Abstract
Our study attempted to verify the effect of lncRNA BST2 interferon-stimulated positive regulator (BISPR) on cell viability, propagation and invasiveness of thyroid papillary carcinoma (TPC) and the interactive relationship between BISPR and miR-21-5p. Microarray analyzed the aberrant expression lncRNA BISPR in TPC. BISPR and miR-21-5p as well as B-cell lymphoma-2 (Bcl-2) expressions in TPC cells were determined by quantitative polymerase chain reaction (qRT-PCR) and Western blot. Cell counting kit-8 (CCK-8) assay, dual luciferase reporter assay, and transwell assay were conducted to manifest cell viability, propagation, and invasiveness of TPC cells. Flow cytometry was performed to determine the apoptosis and cell cycle of TPC cells. Mouse xenograft model was built to testify the effect of BISPR on tumor growth. BISPR in TPC tissues was over-expressed. BISPR knockdown restrained the propagation and invasiveness and enhanced the iodine uptake of TPC cells. The tumor-forming rate reduced after BISPR knockdown. In addition, miR-21-5p was lowly expressed in cancer tissues. BISPR promoted the development of TPC cells by inhibiting miR-21-5p expression. Bcl-2 was suppressed by miR-21-5p and sh-BISPR. BISPR, which was over-expressed in TPC, improved TPC cell viability, propagation, and invasiveness. MiR-21-5p was lowly expressed in TPC which inhibited Bcl-2 expression. BISPR stimulated propagation and invasiveness of TPC cells by depressing miR-21-5p.
Keywords: Bcl-2, BISPR, miR-21-5p, thyroid papillary carcinoma
Introduction
Thyroid cancer (TC), the most common carcinoma of the endocrine organs with enormous heterogeneity, has shown steadily increasing morbidity in recent decades worldwide.1 Thyroid papillary carcinoma (TPC) accounts for approximately 80%–85% of all TC in adults.2 It typically involves an indolent tumor related with a favorable prognosis and therapeutic response.3 Before tumor-cell dissemination, 5-year survival rate was over 95% after comprehensive therapy, such as thyroidectomy, radioactive iodine (RAI), and thyroid-stimulating hormone (TSH) suppression therapy.4 Nevertheless, metastasis of TPC resulted in high recurrence.5 Therefore, it is imperative now to investigate the molecular mechanisms that underlie TPC.
Long noncoding RNAs (lncRNAs) are a subset of transcripts that are more than 200 nucleotides in length and have limited protein-coding potential.6 It is reported that only 2% of human genome is transcribed into mRNAs (messenger RNAs), while 80%–90% of that is transcribed into lncRNAs.7 Increasing evidence showed that lncRNAs were involved in the progression of TPC. LncRNA GAS8-AS1 was found to be the second most frequently mutated gene in patients with TPC.8 Recently, mounting evidence reveals that lncRNAs have multiple functions in tumors over a variety of biological processes, such as cell cycle arrest, apoptosis, and metastasis.9 Previous studies found that lncRNA PTCSC3 inhibited progression of glioma cells and suppressed Wnt/β-catenin signaling pathway.10 LncRNA BST2 interferon-stimulated positive regulator (BISPR) is a promoter-sharing lncRNA of BST2. Overexpression of BISPR resulted in upregulation of BST2;11 therefore, lncBST2 has been renamed BISPR.12 BST2 was proved to promote production of immune-inflammatory mediators13 but may also promote tumorigenesis.14 There have been several researches focusing on the molecular mechanism of BST2 underlying in human cancers. For instance, BST2 promoted cell growth in renal cell carcinoma15 and oral cavity cancer.16 However, few studies reported the biological function of BISPR in TPC.
MicroRNAs (miRNAs) are a class of small, noncoding single-strand RNAs that can regulate the expression of multiple protein-coding genes at the post-transcriptional level by binding to the 3′-untranslated region (3′-UTR) of their target mRNAs (messenger RNAs).17 MiRNA expression profiling in human cancers has revealed signatures that are closely related with the diagnosis, staging, progression, and so on to therapies.18 In particular, miR-21-5p is an oncogenic miRNA that is over-expressed in many solid tumors.19 Previous studies found that miR-21 was up-regulated in TPC cells.20 Dysregulation of miR-21 was associated with BRAFV600E in TPC cells.21 MiR-21-5p down-regulated some tumor suppressors, such as Phosphatase and tensin homolog (PTEN) and B-cell lymphoma-2 (Bcl-2) as an anti-apoptotic gene in various human cancers.22 B-cell lymphoma 2 (Bcl-2) is one of the downstream proteins of miR-21-5p. As an oncogene, Bcl-2 was implicated in cell metastasis and invasion. Previous studies verified that Bcl-2 was expressed in most TC, including TPC, while only a small proportion of undifferentiated TC showed Bcl-2 expression.23 However, the role of miR-21-5p on regulating Bcl-2 in TPC remains unclear.
In this study, we tended to find the impact of lncRNA BISPR on the development of TPC as well as to explore the direct correlation of miR-21-5p and its downstream protein Bcl-2 and their role in TPC cells. Therefore, BISPR and miR-21-5p might be potential important therapeutic targets for TPC treatment.
Materials and methods
Tissue samples
The tissue samples from 14 patients with TPC were provided by Sun Yat-sen Memorial Hospital. There was no radiotherapy or chemotherapy prior to the surgery. All samples were immediately frozen in liquid nitrogen after surgery. The Clinical Ethics Committee of Sun Yat-sen Memorial Hospital approved all aspects. Informed consent was taken from all subjects.
Cell culture
Human TPC cell lines BHT101 and Hth83 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; HyClone, Logan, UT, USA) together with 10% fetal bovine serum (FBS; HyClone), 100 U/mL penicillin, and 100 μg/mL streptomycin.
Cell transfection
BLOCK-iT™ RNAi Designer software (Invitrogen, USA) was used to design nucleotide sequences and to synthesize extraneous nucleic acid sequence with same base groups as negative control (corresponding sequences were listed in Table 1). PRI-sh-BISPR and pRI-sh-NC (Inovogen, Beijing, China) were extracted and sequencing identified. The adherent cells were transfected using Lipofectamine™ 2000 (Invitrogen). The efficiency of transfection was observed after being transfected for 24 and 48 h.
Table 1.
shRNA sequences.
| Name | Sequences (5′-3′) |
|---|---|
| sh-BISPR | |
| Top strand | CACCGTATTTCTCTGTAACCATTTCGAAAAATGGTTACAGAGAAATA |
| Bottom strand | AAAATATTTCTCTGTAACCATTTTTCGAAATGGTTACAGAGAAATAC |
| sh-NC | |
| Top strand | CACCGTATTCCTCTGTGACCATTTCGAATCATGGTTATAGAGAAATA |
| Bottom strand | AAAATATTTCTCTATAACAATCCTTCGAAATGGTCACAGAGGAATAC |
sh-BISPR: short hairpin BST2 interferon-stimulated positive regulator; sh-NC: short hairpin negative control.
Microarray analysis
Total RNA for microarray analyses were prepared from BHT101 and Hth83 cell lines. Affymetrix Human Genome U133 Plus 2.0 Array (HG-U133_Plus_2) was used to analyze gene expression. MiRNA expression was analyzed in RNA samples obtained by Real Total microRNA kit (Real, Valencia, Spain). Labeling, hybridization, and detection were carried out in each case following the recommendations of the manufacturer.
Cell RNA extraction and qRT-PCR
TRIzol™ (Invitrogen) was added to the cells to extract total RNA. These RNA samples were reverse transcribed into cDNAs using PrimeScript® 1st Strand Synthesis Kit (TaKaRa, Japan). Quantitative polymerase chain reaction (QPCR) analysis was conducted by QuantiTect SYBR® Green RT-PCR Kit (QIAGEN, Germany). The primer sequences were listed in Table 2. PCR results were calculated by 2−ΔΔCTmethod.
Table 2.
qRT-PCR primer sequences.
| Name | Sequences (5′-3′) |
|---|---|
| BISPR | |
| Forward primer | GCCAACAAACAATGTCGGTCT |
| Reverse primer | CAGAGACACAGATGCTGCCTAA |
| miR-21-5p | |
| Forward primer | GACTGATGTTGATGTCGGGT |
| Reverse primer | GTCAGACAGCCCATCGACT |
| Bcl-2 | |
| Forward primer | TCTCATGCCAAGGGGGAAAC |
| Reverse primer | CCGGTTATCGTACCCCGTTC |
| GAPDH | |
| Forward primer | AATCCCATCACCATCTTCC |
| Reverse primer | CATCACGCCACAGTTTCC |
BISPR: BST2 interferon-stimulated positive regulator; Bcl-2: B-cell lymphoma-2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Western blot
Cells were harvested and then lysed via radioimmunoprecipitation assay buffer (RIPA buffer, Thermo Fisher Scientific, MA, USA) to obtain proteins. Subsequently, proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene fluoride (PVDF) membranes (Invitrogen). The membranes were incubated with primary antibodies Bcl-2 antibody (rabbit-anti-human, 1:800, R&D Systems, Minneapolis, USA) and glyceraldehyde 3-phosphate dehydrogenase (GADPH) antibody (rabbit-anti-human, 1:800; R&D Systems) overnight, followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:800; SinBio, China). Detection was performed by enhanced chemiluminescence kit (Amersham, Little Chalfont, UK).
Cell counting kit-8 assay
Cells were seeded into 96-well plates (2 × 103cells per well). After culture for 24, 48, 72, and 96 h, each well was supplemented with 10 μL cell counting kit-8 (CCK-8) solutions (Beyotime, Institute of Biotechnology, China) and incubated for 2 h. The absorbance was measured at 450 nm wave length.
Dual luciferase reporter assay
Plasmid pmirGLO was purchased from Promega Corporation (Madison, WI, USA). BHT101 and Hth83 cell lines were seeded into 24-well plates. pmirGLO-BISPR-wt, pmirGLO-BISPR-mut, pmirGLO-Bcl-2 wt, pmirGLO-Bcl-2-mut, miR-21-5p mimics, and miR-21-5p inhibitor (GenePharma, Shanghai, China) were constructed and co-transfected into BHT101 and Hth83 cell lines using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA) for 48 h. Subsequently, dual luciferase reporter assay system kit (Promega Corporation) was applied to examine the luciferase activity.
RNA pull down assay
Cell lysis buffer of 1 mL was added into the cell culture vessel, and the scraped cells were placed in a 1.5-mL Eppendorf tube. The cells were frozen and melted by liquid nitrogen repeatedly for three times, and the supernatant was collected by centrifuging which was added with RNase inhibitor. Bio-miR-21-5p probe was blended with beads and shook by a shaker for 20 min at room temperature. Beads, after washed, were mixed with cell lysis liquid and shook by the shaker for 2 h at 4°C. Thirty micro liters of cold 0.1% SDS buffer liquid was added into the beads and heated for 2 min at 95°C. After that, the supernatant was collected and added into the above wells to perform electrophoresis. The different stripes after staining were taken for sequencing analysis.
Transwell assay
The upper chambers of transwell inserts (8 μM pore-sized, Nalge Nunc Intl, NY, USA) were coated with Matrigel (BD Biosciences, San Jose, CA, USA) for invasion assay. The subsequent experiments were conducted as the protocols described. After 24 h, cells were fixed using 4% paraformaldehyde, and permeabilized with methanol. After removing these cells still in the upper chambers, the cells in lower chambers were stained with 0.1% crystal violet dye and washed by phosphate-buffered saline (PBS; Invitrogen).
Iodine uptake assay in vitro
Cells were seeded into 24-well plates (5 × 104 cells per well) and incubated overnight. After rinsing with 1-mL Hank’s balanced salt solution (HBSS), cells were added with 1-mL HBSS containing 0.1 μCi Na125I and 1 μmol/L NaI. The radioactivity was measured by γ-ray counter (SN-682, Rihuan Equipment Factory of Shanghai Nucleus Research Institute, Chinese Academy of Sciences).
Apoptosis assay
Cells were collected in a 15 mL centrifuge tube and washed with 1 × binding buffer. Then the cells were re-suspended with 200 μL 1 × binding buffer and 5 μL Annexin V and incubated in darkness at room temperature for 30 min. Each tube was added with 200 μL 1 × binding buffer and 5 μL PI and cultured in darkness for 15 min. Flow cytometer was used to examine the proportion of cells in each quadrant.
Cell cycle analysis
After cells were re-suspended by PBS (Invitrogen), 75% precooled ethanol was added drop by drop and fixed at −20°C for over 24 h. After the ethanol was abandoned, the cells were re-suspended by 10 μL 100 U/mL RNase A. In terms of the cell distribution with different DNA contents, the above sample was examined by flow cytometer. The percentages of each cell cycle were matched, and the differences of cell cycle between each group were calculated.
Immunohistochemistry
The pathological sections embedded with paraffin were deparaffinized and dehydrated by gradient ethanol. Endogenous peroxidase was removed and the antigen was restored. Ki67 (100 μL; rabbit-anti-human, 1:800, Abcam, Cambridge, MA, USA) and Bcl-2 (rabbit-anti-human, 1:800, Abcam) antibodies were added and cultured overnight. Samples were cultured with HRP-conjugated goat anti-rabbit IgG secondary antibody (1:800, SinBio) for 40 min. After coloration with 3, 3-diaminobenzidine (DAB) and counterstaining with hematoxylin for 1 min, the samples were dehydrated by gradient ethanol and sealed. The protein levels were evaluated using semi quantitative method. The total score = staining intensity score + staining range score (the proportion of positive cells in cytomembrane and cytoplasm). If the total score difference of two samples was >100, the expression level was considered to have significant change.
Mouse xenograft model
Eight female nude mice (4 weeks old) were obtained from the Department of Laboratory Animal Science of Shanghai Jiao Tong University School of Medicine. BHT101, transfected with short hairpin BST2 interferon-stimulated positive regulator (sh-BISPR), and Hth83, transfected with short hairpin negative control (sh-NC), were injected into the back of nude mice. When the tumors grew to 50–100 mm3, the tumor size was measured in triplicate using digital caliper every week. The protocols for these animal experiments were approved by Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine.
Statistical analysis
All data were presented as means ± standard deviation. If the data obeyed normal distribution, two groups of data were analyzed using paired-sample t-test or independent-sample t-test. If the data did not obey normal distribution, Kruskal–Wallis method was used to test. All analyses were performed with SPSS software package (version 17.0, SPSS Inc). All data were bilaterally tested. Value of P < 0.05 in the analysis was considered statistically significant.
Results
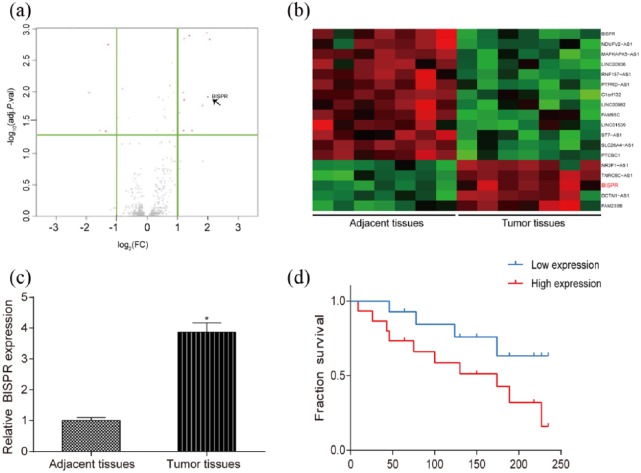
BISPR highly expressed in TPC tissues
The result of microarray showed that BISPR highly expressed in tumor tissues and lowly expressed in adjacent tissues based on the screening criteria of fold change >2 and false-positive rate (FPR) <0.01 (Figure 1(a) and (b)). MRNA expression level of BISPR in tumor tissues was remarkably higher than that in adjacent tissues detected by qRT-PCR (Figure 1(c), P < 0.05). Meanwhile, patients with low expression of BISPR had higher survival time compared with patients with high expression of BISPR (Figure 1(d)).
Figure 1.
BISPR highly expressed in TPC tissues. (a and b) Volcano plot and heat map showed that BISPR expressed significantly in tumor tissues and low in adjacent tissues based on fold change >2 and FPR <0.01. (c) The expression level of BISPR in tumor tissues was higher than that in adjacent tissues. (d) Patients with low expression of BISPR had higher fraction survival compared with patients with high BISPR expression. *P < 0.05, compared with adjacent tissues.
BISPR knockdown counteracted the propagation and invasiveness of TPC cells and stimulated cell apoptosis
QRT-PCR demonstrated that sh-BISPR suppressed BISPR expression in human TPC cell lines BHT101 and Hth83 (Figure 2(a), P < 0.05). Through the iodine uptake assay, we found that sh-BISPR enhanced the iodine uptake ability of BHT101 and Hth83 cells compared with sh-NC group (Figure 2(b), P < 0.05). Moreover, the result of CCK-8 assay indicated that sh-BISPR inhibited the proliferation of BHT101 and Hth83 cells in time-dependent manner (Figure 2(c), P < 0.05). Compared with sh-NC group, more cells of sh-BISPR group were arrested in G0/G1 detected by flow cytometry (Figure 2(d), P < 0.05). The result of cell apoptosis assay indicated that sh-BISPR promoted cell apoptosis of BHT101 and Hth83 cell lines (Figure 2(e), P < 0.05). Similarly, the result of transwell assay suggested that sh-BISPR inhibited invasion ability of BHT101 and Hth83 cells in comparison with sh-NC group (Figure 2(f), P < 0.05). Therefore, BISPR knockdown counteracted the propagation and invasiveness of TPC cells and stimulated cell apoptosis.
Figure 2.
BISPR knockdown inhibited the proliferation and invasion of TPC cells and promoted cells apoptosis. (a) BISPR expression in BHT101 and Hth83 cell lines with sh-BISPR was lower than that in sh-NC group tested by qRT-PCR. (b) The iodine uptake ability of BHT101 and Hth83 cell lines with sh-BISPR was higher than that in sh-NC group. (c) Cell viability in sh-BISPR group was lower than that in sh-NC group examined by CCK-8 assay. (d) Cell cycle of BHT101 and Hth83 cell lines with sh-BISPR was arrested in G0/G1 phase in comparison with sh-NC group measured by flow cytometry. (e) Compared with sh-NC group, cell apoptosis rate in BHT101 and Hth83 cell lines with sh-BISPR was higher than that in sh-NC group detected by cell apoptosis assay. (f) The number of invasive cells in BHT101 and Hth83 cell lines with sh-BISPR was lower than that in sh-NC group tested by transwell assay. *P < 0.05, compared with sh-NC group.
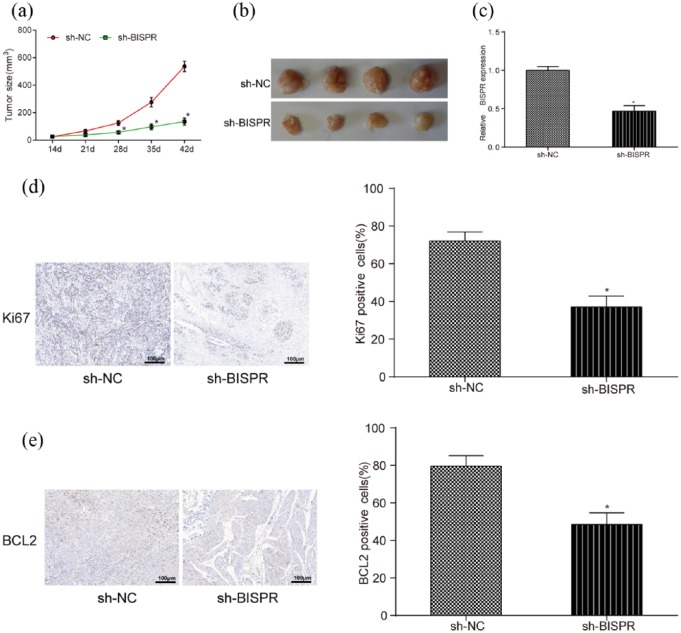
BISPR knockdown inhibited the development of TPC tumors in vivo
In in vivo experiments, sh-BISPR counteracted tumor growth in mice with TPC (Figure 3(a) and (b)). BISPR expression in mice transfected with sh-BISPR was lower than that in mice transfected with sh-NC detected by qRT-PCR (Figure 3(c), P < 0.05). Moreover, the results of immunohistochemistry revealed that sh-BISPR inhibited the expression of Ki67 and Bcl-2, which are maker proteins of cell proliferation in comparison with control group (Figure 3(d) and (e), both P < 0.05).
Figure 3.
The effect of lncRNA BISPR on the growth of TPC cells in vivo. (a and b) The tumor size of mice transfected with sh-BISPR was much smaller than that in sh-NC group since transfection for 28 days. (c) BISPR expression in mice transfected with sh-BISPR was lower than that in sh-NC group. (d and e) Ki67- and Bcl-2-positive cells in mice transfected with sh-BIPSR were less than that in sh-NC group examined by immunohistochemistry. *P < 0.05, compared with sh-NC group.
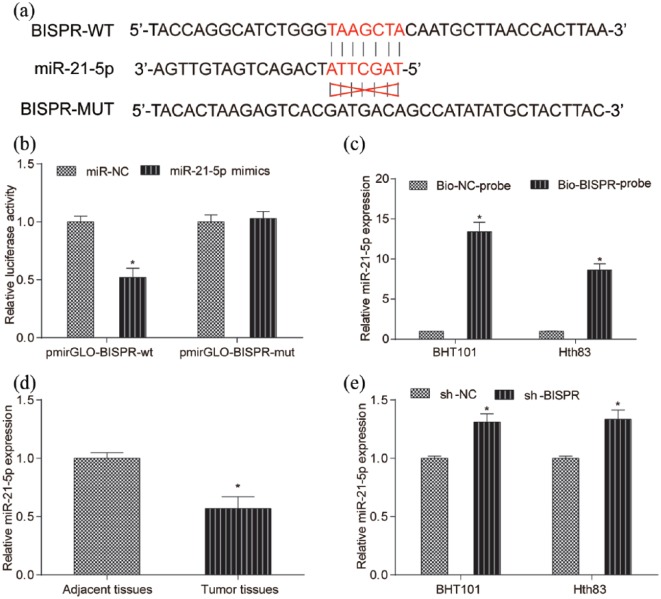
MiR-21-5p directly targeted BISPR
Through bioinformatics, it is predicted that there is target relationship between miR-21-5p and BISPR (Figure 4(a)). Dual luciferase reporter assay and RNA pull down assay verified that miR-21-5p directly targeted BISPR (Figure 4(b) and (c), both P < 0.05). Higher expression of miR-21-5p was observed in tumor tissues through the detection of qRT-PCR (Figure 4(d), P < 0.05). sh-BISPR promoted miR-21-5p expression in BHT101 and Hth83 cell lines (Figure 4(e), P < 0.05). Hence, miR-21-5p directly targeted BISPR.
Figure 4.
MiR-21-5p directly targeted BISPR. (a) TargetScan predicted that there was a binding site of miR-21-5p existing in the 3′-UTR of BISPR. (b) Relative luciferase activity in BISPR-wt and miR-21-5p mimics group was lower than that in BISPR-wt and miR-NC group. There was no significant difference between BISPR-mut and miR-21-5p mimics group and BISPR-mut and miR-NC group. (c) MiR-21-5p expression in BHT101 and Hth83 cell lines with Bio-BISPR-probe was notably higher than that in Bio-NC probe group. (d) MiR-21-5p expression in tumor tissues was lower than that in adjacent tissues. (e) Compared with sh-NC group, miR-21-5p expression in BHT101 and Hth83 cell lines with sh-BISPR was much higher than that in sh-NC group. *P < 0.05, compared with sh-NC group.
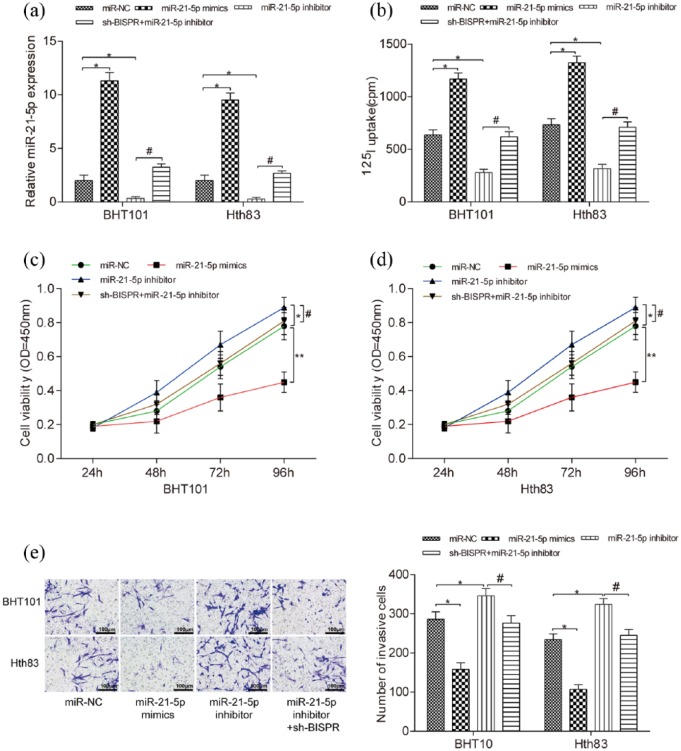
BISPR promoted the development of TPC cells by inhibiting miR-21-5p expression
MiR-21-5p mimics promoted miR-21-5p expression in BHT101 and Hth83 cell lines, while miR-21-5p inhibitor suppressed miR-21-5p expression (Figure 5(a), both P < 0.05). Cell iodine uptake ability was enhanced by miR-21-5p mimics and restrained by miR-21-5p inhibitor compared with miR-NC group, meanwhile sh-BISPR counteracted the negative effect of miR-21-5p on cell iodine uptake ability (Figure 5(b), P < 0.05). CCK-8 assay and transwell assay indicated that miR-21-5p mimics inhibited the propagation (P < 0.01) and invasiveness (P < 0.05) of BHT101 and Hth83 cells, miR-21-5p inhibitor played a positive role on that (Figure 5(c)–(e), P < 0.05). In the meantime, sh-BISPR balanced the positive effect of miR-21-5p inhibitor on the production and aggression of cells. Therefore, BISPR promoted the development of TPC cells by inhibiting miR-21-5p expression.
Figure 5.
BISPR promoted the development of TPC cells by inhibiting miR-21-5p expression. (a) Compared with miR-NC group, the expression level of miR-21-5p in miR-21-5p mimics group was much higher and that in miR-21-5p inhibitor group was much lower. At the meantime, miR-21-5p expression in sh-BISPR + miR-21-5p inhibitor group was much higher than that in miR-21-5p inhibitor group. (b) The iodine uptake of BHT101 and Hth83 cell lines with miR-21-5p mimics was much higher than that in miR-NC group, while that with miR-21-5p inhibitor was lower than that in the miR-NC group. At the same time, the cell iodine uptake in sh-BISPR + miR-21-5p inhibitor group was much higher than that in miR-21-5p inhibitor group. *P < 0.05, compared with miR-NC group; #P < 0.05, compared with miR-21-5p inhibitor group. (c and d) The cell viability of BHT101 and Hth83 cell lines with miR-21-5p mimics was much lower than that in miR-NC group, while that with miR-21-5p inhibitor was higher than that in miR-NC group. However, cell viability in sh-BISPR + miR-21-5p inhibitor group was lower than that in miR-21-5p inhibitor group. *P < 0.05, compared with miR-NC group; **P < 0.01, compared with miR-NC group; #P < 0.05, compared with miR-21-5p inhibitor group. (e) The number of invasive cells in BHT101 and Hth83 cell lines with miR-21-5p mimics was much lower than that in miR-NC group while that with miR-21-5p inhibitor was higher than that in the miR-NC group. However, the number of invasive cells in sh-BISPR + miR-21-5p inhibitor group was lower than that in miR-21-5p inhibitor group. *P < 0.05, compared with miR-NC group; #P < 0.05, compared with miR-21-5p inhibitor group.
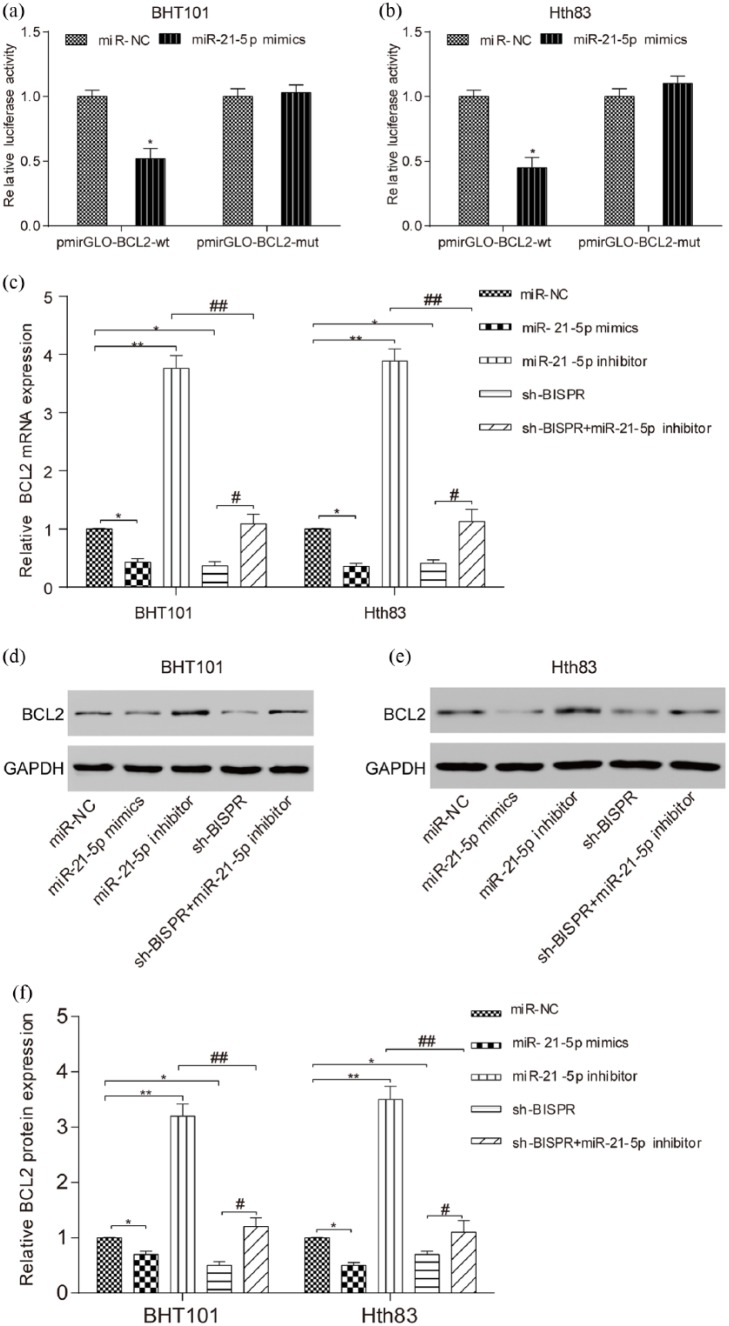
Bcl-2 was suppressed by miR-21-5p and sh-BISPR
The apoptosis-related protein Bcl-2 was predicted to be regulated by miR-21-5p through bioinformatics. Dual luciferase reporter assay manifested the direct relationship between miR-21-5p and Bcl-2 (Figure 6(a) and (b), P < 0.05). In addition, qRT-PCR and Western blot verified that miR-21-5p suppressed Bcl-2 expression, while sh-BISPR inhibited Bcl-2 expression (Figure 6(c) and (d), all P < 0.05, P < 0.01).
Figure 6.
Bcl-2 was suppressed by miR-21-5p and sh-BISPR. (a and b) Relative luciferase activity in Bcl-2 wt and miR-21-5p mimics group was lower than that in Bcl-2 wt and miR-NC group. There was no significant difference between BCL2-mut and miR-21-5p mimics group and Bcl-2-mut and miR-NC group. (c–f) The results of qRT-PCR and Western blot showed that compared with miR-NC group, Bcl-2 mRNA and protein expression in miR-21-5p mimics and sh-BISPR were lower and those in miR-21-5p inhibitor group were much higher. In the meantime, in comparison with sh-BISPR + miR-21-5p inhibitor group, Bcl-2 mRNA and protein expression in miR-21-5p inhibitor group were much higher while those in sh-BISPR group were much lower. *P < 0.05, compared with miR-NC group; **P < 0.01, compared with miR-NC group; #P < 0.05, compared with sh-BISPR + miR-21-5p inhibitor group; ##P < 0.01, compared with sh-BISPR + miR-21-5p inhibitor group.
Discussion
TPC is a common endocrine malignancy with a low morbidity but increasing incidence. Recently, lncRNAs and microRNAs are generally considered to have an effect on TPC cells by aberrant expression. Therefore, in this study, we revealed dysregulation of lncRNA BISPR and miR-21-5p in TPC. We also demonstrated that BISPR stimulated TPC cell growth and weakened their iodine uptake ability, which could be reversed by upregulating miR-21-5p.
LncRNAs have been reported in many researches to aberrantly express in various human cancers, acting as oncogenes or tumor inhibitors. For example, Sun et al.9 revealed that NR_036575.1 was over-expressed in TPC tissues, which restrained cell progression in vitro as well. Some researchers reported the effects of BISPR/BTS-2 on different types of carcinoma. For instance, BTS-2 enhanced cell growth and invasiveness in renal cell carcinoma15 and mammary tumor.14 Concordant with previous research, the results of our experiments showed that BISPR was over-expressed in TPC tissues, which stimulated TPC cell growth. Our study confirmed that lncRNA BISPR improved TPC cell process as a tumor promoter.
In addition, microRNAs were involved in various cellular processes combined with lncRNAs. Accumulating evidence has validated the effect of lncRNAs and microRNAs in various human cancers. According to Wei et al.,24 lncRNA X-inactive specific transcript (XIST) promoted pancreatic cancer proliferation by inhibiting miR-133a. Analogously, Li et al.25 proved that lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) down-regulated miR-146b-5p expression to promote hepatocellular carcinoma cell growth and metastasis. Zhao et al.26 also proved that the effects of TUG1 overexpression on the promotion of Purjinje cell (PC) migration could be reversed by miR-382. MiR-21 has been reported to aid in distinguishing the papillary tumor variants in a selected miRNA panel.27 In our research, experiments were employed to detect the interaction between BISPR and miR-21-5p in TPC cells. The results suggested that miR-21-5p was lowly expressed in TPC tissues and cells, which inhibited TPC cell proliferation and invasion regulated by BISPR. Concordant with our results, Dai et al.28 found that miR-21 expression was significantly lower in patients with TPC recurrence. In contrast, there are some reports stating that miR-21 was up-regulated in TPC cells.20,29 Analogously, Zhang et al.17 verified that miR-21 significantly enhanced proliferation and invasion as well as inhibited the apoptosis of TPC cells by targeting programed cell death 4. On this question, different cell lines may contribute to different results.
Our study identified that lncRNA BISPR and miR-21-5p were responsible for the development of TPC. Nevertheless, there are some limitations existing in this study. First, only two TPC cell lines were selected in our experiments, which is inadequate to determine the negative role of miR-21 in TPC cell process. Second, further studies in the impacts of BISPR and miR-21 on signaling pathway are needed for our comprehensive understandings about the molecular networking of TPC.
In conclusion, our study confirmed that BISPR enhanced the propagation of cancer cells in TPC by inhibiting miR-21-5p expression. The study, therefore, provided new insights into the mechanisms of BISPR in TPC. In the meantime, BISPR and miR-21-5p might be potential diagnostic biomarkers for TPC diagnosis and treatment.
Footnotes
Consent to publish: Consent for publication was obtained from the participants.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the ethical committee of Sun Yat-sen Memorial Hospital, and all participants signed the informed consent.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Zheng H, Wang M, Jiang L, et al. (2016) BRAF-activated long noncoding RNA modulates papillary thyroid carcinoma cell proliferation through regulating thyroid stimulating hormone receptor. Cancer Research and Treatment 48: 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu B, Shao Q, Xie K, et al. (2016) The long non-coding RNA ENST00000537266 and ENST00000426615 influence papillary thyroid cancer cell proliferation and motility. Cellular Physiology and Biochemistry 38: 368–378. [DOI] [PubMed] [Google Scholar]
- 3. Qiu ZL, Shen CT, Sun ZK, et al. (2016) Circulating long non-coding RNAs act as biomarkers for predicting 131I uptake and mortality in papillary thyroid cancer patients with lung metastases. Cellular Physiology and Biochemistry 40: 1377–1390. [DOI] [PubMed] [Google Scholar]
- 4. Yin Y, Hong S, Yu S, et al. (2017) MiR-195 inhibits tumor growth and metastasis in papillary thyroid carcinoma cell lines by targeting CCND1 and FGF2. International Journal of Endocrinology 2017: 6180425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ge MH, Cao J, Wang JY, et al. (2017) Nomograms predicting disease-specific regional recurrence and distant recurrence of papillary thyroid carcinoma following partial or total thyroidectomy. Medicine 96: e7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li T, Yang XD, Ye CX, et al. (2017) Long noncoding RNA HIT000218960 promotes papillary thyroid cancer oncogenesis and tumor progression by upregulating the expression of high mobility group AT-hook 2 (HMGA2) gene. Cell Cycle 16: 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lan X, Sun W, Zhang P, et al. (2016) Downregulation of long noncoding RNA NONHSAT037832 in papillary thyroid carcinoma and its clinical significance. Tumour Biology 37: 6117–6123. [DOI] [PubMed] [Google Scholar]
- 8. Zhang D, Liu X, Wei B, et al. (2017) Plasma lncRNA GAS8-AS1 as a potential biomarker of papillary thyroid carcinoma in Chinese patients. International Journal of Endocrinology 2017: 2645904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun W, Lan X, Wang Z, et al. (2016) Overexpression of long non-coding RNA NR_036575.1 contributes to the proliferation and migration of papillary thyroid cancer. Medical Oncology 33: 102. [DOI] [PubMed] [Google Scholar]
- 10. Xia S, Ji R, Zhan W. (2017) Long noncoding RNA papillary thyroid carcinoma susceptibility candidate 3 (PTCSC3) inhibits proliferation and invasion of glioma cells by suppressing the Wnt/beta-catenin signaling pathway. BMC Neurology 17: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kambara H, Gunawardane L, Zebrowski E, et al. (2014) Regulation of interferon-stimulated gene BST2 by a lncRNA transcribed from a shared bidirectional promoter. Frontiers in Immunology 5: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barriocanal M, Carnero E, Segura V, et al. (2014) Long non-coding RNA BST2/BISPR is induced by IFN and regulates the expression of the antiviral factor Tetherin. Frontiers in Immunology 5: 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galao RP, Le Tortorec A, Pickering S, et al. (2012) Innate sensing of HIV-1 assembly by tetherin induces NFkB-dependent proinflammatory responses. Cell Host & Microbe 12: 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahauad-Fernandez WD, DeMali KA, Olivier AK, et al. (2014) Bone marrow stromal antigen 2 expressed in cancer cells promotes mammary tumor growth and metastasis. Breast Cancer Research 16: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pham QT, Oue N, Yamamoto Y, et al. (2017) The expression of BTS-2 enhances cell growth and invasiveness in renal cell carcinoma. Anticancer Research 37: 2853–2860. [DOI] [PubMed] [Google Scholar]
- 16. Fang KH, Kao HK, Chi LM, et al. (2014) Overexpression of BST2 is associated with nodal metastasis and poorer prognosis in oral cavity cancer. Laryngoscope 124: E354–E360. [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Yang Y, Liu Y, et al. (2014) MicroRNA-21 regulates biological behaviors in papillary thyroid carcinoma by targeting programmed cell death 4. Journal of Surgical Research 189: 68–74. [DOI] [PubMed] [Google Scholar]
- 18. Yeh WL, Lin HY, Huang CY, et al. (2015) Migration-prone glioma cells show curcumin resistance associated with enhanced expression of miR-21 and invasion/anti-apoptosis-related proteins. Oncotarget 6: 37770–37781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeng YL, Zheng H, Chen QR, et al. (2017) Bone marrow-derived mesenchymal stem cells overexpressing MiR-21 efficiently repair myocardial damage in rats. Oncotarget 8: 29161–29173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suresh R, Sethi S, Ali S, et al. (2015) Differential expression of microRNAs in papillary thyroid carcinoma and their role in racial disparity. Journal of Cancer Science and Therapy 7: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang Y, Liao D, Pan L, et al. (2013) Expressions of miRNAs in papillary thyroid carcinoma and their associations with the BRAFV600E mutation. European Journal of Endocrinology 168: 675–681. [DOI] [PubMed] [Google Scholar]
- 22. Sun M, Gao J, Ali T, et al. (2017) Characteristics of Aerococcus viridans isolated from bovine subclinical mastitis and its effect on milk SCC, yield, and composition. Tropical Animal Health and Production 49: 843–849. [DOI] [PubMed] [Google Scholar]
- 23. Gupta A, Jain S, Khurana N, et al. (2016) Expression of p63 and Bcl-2 in malignant thyroid tumors and their correlation with other diagnostic immunocytochemical markers. Journal of Clinical and Diagnostic Research 10: EC04–EC18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei W, Liu Y, Lu Y, et al. (2017) LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. Journal of Cellular Biochemistry 118: 3349–3358. [DOI] [PubMed] [Google Scholar]
- 25. Li C, Miao R, Liu S, et al. (2017) Down-regulation of miR-146b-5p by long noncoding RNA MALAT1 in hepatocellular carcinoma promotes cancer growth and metastasis. Oncotarget 8: 28683–28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao L, Sun H, Kong H, et al. (2017) The lncRNA-TUG1/EZH2 axis promotes pancreatic cancer cell proliferation, migration and EMT phenotype formation through sponging miR-382. Cellular Physiology and Biochemistry 42: 2145–2158. [DOI] [PubMed] [Google Scholar]
- 27. Sheu SY, Grabellus F, Schwertheim S, et al. (2010) Differential miRNA expression profiles in variants of papillary thyroid carcinoma and encapsulated follicular thyroid tumours. British Journal of Cancer 102: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai L, Wang Y, Chen L, et al. (2017) MiR-221, a potential prognostic biomarker for recurrence in papillary thyroid cancer. World Journal of Surgical Oncology 15: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guo Z, Hardin H, Montemayor-Garcia C, et al. (2015) In situ hybridization analysis of miR-146b-5p and miR-21 in thyroid nodules: Diagnostic implications. Endocrine Pathology 26: 157–163. [DOI] [PubMed] [Google Scholar]