Abstract
Reactive oxygen species (ROS) are well-described by-products of cellular metabolic activities, acting as signaling molecules and regulating the redox state of proteins. Solvent exposed thiol residues like cysteines are particularly sensitive to oxidation and their redox state affects structural and biochemical capacities of many proteins. While thiol redox regulation has been largely studied in several cell compartments like in the plant chloroplast, little is known about redox sensitive proteins in the nucleus. Recent works have revealed that proteins with oxidizable thiols are important for the regulation of many nuclear functions, including gene expression, transcription, epigenetics, and chromatin remodeling. Moreover, thiol reducing molecules like glutathione and specific isoforms of thiols reductases, thioredoxins and glutaredoxins were found in different nuclear subcompartments, further supporting that thiol-dependent systems are active in the nucleus. This mini-review aims to discuss recent progress in plant thiol redox field, taking examples of redox regulated nuclear proteins and focusing on major thiol redox systems acting in the nucleus.
Keywords: nucleus, thiol, glutathione, thioredoxin, glutaredoxin, ROS
Introduction
Oxygen is one of the most important molecules for aerobic organisms. It is necessary for cell metabolism, but it also generates reactive oxygen species (ROS) as by-products of oxidoreduction pathways. ROS include free radical species like superoxides (), hydroxyl radicals (OH•), or nitric oxide (NO•), and non-radical species like hydrogen peroxide (H2O2) and peroxynitrite (ONOO-) (Sies et al., 2017). In plants, major sources of ROS are photosynthetic and respiratory chains in chloroplasts and mitochondria. ROS are also generated by plasma membrane NADPH oxidases and peroxisomal xanthine oxidases. Oxidative eustress is playing important signaling functions by inducing post-translational modifications (PTM) and by regulating protein redox state. ROS can also trigger oxidative distress which can damage the cell (Foyer and Noctor, 2016; Choudhury et al., 2017). Plant cells display a large panel of ROS scavenging enzymes like catalases, peroxidases, and superoxide dismutases. They also generate compounds that reverse ROS-induced oxidations. Among these compounds are antioxidant molecules like glutathione and ascorbate, which both play important roles as cofactors for thiol reduction enzymes like peroxidases and reductases (Noctor, 2017; Rahantaniaina et al., 2017). Glutathione and ascorbate are themselves reduced by glutathione reductases (GRs) and dehydroascorbate reductases (DHARs). Thioredoxins (TRXs) and glutaredoxins (GRXs) are key thiol reduction enzymes. They act as reducing power of metabolic enzymes and ROS scavenging systems but they also regulate thiol-based post-transcriptional redox modifications in proteins (Meyer et al., 2012). Oxidized TRXs are generally reduced by NADPH-dependent thioredoxin reductases (NTRs), whereas the reduction of GRXs is dependent on glutathione. Due to their multifunctional thiol reduction capacities, TRXs and GRXs have been involved in many metabolic functions, controlling plant developmental programs and acting as key signaling molecules in response to abiotic and biotic stresses (Meyer et al., 2009; Rouhier et al., 2015). In this mini-review, we aim to give an updated overview of nuclear thiol-based ROS signaling in plants.
Ros and Cys Ox-PTMs in the Nucleus
Some data suggest that ROS are actively generated in the nucleus (Ashtamker et al., 2007), but they principally accumulate in the nucleus through transfer from other cell compartments. Genetically encoded fluorescent H2O2 sensors (e.g., HyPer) have consistently shown that cytosolic H2O2 freely diffuses in the nucleus through nuclear pores (Møller et al., 2007; Rodrigues et al., 2017). It is also transferred from the chloroplasts to the nucleus under pathogen and high light (HL) conditions (Caplan et al., 2015; Exposito-Rodriguez et al., 2017).
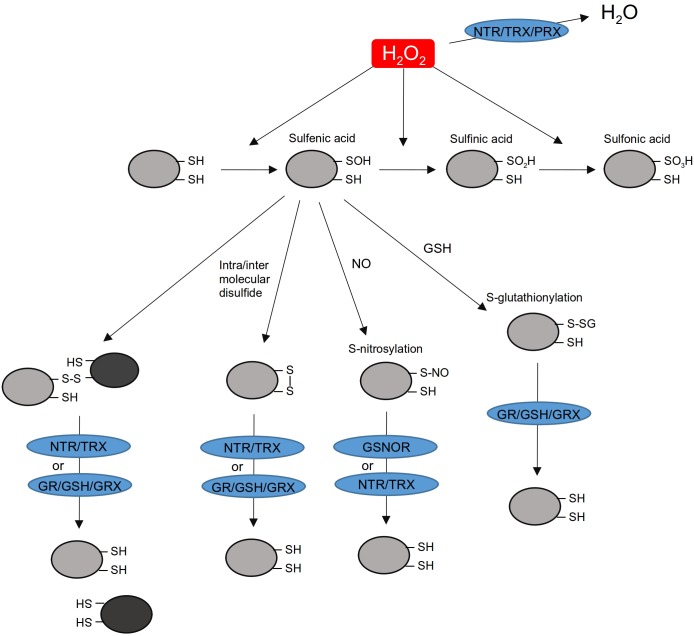
The chemical characteristics of the sulfur atom make Cys and Met residues major sites of oxidation within proteins (Davies, 2005). Depending on their pKa and on the pH of the medium, thiol residues are deprotonated into a thiolate residue (R-S-) which is prone to oxidation. This is leading to successive oxidations to sulfenic (R-SOH), sulfinic (R-SO2H), and sulfonic (R-SO3H) acids (Davies, 2005). Thiol groups can also form a disulfide bridge (S-S) or react with reactive nitrogen species (RNS) or oxidized glutathione (GSSG) resulting in S-nitrosylation (R-SNO) or S-glutathionylation (R-S-SG). Depending on their nature, most of these thiol modifications can be reversed by dedicated thiol reduction systems (TRX, GRX, and GSNO Reductase) which exhibit disulfide bond, deglutathionylation or denitrosylation activities (Figure 1). Thiol modifications can alter the structure and/or the activity of many proteins like transcription factors, MAP kinases, and chromatin modification proteins (see below). Proteomic approaches aiming to identify oxidized thiol targets have been developed in plants. Hundreds of nuclear candidate proteins were found sulfenylated, nitrosylated, or glutathionylated (Supplementary Table 1; Zaffagnini et al., 2012, 2016; Morisse et al., 2014; Waszczak et al., 2014; Chaki et al., 2015; Pérez-Pérez et al., 2017). These approaches also revealed the complexity of the thiol redox modification networks in plants (Pérez-Pérez et al., 2017). However, among all these candidates, redox regulation has been validated only in a few cases (see below).
FIGURE 1.
H2O2-induced thiol modifications and scavenging activities in the nucleus. H2O2 accumulating in the nucleus can be detoxified by a NTR/TRX/PRX system. While many other H2O2 detoxification enzymes are active in plants (e.g., catalases and ascorbate peroxidases), their presence in the nucleus is not demonstrated yet. They are not represented here. H2O2 can oxidize thiol residues in protein. Sulfenic acid reacts with GSH, NO or with adjacent thiol residues. Putative nuclear proteins prone to S-glutathionylated, S-nitrosylated, or disulfide bonds formation have been identified by proteomic and biochemical approaches (see Supplementary Table 1; Delorme-Hinoux et al., 2016; Pérez-Pérez et al., 2017). S-glutathionylated, S-nitrosylated, or intra/intermolecular disulfide bonds can be reduced by NTR/TRX, GR/GSH/GRX, or GSNOR. NTR, NADPH-dependent thioredoxin reductase; TRX, thioredoxin; GR, glutathione reductase; GRX, glutaredoxin; PRX, peroxiredoxin; GSNOR, S-nitrosoglutathione reductase; GSH, glutathione; NO, nitric oxide; H2O2, hydrogen peroxide.
Thiol Redox Systems in the Nucleus
Glutathione
Plants exhibit a large panel of thiol reduction systems (Meyer et al., 2012). Among them is glutathione, a low molecular weight thiol-containing tripeptide (γ-glutamyl-cysteinyl-glycine). Glutathione biosynthesis is performed in chloroplasts and in the cytosol but is found in almost all cell compartments, including the nucleus. Nuclear pores are generally assumed to allow unrestricted bidirectional diffusion of glutathione across the nuclear envelope. Therefore, nuclear glutathione translocation to the nucleus can be passive. Glutathione quantification at the subcellular level is technically challenging due to its highly dynamic compartmentation (Delorme-Hinoux et al., 2016). Thiol-specific dyes and genetically encoded probes such as reduction-oxidation-sensitive green fluorescent proteins (roGFPs) have consistently detected glutathione in the nucleus. Immunocytochemistry (ICC) coupled to electronic microscopy were also used to address its location at a subcompartment level (Zechmann et al., 2008; Zechmann and Müller, 2010). This study found glutathione uniformly spread in the nucleoplasm, without distinction between euchromatin and heterochromatin. In Arabidopsis thaliana, a glutathione reductase (GR1) is found in the nucleus, suggesting that oxidized glutathione (GSSG) is actively reduced in the nucleus (Delorme-Hinoux et al., 2016).
The functions of glutathione in the nucleus are still poorly understood. Thiol-labeling experiments using the 5-chloromethylfluorescein diacetate (CMFDA) dye and glutathione redox state measured by roGFP, suggest that a redox cycle is occurring during the cell cycle progression and is critical for cell cycle progression (Diaz Vivancos et al., 2010; Vivancos et al., 2010; de Simone et al., 2017). Consistently, sustained mild oxidation observed in ascorbate mutants also restricts nuclear functions and impairs progression through the cell cycle (de Simone et al., 2017). More than acting as a general redox buffer, glutathione could also provide reducing moiety for anti-oxidant enzymes like GRXs (i.e., GRXC1, GRXC2, GRXS17, GRXS13, and ROXY1/2/4) and Glutathione S-Transferases (i.e., GSTF5-10, GSTU19/20, and GSTT19/20), several of them having been identified in the nucleus (Rouhier et al., 2015; Palm et al., 2016).
Consistent levels of ascorbate have also been found in the nucleus but little evidence for its nuclear functions has been described (Zechmann et al., 2011; Considine and Foyer, 2014; de Simone et al., 2017; Zechmann, 2017).
Thiols Reductases in the Nucleus
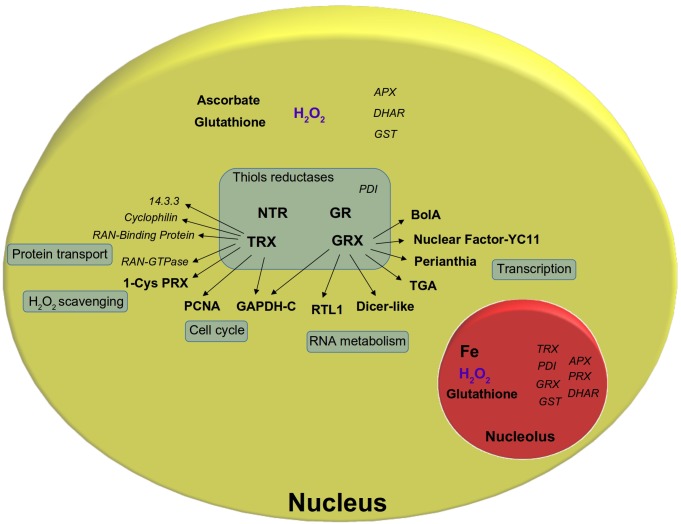
Thioredoxins (TRXs) and Glutaredoxins (GRXs) are major classes of thiols reductases. Plants harbor a complex TRX and GRX network (Meyer et al., 2012). Among the 40 TRXs and 50 GRXs isoforms were found in Arabidopsis, at least 4–8 of them have been assigned to the nucleus, although often in association with a cytosolic localization (Delorme-Hinoux et al., 2016). Moreover, NTR isoforms were also found in the nucleus, where they reduce TRXh, TRXo1, and Nucleoredoxin 1 (NRX1) (Serrato et al., 2001; Serrato and Cejudo, 2003; Marty et al., 2009; Marchal et al., 2014). Some thiol reductases are constitutively located in the nucleus, but others were found to shuttle between the cytosol and nucleus. In tomato subjected to heat stress, the predominant cytosolic GRXS17 was found to relocate in the nucleus (Wu et al., 2012). In wheat and Chlamydomonas, TRXh isoforms accumulate in the nucleus upon oxidative or genotoxic stress (Serrato and Cejudo, 2003; Sarkar et al., 2005). Little is known about the subnuclear localization of these respective proteins. This is due to the low resolution of localization techniques like ICC and GFP-fusion coupled with confocal microscopy analyses. In most cases, these proteins are detected in the nucleoplasm, without distinction between heterochromatin and euchromatin, and are apparently excluded from the nucleolus. Recently, ICC analyses have detected NRX1 and NTRA in the nucleolar cavity, but the functional significance of this localization is still unknown (Marchal et al., 2014). Major thiol redox components found in the nucleus are presented in Figure 2. ROS scavenging enzymes and thiol-containing target proteins are shown as well. Most of these components have been recently reviewed by Delorme-Hinoux et al. (2016) and will not be further discussed here.
FIGURE 2.
Major thiol redox components found in the nucleus. Thiol reduction systems, thiol-containing target proteins were represented as well as ROS scavenging enzymes. Proteins which were only found in proteomic data (according to Montrichard et al., 2009; Waszczak et al., 2014; Delorme-Hinoux et al., 2016; Palm et al., 2016; Montacié et al., 2017; Pérez-Pérez et al., 2017) but not further validated in the nucleus were represented in italic. NTR, NADPH-dependent thioredoxin reductase; TRX, thioredoxin; GR, glutathione reductase; GRX, glutaredoxin; PRX, peroxiredoxin; PDI, protein disulfide isomerase; GST, glutathione S-transferase; APX, ascorbate peroxidase; DHAR, dehydroascorbate reductase; Ran-GTPase, Ran-guanosine triphosphatase; PCNA, proliferating cell nuclear antigen; GAPDH-C, glyceraldehyde 3-phosphate dehydrogenase-C; RTL1, RNAse III-like 1; TGA, TGA-transcription factor; NF-YC11, nuclear factor-YC11; GSH, glutathione; NO, nitric oxide; H2O2, hydrogen peroxide.
The nucleolus, a nuclear subcompartment responsible for rRNA biosynthesis, might also be subjected to redox regulation (Saez-Vasquez and Medina, 2009). Significant accumulation of H2O2 has been detected in the nucleolus in tobacco cell suspension subjected to elicitor treatments (Ashtamker et al., 2007). Intriguingly, the nucleolus also accumulates high amounts of iron, which might provide substrates for ROS generation by Fenton reactions (Roschzttardtz et al., 2011). In addition, glutathione and several isoforms of PRXs, DHARs, APXs, TRXs, and GRX-like proteins were enriched in this compartment (Zechmann et al., 2008; Zechmann and Müller, 2010; Palm et al., 2016; Montacié et al., 2017). Whether redox activities are occurring in the nucleolus will need further investigations.
Redox-Regulated Nuclear Functions
Transcriptomic Control by ROS
Reactive oxygen species causes drastic changes in nuclear gene expression (Gadjev et al., 2006; Willems et al., 2016; Shaikhali and Wingsle, 2017). Oxidative stress affects many pathways involved in RNA processing, including splicing, polyadenylation, exporting, and editing. It is also involved in RNA degradation and protein translation (Van Ruyskensvelde et al., 2018). Under High-light (HL) conditions, ROS originated in chloroplasts are associated with chloroplast-to-nucleus (retrograde) signaling. Among the molecules involved in the retrograde signaling (Suzuki et al., 2012; Vogel et al., 2014; Dietz et al., 2016), singlet oxygen (1O2) induces expression of subsets of 1O2-responsive genes and enhances tolerance to HL and to other abiotic and biotic stress (Wagner et al., 2004; Carmody et al., 2016). H2O2 originating by dismutation of superoxide in the chloroplast has also been recently shown to be involved in retrograde signaling upon HL exposure (Exposito-Rodriguez et al., 2017). Another retrograde signaling was suggested to involve a redox regulation of the chloroplastic cyclophilin Cyp20.3, leading to stimulation of Cys synthesis, accumulation of non-protein thiols and activation of defense gene expression (Dominguez-Solis et al., 2008; Park et al., 2013). Presumably, ROS generated in other cell compartments (mitochondria, peroxisomes, and apoplast) can also exert similar retrograde signaling (Noctor and Foyer, 2016; Rodríguez-Serrano et al., 2016).
Photorespiration produces H2O2 in peroxisomes. In this compartment, catalases play an important role in removing H2O2. The cat2 mutant inactivated in the major peroxisomal catalase accumulates a high level of peroxisomal H2O2 and impacts nuclear gene expression extensively, rapidly inducing subsets of stress and hormonal response genes (Queval et al., 2007). In this case, regulation of gene expression involves glutathione signaling also, as the transcriptomic response is partly abolished in a glutathione-defective (cad2) cat2 cad2 double mutant (Han et al., 2013a,b). A signaling role of glutathione on nuclear gene expression was also suggested by transcriptomic data in genetically or pharmacologically manipulated glutathione backgrounds (Xiang and Oliver, 1998; Ball et al., 2004; Schnaubelt et al., 2015). Other transcriptomic analyses also showed involvement of thiol reduction systems in modulating nuclear gene expression (Bashandy et al., 2009; Martins et al., unpublished data). Whether these actors are directly involved in gene expression needs further investigations.
Redox Regulation of Transcription Factors
A likely impact of ROS on nuclear gene expression relies on the regulation of redox-sensitive transcription factors (Considine and Foyer, 2014; Dietz, 2014; Rouhier et al., 2015; Waszczak et al., 2015). In most cases, redox regulation induces conformation changes in transcription factors or associated proteins. Such modifications can occur in the cytosol and trigger nuclear translocation, e.g., by uncovering of a nuclear localization sequence (NLS). A well-documented example is the thiol redox-dependent nuclear translocation of the glycolytic enzyme Glucose 6-Phosphate Dehydrogenase C (GAPDH-C) which impacts both its metabolic activity and its moonlighting function as a transcriptional activator of glycolytic genes (Holtgrefe et al., 2008; Vescovi et al., 2013; Zaffagnini et al., 2013; Testard et al., 2016; Zhang et al., 2017). The HL- and H2O2-dependent nuclear translocation of Heat-Shock Factors (HSFA1D and HSFA8) is also dependent on specific Cys residues (Miller and Mittler, 2006; Jung et al., 2013; Giesguth et al., 2015; Dickinson et al., 2018). The pathogen-induced Salicylic Acid (SA)-dependent transcriptional response is mediated by redox-dependent nuclear translocation of NON-EXPRESSOR OF PR GENES1 (NPR1). In this particular case, NPR1 is kept in the cytosol in a disulfide-bound oligomeric homocomplex. Upon pathogen attack, SA induces TRXh5 expression which counteracts NPR1 oligomer formation by reducing NPR1 disulfides. Moreover, through its denitrosylase activity, TRXh5 also suppresses the stimulatory effect of Cys156 S-nitrosylation on formation of disulfide-linked NPR1 oligomer (Tada et al., 2008; Kneeshaw et al., 2014). NPR1 is translocated in the nucleus where it promotes PR gene expression through interaction with TGA transcription factors such as TGA1 (Després et al., 2003; Mou et al., 2003; Tada et al., 2008; Kneeshaw et al., 2014).
Indeed, other members of the TGA transcription factors are likely redox regulated in the nucleus. Among the 10 TGA factors found in Arabidopsis, several of them (i.e., TGA1, TGA2, TGA3, TGA7, and Perianthia) interact with type III GRXs (ROXY1 and 2) and are involved in the development of petals, anthers and microspores. Although the redox dependent control of these TGA is not fully established, ROXY/TGA interactions are occurring in the nucleus and affect TGA-regulated gene expression (Xing et al., 2005; Xing and Zachgo, 2008; Li et al., 2009, 2011; Murmu et al., 2010; reviewed by Dietz, 2014 and Delorme-Hinoux et al., 2016).
R2R3-type MYB transcription factors from maize require reducing conditions for DNA binding. Under non-reducing conditions, Cys49 and Cys53 form a disulfide bond that prevents the R2R3 MYB domain from binding DNA (Williams and Grotewold, 1997; Heine et al., 2004). More recently, the structure and the DNA binding activity of a AtMYB30 transcription factor were shown to be influenced by S-nitrosylation (Tavares et al., 2014).
AP2/ethylene response factor (ERF) is another class of transcription factors which undergoes redox regulation (Welsch et al., 2007; Shaikhali et al., 2008; Vogel et al., 2014). One of the most striking examples was described for the Rap2.12-dependent regulation of hypoxia response genes. Under aerobic conditions, Rap2.12 is bound to the plasma membrane within an acyl-CoA binding protein 1 or 2 (ACBP1/2) complex. In low oxygen, Rap2.12 is released from the plasma membrane by a mechanism involving a N-terminal Cys2 residue, and is translocated to the nucleus where it activates hypoxia response genes (Gibbs et al., 2011; Licausi et al., 2011; Licausi, 2013).
Comelli and Gonzalez (2007) also reported a redox regulation of conserved Cys in the homeodomain (HD) DNA of plant class III HD-Zip proteins. Here, DNA binding capacities are only maintained when an intramolecular disulfide bond is reduced by a thioredoxin (Comelli and Gonzalez, 2007). A Cys-dependent redox regulation of the DNA binding activity of basic region leucine zipper (bZIP) transcription factors has also been reported (Shaikhali et al., 2012).
Finally, a subunit of the Nuclear Factor-Y (NF-Y) transcription factor complex (NY-YC11) physically interacts with the iron-sulfur cluster glutaredoxin GRXS17 in the nucleus. It is not known yet if this interaction is redox-dependent (Knuesting et al., 2015). In the cytosol and the nucleus, GRXS17 also interacts with and reduces BolA2, a factor involved in iron metabolism (Couturier et al., 2014; Qin et al., 2015).
Epigenetic Regulation
Redox regulation of epigenetic processes has mostly been addressed in mammals (García-Giménez et al., 2017), but this field is poorly explored in plants (Delorme-Hinoux et al., 2016; Shen et al., 2016). Nevertheless, due to the ubiquity of these basic mechanisms in living organisms, it is likely that such regulation occurs in plants as well. Different enzymes involved in histone methylation are prone to redox regulation, affecting both positive and negative histone marks (e.g., H3K4me2, H3K4me3, H3K79me3, H3K27me2, and H3K9me2) (Chen et al., 2006; Zhou et al., 2008, 2010; Niu et al., 2015). In mammals, nuclear histone acetylation activities are redox sensitive, affecting chromatin conformation and transcription (Ito et al., 2004; Ago et al., 2008; Nott et al., 2008; Doyle and Fitzpatrick, 2010). During brain development, neurotrophic factors induce S-nitrosylation at conserved Cys of HDAC2 in neurons, resulting in changes of histone modification and gene expression (Nott et al., 2008). Upon cardiac hypertrophy, a ROS/TRX-dependent redox switch of key Cys residues affects nuclear trafficking of a class II HDAC and subsequent gene expression (Ago et al., 2008). Within the large family of HDAC identified in plants (Pandey et al., 2002), members of the class I RPD-3 like HDAC (HDAC9, 19) have been shown to be sensitive to oxidation (Liu et al., 2015; Mengel et al., 2017), but the physiological significance of those modifications is still poorly understood. NO-induced HDAC inhibition is proposed to operate in plant stress response by facilitating the stress-induced transcription of genes (Mengel et al., 2017).
In addition to methylation and acetylation, mammalian histone H3 has been shown to be glutathionylated on a conserved and unique Cys residue (García-Giménez et al., 2014). Histone H3 glutathionylation increases during cell proliferation and decreases during aging. This produces structural changes affecting nucleosome stability and leading to a more open chromatin structure (García-Giménez et al., 2013, 2014, 2017; Xu et al., 2014).
Small RNAs (siRNA and miRNA) are key regulators of gene expression, involved in most developmental and stress response processes in eukaryotic cells (Leisegang et al., 2017). Biogenesis of small RNAs is orchestrated by DICER-LIKE (DCL) and RNASE THREE-LIKE (RTL) endonucleases that process almost every class of double-stranded RNA precursors. Charbonnel et al. (2017) have recently demonstrated that members of DCL and RTL families in Arabidopsis are glutathionylated on a conserved Cys which affects their RNase III activity. R-S-SG of RTL1 is reversed by type I GRXs, suggesting that small RNA biogenesis and subsequent gene expression responses are under the control of the cell redox environment (Charbonnel et al., 2017). Indeed, the RNase activity of another member of the family (RTL2) was previously shown to be regulated by its dimerization state through an intermolecular disulfide bond (Comella et al., 2008), showing that a redox switch might regulate small RNA biogenesis.
Epigenetic regulation of gene expression is performed by DNA methylation. Some key metabolic enzymes involved in DNA methylation are suspected to be redox regulated. Among them are enzymes of the S-Adenosyl Methionine (SAM) cycle which provide precursors for DNA and histone methylation (Shen et al., 2016). Other nuclear candidates are the DNA demethylases Repressor of Silencing1 (ROS1) and Demeter-like (DME, DML2, and DML3) enzymes which remove methylated bases from the DNA backbone (Zhu, 2009). All these enzymes contain an iron-sulfur (Fe-S) cluster which might be susceptible to oxidation by ROS. Moreover, different members of the cytosolic Fe-S cluster assembly machinery (i.e., MET18 and AE7) are involved in DNA methylation, likely because they affect the nuclear DNA demethylases Fe-S cluster metabolism (Luo et al., 2012; Duan et al., 2015). Therefore, all these examples show an emerging link between redox regulation and epigenetic regulation.
Conclusion and Perspectives
Data supporting the role of redox regulation in nuclear functions are rapidly increasing. ROS are key actors of this regulation, influencing gene expression at multiple levels (transcription and post-transcription), notably by modulating activities of transcription regulators. While H2O2 has been detected in the nucleus, little is known about ROS metabolism and dynamics in this cell compartment. The recent discovery of a H2O2 flux from the chloroplast to the nucleus opens new perspectives to decipher the role of ROS in gene expression. In this way, the identification of a redox regulation of key transcriptional regulators (e.g., transcription factors, HDAC) will shed light on the ways ROS are acting on gene expression. Key for these questions are the proteomic approaches aiming to identify nuclear PTM occurring on redox-sensitive residues, and the structure biology techniques designed to visualize redox-based modifications on protein structure (Waszczak et al., 2014; Parker et al., 2015; Zaffagnini et al., 2016; Pérez-Pérez et al., 2017). While those redox proteome approaches have identified hundreds of nuclear proteins which could be prone to redox modifications, biochemical and functional evidence is missing to support the biological significance of these redox switches. This will be a major challenge for future research in redox biology.
Author Contributions
LM, JT-H and J-PR wrote the paper. LM and JT-H performed the figures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Centre National de la Recherche Scientifique, the Agence Nationale de la Recherche (ANR-Blanc Cynthiol 12-BSV6-0011). LM was supported by a Ph.D. grant from the Université de Perpignan Via Domitia (Ecole Doctorale Energie et Environnement ED305). JT-H was supported by a Ph.D. grant from Consejo Nacional de Ciencia y Tecnología (CONACYT: 254639/411761).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00705/full#supplementary-material
References
- Ago T., Liu T., Zhai P., Chen W., Li H., Molkentin J. D., et al. (2008). A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133 978–993. 10.1016/j.cell.2008.04.041 [DOI] [PubMed] [Google Scholar]
- Ashtamker C., Kiss V., Sagi M., Davydov O., Fluhr R. (2007). Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells. Plant Physiol. 143 1817–1826. 10.1104/pp.106.090902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L., Accotto G.-P., Bechtold U., Creissen G., Funck D., Jimenez A., et al. (2004). Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16 2448–2462. 10.1105/tpc.104.022608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashandy T., Taconnat L., Renou J.-P., Meyer Y., Reichheld J.-P. (2009). Accumulation of flavonoids in an ntra ntrb mutant leads to tolerance to UV-C. Mol. Plant 2 249–258. 10.1093/mp/ssn065 [DOI] [PubMed] [Google Scholar]
- Caplan J. L., Kumar A. S., Park E., Padmanabhan M. S., Hoban K., Modla S., et al. (2015). Chloroplast stromules function during innate immunity. Dev. Cell 34 45–57. 10.1016/j.devcel.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody M., Crisp P. A., d’Alessandro S., Ganguly D., Gordon M., Havaux M., et al. (2016). Uncoupling high light responses from singlet oxygen retrograde signaling and spatial-temporal systemic acquired acclimation. Plant Physiol. 171 1734–1749. 10.1104/pp.16.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki M., Shekariesfahlan A., Ageeva A., Mengel A., von Toerne C., Durner J., et al. (2015). Identification of nuclear target proteins for S-nitrosylation in pathogen-treated Arabidopsis thaliana cell cultures. Plant Sci. 238 115–126. 10.1016/j.plantsci.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Charbonnel C., Niazi A. K., Elvira-Matelot E., Nowak E., Zytnicki M., de Bures A., et al. (2017). The siRNA suppressor RTL1 is redox-regulated through glutathionylation of a conserved cysteine in the double-stranded-RNA-binding domain. Nucleic Acids Res. 45 11891–11907. 10.1093/nar/gkx820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Ke Q., Kluz T., Yan Y., Costa M. (2006). Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol. Cell. Biol. 26 3728–3737. 10.1128/MCB.26.10.3728-3737.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury F. K., Rivero R. M., Blumwald E., Mittler R. (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J. 90 856–867. 10.1111/tpj.13299 [DOI] [PubMed] [Google Scholar]
- Comella P., Pontvianne F., Lahmy S., Vignols F., Barbezier N., Debures A., et al. (2008). Characterization of a ribonuclease III-like protein required for cleavage of the pre-rRNA in the 3’ETS in Arabidopsis. Nucleic Acids Res. 36 1163–1175. 10.1093/nar/gkm1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli R. N., Gonzalez D. H. (2007). Conserved homeodomain cysteines confer redox sensitivity and influence the DNA binding properties of plant class III HD-Zip proteins. Arch. Biochem. Biophys. 467 41–47. 10.1016/j.abb.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Considine M. J., Foyer C. H. (2014). Redox regulation of plant development. Antioxid. Redox Signal. 21 1305–1326. 10.1089/ars.2013.5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier J., Wu H.-C., Dhalleine T., Pégeot H., Sudre D., Gualberto J. M., et al. (2014). Monothiol glutaredoxin-BolA interactions: redox control of Arabidopsis thaliana BolA2 and SufE1. Mol. Plant 7 187–205. 10.1093/mp/sst156 [DOI] [PubMed] [Google Scholar]
- Davies M. J. (2005). The oxidative environment and protein damage. Biochim. Biophys. Acta 1703 93–109. 10.1016/j.bbapap.2004.08.007 [DOI] [PubMed] [Google Scholar]
- de Simone A., Hubbard R., de la Torre N. V., Velappan Y., Wilson M., Considine M. J., et al. (2017). Redox changes during the cell cycle in the embryonic root meristem of Arabidopsis thaliana. Antioxid. Redox Signal. 27 1505–1519. 10.1089/ars.2016.6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme-Hinoux V., Bangash S. A. K., Meyer A. J., Reichheld J.-P. (2016). Nuclear thiol redox systems in plants. Plant Sci. 243 84–95. 10.1016/j.plantsci.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Després C., Chubak C., Rochon A., Clark R., Bethune T., Desveaux D., et al. (2003). The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15 2181–2191. 10.1105/tpc.012849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Vivancos P., Wolff T., Markovic J., Pallardó F. V., Foyer C. H. (2010). A nuclear glutathione cycle within the cell cycle. Biochem. J. 431 169–178. 10.1042/BJ20100409 [DOI] [PubMed] [Google Scholar]
- Dickinson P. J., Kumar M., Martinho C., Yoo S. J., Lan H., Artavanis G., et al. (2018). Chloroplast signaling gates thermotolerance in Arabidopsis. Cell Rep. 22 1657–1665. 10.1016/j.celrep.2018.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K.-J. (2014). Redox regulation of transcription factors in plant stress acclimation and development. Antioxid. Redox Signal. 21 1356–1372. 10.1089/ars.2013.5672 [DOI] [PubMed] [Google Scholar]
- Dietz K.-J., Mittler R., Noctor G. (2016). Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 171 1535–1539. 10.1104/pp.16.00938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Solis J. R., He Z., Lima A., Ting J., Buchanan B. B., Luan S. (2008). A cyclophilin links redox and light signals to cysteine biosynthesis and stress responses in chloroplasts. Proc. Natl. Acad. Sci. U.S.A. 105 16386–16391. 10.1073/pnas.0808204105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle K., Fitzpatrick F. A. (2010). Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function. J. Biol. Chem. 285 17417–17424. 10.1074/jbc.M109.089250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C.-G., Wang X., Tang K., Zhang H., Mangrauthia S. K., Lei M., et al. (2015). MET18 connects the cytosolic iron-sulfur cluster assembly pathway to active DNA demethylation in Arabidopsis. PLoS Genet. 11:e1005559. 10.1371/journal.pgen.1005559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Rodriguez M., Laissue P. P., Yvon-Durocher G., Smirnoff N., Mullineaux P. M. (2017). Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 8:49. 10.1038/s41467-017-00074-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C. H., Noctor G. (2016). Stress-triggered redox signalling: what’s in pROSpect? Plant Cell Environ. 39 951–964. 10.1111/pce.12621 [DOI] [PubMed] [Google Scholar]
- Gadjev I., Vanderauwera S., Gechev T. S., Laloi C., Minkov I. N., Shulaev V., et al. (2006). Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 141 436–445. 10.1104/pp.106.078717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Giménez J. L., Ibañez-Cabellos J. S., Seco-Cervera M., Pallardó F. V. (2014). Glutathione and cellular redox control in epigenetic regulation. Free Radic. Biol. Med. 75(Suppl. 1):S3. 10.1016/j.freeradbiomed.2014.10.828 [DOI] [PubMed] [Google Scholar]
- García-Giménez J. L., Òlaso G., Hake S. B., Bönisch C., Wiedemann S. M., Markovic J., et al. (2013). Histone h3 glutathionylation in proliferating mammalian cells destabilizes nucleosomal structure. Antioxid. Redox Signal. 19 1305–1320. 10.1089/ars.2012.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Giménez J. L., Romá-Mateo C., Pérez-Machado G., Peiró-Chova L., Pallardó F. V. (2017). Role of glutathione in the regulation of epigenetic mechanisms in disease. Free Radic. Biol. Med. 112 36–48. 10.1016/j.freeradbiomed.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Gibbs D. J., Lee S. C., Isa N. M., Gramuglia S., Fukao T., Bassel G. W., et al. (2011). Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479 415–418. 10.1038/nature10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesguth M., Sahm A., Simon S., Dietz K.-J. (2015). Redox-dependent translocation of the heat shock transcription factor AtHSFA8 from the cytosol to the nucleus in Arabidopsis thaliana. FEBS Lett. 589 718–725. 10.1016/j.febslet.2015.01.039 [DOI] [PubMed] [Google Scholar]
- Han Y., Chaouch S., Mhamdi A., Queval G., Zechmann B., Noctor G. (2013a). Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 18 2106–2121. 10.1089/ars.2012.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Mhamdi A., Chaouch S., Noctor G. (2013b). Regulation of basal and oxidative stress-triggered jasmonic acid-related gene expression by glutathione. Plant Cell Environ. 36 1135–1146. 10.1111/pce.12048 [DOI] [PubMed] [Google Scholar]
- Heine G. F., Hernandez J. M., Grotewold E. (2004). Two cysteines in plant R2R3 MYB domains participate in REDOX-dependent DNA binding. J. Biol. Chem. 279 37878–37885. 10.1074/jbc.M405166200 [DOI] [PubMed] [Google Scholar]
- Holtgrefe S., Gohlke J., Starmann J., Druce S., Klocke S., Altmann B., et al. (2008). Regulation of plant cytosolic glyceraldehyde 3-phosphate dehydrogenase isoforms by thiol modifications. Physiol. Plant. 133 211–228. 10.1111/j.1399-3054.2008.01066.x [DOI] [PubMed] [Google Scholar]
- Ito K., Hanazawa T., Tomita K., Barnes P. J., Adcock I. M. (2004). Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem. Biophys. Res. Commun. 315 240–245. 10.1016/j.bbrc.2004.01.046 [DOI] [PubMed] [Google Scholar]
- Jung H.-S., Crisp P. A., Estavillo G. M., Cole B., Hong F., Mockler T. C., et al. (2013). Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc. Natl. Acad. Sci. U.S.A. 110 14474–14479. 10.1073/pnas.1311632110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneeshaw S., Gelineau S., Tada Y., Loake G. J., Spoel S. H. (2014). Selective protein denitrosylation activity of Thioredoxin-h5 modulates plant Immunity. Mol. Cell 56 153–162. 10.1016/j.molcel.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Knuesting J., Riondet C., Maria C., Kruse I., Bécuwe N., König N., et al. (2015). Arabidopsis glutaredoxin S17 and its partner, the nuclear factor Y subunit C11/negative cofactor 2α, contribute to maintenance of the shoot apical meristem under long-day photoperiod. Plant Physiol. 167 1643–1658. 10.1104/pp.15.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisegang M. S., Schröder K., Brandes R. P. (2017). Redox regulation and noncoding RNAs. Antioxid. Redox Signal. 10.1089/ars.2017.7276 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Li S., Gutsche N., Zachgo S. (2011). The ROXY1 C-terminal L∗∗LL motif is essential for the interaction with TGA transcription factors. Plant Physiol. 157 2056–2068. 10.1104/pp.111.185199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Lauri A., Ziemann M., Busch A., Bhave M., Zachgo S. (2009). Nuclear activity of ROXY1, a glutaredoxin interacting with TGA factors, is required for petal development in Arabidopsis thaliana. Plant Cell 21 429–441. 10.1105/tpc.108.064477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licausi F. (2013). Molecular elements of low-oxygen signaling in plants. Physiol. Plant. 148 1–8. 10.1111/ppl.12011 [DOI] [PubMed] [Google Scholar]
- Licausi F., Kosmacz M., Weits D. A., Giuntoli B., Giorgi F. M., Voesenek L. A., et al. (2011). Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479 419–422. 10.1038/nature10536 [DOI] [PubMed] [Google Scholar]
- Liu P., Zhang H., Yu B., Xiong L., Xia Y. (2015). Proteomic identification of early salicylate- and flg22-responsive redox-sensitive proteins in Arabidopsis. Sci. Rep. 5:8625. 10.1038/srep08625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Bernard D. G., Balk J., Hai H., Cui X. (2012). The DUF59 family gene AE7 acts in the cytosolic iron-sulfur cluster assembly pathway to maintain nuclear genome integrity in Arabidopsis. Plant Cell 24 4135–4148. 10.1105/tpc.112.102608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal C., Delorme-Hinoux V., Bariat L., Siala W., Belin C., Saez-Vasquez J., et al. (2014). NTR/NRX define a new thioredoxin system in the nucleus of Arabidopsis thaliana cells. Mol. Plant 7 30–44. 10.1093/mp/sst162 [DOI] [PubMed] [Google Scholar]
- Marty L., Siala W., Schwarzländer M., Fricker M. D., Wirtz M., Sweetlove L. J., et al. (2009). The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106 9109–9114. 10.1073/pnas.0900206106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel A., Ageeva A., Georgii E., Bernhardt J., Wu K., Durner J., et al. (2017). Nitric oxide modulates histone acetylation at stress genes by inhibition of histone deacetylases. Plant Physiol. 173 1434–1452. 10.1104/pp.16.01734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Belin C., Delorme-Hinoux V., Reichheld J.-P., Riondet C. (2012). Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, crosstalks, and functional significance. Antioxid. Redox Signal. 17 1124–1160. 10.1089/ars.2011.4327 [DOI] [PubMed] [Google Scholar]
- Meyer Y., Buchanan B. B., Vignols F., Reichheld J.-P. (2009). Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu. Rev. Genet. 43 335–367. 10.1146/annurev-genet-102108-134201 [DOI] [PubMed] [Google Scholar]
- Miller G., Mittler R. (2006). Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 98 279–288. 10.1093/aob/mcl107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I. M., Jensen P. E., Hansson A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58 459–481. 10.1146/annurev.arplant.58.032806.103946 [DOI] [PubMed] [Google Scholar]
- Montacié C., Durut N., Opsomer A., Palm D., Comella P., Picart C., et al. (2017). Nucleolar proteome analysis and proteasomal activity assays reveal a link between nucleolus and 26S proteasome in A. thaliana. Front. Plant Sci. 8:1815. 10.3389/fpls.2017.01815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrichard F., Alkhalfioui F., Yano H., Vensel W. H., Hurkman W. J., Buchanan B. B. (2009). Thioredoxin targets in plants: the first 30 years. J. Proteomics 72 452–474. 10.1016/j.jprot.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Morisse S., Zaffagnini M., Gao X.-H., Lemaire S. D., Marchand C. H. (2014). Insight into protein S-nitrosylation in Chlamydomonas reinhardtii. Antioxid. Redox Signal. 21 1271–1284. 10.1089/ars.2013.5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Z., Fan W., Dong X. (2003). Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113 935–944. 10.1016/S0092-8674(03)00429-X [DOI] [PubMed] [Google Scholar]
- Murmu J., Bush M. J., DeLong C., Li S., Xu M., Khan M., et al. (2010). Arabidopsis basic leucine-zipper transcription factors TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly required for anther development. Plant Physiol. 154 1492–1504. 10.1104/pp.110.159111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., DesMarais T. L., Tong Z., Yao Y., Costa M. (2015). Oxidative stress alters global histone modification and DNA methylation. Free Radic. Biol. Med. 82 22–28. 10.1016/j.freeradbiomed.2015.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G. (2017). ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 10.1016/j.semcdb.2017.07.013 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Noctor G., Foyer C. H. (2016). Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 171 1581–1592. 10.1104/pp.16.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A., Watson P. M., Robinson J. D., Crepaldi L., Riccio A. (2008). S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 455 411–415. 10.1038/nature07238 [DOI] [PubMed] [Google Scholar]
- Palm D., Simm S., Darm K., Weis B. L., Ruprecht M., Schleiff E., et al. (2016). Proteome distribution between nucleoplasm and nucleolus and its relation to ribosome biogenesis in Arabidopsis thaliana. RNA Biol. 13 441–454. 10.1080/15476286.2016.1154252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R., Müller A., Napoli C. A., Selinger D. A., Pikaard C. S., Richards E. J., et al. (2002). Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 30 5036–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-W., Li W., Viehhauser A., He B., Kim S., Nilsson A. K., et al. (2013). Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc. Natl. Acad. Sci. U.S.A. 110 9559–9564. 10.1073/pnas.1218872110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Balmant K., Zhu F., Zhu N., Chen S. (2015). cysTMTRAQ-An integrative method for unbiased thiol-based redox proteomics. Mol. Cell. Proteomics 14 237–242. 10.1074/mcp.O114.041772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez M. E., Mauriès A., Maes A., Tourasse N. J., Hamon M., Lemaire S. D., et al. (2017). The deep thioredoxome in Chlamydomonas reinhardtii: new insights into redox regulation. Mol. Plant 10 1107–1125. 10.1016/j.molp.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Qin L., Wang M., Zuo J., Feng X., Liang X., Wu Z., et al. (2015). Cytosolic BolA plays a repressive role in the tolerance against excess iron and MV-induced oxidative stress in plants. PLoS One 10:e0124887. 10.1371/journal.pone.0124887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G., Issakidis-Bourguet E., Hoeberichts F. A., Vandorpe M., Gakière B., Vanacker H., et al. (2007). Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 52 640–657. 10.1111/j.1365-313X.2007.03263.x [DOI] [PubMed] [Google Scholar]
- Rahantaniaina M.-S., Li S., Chatel-Innocenti G., Tuzet A., Issakidis-Bourguet E., Mhamdi A., et al. (2017). Cytosolic and chloroplastic DHARs cooperate in oxidative stress-driven activation of the salicylic acid pathway. Plant Physiol. 174 956–971. 10.1104/pp.17.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues O., Reshetnyak G., Grondin A., Saijo Y., Leonhardt N., Maurel C., et al. (2017). Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. U.S.A. 114 9200–9205. 10.1073/pnas.1704754114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano M., Romero-Puertas M. C., Sanz-Fernández M., Hu J., Sandalio L. M. (2016). Peroxisomes extend peroxules in a fast response to stress via a reactive oxygen species-mediated induction of the peroxin PEX11a. Plant Physiol. 171 1665–1674. 10.1104/pp.16.00648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschzttardtz H., Grillet L., Isaure M.-P., Conéjéro G., Ortega R., Curie C., et al. (2011). Plant cell nucleolus as a hot spot for iron. J. Biol. Chem. 286 27863–27866. 10.1074/jbc.C111.269720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N., Cerveau D., Couturier J., Reichheld J.-P., Rey P. (2015). Involvement of thiol-based mechanisms in plant development. Biochim. Biophys. Acta 1850 1479–1496. 10.1016/j.bbagen.2015.01.023 [DOI] [PubMed] [Google Scholar]
- Saez-Vasquez J., Medina F. J. (2009). The plant nucleolus. Adv. Bot. Res. 47 1–46. [Google Scholar]
- Sarkar N., Lemaire S., Wu-Scharf D., Issakidis-Bourguet E., Cerutti H. (2005). Functional specialization of Chlamydomonas reinhardtii cytosolic thioredoxin h1 in the response to alkylation-induced DNA damage. Eukaryot. Cell 4 262–273. 10.1128/EC.4.2.262-273.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaubelt D., Queval G., Dong Y., Diaz-Vivancos P., Makgopa M. E., Howell G., et al. (2015). Low glutathione regulates gene expression and the redox potentials of the nucleus and cytosol in Arabidopsis thaliana. Plant Cell Environ. 38 266–279. 10.1111/pce.12252 [DOI] [PubMed] [Google Scholar]
- Serrato A. J., Cejudo F. J. (2003). Type-h thioredoxins accumulate in the nucleus of developing wheat seed tissues suffering oxidative stress. Planta 217 392–399. 10.1007/s00425-003-1009-4 [DOI] [PubMed] [Google Scholar]
- Serrato A. J., Crespo J. L., Florencio F. J., Cejudo F. J. (2001). Characterization of two thioredoxins h with predominant localization in the nucleus of aleurone and scutellum cells of germinating wheat seeds. Plant Mol. Biol. 46 361–371. 10.1023/A:1010697331184 [DOI] [PubMed] [Google Scholar]
- Shaikhali J., Heiber I., Seidel T., Ströher E., Hiltscher H., Birkmann S., et al. (2008). The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol. 8:48. 10.1186/1471-2229-8-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikhali J., Norén L., de Dios Barajas-López J., Srivastava V., König J., Sauer U. H., et al. (2012). Redox-mediated mechanisms regulate DNA binding activity of the G-group of basic region leucine zipper (bZIP) transcription factors in Arabidopsis. J. Biol. Chem. 287 27510–27525. 10.1074/jbc.M112.361394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikhali J., Wingsle G. (2017). Redox-regulated transcription in plants: emerging concepts. AIMS Mol. Sci. 4 301–338. 10.3934/molsci.2017.3.301 [DOI] [Google Scholar]
- Shen Y., Issakidis-Bourguet E., Zhou D.-X. (2016). Perspectives on the interactions between metabolism, redox, and epigenetics in plants. J. Exp. Bot. 67 5291–5300. 10.1093/jxb/erw310 [DOI] [PubMed] [Google Scholar]
- Sies H., Berndt C., Jones D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86 715–748. 10.1146/annurev-biochem-061516-045037 [DOI] [PubMed] [Google Scholar]
- Suzuki N., Koussevitzky S., Mittler R., Miller G. (2012). ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 35 259–270. 10.1111/j.1365-3040.2011.02336.x [DOI] [PubMed] [Google Scholar]
- Tada Y., Spoel S. H., Pajerowska-Mukhtar K., Mou Z., Song J., Wang C., et al. (2008). Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 321 952–956. 10.1126/science.1156970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares C. P., Vernal J., Delena R. A., Lamattina L., Cassia R., Terenzi H. (2014). S-nitrosylation influences the structure and DNA binding activity of AtMYB30 transcription factor from Arabidopsis thaliana. Biochim. Biophys. Acta 1844 810–817. 10.1016/j.bbapap.2014.02.015 [DOI] [PubMed] [Google Scholar]
- Testard A., Da Silva D., Ormancey M., Pichereaux C., Pouzet C., Jauneau A., et al. (2016). Calcium- and nitric oxide-dependent nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in response to long chain bases in tobacco BY-2 cells. Plant Cell Physiol. 57 2221–2231. 10.1093/pcp/pcw137 [DOI] [PubMed] [Google Scholar]
- Van Ruyskensvelde V., Van Breusegem F., Van Der Kelen K. (2018). Post-transcriptional regulation of the oxidative stress response in plants. Free Radic. Biol. Med. 10.1016/j.freeradbiomed.2018.02.032 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Vescovi M., Zaffagnini M., Festa M., Trost P., Lo Schiavo F., Costa A. (2013). Nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in cadmium-stressed Arabidopsis roots. Plant Physiol. 162 333–346. 10.1104/pp.113.215194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos P. D., Dong Y., Ziegler K., Markovic J., Pallardó F. V., Pellny T. K., et al. (2010). Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 64 825–838. 10.1111/j.1365-313X.2010.04371.x [DOI] [PubMed] [Google Scholar]
- Vogel M. O., Moore M., König K., Pecher P., Alsharafa K., Lee J., et al. (2014). Fast retrograde signaling in response to high light involves metabolite export, MITOGEN-ACTIVATED PROTEIN KINASE6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell 26 1151–1165. 10.1105/tpc.113.121061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D., Przybyla D., Op den Camp R., Kim C., Landgraf F., Lee K. P., et al. (2004). The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306 1183–1185. 10.1126/science.1103178 [DOI] [PubMed] [Google Scholar]
- Waszczak C., Akter S., Eeckhout D., Persiau G., Wahni K., Bodra N., et al. (2014). Sulfenome mining in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 111 11545–11550. 10.1073/pnas.1411607111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczak C., Akter S., Jacques S., Huang J., Messens J., Van Breusegem F. (2015). Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 66 2923–2934. 10.1093/jxb/erv084 [DOI] [PubMed] [Google Scholar]
- Welsch R., Maass D., Voegel T., Dellapenna D., Beyer P. (2007). Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol. 145 1073–1085. 10.1104/pp.107.104828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems P., Mhamdi A., Stael S., Storme V., Kerchev P., Noctor G., et al. (2016). The ROS wheel: refining ROS transcriptional footprints. Plant Physiol. 171 1720–1733. 10.1104/pp.16.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. E., Grotewold E. (1997). Differences between plant and animal Myb domains are fundamental for DNA binding activity, and chimeric Myb domains have novel DNA binding specificities. J. Biol. Chem. 272 563–571. 10.1074/jbc.272.1.563 [DOI] [PubMed] [Google Scholar]
- Wu Q., Lin J., Liu J.-Z., Wang X., Lim W., Oh M., et al. (2012). Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnol. J. 10 945–955. 10.1111/j.1467-7652.2012.00723.x [DOI] [PubMed] [Google Scholar]
- Xiang C., Oliver D. J. (1998). Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10 1539–1550. 10.1105/tpc.10.9.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S., Rosso M. G., Zachgo S. (2005). ROXY1, a member of the plant glutaredoxin family, is required for petal development in Arabidopsis thaliana. Development 132 1555–1565. 10.1242/dev.01725 [DOI] [PubMed] [Google Scholar]
- Xing S., Zachgo S. (2008). ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J. 53 790–801. 10.1111/j.1365-313X.2007.03375.x [DOI] [PubMed] [Google Scholar]
- Xu Y.-M., Du J.-Y., Lau A. T. Y. (2014). Posttranslational modifications of human histone H3: an update. Proteomics 14 2047–2060. 10.1002/pmic.201300435 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Bedhomme M., Marchand C. H., Morisse S., Trost P., Lemaire S. D. (2012). Redox regulation in photosynthetic organisms: focus on glutathionylation. Antioxid. Redox Signal. 16 567–586. 10.1089/ars.2011.4255 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., De Mia M., Morisse S., Di Giacinto N., Marchand C. H., Maes A., et al. (2016). Protein S-nitrosylation in photosynthetic organisms: a comprehensive overview with future perspectives. Biochim. Biophys. Acta 1864 952–966. 10.1016/j.bbapap.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Fermani S., Costa A., Lemaire S. D., Trost P. (2013). Plant cytoplasmic GAPDH: redox post-translational modifications and moonlighting properties. Front. Plant Sci. 4:450. 10.3389/fpls.2013.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B. (2017). Compartment-specific importance of ascorbate during environmental stress in plants. Antioxid. Redox Signal. 10.1089/ars.2017.7232 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zechmann B., Mauch F., Sticher L., Müller M. (2008). Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. J. Exp. Bot. 59 4017–4027. 10.1093/jxb/ern243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B., Müller M. (2010). Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma 246 15–24. 10.1007/s00709-010-0111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B., Stumpe M., Mauch F. (2011). Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta 233 1–12. 10.1007/s00425-010-1275-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhao Y., Zhou D.-X. (2017). Rice NAD+-dependent histone deacetylase OsSRT1 represses glycolysis and regulates the moonlighting function of GAPDH as a transcriptional activator of glycolytic genes. Nucleic Acids Res. 45 12241–12255. 10.1093/nar/gkx825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Sun H., Chen H., Zavadil J., Kluz T., Arita A., et al. (2010). Hypoxia induces trimethylated H3 lysine 4 by inhibition of JARID1A demethylase. Cancer Res. 70 4214–4221. 10.1158/0008-5472.CAN-09-2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Sun H., Ellen T. P., Chen H., Costa M. (2008). Arsenite alters global histone H3 methylation. Carcinogenesis 29 1831–1836. 10.1093/carcin/bgn063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.-K. (2009). Active DNA demethylation mediated by DNA glycosylases. Annu. Rev. Genet. 43 143–166. 10.1146/annurev-genet-102108-134205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.