Abstract
Space exploration programs have long been interested in the effects of spaceflight on biology. This research is important not only in its relevance to future deep space exploration, but also because it has allowed investigators to ask questions about how gravity impacts cell behavior here on Earth. In the 1980s, scientists designed and built the first rotating wall vessel, capable of mimicking the low shear environment found in space. This vessel has since been used to investigate growth of both microorganisms and human tissue cells in low shear modeled microgravity conditions. Bacterial behavior has been shown to be altered both in space and under simulated microgravity conditions. In some cases, bacteria appear attenuated, whereas in others virulence is enhanced. This has consequences not only for manned spaceflight, but poses larger questions about the ability of bacteria to sense the world around them. By using the microgravity environment as a tool, we can exploit this phenomenon in the search for new therapeutics and preventatives against pathogenic bacteria for use both in space and on Earth.
Keywords: microgravity, virulence, rotating wall vessel, spaceflight, bacteria, host pathogen
Experiments performed in space and in low gravity have provided insight on how bacteria cause disease and this information can be used to develop tools to prevent or treat infections.
INTRODUCTION
For many years, the effects of spaceflight on cell physiology have been explored in experiments performed both on Earth and in orbit. An extensive list of cell types has been investigated, including human cells of the gut, heart muscle and liver, immune cells, cancer cells and organisms such as bacteria and fungi. The results of these experiments have been immensely informative and have directed future research in several different biological fields. Of particular interest to those in vaccine research is the use of microgravity and microgravity analogs to investigate pathogen responses to these environments, and also to model host–pathogen interactions. The aim of this review is to impart some of the history of microgravity research in these fields and to provide perspectives for the future as to the broad application of microgravity to translational research, particularly for the development of vaccines and therapeutics against bacterial pathogens.
SPACEFLIGHT AND LOW SHEAR MODELED MICROGRAVITY
Very early in space exploration, scientists realized that the spaceflight environment had a significant effect on human physiology. Astronauts returning from space often displayed a myriad of health conditions, including decreased immune function, bone loss and anemia (Vogel 1975; Tavassoli 1982; Taylor and Janney 1992). This led to a plethora of spaceflight experiments designed to test the effects of spaceflight stimuli, such as microgravity, radiation and a disrupted circadian rhythm, on tissue cells. Cell samples, including pituitary cells, splenocytes, leukocytes and neurons, were carried aboard spaceflight missions ranging from 8 to 14 days (Grove, Pishak and Mastro 1995; Davis et al. 1996; Hymer et al. 1996; Ichiki et al. 1996; Ishihara et al. 1997). These cells almost universally showed alterations in cell function during spaceflight, when compared to ground controls. This includes generalized changes in cell distribution, morphology and activation, as well as specific effects such as decreased leukocyte levels, decreased proliferation of bone marrow progenitor cells and altered proprioception (the ability to sense stimuli), in rat dorsal root ganglions.
While the use of spaceflight allowed researchers to gain insights into cell function, the cost and physical limitations of performing experiments on space missions made these experiments prohibitively expensive and difficult to conduct. In the 1970s, engineers at the National Aeronautics and Space Administration (NASA) began to develop a cell culture system to model fluid dynamics experienced during space flight. The aim of this project was to design a microgravity simulating culture apparatus, as it was postulated that by removing the effect of gravity on cells in culture, they might be able to form larger aggregates, resembling tissues. This was indeed borne out in experiments using human colorectal carcinoma cells (Goodwin, Jessup and Wolf 1992), and the culture vessel has since been used as a device for tissue engineering, in host–pathogen interaction studies and to assess gene expression in both tissues and microorganisms.
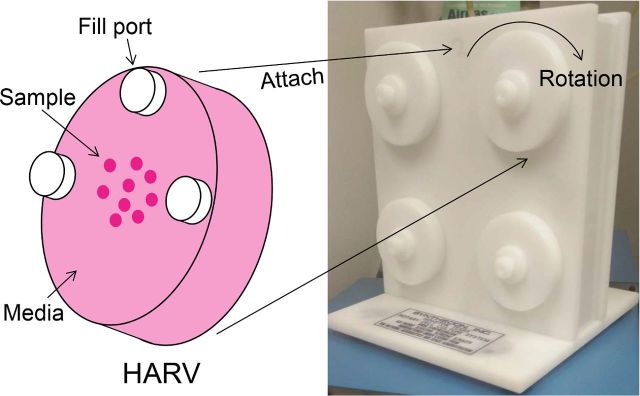
The rotating wall vessel (RWV) utilizes solid body rotation about the horizontal axis to minimize shear stress and decrease the gravitational force on particles in the fluid, thereby mimicking space conditions (Wolf and Schwarz 1991). It is composed of a cylindrical chamber which is completely filled with growth medium (Fig. 1). The cylinder is rotated at a precise speed chosen to limit the shear forces to which particles suspended in the medium are exposed (Wolf and Schwarz 1991; Klaus 2007). To maintain adequate oxygenation while limiting turbulence, the RWV utilizes a gas exchange system which delivers oxygen to the media via axial diffusion of dissolved gases. Gas exchange within the RWV can occur within the chamber core (slow turning lateral vessel; STLV) or around the inner wall of the chamber (high aspect ratio vessel; HARV). Although both create similar low shear growth conditions, STLVs are generally used for anchorage-dependent cells that have less stringent oxygen requirements, while HARVs are used to culture cells in suspension, under higher oxygen saturation.
Figure 1.
Schematic of a rotating wall vessel, using the HARV as an example.
Analysis of the fluid dynamics within RWVs has shown significant correlations with those of fluids in spaceflight. Two parameters of particular importance are the gravitational force and the shear stress. The gravitational force acting on particles within the RWV was measured at 10−2 × g. This is still orders of magnitude greater than the gravitational force acting on particles during spaceflight (10−4 – 10−6 × g), but far less than that occurring on Earth (100 × g). With regard to the shear stress exerted on particles, this was measured at <1 dyne/cm2 (Wolf and Schwarz 1991; Nauman et al. 2007). One dyne is defined as the force required to cause a mass of 1 g to accelerate at 1 cm/s2. As a frame of reference, shear stress in an artery is ∼10–70 dyne/cm2. Therefore, the shear stress experienced by particles in an RWV is very low. For these reasons, cells grown in an RWV are often referred to as being cultured in low shear modeled microgravity (LSMMG).
While growth in the RWV shares many similarities with the environment of spaceflight, it is worth noting that they are not true analogs. The microgravity-like environment of the RWV is maintained by balancing, not removing external forces. Particle size, rotational speed and media density all need to be carefully managed to maintain low shear. As such, it does not truly mimic microgravity, but merely recapitulates a low shear environment which is also found in microgravity. This is perhaps most obviously seen in gene expression patterns of cells cultured in either true spaceflight microgravity or LSMMG. In an experiment using human renal cortical cells, over 1600 genes showed changes in expression in spaceflight, while growth in the RWV induced changes in only 900 (Hammond et al. 2000). Thus, while it is a useful tool for studying LSMMG, the conditions of the RWV do not truly represent those experienced during spaceflight.
MICROORGANISMS IN MICROGRAVITY
While early spaceflight experiments focused on tissue cells, many different bacterial species have also been carried on spacecraft as part of research programs. This includes innocuous laboratory strains, opportunistic pathogens of the normal flora and common gut pathogens (Gasset et al. 1994; McLean et al. 2001; Benoit and Klaus 2007; Wilson et al. 2007, 2008; Hammond et al. 2013). While this research started as a purely academic exercise, the results have opened up an entirely new field focused on the relevance of microgravity as a regulator of bacterial gene expression and virulence.
Initial experiments performed on bacteria subjected to spaceflight showed varying phenotypic responses. Of note were changes in growth rate, resistance to external stresses and effects on bacterial conjugation (Gasset et al. 1994; Klaus et al. 1997; De Boever et al. 2007). Early reports suggested that growth in microgravity or LSMMG increased bacterial growth rates over cultures grown in normal gravity (Klaus et al. 1997; Kacena et al. 1999). However, over time and with increased scrutiny, this observation was found not to be universally true. Instead, growth rates were dependent on growth media, motility and the bacterial species and strain (Benoit and Klaus 2007). Similarly, while several studies have shown increased resistance of LSMMG-grown laboratory Escherichia coli strains to stresses such as low pH, ethanol and hyperosmosis (Gao et al. 2001; Lynch, Brodie and Matin 2004; Lynch et al. 2006), other studies have concluded that the choice of media and nutrient depletion have a more significant effect than gravity (Tucker et al. 2007). This dichotomy was also borne out in gene expression studies, with some researchers identifying many genes associated with LSMMG, while others reported few changes in gene expression (Vukanti, Mintz and Leff 2008; Arunasri et al. 2013). Thus, by looking at these studies as a whole, there is no definable set of microgravity responses, at least among laboratory E. coli strains. The major findings of these and other studies investigating bacterial responses to microgravity are summarized in Table 1.
Table 1.
Responses of bacteria to microgravity.
| Bacterium | Culture condition | Bacterial responses to microgravity | Reference |
|---|---|---|---|
| Bacillus thuringiensis | Spaceflight | Increased bacterial conjugation in spaceflight. | De Boever et al. (2007) |
| E. coli (K-12) | Spaceflight | Decreased doubling time of spaceflight cultures compared to ground cultures, but no significant difference in cell size. | Gasset et al. (1994) |
| Spaceflight | Bacteria grown in spaceflight had shorter lag phase, increased time in exponential phase and higher final cell density than ground controls. | Klaus et al. (1997) | |
| Spaceflight | Higher final cell density and shorter lag phase in spaceflight grown cultures. | Kacena et al. (1999) | |
| HARV | No change in the expression of stress response genes between LSMMG and ground cultures. No consistency between the gene expression profiles of bacteria cultured in different media in response to LSMMG. | Tucker et al. (2007) | |
| HARV | Increased resistance to osmotic and acid stresses. | Lynch, Brodie and Matin (2004) | |
| HARV | Increased biofilm formation in LSMMG and increased resistance to salt, ethanol and antibiotics. | Lynch et al. (2006) | |
| RWV | Increased resistance to ethanol. | Gao et al. (2001) | |
| Random positioning machine (RPM) | Increased growth rate in LSMMG. Gene expression changes in LSMMG dependent on media composition. | Arunasri et al. (2013) | |
| STLV | Increase in expression of genes for nutrient stress. | Vukanti, Mintz and Leff (2008) | |
| Spaceflight and RWV | Correlation between increased cell density and lack of motility in microgravity and LSMMG experiments. | Benoit and Klaus (2007) | |
| ETEC | HARV | Increased expression of the labile toxin virulence factor. | Chopra et al. (2006) |
| EHEC | HARV | Increased intimin adherence factor expression | Carvalho et al. (2005) |
| Salmonella Typhimurium | HARV | Microarray analysis identifies 163 genes belonging to the LSMMG regulon. Identified decreased expression of genes located in the Salmonella pathogenicity islands I and II. | Wilson et al. (2002b) |
| HARV | Responses to LSMMG, including increased resistance to acid, thermal and osmotic stresses, occur in an rpoS-independent manner. | Wilson et al. (2002a) | |
| HARV | Increased macrophage invasion in LSMMG. Increased virulence in an antiorthostatic mouse model. | Chopra et al. (2006) | |
| HARV | Increased virulence of bacteria in the mouse model after exposure to microgravity. | Nickerson et al. (2000) | |
| Spaceflight | Increased virulence of spaceflight-flown Salmonella Typhimurium in a mouse model. Small RNA chaperone Hfq implicated in microgravity regulon. | Wilson et al. (2007) | |
| Spaceflight | Response to LSMMG is dependent on phosphate ion concentration. | Wilson et al. (2008) | |
| Spaceflight | Decreased virulence of three site-directed mutants of Salmonella Typhimurium in a C. elegans model of infection. | Hammond et al. (2013) | |
| S. aureus | HARV | Increased biofilm formation, exopolysaccharide production and antibiotic resistance. Decreased Hfq expression. | Castro et al. (2011) |
| HARV | Decrease in staphyloxanthin and toxin production. | Rosado et al. (2010) | |
| P. aeruginosa | RWV | Biofilm formation is sensitive to changes in shear force in the RWV. | Crabbé et al. (2008) |
| RWV | Increase in expression of genes involved in the stress response. Increased Hfq expression. | Crabbé et al. (2010) | |
| Spaceflight | Differential regulation of 167 genes in spaceflight. Hfq identified as a major transcriptional regulator to spaceflight. | Crabbé et al. (2011) | |
| Spaceflight | Biofilm morphology unchanged in spaceflight. | McLean et al. (2001) | |
| Spaceflight | Increased cell viability, biofilm biomass and thickness during spaceflight. | Kim et al. (2013) | |
| Y. pestis | HARV | Decreased TTSS expression and decreased cytotoxicity in LSMMG. | Lawal, Jejelowo and Rosenzweig (2010) |
| HARV | Increased virulence of LSMMG grown bacteria in a TTSS defective mutant. | Lawal et al. (2013) |
In terms of pathogenic E. coli, however, researchers have observed specific differences in the expression of virulence determinants. Enterotoxigenic E. coli (ETEC) grown under LSMMG had increased expression of heat-labile toxin (LT) over normal gravity controls (Chopra et al. 2006). In enterohemorrhagic E. coli (EHEC), expression of the intimin adherence protein, which mediates the formation of attaching and effacing lesions, was increased when bacteria were grown in LSMMG (Carvalho et al. 2005). Interestingly, however, enteropathogenic E. coli (EPEC), which also uses intimin for adherence, did not increase intimin protein expression under simulated microgravity. This suggests that some pathogenic bacteria can reappropriate the microgravity response to control regulation of virulence factors.
As with E. coli, growth of Salmonella enterica serovar Typhimurium under LSMMG has been shown to increase resistance to acid, heat and osmotic stresses, as well as shortening the generation time of bacterial replication (Wilson et al. 2002a). In gene expression studies, growth of bacteria in LSMMG led to the differential expression of 163 genes when compared to normal gravity controls (Wilson et al. 2002b). Interestingly, more downregulated genes were identified than upregulated genes. This correlated with an earlier study using 2D gel electrophoresis analysis, where more proteins were identified as downregulated in cultures grown in LSMMG, when compared to normal gravity controls (Nickerson et al. 2000). Researchers also saw a similar pattern of gene expression changes in space-flown bacteria when compared to normal gravity controls (Wilson et al. 2007, 2008). By collating and comparing data from these independent experiments, a number of common gene families were identified which may compose the microgravity regulon. Examples of downregulated genes of this regulon include those for flagella, Hyc hydrogenase, ribosome structural components and regulatory RNAs, while the outer membrane porins were largely upregulated and iron utilization genes were differentially regulated (Wilson et al. 2008).
As well as investigating changes in growth rate and gene expression, researchers have also assessed changes in virulence upon exposure to spaceflight and LSMMG. A major virulence determinant for Salmonella is their ability to survive and proliferate in macrophages. In two independent studies, Salmonella Typhimurium grown under LSMMG was found to have increased survival in macrophages, when compared to ground controls (Nickerson et al. 2000; Wilson et al. 2002a). To determine whether this affected their virulence potential in vivo, mice were infected with Salmonella Typhimurium grown in rich media either under LSMMG or under normal gravity. Those infected with LSMMG-grown bacteria had a shorter time to death than those infected with normal gravity controls (Nickerson et al. 2000). In addition, the 50% lethal dose (LD50) for LSMMG-grown bacteria was 5.2-fold lower than for normal gravity-grown bacteria. To confirm this finding, the experiment was later repeated using bacteria grown terrestrially or aboard the Space Shuttle Atlantis, Mission STS-115. The LD50 for spaceflight-grown bacteria (∼4.5 × 106 CFU) was 2.7-fold less than for ground-based controls (∼1.2 × 107 CFU) (Wilson et al. 2007). Mice infected with spaceflight-grown bacteria also had decreased time to death and decreased survival at each dose tested. This experiment has been repeated yet again, with similar findings (6.9-fold decrease in LD50) (Wilson et al. 2008). However, as was seen with E. coli, the difference in phenotype, in this case virulence, could be obliterated by a change of media. In a parallel experiment to that mentioned above, growth of bacteria in minimal medium during spaceflight showed no increase in virulence in mice over normal gravity controls (Wilson et al. 2008). This suggests that there is complex interplay between the different virulence regulators, microgravity and availability of nutrients in Salmonella Typhimurium pathogenesis.
Researchers have also used an anti-orthostatic mouse model to investigate the contribution of LSMMG to Salmonella Typhimurium virulence. In this model, animals are suspended by their tails, causing fluid to shift towards the head and removing weight from the hind limbs. This simulates the effect of microgravity on the host. Mice infected with LSMMG-grown bacteria had significantly higher mortality rates than normal gravity controls across five different dosage levels (Chopra et al. 2006). This led to a 100-fold difference in LD50 between the two groups. Collectively, these data suggest that Salmonella Typhimurium is more virulent under microgravity-like conditions.
The contribution of virulence genes to Salmonella pathogenesis in microgravity in vivo was investigated aboard five spaceflight missions between 2008 and 2010 (Hammond et al. 2013). In these experiments, site-directed mutants of Salmonella Typhimurium were tested for virulence in the Caenorhabditis elegans model of infection. Four separate Salmonella Typhimurium mutants were analyzed: pipA, rhuM, hilA and hilD. Of these, hilA, pipA and rhuM showed significant attenuation in their ability to kill wild-type C. elegans larvae during spaceflight. While the rhuM mutant was also attenuated in ground controls, the other two mutants showed no attenuation when grown under normal gravity conditions. This is the first time that specific bacterial genes were shown to be involved in virulence, while both the bacteria and the host were in microgravity.
The hilA gene is a transcriptional activator of invasion genes located on Salmonella Pathogenicity Island 1 (SPI-1). It is regulated by an array of environmental signals including oxygen, osmolarity and pH (Bajaj et al. 1996). The pipA gene, located on SPI-5, has previously been associated with virulence in terrestrial Salmonella Dublin research. In this serovar, mutations in the pip operon, including pipA, were found to significantly decrease fluid secretion in a calf ileal loop model of gastroenteritis (Wood et al. 1998). The identification of microgravity responsive genes that are activated within the intestinal lumen is intriguing, as it is postulated that the brush border microvilli of the intestines and proximal renal tubules represent a site of microgravity-like low shear (Guo, Weinstein and Weinbaum 2000). It is thus possible that the bacteria can detect microgravity in order to perceive their location and then alter their gene expression patterns accordingly.
Several studies have examined the microgravity-sensing ability of bacteria in detail. In Salmonella Typhimurium, response to microgravity was shown to be independent of the common stress response mediated by the global regulator, rpoS (Wilson et al. 2002a). Instead, microgravity responses in Salmonella Typhimurium (also Staphylococcus aureus and Pseudomonas aeruginosa) were associated with expression of the small non-coding RNA (sRNA) chaperone Hfq (Wilson et al. 2007; Crabbé et al. 2010; Castro et al. 2011). This protein mediates binding of sRNAs to their mRNA targets, stabilizing the structure and facilitating post-translational regulation (Chao and Vogel 2010; De Lay, Schu and Gottesman 2013). In Salmonella, it has been suggested that Hfq is involved in the regulation of up to 20% of all genes (Sittka et al. 2008; Ansong et al. 2009). Relevant genes include those that are involved in the stress response, motility, growth and virulence. How the environmental signal is received by the cell and transmitted via Hfq is yet to be established.
Many other bacterial pathogens have also been studied in LSMMG. Pseudomonas aeruginosa is a nosocomial pathogen that is renowned for its ability to form a biofilm, allowing it to persist on surfaces, and form pellicles on the surface of liquids at the air–liquid interface (O'Toole and Kolter 1998). It commonly infects patients with cystic fibrosis (Davies 2002). By growing P. aeruginosa in LSMMG, researchers found an increase in biofilm factors (such as the extracellular matrix scaffold proteins encoded by the psl locus), in rhamnolipid production and in the rhl quorum-sensing system (Crabbé et al. 2008). The LSMMG-grown bacteria also showed similarities with P. aeruginosa isolated from the lungs of patients with cystic fibrosis, thus providing a novel model to study this interaction. Further experiments into the gene expression and regulation of LSMMG-grown bacteria found links to both Hfq, as described above, and the rpoE-like sigma factor AlgU (Crabbé et al. 2010). When similar gene expression studies were performed on spaceflight-grown bacteria, there was a significant overlap between genes upregulated in LSMMG and spaceflight. One of these genes was Hfq, confirming its involvement in the so-called microgravity regulon across multiple bacterial species. Other P. aeruginosa virulence determinants were also increased in spaceflight, including the lectins, lecA and lecB, which the bacteria use to bind to eukaryotic cells. Additional studies have found that P. aeruginosa biofilms grown during spaceflight have novel architecture and increased viability and biofilm biomass when compared to ground controls (Kim et al. 2013). These phenotypes have significant ramifications not only for ground-based research, but also for spaceflight missions, as P. aeruginosa has previously been isolated from the surfaces of water distribution systems on board the International Space Station (Bruce et al. 2005).
Another human pathogen that has shown an increase in biofilm formation and persistence under LSMMG is S. aureus. In RWV studies, S. aureus was found to decrease its growth rate and increase biofilm formation (Castro et al. 2011). This phenotype was also associated with the sRNA chaperone Hfq; however, here, Hfq was downregulated in LSMMG cultures. In addition to increasing their biofilm formation, the bacteria also downregulated virulence factors such as staphyloxanthin and several hemolysins (Rosado et al. 2010; Castro et al. 2011).
There is one other example of a bacterial pathogen that decreases its virulence upon growth in LSMMG, Yersinia pestis. Early studies with Y. pestis grown in LSMMG showed a downregulation in secretion of type III secretion system effectors, and hence a decrease in virulence in in vitro tissue culture cell assays (Lawal, Jejelowo and Rosenzweig 2010). However, this observation was not consistent with in vivo assays, where mice infected with either LSMMG or normal gravity-grown bacteria succumbed to infection at equivalent rates (Lawal et al. 2013). These studies with both S. aureus and Y. pestis, when compared to previously discussed pathogens such as Salmonella and Pseudomonas, clearly illustrate that the effects of microgravity on a given pathogen will undoubtedly depend on the particular pathogenic strategies and growth requirements of that pathogen.
HOST–PATHOGEN INTERACTIONS
As previously mentioned, the RWV was initially used primarily to generate 3D tissue models. The RWV environment is advantageous in tissue engineering, due largely to the extremely low shear conditions. This allows cells to aggregate (Dintenfass et al. 1986; Hymer et al. 1996), forming large, complex spheroids (organoids). The microgravity environment may also help to promote aggregation, as cells grown under microgravity conditions show higher levels of collagen made by human-derived fibroblasts and integrins and other adhesion molecules generated by splenocytes and lymphocytes (Grove, Pishak and Mastro 1995; Seitzer et al. 1995). This has allowed researchers to cultivate complex aggregates which recreate the structural diversity of living tissue, such as bone (Hwang et al. 2009), cardiac muscle (Freed and Vunjak-Novakovic 1997), gut (Nickerson et al. 2001; Salerno-Goncalves, Fasano and Sztein 2011; Sato et al. 2011) and liver (Zhang et al. 2014). While originally designed to be used in organ transplantation, these organoid models are becoming increasingly used in the areas of host–pathogen interactions.
Organoids have several advantages over other host–pathogen models. First, they are more complex than traditional cell culture monolayers, allowing for more natural interactions between host cells and pathogens. They are also easier to obtain and work with, as opposed to obtaining biopsy material. Finally, they have advantages over animal models in that the cells are human derived and so they better model human–pathogen interactions. A list of organoid models developed to model host–pathogen interactions is shown in Table 2.
Table 2.
Organoid models developed for pathogen infection studies.
| Tissue | Cell type(s) | Pathogen | Reference |
|---|---|---|---|
| Bladder | Human bladder 5637 cells | UPEC | Smith et al. (2006) |
| Lung | A549 lung epithelial cells | P. aeruginosa | Carterson et al. (2005) |
| Lung | A549 lung epithelial cells | Francisella tularensis | David, Sayer and Sarkar-Tyson (2014) |
| Gut | HCT-8 small intestinal epithelial cells | Cryptosporidium parvum | Warren et al. (2008) |
| Gut | HCT-8 small intestinal epithelial cells | EHEC and EPEC | Carvalho et al. (2005) |
| Gut | Int-407 small intestinal epithelial cells | Salmonella Typhimurium | Nickerson et al. (2001) |
| Gut | HCT-8 small intestinal epithelial cells, activated lymphocytes, fibroblasts, endothelial cells | Salmonella Typhi | Salerno-Goncalves, Fasano and Sztein (2011) |
| Gut | HT-29 colorectal epithelial cells | Salmonella Typhimurium | Honer Zu Bentrup et al. (2006) |
| Gut | Caco-2 colorectal epithelial cells | Coxsackievirus B | Drummond, Nickerson and Coyne (2016) |
The majority of work on RWV-developed organoids for host–pathogen interactions has been performed using cells from the gastrointestinal tract. Several authors have shown that culture of immortalized gut epithelial cells in the RWV allows for the differentiation of cells into multiple lineages, including enterocytes, M cells, goblet cells and Paneth cells (Nickerson et al. 2001; Honer Zu Bentrup et al. 2006; Salerno-Goncalves, Fasano and Sztein 2011; Drummond, Nickerson and Coyne 2016). This has been done with cells of the small intestine, such as Int-407 and HCT-8 (Nickerson et al. 2001; Carvalho et al. 2005; Warren et al. 2008; Salerno-Goncalves, Fasano and Sztein 2011), as well as colorectal cell lines Caco-2 and HT-29 (Honer Zu Bentrup et al. 2006; Drummond, Nickerson and Coyne 2016). Not only do cells differentiate into multiple cell types, but they also show other markers of human gut tissue, such as appropriate cell polarization and organization, tight junction formation, apical brush border microvilli and localized mucus secretion. Interestingly, cells grown in LSMMG also show a decrease in tumor markers when compared to cells grown in monolayers (Nickerson et al. 2001). These gut organoids, therefore, represent a novel method for investigation of pathogen virulence, which is more complex and biologically relevant than cell monolayers, while still being derived from human cells.
Infection of gut organoids has been carried out with bacterial pathogens including Salmonella enterica and Escherichia coli, parasites such as Cryptosporidium parvum, as well as the viral pathogen Coxsackievirus B (Nickerson et al. 2001; Carvalho et al. 2005; Honer Zu Bentrup et al. 2006; Warren et al. 2008; Salerno-Goncalves, Fasano and Sztein 2011; Drummond, Nickerson and Coyne 2016). These infections can take place in the RWV under LSMMG, but it is more common for organoids to be removed from the RWV and infected under normal cell culture conditions. Upon infection with Salmonella Typhimurium, organoids were found to be more resistant to bacterial invasion and less prone to apoptosis than infected cell monolayers (Nickerson et al. 2001; Honer Zu Bentrup et al. 2006). Over the course of infection, organoids showed characteristics similar to the normal gut infection process, including membrane ruffling, cytokine production and sloughing of infected enterocytes (Honer Zu Bentrup et al. 2006; Salerno-Goncalves, Fasano and Sztein 2011). Interestingly, a Salmonella Typhimurium type III secretion mutant ΔinvA, which is known to be involved in invasion in vitro and attenuated for virulence in mice, showed no defect in invasion in the organoid model (Honer Zu Bentrup et al. 2006). This suggests a more complex role for the InvA protein in virulence and a novel invA-independent mechanism used by Salmonella to invade the gut epithelium.
A similar contradiction was observed in organoids infected with two E. coli pathotypes: EPEC and EHEC. Both were able to produce the characteristic attaching and effacing (A/E) lesions by expression of the intimin protein; however, an EPEC intimin mutant (Δeae) was unable to adhere to cells (Carvalho et al. 2005). The prevailing dogma states that typical EPEC adhere to the epithelium by using their bundle-forming pili (BFP). Intimin is then expressed to mediate intimate attachment to host cells. This has been shown in tissue culture adherence assays, where BFP mutants are completely non-adherent, while intimin mutants show normal patterns of adherence (Cleary et al. 2004). The results of this experiment are therefore at odds with the current model of EPEC adherence, and may help to resolve outstanding issues surrounding the necessity of BFP and the virulence potential of atypical EPEC, which are BFP negative. The organoid model may therefore enable investigators to refine existing theories and bridge the gap between traditional cell culture and animal models.
The lung organoid model has been used for multiple pathogens, including Pseudomonas aeruginosa and Francisella tularensis. A549 lung epithelial cells grown under LSMMG conditions in the RWV undergo appropriate differentiation, form tight junctions and secrete mucus in a localized fashion similar to natural tissue (Carterson et al. 2005; David, Sayer and Sarkar-Tyson 2014). Transcriptomics on organoid-grown cells also showed greater levels of cell signaling, membrane trafficking and extracellular matrix transcripts when compared to monolayers, suggesting a more in vivo-like transcriptome (David, Sayer and Sarkar-Tyson 2014). As with the Salmonella-infected gut organoids, lung organoids proved more resistant to infection with P. aeruginosa than conventional tissue culture-grown cells (Carterson et al. 2005). Upon P. aeruginosa infection, cytokine secretion from organoids was found to be better regulated than that in monolayers, as evidenced by the induction of anti-inflammatory cytokines such as IL-10 (Carterson et al. 2005). This suggests that the cellular differentiation and transcriptional changes induced in the LSMMG environment allow for a more refined response to bacterial challenge, which better mimics natural infection.
Finally, virulence of uropathogenic E. coli (UPEC) has been investigated using an organoid bladder model. By using the uroepithelial cell line 5637, researchers created organoids that strongly resembled bladder tissue, as indicated by their expression of tight junction, cell adhesion and urothelium differentiation markers (Smith et al. 2006). As with the gut model, organoids infected with UPEC showed characteristics of in vivo infection, such as exfoliation of surface cells, which is consistent with responses seen in the mouse cystitis model (Mulvey et al. 1998).
APPLICATIONS IN RESEARCH AND MANUFACTURING OF VACCINES AND THERAPEUTICS
As discussed above, microgravity and LSMMG have already had a notable impact on the field of tissue engineering, as well as beginning to shed light on the influence of gravity on pathogenicity and host–pathogen interactions. In the future, this technology has the capacity to be implemented in the search for therapeutics and preventatives against infectious diseases.
Vaccine design
The identification of novel virulence genes in microgravity offers a variety of new targets for live-attenuated vaccine design. Conventionally, attenuating mutations are often chosen for their ability to slow or inhibit growth, thus decreasing virulence. By identifying virulence genes that are influenced by microgravity (e.g. when closely adhering to the gut microvilli), we may be able to more specifically attenuate bacteria. This approach could lead to the development of live-attenuated vaccine strains that are more metabolically fit and thus more immunogenic than strains that have mutations targeting only metabolic genes.
Live vector vaccines could also potentially be improved by integrating information gleaned from space experiments. These vaccines differ from live-attenuated vaccines in that they use an attenuated strain to express foreign antigens from an unrelated pathogen. One example of this is the Bacillus anthracis protective antigen from anthrax toxin expressed in an attenuated strain of Salmonella Typhi (Galen et al. 2004). These vaccines have the advantage of being able to stimulate multiple arms of the immune system. There has been a recent shift within the field of live vector vaccines from plasmid-encoded to chromosomal expression of antigens in order to decrease the metabolic burden on bacteria (Galen and Levine 2001). In this scenario, expression of immunogenic foreign antigens is spread out over the chromosome by integrating the gene of choice at several locations. These integrated genes are controlled both by a constitutive promoter and the native promoter of the gene in which the foreign gene has been inserted. By integrating the genes in this manner, one or more foreign antigens can be expressed from different loci at various times. This approach has been used successfully for the expression of the Yersinia pestis F1 capsule and LcrV protective antigens within an attenuated Salmonella Typhi live vector strain (Galen et al. 2015). The advantage of this method is that the bacteria are not overwhelmed by the simultaneous activation and transcription of potentially toxic foreign antigen(s), but can alternate antigen expression as the individual promoters are stimulated during distinct phases of growth. Microgravity-responsive promoters could prove to be excellent new genetic tools for Salmonella and other enteric bacterial live-vector vaccines, due to their specific activation within the gut microvilli environment. By using microgravity-responsive promoters to regulate gene expression, antigens could be expressed specifically upon arrival at the intestinal mucosa, thus providing direct access of the antigen to the mucosal immune system.
Therapeutics research
As well as using microgravity-responsive promoters to alternately regulate foreign antigen expression, microgravity-influenced regulation could also be exploited in the development of novel mucosal therapeutics. If we could identify compounds capable of disrupting a microgravity-sensitive global regulator, then we could potentially inhibit the ability of bacteria to sense their local environment and activate virulence genes. Proof-of-principle experiments using this approach on other global regulators have been completed using the pathogenic Escherichia coli model organism Citrobacter rodentium and also Vibrio cholerae. For Ci. rodentium, mice treated with Regicin, a novel inhibitor compound with activity against the global regulator RegA, showed significant improvement in disease severity when compared to control mice (Yang et al. 2013). When applying this strategy to V. cholerae, researchers used virstatin, an inhibitor of ToxT, to inhibit production of two major virulence determinants (cholera toxin and the toxin coregulated pilus) and protect mice from intestinal colonization (Hung et al. 2005). This intriguing strategy could be extended to other bacteria that show an increase in virulence in response to microgravity.
CONCLUSION
The accessibility of spaceflight and the LSMMG conditions of the RWV is allowing researchers from across the biological sciences to search for medical breakthroughs in previously unanticipated new directions, investigating potential links between microgravity, cell biology and pathogenicity that may have important implications not only for manned spaceflight, but also in a range of medical applications. Further explorations of the effects of microgravity on cell biology will undoubtedly open up new avenues of research and development in biotechnology to investigators, which could in turn have unforeseen benefits to the prevention and treatment of disease both on Earth and in space.
FUNDING
This work was supported in part by the National Aeronautics and Space Administration (grant number NNX13AN80G to MML).
Conflict of interest. None declared.
REFERENCES
- Ansong C, Yoon H, Porwollik S, et al. Global systems-level analysis of Hfq and SmpB deletion mutants in Salmonella: implications for virulence and global protein translation. PLoS One. 2009;4:e4809. doi: 10.1371/journal.pone.0004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunasri K, Adil M, Charan KV, et al. Effect of simulated microgravity on E. coli K12 MG1655 growth and gene expression. PLoS One. 2013;8:e57860. doi: 10.1371/journal.pone.0057860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj V, Lucas RL, Hwang C, et al. Co-ordinate regulation of Salmonella Typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–14. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- Benoit MR, Klaus DM. Microgravity, bacteria, and the influence of motility. Adv Space Res. 2007;39:1225–32. [Google Scholar]
- Bruce RJ, Ott CM, Skuratov VM, et al. SAE Technical Paper 2005-01-2886. SAE International; 2005. Microbial surveillance of potable water sources of the International Space Station. [Google Scholar]
- Carterson A, zu Bentrup KH, Ott C, et al. A549 lung epithelial cells grown as three-dimensional aggregates: alternative tissue culture model for Pseudomonasaeruginosa pathogenesis. Infect Immun. 2005;73:1129–40. doi: 10.1128/IAI.73.2.1129-1140.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho HM, Teel LD, Goping G, et al. A three-dimensional tissue culture model for the study of attach and efface lesion formation by enteropathogenic and enterohaemorrhagic Escherichia coli. Cell Microbiol. 2005;7:1771–81. doi: 10.1111/j.1462-5822.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- Castro SL, Nelman-Gonzalez M, Nickerson CA, et al. Induction of attachment-independent biofilm formation and repression of hfq expression by low-fluid-shear culture of Staphylococcus aureus. Appl Environ Microb. 2011;77:6368–78. doi: 10.1128/AEM.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Vogel J. The role of Hfq in bacterial pathogens. Curr Opin Microbiol. 2010;13:24–33. doi: 10.1016/j.mib.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Chopra V, Fadl A, Sha J, et al. Alterations in the virulence potential of enteric pathogens and bacterial–host cell interactions under simulated microgravity conditions. J Toxicol Env Heal A. 2006;69:1345–70. doi: 10.1080/15287390500361792. [DOI] [PubMed] [Google Scholar]
- Cleary J, Lai L-C, Shaw RK, et al. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology. 2004;150:527–38. doi: 10.1099/mic.0.26740-0. [DOI] [PubMed] [Google Scholar]
- Crabbé A, De Boever P, Van Houdt R, et al. Use of the rotating wall vessel technology to study the effect of shear stress on growth behaviour of Pseudomonasaeruginosa PA01. Environ Microbiol. 2008;10:2098–110. doi: 10.1111/j.1462-2920.2008.01631.x. [DOI] [PubMed] [Google Scholar]
- Crabbé A, Pycke B, Van Houdt R, et al. Response of Pseudomonas aeruginosa PAO1 to low shear modelled microgravity involves AlgU regulation. Environ Microbiol. 2010;12:1545–64. doi: 10.1111/j.1462-2920.2010.02184.x. [DOI] [PubMed] [Google Scholar]
- Crabbé A, Schurr MJ, Monsieurs P, et al. Transcriptional and proteomic responses of Pseudomonas aeruginosa PAO1 to spaceflight conditions involve Hfq regulation and reveal a role for oxygen. Appl Environ Microbiol. 2011;77:1221–30. doi: 10.1128/AEM.01582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J, Sayer NM, Sarkar-Tyson M. The use of a three-dimensional cell culture model to investigate host–pathogen interactions of Francisellatularensis in human lung epithelial cells. Microbes Infect. 2014;16:735–45. doi: 10.1016/j.micinf.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Davies JC. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev. 2002;3:128–34. doi: 10.1016/s1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Davis TA, Wiesmann W, Kidwell W, et al. Effect of spaceflight on human stem cell hematopoiesis: suppression of erythropoiesis and myelopoiesis. J Leukocyte Biol. 1996;60:69–76. doi: 10.1002/jlb.60.1.69. [DOI] [PubMed] [Google Scholar]
- De Boever P, Mergeay M, Ilyin V, et al. Conjugation-mediated plasmid exchange between bacteria grown under space flight conditions. Microgravity Sci Tec. 2007;19:138–44. [Google Scholar]
- De Lay N, Schu DJ, Gottesman S. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J Biol Chem. 2013;288:7996–8003. doi: 10.1074/jbc.R112.441386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintenfass L, Osman P, Maguire B, et al. Experiment on aggregation of red cells under microgravity on STS 51-C. Adv Space Res. 1986;6:81–84. doi: 10.1016/0273-1177(86)90187-0. [DOI] [PubMed] [Google Scholar]
- Drummond CG, Nickerson CA, Coyne CB. A three-dimensional cell culture model to study enterovirus infection of polarized intestinal epithelial cells. mSphere. 2016;1:e00030-15. doi: 10.1128/mSphere.00030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed LE, Vunjak-Novakovic G. Microgravity tissue engineering. In Vitro Cell Dev-An. 1997;33:381–5. doi: 10.1007/s11626-997-0009-2. [DOI] [PubMed] [Google Scholar]
- Galen JE, Levine MM. Can a ‘flawless’ live vector vaccine strain be engineered? Trends Microbiol. 2001;9:372–6. doi: 10.1016/s0966-842x(01)02096-0. [DOI] [PubMed] [Google Scholar]
- Galen JE, Wang JY, Carrasco JA, et al. A bivalent typhoid live vector vaccine expressing both chromosome- and plasmid-encoded Yersinia pestis antigens fully protects against murine lethal pulmonary plague infection. Infect Immun. 2015;83:161–72. doi: 10.1128/IAI.02443-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen JE, Zhao L, Chinchilla M, et al. Adaptation of the endogenous Salmonella enterica serovar Typhi clyA-encoded hemolysin for antigen export enhances the immunogenicity of anthrax protective antigen domain 4 expressed by the attenuated live-vector vaccine strain CVD 908-htrA. Infect Immun. 2004;72:7096–106. doi: 10.1128/IAI.72.12.7096-7106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Fang A, Pierson D, et al. Shear stress enhances microcin B17 production in a rotating wall bioreactor, but ethanol stress does not. Appl Microbiol Biot. 2001;56:384–7. doi: 10.1007/s002530100610. [DOI] [PubMed] [Google Scholar]
- Gasset G, Tixador R, Eche B, et al. Growth and division of Escherichia coli under microgravity conditions. Res Microbiol. 1994;145:111–20. doi: 10.1016/0923-2508(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Goodwin TJ, Jessup JM, Wolf DA. Morphological differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating wall vessels. In Vitro Cell -An. 1992;28:47–60. doi: 10.1007/BF02631079. [DOI] [PubMed] [Google Scholar]
- Grove DS, Pishak SA, Mastro AM. The effect of a 10-day space flight on the function, phenotype, and adhesion molecule expression of splenocytes and lymph node lymphocytes. Exp Cell Res. 1995;219:102–9. doi: 10.1006/excr.1995.1210. [DOI] [PubMed] [Google Scholar]
- Guo P, Weinstein A, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol-Renal. 2000;279:F698–712. doi: 10.1152/ajprenal.2000.279.4.F698. [DOI] [PubMed] [Google Scholar]
- Hammond TG, Benes E, O'Reilly KC, et al. Mechanical culture conditions effect gene expression: gravity-induced changes on the space shuttle. Physiol Genomics. 2000;3:163–73. doi: 10.1152/physiolgenomics.2000.3.3.163. [DOI] [PubMed] [Google Scholar]
- Hammond TG, Stodieck L, Birdsall HH, et al. Effects of microgravity on the virulence of Salmonella toward Caenorhabditis elegans. New Space. 2013;1:123–31. [Google Scholar]
- Honer Zu Bentrup K, Ramamurthy R, Ott C, et al. Three-dimensional organotypic models of human colonic epithelium to study the early stages of enteric salmonellosis. Microbes Infect. 2006;8:1813–25. doi: 10.1016/j.micinf.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Hung DT, Shakhnovich EA, Pierson E, et al. Small-molecule inhibitor of Vibriocholerae virulence and intestinal colonization. Science. 2005;310:670–4. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- Hwang Y-S, Cho J, Tay F, et al. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30:499–507. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Hymer W, Grindeland R, Salada T, et al. Experimental modification of rat pituitary growth hormone cell function during and after spaceflight. J Appl Physiol. 1996;80:955–70. doi: 10.1152/jappl.1996.80.3.955. [DOI] [PubMed] [Google Scholar]
- Ichiki A, Gibson L, Jago T, et al. Effects of spaceflight on rat peripheral blood leukocytes and bone marrow progenitor cells. J Leukocyte Biol. 1996;60:37–43. doi: 10.1002/jlb.60.1.37. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Ohira Y, Roy R, et al. Effects of 14 days of spaceflight and nine days of recovery on cell body size and succinate dehydrogenase activity of rat dorsal root ganglion neurons. Neuroscience. 1997;81:275–9. doi: 10.1016/s0306-4522(97)00097-3. [DOI] [PubMed] [Google Scholar]
- Kacena M, Merrell G, Manfredi B, et al. Bacterial growth in space flight: logistic growth curve parameters for Escherichiacoli and Bacillus subtilis. Appl Microbiol Biot. 1999;51:229–34. doi: 10.1007/s002530051386. [DOI] [PubMed] [Google Scholar]
- Kim W, Tengra FK, Young Z, et al. Spaceflight promotes biofilm formation by Pseudomonas aeruginosa. PLoS One. 2013;8:e62437. doi: 10.1371/journal.pone.0062437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus D, Simske S, Todd P, et al. Investigation of space flight effects on Escherichiacoli and a proposed model of underlying physical mechanisms. Microbiology. 1997;143:449–55. doi: 10.1099/00221287-143-2-449. [DOI] [PubMed] [Google Scholar]
- Klaus DM. Clinostats and bioreactors. Gravitational Space Res. 2007;14:55–64. [PubMed] [Google Scholar]
- Lawal A, Jejelowo OA, Rosenzweig JA. The effects of low-shear mechanical stress on Yersiniapestis virulence. Astrobiology. 2010;10:881–8. doi: 10.1089/ast.2010.0493. [DOI] [PubMed] [Google Scholar]
- Lawal A, Kirtley ML, van Lier CJ, et al. The effects of modeled microgravity on growth kinetics, antibiotic susceptibility, cold growth, and the virulence potential of a Yersinia pestis ymoA-deficient mutant and its isogenic parental strain. Astrobiology. 2013;13:821–32. doi: 10.1089/ast.2013.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S, Brodie E, Matin A. Role and regulation of σs in general resistance conferred by low-shear simulated microgravity in Escherichia coli. J Bacteriol. 2004;186:8207–12. doi: 10.1128/JB.186.24.8207-8212.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S, Mukundakrishnan K, Benoit M, et al. Escherichia coli biofilms formed under low-shear modeled microgravity in a ground-based system. Appl Environ Microb. 2006;72:7701–10. doi: 10.1128/AEM.01294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RJ, Cassanto JM, Barnes MB, et al. Bacterial biofilm formation under microgravity conditions. FEMS Microbiol Lett. 2001;195:115–9. doi: 10.1111/j.1574-6968.2001.tb10507.x. [DOI] [PubMed] [Google Scholar]
- Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic. Escherichia coli. Science. 1998;282:1494–7. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- Nauman EA, Ott CM, Sander E, et al. Novel quantitative biosystem for modeling physiological fluid shear stress on cells. Appl Environ Microb. 2007;73:699–705. doi: 10.1128/AEM.02428-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson CA, Goodwin TJ, Terlonge J, et al. Three-dimensional tissue assemblies: novel models for the study of Salmonella enterica serovar Typhimurium pathogenesis. Infect Immun. 2001;69:7106–20. doi: 10.1128/IAI.69.11.7106-7120.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson CA, Ott CM, Mister SJ, et al. Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect Immun. 2000;68:3147–52. doi: 10.1128/iai.68.6.3147-3152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonasaeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Rosado H, Doyle M, Hinds J, et al. Low-shear modelled microgravity alters expression of virulence determinants of Staphylococcus aureus. Acta Astronaut. 2010;66:408–13. [Google Scholar]
- Salerno-Goncalves R, Fasano A, Sztein MB. Engineering of a multi-cellular organotypic model of the human intestinal mucosa. Gastroenterology. 2011;141:e18. doi: 10.1053/j.gastro.2011.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–72. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Seitzer U, Bodo M, Müller PK, et al. Microgravity and hypergravity effects on collagen biosynthesis of human dermal fibroblasts. Cell Tissue Res. 1995;282:513–7. doi: 10.1007/BF00318883. [DOI] [PubMed] [Google Scholar]
- Sittka A, Lucchini S, Papenfort K, et al. Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLos Genet. 2008;4:e1000163. doi: 10.1371/journal.pgen.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YC, Grande KK, Rasmussen SB, et al. Novel three-dimensional organoid model for evaluation of the interaction of uropathogenic Escherichiacoli with terminally differentiated human urothelial cells. Infect Immun. 2006;74:750–7. doi: 10.1128/IAI.74.1.750-757.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli M. Anemia of spaceflight. Blood. 1982;60:1059–67. [PubMed] [Google Scholar]
- Taylor GR, Janney RP. In vivo testing confirms a blunting of the human cell-mediated immune mechanism during space flight. J Leukocyte Biol. 1992;51:129–32. doi: 10.1002/jlb.51.2.129. [DOI] [PubMed] [Google Scholar]
- Tucker D, Ott C, Huff S, et al. Characterization of Escherichiacoli MG1655 grown in a low-shear modeled microgravity environment. BMC Microbiol. 2007;7:15. doi: 10.1186/1471-2180-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JM. Bone mineral measurement: Skylab experiment M-078. Acta Astronaut. 1975;2:129–39. doi: 10.1016/0094-5765(75)90049-1. [DOI] [PubMed] [Google Scholar]
- Vukanti R, Mintz E, Leff L. Changes in gene expression of E. coli under conditions of modeled reduced gravity. Microgravity Sci Tec. 2008;20:41–57. [Google Scholar]
- Warren CA, Destura RV, Sevilleja JEAD, et al. Detection of epithelial cell injury and quantification of infection in the HCT8 organoid model of Cryptosporidiosis. J Infect Dis. 2008;198:143–9. doi: 10.1086/588819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JW, Ott CM, Höner zu Bentrup K, et al. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. P Natl Acad Sci USA. 2007;104:16299–304. doi: 10.1073/pnas.0707155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JW, Ott CM, Quick L, et al. Media ion composition controls regulatory and virulence response of Salmonella in spaceflight. PLoS One. 2008;3:e3923. doi: 10.1371/journal.pone.0003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JW, Ott CM, Ramamurthy R, et al. Low-shear modeled microgravity alters the Salmonella enterica serovar Typhimurium stress response in an RpoS-independent manner. Appl Environ Microb. 2002a;68:5408–16. doi: 10.1128/AEM.68.11.5408-5416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JW, Ramamurthy R, Porwollik S, et al. Microarray analysis identifies Salmonella genes belonging to the low-shear modeled microgravity regulon. P Natl Acad Sci USA. 2002b;99:13807–12. doi: 10.1073/pnas.212387899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Schwarz RP. NASA Technical Paper 3143. Houston, TX: 1991. Analysis of gravity-induced particle motion and fluid perfusion flow in the NASA-designed rotating zero-head-space tissue culture vessel. [Google Scholar]
- Wood MW, Jones MA, Watson PR, et al. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol Microbiol. 1998;29:883–91. doi: 10.1046/j.1365-2958.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Hocking DM, Cheng C, et al. Disarming bacterial virulence through chemical inhibition of the DNA binding domain of an AraC-like transcriptional activator protein. J Biol Chem. 2013;288:31115–26. doi: 10.1074/jbc.M113.503912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang B, Chen X, et al. Three-dimensional culture in a microgravity bioreactor improves the engraftment efficiency of hepatic tissue constructs in mice. J Mater Sci: Mater M. 2014;25:2699–709. doi: 10.1007/s10856-014-5279-0. [DOI] [PubMed] [Google Scholar]