Abstract
Celastrol is a bioactive compound derived from traditional Chinese medicinal herbs of the Celastraceae family. Celastrol is known to possess anti-inflammatory and anti-oxidant activities. Our studies have highlighted the immunomodulatory attributes of celastrol in adjuvant-induced arthritis (AA), an experimental model of human rheumatoid arthritis (RA). RA is an autoimmune disease characterized by chronic inflammation of the synovial lining of the joints, leading eventually to tissue damage and deformities. Identification of the molecular targets of celastrol such as the NF-κB pathway, MAPK pathway, JAK/STAT pathway and RANKL/OPG pathway has unraveled its strategic checkpoints in controlling arthritic inflammation and tissue damage in AA. The pathological events that are targeted and rectified by celastrol include increased production of pro-inflammatory cytokines; an imbalance between pathogenic T helper 17 and regulatory T cells; enhanced production of chemokines coupled with increased migration of immune cells into the joints; and increased release of mediators of osteoclastic bone damage. Accordingly, celastrol is a promising candidate for further testing in the clinic for RA therapy. Furthermore, the results of other preclinical studies suggest that celastrol might also be beneficial for the treatment of a few other autoimmune diseases besides arthritis.
Keywords: celastrol, inflammation, autoimmune diseases, immune modulation, natural products
Celastrol, a natural herbal product, has anti-arthritic activity and it is a promising candidate for further testing in patients with rheumatoid arthritis and other autoimmune diseases.
INTRODUCTION
Rheumatoid arthritis (RA) is a debilitating autoimmune disease characterized by chronic inflammation of the synovial lining of the joints, damage to cartilage, and bone and deformities of the joints (Harris 1990; Scott, Smith and Kingsley 2003; Lipsky 2005). Both genetic and environmental factors are involved in the initiation and progression of this disease (Harris 1990; Lipsky 2005). RA pathogenesis involves the activation of autoreactive T cells; increased production of pro-inflammatory cytokines; enhanced production of chemokines and increased migration of pathogenic cells into the joints; and increased production of mediators of bone remodeling leading to tissue damage in the joints (Harris 1990; Scott, Smith and Kingsley 2003; Lipsky 2005; Furst and Emery 2014). The non-steroidal anti-inflammatory drugs, the disease-modifying anti-rheumatic drugs and steroids represent the mainstream therapeutics for RA. The long-term use of conventional anti-inflammatory or immunosuppressive agents can have severe adverse effects (Couzin 2004; Kremers et al.2004). Biologics such as anti-tumor necrosis factor-α antibody and anti-interleukin-6 (IL-6) receptor antibody, which target specific pro-inflammatory cytokines involved in the pathogenesis of arthritis, are quite effective in a proportion of RA patients but their use can predispose patients to severe infections (Lee, Yedla and Kavanaugh 2003; Garcia-Hernandez, Gonzalez-Amaro and Portales-Perez 2014). Additional drawbacks of the use of biologics include the unresponsiveness of subsets of patients, inconvenience of administration by injection and high cost (Lee and Kavanaugh 2005; Rosman, Shoenfeld and Zandman-Goddard 2013). Accordingly, there is a need for safer and effective new therapeutic products for RA.
Medicinal plants belonging to the traditional systems of medicine offer many promising herbal extracts and/or purified compounds such as Tripterygium Wilfordii Hook F (Thunder God Vine), Boswellia Serrata and celastrol (Fig. 1) for therapeutic purposes in arthritis (Abdel-Tawab, Werz and Schubert-Zsilavecz 2011; Venkatesha et al.2011; Liu et al.2013; Lv et al.2015). However, certain essential prerequisites for their effective application include: a thorough characterization of the herbal extracts, a reliable quality control of their composition and formulation, defining their mechanisms of action and satisfactorily testing them in preclinical models of human RA. Fulfillment of these criteria would then permit clinical testing of selected natural products in RA patients. Studies on celastrol have validated its anti-arthritic activity as well as defined its mechanisms of action in adjuvant-induced arthritis (AA) model of RA (Venkatesha et al.2011, 2012; Cascao et al.2012, 2015; Nanjundaiah et al.2012; Yu, Venkatesha and Moudgil 2012). Similarly, other studies have unraveled the potential disease-modulating activity of celastrol in experimental models of other major human autoimmune diseases such as multiple sclerosis (MS) (Abdin and Hasby 2014; Wang et al.2015), inflammatory bowel disease (IBD) (Pinna et al.2004; Shaker, Ashamallah and Houssen 2014; Jia et al.2015; Zhao et al.2015) and systemic lupus erythematosus (SLE) (Li et al.2005; Xu et al.2007).
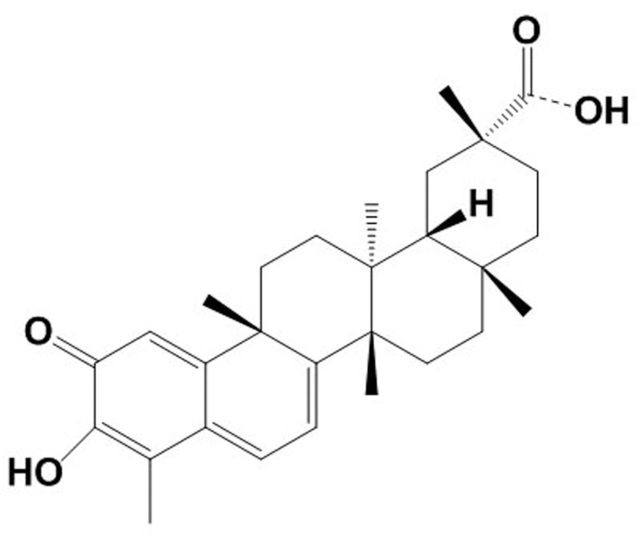
Figure 1.
Structure of celastrol. Celastrol is a pentacyclic triterpenoid with a molecular formula C29H38O4 and molecular weight 450. Celastrol is also known as tripterine/tripterin.
In this article, we have discussed RA as a prototypic autoimmune disease model to highlight the beneficial effects of celastrol against autoimmune inflammation in context of the main pathways involved in RA pathogenesis. A brief summary of the cell signaling pathways shared by various inflammatory and autoimmune diseases and influenced by celastrol is presented in the beginning. Also included is a brief description of the effects of celastrol in models of other autoimmune diseases mentioned above.
MODULATION OF CELL SIGNALING PATHWAYS BY CELASTROL
Celastrol modulates several cell signaling pathways that are associated with chronic inflammatory and autoimmune disease conditions. These pathways include the nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPK), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) and anti-oxidant defense pathways. In addition, celastrol is shown to regulate Toll-like receptor (TLR) pathway. For NF-κB pathway, celastrol blocks I kappa B (IκB) kinase (IKK) activity as well as the degradation and phosphorylation of IκB (Lee et al.2006). This in turn blocks the activation of NF-κB and its nuclear translocation, and thereby, inhibits the expression of NF-κB-regulated target genes (Tak and Firestein 2001; Simmonds and Foxwell 2008). For MAPK pathway, celastrol inhibits the phosphorylation of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) (Jung et al.2007; Venkatesha et al.2011). However, its precise action on p38 MAPK is not clear. For JAK/STAT pathway, celastrol is shown to inhibit the phosphorylation of STAT3 as well as STAT3-mediated interleukin-17 (IL-17) expression, T helper 17 (Th17) differentiation and cell proliferation (Kannaiyan et al.2011; Venkatesha et al.2011; Rajendran et al.2012; Astry et al.2015). However, the effects of celastrol on JAKs and other STATs are not clear. For PI3K/Akt/mTOR pathway, celastrol has been shown to inhibit PI3K activity, Akt phosphorylation, mTOR phosphorylation, as well as the downstream effectors such as ribosomal protein S6 kinase (p70S6K), eukaryotic initiation factor 4E and ERK (Ma et al.2014; Shrivastava et al.2015). The modulation of these processes in turn results in the inhibition of hypoxia-inducible factor-1 (HIF-1) (Ma et al.2014). In regard to TLR pathway, celastrol inhibits lipopolysaccharide (LPS)-induced angiogenesis by suppressing TLR-4-mediated NF-κB activation (Ni et al.2014). Furthermore, celastrol can inhibit TLR activation in macrophages by blocking the binding of LPS to TLR-4/myeloid differentiation factor 2 complex (Lee et al.2015). This effect is thiol-dependent and involves thiol reactivity of celastrol. Taken together, celastrol interferes with multiple cell signaling pathways and induces differential actions depending on the disease processes in various inflammatory and autoimmune conditions.
CELASTROL SUPPRESSES AUTOIMMUNE ARTHRITIS
The anti-arthritic activity of celastrol/ tripterine has been the subject of various in vivo and in vitro studies (Li et al.1997, 2008, 2012, 2013a,b; Venkatesha et al.2011, 2012; Cascao et al.2012; Nanjundaiah et al.2012; Yu, Venkatesha and Moudgil 2012; Xu et al.2013). Celastrol was found to effectively suppress arthritis in experimental models of human RA such as AA and collagen-induced arthritis. Various cellular and molecular mechanisms of action of celastrol have been examined using in vitro experiments based on splenocytes, lymph node cells (LNC) and synovial fibroblast-like cells (FLS) derived from arthritic animals as well as FLS of RA patients (RA-FLS).
Cytokine responses in arthritis and their modulation by celastrol
An imbalance of pro-inflammatory and anti-inflammatory cytokines contributes to RA pathology (Isomaki and Punnonen 1997; Brennan and McInnes 2008; Astry, Venkatesha and Moudgil 2013). Tumor necrosis factor alpha (TNFα), IL-1β, IL-6 and IL-17 are pro-inflammatory cytokines that have extensively been studied in RA (Brennan and McInnes 2008; Astry, Venkatesha and Moudgil 2013). TNFα, IL-1β and IL-6 are produced by cells of myeloid origin including macrophages, monocytes and fibroblasts, whereas IL-17 is predominantly produced by the CD4+ Th17 cells (Isomaki and Punnonen 1997; Brennan and McInnes 2008). Other sources of IL-17 include CD8+ T cells, γδ T cells, natural killer T cells and lymphoid tissue inducer cells (Isomaki and Punnonen 1997; Stockinger and Veldhoen 2007; Brennan and McInnes 2008). These pro-inflammatory cytokines reciprocally influence the production of one another, and they can act independently or synergistically in the course of arthritis. The blocking of these pro-inflammatory cytokines has been shown to be beneficial for the therapeutic control of RA (Adorini 2003; Venkatesha et al.2014). IL-4, IL-10 and IL-13 function mainly as anti-inflammatory cytokines and are mostly produced by Th2 cells (Isomaki and Punnonen 1997; Brennan and McInnes 2008). Apparently, the levels of anti-inflammatory cytokines are not sufficient to neutralize the deleterious effects of pro-inflammatory cytokines during the progression of RA (Isomaki and Punnonen 1997).
In our studies (Venkatesha et al.2011, 2012; Nanjundaiah et al.2012), celastrol altered the balance between pro-inflammatory and anti-inflammatory cytokines primarily via reduction of pro-inflammatory cytokines as tested in the draining LNC and synovial-infiltrating cells (SIC) of arthritic rats treated with celastrol and compared with control rats. We further observed that celastrol treatment inhibited IL-17 expression in the cells of the draining lymph nodes and reduced the level of IL-17 in culture supernatant of SIC (Venkatesha et al.2011; Nanjundaiah et al.2012). In vitro experiments also confirmed the IL-17-inhibitory activity of celastrol observed in vivo. The reduction in IL-17 expression by celastrol was further supported by inhibition of STAT3 phosphorylation and retinoid-related orphan receptor gamma t (RORγt), the key transcription factors involved in Th17 differentiation. Besides inhibiting Th17-derived IL-17, celastrol inhibited the expression and secretion of IL-6, which correlated with pSTAT3 inhibition. Similarly, celastrol inhibited IL-1β and TNFα production (Cascao et al.2012), and this effect was linked with NF-κB-inhibitory activity of celastrol. In regard to inhibition of the release of mature IL-1β, we observed that celastrol reduced both pro-IL-1β as well as mature IL-1β (Astry et al.2015). We attribute these effects to the celastrol-mediated inhibition of NF-κB. There is a likely additional direct effect of celastrol on the conversion of pro-IL-1β into mature IL-1β. In this regard, it has been reported that celastrol can downregulate inflammasome-associated caspase-1, a key enzyme involved in the conversion of pro-IL-1β to mature IL-1β (Cascao et al.2012). The molecular mechanisms of some of these pathways are summarized in Fig. 2.
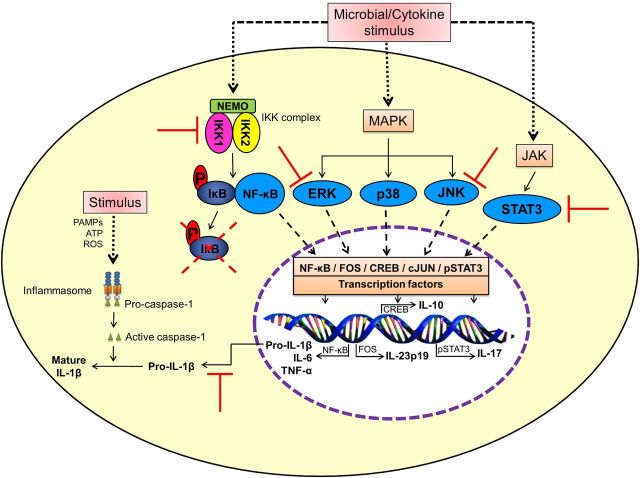
Figure 2.
Celastrol inhibits NF-κB and other cell signaling pathways associated with inflammation. Celastrol inhibits NF-κB activation via inhibition of IKK activity as well as degradation and phosphorylation of IκBα; inhibits the phosphorylation of ERK, JNK and STAT3, which regulate other nuclear transcription factors; and inhibits the production of pro-inflammatory cytokines (e.g. TNFα, IL-1β, IL-6, IL-17). The effect of celastrol on mature IL-1β production is primarily owing to reduction of pro-IL-1β production via inhibition of NF-κB. (CREB—cAMP response element-binding protein; ERK—extracellular signal-regulated kinase; IKK—IκB kinase; JNK—c-Jun N-terminal kinase; MAPK—mitogen-activated protein kinase; NEMO—NF-κB essential modulator; NF-κB—nuclear factor-kappa B; P—phosphorylated; PAMPs—pathogen-associated molecular patterns; STAT3—signal transducer and activator of transcription 3).
Celastrol regulates the expression of chemokines, adhesion molecules and cell migration
The migration of lymphocytes, macrophages and other pathogenic cells into an inflamed joint is controlled by chemokines/chemokine receptors and cell adhesion molecules expressed by the vascular endothelium (Smith et al.2001; Middleton et al.2004; Cascao et al.2010; Szekanecz et al.2010; Moura et al.2011). The resulting cellular trafficking into the joints leads to inflammation, pannus formation and tissue damage locally. The levels of pro-inflammatory chemokines and their receptors are elevated in the serum, synovial fluid and synovial tissue of patients with arthritis (Hampel et al.2013; Zhebrun et al.2014). Chemokines such as ‘regulated on activation, normal T cell expressed and secreted’ (RANTES) and monocyte chemotactic protein 1 (MCP-1) are potent chemoattractants involved in joint inflammation in RA as well as animal models of RA (Shahrara et al.2008; Pavkova Goldbergova et al.2012). Accordingly, neutralizing these and other chemokines leads to inhibition of chemokine-induced chemotaxis and suppression of arthritic inflammation (Shahrara et al.2008; Blanchetot et al.2013). Similarly, blocking of chemokine receptors also suppressed arthritic inflammation (Mohan and Issekutz 2007; Yokoyama et al.2014). Thus, chemokines, chemokine receptors and adhesion molecules serve as attractive targets for arthritis therapy (Barnes et al.1998; Halloran et al.1999; Szekanecz, Kim and Koch 2003; Chen, Oppenheim and Howard 2004). Herbal medicine products that modulate the expression of chemokines and their receptors have been shown to suppress arthritic inflammation in animal models of RA (Chen, Oppenheim and Howard 2004; Marotte et al.2010; Venkatesha et al.2012). The results of our study (Venkatesha et al.2012) on testing the culture supernatant of spleen adherent cells (SAC) and serum prepared from celastrol-treated rats showed reduction in the levels of growth-related oncogene (GRO/KC) and RANTES compared to control samples from untreated arthritic rats. However, the level of macrophage inflammatory protein 1α (MIP-1α) was not altered under these conditions. Furthermore, the chemokine MCP-1 showed an opposite trend in SAC culture supernatant versus serum samples. The level of MCP-1 was decreased by celastrol treatment in the former, but increased in the latter samples. However, in vitro treatment with celastrol of antigen (Mtb)-/IL-1β-stimulated rat synovial fibroblasts-like cells decreased the levels of GRO/KC, RANTES, MCP-1 and MIP-1α. In our another study, the culture supernatant of synovial-infiltrating cells (SIC) of arthritic rats treated with celastrol showed reduction in the levels of both MCP-1 and RANTES (Astry et al.2015). However, there was no significant change in the expression of chemokine receptors. Furthermore, celastrol has been shown to inhibit the expression of E-selectin, vascular cell adhesion molecule, and intercellular adhesion molecule-1 (ICAM-1) on endothelial cells (Zhang et al.2006).
RA is characterized by synovial inflammation (Harris 1990; Scott, Smith and Kingsley 2003; Lipsky 2005). RA-FLS, along with macrophages, are integral components of the synovial lining, which in a normal joint is one to three cell thick (Noss and Brenner 2008; Bartok and Firestein 2010). RA-FLS, upon stimulation, produce increased amounts of cytokines, chemokines and matrix-degrading enzymes (matrix metalloproteinases; MMPs) that can further enhance ongoing inflammation and tissue damage. In addition, FLS in RA become resistant to apoptosis and acquire increased invasive and migratory properties (Noss and Brenner 2008; Bartok and Firestein 2010). These functional alterations contribute to hyperproliferative synovium and spreading of inflammation to unaffected joints. Thus, RA-FLS are an attractive target for controlling arthritic inflammation. In this regard, celastrol has been shown to inhibit the migration and invasiveness of IL-17-stimulated RA-FLS. This effect involved inhibition of NF-κB-mediated expression of MMP-9 by celastrol (Li et al.2012). In another study, celastrol inhibited hypoxia-induced migration and invasion of RA-FLS, which was attributable in part to suppression of HIF-1alpha-mediated expression of CXC chemokine receptor 4 (Li et al.2013b). Another study revealed that celastrol inhibited proliferation of these cells by inducing DNA damage, cell cycle arrest and apoptosis (Xu et al.2013). Celastrol has also been reported to have an anti-proliferative effect on T cells and cancer cells (Chiang et al.2014; Astry et al.2015). Besides its effects on RA-FLS, celastrol has also been shown to reduce the cellular infiltrate into the synovium; the number of sub-lining CD68+ macrophages, which are considered as biomarkers of therapeutic response to RA treatment; and the joint damage in AA (Cascao et al.2015). A similar reduction in infiltration of CD68+ macrophages in the synovium was also observed following treatment with conventional drugs and biologics such as methotrexate and Anankinra (Dolhain et al.1998; Cunnane et al.2001; Smith et al.2001; Thurlings et al.2008). Furthermore, we have observed reduction in Th17 cells in SIC of arthritic rats treated with celastrol (Astry et al.2015).
Th17/T regulatory cell balance in arthritis and its control by celastrol
The major T cell subsets involved in RA include Th1, Th2 and Th17, and T regulatory (Treg) (Mosmann and Sad 1996; Stockinger and Veldhoen 2007). These subsets are identified on the basis of specific transcription factors expressed and the type of cytokines produced by them (Mosmann and Sad 1996; Stockinger and Veldhoen 2007). Th1 cells are identified by the transcription factor T-bet and the production of IFNγ, whereas, Th2 cells express the transcription factor GATA3 and produce IL-4, an anti-inflammatory cytokine. The Th17 subset is characterized by the transcription factor RORγt and the production of pro-inflammatory cytokine IL-17 (Mosmann and Sad 1996; Stockinger and Veldhoen 2007; Roeleveld et al.2013; Astry et al.2015; Venkatesha and Moudgil 2015). On the other hand, Treg cells express both high level of CD25 on cell surface and the transcription factor forkhead box protein 3 (Foxp3) intracellularly, and produce immunomodulatory cytokines IL-10 and TGFβ (Gol-Ara et al.2012). It was believed that activated Th1 cells play a role in the initiation and progression of inflammation, whereas Th2 cells have an anti-inflammatory effect and thereby control inflammation. The identification of Th17 and Treg cells has expanded our understanding of the cellular immune events in various autoimmune diseases, including RA (Bettelli et al.2006; Noack and Miossec 2014). Th17 cells produce IL-17, which is critical for the inflammatory cell infiltration into the synovium, synovial hyperplasia, angiogenesis, and cartilage and bone damage, and hence this subset has become an attractive therapeutic target for the treatment of RA and some other autoimmune diseases (Roeleveld et al.2013; Astry, Venkatesha and Moudgil 2015). Following activation, naïve T cells differentiate into Th17 cells in response to IL-6 and/or IL-1β and TGFβ. The IL-6 signaling activates STAT3 and induces the Th17 lineage-determining transcription factor RORγt (Stockinger and Veldhoen 2007). Suppressor of cytokine signaling 3 (SOCS3) is a negative regulator of Th17 differentiation. In the presence of IL-6, TGFβ inhibits SOCS3 and promotes Th17 differentiation (Qin et al.2009). TGFβ signaling is essential for Treg differentiation. TGFβ enables Treg formation from naïve cells in the absence of IL-6, but in the presence of IL-2 (Chen et al.2003; Peng et al.2004). TGFβ-induced SMAD (a word derived from Sma and Mad (Mothers against decapentaplegic)) and SMAD-independent signals are involved in the development of both Th17 and Treg cells (Fantini et al.2004; Lu et al.2010). In addition, IL-2 and STAT5 are involved in the generation, maintenance and function of Treg (Mahmud, Manlove and Farrar 2013).
Th17/Treg imbalance has been reported in RA and experimental models of this disease (Eisenstein and Williams 2009; Noack and Miossec 2014; Astry, Venkatesha and Moudgil 2015). Therefore, novel therapeutic approaches, particularly those that can reset the Th17/Treg balance in favor of immune regulation, are being sought for RA. The inhibition of Th17 is found to be effective in several models of RA and other autoimmune diseases (Roeleveld et al.2013). Furthermore, new approaches for the manipulation of Treg including ex vivo expansion of these cells for adoptive transfer or the induction of Treg to treat autoimmunity are under consideration (Golshayan et al.2009; Putnam et al.2009; Burmester, Feist and Dorner 2014; Noack and Miossec 2014; Vivar and Van Vollenhoven 2014). In our study on the effect of celastrol on Th17/Treg balance in AA (Astry et al.2015), we observed that celastrol significantly decreased the frequency of IL-17+RORγt+ Th17 cells, but augmented the frequency of CD25+Foxp3+ Treg in the joints of arthritic rats. Furthermore, celastrol inhibited Th17 differentiation, but augmented Treg differentiation of naïve mouse CD4+ T cells under defined cytokine environment conditions (Astry et al.2015). Apparently, celastrol directly inhibits Th17 differentiation and promotes Treg differentiation in part by blocking the IL-6 receptor signaling pathway through the inhibition of STAT3 phosphorylation (Astry et al.2015). Celastrol also has an effect on cytokines required for Th17 differentiation. As mentioned above, TGFβ is required for the differentiation of both Th17 and Treg, whereas the presence of IL-6 and/or IL-1β favors Th17 generation (Noack and Miossec 2014; Astry, Venkatesha and Moudgil 2015). Celastrol treatment of arthritic rats significantly reduced the levels of IL-6 and IL-1β in vivo (Venkatesha et al.2011; Astry et al.2015). Furthermore, celastrol inhibited the expression of activation markers CD80, CD86 and MHCII on antigen-presenting cell (APC), suggesting that it might suppress T cell activation, proliferation and expansion of the Th17 pool of cells (Astry et al.2015). This outcome was supported in part by the observation of decreased recall response of the antigen-primed T cells in vitro following celastrol treatment (Astry et al.2015). The mechanism of celastrol-induced regulation of Th17/Treg balance is summarized in Fig. 3.
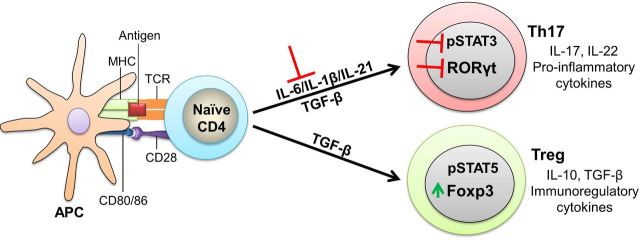
Figure 3.
Celastrol inhibits Th17 differentiation, but promotes Treg differentiation. The intricacies of antigen presentation (e.g. the nature of antigen and the strength of co-stimulation) and the local cytokine environment influence the differentiation of naïve CD4+ into different effector CD4+ T cell subsets. Th17 cells secrete IL-17 and IL-22 and trigger pathogenic events in arthritis, whereas, Treg cells secrete IL-10 and TGF-β, which mediate immunoregulatory activities. For Th17 differentiation, activation of STAT3 in the presence of IL-6/IL-1β and TGF-β induces RORγt. For Treg differentiation, IL-2-induced activation of STAT5 upregulates Foxp3 expression. Celastrol inhibits IL-6/IL-1β as well as STAT3, thereby suppressing Th17 development. Apparently, celastrol-induced decrease in IL-6 provides an environment that facilitates Treg differentiation, while reducing Th17 differentiation. (IL—interleukin; STAT—signal transducer and activator of transcription; TGF—transforming growth factor; Th17—T helper 17 cell; Treg—T regulatory cell).
Modulation of osteoimmune cross-talk by celastrol
Uncontrolled arthritis leads to bone and cartilage damage. Bone remodeling is an outcome of the balance between new bone formation by osteoblasts and bone resorption by osteoclasts (Khosla 2001; Takayanagi 2007; Zhao and Ivashkiv 2011), and this balance is disturbed in RA, with bone resorption exceeding bone formation (Schett 2006; Karmakar, Kay and Gravallese 2010; Maruotti et al.2011; Xu et al.2012). It is increasingly being realized that there is a cross-talk between bone and the immune system at cellular and molecular levels, and this sub-field has been named as ‘osteoimmunology’ (Takayanagi 2007). Pro-inflammatory cytokines such as IL-17 induce the production of other pro-inflammatory cytokines, of receptor activator of NF-κB ligand (RANKL), and of granulocyte macrophage colony-stimulating factor (GM-CSF) (Kotake et al.1999; Kolls and Linden 2004; Takayanagi 2007). RANKL produced by preosteoblastic/stromal cells binds to RANK on monocytes/macrophages (preosteoclasts) and facilitates the differentiation of preosteoclasts into mature osteoclasts (Hadjidakis and Androulakis 2006; Boyce and Xing 2008; Maruotti et al.2011). GM-CSF supports the survival and proliferation of osteoclasts (Takayanagi 2007; Maruotti et al.2011). On the other hand, osteoblasts produce osteoprotegerin (OPG), a decoy receptor for RANKL (Khosla 2001; Hadjidakis and Androulakis 2006; Boyce and Xing 2008; Maruotti et al.2011). The RANKL/OPG ratio determines osteoclast/osteoblast activity in arthritic joints and promotes bone damage (Fig. 4) (Takayanagi 2007; Xu et al.2012). Osteopontin (OPN), insulin-like growth factor (IGF)-I and tissue-degrading enzymes such as MMPs are other osteoclastogenic mediators, whereas osteocalcin (OCN) is an osteoblastic mediator that influences bone remodeling (Hill, Reynolds and Meikle 1995; Strand and Kavanaugh 2004; Franco et al.2011).
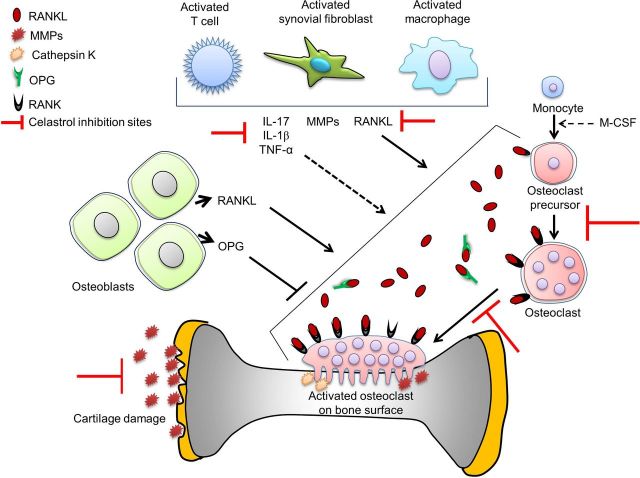
Figure 4.
Celastrol inhibits osteoclastic activity and protects against joint damage. Osteoclast precursors differentiate into osteoclasts under the influence of M-CSF. The RANKL/OPG pathway is the key regulator of bone remodeling. The binding of RANKL to its receptor RANK on the surface of an osteoclast precursor promotes the proliferation and differentiation of osteoclasts. OPG, which is secreted by the osteoblasts, is a soluble decoy receptor for RANKL and it serves as a natural inhibitor of osteoclast activation. Celastrol protects against bone and cartilage damage by regulating pro-inflammatory cytokines, inhibiting RANKL production, modulating RANKL/OPG ratio and reducing the secretion of matrix-degrading enzymes such as MMPs. (M-CSF—macrophage colony-stimulating factor; MMPs—matrix metalloproteinases; OPG—osteoprotegerin; RANK—receptor activator for nuclear factor κB; RANKL—RANK ligand).
Various therapeutic agents may differentially influence inflammation and bone damage (Joosten et al.1999; Gravallese 2002; Smolen et al.2005; Fonseca et al.2009). Thus, novel anti-arthritic agents that can inhibit both inflammation and bone damage in arthritis are being sought. Using the AA model, we (Nanjundaiah et al.2012) and others (Cascao et al.2012, 2015) have shown that celastrol effectively suppressed not only arthritic inflammation but also inflammation-induced bone and cartilage damage in rats. There was significant decrease in infiltration of immune cells into the synovium in celastrol-treated rats compared to control rats (Cascao et al.2012; Nanjundaiah et al.2012). Furthermore, celastrol decreased osteoclast numbers in the hind paw joints, reduced the subchondral bone loss and preserved bone volume (Nanjundaiah et al.2012). Furthermore, celastrol decreased RANKL, but increased OPG, as tested in the sera and culture supernatant of SIC of celastrol-treated arthritic rats. Celastrol also caused reduction in osteoclastogenic GM-CSF. Furthermore, IGF-I and OPN were decreased in culture supernatant of SIC but not in serum. On the other hand, celastrol increased osteoblastic OCN in serum (Nanjundaiah et al.2012). Additional reported effects of celastrol by other investigators include the inhibition of RANKL-induced signaling in osteoclasts, the induction of osteoclast apoptosis and the reduction in osteoclast formation in bone marrow in vitro and in vivo (Idris et al.2010; Gan et al.2015). Furthermore, celastrol inhibited the expression of MMP-9 and the invasion of synovial-like fibroblasts (Li et al.2013a), and it decreased the expression of MMP-1, MMP-3, MMP-13 in primary human osteoarthritic chondrocytes (Ding et al.2013). Above results suggest that celastrol is a promising agent for the concurrent control of inflammation and bone damage in arthritis. The mediators targeted by celastrol for modulating bone remodeling are shown in Fig. 4.
Microarray-based gene expression profiling reveals additional molecular targets of celastrol in arthritis
To gain an insight into the entire spectrum of celastrol-induced actions, we studied microarray-based gene expression of antigen-stimulated immune cells from the draining lymph nodes of the celastrol-treated arthritic rats, and compared the results with those of untreated arthritic rats (Yu, Venkatesha and Moudgil 2012). These cells were restimulated in culture with the disease-related antigen, mycobacterial heat-shock protein 65. The antigen-induced changes in gene expression were compared in different groups. A total of 22 523 genes were analyzed, of which, 76 genes were differentially expressed in arthritic control rats compared to 33 genes in celastrol-treated rats. The two groups shared 19 genes. Celastrol primarily regulated the genes associated with inflammatory and immune responses, including antigen processing and presentation as well as cell-/antibody-mediated immune responses. Cell proliferation, metabolism and oxidative stress represent additional pathways influenced by celastrol. Taken together, these results unraveled new potential targets and additional mechanisms of action of celastrol-mediated suppression of arthritis.
CELASTROL-INDUCED MODULATION OF IMMUNE PATHOLOGY IN MODELS OF OTHER HUMAN AUTOIMMUNE DISEASES
Studies in in vivo/in vitro experimental models of other human autoimmune diseases besides arthritis, such as MS, IBD, SLE, psoriasis, and type 1 diabetes (T1D), further support the potential role of celastrol in suppressing autoimmune inflammation. However, compared to arthritis, currently there are limited numbers of studies conducted in other models of autoimmunity. The results of these studies are summarized below.
Multiple sclerosis
MS is an autoimmune disease that causes demyelination of the central nervous system (CNS), which then leads to fatigue, muscle weakness and cognitive impairments (Compston and Coles 2008). Animal models of MS, such as myelin oligodendrocyte glycoprotein (MOG) peptide-induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice, have contributed significantly to defining the cellular and molecular mechanisms underlying the pathogenesis of this disease (Robinson et al.2014). MS is a predominantly T cell-mediated disease (Compston and Coles 2008; Wu and Alvarez 2011). The initiation and progression of MS involve an altered balance of both pro- versus anti-inflammatory cytokines as well as pathogenic (Th17, Th1) versus protective (Th2, Treg) subsets of T cells; the trafficking of pathogenic cells into the CNS; and tissue damage therein (Compston and Coles 2008; Wu and Alvarez 2011). A few drugs are available for MS, but there is a need for new and more effective therapeutic agents. In EAE, celastrol is able to reduce the clinical severity of the disease by targeting multiple pathways (Abdin and Hasby 2014; Wang et al.2015). In a study in mice with MOG-induced EAE, celastrol treatment attenuated disease development, which was associated with reduction in pathogenic Th17 response in both the periphery and the target organ (Wang et al.2015). The beneficial effect of celastrol in EAE was attributed to inhibition of dendritic cells (DC) rather than to Th17 differentiation. In another study in a relapsing-remitting EAE model in Sprague Dawley rats, celastrol treatment switched the T cell response from a predominantly Th1 (TNFα) to Th2 (IL-10) type, with an increase of IL-10 production. Moreover, NF-κB expression and the T cell counts in the CNS tissue were reduced in celastrol-treated rats compared to controls (Abdin and Hasby 2014). Taken together, these studies demonstrate the potential of celastrol as a therapeutic for MS.
Inflammatory bowel disease
IBD, also known as colitis, involves inflammation of the intestinal tract leading to abdominal pain, recurring diarrhea, vomiting and fatigue (Garud and Peppercorn 2009; Marks et al.2010). IBD can manifest as ulcerative colitis or Crohn's disease. The etiology of IBD is not fully defined, but it is believed to be an outcome of an interplay among genetic, environmental and lifestyle-related factors. Gut microbiota have also been invoked in IBD pathogenesis (Mukhopadhya et al.2012; Hisamatsu et al.2013). Studies on cells from biopsies of Crohn's patients and experimental models of IBD have demonstrated the efficacy of celastrol against colitis. Celastrol was tested on mucosal inflammatory biopsies from patients with Crohn's disease to determine its effects on the production of pro-inflammatory cytokines (Pinna et al.2004). Celastrol significantly reduced the production of multiple pro-inflammatory cytokines, with most notable effect on CD33+ cells. Further analysis using a human monocytic cell line showed that celastrol inhibited LPS-induced NF-κB translocation (Pinna et al.2004). In dextran sulfate sodium (DSS)-induced colitis model (Jia et al.2015), celastrol was shown to reduce the severity of colitis, and decrease cell necroptosis as evident from reduced levels of receptor-interacting protein kinase 3 and mixed lineage kinase domain-like (MLKL), which represent necroptosis factors. Moreover, the levels of pro-inflammatory mediators such as IL-1β and IL-6 were decreased (Jia et al.2015). Similarly, in another study in the same model (DSS-induced colitis), celastrol reduced the disease severity via downregulation of oxidative stress and inflammatory cytokines. Following celastrol administration, there was a decrease in pro-inflammatory cytokines IL-1β, IFNγ, IL-17 and IL-23 levels, and an increase in anti-inflammatory cytokine IL-10 level (Shaker, Ashamallah and Houssen 2014). In an IL-10-deficient experimental model of Crohn's disease, it was demonstrated that celastrol treatment induced protection against colitis by upregulating autophagy via inhibition of the PI3K/Akt/mTOR signaling pathway (Zhao et al.2015). The analysis of colon tissue of celastrol-treated mice showed decreased myeloperoxidase level, reduced neutrophil infiltration, and decreased pro-inflammatory cytokines. The above studies suggest that celastrol might be useful in alleviating the symptoms and controlling the disease pathology in IBD patients.
Systemic lupus erythematosus
SLE, also known as lupus, is a multifactorial disease that affects the kidney and several other organs. Moreover, there are multiple clinical manifestations of lupus, further indicating the complexities of this disease (D'Cruz, Khamashta and Hughes 2007; Kiriakidou 2013; Lisnevskaia, Murphy and Isenberg 2014). SLE is characterized by defective clearance of apoptotic material, which then accumulates and then is taken up by APCs, followed by presentation of the self-antigenic epitopes to autoreactive T/B cells. In addition, there is intense polyclonal B cell activation as well as defective T cell signaling and co-stimulation (D'Cruz, Khamashta and Hughes 2007; Kiriakidou 2013; Lisnevskaia, Murphy and Isenberg 2014). Studies using the BW F1 mouse model of lupus (Xu and Wu 2002) have shown that following celastrol administration, there was a decrease in both anti-dsDNA antibodies in serum as well as excretion of protein in the urine (proteinuria), two common biomarkers of lupus. Furthermore, there was reduced expression of collagen type IV, fibronectin and tissue inhibitor of metalloproteinases 1, but increased expression of MMP-1 and 2 in the kidney. A subsequent study (Xu et al.2007) corroborated these findings and also showed that celastrol treatment decreased renal collagen type IV presumably by suppressing local expression of TGF-β. Another study in BALB/c mice (Li et al.2005), in which lupus was induced by injection of active chromatin, showed that tripterine (celastrol) administration reduced serum autoantibodies and total IgG, and afforded protection against glomerular injury. These effects were associated with decreased IL-10 and nitric oxide production, but without any change in IFN-γ.
Psoriasis
Psoriasis is an autoimmune condition characterized by the formation of red patches on the skin, and these patches are usually itchy and scaly in texture. There are multiple clinical manifestations of psoriasis of varying severity (Lowes, Suárez-Fariñas and Krueger 2014; Boehncke and Schön 2015). The pathogenesis of psoriasis involves a cross-talk between tissue-resident DCs and keratinocytes. The keratinocytes activate the DCs and then the DCs recruit both innate (e.g. neutrophils, macrophages and more DCs) and adaptive immune cells. The latter include Th1, Th17 and Th22 subsets of T cells. The Th22 subset is most notable because the cells of this subset produce the cytokines IL-22 and TNF-α, which play a major role in disease pathogenesis (Michalak-Stoma et al.2013). Using the HaCaT keratinocyte cell line, it was shown that celastrol is a pro-apoptotic mediator (Zhou et al.2011). HaCaT cells cultured in the presence of celastrol showed growth inhibition as well as signs of apoptosis, as evident by phosphatidylserine externalization, depolarization of mitochondrial membrane potential and activation of caspase-3. Furthermore, there was increased expression of B-cell lymphoma 2 (Bcl-2)-associated X protein, a pro-apoptotic factor, and decreased level of Bcl-2, an anti-apoptotic factor. Moreover, celastrol-mediated apoptosis was associated with suppression of the NF-κB pathway. Another set of studies on HaCaT cells showed that celastrol suppressed IFN-γ-induced ICAM-1 expression leading to reduced monocyte adhesiveness (Seo et al.2010, 2011). This effect involved induction of heme oxygenase-1 by celastrol through reactive oxygen species generation and activation of ERK and p38 MAPK. The above results suggest that celastrol might be a promising candidate for further testing in animal models of psoriasis.
Type 1 diabetes
T1D is a multifactorial disease involving an autoimmune attack on the insulin-producing β-islet cells in the pancreas. There is infiltration of autoreactive T cells into the pancreas. These T cells target the β-islet cells with cytokines such as IFNγ, TNFα and IL-1β (Cnop et al.2005). This leads to an increase in blood glucose that can result in polyuria, polydipsia, blindness, kidney failure and other ailments (Atkinson, Eisenbarth and Michels 2014). Using a rat pancreatic beta-cell line RINmSF, it has been shown that celastrol treatment reduced the cytokine-induced cell death; the production of pro-inflammatory mediators such as inducible nitric oxide synthase, cyclooxygenase-2 and CC chemokine ligand 2; and the activation of NF-κB (Ju et al.2015). However, in another study based on the non-obese diabetic mouse model of T1D, celastrol failed to prevent the development of diabetes, although it caused transient lowering of blood glucose (Grant et al.2013).
CONCLUSION
Based on the results of preclinical studies, we suggest that celastrol is a promising candidate for further evaluation in RA patients. If validated, celastrol might serve as a useful adjunct/alternative to conventionally used drugs, whose long-term use is associated with severe adverse effects. In this regard, Tripterygium Wilfordii extract has been tested in RA patients (Liu et al.2013). In a recent study on RA therapy, T. Wilfordii extract was found to be as effective as methotrexate (Liu et al.2013; Lv et al.2015). These studies have set the stage for systematic testing of celastrol and other purified herbal compounds in RA patients in the near future. Furthermore, celastrol has potential therapeutic benefits in several other autoimmune diseases besides RA.
Acknowledgments
We thank Dr Hua Yu, Dr Li Tong, Dr Siddaraju Nanjundaiah and Dr Joseph Stains for their contribution to the original studies based on celastrol reviewed here and for their helpful discussions.
FUNDING
This work was supported by National Institutes of Health (NIH)/National Center for Complementary and Integrative Health (NCCIH) [grant number R01 AT004321 to KDM and F31 AT007278 to BA] and Veterans Affairs (VA) Merit 1 I01 BX002424-01.
Conflict of interest. None declared.
REFERENCES
- Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin Pharmacokinet. 2011;50:349–69. doi: 10.2165/11586800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Abdin A, Hasby E. Modulatory effect of celastrol on Th1/Th2 cytokines profile, TLR2 and CD3+ T-lymphocyte expression in a relapsing-remitting model of multiple sclerosis in rats. Eur J Pharmacol. 2014;42:102–12. doi: 10.1016/j.ejphar.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Adorini L. Cytokine-based immunointervention in the treatment of autoimmune diseases. Clin Exp Immunol. 2003;132:185–92. doi: 10.1046/j.1365-2249.2003.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astry B, Venkatesha SH, Laurence A, et al. Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin Immunol. 2015;157:228–38. doi: 10.1016/j.clim.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astry B, Venkatesha SH, Moudgil KD. Temporal cytokine expression and the target organ attributes unravel novel aspects of autoimmune arthritis. Indian J Med Res. 2013;138:717–31. [PMC free article] [PubMed] [Google Scholar]
- Astry B, Venkatesha SH, Moudgil KD. Involvement of the IL-23/IL-17 axis and the Th17/Treg balance in the pathogenesis and control of autoimmune arthritis. Cytokine. 2015;74:54–61. doi: 10.1016/j.cyto.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DA, Tse J, Kaufhold M, et al. Polyclonal antibody directed against human RANTES ameliorates disease in the Lewis rat adjuvant-induced arthritis model. J Clin Invest. 1998;101:2910–9. doi: 10.1172/JCI2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–55. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Blanchetot C, Verzijl D, Mujic-Delic A, et al. Neutralizing nanobodies targeting diverse chemokines effectively inhibit chemokine function. J Biol Chem. 2013;288:25173–82. doi: 10.1074/jbc.M113.467969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983–94. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–46. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester GR, Feist E, Dorner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:77–88. doi: 10.1038/nrrheum.2013.168. [DOI] [PubMed] [Google Scholar]
- Cascao R, Moura RA, Perpetuo I, et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Ther. 2010;12:R196. doi: 10.1186/ar3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascao R, Vidal B, Lopes IP, et al. Decrease of CD68 synovial macrophages in celastrol treated arthritic rats. PLoS One. 2015;10:e0142448. doi: 10.1371/journal.pone.0142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascao R, Vidal B, Raquel H, et al. Effective treatment of rat adjuvant-induced arthritis by celastrol. Autoimmun Rev. 2012;11:856–62. doi: 10.1016/j.autrev.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Oppenheim JJ, Howard OM. Chemokines and chemokine receptors as novel therapeutic targets in rheumatoid arthritis (RA): inhibitory effects of traditional Chinese medicinal components. Cell Mol Immunol. 2004;1:336–42. [PubMed] [Google Scholar]
- Chiang KC, Tsui KH, Chung LC, et al. Celastrol blocks interleukin-6 gene expression via downregulation of NF-kappaB in prostate carcinoma cells. PLoS One. 2014;9:e93151. doi: 10.1371/journal.pone.0093151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, et al. Mechanisms of pancreatic β-Cell death in type 1 and type 2 diabetes many differences, few similarities. Diabetes. 2005;54:s97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Couzin J. Drug safety. Withdrawal of Vioxx casts a shadow over COX-2 inhibitors. Science. 2004;306:384–5. doi: 10.1126/science.306.5695.384. [DOI] [PubMed] [Google Scholar]
- Cunnane G, Madigan A, Murphy E, et al. The effects of treatment with interleukin-1 receptor antagonist on the inflamed synovial membrane in rheumatoid arthritis. Rheumatology. 2001;40:62–9. doi: 10.1093/rheumatology/40.1.62. [DOI] [PubMed] [Google Scholar]
- D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–96. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- Ding QH, Cheng Y, Chen WP, et al. Celastrol, an inhibitor of heat shock protein 90beta potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur J Pharmacol. 2013;708:1–7. doi: 10.1016/j.ejphar.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Dolhain RJ, Tak PP, Dijkmans BA, et al. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Brit J Rheumatol. 1998;37:502–8. doi: 10.1093/rheumatology/37.5.502. [DOI] [PubMed] [Google Scholar]
- Eisenstein EM, Williams CB. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. 2009;65:26R–31R. doi: 10.1203/PDR.0b013e31819e76c7. [DOI] [PubMed] [Google Scholar]
- Fantini MC, Becker C, Monteleone G, et al. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–53. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- Fonseca JE, Canhao H, Tavares NJ, et al. Persistent low grade synovitis without erosive progression in magnetic resonance imaging of rheumatoid arthritis patients treated with infliximab over 1 year. Clin Rheumatol. 2009;28:1213–6. doi: 10.1007/s10067-009-1207-y. [DOI] [PubMed] [Google Scholar]
- Franco GC, Kajiya M, Nakanishi T, et al. Inhibition of matrix metalloproteinase-9 activity by doxycycline ameliorates RANK ligand-induced osteoclast differentiation in vitro and in vivo. Exp Cell Res. 2011;317:1454–64. doi: 10.1016/j.yexcr.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst DE, Emery P. Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology. 2014;53:1560–9. doi: 10.1093/rheumatology/ket414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan K, Xu L, Feng X, et al. Celastrol attenuates bone erosion in collagen-Induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264.7. Int Immunopharmacol. 2015;24:239–46. doi: 10.1016/j.intimp.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Garcia-Hernandez MH, Gonzalez-Amaro R, Portales-Perez DP. Specific therapy to regulate inflammation in rheumatoid arthritis: molecular aspects. Immunotherapy. 2014;6:623–36. doi: 10.2217/imt.14.26. [DOI] [PubMed] [Google Scholar]
- Garud S, Peppercorn MA. Ulcerative colitis: current treatment strategies and future prospects. Ther Adv Gastroenterol. 2009;2:99–108. doi: 10.1177/1756283X09102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gol-Ara M, Jadidi-Niaragh F, Sadria R, et al. The role of different subsets of regulatory T cells in immunopathogenesis of rheumatoid arthritis. Arthritis. 2012;2012:805875. doi: 10.1155/2012/805875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshayan D, Wyss JC, Abulker CW, et al. Transplantation tolerance induced by regulatory T cells: in vivo mechanisms and sites of action. Int Immunopharmacol. 2009;9:683–8. doi: 10.1016/j.intimp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Grant CW, Moran-Paul CM, Duclos SK, et al. Testing agents for prevention or reversal of type 1 diabetes in rodents. PLoS One. 2013;8:e72989. doi: 10.1371/journal.pone.0072989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl. 2):ii84–6. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjidakis DJ, Androulakis II. Bone remodeling. Ann NY Acad Sci. 2006;1092:385–96. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- Halloran MM, Woods JM, Strieter RM, et al. The role of an epithelial neutrophil-activating peptide-78-like protein in rat adjuvant-induced arthritis. J Immunol. 1999;162:7492–500. [PubMed] [Google Scholar]
- Hampel U, Sesselmann S, Iserovich P, et al. Chemokine and cytokine levels in osteoarthritis and rheumatoid arthritis synovial fluid. J Immunol Methods. 2013;396:134–9. doi: 10.1016/j.jim.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. New Engl J Med. 1990;322:1277–89. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hill PA, Reynolds JJ, Meikle MC. Osteoblasts mediate insulin-like growth factor-I and -II stimulation of osteoclast formation and function. Endocrinology. 1995;136:124–31. doi: 10.1210/endo.136.1.7828521. [DOI] [PubMed] [Google Scholar]
- Hisamatsu T, Kanai T, Mikami Y, et al. Immune aspects of the pathogenesis of inflammatory bowel disease. Pharmacol Therapeut. 2013;137:283–97. doi: 10.1016/j.pharmthera.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Idris AI, Krishnan M, Simic P, et al. Small molecule inhibitors of IkappaB kinase signaling inhibit osteoclast formation in vitro and prevent ovariectomy-induced bone loss in vivo. FASEB J. 2010;24:4545–55. doi: 10.1096/fj.10-164095. [DOI] [PubMed] [Google Scholar]
- Isomaki P, Punnonen J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann Med. 1997;29:499–507. doi: 10.3109/07853899709007474. [DOI] [PubMed] [Google Scholar]
- Jia Z, Xu C, Shen J, et al. The natural compound celastrol inhibits necroptosis and alleviates ulcerative colitis in mice. Int Immunopharmacol. 2015;29:552–9. doi: 10.1016/j.intimp.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Helsen MM, Saxne T, et al. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–55. [PubMed] [Google Scholar]
- Ju SM, Youn GS, Cho YS, et al. Celastrol ameliorates cytokine toxicity and pro-inflammatory immune responses by suppressing NF-κB activation in RINm5F beta cells. BMB Rep. 2015;48:172–7. doi: 10.5483/BMBRep.2015.48.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HW, Chung YS, Kim YS, et al. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Mol Med. 2007;39:715–21. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- Kannaiyan R, Hay HS, Rajendran P, et al. Celastrol inhibits proliferation and induces chemosensitization through down-regulation of NF-kappaB and STAT3 regulated gene products in multiple myeloma cells. Brit J Pharmacol. 2011;164:1506–21. doi: 10.1111/j.1476-5381.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S, Kay J, Gravallese EM. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin N Am. 2010;36:385–404. doi: 10.1016/j.rdc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- Kiriakidou M. Systemic lupus erythematosus. Ann Intern Med. 2013;159:ITC4–1. doi: 10.7326/0003-4819-159-7-201310010-01004. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers HM, Nicola P, Crowson CS, et al. Therapeutic strategies in rheumatoid arthritis over a 40-year period. J Rheumatol. 2004;31:2366–73. [PubMed] [Google Scholar]
- Lee JH, Koo TH, Yoon H, et al. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72:1311–21. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee BH, Kim ND, et al. Celastrol blocks binding of lipopolysaccharides to a Toll-like receptor4/myeloid differentiation factor2 complex in a thiol-dependent manner. J Ethnopharmacol. 2015;172:254–60. doi: 10.1016/j.jep.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kavanaugh A. Adverse reactions to biologic agents: focus on autoimmune disease therapies. J Allergy Clin Immun. 2005;116:900–5. doi: 10.1016/j.jaci.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Yedla P, Kavanaugh A. Secondary immune deficiencies associated with biological therapeutics. Curr Allergy Asthm R. 2003;3:389–95. doi: 10.1007/s11882-003-0072-z. [DOI] [PubMed] [Google Scholar]
- Li G, Liu D, Zhang Y, et al. Celastrol inhibits lipopolysaccharide-stimulated rheumatoid fibroblast-like synoviocyte invasion through suppression of TLR4/NF-kappaB-mediated matrix metalloproteinase-9 expression. PLoS One. 2013a;8:e68905. doi: 10.1371/journal.pone.0068905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Liu D, Zhang Y, et al. Anti-invasive effects of celastrol in hypoxia-induced fibroblast-like synoviocyte through suppressing of HIF-1alpha/CXCR4 signaling pathway. Int Immunopharmacol. 2013b;17:1028–36. doi: 10.1016/j.intimp.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Li GQ, Zhang Y, Liu D, et al. Celastrol inhibits interleukin-17A-stimulated rheumatoid fibroblast-like synoviocyte migration and invasion through suppression of NF-kappaB-mediated matrix metalloproteinase-9 expression. Int Immunopharmacol. 2012;14:422–31. doi: 10.1016/j.intimp.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Li H, Jia YF, Pan Y, et al. Effect of tripterine on collagen-induced arthritis in rats. Zhongguo Yao Li Xue Bao. 1997;18:270–3. [PubMed] [Google Scholar]
- Li H, Zhang YY, Huang XY, et al. Beneficial effect of tripterine on systemic lupus erythematosus induced by active chromatin in BALB/c mice. Eur J Pharmacol. 2005;512:231–7. doi: 10.1016/j.ejphar.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang YY, Tan HW, et al. Therapeutic effect of tripterine on adjuvant arthritis in rats. J Ethnopharmacol. 2008;118:479–84. doi: 10.1016/j.jep.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Lipsky PE. Rheumatoid arthritis. In: Kasper D, Braunwald E, Fauci A, et al., editors. Harrison's Principles of Internal Medicine. 16th edn. New York: McGraw-Hill; 2005. pp. 1968–77. [Google Scholar]
- Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384:1878–88. doi: 10.1016/S0140-6736(14)60128-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tu S, Gao W, et al. Extracts of Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis: a systemic review and meta-analysis of randomised controlled trials. Evid-Based Compl Alt. 2013;2013:410793. doi: 10.1155/2013/410793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of Psoriasis. Annu Rev Immunol. 2014;32:227–55. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang J, Zhang F, et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv QW, Zhang W, Shi Q, et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann Rheum Dis. 2015;74:1078–86. doi: 10.1136/annrheumdis-2013-204807. [DOI] [PubMed] [Google Scholar]
- Ma J, Han LZ, Liang H, et al. Celastrol inhibits the HIF-1alpha pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol Rep. 2014;32:235–42. doi: 10.3892/or.2014.3211. [DOI] [PubMed] [Google Scholar]
- Mahmud SA, Manlove LS, Farrar MA. Interleukin-2 and STAT5 in regulatory T cell development and function. Jakstat. 2013;2:e23154. doi: 10.4161/jkst.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DJ, Rahman FZ, Sewell GW, et al. Crohn's disease: an immune deficiency state. Clin Rev Allerg Immu. 2010;38:20–31. doi: 10.1007/s12016-009-8133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotte H, Ruth JH, Campbell PL, et al. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology. 2010;49:467–79. doi: 10.1093/rheumatology/kep397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruotti N, Grano M, Colucci S, et al. Osteoclastogenesis and arthritis. Clin Exp Med. 2011;11:137–45. doi: 10.1007/s10238-010-0117-2. [DOI] [PubMed] [Google Scholar]
- Michalak-Stoma A, Bartosińska J, Kowal M, et al. Serum levels of selected th17 and th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625–31. doi: 10.1155/2013/856056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J, Americh L, Gayon R, et al. Endothelial cell phenotypes in the rheumatoid synovium: activated, angiogenic, apoptotic and leaky. Arthritis Res Ther. 2004;6:60–72. doi: 10.1186/ar1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan K, Issekutz TB. Blockade of chemokine receptor CXCR3 inhibits T cell recruitment to inflamed joints and decreases the severity of adjuvant arthritis. J Immunol. 2007;179:8463–9. doi: 10.4049/jimmunol.179.12.8463. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Moura RA, Cascao R, Perpetuo I, et al. Cytokine pattern in very early rheumatoid arthritis favours B-cell activation and survival. Rheumatology. 2011;50:278–82. doi: 10.1093/rheumatology/keq338. [DOI] [PubMed] [Google Scholar]
- Mukhopadhya I, Hansen R, El-Omar EM, et al. IBD-what role do Proteobacteria play? Nat Rev Gastroentero. 2012;9:219–30. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- Nanjundaiah SM, Venkatesha SH, Yu H, et al. Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. J Biol Chem. 2012;287:22216–26. doi: 10.1074/jbc.M112.356816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Zhao W, Kong X, et al. Celastrol inhibits lipopolysaccharide-induced angiogenesis by suppressing TLR4-triggered nuclear factor-kappa B activation. Acta Haematol. 2014;131:102–11. doi: 10.1159/000354770. [DOI] [PubMed] [Google Scholar]
- Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–77. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–70. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Pavkova Goldbergova M, Lipkova J, Pavek N, et al. RANTES, MCP-1 chemokines and factors describing rheumatoid arthritis. Mol Immunol. 2012;52:273–8. doi: 10.1016/j.molimm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Peng Y, Laouar Y, Li MO, et al. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. P Natl Acad Sci USA. 2004;101:4572–7. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna GF, Fiorucci M, Reimund JM, et al. Celastrol inhibits pro-inflammatory cytokine secretion in Crohn's disease biopsies. Biochem Bioph Res Co. 2004;322:778–86. doi: 10.1016/j.bbrc.2004.07.186. [DOI] [PubMed] [Google Scholar]
- Putnam AL, Brusko TM, Lee MR, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–62. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Wang L, Feng T, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P, Li F, Shanmugam MK, et al. Celastrol suppresses growth and induces apoptosis of human hepatocellular carcinoma through the modulation of STAT3/JAK2 signaling cascade in vitro and in vivo. Cancer Prev Res. 2012;5:631–43. doi: 10.1158/1940-6207.CAPR-11-0420. [DOI] [PubMed] [Google Scholar]
- Robinson AP, Harp CT, Noronha A, et al. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb Clin Neurol. 2014;122:173–89. doi: 10.1016/B978-0-444-52001-2.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeleveld DM, van Nieuwenhuijze AE, van den Berg WB, et al. The Th17 pathway as a therapeutic target in rheumatoid arthritis and other autoimmune and inflammatory disorders. Biodrugs. 2013;27:439–52. doi: 10.1007/s40259-013-0035-4. [DOI] [PubMed] [Google Scholar]
- Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013;11:88. doi: 10.1186/1741-7015-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G. Rheumatoid arthritis: inflammation and bone loss. Wien Med Wochenschr. 2006;156:34–41. doi: 10.1007/s10354-005-0244-7. [DOI] [PubMed] [Google Scholar]
- Scott DL, Smith C, Kingsley G. Joint damage and disability in rheumatoid arthritis: an updated systematic review. Clin Exp Rheumatol. 2003;21(Suppl. 31):S20–7. [PubMed] [Google Scholar]
- Seo WY, Goh AR, Ju SM, et al. Celastrol induces expression of heme oxygenase-1 through ROS/Nrf2/ARE signaling in the HaCaT cells. Biochem Bioph Res Co. 2011;407:535–40. doi: 10.1016/j.bbrc.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Seo WY, Ju SM, Song HY, et al. Celastrol suppresses IFN-gamma-induced ICAM-1 expression and subsequent monocyte adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. Biochem Bioph Res Co. 2010;398:140–5. doi: 10.1016/j.bbrc.2010.06.053. [DOI] [PubMed] [Google Scholar]
- Shahrara S, Proudfoot AE, Park CC, et al. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180:3447–56. doi: 10.4049/jimmunol.180.5.3447. [DOI] [PubMed] [Google Scholar]
- Shaker ME, Ashamallah SA, Houssen ME. Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokines and intestinal homeostasis. Chem-Biol Interact. 2014;210:26–33. doi: 10.1016/j.cbi.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Jeengar MK, Reddy VS, et al. Anticancer effect of celastrol on human triple negative breast cancer: Possible involvement of oxidative stress, mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp Mol Pathol. 2015;98:313–27. doi: 10.1016/j.yexmp.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology. 2008;47:584–90. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- Smith MD, Slavotinek J, Au V, et al. Successful treatment of rheumatoid arthritis is associated with a reduction in synovial membrane cytokines and cell adhesion molecule expression. Rheumatology. 2001;40:965–77. doi: 10.1093/rheumatology/40.9.965. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Han C, Bala M, et al. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 2005;52:1020–30. doi: 10.1002/art.20982. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Strand V, Kavanaugh AF. The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology. 2004;43(Suppl. 3):iii10–6. doi: 10.1093/rheumatology/keh202. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Kim J, Koch AE. Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol. 2003;15:15–21. doi: 10.1016/s1044-5323(02)00124-0. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Vegvari A, Szabo Z, et al. Chemokines and chemokine receptors in arthritis. Front Biosci. 2010;2:153–67. doi: 10.2741/s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- Thurlings RM, Vos K, Wijbrandts CA, et al. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67:917–25. doi: 10.1136/ard.2007.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha SH, Astry B, Nanjundaiah SM, et al. Suppression of autoimmune arthritis by Celastrus-derived Celastrol through modulation of pro-inflammatory chemokines. Bioorg Med Chem. 2012;20:5229–34. doi: 10.1016/j.bmc.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha SH, Dudics S, Acharya B, et al. Cytokine-modulating strategies and newer cytokine targets for arthritis therapy. Int J Mol Sci. 2014;16:887–906. doi: 10.3390/ijms16010887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha SH, Yu H, Rajaiah R, et al. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem. 2011;286:15138–46. doi: 10.1074/jbc.M111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar N, Van Vollenhoven RF. Advances in the treatment of rheumatoid arthritis. F1000Prime Rep. 2014;6:31. doi: 10.12703/P6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cao L, Xu LM, et al. Celastrol ameliorates EAE induction by suppressing pathogenic T cell responses in the peripheral and central nervous systems. J Neuroimmune Pharm. 2015;10:506–16. doi: 10.1007/s11481-015-9598-9. [DOI] [PubMed] [Google Scholar]
- Wu GF, Alvarez E. The immunopathophysiology of multiple sclerosis. Neurol Clin. 2011;29:257–78. doi: 10.1016/j.ncl.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wu Z. The effect of tripterine in prevention of glomerulosclerosis in lupus nephritis mice. Zhonghua Nei Ke Za Zhi. 2002;41:317–21. [PubMed] [Google Scholar]
- Xu S, Wang Y, Lu J, et al. Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatol Int. 2012;32:3397–403. doi: 10.1007/s00296-011-2175-5. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhong J, Wu Z, et al. Effects of tripterine on mRNA expression of TGF-beta1 and collagen IV expression in BW F1 mice. Cell Biochem Funct. 2007;25:501–7. doi: 10.1002/cbf.1338. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wu G, Wei X, et al. Celastrol induced DNA damage, cell cycle arrest, and apoptosis in human rheumatoid fibroblast-like synovial cells. Am J Chinese Med. 2013;41:615–28. doi: 10.1142/S0192415X13500432. [DOI] [PubMed] [Google Scholar]
- Yokoyama W, Kohsaka H, Kaneko K, et al. Abrogation of CC chemokine receptor 9 ameliorates collagen-induced arthritis of mice. Arthritis Res Ther. 2014;16:445. doi: 10.1186/s13075-014-0445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Venkatesha SH, Moudgil KD. Microarray-based gene expression profiling reveals the mediators and pathways involved in the anti-arthritic activity of Celastrus-derived Celastrol. Int Immunopharmacol. 2012;13:499–506. doi: 10.1016/j.intimp.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Marconi A, Xu LM, et al. Tripterine inhibits the expression of adhesion molecules in activated endothelial cells. J Leukocyte Biol. 2006;80:309–19. doi: 10.1189/jlb.1005611. [DOI] [PubMed] [Google Scholar]
- Zhao B, Ivashkiv LB. Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res Ther. 2011;13:234. doi: 10.1186/ar3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Sun Y, Shi P, et al. Celastrol ameliorates experimental colitis in IL-10 deficient mice via the up-regulation of autophagy. Int Immunopharmacol. 2015;26:221–8. doi: 10.1016/j.intimp.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Zhebrun DA, Totolyan AA, Maslyanskii AL, et al. Synthesis of some CC chemokines and their receptors in the synovium in rheumatoid arthritis. B Exp Biol Med. 2014;158:192–6. doi: 10.1007/s10517-014-2720-9. [DOI] [PubMed] [Google Scholar]
- Zhou LL, Lin ZX, Fung KP, et al. Celastrol-induced apoptosis in human HaCaT keratinocytes involves the inhibition of NF-κB activity. Eur J Pharmacol. 2011;670:399–408. doi: 10.1016/j.ejphar.2011.09.014. [DOI] [PubMed] [Google Scholar]