Abstract
Fertilization is a fundamental process of development and is a prerequisite for successful human reproduction. In mice, although several receptor proteins have been shown to play important roles in the process of fertilization, only three genes have been shown to cause fertilization failure and infertility when deleted in vivo. In clinical practice, some infertility case subjects suffer from recurrent failure of in vitro fertilization and intracytoplasmic sperm injection attempts due to fertilization failure, but the genetic basis of fertilization failure in humans remains largely unknown. Wee2 is a key oocyte-specific kinase involved in the control of meiotic arrest in mice, but WEE2 has not been associated with any diseases in humans. In this study, we identified homozygous mutations in WEE2 that are responsible for fertilization failure in humans. All four independent affected individuals had homozygous loss-of-function missense mutations or homozygous frameshift protein-truncating mutations, and the phenotype of fertilization failure was shown to follow a Mendelian recessive inheritance pattern. All four mutations significantly decreased the amount of WEE2 protein in vitro and in affected individuals’ oocytes in vivo, and they all led to abnormal serine phosphorylation of WEE2 and reduced tyrosine 15 phosphorylation of Cdc2 in vitro. In addition, injection of WEE2 cRNA into affected individuals’ oocytes rescued the fertilization failure phenotype and led to the formation of blastocysts in vitro. This work presents a novel gene responsible for human fertilization failure and has implications for future therapeutic treatments for infertility cases.
Keywords: female infertility, fertilization failure, oocyte maturation, Mendelian disease, genetic mutations
Introduction
In the process of fertilization, the haploid sperm and oocyte fuse to form a diploid zygote that will subsequently develop into a complete individual.1 Fertilization is therefore a fundamental process of development and a prerequisite for successful human reproduction. In in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) treatments, mature oocytes are retrieved from affected individuals and fertilized with sperm in vitro to start early embryonic development, and the ensuing viable embryos are transplanted into the affected individuals’ uterus for establishing pregnancy. However, some affected individuals suffer from recurrent failure of IVF and ICSI attempts. For these affected individuals, although morphologically normal sperm and oocytes can be retrieved, fertilization always fails. Until now, only a single mutation in the TLE6 (MIM: 612399) gene has been associated with this phenotype,2 and the genetic basis of human fertilization failure has remained largely unknown.
Before puberty, oocytes were maintained at a cellular quiescent stage of prophase I termed “dictyate arrest” (also known as germinal vesicle [GV] stage arrest).3 During meiosis, cells undergo two divisions—meiosis I and meiosis II—to create haploid gametes.3 Dictyate-arrested oocytes resume meiosis I upon stimulation by a surge in luteinizing hormone, and meiosis I is completed by extruding the first polar body. Then, oocytes enter meiosis II and arrest at metaphase II (MII) until fertilization.3 Therefore, defects in the process of meiosis will lead to oocyte maturation arrest and fertilization failure. Wee2, also known as Wee1B, has different roles in mouse oocytes at the GV stage and MII stage. In the GV stage, Wee2 is an important oocyte-specific kinase involved in maintaining meiotic arrest4 by inhibiting the maturation-promoting factor (MPF) by inactivating Cdc2 (MIM: 116940).5, 6 In the MII stage, Wee2 is essential for MII exit. It has been documented that when Wee2 is downregulated, mouse oocytes have high MPF activity leading to failure of MII exit and the formation of pronuclei, which is an indicator of fertilization failure.7 To date, WEE2 (MIM: 614084; GenBank: NM_001105558.1) has not been associated with any diseases in humans.
In this study, we identified homozygous mutations in WEE2 that are responsible for human fertilization failure. All four affected individuals had homozygous loss-of-function missense mutations or homozygous frameshift protein-truncating mutations, and the phenotype of fertilization failure in these affected individuals followed a Mendelian recessive inheritance pattern. We investigated the functional mechanism of the corresponding mutations in vitro and in live oocytes, and we explored a potential therapeutic treatment in one of the affected individuals by injecting WEE2 cRNA into her oocytes.
Subjects and Methods
Clinical Samples
Infertility-affected individuals diagnosed with fertilization failure and healthy controls were recruited from the Ninth Hospital affiliated with Shanghai Jiao Tong University. Peripheral blood samples were taken for DNA extraction. The control first polar body (PB1) oocytes used in this study were matured in vitro from oocytes at the GV or metaphase I (MI) stage. Some PB1 oocytes from the affected individuals were donated for investigation after they provided written and informed consent. The study was approved by the ethics committee of the Medical College of Fudan University and the reproductive center of the Ninth Hospital affiliated with Shanghai Jiao Tong University.
Genetic Studies
Genomic DNA was extracted from peripheral blood based on a previously published protocol.8 Whole-exome capture (Agilent) and Illumina sequencing were performed following the standard protocols. Basic bioinformatics analysis included mapping the raw FASTQ files to the human reference sequence (NCBI Genome build GRCh37); variant calling; annotation with the GRCh37, dbSNP (version 138), 1000 Genomes, Exome Aggregation Consortium (ExAC, version 0.3.1) databases, and our in-house exome database; and functional prediction with the SIFT and PolyPhen-2 programs. Homozygosity mapping was performed with HomozygosityMapper.9 Because three of the four families were consanguineous, homozygous and compound heterozygous variants were prioritized in the analysis. All candidate mutations had to meet the following inclusion criteria: (1) be absent in other family members, (2) have a frequency less than 0.1% (for homozygous variants) or 1% (for compound-heterozygous variants) in public databases, and (3) be located in or near a homozygous region greater than 2.0 Mb.
Expression Vector Construction and Mutagenesis
The full-length coding sequence of WEE2 was amplified from control human oocyte cDNA. The WEE2 product was then cloned into the pCMV6-Entry vector to introduce a FLAG-tag (Origene) using AsiSI and MluI (New England Biolabs) dual-enzyme digestion. All FLAG tags were fused in the N termini and were in frame with the frameshifted C termini. Site-directed mutagenesis was performed to introduce four variants—p.Asp234His, p.Glu75Valfs∗6, p.Thr493Asnfs∗39, and p.His337Tyrfs∗24—into the pCMV6-Entry vector according to the instructions of the KOD-Plus-Mutagenesis Kit (Toyobo Life Science).
Immunofluorescence
In order to determine the effects of these mutations on WEE2, immunofluorescence was performed in mouse oocytes, HeLa cells, and affected individual oocytes as previously described.8 Briefly, in mouse oocytes, wild-type or mutant WEE2 cRNAs were microinjected into GV oocytes, which were then cultured for 12 hr and fixed for immunofluorescence. HeLa cells were transfected with wild-type or mutant WEE2 constructs, and cells were fixed for immunofluorescence after culturing for 30 hr. The oocytes of the affected individuals and healthy control subjects were fixed in 2% paraformaldehyde for immunofluorescence. An anti-WEE2 antibody was used at 1:100 dilution (Abcam). DAPI was used at 1:600 dilution (Beyotime) for DNA visualization. An anti-FLAG antibody was used at 1:500 dilution (Sigma). All images were captured on a confocal laser-scanning microscope (Leica).
Western Blotting
To evaluate the effects of the mutations on WEE2 phosphorylation, HeLa cells were transfected with wild-type or mutant WEE2 constructs and incubated for 36 hr. Total protein was extracted by RIPA lysis buffer (Shanghai Wei AO Biological Technology), and immunoprecipitation was performed using anti-FLAG beads. Western blotting was conducted using a phospho-serine antibody (Immunechem Canada) to detect WEE2 phosphorylation. To determine the effect of WEE2 mutations on MPF activity, transfected HeLa cells were used for western blotting. Antibodies against Cdc2 (Abcam) and pY15-Cdc2 (Abcam) were used at 1:1,000 dilution, and mouse anti-vinculin (MIM: 193065) (1:5,000 dilution, Sigma) was used as the internal control. The secondary antibodies were goat anti-rabbit IgG (1:5,000 dilution, Abmart) or goat anti-mouse IgG (1:5,000 dilution, Abmart) conjugated to horseradish peroxidase.
Oocyte Collection and Microinjection
GV oocytes were collected from the ovaries of 7- to 8-week-old ICR mice. GV oocytes were cultured at 37°C in M2 medium (Sigma) supplemented with 200 μM of 3-isobutyl-1-methylxanthine (Sigma) to prevent GV breakdown. For collecting MII oocytes, 7.5 IU of human chorionic gonadotropin was administered 48 hr after injection of pregnant mare’s serum gonadotropin. Approximately 10 pl of the mRNA solution (500 ng/μL) was injected per oocyte. GV oocytes were fixed for immunofluorescence 12 hr after injection. MII oocytes were counted for pronuclear formation 10 hr after injection.
Statistical Analysis
Data are representative of at least three independent experiments. Significance was analyzed by one-way ANOVA, and p < 0.05 was considered statistically significant. Quantitation of immunofluorescence and western blotting results was performed with the ImageJ software.
Results
Clinical Characteristics of the Affected Individuals
All four female affected individuals had normal menstrual cycles and had been diagnosed with primary infertility of unknown cause for several years. The male partners of these affected individuals had normal sperm counts and their sperm had normal morphologic features and motility. To further exclude male factors, we acquired in vitro-matured MII oocytes from other affected individuals and found that these oocytes could be fertilized after injecting sperm of the male partners of the four affected individuals, indicating that the sperm were functionally normal. The affected individuals in family 1, family 2, and family 3 were from consanguineous families. The proband in family 1 was 37 years old and her parents were cousins. She underwent two failed ICSI attempts. Three PB1 oocytes were retrieved, but none of them could be fertilized (Table 1). The proband in family 2 was 34 years old, and based on the result of her homozygosity mapping her parents also had some biological relationship (Figure S1). She had one failed ICSI attempt, in which eight PB1 oocytes were retrieved but could not be fertilized (Table 1). The proband in family 3 was 29 years old. Her grandfather on her father’s side and her grandmother on her mother’s side were cousins. She had one failed IVF attempt and two failed ICSI attempts. Altogether, 18 PB1 oocytes were retrieved but none could be fertilized (Table 1). The proband in family 4 was 27 years old, and she had one failed ICSI attempts. During her first ICSI cycle, 20 PB1 oocytes were retrieved but none could be fertilized (Table 1). The morphology of the retrieved oocytes before ICSI is shown in Figure S2.
Table 1.
Clinical Characteristics of Affected Individuals and Their Retrieved Oocytes
| Case | Age (years) | Duration of Infertility (years) | IVF/ICSI Cycles | Total No. of Oocytes Retrieved | GV Oocytes | MI Oocytes | PB1 Oocytes | Fertilized Oocytes |
|---|---|---|---|---|---|---|---|---|
| Family 1 | 37 | 9 | 2 | 3 | 0 | 0 | 3 | 0 |
| Family 2 | 34 | 7 | 1 | 11 | 2 | 1 | 8 | 0 |
| Family 3 | 29 | 6 | 3 | 19 | 0 | 1 | 18 | 0 |
| Family 4 | 27 | 4 | 1 | 20 | 0 | 0 | 20 | 0 |
Abbreviations: GV, germinal vesicle; MI, metaphase I; PB1, the first polar body.
Identification of Homozygous Mutations in WEE2
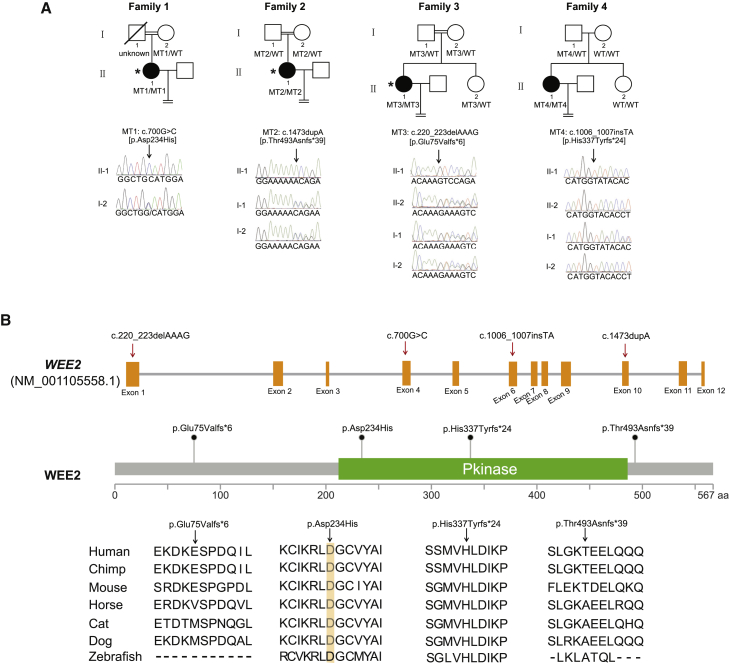
After whole-exome sequencing, bioinformatics filtering analysis, and homozygosity mapping (Figure S1), WEE2 was the only gene that segregated with the phenotype in each of families 1, 2, and 3. Sanger sequencing confirmed mutations in WEE2 in these families (Figure 1A). The proband in family 1 had a homozygous missense mutation c.700G>C (p.Asp234His). The affected individuals in families 2 and 3 both had homozygous frameshift protein-truncating mutations. The proband in family 2 had a c.1473dupA (p.Thr493Asnfs∗39) mutation, while the proband in family 3 had a c.220_223delAAAG (p.Glu75Valfs∗6) mutation. Another homozygous frameshift protein-truncating mutation c.1006_1007insTA (p.His337Tyrfs∗24) was identified in the proband in family 4 by direct sequencing of all exons of WEE2. The allele frequency of p.Glu75Valfs∗6 is 0.00008296 (10/120,538) in the ExAC database, while other three mutations were not found in the ExAC database. The specific information for the mutations is shown in Table 2. The positions of these mutations are shown in Figure 1B along with their conservation in different species.
Figure 1.
Identification of Mutations in WEE2
(A) Four pedigrees presented with the fertilization failure phenotype. Families 1, 2, and 3 were consanguineous families with a recessive inheritance pattern. Sanger sequencing confirmation is shown below the pedigrees. Affected individuals undergoing whole-exome sequencing and homozygosity mapping are labeled with an asterisk. The equal sign indicates infertility. Black circles represent the affected individuals.
(B) Locations and conservation of mutations in the WEE2 gene. The positions of all mutations are indicated in the genomic structure of WEE2, and the conservation of the mutated amino acids is indicated by the alignment of seven mammalian species.
Table 2.
Overview of the WEE2 Mutations Observed in Four Families
| Observed in Families | Genomic Position on chr. 7 (bp) | cDNA Change | Protein Change | Mutation Type | SIFTa | PPH2a | 1KG_easb | ExAC |
|---|---|---|---|---|---|---|---|---|
| Family 1 | 141,418,986 | c.G700C | p.Asp234His | missense | D | D | NA | NA |
| Family 2 | 141,427,183 | c.1473dupA | p.Thr493Asnfs∗39 | frameshift insertion | NA | NA | NA | NA |
| Family 3 | 141,408,777 | c.220_223delAAAG | p.Glu75Valfs∗6 | frameshift deletion | NA | NA | NA | 8.3 × 10−5 |
| Family 4 | 141,423,059 | c.1006_1007insTA | p.His337Tyrfs∗24 | frameshift insertion | NA | NA | NA | NA |
Mutation assessment by SIFT and PolyPhen2 (PPH2). D, damaging.
Frequency of corresponding mutations in the East Asian population of the 1000 Genome (1KG). NA, not available.
Localization and Expression of Mutant Protein in Cells and Oocytes
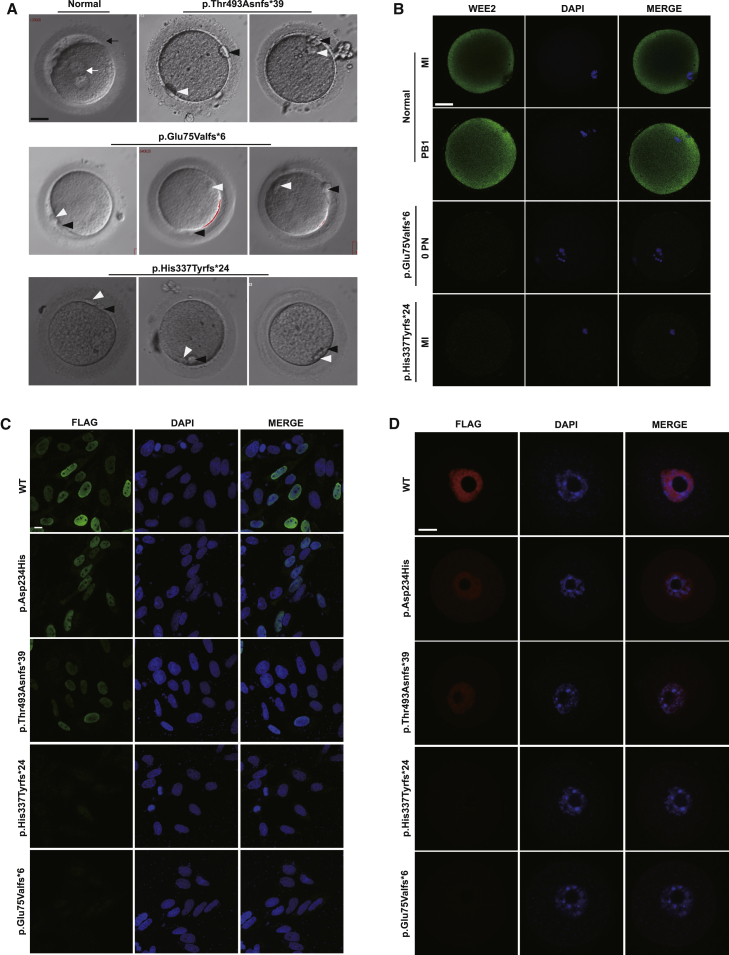
Light microscopy showed that oocytes from affected individuals could not form pronuclei after fertilization (Figure 2A). Immunofluorescence staining in two affected individual oocytes suggested that the mutations caused loss of WEE2 protein or protein degradation (Figures 2B and S3A). To evaluate the effects of the mutations on protein localization, wild-type and mutant constructs were transfected into HeLa cells. As Figure 2C indicates, the nuclear localization of p.Asp234His and p.Thr493Asnfs∗39 was not affected but the protein level was slightly reduced, while p.His337Tyrfs∗24 and p.Glu75Valfs∗6 were significantly degraded as indicated by the very faint immunofluorescence signal (Figures 2C and S3B). This is consistent with the results of immunofluorescence in mouse GV oocytes injected with wild-type or mutant cRNAs (Figures 2D and S3C). To evaluate the functional effects of the mutations in vitro, western blot analysis was performed with cells transfected with wild-type and mutant WEE2 constructs. The amounts of p.Asp234His and p.Thr493Asnfs∗39 altered proteins were significantly lower than wild-type, while the p.His337Tyrfs∗24 and p.Glu75Valfs∗6 altered proteins were nearly undetectable (Figures 3A and S4A). Thus, although the WEE2 antibody (epitope: amino acids 305–396) would not recognize the truncated proteins predicted to be produced by the p.His337Thrfs∗24 and p.Glu75Valfs∗6, considering the corresponding results of Flag-tagged WEE2 in mouse oocytes and HeLa cells, we believe that these two mutations lead to protein degradation.
Figure 2.
Morphology of Normal Zygotes and Retrieved Oocytes after ICSI and the Effects of the Mutations on WEE2 Protein Levels in Oocytes and HeLa Cells
(A) The morphologies of a normal zygote and affected individual oocytes on day 1 after ICSI. The white arrow indicates the pronuclei, and the black arrow indicates the zona pellucida. The white arrowhead indicates the first polar body, and the black arrowhead indicates the second polar body. Scale bar = 40 μm.
(B) Immunofluorescence of healthy control and affected individual oocytes with the p.Glu75Valfs∗6 or p.His337Tyrfs∗24 mutation. Abbreviations: MI, metaphase I; PB1, first polar body; PN, pronuclei. Scale bar = 40 μm.
(C) Protein level and localization of WT and mutant WEE2 in HeLa cells. Scale bar = 10 μm.
(D) Protein level and localization of WT and mutant WEE2 in mouse GV oocytes. Images were captured by confocal microscopy (Leica). Scale bar = 20 μm.
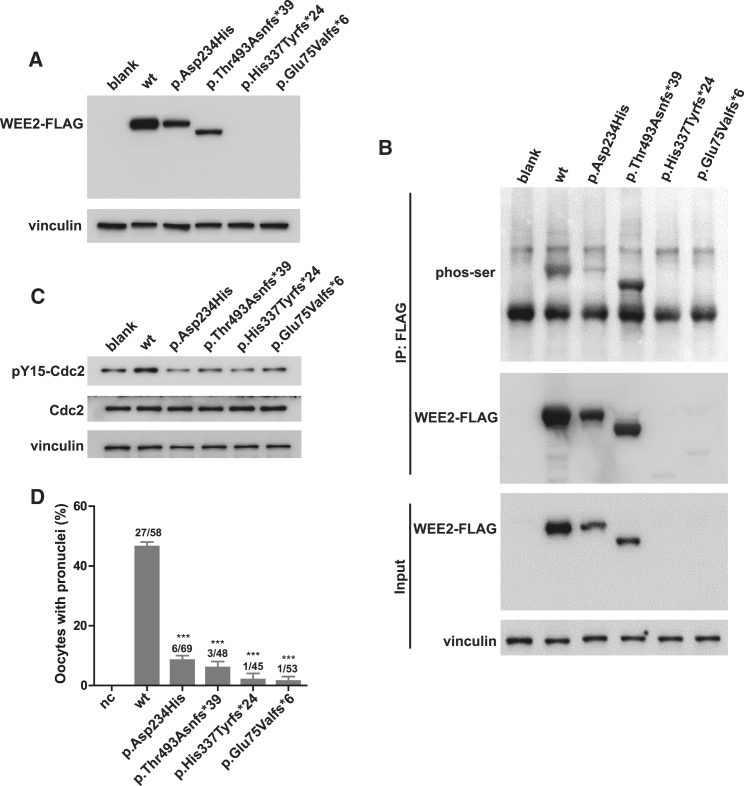
Figure 3.
Effects of the Mutations on WEE2 and Cdc2 Phosphorylation and Pronuclei Formation
(A) The effects of the mutations on WEE2 protein level by western blotting in HeLa cells transfected with WT or mutant constructs.
(B) The effects of the mutations on serine phosphorylation of WEE2 in HeLa cells. WEE2 was immunoprecipitated by FLAG beads, and western blotting was performed using a phospho-serine antibody.
(C) The effects of the mutations on tyrosine 15 phosphorylation of Cdc2 in HeLa cells. HeLa cells were transfected with WT or mutant constructs, and 20 μg whole-cell lysate was used for western blotting.
(D) The effects of the mutations on pronuclei formation. A total of 10 pl of the 500 ng/μL WEE2 cRNA solution was injected into mouse MII oocytes, and the pronuclei formation rate was calculated 10 hr after WEE2 cRNA injection. The total number of oocytes used is listed on top of the column. Significance was compared between WT and mutant groups. Three independent experiments were performed, and results are shown as the mean ± SEM. ∗∗∗p < 0.001.
Phosphorylation of Cdc2 and WEE2 Was Impaired by WEE2 Mutations In Vitro
It has been shown previously that serine phosphorylation of mouse WEE2 is required for egg activation, which in turn is required for successful fertilization.7 We first determined the effects of the mutations on human WEE2 serine phosphorylation. As shown in Figures 3B and S4B, the serine phosphorylation of WEE2 was significantly reduced for the p.Asp234His, p.His337Tyrfs∗24, and p.Glu75Valfs∗6 mutations, while it was significantly increased for the p.Thr493Asnfs∗39 mutation. Compared with the other mutations, p.Thr493Asnfs∗39 resulted in a truncated protein with a lower detection level (Figure 3A), which might further cause abnormally increased phosphorylation. Wee2-mediated phosphorylation of Cdc2 is also required for pronuclei formation,7 and we therefore determined the effects of the WEE2 mutations on tyrosine 15 phosphorylation (pY15) of Cdc2 in vitro. As shown in Figures 3C and S4C, all case-derived mutations in WEE2 decreased the level of pY15-Cdc2. To further confirm the impairment of mutations on pronuclei formation, we injected wild-type (WT) or mutant WEE2 cRNA into mouse MII oocytes. As expected, all mutants significantly reduced the rate of pronuclei formation (Figure 3D). We further demonstrated that oocytes injected with mutant WEE2 cRNAs had low pY15-Cdc2 levels, indicating that they might have high MPF activity (Figure S4D). Thus, we concluded that mutations in WEE2 decreased the amount of WEE2 protein and impaired the phosphorylation of both WEE2 and Cdc2 and that this led to the failure of fertilization.
Phenotypic Rescue by WEE2 cRNA Injection in Affected Individual Oocytes
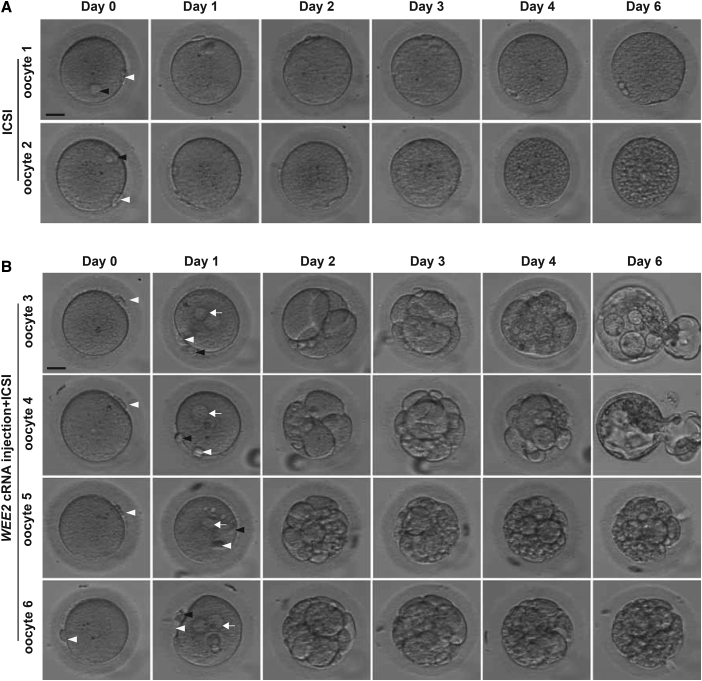
To determine whether the phenotype of fertilization failure could be rescued, we directly injected WEE2 cRNA into oocytes of proband II-1 from family 4 during the recent second ICSI cycle. A total of 13 oocytes were retrieved, of which 11 were PB1 oocytes. Among the 11 PB1 oocytes, 4 were injected with WEE2 cRNA and then used for ICSI, while the remaining PB1 oocytes acted as controls and were used directly for ICSI without cRNA injection. In the control group, all oocytes remained unfertilized after ICSI (Figure 4A). However, all four oocytes injected with WEE2 cRNA were successfully fertilized as indicated by the formation of 2 pronuclei on day 1, and two of the oocytes developed into blastocysts on day 6 (Figure 4B). Preimplantation genetic screening showed that the two blastocysts had normal numbers of chromosomes and had no obvious large repetition/deletion fragments (Figure S5). These results further confirm the effects of WEE2 on fertilization and suggest the possibility for novel treatment options for affected individuals with mutations in WEE2 in the future.
Figure 4.
Phenotypic Rescue by WEE2 cRNA Injection into Oocytes from the Proband from Family 4
(A) In the control group, two retrieved PB1 oocytes underwent ICSI without WEE2 cRNA injection. Day 0 indicates the time point at 4 hr after ICSI. The white arrowhead indicates the first polar body, and the black arrowhead indicates the second polar body. Scale bar = 40 μm.
(B) In the experimental group, four retrieved PB1 oocytes were injected with WEE2 cRNA and cultured for 4 hr and then used for ICSI. Day 0 indicates the time point at 4 hr after cRNA injection and just before ICSI. Oocytes were monitored for 6 days after ICSI. White arrows indicate the pronuclei. Scale bar = 40 μm.
Discussion
In this study, we identified four different homozygous mutations in WEE2 that are responsible for the unique phenotype of fertilization failure. These mutations reduced the WEE2 protein level in the oocytes and affected the phosphorylation state of WEE2 and Cdc2 in vitro, which blocked oocyte MII exit and caused subsequent fertilization failure. Finally, we found that injection of WEE2 cRNA into affected oocytes led to successful fertilization and blastocyst formation in vitro.
During fertilization, the specialized meiotic germ cells, the oocyte and sperm, are transformed into a zygote that is capable of developing into all cells and tissues of human body.1 Fertilization involves several biological changes, including sperm-egg binding,10 Ca2+ oscillations, the release of cortical granules, and the extrusion of the second polar body.11, 12 Mature oocytes are arrested at the MII stage and must undergo MII exit in order to be fertilized.13 Inactivation of the MPF, which is the complex of Cdc2 and Cyclin B1 (MIM: 123836), is imperative for MII exit in mouse oocytes.7 Although several receptor proteins have important roles in the process of fertilization in mice,14, 15 only three genes have been shown to cause failure of fertilization and infertility when deleted in vivo.16, 17, 18, 19
In this study, we identified different homozygous mutations in WEE2 that cause fertilization failure by reducing the WEE2 protein level and impairing the phosphorylation of WEE2 and Cdc2. It has been shown that in Wee2 knockdown mouse oocytes MPF has high activity and causes the failure of MII exit and the subsequent formation of pronuclei.7 In this study, all mutations we identified were truncated or loss of function and had similar effects as Wee2 knockdown in mouse oocytes. In addition, all of these mutations decreased the level of pY15-Cdc2 (Figures 3 and S4C). These results indicate that MPF activity is also presumably high in affected individuals’ oocytes and that this leads to the phenotype of fertilization failure. By direct injection of WEE2 cRNA into oocytes from the proband in family 4, we showed that the oocytes could be successfully fertilized and that normal blastocysts could be formed. Further studies in terms of safety and efficacy still need to be performed, but our present results suggest a novel therapeutic approach for these affected individuals with mutations in WEE2.
Female infertility can result from several factors, including dysfunctions in ovary development, folliculogenesis, gamete maturation and recognition, early embryonic development, etc.20, 21, 22 However, the genetic basis for infertility characterized by abnormalities in human oocyte development, fertilization, and early embryogenesis is not well understood. With the widespread application of IVF for infertile couples, the genetic factors behind the Mendelian phenotypes observed in these procedures are becoming clearer. Recently, we identified mutations in a few novel genes that are responsible for female infertility caused by oocyte maturation arrest (MIM: 616780, 617743) and early embryonic arrest (MIM: 617234).8, 23, 24, 25 For example, mutations in TUBB8 (MIM: 616768), encoding a primate-specific β-tubulin isotype with previously unknown function, cause oocyte MI arrest by impairing oocyte microtubule stability and spindle assembly.8 Different mutations in TUBB8 also cause multiplicity of phenotype in human oocytes and early embryos.23 Biallelic mutations in PATL2 (MIM: 614661) are responsible for oocytes phenotypic variability, including oocyte arrest at the GV stage and MI stage and oocytes with an abnormally large polar body.24 Mutations in PADI6 (MIM: 610363) caused early embryonic arrest by destroying the subcortical maternal complex (SCMC) complex.25 In addition, Alazami et al.2 identified a single missense mutation in TLE6 that caused fertilization failure by impairing TLE6 phosphorylation and affecting SCMC formation.2 Huang et al. identified a mutation in ZP1 (MIM: 195000) that is responsible for a unique form of familial infertility characterized by abnormal eggs that lack a zona pellucida.26 These findings indicate that some phenotypes in early human reproductive processes follow Mendelian patterns. Although more than 5,000 Mendelian disorders have been recorded in humans, most of these are related to traits seen after birth. In the genomic era, systematic genetic analysis and deepened as well as broadened phenotype analysis will help uncover novel genes, pathways, and functions involved in human reproduction. Thus, by investigating the Mendelian phenotypes underlying early human reproduction process, the molecular mechanisms behind the processes can be elucidated, and such findings will facilitate genetic diagnoses and help to guide the therapeutic pathway of infertility affected individuals in the future.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFC1000600 and 2017YFC1001500), the National Basic Research Program of China (2015CB943300), the National Natural Science Foundation of China (81725006, 81771581, 81571501), and the Shanghai Rising-Star Program 17QA1400200.
Published: March 29, 2018
Footnotes
Supplemental Data include five figures and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.02.015.
Contributor Information
Qing Sang, Email: sangqing@fudan.edu.cn.
Lei Wang, Email: wangleiwanglei@fudan.edu.cn.
Web Resources
1000 Genomes, http://www.internationalgenome.org/
ExAC Browser, http://exac.broadinstitute.org/
OMIM, http://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
Supplemental Data
References
- 1.Clift D., Schuh M. Restarting life: fertilization and the transition from meiosis to mitosis. Nat. Rev. Mol. Cell Biol. 2013;14:549–562. doi: 10.1038/nrm3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alazami A.M., Awad S.M., Coskun S., Al-Hassan S., Hijazi H., Abdulwahab F.M., Poizat C., Alkuraya F.S. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol. 2015;16:240. doi: 10.1186/s13059-015-0792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster A., Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017;27:55–68. doi: 10.1016/j.tcb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Han S.J., Chen R., Paronetto M.P., Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr. Biol. 2005;15:1670–1676. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Han S.J., Conti M. New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle. 2006;5:227–231. doi: 10.4161/cc.5.3.2395. [DOI] [PubMed] [Google Scholar]
- 6.Oh J.S., Han S.J., Conti M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J. Cell Biol. 2010;188:199–207. doi: 10.1083/jcb.200907161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh J.S., Susor A., Conti M. Protein tyrosine kinase Wee1B is essential for metaphase II exit in mouse oocytes. Science. 2011;332:462–465. doi: 10.1126/science.1199211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng R., Sang Q., Kuang Y., Sun X., Yan Z., Zhang S., Shi J., Tian G., Luchniak A., Fukuda Y. Mutations in TUBB8 and human oocyte meiotic arrest. N. Engl. J. Med. 2016;374:223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seelow D., Schuelke M., Hildebrandt F., Nürnberg P. HomozygosityMapper--an interactive approach to homozygosity mapping. Nucleic Acids Res. 2009;37:W593–W599. doi: 10.1093/nar/gkp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wassarman P.M., Litscher E.S. Mammalian fertilization: the egg’s multifunctional zona pellucida. Int. J. Dev. Biol. 2008;52:665–676. doi: 10.1387/ijdb.072524pw. [DOI] [PubMed] [Google Scholar]
- 11.Ducibella T., Huneau D., Angelichio E., Xu Z., Schultz R.M., Kopf G.S., Fissore R., Madoux S., Ozil J.P. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev. Biol. 2002;250:280–291. [PubMed] [Google Scholar]
- 12.Horner V.L., Wolfner M.F. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev. Dyn. 2008;237:527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- 13.Jones K.T. Turning it on and off: M-phase promoting factor during meiotic maturation and fertilization. Mol. Hum. Reprod. 2004;10:1–5. doi: 10.1093/molehr/gah009. [DOI] [PubMed] [Google Scholar]
- 14.Ikawa M., Inoue N., Benham A.M., Okabe M. Fertilization: a sperm’s journey to and interaction with the oocyte. J. Clin. Invest. 2010;120:984–994. doi: 10.1172/JCI41585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi E., Wright G.J. Sperm meets egg: the genetics of mammalian fertilization. Annu. Rev. Genet. 2016;50:93–111. doi: 10.1146/annurev-genet-121415-121834. [DOI] [PubMed] [Google Scholar]
- 16.Inoue N., Ikawa M., Isotani A., Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 17.Miller B.J., Georges-Labouesse E., Primakoff P., Myles D.G. Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J. Cell Biol. 2000;149:1289–1296. doi: 10.1083/jcb.149.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaji K., Oda S., Shikano T., Ohnuki T., Uematsu Y., Sakagami J., Tada N., Miyazaki S., Kudo A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 2000;24:279–282. doi: 10.1038/73502. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi E., Doe B., Goulding D., Wright G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matzuk M.M., Lamb D.J. The biology of infertility: research advances and clinical challenges. Nat. Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layman L.C. The genetic basis of female reproductive disorders: etiology and clinical testing. Mol. Cell. Endocrinol. 2013;370:138–148. doi: 10.1016/j.mce.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart R.J. Physiological aspects of female fertility: role of the environment, modern lifestyle, and genetics. Physiol. Rev. 2016;96:873–909. doi: 10.1152/physrev.00023.2015. [DOI] [PubMed] [Google Scholar]
- 23.Feng R., Yan Z., Li B., Yu M., Sang Q., Tian G., Xu Y., Chen B., Qu R., Sun Z. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J. Med. Genet. 2016;53:662–671. doi: 10.1136/jmedgenet-2016-103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen B., Zhang Z., Sun X., Kuang Y., Mao X., Wang X., Yan Z., Li B., Xu Y., Yu M. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am. J. Hum. Genet. 2017;101:609–615. doi: 10.1016/j.ajhg.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y., Shi Y., Fu J., Yu M., Feng R., Sang Q., Liang B., Chen B., Qu R., Li B. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am. J. Hum. Genet. 2016;99:744–752. doi: 10.1016/j.ajhg.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H.L., Lv C., Zhao Y.C., Li W., He X.M., Li P., Sha A.G., Tian X., Papasian C.J., Deng H.W. Mutant ZP1 in familial infertility. N. Engl. J. Med. 2014;370:1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.