Abstract
Objective
To examine the potential for international travel to spread yellow fever virus to cities around the world.
Methods
We obtained data on the international flight itineraries of travellers who departed yellow fever-endemic areas of the world in 2016 for cities either where yellow fever was endemic or which were suitable for viral transmission. Using a global ecological model of dengue virus transmission, we predicted the suitability of cities in non-endemic areas for yellow fever transmission. We obtained information on national entry requirements for yellow fever vaccination at travellers’ destination cities.
Findings
In 2016, 45.2 million international air travellers departed from yellow fever-endemic areas of the world. Of 11.7 million travellers with destinations in 472 cities where yellow fever was not endemic but which were suitable for virus transmission, 7.7 million (65.7%) were not required to provide proof of vaccination upon arrival. Brazil, China, India, Mexico, Peru and the United States of America had the highest volumes of travellers arriving from yellow fever-endemic areas and the largest populations living in cities suitable for yellow fever transmission.
Conclusion
Each year millions of travellers depart from yellow fever-endemic areas of the world for cities in non-endemic areas that appear suitable for viral transmission without having to provide proof of vaccination. Rapid global changes in human mobility and urbanization make it vital for countries to re-examine their vaccination policies and practices to prevent urban yellow fever epidemics.
Résumé
Objectif
Évaluer la capacité des déplacements internationaux à propager le virus de la fièvre jaune dans des villes du monde entier.
Méthodes
Nous avons recueilli des données sur les itinéraires de vols internationaux de voyageurs qui quittaient des zones d'endémie de la fièvre jaune à travers le monde en 2016 pour se rendre dans des villes où la fièvre jaune était endémique ou qui étaient propices à la transmission virale. À l'aide d'un modèle écologique mondial de transmission du virus de la dengue, nous avons prédit le caractère approprié de villes situées dans des zones non endémiques pour la transmission de la fièvre jaune. Nous avons obtenu des informations sur les conditions nationales d'entrée relatives à la vaccination contre la fièvre jaune dans les villes d'arrivée des voyageurs.
Résultats
En 2016, 45,2 millions de passagers de vols internationaux ont quitté des zones d'endémie de la fièvre jaune situées dans le monde entier. Sur 11,7 millions de voyageurs à destination de 472 villes où la fièvre jaune n'était pas endémique, mais qui étaient propices à la transmission du virus, 7,7 millions (65,7%) n'ont pas eu à fournir de preuve de vaccination à leur arrivée. C'est au Brésil, en Chine, aux États-Unis d'Amérique, en Inde, au Mexique et au Pérou que les volumes de voyageurs arrivant de zones d'endémie de la fièvre jaune étaient les plus élevés et que le nombre de personnes vivant dans des villes propices à la transmission de la fièvre jaune était le plus important.
Conclusion
Chaque année, des millions de voyageurs quittent, sans avoir à fournir de preuve de vaccination, des zones d'endémie de la fièvre jaune à travers le monde pour se rendre dans des villes situées dans des zones non endémiques qui s'avèrent propices à la transmission virale. Compte tenu de l'évolution rapide de la mobilité humaine et de l'urbanisation dans le monde entier, il est indispensable que les pays réexaminent leurs politiques et pratiques de vaccination pour prévenir les épidémies de fièvre jaune en milieu urbain.
Resumen
Objetivo
Examinar el potencial de los viajes internacionales de propagar el virus de la fiebre amarilla en ciudades de todo el mundo.
Métodos
Se obtuvieron datos de los itinerarios de vuelo internacionales de viajeros que partieron de áreas endémicas de la fiebre amarilla en el mundo en 2016 hacia ciudades donde la fiebre amarilla era endémica o que eran adecuadas para la transmisión viral. Mediante el uso de un modelo ecológico global de la transmisión del virus del dengue, se predijo la idoneidad de las ciudades en áreas no endémicas para la transmisión de la fiebre amarilla. Se obtuvo información sobre los requisitos nacionales de entrada sobre la vacunación contra la fiebre amarilla en las ciudades de destino de los viajeros.
Resultados
En 2016, 45,2 millones de viajeros de vuelos internacionales salieron de áreas endémicas de fiebre amarilla en el mundo. De los 11,7 millones de viajeros con destinos en 472 ciudades donde la fiebre amarilla no era endémica, pero eran aptas para la transmisión viral, 7,7 millones (65,7%) no estaban obligados a proporcionar una prueba de vacunación al llegar. Brasil, China, la India, México, Perú y los Estados Unidos tenían los mayores volúmenes de viajeros que provenían de áreas endémicas de fiebre amarilla y las mayores poblaciones en ciudades adecuadas para la transmisión de la fiebre amarilla.
Conclusión
Cada año, millones de viajeros abandonan las áreas endémicas de la fiebre amarilla del mundo hacia ciudades de áreas no endémicas que parecen adecuadas para la transmisión viral sin tener que presentar una prueba de vacunación. Los rápidos cambios globales en la movilidad humana y la urbanización hacen que sea vital que los países reexaminen sus políticas y prácticas de vacunación para prevenir las epidemias urbanas de fiebre amarilla.
ملخص
الغرض
فحص احتمالية أن يؤدي السفر حول العالم إلى انتشار فيروس الحمّى الصفراء في مدن العالم.
الطريقة
لقد حصلنا على مجموعة من البيانات بشأن قوائم الرحلات الدولية التي تضم المسافرين الذين غادروا من المناطق التي تعاني من الحمّى الصفراء حول العالم في عام 2016، متجهين إلى مدن كانت تعاني من انتشار الحمّى الصفراء بها أو التي كانت مواتية لانتشار العدوى الفيروسية بها. وباستخدام نموذج بيئي عالمي لانتقال فيروس الضنك، قمنا بالتنبؤ بمدى ملاءمة المدن التي تقع في مناطق غير مصابة بالحمّى لانتقال الحمّى الصفراء إليها. وحصلنا على معلومات عن المتطلبات الوطنية للسماح بإدخال المسافرين فيما يتعلق بتطعيم الحمّى الصفراء في المدن التي يتجه إليها المسافرون.
النتائج
في عام 2016، غادر 45.2 مليون مسافر عبر الرحلات الجوية الدولية من مناطق تعاني من الحمّى الصفراء حول العالم. ومن بين 11.7 مليون مسافر اتجهوا إلى 472 مدينة لم تعاني من انتشار الحمّى الصفراء بها لكنها كانت ملائمة لانتقال الفيروس بها، لم يكن 7.7 مليون من المسافرين (بنسبة 65.7%) مُطالبين بتقديم إثبات حول حصولهم على التطعيم عند الوصول. وقد تلقت البرازيل والصين والهند والمكسيك وبيرو والولايات المتحدة الأمريكية الأعداد الأكبر من المسافرين الذين وصلوا من مناطق مصابة بالحمّى الصفراء وكانوا يمثلون نسبة السكان الأكبر الذي يعيشون في مدن ملائمة لانتقال الحمّى الصفراء إليها.
الاستنتاج
يغادر الملايين من المسافرين في كل عام من مناطق مصابة بالحمّى الصفراء حول العالم إلى مدن تقع في مناطق غير مصابة بالعدوى والتي تبدو أنها ملائمة لانتقال الفيروس إليها دون الحاجة إلى تقديم إثبات على الحصول على التطعيم. إن التغييرات العالمية السريعة في حركة البشر وتكوين المجتمعات الحضرية تحتم على البلدان إعادة النظر في سياسات وممارسات توفير التطعيمات لديها لتفادي حدوث عدوى الحمّى الصفراء.
摘要
目的
审核黄热病病毒通过国际旅行在全球各城市传播的可能性。
方法
我们获取了相关旅客国际航班线路的行程信息。这些旅客于 2016 年从全球黄热病流行区出发前往各个城市。这些目的地城市要么是黄热病流行区,要么是病毒易感区。我们运用登革热病毒传播的全球生态模型预测非病区城市的黄热病易感性。我们获取了旅客抵达的目的地城市针对黄热病疫苗接种的入境要求信息。
结果
2016 年,4520 万搭乘国际航班的旅客从全球黄热病流行区出发。其中,1170 万旅客的目的地位于 472 个非黄热病流行区的城市,但这些城市属于病毒易感区,770 万 (65.7%) 的旅客在抵达时并未被要求提供疫苗接种证明。巴西、秘鲁、墨西哥、美国、印度和中国拥有最多来自黄热病流行区的旅客,同时这些国家城市人口最为密集,是黄热病病毒传播的易感区。
结论
每年都有数百万旅客从全球黄热病流行区离开并前往非病区但易于传播病毒的城市,并且无需提供疫苗接种证明。随着全球人口移动性的增加和城市化进程的加快,于各国而言,重新审视其疫苗接种政策和惯例以预防黄热病爆发至关重要
Резюме
Цель
Изучить потенциальное влияние международных поездок на распространение вируса желтой лихорадки в городах по всему миру.
Методы
Авторы получили данные о маршрутах международных перелетов путешественников, которые в 2016 году отправлялись из районов мира, эндемичных по желтой лихорадке, для тех городов, где желтая лихорадка не была эндемичной или в которых были благоприятные условия для передачи вируса. Используя глобальную экологическую модель передачи вируса денге, авторы предсказали наличие благоприятных условий для передачи желтой лихорадки в городах в неэндемичных районах. Авторы получили информацию о национальных требованиях на въезд для вакцинации против желтой лихорадки в городах назначения путешественников.
Результаты
В 2016 году 45,2 миллиона пассажиров международных авиарейсов совершили вылеты из районов мира, эндемичных по желтой лихорадке. Из 11,7 миллиона путешественников с пунктами назначения в 472 городах, где желтая лихорадка не была эндемичной, но в которых имелись благоприятные условия для передачи вируса, 7,7 миллиона (65,7%) не были обязаны по прибытии предоставлять подтверждающие документы о вакцинации. В Бразилии, Индии, Китае, Мексике, Перу и Соединенных Штатах Америки было наибольшее число путешественников, прибывающих из районов, эндемичных по желтой лихорадке. А также в этих странах наибольшее число людей проживает в городах с благоприятными условиями для передачи вируса желтой лихорадки.
Вывод
Каждый год миллионы путешественников отправляются из районов мира, эндемичных по желтой лихорадке, в города в неэндемичных районах, которые, по-видимому, пригодны для передачи вируса, без необходимости предоставления подтверждающих документов о вакцинации. Стремительные глобальные изменения в мобильности людей и урбанизации делают для стран жизненно важным вопрос пересмотра своей политики и практики вакцинации для предотвращения эпидемий желтой лихорадки в городах.
Introduction
In December 2015, Angola reported its first locally acquired case of yellow fever in nearly a decade. The ensuing epidemic was first recognized in Luanda, then spread across Angola’s 18 provinces, resulting in 4347 suspected or confirmed cases and 377 deaths.1 International travellers departing from Angola then imported yellow fever virus into Kenya and the Democratic Republic of the Congo,2 where another epidemic ensued, causing 2987 suspected or confirmed cases and 121 deaths.1 Furthermore, 11 foreign workers infected in Angola travelled to urban centres in China, the first time imported cases of yellow fever have been reported in Asia.3 Four cases were recently imported into Europe over an 8-month period by travellers returning from South America.4 The time period is in stark contrast to the 27 years during which the previous four cases of travel-associated yellow fever were imported into Europe.4 In early 2018, nine cases were exported from Brazil and led to three deaths.5 Increased air travel and globalization is making it easier for humans to transport yellow fever virus across international borders, potentially catalysing deadly urban epidemics.3
An essential tool in the fight against yellow fever is a live-attenuated vaccine developed in 1937.6 This vaccine is vital for the prevention and control of yellow fever epidemics since no effective antiviral therapy exists.7 However, a substantial proportion of the world’s yellow fever vaccine stock was recently consumed in response to epidemics in Africa8 and Brazil.9 As a stopgap measure, the World Health Organization (WHO) approved fractional dosing to extend the vaccine supply, while recognizing that the duration of immunity may be compromised.10 With only four WHO-qualified yellow fever vaccine manufacturers in the world, rapid replenishment of the global emergency stockpile stretches finite resources, potentially resulting in vaccine shortages for preventive campaigns.11 In late 2017, stocks of YF-VAX® (Sanofi Pasteur, Lyon, France) in North America were depleted because of manufacturing difficulties.5 Should another urban epidemic occur in the near future, vaccine demand could easily exceed the available supply.
Although many countries have vaccination policies to prevent international spread of the yellow fever virus, implementation is inconsistent.12 Most, but not all countries where yellow fever is endemic require arriving international travellers without medical contraindications to provide official documentation of vaccination as a prerequisite for entry. As the vaccine provides protective immunity to 90% and 99% of individuals 10 and 30 days after vaccination, respectively,13 most travellers are protected from acquiring and exporting the yellow fever virus. Furthermore, some countries where the disease is not endemic, but where the competent mosquito vector Aedes aegypti is present require travellers arriving from a yellow fever-endemic country to provide proof of vaccination.14
The confluence of climate change,15 rapid urbanization16 and international air travel17 are accelerating the globalization of mosquito-borne viruses such as dengue, chikungunya and Zika viruses. Here we examined the potential for the yellow fever virus to spread via international air travel into the world’s cities, in order to guide global epidemic prevention efforts.
Methods
To identify gaps in yellow fever vaccination policies around the world, we assessed the potential for the international spread of yellow fever from areas deemed by WHO to be at risk of transmission to areas where conditions are known, or predicted, to be suitable for transmission. Our goal was to provide a global perspective on urban exposure to imported yellow fever virus, irrespective of past or present epidemics.
Global endemicity
We considered places where WHO recommended yellow fever vaccination in 2016, including recently identified parts of Brazil, to be areas where humans were at risk of local infection.18–20 We refer to these areas as yellow fever-endemic areas, although we recognize that they may not have been experiencing yellow fever transmission. We excluded places where yellow fever vaccination was generally not recommended by WHO. For non-holoendemic countries (i.e. where only part of the country was at risk of yellow fever),20 we delineated subnational areas of risk using ArcGIS v. 10.4.1 (Esri, Redlands, United States of America). We then used LandScan (Oak Ridge National Laboratory, Oak Ridge, USA)21 to estimate the total population living within the global range of the yellow fever virus.
International dispersion
To account for the possibility that individuals infected with yellow fever virus within an endemic area might travel by land to a nearby airport in a non-endemic area, we used ArcGIS v. 10.4.1 to identify all commercial airports registered with the International Air Transport Association (IATA): (i) within 200 km of any yellow fever-endemic area worldwide (base scenario); and (ii) within 200 km of any city within a yellow fever-endemic area (urban scenario). In the base scenario, we considered travellers departing from areas of potential sylvatic or urban transmission as possible sources of exported yellow fever virus. In the urban scenario, we focused on travellers departing from airports within 200 km of a city (i.e. an urban centre with more than 300 000 residents, as defined by the United Nations’ World Urbanization Prospects)22 located in a yellow fever-endemic area. We mapped the final destination airports and the number of international travellers (determined from unique trips on commercial flights) departing from airports in each scenario by analysing worldwide tickets sales data from IATA between 1 January and 31 December 2016.23 These data included the travellers’ full itineraries: their initial airport of embarkation, their final destination airport and, where applicable, connecting airports. The data did not detail uncompleted trips due, for example, to cancelled or missed flights. Overall, these data accounted for an estimated 90% of all trips on commercial flights worldwide; the remaining 10% were modelled using airline market intelligence.23 Such data have been used previously to anticipate the global spread of emerging infectious diseases.24
Potential for urban transmission
To identify cities where yellow fever was not endemic, but which may have been suitable for viral transmission, we used a high-resolution, global, ecological model of dengue virus transmission, which was developed using empirical data on the real-world occurrence of dengue fever and associated environmental and climatic predictors of dengue virus transmission.25 We assumed that cities predicted to be suitable for dengue virus transmission were also ecologically suitable for yellow fever virus transmission, because both viruses are primarily transmitted by Aedes aegypti, an anthropophilic mosquito highly adapted to urban settings.25 Adopting a conservative approach, we excluded cities where the predicted probability of dengue-suitability was below 50%. As our analysis focused on urban importation and transmission of yellow fever virus, we did not consider its introduction into rural, sylvatic areas or transmission among non-human primates. We defined a yellow fever-suitable city as a population centre with at least 300 000 residents in an area where the yellow fever virus was not endemic but which was predicted to be suitable for viral transmission. We excluded cities above 2300 m because environmental conditions at these elevations are considered unsuitable for yellow fever virus transmission.26
We assessed the potential for importation of the yellow fever virus by quantifying the volume of airline passengers travelling from yellow fever-endemic areas of the world, according to our base and urban scenarios, to yellow fever-suitable and -endemic cities. We also considered the possibility that individuals infected with the virus might arrive at an airport in a non-endemic area and then travel by land to a neighbouring city within a yellow fever-endemic or -suitable area: in our analysis, we included all commercial airports located within 200 km of these mutually exclusive geographical areas. We then categorized traveller flows according to the official yellow fever travel vaccination policy in each endemic and non-endemic country: (i) no proof of yellow fever vaccination required; (ii) proof of vaccination required if arriving from a yellow fever-endemic country; and (iii) proof of vaccination required if arriving from any country.27 Finally, we aggregated the resident populations of all yellow fever-suitable and -endemic cities.
Results
We estimated that 923 million people lived in areas of the world where yellow fever was endemic in 2016, spanning 25 holoendemic and 17 non-holoendemic countries or territories (Box 1).
Box 1. Countries and territories at risk of yellow fever transmission in 2016, according to the United States’ Centers for Disease Control and Prevention and the World Health Organization1–3.
Countries and territories where yellow fever was endemic (i.e. holoendemic countries)
Angola, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Congo, Côte d'Ivoire, Equatorial Guinea, French Guiana, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Guyana, Liberia, Nigeria, Paraguay, Senegal, Sierra Leone, South Sudan, Suriname, Togo, Uganda
Countries where only a portion were at risk of yellow fever (i.e. non-holoendemic countries)
Argentina, Bolivia (Plurinational State of), Brazil, Chad, Colombia, Democratic Republic of the Congo, Ecuador, Ethiopia, Kenya, Mali, Mauritania, Niger, Panama, Peru, Sudan, Trinidad and Tobago, Venezuela (Bolivarian Republic of)
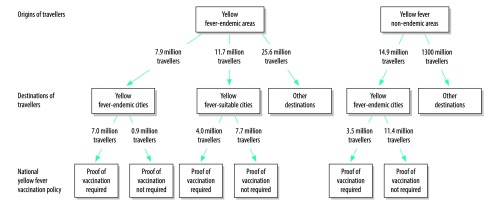
In our base scenario, 45.2 million travellers departed from yellow fever-endemic areas for international destinations in 2016. Of these, 7.9 million (17.4%) had final destinations at airports within or adjacent to yellow fever-endemic cities, 11.7 million (25.8%) had destinations at airports within or adjacent to yellow fever-suitable cities and 25.6 million (57.8%) had other destinations (Fig. 1). Of the 7.9 million travellers with international destinations at or near other yellow fever-endemic cities, 0.86 million (11.0%) landed in a country where proof of yellow fever vaccination was not required upon arrival: one holoendemic country (i.e. South Sudan) and three non-holoendemic countries (i.e. Argentina, Brazil and Peru). Of the 11.7 million travellers with destinations at or near yellow fever-suitable cities, 7.7 million (65.7%) landed in a country where proof of yellow fever vaccination was not required: four non-holoendemic countries (i.e. Argentina, Brazil, Ecuador and Peru) and 12 non-endemic countries (e.g. the United States). Conversely, 14.9 million travellers departed non-endemic areas of the world for airports within or adjacent to yellow fever-endemic cities; 11.4 million (76.4%) of these travellers landed in countries where proof of yellow fever vaccination was not required on arrival.
Fig. 1.
International movements of air travellers between areas that were or were not endemic for yellow fever, 2016
Notes: A yellow fever-endemic area was a national or subnational area where the World Health Organization recommended yellow fever vaccination. A yellow fever-endemic city was a city located in an area where vaccination was recommended. A yellow fever-suitable city was a city that was suitable for dengue virus transmission (see main text for details). Other destinations were: (i) all destinations where yellow fever was not endemic and which were not suitable for yellow fever transmission; and (ii) areas where yellow fever was endemic or which were suitable for yellow fever transmission but did not contain a settlement with a population greater than 300 000.
In our urban scenario, 32.2 million travellers departed airports within or near yellow fever-endemic cities for international destinations in 2016. Of these, 6.1 million (18.9%) arrived at or near yellow fever-endemic cities (Table 1); there was one fewer destination city than in our base scenario. In addition, 8.4 million (26.1%) arrived at or near yellow fever-suitable cities; there were six fewer destination cities than in our base scenario (Table 2). As the urban scenario considered only travellers departing from airports within 200 km of a city within a yellow fever-endemic area, it represents the potential for dispersion during an urban outbreak rather than dispersion secondary to urban or sylvatic transmission, as in the base scenario.
Table 1. International air travellers arriving in cities where yellow fever was endemic from other endemic areas or cities, 2016.
| Destination country or territory,a by rankb | No. travellers arriving from yellow fever-endemic areas |
Urban population of destination country, millionsc | Proof of yellow fever vaccination required upon arrival |
||
|---|---|---|---|---|---|
| Departure airport within 200 km of a yellow fever-endemic area (base scenario)d | Departure airport within 200 km of a city in a yellow fever-endemic area (urban scenario)e | From yellow fever-endemic countries only | From any country | ||
| 1. Colombia | 1 373 439 | 776 317 | 16.4 | Yes | No |
| 2. Panama | 995 941 | 625 764 | 1.7 | Yes | No |
| 3. Brazil | 769 203 | 474 260 | 54.6 | Nof | Nof |
| 4. Nigeria | 532 602 | 485 319 | 46.8 | Yes | No |
| 5. Ghana | 389 242 | 378 893 | 6.1 | No | Yes |
| 6. Côte d'Ivoire | 360 179 | 347 372 | 6.0 | No | Yes |
| 7. Kenya | 357 561 | 291 022 | 5.7 | Yes | No |
| 8. Senegal | 322 374 | 295 805 | 3.5 | Yes | No |
| 9. Cameroon | 280 895 | 272 308 | 7.5 | Yes | No |
| 10. Venezuela (Bolivarian Republic of) | 221 837 | 185 895 | 7.3 | Yes | No |
| 11. Gabon | 199 560 | 197 595 | 0.7 | No | Yes |
| 12. Congo | 195 571 | 178 963 | 2.9 | No | Yes |
| 13. Benin | 189 191 | 186 575 | 1.4 | Yes | No |
| 14. Mali | 161 064 | 151 877 | 2.5 | No | Yes |
| 15. Paraguay | 151 425 | 112 640 | 2.8 | Yes | No |
| 16. Uganda | 149 683 | 135 482 | 1.9 | Yes | No |
| 17. Angola | 125 518 | 92 021 | 7.2 | No | Yes |
| 18. Bolivia (Plurinational State of) | 121 798 | 93 353 | 2.1 | Yes | No |
| 19. Democratic Republic of the Congo | 118 798 | 80 433 | 20.1 | No | Yes |
| 20. Burkina Faso | 105 837 | 97 019 | 3.5 | Yes | No |
| 21. Togo | 104 851 | 102 487 | 1.0 | No | Yes |
| 22. South Sudan | 92 280 | 83 838 | 0.3 | No | No |
| 23. Sudan | 90 271 | 48 908 | 2.1 | Yes | No |
| 24. Guinea | 75 603 | 73 078 | 1.9 | Yes | No |
| 25. Liberia | 65 060 | 64 915 | 1.3 | No | Yes |
| Other countriesg | 315 213 | 284 692 | 7.4 | NA | NA |
| Total | 7 864 996 | 6 116 831 | 214.7 | NA | NA |
NA: not applicable.
a All destination countries and territories were yellow fever-endemic areas.
b Countries and territories were ranked according to the number of travellers arriving from yellow fever-endemic areas, which was determined by examining all outbound international flights from airports within areas where the World Health Organization (WHO) recommended yellow fever vaccination and all airports within 200 km of such areas.17–19
c Nationally aggregated population living in cities.
d The base scenario considered international travellers arriving from airports within areas where WHO recommended yellow fever vaccination and all airports within 200 km of such areas.
e The urban scenario considered international travellers arriving from airports within 200 km of a city (population ≥ 300 000) in an area where WHO recommended yellow fever vaccination.
f We did not take into account Brazil’s temporary yellow fever vaccination requirements for incoming passengers from Angola and the Democratic Republic of the Congo during the 2016 outbreak.
g There were 10 other yellow fever-endemic destination countries with an airport within 200 km of a yellow fever-endemic city with a population of at least 300 000: Argentina, Burundi, Central African Republic, Chad, Ethiopia, Gambia, Guinea-Bissau, Niger, Peru and Sierra Leone. We did not show the 7 countries where there was no city with at least 300 000 residents located in a yellow fever-endemic area: Ecuador, Equatorial Guinea, French Guiana, Guyana, Mauritania, Suriname and Trinidad and Tobago.
Table 2. International air travellers arriving in cities suitable for yellow fever transmission from areas or cities where yellow fever was endemic, 2016.
| Destination country or territory,a by rankb | No. travellers arriving from yellow fever-endemic areas |
Urban population of destination country, millionsc | Proof of yellow fever vaccination required upon arrival |
||
|---|---|---|---|---|---|
| Departure airport within 200 km of a yellow fever-endemic area (base scenario)d | Departure airport within 200 km of a city in a yellow fever-endemic area (urban scenario)e | From yellow fever-endemic countries only | From any country | ||
| 1. United Statesf | 2 762 081 | 1 659 163 | 9.6 | No | No |
| 2. Mexico | 1 166 021 | 874 820 | 33.5 | No | No |
| 3. United Arab Emirates | 890 623 | 717 232 | 0.5 | No | No |
| 4. Peru | 752 113 | 536 161 | 12.1 | No | No |
| 5. Ecuador | 595 181 | 405 106 | 3.0 | No | No |
| 6. Dominican Republic | 538 042 | 322 848 | 3.5 | No | No |
| 7. Brazil | 481 737 | 311 969 | 44.2 | Nog | Nog |
| 8. Venezuela (Bolivarian Republic of) | 461 006 | 376 804 | 7.6 | Yes | No |
| 9. China | 403 683 | 316 588 | 98.7 | Yes | No |
| 10. India | 385 786 | 345 314 | 235.3 | Yes | No |
| 11. Cuba | 372 455 | 237 228 | 3.2 | Yes | No |
| 12. Saudi Arabia | 319 711 | 256 316 | 6.5 | Yes | No |
| 13. Costa Rica | 283 169 | 216 087 | 1.2 | Yes | No |
| 14. United Republic of Tanzania | 268 038 | 247 515 | 7.8 | Yes | No |
| 15. Egypt | 217 597 | 204 251 | 22.8 | Yes | No |
| 16. Argentina | 213 665 | 170 456 | 6.3 | No | No |
| 17. Rwanda | 170 040 | 162 831 | 1.3 | Yes | No |
| 18. Guatemala | 115 834 | 94 882 | 2.9 | Yes | No |
| 19. El Salvador | 103 943 | 85 577 | 1.1 | Yes | No |
| 20. China, Hong Kong SAR | 96 258 | 74 284 | 7.3 | No | No |
| 21. Sudan | 90 037 | 48 723 | 5.6 | Yes | No |
| 22. Thailand | 86 481 | 62 266 | 12.7 | Yes | No |
| 23. Puerto Rico | 77 282 | 57 657 | 2.8 | No | No |
| 24. Jamaica | 76 848 | 19 822 | 0.6 | Yes | No |
| 25. Nicaragua | 68 481 | 59 128 | 1.0 | No | No |
| Other countriesh | 665 455 | 531 709 | 211.0 | NA | NA |
| Total | 11 661 567 | 8 394 737 | 742.1 | NA | NA |
NA: not applicable; SAR: Special Administrative Region.
a Destination cities in these countries and territories were ecologically suitable for yellow fever virus transmission but were not in yellow fever-endemic areas.
b Countries and territories were ranked according to the number of travellers arriving from yellow fever-endemic areas, which was determined by examining all outbound international flights from airports within areas where the World Health Organization (WHO) recommended yellow fever vaccination and all airports within 200 km of such areas.17–19
c Nationally aggregated population living in yellow fever-suitable cities. In the urban scenario, there were six fewer yellow fever-suitable destination cities than in the base scenario: Satna, India (population 0.31 million); Ibb, Yemen (population 0.45 million); Al Hudaydah, Yemen (population 0.57 million); Taiz, Yemen (population 0.69 million); Aden, Yemen (population 0.88 million); and Sana’a, Yemen (population 2.7 million).
d Our base scenario considered international travellers arriving from airports within areas where WHO recommended yellow fever vaccination and all airports within 200 km of such areas.
e Our urban scenario considered international travellers arriving from airports within 200 km of a city (population ≥ 300 000) in an area where WHO recommended yellow fever vaccination.
f United States’ territory included all continental states and Hawaii. Puerto Rico was not included and is listed separately. Other United States territories, such as Guam, American Samoa and the United States Virgin Islands, do not have cities with at least 300 000 residents and are thus not included.
g We did not take into account Brazil’s temporary yellow fever vaccination requirements for incoming passengers from Angola and the Democratic Republic of the Congo during the 2016 outbreak.
h There were 29 other countries or territories suitable for yellow fever transmission (details available from the corresponding author on request).
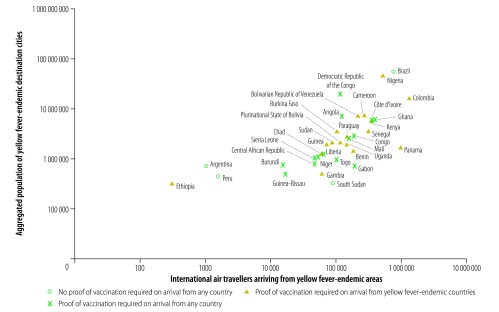
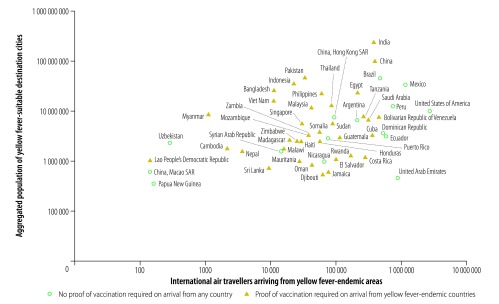
Among countries with yellow fever-endemic cities, Brazil, Colombia and Nigeria had the highest traveller numbers from other yellow fever-endemic areas of the world and the largest populations living in yellow fever-endemic cities (Fig. 2). Colombia and Nigeria required proof of yellow fever vaccination from travellers arriving from other yellow fever-endemic countries but not from non-endemic countries. In contrast, Brazil did not require proof of vaccination from travellers arriving from yellow fever-endemic countries. Among countries with yellow fever-suitable cities, Brazil, China, India, Mexico, Peru and the United States had the highest traveller numbers arriving from yellow fever-endemic areas and the largest populations living in yellow fever-suitable cities (Fig. 3). Of these, Brazil, Mexico, Peru and the United States did not require proof of yellow fever vaccination from travellers arriving from yellow fever-endemic areas. Fig. 4 and Table 3 (available at: http://www.who.int/bulletin/volumes/96/5/17-205658) show the resident populations of yellow fever-endemic cities globally according to national yellow fever travel vaccination policy and Fig. 5 and Table 4 (available at: http://www.who.int/bulletin/volumes/96/5/17-205658) show the corresponding populations of yellow fever-suitable cities.
Fig. 2.
International air travellers arriving from yellow fever-endemic areas and aggregated population of yellow fever-endemic destination cities, by country, 2016
Notes: Both axes have a logarithmic scale. A yellow fever-endemic area was a national or subnational area where the World Health Organization recommended yellow fever vaccination. The symbols indicate the national yellow fever vaccination policy for travellers arriving in the country.
Fig. 3.
International air travellers arriving from yellow fever-endemic areas and aggregated population of yellow fever-suitable destination cities, by country or territory, 2016
SAR: Special Administrative Region.
Notes: Both axes have a logarithmic scale. A yellow fever-suitable city was ecologically suitable for yellow fever virus transmission but was not located in a yellow fever-endemic area, which was defined as an area where the World Health Organization recommended yellow fever vaccination. The symbols indicate the national yellow fever vaccination policy for travellers arriving in the country.
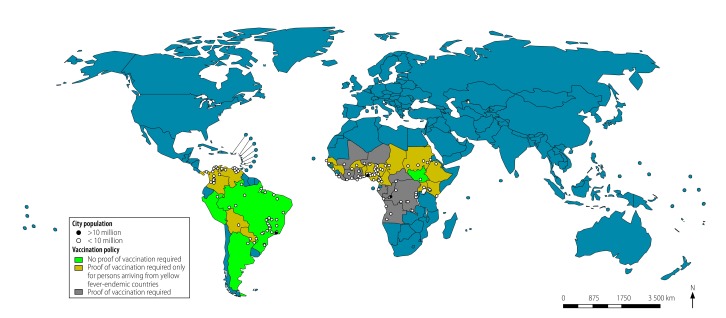
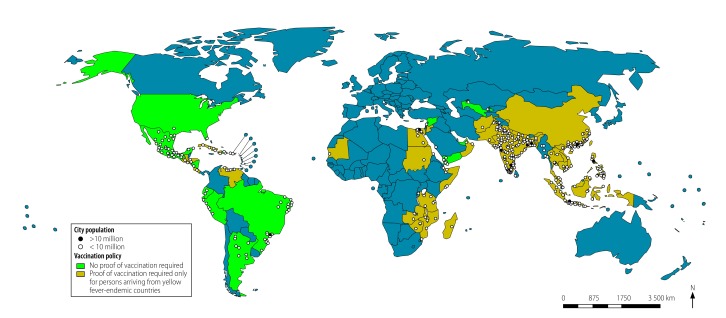
Fig. 4.
Population of yellow fever-endemic cities, by travel vaccination policy, 2016
Notes: In total, there were 170 yellow fever-endemic cities, represented by circles on the map, in 35 countries. Yellow fever-endemic cities were located in areas where the World Health Organization recommended yellow fever vaccination. In the urban scenario (see main text for details), there was one fewer yellow fever-endemic city than in the base scenario: Tshikapa, Democratic Republic of the Congo (population 0.69 million).
Table 3. Top 50 yellow fever - endemic destination cities of air travellers from areas or cities where yellow fever was endemic, by city population, 2016.
| Destination city, country or territory,a by rankb | Populationc | Proof of yellow fever vaccination required upon arrivald | |
|---|---|---|---|
| From yellow fever-endemic countries only | From any country | ||
| 1. Lagos, Nigeria | 13 122 829 | Yes | No |
| 2., Rio de Janeiro Brazil | 12 902 306 | No | No |
| 3. Kinshasa, Democratic Republic of the Congo | 11 586 914 | No | Yes |
| 4. Belo Horizonte, Brazil | 5 716 422 | No | No |
| 5. Luanda, Angola | 5 506 000 | No | Yes |
| 6. Abidjan, Côte d'Ivoire | 4 859 798 | No | Yes |
| 7. Brasília, Brazil | 4 155 476 | No | No |
| 8. Nairobi, Kenya | 3 914 791 | Yes | No |
| 9. Medellín, Colombia | 3 910 989 | Yes | No |
| 10. Porto Alegre, Brazil | 3 602 526 | No | No |
| 11. Kano, Nigeria | 3 587 049 | Yes | No |
| 12. Salvador, Brazil | 3 582 967 | No | No |
| 13. Dakar, Senegal | 3 520 215 | Yes | No |
| 14. Ibadan, Nigeria | 3 160 190 | Yes | No |
| 15. Yaoundé, Cameroon | 3 065 692 | Yes | No |
| 16. Campinas, Brazil | 3 047 102 | No | No |
| 17. Douala, Cameroon | 2 943 318 | Yes | No |
| 18. Ouagadougou, Burkina Faso | 2 741 128 | Yes | No |
| 19. Cali, Colombia | 2 645 941 | Yes | No |
| 20. Kumasi, Ghana | 2 598 789 | No | Yes |
| 21. Bamako, Mali | 2 515 000 | No | Yes |
| 22. Abuja, Nigeria | 2 440 242 | Yes | No |
| 23. Asunción, Paraguay | 2 356 174 | Yes | No |
| 24. Port Harcourt, Nigeria | 2 343 309 | Yes | No |
| 25. Goiânia, Brazil | 2 284 828 | No | No |
| 26. Accra, Ghana | 2 277 298 | No | Yes |
| 27. Maracaibo, Venezuela (Bolivarian Republic of) | 2 196 435 | Yes | No |
| 28. Belém, Brazil | 2 181 607 | No | No |
| 29. Santa Cruz, Bolivia (Plurinational State of) | 2 106 682 | Yes | No |
| 30. Manaus, Brazil | 2 025 379 | No | No |
| 31. Lubumbashi, Democratic Republic of the Congo | 2 015 091 | No | Yes |
| 32. Mbuji-Mayi, Democratic Republic of the Congo | 2 006 641 | No | Yes |
| 33. Barranquilla, Colombia | 1 991 158 | Yes | No |
| 34. Conakry, Guinea | 1 936 045 | Yes | No |
| 35. Kampala, Uganda | 1 935 654 | Yes | No |
| 36. Brazzaville, Congo | 1 887 625 | No | Yes |
| 37. Ciudad de Panama, Panama | 1 672 810 | Yes | No |
| 38. Grande Vitória, Brazil | 1 636 141 | No | No |
| 39. Benin City, Nigeria | 1 495 763 | Yes | No |
| 40. Grande São Luis, Brazil | 1 436 781 | No | No |
| 41. Huambo, Angola | 1 269 211 | No | Yes |
| 42. Monrovia, Liberia | 1 263 800 | No | Yes |
| 43. N'Djaména, Chad | 1 260 146 | Yes | No |
| 44. Bucaramanga, Colombia | 1 215 066 | Yes | No |
| 45 Kananga, Democratic Republic of the Congo | 1 168 687 | No | Yes |
| 46. Onitsha, Nigeria | 1 109 287 | Yes | No |
| 47. Mombasa, Kenya | 1 103 703 | Yes | No |
| 48. Cartagena, Colombia | 1 092 336 | Yes | No |
| 49. Niamey, Niger | 1 089 589 | No | Yes |
| 50. Kaduna, Nigeria | 1 047 815 | Yes | No |
a All destination countries and territories were yellow fever-endemic areas.
b Cities were ranked according to urban population size.
c We obtained population data from United Nations’ World Urbanization Prospects.22
d We did not take into account Brazil’s temporary yellow fever vaccination requirements for incoming passengers from Angola and the Democratic Republic of the Congo during the 2016 outbreak.
Notes: Travel was estimated using our base scenario which considered international travellers arriving from airports within areas where WHO recommended yellow fever vaccination and all airports within 200 km of such areas.Tabulated data reflects cities depicted in Figure 4.
Fig. 5.
Population of yellow fever-suitable cities, by travel vaccination policy, 2016
Notes: In total, there were 472 yellow fever-suitable cities in 54 countries. A yellow-fever-suitable city was ecologically suitable for yellow fever virus transmission but was not located in a yellow fever-endemic area, which was defined as an area where the World Health Organization recommended yellow fever vaccination. In the urban scenario (see main text for details), there were six fewer yellow fever-suitable cities than in the base scenario: Satna, India (population 0.31 million); Ibb, Yemen (population 0.45 million); Al Hudaydah, Yemen (population 0.57 million); Taiz, Yemen (population 0.69 million); Aden, Yemen (population 0.88 million); and Sana’a, Yemen (population 2.7 million).
Table 4. Top 50 yellow fever suitable destinations, by population, of international air travellers from areas or cities where yellow fever was endemic, by city population, 2016.
| Destination city, country or territory,a by rankb | Populationc | Proof of yellow fever vaccination required upon arrivald | Non-holoendemic countrye | |
|---|---|---|---|---|
| From yellow fever-endemic countries only | From any country | |||
| 1. New Delhi; India | 25 703 168 | Yes | No | No |
| 2. São Paulo, Brazil | 21 066 245 | No | No | Yes |
| 3. Mumbai, India | 21 042 538 | Yes | No | No |
| 4. Cairo, Egypt | 18 771 769 | Yes | No | No |
| 5. Dhaka, Bangladesh | 17 598 228 | Yes | No | No |
| 6. Karachi, Pakistan | 16 617 644 | Yes | No | No |
| 7. Kolkata, India | 14 864 919 | Yes | No | No |
| 8. Manila, Philippines | 12 946 263 | Yes | No | No |
| 9. Guangzhou, China | 12 458 130 | Yes | No | No |
| 10. Shenzhen, China | 10 749 473 | Yes | No | No |
| 11. Jakarta, Indonesia | 10 323 142 | Yes | No | No |
| 12. Bangalore, India | 10 087 132 | Yes | No | No |
| 13. Lima, Peru | 9 897 033 | No | No | Yes |
| 14. Chennai, India | 9 890 427 | Yes | No | No |
| 15. Bangkok, Thailand | 9 269 823 | Yes | No | No |
| 16. Hyderabad, India | 8 943 523 | Yes | No | No |
| 17. Lahore, Pakistan | 8 741 365 | Yes | No | No |
| 18. Dongguan, China | 7 434 935 | Yes | No | No |
| 19. Ahmadabad, India | 7 342 850 | Yes | No | No |
| 20. Hong Kong SAR, China | 7 313 557 | No | No | No |
| 21. Ho Chi Minh City, Viet Nam | 7 297 780 | Yes | No | No |
| 22. Foshan, China | 7 035 945 | Yes | No | No |
| 23. Kuala Lumpur, Malaysia | 6 836 911 | Yes | No | No |
| 24. Miami, United States | 5 817 221 | No | No | No |
| 25. Pune, India | 5 727 530 | Yes | No | No |
| 26. Surat, India | 5 650 011 | Yes | No | No |
| 27. Singapore, Singapore | 5 618 866 | Yes | No | No |
| 28. Khartoum, Sudan | 5 129 358 | Yes | No | Yes |
| 29. Dar es Salaam, United Republic of Tanzania | 5 115 670 | Yes | No | No |
| 30. Guadalajara, Mexico | 4 843 241 | No | No | No |
| 31. Yangon, Myanmar | 4 801 930 | Yes | No | No |
| 32. Chittagong, Bangladesh | 4 539 393 | Yes | No | No |
| 33. Monterrey, Mexico | 4 512 572 | No | No | No |
| 34. Xiamen, China | 4 430 081 | Yes | No | No |
| 35. Jiddah, Saudi Arabia | 4 075 803 | Yes | No | No |
| 36. Shantou, China | 3 948 813 | Yes | No | No |
| 37. Fortaleza, Brazil | 3 880 202 | No | No | Yes |
| 38. Recife, Brazil | 3 738 526 | No | No | Yes |
| 39. Zhongshan, China | 3 691 360 | Yes | No | No |
| 40. Hà Noi, Viet Nam | 3 629 493 | Yes | No | No |
| 41. Faisalabad, Pakistan | 3 566 952 | Yes | No | No |
| 42. Curitiba, Brazil | 3 473 681 | No | No | Yes |
| 43. Jaipur, India | 3 460 701 | Yes | No | No |
| 44. Fuzhou, China | 3 282 932 | Yes | No | No |
| 45. Nanning, China | 3 234 379 | Yes | No | No |
| 46. Lucknow, India | 3 221 817 | Yes | No | No |
| 47. Wenzhou, China | 3 207 846 | Yes | No | No |
| 48. Kanpur, India | 3 020 795 | Yes | No | No |
| 49. Sana'a', Yemen | 2 961 934 | No | No | No |
| 50. Santo Domingo, Dominican Republic | 2 945 353 | No | No | No |
SAR: Special Administrative Region.
a Destination cities in these countries and territories were ecologically suitable for yellow fever virus transmission but were not in yellow fever-endemic areas.
b Cities were ranked according to urban population size.
c We obtained population data from United Nations’ World Urbanization Prospects.22
d We did not take into account Brazil’s temporary yellow fever vaccination requirements for incoming passengers from Angola and the Democratic Republic of the Congo during the 2016 outbreak.
e Non-holoendemic countries have subnational areas that are at risk of yellow fever transmission as defined by the WHO and CDC Yellow Book. Cities listed in this table are not located within the YF extent of non-holoendemic countries.
Notes: Travel was estimated using our base scenario which considered international travellers arriving from airports within areas where WHO recommended yellow fever vaccination and all airports within 200 km of such areas.Tabulated data reflects cities depicted in Figure 5.
Discussion
The 2016 yellow fever epidemic in Angola and the associated exportation of cases into urban areas of China exposed shortcomings in existing yellow fever travel vaccination policies and practices. As a holoendemic country, Angola has a policy that requires all international travellers to provide proof of yellow fever vaccination upon arrival. In addition, China has the same requirement for travellers arriving from yellow fever-endemic countries. Yet both lines of defence failed, leading to the first cases of imported yellow fever in Asia. Recent research has confirmed the role played by air travel between Angola and China in increasing the risk of importing the disease.28 This event illustrates that urban areas that have never experienced yellow fever transmission, or have not experienced it in modern times, are increasingly susceptible to epidemics. We elected to study the travel conduits that could facilitate the international spread of yellow fever virus into the world’s cities.
First, our analysis revealed that 89% of travellers departing from yellow fever-endemic areas for yellow fever-endemic cities in other countries (both holoendemic and non-holoendemic) in 2016 were required to provide proof of vaccination upon arrival. This high proportion presumably reflects countries’ desire to protect themselves against importation of yellow fever virus. To reduce the risk of importation, and of the consequent potential for domestic transmission and of possible exportation of yellow fever virus, these countries should focus on implementing existing yellow fever travel vaccination policies effectively. However, some travellers may purchase counterfeit international vaccination certificates,29 which makes this line of defence potentially fallible. Second, we found that less than 35% of travellers departing yellow fever-endemic areas for cities that appeared suitable for yellow fever transmission, were required to provide proof of vaccination upon arrival. Countries that did not require proof of yellow fever vaccination might have assumed that the historical absence of yellow fever was predictive of its future absence. In other instances, nationally implemented vaccination policies may be obfuscated because only a small geographical area within a country may be ecologically suitable for yellow fever transmission; for example, the 9.5 million United States’ residents who live in five urban areas that appear suitable for yellow fever transmission represent less than 3% of the country’s population. Nonetheless, countries should carefully consider whether the risk of yellow fever virus importation and subsequent domestic transmission warrants a change to existing yellow fever travel vaccination policies or practices. Of note, administering yellow fever vaccine at national ports of entry to individuals who do not hold a record of vaccination will increase immunity among susceptible travellers but will not prevent importation of the virus by travellers who are already infected. Third, we found that less than 25% of travellers who departed from areas of the world where yellow fever was not endemic for yellow fever-endemic cities were required to provide proof of vaccination upon arrival. This reveals a policy gap in protecting international travellers against becoming infected and subsequently exporting the virus. This low proportion may reflect the absence of national incentives because countries with entry requirements for yellow fever vaccination are protecting international travellers and the global community without realizing any domestic benefit.
Although broader use of yellow fever vaccine by international travellers could limit dispersion of the virus and reduce the risk of urban epidemics, its use in non-epidemic settings must be carefully weighed against the risk of vaccine-associated neurological and viscerotropic events. Infants younger than 9 months, adults aged 60 years and older and individuals with thymus disorders and weakened immune systems are at an elevated risk of these potentially life-threatening events.30 Furthermore, if international changes in vaccination policy and practice are implemented and enforced, travellers could face difficulties accessing yellow fever vaccine, given current diminished stocks and constrained manufacturing capacity. Even though an estimated 50 million vaccine doses were produced in 2017,11 a new yellow fever epidemic in a populated urban centre could readily deplete global emergency vaccine stockpiles.
We made several important assumptions in our analysis. First, we assumed that the risk of yellow fever virus dispersion across all yellow fever-endemic areas of the world was uniform, because we were not attempting to model the spread of the virus out of a particular geographical area that was experiencing epizootic or epidemic activity. Rather, our goal was to describe global pathways via which the yellow fever virus could disseminate to trigger epidemics in the world’s cities, thereby identifying crucial gaps in existing yellow fever travel vaccination policies and practices. Since the potential for international dispersion of the virus out of rural areas presumably differs from that out of urban areas, our urban scenario focused solely on travellers departing airports in or immediately adjacent to cities in yellow fever-endemic areas. However, the recent case of a traveller who acquired a yellow fever virus infection in rural Suriname and then flew to the Netherlands indicates that there is still a risk of yellow fever exportation from rural areas.4
Our assumptions about the suitability of cities for yellow fever virus transmission were based on a global ecological model of dengue virus transmission. A recently published modelling analysis of suitability for yellow fever transmission globally predicted a similar pattern to the pattern of dengue suitability we assumed,31 especially in urbanized regions, which were the primary focus of our study. However, we may have overestimated the risk of yellow fever transmission in areas where dengue is known to be active but where Ae. albopictus rather than Ae. aegypti is the dominant vector (e.g. in China, Hong Kong Special Administrative Region). On the other hand, although Ae. aegypti is the primary vector for transmission of yellow fever virus, some studies have indicated that Ae. albopictus might also be a competent vector in nature.32 As our analysis focused on the importation of yellow fever virus into cities and ignored downstream transmission among non-human primates in rural sylvatic cycles, we believe our model of urban dengue suitability closely approximates suitability for yellow fever virus transmission.
Our model of dengue suitability represents an annualized view of potential yellow fever transmission. The model does not account for seasonal variability due to changing climatic conditions.33 Furthermore, we did not take into account seasonal patterns in local (i.e. urban–rural) or international travel despite the possibility that interactions between the ecological seasonality of yellow fever transmission and the seasonality of human mobility could influence the risk of yellow fever virus importation. In addition, we did not attempt to quantify variations in the intensity of transmission between tropical and subtropical climates or between industrialized and developing areas of the world. For example, because of differences in climate and the built environment,34 some cities in the southern United States have experienced sporadic transmission of dengue, chikungunya and Zika viruses, whereas cities in Latin America have experienced sustained and intense transmission of the same pathogens. Moreover, we did not attempt to estimate how the underlying level of population immunity influences the potential for epidemics. Although we presumed that populations in yellow fever-suitable cities would have negligible immunity to the yellow fever virus, we made no assumptions about immunity in yellow fever-endemic cities, because high-resolution data on yellow fever vaccination and natural infection were lacking. Lastly, we did not take into account Brazil’s temporary yellow fever vaccination requirements for travellers who came from Angola and the Democratic Republic of the Congo during the 2017 yellow fever outbreak and therefore categorized Brazil as not requiring proof of vaccination upon arrival from yellow fever-endemic countries.
With more than 3 billion domestic and international passengers now boarding commercial flights each year, humans have become the primary agents for the global spread of mosquito-borne viruses such as dengue, chikungunya, Zika and yellow fever. Our findings on yellow fever virus transmission provide countries with insights into contemporary vulnerabilities to international spread of the virus. Our goal was to help countries ensure that their policies and interventions to prevent, or to protect against, the international spread of yellow fever virus are commensurate with existing risks and avoid unnecessary interference with international traffic and trade, as per International Health Regulations (2005).35 At a time when global yellow fever vaccine supplies are diminished, an epidemic in a densely populated city could have substantial health and economic consequences. Hence, the global community needs to carefully re-examine existing yellow fever travel vaccination policies and practices to prevent urban epidemics.
Acknowledgements
We thank Kieran Petrasek at St. Michael's Hospital for significant help with our maps. MUGK is also affiliated to the Department of Zoology, University of Oxford, England and Harvard Medical School, Boston, USA; IIB is also affiliated to the Division of Infectious Diseases, Department of Medicine, University of Toronto, Canada; SIH is also affiliated to the Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, University of Oxford, England; and KK is also affiliated to the Division of Infectious Diseases, Department of Medicine, University of Toronto, Canada.
Funding:
MUGK is supported by The Branco Weiss Fellowship - Society in Science and acknowledges funding from a Training Grant from the National Institute of Child Health and Human Development (T32HD040128) and the National Library of Medicine of the National Institutes of Health (R01LM010812, R01LM011965).
Competing interests:
KK is the founder of BlueDot, a social enterprise that develops digital technologies for public health. SEB, AW, MG, IIB, MIC and KK received employment or consulting income from BlueDot during this research.
References
- 1.Situation report. Yellow fever. 28 October 2016. Geneva: World Health Organization; 2016. Available from: http://apps.who.int/iris/bitstream/10665/250661/1/yellowfeversitrep28Oct16-eng.pdf?ua=1 [cited 2016 Dec 12].
- 2.Rapid risk assessment. Outbreak of yellow fever in Angola, Democratic Republic of Congo and Uganda. Second update, 13 July 2016. Stockholm: European Centre for Disease Prevention and Control; 2016. Available from: http://ecdc.europa.eu/en/publications/Publications/RRA-Yellow%20fever-Angola-China-DRC-Uganda.pdf [cited 2016 Dec 12].
- 3.Wasserman S, Tambyah PA, Lim PL. Yellow fever cases in Asia: primed for an epidemic. Int J Infect Dis. 2016. July;48:98–103. 10.1016/j.ijid.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 4.Rapid risk assessment. Yellow fever among travellers returning from South America. 14 March 2017. Stockholm: European Centre for Disease Prevention and Control; 2017. Available from: http://ecdc.europa.eu/en/publications/Publications/14-03-2017-RRA-Yellow%20fever,%20Flaviviridae-Suriname,%20Southern%20America.pdf [cited 2017 Apr 3].
- 5.Hamer DH, Angelo K, Caumes E, van Genderen PJJ, Florescu SA, Popescu CP, et al. Fatal yellow fever in travelers to Brazil, 2018. MMWR Morb Mortal Wkly Rep. 2018;67(11): ePub 16 March 2018 10.15585/mmwr.mm6711e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theiler M, Smith HH. The effect of prolonged cultivation in vitro upon the pathogenicity of yellow fever virus. J Exp Med. 1937. May 31;65(6):767–86. 10.1084/jem.65.6.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wieten RW, Jonker EF, van Leeuwen EM, Remmerswaal EB, Ten Berge IJ, de Visser AW, et al. A single 17D yellow fever vaccination provides lifelong immunity; characterization of yellow-fever-specific neutralizing antibody and T-cell responses after vaccination. PLoS One. 2016. March 15;11(3):e0149871. 10.1371/journal.pone.0149871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett ADT. Yellow fever in Angola and beyond – the problem of vaccine supply and demand. N Engl J Med. 2016. July 28;375(4):301–3. 10.1056/NEJMp1606997 [DOI] [PubMed] [Google Scholar]
- 9.Outbreak reports on yellow fever. Epidemiological updates [internet]. Stockholm: European Centre for Disease Prevention and Control; 2018. Available from: https://ecdc.europa.eu/en/yellow-fever/threats-and-outbreaks/outbreak-reports [cited 2018 Mar 15].
- 10.Fractional dose yellow fever vaccine as a dose-sparing option for outbreak response. WHO Secretariat information paper. 20 July 2016. Geneva: Department of Vaccines Immunizations and Biologicals, World Health Organization; 2016. Available from: http://apps.who.int/iris/bitstream/10665/246236/1/WHO-YF-SAGE-16.1-eng.pdf [cited 2016 Dec 20].
- 11.Yellow fever vaccine : current supply outlook. May 2016. Copenhagen: UNICEF Supply Division; 2016. Available from: https://www.unicef.org/supply/files/YF_number_3_Supply_Update.pdf [cited 2017 Jan 12].
- 12.Schönenberger S, Hatz C, Bühler S. Unpredictable checks of yellow fever vaccination certificates upon arrival in Tanzania. J Travel Med. 2016. June 13;23(5):taw035. 10.1093/jtm/taw035 [DOI] [PubMed] [Google Scholar]
- 13.Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001. August;1(1):11–20. 10.1016/S1473-3099(01)00016-0 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. CDC health information for international travel 2016. New York: Oxford University Press; 2016. [Google Scholar]
- 15.Rogers DJ, Randolph SE. Climate change and vector-borne diseases. Adv Parasitol. 2006;62:345–81. 10.1016/S0065-308X(05)62010-6 [DOI] [PubMed] [Google Scholar]
- 16.Hassell JM, Begon M, Ward MJ, Fèvre EM. Urbanization and disease emergence: dynamics at the wildlife-livestock-human interface. Trends Ecol Evol. 2017. January;32(1):55–67. 10.1016/j.tree.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasitol. 2006;62:293–343. 10.1016/S0065-308X(05)62009-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Disease outbreak news. Yellow fever – Brazil. 27 January 2017. Geneva: World Health Organization; 2017. Available from: http://www.who.int/csr/don/27-january-2017-yellow-fever-brazil/en/ [cited 2017 Feb 3].
- 19.Disease outbreak news: Yellow fever – Brazil. 4 April 2017. Geneva: World Health Organization; 2017. Available from: http://www.who.int/csr/don/04-april-2017-yellow-fever-brazil/en/ [cited 2017 Apr 10].
- 20.Chapter 3: Infectious diseases related to travel. Yellow fever. Atlanta: Division of Global Migration and Quarantine, Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases; 2017. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/yellow-fever [cited 2017 Jan 16].
- 21. LandScanTM [internet]. Oak Ridge: Oak Ridge National Laboratory; 2016. Available from: http://web.ornl.gov/sci/landscan/ [cited 2018 Mar 11]. [Google Scholar]
- 22.World urbanization prospects. 2014 revision. New York: Population Division, Department of Economic and Social Affairs, United Nations; 2015. Available from: https://esa.un.org/unpd/wup/Publications/Files/WUP2014-Report.pdf [cited 2016 Dec 12].
- 23.Passenger Intelligence Services (PaxIS) [internet]. Montreal: International Air Transport Association; 2017. Available from: http://www.iata.org/services/statistics/intelligence/paxis/Pages/index.aspx [cited 2018 Mar 11].
- 24.Khan K, Arino J, Hu W, Raposo P, Sears J, Calderon F, et al. Spread of a novel influenza A (H1N1) virus via global airline transportation. N Engl J Med. 2009. July 9;361(2):212–4. 10.1056/NEJMc0904559 [DOI] [PubMed] [Google Scholar]
- 25.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013. April 25;496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jentes ES, Poumerol G, Gershman MD, Hill DR, Lemarchand J, Lewis RF, et al. ; Informal WHO Working Group on Geographic Risk for Yellow Fever. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect Dis. 2011. August;11(8):622–32. 10.1016/S1473-3099(11)70147-5 [DOI] [PubMed] [Google Scholar]
- 27.Chapter 3: Infectious diseases related to travel. Yellow fever and malaria information, by country. Atlanta: Division of Global Migration and Quarantine, Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases; 2017. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/yellow-fever-malaria-information-by-country [cited 2016 Dec 12].
- 28.Wilder-Smith A, Leong WY. Importation of yellow fever into China: assessing travel patterns. J Travel Med. 2017. July 1;24(4). 10.1093/jtm/tax008 [DOI] [PubMed] [Google Scholar]
- 29.Siringi S. Fake health certificate racket rife in Kenya. Lancet Infect Dis. 2002. August;2(8):454. 10.1016/S1473-3099(02)00357-2 [DOI] [PubMed] [Google Scholar]
- 30.de Menezes Martins R, da Luz Fernandes Leal ML, Homma A. Serious adverse events associated with yellow fever vaccine. Hum Vaccin Immunother. 2015;11(9):2183–7. 10.1080/21645515.2015.1022700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shearer FM, Longbottom J, Browne AJ, Pigott DM, Brady OJ, Kraemer MUG, et al. Existing and potential infection risk zones of yellow fever worldwide: a modelling analysis. Lancet Glob Health. 2018. March;6(3):e270–8. 10.1016/S2214-109X(18)30024-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amraoui F, Vazeille M, Failloux AB. French Aedes albopictus are able to transmit yellow fever virus. Euro Surveill. 2016. September 29;21(39):30361. 10.2807/1560-7917.ES.2016.21.39.30361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Gallagher N, Marano N, Staples JE. Assessing the risk of international spread of yellow fever virus: a mathematical analysis of an urban outbreak in Asuncion, 2008. Am J Trop Med Hyg. 2012. February;86(2):349–58. 10.4269/ajtmh.2012.11-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, et al. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis. 2003. January;9(1):86–9. 10.3201/eid0901.020220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International health regulations (2005). Third edition. Geneva: World Health Organization; 2016. Available from: http://www.who.int/ihr/publications/9789241580496/en/ [cited 2018 Mar 11].