) and a 12 h fast at 7 weeks of treatment (E) and was used to generate area under the curve (F). Data presented as Mean ± SEM, n = 10. Statistical analysis by 2 way ANOVA, ***p < 0.001 Main effect for db/db vs db/+.
We next measured glucose tolerance by performing oral glucose tolerance test (OGTT; 2 g/kg LBM) at both week 2 and week 7 of the treatment period. Consistent with the failing pancreatic function of both db/db groups and the fasting glycemia observations, MCC950 had no effect on the severe glucose intolerance displayed by db/db relative to db/+ mice during the OGTT at both intervention periods (Figure 3D–F).
3.5. db/db mice do not display inflammasome activation in pancreatic islets
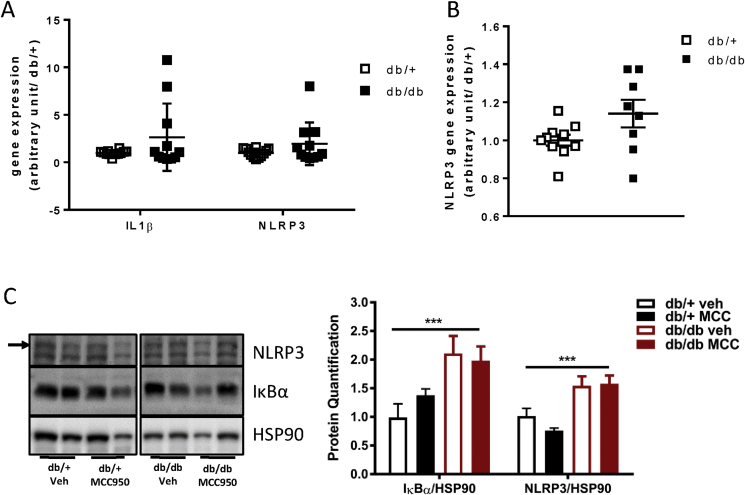
Since MCC950 inhibited NLRP3 activation in vitro (Supplementary Fig. 1) and was clearly bioavailable (Supplementary Fig. 2), our data raised the possibility that, in this particular model, NLRP3 was not activated in the pancreas of db/db mice. Indeed, previous data from our laboratory found no difference in IL-1β expression in pancreatic islets of 6 weeks old db/db compared to db/+ islets [28]. In addition, we isolated pancreatic islets isolated from db/+ and db/db mice at both 6 and 16 weeks and analyzed the mRNA expression of NLRP3 as well as IL-1β in the islets of the 16 weeks old animals (Figure 4A,B). Given the current literature and work from our own group [12], we were surprised to observe no increase in the mRNA expression of either IL-1β or NLRP3 in the cDNA extracted from db/db islets compared with that obtained the db/+ islets (Figure 4A,B). Of note, IL-1β expression at 16 weeks was quite heterogeneous in the db/db group, probably reflecting different stages of the diabetes progression in these mice. Interestingly, and in line with previously published data [29], we were able to detect increased inflammasome components in the renal cortex of db/db mice compared with db/+ mice (Figure 4C). We could not detect a significant effect of MCC950 administration on the NLRP3 component by western blotting despite the accumulation of the drug in the kidney (Figure 4C and Supplementary Fig. 2).
Figure 4.
MCC950 treatment in db/db mice does not impact pancreatic inflammasome components. (A) Gene expression for IL-1β and NLRP3 was measured in isolated pancreatic islet of 16 weeks old db/+ and db/db mice. (B) Gene expression for NLRP3 was measured in isolated pancreatic islet of 6 weeks old db/+ and db/db mice. (C) 16 weeks old db/db mice were given 40 mg/kg MCC950 through their drinking water for an 8 weeks period. IkBa and NLRP3 were measured on renal cortex by western blotting at the end of the treatment period. Data presented as Mean ± SEM, A–B; n = 11–12; C; n = 5–8.
4. Discussion
Activation of the NLRP3 inflammasome has been implicated in the pathology of obesity and insulin resistance [30], [31], [32]. These previous studies demonstrated a role for the NLRP3 inflammasome in the adipose tissue and liver and mostly attributed its activation and the associated secretion of IL-1β to infiltrating macrophages in these tissues.
In addition, the NLRP3 inflammasome has been increasingly studied for its role in pancreatic dysfunction in the context of T2D [5]. The activation of this inflammasome complex leading to the production of the pro-inflammatory cytokine IL-1β has been described in the pancreas in response to several different stimuli. In the pancreatic islet, it appears that both glucose stressed-β cells as well as resident pro-inflammatory macrophages contribute to increased levels of IL-1β and the subsequent insulin secretion impairment [9]. Reports are still unclear whether the myeloid-derived or β cell-produced IL-1β, if not both, is the culprit in β-cell dysfunction. Interestingly, a recent report studying diabetic nephropathy in db/db mice also suggested a strong role for the NLRP3 inflammasome and the subsequent IL-1β secretion in non-myeloid cells. Indeed, mice lacking NLRP3 are protected from diabetic nephropathy, even when transplanted with wild-type bone marrow but conversely, transfer of NLRP3 deficient bone marrow into wild-type animals fails to prevent diabetic nephropathy [29]. This report emphasized a role for IL-1β in the diabetic pathology of db/db mice as they presented a six-fold increase in the cytokine circulating concentration between 4 and 12 weeks of age in these animals [29]. These previous publications prompted us to examine the efficacy of our specific NLRP3 inhibitor in the db/db mice in order to arrest the transition from β-cell compensation to failure. We were, therefore surprised that, in our hands neither NLRP3 nor IL-1β mRNA expression were elevated in the islets from db/db mice. In retrospect, it was not surprising that MCC950 had no effect in this experimental model of T2D.
Our data contrast a number of reports indicative of an NLRP3-dependent mechanism to explain IL-1β activation and its role in pancreatic dysfunction. Youm et al. demonstrated that aged mice fed a high fat diet until reaching one year of age displayed ASC dependent glucose intolerance, but, more importantly, they observed a conserved insulin secretory function in aged mice on a high fat diet lacking the adaptor ASC or NLRP3 itself [33]. It is critically important, however, to note that in this previous study the authors did not use littermate controls for the ASC−/− and NLRP3−/− mice. This could represent an artifact, as it was shown that basal and challenged insulin levels and glucose metabolism are strongly influenced by the strains but also more importantly by the sub-strain of C57Bl/6 mouse used [34], [35]. Indeed, data from our laboratory have shown that metabolic phenotype differences when comparing genetic mouse models are abolished when appropriate littermate control studies are performed [25]. Moreover, the work of others [21] has demonstrated that glucose tolerance and insulin secretion capacity could be significantly different in two sub-strains studied side by side on a high fat diet [35].
Interestingly, Kim et al. also proposed a role for the NLRP3 inflammasome in the same model used herein as they treated db/db mice with the modified form of Vitamin E γ-tocotrienol (γT3) [36]. They report an improved glucose tolerance accompanied by an enhanced glucose stimulated insulin secretion in vivo. Importantly, however, they did not demonstrate the activation of NLRP3 in the db/db pancreatic islet and their γT3 compound appears to have several targets in addition to NLRP3, making it difficult to ascertain the role of this inflammasome in the observed metabolic effects. Indeed, they also report a significantly decreased food intake that could likely mediate the decreased fasting glycemia, given the fasting insulin levels were not different between treated and control db/db animals. In addition, they also observed increased AMPK activation in the γT3-treated mice, a known metabolic activator associated with improved glucose metabolism [37], [38].
Of note, MCC950 targets the NLRP3 inflammasome activation meaning that not only the IL-1β inflammatory pathway but also IL-18 driven inflammation or pyroptosis would be inhibited, ruling out a role for these pathways as well in the db/db model [39].
Finally, recent reports by Donath and colleagues indicate that the role of IL-1β might not be just detrimental in the pancreas as they report that IL-1β is secreted physiologically after a meal. Indeed, mice deficient for IL-1β exhibited decreased insulin levels in response to refeeding or to a high fat diet [40]. This might add some complexity to the pharmaceutical field considering IL-1β as a therapeutic target for diabetes.
In conclusion, it appears that the NLRP3 inflammasome is not responsible for the β-cell insulin secretory failure observed in the db/db mouse model of T2D. The use of a genetic model lacking the leptin receptor such as the db/db mouse might explain part of the discrepancy as leptin signaling and the downstream STAT3 pathway have been implicated in inflammation modulation [41], [42]. However, given the increasing number of human studies targeting IL-1β and other inflammation components for the treatment of T2D [13], [43], [44], [45], [46], our data caution the use of db/db mice as an appropriate pre-clinical model of T2D, particularly when testing the efficacy of drugs that target insulin secretory pathways and islet inflammation.
Acknowledgements
This study was supported by a DART Grant (Y15G-FEBM) and a Senior Principal Research Fellowship (APP1021168) awarded to MAF and Principal Research Fellow (APP1059354) awarded to MAC from the National Health & Medical Research Council of Australia. We would like to thank Ruby Pelingon (IMB, University of Queensland) for assistance with LC-MS/MS studies. Australia-India Strategic Research Fund (AISRF07840) awarded to MAC and AABR.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.02.001.
Contributor Information
H.L. Kammoun, Email: helene.kammoun@baker.edu.au.
M.A. Febbraio, Email: m.febbraio@garvan.org.au.
Conflict of interest
MAC currently holds a fractional Professorial Research Fellow appointment at the University of Queensland with his remaining time as CEO of Inflazome Ltd. a company headquartered in Dublin, Ireland that is developing drugs to address clinical unmet needs in inflammatory disease by targeting the inflammasome.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1. MCC950 inhibits IL-1β secretion in BMDM stimulated with NLRP3 inflammasome activators. Bone marrow derived macrophages were pre-treated with LPS (100 ng/ml) for 3 h before treating the cells with MCC950 (10 or 25 μM as per graph) for 1 h previous to inflammasome activation by ATP (4 mM for 30 min). IL-1β was measured by ELISA in the cell supernatant collected after ATP treatment. Data presented as Mean ± SEM, n = 3 experiments with each experiment performed in triplicate. Statistical analysis by 1 way ANOVA, ***p < 0.001 vs control condition, ###p < 0.001 vs LPS + ATP condition.
Supplementary Figure 2. MCC950 is bioavailable in plasma and tissues of db/+ and db/db mice. 6 weeks old db/db mice were given 40 mg/kg MCC950 through their drinking water for an 8 weeks period. MCC950 was measured by Mass spectrometry in plasma, urine, fat, liver and kidney at the end of the treatment period. Data presented as Mean ± SEM, n = 10 for all data but fat where n = 5–8. Statistical analysis by 2 way ANOVA, ***p < 0.001 Main effect for db/db vs db/+.
Supplementary Figure 3. Taqman Probes' references for real time PCR.
References
- 1.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 3.Boldison J., Wong F.S. Immune and pancreatic beta cell interactions in Type 1 diabetes. Trends in Endocrinology and Metabolism. 2016;27:856–867. doi: 10.1016/j.tem.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Willcox A., Richardson S.J., Bone A.J., Foulis A.K., Morgan N.G. Analysis of islet inflammation in human type 1 diabetes. Clinical and Experimental Immunology. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eguchi K., Nagai R. Islet inflammation in type 2 diabetes and physiology. Journal of Clinical Investigation. 2017;127:14–23. doi: 10.1172/JCI88877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehses J.A., Perren A., Eppler E., Ribaux P., Pospisilik J.A., Maor-Cahn R. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 7.Richardson S.J., Willcox A., Bone A.J., Foulis A.K., Morgan N.G. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52:1686–1688. doi: 10.1007/s00125-009-1410-z. [DOI] [PubMed] [Google Scholar]
- 8.Hasnain S.Z., Borg D.J., Harcourt B.E., Tong H., Sheng Y.H., Ng C.P. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nature Medicine. 2014;20:1417–1426. doi: 10.1038/nm.3705. [DOI] [PubMed] [Google Scholar]
- 9.Herder C., Dalmas E., Boni-Schnetzler M., Donath M.Y. The IL-1 pathway in Type 2 diabetes and cardiovascular complications. Trends in Endocrinology and Metabolism. 2015;26:551–563. doi: 10.1016/j.tem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H.I., Spinas G.A. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. Journal of Clinical Investigation. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eguchi K., Manabe I., Oishi-Tanaka Y., Ohsugi M., Kono N., Ogata F. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metabolism. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Masters S.L., Dunne A., Subramanian S.L., Hull R.L., Tannahill G.M., Sharp F.A. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nature Immunology. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen C.M., Faulenbach M., Vaag A., Volund A., Ehses J.A., Seifert B. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. New England Journal of Medicine. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 14.Rissanen A., Howard C.P., Botha J., Thuren T., Global I. Effect of anti-IL-1beta antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2012;14:1088–1096. doi: 10.1111/j.1463-1326.2012.01637.x. [DOI] [PubMed] [Google Scholar]
- 15.Hensen J., Howard C.P., Walter V., Thuren T. Impact of interleukin-1beta antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: results of secondary endpoints from a randomized, placebo-controlled trial. Diabetes & Metabolism. 2013;39:524–531. doi: 10.1016/j.diabet.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Coll R.C., Robertson A.A., Chae J.J., Higgins S.C., Munoz-Planillo R., Inserra M.C. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nature Medicine. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basiorka A.A., McGraw K.L., Eksioglu E.A., Chen X., Johnson J., Zhang L. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128:2960–2975. doi: 10.1182/blood-2016-07-730556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neudecker V., Haneklaus M., Jensen O., Khailova L., Masterson J.C., Tye H. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. Journal of Experimental Medicine. 2017 doi: 10.1084/jem.20160462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mridha A.R., Wree A., Robertson A.A.B., Yeh M.M., Johnson C.D., Van Rooyen D.M. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. Journal of Hepatology. 2017;66:1037–1046. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dempsey C., Rubio Araiz A., Bryson K.J., Finucane O., Larkin C., Mills E.L. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain, Behavior, and Immunity. 2017;61:306–316. doi: 10.1016/j.bbi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 21.van Hout G.P., Bosch L., Ellenbroek G.H., de Haan J.J., van Solinge W.W., Cooper M.A. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. European Heart Journal. 2017;38:828–836. doi: 10.1093/eurheartj/ehw247. [DOI] [PubMed] [Google Scholar]
- 22.Kraakman M.J., Kammoun H.L., Allen T.L., Deswaerte V., Henstridge D.C., Estevez E. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metabolism. 2015;21:403–416. doi: 10.1016/j.cmet.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Bensellam M., Maxwell E.L., Chan J.Y., Luzuriaga J., West P.K., Jonas J.C. Hypoxia reduces ER-to-Golgi protein trafficking and increases cell death by inhibiting the adaptive unfolded protein response in mouse beta cells. Diabetologia. 2016;59:1492–1502. doi: 10.1007/s00125-016-3947-y. [DOI] [PubMed] [Google Scholar]
- 24.Kammoun H.L., Allen T.L., Henstridge D.C., Kraakman M.J., Peijs L., Rose-John S. Over-expressing the soluble gp130-Fc does not ameliorate methionine and choline deficient diet-induced non alcoholic steatohepatitis in mice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster G.I., Kammoun H.L., Kraakman M.J., Kowalski G.M., Bruce C.R., Febbraio M.A. PKR is not obligatory for high-fat diet-induced obesity and its associated metabolic and inflammatory complications. Nature Communications. 2016;7 doi: 10.1038/ncomms10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura M., Tanaka S., Isoda F., Sekigawa K., Yamakawa T., Sekihara H. T lymphopenia in obese diabetic (db/db) mice is non-selective and thymus independent. Life Sciences. 1998;62:1243–1250. doi: 10.1016/s0024-3205(98)00054-x. [DOI] [PubMed] [Google Scholar]
- 27.Nagareddy P.R., Kraakman M., Masters S.L., Stirzaker R.A., Gorman D.J., Grant R.W. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metabolism. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan J.Y., Luzuriaga J., Bensellam M., Biden T.J., Laybutt D.R. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in beta-cell gene expression and progression to diabetes. Diabetes. 2013;62:1557–1568. doi: 10.2337/db12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahzad K., Bock F., Dong W., Wang H., Kopf S., Kohli S. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney International. 2015;87:74–84. doi: 10.1038/ki.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandanmagsar B., Youm Y.H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Medicine. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stienstra R., van Diepen J.A., Tack C.J., Zaki M.H., van de Veerdonk F.L., Perera D. Inflammasome is a central player in the induction of obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature Immunology. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youm Y.H., Adijiang A., Vandanmagsar B., Burk D., Ravussin A., Dixit V.D. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152:4039–4045. doi: 10.1210/en.2011-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontaine D.A., Davis D.B. Attention to background strain is essential for metabolic research: C57BL/6 and the international knockout mouse consortium. Diabetes. 2016;65:25–33. doi: 10.2337/db15-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hull R.L., Willard J.R., Struck M.D., Barrow B.M., Brar G.S., Andrikopoulos S. High fat feeding unmasks variable insulin responses in male C57BL/6 mouse substrains. Journal of Endocrinology. 2017;233:53–64. doi: 10.1530/JOE-16-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y., Wang W., Okla M., Kang I., Moreau R., Chung S. Suppression of NLRP3 inflammasome by gamma-tocotrienol ameliorates type 2 diabetes. The Journal of Lipid Research. 2016;57:66–76. doi: 10.1194/jlr.M062828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez M., Nogueiras R., Tena-Sempere M., Dieguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nature Reviews Endocrinology. 2016;12:421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 38.Weikel K.A., Ruderman N.B., Cacicedo J.M. Unraveling the actions of AMP-activated protein kinase in metabolic diseases: systemic to molecular insights. Metabolism. 2016;65:634–645. doi: 10.1016/j.metabol.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y., Hara H., Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends in Biochemical Sciences. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dror E., Dalmas E., Meier D.T., Wueest S., Thevenet J., Thienel C. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nature Immunology. 2017;18:283–292. doi: 10.1038/ni.3659. [DOI] [PubMed] [Google Scholar]
- 41.Iikuni N., Lam Q.L., Lu L., Matarese G., La Cava A. Leptin and inflammation. Current Immunology Reviews. 2008;4:70–79. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gove M.E., Rhodes D.H., Pini M., van Baal J.W., Sennello J.A., Fayad R. Role of leptin receptor-induced STAT3 signaling in modulation of intestinal and hepatic inflammation in mice. Journal of Leukocyte Biology. 2009;85:491–496. doi: 10.1189/jlb.0808508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Asseldonk E.J., Stienstra R., Koenen T.B., Joosten L.A., Netea M.G., Tack C.J. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. The Journal of Cinical Endocrinology and Metabolism. 2011;96:2119–2126. doi: 10.1210/jc.2010-2992. [DOI] [PubMed] [Google Scholar]
- 44.Sloan-Lancaster J., Abu-Raddad E., Polzer J., Miller J.W., Scherer J.C., De Gaetano A. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care. 2013;36:2239–2246. doi: 10.2337/dc12-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavelti-Weder C., Babians-Brunner A., Keller C., Stahel M.A., Kurz-Levin M., Zayed H. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35:1654–1662. doi: 10.2337/dc11-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Y.S., Wollam J., Olefsky J.M. An integrated view of immunometabolism. Cell. 2018;172:22–40. doi: 10.1016/j.cell.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.