Abstract
Natural killer T (NKT) cells play a critical role in the host's innate immune response. CD1d-mediated presentation of glycolipid antigens to NKT cells has been established; however, the mechanisms by which NKT cells recognize infected or cancerous cells remain unclear. 5′-AMP activated protein kinase (AMPK) is a master regulator of lipogenic pathways. We hypothesized that activation of AMPK during infection and malignancy could alter the repertoire of antigens presented by CD1d and serve as a danger signal to NKT cells. In this study, we examined the effect of alterations in metabolism on CD1d-mediated antigen presentation to NKT cells and found that an infection with lymphocytic choriomeningitis virus rapidly increased CD1d-mediated antigen presentation. Hypoxia inducible factors (HIF) enhance T-cell effector functions during infection, therefore antigen presenting cells pretreated with pharmacological agents that inhibit glycolysis, induce HIF and activate AMPK were assessed for their ability to induce NKT-cell responses. Pretreatment with 2-deoxyglucose, cobalt chloride, AICAR and metformin significantly enhanced CD1d-mediated NKT-cell activation. In addition, NKT cells preferentially respond to malignant B cells and B-cell lymphomas express HIF-1α. These data suggest that targeting cellular metabolism may serve as a novel means of inducing innate immune responses.
Keywords: CD1d, NKT cells, metabolism, antigen processing, LCMV
Our findings suggest that changes in metabolism due to infection or malignancy serve as danger signals and enhance CD1d-mediated NKT-cell responses.
INTRODUCTION
In contrast to the MHC class I restriction that dictates classical T-cell recognition, NKT cells are CD1d restricted. The CD1d molecule is structurally similar to MHC class I, with a three domain α chain that associates non-covalently with β2-microglobulin (β2m). However, unlike MHC class I, CD1d has a hydrophobic antigen-binding groove that enables selective binding of glycolipid antigens (East, Kennedy and Webb 2014; Zajonc and Girardi 2015). Also, in comparison to the relatively ubiquitous expression of MHC class I on almost all eukaryotic cells, CD1d is expressed mainly on dendritic cells, macrophages, B cells and T cells (Salio et al.2014).
CD1d-mediated antigen presentation begins with the synthesis of the CD1dα chain in the endoplasmic reticulum (ER) (Barral and Brenner 2007). Here, chaperones like calnexin, calreticulin and Erp57 help to ensure that it is properly folded (Kang and Cresswell 2002). The antigen-binding groove of CD1d is occupied by a ‘self’ lipid thought to be loaded by microsomal triglyceride transfer protein (Dougan et al.2005). After association with β2m, the CD1d molecule follows the secretory pathway from the ER to the Golgi and reaches the plasma membrane (PM). Studies from our group and others have shown that in order to present an activating endogenous antigen to NKT cells, CD1d molecules must recycle from the PM to endocytic compartments, due primarily to the presence of a CD1d-localized tyrosine-based targeting motif (Jayawardena-Wolf et al.2001; Chiu et al.2002; Roberts et al.2002) that is analogous to the invariant chain (Ii) for MHC class II molecules. In fact, Ii, which mediates trafficking of MHC class II from the Golgi to the lysosomes, can also associate with CD1d; however, the tyrosine-based motif is necessary for the proper recycling of CD1d molecules and their trafficking to endocytic compartments (Sullivan, Nagarajan and Kronenberg 2005).
Following internalization from the PM, adaptor proteins AP2 and AP3 direct CD1d molecules back to an endocytic compartment, also known as MIIC, where MHC class II molecules are normally loaded with peptide antigens (Cernadas et al.2003; Elewaut et al.2003). Once in the endocytic recycling compartment, the stabilizing self-lipid is exchanged for other activating lipid antigens with the help of saposins (Kang and Cresswell 2004; Zhou et al.2004). These loaded CD1d molecules are then re-expressed on the PM and can be recognized by invariant NKT cells. This complex process of CD1d-mediated antigen processing and presentation has several potential levels of control, yet very few endogenous regulatory factors for this process have been identified.
Some of the factors known to be important for CD1d-mediated antigen processing and presentation are the mitogen-activated protein kinases (MAPK), PKCδ and Rho kinases (Renukaradhya et al.2005, 2008; Brutkiewicz et al.2007; Gallo et al.2012). Altered metabolism is a common feature of both viral infections and cancers, including lymphoma (Platanias and Vakana 2011; Levy and Bartosch 2015; Lovelace and Polyak 2015). Recently, we reported that overexpression or induction of Bcl-xL in mouse B-cell lymphoma cells results in significantly increased CD1d-mediated antigen presentation. In contrast, knockdown of Bcl-xL leads to a significant decrease in level of the intracellular trafficking of CD1d to LAMP+ compartments and a reduction in CD1d-mediated NKT cells activation (Subrahmanyam, Carey and Webb 2014). These data demonstrate that Bcl-xL functions to regulate CD1d-mediated antigen presentation by altering intracellular trafficking of CD1d and support the notion that the regulation of intracellular CD1d-mediated antigen presentation may serve as a strategy that can influence host immune recognition of stressed cells. Moreover, these data implicate a role for alterations in cellular bioenergetics in the modulation of antigen processing and presentation. Thus, we hypothesized that changes in metabolism due to infection or malignancy could serve as danger signals and enhance CD1d-mediated NKT-cell responses.
Classically, two pathways are thought to exist for NKT-cell activation. An antigen-dependent pathway by which NKT cells are activated through direct recognition of microbial glycolipid antigens via their TCR (Kinjo et al.2005, 2006; Mattner et al.2005; Sriram et al.2005) and another cytokine-dependent pathway due to the ability of NKT cells to respond to innate or inflammatory stimuli, which may be associated with self-Ag recognition (Brigl et al.2003; Mattner et al.2005; Paget et al.2007; Salio et al.2007; Brigl and Brenner 2010; Darmoise et al.2010). Given the important role that CD1d-specific NKT cells play in many different types of infections, autoimmunity and transplantation, we investigated NKT-cell responses during an acute viral infection and to cancer cells. These two divergent stimuli were potent inducers of cytokine production by NKT cells, and our studies reveal a novel role for the energy sensor AMP-activated protein kinase (AMPK) in regulating CD1d-mediated antigen processing and presentation.
MATERIALS AND METHODS
Peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque (Amersham Pharmacia Biotek, Uppsala, Sweden) density gradient centrifugation. All donors gave written informed consent before enrolling in the study. The Institutional Review Board of University of Maryland School of Medicine (Baltimore, MD) approved this investigation. Human primary B cells were isolated using the Pan B-cell isolation kit from StemCell Technologies according to the manufacturer's instructions. To generate primary human NKT cells, PBMC were purchased from Biological Specialty Corp and the New York Blood Center. NKT cells were isolated and expanded as previously reported (Webb et al.2012).
Mice
Male and female C57BL/6 wild-type mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were infected between 6 and 12 weeks of age and were age and sex matched. All experiments were performed in accordance with procedures approved by Indiana University School of Medicine animal use and care committee.
Virus and infection of mice
The Armstrong strain of lymphocytic choriomeningitis virus (LCMV) was provided by Dr R. Welsh (University of Massachusetts Medical Center, Worcester, MA). Viral stocks were prepared in BHK cells as previously described (Spence et al.2001). Mice were infected i.p. with 1–2 × 105 plaque forming units PFU of LCMV as previously described (Hobbs et al.2001; Roberts et al.2004). On the indicated days post infection (p.i.), thymi and spleens were harvested and cocultured with NKT-cell hybridomas.
Cell lines, antigens and other reagents
The Vα14+ NKT-cell hybridoma cell lines DN32.D3, N38-2C12, N38-3C3 and the Vα5+ CD1d-specific NKT-cell hybridoma N37-1A12 have all been described (Lantz and Bendelac 1994; Brutkiewicz et al.1995; Roberts et al.2002) and were cultured in IMDM medium supplemented with 5% FBS, penicillin/streptomycin and 2 mM L-glutamine (Gibco Waltham, MA). L-CD1d cells are CD1d1-transfected L cells and the controls, L-vector cells were cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine, penicillin/streptomycin and 500 μg mL−1 G418 as previously reported. Human B-cell lymphoma lines, Farage, Raji, Ramos, SU-DHL-4, SU-DHL-6 and Karpas were generously provided by Dr Ronald Gartenhaus (University of Maryland School of Medicine). The human B lymphoblastoid cell line transfected with human CD1D, C1R-CD1D was generously provided by Dr Mark Exley (Harvard Medical School, Boston, MA). All B-cell lymphoma lines were cultured in RPMI 1640 medium supplemented with non-essential amino acids (Sigma-Aldrich St. Louis, MO), sodium pyruvate (Gibco), vitamin solution (Gibco), 2-mercaptoethanol (Gibco), 10% FBS and penicillin/streptomycin. Pharmacological agents 2-deoxyglucose (2DOG), cobalt chloride and metformin (all from Sigma), A-769662 (Abcam Cambridge, MA) and AICAR (Cell Signaling Technology Danvers, MA) were used as described.
Mononuclear cell isolation
Thymi and spleens were harvested from uninfected and LCMV-infected mice and processed into single-cell suspensions. Erythrocytes were lysed by hypotonic shock in 0.84% NH4Cl. The remaining cells were washed twice with IMDM supplemented with 5% FBS (complete medium), and then resuspended in the same medium.
NKT cell hybridoma assay
To measure endogenous Ag presentation by CD1d1 molecules, LCD1 cells were infected with LCMV (MOI = 5) for 20 h at 37°C. The cells were then washed three times in cold PBS. The NKT-cell hybridomas (5–10 × 104 cells/well) were incubated with 5 × 105 target cells for 24 h at 37°C. Coculture supernatants were harvested, and IL-2 production was measured by ELISA.
Flow cytometry
Cells were stained in PBS containing 0.5% BSA and 2 mM EDTA for 30 min at 4°C with a PE-conjugated antibody to CD1d (clone 1B1) from BD Biosciences (San Jose, CA). Data were collected on an LSR II from BD Biosciences (San Jose, CA) and analyzed using FCS Express Version 3 from De Novo Software (Los Angeles, CA).
Western blotting
Cells were lysed using lysis buffer containing NaCl (150 mM), Tris-Cl (50 mM), EDTA (8.5 mM), sodium azide (0.02%), NP-40 (0.1%) and complete mini protease inhibitor cocktail tablet from Roche Applied Science (Indianapolis, IN) as directed by the manufacturer, and prepared in water. Proteins were resolved by electrophoresis on a 4%–12% gradient polyacrylamide gel and transferred to a PVDF membrane using the iBlot transfer system (Life Technologies, Carlsbad, CA). All gels, equipment, buffers and other materials were from Life Technologies and were used as per the manufacturer's instructions.
Statistical analyses
Two-tailed student's t test, one-way analysis of variance (ANOVA) or two-way ANOVA were used as appropriate. Specific experimental groups were compared with controls using the Sidak's multiple comparisons test. P value less than 0.05 was considered significant. All analyses were performed using Prism 5.02 by GraphPad (La Jolla, CA). # P < 0.0001, *** P < 0.001, ** P < 0.01 and * P < 0.05.
RESULTS
LCMV infection induces NKT-cell responses
We have previously reported a dichotomy in NKT-cell responses between different viral infections. Infection with vaccinia virus or vesicular stomatitis virus resulted in a decrease in CD1d-mediated NKT-cell activation, whereas an acute infection with LCMV did not (Renukaradhya et al.2005). To further investigate the effects of an acute LCMV infection on CD1d-mediated antigen presentation to NKT cells, LCD1 cells (murine L-cell fibroblasts transfected with the cd1d1 cDNA) or murine thymocytes were infected with LCMV for 90 min, washed and cocultured with a panel of murine NKT-cell hybridomas. The production of IL-2 into the supernatants was used as an indication of CD1d-dependent NKT-cell activation. Following infection with LCMV, there was an increase in NKT-cell activation induced by both LCD1 cells and murine thymocytes, suggesting a virus-induced enhancement in CD1d-mediated antigen presentation (Fig. 1A and B). This increase in NKT-cell responses was due to CDd1-dependent antigen presentation, because infection of control L cells and the NKT-cell hybridomas themselves did not result in an increase in cytokine production by NKT cells (data not shown). Importantly, these observations were made with both canonical (Vα14+) and non-canonical NKT cells (Vα5+).
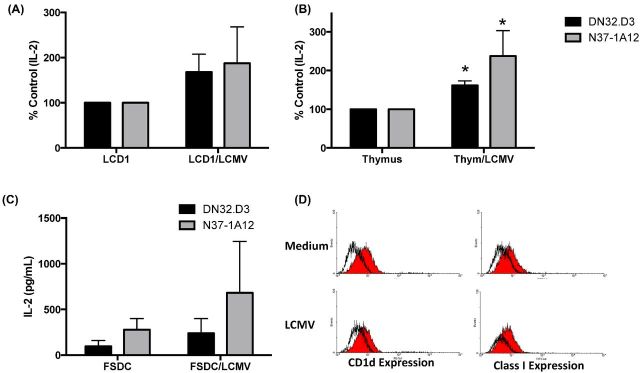
Figure 1.
Acute infection with LCMV enhances CD1d-mediated NKT-cell responses. (A) LCD1 or (B) murine thymocytes were cultured in medium or infected with LCMV (MOI = 5) for 90 min, washed with PBS, then cocultured with NKT cells (DN32.D3 and N37-1A12) for 20–24 h. IL-2 was measured, as an indication of NKT-cell activation, by standard cytokine ELISA. Data shown as % control and are the average of two experiments performed in triplicate. Data are representative of >5 independent experiments. Cytokine release from NKT cells cocultured with uninfected cells was compared to LCMV-infected cells. * P < 0.05. (C) LCMV infection increases NKT-cell responses to FSDC. A dendritic cell line, FSDC, was infected with LCMV as described above, and cocultured with NKT-cell hybridomas. Data shown are the average of two experiments performed in triplicate. (D) LCMV infection does not alter CD1d cell surface expression. Following 24 p.i. FSDC cells were stained for CD1d and MHC class I expression and analyzed by flow cytometry.
To determine whether infection with LCMV could alter CD1d-mediated NKT cells activation in different cell types, we infected the murine dendritic cell line, FSDC, and cocultured these cells with a panel of NKT-cell hybridomas. LCMV infection of FSDC cells resulted in an increase in NKT- cell activation, without an effect on surface CD1d expression (Fig. 1C and D). These data suggest that a viral infection can rapidly modulate the repertoire of endogenous antigens presented by CD1d molecules.
We next investigated whether an acute LCMV infection would alter CD1d-mediated antigen presentation ex vivo. Therefore, C57BL/6 mice were infected with LCMV for the indicated time periods; thymi were then harvested and cocultured with NKT cells. It was found that in vivo infection with LCMV rapidly alters CD1d-mediated antigen presentation (Fig. 2A and B). It has been established that stimulation with naïve murine splenocytes do not activate type I NKT cells (Park, Roark and Bendelac 1998); however, we found that LCMV infection confers recognition of splenocytes by NKT cells in the absence of exogenous antigen (Fig. 2A and B).
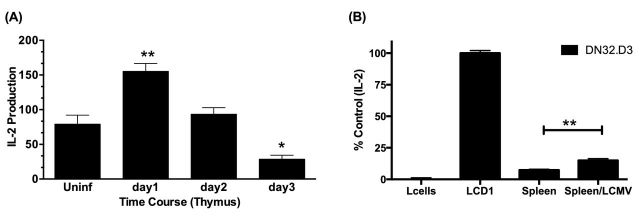
Figure 2.
LCMV infection rapidly increases CD1d-mediated NKT-cell activation. (A) C57BL/6 mice were infected with LCMV for the indicated time periods, thymi were harvested and cocultured DN32.D3. IL-2 was measured, as an indication of NKT-cell activation, by standard cytokine ELISA. Data shown are the average of one experiment performed in triplicate and is representative of two independent experiments. (B) LCMV infection confers recognition of murine splenocytes by NKT-cell hybridomas. Splenocytes were harvested from LCMV-infected C57BL/6 mice (d3 p.i.) and cocultured with DN32.D3 NKT-cell hybridomas. Data shown are the average of one experiment performed in triplicate and are representative of >3 independent experiments. In other experiments, splenocytes were harvested 1, 2, 3 and 8 days p. i. and in each case were able to stimulate NKT-cell hybridomas in the absence of exogenous antigen. Cytokine release from NKT cells cocultured with uninfected cells was compared to LCMV-infected cells. ** P < 0.01 and * P < 0.05.
It has been reported that cytotoxic T cells can recognize very early, minor changes in virus-infected target cells (Jackson et al.1976; Jackson, Ada and Tha Hla 1976); thus, we examined whether alterations in glycolysis (such as those induced by a virus infection) would enhance CD1d-mediated NKT-cell activation. Hypoxia-inducible factor (HIF) transcription factors play an important role in the metabolic changes that drive cellular adaptation to low oxygen availability. HIF expression can be induced by hypoxia, but also by other stress-associated factors such as infections and cancer (Palazon et al.2014). Thus, we hypothesized that induction of HIF-1α or alterations in glycolytic metabolism may the mechanism by which NKT cells are able to distinguish between healthy cells and infected or malignant cells. To examine whether changes in metabolism would alter CD1d-mediated NKT cells, LCD1 cells were incubated with increasing doses of 2DOG, an inhibitor of cellular glycolysis (Zhong et al.2009). It was found that treatment resulted in an increase in CD1d-mediated NKT-cell activation (Fig. 3A and B). Due to the variability in cytokine production by the NKT-cell hybridomas, these experiments were repeated >7 times and the trend demonstrated a reproducible increase in NKT-cell responses. Likewise, pretreatment with cobalt chloride (CoCl2), which also induces HIF-1α and increases oxidative respiration (Li et al.2015), resulted in a concomitant increase in NKT-cell activation (Fig. 3B and C). Importantly, CoCl2 pretreatment did not alter CD1d cell surface expression at any dose examined (Fig. 3D). Collectively, these data suggest that changes in glucose metabolism can alert the innate immune response, by inducing CD1d-mediated NKT-cell activation. This induction is likely due to a change in the repertoire of endogenous glycolipid antigens, as there was no detectable increase in CD1d on the cell surface.
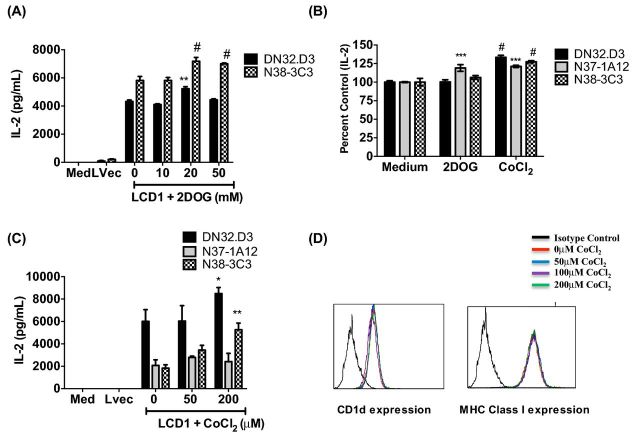
Figure 3.
Pretreatment with 2DOG and cobalt chloride results in increased CD1d-dependent stimulation of NKT cells. (A) LCD1 were incubated with 2DOG or (B and C) cobalt chloride for 4 h at 37˚C at the indicated concentrations, washed and cocultured with NKT hybridomas, DN32.D3, N38-3C3 and N37-1A12. Medium and L cells transfected with an empty vector (Lvec) were included as negative controls. Data shown are the average of one experiment performed in triplicate and are representative of >7 independent experiments. (B) Cumulative data from three experiments shown as % control due to the variability in cytokine production by NKT-cell hybridomas. (D) Pretreatment with cobalt chloride does not affect CD1d cell surface expression. LCD1 were analyzed for surface expression of CD1d or MHC class I by flow cytometry following a 4 h pretreatment with concentrations of CoCl2 indicated above (black histogram is the negative control). Net cytokine production from NKT cells cocultured with vehicle control-treated cells (Med) was compared to drug-treated cells. # P < 0.0001, *** P < 0.001, ** P < 0.01 and * P < 0.05.
Primary cancer cells express HIF-1α and can induce NKT-cell activation
We have found that alterations in metabolism due to treatment with 2DOG and cobalt chloride can enhance CD1d-mediated NKT-cell responses; thus, we hypothesized that this may be a mechanism by which NKT cells also recognize transformed cells. Interestingly, we found that alterations in glucose metabolism, either by inhibiting glycolysis or inducing HIF-1, resulted in enhanced NKT-cell activation (Fig. 3A–C). We confirmed this using a panel of human B-cell lymphoma following treatment with CoCl2-induced HIF-1α (Fig. 4A). Moreover, and in agreement with a prior study (Argyriou et al.2011), we found that HIF-1α was also highly expressed in malignant B cells isolated from a patient with lymphoma (NHL B cells) (Fig. 4A). To investigate whether human NKT cells can recognize primary non-Hodgkin's lymphoma (NHL) cells, B cells were isolated from the peripheral blood of healthy donors and patients with active disease. When cocultured in the presence of the NKT-cell agonist α-galactosylceramide (α-GalCer), stimulation with lymphoma cells induced higher levels of IFN-γ production by human primary NKT cells, compared to healthy B cells (Fig. 4B) (Li et al.2014). Collectively, these data suggest that the upregulation of HIF-1α expression by malignant B cells may modulate CD1d-mediated activation of NKT cells.
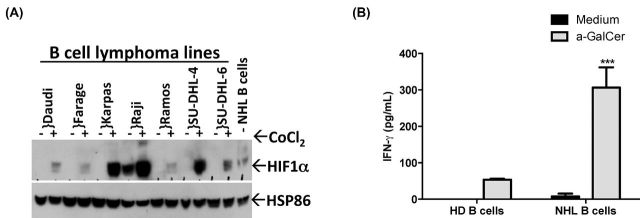
Figure 4.
B-cell lymphomas upregulate HIF-1α. (A) HIF-1α expression was assessed in a panel of B-cell lymphoma lines following 4 h treatment with CoCl2 (100 μM) or in freshly isolated malignant B cells from a patient by western blotting. HSP86 served as a loading control. (B) Primary human NKT cells were cocultured with B cells isolated from the peripheral blood of healthy donors (HD) or patients with lymphoma (NHL) in the absence or presence of antigen- α-GalCer (100 ng mL−1) for 48 h. IFN-γ was measured, as an indication of human NKT-cell activation, by standard cytokine ELISA. Data shown are from one experiment of two similar experiments performed in triplicate. Net cytokine production from NKT cells stimulated with HD B cells was compared to NHL B cells. ** P < 0.01.
Induction of AMPK results in increased CD1d-mediated antigen presentation
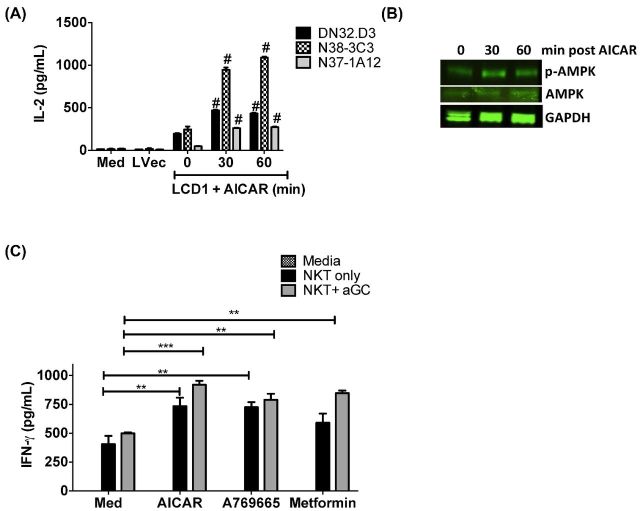
AMPK serves as a fuel-sensing enzyme that is activated by the binding of AMP and subsequent phosphorylation by upstream kinases, such as the tumor suppressor liver kinase B1 (LKB1), when cells sense an increase in the ratio of AMP to ATP (Flavin, Zadra and Loda 2011). Acute activation of AMPK stimulates fatty acid oxidation to generate more ATP and simultaneously inhibits ATP-consuming processes, including fatty acid and protein syntheses. This preserves energy for acute cell-surviving program; in contrast, chronic activation leads to inhibition of cell growth. Studies have shown that AMPK is rapidly activated in vitro by both physiological and pathophysiological low-oxygen conditions (Grahame Hardie 2014). We sought to investigate whether activation of AMPK would have an impact on CD1d-mediated antigen presentation to NKT cells. CD1d expressing cells were pretreated with 5-aminoimidazole-4-carboxamide 1-D-ribonucleoside (AICAR), the prototypical AMPK activator and then cocultured with NKT cells. As shown in Fig. 5A, we found that AMPK activation significantly increases CD1d-mediated NKT cell activation. We confirmed AMPK induction by AICAR by western blot (Fig. 5B). To confirm a role for AMPK activation in being an important regulator of CD1d-mediated NKT-cell responses overall (i.e. not just in murine fibroblasts), we treated the human B lymphoblastoid cell line, C1R-CD1D, with metformin, AICAR or another AMPK activator- A769665. Importantly, the activation of AMPK alone induced NKT-cell activation to levels comparable to stimulation of cells treated with the potent NKT-cell agonist, α-GalCer (Fig. 5C). These data demonstrate that AMPK activation significantly increases CD1d-mediated NKT-cell activation, and further suggests that NKT cells may be uniquely sensitive to changes in glycolytic respiration, as a mechanism used by the innate immune system to control neoplastic transformation and initiate antiviral immune responses.
Figure 5.
Induction of AMPK results in enhanced NKT-cell responses. (A) LCD1 cells were incubated with AICAR (1000 μM) for 0, 30 or 60 h at 37°C, washe, and then cocultured NKT-cell hybridoma lines DN32.D3, N38-3C3 and N37-1A12. Medium and L cells transfected with empty vector (Lvec) are included as negative controls. One experiment performed in triplicate, representative of three independent experiments, is shown. (B) Levels of both p-AMPK and AMPK were found to increase in a time-dependent fashion following treatment with AICAR. GAPDH was included as a loading control. (C) Pretreatment with AICAR increases CD1d-mediated NKT-cell activation. C1R-CD1D B-cell lymphoblasts were pretreated with 0 or 1000 μM AICAR, A79665 or metformin in the presence or absence of 100 ng mL−1 α-GalCer for 2 h, washed and then cocultured with primary human NKT cells. Cytokine release was measured by ELISA. Data shown are from one experiment of two similar experiments performed in triplicate. # P < 0.0001, *** P < 0.001, ** P < 0.01 and * P < 0.05.
DISCUSSION
In this study, we sought to ascertain the mechanisms by which NKT cells recognize virally infected and malignant cells. We found that NKT cells produce higher levels of cytokine when cocultured with LCMV-infected cells or primary B-cell lymphomas, compared to healthy, uninfected control cells. IFN-γ-associated metabolic changes have been observed during viral infection (Wikoff et al.2009) and during malignancy (Flavin, Zadra and Loda 2011). Thus, we subsequently examined the effect of alterations in metabolism on CD1d-mediated Ag presentation to NKT cells. Pretreatment with 2DOG, cobalt chloride and AICAR, which block glycolysis, induce HIF-1α and activate AMPK, respectively, resulted in an increase in CD1d-mediated NKT-cell responses. These data suggest that targeting cellular metabolism may serve as a novel means of inducing innate immune responses.
NKT cells comprise an important subset of T cells that are activated by glycolipid antigens within the context of CD1d (Terabe and Berzofsky 2014). Given their potential high therapeutic significance, NKT-cell-based clinical trials have been conducted in patients with advanced non-small cell lung cancers and with head and neck cancers (reviewed in Fujii et al.2013). However, the mechanisms by which NKT cells discriminate between healthy normal cells and cancerous or infected cells remain unclear. Consequently, in our studies, we have primarily focused our efforts on delineating the underlying mechanism(s) by which NKT cells recognize and destroy these targets, in order to make progress on the development of effective NKT-cell-based therapeutic strategies.
In our previous study examining the role of Bcl-xL in CD1d-mediated antigen presentation, we found that there was increased levels of Rab7 after Bcl-xL knockdown compared to the scrambled control (Subrahmanyam, Carey and Webb 2014). These data suggest that knocking down Bcl-xL leads to upregulation of Rab7 and an expansion of the late endosomal compartment, acting as a depot for CD1d molecules and resulting in impaired trafficking to the lysosomal compartment, and further implicates a role for autophagy in regulating antigen presentation. AMPK promotes autophagy (Dong et al.2016); thus, in this study, we investigated the role of AMPK on CD1d-dependent NKT-cell responses.
While other studies have examined the role of metabolism on T-cell responses (Tamas et al.2006; Palmer et al.2015), we have focused on its role in antigen processing and presentation. Specifically, AMPK suppresses mTOR-dependent transcriptional regulators (such as cyclin D1 and myc) to inhibit cell growth and tumorigenesis. Two mTORC1-regulated transcription factors involved in cell growth are the sterol-regulatory element-binding protein 1 and HIF1. The HIF subunits are stabilized through the hypoxic inactivation of the von Hippel–Lindau E3 ligase that targets them for destruction. Conditions that lower intracellular ATP levels; for example, low glycolytic rates from low glucose concentrations or inhibitors, such as 2DOG, can lead to the activation of AMPK in a LKB1-dependent manner. Importantly, we have found that pretreatment of antigen presenting cells with 2DOG, CoCl2 and AICAR all result in increased NKT-cell activation, highlighting the importance of targeting these pathways. Our proposed model is shown in Fig. 6.
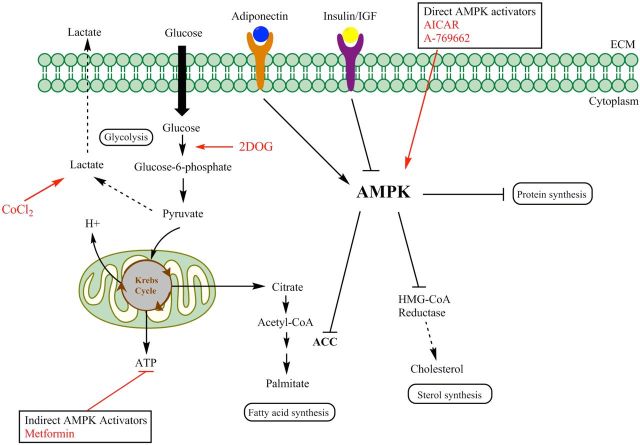
Figure 6.
Proposed model illustrating the mechanisms by which AMPK functions as a central metabolic switch that governs glucose and lipid metabolism and activation of this master switch serves as a danger signal to alert NKT cells to infection and malignancy. Once activated, AMPK reduces plasma insulin levels, suppresses ATP-consuming metabolic functions (such as synthesis of fatty acids, sterols, glycogen and proteins) and increases ATP-producing activities (such as glucose uptake, fatty acid oxidation and mitochondrial biogenesis) to restore energy homeostasis. In this study, antigen presenting cells were pretreated with 2DOG, cobalt chloride, AICAR, A769662 and metformin to targeted several steps in this pathway, as indicated with red arrows, and found an enhanced CD1d-mediated NKT-cell activation. These data suggest that targeting cellular metabolism may serve as a novel means of inducing innate immune responses.
It has been well established that tumor metabolism is significantly different compared to normal cells and is characterized by an increase in the utilization of glycolysis, even in the presence of an adequate oxygen supply (the Warburg Effect)(Semenza 2007). Additionally, the microenvironment surrounding tumors is typically poorly vascularized and causes low levels of oxygen near the tumor. A number of proteins, specifically HIF1-α and AMPK, are upregulated under conditions of hypoxia. These proteins control the transcriptional regulation of over 100 different proteins responsible for glucose metabolism, lipid metabolism and a diverse set of other functions. In order to assess the potential role of these pathways on CD1d-mediated NKT-cell responses, we used pharmacological agents to induce HIF and AMPK, and found that both resulted in an increase in CD1d-dependent NKT-cell activation. Taken together, our data suggest that stressors such as certain viral infections and cancers induce alter cellular bioenergetics leading to a switch in oxidative respiration. We believe that this alters the repertoire of endogenous glycolipid antigens presented by CD1d molecules to NKT cells, thereby acting as a danger signal and enhancing NKT-cell-mediated effector responses. Ongoing studies are focused on characterizing the changes in the lipid profiles, as well as identifying pharmacological agents that enhance CD1d-mediated NKT-cell responses.
It has been reported that patients who are currently taking metformin, a classic activator of AMPK responsible for decreasing circulating glucose levels by blocking complex I of the electron transport chain, are at a significantly decreased risk of developing certain types of cancer (Mayer, Klotz and Venkateswaran 2015). Because this phenomenon correlates with generating a hypoxic state in tumor cells, we wished to investigate if similar hypoxia-inducing compounds would increase immunologic cell activity, specific NKT cells, in vitro. We found that treatment of antigen presenting cells (APCs) with agents that alter metabolism such as, 2DOG, CoCl2, and AICAR, followed by coculture with NKT cells, causes an increase in NKT-cell cytokine secretion. Additionally, we observed an increase in AMPK and well as pAMPK transcription. Taken together, our studies implicate a novel role for metabolism in enhancing the innate immune response. Furthermore, we posit that activating AMPK by repurposing FDA-approved drugs, such as metformin, has the potential to revolutionize treatment regimens, by replacing interventions that have life-threatening toxicities with ones that are safe and effective.
Acknowledgments
The authors would like to thank the patients and healthy donors who allowed their samples to be studied.
FUNDING
This work was supported by the National Institutes of Health [ R21 CA162277, R21 CA199544, R21 CA184469 to T.J.W. and R01 46455 to R.R.B.].
Conflict of interest. None declared.
REFERENCES
- Argyriou P, Papageorgiou SG, Panteleon V, et al. Hypoxia-inducible factors in mantle cell lymphoma: implication for an activated mTORC1–>HIF-1alpha pathway. Ann Hematol. 2011;90:315–22. doi: 10.1007/s00277-010-1070-6. [DOI] [PubMed] [Google Scholar]
- Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7:929–41. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Seminars in Immunology. 2010;22:79–86. doi: 10.1016/j.smim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, et al. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Brutkiewicz RR, Bennink JR, Yewdell JW, et al. TAP-independent, b2-microglobulin-dependent surface expression of functional mouse CD1.1. J Exp Med. 1995;182:1913–9. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutkiewicz RR, Willard CA, Gillett-Heacock KK, et al. Protein kinase C delta is a critical regulator of CD1d-mediated antigen presentation. Eur J Immunol. 2007;37:2390–5. doi: 10.1002/eji.200737124. [DOI] [PubMed] [Google Scholar]
- Cernadas M, Sugita M, van der Wel N, et al. Lysosomal localization of murine CD1d mediated by AP-3 is necessary for NK T cell development. J Immunol. 2003;171:4149–55. doi: 10.4049/jimmunol.171.8.4149. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Park SH, Benlagha K, et al. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nat Immunol. 2002;3:55–60. doi: 10.1038/ni740. [DOI] [PubMed] [Google Scholar]
- Darmoise A, Teneberg S, Bouzonville L, et al. Lysosomal α-Galactosidase Controls the Generation of Self Lipid Antigens for Natural Killer T Cells. Immunity. 2010;33:216–28. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Liang S, Hu J, et al. Autophagy as a target for hematological malignancy therapy. Blood Rev. 2016 doi: 10.1016/j.blre.2016.04.005. http://dx.doi.org/10.1016/j.blre.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Dougan SK, Salas A, Rava P, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–39. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East JE, Kennedy AJ, Webb TJ. Raising the roof: the preferential pharmacological stimulation of Th1 and th2 responses mediated by NKT cells. Med Res Rev. 2014;34:45–76. doi: 10.1002/med.21276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewaut D, Lawton AP, Nagarajan NA, et al. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Valpha14i NKT cells. J Exp Med. 2003;198:1133–46. doi: 10.1084/jem.20030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin R, Zadra G, Loda M. Metabolic alterations and targeted therapies in prostate cancer. J Pathol. 2011;223:283–94. doi: 10.1002/path.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii SI, Shimizu K, Okamoto Y, et al. NKT Cells as an Ideal Anti-Tumor Immunotherapeutic. Front Immunol. 2013;4:409. doi: 10.3389/fimmu.2013.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RM, Khan MA, Shi J, et al. Regulation of the actin cytoskeleton by Rho kinase controls antigen presentation by CD1d. J Immunol. 2012;189:1689–98. doi: 10.4049/jimmunol.1101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame Hardie D. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med. 2014;276:543–59. doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs JA, Cho S, Roberts TJ, et al. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001;75:10746–54. doi: 10.1128/JVI.75.22.10746-10754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Ada GL, Hapel AJ, et al. Changes in the surface of virus-infected cells recognized by cytotoxic T cells. II. A requirement for glycoprotein synthesis in virus-infected target cells. Scand J Immunol. 1976;5:1021–9. doi: 10.1111/j.1365-3083.1976.tb03054.x. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Ada GL, Tha Hla R. Cytotoxic T cells recognize very early, minor changes in ectromelia virus-infected target cells. Aust J Exp Biol Med. 1976;54:349–63. doi: 10.1038/icb.1976.35. [DOI] [PubMed] [Google Scholar]
- Jayawardena-Wolf J, Benlagha K, Chiu YH, et al. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Cresswell P. Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain. J Biol Chem. 2002;277:44838–44. doi: 10.1074/jbc.M207831200. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5:175–81. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Lantz O, Bendelac A. An invariant T cell receptor a chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy P, Bartosch B. Metabolic reprogramming: a hallmark of viral oncogenesis. Oncogene. 2015 doi: 10.1038/onc.2015.479. [DOI] [PubMed] [Google Scholar]
- Li G, Zhao Y, Li Y, et al. Up-regulation of neuronal nitric oxide synthase expression by cobalt chloride through a HIF-1α mechanism in neuroblastoma cells. Neuromol Med. 2015;17:443–53. doi: 10.1007/s12017-015-8373-7. [DOI] [PubMed] [Google Scholar]
- Li J, Sun W, Subrahmanyam PB, et al. NKT Cell Responses to B Cell Lymphoma. Med Sci. 2014;2:82–97. doi: 10.3390/medsci2020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace E, Polyak S. Natural products as tools for defining how cellular metabolism influences cellular immune and inflammatory function during chronic infection. Viruses. 2015;7:2933. doi: 10.3390/v7122933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Mayer MJ, Klotz LH, Venkateswaran V. Metformin and prostate cancer stem cells: a novel therapeutic target. Prostate Cancer P D. 2015;18:303–9. doi: 10.1038/pcan.2015.35. [DOI] [PubMed] [Google Scholar]
- Paget C, Mallevaey T, Speak AO, et al. Activation of Invariant NKT Cells by Toll-like Receptor 9-Stimulated Dendritic Cells Requires Type I Interferon and Charged Glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Palazon A, Goldrath Ananda W, Nizet V, et al. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–28. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CS, Ostrowski M, Balderson B, et al. Glucose metabolism regulates T cell activation, differentiation and functions. Front Immun. 2015;6:1. doi: 10.3389/fimmu.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J Immunol. 1998;160:3128–34. [PubMed] [Google Scholar]
- Platanias L, Vakana E. AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications. Oncotarget. 2011;2:1322–8. doi: 10.18632/oncotarget.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renukaradhya GJ, Khan MA, Shaji D, et al. Vesicular stomatitis virus matrix protein impairs CD1d-mediated antigen presentation through activation of the p38 MAPK pathway. J Virol. 2008;82:12535–42. doi: 10.1128/JVI.00881-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renukaradhya GJ, Webb TJ, Khan MA, et al. Virus-induced inhibition of CD1d1-mediated antigen presentation: reciprocal regulation by p38 and ERK. J Immunol. 2005;175:4301–8. doi: 10.4049/jimmunol.175.7.4301. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Lin Y, Spence PM, et al. CD1d1-dependent control of the magnitude of an acute antiviral immune response. J Immunol. 2004;172:3454–61. doi: 10.4049/jimmunol.172.6.3454. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Sriram V, Spence PM, et al. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J Immunol. 2002;168:5409–14. doi: 10.4049/jimmunol.168.11.5409. [DOI] [PubMed] [Google Scholar]
- Salio M, Silk JD, Jones EY, et al. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–66. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- Salio M, Speak AO, Shepherd D, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proceedings of the National Academy of Sciences. 2007;104:20490–5. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231–4. doi: 10.1007/s10863-007-9081-2. [DOI] [PubMed] [Google Scholar]
- Spence PM, Sriram V, Van Kaer L, et al. Generation of cellular immunity to lymphocytic choriomeningitis virus is independent of CD1d1 expression. Immunology. 2001;104:168–74. doi: 10.1046/j.0019-2805.2001.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram V, Du W, Gervay-Hague J, et al. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam PB, Carey GB, Webb TJ. Bcl-xL regulates CD1d-mediated antigen presentation to NKT cells by altering CD1d trafficking through the endocytic pathway. J Immunol. 2014;193:2096–105. doi: 10.4049/jimmunol.1400155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BA, Nagarajan NA, Kronenberg M. CD1 and MHC II find different means to the same end. Trends Immunol. 2005;26:282–8. doi: 10.1016/j.it.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Tamas P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–70. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M, Berzofsky JA. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol Immun. 2014;63:199–213. doi: 10.1007/s00262-013-1509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TJ, Li X, Giuntoli RL, 2nd, et al. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 2012;72:3744–52. doi: 10.1158/0008-5472.CAN-11-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Kalisak E, Trauger S, et al. Response and recovery in the plasma metabolome tracks the acute LCMV-induced immune response. J Proteome Res. 2009;8:3578–87. doi: 10.1021/pr900275p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc DM, Girardi E. Recognition of microbial glycolipids by Natural Killer T cells. Front Immun. 2015;6:400. doi: 10.3389/fimmu.2015.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong D, Xiong L, Liu T, et al. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J Biol Chem. 2009;284:23225–33. doi: 10.1074/jbc.M109.005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C, 3rd, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]