Abstract
Amyloidosis cutis dyschromica (ACD) is a distinct form of primary cutaneous amyloidosis characterized by generalized hyperpigmentation mottled with small hypopigmented macules on the trunks and limbs. Affected families and sporadic case subjects have been reported predominantly in East and Southeast Asian ethnicities; however, the genetic cause has not been elucidated. We report here that the compound heterozygosity or homozygosity of GPNMB truncating alleles is the cause of autosomal-recessive ACD. Six nonsense or frameshift mutations were identified in nine individuals diagnosed with ACD. Immunofluorescence analysis of skin biopsies showed that GPNMB is expressed in all epidermal cells, with the highest staining observed in melanocytes. GPNMB staining is significantly reduced in the lesional skin of affected individuals. Hyperpigmented lesions exhibited significantly increased amounts of DNA/keratin-positive amyloid deposits in the papillary dermis and infiltrating macrophages compared with hypo- or depigmented macules. Depigmentation of the lesions was attributable to loss of melanocytes. Intracytoplasmic fibrillary aggregates were observed in keratinocytes scattered in the lesional epidermis. Thus, our analysis indicates that loss of GPNMB, which has been implicated in melanosome formation, autophagy, phagocytosis, tissue repair, and negative regulation of inflammation, underlies autosomal-recessive ACD and provides insights into the etiology of amyloidosis and pigment dyschromia.
Keywords: amyloidosis cutis dyschromica, amyloid, GPNMB, Glycoprotein nonmetastatic gene B, hyperpigmentation, hypopigmentation, DBA/2J, glaucoma, pigment dispersion, PMEL
Introduction
Primary localized cutaneous amyloidosis (PLCA) refers to a group of chronic itchy skin conditions, defined as amyloid deposition in previously apparent normal skin in the absence of systemic involvement. The major subtypes include the more common macular and lichen amyloidosis and the rare nodular amyloidosis.1 Most cases are sporadic. Autosomal-dominant PLCA results from heterozygous missense mutations in OSMR (encoding oncostatin M receptor-beta [OSMRβ] [MIM: 105250]) and IL31RA (encoding interleukin [IL]-31 receptor-alpha [MIM: 613955]), both of which belong to the IL-6 cytokine family.2, 3 OSMRβ, a component of both the OSM type II receptor and the IL-31 receptor, combines with IL31RA to form the IL-31 receptor. Amyloidosis cutis dyschromica (ACD) is a distinct variant of PLCA and is predominantly observed in East and Southeast Asian individuals.4 The clinical features include dotted, reticular hyperpigmentation with hypopigmented macules; mild or no pruritus; onset before puberty; and small foci of amyloid deposition in the papillary dermis. The dyschromia was distributed throughout the trunk and limbs, sparing the hands, feet, and face in most case subjects. A clinical and epidemiological review of 48 case subjects by Mahon et al.4 suggested that ACD may exhibit autosomal dominance inherited with incomplete penetrance. Familial case subjects were more common than sporadic case subjects and showed an earlier mean age of onset before 10 years versus common age of onset in the 20s in sporadic case subjects.4 Detection of keratin composition in amyloid deposits of individuals with ACD suggested that the amyloid is derived from degenerative keratinocytes.5, 6, 7, 8 The genetic cause has not yet been identified.
Through whole-exome sequencing of an ACD-affected family and validation in other affected individuals, we identified nine ACD-affected individuals with biallelic truncating mutations in glycoprotein nonmetastatic gene B (GPNMB [MIM: 604368]). GPNMB is a highly glycosylated type I transmembrane protein and was first isolated from weakly metastatic melanoma cells in 1995 as a regulator of tumor growth.9 In normal tissues, GPNMB is expressed in numerous cell types; GPNMB is also known as osteoactivin (OA) in osteoblasts,10 dendritic cell-heparin integrin ligand (DC-HIL) in dendritic and T cells,11 hematopoietic growth factor inducible neurokinin-1 type (HG-FIN) in tumor cells,12 and quail neuroretina cells-71 (QNR-71) in pigment cells of quail neuroretina.13 GPNMB belongs to the premelanosome protein (PMEL)/NMB family owing to its high sequence homology with PMEL (MIM: 155550).9, 13 GPNMB is highly expressed in melanocytes, is partially localized in melanosomes, lysosomes, and early endosomes,14 and is critical for melanosome formation.15 The adhesion of keratinocytes and endothelial cells to GPNMB suggests that GPNMB may contribute to melanocyte adhesion to keratinocytes16 and transendothelial migration of dendritic cells.11 Homozygous premature nonsense mutations leading to loss of function of GPNMB contribute to the iris pigment dispersion phenotype in mouse pigmentary glaucoma harboring mutations in two genes, Gpnmb and Tyrp1.17, 18 Tyrp1 mutations contribute to the iris stromal atrophy phenotype in mutant mice. However, no mutations in GPNMB and TYRP1 have been identified in human pigmentary glaucoma and pigment dispersion syndrome.19, 20 The expression of GPNMB in macrophages serves as a feedback regulator of pro-inflammatory responses.21 GPNMB expressed on dendritic cells inhibits T cell activation by binding to syndecan-4, thus negatively regulating inflammation.22, 23 During the growth of melanoma in mice, GPNMB-expressing myolomonocytic cells (mainly CD11b+Gr1+ cells) expand, suppress T cell proliferation via the GPNMB/syndecan-4 pathway, and promote melanoma growth.24 Blockade of GPNMB in GPNMB-expressing myolomonocytic cells via gene deletion or specific antibodies abrogates their suppressor function and expansion and markedly inhibits melanoma progression in the skin. In an Alzheimer disease model, CD11c+ microglia accumulating around amyloid plaques displayed increased GPNMB level and an expression profile suggesting a potential role in suppressing immune reactivity to amyloid plaques and increasing Aβ-uptake and degradation.25 GPNMB is a phagocytic protein colocalized with autophagic proteins (Atg8/LC3) and phagosomes26, 27 and is essential for recruitment of the autophagy protein LC3 (Atg8) to phagosomes, lysosomal fusion with phagosomes, and degradation in kidney epithelial cells and bone-marrow-derived macrophages.26 In CCl4-induced acute liver injury, kidney ischemia, and dextran sulfate sodium-induced colitis models, GPNMB is markedly upregulated in epithelial cells and infiltrating macrophages of injured tissue and is critical for tissue repair and clearance of apoptotic cell debris by macrophages.26, 28, 29 GPNMB-negative macrophages exhibit significantly reduced phagocytosis of apoptotic cells compared with GPNMB-positive macrophages.26, 28 Macrophages with GPNMB mutations or knockdown express higher levels of pro-inflammatory cytokines.29, 30 According to these functions and mechanisms, GPNMB could be involved in the pathogenesis of ACD involving pigment dyschromia and amyloidosis.
In this study, we identified truncating mutations in GPNMB as the underlying genetic cause of autosomal-recessive ACD. We further explored the effects of GPNMB mutations and expression in human normal skin and ACD lesions and characterized the amyloid deposits in the papillary dermis, keratinocytes, melanocytes, Langerhans cells, and infiltrated immune cells within hyper- and hypo- or depigmented lesional skins. Our findings improve our understanding of the underlying etiology of amyloidosis and pigment dyschromia.
Material and Methods
Sample Collection
Nine individuals diagnosed with ACD were recruited from MacKey Memorial Hospital and Tri-Service General Hospital (Taipei, Taiwan). Normal control subjects were randomly selected from the Taiwan Han Chinese Cell and Genome Bank. This study was approved by the Institutional Review Board of MacKey Memorial Hospital, Tri-Service General Hospital, and Academia Sinica (Taiwan). Informed consent was obtained from all participants prior to enrollment. Genomic DNA was extracted from whole blood according to standard procedures using a Gentra Puregene Blood Kit (QIAGEN). We assessed the purity (A260/A280 and A260/A230 ratio) and integrity of the extracted genomic DNA by using a NanoDrop 2000 spectrophotometer (Thermo Scientific) and gel electrophoresis in a 1% agarose gel. DNA was quantified using a Qubit 2.0 fluorometer (Life Technologies). Quantity and quality of all extracted DNA samples was concentration 72–96 ng/μL (Qubit), A260/A280 ≥ 1.8, A260/A230 ≥ 2, and no DNA degradation. Genomic DNA was used subsequently in exome sequencing.
Exome Sequencing
The genomes of eight individuals in family A were sequenced and analyzed at the National Center for Genome Medicine (NCGM). Indexed, exome-enriched libraries were prepared using a SureSelect Human All Exon V5+UTRs kit (Agilent Technologies) targeting ∼75 Mb of the human genome according to the manufacturer’s protocol. Size distribution of the exome-enriched libraries was evaluated via the Agilent BioAnalyzer 2100 with the High Sensitivity DNA Kit (Agilent Technologies). All exome libraries were quantified by real-time PCR (KAPA Biosystems) with primers specific to the ends of the adapters and pooled at equimolar concentrations. The denaturation, dilution, and cluster amplification of the pooled library were performed according to the manufacturer’s protocol (Illumina). Sequencing was performed with 101-bp paired-end reads on an Illumina HiSeq2000 (Illumina). Multiple-sample alignment and variant calling were performed in the HiPipe (High performance Pipelines for NGS Data Analysis) workspace. Sequencing reads were aligned to the GRCh37 human reference genome using the Burrows-Wheeler Aligner v.0.6.2mt.31 GATK v.2.3-9 (see Web Resources) was used to refine local alignment of reads, mark duplicates, and call variants (single-nucleotide variants and indels) and to calculate exome coverage. Variants were filtered against in-house exome controls and analyzed for homozygous or compound heterozygous mutations in affected individuals. Filtered variants were annotated and analyzed for their functional impact by VarioWatch and Variant Effect Predictor.
Sanger Sequencing
The primers and PCR conditions used for the validation of GPNMB and NIPAL2 variants and for mutation screening of these candidate genes in other familial and sporadic case subjects are given in Table S1. Polymerase chain reaction (PCR) products were sequenced using an ABI3730 DNA sequencer (Life Technologies).
Plasmid Construction and Transfection
Wild-type human GPNMB cDNA was amplified from the glioma cell line S1 and subcloned into the pJET1.2/blunt vector to produce pJET-GPNMB. The cDNA insert of pJET-GPNMB-WT was sequenced on both strands by Sanger sequencing. The c.565C>T (p.Arg189∗) mutation was then generated by site-directed mutagenesis using a QuikChange Lightning Site-directed Mutagenesis Kit (Agilent Technologies) according to the manufacturer’s instructions. Wild-type and mutant cDNA inserts were subcloned into pcDNA3.1 to produce pGPNMB-WT and pGPNMB-Arg189∗. For the expression of N-terminal HA-tagged wild-type and mutant GPNMB cDNA, pJET-GPNMB-WT was inserted an HA-tag at the end of an N-terminal signal sequence by site-directed mutagenesis. The HA-tagged wild-type and mutant p.Arg189∗ cDNA were subcloned into pcDNA3.1 to generate pHA-GPNMB-WT and pHA-GPNMB-Arg189∗. All constructs were verified by Sanger sequencing and transfected into human HeLa cells with JetPRIME reagent (Polyplus-transfection). To determine transfection efficiency, cells were cotransfected with the pEGFP-N1 plasmid (Clontech).
Western Blotting
Human HeLa cells were grown in Dulbecco’s modified Eagle medium (DMEM; GIBCO, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Biological Industries) and 2 mM l-glutamine (GIBCO). MNT-1 human malignant melanoma cells were kindly provided by Dr. Shiau-Chuen Cheah (UCSI University, Malaysia) with permission from Dr. Michael S. Marks (University of Pennsylvania). MNT-1 cells were cultured in DMEM (GIBCO) with 20% fetal bovine serum (Hyclone), 10% AIM-V medium (GIBCO), 0.1 mM nonessential amino acid mix (GIBCO), 1 mM sodium pyruvate (GIBCO), 2 mM l-glutamine (GIBCO), and 100 U/mL penicillin/streptomycin (GIBCO). Forty-eight hours after transfection, cells were lysed in M-PER mammalian protein extraction reagent (Thermo Scientific) containing a protease inhibitor cocktail (Roche) for 30 min on ice and centrifuged at 20,000 × g for 30 min at 4°C to obtain soluble fractions and insoluble pellets. Insoluble pellets were washed once with M-PER reagent and solubilized with 0.5% sodium dodecyl sulfate (SDS) and 1% 2-mercaptoethanol. Insoluble lysates were obtained after boiling for 10 min and centrifugation. Protein concentrations in soluble and insoluble lysates were determined using Bicinchoninic Acid Protein Assays (Thermo Scientific). Equal amounts of proteins (30 μg of total proteins for soluble fractions and 300 μg for insoluble fractions) were separated on 10% SDS polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride membranes and probed with goat polyclonal anti-human GPNMB antibodies (1:1,000; AF2550; R&D Systems), anti-HA antibodies (1:1,000; MMS-101R; Covance), anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies (1:5,000; MAB374; Millipore), or anti-green fluorescent protein (GFP) antibodies (1:1,000; MAB3580; Millipore). Primary antibodies were detected with horseradish peroxidase-coupled anti-goat or anti-mouse secondary antibodies (KPL) and a WesternBright ECL chemiluminescence system (Advansta). Quantification of band intensities was carried out using ImageJ software (NIH).
Real-Time PCR
Total RNA was isolated using an RNeasy Kit (QIAGEN) and treated with DNase (RNase-free DNase Set; QIAGEN) to avoid contamination of genomic DNA. We assessed the purity (A260/A280 and A260/A230 ratio) and quality (RIN ≥ 8.0) of the extracted RNA with a NanoDrop 2000 spectrophotometer (Thermo Scientific) and an Agilent bioanalyzer 2100 (Agilent Technologies). Quantity and quality of all extracted RNA samples was at concentration 386–466 ng/μL (NanoDrop), A260/A280 ≥ 1.9, A260/A230 ≥ 2, and RIN ≥ 8.0. The first-strand cDNA was then synthesized with 1 μg of total RNA, using an oligo d(T) primer and SuperScript III reverse transcriptase (Invitrogen) in accordance with to the manufacturer’s instructions. Real-time PCR was performed in triplicates with 2 μL of 10-fold diluted cDNA, 500 nM each of forward and reverse primer, and 2× SYBR Green PCR Master Mix (Applied Biosystems; Life Technologies) in a total volume of 10 μL in a 384-well pate. The primer sets are given in Table S1. All real-time PCR reactions were run on a ViiA7 Real-time PCR System (Life Technologies). The cycling condition was in accordance with the manufacturer’s recommended cycling parameters: 50°C for 5 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Dissociation curves for each amplicon were then analyzed to verify the specificity of each amplification reaction; the dissociation curve was obtained by heating the amplicon from 60°C to 95°C. Gene expression fold change was calculated using the ΔΔCt method. pEGFP-1 was cotransfected with wild-type or mutant cDNA constructs as internal controls to normalize the transfection efficiency. GPNMB mRNA levels were normalized to those of GFP and to the reference genes, using the geometric mean of four reference genes (GAPDH, ACTB, YWHAZ, and SDHA). The change in GPNMB mRNA levels in wild-type or mutant cDNA-transfected cells was similar on comparing the normalization results of GFP and reference genes.
Immunofluorescence and Imaging
HeLa cells overexpressing GPNMB constructs on 4-well chamber μ-slides (Ibidi, Germany) were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at room temperature for 10 min and permeabilized with 0.1% Triton X-100 in 0.2% bovine serum albumin (BSA)/PBS at room temperature for 10 min. After blocking with 2% BSA/PBS, the cells were stained with goat polyclonal anti-GPNMB (1:100; AF2550; R&D Systems), mouse monoclonal anti-HA (1:1,000; MMS-101R; Covance), or mouse monoclonal anti-calnexin antibodies (1:100; MAB3126; Millipore). Secondary antibodies, i.e., donkey anti-goat IgG Alexa Fluor 488 (1:200; A11055; Molecular Probes) or chicken anti-mouse IgG Alexa Fluor 594 (1:200; A21201; Molecular Probes), were diluted and incubated with cells. Finally, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted in mounting medium. Fluorescence images were acquired using an Olympus 1X71 microscope (Olympus Corporation). The acquired images were processed by Velocity software (PerkinElmer). To label recycled endosomes with transferrin, cells were starved for 30 min at 37°C with serum-free DMEM containing 0.5% BSA and 15 mM HEPES and then incubated for 3 hr at 37°C with starvation medium containing 7.5 μg/mL Alexa Fluor 594 transferrin (Molecular Probes). Cells were immediately fixed and processed for immunofluorescence as described above.
Skin biopsy samples containing both hyperpigmented lesions and de- or hypopigmented lesions were taken from the upper arm of two individuals presenting with ACD. Biopsy samples were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned (6-μm thickness). The slides were subjected to hematoxylin-eosin (HE) staining for general morphology, Congo red staining for amyloids (ab150663; Abcam), and Fontana-Masson staining for melanin (ab150669; Abcam). For immunofluorescence staining, paraffin sections were dewaxed and rehydrated, and antigen retrieval was performed with boiled citrate buffer (pH 6.0). Sections were blocked with 5% normal donkey serum/0.1% saponin in PBS and incubated with goat polyclonal anti-GPNMB (1:100; R&D Systems), mouse monoclonal anti-cytokeratin (1:50; 34βE12, GTX73669; GeneTex), mouse monoclonal anti-melan A/MART1 (1:50; ab187369; Abcam), mouse monoclonal anti-HMB45 (1:50; anti-PMEL, GTX20787; GeneTex), mouse monoclonal anti-CD68 (1:100; MCA5709; Bio-Rad), mouse monoclonal anti-neutrophil elastase (1:50; GTX72042; GeneTex), goat polyclonal anti-langerin (1:100; AF2088; R&D Systems), or rabbit monoclonal anti-galactin7 antibodies (1:100; ab108623; Abcam) in blocking buffer. Donkey anti-goat IgG Alexa Fluor 488 (1:100; 705-546-147; Jackson ImmunoResearch), donkey anti-mouse IgG Alexa Fluor 594 (1:400; 715-586-151), donkey anti-mouse IgG Alexa Fluor 488 (1:100; 715-545-150), donkey anti-mouse IgG Alexa Fluor 488 (1:100; 715-547-003), donkey anti-rabbit IgG Alexa Fluor 594 (1:400; 711-585-152), donkey anti-goat IgG Alexa Fluor 488 (1:100; 705-547-003), or donkey anti-goat IgG Alexa Fluor 647 (1:150; A21447; Invitrogen) were used as secondary antibodies. Sections were then counterstained with DAPI. Slides were mounted and coverslipped with antifade permanent hard aqueous mounting solution (HNG). Negative controls were treated identically but without the primary antibody. Images were acquired using a Pannoramic 250 Flash II scanner (3DHISTECH; Hungary) and processed using Panoramic viewer software 1.15.3 (3DHISTECH). To allow comparisons of GPNMB levels between sections, all Panoramic settings were kept constant between sections for evaluating GPNMB expression. For comparing the numbers of MART1+, PMEL+, and CD68+ cells as well as the proportion of CD68+ area between the hyperpigmented region and de- or hypopigmented region in the same skin section, all setting regarding MART1, PMEL, and CD68 quantification in the same section were kept constant. The area of CD68-positive staining was quantified using ImageJ software (NIH).
Electron Microscopy
Biopsies of ∼1-mm thick skin were fixed for 2 hr at room temperature in 4% paraformaldehyde and 2.5% glutaraldehyde solution (pH 7.4). After wash with PBS, the following procedures including post-fixation in 1% Osmium tetraoxide, dehydration through graded ethanol solutions, embedding in Spurrs’ resin, double-staining with 2% uranyl acetate and lead citrate, and imaging with a transmission election microscopy (JEM-1200EX, Joel) were performed in the Electron Microscope Core Facility of the Institute of Biomedical Sciences, Academia Sinica (Taipei, Taiwan).
Statistical Analysis
Statistical analysis was performed by unpaired, two-tailed Student’s t test. Data are represented as the means ± SDs for bar graphs. All calculations were performed using GraphPad Prism software.
Results
Clinical Features
A total of nine individuals of Han Chinese origin, i.e., eight familial case subjects from four autosomal-recessive inherited families and one sporadic case subject, were recruited after being diagnosed with ACD according to clinical and histological criteria. The clinical features of these individuals are summarized in Table 1. Five individuals originated from one family (family A) with autosomal-recessive ACD (Figures 1A–1D). The mean age of onset was 6 years (2–13 years). Skin dyschromia and the affected area gradually increased with age and then stabilized. The cutaneous manifestations in all individuals were similar, with generalized hyperpigmentation mottled with small hypopigmented macules or patches distributed over the trunks and limbs in a symmetrical pattern (Figures 1B and S1). The skin lesions on the neck and face were much milder with some scattered hyper- and hypopigmented or only hypopigmented spots on the neck and forehead and around the hairline of the face. Five individuals presented with some hypopigmented spots on the dorsa of both hands and feet. Individual II-3 in family A was mildly affected with fewer hypopigmented spots all over the body compared with other individuals (Figure 1B, II-3). Small blisters, not previously reported in the literature, were occasionally found on the arms in four individuals from family A. All individuals had no history of extensive sun exposure and no photosensitivity. Hematology, abdominal ultrasonography, chest X-ray, and vision examination did not reveal any abnormalities. Oral acitretin (retinoids) have been reported to exhibit variable efficacy to ACD. Individual 6 showed a little improvement after 3 months of acitretin treatment and was then noncompliant due to heavily dry, cracked skin and lips.32
Table 1.
Clinical and Genetic Characteristics of Nine Individuals with ACD
|
Family A (Individuals 1–5) |
Family Ba |
Family Ca |
Family D |
Family E |
|||||
|---|---|---|---|---|---|---|---|---|---|
| II-1 | II-3b | II-4 | II-5 | II-7 | Individual 6 | Individual 7 | Individual 8 | Individual 9 | |
| Sex | female | male | female | male | female | male | male | male | female |
| Age at exam (years) | 42 | 38 | 34 | 32 | 24 | 25 | 20 | 33 | 35 |
| Age at onset (years) | 2 | 10 | 3 | 2 | 7 | 8 | 9 | 13 | 6 |
| Disease duration | 40 | 28 | 31 | 30 | 17 | 17 | 11 | 20 | 29 |
| Sites of skin lesions | face, neck, trunk, limbs, hands, feet | neck, trunk, limbs | face, neck, trunk, limbs, hands, feet | face, neck, trunk, limbs, hands, feet | neck, trunk, limbs | neck, trunk, limbs, hands, feet | neck, trunk, limbs, hands, feet | neck, trunk, limbs | face, neck, trunk, limbs |
| Abnormal mucous membrane, nails, teeth, hair, palm, and sole | – | – | – | – | – | – | – | – | – |
| Mild pruritus | – | – | + | – | + | – | – | – | – |
| Blister | + | + | + | + | – | – | – | – | – |
| Xerosis | + | + | + | + | + | + | + | + | + |
| Cytoskeratin in amyloid deositsc | ND | + | ND | ND | + | + | + | + | + |
| GPNMB Mutation | |||||||||
| DNA mutation (exon) | c.565C>T (ex 5) | c.565C>T (ex 5) | c.565C>T (ex 5) | c.296del (ex 3) | c.719_720del (ex 6) | ||||
| c.660T>G (ex 5) | c.1056del (ex 7) | c.565C>T (ex 5) | c.565C>T (ex 5) | c.877_880del (ex 6) | |||||
| Protein consequence | p.Arg189∗ | p.Arg189∗ | p.Arg189∗ | p.Asn99Thrfs∗2 | p.Val240Aspfs∗24 | ||||
| p.Tyr220∗ | p.Pro353Leufs∗20 | p.Arg189∗ | p.Arg189∗ | p.Val293Profs∗6 | |||||
| Protein domain | NTD | NTD; b/w PKD & KRG | NTD | NTD | PKD | ||||
GenBank: NM_002510.2 and NP_002501.1. Abbreviations: plus and minus signs (+/–), with/without the symptom or sign; b/w, between; NTD, N-terminal domain; PKD, polycystic kidney disease-like domain; KRG, kringle-like domain; ND, not determined.
Clinical findings in individual 6 and individual 7 were first partly published by one of us.32
Individual II-3 was mildly affected.
Cytokeratin (34bE12) was detected in amyloid deposits in the papillary dermis.
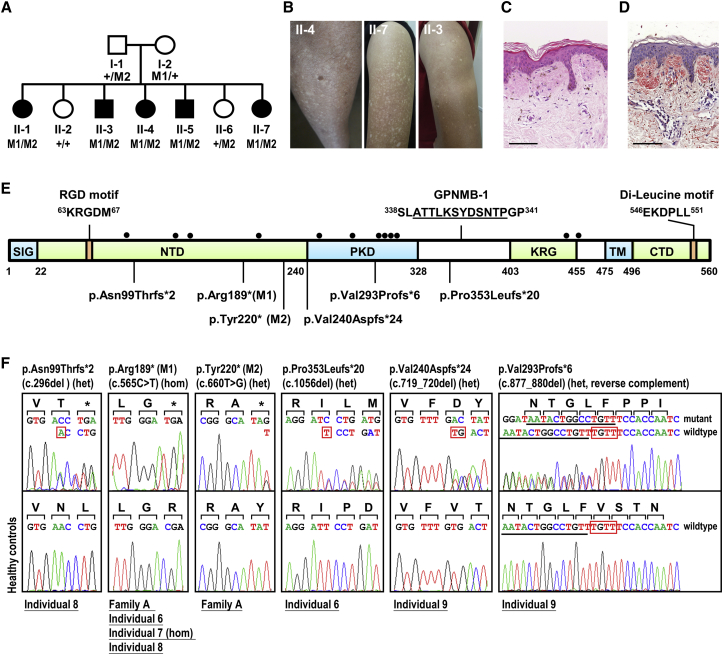
Figure 1.
GPNMB Mutations in Individuals with ACD
(A) Pedigree of family A. WES of family A identified two compound nonsense mutations (M1 and M2) in GPNMB.
(B) Skin lesions on the upper arm of individuals II-3, II-4, and II-7 from family A. The lower part of the upper arm of individual II-3 had a dark tan.
(C) H&E staining showed homogeneous eosinophilic masses in the papillary dermis with sparse subjacent melanophages infiltrated (individual II-7; scale bars: 100 μm).
(D) Congo red staining indicated positivity for amyloid deposits in the papillary dermis (individual II-7; scale bars: 100 μm).
(E) Diagram of human GPNMB and its domains defined as signal sequence (SIG), N-terminal domain (NTD), polycystic kidney disease-like domain (PKD), kringle-like domain (KRG), transmembrane domain (TM), and C-terminal cytoplasmic domain (CTD).29 Numbers correspond to the amino acid residues according to the protein sequence GenBank: NP_002501.1 (NM_002510.2). Solid circles represent N-glycosylation sites.14 A splice isoform of GPNMB (GPNMB-1) was identified with an in-frame 12-amino acid insertion (underlined, GenBank: NP_001005340.1, NM_001005340.1).
(F) Six homozygous or compound-heterozygous GPNMB mutations detected in nine individuals. Family/individual numbers are underlined, and predicted translational changes are indicated. Red boxes present deleted bases.
Truncating Mutations in GPNMB Cause ACD
We then performed whole-exome sequencing for four affected individuals (II-3, II-4, II-5, and II-7), two healthy individuals (II-2 and II-6), and two parents (I-1 and I-2) in family A to identify the underlying genetic cause of ACD in this family. We generated an average of 5.4 Gb of sequence mapping to the target exome per individual, resulting in an average coverage depth of 86× across the target interval. We applied a filtering pathway to select homozygous and compound heterozygous nonsynonymous single-nucleotide variants/splice-site variants/coding indels that segregated with the disease (Table S2). Given that the recessive causative variant may be present as a rare SNP in the 1000 Genomes SNP database, common variants were filtered out if present in our in-house database. Two candidate genes, NIPAL2 and GPNMB, were identified and the variants were confirmed to be segregated with the disease by Sanger sequencing (Figures 1 and S2A). The variant in NIPAL2 was located in the 5′ untranslated region (UTR) of the reference sequence GenBank: NM_024759.2 and in exon 1 of the predicted GenBank: XM_011517302.2, causing a homozygous p.Pro40Leu (c.119C>T) substitution (GenBank: XP_011515604.1) (Figures S2A and S2B). Compound heterozygous variants in GPNMB were both located in exon 5 of the reference sequences GenBank: NM_001005340.1 and NM_002510.2, leading to p.Arg189∗ (c.565C>T) and p.Tyr220∗ (c.660T>G) (Figure 1). We further sequenced all exons of GPNMB isoforms and NIPAL2 isoforms in other affected individuals (individuals 6–9). All of the variants in NIPAL2 identified from individuals 6–9 were present in the dbSNP database with allele frequencies ranging from 17% to 59% in the East Asian population (Figure S2C). After sequencing of GPNMB for individuals 6–9, we confirmed the location of compound heterozygous mutations in two alternative alleles for each individual by examining the transmission of mutations from parents or by cloning and sequencing. We identified six truncating mutations predicted to cause premature termination in the NTD and PKD domains of GPNMB from all nine individuals (Table 1 and Figure 1E). Four mutations were absent among 369 ethnicity-matched controls and not seen in the ExAC browser of the Exome Aggregation Consortium. Two mutations were present with allele frequencies of 0.136% (1 in 738 alleles; ExAC East Asian 0.12%) and 0.132% (1 in 758 alleles; not seen in the ExAC browser) in ethnicity-matched controls (Table S3).
Mutant GPNMB with p.Arg189∗ Is Significantly Degraded and Mislocalized in the Cytoplasm
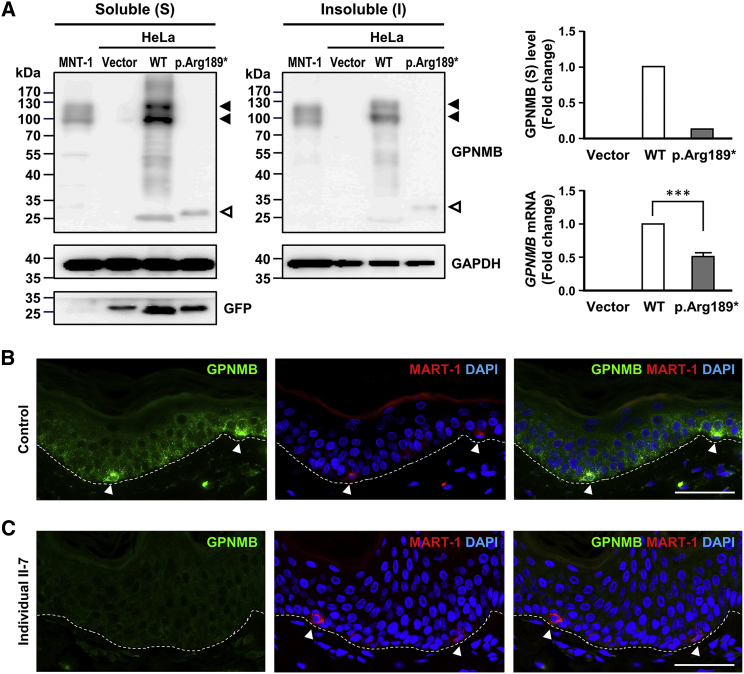
To evaluate the effects of truncating mutations in GPNMB, we transiently transfected HeLa cells with the wild-type or mutant GPNMB cDNA harboring the p.Arg189∗ mutation to examine the amount, solubility, and subcellular localization of GPNMB. A GFP plasmid was cotransfected with each of the cDNA constructs as internal controls to normalize transfection efficiency. HeLa cells with transiently expressed wild-type GPNMB showed two major bands of ∼100 and ∼120 kDa for P1 (full-length) and M (mature) forms, as detected by polyclonal anti-GPNMB antibodies, similar to the endogenous GPNMB expressed in the human melanoma cell line MNT-1 (Figure 2A). Mutant p.Arg189∗ GPNMB was expressed as a distinct band of ∼27 kDa with a dramatically decreased protein level mainly in the soluble fraction of HeLa cells, indicating that mutant GPNMB is degraded and does not form insoluble aggregates (Figure 2A). Similar results were observed for N-terminal HA-tagged wild-type and mutant p.Arg189∗ GPNMB when transiently expressed in HeLa cells (Figures S3A and S3B). Quantitative real-time PCR showed that the mutant p.Arg189∗ GPNMB transcript was detected at a significantly lower level than the wild-type transcript (Figures 2A and S3B). Transiently expressed GPNMB was reported to colocalize partially with the early endosome markers EEA (early endosome antigen) and transferrin in HeLa cells.33 Immunofluorescent staining showed that wild-type GPNMB protein with or without an HA-tag localized to discrete puncta throughout HeLa cells, partially colocalized with transferrin (Figures S3C and S4). Compared with the wild-type GPNMB, mutant p.Arg189∗ GPNMB was expressed at a much lower level, distributed primarily to a reticular network and the nuclear envelope, and colocalized with calnexin in the endoplasmic reticulum (ER), likely leading to degradation (Figures S3C and S4). The low-level expression of the mutant p.Arg189∗ GPNMB transcript and protein as compared with wild-type GPNMB suggested that the mutant p.Arg189∗ GPNMB is degraded through nonsense-mediated mRNA decay and ER-associated degradation.
Figure 2.
Effects of the p.Arg189∗ Mutation on GPNMB Expression In Vitro and In Vivo
(A) Immunoblotting analysis of transiently expressed wild-type and mutant GPNMB in HeLa cells along with human MNT-1 cells as controls. Wild-type and mutant GPNMB in soluble (S) and insoluble (I) lysates detected using anti-GPNMB antibodies were marked with solid arrows and hollow arrows, respectively. GFP was cotransfected with wild-type or mutant cDNA constructs as internal controls to normalize the transfection efficiency. GPNMB mRNA levels were determined through real-time PCR and normalized to the geometric mean of four reference genes (GAPDH, ACTB, YWHAZ, and SDHA). Quantitative PCR data are represented as the means ± SDs of three independent experiments. ∗∗∗p < 0.001.
(B and C) GPNMB expression in the epidermis of normal skin (B) and ACD lesional skin of individual II-7 in family A (C) stained with anti-GPNMB and anti-MART1 antibodies. Nuclei were counterstained with DAPI. The basement membrane is indicated with a white dashed line. MART1-positive melanocytes are marked with arrowheads. Scale bars: 50 μm.
GPNMB Is Expressed in All Epidermal Cells of Healthy Human Skin and Shows Significantly Weaker Staining in ACD Lesional Skin
GPNMB is previously reported to be expressed only in the melanocytes of the skin14 but may also be expressed in keratinocytes and Langerhans cells.16 To clarify the localization of GPNMB in the skin, we performed immunofluorescent staining with anti-GPNMB, anti-melanoma antigen recognized by T cell 1 (MART1) antibodies for detection of melanocytes and anti-langerin for Langerhans cells on normal skin biopsies from healthy control individuals. GPNMB localized to the epidermis of normal skin with a punctate pattern in all keratinocytes and melanocytes (Figure 2B), similar to the punctate pattern observed for transiently expressed wild-type GPNMB in HeLa cells (Figures S3C and S4). Melanocytes showed a stronger GPNMB signal than keratinocytes (Figure 2B) and Langerhans cells (Figure S5). Skin lesions from individuals with ACD exhibited dramatically reduced staining for GPNMB without puncta-like aggregates in all epidermal cells (Figures 2C and 3C).
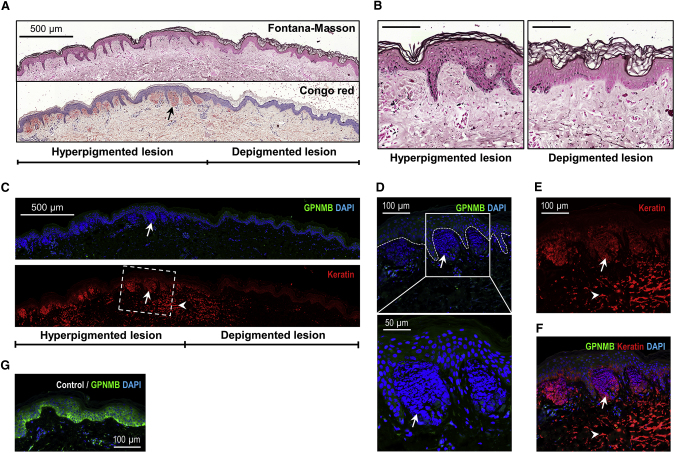
Figure 3.
Histopathological and Immunofluorescent Analyses of Amyloid Deposits
(A and B) The hyperpigmented and depigmented lesions of the severely affected individual (individual II-7) were labeled using Fontana-Masson staining. Congo red staining demonstrated the distribution of amyloid deposits in the papillary dermis. Scale bars: 500 μm (A), 100 μm (B).
(C) Representative images of lesional skin from individual II-7 showed that amyloid deposits (arrows) in the papillary dermis were strongly positive for DAPI (blue) and anti-cytokeratin antibodies (red) and were enriched in hyperpigmented lesions compared with depigmented lesions in ACD.
(D–F) The boxed area in (C) is magnified. The white dashed line represents the basement membrane, demarcating the amyloid deposits in the papillary dermis from the epidermis. No GPNMB signal was detected in the amyloid deposits compared to weak GPNMB staining in the epidermis (D). Large amounts of cytokeratin in the upper dermis are labeled with arrowheads.
(G) Strong staining of GPNMB in normal control skin.
Pathological Changes in Hyperpigmented Lesions Caused by GPNMB Mutations
Skin biopsies half covering the hyperpigmented lesion and half covering the de- or hypopigmented macule were collected from the upper arm of severely affected individual II-7 (Figure S1A) and mildly affected individual II-3 (Figure S1B) in family A. Fontana-Masson staining of hyperpigmented lesions showed hepermelanosis in the epidermis, dropping off of epidermal melanin granules into the papillary dermis and upper dermis, and melanophages as compared with the depigmented (individual II-7, Figures 3A and 3B) or hypopigmented (individual II-3, Figure S6) macules in both individuals. Congo red-positive amyloid deposits were greatly enriched in hyperpigmented lesions compared with that in depigmented (individual II-7, Figure 3A) or hypopigmented (individual II-3, Figure S6) lesions and were surrounded by elongated ridges and infiltrated immune cells. While the borders of the depigmented lesions from individual II-7 still exhibited a few amyloid deposits, the rest of the depigmented lesions showed profoundly reduced Congo red-positive amyloid deposits.
The significant enrichment of amyloid deposits in hyperpigmented lesions compared with that in de- and hypopigmented macules was further confirmed by positive immunostaining of cytokeratin in the amyloid deposits (Figure 3C for individual II-7; Figure S7 for individual II-3). Moreover, immunofluorescence staining revealed a strong accumulation of DAPI-stained DNA in amyloid deposits in the papillary dermis, completely colocalized with high-molecular-weight cytokeratin (34βE12) (Figures 3C and S7) and not detectable by hematoxylin binding to lysine residues of nuclear histones on HE staining (Figure 1C). The DNA signals in amyloid deposits were not as intact as the nuclei inside the cells, suggesting DNA debris in the amyloid deposits (Figure 3D). Large amounts of cytokeratin, not colocalized with DNA (DAPI negative), appeared to drop into the upper dermis and showed a weak Congo-red signal.
Electron micrographs of the affected individuals revealed massive amounts of fibrillar aggregates in close proximity to the epidermal-dermal junction of the hyperpigmented lesion (Figures 4A, 4B, and S8A). Similar fibrillary aggregates, albeit with lower amounts, were obvious in the borders of the depigmented lesion. Macrophages with engulfed melanosomes were detected in the papillary dermis (Figure S8B). Intracytoplasmic fibrillar aggregates were also observed in some keratinocytes of the basal and suprabasal layers (Figures 4C–4H and S8C–S8E).
Figure 4.
Representative Electromicrographs Showing Fibrillary Aggregates in the Papillary Dermis and Inside Keratinocytes
(A) Abundant round deposits of amyloid were located close to the keratinocytes (K) of basal layer (scale bars: 5 μm).
(B) Amyloid deposits (∗) in the papillary dermis exhibited fibrillary material, surrounded by collagen fibers oriented in different directions (scale bars: 1 μm).
(C and D) Similar fibrillary aggregates (∗) were located inside keratinocytes of the suprabasal layer (C) and basal layer (D) (scale bars: 2 μm).
(E–H) Boxed areas in (D) are magnified.
(E and F) Fibrillar aggregates (∗) surrounded by topofilaments (T) were seen in the cytoplasm (scale bars: 0.2 μm).
(G and H) A round amyloid deposit (∗) was located at the dermal-epidermal junction. E, epidermis (scale bars: 0.2 μm).
Desmosomes and melanosomes are labeled with arrows and arrowheads, respectively.
Significant accumulation of CD68+ macrophages was evident adjacent to and beneath amyloid deposits (Figures 5A–5D and S9 for individual II-7; Figures S10A–S10D for individual II-3). Immune cell infiltrates also contained neutrophils (Figure S10E), in addition to macrophages. The number of Langerhans cells in the epidermis of hyperpigmented lesions with immune infiltrates beneath was increased in severely affected individual II-7 (Figure 5E and 5F), whereas they showed no difference between hyperpigmented and hypopigmented lesions in mildly affected individual II-3 (Figure S10D).
Figure 5.
Accumulation of CD68-Positive Macrophages within and Surrounding Amyloid Deposits and Increased Numbers of Epidermal Langerhans Cells in a Hyperpigmented Lesion from Individual II-7 Compared with the Depigmented Macule
(A) A representative image of control skin stained with anti-CD68 antibodies (red) for macrophages and counterstained with DAPI (blue).
(B and C) Representative images of lesional skin sections from individual II-7 stained with anti-cytokeratin (red) and anti-CD68 antibodies (green) for macrophages. Amyloid deposits (arrowheads) in the papillary dermis were positive for DAPI (blue) and anti-cytokeratin antibodies (red). Immune cells, including CD68+ macrophages (arrows), within and surrounding amyloid deposits were observed in hyperpigmented lesions of ACD (B) compared with that in depigmented lesions (C) and control skin (A).
(D) Quantification of CD68+ macrophages and percentage of CD68+ area in the dermal area for 3–7 non-overlapping fields demonstrated in (A), (B), and (C) (n = 3 for controls, n = 7 for hyperpigmented lesions, and n = 5 for depigmented lesions).
(E and F) Quantification of epidermal Langerhans cells (E) and representative images of lesional skin stained with anti-langerin antibodies (green) for Langerhans cells (F). Langerhans cells were counted in four non-overlapping fields for hyperpigmented (Hyper) and depigmented (De) lesions individually. White dotted lines indicate the epidermal-dermal border.
Scale bar: 100 μm. Data (D, E) represent mean ± SD, two-tailed unpaired t test. ∗p < 0.05. ∗∗∗p < 0.001.
Comparing our histopathological, immunofluorescent, and electronic microscopic analyses of lesional skin, these data indicate that hyperpigmented lesions exhibit increased accumulation of DNA/keratin-positive amyloid deposits in papillary dermis, intracellular fibrillary aggregates in epidermal cells, and infiltrated macrophages compared with depigmented and hypopigmented macules.
Significantly Decreased Numbers of Melanocytes in Depigmented Macules
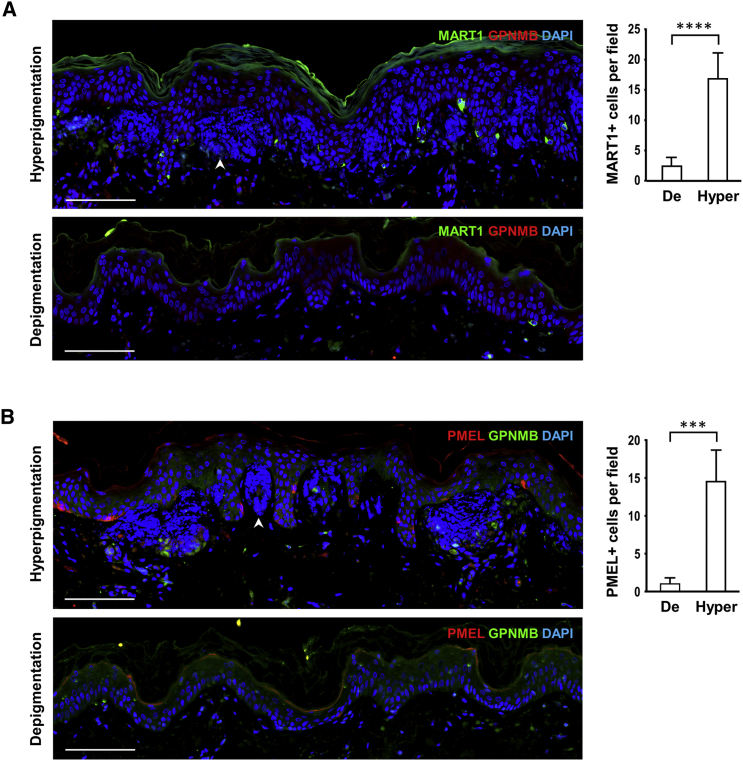
The number and distribution of melanocytes were evaluated using immunostaining with antibodies against the premelanosome proteins PMEL (HMB45) and MART1. The color difference between hyper- and hypopigmented lesions in the mildly affected individual (II-3) was reduced compared with that of severely affected individuals (Figure S1B). The hypopigmented lesion of individual II-3 exhibited decreased melanin levels and slightly reduced numbers of melanocytes compared with the hyperpigmented lesion (Figure S11), suggesting the involvement of melanocytes numbers. The melanocytes were unevenly distributed in the hypopigmented lesion, more often observed at the center of the hypopigmented macule. The depigmented macule of the severely affected individual (II-7) displayed very white skin lesion with residual melanocytes at the border of white macules and very few melanocytes in the remaining area of the white macules, indicating that white skin lesions may result from loss of melanocytes rather than reduced melanogenesis (Figures 6 and S1A).
Figure 6.
Melanocytes in Hyperpigmented Lesions and Depigmented Lesions from Severely Affected Individual II-7
(A) Representative images of lesional skin sections from individual II-7 stained with anti-MART1 antibodies (green) for melanocytes and anti-GPNMB antibodies (red). MART1+ cells were counted in ten non-overlapping fields for hyperpigmented and depigmented lesions individually.
(B) Representative images of lesional skin sections stained with anti-PMEL antibodies (red) for melanocytes and anti-GPNMB antibodies (green). PMEL+ cells were counted in four non-overlapping fields for hyperpigmented and depigmented lesions individually.
The epidermis showed weak GPNMB staining. Nuclei and amyloid deposits (arrowheads) in the papillary dermis were positive for DAPI (blue). The quantification data represent mean ± SD; two-tailed unpaired t test. ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. The numbers of positive cells were markedly decreased in depigmented lesions compared with those in hyperpigmented lesions for MART1 staining (A) and PMEL staining (B). Scale bars: 100 μm.
Discussion
In this study, we establish that compound heterozygosity or homozygosity of GPNMB truncated alleles causes ACD. Six nonsense or frameshift mutations were found, and all were predicted to cause premature termination, in which four of the mutations were absent in 369 ethnicity-matched control subjects and two were present at low allele frequencies. Here, we demonstrate that human GPNMB is expressed in all epidermal cells, with a higher expression in melanocytes than in keratinocytes. In a published transcriptome analysis of skin cells (melanocytes, keratinocytes, and fibroblasts), GPNMB was highly expressed in melanocytes and fibroblasts compared to keratinocytes,34 supporting our observations in the immunofluorescence assay. Moreover, we showed that truncated GPNMB was significantly degraded and mislocalized intracellularly in vitro and in ACD lesional skin. Skin lesions of affected individuals revealed significantly weaker or faint staining for GPNMB as compared with strong expression of GPNMB in normal skin, indicating that loss-of-function mutations in GPNMB cause autosomal-recessive ACD. Mice with homozygous nonsense mutations in Gpnmb (Gpnmb p.Arg150∗) exhibit iris pigment dispersion and compromising ocular immunosuppression in mouse pigmentary glaucoma harboring mutations in Gpnmb and Tyrp1.17, 35 Tyrp1 mutations contribute to the iris stromal atrophy phenotype in mutant mice. The onset in Gpnmb mutant mice without Tyrp1 deficiency is delayed, with early signs of iris pigment dispersion at 16 months.17 The difference of phenotype between human and mouse may be partly due to mouse melanocytes localized in the hair follicles, not in the interfollicular epidermis of mouse skin. GPNMB contributes to the differentiation and function of osteoblasts36 and osteoclasts37, 38 and affects bone mass and bone formation.39, 40 In models of liver fibrosis, colitis, and kidney ischemia, GPNMB-deficient mice showed impaired tissue repair as discussed in the Introduction. In this study, routine health examinations showed that lesions and amyloidosis were restricted to the skin. None of the nine individuals with biallelic GPNMB truncating mutations and detailed medical records available had abnormalities in the eyes, bones, or other tissues. The disease phenotype in mice models might be observed only under stress conditions. Some of the functions of GPNMB were investigated in mice and might not be relevant in humans. Although no causal association of GPNMB and other diseases, including iris pigment dispersion and glaucoma, in human has been reported, further follow-up is recommended for our individuals with ACD when they are older.
Human GPNMB is composed of 560 amino acids (GenBank: NP_002501.1) and is N-glycosylated in the endoplasmic reticulum to produce the ∼100-kDa P1 form. These N-glycans are further modified to produce the ∼115-kDa mature form (M) in the Golgi.14 The domains of GPNMB are illustrated in Figure 1E. GPNMB can also be secreted by ectodomain shedding from the mature, Golgi-modified form of GPNMB.14 GPNMB shows highly sequence homology to PMEL (also known as PMEL17, SILV, and gp100), a melanosomal structural protein constituting amyloid fibrillar sheets in the pre-melanosome after proteolytic maturation of PMEL.41, 42 PMEL fibrils are required for the formation of the pre-melanosome and serve as a template upon which eumelanin are synthesized and deposited. When expressed in Escherichia coli, the N-terminal GPNMB fragment with the NTR-PKD domain becomes insoluble and forms amyloids in vitro.33 However, GPNMB shows different intracellular localization from amyloidogenic PMEL and exhibits no fibril formation owing to the extensive glycosylation of the GPNMB PKD domain.33 In this study, we demonstrated that the truncated p.Arg189∗ GPNMB carrying a large portion of the NTR domain still remained soluble and was significantly degraded when transiently expressed in HeLa cells and in ACD lesional skin. No GPNMB signal was detected in amyloid deposits of ACD skin. Recent reports demonstrated that the amyloid deposits of PLCA comprised keratins, serum amyloid P component, apolipoprotein E, galectin-7, and actin;43 of these, the galectin-7 peptides were found to contain the β strand structure and were amyloidogenic in vitro.44 Galectin-7 fragments from degenerated keratinocytes may contribute to amyloidogenesis in PLCA. However, galectin-7 was not detected in the amyloid deposits in ACD skin (Figure S12). Galectin-7 was mainly localized in the nucleus of keratinocytes in ACD lesional skin compared with both the cytoplasmic and nuclear distribution in keratinocytes of control skin, suggesting that epidermal homeostasis and skin repair may be affected. Although weak staining of galectin-7 was observed in the amyloid deposits in a few regions, it coincided with an increase in cytoplasmic galectin-7 levels in the epidermis (Figure S12D).We found that amyloid deposits contained high amounts of DNA in our affected individuals, in addition to cytokeratin (anti-34βE12). Intriguingly, amyloid peptides have been widely reported to bind DNA in Alzheimer disease.45, 46 Soluble amyloid precursors are able to interact with nucleic acids and convert to amyloid complexes with nucleic acids,47, 48 suggesting the possibility of the conversion of amyloid precursors, such as cytokeratin, from an α helix to β sheet, in ACD.
The coexistence of inflammatory infiltrates, hyperpigmentation, and enriched amyloid deposits suggests the causative relationship between hypermelanosis, amyloidosis, and increased or chronic inflammation in ACD lesional skin. The relation between amyloidosis and inflammation can be bi-directional. In Alzheimer disease, the intracellular and extracellular accumulation of Aβ fibrils causes activation of microglia secreting high levels of pro-inflammatory cytokines,49, 50 further leading to enhancement of Aβ precursor synthesis, amyloidosis, and neuronal damage.51, 52, 53 Chronic inflammation is involved in promoting and accelerating amyloidosis of certain diseases such as familial Mediterranean fever (MIM: 249100), tumor necrosis factor receptor-associated periodic syndrome (MIM: 142680), and Alzheimer disease.54, 55, 56 The causal role of amyloidosis, inflammation, and hypermelanosis in ACD needs to be further established. GPNMB is highly expressed in melanocytes. Loss-of-function truncating mutations in GPNMB resulted in loss of melanocytes in depigmented lesions and slightly decreased numbers of melanocytes in the hypopigmented region. Hyperpigmentation in ACD may due to hypermelanosis, increased numbers of melanocytes, and accumulation of melanin granules/melanosomes in the papillary dermis. Fibrillary amyloid deposits in papillary dermis and accumulation of fibrillary aggregates in epidermal keratinocytes were observed in ACD lesional skin. Amyloid deposits are enriched in the hyperpigmented lesion and contain keratins and DNA, suggesting that degeneration and death of keratinocyte contribute to the formation of amyloid deposits. Thus, GPNMB plays a crucial role in both melanocytes and keratinocytes. It is also possible that GPNMB has a signaling function from melanocytes to keratinocytes.
In summary, our data showed that loss-of-function mutations in GPNMB cause autosomal-recessive ACD. GPNMB was found to have critical roles in melanosome formation, autophagy/phagocytosis, clearance of apoptotic cell debris, and negative regulation of inflammation, making it associated with ACD. The pigment dyschromia and amyloidosis in ACD caused by GPNMB mutations potentially relate to increased or chronic inflammatory responses, misfolded protein aggregates, reduced clearance of cellular debris and protein aggregates, keratinocyte degeneration, and melanocyte survival. Our study expands our understanding to the etiology of amyloidosis and pigment dyschromia in ACD.
Acknowledgments
The authors thank the families and control individuals for their cooperation in this study. This work was supported by Academia Sinica Genomic Medicine Multicenter Study (40-05-GMM), Taiwan. We gratefully acknowledge the support from the National Center for Genome Medicine (NSC106-2319-B-001-001) of the National Core Facility Program for Biotechnology, Ministry of Science and Technology.
Published: January 11, 2018
Footnotes
Supplemental Data include twelve figures and three tables and can be found with this article online at https://doi.org/10.1016/j.ajhg.2017.12.012.
Web Resources
ExAC Browser, http://exac.broadinstitute.org/
gnomAD Browser, http://gnomad.broadinstitute.org/
OMIM, http://www.omim.org/
Variant Effect Predictor, http://useast.ensembl.org/Homo_sapiens/Tools/VEP
VarioWatch, http://grch37.genepipe.ncgm.sinica.edu.tw/variowatch/main.do
Supplemental Data
References
- 1.Mereuta O.M., Dogan A. Cutaneous amyloidosis. In: Picken M.M., Herrera G.A., Dogan A., editors. Amyloid and Related Disorders. Springer; 2015. pp. 469–479. [Google Scholar]
- 2.Lin M.-W., Lee D.-D., Liu T.-T., Lin Y.-F., Chen S.-Y., Huang C.-C., Weng H.-Y., Liu Y.-F., Tanaka A., Arita K. Novel IL31RA gene mutation and ancestral OSMR mutant allele in familial primary cutaneous amyloidosis. Eur. J. Hum. Genet. 2010;18:26–32. doi: 10.1038/ejhg.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arita K., South A.P., Hans-Filho G., Sakuma T.H., Lai-Cheong J., Clements S., Odashiro M., Odashiro D.N., Hans-Neto G., Hans N.R. Oncostatin M receptor-β mutations underlie familial primary localized cutaneous amyloidosis. Am. J. Hum. Genet. 2008;82:73–80. doi: 10.1016/j.ajhg.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahon C., Oliver F., Purvis D., Agnew K. Amyloidosis cutis dyschromia in two siblings and review of the epidemiology, clinical features and management in 48 cases. Australas. J. Dermatol. 2015 doi: 10.1111/ajd.12342. Published online April 12, 2015. 10.1111/ajd.12342. [DOI] [PubMed] [Google Scholar]
- 5.Moriwaki S., Nishigori C., Horiguchi Y., Imamura S., Toda K., Takebe H. Amyloidosis cutis dyschromica. DNA repair reduction in the cellular response to UV light. Arch. Dermatol. 1992;128:966–970. doi: 10.1001/archderm.128.7.966. [DOI] [PubMed] [Google Scholar]
- 6.Ozcan A., Senol M., Aydin N.E., Karaca S. Amyloidosis cutis dyschromica: a case treated with acitretin. J. Dermatol. 2005;32:474–477. doi: 10.1111/j.1346-8138.2005.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes N.F., Mercer S.E., Kleinerman R., Lebwohl M.G., Phelps R.G. Amyloidosis cutis dyschromica associated with atypical Parkinsonism, spasticity and motor weakness in a Pakistani female. J. Cutan. Pathol. 2011;38:827–831. doi: 10.1111/j.1600-0560.2011.01719.x. [DOI] [PubMed] [Google Scholar]
- 8.Qiao J., Fang H., Yao H. Amyloidosis cutis dyschromica. Orphanet J. Rare Dis. 2012;7:95. doi: 10.1186/1750-1172-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weterman M.A., Ajubi N., van Dinter I.M., Degen W.G., van Muijen G.N., Ruitter D.J., Bloemers H.P. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int. J. Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- 10.Safadi F.F., Xu J., Smock S.L., Rico M.C., Owen T.A., Popoff S.N. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J. Cell. Biochem. 2001;84:12–26. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- 11.Shikano S., Bonkobara M., Zukas P.K., Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- 12.Bandari P.S., Qian J., Yehia G., Joshi D.D., Maloof P.B., Potian J., Oh H.S., Gascon P., Harrison J.S., Rameshwar P. Hematopoietic growth factor inducible neurokinin-1 type: a transmembrane protein that is similar to neurokinin 1 interacts with substance P. Regul. Pept. 2003;111:169–178. doi: 10.1016/s0167-0115(02)00288-4. [DOI] [PubMed] [Google Scholar]
- 13.Turque N., Denhez F., Martin P., Planque N., Bailly M., Bègue A., Stéhelin D., Saule S. Characterization of a new melanocyte-specific gene (QNR-71) expressed in v-myc-transformed quail neuroretina. EMBO J. 1996;15:3338–3350. [PMC free article] [PubMed] [Google Scholar]
- 14.Hoashi T., Sato S., Yamaguchi Y., Passeron T., Tamaki K., Hearing V.J. Glycoprotein nonmetastatic melanoma protein b, a melanocytic cell marker, is a melanosome-specific and proteolytically released protein. FASEB J. 2010;24:1616–1629. doi: 10.1096/fj.09-151019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P., Liu W., Zhu C., Yuan X., Li D., Gu W., Ma H., Xie X., Gao T. Silencing of GPNMB by siRNA inhibits the formation of melanosomes in melanocytes in a MITF-independent fashion. PLoS ONE. 2012;7:e42955. doi: 10.1371/journal.pone.0042955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomihari M., Hwang S.H., Chung J.S., Cruz P.D., Jr., Ariizumi K. Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion. Exp. Dermatol. 2009;18:586–595. doi: 10.1111/j.1600-0625.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson M.G., Smith R.S., Hawes N.L., Zabaleta A., Chang B., Wiggs J.L., John S.W. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat. Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- 18.Anderson M.G., Libby R.T., Mao M., Cosma I.M., Wilson L.A., Smith R.S., John S.W. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006;4:20. doi: 10.1186/1741-7007-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch S., Yanagi G., DelBono E., Wiggs J.L. DNA sequence variants in the tyrosinase-related protein 1 (TYRP1) gene are not associated with human pigmentary glaucoma. Mol. Vis. 2002;8:127–129. [PubMed] [Google Scholar]
- 20.Lascaratos G., Shah A., Garway-Heath D.F. The genetics of pigment dispersion syndrome and pigmentary glaucoma. Surv. Ophthalmol. 2013;58:164–175. doi: 10.1016/j.survophthal.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Ripoll V.M., Irvine K.M., Ravasi T., Sweet M.J., Hume D.A. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J. Immunol. 2007;178:6557–6566. doi: 10.4049/jimmunol.178.10.6557. [DOI] [PubMed] [Google Scholar]
- 22.Chung J.S., Dougherty I., Cruz P.D., Jr., Ariizumi K. Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J. Immunol. 2007;179:5778–5784. doi: 10.4049/jimmunol.179.9.5778. [DOI] [PubMed] [Google Scholar]
- 23.Chung J.S., Sato K., Dougherty I.I., Cruz P.D., Jr., Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung J.-S., Tamura K., Cruz P.D., Jr., Ariizumi K. DC-HIL-expressing myelomonocytic cells are critical promoters of melanoma growth. J. Invest. Dermatol. 2014;134:2784–2794. doi: 10.1038/jid.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamphuis W., Kooijman L., Schetters S., Orre M., Hol E.M. Transcriptional profiling of CD11c-positive microglia accumulating around amyloid plaques in a mouse model for Alzheimer’s disease. Biochim. Biophys. Acta. 2016;1862:1847–1860. doi: 10.1016/j.bbadis.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Li B., Castano A.P., Hudson T.E., Nowlin B.T., Lin S.L., Bonventre J.V., Swanson K.D., Duffield J.S. The melanoma-associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J. 2010;24:4767–4781. doi: 10.1096/fj.10-154757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel-Chamberlin M., Wang Y., Satirapoj B., Phillips L.M., Nast C.C., Dai T., Watkins R.A., Wu X., Natarajan R., Leng A. Hematopoietic growth factor inducible neurokinin-1 (Gpnmb/Osteoactivin) is a biomarker of progressive renal injury across species. Kidney Int. 2011;79:1138–1148. doi: 10.1038/ki.2011.28. [DOI] [PubMed] [Google Scholar]
- 28.Kumagai K., Tabu K., Sasaki F., Takami Y., Morinaga Y., Mawatari S., Hashimoto S., Tanoue S., Kanmura S., Tamai T. Glycoprotein nonmetastatic melanoma B (Gpnmb)-positive macrophages contribute to the balance between fibrosis and fibrolysis during the repair of acute liver injury in mice. PLoS ONE. 2015;10:e0143413. doi: 10.1371/journal.pone.0143413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki F., Kumagai K., Uto H., Takami Y., Kure T., Tabu K., Nasu Y., Hashimoto S., Kanmura S., Numata M. Expression of glycoprotein nonmetastatic melanoma protein B in macrophages infiltrating injured mucosa is associated with the severity of experimental colitis in mice. Mol. Med. Rep. 2015;12:7503–7511. doi: 10.3892/mmr.2015.4408. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L., Zhuo H., Ouyang H., Liu Y., Yuan F., Sun L., Liu F., Liu H. Glycoprotein non-metastatic melanoma protein b (Gpnmb) is highly expressed in macrophages of acute injured kidney and promotes M2 macrophages polarization. Cell. Immunol. 2017;316:53–60. doi: 10.1016/j.cellimm.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W.H., Wu C.Y., Yu C.P., Chiang C.P. Amyloidosis cutis dyschromica: four cases from two families. Int. J. Dermatol. 2009;48:518–521. doi: 10.1111/j.1365-4632.2009.03844.x. [DOI] [PubMed] [Google Scholar]
- 33.Theos A.C., Watt B., Harper D.C., Janczura K.J., Theos S.C., Herman K.E., Marks M.S. The PKD domain distinguishes the trafficking and amyloidogenic properties of the pigment cell protein PMEL and its homologue GPNMB. Pigment Cell Melanoma Res. 2013;26:470–486. doi: 10.1111/pcmr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reemann P., Reimann E., Ilmjärv S., Porosaar O., Silm H., Jaks V., Vasar E., Kingo K., Kõks S. Melanocytes in the skin--comparative whole transcriptome analysis of main skin cell types. PLoS ONE. 2014;9:e115717. doi: 10.1371/journal.pone.0115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo J.S., Anderson M.G., Gregory M., Smith R.S., Savinova O.V., Serreze D.V., Ksander B.R., Streilein J.W., John S.W. By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. J. Exp. Med. 2003;197:1335–1344. doi: 10.1084/jem.20022041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdelmagid S.M., Barbe M.F., Rico M.C., Salihoglu S., Arango-Hisijara I., Selim A.H., Anderson M.G., Owen T.A., Popoff S.N., Safadi F.F. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function. Exp. Cell Res. 2008;314:2334–2351. doi: 10.1016/j.yexcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Ripoll V.M., Meadows N.A., Raggatt L.J., Chang M.K., Pettit A.R., Cassady A.I., Hume D.A. Microphthalmia transcription factor regulates the expression of the novel osteoclast factor GPNMB. Gene. 2008;413:32–41. doi: 10.1016/j.gene.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Sheng M.H., Wergedal J.E., Mohan S., Amoui M., Baylink D.J., Lau K.H. Targeted overexpression of osteoactivin in cells of osteoclastic lineage promotes osteoclastic resorption and bone loss in mice. PLoS ONE. 2012;7:e35280. doi: 10.1371/journal.pone.0035280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdelmagid S.M., Belcher J.Y., Moussa F.M., Lababidi S.L., Sondag G.R., Novak K.M., Sanyurah A.S., Frara N.A., Razmpour R., Del Carpio-Cano F.E., Safadi F.F. Mutation in osteoactivin decreases bone formation in vivo and osteoblast differentiation in vitro. Am. J. Pathol. 2014;184:697–713. doi: 10.1016/j.ajpath.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frara N., Abdelmagid S.M., Sondag G.R., Moussa F.M., Yingling V.R., Owen T.A., Popoff S.N., Barbe M.F., Safadi F.F. Transgenic expression of osteoactivin/gpnmb enhances bone formation in vivo and osteoprogenitor differentiation ex vivo. J. Cell. Physiol. 2016;231:72–83. doi: 10.1002/jcp.25020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fowler D.M., Koulov A.V., Alory-Jost C., Marks M.S., Balch W.E., Kelly J.W. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watt B., van Niel G., Raposo G., Marks M.S. PMEL: a pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res. 2013;26:300–315. doi: 10.1111/pcmr.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miura Y., Harumiya S., Ono K., Fujimoto E., Akiyama M., Fujii N., Kawano H., Wachi H., Tajima S. Galectin-7 and actin are components of amyloid deposit of localized cutaneous amyloidosis. Exp. Dermatol. 2013;22:36–40. doi: 10.1111/exd.12065. [DOI] [PubMed] [Google Scholar]
- 44.Ono K., Fujimoto E., Fujimoto N., Akiyama M., Satoh T., Maeda H., Fujii N., Tajima S. In vitro amyloidogenic peptides of galectin-7: possible mechanism of amyloidogenesis of primary localized cutaneous amyloidosis. J. Biol. Chem. 2014;289:29195–29207. doi: 10.1074/jbc.M114.592998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrantes A., Rejas M.T., Benítez M.J., Jiménez J.S. Interaction between Alzheimer’s Abeta1-42 peptide and DNA detected by surface plasmon resonance. J. Alzheimers Dis. 2007;12:345–355. doi: 10.3233/jad-2007-12408. [DOI] [PubMed] [Google Scholar]
- 46.Camero S., Ayuso J.M., Barrantes A., Benítez M.J., Jiménez J.S. Specific binding of DNA to aggregated forms of Alzheimer’s disease amyloid peptides. Int. J. Biol. Macromol. 2013;55:201–206. doi: 10.1016/j.ijbiomac.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Di Domizio J., Zhang R., Stagg L.J., Gagea M., Zhuo M., Ladbury J.E., Cao W. Binding with nucleic acids or glycosaminoglycans converts soluble protein oligomers to amyloid. J. Biol. Chem. 2012;287:736–747. doi: 10.1074/jbc.M111.238477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Domizio J., Dorta-Estremera S., Gagea M., Ganguly D., Meller S., Li P., Zhao B., Tan F.K., Bi L., Gilliet M., Cao W. Nucleic acid-containing amyloid fibrils potently induce type I interferon and stimulate systemic autoimmunity. Proc. Natl. Acad. Sci. USA. 2012;109:14550–14555. doi: 10.1073/pnas.1206923109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sondag C.M., Dhawan G., Combs C.K. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J. Neuroinflammation. 2009;6:1. doi: 10.1186/1742-2094-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhawan G., Floden A.M., Combs C.K. Amyloid-β oligomers stimulate microglia through a tyrosine kinase dependent mechanism. Neurobiol. Aging. 2012;33:2247–2261. doi: 10.1016/j.neurobiolaging.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eikelenboom P., Bate C., Van Gool W.A., Hoozemans J.J., Rozemuller J.M., Veerhuis R., Williams A. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40:232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- 52.Lee J.W., Lee Y.K., Yuk D.Y., Choi D.Y., Ban S.B., Oh K.W., Hong J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doens D., Fernández P.L. Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis. J. Neuroinflammation. 2014;11:48. doi: 10.1186/1742-2094-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demirkaya E., Erer B., Ozen S., Ben-Chetrit E. Efficacy and safety of treatments in Familial Mediterranean fever: a systematic review. Rheumatol. Int. 2016;36:325–331. doi: 10.1007/s00296-015-3408-9. [DOI] [PubMed] [Google Scholar]
- 55.Cantarini L., Lucherini O.M., Muscari I., Frediani B., Galeazzi M., Brizi M.G., Simonini G., Cimaz R. Tumour necrosis factor receptor-associated periodic syndrome (TRAPS): state of the art and future perspectives. Autoimmun. Rev. 2012;12:38–43. doi: 10.1016/j.autrev.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 56.Philippens I.H., Ormel P.R., Baarends G., Johansson M., Remarque E.J., Doverskog M. Acceleration of amyloidosis by inflammation in the amyloid-beta marmoset monkey model of Alzheimer’s disease. J. Alzheimers Dis. 2017;55:101–113. doi: 10.3233/JAD-160673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.