Abstract
The adaptive immune system is expected to protect the host from infectious agents and malignancies, while avoiding robust activation against self-peptides. However, T cells are notoriously inept at protection whenever the pathogen or tumor is persistent in the body for longer periods of time. While this has been thought of as an adaptation to limit the immunopathology from continued effector T-cell responses, it is also likely an extension of the T cell's intrinsic mechanisms which evolved to tolerate self-peptides. Here we deliberate on how the need to tolerate self-peptides might stem from a paradoxical requirement—the utility of such molecules in maintaining a diverse repertoire of pathogen—specific memory T cells in the body. Understanding the mechanisms underlying this intriguing nexus, therefore, has the potential to reveal therapeutic strategies not only for improving immune responses to chronic infections and tumors but also the long-term efficacy of vaccines aimed at cellular immune responses.
Keywords: T-cell memory, tuning, T-cell tolerance, T-cell activation, T-cell exhaustion, immunoregulation
The authors discuss how the immune system maintains host protection by using a potentially dangerous gambit of reacting with the body's own proteins.
INTRODUCTION
The adaptive immune system is quite efficient in controlling the vast majority of infections we face over our lifetime. Typically, the cellular response is initiated by the activation of pathogen-specific T cells within hours to days of an infection (Lanzavecchia and Sallusto 2000). These activated T cells go through rapid waves of cell division, resulting in a clonal expansion of over a 1000-fold within a week. Along the way, some of the expanded cells differentiate to acquire effector functions required for the T cells to either directly kill infected cells or help other cells of the immune system to do so. If all goes according to the evolved plan, the pathogen is controlled and the T-cell response shuts down. In this contraction phase, most of the expanded pathogen-specific T cells undergo apoptosis while some are retained as memory cells to deal with future infections (Kaech, Wherry and Ahmed 2002). This orchestration usually plays out quite well when the pathogen in question is transient—i.e. it causes an acute infection. However, in many of the cases where a pathogen persists in the body for more than a few weeks, the T-cell response intentionally shuts down without sufficiently clearing the target (Moskophidis et al. 1993; Wherry and Kurachi 2015). Such a shutdown of the T-cell response is known in specific models as exhaustion, anergy, tolerance, desensitization, silencing, tuning, etc. Discussions and comparisons of these models are already available the literature, and so are not further elaborated here (Choi and Schwartz 2007; Schietinger and Greenberg 2014; Grossman and Paul 2015; Wherry and Kurachi 2015). But perhaps a common thread that runs through these phenomena is a nearly global silencing of the T cell, such that its ability to make new responses is greatly impaired (Fig. 1). This process is referred to as tuning, to reflect the fact that such silencing can be quantitative and reversible (Rocha, Tanchot and Von Boehmer 1993; Tanchot et al. 2001; Singh and Schwartz 2003). While this behavior of T cells can be a problem in the context of not being able to clear chronic infections, tumors, etc., its beneficial role in the limiting of bystander immunopathology and autoimmunity has also been discussed (Blackburn et al. 2009; Nakamoto et al. 2009; Lucas et al. 2011; Okazaki et al. 2011; Topalian, Drake and Pardoll 2015). Models of lymphocyte tuning have a long history, being developed as a structured theory by Zvi Grossman and the late William E Paul in 1992 (Grossman and Paul 1992). This progression is documented in many publications (Sinclair and Anderson 1994; Grossman and Paul 2015), and is not reviewed here. Instead, we summarize our laboratory's approach to understanding the T cell's curious relationship with persistent proteins using this framework (Singh and Schwartz 2003; Singh, Chen and Schwartz 2006; Singh, Bando and Schwartz 2012; Steinert, Schwartz and Singh 2012) and discuss possible teleological incentives underlying this self-reverence. The conceptual perspective presented here is that self-reactivity in T cells evolved as a mechanism to maintain the diversity of protective memory T cells and that mechanisms which turn off T-cell responses to chronic stimuli were necessary to prevent lethal autoimmunity from such a necessary self-reactivity.
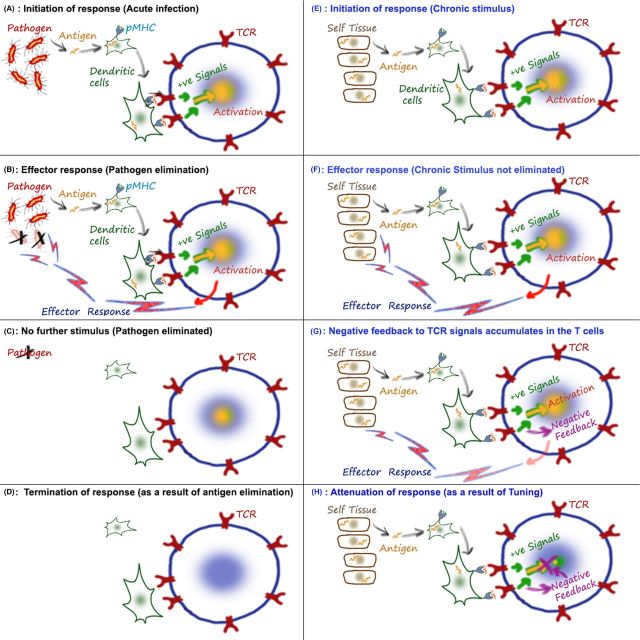
Figure 1.
Tuning is a negative feedback in T cells responding to chronic stimuli. A simplified cartoon summarizing key elements of a T-cell response to acute (left column—A to D) and chronic (right column—E to H) stimulation, focusing only on the cell types and biochemical events that are discussed in this article. Although I use a self-tissue-derived antigen to illustrate a chronic stimulus in E–H, chronic infections can also provide similar sources. An animated version of this cartoon is available at http://nevillab.org/index.php/projects/immune-tolerance. (A) Stimuli that can be eliminated quickly (typically <2–3 weeks) from the host are thought of as acute ones. In this case, the peptides from such agents are carried from the infected tissues to the draining lymph nodes by activated antigen-presenting cells. Typically naïve T cells are activated by dendritic cells when the presented peptide leads to intracellular signaling downstream of the T-cell receptor (TCR). These activating intracellular signals (+ signals) include kinases such as LCK, FYN, ZAP70, etc. and eventually results in gene activation via a network of transcription factors. (B) The genes that are activated for expression include cytokines (such as IFNγ, TNF, IL4) and lytic molecules (including granzyme and perforin) which constitute the effector response of the T cell. As a result of an effective response, the pathogen is cleared from the system. (C) The clearance of the acute infection also results in loss of strong antigenic stimulation. Since the TCR signals are no longer present, the immune activation subsides and the response ends. (D) Therefore, the acute immune response can be thought of as starting and stopping as a consequence of antigen appearance and ‘disappearance’ (or containment). (E) In the case of the chronic antigen, the initial activation of the TCR is broadly similar to A and B. Differences in dendritic cell activation, inflammatory cytokines, action of regulatory T cells, etc. are certainly factors at this stage, but are not shown in the interest of focusing on clarifying the TCR-based tuning process. (F) The continued response of the T cell can lead to effector responses, and tuning may be involved in silencing this process. However, it should be pointed out that the development of an effector response before tuning kicks in is not necessary. And in the case of low-affinity ligands, the silencing by tuning may be recruited even before an effector response is elicited. (G) The central element of tuning is the development of negative feedback downstream of the antigen-sensing machinery (TCR, etc.). The tuning down of the T cell essentially dampens the cellular responsiveness. So although the T cell is around, it does not make robust responses to the stimulation. As a result, this T cell can be categorized as a ‘poor’ memory cell or an exhausted one. This simplified cartoon does not represent many other factors that also contribute to or are markers of the exhausted/tuned/tolerant state of the T cell, but illustrates the cellular negative feedback or tuning, that we expect to be a shared feature of many of these states. Although we and others have shown multiple effects that the negative feedback has on TCR-proximal kinases, the precise molecules sensing chronicity and enforcing tuning are not yet known. Elucidating these will be critical in understanding the commonality between multiple states of T-cell tolerance. (H) The major difference between an acute and chronic stimulus is that, by definition, the first one is cleared from the system with the help of the immune response. As a result of the loss of stimulus from the system, the major effector response from the T cells stops. In the case of chronic stimuli, T cells can stop ‘themselves’ as a result of intracellular negative feedback.
ON THE ORIGINS OF THE T CELL'S PARALYZING FEAR OF PERSISTENT STIMULI
Speculating on the physiological merits or the selective advantages that spurred the evolution of a pathway of T-cell silencing is not trivial. One possibility is that shutting down a T cell's effector response evolved as a host-protective feedback mechanism. In this line of reasoning, continuing inflammatory responses against a non-clearable pathogen could potentially cause accumulated bystander damage to host tissues. Shutting down this seemingly futile response limits the unwelcome immunopathology and improves the fitness of the host. Although intuitive, this postulate is not without its challenges. It can be argued that allowing the pathogen to persist by shutting down the T-cell response before it clears the microbe completely can have potentially deleterious consequences of its own. The morbidity from the infection, the potential for variant microbes that can grow faster and eventually kill the host anyways, etc. are some. Importantly, this persisting pathogen can now use the host as a reservoir to the spread through the local population. Of course, the immediate targets would be the kin (relatives, offspring, etc. which share genetic kinship) of the infected individual. The evolutionary benefits of saving the host whose cells failed to clear a pathogen, while exposing his/her genetic compatriots to a fitness-reducing infection, is challenging to gauge. From a cellular point of view, it is also surprising that the silencing of the T cell is nearly global. Although there are known biological pathways that can be activated to surgically turn down particular effector responses (Hochrein et al. 2000; Owyang et al. 2006; Villarino et al. 2007; Palmer and Restifo 2009), the silencing in T cells seems to operate at a TCR-proximal level (Chiodetti et al. 2006). This would impinge on almost all pathways of T-cell activation by choking off the initial ligand sensing. Silencing does seem to be a progressive state (Wherry 2011) and despite these arguments, the ‘global’ shutdown may be construed as a last-ditch effort to avoid continued responses. But even so, it is surprising that proliferative functions are shut down prior to effector functions, if the goal was to avoid immunopathology from the latter (Ohlen et al. 2002; Wherry et al. 2003). The weight of these arguments does not mean that limiting immunopathology is not an important purpose behind T-cell silencing, but it is worth considering if it is really the preeminent objective behind it.
A second motivation for the evolutionary fixation of pathways of T-cell silencing could be to limit responses to non-pathogen-derived peptide-MHC complexes that T cells are constantly exposed to. There are two major sources of these peptides—proteins from our body (self) and those from commensal microorganisms. As regards the latter, in recent years the critical role of commensals supplementing various physiological functions ranging from digestion to neurodevelopment has been appreciated (Goyal et al. 2015). Given their many advantages, the immune system could have been under pressure to avoid clearing such microbes from the body—even though it is not trivial to distinguish effectively between pathogenic and non-pathogenic microbes. The result is the evolution of a multilayered system that (i) limits commensals beyond epithelial barriers by using specialized innate immune mechanisms and (ii) modifies T-cell responses at these barrier sites to have a non-inflammatory and barrier-promoting function (Hooper, Littman and Macpherson 2012; Belkaid and Hand 2014; Mukherjee and Hooper 2015). So, from the point of view of the T cell, a combination of ignorance (due to the innate barriers) and regulation (specialized responses and regulatory T-cell activity at the barrier sites) serves to maintain a fine balance between sustaining commensals and avoiding their systemic dissemination. Desensitizing a TCR to be insensitive to specific commensal antigens is plausible, but needs further study. In fact when the innate barriers are breached, even by another acute pathogen, the responses mounted by T cells against commensals are not very different from those that they would mount against another pathogen (Hand et al. 2012). This conventional response lasts till the barriers are restored and mirror one to an acute ‘infection’ rather than a chronic one.
However, compared to limiting immunopathology to non-clearable pathogens or avoiding responses to commensals, the arguments for self-tolerance are considerably more engaging. As a source of peptides that cannot be shielded from T cells by way of barriers alone, potential immune responses to self-tissues can be debilitating. Most self-proteins are available in the body for the person's lifetime and are chronic by definition. Of course, the potential for self-reactivity is an abiding concern of the immune system and mechanisms to mitigate that are evident at every stage of T-cell development. Of the over 108 potential TCRs that can be generated randomly in the thymus, strongly self-reactive ones are eliminated by negative selection (Singh and Schwartz 2006). Special mechanisms to ectopically express additional tissue-specific peptides in the thymus using molecules such as Aire and Fezf2 improve upon the efficiency of negative selection (Anderson et al. 2002; Takaba et al. 2015). Even so, it is clear that this step only leads to the elimination of T cells sensing abundant or ubiquitous proteins (Legoux et al. 2015; Malhotra et al. 2016). Many T cells specific for tissue-specific (chronic) proteins complete thymic selection and patrol the periphery (Legoux et al. 2015; Yu et al. 2015). Importantly, while a short-lived (acute) response against these proteins is likely to be tolerated by the host, chronic immune responses to such tissues can be fatal. Therefore, silencing T cells that mount anti-self-responses can be critical to the survival of the species.
Nevertheless, even overt self-reactivity to tissue proteins, as commonly discussed in the literature, is only an issue for a fraction of the TCRs. But the aspect of self-reactivity that can dramatically expand this scope and arguably underscore the importance of evolving robust control mechanisms in every T cell stems from a stage in T-cell development known as positive selection (Lo and Allen 2014). After a developing T cell completes the gene rearrangements needed to generate a receptor, this new TCR is tested for its ability to functionally signal into the cell. This ‘quality control’ step, known as positive selection, involves the TCR engaging a peptide–MHC complex in the thymus. Only those T cells whose new TCRs transmit a prosurvival signal into the cell at this stage are allowed to develop further. In cases where the peptides used in positive selection have been identified, they are invariably derived from broadly and persistently expressed self-proteins (Hogquist et al. 1997; Santori et al. 2002; Ebert et al. 2009; Lo et al. 2009). Therefore, every single T cell that we can find in our peripheral immune system is paradoxically guaranteed to be self-reactive (Davis et al. 2007). Of course, it has been argued that this does not pose a ‘real’ threat of autoimmunity because positively selecting self-peptides are very low affinity ligands. But, as we will discuss further below, the lack of agonistic activity is more a reflection of changes that happen in the T cell rather than a purely biophysical property of the particular peptide–MHC complex. It has also been suggested that positively selecting peptides may be generated using a thymus-specific machinery and therefore not well represented in the periphery (Honey et al. 2002; Xing, Jameson and Hogquist 2013; Sasaki et al. 2015; Takada et al. 2015). Although these pathways, from what is reported so far, only seem to influence a subset of TCRs (Nitta et al. 2010), the identification of additional peptides that positively selecting specific TCRs will allow us to resolve these questions.
THE TUNABLE LYMPHOCYTE
The ability of mature T cells to effectively ‘ignore’ the continued presence of positively selecting peptides in the body often leads us to discount them when discussing potential peptides that TCRs can engage. Typically, any given TCR would have a very low affinity for its positively selecting ligand to start with (since high-affinity ligands are likely to trigger negative selection in the thymus) (Klein et al. 2014). But it is important to note that positively selecting ligands are perfectly capable of signaling via the TCRs they select. Furthermore, it is also emerging that the T cells continue to sense and derive some (poorly understood) signals from these ligands even as mature peripheral cells (Davis et al. 2007; Lo and Allen 2014). The reason these TCRs can avoid making an effector response against such ligands is thought to involve an adaptation process. A series of alterations in the molecular complexes associated with the TCR in developing T cells significantly dampens the sensitivity of the T cell to peptide (Pircher et al. 1991; Davey et al. 1998; Lucas et al. 1999). As a result, even though a TCR on a mature peripheral T cell might structurally bind the self-peptide that once positively selected it, this does not lead to a strong enough intracellular signal required to fully activate the T cell. This adjustment process is the first instance of the T cell invoking ‘tuning’ or receptor-sensitivity modulation to avoid making autoimmune responses (Grossman and Paul 1992; Grossman and Singer 1996).
Although it is not very typical to equate the low-affinity self-peptides that mediate positive selection with the strong agonistic self-peptides that may trigger autoimmune disease, as we understand the mechanisms underlying tuning better, this distinction is being increasingly challenged (Davis et al. 2007; Morris and Allen 2012; Hogquist and Jameson 2014). For instance, one intriguing regulator of the tuning process that follows positive selection is the microRNA mir181a (Li et al. 2007). Mir181a is highly expressed in the double-negative stage of thymocytes at the time they are undergoing positive selection and gets downregulated in later stages of maturation. Since this microRNA downregulates multiple phosphatases including SHP1, SHP2, DUSP1, etc., the activity of kinases that transduce signals downstream of the TCR is further enhanced. This allows the thymocyte to sense the weak signal from the endogenous positively selecting pMHC. As the expression of mir181a wanes by the mature T-cell stage, the levels of multiple phosphatases in the cell are increased. This increased phosphatase activity dampens the activity of TCR proximal kinases, reducing the sensitivity of the TCR at a global level. The idea is that such changes tune the TCR's sensitivity to peptide, effectively minimizing the cell's ability to be activated by the low-affinity positively selective peptide. Importantly, when the authors then manipulated mir181a expression in the fully developed mature T cells, these T cells could in fact sense the endogenous peptide (Li et al. 2007; Ebert et al. 2009). Therefore, the same peptides that mediate positive selection are not only present in the periphery but are also capable of functioning as agonists if the tuning mechanisms in peripheral T cells break down.
In order for tuning to be effective in restraining T cells from autoimmunity, the process has to be dynamic. First, the levels of self-peptide that the T cell encountered in the thymus may not be the same as it will be exposed to in the periphery. Second, these levels may change during the lifetime of the individual. This implies that a T cell must have the ability to constantly calibrate its sensitivity in conversation with the levels of ambient peptide levels. As proposed originally by Zvi Grossman and the late William E Paul (Grossman and Paul 1992), an endogenous sensor in T cells must constantly detect and adjust to the levels of chronic stimulation (Grossman 1993; Grossman and Paul 2000). This of course raises an intriguing question. If peripheral T cells are capable of dynamic tuning to ligands such as their positive selecting ligand, could they also not use the same machinery (linked to the same TCR) tune to a persistent agonist? In other words, since (or if) the same TCR machinery is used to detect positively selecting peptides and a canonical agonist, the machinery can also be activated when the T cells engage an agonist, leading to tuning. Perhaps, this is the underlying reason for the dampening of responses to chronic (and even some acute) agonists that is widely observed.
Indeed, several years ago, we showed that peripheral T cells are quite capable of tuning to different levels of their agonistic peptide in vivo. In these studies, we and others have used adoptive transfer approaches that enable the quantitatively tracking of the fate of mature peripheral T cells in vivo (Rocha, Tanchot and Von Boehmer 1993; Akkaraju et al. 1997; Adler et al. 1998; Pape et al. 1998; Jordan et al. 2000; Singh and Schwartz 2003; Knoechel et al. 2005; Steinert, Schwartz and Singh 2012). Our own strategy involved the adoptive transfer of a model peptide-specific T-cell population to mice where they can then encounter their cognate ligand either as a chronic self-peptide or be immunized acutely using the same peptide (Singh and Schwartz 2003). In such models, otherwise naïve and fully responsive T cells which encounter the chronic stimulation in vivo rapidly downregulate their peptide sensitivity even without the presence of regulatory T cells (Singh, Chen and Schwartz 2006; Singh, Cox and Schwartz 2007). The loss of responsiveness is progressive, over a 5–8-day period and importantly, is reversible. Removal of these tuned T cells from the chronic-stimulation milieu results in a gradual recovery of functionality in these cells. Finally, the same T cell can also be shown to tune its responsiveness at multiple levels, based on the steady-state intensity of peptide presentation in vivo (Tanchot et al. 2001; Singh and Schwartz 2003). Tuning is also evident in altered TCR-proximal signaling events starting with dampened activity of the enzyme ZAP70 which phosphorylates elements of the TCR complex within seconds of pMHC engagement (Singh and Schwartz 2003; Chiodetti et al. 2006; Choi and Schwartz 2007). Similar tuning of the T cell has been reported using other models as well (Marquez et al. 2005; Teague et al. 2008).
Therefore, a process of dynamic tuning can be observed in T cells after undergoing positive selection on self-peptides as well as reacting to chronic conventional high-affinity agonists. Since we do not have a clear molecular understanding of either process, it is of course too early to ask if they are ‘the same’. Indeed, apart from the affinity differences for the TCR-pMHC, the developmental stage of the cells and the peptide-presenting cells involved in positive selection versus peripheral activation are quite different. Although the data suggest that the T cell should be able to maintain tuning to positively selecting ligands even in the mature peripheral naïve T-cell stage, it is still possible that the mechanisms that T cells use to enforce silence to those can be quantitatively or qualitatively different from that they invoke in response to a strong agonist. This may simply stem from the fact the low-affinity ligand may not elicit the same number or type of signaling events in the T cell and therefore can be silenced by a subset of negative feedback processes. Nevertheless, given the engagement of the same TCR, it is quite likely that proximal mechanisms used to sense continued ligand presence are shared between the two pathways. The identification of these and other mechanics in the T cell would be key in further defining how we approach breaking T-cell tolerance to tumors and chronic infections, while restoring tolerance to an ongoing autoimmune response.
THE SELF-REACTIVITY THAT DOESN'T KILL YOU, MAKES YOU STRONGER
The need to avoid autoimmunity is critical for the individual's survival. As discussed above, a robust mechanism of tuning may help in this process. Perhaps the evolutionary impetus for a tuning machinery is in large part driven by the need to restrain this universal self-reactivity of the T-cell repertoire. Following this teleological argument, one then wonders why all T cells had to be universally self-reactive in the first place. In other words, it is challenging to understand why every T cell comes out of the thymus only after being certified to be self-reactive and then necessitate additional mechanisms to ensure that this self-reactivity does not lead to self-destructive autoimmunity. This in essence is a question of why positive selection operates the way it does and has been litigated previously in the literature (Matzinger 1993; Jameson, Hogquist and Bevan 1995; Benoist and Mathis 1997). Indeed, appreciating the dangers of selecting an exclusively self-reactive repertoire during positive selection, classical challenges initially questioned if selection was indeed peptide specific at all (Matzinger 1993). If the purpose of this step in T-cell development was essentially to perform ‘quality control’ – i.e. to ensure that the newly rearranged TCR works, there are potential ways to do that without using peptide specificity. Avoiding specific peptide recognition at that stage also seems more frugal in an evolutionary sense, since it might also reduce selective pressures for maintaining an elaborate tuning machinery in the thymocyte. However, it is now quite clear that positive selection involves a specific TCR-pMHC pairing. While there is some degeneracy, so that one particular peptide can potentially select multiple TCRs, each TCR does require a specific peptide ligand for selection (Liu et al. 1997; Chmielowski, Muranski and Ignatowicz 1999; Ebert et al. 2009; Lo et al. 2009). Indeed, in the intriguing pair of papers where this has been studied, two different T cells that recognize the same foreign ligand in the periphery use different positively selecting peptides to develop in the thymus (Ebert et al. 2009; Lo et al. 2009).
The likely reasons for maintaining peptide-specific positive selection as a critical step in T-cell development have been actively discussed in the recent literature (Paul, Milner and Grossman 2013; Hogquist and Jameson 2014; Lo and Allen 2014; Vrisekoop et al. 2014). An emerging consensus is that the selection on self-peptides anticipates a critical role that these same peptides will play during the activation of the T cell by a peripheral foreign ligand. One such role is that of a co-agonist, wherein, as a naïve T cell is being activated by exceeding low (possibly as low as one) numbers of foreign peptide–MHC complexes, the TCR gets a little help from being able to engage an additional pMHC in order to increase its rate of activation (Wulfing et al. 2002; Krogsgaard et al. 2005; Gascoigne 2008). Since ubiquitous self-peptides are always likely to be displayed on patrolling dendritic cells, positive selection may help ensure the optimal sensitivity of the TCR (Mandl et al. 2013; Persaud et al. 2014; Fulton et al. 2015). Similarly, endogenous peptides may also help promote the ability of naïve T cells to be activated, by keeping them ‘tickled’ at a low level so that the TCR signaling apparatus is always in a poised state, ready for rapid activation at the first sign of a foreign target (Stefanova, Dorfman and Germain 2002; Fischer et al. 2007). This connection between heightened self-recognition and foreign responsiveness has now been reported during responses to immunizations as well as infections (Mandl et al. 2013; Persaud et al. 2014; Fulton et al. 2015). In these studies, T cells which express markers consistent with stronger engagement of self-peptides (e.g. CD5) are preferentially selected during responses to unrelated cognate peptides. Paradoxically, the system prefers to use the exact subset of cells for fighting a pathogen that also presents the greatest threat of triggering autoimmunity (Hogquist and Jameson 2014), if the tuning mechanics were not sufficiently robust.
A SELF-REVERENTIAL MEMORY OF PAST INFECTIONS
So, to revisit the original question then, why positive selection? (Matzinger 1993) i.e. why was a mechanism that ensures universal self-reactivity of the repertoire selected through evolutionary time to become an integral part of T-cell biology? As discussed above, the helpful role that self-reactivity plays toward improving responses to foreign antigens as well as T-cell homeostasis is certainly a consideration. But such functions do beg the hypothetical question i.e. ‘couldn't another (non-self-peptide driven) mechanism have evolved to do the same job and without the associated cost of autoimmunity?’ Indeed, other accessory molecules can be envisioned to play similar roles to the co-agonistic activity of self-pMHC and prosurvival signals for homeostatic purposes could be (and are) provided by non-TCR–ligand interactions. Although it can be an axiomatic trap that evolutionary processes eventually retain only the least costly solution (Hedrick 2004), the argument is raised here because there is potentially a more robust alternate incentive. This relates to a fundamental mandate of the adaptive immune system, that of remembering all the infections that the body encountered over one's lifetime. Importantly, it is unlikely in this context that any other mechanism can replace the diversity of ligands offered by self-pMHC to retain memory T cells.
The ability of the immune system to specifically remember past infections is the basis of the protection we acquire from childhood infections and of course, vaccines (Kaech, Wherry and Ahmed 2002). Over time, an individual is exposed to a wide variety of infections and toxins. As the child is nursed by parental care through these exposures, the immune system also remembers the response it made against each of them. This memory is usually accomplished by keeping few of the antigen-specific cells that responded to the first exposure around to fight the next one—ensuring that the host can survive returning infections with minimal morbidity. In order for this process to work effectively, the immune system must remember most (if not all) of these infections, for the rest of the person's life. In the case of T cells, this implies retaining an extensive complex repertoire of memory T cells that were previously able to repel any of the hundreds of infections that the person is likely to be re-exposed to.
After the initial response to the pathogen is successful, a heterogeneous pool of memory T cells remains either in the tissues, lymphoid organs or circulates through multiple sites. While the factors regulating the pool of tissue resident cells (TRM) are not well understood (Schenkel and Masopust 2014), the long-lived cells in the lymph nodes, spleen and bone marrow are thought to be maintained with the help of trophic factors that support a slow homeostatic turnover of the cells (Schluns and Lefrancois 2003; Grossman et al. 2004; Surh and Sprent 2008). In the case of CD8 T cells, the dominant trophic factors are the cytokines IL-7 and IL-15 (Fig. 2A). Since the levels of these cytokines are thought to be relatively constant in the niches supporting T-cell survival, the system effectively imposes a ceiling on the number of memory cells that can be maintained in the body (Freitas and Rocha 2000). Indeed, while the existence of such a fixed compartment size is not evident for resident or effector memory subsets of CD8 T cells, the size of both central memory CD8s and the CD4 memory pool does appear to be relatively constant over time (Vezys et al. 2009). In this context, if a new memory T cell is generated, it can potentially displace a pre-existing memory T cell—by competing with the latter for the same trophic factor (Fig. 3B and C).
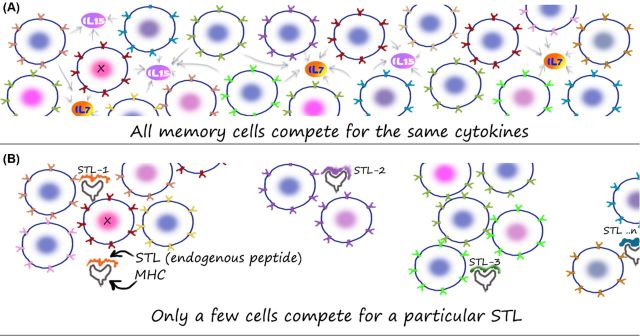
Figure 2.
Structure of the memory T-cell population in vivo. A simplified cartoon comparing how memory T cells competing for cytokines alone (A) or endogenous peptide–MHC complexes (termed subthreshold ligands or STLs after Grossman and Paul) alone (B) may be visualized. An animated version of this cartoon is available at http://nevillab.org/index.php/projects/immune-memory (A) Cytokines such as IL-7 and IL-15 are critical mediators of memory T-cell survival. In the simplistic ‘well-mixed-pot’ model, all memory T cells are able to compete along equal lines for access to such factors. Along the lines of a population biology framework then, the amount of such factors in vivo will limit the total number of T cells that can be maintained in the body. The question of how limiting such factors are and how rigid the population ceilings imposed by such limits are still under investigation. (B) If the T cells compete for a specific STL, then the niche that they can compete in can be quite small—as opposed to a global competition cartooned in (A). Although only STLs are shown in (B), in vivo, it could be a combination of both cytokines and STLs. The important point would be that the diversity element would come from the inclusion of STLs into the competitive framework.
Figure 3.
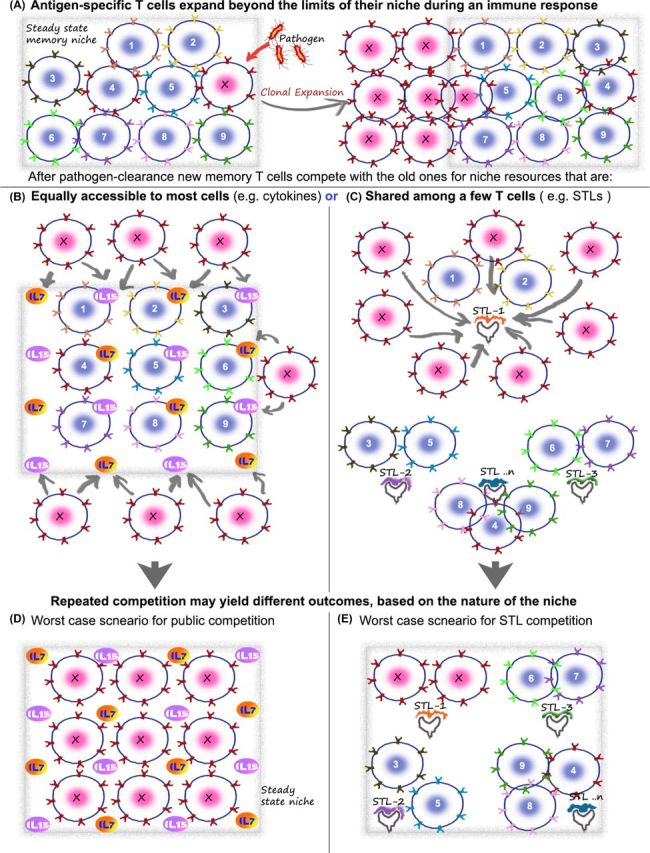
Potential consequences of public versus private competition on the diversity of memory T cells. The basic concept of memory maintenance on the basis of cytokines and STLs is cartooned here, using a hypothetical ‘worst-case’ scenario to illustrate my point that rampant competition between T cells without a restriction of niches can potentially have deleterious consequences on the repertoire. (A) Consider a memory repertoire with a diverse population of memory T cells that have accumulated over time in the individual (cartoon shows T cells 1–9 and X). The frequency of each is relatively low and this allows the total population to exist stably in the niches available in the immune system (niche boundaries marked by gray box). Upon pathogen entry, the appropriate T cell (here marked by X) expands to large numbers, which can even numerically be equal 10%–50% of the total repertoire. The key issue is what happens next. After the pathogen is cleared, this clone of T cells—expanded to large numbers—can go back to competition for resources in the immune niche. The potential drawback of using only public factors for this process is illustrated in (B) and (D), while the solution potentially offered by weak self-ligands (i.e. subthreshold ligands or STLs) is shown in (C) and (E). (B and D) Since X can stochastically compete with all other T cells (provided they have the receptors for the cytokine), it is possible that it can replace some of the rare memory T cells who are at a numerical disadvantage to the larger numbers of X. This can be costly, since the rare memory T cell that was lost in the process could represent a vital protective agency against a future infection by a less frequently appearing but debilitating pathogen. One way the number of new memory T cells that can compete for the cytokine niches (i.e. X competing for IL-7 and IL-15 here) is reduced is by regulating the expression of cytokine receptors on X. But even so, the large numbers of receptor positive cells that are available and the threat of repeated infections do offer a significant threat to the repertoire. Regardless of whether the total memory T-cell number at this point (after multiple infections) stays the same or slowly increases with time, it is important to note that only a small fraction of the effector cells are retained. So, a large number of the new ‘memory recruits’ can potentially compete with pre-existing memory T cells for their survival niches. As a result of this (shown in D), T-cell clones remaining after the recent infection can occupy every niche—theoretically capable of displacing in a stochastic fashion most of the other pre-existing memory T cells in the process. If the pool size can grow a little, this is unlikely to be a serious concern. However, over time the repertoire will be disproportionally biased toward clones remaining from the most repetitive infections—e.g. seasonal flu as opposed to rarer (and potentially more deadly) infections. (C) In the case of competitive niches defined by STLs (in addition to or instead of cytokines), the new recruits (X) only compete with a specific subset of T cells that engage that particular STL. If the diversity of STLs is large enough, each niche is likely to be quite small. Unlike the fixed scope for sources of IL-7/15, etc. in the body, the niche of STLs can be expanded—by using new sources of self-ligands or commensal antigens as the individual's repertoire grows. In this case (cartooned in E), even after repeated responses by a clone, in the ‘worst-case’ scenario, replacement of all the other receptors in a particular niche is unlikely to make a serious dent in the complexity of the total repertoire. As discussed in the text, since multiple TCRs specific for the same antigen can segregate randomly across multiple STLs, the repertoire is likely to retain a broad suite of T cells representative of the individual's pathogen exposures, with this mechanism.
The inherent danger in such a competition is that newly minted memory T cells could eliminate previous memories (Fig. 3C). Since we are likely to have a few different clones of memory T cells for each pathogen and multiple copies of each clone in the pool as well, it can be argued that some degree of such replacement is unlikely to dent the protective potential of the system. But this may not be the case for rarer memory cells, representative of childhood vaccines for rare (yet potentially lethal) diseases. Furthermore, soon after any recent infection, the number of new ‘memory’ T cells for that pathogen is likely to be far in excess of the frequency of any other particular memory cells in the pool (Fig. 3A). Arguably, this numerical advantage can potentially lead to a disproportionate loss of pre-existing cells in favor of new memory T cells (Fig. 3C). With each repeat infection (e.g. seasonal episodes of flu and malaria), the memory pool would thus progressively loose diversity, as the memory T cells for the most recent pathogen expand and contract, erasing cells that help remember other infrequent (yet potentially deadly) diseases. Clearly, this calls for a regulated mechanism to ensure an orderly succession, whereby new memory T cells take their place without indiscriminately eliminating the critical diversity of other memory cells acquired over a lifetime.
A potential solution to this problem arose from studies on the homeostasis of peripheral T-cell populations (Freitas and Rocha 2000). In many models, the survival of peripheral T cells and/or their stem-like turnover is influenced by the diversity of endogenous peptide–MHC complexes (Kieper and Jameson 1999; Viret, Wong and Janeway 1999; Moses et al. 2003; Min et al. 2004; Hataye et al. 2006). A similar phenomenon was also uncovered by our own studies on the mechanisms by which a CD4 T-cell response undergoes contraction (Singh, Bando and Schwartz 2012). Tracking the fate of a monoclonal T-cell cohort in vivo, we found that its frequency was regulated by neighboring polyclonal CD4 T cells. Among the polyclonal T cells, only 1 in 2000 could compete against the monoclonal T cell we studied. Importantly, rather than competing for cytokines or even for the same agonistic antigen, this rare T cell was just better at sensing the same positively selecting ligand as the monoclonal T cell. We interpret these data to suggest that effector T cells compete only against others which recognize the same endogenous (perhaps the same positively selecting) ligand. When applied to memory T cells, this offers an intriguing model for ensuring that the diversity of the repertoire is robustly maintained (Singh, Bando and Schwartz 2012).
Memory T cells are generated from naïve T cells. Each TCR requires its own specific positively selecting ligand in the thymus to develop. This selecting peptide is rather specific such that even two TCRs specific for the same foreign peptide may undergo positive selection of two different thymic self-peptides (Ebert et al. 2009; Lo et al. 2009). At the same time, one thymic self-peptide can support the development of a fairly diverse repertoire of T cells (Ignatowicz, Kappler and Marrack 1996). Taken together, this implies that (i) the peripheral pool of T cells can be divided fairly rigidly into groups based on their thymic positively selecting ligand and (ii) the groups that are so divided are likely to contain a random assortment of unrelated foreign peptide-specific T cells (Fig. 2B). It is estimated that there are thousands of positively selecting peptides in the thymus (Lo and Allen 2014). If maintenance of the memory repertoire is critically dependent on recognition of these pMHCs in the periphery, then we can divide the peripheral memory repertoire into thousands of ‘colonies’ (Fig. 2B). Accordingly, after an antigen-specific response (Fig. 3A) T cells are expected to compete for survival (Freitas and Rocha 2000) only within these small colonies (Fig. 3D and E). Unlike a general competition with the entire T-cell population (Fig. 3B), this limited competition (Fig. 3D) would be expected to affect only a thin slice of the total memory T cell. Even if the same infection were to return seasonally, repeated expansions and contractions of the same clones of memory cells would have no consequence on the broad repertoire. Rather, any loss of other specificities would be limited to the relatively few that share its own niche peptide (Fig. 3C and E).
The robustness of this strategy depends on how many such self-peptides are available in the system and therefore how many colonies the T cells can be fractionated into. Of course, a number >1 (as you would get from a cytokine-competition alone) is still better, but self-peptides clearly are much more diverse (Lo and Allen 2014). In our study, the frequency of competing cells for the one TCR we used was estimated to be 1 in 2000 of the polyclonal repertoire (Singh, Bando and Schwartz 2012) (based on fractionating the polyclonal cells into 100 cell pools and assaying how many of these could compete against one TCR). This matches closely with estimates for the abundance of dominant self-peptides gleaned from studies in which naturally bound peptides were eluted from MHC molecules. Although we have so far discussed positively selecting peptides as the source of such colony-determining ligands, it is also possible that there are other self-peptides that are expressed only in the periphery which fit the bill. The two key features that these peptides have to satisfy for this strategy to work is that they (i) are consistently present in the immune system and (ii) do not activate the T cell fully to trigger effector functions. The latter property prompted us to classify such peptides as subthreshold ligands (STLs) for the T cells (Fig. 2B) (Grossman and Paul 1992; Singh, Bando and Schwartz 2012). Taken together, it is possible that the universe of STLs can be well over our estimates of 2000—suggesting that even if one entire colony of pre-existing memory T cells is displaced by a wave of new memory T cells, >99.99% of the pre-existing repertoire is still preserved. While factors such as the potential for redundant recognition of multiple STLs by different TCRs can affect these calculations, the model offers a preliminary framework for trying to understand how a complex repertoire of memory T cells can be stably maintained in the immune system.
It is important to note that the concept of a fixed population limit in the memory compartment as envisioned in the original population biology models (Freitas and Rocha 2000) is used here for the sake of simplicity. It is possible (and has been documented with aging and with increasing number of pathogen exposures) that the actual size of the memory T-cell pool, at least for some T-cell subsets, can increase over time (Vezys et al. 2009). But of course, even when the population limit has some room for growth, not all effector or (‘pre-memory’) T cells that remain at the end of a response are retained as memory T cells (Williams, Ravkov and Bevan 2008). The few that remain are likely to actively compete with the pre-existing repertoire for the survival factors that they all require. Unlike cytokines such as IL-7 and IL-15 whose cellular sources can be limiting in this context, STLs offer a malleable resource since new sources of ligands (new self-proteins in tissues, commensals, etc.) can be recruited to accommodate the need for an expanding pool. Therefore, even in the context of a somewhat malleable size of the memory T-cell compartment, STL dependence reduces the likelihood of competitive losses of the pre-existing repertoire. Of course, this also offers a strategy to therapeutically manipulate the size of the memory population if the nature and signals emanating from STLs can be understood molecularly.
CONCLUSION
The prospect of using self-peptides to organize the niches of memory T cells, it is argued, offers a teleological reason for peptide-specific positive selection. Since the diversity of memory T cells is best organized by TCR specificity, it is hard to envision an alternate pathway that could have been equally effective in maintaining polyclonality. The strategy does carry the cost of paradoxically ensuring that the entire T-cell repertoire is essentially self-reactive. The ability of T cells to tune their TCR signaling apparatus allows the system to dodge the pathogenic consequences of this self-reactivity. Of course, once self-reactivity was engineered into the framework of a T cell's circuitry it could have found use in many other aspects of T-cell biology including calibrating sensitivity to foreign responses. This suggests that understanding the molecular mechanics of tuning and colonial behavior of T cells can have a significant impact on a wide range of translational contexts. Pharmacological modification of the tuning apparatus in T cells has the potential to not only help rejuvenate responses to chronic infections and tumors but also, conversely, dampen autoimmunity and transplant rejection. Similarly, STLs can offer a new avenue for improving the stability of T-cell memory at the boosting stage of vaccinations. Of course, these are early days and the framework will no doubt evolve as more data emerges about self-reactivity, tuning and the maintenance of the peripheral memory repertoire. Nevertheless, the conceptual framework outlined in this perspective allows us and other laboratories to design experiments that probe a potentially critical yet poorly understood nexus in T-cell biology.
Postscript: Can self-reactivity have evolved to maintain diversity of the naïve T-cell repertoire?
In the interest of including some valuable discussions that I have had with the reviewers of this article, I include below some thoughts on the relative roles of self-reactivity in the maintenance of naïve versus memory T cells. Indeed, much of the what we have learned about the role of self-peptide STLs in T-cell survival are derived from studies primarily examining naïve T cells (Kieper and Jameson 1999; Viret, Wong and Janeway 1999; Moses et al. 2003; Min et al. 2004). Naïve T cells not only compete for access to self-peptide STLs but can also differentiate into a memory phenotype population based on the recognition of such ligands (Tanchot et al. 2002). This leads to a ‘repertoire-filling’ effect, where the absence or loss of specific T cells drives naïve T cells to expand and fill this ‘hole in the repertoire’ (Min et al. 2004). These studies have prompted previous proposals that the teleological incentive for ensuring that the peripheral repertoire is self-reactive is to aid in maintaining the naïve (and in some cases the memory or homeostatically expanded ‘memory’) repertoires (Paul, Milner and Grossman 2013). This article extends this discussion, but argues that the selective pressures underlying the need to preserve the diversity of the pathogen-specific memory repertoire was perhaps a robust driver for evolving mandatory self-reactivity.
Can a similar urgency for maintaining diversity in the naïve repertoire also have contributed to the evolutionary hardwiring of self-reactivity into every TCR? After a new TCR is generated in the thymus, the immature T cell undergoes only a small number of cell divisions before being sent out into the periphery. As this process is repeated for many years, thymic output continues to be a major source of a diverse naïve T-cell repertoire in the periphery (Berzins et al. 1999; McFarland et al. 2000). The frequency of naïve T cells specific for any epitope is typically very low and a wide diversity certainly ensures that the system can react to a broad range of future threats. However, there is no reason to expect that any particular randomly generated TCR-bearing naive T cell currently living in the periphery is likely to be more valuable (i.e. can potentially recognize a future pathogen) than another one that just came out of the thymus. In other words, there is no clear advantage in preserving pre-existing diversity in the naïve repertoire—as long as an overall broad repertoire is maintained by a combination of thymic output and peripheral presence. In fact, it might make more sense to replace older thymic emigrants that are still naïve (and have therefore not proven to be useful against any infection so far) with other new specificities from the thymus (Berzins et al. 1999). Second, naïve T cells also do not typically undergo clonal expansions without an antigenic stimulus (Tough and Sprent 1994) although some basal renewal is observed (Grossman et al. 2004). Thus, the risk of a few clones of naïve T cells outcompeting a diverse repertoire and displacing them with an oligoclonal one is minimal. In light of these arguments, it is not clear that a TCR-centric process for ensuring the diversity of naïve T cells in the periphery is necessary. A mechanism (e.g. survival based on cytokine availability) that simply assures that a large enough number of naïve T cells are maintained might have been sufficient to ensure diversity.
Contrast this with the repertoire of pathogen-specific memory T cells. The TCRs in these cells have proven their ability to recognize (and potentially repel) a pathogenic threat. Therefore, they have a definite advantage against a future infection (i.e. when the same pathogen returns). A mechanism that ensures that this advantage is not lost is likely to confer a selective edge. Second, the most significant threat to the diversity of memory T cells comes from the fact that during each infection, a few clones can expand to large numbers. This large number can then compete against the remaining ‘resting’ memory repertoire and challenge their survival, if the competition was broad in scope. Therefore, although both the naïve and memory repertoire require diversity, it is hard to argue that an STL-based mechanism with its cost of potential autoimmunity was selected for maintaining naïve T cell diversity. However, it is not surprising that once it evolved as a mechanism of choice for maintaining memory T-cell diversity, STL-based-mechanisms are also then used to maintain naïve T cells. Since this is a TCR specificity-based process, and both naïve and memory T cells use the same TCR, it would not only be a thrifty evolutionary strategy, but also a necessary one.
Finally, while the discussion of T-cell intrinsic tuning is the focus of this article, it is important to point out that several other cellular and molecular pathways that enforce self-tolerance are well characterized. These include controls on dendritic cell activation, costimulation, the activity of regulatory T cells, etc. and are extensively reviewed in the literature (Singh and Schwartz 2006).
Acknowledgments
I would like to thank Dr Zvi Grossman (Tel Aviv Univ.), Dr Colin Anderson (U. Alberta) and the anonymous reviewer of this manuscript for constructive critiques and a vigorous debate about the arguments in this article.
FUNDING
NJS is funded by the NIAID through R01AI110719 and R21AI126184.
Conflict of interest. None declared.
REFERENCES
- Adler AJ, Marsh DW, Yochum GS, et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–64. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaraju S, Ho WY, Leong D, et al. A range of CD4 T cell tolerance: partial inactivation to organ-specific antigen allows nondestructive thyroiditis or insulitis. Immunity. 1997;7:255–71. doi: 10.1016/s1074-7613(00)80528-2. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C, Mathis D. Positive selection of T cells: fastidious or promiscuous? Curr Opin Immunol. 1997;9:245–9. doi: 10.1016/s0952-7915(97)80143-4. [DOI] [PubMed] [Google Scholar]
- Berzins SP, Godfrey DI, Miller JF, et al. A central role for thymic emigrants in peripheral T cell homeostasis. P Natl Acad Sci USA. 1999;96:9787–91. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodetti L, Choi S, Barber DL, et al. Adaptive tolerance and clonal anergy are distinct biochemical States. J Immunol. 2006;176:2279–91. doi: 10.4049/jimmunol.176.4.2279. [DOI] [PubMed] [Google Scholar]
- Chmielowski B, Muranski P, Ignatowicz L. In the normal repertoire of CD4+ T cells, a single class II MHC/peptide complex positively selects TCRs with various antigen specificities. J Immunol. 1999;162:95–105. [PubMed] [Google Scholar]
- Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol. 2007;19:140–52. doi: 10.1016/j.smim.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GM, Schober SL, Endrizzi BT, et al. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–74. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Krogsgaard M, Huse M, et al. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–95. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- Ebert PJ, Jiang S, Xie J, et al. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–9. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer UB, Jacovetty EL, Medeiros RB, et al. MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo. P Natl Acad Sci USA. 2007;104:7181–6. doi: 10.1073/pnas.0608299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- Fulton RB, Hamilton SE, Xing Y, et al. The TCR's sensitivity to self peptide-MHC dictates the ability of naive CD8 T cells to respond to foreign antigens. Nat Immunol. 2015;16:107–17. doi: 10.1038/ni.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne NR. Do T cells need endogenous peptides for activation? Nat Rev Immunol. 2008;8:895–900. doi: 10.1038/nri2431. [DOI] [PubMed] [Google Scholar]
- Goyal MS, Venkatesh S, Milbrandt J, et al. Feeding the brain and nurturing the mind: Linking nutrition and the gut microbiota to brain development. P Natl Acad Sci USA. 2015;112:14105–12. doi: 10.1073/pnas.1511465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Z, Min B, Meier-Schellersheim M, et al. Concomitant regulation of T-cell activation and homeostasis. Nat Rev Immunol. 2004;4:387–95. doi: 10.1038/nri1355. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Paul WE. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. P Natl Acad Sci USA. 1992;89:10365–9. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Z, Paul WE. Self-tolerance: context dependent tuning of T cell antigen recognition. Semin Immunol. 2000;12:197–203. doi: 10.1006/smim.2000.0232. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Paul WE. Dynamic tuning of lymphocytes: physiological basis, mechanisms, and function. Annu Rev Immunol. 2015;33:677–713. doi: 10.1146/annurev-immunol-032712-100027. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Singer A. Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. P Natl Acad Sci USA. 1996;93:14747–52. doi: 10.1073/pnas.93.25.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Z. Cellular tolerance as a dynamic state of the adaptable lymphocyte. Immunol Rev. 1993;133:45–73. doi: 10.1111/j.1600-065x.1993.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Hand TW, Dos Santos LM, Bouladoux N, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–6. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hataye J, Moon JJ, Khoruts A, et al. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–6. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- Hedrick SM. The acquired immune system: a vantage from beneath. Immunity. 2004;21:607–15. doi: 10.1016/j.immuni.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Hochrein H, O'Keeffe M, Luft T, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–33. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate 'decisions' and effector function. Nat Immunol. 2014;15:815–23. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Tomlinson AJ, Kieper WC, et al. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6:389–99. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- Honey K, Nakagawa T, Peters C, et al. Cathepsin L regulates CD4+ T cell selection independently of its effect on invariant chain: a role in the generation of positively selecting peptide ligands. J Exp Med. 2002;195:1349–58. doi: 10.1084/jem.20011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–9. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- Jordan MS, Riley MP, Von Boehmer H, et al. Anergy and suppression regulate CD4(+) T cell responses to a self peptide. Eur J Immunol. 2000;30:136–44. doi: 10.1002/1521-4141(200001)30:1<136::AID-IMMU136>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. P Natl Acad Sci USA. 1999;96:13306–11. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Kyewski B, Allen PM, et al. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see) Nat Rev Immunol. 2014;14:377–91. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoechel B, Lohr J, Kahn E, et al. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–86. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard M, Li QJ, Sumen C, et al. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–43. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- Legoux FP, Lim JB, Cauley AW, et al. CD4(+) T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity. 2015;43:896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QJ, Chau J, Ebert PJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–61. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Liu CP, Parker D, Kappler J, et al. Selection of antigen-specific T cells by a single IEk peptide combination. J Exp Med. 1997;186:1441–50. doi: 10.1084/jem.186.9.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WL, Allen PM. Self-peptides in TCR repertoire selection and peripheral T cell function. Curr Top Microbiol Immunol. 2014;373:49–67. doi: 10.1007/82_2013_319. [DOI] [PubMed] [Google Scholar]
- Lo W-L, Felix NJ, Walters JJ, et al. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4(+) T cells. Nat Immunol. 2009;10:1155–61. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas B, Stefanova I, Yasutomo K, et al. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–76. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- Lucas CL, Workman CJ, Beyaz S, et al. LAG-3, TGF-beta, and cell-intrinsic PD-1 inhibitory pathways contribute to CD8 but not CD4 T-cell tolerance induced by allogeneic BMT with anti-CD40L. Blood. 2011;117:5532–40. doi: 10.1182/blood-2010-11-318675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Linehan JL, Dileepan T, et al. Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol. 2016;17:187–95. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Monteiro JP, Vrisekoop N, et al. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–74. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez ME, Ellmeier W, Sanchez-Guajardo V, et al. CD8 T cell sensory adaptation dependent on TCR avidity for self-antigens. J Immunol. 2005;175:7388–97. doi: 10.4049/jimmunol.175.11.7388. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Why positive selection? Immunol Rev. 1993;135:81–117. doi: 10.1111/j.1600-065x.1993.tb00645.x. [DOI] [PubMed] [Google Scholar]
- McFarland RD, Douek DC, Koup RA, et al. Identification of a human recent thymic emigrant phenotype. P Natl Acad Sci USA. 2000;97:4215–20. doi: 10.1073/pnas.070061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Foucras G, Meier-Schellersheim M, et al. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. P Natl Acad Sci USA. 2004;101:3874–9. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13:121–8. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- Moses CT, Thorstenson KM, Jameson SC, et al. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. P Natl Acad Sci USA. 2003;100:1185–90. doi: 10.1073/pnas.0334572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, et al. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–61. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Hooper LV. Antimicrobial defense of the intestine. Immunity. 2015;42:28–39. doi: 10.1016/j.immuni.2014.12.028. [DOI] [PubMed] [Google Scholar]
- Nakamoto N, Cho H, Shaked A, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Murata S, Sasaki K, et al. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity. 2010;32:29–40. doi: 10.1016/j.immuni.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Ohlen C, Kalos M, Cheng LE, et al. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407–18. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Okazaki IM, Wang J, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owyang AM, Zaph C, Wilson EH, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–9. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009;30:592–602. doi: 10.1016/j.it.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Merica R, Mondino A, et al. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:4719–29. [PubMed] [Google Scholar]
- Paul WE, Milner JD, Grossman Z. Pathogen-sensing, regulatory T cells, and responsiveness-tuning collectively regulate foreign- and self-antigen mediated T-cell responses. Cold Spring Harb Symp Quant Biol. 2013;78:265–76. doi: 10.1101/sqb.2013.78.020198. [DOI] [PubMed] [Google Scholar]
- Persaud SP, Parker CR, Lo WL, et al. Intrinsic CD4 T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol. 2014;15:266–74. doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H, Rohrer UH, Moskophidis D, et al. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–5. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- Rocha B, Tanchot C, Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993;177:1517–21. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santori FR, Kieper WC, Brown SM, et al. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity. 2002;17:131–42. doi: 10.1016/s1074-7613(02)00361-8. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Takada K, Ohte Y, et al. Thymoproteasomes produce unique peptide motifs for positive selection of CD8(+) T cells. Nat Commun. 2015;6:7484. doi: 10.1038/ncomms8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–97. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Sinclair NR, Anderson CC. Do lymphocytes require calibration? Immunol Cell Biol. 1994;72:508–12. doi: 10.1038/icb.1994.75. [DOI] [PubMed] [Google Scholar]
- Singh NJ, Bando JK, Schwartz RH. Subsets of nonclonal neighboring CD4+ T cells specifically regulate the frequency of individual antigen-reactive T cells. Immunity. 2012;37:735–46. doi: 10.1016/j.immuni.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NJ, Chen C, Schwartz RH. The impact of T cell intrinsic antigen adaptation on peripheral immune tolerance. PLoS Biol. 2006;4:e430. doi: 10.1371/journal.pbio.0040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NJ, Cox M, Schwartz RH. TLR ligands differentially modulate T cell responses to acute and chronic antigen presentation. J Immunol. 2007;179:7999–8008. doi: 10.4049/jimmunol.179.12.7999. [DOI] [PubMed] [Google Scholar]
- Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198:1107–17. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NJ, Schwartz RH. Primer: mechanisms of immunologic tolerance. Nat Clin Pract Rheum. 2006;2:44–52. doi: 10.1038/ncprheum0049. [DOI] [PubMed] [Google Scholar]
- Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- Steinert EM, Schwartz RH, Singh NJ. At low precursor frequencies, the T-cell response to chronic self-antigen results in anergy without deletion. Eur J Immunol. 2012;42:2875–80. doi: 10.1002/eji.201242518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Takaba H, Morishita Y, Tomofuji Y, et al. Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell. 2015;163:975–87. doi: 10.1016/j.cell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Takada K, Van Laethem F, Xing Y, et al. TCR affinity for thymoproteasome-dependent positively selecting peptides conditions antigen responsiveness in CD8(+) T cells. Nat Immunol. 2015;16:1069–76. doi: 10.1038/ni.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C, Barber DL, Chiodetti L, et al. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J Immunol. 2001;167:2030–9. doi: 10.4049/jimmunol.167.4.2030. [DOI] [PubMed] [Google Scholar]
- Tanchot C, Le Campion A, Martin B, et al. Conversion of naive T cells to a memory-like phenotype in lymphopenic hosts is not related to a homeostatic mechanism that fills the peripheral naive T cell pool. J Immunol. 2002;168:5042–6. doi: 10.4049/jimmunol.168.10.5042. [DOI] [PubMed] [Google Scholar]
- Teague RM, Greenberg PD, Fowler C, et al. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–74. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–35. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, et al. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–9. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Tato CM, Stumhofer JS, et al. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J Exp Med. 2007;204:65–71. doi: 10.1084/jem.20061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret C, Wong FS, Janeway CA., Jr Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–68. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- Vrisekoop N, Monteiro JP, Mandl JN, et al. Revisiting thymic positive selection and the mature T cell repertoire for antigen. Immunity. 2014;41:181–90. doi: 10.1016/j.immuni.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, et al. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–45. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfing C, Sumen C, Sjaastad MD, et al. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–7. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- Xing Y, Jameson SC, Hogquist KA. Thymoproteasome subunit-beta5T generates peptide-MHC complexes specialized for positive selection. P Natl Acad Sci USA. 2013;10:6979–84. doi: 10.1073/pnas.1222244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang N, Ebert PJ, et al. Clonal deletion prunes but does not eliminate self-specific alphabeta CD8(+) T lymphocytes. Immunity. 2015;42:929–41. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]