Abstract
The Gram-negative proteobacteria genus Burkholderia encompasses multiple bacterial species that are pathogenic to humans and other vertebrates. Two pathogenic species of interest within this genus are Burkholderia pseudomallei (Bpm) and the B. cepacia complex (Bcc); the former is the causative agent of melioidosis in humans and other mammals, and the latter is associated with pneumonia in immunocompromised patients. One understudied and shared characteristic of these two pathogenic groups is their ability to persist and establish chronic infection within the host. In this review, we will explore the depth of knowledge about chronic infections caused by persistent Bpm and Bcc. We examine the host risk factors and immune responses associated with more severe chronic infections. We also discuss host adaptation and phenotypes associated with persistent Burkholderia species. Lastly, we survey how other intracellular bacteria associated with chronic infections are combatted and explore possible future applications to target Burkholderia. Our goal is to highlight understudied areas that should be addressed for a more thorough understanding of chronic Burkholderia infections and how to combat them.
Keywords: Burkholderia, B. pseudomallei, Burkholderia cepacia complex, melioidosis, pneumonia, persistent bacteria, chronic infection
This work explores the depth of knowledge about chronic infections caused by persistent Burkholderia pseudomallei and B. cepacia complex.
INTRODUCTION
The Gram-negative Burkholderia genus is composed of obligate aerobic, rod-shaped bacteria that have diverse ecological niches and life cycles (Compant et al. 2008). These niches range from environmental soil and plant reservoirs to mammalian host reservoirs (Coenye and Vandamme 2003). Some Burkholderia species are also mammalian pathogens (Allwood et al. 2011; Valvano 2015), but their pathogenicity varies widely. The duration of an infection depends on a combination of factors, including the causative Burkholderia species, as well as underlying conditions of the infected host. Burkholderia infections are particularly difficult to treat, as most pathogenic species are intrinsically resistant to major classes of antibiotics. There are currently no commercially available vaccines to protect mammals against Burkholderia infections. Thus, an important research area involving medically relevant microorganisms, including those of the pathogenic Burkholderia genus, is to understand the ability of such microorganisms to establish persistent, chronic infections within the host. In this literature review, we discuss chronic infections caused by two pathogenic Burkholderia: Burkholderia pseudomallei (Bpm) and B. cepacia complex (Bcc). We examine the factors that contribute to bacterial persistence in the host and the responses associated with those chronic infections. To conclude, we also discuss theories and putative therapeutics developed for other persistent bacterial pathogens to incite ideas for future research directions to address Burkholderia persistence. And though Bpm and Bcc are the more prevalent and more studied Burkholderia species, it is important to note that there are other pathogenic Burkholderia including B. mallei that are able to cause persistence infections, yet the research of this subject is limited. The literature pertaining to persistence factors in Bpm and Bcc can be used to design experiments and test hypotheses concerning persistence in other Burkholderia species.
OVERVIEW OF MELIOIDOSIS AND BCC INFECTIONS
Bpm is a highly virulent pathogen and causative agent of melioidosis in humans and other mammals. This bacterium is a motile, saprophyte soil-dwelling microorganism (Dance 2000). Bpm species are found in tropical and subtropical regions of the world, and while Thailand and northern Australia are the predominant endemic regions for melioidosis infections, recently other parts of the world have been also declared endemic areas (Limmathurotsakul and Peacock 2011; Khan et al. 2013; Limmathurotsakul et al. 2016). Inhalation, skin inoculation and ingestion are the most common infection routes, with inhalation causing the most severe clinical disease. The incubation period after exposure ranges from 1 to 21 days, with an average incubation time of 9 days (Currie et al. 2000). Localized clinical manifestations may occur at the exposure site, including pneumonia after pulmonary inoculation or localized skin lesion after a cutaneous inoculation. The infection may also translocate from the exposure site to other organs; for example, cutaneous inoculation can lead to the development of pulmonary melioidosis, the most common manifestation of disease (Currie 2003). Arthritis, osteomyelitis, musculoskeletal melioidosis and/or neurological melioidosis are other localized disease manifestations. Moreover, if an active, localized infection is not resolved, a systemic infection or sepsis may result, presenting as acute, recurrent or chronic symptomatic infection, but also as asymptomatic infection (Gan 2005). However, the variety of melioidosis signs and symptoms, some of which are common in other infectious diseases, can often lead to misdiagnosis. Risk factors for developing melioidosis include diabetes mellitus, hazardous alcohol use, chronic lung and/or renal diseases, thalassemia, and occupational and environmental exposure (Suputtamongkol et al. 1999; Currie, Ward and Cheng 2010). Patients with an immunodeficiency, including patients with cystic fibrosis (CF), are also susceptible to developing melioidosis when they visit endemic regions (O'Carroll et al. 2003).
Recent epidemiological studies of melioidosis predict an average of 165 000 human cases per year worldwide, from which 89 000 people die (Limmathurotsakul et al. 2016). In a study in Northern Thailand from 1997 to 2006, the average mortality rate from melioidosis was 42.6% (Limmathurotsakul et al. 2010). With respect to potential existing treatments such as antibiotics, Bpm has been found to be intrinsically resistant to many antibiotics, including penicillin, ampicillin, streptomycin, polymyxin, and first- and second-generation cephalosporins (Wiersinga, Currie and Peacock 2012). Effective antibiotic regimens usually involve an intensive 10–14 days intravenous administration of a third-generation cephalosporin ceftazidime or a carbapenem class antibiotic, such as meropenem, followed by trimethoprim–sulfamethoxazole treatment taken orally for 3 to 6 months (Wiersinga, Currie and Peacock 2012). However, currently no melioidosis vaccines are commercially available. Thus, based on its high virulence, limited effective therapeutics and potential use as a biological weapon, Bpm is classified by the US Departments of Health and Human Services and Agriculture as a Tier 1 select agent. This classification requires that many of the studies performed on this bacterial species be conducted within a high containment facility and in a regulated manner. This Tier 1 status also emphasizes the importance of gaining a better understanding of how to combat this pathogen.
The Bcc is a group made up of at least 20 closely related species (Table 1), which are opportunistic pathogens in individuals with an immunodeficiency, most notably CF and chronic granulomatous disease (CGD) (Vial et al. 2011; Peeters et al. 2013; De Smet et al. 2015). Originally referred to as genomovars, because they are phenotypically indistinguishable but phylogenetically different, these pathogens are now classified by formal species names that began to replace the genomovar designations in the early 2000s (Lipuma 2005). Additionally, Bcc species are ubiquitous soil bacteria that are distantly related to Bpm and other Burkholderia species (Sawana, Adeolu and Gupta 2014). Inhalation of bacteria from the environment is a common infection route and can lead to acute, recurrent, chronic or latent forms of disease in susceptible individuals. Acute pneumonia that can progress into recurrent and/or chronic pneumonia is the most prevalent clinical course of a Bcc infection. Osteomyelitis and meningitis are also other complications. A severe manifestation, known as ‘cepacia syndrome’, occurs in 10% of cases, and is characterized by necrotizing pneumonia and bacteremia, leading to early death (Mahenthiralingam, Urban and Goldberg 2005; Mahenthiralingam, Baldwin and Dowson 2008).
Table 1.
List of Bcc species.
| Species name | Reference |
|---|---|
| Burkholderia ambifaria | Coenye et al. (2001) |
| Burkholderia anthina | Vandamme et al. (2002) |
| Burkholderia arboris | Vanlaere et al. (2008) |
| Burkholderia cenocepacia | Vandamme et al. (2003) |
| Burkholderia cepacia | Palleroni and Holmes (1981) |
| Burkholderiacontaminans | Vanlaere et al. (2009) |
| Burkholderia diffusa | Vanlaere et al. (2008) |
| Burkholderia dolosa | Vermis et al. (2004) |
| Burkholderia lata | Vanlaere et al. (2009) |
| Burkholderia latens | Vanlaere et al. (2008) |
| Burkholderia metallica | Vanlaere et al. (2008) |
| Burkholderia multivorans | Vandamme et al. (1997) |
| Burkholderia pseudomultivorans | Peeters et al. (2013) |
| Burkholderia pyrrocinia | Imanaka et al. (1965); Vandamme et al. (1997, 2002) |
| Burkholderia seminalis | Vanlaere et al. (2008) |
| Burkholderia stabilis | Vandamme et al. (2000) |
| Burkholderia stagnalis | De Smet et al. (2015) |
| Burkholderia territorii | De Smet et al. (2015) |
| Burkholderia ubonensis | Yabuuchi et al. (2000) |
| Burkholderia vietnamiensis | Gillis et al. (1995) |
Multiple studies have elucidated the epidemiology and species ecology of Bcc infections in susceptible populations, especially in patients with CF. For instance, in a microbiological study using a CF registry in the USA from 1995 to 2005, in which the number of patients increased from 19 735 to 23 347, the reported prevalence of Bcc isolates collected significantly declined from 3.6% to 3.1% (P < 0.001) during that time period (Razvi et al. 2009). Despite these encouraging findings indicating a possibly decreasing threat, Bcc is still present in the USA. Meanwhile a European molecular epidemiological study examining chronic bacterial respiratory infections in patients with CF found that Bcc species were isolated from 3.6% of 164 respiratory samples from patients examined at the CF Regional Centre of Florence, Italy from 1998 to 2000 (Campana et al. 2004). In a combination of epidemiological studies performed in a major Portuguese CF center over the span of 16 years, Bcc species were isolated from 41 of 124 patients with CF (33%) (Coutinho et al. 2011b). Of those with Bcc infections, 28 patients (68%) had persistent infections (Coutinho et al. 2011b). There is also evidence that Bcc infections are transmissible among patients with CF (Biddick et al. 2003). Burkholderia multivorans and B. cenocepacia were the most commonly isolated species from Bcc infections in patients with CF in Canada (Lipuma 2010; Zlosnik et al. 2015), United Kingdom (Govan, Brown and Jones 2007) and the United States (Lipuma 2010). More recently, B. contaminans has become the most common species isolated in Argentina (Martina et al. 2013), Portugal (Coutinho et al. 2015) and Spain (Medina-Pascual et al. 2015).
There is currently no commercially available vaccine that protects humans from Bcc infections. Typically, Bcc infections are treated with a combination antibiotic treatment regimen often composed of ceftazidime and meropenem in a similar manner to Bpm infections (Avgeri et al. 2009). Still, Bcc ubiquity in the environment, especially near hospitals and other areas that have a high prevalence of susceptible populations, has made these understudied pathogens a relevant area of research.
CHRONIC INFECTIONS
Chronic Burkholderia infections, like other chronic illnesses, are especially debilitating diseases. A chronic Bpm infection is often defined as a symptomatic infection that lasts longer than 2 months. Further, chronic melioidosis may have localized and/or systemic manifestations. The epidemiology of chronic melioidosis has been examined in endemic regions. In the Darwin 20-year prospective melioidosis study in the Northern Territory of Australia (1989–2009), the epidemiology of 540 culture-confirmed melioidosis cases was examined (Currie et al. 2000; Currie, Ward and Cheng 2010). Of those cases, 11% were considered chronic infections, 4% were relapse infections and the remaining 85% were acute infections (Currie, Ward and Cheng 2010). In that study, patients with chronic melioidosis were significantly less likely to be diabetic than were patients with acute melioidosis (Currie, Ward and Cheng 2010). Also, these chronic infections were more commonly acquired during the wet seasons (Currie, Ward and Cheng 2010). In a retrospective hospital study of 95 culture-confirmed melioidosis cases collected from 2005 to 2010 in the western coastal region of India, 28.4% were chronic infections and 71.6% acute infections (Vidyalakshmi et al. 2012). Of these chronic melioidosis cases, 70.4% presented during the monsoon season. Melioidosis cases overall are seasonal and, therefore, increase of cases during monsoons is linked to endemic populations which might have increased contact with Bpm-contaminated environmental sources (Wiersinga et al. 2006). In the case of rice farmers, they plant at the beginning of the monsoon season and they work in the flooded rice paddies until the rice is harvested (Wiersinga et al. 2006). This puts them in direct contact with soil and water that might contain Bpm. Chronic melioidosis has also been associated with CF (Schulin and Steinmetz 2001). Quite often the melioidosis cases, especially in non-endemic regions, are misdiagnosed for more common chronic infectious diseases, such as tuberculosis (TB) caused by Mycobacterium tuberculosis. This was the case for one diabetic patient in India, who presented with lung fibrosis, small joint arthritis and splenic abscess (Kunnathuparambil et al. 2013). The patient initially received anti-TB therapy before the correct diagnosis and then received effective anti-melioidosis therapy (Kunnathuparambil et al. 2013), providing an example of how misdiagnosis can delay and/or prevent a patient from receiving the proper treatment.
Murine models of melioidosis have been examined to gain a better understanding of the biology of a chronic infection. For example, C57BL/6 mice have been found to live for weeks after receiving intravenous (i.v.) Bpm challenges, making them a useful murine model for studying chronic infections (Leakey, Ulett and Hirst 1998; Hoppe et al. 1999). The i.v. challenge route mimics the systemic disease in human melioidosis cases. Other infection routes have also been used, including intranasal (i.n.) administration to mimic pulmonary exposure in humans. Another chronic infection model includes inoculating C57BL/6 mice i.n. with a low dose of Bpm (Conejero et al. 2011). In that study, the chronically infected C57BL/6 mice survived for over 3 months (Conejero et al. 2011). Additionally, survival is dependent on the infectious dose (Hoppe et al. 1999), with lower infectious doses of Bpm leading to a longer survival of mice. Further studies using C57BL/6 mice indicated that they mount a more protective interferon gamma (IFN-γ)-mediated macrophage response (Breitbach, Kohler and Steinmetz 2008), effective NADPH oxidase (Breitbach, Wongprompitak and Steinmetz 2011), higher immunoglobulin IgG2a/IgG1 ratio (Hoppe et al. 1999) and proinflammatory cytokines (Conejero et al. 2011). Interestingly, BALB/c mice, a strain highly susceptible to infection and more suitable for studying acute infections, produce significantly higher amounts of IFN-γ than C57BL/6 mice during infection (Ulett, Ketheesan and Hirst 2000; Liu et al. 2002). The higher production of IFN-γ in the BALB/c mouse strain than the C57BL/6 mouse strain may indicate that higher IFN-γ production has a deleterious effect on some of the mouse strains examined. The development of a persistent infection also depends on the Bpm strain used. In a study comparing the infection dynamics of Bpm strains 1106a and a more virulent HBPUB10134a in BALB/c mice injected intraperitoneally (i.p.), the more virulent strain caused a higher bacterial burden in the animals’ spleens and induced a higher amount of proinflammatory cytokines in the sera and spleens (Amemiya et al. 2015). Though occurring later in the infection, the detectable cytokines decreased overall and inflammatory cells increased in the spleens for both groups, and all of the mice infected with Bpm strain 1106a survived longer on average (Amemiya et al. 2015). The murine target organs associated with Bpm persistence are often the lungs, spleen and liver. In addition, other infection routes or mouse strains have been used to develop chronic infection models. For instance, a persistent gastric colonization model of melioidosis was developed in BALB/c mice (Goodyear et al. 2012) by inoculating the mice with Bpm clinical strains orally at a low dose, which lead to a chronic gastric infection that disseminated to the spleen and liver (Goodyear et al. 2012). In another study using Taylor Outbred (TO) mice, the animals received sublethal doses of Bpm by i.p. injection, which lead to an early IFN-γ response and development of chronic infection (Santanirand et al. 1999). Those mice survived from 2 to 16 months before succumbing to a reactivated infection (Santanirand et al. 1999). It is important to note that early production of IFN-γ in BALB/c mice appears to be linked to severe susceptibility to infection, which is in contrast to what has been shown in TO mice. These contrasting results ultimately suggest that multiple factors influence the severity and duration of infection in murine models of melioidosis.
Bcc species can also cause chronic infections. Chronic pneumonia is the most common presentation of bacterial persistence. Such infections have mainly been observed and extensively studied in patients with CF. In other populations susceptible to Bcc infections, such as patients with CGD, only recurrent infections have been observed (Greenberg et al. 2009). In particular, the Bcc species distribution during chronic infections has been studied in patients with CF. In one extensive study of patients with CF with chronic Burkholderia infection, 1095 Burkholderia isolates were recovered from serial sputum cultures from a total of 379 patients with CF in 112 US treatment centers. Of those patients with CF, 347 were chronically infected with Bcc species, and the most commonly identified species were Burkholderia cenocepacia (56% of isolates) and B. multivorans (33% of isolates). Burkholderia cenocepacia was also the most common replacement species/strain during chronic infections, in which the subsequent isolate collected from a specific patient was of a different strain/species from those initially collected in the same patient (Bernhardt et al. 2003). Other studies of Bcc species distribution in patients with CF have also shown that the Bcc species causing a chronic infection may remain stable or be replaced by another strain or Bcc species over time (LiPuma et al. 1991).
Long-term Bcc infections have also been characterized in murine models of chronic infection, in which both clinical and environmental B. cenocepacia isolates have been shown to be able to persist in the lungs of infected mice (Pirone et al. 2008). In a BALB/c mouse model, in which the animals were rendered leukopenic by administration of cyclophosphamide prior to i.n. challenge, not all Bcc species and strains were able to persist in the lungs (Chu et al. 2002). Those that did persist in the murine lungs for at least 16 days represented six of seven B. multivorans strains, as well as B. cepacia strain Cep873 and B. vietnamiensis strain FC811 (Chu et al. 2002). In contrast, B. multivorans strain C3430, all of the B. cenocepacia strains and B. stabilis strain C7322 were cleared from the lungs by day 16 post-infection (Chu et al. 2002). A subsequent study in immunocompetent BALB/c mice found that B. multivorans persisted in the lungs by localizing in macrophages, while an increase in interleukin (IL)-1β and neutrophil responses led to the clearance of B. cenocepacia (Chu et al. 2004). This study showed that the lungs, spleen and liver were often target organs of bacterial persistence, findings similar to those with Bpm. In studies using BALB/c and C57BL/6 mice inoculated i.p. with B. cepacia, which models systemic disease, mice developed chronic splenic infections that lasted up to 2 months (Speert et al. 1999). The ability of B. cepacia to persist in a murine model of infection has also been explored in the endotoxin-resistant C3H/HeJ mouse strain, in which sublethal i.n. inoculations led to bacterial persistence in the lungs and cecum and to dissemination to other sites (George et al. 1999). In another study, Swiss CD-1 mice were inoculated intratracheal (i.t.) with B. cepacia enmeshed in agarose beads, and viable bacteria were collected as late as day 21 of infection (Starke et al. 1987). Persistence has also been confirmed and examined without the use of bacterium-immobilizing agents in a murine model of CF (Cftr−/−) for a 9-day infection study (Sajjan et al. 2001). The bacterial mechanisms of persistence that have been discovered for Burkholderia species are discussed in more detail in the next section.
PERSISTENCE AND ADAPTATION
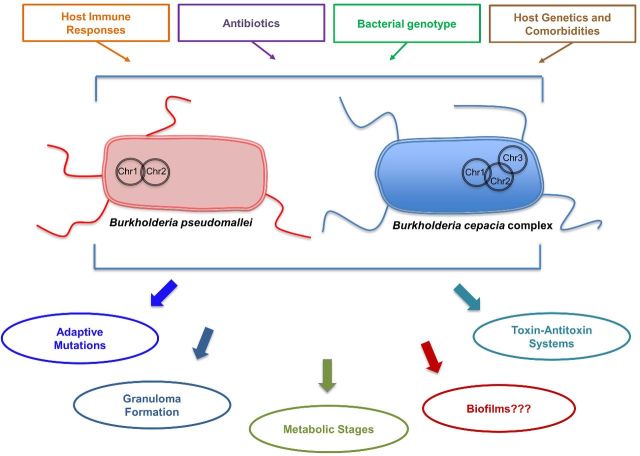
Research groups have identified and started characterizing different strategies that Bpm utilizes to persist in a host (Fig. 1). These persistence factors include toxin–antitoxin (TA) systems, metabolic enzymes and adaptive mutations that lead to persistence. Some of these persistence factors have been associated with chronic, recurrent and latent infections. One such mechanism that has been characterized is the functionality of the HicA toxin of the HicAB TA system in Bpm (Butt et al. 2014). The TA systems have been found in multiple bacteria species and function in the bacteria's ability to survive in a stressful environment (e.g. antibiotic treatment) by entering a slower growth state (Otsuka 2016). Toxin activity results in reduced bacterial growth, and can lead to cell death if prolonged. The antitoxin functions to neutralize the toxin under normal conditions, but is often degraded under stressful conditions, allowing the toxin to affect bacterial cell growth. Five classes of antitoxins have been described according to how they function. The toxins are always protein molecules, while the antitoxins are either protein molecules for types II, IV and V or small non-coding RNA molecules for types I and III (Otsuka 2016). Type II is the most commonly studied and is an apparent protein-to-protein TA system (Otsuka 2016). As with TA systems that have been described in persistent bacteria, the HicAB system in Bpm is possibly involved in the production of antibiotic-tolerant persister cells that cannot be eradicated during antibiotic treatment (Butt et al. 2013, 2014). In this case, overexpression in vitro of HipA increased the number of persister cells tolerant to ciprofloxacin or ceftazidime (Butt et al. 2014). Moreover, some metabolic enzymes and stages have also been shown to be important for persistence. For instance, deletion of kynB in Bpm, which encodes for kynurenine formamidase of the kynurenine pathway, led to increased tolerance and more persister cells tolerant to ciprofloxacin (Butt et al. 2016). Isocitrate lyase, the first enzyme in the glyoxylate shunt, is a persistence factor important for both chronic TB and persistent pulmonary melioidosis in a rat infection model (van Schaik, Tom and Woods 2009). Environmental variables were demonstrated to alter the proportion of persister cells that may develop from a population (Nierman, Yu and Losada 2015). Subpopulations of Bpm have also been found capable of shifting into a physiological state that allows them to live in a moderately acidic anaerobic environment, which is analogous to a host abscess (Hamad et al. 2011). In this metabolic shift, Bpm were able to survive for at least a year in a non-replicative state and were tolerant to conventional antibiotics, but susceptible to nitroimidazole antibiotics (Hamad et al. 2011). Lastly, in two separate studies, whole-genome sequencing analysis of Bpm isolates was used to examine persistent infections that had developed in melioidosis cohorts. One study examined two isolates, collected 139 months apart, from an Australian patient that developed an asymptomatic persistent infection (Price et al. 2013). Over that time period, the bacteria had undergone several adaptive changes, genome reduction and apparent attenuation, leading to the development of long-term colonization (Price et al. 2013). In another study that examined isolates collected between 6 months and 6 years apart from four adult Thai patients with recurrent melioidosis, whole-genome sequencing revealed changes associated with increased antibiotic resistance over the course of the study (Hayden et al. 2012). Additionally, inactivating mutations in a TetR family transcriptional regulator were identified in other clinical isolates analyzed in that study, and it was suggested that TetR is important for bacterial survival and adaptation in the host (Hayden et al. 2012).
Figure 1.
A depiction of Bpm and Bcc predicted mechanisms of persistence and other factors that influence persistence within the host. The number of chromosomes (Chr) in the different species is also illustrated.
For Bcc species, TA systems, metabolism shifts and hypermutation have also been associated with persistence (Fig. 1). One study characterized 16 putative TA systems in the Burkholderia cenocepacia strain J2315, 12 of which were conserved in other strains and 9 of those were identified in other Bcc and Burkholderia species (Van Acker et al. 2014). Five of the putative TA systems were also identified in Bpm (Van Acker et al. 2014). When overexpressed, six of those toxins led to the development of persister cells after treatment with ciprofloxacin, while two of those same toxins also led to the development of persisters after tobramycin treatment (Van Acker et al. 2014). Some toxin overexpression also induced biofilm formation, another factor possibly linked to persistence that is discussed later (Van Acker et al. 2014). Isocitrate lyase has also been shown to be important for the fraction of Bcc-persistent cells present within a biofilm (Van Acker et al. 2013).
To further elucidate the mechanisms of persistence during the course of an infection, extensive analyses were performed on B. cenocepacia clonal isolates collected from the same Portuguese patient with CF over the course of 3.5 years, from the onset of infection until the patient succumbed to cepacia syndrome (Cunha et al. 2003; Coutinho et al. 2011a; Madeira et al. 2013). In a phenotypic comparison study of 11 of these isolates, B. cenocepacia appeared to make an adaptive shift to a fatty acid metabolism. This shift was accompanied by the synthesis of membranes with a different fatty acid composition, more suited for survival in the severely oxygen-limited conditions encountered in the lungs over the time course of the infection (Coutinho et al. 2011a). In a proteomic profiling study of three isolates also collected during this chronic infection, increases in proteins involved in purine and pyrimidine synthesis and iron uptake were identified (Madeira et al. 2013).
Hypermutation has also been associated with persistence in human patients. In a study of the prevalence of hypermutators in 125 Bcc isolates recovered from 48 patients with CF, 10 patients without CF and 15 environmental samples, 17 (13.6%) of isolates were identified as hypermutators (Martina et al. 2014). Of those patients, 27 had chronic Bcc infections, with hypermutator isolates collected from 40.7%; however, hypermutators were not isolated from the patients with acute infections (Martina et al. 2014). Thirteen (76.5%) of the hypermutators identified had defects in mutS and/or mutL, genes encoding proteins that are involved in mismatch repair (Martina et al. 2014). In an agar bead rat model that mimics chronic lung infection, researchers also identified multiple Bcc genes related to cellular metabolism, global regulation, DNA replication and repair, and bacterial surface structures genes that are needed for in vivo survival and persistence (Hunt et al. 2004).
There are other potential persistent strategies that both Bpm and Bcc share, such as intracellular survival within host cells, as well as biofilm formation and related phenotypes (Fig. 1). Intracellular survival within host cells, including epithelial cells and macrophages, has also been explored and discussed as a possible persistence mechanism utilized by Bcc and Bpm species (Allwood et al. 2011; Valvano 2015). However, we do not discuss that information in this review. Biofilm formation, on the other hand, has not been as well discussed for Burkholderia species. Forming biofilms during infection has been linked to the ability of related pathogenic bacterial species, such as Pseudomonas aeruginosa, to cause persistent infections in patients with CF and other susceptible populations (Costerton, Stewart and Greenberg 1999). Both Bpm and Bcc species are able to form biofilms in vitro, which is associated with exopolysaccharide (EPS) production and other factors (Cunha et al. 2004; Mongkolrob, Taweechaisupapong and Tungpradabkul 2015). Both Bpm and Bcc are also able to form small colony variants in vitro, a colony morphology that is associated with biofilm formation, antibiotic resistance and persistence (Haussler, Rohde and Steinmetz 1999; Haussler et al. 2003). Researchers have also explored if forming biofilms is a persistence factor for Burkholderia species infections. Bpm biofilms appear more resistant to antibiotics than are planktonic bacteria (Sawasdidoln et al. 2010; Pibalpakdee et al. 2012). However, no correlation between Bpm biofilms and virulence has been found (Taweechaisupapong et al. 2005), nor it is known if biofilm formation contributes to Bpm survival within a host (Chantratita et al. 2007). More extensive studies have been performed to characterize the importance of biofilm formation in Bcc biology. The Bcc biofilms have been shown to contain persister cells able to survive reactive oxygen species (Van Acker et al. 2013) and neutrophil-like dHL60 cells (Murphy and Caraher 2015). Studies have also shown that Bcc biofilms are sometimes more tolerant to multiple antibiotics than are planktonic cells (Peeters, Nelis and Coenye 2009), though that is not always the case (Caraher et al. 2007). A shiny colony morphology associated with abundant EPS production and piliation has been linked with persistent infection in a pulmonary mouse model of disease (Chung et al. 2003). Overexpression of EPS led to a mucoid colony morphology that was also linked to persistent infection in an animal model, while non-mucoid colony morphology was associated with a more virulent acute infection (Chung et al. 2003; Conway et al. 2004; Zlosnik et al. 2011). Interestingly, Bcc strains isolated from patients with chronic infections have been shown to convert from mucoid phenotypes to non-mucoid phenotypes (Zlosnik et al. 2008). Zlosnik et al. (2008) noted that this result is opposite to what has been found in P. aeruginosa, where it is the mucoid phenotype which was associated with higher virulence (Zlosnik et al. 2008). A subsequent study found that non-mucoid Bcc appear to overexpress multiple virulence factors, thus indicating that this phenotype is associated with virulent acute infection (Zlosnik and Speert 2010). When examining clinical Bcc isolates, no direct association has been established between the ability of the strains to produce EPS and/or to form biofilms in vitro and cause persistent respiratory infections in humans (Cunha et al. 2004). Overall, the importance of such abilities as biofilm formation, bacterial cell morphology and related properties during chronic infection should be explored further.
IMMUNE RESPONSES TO CHRONIC INFECTION
The ability of Bpm and Bcc species to evade the host immune response often leads to a chronic infection, and this has been examined in great detail by multiple groups (Gan 2005; Valvano 2015; Aschenbroich, Lafontaine and Hogan 2016). Immune responses to persistent Bpm infections have been examined in both natural human infections and murine models of disease. As mentioned earlier, persistent Bpm bacteria often survive in localized organ abscesses. Over time, a granuloma containing neutrophils, macrophages, lymphocytes and giant cells will form at the infection site in human patients with melioidosis (Wong, Puthucheary and Vadivelu 1995). The dynamics of granuloma formation around the invading bacteria has not been as thoroughly studied for melioidosis as it has for TB. Strong antibody responses involving the classes IgA, IgM and IgG (especially IgG1 and IgG2) have also been detected during the course of infection in patients with chronic melioidosis (Vasu, Vadivelu and Puthucheary 2003).
The host immune response has been examined in more detail in murine models of disease. In a C57BL/6 mouse model of chronic infection, animals that had active chronic disease produced proinflammatory cytokines, including IFN-γ, tumor necrosis factor-α (TNF-α), IL-6 and monocyte chemotactic protein-1. However, in the same study, uninfected and asymptomatic mice did not produce proinflammatory cytokines. (Conejero et al. 2011). Lesions containing infiltrating lymphocytes, epithelioid macrophages and multinucleated giant cells developed within the mouse lungs, livers and spleens harvested between days 20 and 60 of infection (Conejero et al. 2011). Fibrous capsules, found surrounding lesions with necrotic cores (Conejero et al. 2011), are also associated with granulomas caused by other persistent bacterial infections such as chronic TB (Russell et al. 2009). When examining the role of T cells in host resistance against Bpm infections in C57BL/6 mice, it was found that CD4+ T cells, more so than CD8+ T cells, contributed to host resistance in the later stages of infection (Haque et al. 2006). When comparing the survival of Bpm-infected mice antibody depleted of either CD4+, CD8+ or both subsets of T cells to mice that received a control antibody, the mean survival time (MST) of the control mice was 58 days, the MST of the CD4+ T-cell-depleted mice was 22 days, the MST of the CD8+ T-cell-depleted mice was 32.5 days and the MST of the CD4+/CD8+ T-cell-depleted mice was 20.5 days (Haque et al. 2006). In the study, the MSTs of the CD4+ T-cell-depleted and CD4+/CD8+ T-cell-depleted mice were statistically significant when compared to the control mice, while the MST of the CD8+ T-cell-depleted mice was not statistically significant when compared to the controls (Haque et al. 2006). When examining the CD4+/CD8+ T-cell ratio in mice with persistent Bpm infections, researchers observed a decrease in CD4+ T cells and increase in CD8+ T cells (See et al. 2016). Also, both CD4+ and CD8+ T cells were found to have an increased expression of programmed death-1 protein (PD-1) during chronic infection, whereas expression of cytotoxic, T lymphocyte-associated protein 4 (CTLA-4) remained unchanged. Both proteins are members of the CD28-B7 superfamily and are increased and expressed during chronic TB and persistent human immunodeficiency virus infections (See et al. 2016). Increased expression of PD-1 and/or CTLA-4 may be linked to T-cell exhaustion in TB (See et al. 2016), and the higher expression of PD-1 during Bpm infection suggests that T-cell exhaustion may also occur in chronic melioidosis.
The host immune responses to persistent Bcc infections have also been examined, though it is important to note that Bcc infections often occur in patients with impaired immune systems and/or patients that have contributing comorbidities. Research suggests that the immune response of patients with CF to opportunistic pathogens, like Pseudomonas aeruginosa and Aspergillus fumigatus, is altered and may contribute to the occurrence and immunopathology of disease (Ratner and Mueller 2012). As a result, more research has been focused on gaining a better understanding of the early immune responses to chronic Bcc infections, which may contribute to disease versus immune responses in susceptible populations. It is known that patients with CF with Bcc infections do produce IgG and IgA to Bcc antigens (Mohr, Tomich and Herfst 2001). Additionally, some work has been performed in murine infection models. In one study, mice were inoculated i.p. with Bcc strains leading to persistence in the spleen: though interestingly no consistent histological abnormalities were observed in the spleens, lungs or livers harvested from the mice (Speert et al. 1999). In another study, MyD88−/− mice i.t. challenged with Burkholderia cenocepacia displayed significantly reduced levels of proinflammatory cytokines, including granulocyte-colony stimulating factor, IL-6, keratinocyte-derived cytokine, macrophage inflammatory protein-2 and TNF-α in blood and lung airspace. Additionally, these mice survived longer than wild-type mice despite bacterial persistence (Ventura et al. 2009). It was also suggested that TNF-α may contribute to exacerbated disease (Ventura et al. 2009). Informative data concerning the immune response to persistent Bcc were gathered from a murine model of CF (Cftr−/−) (Sajjan et al. 2001). These knockout mice mounted an ineffective pulmonary inflammatory response to an i.n. challenge with bacteria, resulting in chronic infection (Sajjan et al. 2001). Both macrophages and neutrophils were able to infiltrate the infection site, but further evidence suggested that they were suboptimally activated (Sajjan et al. 2001).
STRATEGIES FOR COMBATING PERSISTENT BACTERIAL INFECTIONS
As mentioned in previous sections, chronic Burkholderia infections share some persistence factors and host immune evasion strategies that are similar to those in other persistent bacteria, such as Mycobacterium tuberculosis and Pseudomonas aeruginosa. Because of this, we think that it is important to consider examining, in chronic Burkholderia infection models and potentially in preclinical studies, infection protection strategies that have worked in other persistent bacteria. We have already discussed the extensive antibiotic regimens used to treat Burkholderia infections, and potential vaccine strategies have also been discussed and reviewed in great detail by other groups (Aschenbroich, Lafontaine and Hogan 2016; Pradenas, Ross and Torres 2016); therefore, we will not discuss that ongoing research here. However, it is important to emphasize that as more persistence factors are identified in Burkholderia species, their immunogenicity and potential use as candidate vaccines should be explored. One approach that is being examined as a potential strategy to combat chronic bacterial pathogens, including M. tuberculosis (Hawn et al. 2013), is using small molecules or other compounds to beneficially modulate the host-cell-mediated response to infection, conferring an advantage to the host. One group has recently identified macrophage signaling pathways and potential targets of modulation that are activated in murine macrophage RAW264.7 cells infected with Bpm clade species (Chiang et al. 2015). The ability of small molecules to act as a direct antimicrobial agent or synergistically with other antibiotics has also been explored for P. aeruginosa (Guo et al. 2016). One study showed that quorum-sensing inhibitors made biofilm-forming P. aeruginosa and Bcc species more susceptible to antibiotic treatment in vitro and in vivo (Brackman et al. 2011). A small molecule called compound 939, which inhibits type III secretion system cluster 3 ATPase, was shown to reduce the intracellular survival of Bpm in infected RAW264.7 cells, thus becoming a potential therapeutic approach for treating melioidosis (Gong et al. 2015). The compound AFN-1252, which was developed to inhibit the fatty acid biosynthesis pathway enzyme enoyl-ACP reductase in Staphylococcus aureus, was found to also inhibit the enoyl-ACP reductase isoform from Bpm (Rao et al. 2015). It is a pending assignment to further examine the potential of these compounds to treat Burkholderia infections. However, the small molecules and/or compounds that are already approved for human use and appear promising during in vitro and in vivo studies could quickly be examined in human trials.
Host responses including the cellular-mediated and subsequent granuloma responses to persistent Burkholderia bacteria, especially Bpm, may also have unique characteristics that treatments can exploit. In the case of modulating a cellular-mediated response, cyclooxygenase-2 inhibition led to growth suppression of Bpm in macrophages and survival in a mouse model of pneumonic melioidosis, when the mice received the inhibitor post-Bpm infection (Asakrah et al. 2013). Examining this pathway further may elucidate new potential therapies for infection. Because there is a wealth of knowledge about granuloma formation in TB, potential therapeutics that target granulomas formed during a Bpm infection should also be explored. Many of the inflammatory cells, cytokine signaling and other factors involved in the granuloma formation process have been identified (Miranda et al. 2012; Ramakrishnan 2012). Some researchers have hypothesized that these pathways involved in granuloma formation might be modulated in a manner beneficial to the host and detrimental to the pathogen (Ramakrishnan 2012). One potential host target associated with granuloma formation is host matrix metalloproteinase 9 (Mmp9). The Mmp9-knockout mice infected with M. tuberculosis were found to have decreased macrophage recruitment to the lungs and poor granuloma development (Taylor et al. 2006; Ramakrishnan 2012). Interestingly, Mmp9 expression was found to be upregulated in C57BL/6 mice infected with Bpm (Conejero et al. 2015). Therefore, Mmp9, a potential therapeutic target for TB should also be explored for Bpm infections.
CONCLUSION
Bpm and Bcc species, like many pathogenic bacteria, are able to cause persistent infections that can lead to symptomatic chronic disease. With this review, we attempted to summarize the literature pertaining to Burkholderia persistence and the host immune response to chronic infection. We gave an overview of the epidemiology of chronic Burkholderia infections, persistent factors of these Burkholderia species, and host humoral and cellular responses to persistent infections. Many Burkholderia species are still relatively understudied when compared to Mycobacterium tuberculosis and Pseudomonas. Accordingly, we have much to learn about persistent Burkholderia species and their ability to cause long-term infections.
Acknowledgments
We thank Mardelle Susman and Laura Muruato for a critical reading of this manuscript.
FUNDING
This study was partially supported by UTMB seed funds.
Conflict of interest. None declared.
REFERENCES
- Allwood EM, Devenish RJ, Prescott M, et al. Strategies for intracellular survival of Burkholderia pseudomallei. Front Microbiol. 2011;2:170. doi: 10.3389/fmicb.2011.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemiya K, Dankmeyer JL, Fetterer DP, et al. Comparison of the early host immune response to two widely diverse virulent strains of Burkholderia pseudomallei that cause acute or chronic infections in BALB/c mice. Microb Pathog. 2015;86:53–63. doi: 10.1016/j.micpath.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Asakrah S, Nieves W, Mahdi Z, et al. Post-exposure therapeutic efficacy of COX-2 inhibition against Burkholderia pseudomallei. PLoS Neglect Trop D. 2013;7:e2212. doi: 10.1371/journal.pntd.0002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbroich SA, Lafontaine ER, Hogan RJ. Melioidosis and glanders modulation of the innate immune system: barriers to current and future vaccine approaches. Expert Rev Vaccines. 2016:1–19. doi: 10.1586/14760584.2016.1170598. [DOI] [PubMed] [Google Scholar]
- Avgeri SG, Matthaiou DK, Dimopoulos G, et al. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int J Antimicrob Ag. 2009;33:394–404. doi: 10.1016/j.ijantimicag.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Bernhardt SA, Spilker T, Coffey T, et al. Burkholderia cepacia complex in cystic fibrosis: frequency of strain replacement during chronic infection. Clin Infect Dis. 2003;37:780–5. doi: 10.1086/377541. [DOI] [PubMed] [Google Scholar]
- Biddick R, Spilker T, Martin A, et al. Evidence of transmission of Burkholderia cepacia, Burkholderia multivorans and Burkholderia dolosa among persons with cystic fibrosis. FEMS Microbiol Lett. 2003;228:57–62. doi: 10.1016/S0378-1097(03)00724-9. [DOI] [PubMed] [Google Scholar]
- Brackman G, Cos P, Maes L, et al. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Ch. 2011;55:2655–61. doi: 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach K, Kohler J, Steinmetz I. Induction of protective immunity against Burkholderia pseudomallei using attenuated mutants with defects in the intracellular life cycle. T R Soc Trop Med H. 2008;102(Suppl 1):S89–94. doi: 10.1016/S0035-9203(08)70022-1. [DOI] [PubMed] [Google Scholar]
- Breitbach K, Wongprompitak P, Steinmetz I. Distinct roles for nitric oxide in resistant C57BL/6 and susceptible BALB/c mice to control Burkholderia pseudomallei infection. BMC Immunol. 2011;12:20. doi: 10.1186/1471-2172-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt A, Halliday N, Williams P, et al. Burkholderia pseudomallei kynB plays a role in AQ production, biofilm formation, bacterial swarming and persistence. Res Microbiol. 2016;167:159–67. doi: 10.1016/j.resmic.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Butt A, Higman VA, Williams C, et al. The HicA toxin from Burkholderia pseudomallei has a role in persister cell formation. Biochem J. 2014;459:333–44. doi: 10.1042/BJ20140073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt A, Muller C, Harmer N, et al. Identification of type II toxin-antitoxin modules in Burkholderia pseudomallei. FEMS Microbiol Lett. 2013;338:86–94. doi: 10.1111/1574-6968.12032. [DOI] [PubMed] [Google Scholar]
- Campana S, Taccetti G, Ravenni N, et al. Molecular epidemiology of Pseudomonas aeruginosa, Burkholderia cepacia complex and methicillin-resistant Staphylococcus aureus in a cystic fibrosis center. J Cyst Fibros. 2004;3:159–63. doi: 10.1016/j.jcf.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Caraher E, Reynolds G, Murphy P, et al. Comparison of antibiotic susceptibility of Burkholderia cepacia complex organisms when grown planktonically or as biofilm in vitro. Eur J Clin Microbiol. 2007;26:213–6. doi: 10.1007/s10096-007-0256-x. [DOI] [PubMed] [Google Scholar]
- Chantratita N, Wuthiekanun V, Boonbumrung K, et al. Biological relevance of colony morphology and phenotypic switching by Burkholderia pseudomallei. J Bacteriol. 2007;189:807–17. doi: 10.1128/JB.01258-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CY, Ulrich RL, Ulrich MP, et al. Characterization of the murine macrophage response to infection with virulent and avirulent Burkholderia species. BMC Microbiol. 2015;15:259. doi: 10.1186/s12866-015-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KK, Davidson DJ, Halsey TK, et al. Differential persistence among genomovars of the Burkholderia cepacia complex in a murine model of pulmonary infection. Infect Immun. 2002;70:2715–20. doi: 10.1128/IAI.70.5.2715-2720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KK, MacDonald KL, Davidson DJ, et al. Persistence of Burkholderia multivorans within the pulmonary macrophage in the murine lung. Infect Immun. 2004;72:6142–7. doi: 10.1128/IAI.72.10.6142-6147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JW, Altman E, Beveridge TJ, et al. Colonial morphology of Burkholderia cepacia complex genomovar III: implications in exopolysaccharide production, pilus expression, and persistence in the mouse. Infect Immun. 2003;71:904–9. doi: 10.1128/IAI.71.2.904-909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye T, Mahenthiralingam E, Henry D, et al. Burkholderia ambifaria sp. nov., a novel member of the Burkholderia cepacia complex including biocontrol and cystic fibrosis-related isolates. Int J Syst Evol Micr. 2001;51:1481–90. doi: 10.1099/00207713-51-4-1481. [DOI] [PubMed] [Google Scholar]
- Coenye T, Vandamme P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ Microbiol. 2003;5:719–29. doi: 10.1046/j.1462-2920.2003.00471.x. [DOI] [PubMed] [Google Scholar]
- Compant S, Nowak J, Coenye T, et al. Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol Rev. 2008;32:607–26. doi: 10.1111/j.1574-6976.2008.00113.x. [DOI] [PubMed] [Google Scholar]
- Conejero L, Patel N, de Reynal M, et al. Low-dose exposure of C57BL/6 mice to Burkholderia pseudomallei mimics chronic human melioidosis. Am J Pathol. 2011;179:270–80. doi: 10.1016/j.ajpath.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejero L, Potempa K, Graham CM, et al. The blood transcriptome of experimental melioidosis reflects disease severity and shows considerable similarity with the human disease. J Immunol. 2015;195:3248–61. doi: 10.4049/jimmunol.1500641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Chu KK, Bylund J, et al. Production of exopolysaccharide by Burkholderia cenocepacia results in altered cell-surface interactions and altered bacterial clearance in mice. J Infect Dis. 2004;190:957–66. doi: 10.1086/423141. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Coutinho CP, Barreto C, Pereira L, et al. Incidence of Burkholderia contaminans at a cystic fibrosis centre with an unusually high representation of Burkholderia cepacia during 15 years of epidemiological surveillance. J Med Microbiol. 2015;64:927–35. doi: 10.1099/jmm.0.000094. [DOI] [PubMed] [Google Scholar]
- Coutinho CP, de Carvalho CC, Madeira A, et al. Burkholderia cenocepacia phenotypic clonal variation during a 3.5-year colonization in the lungs of a cystic fibrosis patient. Infect Immun. 2011a;79:2950–60. doi: 10.1128/IAI.01366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho CP, Dos Santos SC, Madeira A, et al. Long-term colonization of the cystic fibrosis lung by Burkholderia cepacia complex bacteria: epidemiology, clonal variation, and genome-wide expression alterations. Front Cell Infect Microbiol. 2011b;1:12. doi: 10.3389/fcimb.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MV, Leitao JH, Mahenthiralingam E, et al. Molecular analysis of Burkholderia cepacia complex isolates from a Portuguese cystic fibrosis center: a 7-year study. J Clin Microbiol. 2003;41:4113–20. doi: 10.1128/JCM.41.9.4113-4120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MV, Sousa SA, Leitao JH, et al. Studies on the involvement of the exopolysaccharide produced by cystic fibrosis-associated isolates of the Burkholderia cepacia complex in biofilm formation and in persistence of respiratory infections. J Clin Microbiol. 2004;42:3052–8. doi: 10.1128/JCM.42.7.3052-3058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie BJ. Melioidosis: an important cause of pneumonia in residents of and travellers returned from endemic regions. Eur Respir J. 2003;22:542–50. doi: 10.1183/09031936.03.00006203. [DOI] [PubMed] [Google Scholar]
- Currie BJ, Fisher DA, Anstey NM, et al. Melioidosis: acute and chronic disease, relapse and re-activation. T Roy Soc Trop Med H. 2000;94:301–4. doi: 10.1016/s0035-9203(00)90333-x. [DOI] [PubMed] [Google Scholar]
- Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Neglect Trop D. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dance DA. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 2000;74:159–68. doi: 10.1016/s0001-706x(99)00066-2. [DOI] [PubMed] [Google Scholar]
- De Smet B, Mayo M, Peeters C, et al. Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. Int J Syst Evol Micr. 2015;65:2265–71. doi: 10.1099/ijs.0.000251. [DOI] [PubMed] [Google Scholar]
- Gan YH. Interaction between Burkholderia pseudomallei and the host immune response: sleeping with the enemy? J Infect Dis 20051921845–50. [DOI] [PubMed] [Google Scholar]
- George SE, Nelson GM, Kohan MJ, et al. Colonization and clearance of environmental microbial agents upon intranasal exposure of strain C3H/HeJ mice. J Toxicol Env Heal A. 1999;56:419–31. doi: 10.1080/009841099158006. [DOI] [PubMed] [Google Scholar]
- Gillis M, Van Van T, Bardin R, et al. Polyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus and proposition of Burkholderia vietnamiensis sp. nov. for N2-fixing isolates from rice in Vietnam. Int J Syst Evol Micr. 1995;45:274–89. [Google Scholar]
- Gong L, Lai SC, Treerat P, et al. Burkholderia pseudomallei type III secretion system cluster 3 ATPase BsaS, a chemotherapeutic target for small-molecule ATPase inhibitors. Infect Immun. 2015;83:1276–85. doi: 10.1128/IAI.03070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear A, Bielefeldt-Ohmann H, Schweizer H, et al. Persistent gastric colonization with Burkholderia pseudomallei and dissemination from the gastrointestinal tract following mucosal inoculation of mice. PLoS One. 2012;7:e37324. doi: 10.1371/journal.pone.0037324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan JR, Brown AR, Jones AM. Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol. 2007;2:153–64. doi: 10.2217/17460913.2.2.153. [DOI] [PubMed] [Google Scholar]
- Greenberg DE, Goldberg JB, Stock F, et al. Recurrent Burkholderia infection in patients with chronic granulomatous disease:11–year experience at a large referral center. Clin Infect Dis. 2009;48:1577–9. doi: 10.1086/598937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo QY, Wei Y, Xia B, et al. Identification of a small molecule that simultaneously suppresses virulence and antibiotic resistance of Pseudomonas aeruginosa. Sci Rep-UK. 2016;6:19141. doi: 10.1038/srep19141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad MA, Austin CR, Stewart AL, et al. Adaptation and antibiotic tolerance of anaerobic Burkholderia pseudomallei. Antimicrob Agents Ch. 2011;55:3313–23. doi: 10.1128/AAC.00953-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Easton A, Smith D, et al. Role of T cells in innate and adaptive immunity against murine Burkholderia pseudomallei infection. J Infect Dis. 2006;193:370–9. doi: 10.1086/498983. [DOI] [PubMed] [Google Scholar]
- Haussler S, Lehmann C, Breselge C, et al. Fatal outcome of lung transplantation in cystic fibrosis patients due to small-colony variants of the Burkholderia cepacia complex. Eur J Clin Microbiol. 2003;22:249–53. doi: 10.1007/s10096-003-0901-y. [DOI] [PubMed] [Google Scholar]
- Haussler S, Rohde M, Steinmetz I. Highly resistant Burkholderia pseudomallei small colony variants isolated in vitro and in experimental melioidosis. Med Microbiol Immunol. 1999;188:91–7. doi: 10.1007/s004300050110. [DOI] [PubMed] [Google Scholar]
- Hawn TR, Matheson AI, Maley SN, et al. Host-directed therapeutics for tuberculosis: can we harness the host? Microbiol Mol Biol R. 2013;77:608–27. doi: 10.1128/MMBR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden HS, Lim R, Brittnacher MJ, et al. Evolution of Burkholderia pseudomallei in recurrent melioidosis. PLoS One. 2012;7:e36507. doi: 10.1371/journal.pone.0036507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe I, Brenneke B, Rohde M, et al. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect Immun. 1999;67:2891–900. doi: 10.1128/iai.67.6.2891-2900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TA, Kooi C, Sokol PA, et al. Identification of Burkholderia cenocepacia genes required for bacterial survival in vivo. Infect Immun. 2004;72:4010–22. doi: 10.1128/IAI.72.7.4010-4022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka H, Kousaka M, Tamura G, et al. Studies on pyrrolnitrin, a new antibiotic. 3. Structure of pyrrolnitrin. J Antibiot. 1965;18:207–10. [PubMed] [Google Scholar]
- Khan I, Wieler LH, Melzer F, et al. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound Emerg Dis. 2013;60:204–21. doi: 10.1111/j.1865-1682.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- Kunnathuparambil SG, Sathar SA, Tank DC, et al. Splenic abscess due to chronic melioidosis in a patient previously misdiagnosed as tuberculosis. Ann Gastroenterol. 2013;26:77–9. [PMC free article] [PubMed] [Google Scholar]
- Leakey AK, Ulett GC, Hirst RG. BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb Pathog. 1998;24:269–75. doi: 10.1006/mpat.1997.0179. [DOI] [PubMed] [Google Scholar]
- Limmathurotsakul D, Golding N, Dance DAB, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- Limmathurotsakul D, Peacock SJ. Melioidosis: a clinical overview. Brit Med Bull. 2011;99:125–39. doi: 10.1093/bmb/ldr007. [DOI] [PubMed] [Google Scholar]
- Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010;82:1113–7. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipuma JJ. Update on the Burkholderia cepacia complex. Curr Opin Pulm Med. 2005;11:528–33. doi: 10.1097/01.mcp.0000181475.85187.ed. [DOI] [PubMed] [Google Scholar]
- Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma JJ, Fisher MC, Dasen SE, et al. Ribotype stability of serial pulmonary isolates of Pseudomonas cepacia. J Infect Dis. 1991;164:133–6. doi: 10.1093/infdis/164.1.133. [DOI] [PubMed] [Google Scholar]
- Liu B, Koo GC, Yap EH, et al. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect Immun. 2002;70:504–11. doi: 10.1128/IAI.70.2.504-511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira A, dos Santos SC, Santos PM, et al. Proteomic profiling of Burkholderia cenocepacia clonal isolates with different virulence potential retrieved from a cystic fibrosis patient during chronic lung infection. PLoS One. 2013;8:e83065. doi: 10.1371/journal.pone.0083065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahenthiralingam E, Baldwin A, Dowson CG. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol. 2008;104:1539–51. doi: 10.1111/j.1365-2672.2007.03706.x. [DOI] [PubMed] [Google Scholar]
- Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3:144–56. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- Martina P, Bettiol M, Vescina C, et al. Genetic diversity of Burkholderia contaminans isolates from cystic fibrosis patients in Argentina. J Clin Microbiol. 2013;51:339–44. doi: 10.1128/JCM.02500-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina P, Feliziani S, Juan C, et al. Hypermutation in Burkholderia cepacia complex is mediated by DNA mismatch repair inactivation and is highly prevalent in cystic fibrosis chronic respiratory infection. Int J Med Microbiol. 2014;304:1182–91. doi: 10.1016/j.ijmm.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Medina-Pascual MJ, Valdezate S, Carrasco G, et al. Increase in isolation of Burkholderia contaminans from Spanish patients with cystic fibrosis. Clin Microbiol Infec. 2015;21:150–6. doi: 10.1016/j.cmi.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Miranda MS, Breiman A, Allain S, et al. The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin Dev Immunol. 2012;2012:139127. doi: 10.1155/2012/139127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr CD, Tomich M, Herfst CA. Cellular aspects of Burkholderia cepacia infection. Microbes Infect. 2001;3:425–35. doi: 10.1016/s1286-4579(01)01389-2. [DOI] [PubMed] [Google Scholar]
- Mongkolrob R, Taweechaisupapong S, Tungpradabkul S. Correlation between biofilm production, antibiotic susceptibility and exopolysaccharide composition in Burkholderia pseudomalleibpsI, ppk, and rpoS mutant strains. Microbiol Immunol. 2015;59:653–63. doi: 10.1111/1348-0421.12331. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Caraher E. Residence in biofilms allows Burkholderia cepacia complex (Bcc) bacteria to evade the antimicrobial activities of neutrophil-like dHL60 cells. Pathog Dis. 2015;73:ftv069. doi: 10.1093/femspd/ftv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierman WC, Yu Y, Losada L. The in vitro antibiotic tolerant persister population in Burkholderia pseudomallei is altered by environmental factors. Front Microbiol. 2015;6:1338. doi: 10.3389/fmicb.2015.01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll MR, Kidd TJ, Coulter C, et al. Burkholderia pseudomallei: another emerging pathogen in cystic fibrosis. Thorax. 2003;58:1087–91. doi: 10.1136/thorax.58.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka Y. Prokaryotic toxin-antitoxin systems: novel regulations of the toxins. Curr Genet. 2016;62:379–82. doi: 10.1007/s00294-015-0557-z. [DOI] [PubMed] [Google Scholar]
- Palleroni NJ, Holmes B. Pseudomonas cepacia sp. nov., nom. rev. Int J Syst Evol Micr. 1981;31:479–81. [Google Scholar]
- Peeters C, Zlosnik JE, Spilker T, et al. Burkholderia pseudomultivorans sp. nov., a novel Burkholderia cepacia complex species from human respiratory samples and the rhizosphere. Syst Appl Microbiol. 2013;36:483–9. doi: 10.1016/j.syapm.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Peeters E, Nelis HJ, Coenye T. In vitro activity of ceftazidime, ciprofloxacin, meropenem, minocycline, tobramycin and trimethoprim/sulfamethoxazole against planktonic and sessile Burkholderia cepacia complex bacteria. J Antimicrob Chemoth. 2009;64:801–9. doi: 10.1093/jac/dkp253. [DOI] [PubMed] [Google Scholar]
- Pibalpakdee P, Wongratanacheewin S, Taweechaisupapong S, et al. Diffusion and activity of antibiotics against Burkholderia pseudomallei biofilms. Int J Antimicrob Ag. 2012;39:356–9. doi: 10.1016/j.ijantimicag.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Pirone L, Bragonzi A, Farcomeni A, et al. Burkholderia cenocepacia strains isolated from cystic fibrosis patients are apparently more invasive and more virulent than rhizosphere strains. Environ Microbiol. 2008;10:2773–84. doi: 10.1111/j.1462-2920.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- Pradenas G, Ross B, Torres A. Burkholderia cepacia complex vaccines: where do we go from here? Vaccines. 2016;4:10. doi: 10.3390/vaccines4020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price EP, Sarovich DS, Mayo M, et al. Within-host evolution of Burkholderia pseudomallei over a twelve-year chronic carriage infection. MBio. 2013;4:e00388–13. doi: 10.1128/mBio.00388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12:352–66. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- Rao KN, Lakshminarasimhan A, Joseph S, et al. AFN-1252 is a potent inhibitor of enoyl-ACP reductase from Burkholderia pseudomallei–crystal structure, mode of action, and biological activity. Protein Sci. 2015;24:832–40. doi: 10.1002/pro.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner D, Mueller C. Immune responses in cystic fibrosis: are they intrinsically defective? Am J Resp Cell Mol. 2012;46:715–22. doi: 10.1165/rcmb.2011-0399RT. [DOI] [PubMed] [Google Scholar]
- Razvi S, Quittell L, Sewall A, et al. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest. 2009;136:1554–60. doi: 10.1378/chest.09-0132. [DOI] [PubMed] [Google Scholar]
- Russell DG, Cardona PJ, Kim MJ, et al. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–8. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U, Thanassoulis G, Cherapanov V, et al. Enhanced susceptibility to pulmonary infection with Burkholderia cepacia in Cftr(−/−) mice. Infect Immun. 2001;69:5138–50. doi: 10.1128/IAI.69.8.5138-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanirand P, Harley VS, Dance DA, et al. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect Immun. 1999;67:3593–600. doi: 10.1128/iai.67.7.3593-3600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawana A, Adeolu M, Gupta RS. Molecular signatures and phylogenomic analysis of the genus Burkholderia: proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front Genet. 2014;5:429. doi: 10.3389/fgene.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawasdidoln C, Taweechaisupapong S, Sermswan RW, et al. Growing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistance. PLoS One. 2010;5:e9196. doi: 10.1371/journal.pone.0009196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulin T, Steinmetz I. Chronic melioidosis in a patient with cystic fibrosis. J Clin Microbiol. 2001;39:1676–7. doi: 10.1128/JCM.39.4.1676-1677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See JX, Samudi C, Saeidi A, et al. Experimental persistent infection of BALB/c mice with small-colony variants of Burkholderia pseudomallei leads to concurrent upregulation of PD-1 on T cells and skewed Th1 and Th17 responses. PLoS Neglect Trop D. 2016;10:e0004503. doi: 10.1371/journal.pntd.0004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert DP, Steen B, Halsey K, et al. A murine model for infection with Burkholderia cepacia with sustained persistence in the spleen. Infect Immun. 1999;67:4027–32. doi: 10.1128/iai.67.8.4027-4032.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke JR, Edwards MS, Langston C, et al. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987;22:698–702. doi: 10.1203/00006450-198712000-00017. [DOI] [PubMed] [Google Scholar]
- Suputtamongkol Y, Chaowagul W, Chetchotisakd P, et al. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–13. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- Taweechaisupapong S, Kaewpa C, Arunyanart C, et al. Virulence of Burkholderia pseudomallei does not correlate with biofilm formation. Microb Pathog. 2005;39:77–85. doi: 10.1016/j.micpath.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Hattle JM, Dreitz SA, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006;74:6135–44. doi: 10.1128/IAI.02048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulett GC, Ketheesan N, Hirst RG. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect Immun. 2000;68:2034–42. doi: 10.1128/iai.68.4.2034-2042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano MA. Intracellular survival of Burkholderia cepacia complex in phagocytic cells. Can J Microbiol. 2015;61:607–15. doi: 10.1139/cjm-2015-0316. [DOI] [PubMed] [Google Scholar]
- Van Acker H, Sass A, Bazzini S, et al. Biofilm-grown Burkholderia cepacia complex cells survive antibiotic treatment by avoiding production of reactive oxygen species. PLoS One. 2013;8:e58943. doi: 10.1371/journal.pone.0058943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker H, Sass A, Dhondt I, et al. Involvement of toxin-antitoxin modules in Burkholderia cenocepacia biofilm persistence. Pathog Dis. 2014;71:326–35. doi: 10.1111/2049-632X.12177. [DOI] [PubMed] [Google Scholar]
- van Schaik EJ, Tom M, Woods DE. Burkholderia pseudomallei isocitrate lyase is a persistence factor in pulmonary melioidosis: implications for the development of isocitrate lyase inhibitors as novel antimicrobials. Infect Immun. 2009;77:4275–83. doi: 10.1128/IAI.00609-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme P, Henry D, Coenye T, et al. Burkholderia anthina sp. nov. and Burkholderia pyrrocinia, two additional Burkholderia cepacia complex bacteria, may confound results of new molecular diagnostic tools. FEMS Immunol Med Mic. 2002;33:143–9. doi: 10.1111/j.1574-695X.2002.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Vandamme P, Holmes B, Coenye T, et al. Burkholderia cenocepacia sp. nov.–a new twist to an old story. Res Microbiol. 2003;154:91–6. doi: 10.1016/S0923-2508(03)00026-3. [DOI] [PubMed] [Google Scholar]
- Vandamme P, Holmes B, Vancanneyt M, et al. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- Vandamme P, Mahenthiralingam E, Holmes B, et al. Identification and population structure of Burkholderia stabilis sp. nov. (formerly Burkholderia cepacia genomovar IV) J Clin Microbiol. 2000;38:1042–7. doi: 10.1128/jcm.38.3.1042-1047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlaere E, Baldwin A, Gevers D, et al. Taxon K, a complex within the Burkholderia cepacia complex, comprises at least two novel species, Burkholderia contaminans sp. nov. and Burkholderia lata sp. nov. Int J Syst Evol Micr. 2009;59:102–11. doi: 10.1099/ijs.0.001123-0. [DOI] [PubMed] [Google Scholar]
- Vanlaere E, Lipuma JJ, Baldwin A, et al. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int J Syst Evol Micr. 2008;58:1580–90. doi: 10.1099/ijs.0.65634-0. [DOI] [PubMed] [Google Scholar]
- Vasu C, Vadivelu J, Puthucheary SD. The humoral immune response in melioidosis patients during therapy. Infection. 2003;31:24–30. doi: 10.1007/s15010-002-3020-2. [DOI] [PubMed] [Google Scholar]
- Ventura GM, Balloy V, Ramphal R, et al. Lack of MyD88 protects the immunodeficient host against fatal lung inflammation triggered by the opportunistic bacteria Burkholderia cenocepacia. J Immunol. 2009;183:670–6. doi: 10.4049/jimmunol.0801497. [DOI] [PubMed] [Google Scholar]
- Vermis K, Coenye T, LiPuma JJ, et al. Proposal to accommodate Burkholderia cepacia genomovar VI as Burkholderia dolosa sp. nov. Int J Syst Evol Micr. 2004;54:689–91. doi: 10.1099/ijs.0.02888-0. [DOI] [PubMed] [Google Scholar]
- Vial L, Chapalain A, Groleau MC, et al. The various lifestyles of the Burkholderia cepacia complex species: a tribute to adaptation. Environ Microbiol. 2011;13:1–12. doi: 10.1111/j.1462-2920.2010.02343.x. [DOI] [PubMed] [Google Scholar]
- Vidyalakshmi K, Lipika S, Vishal S, et al. Emerging clinico-epidemiological trends in melioidosis: analysis of 95 cases from western coastal India. Int J Infect Dis. 2012;16:e491–7. doi: 10.1016/j.ijid.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. New Engl J Med. 2012;367:1035–44. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- Wiersinga WJ, van der Poll T, White NJ, et al. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4:272–82. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- Wong KT, Puthucheary SD, Vadivelu J. The histopathology of human melioidosis. Histopathology. 1995;26:51–5. doi: 10.1111/j.1365-2559.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Yabuuchi E, Kawamura Y, Ezaki T, et al. Burkholderia uboniae sp. nov., L-arabinose-assimilating but different from Burkholderia thailandensis and Burkholderia vietnamiensis. Microbiol Immunol. 2000;44:307–17. doi: 10.1111/j.1348-0421.2000.tb02500.x. [DOI] [PubMed] [Google Scholar]
- Zlosnik JE, Costa PS, Brant R, et al. Mucoid and nonmucoid Burkholderia cepacia complex bacteria in cystic fibrosis infections. Am J Resp Crit Care. 2011;183:67–72. doi: 10.1164/rccm.201002-0203OC. [DOI] [PubMed] [Google Scholar]
- Zlosnik JE, Hird TJ, Fraenkel MC, et al. Differential mucoid exopolysaccharide production by members of the Burkholderia cepacia complex. J Clin Microbiol. 2008;46:1470–3. doi: 10.1128/JCM.02273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlosnik JE, Speert DP. The role of mucoidy in virulence of bacteria from the Burkholderia cepacia complex: a systematic proteomic and transcriptomic analysis. J Infect Dis. 2010;202:770–81. doi: 10.1086/655663. [DOI] [PubMed] [Google Scholar]
- Zlosnik JE, Zhou G, Brant R, et al. Burkholderia species infections in patients with cystic fibrosis in British Columbia, Canada. 30 years' experience. Ann Am Thorac Soc. 2015;12:70–8. doi: 10.1513/AnnalsATS.201408-395OC. [DOI] [PubMed] [Google Scholar]