Abstract
Early-onset epileptic encephalopathies, including West syndrome (WS), are a group of neurological disorders characterized by developmental impairments and intractable seizures from early infancy. We have now identified biallelic CNPY3 variants in three individuals with WS; these include compound-heterozygous missense and frameshift variants in a family with two affected siblings (individuals 1 and 2) and a homozygous splicing variant in a consanguineous family (individual 3). All three individuals showed hippocampal malrotation. In individuals 1 and 2, electroencephalography (EEG) revealed characteristic fast waves and diffuse sharp- and slow-wave complexes. The fast waves were clinically associated with seizures. CNPY3 encodes a co-chaperone in the endoplasmic reticulum and regulates the subcellular distribution and responses of multiple Toll-like receptors. The amount of CNPY3 in lymphoblastoid cells derived from individuals 1 and 2 was severely lower than that in control cells. Cnpy3-knockout mice exhibited spastic or dystonic features under resting conditions and hyperactivity and anxiolytic behavior during the open field test. Also, their resting EEG showed enhanced activity in the fast beta frequency band (20–35 Hz), which could mimic the fast waves in individuals 1 and 2. These data suggest that CNPY3 and Cnpy3 perform essential roles in brain function in addition to known Toll-like receptor-dependent immune responses.
Keywords: early-onset epileptic encephalopathy, West syndrome, hippocampal malrotation, fast waves, CNPY3, PRAT4A, chaperone, Toll-like receptor
Main Text
Early-onset epileptic encephalopathies (EOEEs) are a group of neurological disorders characterized by developmental impairments and intractable seizures from early infancy.1, 2 EOEEs include Ohtahara syndrome and early myoclonic encephalopathy, characterized by early-onset intractable seizures and the suppression-burst pattern on electroencephalography (EEG).3 With age, they can also transform into West syndrome (WS). One of the most common forms of EOEE, WS is characterized by epileptic spasms, arrest of psychomotor development, and hypsarrhythmia on EEG.4 EOEEs are known to have high genetic heterogeneity, including de novo mutations5 and autosomal-recessive mutations.6, 7
CNPY3 (MIM: 610774) encodes a protein known as Canopy3 or PRAT4A (protein associated with TLR4A).8, 9 It is localized in the endoplasmic reticulum (ER) and functions as a co-chaperone with the general chaperone gp96 to regulate the subcellular distribution and responses of multiple Toll-like receptors (TLRs).9, 10, 11, 12, 13, 14, 15, 16 Multiple TLR-mediated immune responses of dendritic cells and macrophages in bone marrow and those of splenic B cells were impaired in Cnpy3-knockout (Cnpy3−/−) mice.11, 14 Moreover, the birth rate of Cnpy3−/− mice on a C57BL/6 background is very low (approximately 10% of pups), and their growth after birth is severely retarded, leading half of them to die by the end of the weaning period.11 Therefore, it is possible that the chaperone function of Cnpy3 is essential for the maturation of other proteins that regulate growth and survival, at least in mice. In line with this hypothesis, Canopy1, a paralog of Canopy3 in zebrafish, was reported to interact with fibroblast growth factor (FGF) receptor 1 for regulating FGF signaling, supporting the possibility that Cnpy3 might also be involved in growth factor signaling.8
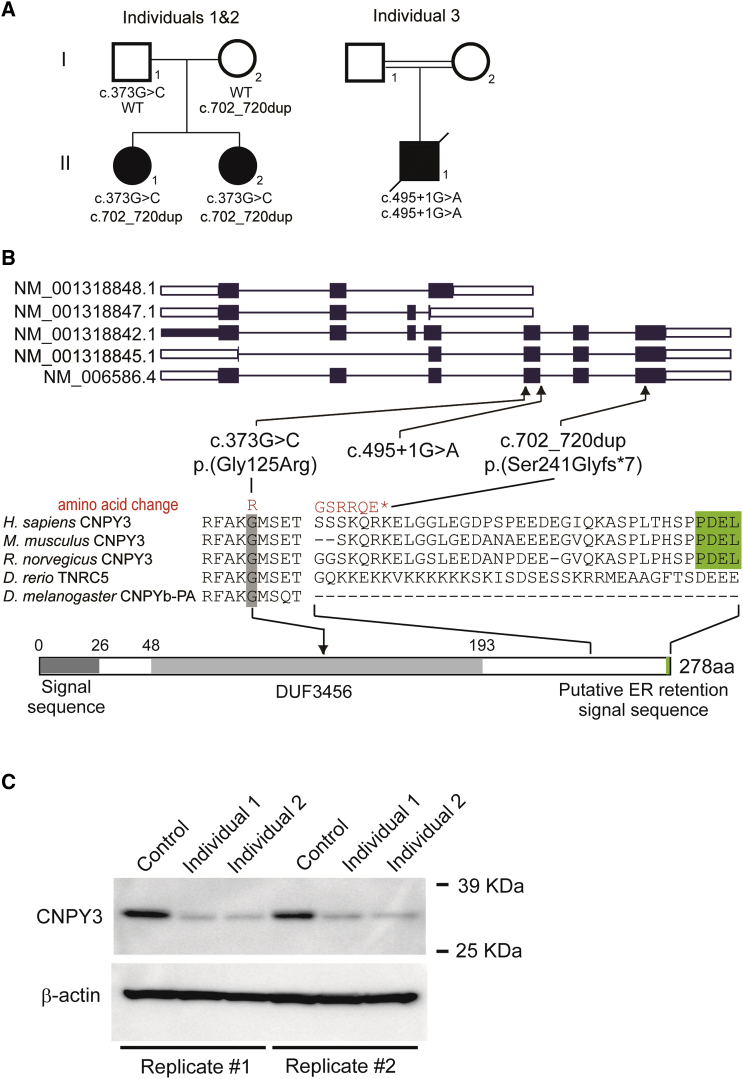
Here, we report on three individuals diagnosed with WS in two families affected by biallelic CNPY3 variants. Clinical information and peripheral blood were obtained from the families after they provided written informed consent. Experimental protocols were approved by the institutional review boards of Yokohama City University and Showa University School of Medicine. We performed whole-exome sequencing (WES) for 700 individuals with epileptic encephalopathy, including WS (n = 171). WES were performed as previously described.17, 18 Singleton WES was performed in 489 individuals. Trio- or quartet-based WES was performed in 211 families, including a family with two affected siblings (Figure 1A). WES of four samples from this family (individuals 1 and 2 and their parents), in which at least 91.9% of RefSeq coding regions were covered by 20 reads or more (Table S1), revealed that CNPY3 harboring compound-heterozygous variants was the sole candidate according to the autosomal recessive model (Table S1). Sanger sequencing confirmed that variants c.373G>C (p.Gly125Arg) and c.702_720dup (p.Ser241Glyfs∗7) (GenBank: NM_006586.4) were transmitted from their father and mother, respectively (Figure S1). Subsequently, we searched for autosomal-recessive CNPY3 variants in our WES data and found an additional individual (individual 3, from consanguineous parents) with a homozygous splice-site variant in CNPY3 (c.495+1G>A [GenBank: NM_006586.4]), which was validated by Sanger sequencing (Figures 1A and S1). In this individual, CNPY3 at 6p21.1 was located within a 20-Mb homozygous stretch, which was identified among 20 regions with a 2-Mb homozygous stretch or more by the Cytogenetics Whole-Genome 2.7M Array (Affymetrix) (Figure S2). Parental samples for segregation analysis and biological samples for mRNA analysis were unavailable for individual 3. Human CNPY3 is 278 aa in length (GenBank: NP_006577.2) and contains an N-terminal signal sequence, a domain of unknown function (DUF3456), and a putative C-terminal ER retention signal.8 Five mRNA transcripts of CNPY3 are registered in RefSeq, and all three variants are located in the region with only longer transcripts (GenBank: NM_006586.4, NM_001318842.1, and NM_001318845.1; Figure 1B). The biological significance of shorter transcripts (GenBank: NM_001318848.1 and NM_001318847.1), which lack the DUF3456 domain and the putative C-terminal ER retention signal, is currently unknown. The c.373G>C variant causes a Gly125 substitution that is evolutionarily conserved from fruit flies to humans, and the c.702_720dup variant leads to the deletion of the putative C-terminal ER retention signal conserved in mammals (Figure 1B).8 All of the variants are absent from GnomAD. Web-based prediction tools suggest that a missense variant (c.373G>C) would be deleterious and that c.495+1G>A would disrupt the splice donor site (Table S2).
Figure 1.
Biallelic CNPY3 Variants in Individuals with WS
(A) Familial pedigrees of three individuals with CNPY3 variants. The segregation of each variant is shown.
(B) CNPY3 transcripts (the UTR and coding region are represented as open and filled rectangles, respectively). Five mRNA transcripts of CNPY3 are registered in RefSeq. Human CNPY3 is 278 aa in length (GenBank: NP_006577.2 translated from GenBank: NM_006586.4) and contains an N-terminal signal sequence, a domain of unknown function (DUF3456), and a putative C-terminal endoplasmic reticulum (ER) retention signal.8 The three variants identified in our individuals were a canonical splice-site variant (c.495+1G>A), a missense variant (c.373G>C) that substituted the evolutionarily conserved glycine residue in the DUF3456 domain, and a frameshift variant (c.702_720dup) that removed the putative ER retention signal conserved among mammals (highlighted in green). Homologous sequences were aligned with CLUSTALW.
(C) Top: immunoblot analysis using anti-human CNPY3 (HPA016560 against 140–249 aa of human CNPY3; Sigma Aldrich) and anti-β-actin (Abcam) antibodies. The amount of CNPY3 was severely lower in LCLs derived from two affected individuals than in control LCLs. Bottom: the amount of β-actin was similar in all individuals. Data of replicated experiments are shown.
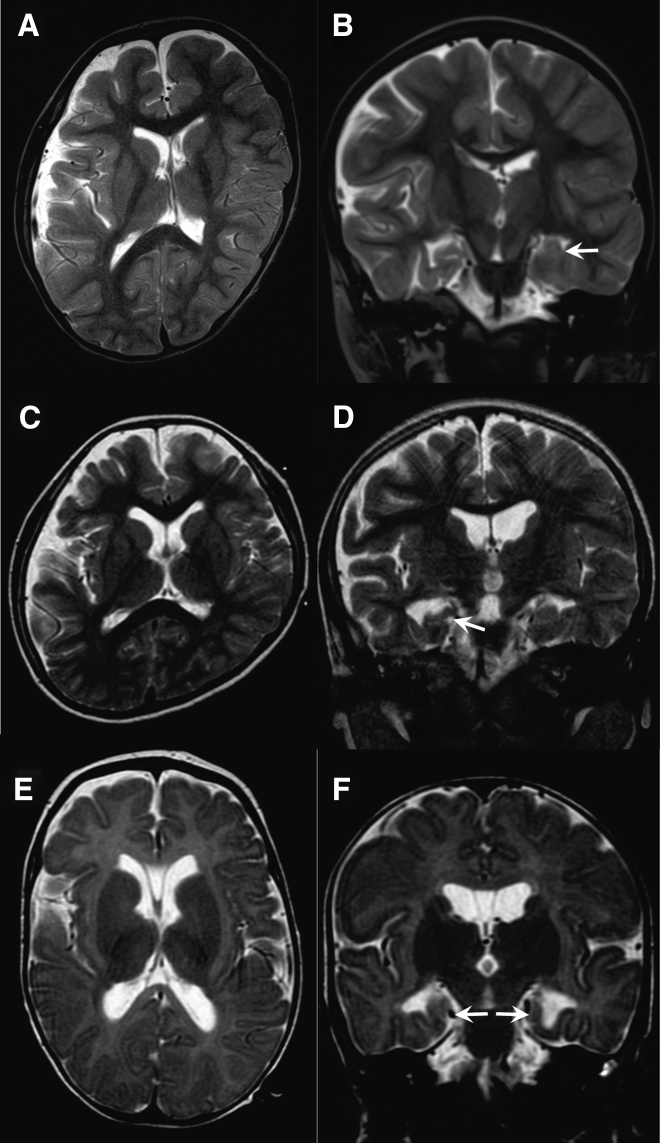
Neurological features of the three individuals with biallelic CNPY3 variants are shown in Table 1, and clinical details are available in the Supplemental Note. Intractable seizures mainly consisting of epileptic spasms and myoclonus developed within 4 months of age in these individuals. Initial diagnoses were WS in individuals 1 and 2 and early myoclonic encephalopathy in individual 3. All three showed hypsarrhythmia on EEG during their clinical course, leading to a diagnosis of WS (Figure S3A). Individuals 1 and 2 later showed characteristic fast waves and diffuse sharp- and slow-wave complexes on EEG. In individual 2, the fast waves were associated with tonic seizures (Figures S3B and S3C). The development of these individuals was markedly delayed. They could not roll over or utter meaningful words. Spastic quadriplegia or diplegia was also observed in all. Brain magnetic resonance imaging (MRI) showed diffuse atrophic changes and malrotation of the hippocampus in all three individuals (Figure 2). These data suggest that biallelic CNPY3 variants can cause EOEE, primarily diagnosed as WS, and hippocampal malrotation might be a characteristic MRI finding.
Table 1.
Clinical Features of Individuals with Biallelic CNPY3 Variants
| Individual 1 | Individual 2 | Individual 3 | |
|---|---|---|---|
| Familial or simplex | familial; elder sister of individual 2 | familial; younger sister of individual 1 | simplex |
| Consanguinity | no | no | second cousin |
| Current age | 14 years | 12 years | died at 13 months |
| Sex | female | female | male |
| Clinical diagnosis | WS at 3 months followed by progressive myoclonic epilepsy | WS at 4 months followed by progressive myoclonic epilepsy | early myoclonic encephalopathy at 3 months; WS at 4 months |
| Prenatal episodes | no | no | no |
| Gestation | 39 weeks | 37 weeks | 40 weeks |
| Birth weight | 2,800 g (−0.2 SD) | 2,510 g (−0.3 SD) | 2,704 g (−1.2 SD) |
| Dysmorphism | no | no | no |
| Seizure onset | 3 months | 4 months | 1 month |
| Seizure types | epileptic spasms followed by myoclonic seizure and focal impaired-awareness seizure | epileptic spasms followed by myoclonic seizure and focal impaired-awareness seizure | tonic seizures and erratic myoclonus followed by epileptic spasms |
| EEG findings | hypsarrhythmia at 3 months; diffuse slow spike-and-wave complex at 10 years | modified hypsarrhythmia at 4 months; diffuse slow spike-and-wave complex at 8 years | suppression burst at 3 months; hypsarrhythmia at 4 months |
| Seizure prognosis | intractable | intractable | intractable |
| Development | bed ridden | bed ridden | bed ridden |
| Neurological signs | spastic quadriplegia and profound intellectual disability | spastic quadriplegia and profound intellectual disability | spastic diplegia |
| Laboratory examination | normal | normal | mild anemia (Hb 10.7 g/dL) |
| MRI findings | progressive diffuse brain atrophy; hippocampal malrotation | progressive diffuse brain atrophy; hippocampal malrotation | diffuse brain atrophy at 1 month; hippocampal malrotation |
| Compromised immunity | no | no | pneumonia at birth |
Abbreviations are as follows: EEG, electroencephalography; Hb, hemoglobin; MRI, magnetic resonance imaging; SD, standard deviation; and WS, West syndrome.
Figure 2.
Brain MRI of Individuals with CNPY3 Variants
Individual 1 at 7 years (A and B), individual 2 at 4 years (C and D), and individual 3 at 4 months (E and F). Axial T2-weighted images (A, C, and E) show mildly enlarged lateral ventricles in all individuals. Individuals 1 and 2 show a deformed cranium with left occipital flatness, caused by a bedridden posture and facing toward the left. Individual 3 shows cavum septi pellucidi and cavum vergae, which are usually considered normal variants. Coronal T2-weighted images (B, D, and F) show malrotation of the left, right, and bilateral hippocampus in individuals 1, 2, and 3, respectively (white arrows).
To examine the effect of biallelic CNPY3 variants, we first evaluated the amount of CNPY3 in lymphoblastoid cell lines (LCLs) derived from individuals 1 and 2. LCLs were treated with a lysis buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM phenylmethanesulfonyl fluoride, and cOmplete EDTA-free protease inhibitor cocktail [Roche]) and then analyzed by SDS-PAGE and immunoblotting. The amount of protein was much lower in LCLs derived from individuals 1 and 2 than in control LCLs, suggesting that c.373G>C and c.702_720dup are loss-of-function variants (Figure 1C). Loss of function is also very likely for the c.495+1G>A variant in individual 3 because it is predicted to cause abnormal splicing (Table S2); however, it could not be experimentally confirmed because of a lack of suitable samples from individual 3. These data suggest that loss of CNPY3 function might have caused EOEE in the three individuals.
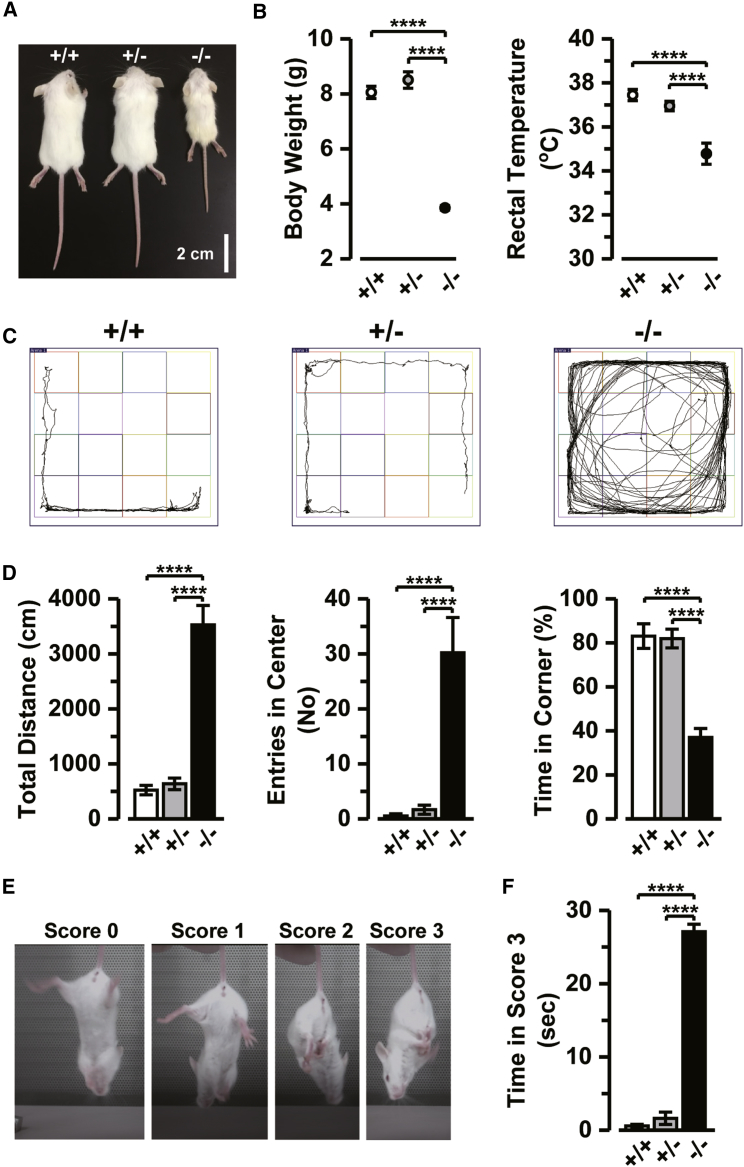
To test the hypothesis that loss of CNPY3 function leads to this disease condition, we analyzed the neurological phenotypes of Cnpy3−/− mice on a BALB/c background11, 12, 13 in detail. All animal experiments were performed in accordance with the guideline of the Physiological Society of Japan and approved by the Institutional Animal Care and Use Committee of the Hamamatsu University School of Medicine. Male and female wild-type (Cnpy3+/+), Cnpy3−/−, and heterozygous (Cnpy3+/−) mice at the age of 18–24 days were used for this study because of the low survival rate of Cnpy3−/− mice after 3 weeks.11 As we previously reported,11 the postnatal development of Cnpy3−/− mice was much slower than that of Cnpy3+/+ or Cnpy3+/− littermates (Figure 3A). We also recognized in this study that not only the body weight but also the rectal temperature of Cnpy3−/− mice was significantly lower than in the other mice (Figure 3B). Moreover, the Cnpy3−/− mice were found to exhibit mild tremors and spastic or dystonic erection of their tails under resting conditions (Movie S1). However, we did not see spontaneous or handling-induced seizures in these mice. Histological examination of Cnpy3−/− pups revealed no apparent structural abnormalities (Figure S4).
Figure 3.
Physical and Behavioral Characteristics of Cnpy3−/− Mice
(A) Physical appearance of Cnpy3+/+, Cnpy3+/−, and Cnpy3−/− littermate mice at 20 days of age.
(B) Differences in body weight (left) and rectal temperature (right) between Cnpy3+/+ (white circles; n = 15), Cnpy3+/− (gray circles; n = 16), and Cnpy3−/− mice (black circles; n = 11).
(C) Representative movement trajectories of Cnpy3+/+, Cnpy3+/−, and Cnpy3−/− mice during the open field test for 5 min.
(D) Comparisons of the total distances traveled (left), number of entries into the center area (middle), and proportions of the time spent in corners (right) during the test period by Cnpy3+/+ (white bar; n = 11), Cnpy3+/− (gray bar; n = 12), and Cnpy3−/− mice (black bar; n = 10).
(E) Representative hindlimb positions and corresponding scores of the hindlimb clasping test.
(F) Comparison of the times spent in the score 3 position during the 30-s test period of Cnpy3+/+ (white bar; n = 11), Cnpy3+/− (gray bar; n = 19), and Cnpy3−/− mice (black bar; n = 8).
∗∗∗∗p < 0.0001 by one-way ANOVA followed by Tukey’s post hoc test. Data are presented as means ± SEM.
Next, we assessed general locomotor activity by using the open field test. Each mouse was first placed in the center of a test box (42 × 42 × 22 cm3 composed of transparent acrylic walls) in a brightly lit room. They were then allowed to explore the box for 5 min, during which time the experimenter was out of their view. The total distance traveled, the number of entries into the center area (21 × 21 cm), and the time spent in the four corner areas (10.5 × 10.5 cm each) were automatically recorded by the SMART 3.0 video tracking system (PanLab). We found that Cnpy3−/− mice tended to move continuously during the 5-min test period (Figures 3C and 3D, Movie S2 [Cnpy3+/+ mice], and Movie S3 [Cnpy3−/− mice]). The total distance traveled by the Cnpy3−/− mice during the test period was more than 5-fold the distance of the Cnpy3+/+ and Cnpy3+/− mice (Figure 3D, left). Moreover, entries into the center area by Cnpy3−/− mice were much more frequent (Figure 3D, middle), and the time spent in the corners was significantly shorter than for Cnpy3+/+ and Cnpy3+/− mice (Figure 3D, right). These results suggest hyperactivity and anxiolytic behavior of Cnpy3−/− mice.
We further assessed motor function by using the hindlimb clasping test. Mice were suspended by their tails at a height of 20 cm from a table, and the positions of the hindlimbs were observed for 30 s. Scores on a scale of 0–3 were then assigned as follows: 0, both hindlimbs consistently thrashed; 1, both hindlimbs consistently splayed outward away from the abdomen; 2, one or both hindlimbs were partially retracted toward the abdomen; and 3, both hindlimbs were entirely clasped and touching the abdomen.19 The hindlimbs of normal mice consistently splayed out and moved as a result of extension reflexes and escape responses (Figure 3E, scores 0 and 1; Movie S4). The hindlimbs of the Cnpy3−/− mice, however, were immediately retracted and clasped to their abdomens, and this posture (score 3) was maintained for most of the 30-s test period (Figure 3F and Movie S5). Thus, motor control in Cnpy3−/− mice seemed to be considerably impaired.
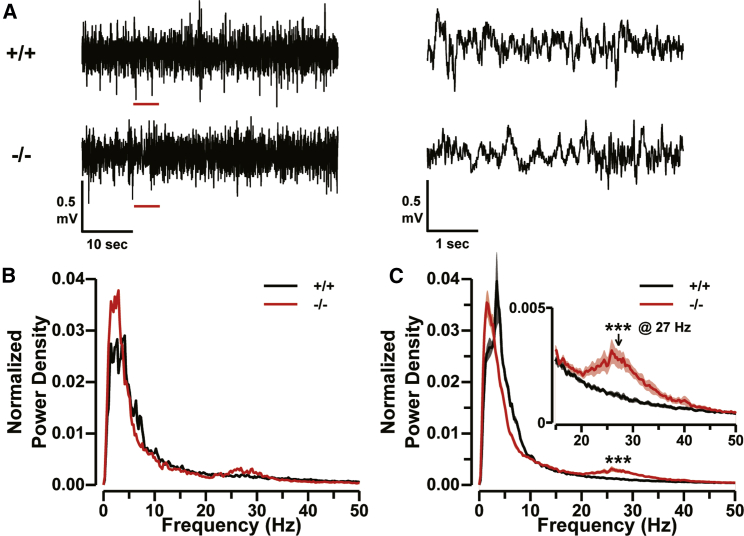
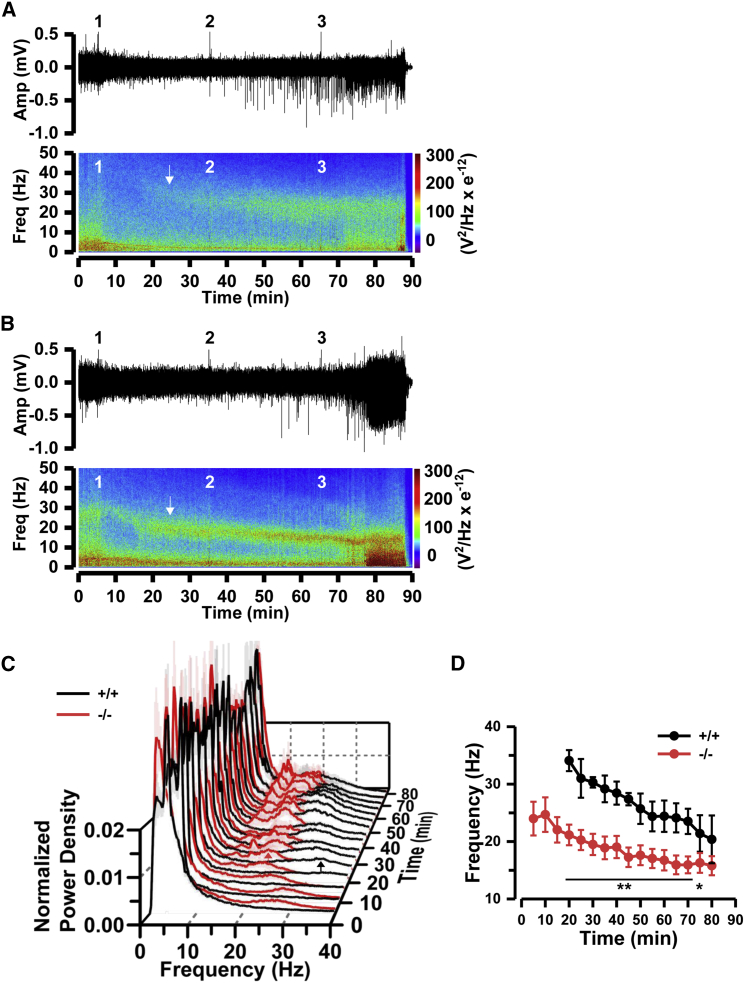
The tremors and abnormal tail movements of Cnpy3−/− mice suggested that they might be prone to seizures, despite the absence of spontaneous seizures, and the hyperactive behavior exhibited by the mice during the open field test is frequently seen in general after status epilepticus. Thus, we next analyzed the resting EEG of the Cnpy3−/− mice. The mice were anesthetized with 0.1%–2.0% isoflurane mixed with room air, and a scalp incision was made to expose the skull. Small gold pin electrodes (1 mm in diameter) were cemented on the skull above the left sensorimotor cortex (signal electrode) and on the right nasal bone (reference electrode). A stainless-steel screw was also cemented on the interparietal bone to act as the ground electrode. After a recovery period of more than 1 hr from surgery and anesthesia on a heating pad, the head was fixed via the stainless-steel screw, and the mouse was connected to the EEG recording system. EEG signals were amplified 800× (Demultiplexed Base Station, Triangel BioSystems International) and digitized at 4 kHz through an A/D converter (PowerLab 4/35, AD Instruments). The resting EEG recordings of the Cnpy3−/− mice did not display clear spikes, waves, or discharges (Figure 4A, bottom). Power spectral analysis of the EEG signal, however, revealed a small hump of increases in power density in the frequency range of 20–35 Hz (Figures 4B and 4C). Such a hump was absent in the spectra of the resting EEG recorded in Cnpy3+/+ (Figures 4B and 4C, black) and Cnpy3+/− (Figure S5) mice. On average, the peak of the hump was present in the fast beta frequency band (25–30 Hz), and the power density at 27 Hz normalized to the total signal power significantly differed from the density at the same frequency in the spectra of Cnpy3+/+ mice (Figure 4C). Thus, the fast waves due to beta-band activity were significantly increased in Cnpy3−/− mice under resting conditions.
Figure 4.
Enhancement in Beta-Band Power in Resting EEG of Cnpy3−/− Mice
(A) Representative EEG traces during normal waking states from Cnpy3+/+ (top) and Cnpy3−/− (bottom) mice. The sections underlined in red (left) are shown to the right on an expanded scale.
(B) Power density spectra of EEG traces in (A). The densities were normalized to the total signal power.
(C) Averaged spectra of EEG traces from Cnpy3+/+ (black line; n = 8) and Cnpy3−/− (red line; n = 9) mice. The inset indicates a magnified view of the spectra in the range of 15–50 Hz.
Data are presented as means ± SEM. ∗∗∗p < 0.001 at 27 Hz by the Mann-Whitney test.
To determine whether enhanced resting beta-band EEG activity in Cnpy3−/− mice is related to their susceptibility to seizures, we next compared the sensitivity to pilocarpine for seizure induction and the time evolution of EEG during seizure development between Cnpy3+/+ and Cnpy3−/− mice. Scopolamine methyl nitrate (1 mg/kg; Tokyo Chemical Industry) was subcutaneously administered to the mice 30 min before the first pilocarpine injection to reduce peripheral effects of the pilocarpine. We injected 100 mg/kg pilocarpine up to three times at 30-min intervals and continuously monitored the EEG during the pilocarpine challenges. A single pilocarpine injection induced neither apparent seizures nor death within 30 min for either Cnpy3+/+ (n = 7) or Cnpy3−/− (n = 8) mice (Table S3). EEG records showed a transient decrease in the total signal power immediately after the first pilocarpine injection in both types of mice (Figures 5A and 5B). Around 20 min after the first pilocarpine injection, however, a hump of beta-band power emerged even in the records of the Cnpy3+/+ mice (Figure 5A, bottom, white arrow; Figure 5C, black arrow). The peak frequency of the hump in the Cnpy3+/+ mice was higher (by ∼10 Hz) than that in the Cnpy3−/− mice (Figure 5D). The hump for the Cnpy3−/− mice also enlarged at the same time, but its peak shifted to a lower frequency range by ∼5 Hz (Figure 5B, bottom, white arrow; Figure 5C, red arrow; and Figure 5D). The second pilocarpine challenge further increased the amplitude of the humps and their proportions in total signal power in both types of mice (Figures 5A–5C), and the challenge facilitated a shift of the humps toward the slow beta-band range (15–25 Hz) (Figures 5C and 5D). At this stage, clear spike events appeared in the records (Figures 5A and 5B, upper traces). One mouse in each group died as a result of status epilepticus seizures after the second challenge (Table S3). The third pilocarpine injection eventually caused epileptic discharges (Figures 5A and 5B) and the death of all mice in both groups, except for one Cnpy3+/+ mouse (Table S3). These results strongly suggest that, although the sensitivity to pilocarpine did not greatly differ between Cnpy3+/+ and Cnpy3−/− mice in terms of mortality, the beta-band hump in the EEG signal reflected abnormal neuronal activities linked to seizure development and that its peak frequency and proportion to the total signal power correlated with the stage of seizure development.
Figure 5.
Enhancement and Leftward Shift of the Beta-Band Power Hump on EEG during Pilocarpine-Induced Seizure Development
(A and B) Representative EEG traces during pilocarpine-induced seizure development for 90 min (top) and spectrograms of the traces (bottom), indicating the time evolution of power spectra from Cnpy3+/+ (A) and Cnpy3−/− (B) mice. Pilocarpine was administered intraperitoneally at points 1 (first 100 mg/kg at 5 min), 2 (second 100 mg/kg at 35 min), and 3 (third 100 mg/kg at 65 min). The white arrow in the spectrogram in (A) indicates the emergence of a beta-band hump 20 min after the first pilocarpine injection in a Cnpy3+/+ mouse. The arrow in the spectrogram in (B) indicates significant enhancement of the hump in a Cnpy3−/− mouse at the corresponding time.
(C) Average power spectra of EEG during the seizures over the groups of Cnpy3+/+ (black line; n = 6) and Cnpy3−/− (red line; n = 7) mice were calculated and aligned at 5-min intervals. Power densities were normalized to the total power. Timing of pilocarpine injections was the same as in (A) and (B). Black and red arrows indicate the peaks of beta-band humps in Cnpy3+/+ and Cnpy3−/− mice, respectively, 20 min after the first pilocarpine injection. The humps gradually enlarged, and the peaks shifted to the slow beta-band range afterward in both types of mice. Data are presented as means ± SEM.
(D) Time courses of the peak frequency of the spectral hump in the range of 10–40 Hz during seizures in Cnpy3+/+ (black line and circles; n = 6) and Cnpy3−/− (red line and circles; n = 7) mice. ∗p < 0.05, ∗∗p < 0.001 by the Mann-Whitney test. Data are presented as means ± SEM.
We identified biallelic CNPY3 variants as a cause of WS in three individuals. All of the variants would greatly reduce the amount of protein and/or impair the function of CNPY3, and the loss of CNPY3 function would cause the epileptic features in these individuals, which is consistent with autosomal recessive inheritance. Individual 1 had an additional de novo missense variant (c.638C>T [p.Thr213Ile], which substitutes the threonine residue located between the AT hook DNA binding domain and the zinc finger domain) in KMT2C (MIM: 606833; Table S2). Loss-of-function variants of KMT2C have been reported in individuals with intellectual disability.20, 21 However, how the missense variant contributes to the phenotype of individual 1 is unclear because no obvious phenotypic difference was observed between individuals 1 and 2.
Brain MRI showed hippocampal malrotation with nonspecific brain atrophy in the three individuals, but Cnpy3−/− mice showed no apparent structural brain abnormalities. Given the fact that the location of the hippocampus in the brain is considerably different between humans and mice, the loss of CNPY3 function could specifically affect morphogenesis of the human hippocampus.
In addition to the epileptic spasms of WS, all three individuals had spastic diplegia or quadriplegia and myoclonus, the indicators of upper-motor-neuron dysfunction. Cnpy3−/− mice also exhibited spastic movements of their tails and hindlimb clasping, suggesting similar motor dysfunction. Because the mice were rather hyperactive, however, as indicated by the open field test, they seemed to have no limb paralysis. The resting tremor, as seen in parkinsonism, might also suggest the presence of dysfunction of the basal ganglia in Cnpy3−/− mice. The low body weight and temperature of the mice might be due to some impairment in the hypothalamic neuroendocrine system, which might also explain the severe growth retardation in individuals 1 and 2 (see Supplemental Note).
Although we did not recognize spontaneous seizures in Cnpy3−/− mice, the presence of abnormally activated neuronal circuits in their brains was indicated by the enhanced fast beta activity on resting EEG, which might provide a basis for their resting tremors, abnormal tail movements, and hyperactivity. The enhanced fast beta activity in mice was not evidently related to susceptibility to pilocarpine-induced seizures under our experimental conditions. This activity, then, must be related to seizure development because similar activity was induced in Cnpy3+/+ mice in response to a pilocarpine injection. In this context, it is interesting to note that individuals 1 and 2 characteristically developed fast waves on EEG, and these fast waves were clinically associated with seizures. Our study therefore suggests that the appearance of fast waves (10 Hz in humans and 25–30 Hz in mice) is a characteristic finding caused by loss of CNPY3 and Cnpy3 function in humans and mice, respectively, and it could be related to seizure activity in humans.
The mechanism by which the loss of function of CNPY3 causes abnormal neuronal activity is a critical issue to be addressed in future studies. Given the CNPY3 expression in TLR-expressing immune cells in the periphery, microglia are likely to play a role. Impaired cytokine production by microglia, as by peripheral immune cells,11 might lead to an excitatory–inhibitory imbalance of neuronal circuits in the brain.22 In addition, expression of Cnpy3 has been recognized in mouse neurons on the basis of Allen Brain Atlas data, suggesting that abnormal chaperone activity of Cnpy3 in both neurons and microglia might be associated with abnormal neuronal activities.
In conclusion, biallelic CNPY3 variants were identified in three individuals with EOEE. Further understanding of abnormal chaperone activity of CNPY3 might provide new insights into the pathogenesis and treatment of epileptic encephalopathy.
Acknowledgments
We thank the individuals and their families for participating in this study. We also thank Nobuko Watanabe, Mai Sato, Kaori Takabe, Miyako Seto, Kaori Shibasaki, Masumi Tsujimura, Chihiro Ogawa, and Akane Kosugi for their technical assistance. This work was supported by the following: grants for Research on Measures for Intractable Diseases, Comprehensive Research on Disability Health and Welfare, the Strategic Research Program for Brain Science, the Initiative on Rare and Undiagnosed Diseases (IRUD) in pediatrics, IRUD for adults, and IRUD Beyond (17ek0109297h0001) from the Japan Agency for Medical Research and Development; Grants-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle, 24118007; Non-linear Neuro-oscillology, 15H05872) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; Grants-in-Aid for Scientific Research (A) (17H01539), (B) (16H05160, 16H05357, and 17H04025), and (C) (15K10367, 16K09975, 17K10080, 17K08534, and 17K00918) from the Japan Society for the Promotion of Science; the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program of the Project for Developing Innovation Systems from the Japan Science and Technology Agency; grants from the Ministry of Health, Labour, and Welfare (27-5 and 27-6); the Takeda Science Foundation; and the Mochida Memorial Foundation for Medical and Pharmaceutical Research. We thank Nancy Schatken from the Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Published: January 25, 2018
Footnotes
Supplemental Data include a Supplemental Note, five figures, three tables, and five movies and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.01.004.
Contributor Information
Naomichi Matsumoto, Email: naomat@yokohama-cu.ac.jp.
Hirotomo Saitsu, Email: hsaitsu@hama-med.ac.jp.
Web Resources
Allen Brain Atlas data, http://mouse.brain-map.org/
CLUSTALW, http://www.genome.jp/tools-bin/clustalw
OMIM, http://www.omim.org/
Supplemental Data
Cnpy3−/− mice occasionally exhibited spastic tremor and tail erection in poor physical condition.
Open field activity was especially low in preweaning Cnpy3+/+ mice.
Open field activity was hyper and persistent during the test period even so preweaning Cnpy3−/− mice.
Cnpy3+/+ mice showed a regular hindlimb extension reflex with constant body torsion.
Both hindlimbs were fully clasped and touching the abdomen just after the test started, suggesting motor dysfunction in Cnpy3−/− mice.
References
- 1.Berg A.T., Berkovic S.F., Brodie M.J., Buchhalter J., Cross J.H., van Emde Boas W., Engel J., French J., Glauser T.A., Mathern G.W. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 2.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohtahara S., Yamatogi Y. Ohtahara syndrome: with special reference to its developmental aspects for differentiating from early myoclonic encephalopathy. Epilepsy Res. 2006;70(Suppl 1):S58–S67. doi: 10.1016/j.eplepsyres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Kato M. A new paradigm for West syndrome based on molecular and cell biology. Epilepsy Res. 2006;70(Suppl 1):S87–S95. doi: 10.1016/j.eplepsyres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Allen A.S., Berkovic S.F., Cossette P., Delanty N., Dlugos D., Eichler E.E., Epstein M.P., Glauser T., Goldstein D.B., Han Y., Epi4K Consortium. Epilepsy Phenome/Genome Project De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaud J.L., Lachance M., Hamdan F.F., Carmant L., Lortie A., Diadori P., Major P., Meijer I.A., Lemyre E., Cossette P. The genetic landscape of infantile spasms. Hum. Mol. Genet. 2014;23:4846–4858. doi: 10.1093/hmg/ddu199. [DOI] [PubMed] [Google Scholar]
- 7.Pavone P., Striano P., Falsaperla R., Pavone L., Ruggieri M. Infantile spasms syndrome, West syndrome and related phenotypes: what we know in 2013. Brain Dev. 2014;36:739–751. doi: 10.1016/j.braindev.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Hirate Y., Okamoto H. Canopy1, a novel regulator of FGF signaling around the midbrain-hindbrain boundary in zebrafish. Curr. Biol. 2006;16:421–427. doi: 10.1016/j.cub.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi Y., Kobayashi M., Akashi-Takamura S., Tanimura N., Konno K., Takahashi K., Ishii T., Mizutani T., Iba H., Kouro T. A protein associated with toll-like receptor 4 (PRAT4A) regulates cell surface expression of TLR4. J. Immunol. 2006;177:1772–1779. doi: 10.4049/jimmunol.177.3.1772. [DOI] [PubMed] [Google Scholar]
- 10.Liu B., Yang Y., Qiu Z., Staron M., Hong F., Li Y., Wu S., Li Y., Hao B., Bona R. Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat. Commun. 2010;1:79. doi: 10.1038/ncomms1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi K., Shibata T., Akashi-Takamura S., Kiyokawa T., Wakabayashi Y., Tanimura N., Kobayashi T., Matsumoto F., Fukui R., Kouro T. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J. Exp. Med. 2007;204:2963–2976. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata T., Takemura N., Motoi Y., Goto Y., Karuppuchamy T., Izawa K., Li X., Akashi-Takamura S., Tanimura N., Kunisawa J. PRAT4A-dependent expression of cell surface TLR5 on neutrophils, classical monocytes and dendritic cells. Int. Immunol. 2012;24:613–623. doi: 10.1093/intimm/dxs068. [DOI] [PubMed] [Google Scholar]
- 13.Shibata T., Motoi Y., Tanimura N., Yamakawa N., Akashi-Takamura S., Miyake K. Intracellular TLR4/MD-2 in macrophages senses Gram-negative bacteria and induces a unique set of LPS-dependent genes. Int. Immunol. 2011;23:503–510. doi: 10.1093/intimm/dxr044. [DOI] [PubMed] [Google Scholar]
- 14.Kiyokawa T., Akashi-Takamura S., Shibata T., Matsumoto F., Nishitani C., Kuroki Y., Seto Y., Miyake K. A single base mutation in the PRAT4A gene reveals differential interaction of PRAT4A with Toll-like receptors. Int. Immunol. 2008;20:1407–1415. doi: 10.1093/intimm/dxn098. [DOI] [PubMed] [Google Scholar]
- 15.Hart B.E., Tapping R.I. Cell surface trafficking of TLR1 is differentially regulated by the chaperones PRAT4A and PRAT4B. J. Biol. Chem. 2012;287:16550–16562. doi: 10.1074/jbc.M112.342717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saitoh S., Miyake K. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol. Rev. 2009;227:32–43. doi: 10.1111/j.1600-065X.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 17.Saitsu H., Nishimura T., Muramatsu K., Kodera H., Kumada S., Sugai K., Kasai-Yoshida E., Sawaura N., Nishida H., Hoshino A. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat. Genet. 2013;45:445–449. doi: 10.1038/ng.2562. e1. [DOI] [PubMed] [Google Scholar]
- 18.Fukai R., Saitsu H., Tsurusaki Y., Sakai Y., Haginoya K., Takahashi K., Hubshman M.W., Okamoto N., Nakashima M., Tanaka F. De novo KCNH1 mutations in four patients with syndromic developmental delay, hypotonia and seizures. J. Hum. Genet. 2016;61:381–387. doi: 10.1038/jhg.2016.1. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J.W., Li Y.F., Wang Z.T., Jia W.Q., Xu R.X. Toll-Like Receptor 4 Deficiency Impairs Motor Coordination. Front. Neurosci. 2016;10:33. doi: 10.3389/fnins.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleefstra T., Kramer J.M., Neveling K., Willemsen M.H., Koemans T.S., Vissers L.E., Wissink-Lindhout W., Fenckova M., van den Akker W.M., Kasri N.N. Disruption of an EHMT1-associated chromatin-modification module causes intellectual disability. Am. J. Hum. Genet. 2012;91:73–82. doi: 10.1016/j.ajhg.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koemans T.S., Kleefstra T., Chubak M.C., Stone M.H., Reijnders M.R.F., de Munnik S., Willemsen M.H., Fenckova M., Stumpel C.T.R.M., Bok L.A. Functional convergence of histone methyltransferases EHMT1 and KMT2C involved in intellectual disability and autism spectrum disorder. PLoS Genet. 2017;13:e1006864. doi: 10.1371/journal.pgen.1006864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squarzoni P., Oller G., Hoeffel G., Pont-Lezica L., Rostaing P., Low D., Bessis A., Ginhoux F., Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cnpy3−/− mice occasionally exhibited spastic tremor and tail erection in poor physical condition.
Open field activity was especially low in preweaning Cnpy3+/+ mice.
Open field activity was hyper and persistent during the test period even so preweaning Cnpy3−/− mice.
Cnpy3+/+ mice showed a regular hindlimb extension reflex with constant body torsion.
Both hindlimbs were fully clasped and touching the abdomen just after the test started, suggesting motor dysfunction in Cnpy3−/− mice.