Abstract
Objective
The incidence of depression is significantly compounded by obesity. Obesity arising from excessive intake of high-fat food provokes anxiodepressive behavior and elicits molecular adaptations in the nucleus accumbens (NAc), a region well-implicated in the hedonic deficits associated with depression and in the control of food-motivated behavior. To determine the etiology of diet-induced depression, we studied the impact of different dietary lipids on anxiodepressive behavior and metabolic and immune outcomes and the contribution of NAc immune activity.
Methods
Adult C57Bl/6 mice were subjected to isocaloric high-fat/high-sucrose diets (HFD), enriched in either saturated or monounsaturated fat, or a control low-fat diet (LFD). Metabolic responses, anxiodepressive behavior, and plasma and NAc inflammatory markers were assessed after 12 weeks. In separate experiments, an adenoviral construct inhibiting IKKβ, an upstream component of the nuclear factor kappa-b (NFkB) pathway, was a priori injected into the NAc.
Results
Both HFDs resulted in obesity and hyperleptinemia; however, the saturated HFD uniquely triggered anxiety-like behavior, behavioral despair, hyperinsulinemia, glucose intolerance, peripheral inflammation, and multiple pro-inflammatory signs in the NAc, including reactive gliosis, increased expression of cytokines, antigen-presenting markers and NFкB transcriptional activity. Selective NAc IKKβ inhibition reversed the upregulated expression of inflammatory markers, prevented anxiodepressive behavior and blunted compulsive sucrose-seeking in mice fed the saturated HFD.
Conclusions
Metabolic inflammation and NFкB-mediated neuroinflammatory responses in the NAc contribute to the expression of anxiodepressive behavior and heightened food cravings caused by a diet high in saturated fat and sugar.
Keywords: Diet-induced obesity, Dietary fatty acids, Nuclear factor kappa-b, Neuroinflammation, Depression, Anxiety, Food reward
Abbreviations: Ad, adenovirus; βgal, beta-galactosidase; CORT, corticosterone; CRP, C-reactive protein; DIO, diet-induced obesity; EPM, elevated-plus maze; FST, forced swim test; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; HFD, high-fat diet; HPA, hypothalamic-pituitary adrenal; IKKβ, inhibitor of kappa-b kinase bêta; IKKdn, inhibitor of kappa-b kinase beta dominant negative; ITT, insulin tolerance test; IFN-γ, interferon-gamma; IL-1β, interleukin-1bêta; i.p., intraperitoneal; Iba-1, ionized calcium binding adaptor molecule 1; LPS, lipopolysaccharide; LFD, low-fat diet; MHC, major histocompatibility complex; NFkB, nuclear factor kappa-b; NAc, nucleus accumbens; OGTT, oral glucose tolerance test; PR, progressive ratio; TNF, tumor necrosis factor
Graphical abstract
Highlights
-
•
Saturated HFD uniquely triggers metabolic impairments & depressive behavior.
-
•
Nucleus accumbens (NAc) inflammation & gliosis are distinct features of the saturated HFD.
-
•
Depressive behavior & NAc immune activation are mediated by IKKβ/NFκB signaling.
-
•
Inhibiting NAc IKKβ/NFκB suppresses compulsive sucrose seeking in mice fed the saturated HFD.
1. Introduction
Obesity considerably elevates the odds of developing depression [1]. Depressed mood not only affects psychological well-being but also considerably increases the risks of obesity complications and diminishes adherence to treatment strategies. In turn, higher body mass index in depressed individuals is associated with poorer prognosis [2]. Obesity in humans and murine models is characterized by a state of chronic low-grade inflammation including elevated circulating inflammatory cytokines, adipokines and triglycerides [3]. Several lines of evidence implicate a neuroimmune etiology in a subset of depressed individuals; thus, persistent immune activation in obesity may give rise to mood impairments. Indeed, an elevation in plasma levels of the inflammatory marker C-reactive protein (CRP), which correlates with depressive symptoms [4], [5], [6], is one of the best predictors of depression onset in obese individuals [6]. Moreover, increased brain expression of interleukin-1 beta (IL-1β) [7], [8], [9], tumor-necrosis factor (TNF) [8], [10], and interferon-gamma (IFN-γ) [10], characteristic of rodent models of lipopolysaccharide (LPS)-induced depressive-like behavior [7], [8], [9], [10], is also evident in mice consuming a high-fat diet (HFD) [11], [12].
As a neuroanatomical substrate integrating signals from diverse inputs, several lines of evidence highlight a major role for the nucleus accumbens (NAc) in the control of motivation and reward-seeking [13], [14], the response to stress [15], and the regulation of anxiodepressive behavior [16], [17]. Anhedonia and motivational deficits are key symptoms of depression that are well-tied to neuroplastic changes in the NAc [18]. We previously found that prolonged high-fat feeding resulting in diet-induced obesity (DIO) elicits anxiodepressive behavior and NAc molecular adaptations that correlate with the extent of behavioral despair [19]. Dietary-derived changes in the NAc may also underlie enhanced palatable food-seeking exhibited by obese individuals and rodent models of obesity [18], [19], [20]. Indeed, as a means to offset the negative emotional state, depressed mood can stimulate food cravings [18], [20] and thereby enable a detrimental cycle whereby caloric overload intensifies metabolic disturbances and strengthens risk of depression comorbidity.
Obese individuals characterized as ‘metabolically healthy’ (without hypertriglyceridemia, inflammation, insulin resistance) do not present with increased risk of depression [21]. Likewise, the anxiogenic effects of a HFD are absent in conditions that preclude obesity and associated metabolic impairments in rodents [22], [23]. Dietary fats can have distinct metabolic, endocrine, and behavioral effects according to their predominant lipid class. In contrast to monounsaturated lipids, excess intake of saturated fat is tied to greater metabolic dysfunction that includes increased visceral fat accumulation and peripheral inflammation [23], [24], [25], [49]. Consistently, depressive symptoms are positively associated with total and saturated fat consumption while inversely correlated with monounsaturated fat intake [26]. Along these lines, we demonstrated that a saturated, but not a monounsaturated, HFD blunts NAc dopaminergic tone and function [27] and perturbs hypothalamo-pituitary-adrenal (HPA) activity in rats [23]; however, the mechanisms mediating saturated fat intake and neurobehavioral impairments are unknown.
Consuming a HFD can elicit immune activation in hypothalamus and hippocampus in rodents [11], [12], [28], [29], [30] whereas human obesity is associated with signs of hypothalamic gliosis and injury [11]. It remains to be elucidated however, if excessive dietary fat can stimulate immune activity in the NAc to gives rise to mood impairments. Given the involvement of the NAc in the hedonic deficits associated with mood disorders [18], as well as its vulnerability to neural adaptations following high-fat feeding [19], the present study sought to identify neuroinflammatory responses in the NAc and determine if they underlie anxiodepressive behavior provoked by DIO. We investigated the impact of two isocaloric HFDs, enriched either in palm oil (saturated) or olive oil (monounsaturated), on energy metabolism, immune activation, and anxiodepressive behavior in mice. A secondary objective was to determine if neuroinflammation propagated by the inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ)-NFкB pathway – a transcription factor playing a key role in driving cytokine gene expression [31] - contributes to anxiodepressive behavior and palatable food-seeking elicited by a HFD. Contrary to the largely protective effect of the monounsaturated HFD, we found a saturated HFD enhances weight gain, metabolic complications, systemic inflammation, and anxiety-like and despair responses in behavioral tests. Concurrently, the saturated HFD was found to increase the expression of several immune markers, trigger reactive gliosis, and stimulate NFкB transcriptional activity in the NAc. Exposing a functional role of NAc NFкB in obesity-induced anxiodepressive and food-motivated behavior, viral-mediated inhibition of IKKβ/NFкB in the NAc prevented neuroinflammation and anxiodepressive behavior and suppressed cue-induced compulsive sucrose-seeking in mice fed the saturated HFD.
2. Material and methods
2.1. Animals and diets
All procedures involving the use of animals were approved by the CRCHUM Animal Care Committee in accordance with Canadian Council on Animal Care guidelines. Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) and NFкB-LacZ reporter mice [32] (C57BL/6 background; in-house colony) were housed in a reverse 12 h light–dark cycle (lights off at 10 AM) with ad libitum access to water and food. Colony-derived heterozygous NFкB-LacZ reporter mice and wildtype littermates were weaned at P21-23 and genotyped as described [32].
Beginning at 8 weeks of age, singly-housed (for food intake measures) or group-housed (2–4/cage; for all remaining experiments) mice were given free access to one of three custom, ingredient-matched diets (Table 1; Dyets, Bethlehem, PA, USA) described in detail previously [27]: a 50% kcal palm oil saturated high-fat diet (“Palm”), an isocaloric 50% kcal olive oil monounsaturated high-fat diet (“Olive”) or a 16.8% kcal soybean oil low-fat diet (“Control”). Eight cohorts of adult male C57BL/6 wildtype and NFКB-LacZ colony mice were employed to investigate the metabolic, behavioral, and biochemical consequences of each diet as described in supporting information. All measures were taken after 12 weeks on the diets.
Table 1.
Diet composition.
| Control | Olive | Palm | |
|---|---|---|---|
| Fat source | Soybean oil | Olive oil | Palm oil |
| Fat (g/kg) | 70 | 270 | 270 |
| Casein (g/kg) | 200 | 200 | 200 |
| l-Cystine (g/kg) | 3 | 3 | 3 |
| Sucrose (g/kg) | 100 | 100 | 100 |
| Cornstarch (g/kg) | 397.5 | 197.5 | 197.5 |
| Dyetrose (g/kg) | 132 | 132 | 132 |
| Mineral Mix (g/kg) | 35 | 35 | 35 |
| Vitamine Mix (g/kg) | 10 | 10 | 10 |
| % Kcal Fat | 17 | 50 | 50 |
| % Kcal carbohydrates | 62 | 43 | 43 |
| % Kcal proteins | 21 | 7 | 7 |
| Total Kcal/g | 3.8 | 4.8 | 4.8 |
| % palmitic acid (C16:0) | 10.2 | 10.512 | 44.5 |
| % stearic acid (C18:0) | 4.5 | 3.0 | 4.2 |
| % oleic acid (C18:1) | 22.7 | 77 | 39.4 |
| % linoleic acid (C18:2) | 54.8 | 7 | 9.5 |
| % linolenic acid (C18:3) | 7.8 | 0.6 | N/A |
| % saturated fat | 15 | 17.1 | 51.1 |
| % monounsaturated fat | 23.4 | 72.3 | 38.8 |
| % polyunsaturated fat | 61.2 | 10.6 | 9.7 |
N/A: data not available.
2.2. Metabolic profiling
Mice from each of the three diet conditions (n = 8/group) were fasted overnight for basal blood glucose measurements. For oral glucose tolerance test (OGTT), mice were fasted overnight followed by oral gavage of dextrose (2 g/kg of body weight). For intraperitoneal insulin tolerance tests (ITT), food was removed from the cage at 9 AM for 4 h fasting prior to intraperitoneal (i.p.) injection of insulin. For both OGTT and ITT, blood glucose was measured at 0, 15, 30, 45, 60, 90, and 120 min. Body mass composition was measured via Echo MRI and subcutaneous, perigonadal, and perirenal fat depots were extracted at sacrifice and weighed. Blood was collected from a new cohort of mice (n = 7–13/group) at the time of sacrifice to measure plasma insulin (Cedarlane Labs, Burlington, Canada), corticosterone (at circadian peak; Enzo Life Sciences, Farmingdale, NY, USA), TNF (Qiagen, Hilden, Germany), IL-1β (Qiagen) and CRP (Life Diagnostics, Inc., WestChester, PA, USA) levels via ELISA. Plasma insulin and leptin from adenovirus treated mice was measured by alpha-ELISA (Perkin–Elmer, MA, USA).
2.3. Behavioral testing
2.3.1. Elevated-plus maze (EPM)
Separate cohorts of mice were used to measure changes in anxiodepressive behavior induced by diet and in response to diet ± NAc IKKβ/NFкB inhibition. For the EPM test, mice were placed in the center of an elevated-plus platform composed of two open arms and two closed arms. Anxiety-like behavior was assessed by quantifying the proportion of time spent and number of entries in the open arms over a 5-minute trial. Each trial was video recorded and analyzed using the EthoVision software as described previously [19].
2.3.2. Forced swim test (FST)
Assessment of behavioral despair using the FST was carried out as described previously [19]. Briefly, mice were placed in a water-filled glass container for 6 min. The first 2 min of the trial served as habituation and velocity was measured in order to assess locomotor capacity. Immobility time during the last 4 min was indicative of behavioral despair. Each trial was video recorded and analyzed using the EthoVision software.
2.4. Quantitative PCR
Tissue punches of NAc (core and shell) were obtained from frozen coronal sections (200 μm) and RNA extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). cDNA was synthetized from 700 ng of total RNA using random hexamers and M-MLV Reverse Transcriptase (Invitrogen). Real-time PCR was performed using the Rotor Gene SYBR Green PCR kit (Qiagen). Primers were designed using BLAST (U.S. National Library of Medicine) and synthesized by Integrated DNA Technologies, Inc. based on the sequences listed in Table 2. Data were extrapolated from standard curves and normalized to the housekeeping genes (Beta-actin, Cyclophilin or HPRT), which were not affected by diet (Fig. S1). The most stable housekeeping gene across experimental groups was selected according to GeNorm and NormFinder algorithms. Mean ± standard error of mean values for each group are expressed in fold changes relative to Control (Figure 3A) or ControlGFP (Figure 4H) normalized at 1.0.
Table 2.
List of primer sequences.
| Genes | Forward sequence | Reverse sequence |
|---|---|---|
| β-actin | TTCTTGGGTATGGAATCCTGTGGCA | ACCAGACAGCACTGTGTTGGCAT |
| Cyclophilin | GCTTTTCGCCGCTTGCTGCA | TGCAAACAGCTCGAAGGAGACGC |
| Hprt | AGTCCCAGCGTCGTGATTAG | TCTCGAGCAAGTCTTTCAGTCC |
| Gfap | AACGACTATCGCCGCCAACTG | CTCTTCCTGTTCGCGCATTTG |
| Iba-1 | GGATTTGCAGGGAGGAAAAG | TGGGATCATCGAGGAATTG |
| Tnf | CACGCTCTTCTGTCTACTG | AAGATGATCTGAGTGTGAGG |
| Il-1β | CTTGTGCAAGTGTCTGAAG | GAACAGGTCATTCTCATCAC |
| Ifn-γ | AAGTTTGAGGTCAACAACCCAC | AATCTCTTCCCCACCCCGAA |
| Cd45 | TGAGCACAACAGAGAATGCCC | AACACACCTGGATGATATGTGGT |
| Cd11b | CCCAGAACCTCTCAAGTGCC | CTGCAACAGAGCAGTTCAGC |
| Hsp-72 | CAGAGGCCAGGGCTGGATTA | ACACATGCTGGTGCTGTCACTTC |
| βgal | CCTCGAATCAGCAACGGCTT | TGAAGTTCAGATGTGCGGCG |
| Ikkβ | GGCACCTTGGATGACCTAGA | CCATATCCTGGCTGTCACCT |
| Vimentin | GGCTCGTCACCTTCGTGAAT | AGAAAAGGTTGGCAGAGGCA |
| Mhc-i(H2kb) | GTGATCTCTGGCTGTGAAGT | GTCTCCACAAGCTCCATGTC |
| Mhc-ii(H2aa) | CAACCGTGACTATTCCTTCC | CCACAGTCTCTGTCAGCTC |
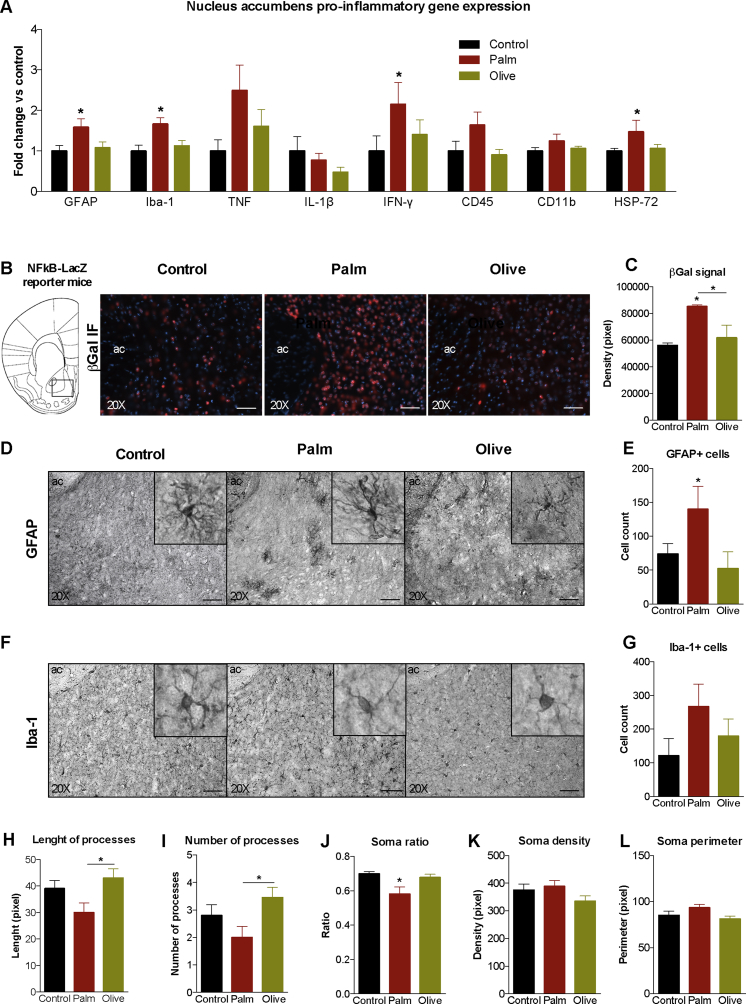
Figure 3.
Saturated, high-fat feeding triggers inflammation in the nucleus accumbens. (A) Relative nucleus accumbens gene expression of glial fibrillary acidic protein (GFAP), ionized calcium binding adaptor molecule-1 (Iba-1), tumor necrosis factor (TNF), interleukin-1bêta (IL-1β), interferon-gamma (IFN-γ), CD45, CD11b, and heat-shock protein-72 (HSP-72). (B) βgal (red) immunofluorescence on nucleus accumbens coronal slices of NFkB-LacZ (βgal) reporter mice fed one of 3 diets. 20× magnification; 50 μm scale bars. (C) Quantification of βgal signal density in nucleus accumbens (n = 4/diet). (D, E) Staining and quantification of GFAP + cells in the nucleus accumbens (n = 7–10/diet). (F, G) Staining and quantification of Iba-1+ cells in the nucleus accumbens (n = 8–14/diet). (H, I) Length and number of processes of Iba-1+ cells (n = 9–11/diet). (J–L) Ratio (minimum ferret/maximum ferret), density and perimeter of Iba-1+ cells (n = 10–11/diet). Cell quantification per surface area of 0.078 mm2; magnification of 20× and 63× (inserts); 50 μm scale bars. Group mean ± SEM; one-way analysis of variance, Bonferroni post hoc; *p < 0.05, **p < 0.01.
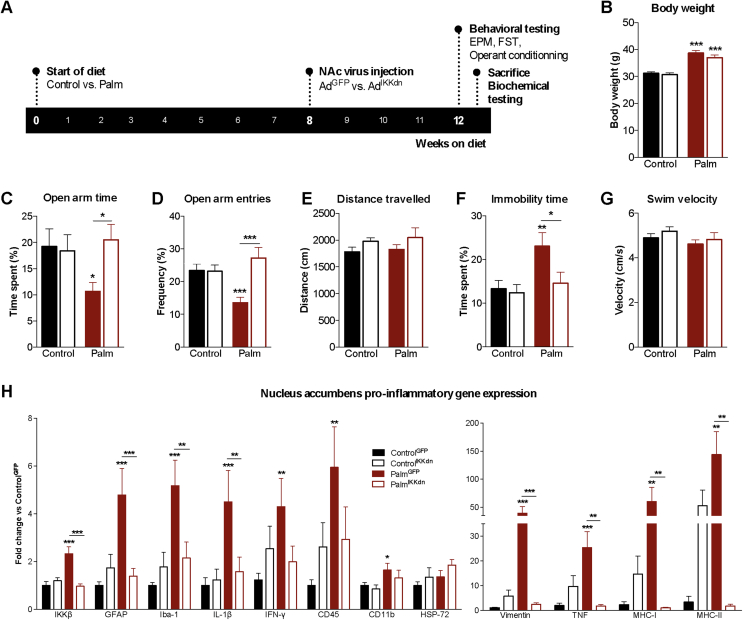
Figure 4.
Inhibition of IKKβ/NFkB in the nucleus accumbens prevents diet-induced anxiodepressive behavior and neuroinflammation. (A) Experimental layout depicting start of diets, viral injection and testing. (B) Final body weights after testing (n = 21–25/group). (C, D) Time spent and entries in open arms of the elevated-plus maze (n = 13–20/group). (E) Distance traveled in the elevated-plus maze. (F) Immobility time during the last 4 min of the forced swim test (n = 13–19/group). (G) Swimming velocity during the first 2 min of the forced swim test (n = 13–19/group). (H) Nucleus accumbens relative gene expression of inhibitor of kappa-B kinase-bêta (IKKβ), glial fibrillary acidic protein (GFAP), ionized calcium binding adaptor molecule-1 (Iba-1), interleukin-1bêta (IL-1β), interferon-gamma (IFN-γ), CD45, CD11b, heat shock protein-72 (HSP-72) vimentin, tumor necrosis factor (TNF), major histocompatibility complex (MHC)-1 and MHC-II (n = 7–9/group). Group mean ± SEM; two-way analysis of variance, Bonferroni post hoc; *p < 0.05, **p < 0.01, ***p < 0.005.
2.5. Beta-galactosidase histochemistry
Heterozygous NFκB-LacZ mice subjected to the diet protocol (n = 4/group) were injected with a lethal dose of sodium pentobarbital prior to cardiac perfusion with 30 mL of ice-cold phosphate buffered saline (PBS), followed by 30 mL of 10% buffered formalin. Following 4 h immersion in 10% buffered formalin, brains were transferred to 30% sucrose for 24–48 h. Coronal brain sections (30 μm) containing the rostro-caudal extent of the NAc were sliced with a microtome (Leica SM2000R). Free floating slices were washed in PBS and incubated in a blocking buffer (3% normal goat serum + 0.1% Triton + 0.02% PBS-azide) for 2 h at room temperature. Cell membranes were then permeabilized by incubating sections in 0.2% Tween in PBS solution for 9 min and then rinsed with PBS prior to overnight incubation in blocking solution with a primary polyclonal chicken antibody against βgal, Ab9361 (1:1000), Abcam (Cambridge, UK) at 4 °C. Following washes, sections were incubated with an Alexa Fluor® 568 conjugated goat anti-chicken IgG antibody (Abcam, 1:1000) for 2 h, at room temperature. Following washes, sections were mounted onto microscope slides with DAPI aqueous mounting media (Vectashield, Vector Labs, Burlingham, CA, USA) and visualized (Zeiss AxioImager.M2 ApoTome.2).
2.6. Glia immunostaining
Following the diet protocol, wildtype mice (n = 3/group) were perfused and brains post-fixed, cryoprotected, and sliced as above. Free-floating slices (30 μm) were incubated with a primary polyclonal rabbit antibody against GFAP (Ab5804), Millipore (Darmstadt, Germany) or Iba-1 (019–19741), Wako Chemicals USA (Richmond, VA, USA) at a dilution of 1:500. Following washes, sections were incubated with the horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody provided in Vectastain Elite ABC HRP kit (Vector Labs) at a dilution of 1:1000 for 2 h at room temperature. Sections were immersed in avidin/biotin complex solution for 1 min, rinsed with PBS, and mounted onto microscope slides. Prior to application of mounting media (Vectashield, Vector Labs) and coverslip, slides were dehydrated in 95% ethanol solution in PBS and 100% ethanol for 30 s and 1 min, respectively.
2.6.1. Morphometric analysis of microglia
Images acquired (Zeiss AxioImager.M2 ApoTome.2) were converted to 8-bit using ImageJ. Density, perimeter, maximum ferret, and minimum ferret for the soma of each microglia (n = 3–4/mouse) were measured using the “analyze particles” function of ImageJ. Soma ratio was calculated by dividing the minimum ferret value by the maximum ferret value, giving an indicator of circularity from 0.00 to 1.00. The total number of visible processes per cell was counted and the length of each process was measured as distance from the border of the soma to the end of the process.
2.7. Viral-mediated inhibition of IKKβ/NFkB
2.7.1. Viral construct
Adenoviral constructs consisted of a sequence coding for a dominant negative mutation of the inhibitor of kappa-B kinase beta subunit (AdIKKdn) [33] or green fluorescent protein (AdGFP) under the control of a cytomegalovirus promoter. In view of the modulatory role of NFкB in inflammatory gene expression in glia and neurons, a recombinant adenovirus (serotype 5) was selected to enable high transduction efficiency and broad host range. Adenoviruses were amplified and purified by Vector Biolabs (Malvern, PA, USA).
2.7.2. Validation of viral inhibition
Viral titer and placement validation was performed in heterozygous NFкB-LacZ mice microinjected with AdIKKdn in NAc of right hemisphere and AdGFP in NAc of left hemisphere. Mice were sacrificed 2 weeks after surgery and 6 h following intraperitoneal lipopolysaccharide (LPS; Escherichia Coli 0127:B8, Millipore Sigma) injection (500 μg/kg). Coronal NAc slices were subjected to the βgal immunofluorescence protocol to visualize changes in LPS-induced NFкB transcriptional.
2.7.3. Stereotaxic surgery
Mice were anaesthetized with isoflurane and placed into a mouse ultraprecise stereotaxic instrument (Kopf, Inc.) with Bregma and Lambda in the same horizontal plane. AdIKKdn or AdGFP (0.5μL/side; 3.0 × 107 PFU/side) was delivered bilaterally into the NAc (AP:+1.7 mm. ML:±1.1 mm, DV: −4.5 mm, relative to bregma and skull surface) using a 0.5 μL NeuroSyringe (Hamilton, Reno, NV, USA).
Bearing in mind the observed distinct neurobehavioral impact of the palm HFD, viral manipulations were carried out in mice submitted to the palm HFD and control diet, providing four groups: ControlGFP; ControlIKKdn; PalmGFP and PalmIKKdn. Mice (n = 17–21/group) were injected with adenoviruses following 8 weeks on their respective diets and then continued on these diets for an additional 4 weeks post-surgery before behavioral and biochemical testing. Mice were individually housed after surgery.
2.8. Operant conditioning
2.8.1. Sucrose motivation
Training mice to respond for sucrose on a progressive ratio (PR) schedule was as described [22], [34]. Briefly, chow-fed mice were food restricted to maintain 90% of their body weight and trained daily in an operant task to press one of two levers to receive a sucrose pellet. Once stable responding in the PR task was achieved, all mice were provided ad libitum access to the Control or Palm HFD and subjected to operant training at least once a week to maintain PR responding. After 8 weeks, baseline breakpoint values were measured over 3 consecutive days followed by stereotaxic microinjections of AdIKKdn or AdGFP adenovirus in to the NAc as described above. Two weeks post-surgery, ControlGFP, ControlIKKdn, PalmGFP, and PalmIKKdn mice were reintroduced into the operant chambers for PR training and testing. Breakpoint thresholds (the last response ratio successfully completed) obtained over 3–5 consecutive days during the 11th week of diet were used in the analysis. Data are presented as breakpoint values before (8th week of diet) and after stereotaxic microinjection (11th week).
2.8.2. Conditioned suppression test
To assess compulsive sucrose seeking, we adapted a protocol of cue-induced suppression of feeding [35] to the operant setting (Figure 5D). At the end of the operant protocol above, PalmGFP and PalmIKKdn mice were transitioned from the palm HFD to standard chow, a manipulation which stimulates craving and food-seeking [22]. Mice were trained to lever-press for sucrose pellets on a fixed-ratio 5 schedule of reinforcement in operant cages equipped with grid floors capable of delivering electrical current (Med Associates, Inc., St Albans, VT, USA). During each 30-minute operant session, in the first 10-minute block the house light was illuminated, in the second 10 min block the house light was off, and in the last 10-minutes the house light was illuminated. Baseline measures of lever pressing represented the mean of 3 consecutive daily sessions. The conditioning phase consisted of 4 consecutive daily sessions with delivery of a foot-shock paired to the illuminated house light (1s 0.45 mA/min for 20 min; total of 20 shocks), active lever, and the availability of sucrose reward. On the test day (Day 5), lever responses were measured under the same conditions except that no foot-shocks were delivered. Data are presented as the total number of lever-presses per session.
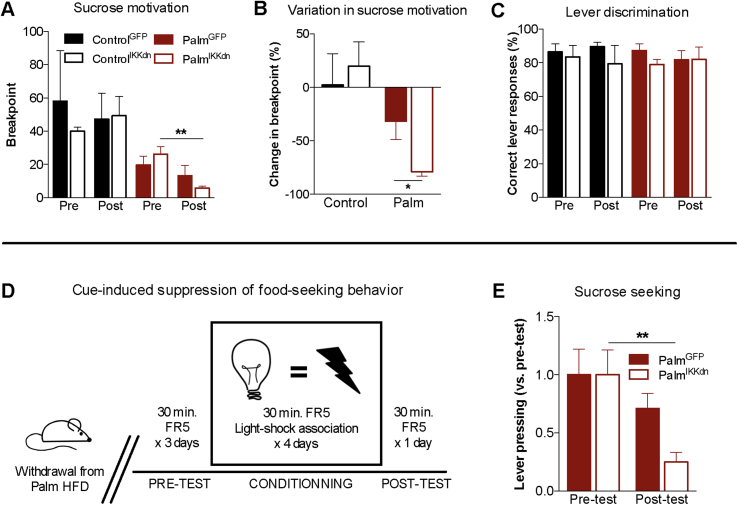
Figure 5.
Nucleus accumbens IKKβ/NFkB inhibition decreases sucrose reward and compulsive sucrose seeking in mice fed the saturated high-fat diet. (A) Breakpoint values in operant responding for sucrose pellets before (Pre) and after (Post) viral injection (n = 3–6/group); two-way analysis of variance, Bonferonni post hoc. (B) Variation in breakpoint ratio before and after viral injection; unpaired two-tailed t-test (PalmGFP vs. PalmIKKdn). (C) Preference for correct over incorrect lever in operant responding for sucrose pellets before and after viral injection. (D) Protocol for cue-induced suppression of sucrose seeking. (E) Variation in lever pressing for sucrose before and after aversive conditioning (n = 5–6/group); two-way analysis of variance, Bonferonni post hoc. Group mean ± SEM; *p < 0.05, **p < 0.01.
2.9. Statistical analyses
Data were analyzed with GraphPad Prism 6 and are presented as mean ± standard error of the mean (SEM). A one-way ANOVA was used to compare the three diet groups. Viral intervention results were analysed via 2-way ANOVA (diet × genotype) with Bonferonni post-hoc as well as differences in sucrose motivation (time × virus). Criterion for significance was set to p < 0.05 in all comparisons.
3. Results
3.1. Saturated high-fat feeding potentiates metabolic impairments and inflammation
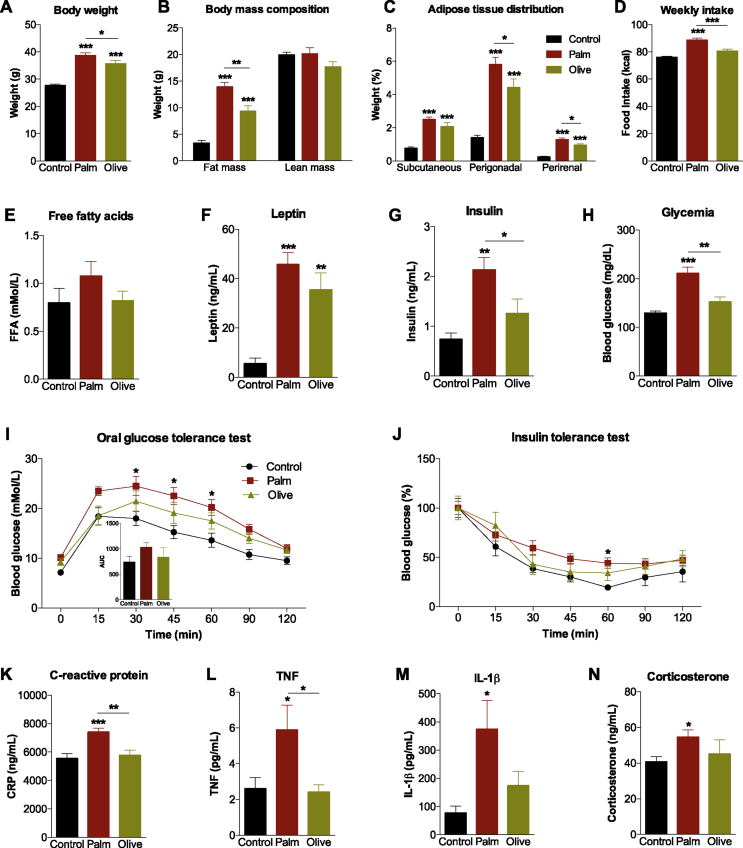
We first sought to determine the impact of prolonged intake of HFDs enriched in either palm or olive oil on multiple metabolic endpoints. Both the palm and olive HFDs resulted in greater weight gain relative to controls; however, mice fed the palm HFD gained an average of 3.0 g ± 1.3 g (equating 8.5% ± 2.8%) more body weight than the olive HFD group (Figure 1A). Similarly, significant fat accumulation was exhibited by both HFD groups, but total fat mass (Figure 1B), and visceral (perirenal and perigonadal) fat deposition were more pronounced in the palm HFD mice (Figure 1C). This was accompanied by greater caloric intake in the palm HFD group compared to controls and olive HFD (Figure 1D). While no changes were observed in plasma total non-esterified free fatty acid levels (Figure 1E), plasma leptin levels increased in both the palm and olive HFD groups with no difference between the two HFDs (Figure 1F). As compared to both the control and olive HFD groups, mice fed palm HFD had higher insulin (Figure 1G) and glucose levels (Figure 1H). Consistently, oral glucose tolerance tests demonstrate that the palm HFD provoked glucose intolerance (30, 45 and 60 min) (Figure 1I). Moreover, mice fed the palm HFD demonstrated a trend towards reduced insulin sensitivity relative to controls (Figure 1J). Plasma CRP and TNF levels were elevated by palm HFD relative to the olive HFD and control diet (Figure 1K,L). Finally, plasma IL-1β and corticosterone concentrations increased in the palm HFD group relative to controls (Figure 1M, N).
Figure 1.
Saturated high-fat feeding potentiates metabolic impairments and inflammation. (A–C) Final body weights (n = 23–24/diet), body mass composition (n = 7–8/diet), and adipose tissue depositions (n = 8/diet) following 12 weeks of low-fat diet (Control), saturated (Palm) or monounsaturated (Olive) high-fat feeding. (D) Average weekly caloric intake on each diet (n = 8/diet). (E–H) Plasma levels of free fatty acids, leptin, insulin and glucose at time of sacrifice (n = 6–8/diet). (I) Oral glucose tolerance test in overnight fasted mice receiving a dose of dextrose (2 g/kg) (n = 7–8/diet). (J) Insulin tolerance test in 4 h fasted mice injected with insulin (n = 4–8/diet). (K–N) Plasma levels of C-reactive protein, tumor necrosis factor (TNF), interleukin-1bêta (IL-1β) and corticosterone at time of sacrifice (n = 7–13/diet). Group mean ± SEM, One-way analysis of variance, Bonferroni post hoc; *p < 0.05, **p < 0.01, ***p < 0.005.
3.2. Stimulation of anxiodepressive behavior by a saturated (but not monounsaturated) high-fat diet
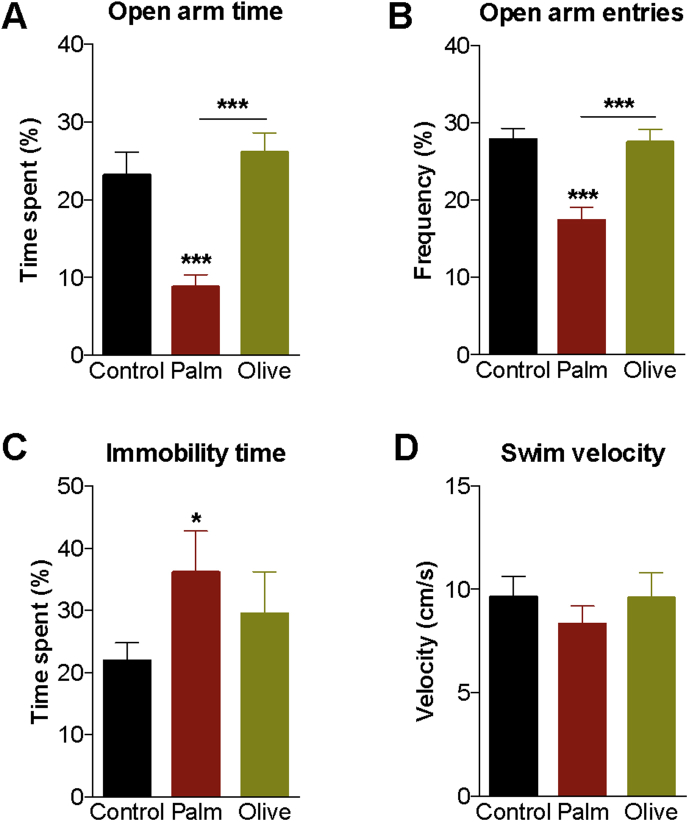
To establish if the development of anxiodepressive behavior relies on diet lipid composition and associated metabolic consequences, naïve mice were tested in the elevated-plus maze (EPM) and forced swim test (FST) following the 12-week diet period. The palm HFD reduced the proportion of time spent and number of entries into the open arm of the EPM as compared to controls and olive HFD (Figure 2A,B). Mice fed the palm, but not the olive, HFD had increased immobility time in the FST compared to controls (Figure 2C). Swim speed (velocity) was similar across the three diet conditions (Figure 2D), suggesting that changes in locomotor capacity do not account for reduced mobility exhibited by palm HFD mice in the EPM and FST. In addition, locomotor activity measured during the last 2 h of light phase (period when behavioral testing was carried out) was similar between palm HFD and control mice (data not shown).
Figure 2.
Stimulation of anxiodepressive behavior by a saturated, high-fat diet. (A, B) Time spent and number of entries into the open arms of the elevated-plus maze (n = 15–16/diet). (C) Immobility time during the 4 last minutes of the forced swim test (n = 6–8/diet). (D) Swimming velocity during the first 2 min of the forced swim test (n = 6–8/diet). Group mean ± SEM; one-way analysis of variance, Bonferroni post hoc; *p < 0.05, ***p < 0.005.
3.3. Saturated high-fat feeding triggers inflammation in the nucleus accumbens
Structural and functional alterations in the NAc have been well associated with anhedonia, anxiodepressive behavior, and manifestation of depressive disorders [18], [36], [37]. Thus, we next sought to determine if NAc inflammation accompanies peripheral immune activation evoked by the palm HFD. NAc mRNA levels of glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba-1), astrocyte and microglia/macrophage markers respectively, were potentiated by the palm HFD (Figure 3A). In addition, IFN-γ and heat shock protein-72 (HSP-72; marker of neural injury) expression increased and there was a trend (p = 0.08) for elevated expression of CD45 (leukocyte common antigen) in palm HFD mice relative to controls (Figure 3A).
Considering the key contribution of the NFкB pathway in the expression of pro-inflammatory genes, we assessed NFкB activity in response to the three diets. For effective readout of NFкB transcriptional activity [32], we next submitted NFkB-LacZ (beta-galactosidase, βgal) reporter mice to the diets to measure and visualize changes of NAc NFкB activation. βgal immunolabeling revealed greater βgal expression in the palm HFD group (Figure 3B). The palm HFD produced a significant increase in βgal signal intensity relative to the control diet and olive HFD (Figure 3C).
Heightened GFAP and Iba-1 mRNA levels observed in palm HFD mice persuaded further examination of diet-induced reactive gliosis in the NAc. Consistent with gene expression data, GFAP immunohistochemistry revealed an elevation in the number of GFAP + astrocytes in palm HFD mice compared to controls (Figure 3D,E). Although increases in Iba-1+ microglia/macrophage number were not significant (Figure 3F,G), Iba-1+ microglia of palm HFD mice displayed greater amoeboid (active state) morphology as evidenced by fewer (Figure 3H) and shorter (Figure 3I) processes as well as reduced soma ratio (Figure 3J). Retraction of microglia processes, characteristic of inflammatory conditions, is a morphological change associated with greater mobility and phagocytic activity in response to immune threat. Density and perimeter of soma (Figure 3K,L) remained unchanged across all three diets.
3.4. Nucleus accumbens viral inhibition of IKKβ/NFκB prevents diet-induced anxiodepressive behavior and neuroinflammation
In order to evaluate the involvement of NFкB signaling in diet-induced NAc neuroinflammation and anxiodepressive behavior, we applied a viral strategy for targeted delivery of a dominant negative form of IKKβ, an upstream component of the canonical IKK complex conferring NFкB activation (AdIKKdn), or a control adenovirus expressing green fluorescent protein (AdGFP) in mice fed the control diet or the palm HFD (Figure 4A). Histology and βgal immunofluorescence in LPS-treated NFkB-LacZ reporter mice injected with AdIKKdn or control (AdGFP) confirmed NAc injection coordinates and the effectiveness of AdIKKdn to reduce NFкB activity (Figs. S2A and B). βgal immunofluorescence was unchanged in the dorsal and ventrolateral areas of the striatum (data not shown). βgal gene expression was also compared between the AdGFP-infected and AdIKKdn-infected hemispheres of LPS-treated NFкB-LacZ reporter mice (Fig. S2C). While the palm HFD increased body weight relative to control as expected, no effect of the virus injected (AdGFP vs. AdIKKdn) was observed (Figure 4B). Accordingly, viral inhibition of IKKβ/NFкB in the NAc did not affect locomotor activity, energy expenditure and respiratory exchange (Fig. S3). In agreement with our previous results, Palm HFD mice receiving AdGFP (“PalmGFP”) showed a significant reduction in the proportion of time spent (Figure 4C) and frequency of entries (Figure 4D) into the open arm of the EPM as compared to ControlGFP mice. Targeted inhibition of IKKβ/NFкB in the NAc of Palm HFD mice (“PalmIKKdn”) normalized EPM anxiety-like behavior to that of controls (Figure 4C,D) without differences in distance travelled (Figure 4E). Similarly, the effect of Palm HFD to increase behavioral despair in the FST was reproduced as shown by increased immobility time in PalmGFP mice as compared to ControlGFP mice (Figure 4F), an effect that was blocked in PalmIKKdn mice (Figure 4F). These changes occurred without any differences between groups in swim velocity during the FST (Figure 4G). Moreover, the 24 h assessment of locomotor activity in metabolic chambers, notably during the light phase when behavioral testing occurred, suggests that normalization of behavioral despair in PalmIKKdn mice is not attributed to increased locomotion (Fig. S3).
NAc IKKβ mRNA expression was reduced by 58.5% ± 4.4% in PalmIKKdn mice (0.97 fold change vs. ControlGFP) relative to PalmGFP mice (2.33 fold change vs. ControlGFP) (Figure 4H). PalmGFP mice demonstrated enhanced NAc mRNA expression of GFAP, Iba-1, vimentin, TNF, IL-1β, IFN-γ, CD45, and CD11b (macrophage and microglia marker) compared to ControlGFP mice (Figure 4H). As these results suggested recruitment of immune cells, major histocompatibility complex class I (MHC-I) and class II (MHC-II) genes were quantified and found to be highly elevated in PalmGFP relative ton ControlGFP mice (Figure 4H). On the other hand, HFDIKKdn mice showed marked reductions in the expression of GFAP, Iba-1, vimentin, TNF, IL-1β, MHC-I, and MHC-II compared to PalmGFP mice (Figure 4H). In contrast, mRNA levels of vimentin, TNF, MHC-I and MHC-II tended to increase in ControlIKKdn mice compared to ControlGFP controls, a finding that likely reflects the bimodal influence of NFкB on inflammatory gene expression [38].
3.5. Nucleus accumbens IKKβ/NFκB signaling modulates sucrose reward and compulsive seeking
Obesity is strongly tied to heightened food reward and food cue reactivity [39], [40], and depressed mood can trigger overconsumption of palatable foods as a means to offset the negative emotional state [18], [20]. Moreover, numerous lines of evidence implicate the NAc in the control of food-motivated behavior and cue-induced food-seeking [13], [14], [41]. To determine if NAc IKKβ/NFкB inhibition blunts sucrose-motivated behavior, PR operant testing was carried out in ControlGFP, ControlIKKdn, PalmIKKdn and PalmGFP immediately before and after adenoviral injections. There were no differences in breakpoint thresholds between ControlGFP and ControlIKKdn as well as between PalmIKKdn and PalmGFP mice before surgery (Figure 5A); however, PalmIKKdn mice breakpoint values significantly dropped after adenoviral injections relative to pre-surgery baseline (Figure 5A,B) despite no differences in preference for correct lever between ControlGFP, ControlIKKdn, PalmIKKdn and PalmGFP mice (Figure 5C). Viral injections had no impact on post-injection body weights, food intake, plasma insulin and leptin and the capacity of mice to discriminate between correct and incorrect levers (Fig. S4). As a final step, we sought to investigate if NAc IKKβ/NFкB inhibition could counteract heightened sucrose seeking elicited by withdrawing access to HFD (transition to chow [19]) using the cue conditioned suppression test of compulsive food seeking (Figure 5D) [35], [42]. PalmGFP mice transitioned to a normal chow diet failed to show a significant reduction in sucrose responding despite exposure to aversive conditioning (Figure 5E) to suggest the presence of compulsive sucrose seeking. Conversely, lever-pressing was markedly suppressed by aversive conditioning in PalmIKKdn mice (Figure 5E), providing evidence that moderating palm HFD-evoked NFkB/IKKβ signaling can diminish compulsive sucrose-seeking.
4. Discussion
Obesity and mood disorder comorbidity poses a major threat to the management of obesity, depression, and overall health. Obesity is estimated to escalate the risk of major depressive disorder by 58% [1], a relationship that is absent when obesity presents without major metabolic deficits [21], [43]. Dietary lifestyle is a key determinant of metabolic outcomes and is increasingly linked to changes in brain function and the development of neuropsychiatric and neurodegenerative diseases. Unraveling the complex interactions between nutrient intake and metabolism, endocrine output, immune activity, and neural function is germane to identifying the pathophysiological mechanisms through which diet and obesity can perturb emotional and motivational processes.
The present findings demonstrate that overconsumption of saturated fat (in combination with excess sugar) that leads to the development of DIO stimulates anxiodepressive behavior. The use of an olive oil-based HFD permitted identification of the unique impact of saturated lipids on behavioral indices and anxiety and despair. Stimulation of anxiodepressive behavior by the palm HFD was accompanied by elevated adiposity, hyperinsulinemia, glucose intolerance, hypercorticosteronemia and circulating pro-inflammatory markers - features of type 2 diabetes and the metabolic syndrome [44]. As we reported previously, the palm HFD also raises plasma concentrations of the long chain fatty acid palmitate in a manner proportional to diet content [23]. Largely protective effects of the isocaloric olive HFD on glucose homeostasis, peripheral and central immune activity and anxiodepressive behavior, despite significant increases in body weight, fat mass, and leptin levels, underscore the distinct action of the saturated HFD. Consistent with these observations, monounsaturated fatty acids can provide cardio-metabolic [25] and mood benefits [26] while Mediterranean diets characterized by a higher proportion of monounsaturated fats confer reduced risk of metabolic [45] and mood disorders [46], [47]. Moreover, a large component of the olive oil HFD and Mediterranean diets is oleate, a fatty acid reported to have anti-inflammatory properties [48]. Thus, oleate may help protect against the priming of microglia observed in the olive group of the current study. This observation is consistent with oleic acid inhibition of LPS-induced NFκB activation in cultured microglia [49]. On the other hand, plasma levels of the saturated fatty acid palmitate are associated with depression severity in humans [50] whereas acute palmitate treatment in mice, a manipulation tied to increase hypothalamic inflammation [51], [52], has anxiogenic actions [53]. Collectively, the present results are largely consistent with clinical and epidemiological observations and with rodent studies from our lab and others suggesting that metabolic impairments underlie the expression of anxiodepressive behavior in obesity [21], [43], [44], [46], [47], [54], [55], [56], [57], [58], [59], [60].
Multiple lines of evidence point to the immune-activating actions of saturated fats in the development of anxiodepressive behavior. Increased visceral adiposity, which correlates with inflammatory profile and metabolic dysfunction [55], manifested in mice fed the saturated but not the monounsaturated HFD. In addition, as seen here and in previous studies [23], [61], the saturated HFD caused a substantial rise in CORT which may have well generated heightened peripheral and central immune responses. These changes were accompanied by significantly higher circulating levels of CRP, TNF, and IL-1β, inflammatory signals well-associated with depression risk and severity. CRP, in particular, was highlighted as a primary variable predicting depression onset in obesity [6]. Concomitantly, several indicators of inflammation in the NAc were distinguished in saturated HFD mice, including heightened NFкB activation, reactive gliosis, and elevated expression of pro-inflammatory cytokines and immune markers. Of key importance, reversal of all of these upregulated genes by the saturated HFD mice was accomplished by selective NAc IKKβ/NFкB inhibition that was sufficient to protect from diet-induced anxiodepressive behavior.
Peripheral cytokines can access the central nervous system and increase production of local inflammatory mediators including cytokines and chemokines by endothelial cells, perivascular macrophages, microglia, and astrocytes. The saturated HFD increased mRNA expression of microglia and astrocyte markers (Iba-1 and GFAP) in the NAc and augmented their activity and number, respectively. This result is in line with reports of reactive gliosis in the hypothalamus in response to a HFD [11], [28]. Also evident was increased gene expression of IFN-γ, a cytokine promoting immune cell infiltration produced by resident microglia and macrophages as well as astrocytes and lymphoid cells [62], [63]. IFN-γ is also an important activator of macrophages and inducer of MHC-I and MHC-II expression, molecules that were substantially increased in PalmGFP mice. The trend for increased CD45 expression observed could additionally suggest macrophage recruitment; however, while it was previously shown that DIO can provoke infiltration of peripheral immune cells [64] establishing whether or not they are increased in the NAc would require local cell quantification. Elevated HSP-72 expression serves as added evidence of NAc cellular insult and inflammation by the saturated HFD as this protein protects from apoptotic and necrotic cell death. Finally, immunohistochemistry data revealed heightened transcriptional activity of NFкB. Taken together, these findings uncover NAc immune activation in response to prolonged saturated high-fat feeding and obesity.
Increases in the expression of pro-inflammatory genes by palm HFD was more pronounced in mice receiving adenovirus injections (magnitude of gene expression increases more pronounced in PalmGFP vs. ControlGFP), owing perhaps to the combined immune-stimulating effect of the diet and the injection and/or virus. In view of this, we measured the expression of additional immune markers, which prove more difficult to quantify in less inflammatory conditions. In agreement with the role of IKKβ/NFкB pathway, saturated HFD increased IKKβ mRNA expression and this was normalized by viral-mediated inhibition of IKKβ in PalmIKKdn mice. Additionally, NAc inhibition of the IKKβ/NFкB signaling pathway reduced signs of reactive gliosis (GFAP, Iba-1, vimentin) as well expression of cytokines (TNF, IL-1β) and antigen markers (CD11b, MHC-II). These molecular changes corresponded with a reversal of behavioral signs of despair and anxiety. Importantly, viral injections did not alter metabolic measures and thus cannot explain the biochemical and behavioral changes found. Together, these observations resonate with the link between peripheral immune cells infiltration and anxiodepressive behavior [9], [65] as well as reduced blood–brain barrier permeability by high-fat feeding [66], [67]. In contrast, IKKβ/NFкB inhibition failed to reduce the expression of inflammatory markers in mice fed the control LFD given that markers of astrogliosis (vimentin), inflammation (TNF) and immune responses (MHC-I, MHC-II) had a tendency to be higher in ControlIKKdn as compared to ControlGFP mice. We suspect this to be related to the additional functions of the NFкB signaling pathway outside of inflammatory regulation [32]. As NFкB is constitutionally active in neurons, attempts to inhibit this pathway under basal conditions may be challenged by homeostatic mechanisms. These findings suggest that improvements in anxiodepressive behavior in this metabolic inflammation model can be explained, at least in part, by reduction of the NFкB pathway in the NAc, and are consistent with reports of neuroinflammation-induced anxiodepressive behavior in rodents [65], [68], [69], [70] and impairments in NAc function following peripherally-induced inflammation in humans [71].
Depression and anxiety can stimulate palatable food cravings and hyperphagia in a subset of individuals [44], [72]. Mice fed the saturated HFD and then subjected to a dieting manipulation known to increase palatable food craving (transition to a low-fat diet) [22] were resistant to an aversive conditioning procedure that suppresses food-seeking under normal conditions [35], [42], [73]. NAc-specific inhibition of the NFкB signaling pathway not only reduced sucrose motivation in PalmIKKdn, but not ControlIKKdn, mice but also prevented compulsive sucrose-seeking behavior upon withdrawal of the palm HFD. These data are in agreement with the involvement of the NFкB signaling pathway in reinforcement and motivated behaviors [74], [75], [76]. As the NFкB signaling pathway has been shown to mediate morphological and molecular changes in NAc medium spiny neurons [36], [37], [75], [77], [78], we posit that saturated HFD-induced NFкB transcriptional activity in the NAc favors neural adaptations underlying changes in mood and motivation. Despite observations of diminished sucrose-seeking by NAc NFкB inhibition there were no changes in body weight in PalmIKKdn mice that could be anticipated if mice are less motivated to consume palatable food. We speculate that this is due to the fact that ad libitum access to HFD (as was provided in home cage where majority of daily calories were consumed) demands little effort to consume and thus does not engage the NAc-mediated motivational processes at play in the operant task, a view that is consistent with NAc interventions differentially modulating free-feeding vs. effort-based food seeking [41]. Nevertheless, identifying high-fat feeding-related neural adaptations underlying the pervasive nature of palatable food consumption in typical environmental conditions in which there is a cost for food is likely to provide important insights for hyperphagia and obesity treatment.
5. Conclusions
Collectively, this work identifies the capacity of excess saturated dietary fat to elicit metabolic impairments, peripheral and neural immune activation, anxiodepressive behavior, and sucrose motivation. The link between obesity and depression may thus involve overconsumption of saturated lipids (and perhaps other pro-inflammatory food/diets) and ensuing NFкB-mediated inflammatory processes in the NAc, and thereby suggests that anti-inflammatory strategies may prove valuable for treating depression in obese individuals. Resulting immune activation in the NAc is suspected to induce neuroplastic adaptations that generate mood impairments, although further work is needed to identify the neural modifications and NAc output pathways involved in this phenomenon.
Acknowledgments
This work was supported by a MDRC/Sunlife grant to SF and a CIHR grant (MOP115042). LDS was supported by a doctoral fellowship from FRQS, SS by a post-doctoral fellowship from the CIHR Neuroinflammation Training Program, VIG by a MITACS Summer Internship, TA by a FRQS Salary Award and SF (2013–18) and NA (2012–2017) a CIHR New Investigator Award. The authors thank the Rodent Phenotyping Platform at CRCHUM for measures in metabolic cages and plasma analysis of insulin and leptin.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.01.018.
Conflict of interest
Declarations of interest: None.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Luppino F.S., de Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W. Overweight, obesity and depression: a systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry. 2010;67(3):10. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 2.Opel N., Redlich R., Grotegerd D., Dohm K., Heindel W., Kugel H. Obesity and major depression: body-mass index (BMI) is associated with a severe course of disease and specific neurostructural alterations. Psychoneuroendocrinology. 2015;51:219–226. doi: 10.1016/j.psyneuen.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. Journal of Clinical Investigation. 2011:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Al-Sayegh H., Jabrah R., Wang W., Yan F., Zhang J. Association between C-reactive protein and depression: modulated by gender and mediated by body weight. Psychiatry Research. 2014;219(1):103–108. doi: 10.1016/j.psychres.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Daly M. The relationship of C-reactive protein to obesity-related depressive symptoms: a longitudinal study. Obesity (Silver Spring) 2013;21(2):248–250. doi: 10.1002/oby.20051. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Liu L., Peng Y.L., Liu Y.Z., Wu T.Y., Shen X.L. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neuroscience and Therapeutics. 2014;20(2):119–124. doi: 10.1111/cns.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazareth J., Guyon A., Heurteaux C., Chabry J., Petit-Paitel A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: importance of CCR2/CCL2 signaling. Journal of Neuroinflammation. 2014;11:132. doi: 10.1186/1742-2094-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguliar-Valles A., Kim J., Jung S., Woodside B., Luheshi G.N. Role of brain transmigrating neutrophils in depression-like behavior during systemic infection. Molecular Psychiatry. 2013 doi: 10.1038/mp.2013.137. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor J.C., Lawson M.A., Andre C., Briley E.M., Szegedi S.S., Lestage J. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. The Journal of Immunology. 2009;182(5):3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morselli E., Fuente-Martin E., Finan B., Kim M., Frank A., Garcia-Caceres C. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Reports. 2014;9(2):633–645. doi: 10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunes E.J., Randall P.A., Podurgiel S., Correa M., Salamone J.D. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neuroscience & Biobehavioral Reviews. 2013:2015–2025. doi: 10.1016/j.neubiorev.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario C.R., Labouèbe G., Liu S., Nieh E.H., Routh V.H., Xu S. Homeostasis meets motivation in the battle to control food intake. The Journal of Neuroscience. 2016;36(45):11469–11481. doi: 10.1523/JNEUROSCI.2338-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold P.W. The organization of the stress system and its dysregulation in depressive illness. Molecular Psychiatry. 2015;20(1):32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 16.Francis T.C., Lobo M.K. Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biological Psychiatry. 2017:645–653. doi: 10.1016/j.biopsych.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams Z.M. Good vibrations with deep brain stimulation. Nature Neuroscience. 2015;18(103):1613–1622. doi: 10.1038/nn.4007. [DOI] [PubMed] [Google Scholar]
- 18.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nature Reviews Neuroscience. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S., Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. International Journal of Obesity (London) 2013;37(3):382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- 20.Volkow N.D., Wang G.J., Tomasi D., Baler R.D. The addictive dimensionality of obesity. Biological Psychiatry. 2013;73(9):811–818. doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamer M., Batty G.D., Kivimaki M. Risk of future depression in people who are obese but metabolically healthy: the English longitudinal study of ageing. Molecular Psychiatry. 2012;17(9):940–945. doi: 10.1038/mp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S., Fernandes M.F., Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. International Journal of Obesity (London) 2013;37(9):1183–1191. doi: 10.1038/ijo.2012.197. [DOI] [PubMed] [Google Scholar]
- 23.Hryhorczuk C., Décarie-Spain L., Sharma S., Daneault C., Rosiers C. Des., Alquier T. Saturated high-fat feeding independent of obesity alters hypothalamus-pituitary-adrenal axis function but not anxiety-like behaviour. Psychoneuroendocrinology. 2017;83(November 2016):142–149. doi: 10.1016/j.psyneuen.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Sartorius T., Ketterer C., Kullmann S., Balzer M., Rotermund C., Binder S. Monounsaturated fatty acids prevent the aversive effects of obesity on locomotion, brain activity, and sleep behavior. Diabetes. 2012;61(7):1669–1679. doi: 10.2337/db11-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriguer F., Rojo-Martínez G., Goday A., Bosch-Comas A., Bordiú E., Caballero-Díaz F. Olive oil has a beneficial effect on impaired glucose regulation and other cardiometabolic risk factors. Di@bet.es study. European Journal of Clinical Nutrition. 2013;67(9):911–916. doi: 10.1038/ejcn.2013.130. [DOI] [PubMed] [Google Scholar]
- 26.Lai J.S., Oldmeadow C., Hure A.J., McEvoy M., Hiles S.A., Boyle M. Inflammation mediates the association between fatty acid intake and depression in older men and women. Nutrition Research. 2016;36(3):234–245. doi: 10.1016/j.nutres.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Hryhorczuk C., Florea M., Rodaros D., Poirier I., Daneault C., Des Rosiers C. Dampened mesolimbic dopamine function and signaling by saturated but not monounsaturated dietary lipids. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdearcos M., Robblee M.M., Benjamin D.I., Nomura D.K., Xu A.W., Koliwad S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Reports. 2014;9(6):2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erion J.R., Wosiski-Kuhn M., Dey A., Hao S., Davis C.L., Pollock N.K. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. Journal of Neuroscience. 2014;34(7):2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cazettes F., Cohen J.I., Yau P.L., Talbot H., Convit A. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Research. 2011;1373:101–109. doi: 10.1016/j.brainres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oeckinghaus A., Hayden M.S., Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nature Immunology. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 32.Bhakar A.L., Tannis L.-L., Zeindler C., Russo M.P., Jobin C., Park D.S. Constitutive NFkB essential for neuronal survival. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(19):8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christoffel D.J., Golden S.A., Heshmati M., Graham A., Birnbaum S., Neve R.L. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37(12):2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S., Hryhorczuk C., Fulton S. Progressive-ratio responding for palatable high-fat and high-sugar food in mice. Journal of Visualized Experiments: JoVE. 2012;63 doi: 10.3791/3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes M.F.A., Matthys D., Hryhorczuk C., Sharma S., Mogra S., Alquier T. Leptin suppresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metabolism. 2015 doi: 10.1016/j.cmet.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan V., Han M.H., Graham D.L., Berton O., Renthal W., Russo S.J. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Christoffel D.J., Golden S.A., Dumitriu D., Robison A.J., Janssen W.G., Ahn H.F. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. Journal of Neuroscience. 2011;31(1):314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology. 2009;1(6) doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stice E., Spoor S., Bohon C., Veldhuizen M.G., Small D.M. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. Journal of Abnormal Psychology. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweet L.H., Hassenstab J.J., McCaffery J.M., Raynor H.A., Bond D.S., Demos K.E. Brain response to food stimulation in obese, normal weight, and successful weight loss maintainers. Obesity (Silver Spring) 2012;20(11):2220–2225. doi: 10.1038/oby.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelley A.E. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience & Biobehavioral Reviews. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Johnson P.M., Kenny P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinnouho G.M., Singh-Manoux A., Gueguen A., Matta J., Lemogne C., Goldberg M. Metabolically healthy obesity and depressive symptoms: 16-year follow-up of the Gazel cohort study. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lasserre A.M., Strippoli M.-P.F., Glaus J., Gholam-Rezaee M., Vandeleur C.L., Castelao E. Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Molecular Psychiatry. 2016 doi: 10.1038/mp.2016.178. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-González M.A., Salas-Salvadó J., Estruch R., Corella D., Fitó M., Ros E. Benefits of the mediterranean diet: insights from the PREDIMED study. Progress in Cardiovascular Diseases. 2015;58(1):50–60. doi: 10.1016/j.pcad.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez-Villegas A., Martínez-González M., Estruch R., Salas-Salvadó J., Corella D., Covas M. Mediterranean dietary pattern and depression: the PREDIMED randomized trial. BMC Medicine. 2013;11(1):208. doi: 10.1186/1741-7015-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García-Toro M., Vicens-Pons E., Gili M., Roca M., Serrano-Ripoll M.J., Vives M. Obesity, metabolic syndrome and Mediterranean diet: impact on depression outcome. Journal of Affective Disorders. 2016;194:105–108. doi: 10.1016/j.jad.2015.12.064. [DOI] [PubMed] [Google Scholar]
- 48.Carrillo C., Cavia Mdel M., Alonso-Torre S. Role of oleic acid in immune system; mechanism of action; a review. Nutricion Hospitalaria. 2012;27(4):978–990. doi: 10.3305/nh.2012.27.4.5783. [DOI] [PubMed] [Google Scholar]
- 49.Oh Y.T., Lee J.Y., Lee J., Kim H., Yoon K.S., Choe W. Oleic acid reduces lipopolysaccharide-induced expression of iNOS and COX-2 in BV2 murine microglial cells: possible involvement of reactive oxygen species, p38 MAPK, and IKK/NF-κB signaling pathways. Neuroscience Letters. 2009;464(2):93–97. doi: 10.1016/j.neulet.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 50.Tsuboi H., Watanabe M., Kobayashi F., Kimura K., Kinae N. Associations of depressive symptoms with serum proportions of palmitic and arachidonic acids, and alpha-tocopherol effects among male population–a preliminary study. Clinical Nutrition. 2013;32(2):289–293. doi: 10.1016/j.clnu.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Benoit S.C., Kemp C.J., Elias C.F., Abplanalp W., Herman J.P., Migrenne S. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. Journal of Clinical Investigation. 2009;119(9):2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng L., Yu Y., Szabo A., Wu Y., Wang H., Camer D. Palmitic acid induces central leptin resistance and impairs hepatic glucose and lipid metabolism in male mice. The Journal of Nutritional Biochemistry. 2015;26(5):541–548. doi: 10.1016/j.jnutbio.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Moon M.L., Joesting J.J., Lawson M.A., Chiu G.S., Blevins N.A., Kwakwa K.A. The saturated fatty acid, palmitic acid, induces anxiety-like behavior in mice. Metabolism: Clinical and Experimental. 2014;63(9):1131–1140. doi: 10.1016/j.metabol.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada N., Katsuura G., Ochi Y., Ebihara K., Kusakabe T., Hosoda K. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152(7):2634–2643. doi: 10.1210/en.2011-0004. [DOI] [PubMed] [Google Scholar]
- 55.Hryhorczuk C., Sharma S., Fulton S.E. Metabolic disturbances connecting obesity and depression. Frontiers in Neuroscience. 2013;7:177. doi: 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jokela M., Hamer M., Singh-Manoux A., Batty G.D., Kivimaki M. Association of metabolically healthy obesity with depressive symptoms: pooled analysis of eight studies. Molecular Psychiatry. 2014;19(8):910–914. doi: 10.1038/mp.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmitz N., Deschênes S., Burns R., Smith K., Lesage A., Strychar I. Depression and risk of type 2 diabetes: the potential role of metabolic factors. Molecular Psychiatry. 2016;217(12):1726–1732. doi: 10.1038/mp.2016.7. [DOI] [PubMed] [Google Scholar]
- 58.Dunbar J.A., Reddy P., Davis-Lameloise N., Philpot B., Laatikainen T., Kilkkinen A. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care. 2008;31(12):2368–2373. doi: 10.2337/dc08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chirinos D.A., Murdock K.W., LeRoy A.S., Fagundes C. Depressive symptom profiles, cardio-metabolic risk and inflammation: results from the MIDUS study. Psychoneuroendocrinology. 2017;82:17–25. doi: 10.1016/j.psyneuen.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreira F.P., Jansen K., Cardoso T.A., Mondin T.C., Magalhães P.V.S., Kapczinski F. Metabolic syndrome in subjects with bipolar disorder and major depressive disorder in a current depressive episode: population-based study. Journal of Psychiatric Research. 2017;92:119–123. doi: 10.1016/j.jpsychires.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 61.Oh Y.T., Kim J., Kang I., Youn J.H. Regulation of hypothalamic-pituitary-adrenal axis by circulating free fatty acids in male Wistar rats: role of individual free fatty acids. Endocrinology. 2014;155(3):923–931. doi: 10.1210/en.2013-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawanokuchi J., Mizuno T., Takeuchi H., Kato H., Wang J., Mitsuma N. Production of interferon-γ by microglia. Multiple Sclerosis Journal. 2006;12(5):558–564. doi: 10.1177/1352458506070763. [DOI] [PubMed] [Google Scholar]
- 63.Monteiro S., Roque S., Marques F., Correia-Neves M., Cerqueira J.J. Brain interference: revisiting the role of IFNγ in the central nervous system. Progress in Neurobiology. 2017 doi: 10.1016/j.pneurobio.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Buckman L.B., Hasty A.H., Flaherty D.K., Buckman C.T., Thompson M.M., Matlock B.K. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain, Behavior, and Immunity. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber M.D., Godbout J.P., Sheridan J.F. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology. 2016:1–16. doi: 10.1038/npp.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elahy M., Lam V., Pallebage-Gamarallage M.M., Giles C., Mamo J.C.L., Takechi R. Nicotine attenuates disruption of blood-brain barrier induced by saturated-fat feeding in wild-type mice. Nicotine & Tobacco Research. 2015:1436–1441. doi: 10.1093/ntr/ntv044. [DOI] [PubMed] [Google Scholar]
- 67.Menard C., Pfau M.L., Hodes G.E., Kana V., Wang V.X., Bouchard S. Social stress induces neurovascular pathology promoting depression. Nature Neuroscience. 2017;20(12):1752–1760. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Couch Y., Anthony D.C., Dolgov O., Revischin A., Festoff B., Santos A.I. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain, Behavior, and Immunity. 2013;29:136–146. doi: 10.1016/j.bbi.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 69.Campos A.C., Vaz G.N., Saito V.M., Teixeira A.L. Further evidence for the role of interferon-gamma on anxiety- and depressive-like behaviors: involvement of hippocampal neurogenesis and NGF production. Neuroscience Letters. 2014;578:100–105. doi: 10.1016/j.neulet.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 70.Patki G., Solanki N., Atrooz F., Allam F., Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Research. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisenberger N.I., Berkman E.T., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological Psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibson E.L. The psychobiology of comfort eating: implications for neuropharmacological interventions. Behavioural Pharmacology. 2012;23(5–6):442–460. doi: 10.1097/FBP.0b013e328357bd4e. [DOI] [PubMed] [Google Scholar]
- 73.Velazquez-Sanchez C., Santos J.W., Smith K.L., Ferragud A., Sabino V., Cottone P. Seeking behavior, place conditioning, and resistance to conditioned suppression of feeding in rats intermittently exposed to palatable food. Behavioral Neuroscience. 2015;129(2):219–224. doi: 10.1037/bne0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ang E., Chen J., Zagouras P., Magna H., Holland J., Schaeffer E. Induction of nuclear factor-κB in nucleus accumbens by chronic cocaine administration. Journal of Neurochemistry. 2008;79(1):221–224. doi: 10.1046/j.1471-4159.2001.00563.x. [DOI] [PubMed] [Google Scholar]
- 75.Russo S.J., Wilkinson M.B., Mazei-Robison M.S., Dietz D.M., Maze I., Krishnan V. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. Journal of Neuroscience. 2009;29(11):3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X., Cui Y., Jing J., Cui Y., Xin W., Liu X. Involvement of p38/NF-kappaB signaling pathway in the nucleus accumbens in the rewarding effects of morphine in rats. Behavioural Brain Research. 2011;218(1):184–189. doi: 10.1016/j.bbr.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 77.Walsh J.J., Friedman A.K., Sun H., Heller E.A., Ku S.M., Juarez B. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nature Neuroscience. 2014;17(1):27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koo J.W., Russo S.J., Ferguson D., Nestler E.J., Duman R.S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.