Abstract
Objective
Decreasing duodenal contraction is now considered as a major focus for the treatment of type 2 diabetes. Therefore, identifying bioactive molecules able to target the enteric nervous system, which controls the motility of intestinal smooth muscle cells, represents a new therapeutic avenue. For this reason, we chose to study the impact of oral galanin on this system in diabetic mice.
Methods
Enteric neurotransmission, duodenal contraction, glucose absorption, modification of gut–brain axis, and glucose metabolism (glucose tolerance, insulinemia, glucose entry in tissue, hepatic glucose metabolism) were assessed.
Results
We show that galanin, a neuropeptide expressed in the small intestine, decreases duodenal contraction by stimulating nitric oxide release from enteric neurons. This is associated with modification of hypothalamic nitric oxide release that favors glucose uptake in metabolic tissues such as skeletal muscle, liver, and adipose tissue. Oral chronic gavage with galanin in diabetic mice increases insulin sensitivity, which is associated with an improvement of several metabolic parameters such as glucose tolerance, fasting blood glucose, and insulin.
Conclusion
Here, we demonstrate that oral galanin administration improves glucose homeostasis via the enteric nervous system and could be considered a therapeutic potential for the treatment of T2D.
Keywords: Galanin, Enteric nervous system, Diabetes
Graphical abstract
Highlights
-
•
Targeting the enteric nervous system (ENS) is an innovative solution to treat diabetes.
-
•
The ENS controls duodenal contractions to modulate glycemia via the gut–brain axis.
-
•
ENS/contractions are targeted by the neuropeptide galanin in the intestine.
-
•
Oral galanin treatment decreases duodenal hyper-contractility in diabetic mice.
-
•
Oral galanin restores the gut–brain axis to improve glycemia in diabetic mice.
1. Introduction
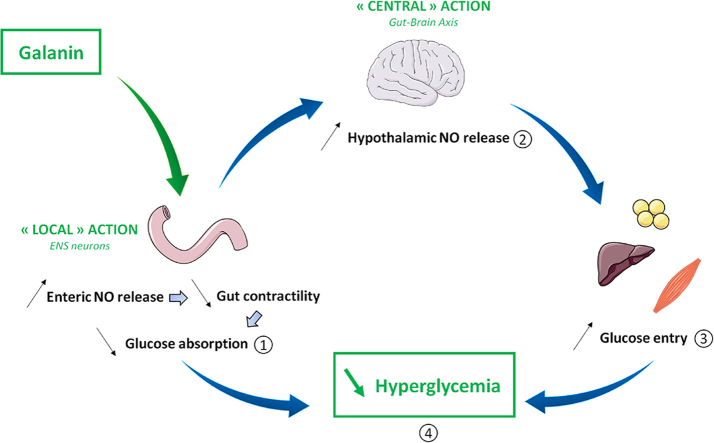
Inter-organ communication is of crucial importance for the maintenance of glucose homeostasis. Inter-organ communication comprises hormonal and nervous regulation, including the hypothalamus, playing a major role as a center of integration of various nervous, and peripheral signals, including nutrients and afferent signal from the intestine [1], [2]. Thus, the modification of hypothalamic neuronal activity via the gut induces large variations of peripheral glucose utilization in organs such as muscles, liver, and white adipose tissues [3]. Hence, it is clear that new therapeutic strategies for type 2 diabetes (T2D) should take into consideration the gut-to-brain axis as major primary site of regulation of glucose homeostasis [4].
Recently, a new concept has emerged demonstrating that the enteric nervous system (ENS) could be targeted by bioactive factors to control glucose utilization in skeletal muscle via a hypothalamic relay [5]. The ENS is mostly composed of Choline Acetyl Transferase (ChAT) and neuronal Nitric Oxide Synthase (nNOS) neurons that respectively stimulate or inhibit intestinal smooth muscle cells. In fact, the ENS alteration observed in proximal intestine of obese/diabetic mice and human generates duodenal hyper-contractility in the fed state [6]. Thus, this aberrant duodenal hyper-contractility first leads to an increase of glucose absorption that contributes to chronic hyperglycemia observed during T2D and then to the genesis of an afferent message from the gut to the hypothalamus that results in an insulin resistant state [5]. Therefore, discovering new molecular actors that able to modulate ENS activity in order to decrease duodenal hyper-contractility represents an original therapeutic approach to improve T2D [7]. Among all potential signaling factors, bioactive peptides could be considered preferential therapeutic targets. In this context, numerous bioactive peptides (e.g., leptin, apelin) present in the intestinal lumen can reach the myenteric plexus to modulate the activity of ENS neurons [5], [8].
Galanin is a neuropeptide largely expressed in the brain but also in ENS neurons [9]. Galanin is known to have beneficial effects on glucose metabolism via central and peripheral actions [10], [11]. So far, three galanin receptors have been identified (Gal-R1, Gal-R2, and Gal-R3), and their expression differs according to the cell type in which they are expressed and the development state [12]. In the intestine, the majority of galanin effects are mediated by Gal-R1 predominantly expressed in ENS neurons [13] and more particular in neurons that express acetylcholine, nNOS, or vasoactive intestinal peptide [14]. More precisely, galanin is known to be a neuropeptide that exerts an inhibitory action on myenteric cholinergic neurons [15]. Galanin is also expressed in enteric neurons, which receive inhibitory and stimulatory information from noradrenergic and cholinergic nerves fibers respectively [16].
During T2D, the expression of galanin is decreased in the duodenum of mice [17], thereby suggesting a potential benefic impact of this peptide on gut-to-brain axis and the control of glucose metabolism. This hypothesis is supported by data showing that galanin inhibits cholinergic transmission in the ENS to favor the relaxation of intestinal smooth muscle cells [18] and that Gal-R1 knockout mice have a significant increase in glycemia in the fed state [19].
Whether galanin is able to modulate the ENS/duodenal contractions to improve glucose homeostasis remains to be determined. Here, our main objective is to unravel the effect of galanin on nitric oxide (NO) release from enteric nNOS neurons, which represent the major population of inhibitory neurons of intestinal contraction, and to evaluate the consequence of this intestinal effect of galanin on gut-to-hypothalamus axis in the control of glucose metabolism.
2. Materials and methods
2.1. Mice
Nine-week-old male C57BL/6J mice (Charles River Laboratory, l’Arbresle, France) were housed in specific pathogen free conditions and in a controlled environment (room temperature of 23 ± 2 °C, 12 h daylight cycle) with free access to food and water. Experiments were conducted according to the European Community regulations concerning the protection of experimental animals and were approved by the local Animal Care and Use Committee. Mice were fed on normal chow (NC) diet or high-fat diet (HFD) containing 20% protein, 35% carbohydrate, and 45% fat (Research Diet, New Brunswick, NJ, USA).
2.2. Isotonic contraction
Mice were euthanized in fed conditions. After dissection, duodenum segments were washed in Krebs–Ringer bicarbonate/glucose buffer (pH 7.4) in 95% O2 and 5% CO2. Segments of duodenum were then incubated in oxygenated Krebs–Ringer solution for 30 min at 37 °C, attached to the isotonic transducer (Lever Transducer, B40 type 373, Hugo Sachs Elektronik, Grünstraße, Germany), and immersed in Falcon tubes containing 25 mL of the same medium maintained at 37 °C. The load applied to the lever was 2g (20 mN). Isotonic contractions were recorded on BDAS software (Hugo Sachs Elektronik) following the transducer displacement. After attaching the duodenum segments, basal contractions were recorded for 10 min. Subsequently, 100 μL of Krebs–Ringer solution or specific drugs (Galanin 100 nM ± galantide 100 nM) were added to the survival medium, and contractions were recorded for 10 min. Amplitudes were recorded for 10 min at 10-second intervals and the average was compared to the average basal contractions. Contraction amplitudes are presented as percentage relative to the basal response whilst contraction frequencies are presented as number of contraction per minute as previously described [5]. For the one-week chronic oral galanin gavage protocol, basal contractions were recorded for 5 min and are presented as average basal contractions. Here, the mice were treated daily with an oral gavage of 100 μL of each drug (Control or galanin 100 nM) during the last week of HFD treatment as previously used [5]. These mice became obese and insulin resistant after 3 months of HFD [5]. To evaluate duodenal contractility, data have been recorded in fed mice, corresponding to the phase in which intestinal segmental waves are generated to increase the rate of nutrients absorption [5], [20]. Data representation in % of basal contraction refers to a basal value obtained before the actual experiment.
2.3. Telemetry
Under anesthesia with isoflurane, a needle attached to a catheter was placed in the stomach for intragastric injection of H2O or galanin. Two electrodes were sutured 5 mm from each other, on the proximal duodenum, 1.5 cm caudal to the pylorus. The signal, corresponding to the electrical activity of duodenum, was received by an RMC-1 receiver (DSI) placed under the cage. The signal obtained during 40 min of continuous recording was analyzed off-line to obtain the integral of the rectified signal over the 100 ms integration interval. The electric signal was allowed to stabilize during 5 min [5]. The next 5 min were considered as basal signal, and then the mice received H2O or drugs (galanin 100 nM ± galantide 100 nM) by intragastric injection of 100 μl at a rate of 10 μl/min using a syringe pump. Data representation in % of basal electrical signal refers to a basal value obtained before the actual experiment.
2.4. Intestinal NO real-time measurement
Mice were euthanized in fed conditions. After dissection, duodenum fragments were washed in Krebs–Ringer bicarbonate/glucose buffer (pH 7.4) in 95% O2 and 5% CO2 and then immersed in Eppendorf tubes containing 400 μL of the same medium. After a 10 min recovery period, the spontaneous NO release was measured at 37 °C for 5 min by using a NO-specific amperometric probe (ISO-NOPF, 100 μm diameter, 5 mm length, World Precision Instruments, Aston Stevenage, UK) implanted directly in the duodenum and in response to each drugs (Control, galanin 100 nM ± galantide 100 nM) [5].
2.5. Hypothalamic NO real-time measurement
Mice in fed conditions were anesthetized with isoflurane. A 1-cm midline incision was made across the top of the skull, and the animal was placed on a stereotaxic apparatus, as described previously [5]. During amperometric measurement, animals received H2O or drugs via an intragastric injection of 10 μl/min rate for 10 min, using a syringe pump. We previously demonstrated that H2O is the vehicle of choice for glucose administration [1], [21]. Furthermore, we have also demonstrated that intragastric perfusion of H2O did not modify c-Fos expression (a marker of neuronal activity) in the hypothalamus, as compared with NaCl [21]. Therefore, all control groups were infused with H2O, as previously described in detail [1].
2.6. Intestinal glucose absorption
Two-hour fasted mice were euthanized. The duodenum was then harvested, washed, everted, and filled with in a Krebs–Ringer solution without glucose. Everted duodenal sacs were incubated in Krebs–Ringer with 10 g/L of glucose alone or with drugs, for 2 min at 37 °C [5]. The media of each sac was then collected and immediately frozen for subsequent galanin and glucose quantification studies. Glucose was measured using Glucose GOD FS 10'kit (DiaSys, France).
2.7. Glucose utilization
Two-hour-fasted mice were orally loaded with 50 μL of [3-3H]glucose and 100 μL of H2O or drugs. Thirty minutes after the oral load, mice were euthanized and blood, liver, muscle, and adipose tissue were collected and immediately frozen. [3-3H] glucose content in tissues was determined by radioactivity counting of NaOH hydrolyzed liver, muscle and adipose tissue [5].
2.8. Immunohistochemistry
Immediately after dissection, duodenal tissues were fixed in 4% formalin solution (Sigma Aldrich) for 24 h and maintained at 4 °C in 70% ethanol until paraffin embedding. Ten-micrometer-thin sections were incubated with goat anti-nNOS (ab1376, Abcam) and anti-Gal-R1 (SAB4501089, Sigma–Aldrich) primary antibodies for 12 h at 4 °C. Autofluorescence and single labeled controls were performed for each of the primary antibodies with both secondary antibodies. After washing with PBS 1X, sections were incubated with Cyanine 3-labeled anti-goat (395570, Interchim) and Dylight 488-labeled anti-rabbit (FES062, Interchim) secondary antibodies for 2 h at 4 °C room temperature. Sections were washed with PBS 1X and then incubated with DAPI (D8417, Sigma Aldrich) in PBS 1X for 1 h at 4 °C. After a final washing step, sections were mounted with Mounting media (Sigma Aldrich). Samples were imaged with a Zeiss LSM 710 confocal microscope (Jena, Germany) using the 63× oil objective (NA 1,4).
2.9. Oral glucose tolerance test (OGTT)
Six-hour-fasted mice were orally loaded with glucose (3 g/kg of body weight). Glycemia was measured at −30, 0, +15, +30, +60, +90, and +120 with a gluco-meter (Accu-Chek Active, Roche), and blood was collected from the tail vein at −30 and + 15 min to measure plasma insulin and glucagon [5]. As described above, the mice were treated daily with an oral gavage of 100 μL of each drug (Control or galanin 100 nM) during the last week of HFD treatment as previously described [5].
2.10. Insulin and glucagon assays
Insulin and glucagon were analyzed by HTRF serum kits (Cisbio, France). Briefly, insulin and glucagon were detected in a sandwich assay format using 2 different specific monoclonal antibodies, one labeled with Terbium Cryptate (donor) and the second with d2 (acceptor). 5 μL of serum was incubated overnight at 4 °C with the 2 corresponding monoclonal antibodies. When the dyes are in close proximity, the excitation of the donor with a light source triggers a Fluorescence Resonance Energy Transfer (FRET). Fluorescence energy transfer was measured on Tecan Infinite 500 plate reader (Tecan, France). Results were analyzed against standard curve fit with the 4 parameter logistic (4 PL) model according kit instructions (GraphPad Prism, USA).
2.11. Gene expression
Tissues were homogenized using a Precellys tissue homogenizer (Bertin Technol., Montigny-le-Bretonneux, France), and total RNA from tissues was prepared using TRIzol (Invitrogen) and GenElute™ Mammalian Total RNA Miniprep Kit (Sigma–Aldrich), according to the manufacturer's instructions. cDNAs were generated using M-MLV Reverse transcriptase kit (Invitrogen) and random hexamers (Invitrogen). Real-time PCR was performed with LightCycler 480 (Life Technologies) using SYBR Green Real-Time PCR Master Mixes (ThermoFisher Scientific) with primers validated by testing the PCR efficiency. Sequences of the primers used for cDNA amplification in the quantitative RT-PCR experiments are detailed in Supplementary Table 1. Gene expression was quantified using the comparative Ct (threshold cycle) method. Results were normalized to beta-2-microglobulin expression. The identity and purity of the amplified product were assessed by analysis of the melting curve, which was performed at the end of amplification.
2.12. Western blot
Vastus lateralis tissues were homogenized in a buffer containing 50 mmol/L HEPES, pH 7.4, 2 mmol/L EDTA, 150 mmol/L NaCl, 30 mmol/L NaPO4, 10 mmol/L NaF, 1% Triton X-100, 10 mg/mL protease inhibitor (Sigma–Aldrich), 10 mg/mL phosphatase I inhibitor (Sigma–Aldrich), 10 mg/mL phosphatase II inhibitor (Sigma–Aldrich), and 1.5 mg/mL benzamidine HCl. Tissue homogenates were centrifuged for 25 min at 15.000g, and supernatants were stored at −80 °C. Solubilized proteins from muscle tissue were run on a 4–20% Criterion SFX Tris-HCl (Bio-Rad, Hercules, CA), transferred onto nitrocellulose membrane (Hybond ECL, GE Healthcare, Buckinghamshire, U.K.), and incubated with the primary antibodies for P-Akt (#4060, Cell Signaling), Akt (#4691, Cell Signaling), P-AMPK (#2535, Cell Signaling), AMPK (#2532S, Cell Signaling). Subsequently, immunoreactive proteins were determined by enhanced chemiluminescence reagent (Clarity Western ECL, Biorad) and visualized by exposure to CCD Imaging Chemidoc MP (Biorad, Hercules, CA). Volume density was analyzed for each band and normalized to total transferred proteins determined by stain free.
2.13. Galanin assay
Plasma galanin assay was performed by ELISA under manufacturer instructions (CliniSciences, Nanterre, France).
2.14. Statistics
The data are expressed as the mean ± SEM. Differences between the experimental groups were assessed where appropriate using by unpaired Student's and one or two way ANOVA, followed by post-hoc test. Data were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). The results were considered statistically significant at P < 0.05.
3. Results
3.1. Galanin stimulates duodenal NO release
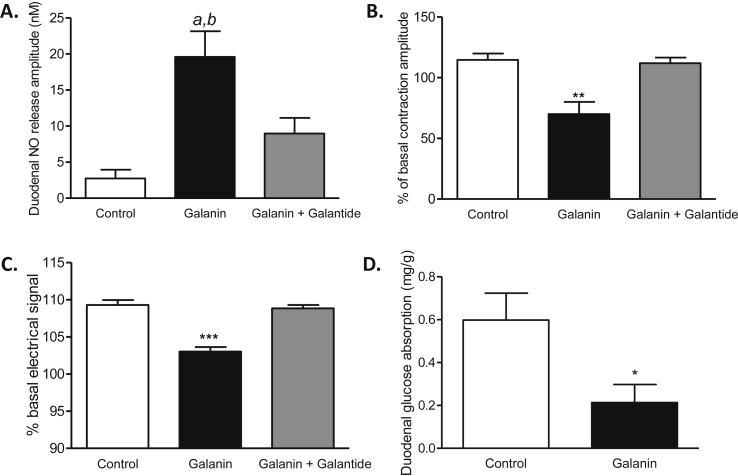
We first assessed whether Gal-R1 was expressed in enteric neurons. In addition to the presence of Gal-R1 on ChAT neurons [22], we found that myenteric nitrergic neurons express Gal-R1 (Supplementary Fig. 1A). Amperometric measurements show that the addition of galanin on ex vivo duodenum stimulates release of NO, a major neurotransmitter of the ENS that inhibits intestinal contractions [5], and this effect is blocked by galantide, a galanin receptor antagonist (Figure 1A and Supplementary Fig. 1B).
Figure 1.
Galanin stimulates duodenal NO release, decreases duodenal contractions to decrease glucose absorption. (A)Ex vivo measurement of duodenal nitric oxide (NO) release amplitude during 5 min in response to Krebs–Ringer (Control), galanin 100 nM and galanin 100 nM plus galantide 100 nM n = 6–12 per group. a, p < 0.01 vs Control; b, p < 0.05 vs galanin + galantide. (B)Ex vivo measurement of duodenal mechanical contraction amplitude during 10 min in response to Krebs–Ringer solution (Control), galanin 100 nM and galanin 100 nM plus galantide 100 nM n = 5 per group. **p < 0.01 vs other groups. (C)In vivo telemetric measurement of duodenal electrical activity during 10 min in response to water (Control), galanin 100 nM and galanin 100 nM plus galantide 100 nM, n = 5 per group. ***p < 0,001 vs other groups. (D)Ex vivo glucose absorption in duodenal everted sacs in response to Krebs–Ringer (Control) or galanin 100 nM n = 8 per group. *p < 0.05 vs Control.
3.2. Galanin decreases duodenal contraction to decrease glucose absorption
Consequently, we measured the effect of galanin on duodenal contraction in both ex vivo and in vivo conditions. Using isotonic sensor, we discovered that galanin inhibits the mechanical contractions of the duodenum (Figure 1B and Supplementary Fig. 1C). In addition, we measured duodenal electrical activity, which reflects the mechanical contraction [5]. We found that the electrical signal was significantly decreased in mice infused with galanin (Figure 1C), thereby confirming the inhibitory effect of galanin on contraction. A positive correlation exists between intestinal contraction and glucose absorption [20]. Here, all the effects of galanin were blocked by galantide (Figure 1A–C). In our model, the decrease of intestinal contraction was associated with a decrease of glucose absorption by the proximal intestine (Figure 1D).
3.3. Intestinal galanin modulates the gut–brain axis to control glucose utilization in tissues
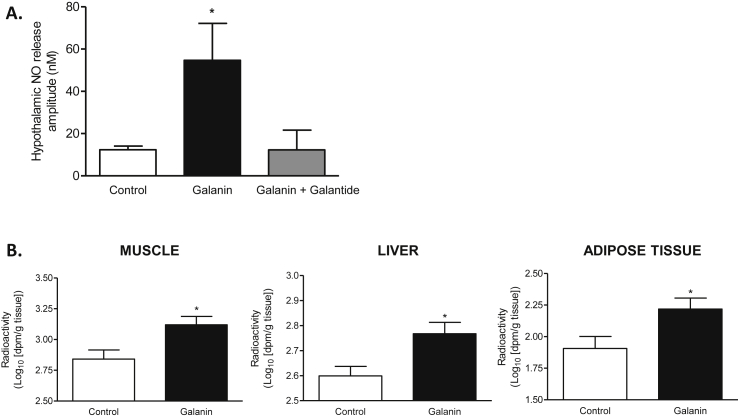
We recently identified a new mode of communication between the gut and the brain involved in the control of glucose utilization and glycemia [5]. In fact, we discovered that an increase of duodenal contraction is associated with a decrease of hypothalamic NO release that blocks glucose entry in metabolic tissues [5]. Therefore, to find a bioactive peptide able to stop duodenal hyper-contractility by a mechanism restoring the gut–brain axis, and eventually glucose utilization, is considered as a new potential therapeutic approach [7]. Interestingly, here we found that by its effect on duodenal contraction, galanin increased the release of NO in the hypothalamus of mice (Figure 2A and Supplementary Fig. 2A) and promoted glucose entry in soleus muscle, liver, and subcutaneous adipose tissue (Figure 2B), but not in the heart, vastus lateralis, and epididymal adipose tissue (Supplementary Figs. 2C–E). We did not show variation of glycogen content in all tissue tested (Supplementary Figs. 2B–D), suggesting that glucose is not stored in these tissues but could directly enter into glycolysis.
Figure 2.
Intestinal galanin modulates the gut-brain axis to control glucose utilization in tissue. (A)In vivo effect of intragastric perfusion of water (Control), galanin 100 nM and galanin 100 nM + galantide 100 nM on nitric oxide (NO) hypothalamic release amplitude. n = 4–7 per group. *p < 0.05 vs other groups. (B)In vivo measurement of glucose entry in muscle, liver and subcutaneous adipose tissue in response to oral gavage of radiolabeled glucose in combination with water (Control) or galanin 100 nM n = 5 per group. *p < 0.05 vs Control.
3.4. Galanin decreases the duodenal hyper-contractility of diabetic mice
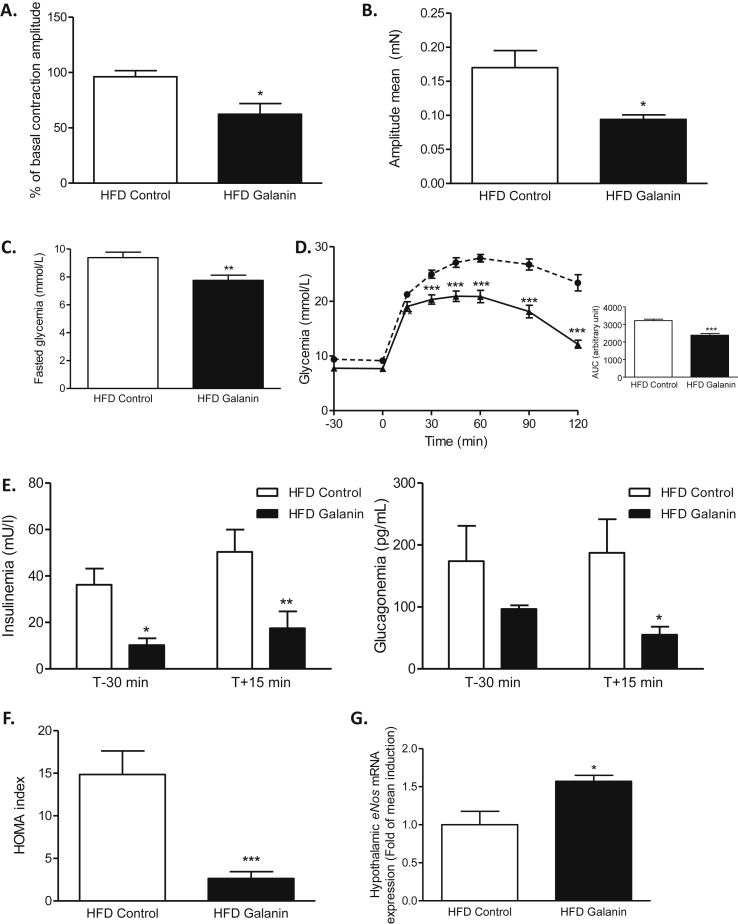
Diabetes is associated with an intestinal hyper-contractility [6] that favors hyperglycemia and insulin-resistance [5]. Similar to what was observed in normal mice, in diabetic mice, galanin had the capacity to decrease duodenal contraction in ex vivo condition (Figure 3A and Supplementary Fig. 3A). Moreover, chronic treatment with oral galanin significantly decreased the basal contraction of the duodenum from diabetic mice (Figure 3B and Supplementary Fig. 3B).
Figure 3.
Oral galanin treatment decreases the duodenal hyper-contractility of diabetic mice and improves diabetic state. (A)Ex vivo measurement of duodenal mechanical contraction amplitude in response to Krebs–Ringer (Control) or galanin 100 nM in high-fat diet (HFD) mice. n = 5 per group. *p < 0.05 vs HFD Control. (B)In vivo measurement of duodenal mechanical contraction amplitude in response to an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 5–8 per group. *p < 0.05 vs HFD Control. (C) Effects of an oral administration of water (HFD Control) or galanin 100 nM during one week on fasted glycemia in HFD mice. n = 12–14 per group. **p < 0.01 vs HFD Control. (D) Oral glucose tolerance test (OGTT) in 6 h fasted HFD mice, after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 12–14 per group. The adjacent graph represents the average area under the curve (AUC) ***p < 0.001 vs HFD Control. (E) OGTT-associated plasma insulin and glucagon 30 min before and 15 min after oral load of glucose after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 5 per group. *p < 0.05, **p < 0.01 vs HFD Control. (F) OGTT-associated insulin resistance index (HOMA-IR) in 6 h fasted HFD mice after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 5 per group. ***p < 0.001 vs HFD Control. (G) Relative expression of endothelial Nitric Oxide Synthase (eNOS) mRNA in hypothalamus of HFD mice after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 4–5 per group.
3.5. Oral galanin treatment improves the diabetic state
After 7 days of oral administration of galanin, diabetic mice have decreased fasting hyperglycemia (Figure 3C) and an improvement in glucose tolerance (Figure 3D) associated with a decrease in plasma insulin and glucagon levels in the fasted state (only for insulin) and in response to an oral glucose load (Figure 3E). Consequently, the HOMA index was significantly lower in galanin-treated mice than in controls (Figure 3F). We did not observe significant variation of plasma galanin in response to this oral treatment (1.82 ± 0.13 ng/mL for HFD Control vs 1.64 ± 0.04 ng/mL for HFD Galanin). This strongly suggests that the major target of galanin effect on glucose metabolism in our model is the intestine.
To explain that this effect could imply the gut–brain axis, we measured the variation of eNOS mRNA expression in the hypothalamus of oral galanin treated mice. Here, oral galanin treatment increased the expression of eNOS mRNA which is a major hypothalamic enzyme implicated in the control of glucose homeostasis [23], [24].
3.6. Oral galanin improves insulin sensitivity via an AMPK signaling
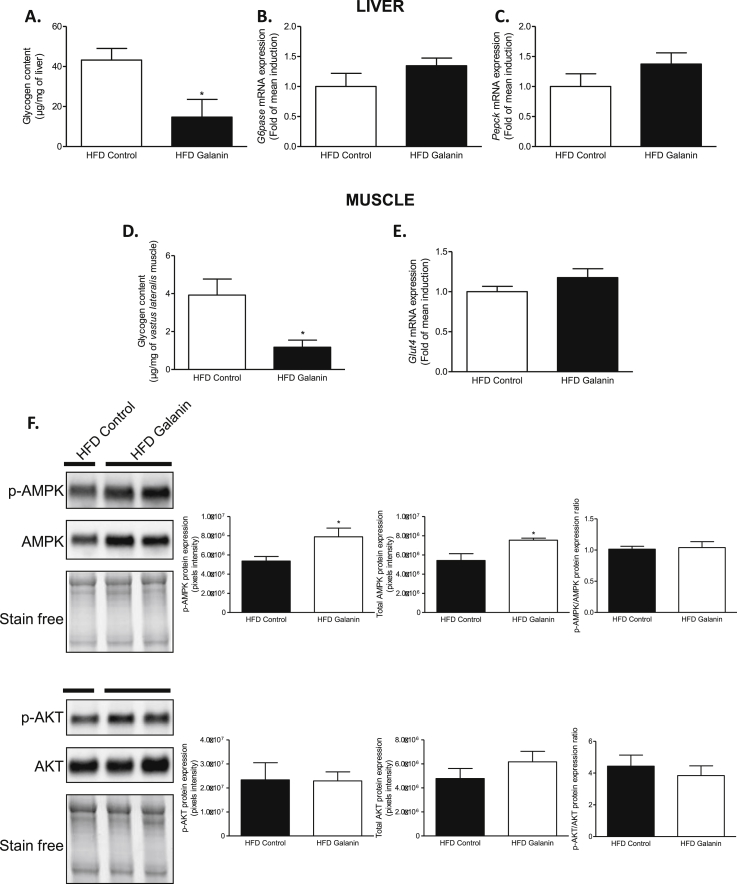
To determine whether oral galanin modulates the gut-to-brain-to peripheral axis, we studied the effects of chronic galanin gavage on tissues. We did not see any modification of Sglt1 and Glut2 mRNA expression in the duodenum (Supplementary Fig. 4A), reinforcing the fact that the majority of the effects observed to impact glycemia (absorption and gut–brain axis stimulation) are likely due to a mechanical phenomenon. In the liver of fed mice, oral galanin treatment decreased glycogen content (Figure 4A). This phenotype was not associated with modifications of hepatic Pepck and G6pase mRNA expression (Figure 4B–C). In the vastus lateralis muscle, glycogen content was also significantly lower in galanin treated mice than control (Figure 4D). The improvement of insulin sensitivity of galanin treatment was not associated with increased muscle Glut4 mRNA expression (Figure 4E), but it was associated with a significant increase of total and phosphorylated AMPK (but not Akt) protein expression (Figure 4F). In fact, AMPK activation is associated with stimulation of glucose uptake and inhibition of glycogen synthase to favor glucose flux through glycolysis [25]; thus, the biological effects of galanin observed here in liver and muscles are consistent with an activation of AMPK signaling. In fasted mice, oral galanin treatment could modify soleus muscle but not hepatic glycogen content (Supplementary Figs. 4B–C).
Figure 4.
Oral galanin improves insulin sensitivity via an AMPK signaling. (A) Hepatic glycogen content of fed mice after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 4–10 per group. *p < 0.05 vs HFD Control. (B) Relative expression of glucose-6-phosphatase (G6pase) mRNA in liver of HFD mice after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 4–5 per group. (C) Relative expression of Phosphoenolpyruvate carboxykinase (Pepck) mRNA in liver of HFD mice after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 4–5 per group. (D)Vastus lateralis muscle glycogen content of fed HFD mice after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 4–10 per group. *p < 0.05 vs HFD Control. (E) Relative expression of glucose transporter type 4 (Glut-4) mRNA in vastus lateralis muscle of HFD mice after an oral administration of water (HFD Control) or galanin 100 nM during one week. n = 5 per group. (F)Vastus lateralis muscle AMPK and Akt protein expression of HFD mice after an oral administration of water (HFD Control) or galanin 100 nM during one week and the relative quantification. n = 4–5 per group. *p < 0.05 vs Control.
4. Discussion
Controlling the coupling of ENS/smooth muscle is now considered a promising way to treat T2D via the gut-to-brain axis [7]. We demonstrate here that oral galanin, a gastrointestinal hormone, is able to stimulate the gut–brain axis by modulating the activity of ENS neurons to improve insulin sensitivity.
Galanin is a peptide that has a beneficial impact on the maintenance of whole body glucose homeostasis. As opposed to numerous bioactive peptides such as apelin [24], [26], [27], intravenous and intracerebroventricular injections of galanin improve insulin sensitivity in different models [11], [28]. This total absence of deleterious effects on glycemia renders galanin as a player of interest for the treatment of T2D. In this context, oral bioactive peptides treatment in diabetic rodents is now considered as an innovative approach [7], and this new mode administration has never been investigated for galanin. Here, we show that galanin increases the release of NO from ENS neurons to favor duodenum relaxation. This result is in line with the inhibitory action of galanin on ChAT neurons previously discovered [22]. Numerous peptides (i.e. leptin, apelin) present in the intestinal lumen can reach the ENS via transcytosis [5], [8]. In this context, these peptides could stimulate and/or inhibit the two types of motoneurons (i.e. nNOS and ChAT neurons) that control intestinal smooth cells [5]. This coupled with the total absence of deleterious effect in the body (including the brain) render oral galanin as the most promising candidate for therapeutic approach.
In fed states, intestinal segmental waves are generated to increase the rate of nutrients absorption [20]. In these conditions, we have previously discovered that intestinal apelin can control glucose absorption by a local action and insulin sensitivity via the gut–brain axis [5]. In fact, oral apelin treatment favors glucose entry in muscles of diabetic mice in fed conditions [5]. Interestingly, we have shown that, in addition to target the muscles, oral galanin has a long term action that could have an impact on fasting blood glucose in diabetic mice. This unsuspected effect of galanin on fasting blood glucose suggests that the modification of the gut–brain axis observed in fed states could have physiological consequences in fasted states. However, the intestinal, central, and peripheral molecular actors involved in this phenomenon remain to be elucidated. Our data support a “local” intestinal action of galanin, but we cannot totally exclude that a part of galanin and/or other releasing factors in response to oral galanin could reach the circulation to target directly the brain and/or peripheral tissue. This point has to be taken into consideration for therapeutic strategies with peptides other than galanin that could have well known deleterious effects on tissues.
Discovering the potential molecular actors implicated in the control of galanin expression in enteric neurons could bring new information to decipher the operating mode of these neurons in normal and pathological conditions. In fact, the proportion of galanin nerve fibers is significantly decreased in the duodenum of type 2 diabetic mice [17]. As opposed to the ENS, data relative to metabolite factors able to modulate galaninergic signaling are well documented. For instance, glucose deprivation increases GalR-1 expression [29], and injection of insulin analogues in type 2 diabetic rats decreases the expression of galanin [30] in the hypothalamus. Thus, there is a lack of studies which could make the link between “glucose, insulin and/or metabolites” and the alteration of galanin expression in the duodenum observed during type 2 diabetes. Here, we find bioactive factors able to restore duodenal galanin level to normal values could represent another alternative to treat hyperglycemia.
Nowadays, therapeutic strategies to treat T2D are multiple and include various pharmacological approaches such as the use of biguanides, sulfonylureas, thiazolidinediones, dipeptidyl peptidase IV inhibitors, and SGLT2 inhibitors alone or in combination [31]. In this therapeutic context and despite the beneficial effects observed with these different oral treatments, diabetic patients may suffer from potential side effects [31]. This raises the question of the medication risk–benefit profiles to adapt the therapeutic treatment to each individual. Therefore, our proposed strategy using natural bioactive peptides given orally to treat T2D represents a major innovative approach [7]. In fact, we and others have previously demonstrated that bioactive peptides present in the gut lumen can reach the ENS in the duodenum via transcytosis [5], [8]. Most importantly, their actions are limited to the duodenum wall. This new therapeutic strategy presents the advantage of limiting the potential iatrogenic and side effects of classical anti-diabetics. In addition, we have demonstrated here that galanin modulates the activity of the ENS/contraction coupling to restore the gut–brain axis to improve glucose homeostasis in diabetic mice. Therefore, and in accordance with literature which does not demonstrate deleterious effect of galanin in the whole body, oral galanin could meet all the criteria to be a new anti-diabetic agent. In the future, the galenic formulation in human has to take into account the fact that galanin has to be specifically released in the duodenum and not in the stomach. In fact, galanin is also released by myenteric neurons in the stomach and thus decreases gastric emptying [32]. This could have repercussions on food intake and aversion leading to malnutrition, similar to what is observed in diabetic patients with gastroparesis [33]. In contrast, specific blockade of galanin receptors in the stomach could improve gastric emptying and its secondary effects.
Here, we clearly show that galantide abolished the galanin effects. However, one potential limitation of our study is that this pharmacological tool does not allow us to fully demonstrate that the effects are mediates only via galanin receptors present on the ENS (Supplementary Fig. 1A). Further investigation using mice with an inducible ENS specific receptor galanin deletion would be useful to further delineate any additional role of the galanin receptor present on the ENS on metabolism.
5. Conclusions
Our preclinical study demonstrates that galanin targets the ENS to restore the gut–brain axis of diabetic mice by decreasing duodenal hyper-contractility. Oral therapeutic strategy offers multiple possibilities to diabetic patients, and oral galanin could be tested alone or in combination with well-characterized anti-diabetics.
Acknowledgments
PDC is a senior research associate at FRS-FNRS (Fonds de la Recherche Scientifique), Belgium. PDC was the recipient of grants from FNRS. This work was supported by the FRFS-WELBIO under grant: WELBIO-CGR-2017, and the Funds Baillet Latour (Grant for Medical Research 2015). PDC was a recipient of an ERC Starting Grant in 2013 (European Research Council, Starting grant 336452-ENIGMO). CK was the recipient of grants from the Société Francaise de Nutrition (SFN), the Fondation Recherche Médicale (FRM) (Grant ING20150532586), and the Société Francophone du Diabète (Allocations Exceptionnelles 2016). We thank A. Marlin and A. Drougard for excellent technical assistance at the beginning of this project, the platform TRI de Purpan for immunohistochemistry study and the Cellular Protein Analysis (APC) platform (Inserm U1048) for HTRF analysis, advice and assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.01.020.
Contributor Information
Patrice D. Cani, Email: patrice.cani@uclouvain.be.
Claude Knauf, Email: claude.knauf@inserm.fr.
Conflicts of interest
None.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Fig. 1: (A) Galanin receptor 1 (GAL-R1) is expressed in myenteric neurons. Duodenal sections from mice stained with anti-GAL-R1 antibody ((left panels, green arrows), anti-neuronal nitric oxide synthase (nNOS) antibodies (middle panels, red arrows) and merge (right panels, yellow arrows). Pictures are representative of four mice per groups. (B) Ex vivo measurement of duodenal nitric oxide (NO) release frequency during 5 minutes in response to Krebs–Ringer (Control), galanin 100nM and galanin 100nM + galantide 100nM. n=6–12 per group. (C) Ex vivo measurement of duodenal mechanical contraction frequency during 10 minutes in response to Krebs–Ringer solution (Control), galanin 100nM and galanin 100nM + galantide 100nM. n=5 per group. a, p<0.01 vs basal contractions before acute injection and vs Control, b, p<0.05 vs galanin + galantide.
Supplementary Fig. 2: (A) In vivo effect of intragastric perfusion of water (Control), galanin 100nM and galanin 100nM + galantide 100nM on nitric oxide (NO) hypothalamic release frequency. n=4–7 per group. (B) In vivo measurement of glycogen content in soleus muscle and liver in response to oral gavage of radiolabeled glucose in combination with water (Control) or galanin 100nM. n=5 per group. (C) In vivo measurement of glucose entry and glycogen content in heart in response to oral gavage of radiolabeled glucose in combination with water (Control) or galanin 100nM. n=5 per group. (D) In vivo measurement of glucose entry and glycogen content in vastus lateralis muscle in response to oral gavage of radiolabeled glucose in combination with water (Control) or galanin 100nM. n=5 per group. (E) In vivo measurement of glucose entry in epididymal adipose tissue in response to oral gavage of radiolabeled glucose in combination with water (Control) or galanin 100nM. n=5 per group.
Supplementary Fig. 3: (A) Ex vivo measurement of duodenal mechanical contraction frequency in response to Krebs–Ringer (Control) or galanin 100nM in high-fat diet (HFD) mice. n=5 per group. (B) In vivo measurement of duodenal mechanical contraction frequency in response to an oral administration of water (HFD Control) or galanin 100nM during one week. n=5–8 per group.
Supplementary Fig. 4: (A) Relative expression of Sodium Glucose Cotransporter 1 (Sglt1) and glucose transporter type 2 (Glut-2) mRNA in duodenum of HFD mice after an oral administration of water (HFD Control) or galanin 100nM during one week. n=5 per group. (B) In vivo measurement of glycogen content in liver in fasted mice in response to an oral administration of water (HFD Control) or galanin 100nM during one week. n=5 per group. (C) In vivo measurement of glycogen content in soleus muscle in fasted mice in response to an oral administration of water (HFD Control) or galanin 100nM during one week. n=4–5 per group. **p<0.01 vs HFD Control.
Primer sequences for targeted mouse genes used for real-time RT-qPCR.
References
- 1.Knauf C., Cani P.D., Perrin C., Iglesias M.A., Maury J.F., Bernard E. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. Journal of Clinical Investigation. 2005;115:3554–3563. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cani P.D., Knauf C. How gut microbes talk to organs: the role of endocrine and nervous routes. Molecular metabolism. 2016;5:743–752. doi: 10.1016/j.molmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz M.W., Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 4.Fournel A., Marlin A., Abot A., Pasquio C., Cirillo C., Cani P.D. Glucosensing in the gastrointestinal tract: impact on glucose metabolism. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2016;310:G645–G658. doi: 10.1152/ajpgi.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournel A., Drougard A., Duparc T., Marlin A., Brierley S.M., Castro J. Apelin targets gut contraction to control glucose metabolism via the brain. Gut. 2017;66:258–269. doi: 10.1136/gutjnl-2015-310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrasekharan B., Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society. 2007;19:951–960. doi: 10.1111/j.1365-2982.2007.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber C. Neurogastroenterology: improving glucose tolerance via the gut-brain axis. Nature Reviews Gastroenterology & Hepatology. 2015 doi: 10.1038/nrgastro.2015.204. [DOI] [PubMed] [Google Scholar]
- 8.Cammisotto P.G., Gingras D., Bendayan M. Transcytosis of gastric leptin through the rat duodenal mucosa. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2007;293:G773–G779. doi: 10.1152/ajpgi.00260.2007. [DOI] [PubMed] [Google Scholar]
- 9.Belai A., Calcutt N.A., Carrington A.L., Diemel L.T., Tomlinson D.R., Burnstock G. Enteric neuropeptides in streptozotocin-diabetic rats; effects of insulin and aldose reductase inhibition. Journal of the Autonomic Nervous System. 1996;58:163–169. doi: 10.1016/0165-1838(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 10.Fang P., Yu M., Shi M., Zhang Z., Sui Y., Guo L. Galanin peptide family as a modulating target for contribution to metabolic syndrome. General and Comparative Endocrinology. 2012;179:115–120. doi: 10.1016/j.ygcen.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Fang P., Yu M., Shi M., He B., Zhang Z., Bo P. The neuropeptide galanin benefits insulin sensitivity in subjects with type 2 diabetes. Current Protein & Peptide Science. 2013;14:669–673. [PubMed] [Google Scholar]
- 12.Webling K.E., Runesson J., Bartfai T., Langel U. Galanin receptors and ligands. Frontiers in Endocrinology. 2012;3:146. doi: 10.3389/fendo.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham T., Guerrini S., Wong H., Reeve J., Jr., Sternini C. Distribution of galanin receptor 1 immunoreactivity in the rat stomach and small intestine. Journal of Comparative Neurology. 2002;450:292–302. doi: 10.1002/cne.10311. [DOI] [PubMed] [Google Scholar]
- 14.Sternini C., Anselmi L., Guerrini S., Cervio E., Pham T., Balestra B. Role of galanin receptor 1 in peristaltic activity in the Guinea pig ileum. Neuroscience. 2004;125:103–112. doi: 10.1016/j.neuroscience.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 15.Yau W.M., Dorsett J.A., Youther M.L. Evidence for galanin as an inhibitory neuropeptide on myenteric cholinergic neurons in the Guinea pig small intestine. Neuroscience Letters. 1986;72:305–308. doi: 10.1016/0304-3940(86)90531-8. [DOI] [PubMed] [Google Scholar]
- 16.Messell T., Harling H., Poulsen S.S., Bersani M., Holst J.J. Extrinsic control of the release of galanin and VIP from intrinsic nerves of isolated, perfused, porcine ileum. Regulatory Peptides. 1992;38:179–198. doi: 10.1016/0167-0115(92)90101-y. [DOI] [PubMed] [Google Scholar]
- 17.Spangeus A., El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes) Histology & Histopathology. 2001;16:159–165. doi: 10.14670/HH-16.159. [DOI] [PubMed] [Google Scholar]
- 18.Akehira K., Nakane Y., Hioki K., Taniyama K. Site of action of galanin in the cholinergic transmission of Guinea pig small intestine. European Journal of Pharmacology. 1995;284:149–155. doi: 10.1016/0014-2999(95)00393-y. [DOI] [PubMed] [Google Scholar]
- 19.Zorrilla E.P., Brennan M., Sabino V., Lu X., Bartfai T. Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiology & Behavior. 2007;91:479–485. doi: 10.1016/j.physbeh.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sababi M., Bengtsson U.H. Enhanced intestinal motility influences absorption in anaesthetized rat. Acta Physiologica Scandinavica. 2001;172:115–122. doi: 10.1046/j.1365-201X.2001.00849.x. [DOI] [PubMed] [Google Scholar]
- 21.Knauf C., Cani P.D., Kim D.H., Iglesias M.A., Chabo C., Waget A. Role of central nervous system glucagon-like Peptide-1 receptors in enteric glucose sensing. Diabetes. 2008;57:2603–2612. doi: 10.2337/db07-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anselmi L., Stella S.L., Jr., Brecha N.C., Sternini C. Galanin inhibition of voltage-dependent Ca(2+) influx in rat cultured myenteric neurons is mediated by galanin receptor 1. Journal of Neuroscience Research. 2009;87:1107–1114. doi: 10.1002/jnr.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabou C., Cani P.D., Campistron G., Knauf C., Mathieu C., Sartori C. Central insulin regulates heart rate and arterial blood flow: an endothelial nitric oxide synthase-dependent mechanism altered during diabetes. Diabetes. 2007;56:2872–2877. doi: 10.2337/db07-0115. [DOI] [PubMed] [Google Scholar]
- 24.Duparc T., Colom A., Cani P.D., Massaly N., Rastrelli S., Drougard A. Central apelin controls glucose homeostasis via a nitric oxide-dependent pathway in mice. Antioxidants and Redox Signaling. 2011;15:1477–1496. doi: 10.1089/ars.2010.3454. [DOI] [PubMed] [Google Scholar]
- 25.Halse R., Fryer L.G., McCormack J.G., Carling D., Yeaman S.J. Regulation of glycogen synthase by glucose and glycogen: a possible role for AMP-activated protein kinase. Diabetes. 2003;52:9–15. doi: 10.2337/diabetes.52.1.9. [DOI] [PubMed] [Google Scholar]
- 26.Drougard A., Fournel A., Marlin A., Meunier E., Abot A., Bautzova T. Central chronic apelin infusion decreases energy expenditure and thermogenesis in mice. Scientific Reports. 2016;6:31849. doi: 10.1038/srep31849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drougard A., Duparc T., Brenachot X., Carneiro L., Gouaze A., Fournel A. Hypothalamic apelin/reactive oxygen species signaling controls hepatic glucose metabolism in the onset of diabetes. Antioxidants and Redox Signaling. 2014;20:557–573. doi: 10.1089/ars.2013.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang P.H., Yu M., Ma Y.P., Li J., Sui Y.M., Shi M.Y. Central nervous system regulation of food intake and energy expenditure: role of galanin-mediated feeding behavior. Neuroscience Bulletin. 2011;27:407–412. doi: 10.1007/s12264-011-1841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorbatyuk O., Hokfelt T. Effect of inhibition of glucose and fat metabolism on galanin-R1 receptor mRNA levels in the rat hypothalamic paraventricular and supraoptic nuclei. NeuroReport. 1998;9:3565–3569. doi: 10.1097/00001756-199811160-00005. [DOI] [PubMed] [Google Scholar]
- 30.Zafar M.I., Hu C., Liu D., Shafqat R.A., Gao F. Insulin detemir causes lesser weight gain in comparison to insulin glargine: role on hypothalamic NPY and galanin. Journal of Diabetes Research. 2014;2014:458104. doi: 10.1155/2014/458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zonszein J., Groop P.H. Strategies for diabetes management: using newer oral combination therapies early in the disease. Diabetes Therophy. 2016;7:621–639. doi: 10.1007/s13300-016-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holzer-Petsche U., Moser R.L. Pharmacological evidence for the release of galanin from rat stomach. Neuropeptides. 1993;25:47–50. doi: 10.1016/0143-4179(93)90067-k. [DOI] [PubMed] [Google Scholar]
- 33.Parkman H.P., Yates K.P., Hasler W.L., Nguyan L., Pasricha P.J., Snape W.J. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology. 2011;141 doi: 10.1053/j.gastro.2011.04.045. 486–498, 498 e481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 4: (A) Relative expression of Sodium Glucose Cotransporter 1 (Sglt1) and glucose transporter type 2 (Glut-2) mRNA in duodenum of HFD mice after an oral administration of water (HFD Control) or galanin 100nM during one week. n=5 per group. (B) In vivo measurement of glycogen content in liver in fasted mice in response to an oral administration of water (HFD Control) or galanin 100nM during one week. n=5 per group. (C) In vivo measurement of glycogen content in soleus muscle in fasted mice in response to an oral administration of water (HFD Control) or galanin 100nM during one week. n=4–5 per group. **p<0.01 vs HFD Control.
Primer sequences for targeted mouse genes used for real-time RT-qPCR.