Abstract
Macrophages play a central role in mycobacterial pathogenesis. Recent work has highlighted the importance of diverse macrophage types and phenotypes that depend on local environment and developmental origins. In this review, we highlight how distinct macrophage phenotypes may influence disease progression in tuberculosis. In addition, we draw on work investigating specialized macrophage populations important in cancer biology and atherosclerosis in order to suggest new areas of investigation relevant to mycobacterial pathogenesis. Understanding the mechanisms controlling the repertoire of macrophage phenotypes and behaviors during infection may provide opportunities for novel control of disease through modulation of macrophage form and function.
Keywords: macrophage, granuloma, tuberculosis, mycobacteria
In this review, we highlight the influence of distinct macrophage phenotypes on disease progression in tuberculosis, including similarities to findings about macrophages in cancer biology and atherosclerosis.
INTRODUCTION
Tuberculosis (TB) is the leading cause of morbidity and mortality associated with a single infectious agent (WHO 2015). Mycobacterium tuberculosis (Mtb) infection is acquired by inhalation of aerosolized droplets containing infectious bacilli. Bacilli are phagocytosed by alveolar macrophages, tissue-specialized macrophages residing in and acting to clear air spaces of infectious, toxic, or allergenic particles that have evaded the mechanical defenses of the respiratory tract (Rubins 2003). Mycobacteria can also be taken up in the air spaces by recruited monocyte-derived macrophages (Thurnher et al.1997) or by dendritic cells, which can carry the organisms to draining lymph nodes initiating an adaptive response to infection that is abnormally delayed (Henderson, Watkins and Flynn 1997; Marino et al.2004; Wolf et al.2007). Pathogenic mycobacteria have achieved broad evolutionary success through specialization as intracellular pathogens able to achieve persistent infection by manipulating host macrophages in order to reside and replicate within them rather than be destroyed by innate defense mechanisms (Eum et al.2010; Philips and Ernst 2012; Repasy et al.2013). Recruitment of different phagocyte types and subtypes during early infection results in different disease outcomes as diverse phagocyte classes play different roles in advancing or controlling infection (Cadena, Flynn and Fortune 2016). Mtb-infected macrophages and dendritic cells migrate from alveolar spaces and into the lung interstitium and surrounding tissues, where they are able to either enter the lymphatic or hematogenous systems spreading to other organs and causing primary progressive disease, migrate to draining lymph nodes initiating the adaptive response, or aggregate into characteristic structures called granulomas (Frieden et al.2003). After the initial stages of infection, Mtb can be distributed among alveolar macrophages, dendritic cells, and neutrophils. With additional time, dendritic cells and recruited monocyte-derived macrophages become the prominent infected cell types in the airways (Wolf et al.2007; Srivastava, Ernst and Desvignes 2014). As recent reviews have covered the diversity of phagocytic cells that are engaged during mycobacterial infection (Srivastava, Ernst and Desvignes 2014), we will limit our discussion to the specific roles of different macrophage subtypes during mycobacterial infection, how recent understanding of macrophage heterogeneity and subtypes will impact our understanding of mycobacterial infections, and how macrophage diversity may drive infection trajectory.
Granulomas as macrophage-driven structures
Granulomas are compact organized aggregates of mature macrophages that arise in response to Mtb infection as well as other inflammatory stimuli and can allow bacilli to persist over time (Russell 2007; Ramakrishnan 2012). Infected macrophages arrange into granulomas characterized by concentric rings of specialized macrophages surrounding a lipid-rich core of central necrosis, referred to as caseum (Russell 2007; Ramakrishnan 2012). Within the granuloma, macrophages can fuse to form multinucleated giant cells (Helming and Gordon 2007b), accumulate lipid, becoming foam cells (Russell et al.2009), transition to epithelioid histiocytes (Adams 1974), or take on diverse chemokine and cytokine expression profiles (Monin and Khader 2014). Cytokines and chemokines produced by granuloma macrophages result in recruitment and aggregation of a diverse cell type complement including neutrophils, dendritic cells, B and T cells, natural killer (NK) cells, and fibroblasts around and within the forming granuloma (Cosma, Sherman and Ramakrishnan 2003; Ehlers and Schaible 2012). There has been growing appreciation for the diverse roles that neutrophils play in driving both early protection as well as mediating detrimental excessive host inflammation and lung damage (Yang et al.2012; Monin and Khader 2014; Dallenga and Schaible 2016). The developing granuloma is dynamic and may have both host-protective and bacteria-beneficial effects, providing a niche in which the bacteria may infect recruited macrophages, grow extracellularly after necrosis of infected macrophages, persist, and disseminate (Davis and Ramakrishnan 2009; Lin et al.2014; Gideon et al.2015). Macrophages can participate in promoting this dissemination by leaving the primary granuloma, while infecting and establishing secondary granulomas at new tissue sites (Davis and Ramakrishnan 2009; Schreiber et al.2011; Welsh et al.2011; Guirado, Schlesinger and Kaplan 2013).
Given the central roles of macrophages during early mycobacterial infection, granuloma development and maintenance, recruitment of diverse cell types, and the eventual dissemination of infection, an understanding of the heterogeneous phenotypes of macrophages at baseline and in response to infection is essential for understanding the mechanisms of mycobacterial infection initiation and progression. In this review, we discuss recent understanding gained about macrophage development, specialization, and activation. We additionally describe how these new insights relate to observations of macrophage diversity during mycobacterial infection and the effect of this diversity on disease progression.
REFINED UNDERSTANDING OF MACROPHAGE DIVERSITY AND FUNCTION
Macrophages are professional phagocytes adapted to kill engulfed microbes but also involved in regulating early organism development and coordinating tissue repair (Tauber 2003; Medzhitov 2010). Initial understandings of macrophage biology are giving way to more nuanced appreciation of the many roles and responsibilities of macrophages during organism homeostasis and disease (Gordon, Pluddemann and Martinez Estrada 2014; Varol, Mildner and Jung 2015). Recent paradigm shifts have changed the dialog around macrophage diversity, opening new pathways of investigation in macrophage biology as a result of a more complex understanding of macrophage heterogeneity and specialization. The importance of macrophage ontogeny, or developmental origin, and specific activation phenotypes are becoming clearer and are relevant to an understanding of the varied roles of macrophages during mycobacterial infection. The baseline heterogeneity of macrophages can help to explain the variation in interaction between pathogenic mycobacteria and macrophages depending on the local microenvironment in which the interaction occurs (Guirado, Schlesinger and Kaplan 2013) and the different macrophage subsets involved in disease (Cambier et al.2014). As has been noted in recent reviews, other infected cell types may also affect the trajectory of disease (Srivastava, Ernst and Desvignes 2014; Cadena, Flynn and Fortune 2016).
Developmental origins and tissue heterogeneity
Macrophage phenotypes can be dramatically influenced by developmental origin, and macrophages of different lineages may contribute to the progression of mycobacterial infection (schematized in Fig. 1). Macrophages were initially considered in the context of differentiated bone marrow-derived blood monocytes exclusively (van Furth et al.1972). However, early work demonstrated that there was a distinct yolk sac-derived population of macrophages seeding the brain in mice (Alliot, Godin and Pessac et al.1999) and that Kupffer cell populations were maintained even after destruction of blood monocytes (Yamada, Naito and Takahashi 1990). In zebrafish, yolk sac-derived macrophages were observed prior to the development of later lineages and shown to distribute throughout body tissues indicating that macrophages were capable of developing from an additional developmental source (Herbomel, Thisse and Thisse 1999). Later, lineage-tracing studies following macrophage sources in animals developing into early adulthood (Ginhoux et al.2010; Schulz et al.2012; Hettinger et al.2013; Jakubzick et al.2013; Yona et al.2013) as well as examination of transcription factor regulatory networks (Lavin et al.2014) demonstrated that most macrophages in adult systems are maintained independent of blood monocytes through self-renewal from precursors established in tissue locations during embryonic development (Hoeffel et al.2012; Guilliams et al.2013; Hashimoto et al.2013; Ginhoux and Jung 2014). Only macrophages resident in the intestine are constantly replenished from blood monocytes (Bain et al.2014), though macrophages and inflammatory dendritic cells (iDCs) can develop in all adult tissues from tissue-infiltrating monocytes (Segura and Amigorena 2013; Yona et al.2013).
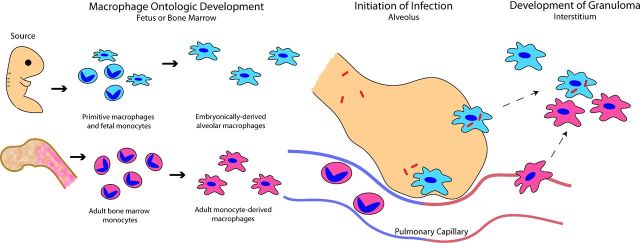
Figure 1.
Ontologic sources of macrophages during mycobacterial infection. Macrophages present in adult tissues are derived from embryonic and bone marrow sources. Embryonically derived macrophages establish residence in tissues during early development and are continuously derived from established tissue-specific precursors. Alternatively, adult bone marrow continuously gives rise to monocytes which differentiate into monocyte-derived macrophages in response to stimuli and migrate into tissues. During infection, mycobacteria are phagocytosed by alveolar macrophages, an embryonically derived lineage, shortly after inhalation. Infected alveolar macrophages migrate into the lung interstitum and are joined by recruited monocyte-derived macrophages, which aggregate together in order to form the granuloma structure. In this way, diverse macrophage populations collaborate to respond to infection and develop the granuloma.
Embryonically derived tissue-resident macrophages display remarkable diversity of gene expression (Li et al.2007; Gautier et al.2012) adapting to the specific microenvironment of residence through both their ontological lineage as well as specific chromatin modification enacted through environment-specific enhancer landscapes and maintained in lineages (Lavin et al.2014). Alternatively, monocyte-derived macrophages and iDCs display distinct behaviors and are shorter lived than their embryonically derived counterparts but are able to dynamically respond to specific changing stimuli allowing for on demand activity during times of inflammation or infection regardless of lineage-specific actions (Yona et al.2013). Relatively few studies have specifically addressed the role of monocyte-derived macrophages in the lung compartment (Kopf, Schneider and Nobs 2015). However, recent work has demonstrated that monocyte subsets and their macrophage derivatives are altered in the context of mycobacterial infection (Tung, Ou and Tsai 2013; Lugo-Villarino and Neyrolles 2014; Lastrucci et al.2015). The existence of parallel systems of macrophage derivation allows for the presence of diverse macrophage phenotypes in tissues at baseline or in response to specific stimuli, such as infection, and therefore the possibility of diverse macrophage behavior in response to that stimulus.
Particularly relevant to mycobacterial infections in humans are the identity, diversity and properties of alveolar macrophages. Both alveolar macrophages and dendritic cells have been shown to be organ-specific lineages (Kopf, Schneider and Nobs 2015) which differentiate from fetal monocytes in the very early stages of development under the control of granulocyte/macrophage colony-stimulating factor (GM-CSF), PPAR γ and the lung microenvironment (Guilliams et al.2013; Hussell and Bell 2014; Schneider et al.2014). In contrast to other populations, alveolar macrophages have the ability to self-renew (Hashimoto et al.2013), and have recently been shown to naturally express low levels of MafB and cMaf, transcription factors that generally repress a suite of genes regulating macrophage self-renewal (Soucie et al.2016). In addition, alveolar macrophages appear to undergo tissue imprinting in the lung, and, as a result of tissue environment and context, and in contrast to other tissue-resident macrophages, naturally produce a number of generally immunosuppressive gene products, including transforming growth factor beta (TGFβ), IL-10 and specific prostaglandins (Guirado, Schlesinger and Kaplan 2013; Hussell and Bell 2014). These properties in the context of mycobacterial infections are discussed further below. The unique tissue context and developmental origin of alveolar macrophages help define the properties and outcomes of the initial interactions between infecting mycobacteria and the host.
Heterogeneity and the activation spectrum
In addition to ontogeny, recent insights into heterogeneity and activation state will also impact our understanding of TB disease progression. Macrophage ‘activation state’ has canonically been classified on the basis of the differential effect of interferon γ (IFNγ) and interleukin-4 (IL-4) on in vitro cultured macrophage gene expression (Stein et al.1992; Martinez and Gordon 2014). These differential activation or polarization states have been termed classically and alternatively activated or M1- and M2-activated macrophages, respectively. This understanding of macrophage ‘polarization’ was an effort to understand macrophages as belonging to cell lineages defined by particular markers in the manner which T cells have been classified as Th1 or Th2 (Martinez and Gordon 2014). However, macrophage activation by diverse stimuli is characterized by shifts in the expression of hundreds of genes without any single gene serving as a definitive marker of a particular activation state in contrast to T cells (Xue et al.2014) . In order to reflect this difference in mechanism of heterogeneity, recently suggested nomenclature systems present the argument that macrophage diversity is more appropriately classified on the basis of similarity of the expression profile of the macrophage with expression profiles observed during in vitro stimulation with IL-4, immune complexes, IL-10, TGFβ, glucocorticoids, lipopolysaccharide (LPS), LPS and IFNγ, and IFNγ alone. These different activation or polarization states exist along a continuum between IFNγ stimulation-type macrophages and IL-4 stimulation-type macrophages, defining a spectrum of activation between the previous binary categorization of M1 and M2 macrophages (Table 1) (Murray et al.2014). However, even this graded categorization, while useful, may not be fully adequate to reflect macrophage heterogeneity. It is therefore important that work to define the activation state of macrophages during mycobacterial infection strives to determine the gene expression profile of particular macrophages under study rather than relying on a limited number of markers that have been shown to be expressed at varying levels across the macrophage activation ‘spectrum’. In this way, an understanding of macrophages that considers altered expression of genes known to be important to immune activity on the basis of infection or during different stages and locations of infection is particularly relevant during mycobacterial infection.
Table 1.
Macrophage activation spectrum on the basis of stimulation and resulting expression of markers involved in mycobacterial infection. Macrophages are divided into functional subsets on the basis of classic sources of stimulation used in ex vivo experimentation. Sources of stimulation are IL-4, immune complexes (Ic), IL-10, transforming growth factor β (TGFβ), glucocorticoids (GC), lipopolysaccharide (LPS), LPS and interferon gamma (IFNγ) and IFNγ alone. Expression of markers relevant to mycobacterial infection is annotated for each stimulation type demonstrating that stimulation types are determined on the basis of expression of sets of markers rather than single markers. This allows for a more nuanced identification of macrophage activation state in vivo on the basis of marker repertoire expression or absence and placement along a spectrum. Table is summarized from data collected in Murray et al.2014.
| ‘Alternative’ | ‘Classical’ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M2 | Stimulation | M1 | ||||||||
| anti-inflammatory | pro-inflammatory | |||||||||
| IL-4 | Ic | IL-10 | TGFβ | GC | (–) | LPS | LPS + IFNγ | IFNγ | ||
| Expression | CD163 | + | + | |||||||
| IL-10 | + | + | ||||||||
| TGFβ | + | |||||||||
| Arg1 | ++++ | ++ | ++ | |||||||
| IL-17 | + | |||||||||
| IL-6 | + | |||||||||
| Nos2 | + | + | ++++ | ++++ | ||||||
| IL-4ra | + | |||||||||
| TNF | + | |||||||||
| IL-1β | + | |||||||||
| IL-23 | + | |||||||||
| IL-12 | + | |||||||||
In vivo complexity
In vivo, macrophage activation states are dynamic, responding to initial stimulation, removal of stimulus, feedback or feed-forward signaling, autocrine production of cytokines, as well as the epigenetic or developmental lineage effects built into the life history of the macrophage (Porta et al.2009; Lawrence and Natoli 2011; Ivashkiv 2013). Identification of macrophage polarization states in vivo is more complicated than in in vitro systems, as individual macrophages are subjected to complex stimuli, and macrophage phenotypes are often mixed within a particular tissue region or at different times during disease (Sica and Mantovani 2012). As motile cells, macrophages can move through varying stimuli in vivo acquiring unique gene expression profiles through sequential alteration. The classically described macrophage phenotypes break down, as macrophages in vivo display wide variation in marker expression and mixed phenotypes. Thus, the particular contribution of coexisting cell types, temporal changes in stimuli during disease progression, and the particular molecular landscapes influencing individual macrophage phenotypes during TB infection in vivo must be closely dissected to gain understanding of the contributions of different macrophage expression profiles or activation states to disease progression in vivo.
Other macrophage forms
Epithelioid histiocytes are macrophages activated to develop characteristics of epithelial cells and form tight interdigitated junctions between their cell membranes (Ramakrishnan 2012). They are a hallmark of granuloma formation and TB disease progression, and were characterized in early reports on the basis of their pathological appearance (Adams 1974; Williams and Williams 1983). It is believed that these epithelioid cells are central to granuloma formation and dynamics. Epithelioid macrophages are not yet able to be fully classified on the basis of their activation state or gene expression profile and relatively little is known about the specific signals that promote their formation during mycobacterial granuloma development and maintenance. Although it was previously believed that macrophages were induced to convert to an epithelioid phenotype through the action of T cells secreting lymphokines, experiments in athymic mice (Epstein et al.1979; Tanaka et al.1982), larval zebrafish void of functional adaptive immunity (Davis et al.2002) and in in vitro conditions absent lymphokine stimulation (Feng et al.2014; Huang et al.2015) have demonstrated epithelioid conversion and granuloma formation independent of classical adaptive immunity. Other processes leading to development of epithelioid macrophages remain unknown. Given the importance of epithelioid macrophages to TB progression and granuloma formation and the growing understanding of the diversity of macrophage phenotypes at baseline, it is important to consider both the cytokine influences and the baseline activation or ontological states of macrophages that might influence their conversion to the epithelioid macrophage phenotype during mycobacterial infection.
Turnover and replenishment of epithelioid and other macrophages in the granuloma has important implications for granuloma dynamics, but has been relatively inaccessible to experimental analysis. 3H-thymidine-labeling experiments indicated that granuloma macrophage lifetimes were on the order of days, although the exact turnover rate could not be calculated due to replenishment of granuloma macrophage populations by labeled monocytes (Tsuda et al.1976). In addition, adoptive transfer of bone marrow monocytes in the presence and absence of mycobacterial infection has demonstrated that recruited macrophage and dendritic cell populations were derived from monocytes (Skold and Behar 2008). These reports, however, do not rule out the involvement of alveolar or tissue-resident macrophages in granuloma formation and their differentiation to epithelioid macrophages.
Multinucleate giant cells (MGCs) form as a result of the fusion of macrophages recruited to the site of granulomatous inflammation such as during mycobacterial infection (Chambers and Spector 1982). The role of MGCs during granuloma formation and mycobacterial infection remains incompletely explored (Helming and Gordon 2007b). In the context of mycobacterial infection, MGCs generally contain only a few bacteria, if any (Ramakrishnan 2012), and it has been reported that they are deficient in the ability to phagocytose infecting bacilli (Lay et al.2007). Formation of MGCs in vitro has been accomplished by exposing macrophages to IL-4 (McInnes and Rennick 1988; McNally and Anderson 1995) or IL-13 (DeFife et al.1997). IL-4 has also been shown to be important for the formation of MGCs during granulomatous schistosomiasis infection (Chensue et al.1992). However, IL-4 alone does not induce MGC formation, but rather it has been demonstrated that macrophages respond to IL-4 stimulation differentially on the basis of their endogenous inflammatory status and the presence of bacterial signals (Helming and Gordon 2007a,b). While the function of this prominent cell type in mycobacterial infection is not well understood, recent work has suggested that MGCs have specialized capacity to phagocytose large and complement-opsonized particles via activation of CR3 during the process of macrophage fusion (Milde et al.2015).
Foam cells or foamy macrophages are lipid-loaded macrophages which form as low density lipoproteins are taken up by LDL receptors and the scavenger receptors SRA and CD36 following stimulation of Toll-like receptors (TLRs) by mycobacterial-derived agonists (Russell et al.2009; Mahajan et al.2012, Singh et al.2012). Macrophages also accumulate lipids by phagocytosis of lipid-loaded platelets (Feng et al.2014). Once internalized, LDL and lipid-loaded platelets are broken down into their constituent parts including phospholipids, triacylglycerides and cholesterol. The triacylglycerides and phospholipids are mainly metabolized by the macrophage, but the cholesterol is esterified and retained by the macrophages stored in lipid droplets (LDs) (Walther and Farese 2012). Under normal conditions, macrophages function to export retained cholesterol through the action of ATP-binding cassette (ABC) transporters secreting high-density lipoproteins (HDL) to the serum in the process (Cuchel and Rader 2006). During mycobacterial infection, either as a result of inflammatory stimulus or through direction of infecting bacilli, cholesterol accumulates within macrophages (Kondo and Kanai 1976). The presence of foamy macrophages has been demonstrated to modulate the inflammatory response through production of prostaglandin E2 and leukotrienes (D'Avila et al.2006; Baldan et al.2008; D'Avila, Maya-Monteiro and Bozza 2008; Silva et al.2009). Mycobacteria associate with LDs within the foamy macrophages, utilizing these lipids as nutritional sources (Peyron et al.2008). This source of cholesterol has been demonstrated to be required for bacterial growth during the chronic phase of infection (Pandey and Sassetti 2008), and the lipid-rich substances observed in the caseous centers of necrotic granulomas, where rich extracellular growth occurs, are thought to derive from the accumulated lipids within foam cells involved in granuloma formation (Russell et al.2009). The many important roles of foam cells in mycobacterial infection make a more detailed understanding of their development during mycobacterial infection, the specific macrophage subsets from which they develop, and the ways in which they are sustained or degrade during infection important.
MACROPHAGE DIVERSITY IN MYCOBACTERIAL INFECTION
During mycobacterial infection, distinct macrophage subsets are recruited to infection, exist within the granuloma and carry out diverse roles within the organism (Guirado, Schlesinger and Kaplan 2013). Recent work has shown that diverse macrophage subsets (and indeed diverse phagocytes e.g. Srivastava, Ernst and Desvignes 2014) are important in initiation of infection, development of the granuloma and cytokine expression and response. Additional environmental influences such as hypoxia (Galagan et al.2013) and lipid environment (Russell et al.2009) are also now understood to direct macrophage phenotype alteration and to affect the course of mycobacterial infection. These studies highlight the importance of macrophage heterogeneity in management and response to diverse stimuli, as well as the ability of infecting mycobacteria to exploit the endogenous heterogeneity of macrophage populations. Mycobacteria have developed strategies to preferentially recruit and infect macrophages more amenable to their growth, to alter the activation state of recruited macrophages, and to influence cytokine production by macrophages. With this understanding, the underlying heterogeneity of macrophage populations becomes increasingly important in an understanding of mycobacterial infection processes.
Heterogeneous macrophage recruitment and infection
Some of the earliest cells infected during initiation of TB are macrophages residing in the air spaces. Alveolar macrophages are classically described in ex vivo experiments as most similar to IL-4-stimulated-type macrophages that express high levels of the pattern recognition receptors mannose receptor, scavenger receptor-A and the β-glucan receptor, while also constitutively secreting lysozyme into the extracellular milieu, where it acts non-specifically to damage bacterial cell walls (Gordon 2003). In vitro studies have demonstrated that AMs respond to Mtb exposure by producing TNF and other pro-inflammatory cytokines (Keane et al.1997). However, these studies have been limited in their ability to fully classify in vivo alveolar macrophage cytokine expression on the basis of their ex vivo nature.
In the context of human mycobacterial infection, circulating monocytes are skewed significantly toward the non-classical or CD16+ phenotype as compared to uninfected individuals, where greater than 90% of circulating monocytes are of the ‘classical’ or CD16− phenotype (Balboa et al.2011). The CD16+ subset of monocytes gives rise to a subset of macrophages that are atypical in secreting elevated levels of TNFα, IL-1 and IL-6 but reduced IL-10 (Frankenberger et al.1996; Aguilar-Ruiz et al.2011), while also displaying increased pro-angiogenic behavior and motility (Wong et al.2012). Specifically, monocytes isolated during TB infection demonstrate elevated cell surface expression of CD11b, TLR2, TLR5, CCR1, CCR2 and CCR5, are deficient in their ability to differentiate into DCs, and demonstrate reduced T-cell activation (Balboa et al.2011). Macrophages differentiated from these monocytes have further been demonstrated to be skewed toward classical markers of ‘alternative’ or M2-like activation with elevated expression of CD163, MerTK, and STAT3 (Lastrucci et al.2015).
Recent work in both zebrafish and mice has shown that mycobacteria are able to preferentially recruit and infect macrophages more amenable to their intracellular growth by masking pathogen-associated molecular patterns through the action of the cell-surface-associated virulence-associated lipid phthiocerol dimycocerosate (PDIM). This action results in mycobacteria evading MyD88-dependent macrophages that would recognize their cell surface antigens through the action of TLRs. Instead, mycobacteria are able to recruit MyD88-independent permissive macrophages through a CCL2-mediated mechanism, in which the mycobacterial phenolic glycolipids (PGL) allow mycobacteria to be preferentially taken up by these macrophages. MyD88-independent macrophages were shown to produce less inducible nitric oxide synthase (iNOS) compared to MyD88-dependent macrophages making them more permissive for mycobacterial growth (Cambier et al.2014). This reduced iNOS phenotype makes these macrophages similar to IL-4-stimulated-type macrophages in this regard, indicating that mycobacteria are able to preferentially recruit these ‘alternatively activated’ alveolar macrophage types and survive within (Gordon 2003; Mosser 2003). The mycobacterially induced skew of monocyte-derived macrophages toward a phenotype expressing CCR2 and displaying an ‘alternatively activated’ like phenotype indicates that mycobacteria may also be able to selectively recruit these derived macrophages and thrive within them. These findings indicate that even at the earliest stages of infection there are different populations of macrophages circulating within an organism or particular tissue at baseline, and these different populations play different roles in progression or control of mycobacterial infection.
Macrophage diversity within the granuloma
Work has divided macrophage populations within the granuloma into generalized regions on the basis of studies in human tissue and non-human primates. In mature granulomas, the macrophage aggregates are surrounded by a lymphocyte cuff composed of B cells, T cells and plasma cells (Ramakrishnan 2012). Granuloma macrophages have been shown to have both pro- and anti-inflammatory phenotypes (Gideon and Flynn 2011; Duell et al.2012; Cilfone et al.2013; Marakalala et al.2016) and have been shown to take on different activation states or accumulate on the basis of activation states within different granuloma microenvironments (Mattila et al.2013; Marino et al.2015). The most peripheral macrophages, closest to the lymphocyte cuff, have been generally shown to be more similar to IL-4-stimulated or M2 macrophages with elevated expression of CD163 and arginase (Mattila et al.2013) and generally anti-inflammatory eicosanoid profiles (Marakalala et al.2016) (Fig. 2). The intermediate region of the granuloma can largely be thought of as containing mainly epithelioid macrophages with the occasional MGC. These macrophages have been shown to have low CD163 expression and elevated CD11c and iNOS expression, a phenotype more similar to IFNγ-simulated or M1 macrophages (Mattila et al.2013). Macrophages within the most central region in non-necrotic granulomas or just adjacent to the central necrotic core are frequently foam cells as identified by Oil Red O-staining (Peyron et al.2008; Russell et al.2009) and show elevated HAM56 staining, a reported marker for foam cell formation (Ihling et al.1996; Cummings et al.2001; Mattila et al.2013). The caseous granuloma center has also been demonstrated to accumulate pro-inflammatory regulators including TNF and the eicosanoid biosynthetic enzyme LTA4H (Marakalala et al.2016).
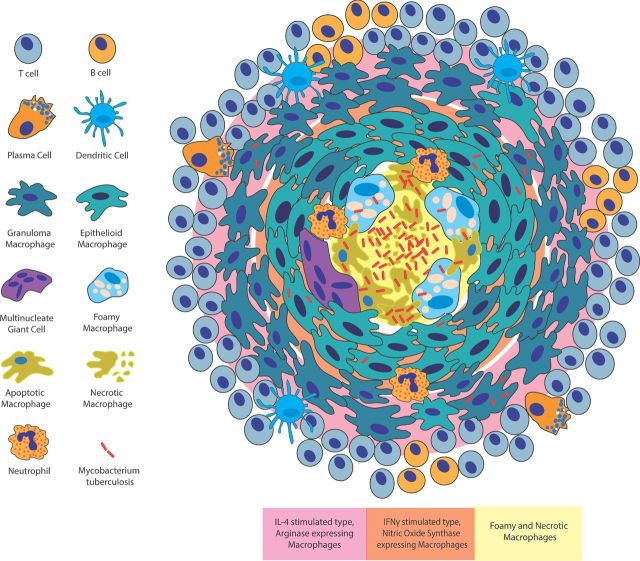
Figure 2.
Structure and inflammatory arrangement of the tuberculosis granuloma. The granuloma is an organized aggregate of macrophages surrounded by a cuff of B and T lymphocytes. Plasma cells and dendritic cells are also granuloma associated. Within the granuloma, macrophages differentiate into additional cell types including epithelioid macrophages, MGCs and foamy macrophages. Different cell types are spatially organized within the granuloma with foamy macrophages found surrounding the necrotic lipid-rich core, epithelioid macrophages forming tight junctions around the central core and additional macrophages aggregating at the outer edge of the structure. The activation state of macrophages as well as the presence of pro-inflammatory and anti-inflammatory markers is also spatially arranged with more anti-inflammatory phenotypes at the periphery of the granuloma structure (pink) and more pro-inflammatory phenotypes in central regions (orange).
Other studies of human tissue have demonstrated an increased ratio of IL-4-type macrophages within granulomas as compared to normal tissue but coexistence of both IL-4- and IFNγ-type macrophages (Huang et al.2015). These two macrophage types compete for the same substrate, L-arginine, metabolized respectively through the action of arginase-1 or Nos2 and influencing macrophage polarization toward the IL-4 or IFNγ type. The availability of each enzyme locally therefore affects the availability of substrate directing macrophage polarization to follow the spatial type (Duque-Correa et al.2014) and indicating a mechanism for possible variation between granulomas and patients in macrophage activation states. Although C57BL/6 mice generally form disorganized granulomas, recent work has demonstrated that Nos2 knockout mice are able to form necrotic granulomas and that these granulomas demonstrate increased macrophage arginase-1 expression that is associated with necrosis, suggesting an important role for IL-4-type macrophages (Reece et al.2010; Duque-Correa et al.2014). In vitro models of granuloma development have identified a shift from the IFNγ-stimulated-type to IL-4-stimulated-type macrophages over time following mycobacterial infection and implicating temporal variation as an important component of macrophage diversity during mycobacterial infection (Huang et al.2015). These in vitro observations have also been noted in murine models and human samples where more IL-4 positive cells and more IL-4-type lung macrophages have been observed during late infection stages (Hernandez-Pando et al.1996; Kahnert et al.2006).
These results highlight the diversity of macrophages on the basis of activation state within the granuloma and during mycobacterial infection. To date, studies have been limited to identification of macrophage activation states in particular regions of the granuloma or areas of tissue rather than being able to determine the precise phenotype of individual cells and cell types within the granuloma though multiple activation phenotypes are known to exist simultaneously. An understanding of potentially different ontological sources of macrophages present in the granuloma has also not been determined but might be relevant to understanding disease progression as different macrophage sources might give rise to diverse activation phenotypes under control of similar stimuli.
Diverse cytokine expression and response profiles
The central role of cytokine production and signaling by and to macrophages and the particular effects of a set of key cytokines on macrophage phenotype during mycobacterial infection has been well established (Cooper and Khader 2008). Studies have demonstrated that bone marrow-derived macrophages, necessarily of the monocyte-derived lineage, develop functional phenotypes following initial cytokine exposure that are not altered on exposure to subsequent cytokines (Erwig et al.1998). Other work in both peripheral blood mononuclear cells and tissue-resident macrophages has suggested that macrophages are able to continuously alter their activation phenotype as their environment and the cytokine exposures in the environment change (Stout and Suttles 2004; Porcheray et al.2005; Murray and Wynn 2011). This work highlights the importance of understanding the ontological contributions to macrophage populations in both early and granuloma stage infection and the effects that ontogeny may have on development of activation state in response to stimuli. The contributions of different macrophage subsets to cytokine production demonstrate the important role of macrophages in autocrine and paracrine signaling during mycobacterial infection.
One of the most central cytokines in mycobacterial infection is IFNγ, normally produced in response to IL-12p70 stimulation and central to control of mycobacterial infection (Bustamante et al.2014). IFNγ is produced largely by CD4+ and CD8+ T cells and secondarily by restricted or invariant receptor repertoire innate lymphocytes, γδ T cells, NK T cells and NK cells, when adaptive responses are restrained or absent (Junqueira-Kipnis et al.2003; Feng et al.2006; Cooper and Khader 2008). IL-23, composed of IL-12p40 and IL-23p19 subunits, is produced by lung macrophages early in infection (Silver et al.2009; Riol-Blanco et al.2010). Differential stimulation of monocytes with either GM-CSF or M-CSF (monocyte colony-stimulating factor) followed by exposure to mycobacteria results in differential expression of IL-23 or IL-10 respectively by these macrophage types (Verreck et al.2004) and therefore differential stimulation of IFNγ production. Short-term production of IFNγ can be accomplished through stimulation by macrophage-produced IL-23, but this production is not sufficient to control bacterial growth in long term (Khader et al.2005). Lung expression of IL-12p70 is low, with major production occurring by cell types in the draining lymph nodes (Cooper et al.2002) and resulting in strong IFNγ production later in infection (Feng et al.2005) IL-23 production by macrophages also induces production of IL-17 by γδ T cells resulting in induction of adhesion molecule expression and contributing to induction of granuloma formation (Lockhart, Green and Flynn 2006; Umemura et al.2007; Okamoto et al.2008).
IL-10 has been demonstrated to act as a mediator of macrophage phenotype during both early and late mycobacterial infection. Strong autocrine induction of IL-10 during early infection has been shown to reduce protective responses to Mtb such as IL-12p40 and TNF production in animal models (Turner et al.2002; Beamer et al.2008) and to be involved in blocking phagosome maturation in human alveolar macrophages (O'Leary, O'Sullivan and Keane 2011). IL-10 is also required for long-term control of inflammation (Higgins et al.2009) and evidence indicates elevated IL-10 signaling in macrophages just interior to the lymphocyte cuff consistent with an increased prevalence of IL-4-type macrophages and possibly indicative of an important anti-inflammatory role of peripheral granuloma macrophages (Mattila et al.2013). Intriguingly, IL-10 deletion in CBA/J mice results in mature fibrotic granuloma formation (Cyktor et al.2013). The balance of action of macrophages in promoting and controlling inflammation within the granuloma is an active area of research, and the balance of anti-inflammatory and pro-inflammatory cytokines may play a key regulatory role in granuloma organization and trajectory (Gideon et al.2015).
TNF is a key determinant of susceptibility to mycobacterial infection and has been shown to be robustly induced in granuloma macrophages (Flynn et al.1995; Roach et al.2002; Capuano et al.2003; Clay, Volkman and Ramakrishnan 2008). Recent characterization of human granulomas suggests a pro-inflammatory central milieu associated with necrotic cores and with pro-inflammatory eicosanoids that impact TNF production (Marakalala et al.2016). The eiconsanoids are lipid mediators derived from arachidonic acid and include of prostaglandins, lipoxins and leukotrienes. The balance of these lipid mediators both in cell culture and in vivo affects the outcome of infection, via influence on mechanisms of macrophage cell death (Bafica et al.2005; Chen et al.2008; Behar, Divangahi and Remold 2010; Divangahi et al.2010). Necrotic outcomes result in more effective mycobacterial replication. Polymorphisms in human populations at the LTA4H locus, altering production of a key enzyme determining the balance of lipoxin A4 and leukotriene B4 production, have been identified and associated with differential disease outcomes in patients (Tobin et al.2010, 2012). In addition, modulation of more upstream components of these arachidonic acid-derived eicosanoids, including targeting of the upstream 5-lipooxygenase and manipulation of PGE2 levels have strong effects on TB progression in mice, with PGE2 serving to inhibit the Type I IFN response and increase host resistance to Mtb (Mayer-Barber et al.2014; Mayer-Barber and Sher 2015).
ANALOGIES TO MACROPHAGES IN OTHER SYSTEMS
Hypoxia and macrophage heterogeneity: lessons from tumors
Hypoxia has been recognized as a mechanism by which specific types of macrophages are recruited to cancerous tumors and by which macrophages are influenced to take on diverse phenotypes within the tumor environment (Biswas, Sica and Lewis et al.2008; Tripathi et al.2014) The diverse phenotypes of these tumor-associated macrophages (TAMs) have begun to be characterized. Hypoxia has also been identified as an important factor controlling progression during mycobacterial infection. Previous work has focused largely on examining the molecular signatures of the bacteria adapted to hypoxic conditions, attributing changes observed during hypoxia to the influence of mycobacterial products on host processes. A more detailed examination of the influences of local hypoxia on alterations in host immune response would be important for better understanding mycobacterial infection as it relates to this important environmental influence.
Tumors are abundantly populated with macrophages and these TAMs are known to promote tumor initiation, progression and metastasis partly through the production of inflammatory cytokines that generate a chronic inflammatory environment permissive for tumor promotion (Noy and Pollard 2014). Tumor conditions switch infiltrating macrophages from an immunologically active state to a trophic immune-suppressive phenotype that promotes tumor progression and malignancy partly through the action of myeloid-derived suppressor cells (MDSC) (Ostrand-Rosenberg et al.2012). MDSCs have also been reported in TB, where they may play a role in disease severity (du Plessis et al.2013; Knaul et al.2014; Tsiganov et al.2014). Infiltrating macrophages are recruited by hypoxic conditions and take on a variety of phenotypes including a more IL-4-stimulated-type phenotype with low expression of MHC class II (Lin and Pollard 2007). These M2-like cells express growth factors orchestrating the development of angiogenesis into tumors and resulting in promotion of malignancy on the basis of exposure to hypoxia, whereas other macrophages identified in tumors are not involved in this production (Laoui et al.2014). It is believed that most TAMs are monocyte-derived macrophages that are recruited to the developing tumor as a result of identification of danger signals and that these macrophages take on distinct phenotypes on the basis of stimuli at the site of tumor development and as a result of their baseline activation state. This system of macrophage infiltration results in the development of distinct subsets of macrophages in different tumor macrophage microenvironments (Movahedi et al.2010). These microenvironments, including hypoxic conditions and areas of macrophage-directed angiogenesis, are similar to conditions found in TB granulomas.
The necrotic core of the granuloma has long been believed to be a hypoxic environment (Barry et al.2009) and work in representative model organisms has confirmed this suspicion (Via et al.2008; Datta et al.2015; Matty et al.2015; Oehlers et al.2015). Studies have identified characteristic metabolic shifts that occur in infecting mycobacteria during hypoxic conditions (Ohno et al.2003; Kumar et al.2008; Shiloh, Manzanillo and Cox 2008; Galagan et al.2013), and it has been observed that reactivation most commonly occurs in more highly oxygenated locations within the human lung (Meylan, Richman and Kornbluth 1992), indicating both an ability and a need for reactivation to be directed toward well-oxygenated regions. Recent work has also identified host angiogenesis into the early developing granuloma as an important determinant of mycobacterial survival (Oehlers et al.2015). This granuloma angiogenesis is driven by the expression of vascular endothelial growth factor A by granuloma macrophages, indicating a particular role of granuloma macrophages in promoting control of hypoxia and directing increases in mycobacterial burden within developing granulomas as a consequence. Trehalose 6,6′-dimycolate purified from Mtb has been demonstrated to induce angiogenesis in experimental models (Saita et al.2000) and VEGF has been shown to be induced during human mycobacterial infections and in granuloma macrophages (Alatas et al.2004; Datta et al.2015). Work in TB has not identified the activation state or otogeny of macrophages involved in induction of angiogenesis. Given the findings that only particular macrophage subsets within tumors are involved in induction of angiogenesis, it is reasonable to assume that a similar subset of macrophages might be responsible for induction of angiogenesis in response to the hypoxic conditions of the granuloma. Identification of this macrophage subset and control of development of this phenotype during mycobacterial infection might be a step toward adjunctive control of disease.
It has been observed that dissemination of mycobacterial infection occurs from the granuloma (Davis and Ramakrishnan 2009) and that iDCs migrate in and out of the granuloma acting to disseminate infection widely while also priming the immune response (Schreiber et al.2011). This indicates a possible specific role for these monocyte-derived macrophage and dendritic cell subsets in mycobacterial infection that is distinct from the potential roles of tissue-resident embryonically derived macrophage subsets. Given the known role of monocyte-derived macrophages in the hypoxic environment of the tumor, it is possible that this particular macrophage population is also important for both promotion of mycobacterial growth through induction of angiogenesis, and later dissemination of disease when hypoxic conditions can no longer be overcome. The particular role of hypoxia in recruiting monocytes for differentiation into iDCs in the setting of infection is unknown. Investigation of this mechanism would add understanding to the picture of granuloma development and maintenance as it has previously been thought that most macrophages resulting in granuloma development in the lungs were of the alveolar macrophage subset, a population of embryonically derived tissue-resident macrophages. Understanding the mechanisms at play and developing strategies to block the differentiation of monocytes into macrophages and dendritic cells at the sites of granuloma development might be relevant to controlling spread of mycobacterial infection from established granulomas to new infection sites.
Cholesterol and macrophage heterogeneity: lessons from atherosclerosis
Macrophage heterogeneity is developing as an important area of inquiry toward understanding the processes of atherosclerosis. Atherosclerosis and the immunological response that ensues results in the development of foamy macrophages, which persist in plaques and are thought to promote disease progression (Moore, Sheedy and Fisher 2013). A second important parallel to TB infection is in the development of foamy macrophages within the granuloma environment. As previously described, foamy macrophages are also considered important cell types in TB, developing in response to infection and persisting or dying in granulomatous tissues providing lipid-rich contents in central granuloma regions, where exuberant extracellular mycobacterial growth can occur. The concentration of cholesterol within foamy macrophages during mouse and guinea pig Mtb infections has also been shown to lead to the development of cholesterol crystals during TB (Caceres et al.2009). In mouse models of atherosclerosis, cholesterol crystals can result in lysosomal destabilization and activate the NLRP3 inflammasome and IL-1 secretion (Duewell et al.2010; Sheedy et al.2013). The role of inflammasome activation in mycobacterial infections is complex and the subject of more in-depth treatment in a number of reviews (Briken, Ahlbrand and Shah 2013; Mayer-Barber and Yan 2016). There appears to be ESX-1-dependent activation of the AIM2 and NLRP3 inflammasomes as well as counterregulation via NO, Type I IFN production and additional mechanisms (Mishra et al.2010; Saiga et al.2012; Mishra et al.2013; Shah et al.2013; Wassermann et al.2015; Kupz et al.2016) However, the sufficiency of cholesterol crystals in atherosclerosis to activate the NLRP3 inflammasome may provide additional insights into macrophage phenotypes and activation states during mycobacterial infection. Further work to understand the reason that particular macrophages and not others develop the foamy phenotype would also add value to our understanding of this important disease process.
Atherosclerosis occurs when, following intramural retention of cholesterol-rich lipoproteins in areas of the vascular wall, monocyte-derived phagocytes migrate into the subendothelial space where they take up accumulated lipids at an abnormally elevated rate and transform into cholesterol-laden foamy macrophages. It has been observed that hypercholesterolemia results in an increase in circulating monocytes (Averill, Meagher and Gerrity et al.1989; Feldman et al.1991) particularly of the inflammatory Ly6Chigh subset (Swirski et al.2007; Tacke et al.2007) due to an increase in hematopoietic stem and progenitor cell proliferation (Yvan-Charvet et al.2010). This results in development of more pro-inflammatory IFNγ-stimulated-type macrophages in atherosclerotic lesions following infiltration of these circulating inflammatory monocytes into developing atherosclerotic lesions (Ley, Miller and Hedrick 2011). It is these monocyte-derived macrophages that go on to develop into foam cells during atherosclerosis (Moore and Freeman 2006; Miller et al.2011) highlighting the possibility that particularly monocyte-derived macrophages are responsible for foam cell development during TB infection as well. Similar to findings in granulomas, atherosclerotic plaques have demonstrated distinct subsets of IL-4-type- and IFNγ-type-activated macrophages that exist simultaneously and are dynamically altered over time and in response to environmental stimuli (Gallardo-Soler et al.2008; Chinetti-Gbaguidi et al.2011). However, the factors that influence differential macrophage activation state in vivo during atherosclerosis are incompletely defined.
The scavenger receptors SRA and CD36, known to be expressed on macrophages during both atherosclerosis and mycobacterial infection, have been demonstrated to account for 75%–90% of uptake and breakdown of LDL in atherosclerotic plaques resulting in foam cell formation during atherosclerosis (Kunjathoor et al.2002; Kzhyshkowska, Neyen and Gordon 2012). Excessive cholesterol uptake during atherogenesis results in elevated levels of free cholesterol in cell membranes and defects in the ability of macrophages to process free cholesterol to cholesterol esters for safe intracellular storage. This accumulation results in increased inflammatory signaling from lipid rafts including increased action of TLRs (Yvan-Charvet et al.2008; Zhu et al.2010; Mogilenko et al.2012) and also inhibits controlled apoptosis of lipid-loaded cells (Feng et al.2003). Eventually, the cholesterol accumulation becomes toxic to cells resulting in their death through secondary necrosis and release of cellular contents resulting in formation of a necrotic core within atherosclerotic plaques (Tabas 2005). The development of the necrotic core is thought to make atherosclerotic plaques more dynamic, leading to thinning of the fibrous cap surrounding the lesion and increasing risk of plaque rupture and dissemination of atherosclerotic products into the bloodstream.
The observed processes by which foamy macrophages are killed during atherogenesis, initiate necrosis and ultimately influence instability of the atherosclerotic plaque that might also be relevant to mycobacterial infection, and might represent a pathogen-directed protective mechanism through which mycobacteria are released extracellularly and provided with rich nutritional content for growth, initiating the central necrosis and caseation observed in mature granulomas, and ultimately influencing later dissemination of mycobacteria by inflammatory macrophage and dendritic cell subsets that respond to the development of necrosis. As research has demonstrated a reliance on the cholesterol accumulated within foamy macrophages to sustain mycobacterial infection over the long term, the particular role of monocyte-derived versus tissue-resident macrophage populations in contributing to the development of foamy macrophages in granulomatous regions might prove a fruitful area of investigation in order to understand and abrogate this important pathological process in TB disease progression. Foamy macrophages are found in granulomas of infectious origin but are rarely reported in sterile or autoimmune granulomas (James 2000). Foamy macrophages are also observed in other chronic intracellular infections including chlamydia (Kalayoglu and Byrne 1998) and toxoplasma (Portugal et al.2008) species and can be observed as part of an inflammatory response to accumulated lipids during endogenous lipoid pneumonia caused by structural lung disease (Harris et al.2011). Future work should lead to improved understanding of the unique triggers that encourage particular macrophage subsets to incorporate into granulomas and, upon arrival or infection, accumulate lipids and adopt this phenotype.
CONCLUSION
Diverse macrophage repertoires in vivo contribute to wide variation in macrophage phenotype during homeostasis. Developing understanding of the differential roles of monocyte-derived and tissue-resident macrophage populations in disease processes such as cancer and atherosclerosis are contributing to an improved understanding of the importance of macrophage ontogeny in determining responses to cytokine stimulation and initial as well as ultimate activation state of macrophages. In the context of mycobacterial infection, diverse macrophage phenotypes respond to and influence an array of stimuli allowing dynamic infection to result in control, low-level infection or dissemination of infection. An improved understanding of the particular role of individual macrophages and macrophage subsets, the stimuli which cause their development and recruitment, and their effect on mycobacterial development is essential to the improved understanding of the role of the macrophage in mycobacterial infection. Future work toward understanding this variability may be facilitated by recently developed techniques allowing for analysis of single cells by infection phenotype, RNA-seq expression profile and cytokine production profile (Avraham et al.2015; Xue et al.2015). Ultimately, an understanding of the complex cell-to-cell variation among macrophages during mycobacterial infection will help us to better understand the diversity of response to infection in patients and the processes that occur during TB infection. This understanding may allow for the development of novel approaches to manipulating disease progression via modulation of macrophage phenotype, form and function.
Acknowledgments
We thank members of the Tobin laboratory for helpful discussions.
FUNDING
This work was supported by the National Institutes of Health T32 GM007171—Medical Scientist Training Program (CMM), and a Mallinckrodt Scholar Award, a Searle Scholar Award, a Vallee Foundation Young Investigator Award, an NIH Director’s New Innovator Award 1DP2-OD008614 and NIAID 1R21AI111067 (DMT).
Conflict of interest. None declared.
REFERENCES
- Adams DO. The structure of mononuclear phagocytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of Bacillus Calmette-Guerin (BCG) Am J Pathol. 1974;76:17–48. [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Ruiz SR, Torres-Aguilar H, Gonzalez-Dominguez E, et al. Human CD16+ and CD16- monocyte subsets display unique effector properties in inflammatory conditions in vivo. J Leukocyte Biol. 2011;90:1119–31. doi: 10.1189/jlb.0111022. [DOI] [PubMed] [Google Scholar]
- Alatas F, Alatas O, Metintas M, et al. Vascular endothelial growth factor levels in active pulmonary tuberculosis. Chest. 2004;125:2156–9. doi: 10.1378/chest.125.6.2156. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. 1999;117:145–52. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Averill LE, Meagher RC, Gerrity RG. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am J Pathol. 1989;135:369–77. [PMC free article] [PubMed] [Google Scholar]
- Avraham R, Haseley N, Brown D, et al. Pathogen cell-to-cell variability drives heterogeneity in host immune responses. Cell. 2015;162:1309–21. doi: 10.1016/j.cell.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafica A, Scanga CA, Serhan C, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115:1601–6. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–37. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa L, Romero MM, Basile JI, et al. Paradoxical role of CD16+CCR2+CCR5+ monocytes in tuberculosis: efficient APC in pleural effusion but also mark disease severity in blood. J Leukocyte Biol. 2011;90:69–75. doi: 10.1189/jlb.1010577. [DOI] [PubMed] [Google Scholar]
- Baldan A, Gomes AV, Ping P, et al. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–8. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–55. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer GL, Flaherty DK, Assogba BD, et al. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J Immunol. 2008;181:5545–50. doi: 10.4049/jimmunol.181.8.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–74. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–7. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- Briken V, Ahlbrand SE, Shah S. Mycobacterium tuberculosis and the host cell inflammasome: a complex relationship. Front Cell Infect Microbiol. 2013;3:62. doi: 10.3389/fcimb.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J, Boisson-Dupuis S, Abel L, et al. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol. 2014;26:454–70. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres N, Tapia G, Ojanguren I, et al. Evolution of foamy macrophages in the pulmonary granulomas of experimental tuberculosis models. Tuberculosis. 2009;89:175–82. doi: 10.1016/j.tube.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Cadena AM, Flynn JL, Fortune SM. The importance of first impressions: early events in mycobacterium tuberculosis infection influence outcome. mBio. 2016;7:e00342–16. doi: 10.1128/mBio.00342-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier CJ, Takaki KK, Larson RP, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–22. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano SV, 3rd, Croix DA, Pawar S, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–44. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers TJ, Spector WG. Inflammatory giant cells. Immunobiology. 1982;161:283–9. doi: 10.1016/S0171-2985(82)80084-3. [DOI] [PubMed] [Google Scholar]
- Chen M, Divangahi M, Gan H, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008;205:2791–801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue SW, Terebuh PD, Warmington KS, et al. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992;148:900–6. [PubMed] [Google Scholar]
- Chinetti-Gbaguidi G, Baron M, Bouhlel MA, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res. 2011;108:985–95. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilfone NA, Perry CR, Kirschner DE, et al. Multi-scale modeling predicts a balance of tumor necrosis factor-alpha and interleukin-10 controls the granuloma environment during Mycobacterium tuberculosis infection. PloS One. 2013;8:e68680. doi: 10.1371/journal.pone.0068680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–94. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, Kipnis A, Turner J, et al. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–7. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- Cosma CL, Sherman DR, Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Annu Rev Microbiol. 2003;57:641–76. doi: 10.1146/annurev.micro.57.030502.091033. [DOI] [PubMed] [Google Scholar]
- Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–55. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- Cummings TJ, Hulette CM, Bigner SH, et al. Ham56-immunoreactive macrophages in untreated infiltrating gliomas. Arch Pathol Lab Med. 2001;125:637–41. doi: 10.5858/2001-125-0637-HIMIUI. [DOI] [PubMed] [Google Scholar]
- Cyktor JC, Carruthers B, Kominsky RA, et al. IL-10 inhibits mature fibrotic granuloma formation during Mycobacterium tuberculosis infection. J Immunol. 2013;190:2778–90. doi: 10.4049/jimmunol.1202722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallenga T, Schaible UE. Neutrophils in tuberculosis–first line of defence or booster of disease and targets for host-directed therapy? Pathog Dis. 2016;74 doi: 10.1093/femspd/ftw012. [DOI] [PubMed] [Google Scholar]
- Datta M, Via LE, Kamoun WS, et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. P Natl Acad Sci USA. 2015;112:1827–32. doi: 10.1073/pnas.1424563112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avila H, Maya-Monteiro CM, Bozza PT. Lipid bodies in innate immune response to bacterial and parasite infections. Int Immunopharmacol. 2008;8:1308–15. doi: 10.1016/j.intimp.2008.01.035. [DOI] [PubMed] [Google Scholar]
- D'Avila H, Melo RC, Parreira GG, et al. Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 2006;176:3087–97. doi: 10.4049/jimmunol.176.5.3087. [DOI] [PubMed] [Google Scholar]
- Davis JM, Clay H, Lewis JL, et al. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFife KM, Jenney CR, McNally AK, et al. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol. 1997;158:3385–90. [PubMed] [Google Scholar]
- Divangahi M, Desjardins D, Nunes-Alves C, et al. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol. 2010;11:751–8. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis N, Loebenberg L, Kriel M, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent mycobacterium tuberculosis infection suppresses T-cell function. AM J Resp Crit Care. 2013;188:724–32. doi: 10.1164/rccm.201302-0249OC. [DOI] [PubMed] [Google Scholar]
- Duell BL, Tan CK, Carey AJ, et al. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol Med Mic. 2012;64:295–313. doi: 10.1111/j.1574-695X.2012.00931.x. [DOI] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Correa MA, Kuhl AA, Rodriguez PC, et al. Macrophage arginase-1 controls bacterial growth and pathology in hypoxic tuberculosis granulomas. P Natl Acad Sci USA. 2014;111:E4024–32. doi: 10.1073/pnas.1408839111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S, Schaible UE. The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Front Immunol. 2012;3:411. doi: 10.3389/fimmu.2012.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein WL, Fukuyama K, Danno K, et al. Granulomatous inflammation in normal and athymic mice infected with schistosoma mansoni: an ultrastructural study. J Pathol. 1979;127:207–15. doi: 10.1002/path.1711270408. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Kluth DC, Walsh GM, et al. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–8. [PubMed] [Google Scholar]
- Eum SY, Kong JH, Hong MS, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–8. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DL, Mogelesky TC, Liptak BF, et al. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb. 1991;11:985–94. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Feng CG, Jankovic D, Kullberg M, et al. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005;174:4185–92. doi: 10.4049/jimmunol.174.7.4185. [DOI] [PubMed] [Google Scholar]
- Feng CG, Kaviratne M, Rothfuchs AG, et al. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol. 2006;177:7086–93. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- Feng Y, Dorhoi A, Mollenkopf HJ, et al. Platelets direct monocyte differentiation into epithelioid-like multinucleated giant foam cells with suppressive capacity upon mycobacterial stimulation. J Infect Dis. 2014;210:1700–10. doi: 10.1093/infdis/jiu355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Frankenberger M, Sternsdorf T, Pechumer H, et al. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87:373–7. [PubMed] [Google Scholar]
- Frieden TR, Sterling TR, Munsiff SS, et al. Tuberculosis. Lancet. 2003;362:887–99. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- Galagan JE, Minch K, Peterson M, et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature. 2013;499:178–83. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo-Soler A, Gomez-Nieto C, Campo ML, et al. Arginase I induction by modified lipoproteins in macrophages: a peroxisome proliferator-activated receptor-gamma/delta-mediated effect that links lipid metabolism and immunity. Mol Endocrinol. 2008;22:1394–402. doi: 10.1210/me.2007-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gideon HP, Flynn JL. Latent tuberculosis: what the host “sees”? Immunol Res. 2011;50:202–12. doi: 10.1007/s12026-011-8229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gideon HP, Phuah J, Myers AJ, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015;11:e1004603. doi: 10.1371/journal.ppat.1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, De Kleer I, Henri S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–92. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol. 2013;35:563–83. doi: 10.1007/s00281-013-0388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K, Chalhoub M, Maroun R, et al. Lipoid pneumonia: a challenging diagnosis. Heart Lung. 2011;40:580–4. doi: 10.1016/j.hrtlng.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007a;37:33–42. doi: 10.1002/eji.200636788. [DOI] [PubMed] [Google Scholar]
- Helming L, Gordon S. The molecular basis of macrophage fusion. Immunobiology. 2007b;212:785–93. doi: 10.1016/j.imbio.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Henderson RA, Watkins SC, Flynn JL. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–43. [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–45. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pando R, Orozcoe H, Sampieri A, et al. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- Hettinger J, Richards DM, Hansson J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–30. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- Higgins DM, Sanchez-Campillo J, Rosas-Taraco AG, et al. Lack of IL-10 alters inflammatory and immune responses during pulmonary Mycobacterium tuberculosis infection. Tuberculosis. 2009;89:149–57. doi: 10.1016/j.tube.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–81. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Luo Q, Guo Y, et al. Mycobacterium tuberculosis-induced polarization of human macrophage orchestrates the formation and development of Tuberculous Granulomas in vitro. PloS One. 2015;10:e0129744. doi: 10.1371/journal.pone.0129744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- Ihling C, Gobel HR, Lippoldt A, et al. Endothelin-1-like immunoreactivity in human atherosclerotic coronary tissue: a detailed analysis of the cellular distribution of endothelin-1. J Pathol. 1996;179:303–8. doi: 10.1002/(SICI)1096-9896(199607)179:3<303::AID-PATH585>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–23. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Gautier EL, Gibbings SL, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DG. A clinicopathological classification of granulomatous disorders. Postgrad Med J. 2000;76:457–65. doi: 10.1136/pmj.76.898.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira-Kipnis AP, Kipnis A, Jamieson A, et al. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol. 2003;171:6039–45. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- Kahnert A, Seiler P, Stein M, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–47. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- Kalayoglu MV, Byrne GI. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177:725–9. doi: 10.1086/514241. [DOI] [PubMed] [Google Scholar]
- Keane J, Balcewicz-Sablinska MK, Remold HG, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Pearl JE, Sakamoto K, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- Knaul JK, Jorg S, Oberbeck-Mueller D, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. AM J Resp Crit Care. 2014;190:1053–66. doi: 10.1164/rccm.201405-0828OC. [DOI] [PubMed] [Google Scholar]
- Kondo E, Kanai K. Accumulation of cholesterol esters in macrophages incubated with mycobacteria in vitro. Jpn J Med Sci Biol. 1976;29:123–37. doi: 10.7883/yoken1952.29.123. [DOI] [PubMed] [Google Scholar]
- Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- Kumar A, Deshane JS, Crossman DK, et al. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem. 2008;283:18032–9. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–8. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- Kupz A, Zedler U, Staber M, et al. ESAT-6-dependent cytosolic pattern recognition drives noncognate tuberculosis control in vivo. J Clin Invest. 2016;126:2109–22. doi: 10.1172/JCI84978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Laoui D, Van Overmeire E, Di Conza G, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- Lastrucci C, Benard A, Balboa L, et al. Tuberculosis is associated with expansion of a motile, permissive and immunomodulatory CD16(+) monocyte population via the IL-10/STAT3 axis. Cell Res. 2015;25:1333–51. doi: 10.1038/cr.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–26. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Lay G, Poquet Y, Salek-Peyron P, et al. Langhans giant cells from M. tuberculosis-induced human granulomas cannot mediate mycobacterial uptake. J Pathol. 2007;211:76–85. doi: 10.1002/path.2092. [DOI] [PubMed] [Google Scholar]
- Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscl Throm Vas Biol. 2011;31:1506–16. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pritchard DK, Wang X, et al. cDNA microarray analysis reveals fundamental differences in the expression profiles of primary human monocytes, monocyte-derived macrophages, and alveolar macrophages. J Leukocyte Biol. 2007;81:328–35. doi: 10.1189/jlb.0206124. [DOI] [PubMed] [Google Scholar]
- Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–6. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- Lin PL, Ford CB, Coleman MT, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med. 2014;20:75–9. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- Lugo-Villarino G, Neyrolles O. Manipulation of the mononuclear phagocyte system by Mycobacterium tuberculosis. Cold Spring Harbor Perspect Med. 2014;4:a018549. doi: 10.1101/cshperspect.a018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Dkhar HK, Chandra V, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Immunol. 2012;188:5593–603. doi: 10.4049/jimmunol.1103038. [DOI] [PubMed] [Google Scholar]
- Marakalala MJ, Raju RM, Sharma K, et al. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat Med. 2016;22:531–8. doi: 10.1038/nm.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Cilfone NA, Mattila JT, et al. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect Immun. 2015;83:324–38. doi: 10.1128/IAI.02494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Pawar S, Fuller CL, et al. Dendritic cell trafficking and antigen presentation in the human immune response to Mycobacterium tuberculosis. J Immunol. 2004;173:494–506. doi: 10.4049/jimmunol.173.1.494. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila JT, Ojo OO, Kepka-Lenhart D, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191:773–84. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matty MA, Roca FJ, Cronan MR, et al. Adventures within the speckled band: heterogeneity, angiogenesis, and balanced inflammation in the tuberculous granuloma. Immunol Rev. 2015;264:276–87. doi: 10.1111/imr.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber KD, Andrade BB, Oland SD, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber KD, Sher A. Cytokine and lipid mediator networks in tuberculosis. Immunol Rev. 2015;264:264–75. doi: 10.1111/imr.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber KD, Yan B. Clash of the cytokine titans: counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell Mol Immunol. 2016 doi: 10.1038/cmi.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes A, Rennick DM. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988;167:598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147:1487–99. [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–6. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Meylan PR, Richman DD, Kornbluth RS. Reduced intracellular growth of mycobacteria in human macrophages cultivated at physiologic oxygen pressure. Am Rev Respir Dis. 1992;145:947–53. doi: 10.1164/ajrccm/145.4_Pt_1.947. [DOI] [PubMed] [Google Scholar]
- Milde R, Ritter J, Tennent GA, et al. Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell Rep. 2015;13:1937–48. doi: 10.1016/j.celrep.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–48. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BB, Moura-Alves P, Sonawane A, et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–63. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Rathinam VA, Martens GW, et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilenko DA, Orlov SV, Trulioff AS, et al. Endogenous apolipoprotein A-I stabilizes ATP-binding cassette transporter A1 and modulates Toll-like receptor 4 signaling in human macrophages. FASEB J. 2012;26:2019–30. doi: 10.1096/fj.11-193946. [DOI] [PubMed] [Google Scholar]
- Monin L, Khader SA. Chemokines in tuberculosis: the good, the bad and the ugly. Semin Immunol. 2014;26:552–8. doi: 10.1016/j.smim.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscl Throm Vas Biol. 2006;26:1702–11. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–21. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukocyte Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers SH, Cronan MR, Scott NR, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517:612–5. doi: 10.1038/nature13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Zhu G, Mohan VP, et al. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol. 2003;5:637–48. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Takeda K, Joetham A, et al. Essential role of Notch signaling in effector memory CD8+ T cell-mediated airway hyperresponsiveness and inflammation. J Exp Med. 2008;205:1087–97. doi: 10.1084/jem.20072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary S, O'Sullivan MP, Keane J. IL-10 blocks phagosome maturation in mycobacterium tuberculosis-infected human macrophages. Am J Resp Cell Mol. 2011;45:172–80. doi: 10.1165/rcmb.2010-0319OC. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P, Beury DW, et al. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–81. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. P Natl Acad Sci USA. 2008;105:4376–80. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron P, Vaubourgeix J, Poquet Y, et al. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Annu Rev Pathol. 2012;7:353–84. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C, Rimoldi M, Raes G, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. P Natl Acad Sci USA. 2009;106:14978–83. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal LR, Fernandes LR, Pietra Pedroso VS, et al. Influence of low-density lipoprotein (LDL) receptor on lipid composition, inflammation and parasitism during Toxoplasma gondii infection. Microbes Infect. 2008;10:276–84. doi: 10.1016/j.micinf.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12:352–66. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- Reece ST, Loddenkemper C, Askew DJ, et al. Serine protease activity contributes to control of Mycobacterium tuberculosis in hypoxic lung granulomas in mice. J Clin Invest. 2010;120:3365–76. doi: 10.1172/JCI42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repasy T, Lee J, Marino S, et al. Intracellular bacillary burden reflects a burst size for Mycobacterium tuberculosis in vivo. PLoS Pathog. 2013;9:e1003190. doi: 10.1371/journal.ppat.1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riol-Blanco L, Lazarevic V, Awasthi A, et al. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J Immunol. 2010;184:1710–20. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach DR, Bean AG, Demangel C, et al. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- Rubins JB. Alveolar macrophages: wielding the double-edged sword of inflammation. Am J Resp Crit Care. 2003;167:103–4. doi: 10.1164/rccm.2210007. [DOI] [PubMed] [Google Scholar]
- Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- Russell DG, Cardona PJ, Kim MJ, et al. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–8. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiga H, Kitada S, Shimada Y, et al. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol. 2012;24:637–44. doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- Saita N, Fujiwara N, Yano I, et al. Trehalose 6,6'-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infect Immun. 2000;68:5991–7. doi: 10.1128/iai.68.10.5991-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Nobs SP, Kurrer M, et al. Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol. 2014;15:1026–37. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- Schreiber HA, Harding JS, Hunt O, et al. Inflammatory dendritic cells migrate in and out of transplanted chronic mycobacterial granulomas in mice. J Clin Invest. 2011;121:3902–13. doi: 10.1172/JCI45113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–5. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Shah S, Bohsali A, Ahlbrand SE, et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-beta and AIM2 inflammasome-dependent IL-1beta production via its ESX-1 secretion system. J Immunol. 2013;191:3514–8. doi: 10.4049/jimmunol.1301331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–20. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3:323–30. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AR, Pacheco P, Vieira-de-Abreu A, et al. Lipid bodies in oxidized LDL-induced foam cells are leukotriene-synthesizing organelles: a MCP-1/CCL2 regulated phenomenon. Biochim Biophys Acta. 2009;1791:1066–75. doi: 10.1016/j.bbalip.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Silver RF, Walrath J, Lee H, et al. Human alveolar macrophage gene responses to Mycobacterium tuberculosis strains H37Ra and H37Rv. Am J Resp Cell Mol. 2009;40:491–504. doi: 10.1165/rcmb.2008-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Jamwal S, Jain R, et al. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe. 2012;12:669–81. doi: 10.1016/j.chom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Skold M, Behar SM. Tuberculosis triggers a tissue-dependent program of differentiation and acquisition of effector functions by circulating monocytes. J Immunol. 2008;181:6349–60. doi: 10.4049/jimmunol.181.9.6349. [DOI] [PubMed] [Google Scholar]
- Soucie EL, Weng Z, Geirsdottir L, et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science. 2016;351:aad5510. doi: 10.1126/science.aad5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev. 2014;262:179–92. doi: 10.1111/imr.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, et al. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukocyte Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscl Throm Vas Biol. 2005;25:2255–64. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Emori K, Nagao S, et al. Epithelioid granuloma formation requiring no T-cell function. Am J Pathol. 1982;106:165–70. [PMC free article] [PubMed] [Google Scholar]
- Tauber AI. Metchnikoff and the phagocytosis theory. Nat Rev Mol Cell Bio. 2003;4:897–901. doi: 10.1038/nrm1244. [DOI] [PubMed] [Google Scholar]
- Thurnher M, Ramoner R, Gastl G, et al. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. Int J Cancer. 1997;70:128–34. doi: 10.1002/(sici)1097-0215(19970106)70:1<128::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tobin DM, Roca FJ, Oh SF, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–46. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, Vary JC, Jr, Ray JP, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–30. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi C, Tewari BN, Kanchan RK, et al. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget. 2014;5:5350–68. doi: 10.18632/oncotarget.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganov EN, Verbina EM, Radaeva TV, et al. Gr-1dimCD11b+ immature myeloid-derived suppressor cells but not neutrophils are markers of lethal tuberculosis infection in mice. J Immunol. 2014;192:4718–27. doi: 10.4049/jimmunol.1301365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T, Dannenberg AM, Ando M, et al. Mononuclear cell turnover in chronic inflammation: studies on tritiated thymidine-labeled cells in blood, tuberculin traps, and dermal BCG lesions of rabbits. Am J Pathol. 1976;83:255–68. [PMC free article] [PubMed] [Google Scholar]
- Tung YC, Ou TT, Tsai WC. Defective Mycobacterium tuberculosis antigen presentation by monocytes from tuberculosis patients. Int J Tuberc Lung D. 2013;17:1229–34. doi: 10.5588/ijtld.12.0984. [DOI] [PubMed] [Google Scholar]
- Turner J, Gonzalez-Juarrero M, Ellis DL, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–51. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–96. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- van Furth R, Cohn ZA, Hirsch JG, et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. B World Health Organ. 1972;46:845–52. [PMC free article] [PubMed] [Google Scholar]
- Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–75. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- Verreck FA, de Boer T, Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. P Natl Acad Sci USA. 2004;101:4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via LE, Lin PL, Ray SM, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–40. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann R, Gulen MF, Sala C, et al. Mycobacterium tuberculosis differentially activates cgas- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17:799–810. doi: 10.1016/j.chom.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Welsh KJ, Risin SA, Actor JK, et al. Immunopathology of postprimary tuberculosis: increased T-regulatory cells and DEC-205-positive foamy macrophages in cavitary lesions. Clin Dev Immunol. 2011;2011:307631. doi: 10.1155/2011/307631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Health Organization. Global Tuberculosis Report. Geneva, Switzerland: WHO Press; 2015. [Google Scholar]
- Williams GT, Williams WJ. Granulomatous inflammation–a review. J Clin Pathol. 1983;36:723–33. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AJ, Linas B, Trevejo-Nunez GJ, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- Wong KL, Yeap WH, Tai JJ, et al. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–88. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Lu Y, Eisele MR, et al. Analysis of single-cell cytokine secretion reveals a role for paracrine signaling in coordinating macrophage responses to TLR4 stimulation. Sci Signal. 2015;8:ra59. doi: 10.1126/scisignal.aaa2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Naito M, Takahashi K. Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. J Leukocyte Biol. 1990;47:195–205. [PubMed] [Google Scholar]
- Yang CT, Cambier CJ, Davis JM, et al. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012;12:301–12. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–93. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Welch C, Pagler TA, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–47. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Owen JS, Wilson MD, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]