Abstract
BACKGROUND AND PURPOSE:
Childhood arteriopathies are rare but heterogenous, and difficult to diagnose and classify, especially by nonexperts. We quantified clinical and imaging characteristics associated with childhood arteriopathy subtypes to facilitate their diagnosis and classification in research and clinical settings.
MATERIALS AND METHODS:
The Vascular Effects of Infection in Pediatric Stroke (VIPS) study prospectively enrolled 355 children with arterial ischemic stroke (2010–2014). A central team of experts reviewed all data to diagnose childhood arteriopathy and classify subtypes, including arterial dissection and focal cerebral arteriopathy–inflammatory type, which includes transient cerebral arteriopathy, Moyamoya disease, and diffuse/multifocal vasculitis. Only children whose stroke etiology could be conclusively diagnosed were included in these analyses. We constructed logistic regression models to identify characteristics associated with each arteriopathy subtype.
RESULTS:
Among 127 children with definite arteriopathy, the arteriopathy subtype could not be classified in 18 (14%). Moyamoya disease (n = 34) occurred mostly in children younger than 8 years of age; focal cerebral arteriopathy–inflammatory type (n = 25), in children 8–15 years of age; and dissection (n = 26), at all ages. Vertigo at stroke presentation was common in dissection. Dissection affected the cervical arteries, while Moyamoya disease involved the supraclinoid internal carotid arteries. A banded appearance of the M1 segment of the middle cerebral artery was pathognomonic of focal cerebral arteriopathy–inflammatory type but was present in <25% of patients with focal cerebral arteriopathy–inflammatory type; a small lenticulostriate distribution infarct was a more common predictor of focal cerebral arteriopathy–inflammatory type, present in 76%. It remained difficult to distinguish focal cerebral arteriopathy–inflammatory type from intracranial dissection of the anterior circulation. We observed only secondary forms of diffuse/multifocal vasculitis, mostly due to meningitis.
CONCLUSIONS:
Childhood arteriopathy subtypes have some typical features that aid diagnosis. Better imaging methods, including vessel wall imaging, are needed for improved classification of focal cerebral arteriopathy of childhood.
Approximately 2500 children in the United States have an arterial ischemic stroke each year.1 Childhood arteriopathies are the most common identifiable cause of arterial ischemic stroke (in a previously healthy child, present in up to 64%).2–6 They represent a strong predictor of recurrent stroke, with rates exceeding 30% within 12 months for some arteriopathy subtypes,2,7,8 and published guidelines for the prevention of recurrence are specific to type (eg, dissection, Moyamoya disease, transient cerebral arteriopathy, and so forth).9 Nonetheless, childhood arteriopathies remain difficult to not only diagnose but classify; they are rare but heterogeneous, and MRA imaging, frequently substituted for conventional angiography, is technically limited. Publication of consensus-based definitions of childhood arteriopathy in 2004 (adapted for the Vascular Effects of Infection in Pediatric Stroke [VIPS] study in 2009) and the development of the Interbody Fusion Devices in the Treatment of Cervicobrachial Syndrome (CASCADE)10 system in 2012 (which provided a novel approach to classifying the “anatomic site of disease” in childhood arterial ischemic stroke) have largely been addressed to pediatric stroke specialists.
These tools are less useful for nonexperts, however, who are frequently responsible for making timely decisions crucial to the prevention of stroke recurrence. It is this gap that in large part, we seek to address. In the prospective, international, National Institutes of Health–funded Vascular Effects of Infection in Pediatric Stroke study, a 4-person team of pediatric stroke experts classified the etiology of 355 cases of pediatric arterial ischemic stroke (based on rigorous central review of neuroimaging and clinical data). With this “expert opinion” as the criterion standard, the goal of the current analysis was to guide the classification of childhood arteriopathies by quantifying the prevalence and odds ratios for clinical and imaging biomarkers that were used in the expert review to do the following: 1) distinguish arteriopathy from cardioembolism, and 2) distinguish among the most common subtypes of childhood arteriopathy. In other words, we aimed to identify and quantify biomarkers that could allow trained neuroradiologists and neurologists who are nonexperts in childhood arteriopathies to generate a reasonable differential diagnosis for a child with stroke.
Materials and Methods
Study Design
Ethics committee approvals were obtained at all sites. From 2009 to 2014, the VIPS study enrolled 355 children (29 days to 18 years of age) with arterial ischemic stroke at 37 international sites, collected detailed clinical data (eg, medical history such as cardiac disease and sickle cell anemia, recent exposures such as infection, and head trauma), and performed central review of brain and cerebrovascular imaging (by M.W., H.J.F., G.A.D., and A.J.B.). Details of VIPS methods have been published.11 As a part of the VIPS study, an exhaustive and systematic centralized review of baseline and follow-up vascular imaging and clinical data was performed to first arrive at a diagnosis of arteriopathy and then to classify the arteriopathy subtype.12 For this study, we included all children with abnormal vascular imaging findings that could be definitively classified as due to arteriopathy or cardioembolism.
Imaging Review
In our review of brain parenchymal imaging, we recorded infarct size (using ABC/2),13 laterality, location, acuity, and associated hemorrhage. Vascular imaging findings were first classified as normal or abnormal and then were completely described with respect to type of abnormality (eg, hypoplasia, irregularity, banding, stenosis, occlusion, and so forth), vascular territories and sides affected, number and type of arterial segments affected, and degree of collateral flow. Details of the VIPS imaging review have been published.12
Childhood Arteriopathy Classification
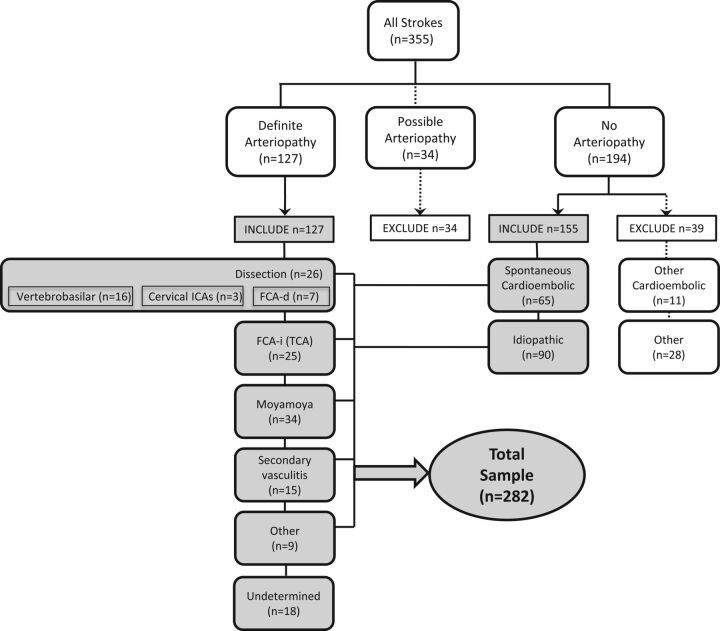
Two primary reviewers (M.W., H.J.F.) independently used clinical data and parenchymal and vascular imaging features to determine a diagnosis of either definite, possible, or no arteriopathy (“primary diagnosis”).12 Disagreements were resolved through consensus discussion by the full review team (M.W., H.J.F., G.A.D., and A.J.B.). We defined arteriopathy as “the imaging appearance of an in situ arterial abnormality (stenosis, irregularity, occlusion, banding, pseudoaneurysm, dissection flap) not attributable to an exogenous thrombus (eg, cardioembolism) and not considered a normal developmental variant.”12 The imaging finding of an isolated arterial occlusion could be classified as “no arteriopathy” (eg, if the clinical history and/or the parenchymal imaging typified cardioembolism), “possible arteriopathy” (eg, if the differential diagnosis included both cardioembolism and arterial dissection), or “definite arteriopathy” (eg, if the imaging was definitive for Moyamoya disease or dissection). The reviewers then classified the arteriopathies into subtypes (“secondary diagnosis”) using pre-established definitions for childhood arteriopathies10,14: arterial dissection, including unilateral focal cerebral arteriopathy–dissection type (FCA-d, further defined below); unilateral focal cerebral arteriopathy–inflammatory type (FCA-i), which includes transient cerebral arteriopathy (TCA); primary and secondary Moyamoya disease (bilateral cerebral arteriopathy of childhood); genetic or syndromic arteriopathies, such as PHACES (posterior fossa malformations, hemangiomas, arterial anomalies, cardiac defects, eye abnormalities, and sternal or supraumbilical defects), a cutaneous condition characterized by multiple congenital abnormalities15,16; primary and secondary diffuse/multifocal vasculitis; fibromuscular dysplasia17; iatrogenic; and others. The primary reviewers independently classified the secondary diagnosis; disagreements were resolved through consensus discussion by the full review team. The final conclusion (the expert opinion regarding the stroke etiology of that case) constituted the criterion standard diagnosis. The diagnoses in the children included in this study are shown in Fig 1.
Fig 1.
Classification of stroke subtype among 355 children with arterial ischemic stroke enrolled in the VIPS study. The cases used for the current study are highlighted in gray.
The original definition of focal cerebral arteriopathy of childhood (FCA) consisted of “stenosis [of intracranial arteries] on vascular imaging not otherwise classified as dissection, Moyamoya disease, sickle cell arteriopathy, postvaricella arteriopathy, vasculitis, or other specific diagnoses (such as postirradiation arteriopathy)” and included “unifocal or multifocal, unilateral, or bilateral lesions of the large and/or medium-sized vessels visualized on angiography.”7 Use of this term has evolved in the pediatric stroke literature, and in North American pediatric stroke centers, it is typically used to describe a specific angiographic appearance of unilateral stenosis and/or irregularity of the intracranial anterior circulation; it has a differential diagnosis including TCA, intracranial dissection, unilateral Moyamoya disease, and the other diagnoses listed above.18 Hence, we implemented an updated definition of FCA: unifocal and unilateral stenosis/irregularity of the large intracranial arteries of the anterior circulation (distal internal carotid artery and/or its proximal branches). FCA-dissection type (FCA-d) referred to intracranial arterial dissection of the anterior circulation, typically with trauma.19 FCA-inflammation type (FCA-i) referred to FCA that is presumed inflammatory (ie, thought to represent a focal vasculitis). This could be diagnosed, for instance, because of marked enhancement of the abnormal arterial segment on vessel wall imaging20 or preceding varicella zoster infection (if considered clinically relevant by the local pediatric stroke neurologist for a diagnosis of postvaricella arteriopathy).21,22 FCA-i was also diagnosed when the evolution of the arteriopathy was typical of TCA: a stereotyped, monophasic natural history characterized by frequent early progression (from days to weeks), a plateau with nonprogression by 6 months, and subsequent improvement in some, with complete resolution in a minority.14,23 FCA that could not be further classified was considered “undetermined” arteriopathy subtype (in which case the reviewers created a differential diagnosis).
Statistical Analysis
The outcome variables for our analyses were the stroke etiology (primary and secondary diagnoses) as classified by the VIPS team. In children with abnormal vascular imaging findings, arteriopathy must first be distinguished from cardioembolism (primary diagnosis); to this end, we first developed a predictive model for cardioembolic stroke. We then addressed our primary goal, modeling clinical and imaging biomarkers associated with the most commonly diagnosed arteriopathy subtypes (secondary diagnosis): dissection, FCA-i, Moyamoya disease, and secondary diffuse/multifocal vasculitis. By design, we evaluated as predictors biomarkers that were used by the reviewers in the classification process; though circular, this evaluation allowed the quantification of the prevalence of the biomarker and the strength of its association with a specific subtype.
For our preliminary model (cardioembolic versus arteriopathic stroke), we compared 65 children classified as having spontaneous cardioembolism (excluding strokes attributed to cardiac surgery) with 109 with definite arteriopathy (excluding those with possible arteriopathy, but including those whose definite arteriopathy could not be further classified; Fig 1). We first used univariate logistic regression models to identify clinical and/or parenchymal and/or vascular imaging characteristics associated either positively or negatively with these 2 broad categories. We then constructed a multivariable model by entering all predictors significant at the .10 level in univariate analysis. Backward-selection logistic regression analysis was used to estimate adjusted odds ratios, with a significance level of .05 specified for removal of a variable from the model.
We followed a similar process to create models predictive of each individual arteriopathy subtype. Univariate logistic regression models were first used to identify characteristics associated with each subtype individually. For these models, we compared each subtype with the group of all other subtypes combined (excluding the 18 subjects with definite arteriopathy that could not be further classified). In addition to calculating odds ratios and 95% confidence intervals for each potential predictor, we determined the frequency with which the predictor was observed within the subtype. We then constructed multivariable models for each subtype as described for the preliminary model above. All models were assessed with postestimation techniques, and C-statistics were compared among potential models. Adjustments were made when necessary to improve the model fit before a final model was determined. All analyses were performed with STATA, Version 14 (StataCorp, College Station, Texas).
Results
All 355 patients with VIPS had initial brain vascular imaging—MRA (91%), CTA (24%), and/or conventional angiography (14%); 53% had cervical vascular imaging; and 3.9% had vessel wall imaging. Overall, 41% had at least 1 follow-up brain vascular imaging study; the last follow-up was a median of 277 days (interquartile range, 172–408 days) poststroke. Figure 1 demonstrates the results of the stroke subtype classification. Characteristics that distinguish cardioembolism from arteriopathic stroke (with a P value < .10 on univariate analysis) are shown in On-line Table 1. Characteristics associated with arteriopathy subtype (with a P value < .10 on univariate analysis) are shown On-line Tables 2–4. All the variables tested are shown in On-line Tables 5–11. Independent predictors are summarized in the Table and shown in detail in On-line Table 11.
Summary results of independent predictors of arteriopathy subtypes
| Arteriopathy Subtype | Cardioembolic | Dissection | FCA-i | Moyamoya | Secondary Vasculitis | Other Definite |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Black race vs other | +++ | |||||
| Clinical characteristics | ||||||
| Congenital or acquired heart disease | +++ | |||||
| Down syndrome | +++ | |||||
| Meningitis | +++ | |||||
| Sepsis/bacteremia | +++ | |||||
| Head trauma | +++ | |||||
| Presentation | ||||||
| Dysarthria | ++ | |||||
| Nausea/vomiting | + | |||||
| Vertigo | +++ | |||||
| Decreased level of consciousness | − | +++ | ||||
| Infarct characteristics: location | ||||||
| Lenticulostriate artery territory | +++ | |||||
| Infarct characteristics: volume | ||||||
| Infarct volume (smaller) | ++ | |||||
| Vascular imaging abnormal findings | ||||||
| Occlusion | − | |||||
| Stenosis | − | |||||
| Irregularity | − | |||||
| Banding | +++ | |||||
| >1 vascular territory | − | +++ | ||||
| >1 arterial segment | − | |||||
| Affected artery | ||||||
| Proximal MCA (M1) | +++ | |||||
| Distal ICA (supraclinoid) | +++ | +++ | ||||
| Cervical artery | +++ |
Note:—Positive association: +++ indicates odds ratio > 20; ++, OR 5–15; +, 1 < OR < 5; P < .05 in all. Negative association: −, OR < 1 and P < .05.
Cardioembolic versus Arteriopathic Stroke
In multivariable analysis, characteristics determined to best distinguish cardioembolic from arteriopathic stroke were the presence of congenital heart disease and involvement of multiple vascular territories (both positively associated with cardioembolic stroke); the presence of vascular stenosis or irregularity spoke against the possibility of cardioembolic stroke (Table and On-line Table 11). All cases of cardioembolic stroke had underlying congenital or acquired cardiac disease (On-line Tables 1 and 5). Having multiple or bilateral arterial segments affected unexpectedly decreased the odds of cardioembolism because these features were seen more frequently in arteriopathy. On vascular imaging, the most common abnormality in cardioembolic stroke was arterial occlusion, present in almost half; however, arterial occlusion was a nonspecific finding observed commonly in the arteriopathy group (61.5% of patients with arteriopathy). Arterial irregularities and stenosis reduced the odds of cardioembolism, though each was observed in about 10% of patients with cardioembolic strokes.
Characteristics of Childhood Arteriopathy Subtypes
Our final multivariable model to distinguish arterial dissection (intracranial, which includes FCA dissection, or extracranial) from other arteriopathy subtypes included a history of head trauma and involvement of the cervical arteries (Table and On-line Table 11). Arterial dissection was associated with a history of head trauma in 39% of cases (On-line Tables 2 and 6). Dissections were equally distributed between the anterior and posterior circulation. Dissections tended to present as unilateral occlusions.
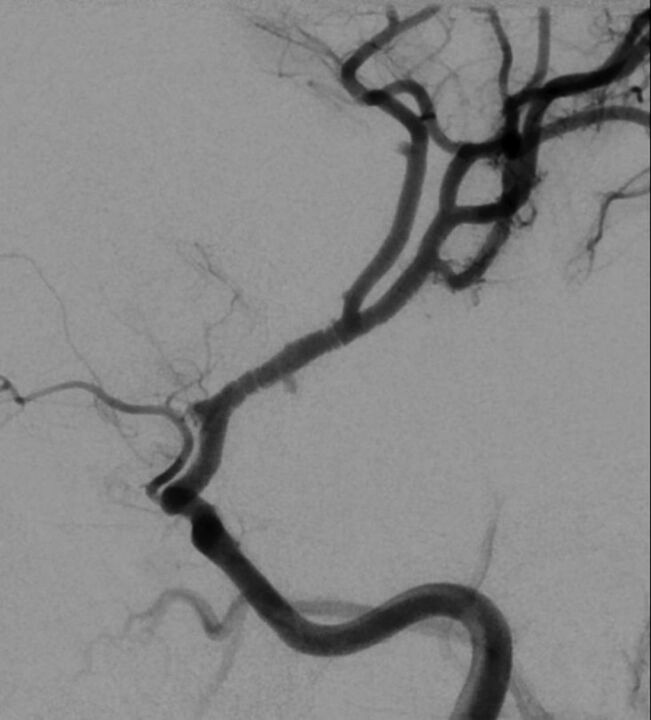
Characteristics associated with FCA-i in multivariable analysis included presentation with dysarthria, smaller infarcts in the lenticulostriate territory, infarct volume of <25 cm3, arterial banding, and isolated involvement of the M1 segment of the middle cerebral artery (Table and On-line Table 11). FCA-i tended to occur more often in children between 8 and 15 years of age (On-line Tables 3 and 7). The banding pattern (Fig 2), while pathognomonic, was uncommon (24% of patients with FCA-i).
Fig 2.
Banding pattern observed in 24% of patients with FCA-i.
Forty-one children met the criteria for FCA: 7 with FCA-d, 25 with FCA-i, and 9 who could not be further classified. Our analysis of characteristics that distinguish FCA-i from FCA-d (On-line Tables 8 and 9) was limited by small sample sizes and the availability of vessel wall imaging in only 8 of the 25 children with FCA-i. The expert review team used a history of head trauma to make a diagnosis of FCA-d; it was present in 5 of the 7 patients with FCA-d (and in none of those with FCA-i). Infarct volumes were larger for FCA-d (median, 88 cm3; interquartile range, 3.3–20 cm3) than for FCA-i (median, 14 cm3; interquartile range, 8.6–99 cm3; P = .05). Arterial occlusion was seen in 86% of subjects with FCA-d versus 40% of those with FCA-i (P = .06). Banding was seen in 24% of subjects with FCA-i but in none with FCA-d (P = .28). In contrast, all 7 patients with FCA-d had involvement of both the supraclinoid ICA and M1. A coincident cervical artery abnormality was more suggestive of FCA-d (4/7 with FCA-d versus 1/25 with FCA-i, P = .007).
Our multivariable model for a Moyamoya disease diagnosis included both primary and secondary forms (Table and On-line Table 11). Of 34 children with Moyamoya disease, 17 were diagnosed with primary (idiopathic) Moyamoya disease, and 17, with secondary Moyamoya disease syndrome, most commonly caused by sickle cell anemia (n = 9) or Down syndrome (n = 6). An association between black race and Moyamoya disease was almost entirely explained by sickle cell anemia; Asian race did not affect the risk of Moyamoya disease in our cohort (On-line Tables 4 and 10). Imaging characteristics included bilateral distal ICA occlusion or stenosis and infarcts involving multiple vascular territories. Involvement of the posterior circulation was present in 21%. Although Moyamoya disease is an intracranial arteriopathy, abnormalities of the cervical arteries were noted in 6 patients (18%) with this condition, likely representing the MRA finding of small cervical internal carotid arteries due to reduced intracranial flow. Patients with Moyamoya disease typically did not present with a decreased level of consciousness, differentiating them from children with secondary vasculitis who had similar distal ICA involvement.
There were no cases of primary diffuse/multifocal vasculitis in VIPS, but there were 15 cases of secondary diffuse/multifocal vasculitis due to meningitis (n = 11), other infection (cavernous sinus thrombophlebitis, n = 1; mycotic aneurysm, n = 1), autoimmune disease (n = 1), or other (n = 1). Hence, clinical characteristics associated with this diagnosis included meningitis and bacteremia/sepsis and presentation with a decreased level of consciousness (Table and On-line Table 11). Complete occlusion of the affected artery reduced the odds of diffuse/multifocal vasculitis. Diffuse/multifocal vasculitis was seen more frequently in Asian patients: There were 6 cases of stroke due to tubercular meningitis enrolled in the Philippines.
Among 7 children with Down syndrome in our cohort, 6 had Moyamoya disease and 1 had a cardioembolic stroke.
Discussion
The diagnosis of childhood arteriopathy is complex, and pediatric stroke experts have developed their diagnostic acumen across the years through the cumulative exposure to many cases. However, because these diseases are rare, pediatric patients with childhood arteriopathy are often seen by health care professionals who have not developed this expertise. The VIPS study presented a unique opportunity to help nonexperts in not only accurately diagnosing pediatric patients with childhood arteriopathy but also distinguishing among specific types based on objectively defined clinical and imaging parameters. The VIPS study previously demonstrated that arteriopathies can be more accurately classified when clinical data are used than when imaging findings are used alone, and when follow-up vascular imaging is performed.11 The current analysis adds to our prior publication by defining the prevalence and predictive value of the individual characteristics that a pediatric stroke expert uses to diagnose childhood arteriopathy. In addition, it allows identification of patterns (ie, combinations of characteristics that distinguish certain arteriopathies) and assessment of the relative importance of each of these characteristics. This analysis assumes the availability of complete and accurate clinical and imaging data at the time of the arteriopathy classification; in real clinical situations, arteriopathy classification should be revisited as new data become available with time.
As a first step in the approach to vascular imaging abnormalities in a child with arterial ischemic stroke, radiologists and clinicians should consider whether the abnormality represents inherent arterial disease (ie, arteriopathy) versus thrombus from a proximal source (cardioembolism or artery-to-artery embolism). Underlying cardiac disease strongly favors cardioembolism; however, 4 children with congenital heart disease had an arteriopathic stroke (1 with a dissection, 3 with Moyamoya disease), indicating that arteriopathic stroke should be considered even in patients with cardiac disease.
Arterial stenosis or irregularity reduces the odds of cardioembolism but can be seen with a recanalizing thrombus. Complete arterial occlusion appeared to reduce the odds of cardioembolism in our model but only because it is a common feature of arteriopathies like Moyamoya disease and dissection; it was still the most common vascular imaging finding in cardioembolism. Distinguishing arteriopathy from thrombus remains challenging; our expert team could not make the distinction in 34 cases (designated possible arteriopathy, Fig 1), highlighting the need for an echocardiogram as part of the work-up of pediatric patients suspected of having childhood arteriopathy.
The most common childhood arteriopathies in our cohort of children presenting with acute arterial ischemic stroke were Moyamoya disease, arterial dissection (intracranial and extracranial), and FCA-i. This distribution would likely be different in a cohort including all children with cerebral or cervical arteriopathy; primary small-vessel CNS vasculitis, for example, typically presents with only headache or cognitive decline and no focal signs or symptoms. Although atherosclerosis may begin in childhood, it was not seen as a cause of stroke in VIPS, consistent with findings in prior reports.3,24
Age was the one demographic characteristic that helped distinguish among arteriopathy subtypes. FCA-i tended to affect older school-aged children, while Moyamoya disease affected younger children; dissection had no age predilection. Sex and race did not correlate with arteriopathy subtype (after accounting for sickle cell disease). Although primary Moyamoya disease occurs more commonly in Korean and Japanese populations,25 we had no enrolling sites in those countries and saw a broad distribution of ethnicities among our subjects with Moyamoya disease.
A diagnosis of dissection is suggested by the involvement of cervical arteries. On the basis of current definitions, FCA-i does not include arteriopathies affecting the posterior circulation. Moyamoya disease predominantly affects the anterior circulation; posterior circulation involvement, when present, is rarely symptomatic. Hence, dissection is high on the differential in a previously healthy child presenting with a posterior circulation arterial ischemic stroke. In addition, FCA-i, Moyamoya disease, and secondary vasculitis are intracranial arteriopathies; involvement of the cervical arteries is strongly suggestive of dissection.
Distinguishing the etiologies of FCA—focal stenosis or irregularity of the distal ICA or proximal MCA—remains a challenge even to pediatric stroke experts. The differential diagnosis includes FCA-i, FCA-d (intracranial dissection of the anterior circulation), and early, unilateral Moyamoya disease. All typically present with hemiparesis, but headache at the stroke ictus is common in FCA-i and FCA-d, but not in Moyamoya disease. Banding was considered a pathognomonic feature for FCA-i but was present in less than one-quarter of cases (being more conspicuous on conventional angiograms compared with CTAs and MRAs); hence, it was useful when present but not a sensitive feature of FCA-i. Infarct location in the lenticulostriate territory and smaller infarct size correlated with FCA-i and were more prevalent biomarkers. However, FCA-i and FCA-d are, in general, difficult to distinguish from each other as shown in postmortem cases.26
An infarct in the superficial middle cerebral artery territories (ie, cerebral convexities) was more suggestive of Moyamoya disease. Chronic deep borderzone infarcts, also common in Moyamoya disease, do not result in focal deficits; because this is a cohort of children with acute arterial ischemic stroke, such infarcts were not included in this analysis. A history of head trauma and/or coincident cervical artery abnormalities suggests dissection. Improved neuroimaging techniques, including vessel wall imaging,27 are needed to distinguish forms of FCA with greater certainty, though vessel wall imaging may not be 100% specific and there may be some overlap, with FCA-d showing minimal enhancement on vessel wall imaging and FCA-i typically presenting with marked enhancement on vessel wall imaging. The distinction between FCA-d and FCA-i is particularly important because their management strategies differ. FCA-i and FCA-d are currently treated with antiplatelet therapy. In addition, life-long restriction of activities (eg, no contact sports) is often recommended after an arterial dissection,28 and clinical trials of corticosteroids for the treatment of FCA-i are under development.
In this article, the definition of FCA-i was restricted to focal disease of the distal ICA and its proximal branches, including but not limited to TCA.14,23 However, we anticipate that increased use of vessel wall imaging,27 allowing the delineation of enhancing arterial segments, will necessitate a broader definition of FCA. For example, we observed cases of focal stenosis of the petrous carotid or posterior circulation arteries that we diagnosed as having a definite arteriopathy that could not be further classified. If such cases had vessel wall imaging demonstrating enhancement of the affected vessel, it may be reasonable to expand the definition of FCA-i to include these cases. In addition, we identified 1 case of FCA-i that demonstrated arteriopathy progression after 6 months, contrary to the traditional definition of TCA; this highlights the fact that while FCA-i includes TCA, not all cases FCA-i are TCA.
The main limitation of our study is that there is notrue criterion standard for the diagnosis of childhood arteriopathies. Our expert review team was uncertain about the classification in 52 cases: 34 with possible arteriopathy and 18 with a definite arteriopathy that could not be further classified (Fig 1). Even among the arteriopathies that the review team classified with high certainty, there was likely some misclassification that cannot be measured. Because all imaging was performed on a clinical basis, there was variability in both the type and timing of imaging performed. As noted in our prior study, follow-up vascular imaging was helpful for classification, yet it was available in only a minority of patients.14 The circularity of some analyses—biomarkers used to classify a subtype and then evaluated as predictors of that subtype—must be emphasized; head trauma, for example, was an anticipated predictor of arterial dissection because it was used in the classification process. In such cases, the value of the analysis is in the prevalence of the predictor, such as noting that a minority of dissection cases had trauma, so an absence of trauma does not rule out this diagnosis.
Last, analyses of arteriopathy subtypes were underpowered (as reflected by large confidence intervals of coefficients in the multivariable models), so they should be interpreted with caution. However, advantages of our study include a prospectively collected cohort, a large sample size relative to most pediatric stroke studies, and rigorous classification methods based on independent, central expert reviews and adjudication. Our study allows the quantification of the prevalence of the predictors, the strength of their correlations with specific diagnoses, and patterns of multiple predictors. These results should provide a guide for clinicians and neuroradiologists to generate a reasonable differential for an arteriopathic stroke in a child and to prioritize diagnoses on that list. The application of these findings will depend, however, on the accurate characterization of the imaging biomarkers by the interpreting neuroradiologist.
Conclusions
The different types of childhood arteriopathies are associated with typical clinical and parenchymal and vascular imaging features that can help narrow the differential diagnosis in pediatric patients with stroke with vascular anomalies (Table).
Supplementary Material
Acknowledgments
The authors acknowledge the important contributions of the research coordinators at VIPS sites and of the patients and their families.
ABBREVIATIONS:
- FCA
focal cerebral arteriopathy of childhood
- FCA-d
focal cerebral arteriopathy–dissection type
- FCA-i
focal cerebral arteriopathy–inflammatory type
- TCA
transient cerebral arteriopathy
- VIPS
Vascular Effects of Infection in Pediatric Stroke
Appendix
Coauthors within the VIPS Investigators Group
Michael M. Dowling (University of Texas Southwestern Medical Center, Dallas); Susan L. Benedict (Primary Children's Medical Center, Salt Lake City); Timothy J. Bernard (Denver Children's Hospital); Christine K. Fox (University of California, San Francisco); Gabrielle A. DeVeber (The Hospital for Sick Children, Toronto); Neil R. Friedman (Cleveland Clinic Children's Hospital); Warren D. Lo (Ohio State University and Nationwide Children's Hospital, Columbus); Rebecca N. Ichord (Children's Hospital of Philadelphia); Marilyn A. Tan (University of the Philippines–Philippine General Hospital, Manila); Mark T. Mackay (Royal Children's Hospital Melbourne); Adam Kirton (Alberta Children's Hospital and University of Calgary, Calgary, Alberta); Marta I. Hernandez-Chavez (Pontificia Universidad Catolica de Chile, Santiago); Peter Humphreys (Children's Hospital of Eastern Ontario, Ottawa); Lori C. Jordan (Vanderbilt University Medical Center, Nashville); Sally Sultan (Columbia University Medical Center, New York); Michael J. Rivkin (Boston Children's Hospital); Mubeen F. Rafay (Children's Hospital, Winnipeg, University of Manitoba); Luigi Titomanlio (Hôpital Robert Debré-Paris); Gordana S. Kovacevic (Mother and Child Health Care Institute, New Belgrade, Serbia); Jerome Y. Yager (Stollery Children's Hospital, Edmonton); Catherine Amlie-Lefond (Seattle Children's Hospital); Nomazulu Dlamini (Evelina London Children's Hospital); John Condie (Phoenix Children's Hospital); Ann Yeh (Women and Children's Hospital of Buffalo); Rachel Kneen (Alder Hey Children's Hospital, Liverpool); Bruce Bjornson (British Columbia Children's Hospital, Vancouver); Paola Pergami (West Virginia University, Morgantown); Li Ping Zou (Chinese PLA General Hospital, Beijing); Jorina M. Elbers (Stanford Children's Health, Palo Alto); Abdalla Abdalla (Akron Children's Hospital); Anthony K. Chan (McMaster University, Hamilton); Osman Farooq (Women and Children's Hospital of Buffalo); Mingming J. Lim (Evelina London Children's Hospital); Jessica L. Carpenter (Children's National Medical Center, Washington, DC); Steven Pavlakis (Maimonides Medical Center, Brooklyn); Virginia C. Wong (Queen Mary Hospital, Hong Kong); and Robert Forsyth (Institute of Neuroscience, Newcastle University, Newcastle upon Tyne, UK).
Footnotes
Disclosures: Max Wintermark—RELATED: Grant: National Institutes of Health*; UNRELATED: Board Membership: GE NFL Advisory Board. Nancy K. Hills—RELATED: Grant: National Institute of Health*, Bellaflies Foundation and Marc and Lynne Benioff. Gabrielle A. DeVeber—RELATED: Grant: National Institute of Health*. Anthony J. Barkovich—RELATED: Grant: National Institutes of Health*. Tim J. Bernard—RELATED: Grant: National Institute of Health*. Adam Kirton—RELATED: Grant: National Institute of Health*; UNRELATED: Expert Testimony: medicolegal consults, Comments: multiple expert opinions; Royalties: Elsevier, Comments: small values for editing a textbook on pediatric brain stimulation. Heather J. Fullerton—RELATED: Grant: National Institutes of Health. *Money paid to the institution.
This work was supported by National Institutes of Health R01 NS062820 (Principal Investigators H.J. Fullerton, G.A. DeVeber), Bellaflies Foundation, and Marc and Lynne Benioff for statistical support.
See the Appendix for a list of investigators.
Contributor Information
Michael M. Dowling, University of Texas Southwestern Medical Center, Dallas
Susan L. Benedict, Primary Children's Medical Center, Salt Lake City
Timothy J. Bernard, Denver Children's Hospital
Christine K. Fox, University of California, San Francisco
Gabrielle A. DeVeber, The Hospital for Sick Children, Toronto
Neil R. Friedman, Cleveland Clinic Children's Hospital
Warren D. Lo, Ohio State University and Nationwide Children's Hospital, Columbus
Rebecca N. Ichord, Children's Hospital of Philadelphia
Marilyn A. Tan, University of the Philippines–Philippine General Hospital, Manila
Mark T. Mackay, Royal Children's Hospital Melbourne
Adam Kirton, Alberta Children's Hospital and University of Calgary, Calgary, Alberta.
Marta I. Hernandez-Chavez, Pontificia Universidad Catolica de Chile, Santiago
Peter Humphreys, Children's Hospital of Eastern Ontario, Ottawa.
Lori C. Jordan, Vanderbilt University Medical Center, Nashville
Sally Sultan, Columbia University Medical Center, New York.
Michael J. Rivkin, Boston Children's Hospital
Mubeen F. Rafay, Children's Hospital, Winnipeg, University of Manitoba
Luigi Titomanlio, Hôpital Robert Debré-Paris.
Gordana S. Kovacevic, Mother and Child Health Care Institute, New Belgrade, Serbia
Jerome Y. Yager, Stollery Children's Hospital, Edmonton
Catherine Amlie-Lefond, Seattle Children's Hospital.
Nomazulu Dlamini, Evelina London Children's Hospital.
John Condie, Phoenix Children's Hospital.
Ann Yeh, Women and Children's Hospital of Buffalo.
Rachel Kneen, Alder Hey Children's Hospital, Liverpool.
Bruce Bjornson, British Columbia Children's Hospital, Vancouver.
Paola Pergami, West Virginia University, Morgantown.
Li Ping Zou, Chinese PLA General Hospital, Beijing.
Jorina M. Elbers, Stanford Children's Health, Palo Alto
Abdalla Abdalla, Akron Children's Hospital.
Anthony K. Chan, McMaster University, Hamilton
Osman Farooq, Women and Children's Hospital of Buffalo.
Mingming J. Lim, Evelina London Children's Hospital
Jessica L. Carpenter, Children's National Medical Center, Washington, DC
Steven Pavlakis, Maimonides Medical Center, Brooklyn.
Virginia C. Wong, Queen Mary Hospital, Hong Kong
Robert Forsyth, Institute of Neuroscience, Newcastle University, Newcastle upon Tyne, UK.
Collaborators: Coauthors within the VIPS Investigators Group, Michael M. Dowling, Susan L. Benedict, Timothy J. Bernard, Christine K. Fox, Gabrielle A. DeVeber, Neil R. Friedman, Warren D. Lo, Rebecca N. Ichord, Marilyn A. Tan, Mark T. Mackay, Adam Kirton, Marta I. Hernandez-Chavez, Peter Humphreys, Lori C. Jordan, Sally Sultan, Michael J. Rivkin, Mubeen F. Rafay, Luigi Titomanlio, Gordana S. Kovacevic, Jerome Y. Yager, Catherine Amlie-Lefond, Nomazulu Dlamini, John Condie, Ann Yeh, Rachel Kneen, Bruce Bjornson, Paola Pergami, Li Ping Zou, Jorina M. Elbers, Abdalla Abdalla, Anthony K. Chan, Osman Farooq, Mingming J. Lim, Jessica L. Carpenter, Steven Pavlakis, Virginia C. Wong, and Robert Forsyth
References
- 1. Agrawal N, Johnston SC, Wu YW, et al. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke 2009;40:3415–21 10.1161/STROKEAHA.109.564633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fullerton HJ, Wu YW, Sidney S, et al. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics 2007;119:495–501 10.1542/peds.2006-2791 [DOI] [PubMed] [Google Scholar]
- 3. Ganesan V, Prengler M, McShane MA, et al. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol 2003;53:167–73 10.1002/ana.10423 [DOI] [PubMed] [Google Scholar]
- 4. Sträter R, Becker S, von Eckardstein A, et al. Prospective assessment of risk factors for recurrent stroke during childhood: a 5-year follow-up study. Lancet 2002;360:1540–45 10.1016/S0140-6736(02)11520-0 [DOI] [PubMed] [Google Scholar]
- 5. Chabrier S, Husson B, Lasjaunias P, et al. Stroke in childhood: outcome and recurrence risk by mechanism in 59 patients. J Child Neurol 2000;15:290–94 10.1177/088307380001500504 [DOI] [PubMed] [Google Scholar]
- 6. Zimmer JA, Garg BP, Williams LS, et al. Age-related variation in presenting signs of childhood arterial ischemic stroke. Pediatr Neurol 2007;37:171–75 10.1016/j.pediatrneurol.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 7. Amlie-Lefond C, Bernard TJ, Sébire G, et al. ; International Pediatric Stroke Study Group. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation 2009;119:1417–23 10.1161/CIRCULATIONAHA.108.806307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danchaivijitr N, Cox TC, Saunders DE, et al. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol 2006;59:620–26 10.1002/ana.20800 [DOI] [PubMed] [Google Scholar]
- 9. Roach ES, Golomb MR, Adams R, et al. ; American Heart Association Stroke Council, Council on Cardiovascular Disease in the Young. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 2008;39:2644–91 10.1161/STROKEAHA.108.189696 [DOI] [PubMed] [Google Scholar]
- 10. Bernard TJ, Manco-Johnson MJ, Lo W, et al. Towards a consensus-based classification of childhood arterial ischemic stroke. Stroke 2012;43:371–77 10.1161/STROKEAHA.111.624585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fullerton HJ, Elkind MS, Barkovich AJ, et al. The Vascular Effects of Infection in Pediatric Stroke (VIPS) study. J Child Neurol 2011;26:1101–10 10.1177/0883073811408089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wintermark M, Hills NK, deVeber GA, et al. ; VIPS Investigators. Arteriopathy diagnosis in childhood arterial ischemic stroke: results of the Vascular Effects of Infection in Pediatric Stroke study. Stroke 2014;45:3597–605 10.1161/STROKEAHA.114.007404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009;72:2104–10 10.1212/WNL.0b013e3181aa5329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sébire G, Fullerton H, Riou E, et al. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr 2004;16:617–22 10.1097/01.mop.0000144441.29899.20 [DOI] [PubMed] [Google Scholar]
- 15. Hess CP, Fullerton HJ, Metry DW, et al. Cervical and intracranial arterial anomalies in 70 patients with PHACE syndrome. AJNR Am J Neuroradiol 2010;31:1980–86 10.3174/ajnr.A2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frieden IJ, Reese V, Cohen D. PHACE syndrome: the association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol 1996;132:307–11 [DOI] [PubMed] [Google Scholar]
- 17. Kirton A, Crone M, Benseler S, et al. Fibromuscular dysplasia and childhood stroke. Brain 2013;136:1846–56 10.1093/brain/awt111 [DOI] [PubMed] [Google Scholar]
- 18. Tolani AT, Yeom KW, Elbers J. Focal cerebral arteriopathy: the face with many names. Pediatr Neurol 2015;53:247–52 10.1016/j.pediatrneurol.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 19. Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology 2001;57:1155–60 10.1212/WNL.57.7.1155 [DOI] [PubMed] [Google Scholar]
- 20. Swartz R, Bhuta S, Farb R, et al. Intracranial arterial wall imaging using high-resolution 3-Tesla contrast-enhanced MRI. Neurology 2009;72:627–34 10.1212/01.wnl.0000342470.69739.b3 [DOI] [PubMed] [Google Scholar]
- 21. Lanthier S, Armstrong D, Domi T. Post-varicella arteriopathy of childhood: natural history of vascular stenosis. Neurology 2005;64:660–63 10.1212/01.WNL.0000151851.66154.27 [DOI] [PubMed] [Google Scholar]
- 22. Chabrier S, Sébire G, Fluss J. Transient cerebral arteriopathy, postvaricella arteriopathy, and focal cerebral arteriopathy or the unique susceptibility of the M1 segment in children with stroke. Stroke 2016;47:2439–41 10.1161/STROKEAHA.116.014606 [DOI] [PubMed] [Google Scholar]
- 23. Chabrier S, Rodesch G, Lasjaunias P, et al. Transient cerebral arteriopathy: a disorder recognized by serial angiograms in children with stroke. J Child Neurol 1998;13:27–32 10.1177/088307389801300105 [DOI] [PubMed] [Google Scholar]
- 24. Sträter R, Becker S, von Eckardstein A, et al. Prospective assessment of risk factors for recurrent stroke during childhood: a 5-year follow-up study. Lancet 2002;360:1540–45 10.1016/S0140-6736(02)11520-0 [DOI] [PubMed] [Google Scholar]
- 25. Kim JS. Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke 2016;18:2–11 10.5853/jos.2015.01627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dlamini N, Freeman JL, Mackay MT, et al. Intracranial dissection mimicking transient cerebral arteriopathy in childhood arterial ischemic stroke. J Child Neurol 2011;26:1203–06 10.1177/0883073811408904 [DOI] [PubMed] [Google Scholar]
- 27. Mandell D, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38:218–29 10.3174/ajnr.A4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernard TJ, deVeber GA, Benke TA. Athletic participation after acute ischemic childhood stroke: a survey of pediatric stroke experts. J Child Neurol 2007;22:1050–53 10.1177/0883073807306271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.