Abstract
The recognition and phagocytosis of free-swimming (planktonic) bacteria by polymorphonuclear neutrophils have been investigated in depth. However, less is known about the neutrophil response towards bacterial biofilms. Our previous work demonstrated that neutrophils recognize activating entities within the extracellular polymeric substance (EPS) of biofilms (the bacterial heat shock protein GroEL) and that this process does not require opsonization. Aim of this study was to evaluate the release of DNA by neutrophils in response to biofilms, as well as the release of the inflammatory cytokine MRP-14. Neutrophils were stimulated with Staphylococcus epidermidis biofilms, planktonic bacteria, extracted EPS and GroEL. Release of DNA and of MRP-14 was evaluated. Furthermore, tissue samples from patients suffering from biofilm infections were collected and evaluated by histology. MRP-14 concentration in blood samples was measured. We were able to show that biofilms, the EPS and GroEL induce DNA release. MRP-14 was only released after stimulation with EPS, not GroEL. Histology of tissue samples revealed MRP-14 positive cells in association with neutrophil infiltration and MRP-14 concentration was elevated in blood samples of patients suffering from biofilm infections. Our data demonstrate that neutrophil-activating entities are present in the EPS and that GroEL induces DNA release by neutrophils.
Keywords: bacterial heat shock protein, GroEL, MRP-14, biofilms, neutrophils, extracellular polymeric substance
We were able to identify the bacterial heat shock protein GroEL as a neutrophil-activating entity within the biofilm matrix and for the first time demonstrate its effect on DNA release by neutrophils.
INTRODUCTION
Polymorphonuclear neutrophils (PMN) are considered the first line of defence in bacterial infection. Chemotaxis towards infectious sites, phagocytosis and killing of bacteria are key events, as is release of potentially cytotoxic and bactericidal entities. (for review see Witko-Sarsat et al.2000; Underhill and Ozinsky 2002; Segal 2005).
The majority of data are derived from experiments with free-swimming (planktonic) bacteria, the response of neutrophils to bacterial biofilms is less understood, although there are data showing infiltration of PMN into sites of biofilm infection (Wagner et al.2003; Gaida et al.2012; Dapunt et al.2014). Moreover, in vitro studies show susceptibility of bacterial biofilms to killing by PMN (Leid et al.2002; Guenther et al.2009; Stroh et al.2011).
Efficient activation of PMN requires recognition of the bacterial target. For planktonic bacteria ‘opsonization’—the coating with antibody and complement C3—leads to an optimal ensuing triggering of PMN via the corresponding receptors Fc-gamma and the complement receptors CR1 or CR3. (reviewed in Witko-Sarsat et al.2000; Underhill and Ozinsky 2002; Segal 2005).
Our previous studies with staphylococcal biofilms, however, revealed that PMN attach to biofilms also in the absence of antibodies and complement, and also that phagocytosis of biofilms by PMN did not require opsonization (Stroh et al.2011). These findings lead to the question, how PMN recognize biofilms and how activation of cells ensues. In that context, we discovered that the extracellular polymeric substance (EPS) of Staphylococcus epidermidis contained activating entities (Meyle et al.2012).
In addition to the well-characterized entities within the EPS that have the potential to activate PMN, such as lipoteichoic acid (LTA) or peptidoglycan, we identified the bacterial heat shock protein GroEL as a possible candidate (Maurer et al.2015).
GroEL is the bacterial homologue of the human heat shock protein (HSP) 60, and shares with HSP60 structural features as well as biological activities (Argueta et al.2006; Davies et al.2006; Osterloh et al.2009).
In this study, we analysed effects of EPS and of GroEL on PMN with the focus on release of the multifunctional cytokine MRP-14 (S100A9/calgranulin) because MRP-14 is generated during implant-associated infection, a prototype of biofilm infection (Dapunt et al.2015). Moreover, we looked for release of DNA, because there is the notion that neutrophils form DNA nets in response to infection, and that this ‘netosis’ is a further means to control bacterial infection (Remijsen et al.2011; Brinkmann and Zychlinsky 2012).
MATERIALS AND METHODS
Isolation of neutrophils (PMN)
Peripheral blood from healthy human volunteers was collected in heparin-coated tubes (Sarstedt, Nümbrecht, Germany). Neutrophils were isolated by centrifugation on PolymorphPrep (Axis-Shield PoC AS, Oslo, Norway). Neutrophils were suspended in Hanks balanced salt solution (HBSS) with 0.5% bovine serum albumin (Sigma-Aldrich, Munich, Germany), and characterized by cytofluorometry using CD66b expression as marker for PMN. The local ethic committee approved the use of peripheral blood from healthy individuals for this study; informed consent was obtained from the volunteers, and the institutional guidelines were observed.
Generation of S. epidermidis biofilms and extraction of EPS
Staphylococcus epidermidis (strain RP64A; purchased from ATCC, No. 35 984, Manassas, VA, USA) was added to 1.5 L of pre-warmed Trypticase Soy Broth to reach a final density of 3 × 106 CFU mL−1, then transferred to 30 polysterol dishes (Nunc 150 × 20, Thermo Fisher Scientific, Roskilde, Denmark) with a final volume of 50 mL per dish. After incubation for 2 days at 37°C without shaking, the medium was removed and the remaining biofilm was scrapped off. The following treatment was adapted from Liu and Fang (2002): per 10 mL of slime, 60 μL of 37% formaldehyde was added and mixed for 1 h at 4°C, followed by the addition of 4 mL 1 M NaCl and mixing for another 3 h at 4°C. The resulting suspension was then centrifuged (Sorvall 5B Plus) for 15 min (18 000 rpm at 4°C). The pellet was discarded, the supernatant filtrated (Millex Syringe-driven Filter Unit 0.22 μm, Merck Millipore Ltd, Tullagreen, Ireland) and then dialysed overnight against Millipore water at 4°C (membrane cut off 3600 Da; Spectrum Labs, Rancho Dominguez, CA, USA). The water was replaced and the isolated EPS was again dialysed for another 3 h, then concentrated using Vivaspin 20 (Sartorius Stedim Biotech, Göttingen, Germany) to a final volume of 4 mL. Proteins were precipitated using trichloroacetic acid, resuspended in water, dialysed against HBSS without BSA and frozen at –20°C until use. To avoid contamination of the preparation, only sterile equipment and materials were used.
Limulus assay and adsorption of lipopolysaccharides
To detect and to eliminate possible contamination by lipopolysaccharides (LPS) which might have occurred during the isolation procedure, the Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, Bonn, Germany) was used following the instructions provided by the manufacturer. The adsorption of LPS was accomplished using Pierce High Capacity Endotoxin Removal Spin Column following the instructions provided but adjusting the incubation time to 2 h in order to maximize LPS removal.
Stimulants
Recombinant GroEL and DnaK were purchased from Enzo Life Sciences, Lörrach, Germany, LTA and phorbol ester (PMA) from Sigma, Munich, Germany.
SDS-PAGE and western blotting
A Lämmli system was used with 9% or 12% bisacrylamid-gels. Of EPS extracts, 25 μL were mixed with 5 μL 5× loading buffer and incubated at 95°C for 10 min. Then 25 μL of the sample were applied. After separation, the samples were blotted to a PVDF membrane (Millipore, Eschborn, Germany). Anti-GroEL and anti-DnaK were added overnight, as secondary antibody peroxidase-labelled anti-mouse IgG (Jackson Immuno Research, Pennsylvania, USA) was used. The reaction was visualized with ECL Prime Western Blot Detection Reagent (GE Healthcare, Buckinghamshire, UK).
Binding of biotinylated EPS to isolated PMN using crosslinker and sepharose beads
PMN were isolated as described and 5%–20% EPS-Biotin or 5%–20% EPS was added. Samples were placed for 30 min at 4°C or 37°C on the tumbling shaker (Reax 3, Heidolp, Schwabach, Germany), after that 35 μL 100 mM bis(sulfosuccinimidyl) suberate (BS)-Crosslinker (No-Weigh Format, Thermo Scientific, Rockford, IL, USA) was added, followed by 30 min on the tumbling shaker at room temperature. A total of 10 μL 10 mM tris-solution was added, followed by 15 min on the tumbling shaker at room temperature. Samples were washed three times in PBS (centrifuged for 5 min at 2200 rpm). Membrane extraction was performed using ProteoExtract Transmembrane Protein Extraction Kit (Novagen, Merck Millipore, Darmstadt, Germany). The protocols provided by the manufacturer were followed. For each sample 25 μL streptavidin sepharose beads (Cell Signaling, Beverly, Massachusetts, USA) were washed in PBS twice (10 min, 4°C, 5000 rpm). Membrane fractions were centrifuged (15 min, 4°C, 15 000 rpm) and supernatants were incubated with the beads for 2 h at 4°C on a shaker. The beads were centrifuged (5000 rpm, 10 min, 4°C). This process was repeated three times and pellets were first resuspended in 500 μL 0.5 M sodium chloride, then in 500 μL 0.1% tween solution and finally in 500 μL PBS. The beads were taken up in 60 μL 2× Lämmli-buffer, incubated for 10 min at 95°C and centrifuged (14 000 rom, 4°C, 1 min). Supernatants were stored at 4°C. The following day western blotting was performed.
Experimental protocols for cell activation
Activation of neutrophils by biofilms
Biofilms were cultivated in 6-well dishes for 2 days. Then non-adherent bacteria were removed by gently washing the wells with HBSS. For opsonization, the biofilm was incubated for 20 min with normal human serum (pooled from five healthy donors and incubated for 30 min at 56°C to prevent complement activation) in a final concentration of 10% (v/v). Following washing, neutrophils (5 × 106/well in 2 mL HBSS) were added. After various times (1–24 h), supernatants were harvested and used to determine DNA release and release of MRP-14.
Activation of neutrophils by planktonic bacteria
The bacteria were suspended in HBSS (109/mL) and for opsonization incubated for 20 min with heat-inactivated normal human serum (10% v/v), then washed and added to neutrophils at different ratios ranging from 20 to 100 bacteria per neutrophil. Following incubation for 1 h, the neutrophils were washed to remove bacteria that had not been ingested. Vancomycin (200 μg mL−1) was added to kill adherent, but not ingested bacteria. Then cell culture was continued for 3–24 h. Supernatants were harvested to determine DNA and MRP-14 release.
Activation of neutrophils by EPS, GroEL or DnaK
Neutrophils were placed in 24-well dishes (1–2 × 106 cells in 2 mL HBSS). EPS was added to yield a final concentration of 0.5%–10% (v/v). Of GroEL and DnaK, 1–5 μg mL−1 were used. For comparison, PMA (1 mg mL−1) and lipoteichoic acid (LTA 0.1 and 1 μg mL−1) were used. Following incubation for up to 24 h, supernatants were harvested.
Quantification of DNA and MRP-14
Commercially available kits were used (Quant-iT Pico Green DNA Assay Kit, Life Technologies, Darmstadt, Germany; MRP-14 Elisa Kit, Biozol, Eching, Germany) and the protocols were supplied by the manufacturer.
Laser scan microscopy
Bacteria (2.5 × 107) were grown in Lab-Tek chamber slides for 48 h. Growth medium was discarded, and planktonic bacteria removed by washing with HBSS (0.5% BSA). Neutrophils were added (5 × 105/well in 400 μL RPMI). After various times (1–6 h) the slides were washed carefully, and fixed with 4% paraformaldehyde. Neutrophils were visualized using an antibody to CD66b and anti-mouse IgG-coupled to Cy3 (Beckman-Coulter, Krefeld, Germany, Dako, Glostrup, Denmark respectively), and DNA was stained with Sytox Green nucleic acid stain (Life Technologies). The samples were mounted with Moviol (Sigma) and examined by laser scan microscopy (40×; Leica DMRBE, Software: Leica TCS).
Patients
Patients who underwent revision surgery due to an implant infection were included in the study. Diagnosis of loosening was based on patients’ complaints, clinical examination and examination by conventional x-ray and/or CT-scan. Bacterial growth was assessed by conventional methods; leukocyte count and C-reactive protein serum concentrations were determined by standard clinical laboratory methods.
Collection of tissue and blood samples
From 28 patients with implant infection, blood samples were taken before surgery, and for comparison blood from healthy volunteers (n = 10). Blood was collected into heparinized tubes. Informed consent was obtained from all patients and volunteers. The local ethic committee approved the study, and the institutional guidelines were observed.
Immunohistochemistry
Human tissue biopsies from patients with implant infections were collected and embedded in paraffin. For visualization of neutrophils, the naphtol-ASD-chloracetate-esterase (NADS-CL) kit (Sigma, Munich, Germany) was used according to the manufacturer's protocol and anti-MRP-14 (LSBio, LS-C105751, 1:100, retrieval condition: pH 9.0). To show osteoclasts, anti-cathepsin K (Santa Cruz, sc-48353, 1:50, retrieval condition: pH 9.0) was used. As secondary antibody the Histofine, Simple Stain universal polymer was used (Nichirei, Tokyo, Japan) followed by the colour reaction with liquid permanent red (Zytomed, Berlin, Germany), and counter stain with haematoxylin.
RESULTS
Release of DNA from human neutrophils following contact with S. epidermidis biofilms
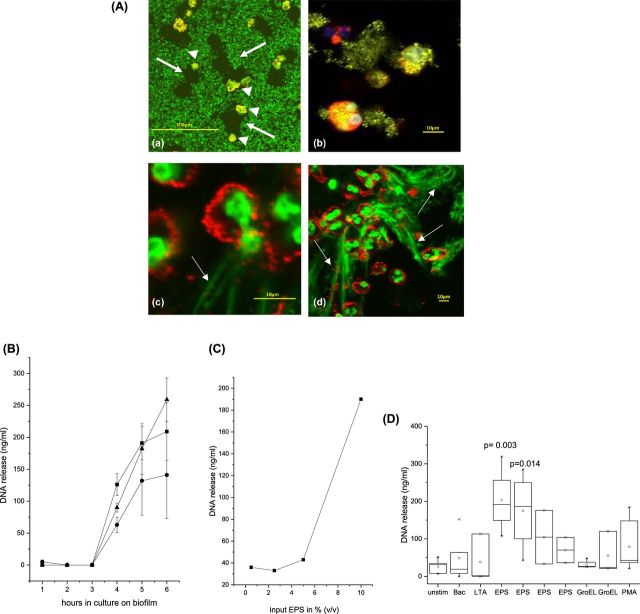
Neutrophils were placed on S. epidermidis biofilms for 2–4 h, and then examined by laser scan microscopy. Uptake of bacteria by neutrophils was seen, as was release of DNA (Fig. 1A). For quantitative analysis, supernatants were harvested at various times after contact of neutrophils with the biofilm. Release of DNA was seen after 4–6 h (data with cells of three individuals are shown in Fig. 1B). Of note, DNA release occurred whether or not the biofilm was opsonized with human serum. These data are in line with the observation that opsonization is required for phagocytosis of planktonic bacteria, but not for phagocytosis of biofilms (Stroh et al.2011).
Figure 1.
Biofilms induce release of DNA: (A) Neutrophils were placed on S. epidermidis biofilms for 2 h. Then neutrophils were visualized using anti-CD66b and anti-mouse-IgG-Cyr (red), and DNA was stained with SytoBC, which stains the DNA in neutrophils and in bacteria as well. (a) Low magnification (×200) shows biofilm-depleted areas around the neutrophils (neutrophils are marked by arrowheads; depleted areas by arrows). (b) Higher magnification (×400) and digital zoom revealed uptake of bacteria, and (c, d) discharge of the nucleus and release of DNA from the neutrophils. (B) Biofilms were grown in multiwell plates, neutrophils were added and after 1–6 h aliquots were taken from the supernatants and DNA was quantified. The values represent replicates of 10 parallel wells; data of three individuals (each represented by a different symbol) are shown. (C) Neutrophils were incubated with various amounts of EPS, and DNA in the supernatant was determined (cells of one individual are shown). (D) Neutrophils were stimulated with S. epidermidis (100 bacteria/cell), LTA (1 μg mL−1), EPS (1–10% v/v), GroEL or PMA (1 μM), and DNA in the supernatant was determined after 6 h. The data are summarized from independent experiments using cells of different individuals (at least n = 4; differences between groups were calculated using ANOVA).
Because the data implied that the biofilm by itself stimulated neutrophils, the EPS was extracted, and its effect on DNA release was compared to that of planktonic S. epidermidis (ratio 100 bacteria/cell), and that of the established activators of PMN, LTA and PMA, respectively. DNA release varied among cells of individual donors, but in all EPS induced DNA release (data of individual donors and summary of all donors are shown in Fig. 1C and D).
Identification of the bacterial stress proteins GroEL and Dnak in the EPS of S. epidermidis biofilms
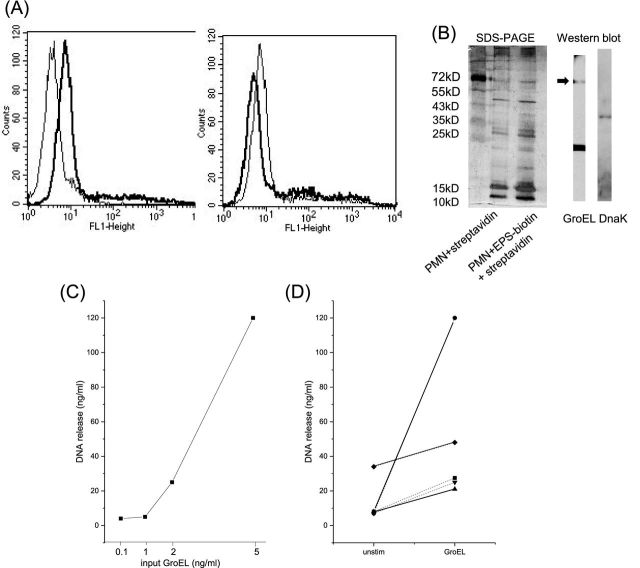
To further analyse the entities within the EPS that activate neutrophils, its interaction with neutrophils was examined. EPS contains more than 30 proteins as seen by SDS-PAGE. The proteins were labelled with biotin; and the binding to neutrophils was determined by cytofluorometry. Binding occurred and could be inhibited by unlabelled EPS, suggesting that there are specific or at least selective receptors for EPS-proteins on the cells (Fig. 2A). To look for potential receptors, a pull-down assay was performed with streptavidin coated to beads and isolated neutrophil membrane proteins that had been incubated for 1 h with EPS-biotin. For comparison, untreated neutrophil membranes were used. The eluates from the streptavidin beads were separated by SDS-PAGE. Both, untreated membranes and membranes that had been incubated with EPS showed multiple protein bands with a rather similar pattern, which was due to the fact that streptavidin binds readily to neutrophils (data not shown). In parallel experiments, however, we could show by western blotting that the EPS contained GroEL and DnaK (Fig. 2B).
Figure 2.
Binding of EPS to neutrophils and identification of GroEL and Dnak: (A) EPS was labelled with biotin, and binding to neutrophils was assessed by cytofluorometry using streptavidin-FITC (left-hand panel: the thick line shows EPS binding, the thin line shows the autofluorescence of the neutrophil). Right-hand panel: neutrophils were pre-incubated with unlabelled EPS (thick line) or not (thin line), then biotin-labelled EPS was added. Binding of biotin-EPS was inhibited by 50%. (B) PMN membranes were incubated without (lane 2) or with EPS-biotin (lane 3), and then with immobilized streptavidin. Proteins bound to streptavidin were eluated, and subjected to SDS-PAGE. Numerous bands were seen, intensity of three, marked by arrows, was higher in membranes pre-exposed to EPS. By western blotting two of the proteins were identified as GroEL and DnaK, respectively. (C, D) Cells were incubated with GroEL, and after 6 h, DNA in the supernatant was determined. Dose response curve for one donor is shown in C and D experiments with cells of five donors, stimulated with GroEL (5 ng (thick lines) or 1 ng (thin lines)).
Effects of GroEL and DnaK on human neutrophils
First experiments with recombinant GroEL and DnaK revealed that GroEL, but not DnaK, activated neutrophils as seen by induction of oxygen radical production (increase of 300–540 arbitrary units; mean of nine individuals; P < 10−4) and up-regulation of the adhesion protein CD11b (increase of 100–440 MFI; mean of nine individuals; P < 10−4). Moreover, GroEL induced release of DNA, again with some variation among individual donors, but in all GroEL and EPS induced DNA release (data of an individual donor and summary of all donors are shown in Figs 2C and 1D, respectively).
Release of MRP-14 from neutrophils
MRP-14 is highly expressed in PMN and it is found locally at infected sites (Fig. 3A), and in the peripheral blood of patients with implant infections (Fig. 3B). In vitro data showed that PMN release MRP-14 into the supernatant when co-incubated with bacterial biofilms, or planktonic opsonized staphylococci, respectively. In extension of these studies we now found that extracted EPS induced MRP-14 release, but not recombinant GroEL (Abb. 3C and D).
Figure 3.
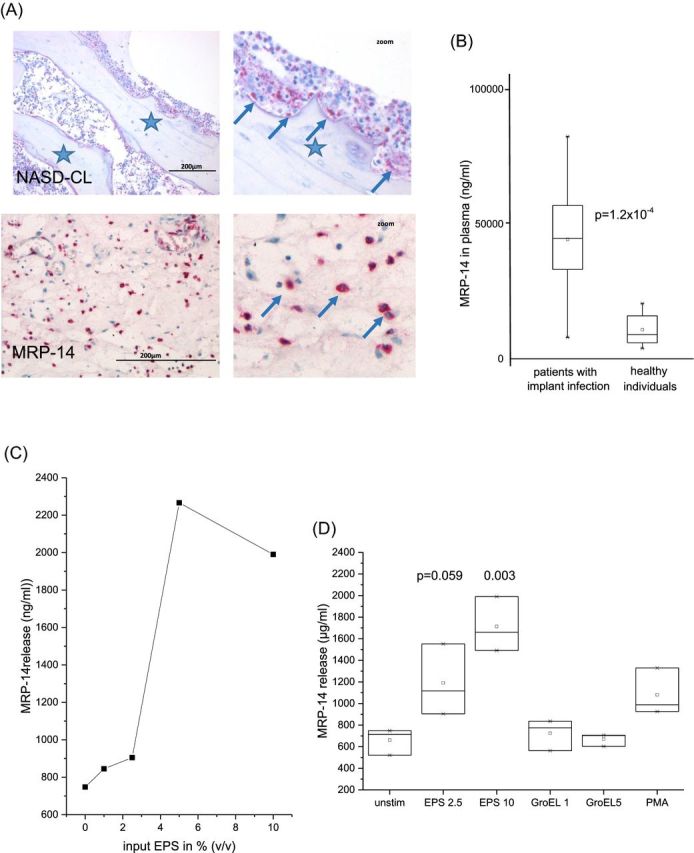
Induction of MRP-14: (A) Biopsies of patients with implant-associated osteomyelitis are shown. The upper panel shows the partially degraded bone (*) and a cellular infiltrate (arrows). The reddish stained cells are neutrophils (the right panel shows a digital zoom of a selected area). The lower panel shows MRP-14 positive cells (red). (B) MRP-14 was measured in the serum of patients with implant infection (n = 26), and for comparison in healthy individuals (n = 10) (the groups differed significantly according to Mann–Whitney Test with P = 1.2 × 10−5). (C) Cells were incubated with increasing amounts of EPS; release of MRP-14 into the supernatant was determined (data of one individual are shown). (D) The summary of four individuals (difference between groups were calculated by ANOVA).
DISCUSSION
PMN are the first responders in bacterial infection. They are equipped with an abundance of preformed bactericidal and cytotoxic entities, the capacity to generate reactive oxygen species and to engulf bacteria for intracellular destruction (Segal 2005; Borregaard, Sorensen and Theilgaard-Monch 2007). Bactericidal activity of neutrophils was studied mainly with planktonic bacteria, and there is the notion that bacteria organized in biofilms might escape the innate host defence or modulate the immune response (Vuong et al.2004; Cheung et al.2010; Schommer et al.2011; Spiliopoulou et al.2012).
Our previous studies, however, provided evidence that neutrophils attack bacteria in biofilms and are able to destroy biofilms of either S. aureus or S. Epidermidis (Guenther et al.2009; Meyle et al.2010; Stroh et al.2011).
How neutrophils recognize biofilms, however, is still under investigation. There is evidence that recognition is not dependent on opsonization, the latter defined as coating of bacteria with antibodies, particularly IgG, and with the activated complement C3, C3b and C3bi. For planktonic bacteria opsonization is an absolute requirement for efficient phagocytosis, because the signal is mediated via receptors for the invariable part of the antibody, the Fc gamma receptors CD16, CD32 and CD64, and the complement receptors CD11b/CD18 and CD35, respectively (Fallman, Andersson and Andersson 1993; Underhill and Ozinsky 2002; Spaan et al.2013).
In case of biofilms as a target, neutrophils apparently recognize entities within the biofilm. The fact that biofilm ‘slime’ contains bioactive substances has long been known (Gray et al.1984) and previous studies by our group confirmed that the EPS activates neutrophils (Meyle et al.2012).
Likely candidates are LTA or peptidoglycan, which are all present in the EPS and—at least as soluble molecules—activate a variety of neutrophil functions (Sabroe et al.2003; Jabbouri and Sadovskaya 2010).
We found that in addition to these entities also the protein fraction of the extracted EPS stimulated neutrophils: up-regulation of CD11b and induction of ROS were seen (Maurer et al.2015) and as we described now, release of DNA and of MRP-14. To identify the activating entities, biotin-labelled EPS was attached to neutrophils, and bound proteins were extracted by immobilized streptavidin. By SDS-PAGE multiple proteins bands were seen. The high non-specific binding was due to the fact that numerous neutrophil membrane proteins bound directly streptavidin, thus precluding the use of this method for direct sequencing of the candidate proteins. As an alternative approach, western blotting using an array of antibody directed towards bacterial proteins was used. We identified two of the proteins as GroEL and DnaK. Both, GroEL and DnaK, are produced by nearly all bacteria species as chaperones, but similar to their human homologues, the heat shock proteins HSP60 and HSP70, respectively, they are also released from the cells. As exogenous stimulators they affect numerous cell functions on a wide variety of cells (reviewed in Calderwood 2007).
Under our experimental conditions, recombinant GroEL, but not DnaK, activated neutrophils. GroEL, as well as the intact biofilm and the protein fraction of EPS, induced the release of DNA from neutrophils. Release of DNA, a process also termed ‘netosis’ or ‘NET formation’—the latter stands for ‘neutrophil extracellular traps’—is presumably an additional means of bactericidal activity which works by trapping bacteria in the ejected DNA strands and fixing them for extracellular killing by bactericidal peptides (reviewed in Brinkmann and Zychlinsky 2012).
When neutrophils were placed on biofilms, within hours depletion of the biofilm was seen in the immediate vicinity. Moreover, DNA was released, apparent as elongated strands. Of note, EPS and GroEL also induced DNA release, as determined by staining of DNA in the cell supernatant. Whether or not DNA participates in the defence against biofilm infection—as it has been implied for planktonic bacteria—is questionable, because there are numerous data describing that extracellular DNA promotes biofilm formation, and that degradation of DNA destroys biofilms (Whitchurch et al.2002; Qin et al.2007; Kaplan et al.2012). Although these data are derived from experiments with bacterial DNA, comparable effects with mammalian DNA are feasible, and hence, the biological consequence of DNA release is still a matter of debate.
In parallel to DNA release, we also observed release into the cell supernatant of MRP-14 in response to biofilms, or EPS, but not to recombinant GroEL. MRP-14 is a multifunctional, rather abundant cytokine of neutrophils with intra- and extracellular functions (Hessian, Edgeworth and Hogg 1993). We found expression of MRP-14 in tissue samples of patients with severe bacterial biofilm infections, and also considerable serum concentrations (Dapunt et al.2015), indicating a link between infection and MRP-14 release.
Taken together, our data provide evidence that neutrophils recognize entities within the EPS of biofilms, and among those—not to the exclusion of others—the heat shock protein GroEL. The fact that we did not see a biological activity of DnaK does not rule out that it participates in neutrophil activation. Priming and synergistic effects have not yet been assessed systematically. Moreover, recombinant protein might lack biological activity, because of their different tertiary structure.
How EPS or GroEL, respectively, activate neutrophils, is still under investigation.
Likely candidates are the receptors for the so-called ‘pathogen-associated molecular patterns’, defined as molecules that are shared by various bacteria species, for example LPS, LTA, flagellin or bacteria DNA (reviewed in Medzhitov 2013; Thomas et al.2013). Our previous data with total EPS indicated involvement of the toll-like receptor TLR4 (Maurer et al.2015), which is also described as receptor for GroEL (Argueta et al.2006).
On the other hand, for the human homologue of GroEL, HSP-60, which also activates neutrophils (Osterloh et al.2009), alternative receptors have been described including scavenger receptors (Baranova et al.2012) or CD40 (Calderwood 2007).
That neutrophils, though expressing TLRs, may use other pattern recognition receptors for activation would be not unique to GroEL; also LTA, peptidoglycan or bacterial DNA, which are known ligands for TLR2, TLR4 or TLR9, respectively, use alternative receptors (Lipford, Heeg and Wagner 1998; Peiser, Mukhopadhyay and Gordon 2002).
Irrespective of the activation pathways, our data provide evidence for the presence of neutrophil-activating entities within the EPSs of S. epidermidis biofilms. The analysis of the signalling pathways suggests that there is more than one activating entity. The one we identified as GroEL is present in nearly all bacteria strains, which means that the activating effect of EPS might not be limited to S. epidermidis.
FUNDING
This study was supported by the University of Heidelberg. Ulrike Dapunt was supported by the Olympia-Morata scholarship of the Faculty of Medicine of Heidelberg University.
Conflict of interest. None declared.
REFERENCES
- Argueta JG, Shiota S, Yamaguchi N, et al. Induction of Porphyromonasgingivalis GroEL signaling via binding to toll-like receptors 2 and 4. Oral Microbiol Immun. 2006;21:245–51. doi: 10.1111/j.1399-302X.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- Baranova IN, Vishnyakova TG, Bocharov AV, et al. Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. J Immunol. 2012;188:1371–80. doi: 10.4049/jimmunol.1100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–5. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198:773–83. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK. Heat shock proteins in extracellular signaling. Methods. 2007;43:167. doi: 10.1016/j.ymeth.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Rigby K, Wang R, et al. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapunt U, Giese T, Maurer S, et al. Neutrophil-derived MRP-14 is up-regulated in infectious osteomyelitis and stimulates osteoclast generation. J Leukocyte Biol. 2015;98:575–82. doi: 10.1189/jlb.3VMA1014-482R. [DOI] [PubMed] [Google Scholar]
- Dapunt U, Giese T, Lasitschka F, et al. Osteoclast generation and cytokine profile at prosthetic interfaces: a study on tissue of patients with aseptic loosening or implant-associated infections. Eur J Inflamm. 2014;12:147–59. [Google Scholar]
- Davies EL, Bacelar MM, Marshall MJ, et al. Heat shock proteins form part of a danger signal cascade in response to lipopolysaccharide and GroEL. Clin Exp Immunol. 2006;145:183–9. doi: 10.1111/j.1365-2249.2006.03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallman M, Andersson R, Andersson T. Signaling properties of CR3 (CD11b/CD18) and CR1 (CD35) in relation to phagocytosis of complement-opsonized particles. J Immunol. 1993;151:330–8. [PubMed] [Google Scholar]
- Gaida MM, Mayer B, Stegmaier S, et al. Polymorphonuclear neutrophils in osteomyelitis: link to osteoclast generation and bone resorption. Eur J Inflamm. 2012;10:413–26. [Google Scholar]
- Gray ED, Peters G, Verstegen M, et al. Effect of extracellular slime substance from Staphylococcusepidermidis on the human cellular immune response. Lancet. 1984;1:365–7. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Guenther F, Stroh P, Wagner C, et al. Phagocytosis of staphylococci biofilms by polymorphonuclear neutrophils: S.aureus and S.epidermidis differ with regard to their susceptibility towards the host defense. Int J Artif Organs. 2009;32:565–73. doi: 10.1177/039139880903200905. [DOI] [PubMed] [Google Scholar]
- Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukocyte Biol. 1993;53:197–204. [PubMed] [Google Scholar]
- Jabbouri S, Sadovskaya I. Characteristics of the biofilm matrix and its role as a possible target for the detection and eradication of Staphylococcusepidermidis associated with medical implant infections. FEMS Immunol Med Mic. 2010;59:280–91. doi: 10.1111/j.1574-695X.2010.00695.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, LoVetri K, Cardona ST, et al. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J Antibiot. 2012;65:73–7. doi: 10.1038/ja.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid JG, Costerton JW, Shirtliff ME, et al. Immunology of Staphylococcal biofilm infections in the eye: new tools to study biofilm endophthalmitis. DNA Cell Biol. 2002;21:405–13. doi: 10.1089/10445490260099692. [DOI] [PubMed] [Google Scholar]
- Lipford GB, Heeg K, Wagner H. Bacterial DNA as immune cell activator. Trends Microbiol. 1998;6:496–500. doi: 10.1016/s0966-842x(98)01408-5. [DOI] [PubMed] [Google Scholar]
- Liu H, Fang HH. Extraction of extracellular polymeric substances (EPS) of sludges. J Biotechnol. 2002;95:249–56. doi: 10.1016/s0168-1656(02)00025-1. [DOI] [PubMed] [Google Scholar]
- Maurer S, Fouchard P, Meyle E, et al. Activation of neutrophils by the extracellular polymeric substance of S.Epidermidis biofilms is mediated by the bacterial heat shock protein groel. J Biotechnol Biomater. 2015;5:176. [Google Scholar]
- Medzhitov R. Pattern recognition theory and the launch of modern innate immunity. J Immunol. 2013;191:4473–4. doi: 10.4049/jimmunol.1302427. [DOI] [PubMed] [Google Scholar]
- Meyle E, Brenner-Weiss G, Obst U, et al. Immune defense against S.epidermidis biofilms: components of the extracellular polymeric substance activate distinct bactericidal mechanisms of phagocytic cells. Int J Artif Organs. 2012;35:700–12. doi: 10.5301/ijao.5000151. [DOI] [PubMed] [Google Scholar]
- Meyle E, Stroh P, Gunther F, et al. Destruction of bacterial biofilms by polymorphonuclear neutrophils: relative contribution of phagocytosis, DNA release, and degranulation. Int J Artif Organs. 2010;33:608–20. doi: 10.1177/039139881003300906. [DOI] [PubMed] [Google Scholar]
- Osterloh A, Geisinger F, Piedavent M, et al. Heat shock protein 60 (HSP60) stimulates neutrophil effector functions. J Leukocyte Biol. 2009;86:423–34. doi: 10.1189/jlb.0109011. [DOI] [PubMed] [Google Scholar]
- Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–8. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- Qin Z, Ou Y, Yang L, et al. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcusepidermidis. Microbiology. 2007;153(Pt 7):2083–92. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- Remijsen Q, Kuijpers TW, Wirawan E, et al. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–8. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabroe I, Prince LR, Jones EC, et al. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- Schommer NN, Christner M, Hentschke M, et al. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun. 2011;79:2267–76. doi: 10.1128/IAI.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan AN, Surewaard BG, Nijland R, et al. Neutrophils versus Staphylococcusaureus: a biological tug of war. Annu Rev Microbiol. 2013;67:629–50. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- Spiliopoulou AI, Kolonitsiou F, Krevvata MI, et al. Bacterial adhesion, intracellular survival and cytokine induction upon stimulation of mononuclear cells with planktonic or biofilm phase Staphylococcusepidermidis. FEMS Microbiol Lett. 2012;330:56–65. doi: 10.1111/j.1574-6968.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- Stroh P, Gunther F, Meyle E, et al. Host defence against Staphylococcusaureus biofilms by polymorphonuclear neutrophils: oxygen radical production but not phagocytosis depends on opsonisation with immunoglobulin G. Immunobiology. 2011;216:351–7. doi: 10.1016/j.imbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Thomas N, Heather J, Pollara G, et al. The immune system as a biomonitor: explorations in innate and adaptive immunity. Interface Focus. 2013;3:20120099. doi: 10.1098/rsfs.2012.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- Vuong C, Voyich JM, Fischer ER, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcusepidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6:269–75. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Wagner C, Kondella K, Bernschneider T, et al. Post-traumatic osteomyelitis: analysis of inflammatory cells recruited into the site of infection. Shock. 2003;20:503–10. doi: 10.1097/01.shk.0000093542.78705.e3. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, et al. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Rieu P, Descamps-Latscha B, et al. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–53. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]