Abstract
Objective:
To investigate the extent of asbestos exposure among patients with primary lung cancer in Japan.
Methods:
A retrospective estimation of potential asbestos-exposed individuals, as determined by the presence of pleural plaques identified on chest computed tomography (CT), was conducted on 885 pathologically confirmed primary lung cancer patients (mean age 71.3 years, 641 males). All patients were diagnosed at 29 hospitals across Japan between 2006 and 2007. Since these hospitals belong to the Japan Federation of Democratic Medical Institutions (MIN-IREN), an organization of medical institutions for workers, the study subjects may contain a higher proportion of workers than the general population.
Results:
Pleural plaques were identified in 12.8% of subjects (15.8% in males and 4.9% in females), consisting exclusively of cases older than 50 years. They were found most frequently on the chest wall pleura (96.5%), followed by the diaphragm (23.9%) and mediastinum (9.7%). Calcifications were seen in 47 cases (41.6%). The highest prevalence of pleural plaques was seen among workers from construction-related fields (37.7%). No distinct lung cancer histology was observed in patients with pleural plaques. Coexistence of pleural plaques and small irregular opacities was observed in 2.5% of subjects.
Conclusion:
In a Japanese population representing more workers than general Japanese, 12.8% of patients with primary lung cancer may have experienced asbestos exposure at some time in the past. Special medical attention should be paid to individuals with a history of employment in construction-related occupations, as workers in this sector showed the highest prevalence of pleural plaques.
Keywords: Asbestos, Computed tomography, Lung cancer, Pleural plaques, Prevalence
Introduction
Japan was one of the largest asbestos-consuming countries. The importation and use of asbestos in Japan increased after the Second World War, and peaked in the 1970s and 1980s. Total importation between 1930-2005 was estimated at roughly 9.9 million tons1). It is well established that either occupational or environmental exposure to asbestos is associated with both malignant and non-malignant respiratory disease. The major malignant diseases of concern are lung cancer and pleural mesothelioma. More than 70% of mesothelioma cases in Japan are believed to be associated with past asbestos exposure2). It is difficult to estimate the etiological fraction of lung cancers in a population that is attributable to asbestos exposure. While research linking asbestos exposure and malignancies has customarily followed an asbestos-exposed population3,4), no published study has yet investigated the proportion of lung cancer cases that are attributable to asbestos exposure in Japan. Additionally, to our knowledge no English-language paper has yet discussed radiologically identified asbestos-related pleural abnormalities in a cohort of primary lung cancer patients. Accordingly, we identified potential asbestos-exposed individuals in a population of patients with primary lung cancer from a group of hospitals in the Japan Federation of Democratic Medical Institutions (MIN-IREN). Asbestos exposure was determined by the presence of pleural plaques, which are the most common and relatively early radiographic manifestation of benign asbestos-related pleural disease, and are considered indicators of past exposure to asbestos5-7).
Subjects and Methods
Subjects
This hospital-based multicenter study was designed to retrospectively investigate potentially asbestos-exposed individuals among patients with primary lung cancer in Japan. In 2008, we invited a group of hospitals belonging to MIN-IREN to participate in the present study. MIN-IREN was established primarily as an organization of medical institutions for workers. Accordingly, in addition to the healthcare delivered by most hospitals, hospitals of this group also operate clinics specializing in occupational medicine and actively provide care for work-related diseases. Consequently, patients seeking consultation in these hospitals may represent a higher percentage of workers than occurs in the general Japanese population. We asked the participating hospitals to provide medical information, including medical records, chest radiographs (CXR), and chest computed tomography scans (CT) of all patients who were newly diagnosed with primary lung cancer. Initially, information was obtained for 947 cases, diagnosed between Jan 1, 2006 and Dec 31, 2007 in 29 hospitals from 19 prefectures across Japan. All cases were consecutive patients whose diagnosis was confirmed histologically or cytologically. After exclusion of 62 cases with uninterpretable chest images due to metastasis or an advanced stage of the disease, we included information from 885 cases (641 males and 244 females, ages 26 - 94 years) in our final analysis. Written informed consent from the patients was waived, because this was a retrospective study and used anonymized data and images. The study protocol was approved by the ethical committee of Chiba Kensei Hospital.
Collection of clinical and occupational history
Clinical history, history of smoking, and occupational information was retrieved by reviewing medical charts. Information on smoking was available for 692 cases (78.2%). Occupational information was recorded in 615 cases (69.5%); each of these cases was assigned a particular occupational category according to the "Classification of occupations for employment service (ESCO) " 8), defined by the then Ministry of Labor of Japan. If a subject had engaged in multiple occupations, work with the highest potential for asbestos exposure was selected9).
Evaluation of chest radiographs and CT scans
Of all the radiological images, those closest to the date of diagnosis of primary lung cancer were reviewed. The images were assessed for the presence of benign asbestos-related parenchymal and pleural abnormalities by a panel of experts, consisting of occupational physicians, chest physicians and radiologists, including one B-reader (NS), who received certification from the National Institute for Occupational Safety and Health (NIOSH) of the United States. The panel members were from the participating hospitals, all of whom have many years of experience in interpreting abnormalities in the chest of dust-exposed workers. In discordant cases, a final decision was reached by consensus with the B-reader (NS).
In brief, we evaluated dust-induced parenchymal and pleural changes identified in the CXR and CT as described in the ILO 2000 International Classification of Radiographs of Pneumoconioses (ILO/ICRP)10), and the International Classification of HRCT for Occupational and Environmental Respiratory Diseases (ICOERD)11), respectively. Parenchymal changes include both small opacities and large opacities with a radiographic appearance consistent with dust and fiber inhalation. Small opacities, i.e. of up to 1 cm in width on CXR, were accordingly interpreted as small rounded opacities or small irregular opacities. On CT, all measureable, well-defined rounded opacities of up to 1 cm in breadth were recorded as small rounded opacities; while intralobular dot-like lesions or subpleural curvilinear opacities were recorded as irregular and/or linear opacities. A large opacity was defined as an opacity with the longest dimension exceeding 1 cm on CXR or chest CT, as described in the ILO/ICRP and ICOERD, respectively. Pleural plaques represent localized pleural thickening, generally of the parietal pleura. On CXR, pleural plaques may be seen on the chest wall (in-profile plaque or face-on plaque), on the diaphragm, and at other sites. A minimum width of about 3 mm is required for an in-profile plaque to be recorded as present. The ILO/ICRP records pleural plaques on a CXR as absent or present, with the right and left sides assessed separately10). On the other hand, the ICOERD differentiates pleural abnormalities on CT as "parietal type", which includes the typical tableland shape as well as the flat (less elevated) thickening of the pleura without subpleural fibrosis; and "visceral type" (also described as diffuse pleural thickening), which is always associated with the presence of subpleural fibrosis or parenchymal bands and rounded atelectasis12).
In the present study, we considered only CT-confirmed pleural plaques as a surrogate for past asbestos exposure. Since the CT shows a cross-section of the thorax and gives a clear view of the anterior and posterior walls of the thorax, CT is more sensitive and specific than CXR in characterizing dust and fiber-induced pleural changes13,14). To increase specificity, we classified pleural lesions on CT into one of four categories: "definite," "probable," "possible," and "none". In practice, we applied "definite" to typical tableland shape lesions as well as less elevated lesions with prominent thickness and extent; "probable" to lesions whose thickness or extent was less well-defined; "possible" if there was a non-specific pleural thickening, or a lesion unlikely to be a pleural plaque; and "none" for CTs that were clear of pleural lesions. The presence of a pleural abnormality was defined by the categories "definite" and "probable." Additionally, only "parietal type" lesions were classified as pleural plaques, while diffuse pleural thickening mainly involving the visceral pleura was omitted.
Statistical analysis
Analyses mainly focused on estimating the portion of potential asbestos-exposed individuals among patients with primary lung cancer; thus, we enumerated the frequency of asbestos-related findings as determined by the presence of CT-identified pleural plaques. In the analyses, we simply defined pleural plaques and parenchymal lung abnormalities as present or absent. We divided age into five categories: younger than 50 years, 50 - 59 years, 60 - 69 years, 70 - 79 years, and 80 years and older. We grouped smoking status into "ever-smokers," which combined current and ex-smokers, and "non-smokers." Occupation was categorized based on the classification of occupations for employment service (ESCO). Comparisons between groups were conducted using the Chi-squared test or Fisher's exact test as appropriate. Continuous variables were compared using the Student t-test. Differences were considered statistically significant at p < 0.05. Analyses were performed using Stata 13.1 Special Edition (StataCorp LP, College Station, Texas, USA).
Results
Characteristics of patients with primary lung cancer
Table 1 describes the characteristics of the 885 patients with primary lung cancer. Males made up the majority with 72.4%. Mean age at diagnosis was 71.3±9.9 years (mean±standard deviation), with no observed difference in age between sexes (p = 0.347). There was a significant sex difference in the distribution of cases across age categories (p < 0.005). Frequency of smoking was 74.4%, with a significant difference in distribution between sexes (p < 0.001). With the available information, 43.7% of males were engaged in manufacturing or as laborers, while 29.3% of females were housewives.
Table 1.
Characteristics of the 885 patients with primary lung cancer according to sex
| Number (%) | p | |||
|---|---|---|---|---|
| All | Male | Female | ||
|
a Information on smoking was available for 692 cases (78.2%). b Information on occupation was available for 615 cases (69.5%). p = p-value of Student t-test or Chi-squared test; M ± SD = mean ± standard deviation. | ||||
| Number of patients | 885 | 641 (72.4) | 244 (27.6) | |
| Age, years, M ± SD | 71.3 ± 9.9 | 71.1 ± 9.4 | 71.8 ± 11.3 | .347 |
| Age groups, years | <.005 | |||
| ≤49 | 19 (2.2) | 10 (1.6) | 9 (3.7) | |
| 50 – 59 | 94 (10.6) | 66 (10.3) | 28 (11.5) | |
| 60 – 69 | 225 (25.4) | 173 (27.0) | 52 (21.3) | |
| 70 – 79 | 358 (40.5) | 272 (42.4) | 86 (35.3) | |
| ≥80 | 189 (21.4) | 120 (18.7) | 69 (28.3) | |
| Smokinga | <.001 | |||
| Ever-smoker | 515 (74.4) | 455 (87.7) | 60 (34.7) | |
| Non-smoker | 177 (25.6) | 64 (12.3) | 113 (65.3) | |
| Occupationb | ||||
| Professional & engineering | 44 (7.2) | 34 (7.4) | 10 (6.4) | |
| Administrative & managerial | 10 (1.6) | 4 (0.9) | 6 (3.8) | |
| Clerical | 55 (8.9) | 43 (9.4) | 12 (7.6) | |
| Sales | 43 (7.0) | 26 (5.7) | 17 (10.8) | |
| Service | 27 (4.4) | 11 (2.4) | 16 (10.2) | |
| Security | 9 (1.5) | 9 (2.0) | – | |
| Agriculture, forestry & fishery | 26 (4.2) | 21 (4.6) | 5 (3.2) | |
| Transport & communication | 36 (5.9) | 34 (7.4) | 2 (1.3) | |
| Manufacturing and general labor | 215 (35.0) | 200 (43.7) | 15 (9.6) | |
| Housewife | 46 (7.5) | – | 46 (29.3) | |
| Jobless | 54 (8.8) | 34 (7.4) | 20 (12.7) | |
| Others | 50 (8.1) | 42 (9.2) | 8 (5.1) | |
Parenchymal lung and pleural abnormalities
Radiographic findings of the 885 patients are presented in Table 2. Of 125 cases (14.1%) found to have pleural abnormalities on CT, we excluded 12 cases with changes mainly in the visceral pleura and recognized 113 cases (12.8%) as having pleural plaques. Chest radiographs identified pleural plaques in 48 cases, or 5.4% (44 males, 6.9%; and 4 females, 1.6%); both methods agreed on 38 cases (4.3%). Examples of pleural plaques detected on chest CT and CXR are presented in Fig. 1. The number of cases with small irregular opacities detected by CT and CXR was 107 cases (12.1%) and 71 cases (8.0%), respectively. The number of concordant findings was 56 cases (6.3%). The two methods showed small rounded opacities in 15 cases (1.7%), with concordance in 13 cases (1.5%). Chest CT identified large opacities in 8 cases (0.9%) while CXR indicated these in 6 cases (0.7%); and the two agreed on 5 cases (0.6%).
Table 2.
Radiological findings of the 885 patients with primary lung cancer
| Number (%) | ||||||
|---|---|---|---|---|---|---|
| CT | CXR | CT & CXR | ||||
|
a The presence of pleural plaques on CT was defined by the categories “definite” and “probable”. CT = computed tomography, CXR = chest radiography, CT & CXR = identified using both methods | ||||||
| Pleural plaquesa | 113 | (12.8) | 48 | (5.4) | 38 | (4.3) |
| Pleural abnormalities | ||||||
| Definite | 80 | (9) | ||||
| Probable | 33 | (3.7) | ||||
| Possible | 52 | (5.9) | ||||
| None | 720 | (81.4) | ||||
| Parenchymal abnormalities | ||||||
| Irregular opacities | 107 | (12.1) | 71 | (8.0) | 56 | (6.3) |
| Small rounded opacities | 15 | (1.7) | 15 | (1.7) | 13 | (1.5) |
| Large opacities | 8 | (0.9) | 6 | (0.7) | 5 | (0.6) |
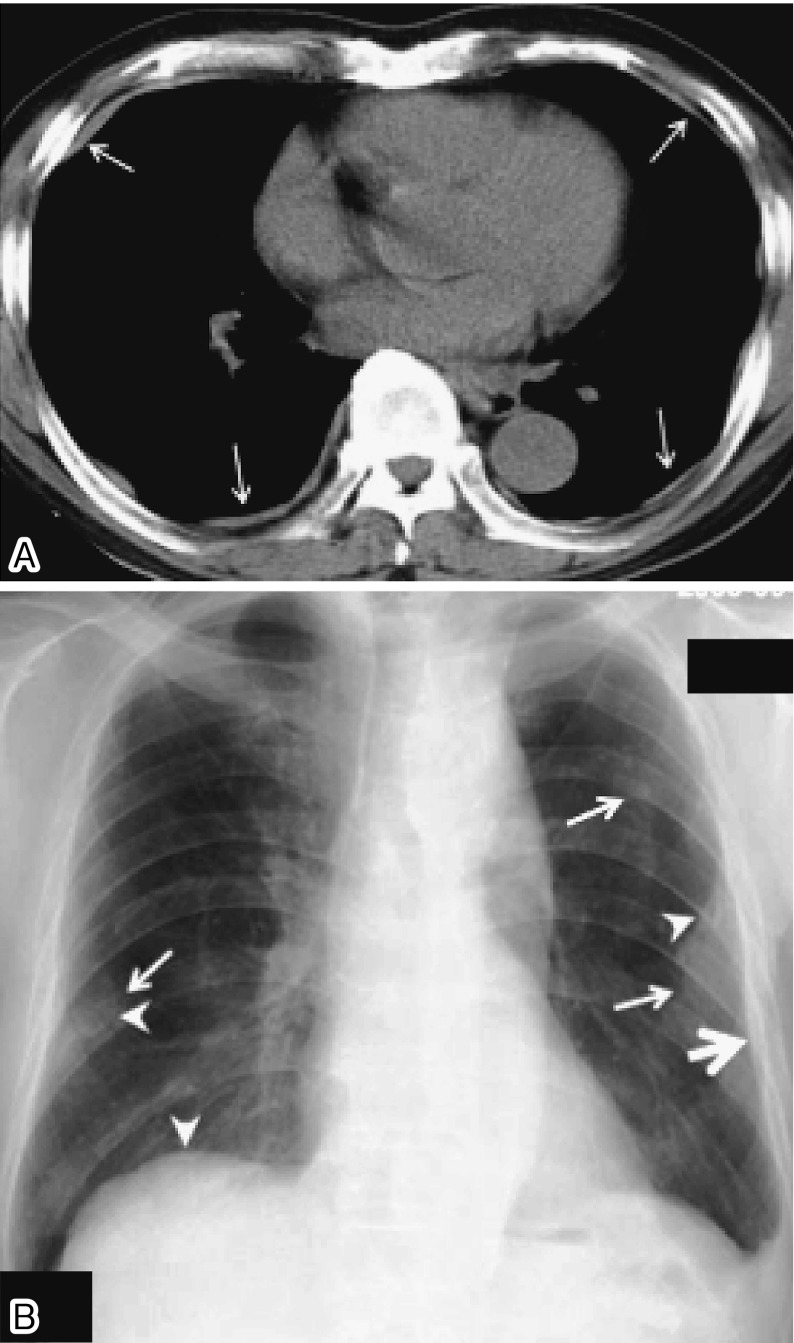
Fig. 1.
Examples of pleural plaques on (A) a chest CT (arrows indicate pleural plaques) and (B) a chest radiograph (arrows indicate face-on plaques, thick arrow indicates in-profile plaque, and arrowheads indicate calcified plaques)
Characteristics of 113 patients with pleural plaques on their CT are found in Table 3. A higher prevalence of pleural plaques was seen in males, older age groups, and among smokers. Anatomically, involvement of the chest wall pleura was frequent (109 cases, 96.5%) and the left side predominated (81 cases, 71.7%). Calcification of plaques was detected in 47 cases (41.6%), and the coexistence of irregular opacities in 22 cases (19.5%).
Table 3.
Characteristics of 113 patients with pleural plaques on chest CT
| Number | (%) | ||
|---|---|---|---|
|
a Information on smoking was available for 692 cases (78.2%). b Only plaques on the chest wall pleura were considered. | |||
| Gender | Male | 101 | (15.8) |
| Female | 12 | (4.9) | |
| Age, year | ≤49 | – | – |
| 50 – 59 | 8 | (8.5) | |
| 60 – 69 | 32 | (14.2) | |
| 70 – 79 | 51 | (14.3) | |
| ≥80 | 22 | (11.6) | |
| Smokinga | Ever-smoker | 79 | (15.3) |
| Non-smoker | 16 | (9.0) | |
| Location | Chest wall | 109 | (96.5) |
| Diaphragm | 27 | (23.9) | |
| Mediastinum | 11 | (9.7) | |
| Chest involvementb | Bilateral | 43 | (38.1) |
| Left | 81 | (71.7) | |
| Right | 67 | (59.3) | |
| Calcification | 47 | (41.6) | |
| Irregular opacities | 22 | (19.5) | |
| Histology of lung cancer | Adenocarcinoma | 46 | (40.7) |
| Squamous Cell | 37 | (32.7) | |
| Large Cell | 3 | (2.7) | |
| Small Cell | 15 | (13.3) | |
| Others/Unidentified | 12 | (10.6) | |
Histologically, the most frequent cell type was adenocarcinoma, which was found in 408 cases (46.1%), followed by squamous cell (264 patients, 29.8%), and small cell (106 patients, 12%) (Table 4). The distribution of cell types differed significantly by sex (p < 0.001) and smoking habit (p < 0.001); however, no distinct histology of primary lung cancer was observed between patients with or without pleural plaques.
Table 4.
Histological cell types of 885 patients with primary lung cancer according to sex, smoking status, and the presence of pleural plaques
| Number (%) | |||||||
|---|---|---|---|---|---|---|---|
| All | Gender | Smokinga | Pleural plaque | ||||
| 885 | Male | Female | Ever | Non | Present | Absent | |
| 641 | 244 | 515 | 177 | 113 | 772 | ||
| Type | (72.4) | (27.6) | (74.4) | (25.6) | (12.8) | (87.2) | |
|
a Information on smoking was available for 692 cases (78.2%); Ever = Ever-smoker, Non = Non-smoker. p = p-value for Chi-squared test or Fisher's exact test as appropriate. | |||||||
| Adenocarcinoma | 408 | 242 | 166 | 194 | 120 | 46 | 362 |
| (46.1) | (37.8) | (68.0) | (37.7) | (67.8) | (40.7) | (46.9) | |
| Squamous cell | 264 | 234 | 30 | 186 | 30 | 37 | 227 |
| (29.8) | (36.5) | (12.3) | (36.1) | (17.0) | (32.7) | (29.4) | |
| Large cell | 22 | 14 | 8 | 15 | 3 | 3 | 19 |
| (2.5) | (2.2) | (3.3) | (2.9) | (1.7) | (2.7) | (2.5) | |
| Small cell | 106 | 90 | 16 | 74 | 10 | 15 | 91 |
| (12.0) | (14) | (6.6) | (14.4) | (5.7) | (13.3) | (11.8) | |
| Others/unidentified | 85 | 61 | 24 | 46 | 14 | 12 | 73 |
| (9.6) | (9.5) | (9.8) | (8.9) | (7.9) | (10.6) | (9.5) | |
| p | <.001 | <.001 | .768 | ||||
As shown in Table 5, among the major occupational categories, the prevalence of pleural plaques was highest among security workers (4 cases, 44.4%), manufacturing and general laborers (52 cases, 24.2%), and transport and communication workers (5 cases, 13.9%); whereas the prevalence of irregular opacities was highest among security workers (3 cases, 33.3%), manufacturing and general laborers (45 cases, 20.9%), and among the jobless (10 cases, 18.5%). A further analysis indicated that, among subcategories of manufacturing and general laborers with more than 10 patients with primary lung cancer, the prevalence of pleural plaques was highest among construction-related workers (37.7%), followed by metal processing (26.7%), civil construction laborers (26.7%), foundry workers (23.5%), miners (22.2%), and electricians (21.4%).
Table 5.
Prevalence of CT identified pleural plaques or irregular opacities by occupation
| All | Pleural plaques | Irregular opacities | |
|---|---|---|---|
| Number of casesa | 615† | 80¶ | 86§ |
| Occupation | Number (%) | ||
|
a Number of cases which can be allocated into a particular occupation. † Among the 885 patients with primary lung cancer. ¶ Among the 113 cases with pleural plaques on chest CT. § Among the 107 cases with irregular opacities on chest CT. | |||
| Professional & engineering | 44 | 2 (4.6) | 6 (13.6) |
| Administrative & managerial | 10 | – | 1 (10.0) |
| Clerical | 55 | 4 (7.3) | 5 (9.1) |
| Sales | 43 | 2 (4.7) | 3 (7.0) |
| Service | 27 | 2 (7.4) | – |
| Security | 9 | 4 (44.4) | 3 (33.3) |
| Agriculture, forestry & fishery | 26 | – | 3 (11.5) |
| Transport & communication | 36 | 5 (13.9) | 3 (8.3) |
| Manufacturing and general labor | 215 | 52 (24.2) | 45 (20.9) |
| Housewife | 46 | 3 (6.5) | 3 (6.5) |
| Jobless | 54 | 2 (3.7) | 10 (18.5) |
| Other | 50 | 4 (8.0) | 4 (8.0) |
Discussion
Many investigators have evaluated the relationship between CT-identified pleural plaques and respiratory impairment or associated long-term health risks among asbestos-exposed populations15-18); however, no published study has yet documented CT-identified pleural plaques among primary lung cancer patients. To our knowledge, this is the first report concerning CT-identified asbestos-related pleural findings among a large number of subjects with primary lung cancer. The main finding of this study is that 12.8% of the study population has pleural plaques on chest CT. Since pleural plaques are the most common radiological manifestation of benign asbestos-related pleural disease and considered an indicator of past asbestos exposure5-7), we suggest that at least 12.8% of our patients have been potentially exposed to asbestos, either occupationally or environmentally.
Since the prevalence of pleural plaques varies widely between studies, our findings must be considered alongside evidence from other epidemiological studies. In one such study, the reported prevalence of pleural abnormalities, pleural plaques and diffuse pleural thickening was 1.5% among 2633 chest CTs taken between 2009 and 2011 in the United States19). That study considered a general population of white, middle-class Americans, compared to our study of Japanese lung cancer patients, and included a younger age group (59.2±12.1 years compared to 71.3±9.9 years) and a higher proportion of females (50.3% compared to 27.6%). Another study documented pleural plaques on 5.1% of 1482 chest CTs from a radiological database of a university hospital in Italy20). Although this hospital-based Italian study reviewed chest CTs which were taken for various clinical indications including suspected pulmonary embolism or neoplasms, the investigators did not provide information such as age, gender, asbestos exposure, or the period in which the CT scans were obtained for the entire cohort. A large CT screening, conducted between 2003 and 2005 in France, detected pleural plaques in 15.9% of 5545 chest CTs of asbestos-exposed workers21). In the French study, the population consisted exclusively of retired male asbestos-exposed workers; the average age (63.5±5.7 years) was younger than ours (71.1±9.4 years). These discrepancies among these studies might be attributable to any of several factors related to the subjects enrolled (such as age, sex, exposure to risk factors), the technology employed in CT imaging, and the definition applied for pleural plaques in each study.
Our findings were derived from the information of relatively large cohort (n = 885), pathological confirmation of primary lung cancer, and accurate determination of pleural plaques based on CT interpretation by a panel of experts. The cases in our study were from a group of hospitals that also operate clinics specializing in occupational medicine; accordingly, patients attending these hospitals may represent a higher proportion of workers than the general Japanese population. Our study may have included a greater percentage of asbestos-exposed individuals than occurs in the general population. Nevertheless, the gender distribution in our cases was close to that seen in the lung cancer registry of the general Japanese population22). In contrast, males dominate in asbestos-related23) or occupationally acquired lung cancer cases24).
In the cases we analyzed, the frequency of lung cancer cell types was lower for adenocarcinoma and higher for squamous cell and small cell cancers than that seen in a nationwide Japanese primary lung cancer population22). The average age and gender distribution of the cases were comparable between the two studies. In our study, no distinct cell type was observed between patients with and without pleural plaques. All major histological types of lung cancer can be related to asbestos5); accordingly, the difference in histology distribution from the nationwide report may not affect the prevalence of pleural plaques in our patients. Although smoking information was not reported in the nationwide study, this difference in cell type was thought to be attributable to the smoking habits found in our population.
We found that 12.8% of our study population has pleural plaques; frequency was higher among smokers, 15.3%, compared to 9% among non-smokers. Some investigators consider smoking to be associated with the higher prevalence of pleural plaques25); however, there was no support for a causal relationship between smoking habits and pleural plaques26). We believe that the higher prevalence of smoking among our cases will not significantly affect the result of this study.
Pleural plaques solely involve the parietal pleura and are considered highly specific for asbestos exposure. Diffuse pleural thickening, on the other hand, involves visceral pleura and is less specific, and may have various etiologies5-7). In some cases, it is difficult to distinguish the two conditions based on CXR. However, in this study, we determined pleural plaques based on CT interpretation by a panel of experts, employed a strict CT definition in diagnosis, and considered only those lesions that originated from the parietal pleura and omitted lesions involving the visceral pleura. Thus, it is possible that our study underestimated the actual proportion of pleural plaques and hence asbestos exposure. Since CT has higher sensitivity and specificity than CXR in evaluating pleural abnormalities13,14), misclassification of pleural plaques in favor of sensitivity is unlikely. On the other hand, CT could have missed some cases of pleural plaques. As Yusa et al.27) reported in 30 cases with surgically confirmed pleural plaques, 12 cases were undetected in a retrospective CT-examination study. Moreover, the absence of pleural plaques does not necessarily preclude previous asbestos exposure.
Given that 80 - 90% of radiologically identified plaques are attributable to occupational asbestos exposure in non-endemic regions5), we considered that occupation has an impact on the occurrence of pleural plaques in our patients. Due to the retrospective nature of the present study, occupational information of the cases was limited. However, we could assign 69% of the patients into a particular occupation, allowing us to examine the frequency of pleural plaques on the basis of occupational categories. We found that more than half of our cases with pleural plaques (65%, 52 of the 80 cases of whom occupation could be allocated) consisted of manufacturing and general laborers. Subgroup-analysis of these manufacturing and general laborers revealed that the prevalence of pleural plaques was highest among construction-related workers (37.7%), followed by metal processing (26.7%) and civil construction laborers (26.7%). Since the majority of asbestos-containing products were used in construction materials, automobiles, and industrial machines1); special medical attention should be paid to individuals with a history of employment in these occupations as they also are listed under occupations with high risk of asbestos exposure, published by Ministry of Health, Labor and Welfare9). Additionally, we have noted that the prevalence of pleural plaques was considerably higher among security workers (4 cases, 44.4%), and transport and communication workers (5 cases, 13.9%). However, the small number of cases in these categories make it difficult to draw any meaningful conclusions.
Regarding our imaging methods, CT can identify pleural plaques in locations that are hidden in CXR, such as the anterior chest wall and paravertebral regions. In addition, CT can distinguish rib fractures, extrapleural fat, or thoracic muscle which may mimic pleural plaques and give false positives in CXR13). In this study, among the 885 patients with primary lung cancer, CXR identified pleural plaques in 48 cases (5.4 %), while CT detected plaques in 113 cases (12.8%). Of the 48 cases positive for pleural plaques with CXR, 10 were found to be for other causes, such as pleurisy, diffuse pleural thickening, extrapleural fat, or muscle on CT evaluation. This showed that CT is more sensitive and specific than CXR in characterizing pleural abnormalities. The use of CT for better characterization of pleural abnormalities has important medico-legal implications. Pleural plaques are considered to be an early manifestation of asbestos-related diseases6,7), and could be an independent risk factor in asbestos-related lung cancer16). Early recognition in individuals with likely asbestos exposure is vital to health care. Moreover, identification of pleural plaques in individuals without an established history of occupational exposure could be a trigger for authorities to initiate epidemiological surveillance. Additionally, the compensation system for asbestos-related lung cancer in Japan requires, in addition to occupational history of asbestos exposure, the presence of either radiologically confirmed asbestosis or pleural plaques28).
From the present study, we cannot postulate a causal relationship between asbestos exposure and the development of lung cancer in our cases with pleural plaques. The proportion of smokers was fairly high among our cases (74.4%) compared to the general population29), and tobacco smoking is a well-known risk factor for lung cancer. In addition, asbestos-exposed workers have frequently been exposed to other occupational carcinogens, such as welding fumes and polycyclic aromatic hydrocarbons. In female cases, exposure to environmental radon and air pollutants is more likely to occur than to asbestos. It is difficult to allocate the relative contributions of exposure to asbestos or other carcinogens and smoking in the pathogenesis of lung cancer. Despite the reported independent risk of pleural plaques in lung cancer16), the issue remains controversial30), and more importantly, pleural plaques may develop in situations with relatively low asbestos exposure. In addition, there is no distinct cell type to distinguish asbestos-related lung cancer from other lung cancers6,23). However, this study showed that more than one-tenth of patients with primary lung cancer may have experienced a possible occupational asbestos exposure.
Since the import and use of asbestos in Japan peaked in the 1970s and 1980s, and given a latency of more than 40 years23), a considerable number of asbestos-related lung cancers is expected at present, and increasingly in the coming decades. In Japan, lung cancer incidence has steadily increased in both sexes31), and accounted for 15.3% of all new cancers in males and 10.0% in females in 201229). In the year 2012, there were 113,047 incident lung cancer cases. The number of these cases that can be attributed to asbestos exposure is unknown.
Japan imposed a total ban on asbestos use since 2012; however, there is a growing concern about environmentally acquired asbestos-related diseases. Given that the majority (roughly 70 - 90%) of imported asbestos was used in the production of asbestos-containing cement products for building materials and in construction1), the main potential source of public exposure of concern today is from the demolition of old asbestos-containing buildings. It is anticipated that the number of demolished buildings containing asbestos will continue to increase until 20301). There is a high risk of significant asbestos dispersal in work areas, and also of environmental pollution. It is crucial to implement safe practices for the management and removal of asbestos to safeguard against the dispersal of asbestos into environment.
Conclusion
The prevalence of pleural plaques in our study differs to some extent from other population studies. Results must be cautiously interpreted due to the fact that this hospital-based study was comprised solely of patients with primary lung cancer, as well as a population representing a higher percentage of workers than the general Japanese population. Our results show that 12.8% of patients with primary lung cancer have pleural plaques on chest CT. Notably, frequency was highest among individuals with an employment history related to construction, suggesting that these patients have experienced a possible occupational asbestos exposure. Given that pleural plaques are early manifestations of asbestos exposure, and that asbestos exposure is associated with an increased risk of malignancies, early recognition of pleural plaques in at-risk populations is important to initiate proper health surveillance and early detection of malignancies.
Conflicts of interest: None declared.
References
- 1). Furuya S, Takahashi K, Movahed M, et al. National asbestos profile of Japan. Based on the National asbestos profile by the ILO and the WHO. [Online]. [cited 2013 ]; Available from: URL: http://envepi.med.uoeh-u.ac.jp/NAPJ.pdf
- 2). Gemba K, Fujimoto N, Kato K, et al. National survey of malignant mesothelioma and asbestos exposure in Japan. Cancer Science 2012; 103 (3): 483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Wang X, Yano E, Qiu H, et al. A 37-year observation of mortality in Chinese chrysotile asbestos workers. Thorax 2012; 67 (2): 106-110. [DOI] [PubMed] [Google Scholar]
- 4). Tomioka K, Natori Y, Kumagai S, et al. An updated historical cohort mortality study of workers exposed to asbestos in a refitting shipyard, 1947-2007. International Archives of Occupational and Environmental Health 2011; 84 (8): 959-967. [DOI] [PubMed] [Google Scholar]
- 5). Wolff H, Vehmas T, Oksa P, et al. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scandinavian Journal of Work, Environment & Health 2015; 41 (1): 5-15. [DOI] [PubMed] [Google Scholar]
- 6). Nielsen LS, Bælum J, Rasmussen J, et al. Occupational asbestos exposure and lung cancer--a systematic review of the literature. Archives of Environmental & Occupational Health 2014; 69 (4): 191-206. [DOI] [PubMed] [Google Scholar]
- 7). Cugell DW, Kamp DW. Asbestos and the pleura: a review. Chest 2004; 125 (3): 1103-1117. [DOI] [PubMed] [Google Scholar]
- 8). Employment Service Bureau. Classification of occupations for employment services (ESCO)-1999 revision: Employment Information Center, Ministry of Labor, Japan: (In Japanese). [Google Scholar]
- 9). Ministry of Health, Labour and Welfare. Asbestos-related works. In: Guidance for identifying asbestos exposure history. Tokyo, Japan: Government of Japan; 2006. p. 10-54. [Online]. [cited 2017 Dec. 16]; Available from: URL: http://www.jaish.gr.jp/information/mhlw/sekimen/h18_tebiki.html (In Japanese).
- 10). ILO. Guidelines for the use of the ILO International Classification of Radiographs of Pneumoconioses, Revised Edition 2000. In: Occupational Safety and Health Series No.22. Geneva: International Labour Office. [Google Scholar]
- 11). Kusaka Y, Hering KG, Parker JE. International classification of HRCT for occupational and environmental respiratory diseases. Tokyo: Springer; 2005. [Google Scholar]
- 12). Suganuma N, Kusaka Y, Hering KG, et al. Reliability of the proposed international classification of high-resolution computed tomography for occupational and environmental respiratory diseases. Journal of Occupational Health 2009; 51 (3): 210-222. [DOI] [PubMed] [Google Scholar]
- 13). Norbet C, Joseph A, Rossi SS, et al. Asbestos-related lung disease: a pictorial review. Current Problems in Diagnostic Radiology 2015; 44 (4): 371-382. [DOI] [PubMed] [Google Scholar]
- 14). Terra-Filho M, Bagatin E, Nery LE, et al. Screening of miners and millers at decreasing levels of asbestos exposure: comparison of chest radiography and thin-section computed tomography. PLoS One 2015; 10 (3): e0118585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Kopylev L, Christensen KY, Brown JS, et al. A systematic review of the association between pleural plaques and changes in lung function. Occupational and Environmental Medicine 2015; 72 (8): 606-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Pairon JC, Andujar P, Rinaldo M, et al. Asbestos exposure, pleural plaques, and the risk of death from lung cancer. American Journal of Respiratory and Critical Care Medicine 2014; 190 (12): 1413-1420. [DOI] [PubMed] [Google Scholar]
- 17). Vehmas T, Oksa P, Kivisaari L. Lung and pleural CT signs predict deaths: 10-year follow-up after lung cancer screening of asbestos-exposed workers. International Archives of Occupational and Environmental Health 2012; 85 (2): 207-213. [DOI] [PubMed] [Google Scholar]
- 18). Clin B, Paris C, Ameille J, et al. Do asbestos-related pleural plaques on HRCT scans cause restrictive impairment in the absence of pulmonary fibrosis? Thorax 2011; 66 (11): 985-991. [DOI] [PubMed] [Google Scholar]
- 19). Araki T, Yanagawa M, Sun FJ, et al. Pleural abnormalities in the Framingham Heart Study: prevalence and CT image features. Occupational and Environmental Medicine 2017; 74 (10): 756-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Mazzei MA, Contorni F, Gentili F, et al. Incidental and Underreported Pleural Plaques at Chest CT: Do Not Miss Them-Asbestos Exposure Still Exists. BioMed Research International 2017; 2017: 6797826. (doi: 10.1155/2017/6797826). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Paris C, Thierry S, Brochard P, et al. Pleural plaques and asbestosis: dose- and time-response relationships based on HRCT data. European Respiratory Journal 2009; 34 (1): 72-79. [DOI] [PubMed] [Google Scholar]
- 22). Sawabata N, Asamura H, Goya T, et al. Japanese Lung Cancer Registry Study: first prospective enrollment of a large number of surgical and nonsurgical cases in 2002. Journal of Thoracic Oncology 2010; 5 (9): 1369-1375. [DOI] [PubMed] [Google Scholar]
- 23). Kishimoto T, Gemba K, Fujimoto N, et al. Clinical study of asbestos-related lung cancer in Japan with special reference to occupational history. Cancer Science 2010; 101 (5): 1194-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Ahn YS, Jeong KS. Epidemiologic characteristics of compensated occupational lung cancers among Korean workers. Journal Of Korean Medical Science 2014; 29 (11): 1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). McMillan GH, Pethybridge RJ, Sheers G. Effect of smoking on attack rates of pulmonary and pleural lesions related to exposure to asbestos dust. British Journal of Industrial Medicine 1980; 37 (3): 268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Edelman DA. Asbestos exposure, pleural plaques and the risk of lung cancer. International Archives of Occupational and Environmental Health 1988; 60 (6): 389-393. [DOI] [PubMed] [Google Scholar]
- 27). Yusa T, Yasukawa T, Moriya Y, et al. Detectability of pleural plaque by chest imaging in patients with pleural plaques which were identified from surgical findings. Japanese Journal of Occupational Medicine and Traumatology 2008; 56 (6): 215-220 (English abstract, Article in Japanese). [Google Scholar]
- 28). Ministry of Health, Labour and Welfare. Criteria of asbestos-related lung cancer. In: Recognition standards for asbestos-related diseases (Revised 2012). Tokyo, Japan: Government of Japan. [Online]. [cited 2017 Dec. 18]; Available from: URL: http://www.mhlw.go.jp./houdou/2006/02/h0209-1.html (In Japanese).
- 29). Center for Cancer Control and Information Services. Cancer statistics in Japan-2016. National Cancer Center, Japan. [Online]. [cited 2017 Dec. 18]; Available from: URL: http://ganjoho.jp/reg_stat/statistics/brochure/backnumber/2016_jp.html
- 30). Maxim LD, Niebo R, Utell MJ. Are pleural plaques an appropriate endpoint for risk analyses? Inhalation Toxicology 2015; 27 (7): 321-334. [DOI] [PubMed] [Google Scholar]
- 31). Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958-2013. Japanese Journal of Clinical Oncology 2015; 45 (4): 390-401. [DOI] [PubMed] [Google Scholar]