Abstract
Shigella flexneri is a leading cause of diarrheal disease in children under five in developing countries. There is currently no licensed vaccine and broad spectrum protection requires coverage of multiple serotypes. The live attenuated vaccines CVD 1213 and CVD 1215 were derived from two prominent S. flexneri serotypes: S. flexneri 3a and S. flexneri 6. To provide broad-spectrum immunity, they could be combined with CVD 1208S, a S. flexneri 2a strain that demonstrated promising results in phase I and II clinical trials. Each strain contains a mutation in the guaBA operon. These vaccine candidates were tested in vitro and in vivo and were found to be auxotrophic for guanine and defective in intracellular replication, but capable of inducing cytokine production from both epithelial cells and macrophages. Both strains were attenuated for virulence in the guinea pig Serény test and induced robust serotype-specific antibody responses following immunization. Each strain induced homologous serotype protection against challenge and a mixed inoculum of the three S. flexneri vaccines conferred protection against all three virulent wild-type strains. These data support the use of CVD 1213, CVD 1215 and CVD 1208S in a multivalent vaccine to confer broad protection against disease caused by Shigella flexneri.
Keywords: Shigella flexneri, vaccine, live attenuated, diarrheal disease
Two new Shigella flexneri vaccine candidates were characterized in vitro and in vivo as part of a multicomponent vaccine.
INTRODUCTION
The bacterial pathogen Shigella causes ∼150 million shigellosis cases per year in residents of endemic regions, travelers and military personnel, leading to hundreds of thousands of deaths worldwide (Centers for Disease & Prevention 2006; Shah, DuPont and Ramsey 2009; Wikswo and Hall 2012). During the large scale Global Enteric Multicenter Study (GEMS) conducted at seven sites in Africa and Asia, Shigella was identified as the most predominant cause of moderate-to-severe diarrhea (MSD) in children ages 2–5 years old at all sites and among the top four diarrheal pathogens in the younger cohorts (Kotloff et al.2013). In addition to current calculations of disease burden, advanced molecular detection methods indicate that Shigella is the cause of more MSD cases than previously estimated by rapid detection during the large-scale studies (Lindsay et al.2013). An extensive birth cohort analysis project called the Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project, or MAL-ED was conducted in Africa, Asia and South America to identify the pathogens causing the largest diarrheal burden in these populations (Platts-Mills et al.2015). In this study, Shigella was found to be a significant cause of diarrhea in 2-year-old children. With growing antibiotic resistance reducing therapeutic options and an increased appreciation for long term sequelae associated with MSD, there is great need for an effective vaccine (Kotloff et al.1999; Bardhan et al.2010; Moore et al.2010).
There are four Shigella species that cause human disease: S. dysenteriae, S. flexneri, S. sonnei and S. boydii, and each (except for S. sonnei) includes multiple serotypes. Shigella flexneri, the most common of the Shigella species in endemic regions, disproportionately affects young children (Bennish and Wojtyniak 1991; WHO 2005). Field studies have demonstrated that natural immunity is serotype-specific (Cohen et al.1991; Ferreccio et al.1991; Rasolofo-Razanamparany et al.2001). For this reason, vaccine development must address the challenge of the multitude of Shigella serotypes. Shigella isolates collected during the GEMS were serotyped; 65% of the Shigella isolates were comprised of the S. flexneri species, and three of the most commonly isolated flexneri serotypes were 2a, 3a and 6 (Livio et al.2014). In addition to their prevalence as clinical isolates, together, these three serotypes express each type- and group-specific antigen found in the 15 S. flexneri serotypes. Based on this observation, researchers at the Center for Vaccine Development have proposed a strategy to create a broad spectrum, live attenuated S. flexneri vaccine including these three serotypes. Antibody cross-reactivity and animal studies support the potential for cross protection using vaccines derived from these specific serotypes (Van De Verg et al.1996; Noriega et al.1999; Mukhopadhaya et al.2003; Levine et al.2007). It has been estimated that a vaccine composed of a combination of S. flexneri serotypes 2a, 3a and 6 could provide direct coverage against 41% of Shigella strains and cross protection could extend this to 66% overall coverage (Livio et al.2014).
Shigella flexneri 2a strain CVD 1208S, one vaccine component, has advanced to human clinical studies and served as a reference vaccine candidate for the current experiments (Kotloff et al.2007). CVD 1208S is derived from wild-type strain 2457T and contains deletions in the guaBA operon as well as set and sen genes. The guaBA operon encodes enzymes essential for de novo guanine nucleotide biosynthesis and intracellular survival in the host. The set and sen genes encode two enterotoxins, found on the bacterial chromosome and the virulence plasmid respectively (Kotloff et al.2004). CVD 1208S was demonstrated to be safe, well tolerated and highly immunogenic in volunteers in a Phase I study (Kotloff et al.2007). Based on these promising results, the guaBA mutation was introduced to S. flexneri serotypes 3a and 6 (wild-type strains J17B and CCH060) to generate CVD 1213 and CVD 1215. In these studies, the two new candidates were evaluated in vitro and in vivo for attenuation of virulence, immunogenicity and protection. Epithelial and macrophage cell culture systems were used to assess host cell invasion, cytotoxicity and cytokine secretion as measures of pathogenic potential and host cell engagement (Zychlinsky, Prevost and Sansonetti 1992; Noriega et al.1994; Fernandez-Prada et al.1997, 2000; Koterski et al.2005; Fiorentino et al.2014). The guinea pig model was used to confirm the safety of the vaccines, the induction of relevant immune responses, and the ability to confer protection against wild-type challenge in the Serény Test. Each vaccine was immunogenic when administered in a monovalent formulation and when a trivalent mixed inoculum was used. Furthermore, the trivalent vaccine composed of CVD 1208S, CVD 1213 and CVD 1215 conferred protection against all three wild-type S. flexneri serotypes. These results support the potential for using these three live attenuated strains as a vaccine to confer broad coverage against S. flexneri.
MATERIALS AND METHODS
Bacterial strains
The Shigella strains used in these studies are listed in Table 1. Shigella strains (wild type and vaccines) were grown on trypticase soy agar (TSA; Becton Dickinson, Sparks, MD) and containing 0.01% Congo red dye and 0.005% guanine (Sigma-Aldrich, St. Louis, MO).
Table 1.
Bacterial strains and plasmids.
| Strain | Description | |
|---|---|---|
| 2457T | Wild-type S. flexneri 2a | |
| CVD 1208S | ΔguaBA, Δsen, Δset S. flexneri 2a vaccine derived from 2457T | |
| J17B | Wild-type S. flexneri 3a | |
| CVD 1213 | ΔguaBA, Δsen S. flexneri 3a vaccine, derived from J17B | |
| CCH060 | Wild-type S. flexneri 6 | |
| CVD 1215 | ΔguaBA S. flexneri 6 vaccine derived from CCH060 | |
| 4243A | Plasmid cured S. flexneri 2a derived from 2457T | |
| Plasmid | Description | Reference |
| pFM726A.02 | Suicide plasmid containing kanamycin resistance cassette, guaA and guaB fragments | Noriega et al. (1996) |
| pKD46 | Plasmid encodes lambda red genes; temperature sensitive replication; ampicillin resistance | Datsenko and Wanner (2000) |
| pCP20 | FLP recombinase expression plasmid (temperature dependent), ampicillin and chloramphenicol resistance | Datsenko and Wanner (2000) |
| pKD3 | Plasmid used for gene disruption; flanks FRT sites; temperature sensitive; chloramphenicol resistance | Datsenko and Wanner (2000) |
| pKD4 | Temperature sensitive, kanamycin resistance | Datsenko and Wanner (2000) |
Molecular genetic techniques
All the primers used for PCR are listed in Table 2 were created by Integrated DNA Technology. Reagents for mutagenesis and PCR, including restriction enzymes, ligase and polymerases, were purchased from New England Biolabs (Beverly, MA) or Roche (Basel, Switzerland) and used according to the manufacturers’ instructions.
Table 2.
Primers used in this study.
| Primer | Sequence |
|---|---|
| Red-Sen-F | TTGGATTAGCTCAGTCAATCCCATACGCATCGCGTGATAAATAACTTTGGGGTGAAGTAAATTATCAGGCGTGTAGGCTGGAGCTGCTTC |
| Red-Sen-R | CAACAACACTAAGTCTGCGTCACAACCCATCAATGAAAGGAATATATACATATGCCATCAGTAAATTTAACATATGAATATCCTCCTTA |
| Wu048 | GTGAAGGTGAAGCCCGTGAAGT |
| Wu049 | TGCAGCAGCATTGCGGTTACG |
| EB1 | TACACGTCCATTATGCAAGGCT |
| EB2 | TGCCATCAGTAAATTTAATCCCATC |
| set-N153 | ATAACGTATTCATTGGTGCCATAACCGGTGGC |
| set-N161 | CTGGGCCCCCTGAACTGGACATACGACAAAACAT |
| Gua446 | TCCGCAGCAATGGATACCGTAAC |
| Gua3226 | CATTACGCCAACGGAACGTAC |
| Wu060 | GTTCACTGCGTGTCTGGGTT |
| Wu061 | CGTTGTAGCGGTAGTCGAGCTGGA |
Construction of vaccine strains
CVD 1208S was created as a derivative of CVD 1204 by introducing deletions in the ShET1 and ShET2 enterotoxin genes set and sen as previously described (Noriega et al.1996; Kotloff et al.2004). CVD 1213 was created from wild-type S. flexneri 3a strain J17B and CVD 1215 was generated from S. flexneri 6 strain CCH060. The guaBA deletion in CVD 1213 and CVD 1215 encompassed the same deleted region described for S. flexneri 2a and was introduced using λ-red mutagenesis (Datsenko and Wanner 2000). pKD46, a plasmid expressing λ-red recombinase, was electroporated into S. flexneri 3a and 6 wild-type strains to generate Sf3a(pKD46) and Sf6(pKD46) respectively. The guaBA genes are contained in an operon with guaB proximal to guaA. A single deletion mutates both genes. The mutagenic fragment, guaB-Kan-guaA, was amplified by PCR (using primers Gua446 and Gua3226) from pFM726A.02, a plasmid derived from pFM726A that contains a kanamycin resistance cassette with FRT flanking sequences between the guaB and guaA flanks (using Roche Long Template PCR kit). The PCR product was electroporated into Sf3a(pKD46) and Sf6(pKD46) to allow the deleted guaBA operon (guaB-Kan-guaA) to replace the intact operon in the bacterial chromosome. Bacteria were grown on kanamycin to select for fragment integration at 30°C. A secondary screen was performed to identify colonies that were auxotrophic for guanine by the ability to grow on minimum medium without supplementation of 0.1% guanine. The guaBA deletion was further confirmed by PCR using primers Wu048 and Wu049 to indicate a deleted fragment in the chromosome. The temperature sensitive plasmid pKD46 was cured from the strain by switching growth temperature from 30°C to 37°C. To remove the kanamycin cassette, plasmid pCP20 was electroporated into each strain. Plasmid pCP20 was then cured by switching the temperature from 30°C to 37°C.
The sen deletion was engineered in the S. flexneri 3a ΔguaBA mutant strain via λ-red mutagenesis as described above. Primers Red-Sen-F and Red-Sen-R were designed with primer tail sequences homologous to up-stream (nucleotides 74279–74348, Genbank #AF348706) and down-stream (nucleotides 73530–73599, Genbank #AF348706) flanks of sen, encoding enterotoxin ShET2. These primers were used to amplify the chloramphenicol resistance cassette, cml, from pKD3. The resulting PCR product was electroporated into Sf3aΔgua(pKD46). Bacteria were grown on soy agar with chloramphenicol (50 μg/ml) at 30°C and colonies were screened for the loss of sen using primers EB1 and EB2. The sen deletion encompasses a 678 bp region. The final strain was named CVD 1213.
Complementation studies
Complementation of guanine auxotrophy was performed with pGuaBA containing a minimal fragment encoding guaBA controlled by the lactose promoter (PlacZ) and a chloramphenicol cassette (Wu et al.2011; Vindurampulle et al.2013). The plasmid was electroporated into competent CVD 1208S, CVD 1213 and CVD 1215. The bacteria were plated on TSA with chloramphenicol (25 μg/ml) and incubated 18 h (hours) at 37°C. Resulting colonies were plated again on TSA with chloramphenicol, and incubated 18 h at 37°C.
PCR analysis
Diagnostic PCR assays were performed to confirm the genetic mutations introduced into each strain. Primers used for confirmation of each altered gene in CVD 1208S, CVD 1213 and CVD 1215 were as follows. Primers Wu048 and Wu049 bind to sequences up- and down-stream of guaBA respectively and amplify a 1.8 kb product from wild-type strains and a 1 kb product from the vaccines due to the deletion of guaBA. Primers Wu060 and Wu061 bind sequences up- and downstream of sen respectively and amplify a 1.8 kb product in WT and a 1.1 kb product due to the deletion of sen in CVD 1213. Template DNA consisted of a single bacterial colony added to the reaction mixture. Reactions were performed with parameters specific for the primer length and composition as well as the length of the product.
Growth curves
To assess replication defects caused by guanine auxotrophy, all vaccines and the parental wild-type strains were grown in minimal media with and without supplemental guanine. An initial inoculum of 107 colony forming units per ml (CFU/ml) was generated by suspending bacteria isolated from TSA plates in minimal media. Growth curve studies were performed using M9 Minimal Salts (Sigma-Aldrich) with the addition of aspartic acid (0.05 g/L), L-serine (0.05 g/L), nicotinic acid (0.05 g/L), casamino acid (0.05/L), cysteine (0.05g/L) and glucose (0.05 g/L). The starting concentration of each culture was adjusted to 107 CFU/ml and the optical density was monitored over 8 h.
Macrophage infections
THP-1 human monocytes (ATCC TIB-202) were cultured in suspension in RPMI (Roswell Park Memorial Institute) medium (Invitrogen, Carlsbad, CA) containing phenol red and supplemented with 10% fetal bovine serum (FBS) and 0.5% 2-mercaptoethanol. One-ml of THP-1 cells, at a density of 106 cells per ml suspended in RPMI with 10% FBS and 40 ng/ml of phorbol 12-myristate 13-acetate (PMA), was added to each well in 12-well tissue culture plates to allow the cells to differentiate into macrophages and adhere to form a monolayer. The cells were incubated 48 h, washed with Dulbecco's phosphate buffered saline (DPBS) (Corning, NY), and fresh RPMI (without PMA) was added for an additional 24 h incubation. For assays conducted with J774 mouse macrophages (ATCC TIB-67), the cells were expanded in Dulbecco's Minimum Essential Medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, seeded in 96-wells plates at a density of 5 × 105 cells per well, and incubated for 24 h to allow the cells to adhere. To perform the assay, bacteria isolated from an overnight TSA plate were suspended in RPMI or DMEM for a multiplicity of infection (MOI) of 100 bacteria per macrophage. Bacterial suspensions were added to each well (1 ml for THP-1 cells and 100 μl for J774 cells), the plates were centrifuged 5 min at 1000 × g at room temperature, and incubated 30 min in 5% CO2 at 37°C to allow for bacterial uptake. The macrophages were washed two times with PBS then RPMI or DMEM (without phenol red) (Corning), supplemented with 5% FBS and 4 μl/ml of cell proliferation WST-1 reagent (Sigma-Aldrich), was added to each well (2 ml for THP-1 cells and 100 μl for J774 cells). WST-1 is a tetrazolium salt that is cleaved to a soluble formazan by the glycolytic production of NAD(P)H in viable cells and is thus a measure of cellular proliferation. The cells were incubated at 37°C in 5% CO2 and the optical density (calculated difference between OD450 nm and OD600 nm) was measured once an hour for 5 h. In parallel, supernatants were removed to quantify cytokine secretion.
HT-29 cell invasion assays
Human HT-29 (ATCC HTB-38) monolayers were cultured in DMEM supplemented with 10% FBS in 150 cm2 flasks (Corning). The cells were incubated in 5% CO2 at 37°C and passaged once a week. The bacterial inoculum was prepared by resuspending colonies from a TSA plate in DPBS, washed and suspended in cell culture media adjusted for bacterial concentration of 5 × 107 CFU per ml. For the invasion assay, HT-29 cells were seeded at a density of 5 × 105 cells per well in a 12-well plate and incubated overnight. A 1 ml suspension of 5 × 107/ml bacteria was added to the HT-29 cells in triplicate wells. The plates were centrifuged 5 min at 1000 × g at room temperature and then incubated at 37°C with 5% CO2 for 90 min. The cells were washed and incubated with media containing 50 μg/ml gentamicin. At each time point, two replicates of each sample were washed with DBPS to remove gentamicin and lysed with 1 ml/well of 1% Triton X-100 at each time point. Serial dilutions were plated to determine the number of intracellular bacteria.
Cytokine assays
Prior to lysis, media from each well of the infected HT-29 cell monolayers and THP-1 macrophages was collected for cytokine analysis using DuoSet® enzyme-linked immunosorbent assay (ELISA) kits for human IL-8, CXCL-1, TNF-α and IL-1β (R&D Systems). Supernatants were collected at 4 h and 8 h post infection to quantify cumulative cytokine induction. To assess interval secretion, cell supernatants were collected from the wells 4 h post invasion; cells were washed once with PBS and bathed in fresh DMEM (with gentamicin) for an additional 4 h (4 to 8 h time point). The supernatant of uninfected cells was used as a control and when cytokines values were below detectable levels, a value of ‘1’ was assigned for analysis.
Immunizations and sample collection
Female, Dunkin-Hartley guinea pigs (six to eight weeks old) were sedated with a subcutaneous injection of ketamine HCl (40 mg/kg of body weight) and xylazine (5 mg/kg). The animals were immunized intranasally with 100 μl of bacterial suspension containing 109 CFU of CVD 1208S, CVD 1213 or CVD 1215 on day zero. The mixed inoculum contained an equal number of CVD 1208S, CVD 1213 and CVD 1215 for a total of 1010 CFU per animal. Approximately two weeks later, an identical booster dose was administered. Prior to immunization and approximately two weeks following each dose, tears and serum were collected from each animal. Tears were collected from guinea pigs as previously described in 50 μl capillary tubes (Noriega et al.1996). Blood was collected from anaesthetized animals via the anterior vena cava and processed to obtain sera.
Serény test
The Serény test was performed (Sereny 1955) to assess the level of attenuation of CVD 1213 and CVD1215 in comparison with wild-type S. flexneri serotypes 3a and 6 and protection against wild-type challenge. This method was also used to assess the protection against challenge with wild-type S. flexneri serotypes 2a, 3a and 6 following combined immunization with CVD 1208S, CVD 1213 and CVD 1215. A total of 10 μl aliquots of each test strain were instilled into the guinea pigs’ right eye. Animals were monitored daily for five days for signs of infection and inflammation and scored as follows: 0 = normal eye indistinguishable from contralateral uninoculated eye; 1 = lacrimation or eyelid edema; 2 = 1 plus mild conjunctival hyperemia; 3 = 2 plus slight exudates; and 4 = full blown purulent keratoconjunctivitis. All animal procedures were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee. Efficacy was calculated using the formula: Vaccine Efficacy = 100 × (Unvaccinated attack rate – Vaccinated attack rate) / Unvaccinated attack rate. Animals with a score of 2 or more were considered in the attack rate calculation.
ELISA assays for antibody titers
Antigens used in ELISAs included S. flexneri LPS from serotypes 2a, 3a and 6 and purified invasion plasmid antigen B (IpaB) protein (5 μg/ml). Specific IgG and IgA anti-LPS endpoint titers were determined from guinea pig sera and tears. All samples were run in duplicate. Threefold dilutions of sera or tears in 10% non-fat dry milk in PBS with 0.05% Tween 20 (Sigma-Aldrich) were added to pre-coated plates and incubated for 1 h at 37°C. Secondary peroxidase-labeled antibodies were followed by TMB substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) at room temperature for 15 min. A total of 100 μl of H2PO4 was used to stop the reaction and the OD450 was determined in a micro plate reader (Versamax). Linear regression curves were plotted and titers were calculated as the inverse of the dilution that produced an OD450 of 0.2 above the blank.
Complement-mediated serum bactericidal antibody assays
Seventy-five micro-liters of serum from immunized and control guinea pigs was added to a sterile, round bottom 96-well plate and diluted 2-fold (performed in duplicate). Baby rabbit complement (25 μl) and wild-type bacteria (diluted in PBS to ∼5000 CFU per 100 μl) were added to each well and incubated, shaking at 200 rotations per minute, for 1 h at 37°C. After the incubation, samples from each well were plated on TSA to count CFU and calculate percent killing. The end point of the assay was the titer at which 50% of the bacteria are killed and was determined with a line of best fit, averaging the bacterial killing of the sera samples at each dilution.
Statistical analysis
Three, independent replicate experiments were conducted for the macrophage infections, epithelial cells invasion assays and cytokine ELISAs. Technical duplicates were performed for guinea pig antibody ELISAs. Differences between groups were analyzed with Student's t-test, or two-way ANOVA. Significance was determined by P-values <0.05.
RESULTS
Shigella flexneri guaBA mutants are auxotrophic for guanine
Following the successful attenuation of S. flexneri 2a strain CVD 1208S by guaBA mutation, we applied a similar technique to create the new S. flexneri vaccine candidates CVD 1213 and CVD 1215 from S. flexneri 3a strain J17B and S. flexneri 6 strain CCH060. The genes guaB and guaA are contained within an operon allowing a single deletion to inactivate both genes. CVD 1208S contains three mutations, guaBA, set and sen, that have each been confirmed with PCR. The guaBA and sen deletions in CVD 1213 and guaBA in CVD 1215 were confirmed using PCR with gene specific primers to amplify chromosomal fragments demonstrating the targeted deletions (Fig. 1).
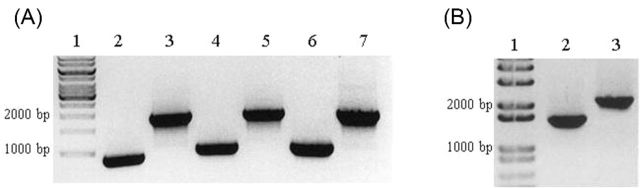
Figure 1.
PCR confirms deletions in vaccine strains. Single colonies of vaccine or wild-type strains were used as templates for amplification of the guaBA (A) or sen (B) loci by PCR. (A) Lanes 1, molecular weight marker; 2, CVD 1208S; 3, 2457T; 4, CVD 1213; 5, J17B; 6, CVD 1215; 7, CCH060. The expected size of the guaBA band in the vaccine strains is ∼900 and ∼1800 bp in the wild-type strains using the primers Wu048 and Wu049. (B) Lanes 1, molecular weight marker; 2, CVD 1213; 3, J17B. The expected size of the sen band for CVD 1213 is ∼1400 and ∼1800 bp for J17B using the primers Wu060 and Wu061.
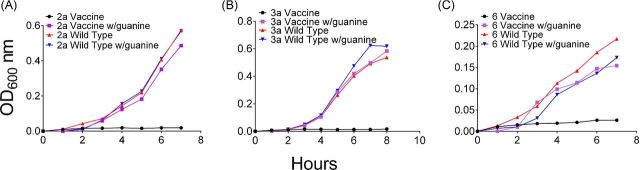
To confirm auxotrophy, the vaccine strains CVD 1213 and CVD 1215, were grown in minimal broth cultures or on minimal agar with and without guanine. On the minimal agar plates and in minimal media, the vaccines strains were unable to grow without additional guanine (Fig. 2 and Fig. S1, Supporting Information). The parental wild-type strains for each vaccine grew efficiently regardless of the presence or absence of guanine. Although optimal growth conditions for S. flexneri 6 also requires pantothenic acid, thymine and vitamin B, the strain was able to grow with guanine, albeit at a slower rate than serotypes 2a and 3a.
Figure 2.
Guanine auxotrophy is confirmed in growth curves. Each vaccine strain and its parental wild-type strain were grown in minimal media with and without supplemental guanine. (A) Shigella flexneri 2a, (B) S. flexneri 3a and (C) S. flexneri 6.
The guaBA deletion was complemented by the plasmid pATGguaBA containing the fragment of the guaBA operon (Wu et al.2011; Vindurampulle et al.2013). This plasmid confers chloramphenicol resistance and allows the vaccines to grow on minimal agar without guanine (Fig. S1, Supporting Information).
Vaccine strains have a cytotoxic effect in human macrophages
Based on previous results demonstrating the capacity of wild-type Shigella to kill macrophages, we tested the effects of the vaccines on differentiated human THP-1 macrophage-like cells (Zychlinsky, Prevost and Sansonetti 1992; Hilbi, Zychlinsky and Sansonetti 1997). Wild-type S. flexneri 2a strain 2457T and the vaccine derivative, CVD 1208S, exhibited significantly higher macrophage cytotoxicity than the plasmid cured strain, 4243A 4 to 5 h following uptake (Fig. 3A); the viability of macrophages exposed to 4243A was similar to that of uninfected cells. This was not due to differences in initial uptake of bacteria as all strains were present at similar levels within macrophages (Fig. S2, Supporting Information). CVD 1213 and CVD 1215 were equally cytotoxic as their parental wild-type strain on THP-1 cells (Fig. 3B and C). CVD 1215 and CCH060 as well as CVD 1213 and J17B were similar to each other at all time points and significantly reduced macrophage viability compared to uninfected macrophages at 4 and 5 h after uptake.
Figure 3.
Cytotoxicity in THP-1 macrophages. Vaccine strains and the parental wild-type strains have cytotoxic effects on THP-1 macrophages. THP-1 cells were infected with wild-type or vaccine strains and cell viability was measured for 5 h and compared to uninfected cells: (A) S. flexneri 2a, (B) S. flexneri 3a and (C) S. flexneri 6. Error bars depict standard deviation of three biological replicates. Optical density readings represent the colorimetric change associated with WST-1 reagent cleavage which correlates with cellular viability. Optical density was calculated by subtracting the OD600 nm value from the OD450 nm value.
Cytotoxicity was also assessed in J774 mouse macrophages and revealed that S. flexneri wild-type and vaccine strain pairs were equally cytotoxic in this cell type. As has been previously reported, wild-type S. flexneri leads to rapid and complete cell death in mouse macrophages (Fig. S3, Supporting Information) (Fernandez-Prada et al.2000). This is unlike the effect in human macrophages in which the vaccine and wild-type strains decrease their viability at a slower rate.
The impact of vaccines on IL-1β and TNF-α cytokine secretion from infected THP-1 cells was assessed as an in vitro marker of the inflammatory response (Sansonetti et al.2000). IL-1β secretion was elevated within 2 h following infection with CVD 1208S and its wild-type strain when compared to uninfected cells and cells infected with a non-virulent Shigella derivative strain 4243A (Fig. S4, Supporting Information). Secretion of IL-1β from THP-1 cells infected with CVD 1213 and CVD 1215 was also elevated compared to uninfected cells (P < 0.05 compared to uninfected cells), although not as much as the S. flexneri 2a infected cells. TNF-α secretion also increased in cells infected with each vaccine strain relative to uninfected cells, but TNF-α produced from 4243A-infected cells was significantly higher than that produced by cells infected with all other vaccine and wild-type strains (P < 0.05). TNF-α secretion from serotypes 3a and 6 infected macrophages was similar but higher than serotype 2a-infected cells (P < 0.05) (Fig. S4, Supporting Information).
Vaccines display attenuated virulence in human cell culture systems
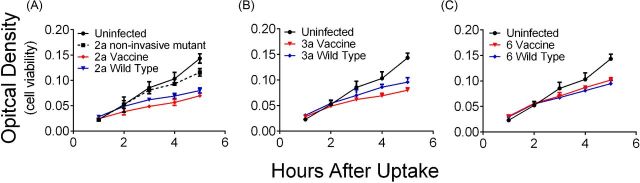
Invasion and intracellular replication of the vaccine strains was measured in the human intestinal epithelial cell line HT-29. Intracellular bacteria were quantified at numerous time points after invasion by counting CFU in cell lysates. CVD 1208S and CVD 1215 were able to invade cells at equivalent levels to their wild-type parental strain, while CVD 1213 was slightly less invasive than the wild type (P < 0.05) (Fig. 4). Wild-type strains proceeded to replicate over the following 8 h. In contrast, the vaccine strains did not replicate and remained at a constant concentration over the 8 h period (Fig. 4).
Figure 4.
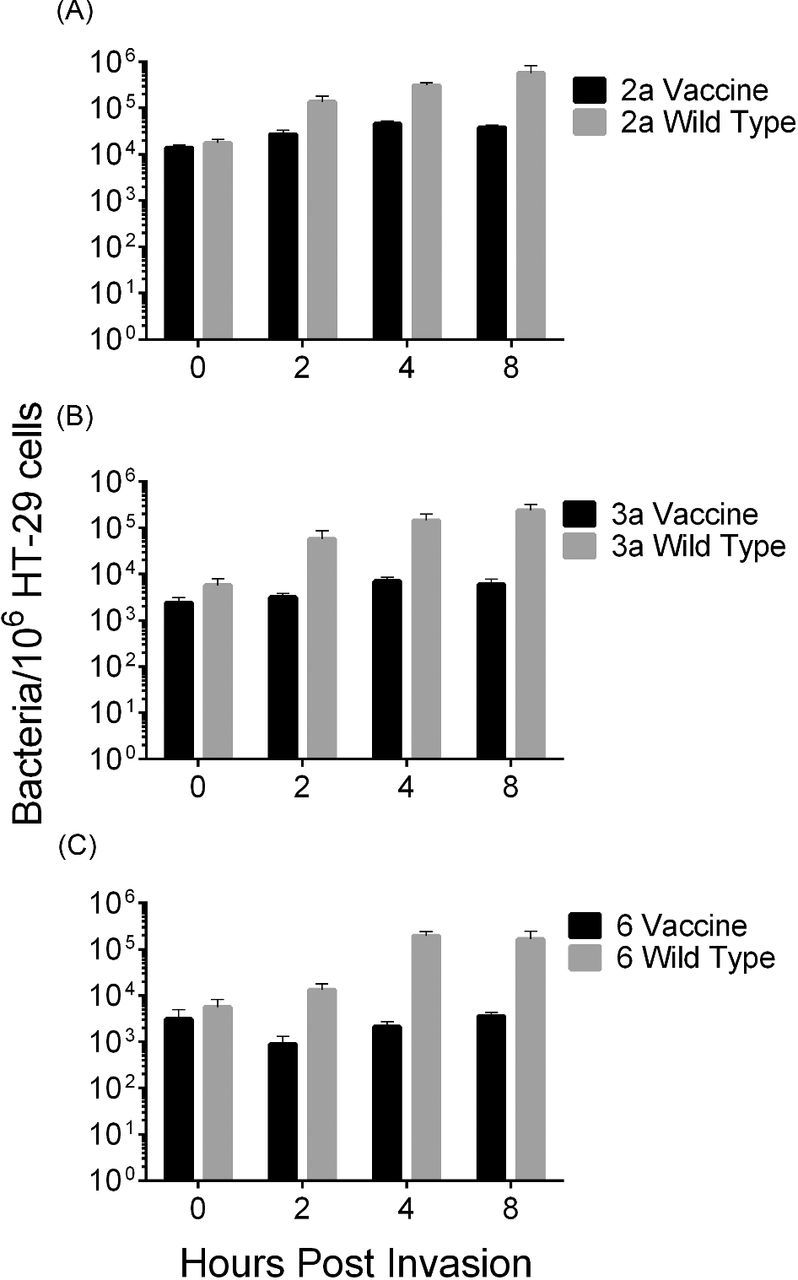
Vaccine strains are unable to replicate intracellularly in HT-29 cells. Shigella flexneri vaccine or wild-type strains was used to infect HT-29 cells (MOI of 100) for 90 min. Cells were lysed to enumerate intracellular bacteria at 0, 2, 4 and 8 h post infection. (A) Shigella flexneri 2a, (B) S. flexneri 3a and (C) S. flexneri 6. Error bars represent deviation of biological duplicates.
Upon entry into intestinal epithelial cells, Shigella triggers the production and release of interleukin 8 (IL-8) (Eckmann, Kagnoff and Fierer 1993; Philpott et al.2000), which is associated with inflammation by causing an influx of neutrophils to the site of infection. CXCL-1, or Gro-α, is also a neutrophil chemoattractant secreted by intestinal cells (Cuenca, Azizkhan and Haskill 1992). For all strains tested, similar patterns of induction of both cytokines were observed. During the initial 4 h incubation period, significantly greater amounts of IL-8 and CXCL-1 were secreted from infected cells over uninfected cells, but secretion from wild-type and vaccine-infected cells was similar (P < 0.05) (Fig. 5). These levels increased over the following 8 h period. The cumulative levels of IL-8 produced by the wild-type S. flexneri 3a (J17B) and 6 (CCH060) strains during the first 8 h period were higher than those induced by their respective vaccine strain derivatives. To determine if a different secretion pattern would emerge if time intervals were assessed, supernatants were removed at the 4-h time point, cells were washed and fresh media was added. This provides information about the cell secretion response to intracellular bacteria without the compounding effect of positive feedback on the cells from other secreted factors. When the cells were washed at 4 h and allowed to incubate an additional 4 h, there was a detectable difference in IL-8 secretion between cells infected with all vaccine and wild-type pairs which reached significance for S. flexneri 3a and 6 (Fig. 5).
Figure 5.
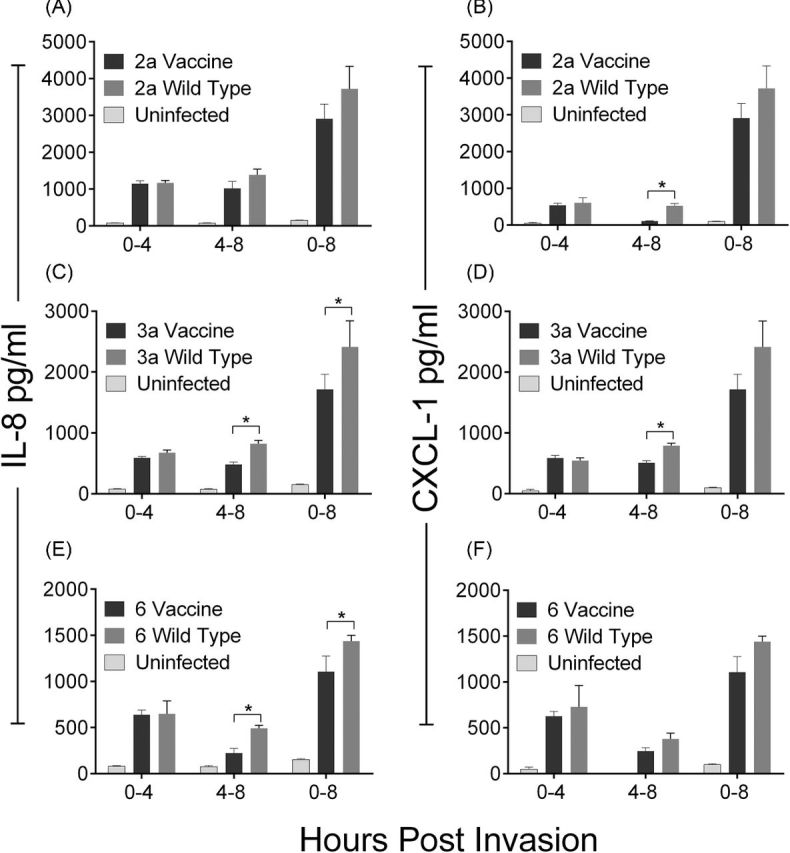
IL-8 and CXCL-1 are secreted from host cells in response to bacterial invasion. Shigella flexneri wild-type and vaccine strains were used to infect HT-29 cells for 90 min and washed with media containing gentamicin. Supernatants were collected at the indicated time points and concentrations of IL-8 (A, C, E) or CXCL-1 (B, D, F) were quantified by ELISA. Each bar shows the mean of four biological replicates and the standard deviation. Significant differences between wild-type and vaccine strains (P < 0.05) are indicated by *.
CVD 1213 and CVD 1215 are attenuated and immunogenic in vivo
The guinea pig Serény test was used to assess attenuation of the new vaccine strains. The guinea pig eye was inoculated with ∼107 CFU of each wild-type or vaccine strain and monitored for inflammation for 5 days (Sereny 1955). Wild-type S. flexneri serotypes 2a, 3a and 6 induced inflammation in 100% of the animals by 24 h post inoculation which progressed to full blown keratoconjunctivitis by 48–72 h. Animals that received CVD 1208S, CVD 1213 and CVD 1215 did not show signs of inflammation; all animals maintained scores of ‘0’ throughout the observation period.
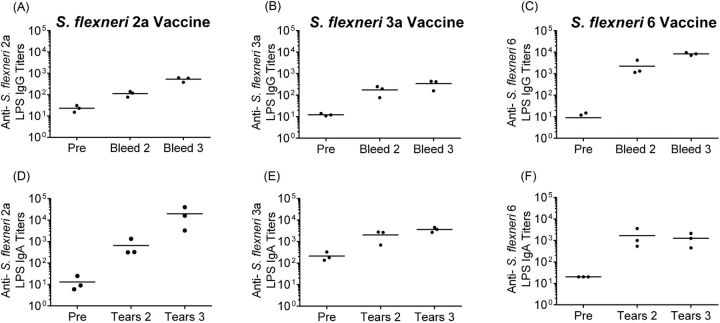
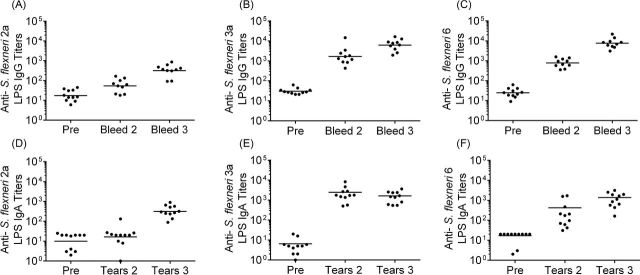
Intranasal immunization of guinea pigs is used as a mucosal route and allows measurement of vaccine-induced immune responses. Guinea pigs were immunized with two doses of each vaccine strain spaced two weeks apart. Serum and tears were collected prior to immunization and two weeks following each dose and assayed by ELISA to quantify anti-serotype-specific LPS antibody responses. All of the animals immunized with each individual vaccine developed anti-LPS mucosal IgA and serum IgG in responses following the first vaccine dose and titers were boosted to higher levels following the second dose (Fig. 6). Following two doses, IgG titers in CVD 1208S inoculated animals increased 20-fold compared to pre-immune levels and IgA titers increased 1000-fold (Fig. 6). The IgG titers of CVD 1213 inoculated animals increased at least 65-fold in all animals and IgA titers increased greater than 2-fold in two animals (Fig. 6). Animals administered CVD 1215 responded with titers that were 100-fold over pre-inoculation IgA and IgG titers (Fig. 6). The second dose yielded increased anti-LPS IgG titers in all animals and an increase in IgA responses in 1208S and 1215 inoculated animals (CVD 1213 titers remained the same as the second collection). All immunized animals were challenged via intraocular inoculation with the homologous wild-type parent strain following two vaccine doses. When challenged via conjunctival inoculation with the homologous wild-type strain, CVD 1208S, CVD 1213 and CVD 1215 vaccinated animals were fully protected (Table 3).
Figure 6.
Antibody titers in guinea pigs immunized with S. flexneri vaccine strains. Guinea pigs were immunized with two doses of CVD 1208S (A and D), CVD 1213 (B and E) or CVD 1215 (C and F). Antibodies to S. flexneri serotype specific LPS were measured by ELISA in serum (panels A, B, and C) and tears (panels D, E, and F) prior to immunization (labeled ‘Pre’) and two weeks following each dose.
Table 3.
Wild-type challenge attack rate, vaccine efficacy and complement-mediated bactericidal activity following two immunizations.
| Attack rate following challenge | Efficacy against wild-type | 50% serum bactericidal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccination | with wild-type strain | challenge | titer | ||||||
| 2457T | J17B | CCH060 | 2457T | J17B | CCH060 | 2457T | J17B | CCH060 | |
| CVD 1208S | 0/4 | – | – | 100% | – | – | 1917 | – | – |
| CVD 1213 | – | 0/4 | – | – | 100% | – | – | 1356 | – |
| CVD 1215 | – | – | 0/4 | – | – | 100% | – | – | 2700 |
| Combination | 1/3 | 0/4 | 0/4 | 67% | 100% | 100% | 4395 | 2344 | 742 |
Serum antibodies from immunized animals have bactericidal activity
Sera collected from the immunized guinea pigs were used to determine the functional characteristics of the Shigella specific antibodies. Antibody and complement-mediated killing of wild-type strains 2457T, J17B and CCH060 (serotypes 2a, 3a and 6) was demonstrated in sera collected from individually immunized animals. Bactericidal activity was reported as the serum titer at which 50% of the starting bacterial inoculum is killed. There was no bactericidal activity in pre-immune serum samples. Animals immunized with CVD 1208S, CVD 1213 and CVD 1215 had a 50% killing titers against the homologous serotype of 1917, 1356 and 2700 respectively (Fig. 7 and Table 3). There was no bactericidal activity against heterologous S. flexneri serotypes.
Figure 7.
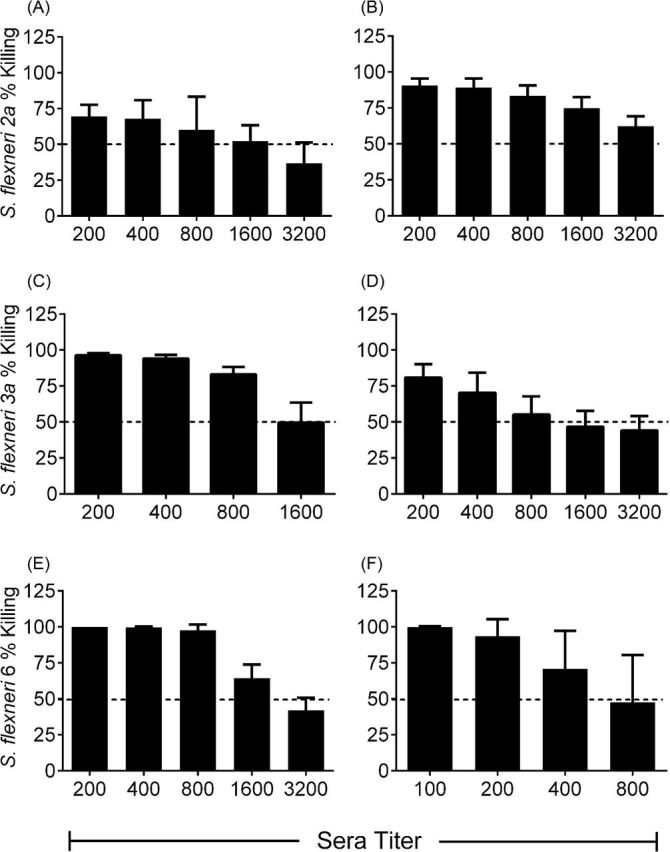
Serum from immunized guinea pigs has bactericidal activity. Sera from animals immunized with two doses of (A) CVD 1208S, (C) CVD 1213, or (E) CVD 1215 or with two doses of a mixture of CVD 1208S, CVD 1213 and CVD 1215 (B, D and F) was assessed for the ability to kill wild-type strains of S. flexneri. The mean percent bactericidal activity against parental wild-type strains 2457T (A and B), J17B (C and D) and CCH060 (E and F) is shown for each serum dilution tested from immunized guinea pigs. The horizontal dashed line indicates the 50% bactericidal titer.
We also investigated bactericidal activity against each parental strain in guinea pigs immunized with CVD 1208S, CVD 1213 and CVD 1215 combined. Sera from these animals also exhibited bactericidal activity against each of the wild-type parental strains. The average 50% bactericidal titers of serum from animals immunized with the mixed inoculum were 4395, 2344 and 742 against S. flexneri 2a, 3a and 6 respectively (Fig. 7 and Table 3).
Combined immunization with CVD 1208S, CVD 1213 and CVD 1215 protects against wild-type challenge with each S. flexneri component
A multivalent strategy can provide broad-spectrum coverage against Shigella species. Ideally, the three vaccine candidates, CVD 1208S, CVD 1213 and CVD 1215, would be components of a multivalent vaccine. To determine if mixed immunization can provide protection against the individual parental wild-type strains, 11 guinea pigs were given two doses of a mixture containing equal parts of each vaccine. IgG and IgA specific for S. flexneri serotypes 2a, 3a and 6 specific LPS were measured in serum and tears respectively. All animals developed IgG and IgA to all three components of the mixed inoculum in the following increasing order: serotype 2a, 6 and 3a LPS (Fig. 8). Animal sera were also pooled to determine IgG antibody responses to IpaB, a type 3 secretion system antigen associated with immunity (Wahid et al.2013; Heine et al.2014). Animals immunized with the individual vaccines as well as the mixed vaccine inoculum responded with titers greater than 10 000 to IpaB (pooled pre-immunization titers from animals given the mixed vaccine was 33).
Figure 8.
Guinea pigs immunized with a mixture of CVD 1208S, CVD 1213 and CVD 1215 produce antibodies to all three serotypes. Guinea pigs were immunized with two doses of a mixture of CVD 1208S, CVD 1213 and CVD 1215. Antibodies to S. flexneri 2a (A and D), 3a (B and E) and 6 (C and F) LPS were measured by ELISA in serum (panels A, B, and C) and tears (panels D, E, and F) prior to immunization (labeled ‘Pre’) and two weeks following each dose.
The vaccinated animals were split into three groups for the challenge study; three were given 5 × 107 CFU 2457T, four were given 2 × 108 CFU J17B and four given 9 × 107 CFU CCH060. All animals challenged with S. flexneri 6 strain CCH060 or S. flexneri 3a strain J17B were protected against keratoconjunctivitis, demonstrating 100% vaccine efficacy (all control animals developed keratoconjunctivitis). Two of the four animals challenged with J17B developed mild swelling (scored 1) 48 h after challenge, but were protected against full-blown keratoconjunctivitis (Table 3). The mild inflammation was resolved by 72 h and 96 h following the initial challenge. Two of the three animals challenged with S. flexneri 2a strain 2457T were protected against wild-type challenge; one animal developed a severe reaction (score 3) and was euthanized three days after challenge (Table 3).
DISCUSSION
Several recent studies have quantified the burden of Shigella, a significant diarrheal pathogen in young children in low income regions (Kotloff et al.2012; Platts-Mills et al.2015). Specifically, S. flexneri including all serotypes, along with S. sonnei, make up 89% of Shigella isolates identified in children under five years of age in endemic regions (Livio et al.2014). Based on this data, we proposed the use of a multispecies and serotype vaccine, composed of attenuated strains of S. sonnei, and S. flexneri serotypes 2a, 3a and 6, to confer broad protection against multiple Shigella serotypes. Although protection against Shigella is based on O-antigen specificity, we believe this is a feasible strategy based on the pre-clinical demonstration of cross protection between serotypes in guinea pigs (Noriega et al.1999). In this study, we report the development of candidate strains for the S. flexneri component of a broad-spectrum vaccine.
CVD 1208S is an ideal comparator for any new vaccine candidate against S. flexneri because it has been extensively tested in vitro, in animals and in human adult volunteers. The guaBA metabolic mutation is a proven attenuation method and was therefore applied to S. flexneri 3a and S. flexneri 6. This mutation prevents vaccine replication and intracellular spread, therefore decreasing the reactogenicity associated with Shigella. By leaving the Type-3 Secretion System (T3SS) intact, the vaccines have maximal interaction with macrophages and intestinal cells. We believe this interaction increases the opportunity for Shigella antigen uptake by macrophages and dendritic cells, and their subsequent interaction with T cells in lymphoid tissues. Because of their conserved nature, T3SS antigens (i.e. IpaB) may also contribute to the induction of cross protective immunity (Martinez-Becerra et al.2013; Heine et al.2014, 2015). The data presented herein demonstrates that the new vaccine strains are properly attenuated and still able to elicit cytokine responses in vitro and functional antibody responses in vivo.
Host cell invasion of both macrophages and intestinal cells is a first and vital step to achieve protective immunity. Several studies have shown that Shigella causes death of macrophages following its escape from the phagosome (Zychlinsky, Prevost and Sansonetti 1992). It has been demonstrated more recently that IpaB plays a role in caspase-1 activation and the subsequent release of IL-1β that leads to an inflammatory response (Sansonetti et al.2000). An additional T3SS protein called MxiI also leads to downstream inflammasome activation (Suzuki et al.2014). In this study, the benchmark strain CVD 1208S had a cytotoxic effect on human macrophages that was comparable to 2457T infected cells. CVD 1208S also induced similar levels of TNF-α and IL-1β release from THP-1 macrophages when compared to wild-type infected cells. The increased secretion of TNF-α from human monocyte derived macrophages following infection with a non-virulent Shigella strain has been previously documented relative to wild-type Shigella (Fernandez-Prada et al.2000). Our results in human macrophages infected with the virulence plasmid-cured strain 4243A as well as wild-type S. flexneri 2a are consistent with previous report. The observed secretion of cytokines from vaccine infected cells indicates the vaccines induce responses similar to those of wild-type, as opposed to non-virulent strains. The new vaccine strains, CVD 1213 and CVD 1215, and their parental wild-type strain had the same effects on macrophage viability and cytokine induction patterns. This was expected because the vaccine strains maintain virulence plasmid effectors that play a dominant role in macrophage interaction.
Following escape from macrophages, the vaccines are expected to invade intestinal epithelial cells. Epithelial cell invasion and cytosolic replication are key steps in Shigella pathogenesis. For a live, attenuated oral vaccine, invasion or translocation across the intestinal epithelial lining is likely necessary to stimulate mucosal O-antigen specific IgA antibody secreting cells (Barry et al.2013). As shown in Fig. 4, each of the three candidates was able to invade intestinal HT-29 cells with similar efficiency to the wild-type strain but were unable to survive and/or replicate at wild-type levels. Previous studies have indicated that although vaccines cannot replicate, they should be able to invade the epithelial cells to induce pro-inflammatory cytokine secretion and specific mucosal immunity (Jennison and Verma 2004). A key cytokine induced in epithelial cells following invasion by Shigella is IL-8, a neutrophil chemoattractant and indicator of host cell activation. Although neutrophil infiltration ultimately leads to the clearance of Shigella, IL-8 secretion is a useful marker of the host cell response to infection. In fact, IL-8 secretion in vitro is used as a correlate of neutrophil recruitment to the colon in vivo (Singer and Sansonetti 2004). CXCL-1 (or Gro-α) is a neutrophil recruiting cytokine similar to IL-8. Interestingly, the cumulative amount of IL-8 (and CXCL-1) secretion measured after 4 h of infection was similar between CVD 1208S and wild-type Shigella-infected cells (Fig. 5). In contrast, during the 4 to 8 h interval when cells are incubated in fresh media, there was a detectable difference in IL-8 secretion between wild-type and vaccine strains (Fig. 5). The decreased cytokine secretion in vaccine strain infected cells during this interval is most likely due to gradual decreases in intracellular bacterial numbers. Similar results were seen for CVD 1213 and CVD 1215 infected cells, suggesting that the new candidates performed similarly to the benchmark strain CVD 1208S.
The guinea pig model is widely accepted for studying safety and protective immunity of Shigella vaccine candidates. Using this model, we demonstrate that the new vaccines are safe and non-inflammatory in vivo. CVD 1213 and CVD 1215 were equally immunogenic in guinea pigs; after two doses, 100% of the animals were protected against wild-type challenge with S. flexneri 3a or 6 respectively. CVD 1208S has progressed through animal and clinical trials and was shown to be safe and immunogenic in adult human populations.
When guinea pigs were given two doses of an equal mixture of CVD 1208S, CVD 1213 and CVD 1215, all animals seroconverted (>4-fold increase over pre-immune titers) for IgA and IgG specific to serotypes 2a, 3a and 6 LPS. Although the responses to each serotype-specific LPS varied, they were all robust and the combination of strains did not impair the ability of the vaccine to confer protection against wild-type challenge against any serotype. The demonstration of antibodies that mediate complement bactericidal activity further strengthen the potential of this vaccine to induce functionally active immune components that can mediate bacterial clearance and contribute to protective immunity.
This study advances the field by reporting the creation of two new vaccine strains as part of a broad-spectrum, multistrain vaccine targeting the Shigella species. CVD 1208S, CVD 1213, CVD 1215 could be used as vaccine strains individually and combined as a multivalent formula. Future studies will assess the new vaccines for use as carriers of antigens from similar enteric pathogens for the possible creation of a broad-spectrum diarrheal vaccine.
Supplementary Material
SUPPLEMENTARY DATA
FUNDING
This work was supported by the at the [grant numbers to E.M.B. and to M.F.P.]; and the to [B.C.D].
Conflict of interest. None declared.
REFERENCES
- Bardhan P, Faruque AS, Naheed A, et al. Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg Infect Dis. 2010;16:1718–23. doi: 10.3201/eid1611.090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry EM, Pasetti MF, Sztein MB, et al. Progress and pitfalls in Shigella vaccine research. Nat Rev Gastroentero. 2013;10:245–55. doi: 10.1038/nrgastro.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennish ML, Wojtyniak BJ. Mortality due to shigellosis: community and hospital data. Rev Infect Dis. 1991;13(Suppl 4):S245–51. doi: 10.1093/clinids/13.supplement_4.s245. [DOI] [PubMed] [Google Scholar]
- Centers for Disease C & Prevention. Outbreaks of multidrug-resistant Shigella sonnei gastroenteritis associated with day care centers–Kansas, Kentucky, and Missouri, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1068–71. [PubMed] [Google Scholar]
- Cohen D, Green MS, Block C, et al. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–9. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca RE, Azizkhan RG, Haskill S. Characterization of GRO alpha, beta and gamma expression in human colonic tumours: potential significance of cytokine involvement. Surg Oncol. 1992;1:323–9. doi: 10.1016/0960-7404(92)90094-2. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. P Natl Acad Sci USA. 2000;97:6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–74. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Prada CM, Hoover DL, Tall BD, et al. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infect Immun. 1997;65:1486–96. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Prada CM, Hoover DL, Tall BD, et al. Shigella flexneri IpaH(7.8) facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human macrophages. Infect Immun. 2000;68:3608–19. doi: 10.1128/iai.68.6.3608-3619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, Prado V, Ojeda A, et al. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134:614–27. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- Fiorentino M, Levine MM, Sztein MB, et al. Effect of wild-type Shigella species and attenuated Shigella vaccine candidates on small intestinal barrier function, antigen trafficking, and cytokine release. PLoS One. 2014;9:e85211. doi: 10.1371/journal.pone.0085211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine SJ, Diaz-McNair J, Andar AU, et al. Intradermal delivery of Shigella IpaB and IpaD type III secretion proteins: kinetics of cell recruitment and antigen uptake, mucosal and systemic immunity, and protection across serotypes. J Immunol. 2014;192:1630–40. doi: 10.4049/jimmunol.1302743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine SJ, Franco-Mahecha OL, Chen X, et al. Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice. Immunol Cell Biol. 2015;93:641–52. doi: 10.1038/icb.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi H, Zychlinsky A, Sansonetti PJ. Macrophage apoptosis in microbial infections. Parasitology. 1997;115(Suppl):S79–87. doi: 10.1017/s0031182097001790. [DOI] [PubMed] [Google Scholar]
- Jennison AV, Verma NK. Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol Rev. 2004;28:43–58. doi: 10.1016/j.femsre.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Koterski JF, Nahvi M, Venkatesan MM, et al. Virulent Shigella flexneri causes damage to mitochondria and triggers necrosis in infected human monocyte-derived macrophages. Infect Immun. 2005;73:504–13. doi: 10.1128/IAI.73.1.504-513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Blackwelder WC, Nasrin D, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55(Suppl 4):S232–45. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Pasetti MF, Barry EM, et al. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J Infect Dis. 2004;190:1745–54. doi: 10.1086/424680. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Simon JK, Pasetti MF, et al. Safety and immunogenicity of CVD 1208S, a live, oral DeltaguaBA Deltasen Deltaset Shigella flexneri 2a vaccine grown on animal-free media. Hum Vaccines. 2007;3:268–75. doi: 10.4161/hv.4746. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Winickoff JP, Ivanoff B, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651–66. [PMC free article] [PubMed] [Google Scholar]
- Levine MM, Kotloff KL, Barry EM, et al. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–53. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay B, Ochieng JB, Ikumapayi UN, et al. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J Clin Microbiol. 2013;51:1740–6. doi: 10.1128/JCM.02713-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livio S, Strockbine N, Panchalingam S, et al. Shigella isolates from the Global Enteric Multicenter Study (GEMS) inform vaccine development. Clin Infect Dis. 2014;59:933–41. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Becerra FJ, Chen X, Dickenson NE, et al. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect Immun. 2013;81:4470–7. doi: 10.1128/IAI.00859-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SR, Lima NL, Soares AM, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139:1156–64. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhaya A, Mahalanabis D, Khanam J, et al. Protective efficacy of oral immunization with heat-killed Shigella flexneri 2a in animal model: study of cross protection, immune response and antigenic recognition. Vaccine. 2003;21:3043–50. doi: 10.1016/s0264-410x(03)00111-7. [DOI] [PubMed] [Google Scholar]
- Noriega FR, Liao FM, Maneval DR, et al. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67:782–8. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FR, Losonsky G, Lauderbaugh C, et al. Engineered deltaguaB-A deltavirG Shigella flexneri 2a strain CVD 1205: construction, safety, immunogenicity, and potential efficacy as a mucosal vaccine. Infect Immun. 1996;64:3055–61. doi: 10.1128/iai.64.8.3055-3061.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FR, Wang JY, Losonsky G, et al. Construction and characterization of attenuated delta aroA delta virG Shigella flexneri 2a strain CVD 1203, a prototype live oral vaccine. Infect Immun. 1994;62:5168–72. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott DJ, Yamaoka S, Israel A, et al. Invasive Shigella flexneri activates NF-kappa B through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J Immunol. 2000;165:903–14. doi: 10.4049/jimmunol.165.2.903. [DOI] [PubMed] [Google Scholar]
- Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob Health. 2015;3:e564–75. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasolofo-Razanamparany V, Cassel-Beraud AM, Roux J, et al. Predominance of serotype-specific mucosal antibody response in Shigella flexneri-infected humans living in an area of endemicity. Infect Immun. 2001;69:5230–4. doi: 10.1128/IAI.69.9.5230-5234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti PJ, Phalipon A, Arondel J, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–90. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- Sereny B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Hung. 1955;2:293–6. [PubMed] [Google Scholar]
- Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers' diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609–14. [PubMed] [Google Scholar]
- Singer M, Sansonetti PJ. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J Immunol. 2004;173:4197–206. doi: 10.4049/jimmunol.173.6.4197. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Franchi L, He Y, et al. Shigella type III secretion protein MxiI is recognized by Naip2 to induce Nlrc4 inflammasome activation independently of Pkcdelta. PLoS Pathog. 2014;10:e1003926. doi: 10.1371/journal.ppat.1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Verg LL, Bendiuk NO, Kotloff K, et al. Cross-reactivity of Shigella flexneri serotype 2a O antigen antibodies following immunization or infection. Vaccine. 1996;14:1062–8. doi: 10.1016/0264-410x(96)00006-0. [DOI] [PubMed] [Google Scholar]
- Vindurampulle C, Barry EM, Levine MM. Attenuated Salmonella enterica serovar paratyphi and uses thereof July 2, 2013US Patent: 8475810 [Google Scholar]
- Wahid R, Simon JK, Picking WL, et al. Shigella antigen-specific B memory cells are associated with decreased disease severity in subjects challenged with wild-type Shigella flexneri 2a. Clin Immunol. 2013;148:35–43. doi: 10.1016/j.clim.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Epidemiology. In: World Health Organization, editor. Guidelines For The Control Of Shigellosis, Including Epidemics Due To Shigella Dysenteriae Type 1. Geneva: WHO Press; 2005. pp. 2–4. [Google Scholar]
- Wikswo ME, Hall AJ. Outbreaks of acute gastroenteritis transmitted by person-to-person contact–United States, 2009–2010. MMWR Surveill Summ. 2012;61:1–12. [PubMed] [Google Scholar]
- Wu T, Grassel C, Levine MM, et al. Live attenuated Shigella dysenteriae type 1 vaccine strains overexpressing shiga toxin B subunit. Infect Immun. 2011;79:4912–22. doi: 10.1128/IAI.05814-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.