Abstract
Colorectal cancer (CRC) heritability has been estimated to be around 30%. However, mutations in the known CRC-susceptibility genes explain CRC risk in fewer than 10% of affected individuals. Germline mutations in DNA-repair genes (DRGs) have recently been reported in CRC, but their contribution to CRC risk is largely unknown. We evaluated the gene-level germline mutation enrichment of 40 DRGs in 680 unselected CRC individuals and 27,728 ancestry-matched cancer-free adults. Significant findings were then examined in independent cohorts of 1,661 unselected CRC individuals and 1,456 individuals with early-onset CRC. Of the 680 individuals in the discovery set, 31 (4.56%) individuals harbored germline pathogenic mutations in known CRC-susceptibility genes, and another 33 (4.85%) individuals had DRG mutations that have not been previously associated with CRC risk. Germline pathogenic mutations in ATM and PALB2 were enriched in both the discovery (OR = 2.81 and p = 0.035 for ATM and OR = 4.91 and p = 0.024 for PALB2) and validation (OR = 2.97 and adjusted p = 0.0013 for ATM and OR = 3.42 and adjusted p = 0.034 for PALB2) sets. Biallelic loss of ATM was evident in all individuals with matched tumor profiling. CRC individuals also had higher rates of actionable mutations in the HR pathway, which can substantially increase the risk of developing cancers other than CRC. Our analysis provides evidence for ATM and PALB2 as CRC-risk genes, underscoring the importance of the homologous recombination pathway in CRC. In addition, we identified frequent complete homologous recombination deficiency in CRC tumors, representing a unique opportunity to explore targeted therapeutic interventions such as poly-ADP ribose polymerase inhibitor (PARPi).
Keywords: colorectal cancer genetics, germline genetics, cancer heritability, ATM mutations, PALB2 mutations, homologous recombination, DNA-repair deficiency, CRC, actionable mutations
Introduction
Colorectal cancer (CRC [MIM: 114500]) is the third most common malignancy in the US.1 Although most CRC cases are thought to be sporadic, recent twin studies have estimated that 30% of the inter-individual variability in CRC risk is attributable to inherited genetic factors.2 Over the past few decades, several CRC-predisposition genes—including APC (MIM: 611731), MLH1 (MIM: 120436), MSH2 (MIM: 609309), MSH6 (MIM: 600678), PMS2 (MIM: 600259), STK11 (MIM: 602216), MUTYH (MIM: 604933), SMAD4 (MIM: 600993), BMPR1A (MIM: 601299), PTEN (MIM: 601728), TP53 (MIM: 191170), CHEK2 (MIM: 604373), POLD1 (MIM: 174761), and POLE (MIM: 174762)—have been described.3, 4, 5 Collectively, mutations in these Mendelian CRC-risk genes explain the increased risk of CRC in 5%–10% of unselected cases.6, 7, 8, 9 The discrepancy between the proportion of CRC cases explained by these genetic risk factors and the estimated degree of heritability, known as “missing heritability,” indicates that one or more undiscovered inherited risk factors contribute to CRC risk.
DNA repair is a critical biological process that prevents permanent DNA damage and ensures genomic stability. Although defects in DNA mismatch repair and certain DNA polymerases have been implicated in CRC risk, the role of other canonical DNA-repair pathways is less defined. Our group and others have reported several observational studies in which some CRC individuals were found to have germline mutations in DNA-repair genes (DRGs), such as ATM (MIM: 607585), BRCA1 (MIM: 113705), BRCA2 (MIM: 600185), and PALB2 (MIM: 610355), that have classically been associated with susceptibility to cancers other than CRC.6, 10, 11 Because these DRG mutations are also present in the general population at a very low frequency, it is still unclear whether these DRG defects are truly associated with a higher CRC risk or merely represent incidental findings in these CRC individuals.12 To date, there has not been a case-control study on CRC individuals to systematically examine candidate DRGs for potential enrichment of germline mutations.
Here, we build upon our previous observations by conducting a case-cohort study on CRC and cancer-free control individuals to evaluate the role of gene-level DRG defects in CRC susceptibility, as well as perform complementary somatic analyses of candidate genes. We hypothesized that germline mutations in DRGs that have been previously linked to other Mendelian forms of inherited cancer predisposition account for a significant fraction of the missing CRC heritability. To investigate this hypothesis, we studied germline whole-exome sequencing data in a large discovery set of CRC individuals who were not preselected for early-onset disease or positive family history and subsequently validated our findings in an independent large validation set of similarly unselected CRC individuals. For CRC individuals who had disruptive germline mutations in genes related to homologous recombination (HR), we also examined somatic tumor DNA for biallelic inactivation so as to explore whether such CRCs might theoretically be treated by agents that target deficient double-strand DNA repair (e.g., poly-ADP ribose polymerase inhibitor [PARPi]).
Material and Methods
Study Subjects
Discovery Set
Two independent cohorts that included a total of 680 CRC persons were examined in the discovery phase (Figure S1). Of these, 591 CRC persons came from the population-based Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) cohorts.13 Only individuals with available self-reported ancestry information were included in this case series. CRC individuals from the NHS and HPFS were not selected on the basis of their age of presentation, stage of their disease, or presence of a positive family history of CRC or other cancers.13 In addition, 89 CRC persons from the CanSeq study at Dana-Farber Cancer Institute (DFCI) were included in the discovery set.14 The CanSeq study is a single-arm prospective study that aims to evaluate the clinical utility of using paired (tumor and normal) whole-exome sequencing in the clinical care of individuals with advanced cancer without pre-selection for early age at diagnosis or high-risk family histories (these individuals are hereafter referred to as unselected individuals).15 Both studies were approved by the Partners Human Research Committee institutional review board (IRB) (Brigham and Women’s Hospital IRB no. 2001-P-001945 for NHS and HPFS; DFCI IRB no. 12-078 for CanSeq), and informed consent was obtained from all subjects.
Validation Set
Germline data of 1,661 individuals from two independent cohorts of unselected CRC individuals—The Cancer Genome Atlas (TCGA; n = 603) and the cohort reported by Yurgelun et al. (n = 1,058)—were used for validating the main findings detected in the discovery phase (hereafter called the validation set).16, 17 Neither cohort was selected for early-onset disease or positive family history. We used similar variant-calling and pathogenicity-assessment pipelines to evaluate germline variants in both cohorts.
Early-Onset-CRC Set
For further delineation of the penetrance of DRGs with significant enrichment of germline mutations in the discovery and validation sets in CRC individuals, the enrichment of germline mutations was evaluated in 1,456 individuals with early-onset CRC (age < 56 years). These individuals were part of two large CRC studies.10, 18 In total, our study evaluated relevant germline sequencing data of 3,797 CRC individuals and cancer-free adult control individuals (Figure S1).
Sequencing and Bioinformatics Analysis
Germline DNA from the CRC subjects in the discovery set was obtained from whole blood or adjacent normal colon tissue that was dissected after pathology review. DNA was extracted from formalin-fixed, paraffin-embedded (FFPE) blocks according to commonly used practices.19 All germline variants in the validation and early-onset-CRC sets were detected from whole blood. Production pipelines of the germline variants of these cohorts are described in Table S1 and elsewhere.10, 13, 16, 17, 18 Partial- or whole-gene deletions were not evaluated in this study.
Selection of DRGs and Gene Sets
Only genes that have been clearly associated with a Mendelian cancer-predisposition syndrome in humans were examined. A total of 14 well-known CRC-risk genes, as well as 40 DRGs that have been associated with cancer phenotypes other than CRC, were evaluated (Tables S2 and S3). Some of these DRGs, such as BLM (MIM: 210900) and NTHL1 (MIM: 602656), have been recently linked to CRC susceptibility; however, so far these observations have not been independently validated, so these genes were included in the DRG set to be evaluated here. Analysis of the germline variants in POLE and POLD1 was restricted to the known pathogenic missense mutations in the exonuclease domain of the protein.
Of the examined DRGs, 14 play an important part in the HR pathway: ATM, BARD1 (MIM: 601593), BLM, BRCA1, BRCA2, BRIP1 (MIM: 605882), MRE11 (MIM: 600814), NBN (MIM: 602667), PALB2, RAD51 (MIM: 179617), RAD51C (MIM: 602774), RAD51D (MIM: 602954), RAD54L (MIM: 603615), and XRCC3 (MIM: 600675).20 “Actionable DRGs” were defined as established cancer-predisposition genes that confer a 3-fold or higher increase in the risk of cancer phenotypes other than CRC and for which enhanced screening and family genetic testing are recommended. Out of the examined DRGs, ATM, BRCA1, BRCA2, BRIP1, PALB2, RAD51C, and RAD51D were considered clinically actionable.21, 22, 23, 24, 25
Variant Interpretation
An identical workflow for variant inclusion and pathogenicity assessment was used for evaluating the germline variants in both CRC and control individuals (Table S1). The clinically oriented American College of Medical Genetics and Genomics guidelines for assessing germline variants were used for evaluating germline variants in CRC and control individuals. On the basis of the available evidence, germline variants were classified into five categories: benign, likely benign, variants of unknown significance, likely pathogenic, and pathogenic.26 Only germline variants that had sufficient evidence of pathogenicity to be classified as pathogenic or likely pathogenic variants (hereafter collectively referred to as pathogenic mutations) were included. All variants of unknown significance were excluded from all analyses.
Frequency of Mutations in the General Population
Annotated germline variants in the examined genes in 53,105 cancer-free adults from the Exome Aggregation Consortium (ExAC) Browser (release 0.3.1 on March 16, 2016), excluding the TCGA cohort, were also evaluated with an identical workflow to the one used for CRC individuals.27 Frequencies of germline pathogenic mutations in the genes of interest were calculated for each of the continental populations in the ExAC Browser. Gene-mutation frequencies for the ExAC non-Finnish European (n = 27,173) and African and African American (n = 4,533) cohorts were then used for calculating the predicted pathogenic gene-mutation frequency in an ancestry-matched control cohort of 27,728 individuals (27,173 non-Finnish Europeans [98%] and 555 African Americans [2%]) (Figure S2).28 Population-specific common-variant frequencies were similar in CRC and control individuals, decreasing the likelihood of a significant population structure (Figure S3). Ancestry information for some individuals in the validation set was not readily available. Because the majority of the CRC individuals included in these studies are expected to have European ancestry, non-Finnish European individuals from the ExAC cohort (ExAC_NFE; n = 27,173) were used as a control group.
Tumor Loss of Heterozygosity (LOH) Analysis
MuTect was applied to identify somatic single-nucleotide variants.29 Strelka was used to detect small insertions and deletions. Individual sites were reviewed with the Integrated Genomics Viewer.30 Artifacts from DNA oxidation during sequencing were removed with a filter-based method.31, 32 Annotation of identified variants was performed with Oncotator.33 Probability distributions of possible cancer cell fractions (CCFs) of mutations were calculated with ABSOLUTE on the basis of local copy number and the estimated sample purity.34
Statistical Analysis
A logistic regression model was used for examining the clinical characteristics of CRC individuals with germline pathogenic mutations. Two-sided Fisher’s exact tests were used for calculating the odds ratios (ORs) and confidence intervals (CIs) (with “minimum-likelihood correction”) for the enrichment of germline pathogenic mutations in each of the examined DRGs. In addition, an exact binomial test of proportions was used for calculating the p value for the measured enrichment of each gene in CRC individuals versus in the reference population. Consistent with established statistical methods for two-stage association studies, we implemented a permissive first discovery-stage analysis where genes with p values smaller than 0.05 were considered significant. Before performing secondary analyses, we tested these top candidate genes in a subsequent validation phase in an independent cohort with appropriate Bonferroni correction.35, 36, 37
Results
Cohort Characteristics and Sequencing Metrics of CRC Cohorts
Demographic characteristics of all 680 CRC individuals from the discovery cohort are summarized in Table 1 and Table S4. The average target coverage for germline whole-exome sequencing (WES) for the discovery set was 71.69× (NHS and HPFS) and 137.11× (CanSeq). DRGs, in which significant enrichment of germline pathogenic mutations was seen in the discovery set, were subsequently examined in 1,661 unselected CRC individuals and 1,456 individuals with early-onset CRC (Material and Methods).10, 16 Examined DRGs had an average coverage of 58.67× in the ExAC cohort (Figure S4 and Table S5).
Table 1.
Clinical, Pathological, and Molecular Characteristics of 680 CRC Individuals Who Were Examined in the Discovery Set
| Characteristica | All Individuals (n = 680) |
Mutations in Known CRC-Susceptibility Genes (High Penetrance)b,c |
pd |
Mutations in Known CRC-Susceptibility Genes (Low Penetrance)c,e |
pd |
Mutations in DNA-Repair Genesc,f |
pd |
Mutations in the Homologous Recombination Pathwayc,g |
pd |
Mutations in ATMc |
pd |
Mutations in PALB2c |
pd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent (n = 616) | Present (n = 12) | Absent (n = 616) | Present (n = 19) | Absent (n = 616) | Present (n = 33) | Absent (n = 616) | Present (n = 19) | Absent (n = 616) | Present (n = 5) | Absent (n = 616) | Present (n = 3) | |||||||||
| Sex | female | 414 (61%) | 376 (61%) | 7 (58%) | 0.99 | 376 (61%) | 9 (47%) | 0.24 | 376 (61%) | 22 (67%) | 0.59 | 376 (61%) | 11 (58%) | 0.81 | 376 (61%) | 4 (80%) | 0.65 | 376 (61%) | 1 (33%) | 0.56 |
| male | 266 (39%) | 240 (39%) | 5 (42%) | 240 (39%) | 10 (53%) | 240 (39%) | 11 (33%) | 240 (39%) | 8 (42%) | 240 (39%) | 1 (20%) | 240 (39%) | 2 (67%) | |||||||
| Age of presentation | mean ± SD (years) | 68.8 ± 10.3 | 68.9 ± 10.2 | 58.4 ± 13.8 | 0.0005 | 68.9 ± 10.2 | 72.2 ± 6.2 | 0.16 | 68.9 ± 10.2 | 69.7 ± 10.8 | 0.66 | 68.9 ± 10.2 | 68.2 ± 10.4 | 0.77 | 68.9 ± 10.2 | 75.6 ± 6.6 | 0.14 | 68.9 ± 10.2 | 64.7 ± 18.8 | 0.47 |
| missing | 12 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | |||||||
| Race or ethnicity | white | 667 (98%) | 604 (98%) | 12 (100%) | 0.99 | 604 (98%) | 19 (100%) | 0.99 | 604 (98%) | 32 (97%) | 0.50 | 604 (98%) | 18 (95%) | 0.33 | 604 (98%) | 4 (80%) | 0.10 | 604 (98%) | 3 (100%) | 0.99 |
| black | 13 (1.9%) | 12 (2.0%) | 0 | 12 (2.0%) | 0 | 12 (2.0%) | 1 (3.0%) | 12 (2.0%) | 1 (5.3%) | 12 (2.0%) | 1 (20%) | 12 (2.0%) | 0 | |||||||
| Ashkenazi Jewish | no | 155 (86%) | 144 (88%) | 2 (100%) | 0.99 | 144 (88%) | 3 (38%) | 0.0015 | 144 (88%) | 6 (86%) | 0.59 | 144 (88%) | 4 (100%) | 0.99 | 144 (88%) | 1 (100%) | 0.99 | 144 (88%) | 1 (100%) | 0.99 |
| yes | 25 (14%) | 19 (12%) | 0 | 19 (12%) | 5 (62%) | 19 (12%) | 1 (14%) | 19 (12%) | 0 | 19 (12%) | 0 | 19 (12%) | 0 | |||||||
| missing | 500 | 453 | 10 | 453 | 11 | 453 | 26 | 453 | 15 | 453 | 4 | 453 | 2 | |||||||
| Family history of colorectal cancer in first-degree relative(s) | absent | 501 (75%) | 461 (76%) | 8 (73%) | 0.73 | 461 (76%) | 11 (61%) | 0.16 | 461 (76%) | 21 (64%) | 0.099 | 461 (76%) | 12 (63%) | 0.18 | 461 (76%) | 3 (60%) | 0.34 | 461 (76%) | 2 (67%) | 0.55 |
| present | 164 (25%) | 142 (24%) | 3 (27%) | 142 (24%) | 7 (39%) | 142 (24%) | 12 (36%) | 142 (24%) | 7 (37%) | 142 (24%) | 2 (40%) | 142 (24%) | 1 (33%) | |||||||
| missing | 15 | 13 | 1 | 13 | 1 | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 | |||||||
| Family history of breast cancer in first-degree relative(s) | absent | 359 (81%) | 329 (82%) | 4 (50%) | 0.044 | 329 (82%) | 9 (90%) | 0.99 | 329 (82%) | 17 (71%) | 0.18 | 329 (82%) | 9 (69%) | 0.27 | 329 (82%) | 3 (75%) | 0.55 | 329 (82%) | 2 (100%) | 0.99 |
| present | 85 (19%) | 73 (18%) | 4 (50%) | 73 (18%) | 1 (10%) | 73 (18%) | 7 (29%) | 73 (18%) | 4 (31%) | 73 (18%) | 1 (25%) | 73 (18%) | 0 | |||||||
| missing | 236 | 214 | 4 | 214 | 9 | 214 | 9 | 214 | 6 | 214 | 1 | 214 | 1 | |||||||
| Family history of ovarian cancer in first-degree relative(s) | absent | 425 (96%) | 384 (96%) | 8 (100%) | 0.99 | 384 (96%) | 10 (100%) | 0.99 | 384 (96%) | 23 (96%) | 0.99 | 384 (96%) | 13 (100%) | 0.99 | 384 (96%) | 4 (100%) | 0.99 | 384 (96%) | 2 (100%) | 0.99 |
| present | 19 (4.3%) | 18 (4.5%) | 0 | 18 (4.5%) | 0 | 18 (4.5%) | 1 (4.2%) | 18 (4.5%) | 0 | 18 (4.5%) | 0 | 18 (4.5%) | 0 | |||||||
| missing | 236 | 214 | 4 | 214 | 9 | 214 | 9 | 214 | 6 | 214 | 1 | 214 | 1 | |||||||
| Family history of any cancer in first-degree relative(s) | absent | 270 (41%) | 249 (41%) | 3 (27%) | 0.54 | 249 (41%) | 6 (33%) | 0.63 | 249 (41%) | 12 (36%) | 0.72 | 249 (41%) | 7 (37%) | 0.81 | 249 (41%) | 2 (40%) | 0.99 | 249 (41%) | 2 (67%) | 0.57 |
| present | 395 (59%) | 354 (59%) | 8 (73%) | 354 (59%) | 12 (67%) | 354 (59%) | 21 (64%) | 354 (59%) | 12 (63%) | 354 (59%) | 3 (60%) | 354 (59%) | 1 (33%) | |||||||
| missing | 15 | 13 | 1 | 13 | 1 | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 | |||||||
| Tumor locationh | cecum | 129 (19%) | 117 (19%) | 1 (9.1%) | 0.48 | 117 (19%) | 1 (5.6%) | 0.34 | 117 (19%) | 10 (30%) | 0.31 | 117 (19%) | 7 (37%) | 0.18 | 117 (19%) | 2 (40%) | 0.31 | 117 (19%) | 1 (33%) | 0.062 |
| ascending to transverse colon | 202 (30%) | 184 (30%) | 2 (18%) | 184 (30%) | 5 (28%) | 184 (30%) | 11 (33%) | 184 (30%) | 4 (21%) | 184 (30%) | 2 (40%) | 184 (30%) | 0 | |||||||
| splenic flexure to sigmoid colon | 201 (30%) | 183 (30%) | 6 (55%) | 183 (30%) | 6 (33%) | 183 (30%) | 6 (18%) | 183 (30%) | 3 (16%) | 183 (30%) | 0 | 183 (30%) | 0 | |||||||
| rectum | 136 (20%) | 122 (20%) | 2 (18%) | 122 (20%) | 6 (33%) | 122 (20%) | 6 (18%) | 122 (20%) | 5 (26%) | 122 (20%) | 1 (20%) | 122 (20%) | 2 (67%) | |||||||
| missing | 12 | 10 | 1 | 10 | 1 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | |||||||
| Tumor differentiation | well to moderate | 534 (90%) | 483 (90%) | 6 (67%) | 0.062 | 483 (90%) | 17 (100%) | 0.40 | 483 (90%) | 28 (90%) | 0.99 | 483 (90%) | 17 (94%) | 0.99 | 483 (90%) | 5 (100%) | 0.99 | 483 (90%) | 1 (50%) | 0.20 |
| poor | 62 (10%) | 56 (10%) | 3 (33%) | 56 (10%) | 0 | 56 (10%) | 3 (9.7%) | 56 (10%) | 1 (5.6%) | 56 (10%) | 0 | 56 (10%) | 1 (50%) | |||||||
| missing | 84 | 77 | 3 | 77 | 2 | 77 | 2 | 77 | 1 | 77 | 0 | 77 | 1 | |||||||
| AJCC disease stage | I | 148 (24%) | 135 (24%) | 4 (40%) | 0.35 | 135 (24%) | 1 (5.9%) | 0.020 | 135 (24%) | 8 (25%) | 0.74 | 135 (24%) | 3 (16%) | 0.40 | 135 (24%) | 0 | 0.051 | 135 (24%) | 0 | 0.88 |
| II | 188 (30%) | 171 (30%) | 1 (10%) | 171 (30%) | 8 (47%) | 171 (30%) | 8 (25%) | 171 (30%) | 4 (21%) | 171 (30%) | 0 | 171 (30%) | 1 (33%) | |||||||
| III | 177 (28%) | 157 (28%) | 4 (40%) | 157 (28%) | 8 (47%) | 157 (28%) | 8 (25%) | 157 (28%) | 6 (32%) | 157 (28%) | 3 (60%) | 157 (28%) | 1 (33%) | |||||||
| IV | 110 (18%) | 101 (18%) | 1 (10%) | 101 (18%) | 0 | 101 (18%) | 8 (25%) | 101 (18%) | 6 (32%) | 101 (18%) | 2 (40%) | 101 (18%) | 1 (33%) | |||||||
| missing | 57 | 52 | 2 | 52 | 2 | 52 | 1 | 52 | 0 | 52 | 0 | 52 | 0 | |||||||
| MSI status | MSS or MSI-low | 475 (84%) | 428 (84%) | 5 (62%) | 0.13 | 428 (84%) | 16 (89%) | 0.75 | 428 (84%) | 26 (87%) | 0.80 | 428 (84%) | 16 (94%) | 0.50 | 428 (84%) | 5 (100%) | 0.99 | 428 (84%) | 2 (100%) | 0.99 |
| MSI-high | 92 (16%) | 83 (16%) | 3 (38%) | 83 (16%) | 2 (11%) | 83 (16%) | 4 (13%) | 83 (16%) | 1 (5.9%) | 83 (16%) | 0 | 83 (16%) | 0 | |||||||
| missing | 113 | 105 | 4 | 105 | 1 | 105 | 3 | 105 | 2 | 105 | 0 | 105 | 1 | |||||||
| CIMP status | CIMP-low or -negative | 382 (80%) | 342 (79%) | 5 (83%) | 0.99 | 342 (79%) | 13 (87%) | 0.75 | 342 (79%) | 22 (88%) | 0.44 | 342 (79%) | 12 (86%) | 0.74 | 342 (79%) | 4 (100%) | 0.59 | 342 (79%) | 1 (100%) | 0.99 |
| CIMP-high | 95 (20%) | 89 (21%) | 1 (17%) | 89 (21%) | 2 (13%) | 89 (21%) | 3 (12%) | 89 (21%) | 2 (14%) | 89 (21%) | 0 | 89 (21%) | 0 | |||||||
| missing | 203 | 185 | 6 | 185 | 4 | 185 | 8 | 185 | 5 | 185 | 1 | 185 | 2 | |||||||
Abbreviations are as follows: AJCC, American Joint Committee on Cancer; CIMP, CpG island methylator phenotype-specific promoter; MSI, microsatellite instability; MSS, microsatellite stable; and SD, standard deviation.
Percentage indicates the proportion of individuals with a specific clinical, pathological, or molecular characteristic in all cases or in strata of germline pathogenic mutations.
High-penetrance CRC-risk genes include APC (excluding c.3920T>A [p.Ile1307Lys]), BMPR1A, CHEK2, MLH1, MSH2, MSH6, MUTYH (biallelic inactivation), PMS2, POLD1, POLE, PTEN, SMAD4, STK11, and TP53.
Individuals who had mutations in the other CRC-risk genes or DNA-repair genes (DRGs) were excluded.
To compare characteristics between subgroups according to the germline mutation status, we used Fisher’s exact test for categorical variables and unpaired t tests for continuous variables.
Low-penetrance CRC-risk mutations include APC c.3920T>A (p.Ile1307Lys) and monoallelic inactivation of MUTYH.
This gene set includes the 40 DRGs listed in Table S2.
Homologous recombination (HR) DRGs included in this analysis are ATM, BARD1, BLM, BRCA1, BRCA2, BRIP1, MRE11, NBN, PALB2, RAD51, RAD51C, RAD51D, RAD54L, and XRCC3.
One individual who had two lesions (cecum and sigmoid colon) was excluded from the analysis.
Germline Pathogenic Mutations in Known CRC-Risk Genes
In the discovery set (n = 680), 31 (4.56%) individuals had germline CRC-risk mutations. Of these, 12 (1.76%) harbored highly or moderately penetrant germline pathogenic mutations in APC (n = 2), CHEK2 (n = 4), MSH2 (n = 1), MSH6 (n = 1), PMS2 (n = 2), and TP53 (n = 2) (Figure 1A, Figure S5, and Table S6). In addition, 19 (2.79%) individuals carried heterozygous germline pathogenic mutations in MUTYH (n = 11 [1.62%]) or the Ashkenazi founder low-penetrance variant c.3920T>A (p.Ile1307Lys) in APC (n = 8 [1.18%]). Of 1,661 unselected CRC individuals in the validation set, 93 (5.6%) individuals had at least one germline mutation in the CRC-susceptibility genes (Figure 1A and Tables S7 and S8). The frequency of germline mutations in the mismatch-repair genes (MLH1, MSH2, MSH6, and PMS2) in the discovery CRC set (four individuals [0.6%]) was considerably lower than the frequency of these gene mutations in other studies.16 This underrepresentation of individuals with Lynch syndrome in our discovery cohort could be attributed to the population-based nature of the NHS and HPFS cohorts as well as the fact that these studies only enrolled cancer-free subjects, sometimes at a more advanced age.
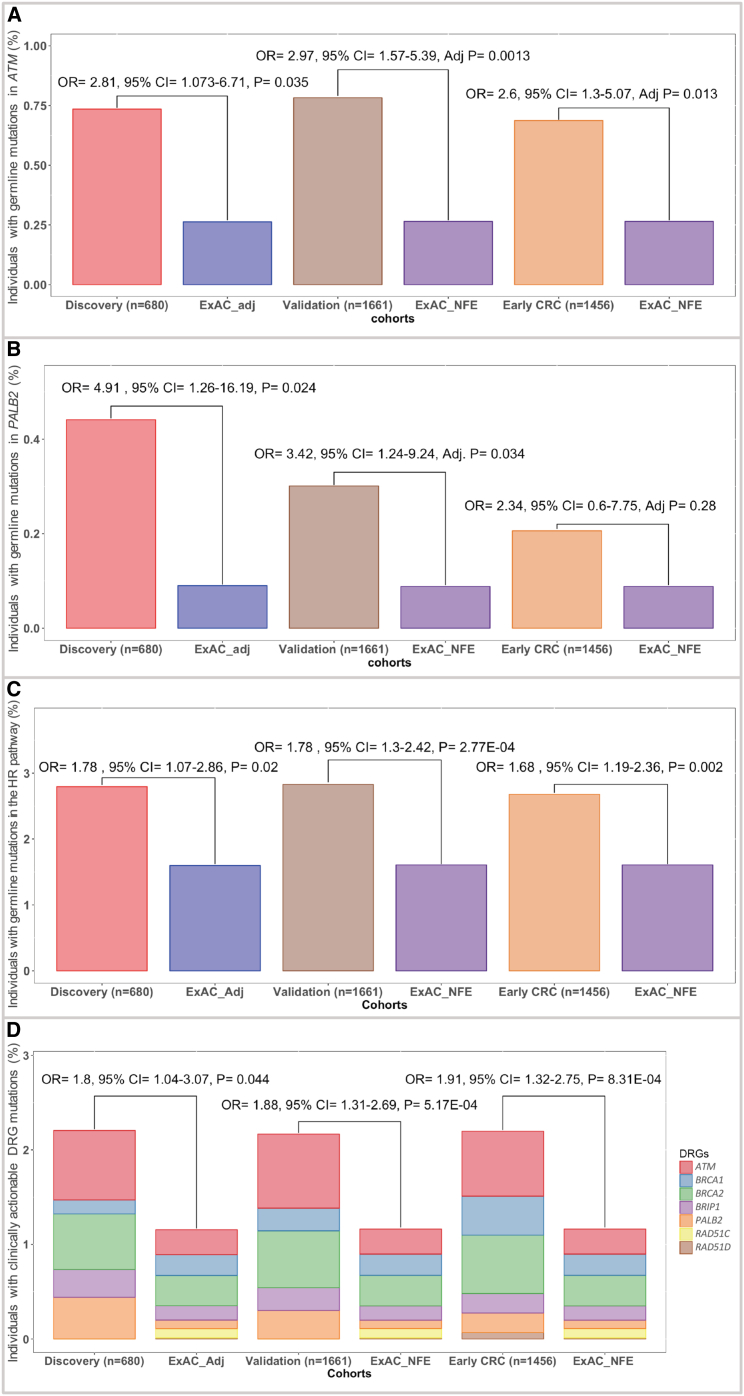
Figure 1.
Germline Pathogenic Mutations in the Known CRC-Predisposition Genes and Additional DRGs
(A) Proportions of individuals with germline pathogenic mutations in the CRC-risk genes in 680 CRC individuals in the discovery set and 1,661 CRC individuals in the validation set.
(B) Number and class of the detected germline pathogenic mutations in the DRGs in the discovery set (n = 680). DRGs where no mutations were detected (n = 19) are not shown here.
(C) Enrichment of germline pathogenic DRG mutations in 680 CRC individuals in the discovery set. Fisher’s exact test was used to calculate the ORs and 95% CIs. A two-sided binomial test was used to calculate the p values.
(D) A total of 18 germline pathogenic ATM mutations were seen in the discovery and validation sets in our study. This included seven (38.9%) nonsense mutations, six (33.3%) frameshift mutations, three (16.6%) splice-site mutations, one (5.6%) known pathogenic in-frame deletion, and one (5.6%) known pathogenic missense mutation.
Germline Pathogenic Mutations in Additional DRGs
Next, germline variants in 40 DRGs in the discovery CRC set (n = 680) were evaluated for pathogenicity. 33 (4.85%) subjects had at least one germline pathogenic mutation in 21 of these DRGs (Figure 1B). Four (0.59%) individuals had two germline pathogenic mutations, each in different DRGs (Table S9). No individuals had germline pathogenic mutations in both sets of known CRC-risk genes and the additional DRGs. Enrichment analysis of the discovery CRC set, relative to cancer-free individuals, showed significant enrichment of germline pathogenic mutations in ATM and PALB2 (Figure 1C and Table 2).
Table 2.
Prevalence of Germline Pathogenic Mutations in 680 CRC Individuals (Discovery Set) and 27,728 Ancestry-Matched Cancer-free Adults from the ExAC Cohort
| Gene | Individuals with Mutations in the Discovery Cohort (n = 680) | Prevalence of Individuals with Mutations in the Discovery Cohort | Individuals with Mutations in Ancestry-Matched Control Group (n = 27,728) | Prevalence of Mutations in the Control Group | Enrichment of Pathogenic Mutations in the Discovery Cohort (Odds Ratio; Fisher’s Exact Test) | 95% Confidence Intervals (Fisher’s Exact Test) | p Value (Two-Sided Exact Binomial Test) |
|---|---|---|---|---|---|---|---|
| ATM | 5 | 0.74% | 73 | 0.26% | 2.81 | 1.07–6.71 | 0.035 |
| BAP1 | 1 | 0.15% | 10 | 0.04% | 4.08 | 0.19–27.85 | 0.218 |
| BARD1 | 1 | 0.15% | 13 | 0.05% | 3.14 | 0.15–19.04 | 0.273 |
| BLM | 3 | 0.44% | 40 | 0.14% | 3.07 | 0.8–9.28 | 0.077 |
| BRCA1 | 1 | 0.15% | 61 | 0.22% | 0.67 | 0.03–3.86 | 1 |
| BRCA2 | 4 | 0.59% | 89 | 0.32% | 1.84 | 0.61–4.89 | 0.177 |
| BRIP1 | 2 | 0.29% | 42 | 0.15% | 1.94 | 0.33–7.57 | 0.275 |
| ERCC2 | 2 | 0.29% | 40 | 0.14% | 2.04 | 0.35–8.00 | 0.25 |
| ERCC3 | 1 | 0.15% | 80 | 0.29% | 0.51 | 0.03–2.89 | 1 |
| ERCC4 | 1 | 0.15% | 16 | 0.06% | 2.55 | 0.12–16.55 | 0.325 |
| FANCC | 1 | 0.15% | 48 | 0.17% | 0.85 | 0.04–5.0 | 1 |
| FANCE | 1 | 0.15% | 5 | 0.02% | 8.16 | 0.35–58.6 | 0.115 |
| FANCL | 1 | 0.15% | 10 | 0.04% | 4.08 | 0.19–27.85 | 0.218 |
| GEN1 | 2 | 0.29% | 18 | 0.06% | 4.54 | 0.75–19.35 | 0.073 |
| MRE11 | 2 | 0.29% | 18 | 0.06% | 4.54 | 0.75–19.35 | 0.073 |
| PALB2 | 3 | 0.44% | 25 | 0.09% | 4.91 | 1.26–16.19 | 0.024 |
| POLH | 1 | 0.15% | 7 | 0.03% | 5.83 | 0.26–40.98 | 0.158 |
| RECQL4 | 2 | 0.29% | 50 | 0.18% | 1.63 | 0.28–6.23 | 0.347 |
| SLX4 | 1 | 0.15% | 23 | 0.08% | 1.77 | 0.09–10.49 | 0.431 |
| XPA | 1 | 0.15% | 19 | 0.07% | 2.15 | 0.1–13.26 | 0.373 |
| XRCC3 | 1 | 0.15% | 6 | 0.02% | 6.8 | 0.3–50.67 | 0.137 |
Only genes with detected germline pathogenic mutations in affected individuals are shown.
Germline Pathogenic Mutations in ATM
Among 680 unselected CRC individuals, five (0.74%) had mutations in ATM. Germline mutations in ATM were significantly more prevalent in the CRC discovery set than in cancer-free individuals (OR = 2.81; 95% CI = 1.07–6.71; p = 0.035) (Table S9). The frequency of ATM germline pathogenic mutations in the CanSeq cohort was not significantly higher than that in the NHS/HPFS cohort (p = 0.5) (Figure S6). Analysis of ATM mutation frequency in another 1,661 unselected CRC individuals from the validation set also identified significant enrichment of ATM germline pathogenic mutations (13 individuals [0.78%]; OR = 2.97; 95% CI = 1.57–5.39; adjusted p = 0.0013) (Figures 1D and 2A and Tables S10 and S11). Evaluation of an independent cohort of 1,456 individuals with early-onset CRC similarly showed significant enrichment of germline ATM mutations in these individuals (ten individuals [0.69%]; OR = 2.6; 95% CI = 1.3–5.07; adjusted p = 0.013) (Figure 2A).
Figure 2.
Enrichment of DRG Mutations in Various Cohorts
(A) Inherited pathogenic germline mutations in ATM were more commonly seen in individuals with CRC in the discovery (n = 680), validation (n = 1,661), and early-onset-CRC (1,456) sets than in cancer-free individuals.
(B) Germline pathogenic mutations in PALB2 were significantly enriched in unselected CRC individuals from the discovery and validation sets. However, no significant enrichment was seen in the individuals with early-onset CRC.
(C) A secondary analysis of the HR pathway showed significant enrichment of germline HR gene mutations, as an aggregate, in all CRC cohorts.
(D) Individuals with CRC were also almost twice as likely to carry a clinically actionable mutation for which screening recommendations exist, which can greatly affect the clinical care offered to these individuals and their families.
Although most of the individuals included in our study were of European ancestry, self-reported ancestry information, as previously shown, can be inaccurate.38 To evaluate for spurious ATM mutation enrichment that could have resulted from inadequate population stratification, we next blinded the ancestry data of the CRC subjects from the validation cohort and examined ATM mutation enrichment in relation to that in cancer-free control individuals from various continental populations in the ExAC Browser. Our analysis showed that regardless of the selected control population, rates of germline ATM mutations were significantly higher in the CRC validation set (n = 1,661; OR = 2.4–6.5; adjusted p < 0.05 for all pairwise comparisons; binomial exact test with Bonferroni correction for six independent tests) (Figure S7).
Germline Pathogenic Mutations in PALB2
Three individuals in our discovery cohort were found to have germline PALB2 mutations, which represents a significant enrichment over the frequency of these mutations in cancer-free control individuals (0.44%; OR = 4.91; 95% CI = 1.26–16.19; p = 0.024) (Table S9). This enrichment was also evident in 1,661 unselected CRC individuals from the validation cohort (five individuals [0.3%]; OR = 3.42; 95% CI = 1.24–9.24; adjusted p = 0.034) (Figure 2B and Tables S10 and S11). Interestingly, no significant enrichment of germline PALB2 mutations was seen in 1,456 individuals with early-onset CRC (three individuals [0.2%]; OR 2.34; 95% CI = 0.6–7.75; adjusted p = 0.28), suggesting late-onset penetrance of PALB2 mutations in CRC individuals.
Somatic LOH
Matched-tumor WES for most of the individuals with germline mutations in the discovery set (n = 64) were available and examined for somatic LOH (Table S12). Among the CRC-risk genes, somatic inactivation of the wild-type allele was seen in APC (eight individuals [80%]), CHEK2 (one individual [25%]), ERCC2 (two individuals [100%]), MSH2 (one individual [100%]), MSH6 (one individual [100%]), MUTYH (two individuals [18%]), PMS2 (two individuals [100%]), and TP53 (two individuals [100%]). Out of the examined DRGs, all individuals with germline pathogenic mutations in ATM (five [100%]) had evidence of somatic inactivation of the wild-type allele in the matched-tumor samples (Figure S8). The somatic inactivation of the ATM wild-type allele in all tumors with germline ATM events provides compelling evidence for ATM to be etiologic for the development of CRC in these individuals. No somatic LOH was detected in any of the tumors in individuals with germline PALB2 mutations, although disruptive non-coding genetic and epigenetic events are not captured by tumor WES.
Germline Pathogenic Mutations in the HR Pathway
Given the mutations specifically observed in HR genes (ATM and PALB2), we next examined the frequency of inherited mutations affecting any of the HR cancer-predisposition genes (Material and Methods). Unselected CRC individuals in the discovery set had a higher rate of germline pathogenic mutations in the HR genes than cancer-free individuals (19 individuals [2.8%]; OR = 1.78; 95% CI = 1.07–2.86; p = 0.02) (Table S9). Evaluation of the validation and early-onset-CRC sets also showed that CRC individuals were more likely to have inherited HR mutations (validation set: 47 individuals [2.8%]; OR = 1.78; 95% CI = 1.30–2.42; p = 2.77E−4; early-onset set: 39 individuals [2.68%]; OR = 1.68; 95% CI = 1.19–2.36; p = 0.002) (Figure 2C and Tables S10 and S11). This effect did not seem to be purely driven by ATM and PALB2 mutations, given that when they were excluded, there was a trend (which did not reach statistical significance) whereby germline disruptive events in other HR genes were more prevalent in the CRC validation set than in cancer-free adults (OR = 1.4; 95% CI = 0.95–2.06; p = 0.077) (Figure S9).
Clinical Actionability and Risk of Other Cancers in CRC Individuals
Analysis of mutations in actionable DRGs (ATM, BRCA1, BRCA2, BRIP1, PALB2, RAD51C, and RAD51D) in the discovery set identified a total of 15 germline pathogenic mutations in 14 (2.1%) CRC individuals. One person had two actionable mutations in BRCA2 and PALB2. Compared with mutations in cancer-free individuals, actionable cancer-risk mutations were approximately twice as prevalent in CRC individuals from the discovery set (OR = 1.8; 95% CI = 1.04–3.07; p = 0.044), the validation set (36 individuals [2.17%]; OR = 1.88; 95% CI = 1.31–2.69; p = 5.17E−4), and the early-onset-CRC set (32 individuals [2.2%]; OR = 1.91; 95% CI = 1.32–2.75; p = 8.31E−4) (Figure 2D).
Utility of Testing Relevant DRGs in CRC
Collectively, CRC heritability in up to about 1.2% of unselected CRC individuals can be explained by higher rates of mutations in ATM and PALB2. To explore the potential impact of performing germline testing of ATM and PALB2 on diagnostic yield, we examined the CRC-specific germline panels offered by eight of the largest commercial laboratories in the US (as of September 2017). Our evaluation showed that these CRC-specific gene panels, which typically evaluate known CRC risk genes, only occasionally evaluate germline mutations in ATM, whereas PALB2 and other actionable DRGs are not captured by these clinical tests (Figure S10).
Clinical Characteristic of Mutation Carriers in the Discovery Set
Overall, there were no significant differences in clinical characteristics between DRG mutant and non-mutant CRC individuals (Table 1). Although CRC individuals with high-penetrance germline CRC-risk mutations presented 10.5 years earlier on average than mutation-negative individuals (p = 0.0005), CRC individuals with germline pathogenic mutations in ATM, PALB2, the HR genes, or DRGs were not more likely to present earlier that mutation-negative persons. All five germline ATM mutation carriers presented with stage III or IV disease (in comparison with 46% of mutation-negative CRC individuals; p = 0.051) (Figure 3). Individuals with germline pathogenic mutations in CRC-risk genes, the DRGs, ATM, or PALB2 were not more likely to report a first-degree family member with CRC or another type of cancer (Figure S11). Interestingly, individuals carrying high-penetrance CRC-risk mutations were more likely to report a positive family history of breast cancer.
Figure 3.
Clinical and Molecular Characteristics of All Individuals with Germline Pathogenic Mutations in CRC-Risk Genes and DRGs in the CRC Discovery Set
All individuals with germline pathogenic mutations in ATM had somatic LOH in their tumor samples. Two of these individuals had large deletions that affected the wild-type ATM allele, whereas three had truncating point mutations leading to the loss of the ATM wild-type allele as well. Abbreviations are as follows: AC-TC, ascending colon to transverse colon; SF-SC, splenic flexure to sigmoid colon; MSI, microsatellite instability; MSS, microsatellite stable; CIMP, CpG island methylator phenotype-specific promoter; and LOH, loss of heterozygosity.
Discussion
Most of CRC heritability is still incompletely characterized. Mutations of several cancer-predisposition DRGs that are not typically associated with CRC have been recently reported in individuals with CRC; however, the clinical significance of these results has not been firmly established. Here, we present a systematic analysis of DRG mutations in large independent CRC cohorts and a cancer-free cohort to evaluate novel observations in known CRC-susceptibility genes and to identify new CRC-susceptibility genes.
We found that a gene-level analysis of DRGs revealed significantly higher rates of ATM mutations in CRC individuals than in cancer-free control individuals, going beyond observational studies to implicate ATM as a CRC-susceptibility gene. ATM is a master regulating kinase that is activated in response to DNA damage. Heterozygous carriers of ATM mutations have been reported to have a higher risk of breast cancer (MIM: 114480) and potentially pancreatic cancer (MIM: 260350).11 A previous cohort-based study that evaluated the risk of various cancers in families of individuals with ataxia telangiectasia (MIM: 208900), which results from biallelic loss of ATM, showed no increased risk of CRC in the obligate carrier parents of these individuals. However, a secondary analysis in that study showed that, collectively, there was an increased risk of CRC when all the heterozygous ATM carrier relatives were evaluated (relative risk = 2.54; 95% CI = 1.06–6.09), although this association was not statistically significant once corrected for multiple-hypothesis testing.11 A larger subsequent study on ATM carriers also failed to detect any enrichment of CRC events in heterozygous ATM carriers.39 However, a recent genome-wide association study that evaluated three loss-of-function ATM variants in several cancer phenotypes showed a higher risk of CRC in ATM-mutation carriers (OR = 1.97; 95% CI = 1.20–3.23), although this study was underpowered for the CRC phenotype (corrected p = 0.18 for 25 tested cancer types).40 Given these underpowered and contradictory observations, the most recent National Comprehensive Cancer Network (NCCN) guidelines for genetic and familial CRC syndromes (version 2.2017; released on August 9, 2017) concluded that the evidence supporting ATM as a CRC-risk gene is deficient and that the risk of CRC in ATM mutation carriers is largely unknown.12 This is the first association study, to our knowledge, that confirmed and independently validated ATM as a moderately penetrant CRC-susceptibility gene that explains the increased risk of colorectal cancer in around 0.74% of all unselected CRC individuals. Furthermore, complete loss of ATM as a result of acquired deleterious somatic events suggests a critical role for ATM in the CRC tumorigenesis in individuals with inherited ATM haploinsufficiency.
Our analysis showed validated evidence supporting germline mutations in PALB2, in addition to those in ATM, as CRC-risk events. PALB2 plays a critical role in DNA HR by recruiting BRCA2 and RAD51 to DNA breaks to initiate DNA repair. Germline defects in PALB2 have been associated with breast and pancreatic cancers.25, 41 Although germline PALB2 mutations have been observed in several CRC cohorts, so far it has been unclear whether these events contribute to CRC risk or merely represent coincidental findings. So far, there has not been any study to evaluate the role of PALB2 mutations in CRC individuals; hence, PALB2 has not been part of the recent NCCN recommendations (version 2.2017) for germline testing in CRC.12 Our analysis showed evidence for higher-than-expected germline pathogenic PALB2 mutation rates in around 0.44% of unselected CRC individuals, although this effect was not observed in cohorts with early-onset CRC. Tumors in individuals with germline mutations in PALB2 did not show biallelic inactivation of the gene; however, our analysis was not designed to capture potential pathogenic non-coding variants or epigenetic gene-silencing events. Although ATM and PALB2 might only explain a small fraction of the CRC heritability in unselected individuals, this represents a 20% increase in the diagnostic yield once these two genes are included.
Both ATM and PALB2 are members of the HR pathway, which restores the integrity of double-strand DNA breaks.42 Inherited mutations in HR genes have long been known to increase the risk of several cancers, including breast, ovarian (MIM: 167000), prostate (MIM: 176807), and pancreatic cancers.23, 43, 44 Here, we showed evidence that germline pathogenic mutations in HR-pathway genes, in aggregate, confer a relative 60%–80% increase in the baseline risk of CRC. In addition, biallelic inactivation of HR genes, observed in CRC individuals with various germline HR gene mutations in this study (particularly carriers of ATM mutations), suggests new venues for exploring targeted therapeutic intervention in CRC individuals. Unlike cancers from mutation-negative individuals, breast, ovarian, and prostate cancers from individuals with germline mutations in canonical HR genes have been shown to have a substantial response to PARPi and platinum-based chemotherapy.45, 46, 47 Given that preclinical studies have shown substantial sensitivity of the HR- and ATM-deficient CRC cell lines to PARPi and that clinical trials to evaluate the efficacy of PARPi in CRC are underway (NCT: NCT00912743, NCT02305758, NCT01589419, and NCT02921256), universal screening of CRC individuals for germline HR mutations could provide very informative data that could expand treatment options for these individuals.48
The detection of mutations in actionable DRGs has significant ramifications for probands and their families. First, these mutations significantly increase the person’s risk of developing cancers other than CRC, several of which have effective screening options available. Furthermore, identifying such mutations in an individual represents a unique opportunity to screen other family members to identify asymptomatic at-risk individuals and implement early surveillance measures. In total, our study estimates that approximately 2.1% (95% CI = 1.1%–3.4%; binomial exact test) of all CRC individuals carry actionable mutations in genes that have not been previously associated with increased CRC risk; this percentage is significantly higher than the combined rate of these mutations in cancer-free control individuals. In addition, this small but significant subset of CRC individuals is, as a result of carrying these mutations, at a substantially higher risk of developing several cancers other than CRC. Importantly, these actionable genes are not part of the recommended germline testing for individuals with CRC.12
Offering clinical germline molecular testing to individuals with cancer for the evaluation of an inherited cancer-predisposition syndrome relies heavily on several factors, such as the individual’s age of presentation and the presence of a positive family history of cancer. Intriguingly, our analysis of large CRC cohorts showed that these factors might not reliably predict the likelihood of identifying a germline cancer-predisposition mutation in individuals with CRC. First, our study showed that, except for individuals with germline high-penetrance CRC-risk mutations, CRC individuals with low-penetrance CRC-risk mutations and those with germline mutations in ATM or PALB2 were not more likely to present at an earlier age than individuals with presumed sporadic CRC. In addition, our study showed that a positive family history of CRC was not more commonly reported in CRC individuals who carried high-penetrance CRC-risk mutations, low-penetrance CRC-risk mutations, or DRG mutations. This is consistent with prior similar observations in the prostate and pediatric cancer spaces.49, 50 These findings underscore the importance of considering the possibility that an inherited CRC-risk mutation is carried in individuals with late-onset CRC as well as in those without a strong family history of CRC. These observations are also relevant to the evaluation of the potential utility of implementing early CRC screening measures. However, larger studies are still needed to further delineate the penetrance of these germline mutations.
Our study has several limitations. Although we performed population stratification, our CRC and control individuals did not come from the same cohort, so enrichment of mutations secondary to non-CRC-related factors cannot be completely ruled out. Also, because the raw sequencing data of the control (ExAC) cohort are not publically available, we did not jointly call germline variants in CRC and control individuals to limit potential sequencing- or pipeline-related variant-calling biases. However, we mitigated this potential source of bias by using the same parameters, tools, and platforms that were used for analyzing the ExAC cohort. In addition, individual-level clinical information relevant to our control group as well as the validation sets was not available, which limited our ability to correct for potential confounders. However, evaluating several independent CRC cohorts makes it unlikely for a confounder to be shared across all cohorts. Finally, larger case-control studies are still necessary to confirm these clinically relevant findings and inform future updates to clinical germline-testing guidelines in CRC individuals.
Broadly, our study of large CRC cohorts showed enrichment of disruptive germline pathogenic mutations in the HR pathway, suggesting its important role in CRC susceptibility and management. In addition, we presented evidence to support ATM and PALB2 as CRC-susceptibility genes by explaining the missing CRC heritability in 1.2% of unselected CRC individuals. We also illustrated that a relatively large proportion of all CRC individuals have germline pathogenic mutations in HR genes, which could greatly affect their clinical care and inform molecularly driven treatment strategies for individuals with mutations in these genes. Finally, because these genes are not routinely tested clinically, these results could inform revisions to CRC testing guidelines.
Acknowledgments
We thank all individuals who participated in this study. Drs. Van Allen, Ogino, Garraway, and Fuchs had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was conducted with support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (award UL1 TR001102 from the NIH National Center for Research Resources and National Center for Advancing Translational Sciences), and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the NIH. This work was also supported by K08 CA188615-02 (E.M.V.); the Damon Runyon Clinical Investigator Award (E.M.V.); KL2 TR001100 (M.G.); R01CA169141-04 (C.S.F.); R01CA118553-07 (C.S.F.); P01 CA87969, UM1 CA186107, P01 CA55075, UM1 CA167552, and R35 CA197735 (S.O); the Stand Up to Cancer (SU2C) Colorectal Cancer Dream Team Translational Research Grant (grant SU2C-AACR-DT22-17 to M.G. and C.S.F.), and the Project P Fund. SU2C is a program of the Entertainment Industry Foundation, and the research grant is administered by the American Association for Cancer Research, a scientific partner of SU2C. N.D.M. is a Howard Hughes Medical Institute Medical Research Fellow. E.M.V.A. is an advisor to Genome Medical and consultant to Invitae. S.S. is a consultant to Myriad Genetics.
Published: February 22, 2018
Footnotes
Supplemental Data include Supplemental Acknowledgments, 11 figures, and 12 tables and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.01.018.
Accession Numbers
All BAM files of the CanSeq study and raw sequencing files of the NHS-HPFS study were deposited in the Database of Genotypes and Phenotypes (dbGaP) under accession numbers dbGaP: phs001075.v1.p1 and phs000722, respectively. TCGA data are available at dbGaP: phs000178.v9.p8. Raw sequencing data of the National Study of Colorectal Cancer Genetics were not available for analysis, although data on downstream variants can be accessed from the CanVar Browser.
Web Resources
CanVar Browser, https://canvar.icr.ac.uk/
Exome Aggregation Consortium (ExAC) Browser, http://exac.broadinstitute.org/
OMIM, http://www.omim.org
Supplemental Data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Mucci L.A., Hjelmborg J.B., Harris J.R., Czene K., Havelick D.J., Scheike T., Graff R.E., Holst K., Möller S., Unger R.H., Nordic Twin Study of Cancer (NorTwinCan) Collaboration Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA. 2016;315:68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishel R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1994;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 4.Bronner C.E., Baker S.M., Morrison P.T., Warren G., Smith L.G., Lescoe M.K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994;368:258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tassan N., Chmiel N.H., Maynard J., Fleming N., Livingston A.L., Williams G.T., Hodges A.K., Davies D.R., David S.S., Sampson J.R., Cheadle J.P. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat. Genet. 2002;30:227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 6.Yurgelun M.B. Identification of a Variety of Mutations in Cancer Predisposition Genes in Patients With Suspected Lynch Syndrome. Gastroenterology. 2015;149:604–613. doi: 10.1053/j.gastro.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch H.T., de la Chapelle A. Hereditary colorectal cancer. N. Engl. J. Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 8.Susswein L.R., Marshall M.L., Nusbaum R., Vogel Postula K.J., Weissman S.M., Yackowski L., Vaccari E.M., Bissonnette J., Booker J.K., Cremona M.L. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet. Med. 2016;18:823–832. doi: 10.1038/gim.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M., Aldubayan S., Connor A.A., Wong B., Mcnamara K., Khan T., Semotiuk K., Khalouei S., Holter S., Aronson M. Genetic testing for Lynch syndrome in the province of Ontario. Cancer. 2016;122:1672–1679. doi: 10.1002/cncr.29950. [DOI] [PubMed] [Google Scholar]
- 10.Pearlman R., Frankel W.L., Swanson B., Zhao W., Yilmaz A., Miller K., Bacher J., Bigley C., Nelsen L., Goodfellow P.J., Ohio Colorectal Cancer Prevention Initiative Study Group Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017;3:464–471. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson D., Duedal S., Kirner J., McGuffog L., Last J., Reiman A., Byrd P., Taylor M., Easton D.F. Cancer risks and mortality in heterozygous ATM mutation carriers. J. Natl. Cancer Inst. 2005;97:813–822. doi: 10.1093/jnci/dji141. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network (2017). Familial and Genetic High-Risk Assessment: Colorectal, version 2.2017, p. 96. https://www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=294.
- 13.Giannakis M., Mu X.J., Shukla S.A., Qian Z.R., Cohen O., Nishihara R., Bahl S., Cao Y., Amin-Mansour A., Yamauchi M. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;17:1206. doi: 10.1016/j.celrep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghazani A.A., Oliver N.M., St Pierre J.P., Garofalo A., Rainville I.R., Hiller E., Treacy D.J., Rojas-Rudilla V., Wood S., Bair E. Assigning clinical meaning to somatic and germ-line whole-exome sequencing data in a prospective cancer precision medicine study. Genet. Med. 2017;19:787–795. doi: 10.1038/gim.2016.191. [DOI] [PubMed] [Google Scholar]
- 15.Gray S.W., Park E.R., Najita J., Martins Y., Traeger L., Bair E., Gagne J., Garber J., Jänne P.A., Lindeman N. Oncologists’ and cancer patients’ views on whole-exome sequencing and incidental findings: results from the CanSeq study. Genet. Med. 2016;18:1011–1019. doi: 10.1038/gim.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yurgelun M.B., Kulke M.H., Fuchs C.S., Allen B.A., Uno H., Hornick J.L., Ukaegbu C.I., Brais L.K., McNamara P.G., Mayer R.J. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J. Clin. Oncol. 2017;35:1086–1095. doi: 10.1200/JCO.2016.71.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chubb D., Broderick P., Dobbins S.E., Frampton M., Kinnersley B., Penegar S., Price A., Ma Y.P., Sherborne A.L., Palles C. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat. Commun. 2016;7:11883. doi: 10.1038/ncomms11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Allen E.M., Wagle N., Stojanov P., Perrin D.L., Cibulskis K., Marlow S., Jane-Valbuena J., Friedrich D.C., Kryukov G., Carter S.L. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat. Med. 2014;20:682–688. doi: 10.1038/nm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Os N.J., Roeleveld N., Weemaes C.M., Jongmans M.C., Janssens G.O., Taylor A.M., Hoogerbrugge N., Willemsen M.A. Health risks for ataxia-telangiectasia mutated heterozygotes: a systematic review, meta-analysis and evidence-based guideline. Clin. Genet. 2016;90:105–117. doi: 10.1111/cge.12710. [DOI] [PubMed] [Google Scholar]
- 22.Goldgar D.E., Healey S., Dowty J.G., Da Silva L., Chen X., Spurdle A.B., Terry M.B., Daly M.J., Buys S.M., Southey M.C., BCFR. kConFab Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Res. 2011;13:R73. doi: 10.1186/bcr2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavaddat N., Peock S., Frost D., Ellis S., Platte R., Fineberg E., Evans D.G., Izatt L., Eeles R.A., Adlard J., EMBRACE Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013;105:812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 24.Rafnar T., Gudbjartsson D.F., Sulem P., Jonasdottir A., Sigurdsson A., Jonasdottir A., Besenbacher S., Lundin P., Stacey S.N., Gudmundsson J. Mutations in BRIP1 confer high risk of ovarian cancer. Nat. Genet. 2011;43:1104–1107. doi: 10.1038/ng.955. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou A.C., Foulkes W.D., Tischkowitz M. Breast-cancer risk in families with mutations in PALB2. N. Engl. J. Med. 2014;371:1651–1652. doi: 10.1056/NEJMc1410673. [DOI] [PubMed] [Google Scholar]
- 26.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnholtz-Sloan J.S., McEvoy B., Shriver M.D., Rebbeck T.R. Ancestry estimation and correction for population stratification in molecular epidemiologic association studies. Cancer Epidemiol. Biomarkers Prev. 2008;17:471–477. doi: 10.1158/1055-9965.EPI-07-0491. [DOI] [PubMed] [Google Scholar]
- 29.Cibulskis K., Lawrence M.S., Carter S.L., Sivachenko A., Jaffe D., Sougnez C., Gabriel S., Meyerson M., Lander E.S., Getz G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders C.T., Wong W.S., Swamy S., Becq J., Murray L.J., Cheetham R.K. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics. 2012;28:1811–1817. doi: 10.1093/bioinformatics/bts271. [DOI] [PubMed] [Google Scholar]
- 32.Costello M., Pugh T.J., Fennell T.J., Stewart C., Lichtenstein L., Meldrim J.C., Fostel J.L., Friedrich D.C., Perrin D., Dionne D. Discovery and characterization of artifactual mutations in deep coverage targeted capture sequencing data due to oxidative DNA damage during sample preparation. Nucleic Acids Res. 2013;41:e67. doi: 10.1093/nar/gks1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos A.H., Lichtenstein L., Gupta M., Lawrence M.S., Pugh T.J., Saksena G., Meyerson M., Getz G. Oncotator: cancer variant annotation tool. Hum. Mutat. 2015;36:E2423–E2429. doi: 10.1002/humu.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter S.L., Cibulskis K., Helman E., McKenna A., Shen H., Zack T., Laird P.W., Onofrio R.C., Winckler W., Weir B.A. Absolute quantification of somatic DNA alterations in human cancer. Nat. Biotechnol. 2012;30:413–421. doi: 10.1038/nbt.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Thomas D.C., Pe’er I., Stram D.O. Optimal two-stage genotyping designs for genome-wide association scans. Genet. Epidemiol. 2006;30:356–368. doi: 10.1002/gepi.20150. [DOI] [PubMed] [Google Scholar]
- 36.Guo X., Elston R.C. One-stage versus two-stage strategies for genome scans. Adv. Genet. 2001;42:459–471. doi: 10.1016/s0065-2660(01)42036-0. [DOI] [PubMed] [Google Scholar]
- 37.Barrett J.C., Buxbaum J., Cutler D., Daly M., Devlin B., Gratten J., Hurles M.E., Kosmicki J.A., Lander E.S., MacArthur D.G. New mutations, old statistical challenges. bioRxiv. 2017 [Google Scholar]
- 38.Mersha T.B., Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum. Genomics. 2015;9:1. doi: 10.1186/s40246-014-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen J.H., Hahnemann J.M., Børresen-Dale A.L., Tretli S., Kleinerman R., Sankila R., Hammarström L., Robsahm T.E., Kääriäinen H., Bregård A. Breast and other cancers in 1445 blood relatives of 75 Nordic patients with ataxia telangiectasia. Br. J. Cancer. 2005;93:260–265. doi: 10.1038/sj.bjc.6602658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helgason H., Rafnar T., Olafsdottir H.S., Jonasson J.G., Sigurdsson A., Stacey S.N., Jonasdottir A., Tryggvadottir L., Alexiusdottir K., Haraldsson A. Loss-of-function variants in ATM confer risk of gastric cancer. Nat. Genet. 2015;47:906–910. doi: 10.1038/ng.3342. [DOI] [PubMed] [Google Scholar]
- 41.Zhen D.B., Rabe K.G., Gallinger S., Syngal S., Schwartz A.G., Goggins M.G., Hruban R.H., Cote M.L., McWilliams R.R., Roberts N.J. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet. Med. 2015;17:569–577. doi: 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mladenov E., Magin S., Soni A., Iliakis G. DNA double-strand-break repair in higher eukaryotes and its role in genomic instability and cancer: Cell cycle and proliferation-dependent regulation. Semin. Cancer Biol. 2016;37-38:51–64. doi: 10.1016/j.semcancer.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Ford D., Easton D.F., Bishop D.T., Narod S.A., Goldgar D.E., Breast Cancer Linkage Consortium Risks of cancer in BRCA1-mutation carriers. Lancet. 1994;343:692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 44.Iqbal J., Ragone A., Lubinski J., Lynch H.T., Moller P., Ghadirian P., Foulkes W.D., Armel S., Eisen A., Neuhausen S.L., Hereditary Breast Cancer Study Group The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br. J. Cancer. 2012;107:2005–2009. doi: 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mateo J., Carreira S., Sandhu S., Miranda S., Mossop H., Perez-Lopez R., Nava Rodrigues D., Robinson D., Omlin A., Tunariu N. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fong P.C., Boss D.S., Yap T.A., Tutt A., Wu P., Mergui-Roelvink M., Mortimer P., Swaisland H., Lau A., O’Connor M.J. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 47.Tutt A., Robson M., Garber J.E., Domchek S.M., Audeh M.W., Weitzel J.N., Friedlander M., Arun B., Loman N., Schmutzler R.K. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 48.Wang C., Jette N., Moussienko D., Bebb D.G., Lees-Miller S.P. ATM-Deficient Colorectal Cancer Cells Are Sensitive to the PARP Inhibitor Olaparib. Transl. Oncol. 2017;10:190–196. doi: 10.1016/j.tranon.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pritchard C.C., Mateo J., Walsh M.F., De Sarkar N., Abida W., Beltran H., Garofalo A., Gulati R., Carreira S., Eeles R. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J., Walsh M.F., Wu G., Edmonson M.N., Gruber T.A., Easton J., Hedges D., Ma X., Zhou X., Yergeau D.A. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015;373:2336–2346. doi: 10.1056/NEJMoa1508054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.